Indoleamine 2,3-Dioxygenase Regulates Placental Trophoblast Cell Invasion
Abstract
1. Introduction
2. Results
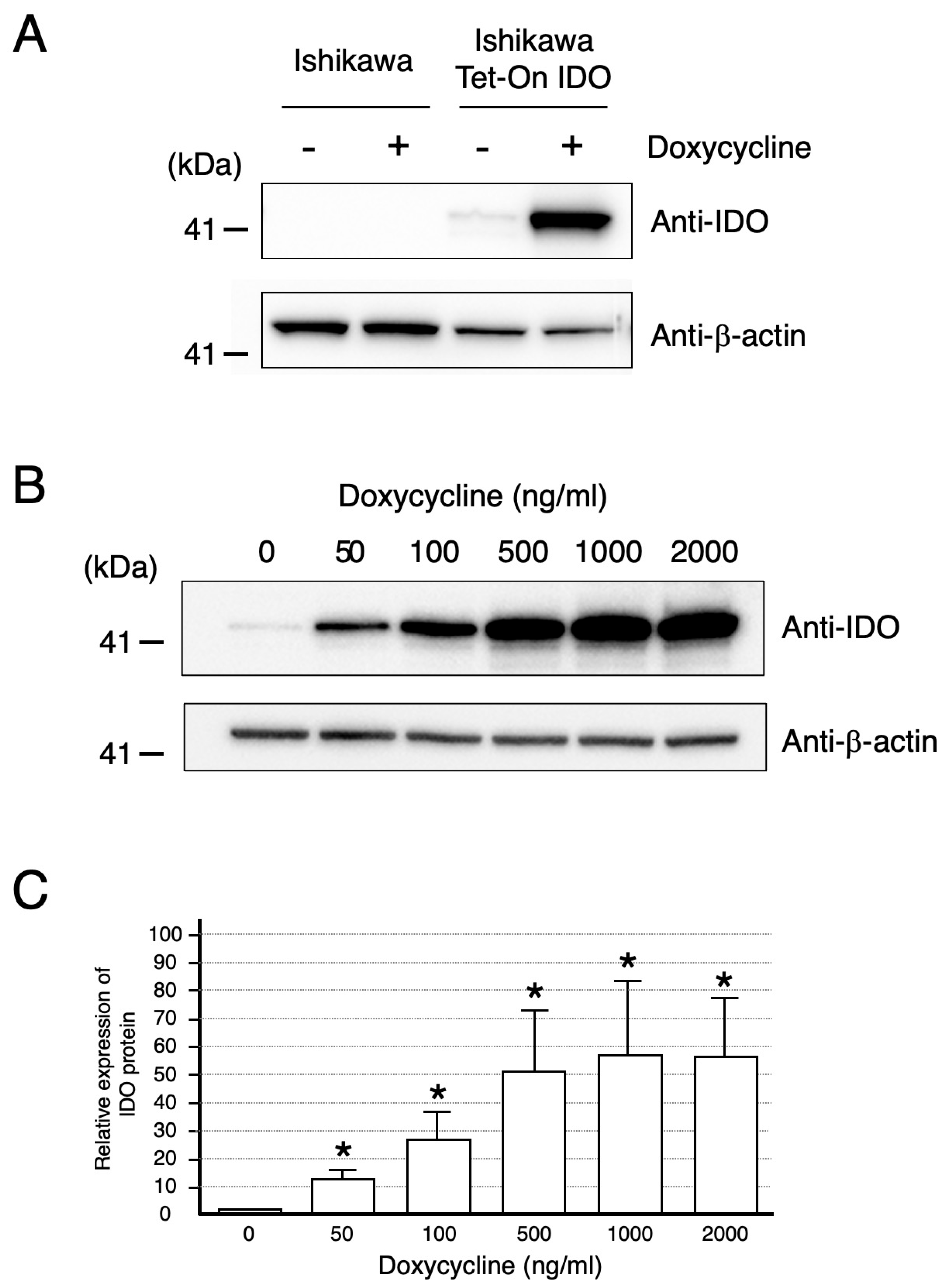
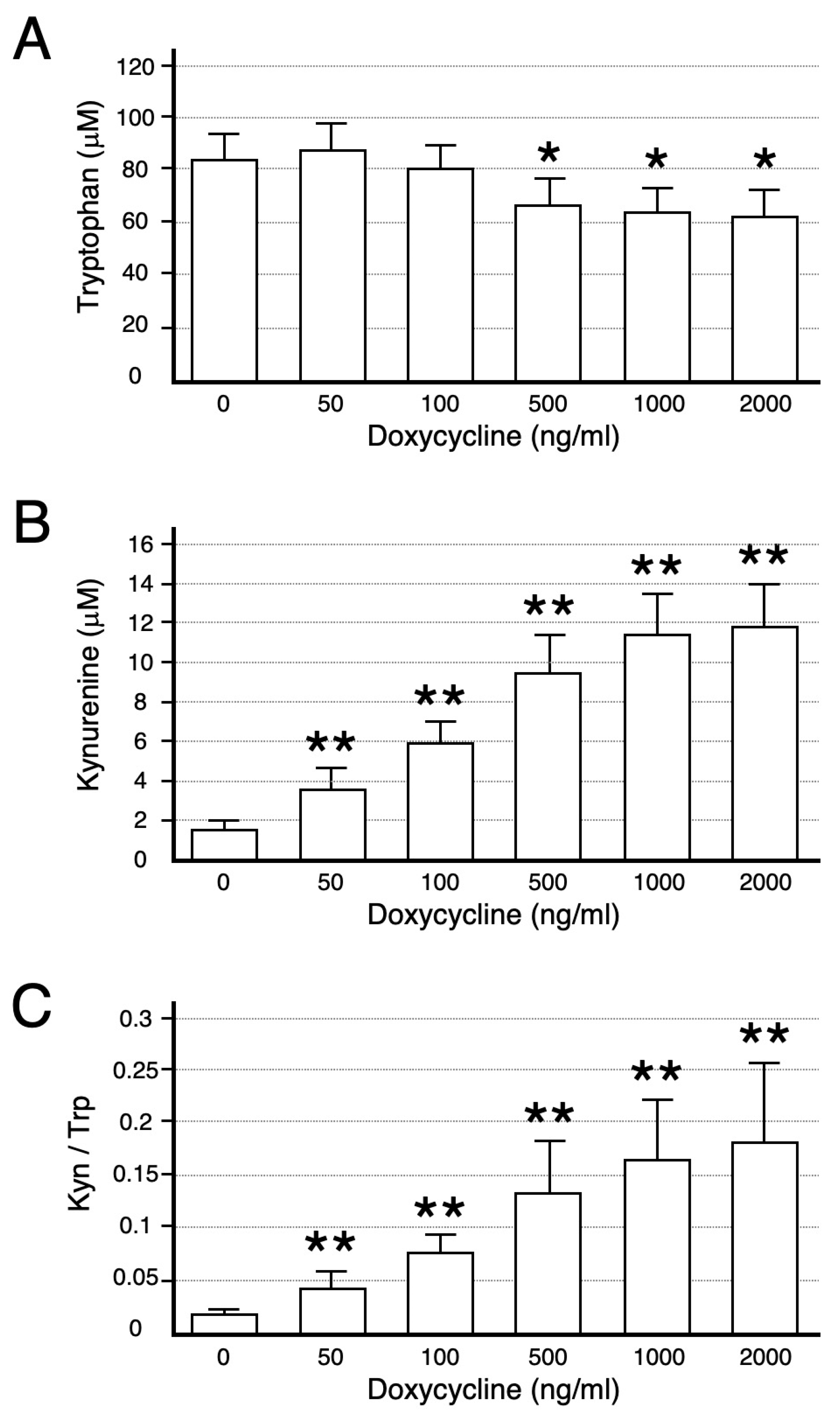
2.1. Ishikawa Cells Expressing IDO
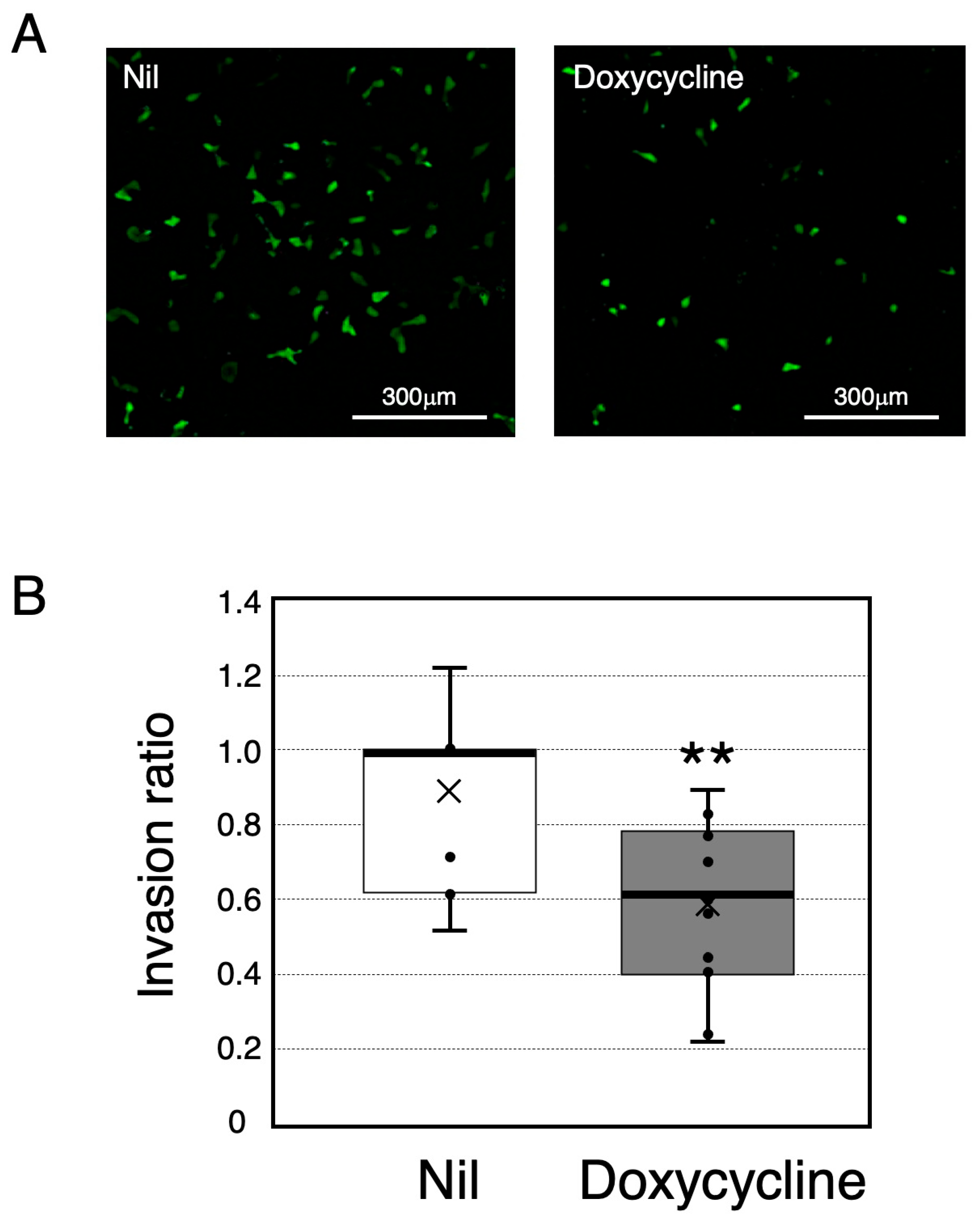
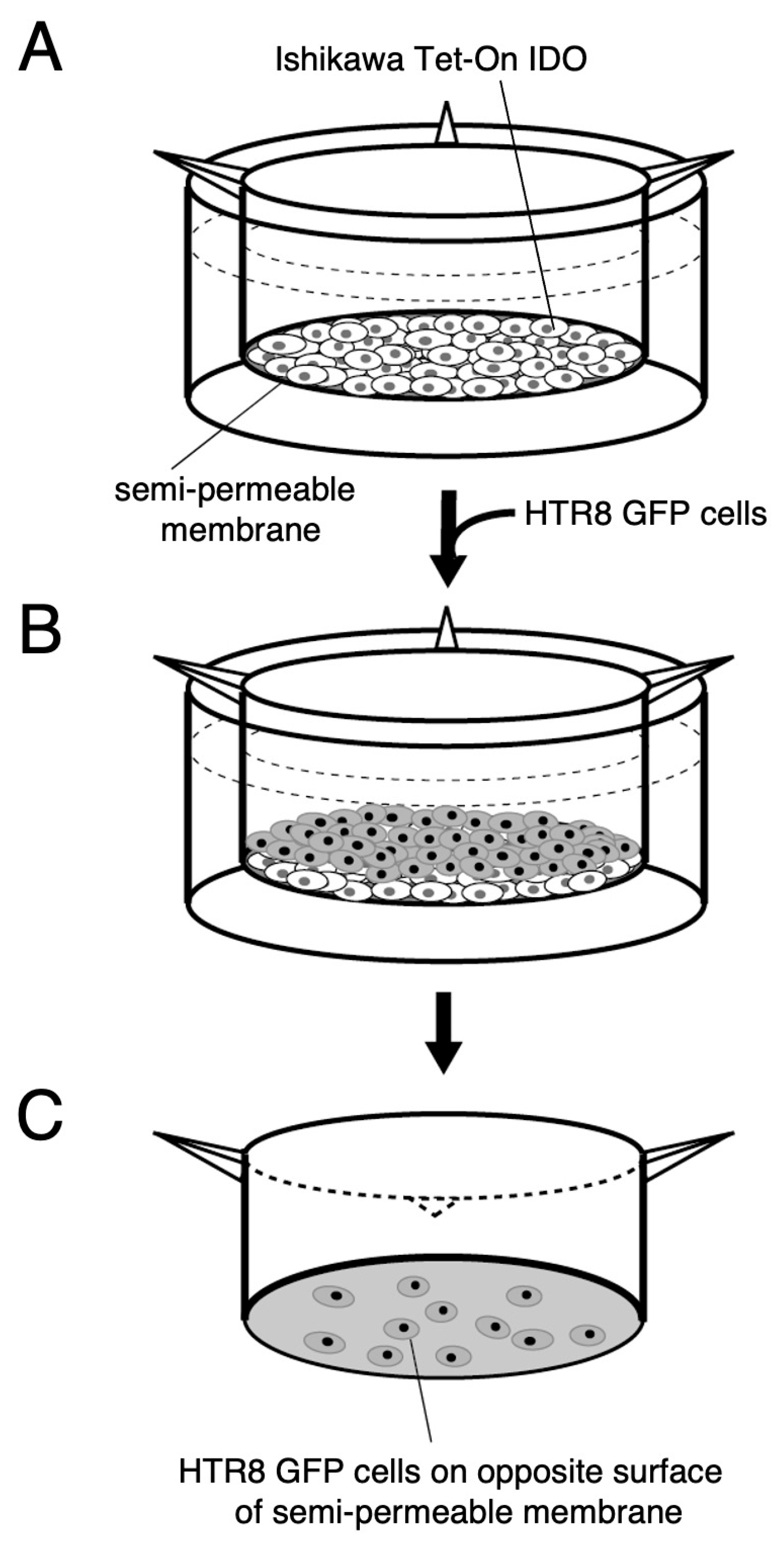
2.2. An Assay for HTR-8/SVneo Cell Invasion
3. Discussion
4. Material and Methods
4.1. Sub-Cloning of IDO Gene into Tet-On Inducible Gene Expression Vector System
4.2. Cell Lines and Stable Transfection
4.3. Western Blot
4.4. Tryptophan Catabolism by IDO
4.5. Invasion Assay
4.6. Statistical Analysis
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jauniaux, E.; Collins, S.; Burton, G.J. Placenta accreta spectrum: Pathophysiology and evidence-based anatomy for prenatal ultrasound imaging. Am. J. Obstet. Gynecol. 2018, 218, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Jauniaux, E.; Jurkovic, D.; Hussein, A.M.; Burton, G.J. New insights into the etiopathology of placenta accreta spectrum. Am. J. Obstet. Gynecol. 2022, 227, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Jauniaux, E.; Burton, G.J. Placenta accreta spectrum: A need for more research on its aetiopathogenesis. BJOG Int. J. Obstet. Gynaecol. 2018, 125, 1449–1450. [Google Scholar] [CrossRef]
- Reister, F.; Frank, H.G.; Kingdom, J.C.; Heyl, W.; Kaufmann, P.; Rath, W.; Huppertz, B. Macrophage-induced apoptosis limits endovascular trophoblast invasion in the uterine wall of preeclamptic women. Lab. Investig. 2001, 81, 1143–1152. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, Y.; Hayaishi, O. Indoleamine 2,3-dioxygenase. Methods Enzymol. 1987, 142, 188–195. [Google Scholar]
- Munn, D.H.; Zhou, M.; Attwood, J.T.; Bondarev, I.; Conway, S.J.; Marshall, B.; Brown, C.; Mellor, A.L. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science 1998, 281, 1191–1193. [Google Scholar] [CrossRef]
- Grohmann, U.; Fallarino, F.; Puccetti, P. Tolerance, DCs and tryptophan: Much ado about IDO. Trends Immunol. 2003, 24, 242–248. [Google Scholar] [CrossRef]
- Mellor, A.L.; Munn, D.H. IDO expression by dendritic cells: Tolerance and tryptophan catabolism. Nat. Rev. Immunol. 2004, 4, 762–774. [Google Scholar] [CrossRef]
- Muller, A.J.; DuHadaway, J.B.; Donover, P.S.; Sutanto-Ward, E.; Prendergast, G.C. Inhibition of indoleamine 2,3-dioxygenase, an immunoregulatory target of the cancer suppression gene Bin1, potentiates cancer chemotherapy. Nat. Med. 2005, 11, 312–319. [Google Scholar] [CrossRef]
- Takikawa, O. Biochemical and medical aspects of the indoleamine 2,3-dioxygenase initiated L-tryptophan metabolism. Biochem. Biophys. Res. Commun. 2005, 338, 12–19. [Google Scholar] [CrossRef]
- Yamazaki, F.; Kuroiwa, T.; Takikawa, O.; Kido, R. Human indolylamine 2,3-dioxygenase. Its tissue distribution, and characterization of the placental enzyme. Biochem. J. 1985, 230, 635–638. [Google Scholar] [CrossRef] [PubMed]
- Kudo, Y.; Boyd, C.A.R.; Spyropoulou, I.; Redman, C.W.G.; Takikawa, O.; Hara, T.; Ohama, K.; Sargent, I.L. Indoleamine 2,3-dioxygenase: Distribution and function in the developing human placenta. J. Reprod. Immunol. 2004, 61, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Sedlmayr, P.; Blaschitz, A.; Wintersteiger, R.; Semlitsch, M.; Hammer, A.; MacKenzie, C.R.; Walcher, W.; Reich, O.; Takikawa, O.; Dohr, G. Localization of indoleamine 2,3-dioxygenase in human female reproductive organs and the placenta. Mol. Hum. Reprod. 2002, 8, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Kudo, Y.; Koh, I.; Yamazaki, T.0; Oomori, Y.; Mukai, Y.; Sugimoto, J. Indoleamine 2,3-dioxygenase and trophoblast invasion in caesarean scar pregnancy: Implications for the aetiopathogenesis of placenta accreta spectrum. J. Reprod. Immunol. 2020, 138, 103099. [Google Scholar] [CrossRef]
- Graham, C.H.; Hawley, T.S.; Hawley, R.G.; MacDougall, J.R.; Kerbel, R.S.; Khoo, N.; Lala, P.K. Establishment and characterization of first trimester human trophoblast cells with extended lifespan. Exp. Cell Res. 1993, 206, 204–211. [Google Scholar] [CrossRef]
- Abbas, Y.; Turco, M.Y.; Burton, G.J.; Moffett, A. Investigation of human trophoblast invasion in vitro. Hum. Reprod. Update 2020, 26, 501–513. [Google Scholar] [CrossRef]
- Nishida, M. The Ishikawa cells from birth to the present. Hum. Cell 2002, 15, 104–117. [Google Scholar] [CrossRef]
- Stone, T.W. Neuropharmacology of quinolinic and kynurenic acids. Pharmacol. Rev. 1993, 45, 309–379. [Google Scholar] [CrossRef]
- Badawy, A.A. The tryptophan utilization concept in pregnancy. Obstet. Gynecol. Sci. 2014, 57, 249–259. [Google Scholar] [CrossRef]
- Badawy, A.A.; Namboodiri, A.M.; Moffett, J.R. The end of the road for the tryptophan depletion concept in pregnancy and infection. Clin. Sci. 2016, 130, 1327–1333. [Google Scholar] [CrossRef]
- King, A.; Burrows, T.; Verma, S.; Hiby, S.; Loke, Y.W. Human uterine lymphocytes. Hum. Reprod. Update 1998, 4, 480–485. [Google Scholar] [CrossRef] [PubMed]
- Jokhi, P.P.; King, A.; Sharkey, A.M.; Smith, S.K.; Loke, Y.W. Screening for cytokine messenger ribonucleic acids in purified human decidual lymphocyte populations by the reverse-transcriptase polymerase chain reaction. J. Immunol. 1994, 153, 4427–4435. [Google Scholar] [CrossRef] [PubMed]
- Ho, H.N.; Chao, K.H.; Chen, C.K.; Yang, Y.S.; Huang, S.C. Activation status of T and NK cells in the endometrium throughout menstrual cycle and normal and abnormal early pregnancy. Hum. Immunol. 1996, 49, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.W.; Feng, G.S. Relationship between interferon-gamma, indoleamine 2,3-dioxygenase, and tryptophan catabolism. FASEB J. 1991, 5, 2516–2522. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kudo, Y.; Sugimoto, J. Indoleamine 2,3-Dioxygenase Regulates Placental Trophoblast Cell Invasion. Int. J. Mol. Sci. 2025, 26, 5889. https://doi.org/10.3390/ijms26125889
Kudo Y, Sugimoto J. Indoleamine 2,3-Dioxygenase Regulates Placental Trophoblast Cell Invasion. International Journal of Molecular Sciences. 2025; 26(12):5889. https://doi.org/10.3390/ijms26125889
Chicago/Turabian StyleKudo, Yoshiki, and Jun Sugimoto. 2025. "Indoleamine 2,3-Dioxygenase Regulates Placental Trophoblast Cell Invasion" International Journal of Molecular Sciences 26, no. 12: 5889. https://doi.org/10.3390/ijms26125889
APA StyleKudo, Y., & Sugimoto, J. (2025). Indoleamine 2,3-Dioxygenase Regulates Placental Trophoblast Cell Invasion. International Journal of Molecular Sciences, 26(12), 5889. https://doi.org/10.3390/ijms26125889




