Overexpression of a Malus baccata (L.) Borkh WRKY Factor Gene MbWRKY33 Increased High Salinity Stress Tolerance in Arabidopsis thaliana
Abstract
1. Introduction
2. Results
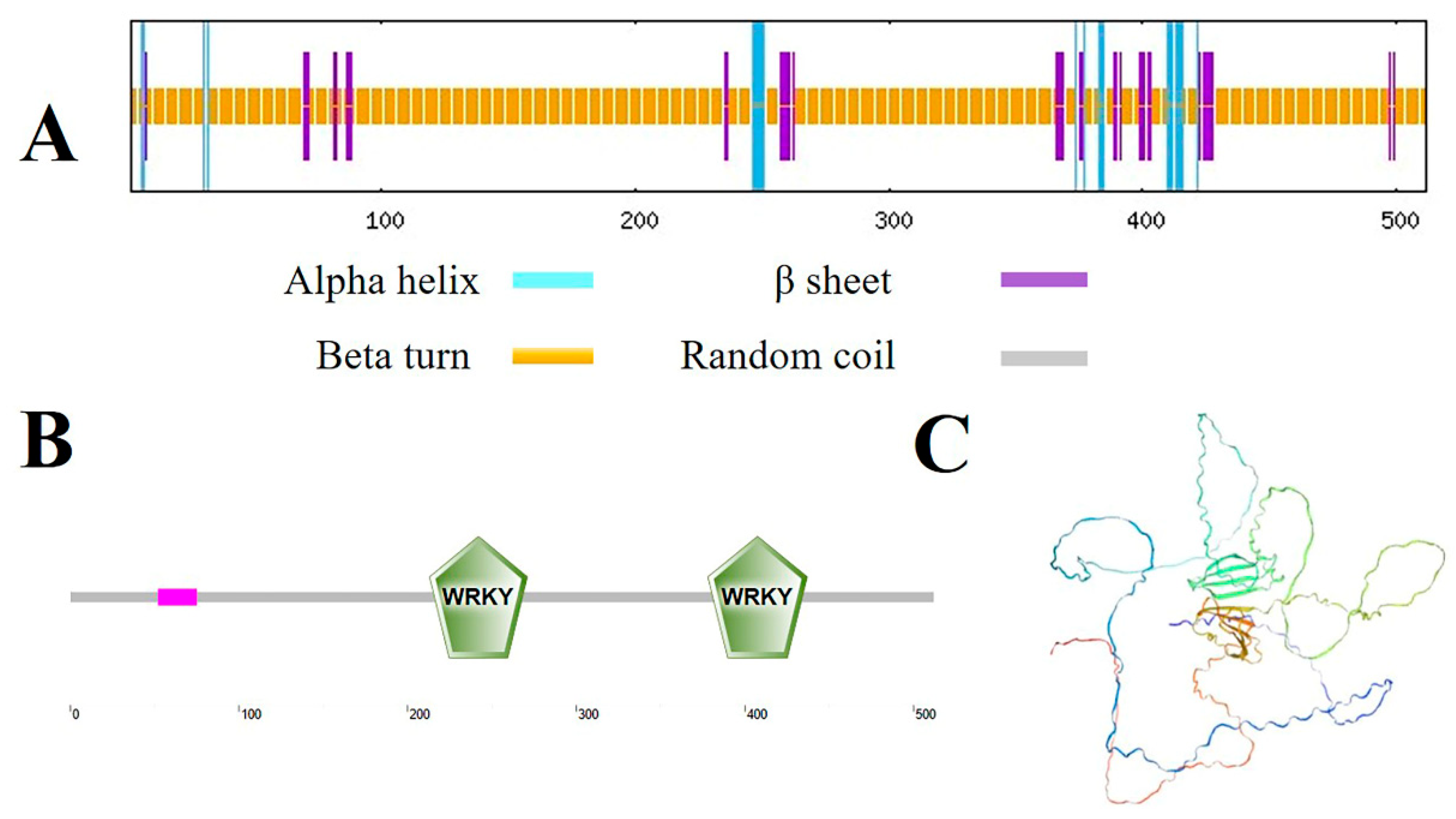
2.1. Cloning and Bioinformatics Analysis of the MbWRKY33 Gene
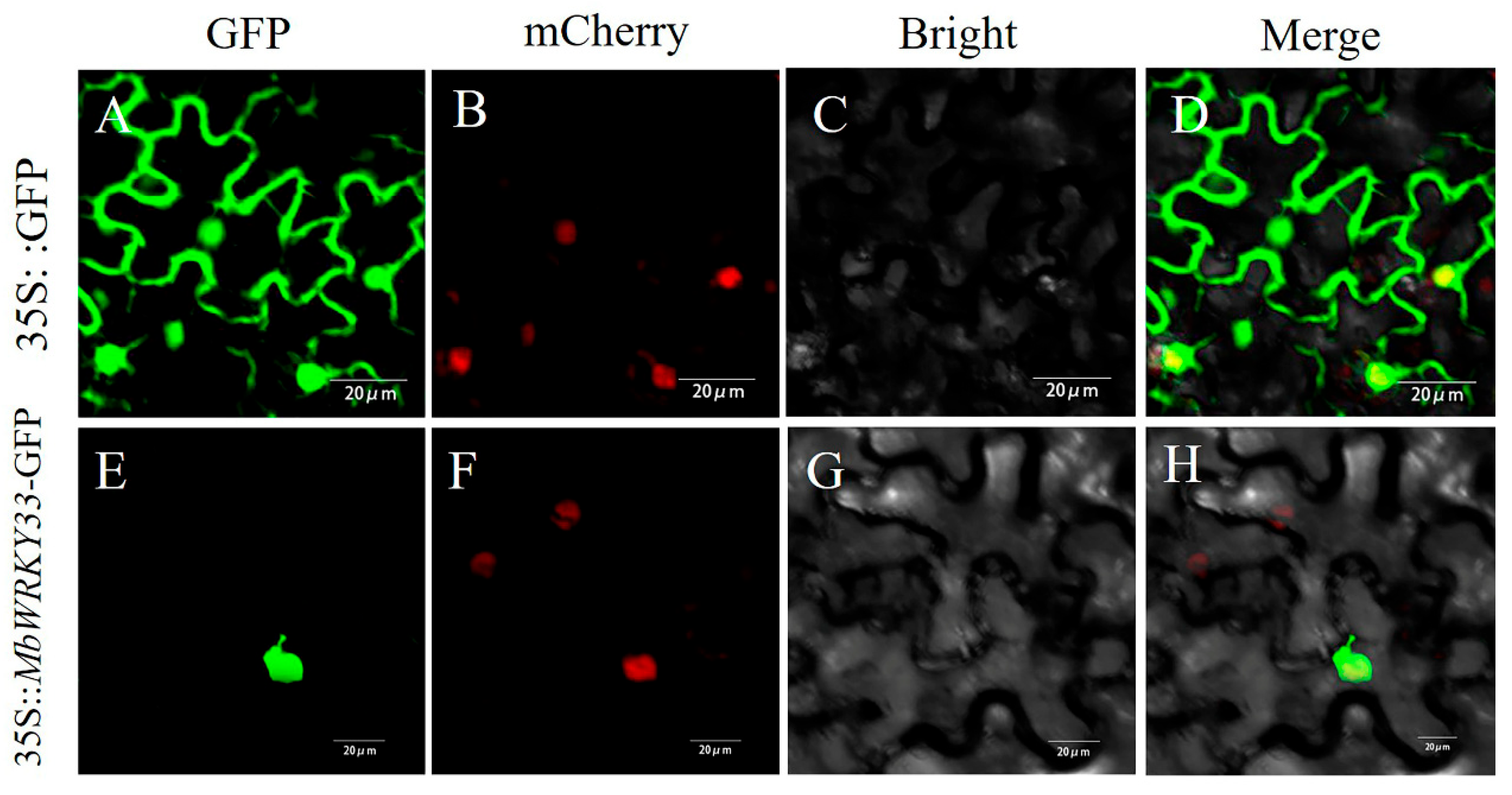
2.2. MbWRKY33 Protein Was Located in the Nucleus
2.3. Analysis of the Expression Level of MbWRKY33 in Malus baccata
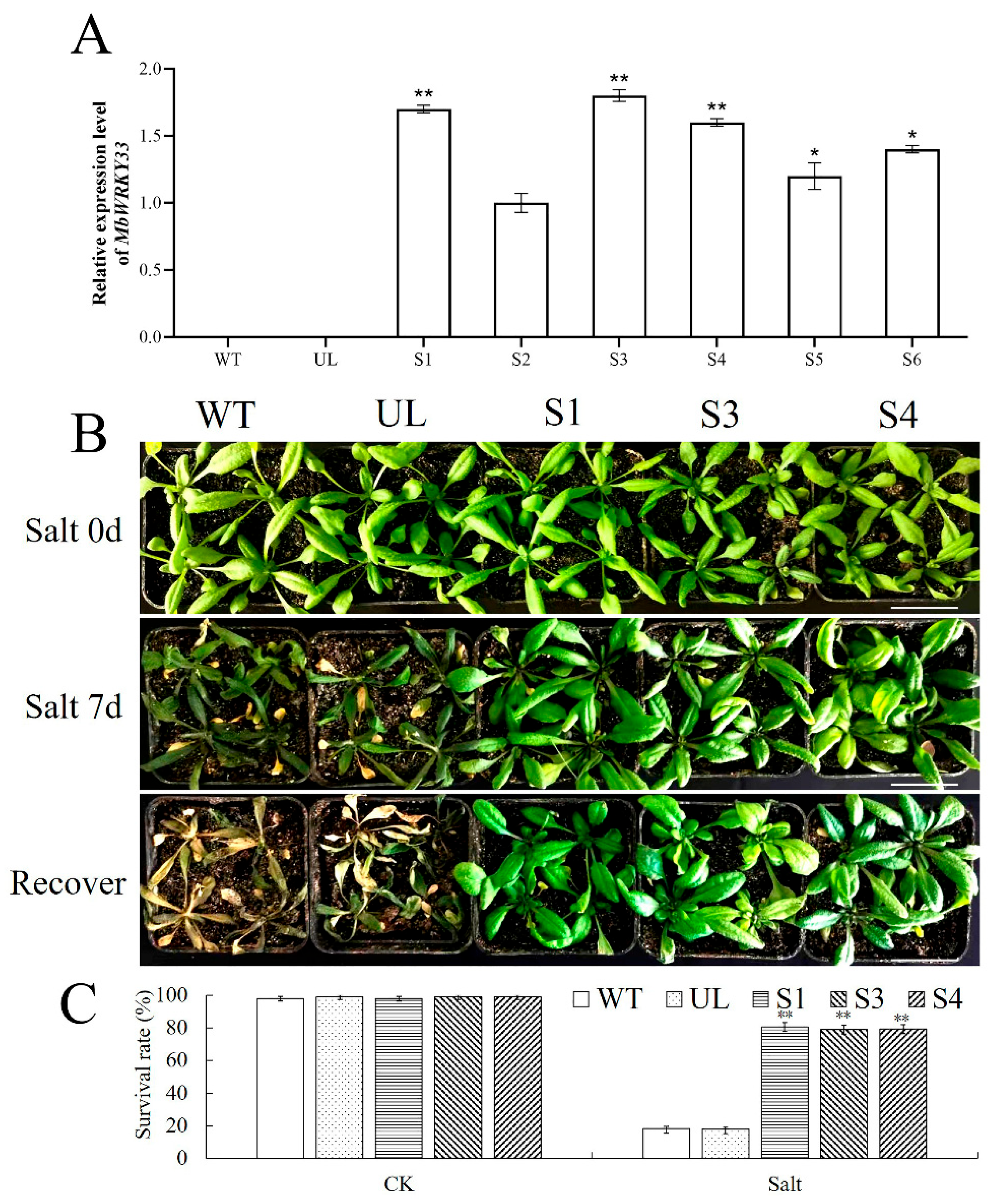
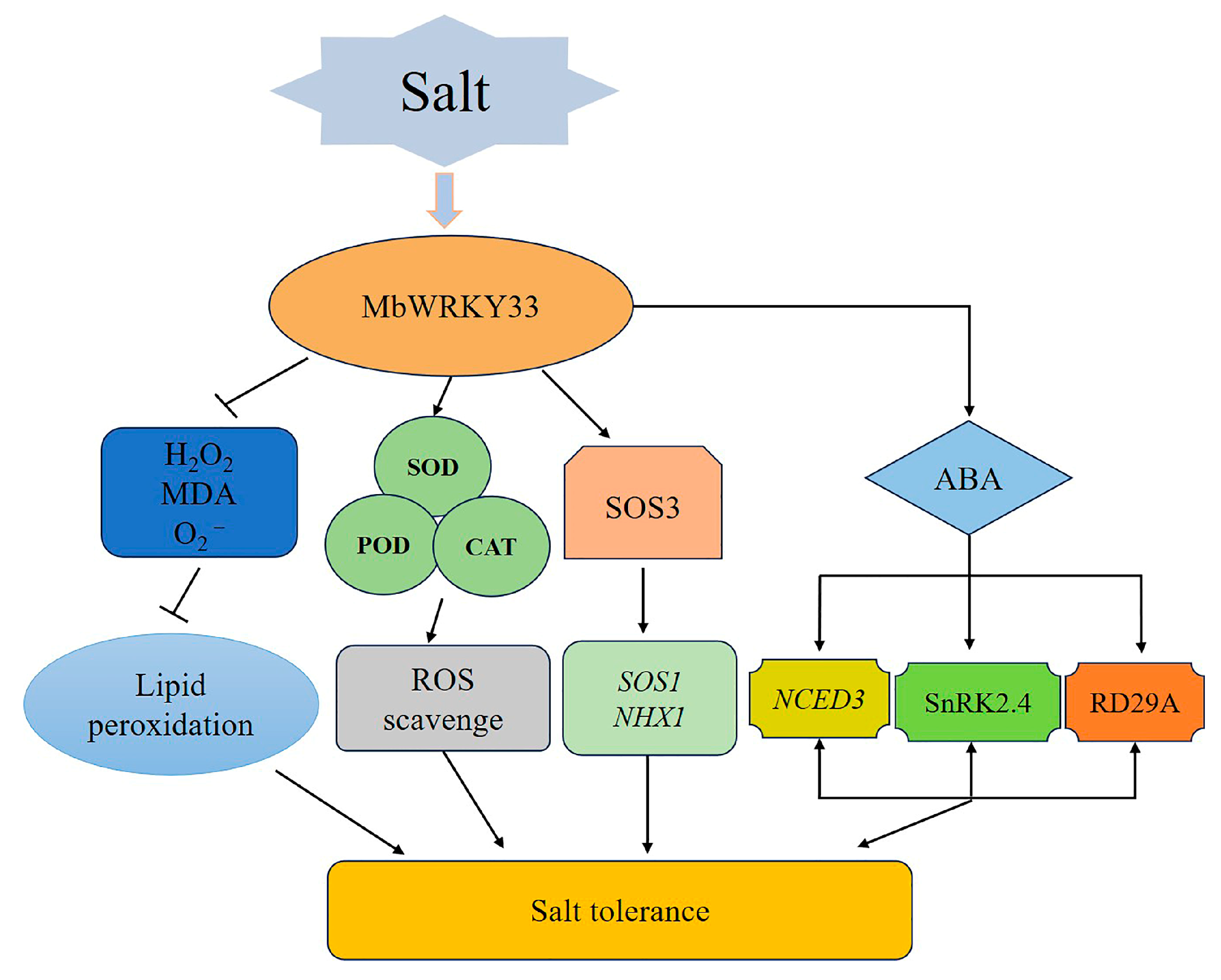
2.4. Overexpression of MbWRKY33 Enhances the Tolerance of Arabidopsis thaliana to Salt Stress
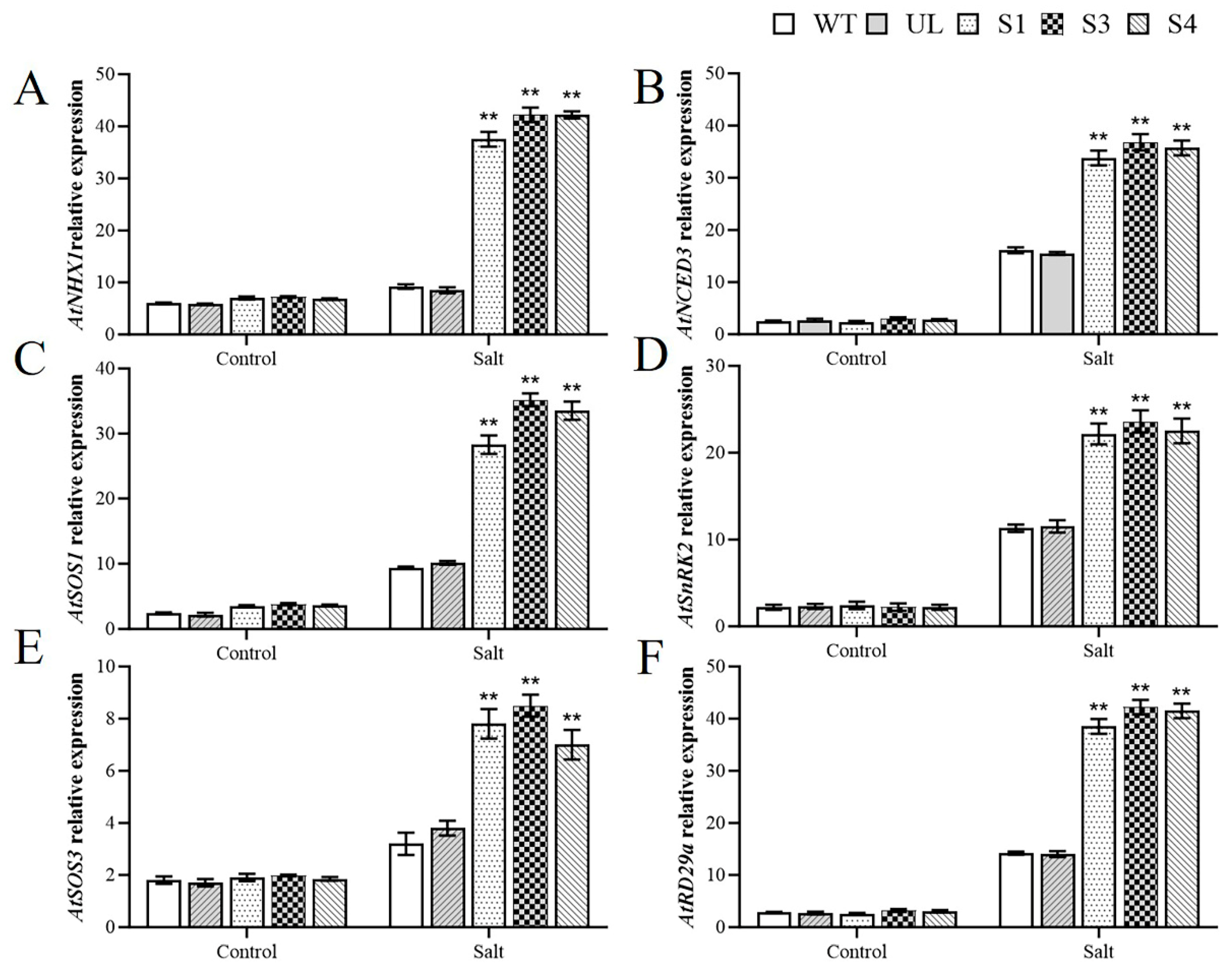
2.5. MbWRKY33 Activates Downstream Salt-Tolerance Related Genes in A. thaliana
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Analysis of the Expression Pattern of MbWRKY33
4.3. RNA Extraction and Cloning of MbWRKY33
4.4. Subcellular Localization Analysis of MbWRKY33
4.5. Sequence Alignment and Structure Prediction of MbWRKY33
4.6. Expression Analysis of the MbWRKY33 Gene
4.7. Obtaining Transgenic Arabidopsis thaliana Lines
4.8. High-Salt Stress Treatment
4.9. Measurement of Physiological Indices
4.10. Expression Analysis of Salt Stress-Related Genes in Arabidopsis thaliana Lines Overexpressing MbWRKY33
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alscher, R.G.; Donahue, J.L.; Cramer, C.L. Reactive oxygen species and antioxidants: Relationships in green cells. Physiol. Plant. 1997, 100, 224–233. [Google Scholar] [CrossRef]
- Han, D.; Shi, Y.; Yu, Z.; Liu, W.; Lv, B.; Wang, B.; Yang, G. Isolation and functional analysis of MdCS1: A gene encoding a citrate synthase in Malus domestica (L.) Borkh. Plant Growth Regul. 2015, 75, 209–218. [Google Scholar] [CrossRef]
- Thakur, P.; Nayyar, H. Facing the Cold Stress by Plants in the Changing Environment: Sensing, Signaling, and Defending Mechanisms. In Plant Acclimation to Environmental Stress; Springer: New York, NY, USA, 2013; pp. 29–69. [Google Scholar]
- Farooq, M.; Hussain, M.; Wakeel, A.; Siddique, K.H.M. Salt stress in maize: Effects, resistance mechanisms, and management. A review. Agron. Sustain. Dev. 2015, 35, 461–481. [Google Scholar] [CrossRef]
- Zhu, J.K. Abiotic Stress Signaling and Responses in Plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, I.; Shin, T.; Peter, N. Life and death under salt stress: Same players, different timing? J. Exp. Bot. 2014, 65, 2963–2979. [Google Scholar]
- Liu, Y.; Hou, J.; Wang, X.; Li, T.; Majeed, U.; Hao, C.; Zhang, X. The NAC transcription factor NAC019-A1 is a negative regulator of starch synthesis in wheat developing endosperm. J. Exp. Bot. 2020, 71, 5794–5807. [Google Scholar] [CrossRef]
- Siddiqua, M.; Nassuth, A. Vitis CBF1 and Vitis CBF4 differ in their effect on Arabidopsis abiotic stress tolerance, development and gene expression. Plant Cell Environ. 2011, 34, 1345–1359. [Google Scholar] [CrossRef]
- Xiao, H.; Siddiqua, M.; Braybrook, S.; Nassuth, A. Three grape CBF/DREB1 genes respond to low temperature, drought, and abscisic acid. Plant Cell Environ. 2010, 29, 1410–1421. [Google Scholar] [CrossRef]
- Erpen, L.; Devi, H.S.; Grosser, J.W.; Dutt, M. Potential use of the DREB/ERF, MYB, NAC, and WRKY transcription factors to improve abiotic and biotic stress in transgenic plants. Plant Cell Tiss. Organ Cult. 2018, 132, 1–25. [Google Scholar] [CrossRef]
- Li, P.; Zheng, T.; Domingues, D.S.; Liu, Y.; Ahmad, S. Editorial: Low-temperature stress in plants: Molecular responses, tolerance mechanisms, plant biodesign, and breeding applications. Front. Plant Sci. 2024, 15, 1411636. [Google Scholar] [CrossRef]
- Johnson, C.S.; Kolevski, B.; Smyth, D.R. TRANSPARENT TESTA GLABRA2, a Trichome and Seed Coat Development Gene of Arabidopsis, Encodes a WRKY Transcription Factor. Plant Cell 2002, 14, 1359–1375. [Google Scholar] [CrossRef] [PubMed]
- Robatzek, S.; Somssich, I.E. Targets of AtWRKY6 regulation during plant senescence and pathogen defense. Genes Dev. 2002, 16, 1139–1149. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Brader, G.n.; Palva, E.T. The WRKY70 Transcription Factor: A Node of Convergence for Jasmonate-Mediated and Salicylate-Mediated Signals in Plant Defense. Plant Cell 2004, 16, 319–331. [Google Scholar] [CrossRef] [PubMed]
- Ülker, B.; Somssich, I.E. WRKY transcription factors: From DNA binding towards biological function. Curr. Opin. Plant Biol. 2004, 7, 491–498. [Google Scholar] [CrossRef]
- Marè, C.; Mazzucotelli, E.; Crosatti, C.; Francia, E.; Stanca, A.M.; Cattivelli, L. Hv-WRKY38: A new transcription factor involved in cold- and drought-response in barley. Plant Mol. Biol. 2004, 55, 399–416. [Google Scholar] [CrossRef]
- Li, S.; Fu, Q.; Huang, W.; Yu, D. Functional analysis of an Arabidopsis transcription factor WRKY25 in heat stress. Plant Cell Rep. 2009, 28, 683–693. [Google Scholar] [CrossRef]
- Li, W.; Wei, Y.; Zhang, L.; Wang, Y.; Song, P.; Li, X.; Han, D. FvMYB44, a strawberry R2R3-MYB transcription factor, improved salt and cold stress tolerance in transgenic Arabidopsis. Agronomy 2023, 13, 1051. [Google Scholar] [CrossRef]
- Doll, J.; Muth, M.; Riester, L.; Nebel, S.; Bresson, J.; Lee, H.C.; Zentgraf, U. Arabidopsis thaliana WRKY25 Transcription Factor Mediates Oxidative Stress Tolerance and Regulates Senescence in a Redox-Dependent Manner. Front. Plant Sci. 2020, 10, 1734. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, J. Multiple functions and regulatory networks of WRKY33 and its orthologs. Gene 2024, 931, 148899. [Google Scholar] [CrossRef]
- Qiu, Y.; Yu, D. Over-expression of the stress-induced OsWRKY45 enhances disease resistance and drought tolerance in Arabidopsis. Environ. Exp. Bot. 2009, 65, 35–47. [Google Scholar] [CrossRef]
- Rushton, P.J.; Macdonald, H.; Huttly, A.K.; Lazarus, C.M.; Hooley, R. Members of a new family of DNA-binding proteins bind to a conserved cis-element in the promoters of α-Amy2 genes. Plant Mol. Biol. 1995, 29, 691–702. [Google Scholar] [CrossRef] [PubMed]
- Eulgem, T.; Rushton, P.J.; Schmelzer, E.; Hahlbrock, K.; Somssich, I.E. Early nuclear events in plant defense signaling: Rapid gene activation by WRKY transcription factors. EMBO J. 1999, 18, 4689–4699. [Google Scholar] [CrossRef] [PubMed]
- Berri, S.; Abbruscato, P.; Faivre-Rampant, O.; Brasileiro, A.C.M.; Fumasoni, I.; Satoh, K.; Kikuchi, S.; Mizzi, L.; Morandini, P.; Pè, M.E.; et al. Characterization of WRKY co-regulatory networks in rice and Arabidopsis. BMC Plant Biol. 2009, 9, 120. [Google Scholar] [CrossRef]
- Wang, L.; Zhu, W.; Fang, L.; Sun, X.; Su, L.; Liang, Z.; Wang, N.; Londo, J.P.; Li, S.; Xin, H. Genome-wide identification of WRKY family genes and their response to cold stress in Vitis vinifera. BMC Plant Biol. 2014, 14, 103. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi-Shinozaki, K.; Shinozaki, K. Transcriptional regulatory networks in cellular responses and tolerance to dehydration and cold stresses. Annu. Rev. Plant Biol. 2006, 57, 781–803. [Google Scholar] [CrossRef]
- Zheng, Z.; Mosher, S.L.; Fan, B.; Klessig, D.F.; Chen, Z. Functional analysis of Arabidopsis WRKY25 transcription factor in plant defense against Pseudomonas syringae. BMC Plant Biol. 2007, 7, 2. [Google Scholar] [CrossRef]
- Jiang, Y.; Deyholos, M.K. Functional characterization of Arabidopsis NaCl-inducible WRKY25 and WRKY33 transcription factors in abiotic stresses. Plant Mol. Biol. 2009, 69, 91–105. [Google Scholar] [CrossRef]
- Li, S.; Fu, Q.; Chen, L.; Huang, W.; Yu, D. Arabidopsis thaliana WRKY25, WRKY26, and WRKY33 coordinate induction of plant thermotolerance. Planta 2011, 233, 1237–1252. [Google Scholar] [CrossRef]
- Zheng, Z.; Qamar, S.A.; Chen, Z.; Mengiste, T. Arabidopsis WRKY33 transcription factor is required for resistance to necrotrophic fungal pathogens. Plant J. 2006, 48, 592–605. [Google Scholar] [CrossRef]
- Pandey, S.P.; Somssich, I.E. The Role of WRKY Transcription Factors in Plant Immunity. Plant Physiol. 2009, 150, 1648–1655. [Google Scholar] [CrossRef]
- Wang, Y.; Shu, Z.; Wang, W.; Jiang, X.; Li, D.; Pan, J.; Li, X. CsWRKY2, a novel WRKY gene from Camellia sinensis, is involved in cold and drought stress responses. Biol. Plant. 2016, 60, 443–451. [Google Scholar] [CrossRef]
- Ma, Y.; Xue, H.; Zhang, F.; Jiang, Q.; Yang, S.; Yue, P.; Wang, F.; Zhang, Y.; Li, L.; He, P.; et al. The miR156/SPL module regulates apple salt stress tolerance by activating MdWRKY100 expression. Plant Biotechnol. J. 2021, 19, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Volk, G.M.; Chao, C.T.; Norelli, J.; Brown, S.K.; Fazio, G.; Peace, C.; McFerson, J.; Zhong, G.Y.; Bretting, P. The vulnerability of US apple (Malus) genetic resources. Genet. Resour. Crop Evol. 2015, 62, 765–794. [Google Scholar] [CrossRef]
- Tham, S.Y.; Loke, W.H. WTO and Regionalism: Opportunities and Challenges for Malaysian Industries. Humanomics 2001, 17, 40–53. [Google Scholar]
- Han, D.G.; Wang, Y.; Zhang, L.; Ma, L.; Zhang, X.Z.; Xu, X.F.; Han, Z.H. Isolation and functional characterization of MxCS1: A gene encoding a citrate synthase in Malus xiaojinensis. Biol. Plant. 2012, 56, 50–56. [Google Scholar] [CrossRef]
- Melino, V.; Tester, M. Salt-Tolerant Crops: Time to Deliver. Annu. Rev. Plant Biol. 2023, 74, 671–696. [Google Scholar] [CrossRef]
- Li, W.; Li, P.; Chen, H.; Zhong, J.; Liang, X.; Wei, Y.; Zhang, L.; Wang, H.; Han, D. Overexpression of a Fragaria vesca 1R-MYB Transcription Factor Gene (FvMYB114) Increases Salt and Cold Tolerance in Arabidopsis thaliana. Int. J. Mol. Sci. 2023, 24, 5261. [Google Scholar] [CrossRef]
- Ren, X.; Chen, Z.; Liu, Y.; Zhang, H.; Zhang, M.; Liu, Q.; Hong, X.; Zhu, J.K.; Gong, Z. ABO3, a WRKY transcription factor, mediates plant responses to abscisic acid and drought tolerance in Arabidopsis. Plant J. 2010, 63, 417–429. [Google Scholar] [CrossRef]
- Chen, L.; Song, Y.; Li, S.; Zhang, L.; Zou, C.; Yu, D. The role of WRKY transcription factors in plant abiotic stresses. Biochim. Biophys. Acta 2012, 1819, 120–128. [Google Scholar] [CrossRef]
- Yang, P.; Chen, C.; Wang, Z.; Fan, B.; Chen, Z. A pathogen- and salicylic acid-induced WRKY DNA-binding activity recognizes the elicitor response element of the tobacco class I chitinase gene promoter. Plant J. 2010, 18, 141–149. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, Y.; Luo, Z.; Lyu, L.; Wang, C.; Zhu, L.; Ma, F.; Li, M.; Han, D. Overexpression of a transcription factor MdWRKY126 altered soluble sugar accumulation in apple and tomato fruit. Hortic. Plant J. 2025, 11, 989–998. [Google Scholar] [CrossRef]
- Guo, C.; Guo, R.; Xu, X.; Gao, M.; Li, X.; Song, J.; Zheng, Y.; Wang, X. Evolution and expression analysis of the grape (Vitis vinifera L.) WRKY gene family. J. Exp. Bot. 2014, 65, 1513–1528. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z. Transcription Factors Indirectly Regulate Genes through Nuclear Colocalization. Cells 2019, 8, 754. [Google Scholar] [CrossRef]
- Bai, X.; Gu, C.; Zhang, X.; Jiang, J. Gene Cloning and Specific Tissue Expression of UlGLK from Ulmus laevis. Bull. Bot. Res. 2022, 42, 848–854. [Google Scholar]
- Noctor, G.; Mhamdi, A.; Foyer, C.H. The Roles of Reactive Oxygen Metabolism in Drought: Not So Cut and Dried. Plant Physiol. 2014, 164, 1636–1648. [Google Scholar] [CrossRef]
- Wang, Y.; Branicky, R.; Noë, A.; Hekimi, S. Superoxide dismutases: Dual roles in controlling ROS damage and regulating ROS signaling. J. Cell Biol. 2018, 217, 1915–1928. [Google Scholar] [CrossRef]
- Xie, Z.; Zhang, Z.L.; Zou, X.; Huang, J.; Ruas, P.; Thompson, D.; Shen, Q.J. Annotations and Functional Analyses of the Rice WRKY Gene Superfamily Reveal Positive and Negative Regulators of Abscisic Acid Signaling in Aleurone Cells. Plant Physiol. 2005, 137, 176–189. [Google Scholar] [CrossRef] [PubMed]
- Dumanović, J.; Nepovimova, E.; Natić, M.; Kuča, K.; Jaćević, V. The Significance of Reactive Oxygen Species and Antioxidant Defense System in Plants: A Concise Overview. Front. Plant Sci. 2021, 11, 552969. [Google Scholar] [CrossRef]
- Chan, Z.; Wang, Y.; Cao, M.; Gong, Y.; Mu, Z.; Wang, H.; Hu, Y.; Deng, X.; He, X.J.; Zhu, J.K. 4 modulates cold stress resistance in Arabidopsis partially through the CBF-mediated pathway. New Phytol. 2016, 209, 1527–1539. [Google Scholar] [CrossRef]
- Rubio, M.C.; Bustos-Sanmamed, P.; Clemente, M.R.; Becana, M. Effects of salt stress on the expression of antioxidant genes and proteins in the model legume Lotus japonicus. New Phytol. 2009, 181, 851–859. [Google Scholar] [CrossRef]
- Zhang, L.; Pei, Y.; Wang, H.; Jin, Z.; Liu, Z.; Qiao, Z.; Fang, H.; Zhang, Y. Hydrogen Sulfide Alleviates Cadmium-Induced Cell Death through Restraining ROS Accumulation in Roots of Brassica rapa L. ssp. pekinensis. Oxid. Med. Cell. Longev. 2015, 2015, 804603. [Google Scholar] [PubMed]
- Song, Y.; Chen, L.; Zhang, L.; Yu, D. Overexpression of OsWRKY72 gene interferes in the abscisic acid signal and auxin transport pathway of Arabidopsis. J. Biosci. 2010, 35, 459–471. [Google Scholar] [CrossRef]
- Parvaiz, A.; Satyawati, S. Salt stress and phyto-biochemical responses of plants-A review. Plant Soil Environ. 2018, 54, 88–99. [Google Scholar] [CrossRef]
- Bazihizina, N.; Colmer, T.D.; Cuin, T.A.; Mancuso, S.; Shabala, S. Friend or Foe? Chloride Patterning in Halophytes. Trends Plant Sci. 2019, 24, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Wang, Q.Y.; Cheng, X.G.; Xu, Z.S.; Li, L.C.; Ye, X.G.; Xia, L.Q.; Ma, Y.Z. GmDREB2, a soybean DRE-binding transcription factor, conferred drought and high salt tolerance in transgenic plants. Biochem. Biophys. Res. Commun. 2007, 353, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wu, S.; Liu, S.; Takano, T. The Arabidopsis sucrose non-fermenting-1-related protein kinase AtSnRK2.4 interacts with a transcription factor, AtMYB21, that is involved in salt tolerance. Plant Sci. 2021, 303, 110685. [Google Scholar] [CrossRef]
- Long, T.; Xu, B.; Hu, Y.; Wang, Y.; Mao, C.; Wang, Y.; Zhang, J.; Liu, H.; Huang, H.; Liu, Y.; et al. Genome-wide identification of ZmSnRK2 genes and functional analysis of ZmSnRK2.10 in ABA signaling pathway in maize (Zea mays L.). BMC Plant Biol. 2021, 21, 309. [Google Scholar] [CrossRef]
- Bello, B.; Zhang, X.; Liu, C.; Yang, Z.; Yang, Z.; Wang, Q.; Zhao, G.; Li, F. Cloning of Gossypium hirsutum Sucrose Non-Fermenting 1-Related Protein Kinase 2 Gene (GhSnRK2) and Its Overexpression in Transgenic Arabidopsis Escalates Drought and Low-Temperature Tolerance. PLoS ONE 2014, 9, e112269. [Google Scholar] [CrossRef][Green Version]
- Han, D.; Wang, Y.; Zhang, Z.; Pu, Q.; Ding, H.; Han, J.; Fan, T.; Bai, X.; Yang, G. Isolation and functional analysis of MxCS3: A gene encoding a citrate synthase in Malus xiaojinensis, with functions in tolerance to iron stress and abnormal flower in transgenic Arabidopsis thaliana. Plant Growth Regul. 2017, 82, 479–489. [Google Scholar] [CrossRef]
- Goujon, T.; Minic, Z.; El Amrani, A.; Lerouxel, O.; Aletti, E.; Lapierre, C.; Joseleau, J.P.; Jouanin, L. AtBXL1, a novel higher plant (Arabidopsis thaliana) putative beta-xylosidase gene, is involved in secondary cell wall metabolism and plant development. Plant J. 2003, 33, 677–690. [Google Scholar] [CrossRef]
- Han, D.; Wang, L.; Wang, Y.; Yang, G.; Gao, C.; Yu, Z.; Li, T.; Zhang, X.; Ma, L.; Xu, X.; et al. Overexpression of Malus xiaojinensis CS1 gene in tobacco affects plant development and increases iron stress tolerance. Sci. Hortic. 2013, 150, 65–72. [Google Scholar] [CrossRef]
- Miller, G.A.D.; Suzuki, N.; Ciftci-Yilmaz, S.; Mittler, R.O.N. Reactive oxygen species homeostasis and signaling during drought and salinity stresses. Plant Cell Environ. 2010, 33, 453–467. [Google Scholar] [CrossRef]
- Tang, J.; Wang, F.; Wang, Z.; Huang, Z.; Xiong, A.; Hou, X. Characterization and co-expression analysis of WRKY orthologs involved in responses to multiple abiotic stresses in Pak-choi (Brassica campestris ssp. chinensis). BMC Plant Biol. 2013, 13, 188. [Google Scholar] [CrossRef]
- Acosta-Motos, J.; Ortuo, M.; Bernal-Vicente, A.; Diaz-Vivancos, P.; Sanchez-Blanco, M.; Hernandez, J. Plant responses to salt stress: Adaptive mechanisms. Agronomy 2017, 7, 18. [Google Scholar] [CrossRef]
- Liu, W.; Wang, T.; Wang, Y.; Liang, X.; Han, J.; Han, D. MbMYBC1, a M. baccata MYB transcription factor, contribute to cold and drought stress tolerance in transgenic Arabidopsis. Front. Plant Sci. 2023, 14, 1141446. [Google Scholar] [CrossRef]
- Ren, C.; Li, Z.; Song, P.; Wang, Y.; Liu, W.; Zhang, L.; Li, X.; Li, W.; Han, D. Overexpression of a grape MYB transcription factor gene VhMYB2 increases salinity and drought tolerance in Arabidopsis thaliana. Int. J. Mol. Sci. 2023, 24, 10743. [Google Scholar] [CrossRef]
- Deinlein, U.; Weber, M.; Schmidt, H.; Rensch, S.; Trampczynska, A.; Hansen, T.H.; Husted, S.; Schjoerring, J.K.; Talke, I.N.; Krämer, U.; et al. Elevated Nicotianamine Levels in Arabidopsis halleri Roots Play a Key Role in Zinc Hyperaccumulation. Plant Cell 2012, 24, 708–723. [Google Scholar] [CrossRef]
- Tsednee, M.; Yang, S.C.; Lee, D.C.; Yeh, K.C. Root-Secreted Nicotianamine from Arabidopsis halleri Facilitates Zinc Hypertolerance by Regulating Zinc Bioavailability. Plant Physiol. 2014, 166, 839–852. [Google Scholar] [CrossRef]
- Han, D.G.; Yang, G.H.; Xu, K.D.; Shao, Q.; Yu, Z.Y.; Wang, B.; Ge, Q.L.; Yu, Y. Overexpression of a Malus xiaojinensis Nas1 Gene Influences Flower Development and Tolerance to Iron Stress in Transgenic Tobacco. Plant Mol. Biol. Rep. 2013, 31, 802–809. [Google Scholar] [CrossRef]
- Xiao, L.Y.; Shi, Y.Y.; Wang, R.; Feng, Y.; Wang, L.S.; Zhang, H.S.; Shi, X.Y.; Jing, G.Q.; Deng, P.; Song, T.Z. The transcription factor OsMYBc and an E3 ligase regulate expression of a K+ transporter during salt stress. Plant Physiol. 2022, 190, 843–859. [Google Scholar] [CrossRef]
- Chen, K.Q.; Song, M.G.; Guo, Y.N.; Liu, L.F.; Xue, H.; Dai, H.Y.; Zhang, Z.L. MdMYB46 could enhance salt and osmotic stress tolerance in apple by directly activating stress-responsive signals. Plant Biotechnol. J. 2019, 17, 2341–2355. [Google Scholar] [CrossRef]
- Imran, M.; Amanullah, S.; Rothenberg, D.O.; Wang, Q.; Wang, L.; Fan, Y. Functional characterization of Hedychium coronarium J. Koenig MYB132 confers the potential role in floral aroma synthesis. Plants 2021, 10, 2014. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Chen, Z.; Li, L.; Li, Q.; Geng, Z.; Liu, W.; Hou, R.; Zhang, L.; Han, D. MbWRKY50 confers cold and drought tolerance through upregulating antioxidant capacity associated with ROS scavenging. J. Plant Physiol. 2025, 310, 154526. [Google Scholar] [CrossRef]
- Xiao, L.; He, Q.; Guo, Y.; Zhang, J.; Nie, F.; Li, Y.; Ye, X.; Zhang, L. Cyanea capillata tentacle-only extract as a potential alternative of nematocyst venom: Its cardiovascular toxicity and tolerance to isolation and purification procedures. Toxicon 2009, 53, 146–152. [Google Scholar] [CrossRef]
- Han, D.; Zhang, Z.; Ni, B.; Ding, H.; Liu, W.; Li, W.; Chai, L.; Yang, G. Isolation and functional analysis of MxNAS3 involved in enhanced iron stress tolerance and abnormal flower in transgenic Arabidopsis. J. Plant Interact. 2018, 13, 433–441. [Google Scholar] [CrossRef]
- Zhao, F.G.; Zheng, T.; Liu, Z.J.; Fu, W.H.; Fang, J.G. Transcriptomic Analysis Elaborates the Resistance Mechanism of Grapevine Rootstocks against Salt Stress. Plants 2022, 11, 1167. [Google Scholar] [CrossRef]
- Ren, C.; Luo, G.; Li, X.; Yao, A.; Liu, W.; Zhang, L.; Wang, Y.; Li, W.; Han, D. MxFRO4 confers iron and salt tolerance through up-regulating antioxidant capacity associated with the ROS scavenging. J. Plant Physiol. 2023, 285, 154001. [Google Scholar] [CrossRef]
- Qin, Z.; Hou, F.Y.; Li, A.X.; Dong, S.X.; Wang, Q.M.; Zhang, L.M. Transcriptome-wide identification of WRKY transcription factor and their expression profiles under salt stress in sweetpotato (Ipomoea batatas L.). Plant Biotechnol. Rep. 2020, 14, 599–611. [Google Scholar] [CrossRef]
- Han, D.G.; Shi, Y.; Wang, B.; Liu, W.; Yu, Z.Y.; Lv, B.Y.; Yang, G.H. Isolation and Preliminary Functional Analysis of MxCS2: A Gene Encoding a Citrate Synthase in Malus xiaojinensis. Plant Mol. Biol. Rep. 2015, 33, 133–142. [Google Scholar] [CrossRef]
- Duan, Y.; Han, J.; Guo, B.; Zhao, W.; Zhou, S.; Zhou, C.; Zhang, L.; Li, X.; Han, D. MbICE1 confers drought and cold tolerance through up-regulating antioxidant capacity and stress-resistant genes in Arabidopsis thaliana. Int. J. Mol. Sci. 2022, 23, 16072. [Google Scholar] [CrossRef]
- Long, T.A.; Tsukagoshi, H.; Busch, W.; Lahner, B.; Salt, D.E.; Benfey, P.N. The bHLH transcription factor POPEYE regulates response to iron deficiency in Arabidopsis roots. Plant Cell 2010, 22, 2219–2236. [Google Scholar] [CrossRef]
- Julkowska, M.; Testerink, C. Tuning plant signaling and growth to survive salt. Trends Plant Sci. 2015, 20, 586–594. [Google Scholar] [CrossRef]
- Xu, Y.; Li, Y.; Chen, Z.; Chen, X.; Li, X.; Li, W.; Li, L.; Li, Q.; Geng, Z.; Shi, S.; et al. Malus xiaojinensis MxbHLH30 Confers Iron Homeostasis Under Iron Deficiency in Arabidopsis. Int. J. Mol. Sci. 2025, 26, 368. [Google Scholar] [CrossRef]
- Ming, L.; Zheng, H.; Jiang, Q.; Sun, X.; Yuan, G.; Qi, J.; Zhang, H. GmNAC15 overexpression in hairy roots enhances salt tolerance in soybean. J. Integr. Agric. 2018, 17, 530–538. [Google Scholar]
- An, J.P.; Yao, J.F.; Xu, R.R.; You, C.X.; Wang, X.F.; Hao, Y.J. An apple NAC transcription factor enhances salt stress tolerance by modulating the ethylene response. Physiol. Plant. 2018, 164, 9–289. [Google Scholar] [CrossRef]
- Per, T.S.; Khan, N.A.; Reddy, P.S.; Masood, A.; Hasanuzzaman, M.; Khan, M.I.R.; Anjum, N.A. Approaches in modulating proline metabolism in plants for salt and drought stress tolerance: Phytohormones, mineral nutrients and transgenics. Plant Physiol. Biochem. 2017, 115, 126–140. [Google Scholar] [CrossRef]
- Zhao, J.; Wu, Q.; Wu, H.; Wang, A.; Wang, X.; Li, C.; Zhao, H.; Wu, Q. FtNAC31, a Tartary buckwheat NAC transcription factor, enhances salt and drought tolerance in transgenic Arabidopsis. Plant Physiol. Biochem. 2022, 191, 20–33. [Google Scholar] [CrossRef]
- Li, Y.; Zhong, J.; Huang, P.; Shao, B.; Li, W.; Liu, W.; Wang, Y.; Xie, L.; Han, M.; Han, D. Overexpression of MxFRO6, a FRO gene from Malus xiaojinensis, increases iron and salt tolerance in Arabidopsis thaliana. Cell. Dev. Biol. Plant 2022, 58, 189–199. [Google Scholar] [CrossRef]
- Zhang, M.; Xie, B.; Tan, F.; Zhang, Q. Relationship Between Soluble Protein, Chlorophyll and ATP in Drought-Resistant Sweet Potato Under Water Stress. Agric. Sci. China 2002, 1, 1329–1333. [Google Scholar]
- Li, P.; Zheng, X.; Liu, Y.; Zhu, Y. Pre-storage application of oxalic acid alleviates chilling injury in mango fruit by modulating proline metabolism and energy status under chilling stress. Food Chem. 2014, 142, 72–78. [Google Scholar] [CrossRef]
- Han, Y.; Yang, H.; Wu, M.; Yi, H. Enhanced drought tolerance of foxtail millet seedlings by sulfur dioxide fumigation. Ecotoxicol. Environ. Saf. 2019, 178, 9–16. [Google Scholar] [CrossRef]
- Malnoy, M.; Jin, Q.; Borejsza-Wysocka, E.; He, S.; Aldwinckle, H. Overexpression of the Apple MpNPR1 Gene Confers Increased Disease Resistance in Malus × domestica. Mol. Plant. Microbe Interact. 2007, 20, 1568–1580. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Song, A.; Gao, C.; Wang, L.; Wang, Y.; Sun, J.; Jiang, J.; Chen, F.; Chen, S. Chrysanthemum WRKY gene CmWRKY17 negatively regulates salt stress tolerance in transgenic chrysanthemum and Arabidopsis plants. Plant Cell Rep. 2015, 34, 1365–1378. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xing, L.; Dai, J.; Li, Z.; Zhang, A.; Wang, T.; Liu, W.; Li, X.; Han, D. Overexpression of a grape WRKY transcription factor VhWRKY44 improves the resistance to cold and salt of Arabidopsis thaliana. Int. J. Mol. Sci. 2024, 25, 7437. [Google Scholar] [CrossRef] [PubMed]
- Bartels, D.; Sunkar, R. Drought and salt tolerance in plants. CRC Crit. Rev. Plant Sci. 2005, 24, 23–58. [Google Scholar] [CrossRef]
- Ma, L.; Hou, C.W.; Zhang, X.Z.; Li, H.L.; Han, D.G.; Wang, Y.; Han, Z.H. Seasonal growth and spatial distribution of apple tree roots on different rootstocks or interstems. J. Am. Soc. Hort. Sci. 2013, 138, 79–87. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Gao, M.; Kong, Y.; Yu, Q.; Yao, L.; Li, X.; Li, W.; Liu, W.; Hou, R.; Zhang, L.; et al. Overexpression of a Malus baccata (L.) Borkh WRKY Factor Gene MbWRKY33 Increased High Salinity Stress Tolerance in Arabidopsis thaliana. Int. J. Mol. Sci. 2025, 26, 5833. https://doi.org/10.3390/ijms26125833
Wang X, Gao M, Kong Y, Yu Q, Yao L, Li X, Li W, Liu W, Hou R, Zhang L, et al. Overexpression of a Malus baccata (L.) Borkh WRKY Factor Gene MbWRKY33 Increased High Salinity Stress Tolerance in Arabidopsis thaliana. International Journal of Molecular Sciences. 2025; 26(12):5833. https://doi.org/10.3390/ijms26125833
Chicago/Turabian StyleWang, Xinhui, Ming Gao, Yihan Kong, Qian Yu, Lu Yao, Xingguo Li, Wenhui Li, Wanda Liu, Ruining Hou, Lihua Zhang, and et al. 2025. "Overexpression of a Malus baccata (L.) Borkh WRKY Factor Gene MbWRKY33 Increased High Salinity Stress Tolerance in Arabidopsis thaliana" International Journal of Molecular Sciences 26, no. 12: 5833. https://doi.org/10.3390/ijms26125833
APA StyleWang, X., Gao, M., Kong, Y., Yu, Q., Yao, L., Li, X., Li, W., Liu, W., Hou, R., Zhang, L., & Han, D. (2025). Overexpression of a Malus baccata (L.) Borkh WRKY Factor Gene MbWRKY33 Increased High Salinity Stress Tolerance in Arabidopsis thaliana. International Journal of Molecular Sciences, 26(12), 5833. https://doi.org/10.3390/ijms26125833





