An Experimental Rat Model for Simultaneous Induction of Peripheral Neuropathy and Myelotoxicity by Docetaxel Administration: Evaluating the Protective Role of Dimethyl Fumarate
Abstract
1. Introduction
2. Results
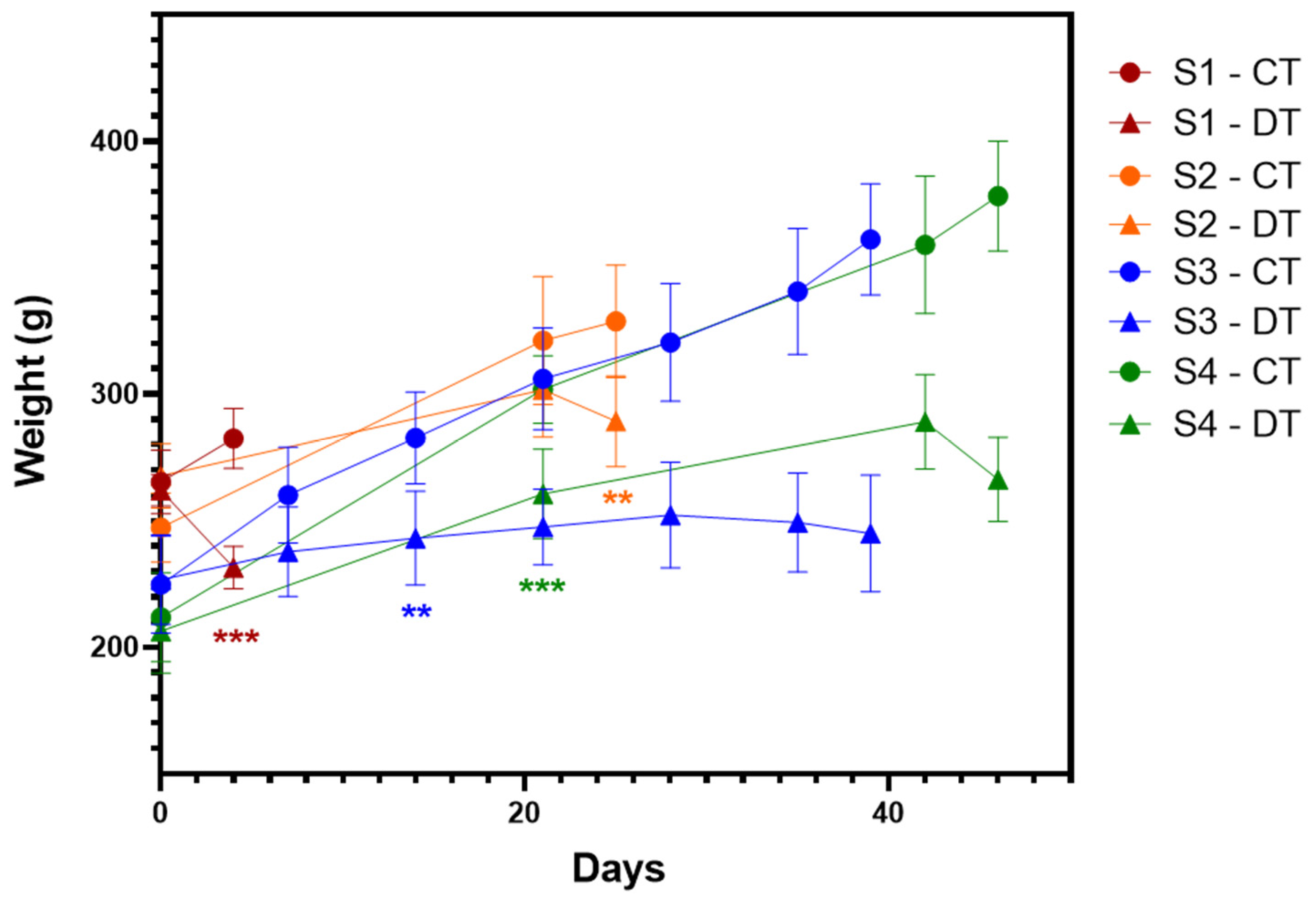
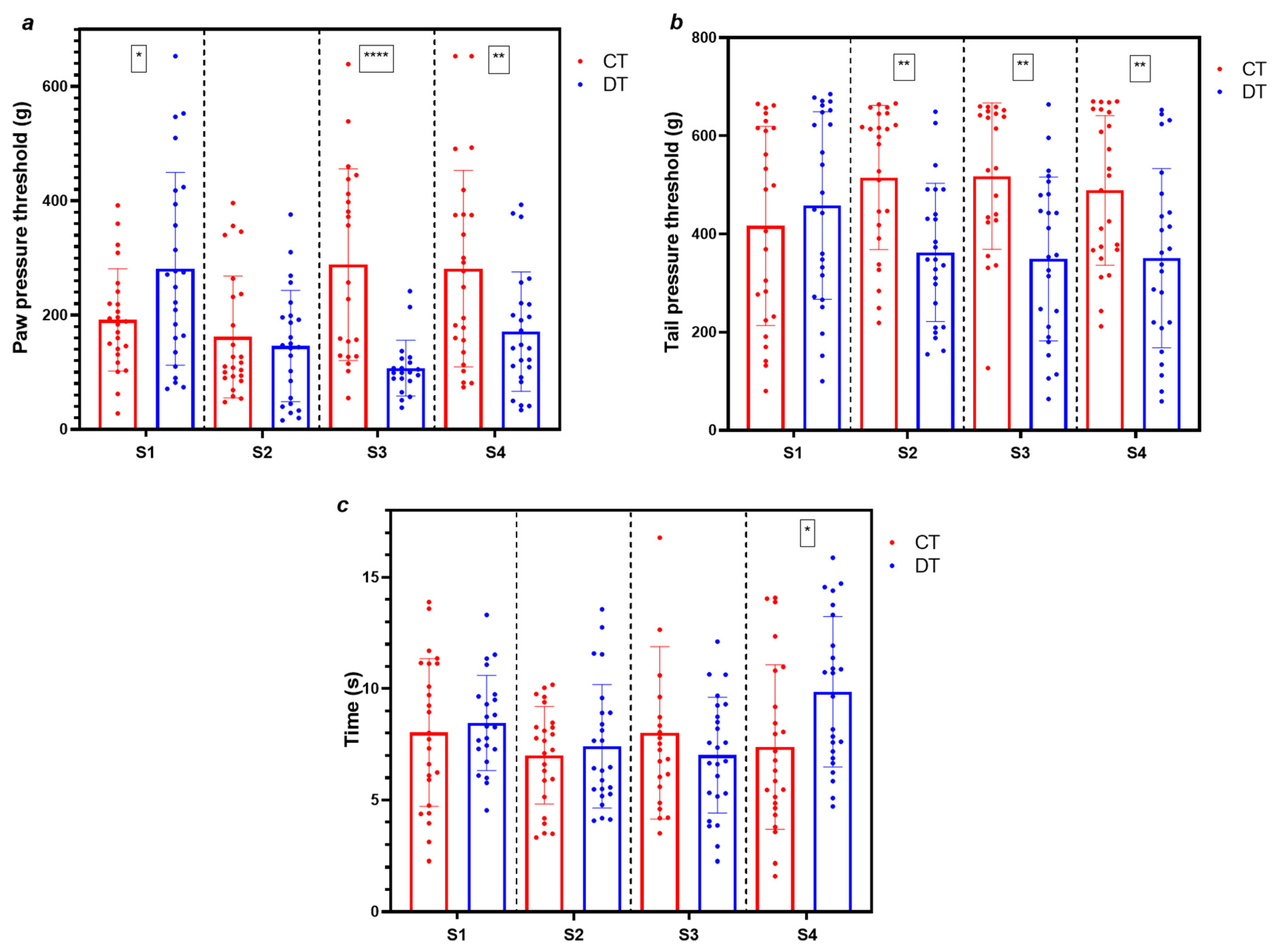
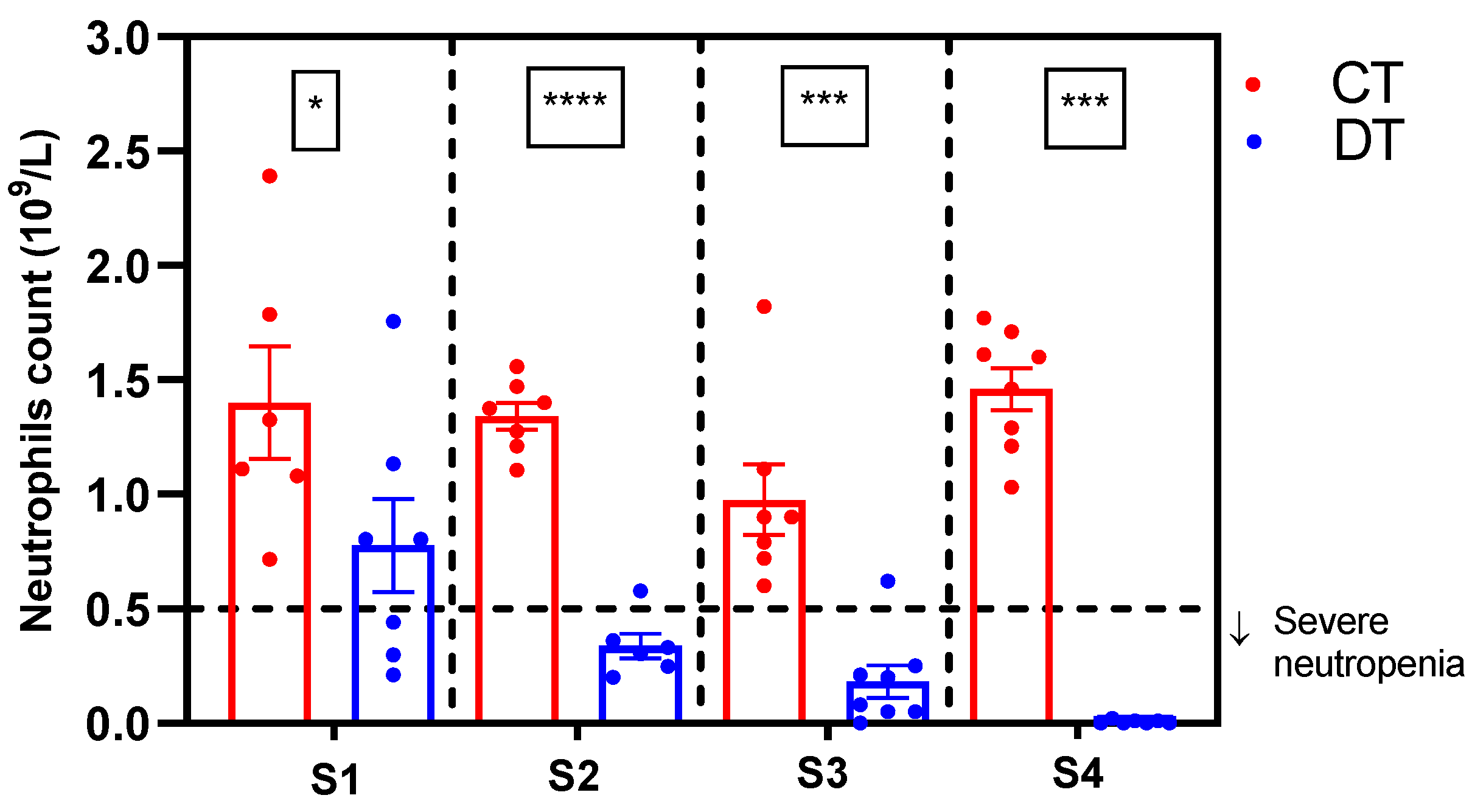
2.1. Simultaneous Induction of Nociceptive Alterations and Neutropenia Using Docetaxel
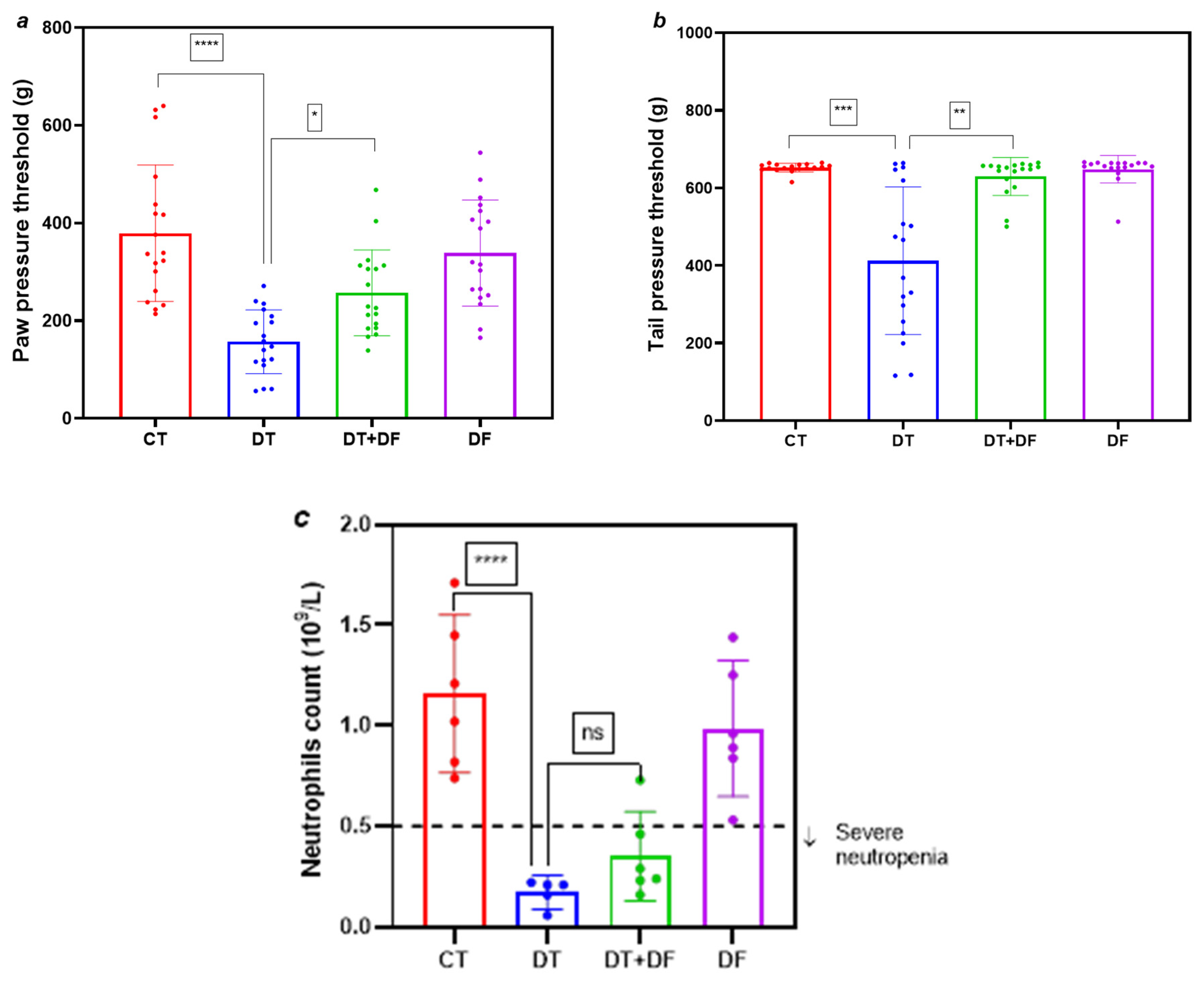
2.2. Protective Role of Dimethyl Fumarate Against Docetaxel-Induced Nociceptive Alterations and Neutropenia
2.2.1. Nociceptive Alterations and Neutropenia
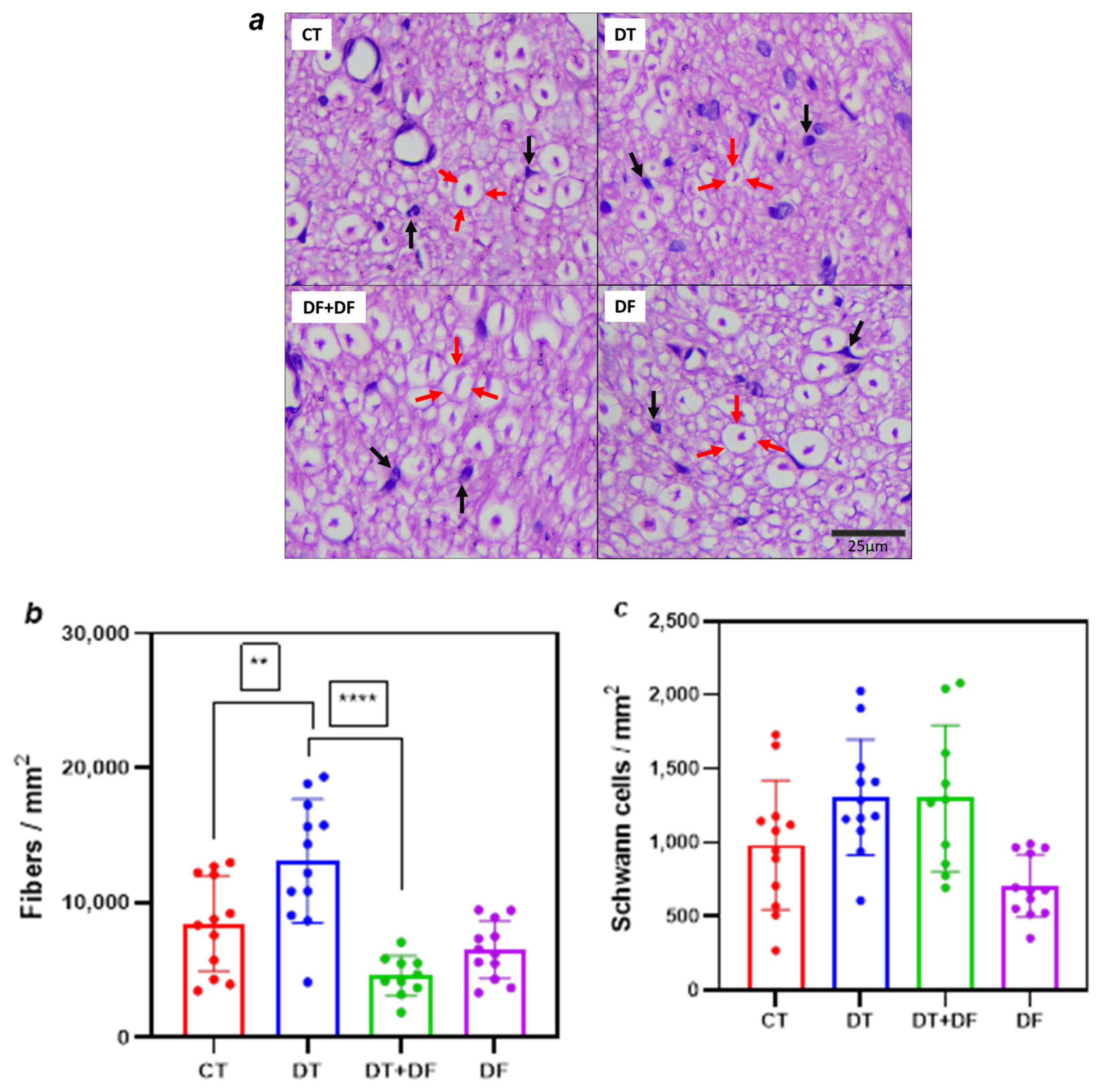
2.2.2. Histopathological Examination
2.3. Effect of Dimethyl Fumarate and Docetaxel Combinations in Prostate Cancer Cell Lines
3. Discussion
4. Materials and Methods
4.1. Drugs
4.2. Animals
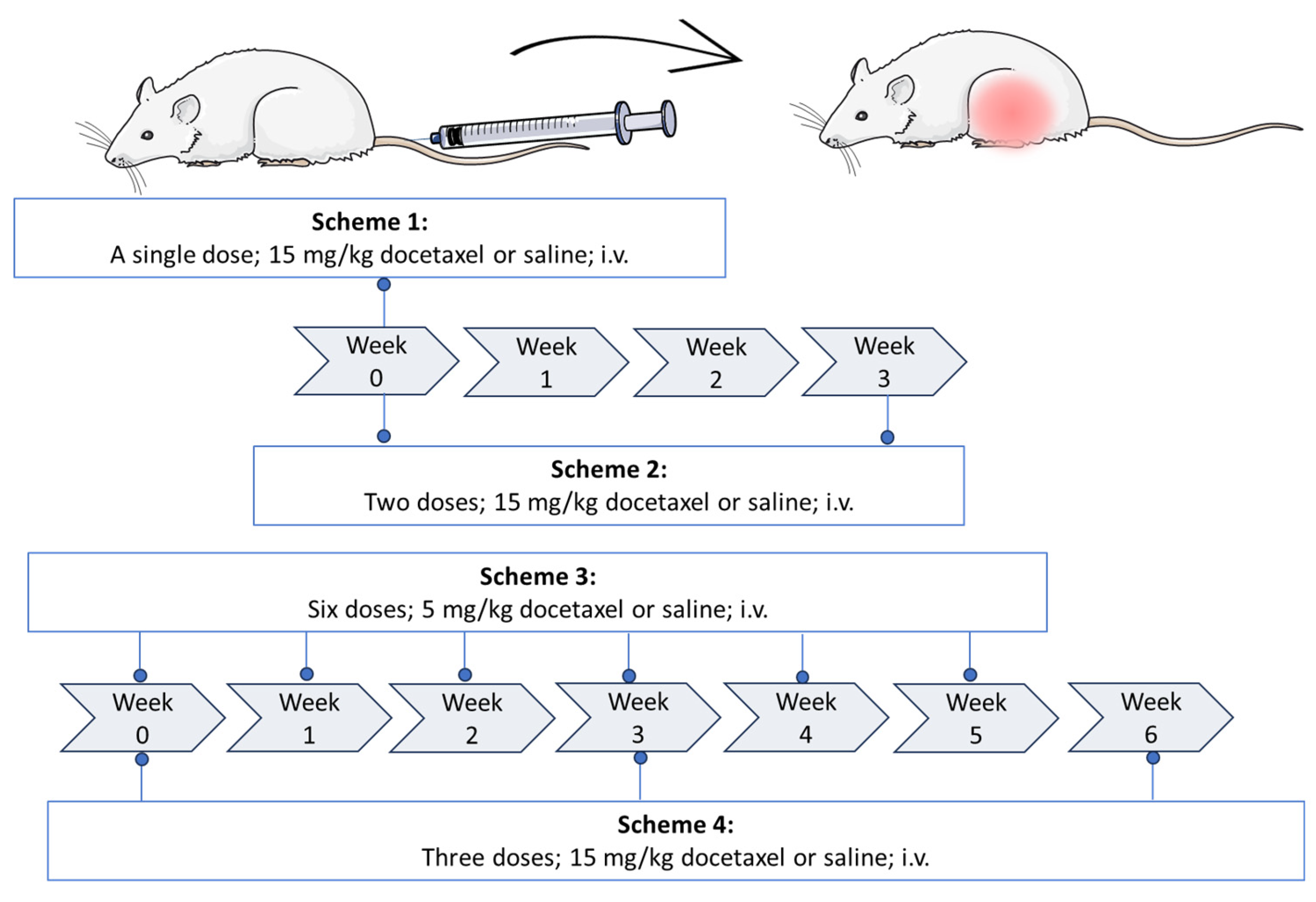
4.3. Establishment of the Docetaxel Damage Model
4.4. Behavioral Testing, Euthanasia, and Blood Cell Count
4.5. Hemograms and Blood Biochemistry
4.6. Assessment of Dimethyl Fumarate Protection
4.7. Histopathology
4.8. Cell Culture
4.9. Crystal Violet Assay
4.10. Drug Combination Schemes
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- New, P.Z.; Jackson, C.E.; Rinaldi, D.; Burris, H.; Barohn, R.J. Peripheral neuropathy secondary to docetaxel (Taxotere). Neurology 1996, 46, 108–111. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Zhang, W.; Dong, B.; Xin, Z.; Ji, Y.; Su, R.; Shen, K.; Pan, J.; Wang, Q.; Xue, W. Docetaxel remodels prostate cancer immune microenvironment and enhances checkpoint inhibitor-based immunotherapy. Theranostics 2022, 12, 4965–4979. [Google Scholar] [CrossRef] [PubMed]
- Cortes, J.E.; Pazdur, R. Docetaxel. J. Clin. Oncol. 1995, 13, 2643–2645. [Google Scholar] [CrossRef] [PubMed]
- Schweizer, M.; Antonarakis, E. Chemotherapy and its evolving role in the management of advanced prostate cancer. Asian J. Androl. 2014, 16, 334–340. [Google Scholar] [CrossRef]
- Ho, M.Y.; Mackey, J.R. Presentation and management of docetaxel-related adverse effects in patients with breast cancer. Cancer Manag. Res. 2014, 6, 253–259. [Google Scholar] [CrossRef]
- Perez, A.M.; Haberland, N.I.; Miteva, M.; Wikramanayake, T.C. Chemotherapy-Induced Alopecia by Docetaxel: Prevalence, Treatment and Prevention. Curr. Oncol. 2024, 31, 5709–5721. [Google Scholar] [CrossRef]
- Velasco, R.; Bruna, J. Taxane-induced peripheral neurotoxicity. Toxics 2015, 3, 152–169. [Google Scholar] [CrossRef]
- Schneider, B.P.; Zhao, F.; Ballinger, T.J.; Garcia, S.F.; Shen, F.; Virani, S.; Cella, D.; Bales, C.; Jiang, G.; Hayes, L.; et al. ECOG-ACRIN EAZ171: Prospective Validation Trial of Germline Predictors of Taxane-Induced Peripheral Neuropathy in Black Women with Early-Stage Breast Cancer. J. Clin. Oncol. 2024, 42, 2899–2907. [Google Scholar] [CrossRef]
- Desforges, A.D.; Hebert, C.M.; Spence, A.L.; Reid, B.; Dhaibar, H.A.; Cruz-Topete, D.; Cornett, E.M.; Kaye, A.D.; Urits, I.; Viswanath, O. Treatment and diagnosis of chemotherapy-induced peripheral neuropathy: An update. Biomed. Pharmacother. 2022, 147, e112671. [Google Scholar] [CrossRef]
- Jordan, B.; Margulies, A.; Cardoso, F.; Cavaletti, G.; Haugness, H.S.; Jahn, P.; Le Rhun, E.; Preusser, M.; Scotté, F.; Taphoorn, M.J.B.; et al. Systemic anticancer therapy-induced peripheral and central neurotoxicity: ESMOeEONSeEANO Clinical Practice Guidelines for diagnosis, prevention, treatment and follow-up. Ann. Oncol. 2020, 31, 1306–1319. [Google Scholar] [CrossRef]
- Loprinzi, C.L.; Lacchetti, C.; Bleeker, J.; Cavaletti, G.; Chauhan, A.; Hertz, D.L.; Kelley, M.R.; Lavino, A.; Lustberg, M.B.; Paice, J.A.; et al. Prevention and Management of Chemotherapy-Induced Peripheral Neuropathy in Survivors of Adult Cancers: ASCO Guideline Update. J. Clin. Oncol. 2020, 38, 3325–3348. [Google Scholar] [CrossRef] [PubMed]
- Blayney, D.W.; Mohanlal, R.; Adamchuk, H.; Kirtbaya, D.V.; Chen, M.; Du, L.; Ogenstad, S.; Ginn, G.; Huang, L.; Zhang, Q. Efficacy of Plinabulin vs Pegfilgrastim for Prevention of Docetaxel-Induced Neutropenia in Patients with Solid Tumors: A Randomized Clinical Trial. JAMA Netw. Open 2022, 5, e2145446. [Google Scholar] [CrossRef] [PubMed]
- Dale, D.C. How I diagnose and treat neutropenia. Curr. Opin. Hematol. 2016, 23, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Dale, D.C. Advances in the treatment of neutropenia. Curr. Opin. Support. Palliat. Care 2009, 3, 207–212. [Google Scholar] [CrossRef]
- Lee, J.; Lee, J.E.; Kim, Z.; Han, S.W.; Hur, S.M.; Yong, S.Y.; Lee, M.H.; Lim, C.W. Pegfilgrastim for primary prophylaxis of febrile neutropenia in breast cancer patients undergoing TAC chemotherapy. Ann. Surg. Treat. Res. 2018, 94, 223–228. [Google Scholar] [CrossRef]
- Tonra, J.R.; Lloyd, G.K.; Mohanlal, R.; Huang, L. Plinabulin ameliorates neutropenia induced by multiple chemotherapies through a mechanism distinct from G-CSF therapies. Cancer Chemother. Pharmacol. 2020, 85, 461–468. [Google Scholar] [CrossRef]
- Sangineto, M.; Grabherr, F.; Adolph, T.E.; Grander, C.; Reider, S.; Jaschke, N.; Mayr, L.; Schwärzler, J.; Dallio, M.; Moschen, A.R.; et al. Dimethyl fumarate ameliorates hepatic inflammation in alcohol related liver disease. Liver Int. 2020, 40, 1610–1619. [Google Scholar] [CrossRef]
- Jiahe, L.; Ma, J.; Lacagnina, M.J.; Sabina, L.; Odem, M.A.; Walters, E.T.; Kavelaars, A.; Grace, P.M. Oral dimethyl fumarate reduces peripheral neuropathic pain in rodents via NFe2L2 antioxidant signaling. Anesthesiology 2020, 132, 343–356. [Google Scholar] [CrossRef]
- Sánchez-Sanz, A.; Coronado-Albi, M.J.; Muñoz-Viana, R.; García-Merino, A.; Sánchez-López, A.J. Neuroprotective and Anti-Inflammatory Effects of Dimethyl Fumarate, Monomethyl Fumarate, and Cannabidiol in Neurons and Microglia. Int. J. Mol. Sci. 2024, 25, 13082. [Google Scholar] [CrossRef]
- Al-Jaderi, Z.; Maghazachi, A.A. Utilization of dimethyl fumarate and related molecules for treatment of multiple sclerosis, cancer, and other diseases. Front. Inmunol. 2016, 7, e278. [Google Scholar] [CrossRef]
- Singh, J.; Thapliyal, S.; Kumar, A.; Paul, P.; Kumar, N.; Bisht, M.; Naithani, M.; Rao, S.; Handu, S.S. Dimethyl Fumarate Ameliorates Paclitaxel-Induced Neuropathic Pain in Rats. Cureus 2022, 14, e28818. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Li, H.; Fan, S.; Wang, K.; Cui, Z.; Zhao, X.; Sun, X.; Lin, M.; Li, J.; Gao, Y.; et al. Dimethyl fumarate promotes the degradation of HNF1B and suppresses the progression of clear cell renal cell carcinoma. Cell Death Dis. 2025, 16, 71. [Google Scholar] [CrossRef] [PubMed]
- Barut, E.N.; Engin, S.; Öz, E.; Reis, R. Dimethyl Fumarate Attenuates Cyclophosphamide-Induced Bladder Damage and Enhances Cytotoxic Activity Against SH-SY5Y Cells. J. Biochem. Mol. Toxicol. 2025, 39, e70212. [Google Scholar] [CrossRef]
- Nicolay, J.P.; Melchers, S.; Albrecht, J.D.; Assaf, C.; Dippel, E.; Stadler, R.; Wehkamp, U.; Wobser, M.; Zhao, J.; Burghaus, I.; et al. Dimethyl fumarate treatment in relapsed and refractory cutaneous T-cell lymphoma: A multicenter phase 2 study. Blood 2023, 142, 794–805. [Google Scholar] [CrossRef] [PubMed]
- Goñi, A.C.L.; Blanco, D.; Peña, A.; Ronda, M.; Gonzales, B.O.; Arteaga, M.E.; Bada, A.M.; Gonzales, Y.; Mancebo, A. Hematological and biochemical parameters in Sprague Dawley laboratory rats breed in CENPALAB, Cenp: SPRD. Rev. Electron. Vete. 2011, 12, 1–10. [Google Scholar]
- Giknis, M.L.A.; Clifford, C.B. Clinical Laboratory Parameters for CrI: WI (Han) Rats; Charles River Laboratories: Wilmington, MA, USA, 2008; pp. 1–17. [Google Scholar]
- Martini, A.; Parikh, A.B.; Sfakianos, J.P.; Montorsi, F.; Galsky, M.D.; Oh, W.K.; Tsao, C.-K. Predicting toxicity-related docetaxel discontinuation and overall survival in metastatic castration-resistant prostate cancer: A pooled analysis of open phase 3 clinical trial data. Prostate Cancer Prostatic Dis. 2021, 24, 743–749. [Google Scholar] [CrossRef]
- Zhao, M.; Su, M.; Lin, X.; Luo, Y.; He, H.; Cai, C.; Tang, X. Evaluation of docetaxel-loaded intravenous lipid emulsion: Pharmacokinetics, tissue distribution, antitumor activity, safety and toxicity. Pharm. Res. 2010, 27, 1687–1702. [Google Scholar] [CrossRef]
- Kim, S.T.; Kyung, E.J.; Suh, J.S.; Lee, H.S.; Lee, J.H.; Chae, S.I.; Park, E.S.; Chung, Y.H.; Bae, J.; Lee, T.J.; et al. Phosphatidylcholine attenuated docetaxel-induced peripheral neurotoxicity in rats. Drug Chem. Toxicol. 2018, 41, 476–485. [Google Scholar] [CrossRef]
- Hirata, T.; Ozaki, S.; Tabata, M.; Iwamoto, T.; Hinotsu, S.; Hamada, A.; Motoki, T.; Nogami, T.; Shien, T.; Taira, N.; et al. A multicenter study of docetaxel at a dose of 100 mg/m2 in japanese patients with advanced or recurrent breast cancer. Intern. Med. 2021, 60, 1183–1190. [Google Scholar] [CrossRef]
- Feng, S.-Q.; Wang, G.-J.; Zhang, J.-W.; Xie, Y.; Sung, R.-B.; Fei, F.; Huang, J.-Q.; Wang, Y.; AA, J.-Y.; Zhou, F. Combined treatment with apatinib and docetaxel in A549 xenograft mice and its cellular pharmacokinetic basis. Acta Pharmacol. Sin. 2018, 39, 1670–1680. [Google Scholar] [CrossRef]
- Semb, K.A.; Aamdal, S.; Oian, P. Capillary protein leak syndrome appears to explain fluid retention in cancer patients who receive docetaxel treatment. J. Clin. Oncol. 1998, 16, 3426–3432. [Google Scholar] [CrossRef]
- Deuis, J.R.; Dvorakova, L.S.; Vetter, I. Methods used to evaluate pain behaviors in rodents. Front. Mol. Neurosci. 2017, 10, e284. [Google Scholar] [CrossRef] [PubMed]
- Frederiks, C.N.; Lam, S.W.; Guchelaar, H.J.; Boven, E. Genetic polymorphisms and paclitaxel- or docetaxel-induced toxicities: A systematic review. Cancer Treat. Rev. 2015, 41, 935–950. [Google Scholar] [CrossRef] [PubMed]
- Branda, R.F.; Powden, C.; Brooks, E.M.; Yildirim, Z.; Naud, S.J.; McCormack, J.J. Vitamin E but not St. John’s wort mitigates leukopenia caused by cancer chemotherapy in rats. Transl. Res. 2006, 148, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Miyagi, A.; Kawashiri, T.; Shimizu, S.; Shigematsu, N.; Kobayashi, D.; Shimazoe, T. Dimethyl Fumarate Attenuates Oxaliplatin-Induced Peripheral Neuropathy without Affecting the Anti-tumor Activity of Oxaliplatin in Rodents. Biol. Pharm. Bull. 2019, 42, 638–644. [Google Scholar] [CrossRef]
- Persohn, E.; Canta, A.; Schoepfer, S.; Traebert, M.; Mueller, L.; Gilardini, A.; Galbiati, S.; Nicolini, G.; Scuteri, A.; Lanzami, F.; et al. Morphological and morphometric analysis of paclitaxel and docetaxel-induced peripheral neuropathy in rats. Eur. J. Cancer 2005, 41, 1460–1466. [Google Scholar] [CrossRef]
- Kawashiri, T.; Miyagi, A.; Shimizu, S.; Shigematsu, N.; Kobayashi, D.; Shimazoe, T. Dimethyl fumarate ameliorates chemotherapy agent-induced neurotoxicity in vitro. J. Pharmacol. Sci. 2018, 137, 202–211. [Google Scholar] [CrossRef]
- Jindam, A.; Kalvala, A.K.; Arruri, V.K.; Das, S.; Kumar, A. Dimethyl fumarate improves nuclear factor erythroid-related factor 2-mediated antioxidant response to ameliorate functional and molecular deficits in experimental diabetic neuropathy. Indian J. Pharmacol. 2024, 56, 386–395. [Google Scholar] [CrossRef]
- Hassanzadeh, E.; Pashaki, A.S.; Hamed, E.A.; Mehrpooya, M.; Mohammadian, K.; Bayani, R.; Sheikhi, K.; Ranjbar, H.; Abbasi, M. Evaluating N-acetylcysteine as a Protective Agent Against Chemotherapy-induced Neuropathy in Breast Cancer. Am. J. Clin. Oncol. 2025, 48, 122–126. [Google Scholar] [CrossRef]
- Petrikonis, K.; Bernatoniene, J.; Kopustinskiene, D.M.; Casale, R.; Davinelli, S.; Saso, L. The Antinociceptive Role of Nrf2 in Neuropathic Pain: From Mechanisms to Clinical Perspectives. Pharmaceutics 2024, 16, 1068. [Google Scholar] [CrossRef]
- Smith, J.A.; Slusher, B.S.; Wozniak, K.M.; Farah, M.H.; Smiyun, G.; Wilson, L.; Feinstein, S.; Jordan, M.A. Structural basis for induction of peripheral neuropathy by microtubule-targeting cancer drugs. Cancer Res. 2016, 76, 5115–5123. [Google Scholar] [CrossRef] [PubMed]
- Betticher, D.C.; Delmore, G.; Breitenstein, U.; Anchisi, S.; Zimmerli-Schwab, B.; Müller, A.; Moos, R.V.; Hügli-Dayer, A.M.; Schefer, H.; Bodenmann, S.; et al. Efficacy and tolerability of two scalp cooling systems for the prevention of alopecia associated with docetaxel treatment. Support. Care Cancer 2013, 21, 2565–2573. [Google Scholar] [CrossRef] [PubMed]
- Haslam, I.S.; Jadkauskaite, L.; Szabó, I.L.; Staege, S.; Hesebeck-Brinckmann, J.; Jenkins, G.; Bhogal, R.K.; Lim, F.-L.; Farjo, N.; Farjo, B.; et al. Oxidative Damage Control in a Human (Mini-) Organ: Nrf2 Activation Protects against Oxidative Stress-Induced Hair Growth Inhibition. J. Investig. Dermatol. 2017, 137, 295–304. [Google Scholar] [CrossRef] [PubMed]
- National Research Council (US). Guide for the Care and Use of Laboratory Animals. In Guide for the Care and Use of Laboratory Animals, 8th ed.; The National Academies Press: Washington, DC, USA, 2011. [Google Scholar] [CrossRef]
- Tabernero, J.; Climent, M.A.; Lluch, A.; Albanell, J.; Vermorken, J.B.; Barnadas, A.; Antón, A.; Laurent, C.; Mayordomo, J.I.; Estaun, N.; et al. A multicentre, randomised phase II study of weekly or 3-weekly docetaxel in patients with metastatic breast cancer. Ann. Oncol. 2004, 15, 1358–1365. [Google Scholar] [CrossRef]
- Bria, E.; Cuppone, F.; Ciccarese, M.; Nistico, C.; Facciolo, F.; Milella, M.; Izzo, F.; Terzoli, E.; Cognetti, F.; Giannarelli, D. Weekly docetaxel as second line chemotherapy for advanced non-small-cell lung cancer: Meta-analysis of randomized trials. Cancer Treat. Rev. 2006, 32, 583–587. [Google Scholar] [CrossRef]
- Food and Drug Administration (FDA). Estimating the Maximum Safe Starting Dose in Initial Clinical Trials for Therapeutics in Adult Healthy Volunteers; FDA: Silver Spring, MD, USA, 2005. Available online: https://www.fda.gov/media/72309/download (accessed on 24 May 2023).
- Charan, J.; Kantharia, N. How to calculate sample size in animal studies? J. Pharmacol. Pharmacother. 2013, 4, 303–306. [Google Scholar] [CrossRef]
- Dwivedi, D.K.; Jena, G.B. Diethylnitrosamine and thioacetamide-induced hepatic damage and early carcinogenesis in rats: Role of Nrf2 activator dimethyl fumarate and NLRP3 inhibitor glibenclamide. Biochem. Biophys. Res. Commun. 2020, 522, 381–387. [Google Scholar] [CrossRef]
- Lal, R.; Dhaliwal, J.; Dhaliwal, N.; Dharavath, R.N.; Chopra, K. Activation of the Nrf2/HO-1 signaling pathway by dimethyl fumarate ameliorates complete Freund’s adjuvant-induced arthritis in rats. Eur. J. Pharmacol. 2021, 899, e174044. [Google Scholar] [CrossRef]
- Negrette-Guzmán, M.; Huerta-Yepez, S.; Vega, M.I.; León-Contreras, J.C.; Hernández-Pando, R.; Medina-Campos, O.N.; Rodríguez, E.; Tapia, E.; Pedraza-Chaverri, J. Sulforaphane induces differential modulation of mitochondrial biogenesis and dynamics in normal cells and tumor cells. Food Chem. Toxicol. 2017, 100, 90–102. [Google Scholar] [CrossRef]
- Chou, T.C. Theoretical Basis, Experimental Design, and Computerized Simulation of Synergism and Antagonism in Drug Combination Studies. Pharmacol. Rev. 2006, 58, 621–681. [Google Scholar] [CrossRef]
- Chou, T.C.; Talalay, P. Quantitative analysis of dose-effect relationships: The combined effects of multiple drugs or enzyme inhibitors. Adv. Enzym. Regul. 1984, 22, 27–55. [Google Scholar] [CrossRef] [PubMed]

| Scheme 3 | |||||||
|---|---|---|---|---|---|---|---|
| Test | Unit | Group | Normal Values | ||||
| CT | DT | ||||||
| Mean (95% CI) | SD | Mean (95% CI) | SD | p vs. CT | |||
| WBC | 109/L | 4.809 (3.586–6.031) | 1.463 | 1.164 (0.892–1.435) | 0.324 | <0.0001 | 1.96–8.25 |
| #LYM | 109/L | 3.420 (2.422–4.418) | 1.194 | 0.591 (0.051–1.131) | 0.646 | <0.0001 | 1.41–7.11 |
| #MON | 109/L | 0.313 (0.359–0.389) | 0.110 | 0.026 (−0.003–0.055) | 0.035 | <0.0001 | 0.03–0.18 |
| #EOS | 109/L | 0.029 (0.018–0.039) | 0.012 | 0.006 (0–0.012) | 0.007 | 0.0047 | 0.01–0.16 |
| #BAS | 109/L | 0.008 (0.001–0.013) | 0.007 | 0.004 (−0.0005–0.008) | 0.005 | >0.9999 | 0–0.005 |
| RBC | 1012/L | 7.893 (7.620–8.165) | 0.326 | 5.191 (4.821–5.563) | 0.443 | <0.0001 | 7.27–9.65 |
| HGB | g/dL | 16.313 (15.890–16.740) | 0.511 | 11.888 (11.100–12.670) | 0.942 | <0.0001 | 13.7–17.6 |
| Crea | mg/dL | 0.733 (0.589–0.877) | 0.142 | 0.568 (0.524–0.615) | 0.041 | 0.0247 | 0.46–0.88 |
| ALP | U/L | 78.670 (58.480–98.870) | 19.512 | 28.711 (25.710–31.710) | 2.669 | 0.0002 | 85.4–311.7 |
| AST | U/L | 75.613 (55.070–96.160) | 18.870 | 50.940 (41.130–60.740) | 9.319 | 0.0416 | 65.8–266.2 |
| ALT | U/L | 29.682 (23.170–36.190) | 6.569 | 19.337 (15.400–36.190) | 3928 | 0.0165 | 26.3–68.5 |
| Urea | mg/dL | 31.899 (24.150–39.800) | 7.722 | 30.411 (24.020–36.800) | 6.424 | >0.9999 | 19.62–23.02 |
| CK-MB | U/L | 2473.161 (1643–3303) | 815.850 | 1989.088 (1211–2216) | 784.760 | 0.648 | 233–4367 |
| Analysis | GROUP | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CT | DT | DT+DF | DF | |||||||
| Mean (95% CI) | SD | Mean (95% CI) | SD | p vs. CT | Mean (95% CI) | SD | p vs. DT (95% CI) | Mean (95% CI) | SD | |
| Fibre density/mm2 | 8433 (6191–10,675) | 3529 | 13,062 (10,143–15,980) | 4.593 | 0.0034 | 4557 (3500–5614) | 1478 | <0.0001 | 6465 (5122–7808) | 2114 |
| Fascicle area (μm2) | 198,591 (−53,588–450,770) | 301,642 | 51,480 (25,364–77,596) | 41,104 | 0.2254 | 80,093 (43,776–116,411) | 34,607 | 0.1969 | 61,787 (17,00–121,874) | 57,257 |
| Schwann cell density/mm2 | 984.0 (706.5–1261) | 436.8 | 1306 (1059–1555) | 390.4 | 0.2666 | 1300 (946.4–1654) | 494.5 | >0.9999 | 705.7 (571.3–840.1) | 211.6 |
| Fibroblast density/mm | 25.00 (14.14–35.86) | 8.746 | 26.75 (15.11–38.39) | 13.93 | >0.9999 | 26.00 (20.62–31.38) | 6.437 | >0.9999 | 25.00 (20.35–20.65) | 3.742 |
| Nerve fiber to Schwann cell ratio | 9.167 (7.313–11.02) | 2.918 | 10.00 (8.352–11.65) | 2.594 | 0.9520 | 3.800 (2.921–4.679) | 1.229 | <0.0001 | 9.500 (7.819–11.18) | 2.646 |
| DT:DF Ratio | Group | DT [nM] | DF [μM] | Effect | CIX | 95% CI | Range of CIX | Description |
|---|---|---|---|---|---|---|---|---|
| 5:1 | 1 × CL50 | 171 | 2.9 | 0.84959 | 0.06572 | (0.039–0.093) | <0.1 | Very strong synergism |
| 0.5 × CL50 | 85.5 | 1.46 | 0.78708 | 0.05924 | (0.035–0.083) | <0.1 | Very strong synergism | |
| 2:1 | 1 × CL50 | 171 | 7.31 | 0.75420 | 0.29597 | (0.173–0.419) | 0.3–0.7 | Synergism |
| 0.5 × CL50 | 85.5 | 3.66 | 0.72731 | 0.18913 | (0.110–0.268) | 0.1–0.3 | Strong synergism | |
| 1:1 | 1 × CL50 | 171 | 14.6 | 0.80724 | 0.34583 | (0.241–0.451) | 0.3–0.7 | Synergism |
| 0.5 × CL50 | 85.5 | 7.31 | 0.79732 | 0.20048 | (0.102–0.299) | 0.1–0.3 | Strong synergism | |
| 1:2 | 1 × CL50 | 85.5 | 14.6 | 0.79298 | 0.34551 | (0.294–0.397) | 0.3–0.7 | Synergism |
| 0.5 × CL50 | 42.8 | 7.31 | 0.60237 | 0.44769 | (0.383–0.513) | 0.3–0.7 | Synergism | |
| 1:5 | 1 × CL50 | 34.2 | 14.6 | 0.61317 | 0.77660 | (0.611–0.942) | 0.90–1.10 | Nearly additive |
| 0.5 × CL50 | 17.1 | 7.31 | 0.38281 | 1.63338 | (0.199–3.068) | 1.45–3.3 | Antagonism |
| DT:DF Ratio | Group | DT [nM] | DF [μM] | Effect | CIX | 95% CI | Range of CIX | Description |
|---|---|---|---|---|---|---|---|---|
| 5:1 | 1 × CL50 | 61.0 | 6.59 | 0.75665 | 0.09356 | (0.073–0.114) | 0.1–0.3 | Strong synergism |
| 0.5 × CL50 | 30.5 | 3.29 | 0.67837 | 0.11546 | (0.037–0.194) | 0.1–0.3 | Strong synergism | |
| 2:1 | 1 × CL50 | 61.0 | 11.0 | 0.73709 | 0.17877 | (0.126–0.231) | 0.1–0.3 | Strong synergism |
| 0.5 × CL50 | 30.5 | 5.49 | 0.64662 | 0.47484 | (0.023–0.926) | 0.90–1.10 | Nearly additive | |
| 1:1 | 1 × CL50 | 61.0 | 32.9 | 0.86485 | 0.23696 | (0.154–0.320) | 0.3–0.7 | Synergism |
| 0.5 × CL50 | 30.5 | 16.5 | 0.81565 | 0.16043 | (0.116–0.205) | 0.1–0.3 | Strong synergism | |
| 1:2 | 1 × CL50 | 30.5 | 32.9 | 0.64410 | 0.79257 | (0.523–1.062) | 0.90–1.10 | Nearly additive |
| 0.5 × CL50 | 15.2 | 16.5 | 0.10335 | 217,217,810 | NA | NA | Lack of effect | |
| 1:5 | 1 × CL50 | 12.2 | 32.9 | 0.33615 | 22.00306 | NA | NA | Lack of effect |
| 0.5 × CL50 | 6.10 | 16.5 | 0.01 | 242,900,000 | NA | NA | Lack of effect |
| Rate 5:1 | Rate 2:1 | Rate 1:1 | Rate 1:2 | Rate 1:5 |
|---|---|---|---|---|
| 1 CL50 a:b | 1 CL50 a:b | 1 CL50 a:b | 1 CL50 a:b | 1 CL50 a:b |
| 0.5 CL50 a:b | 0.5 CL50 a:b | 0.5 CL50 a:b | 0.5 CL50 a:b | 0.5 CL50 a:b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cubides-Cely, S.; David Castro, A.; Prado-Guevara, P.; Mantilla-Hernández, J.C.; Negrette-Guzmán, M. An Experimental Rat Model for Simultaneous Induction of Peripheral Neuropathy and Myelotoxicity by Docetaxel Administration: Evaluating the Protective Role of Dimethyl Fumarate. Int. J. Mol. Sci. 2025, 26, 5859. https://doi.org/10.3390/ijms26125859
Cubides-Cely S, David Castro A, Prado-Guevara P, Mantilla-Hernández JC, Negrette-Guzmán M. An Experimental Rat Model for Simultaneous Induction of Peripheral Neuropathy and Myelotoxicity by Docetaxel Administration: Evaluating the Protective Role of Dimethyl Fumarate. International Journal of Molecular Sciences. 2025; 26(12):5859. https://doi.org/10.3390/ijms26125859
Chicago/Turabian StyleCubides-Cely, Sebastian, Alexander David Castro, Pablo Prado-Guevara, Julio César Mantilla-Hernández, and Mario Negrette-Guzmán. 2025. "An Experimental Rat Model for Simultaneous Induction of Peripheral Neuropathy and Myelotoxicity by Docetaxel Administration: Evaluating the Protective Role of Dimethyl Fumarate" International Journal of Molecular Sciences 26, no. 12: 5859. https://doi.org/10.3390/ijms26125859
APA StyleCubides-Cely, S., David Castro, A., Prado-Guevara, P., Mantilla-Hernández, J. C., & Negrette-Guzmán, M. (2025). An Experimental Rat Model for Simultaneous Induction of Peripheral Neuropathy and Myelotoxicity by Docetaxel Administration: Evaluating the Protective Role of Dimethyl Fumarate. International Journal of Molecular Sciences, 26(12), 5859. https://doi.org/10.3390/ijms26125859








