Cyclodextrin-Modified Capillary Zone Electrophoresis for the Chiral Analysis of Proline and Hydroxyproline Stereoisomers in Chicken Collagen Hydrolysates
Abstract
1. Introduction
2. Results and Discussion
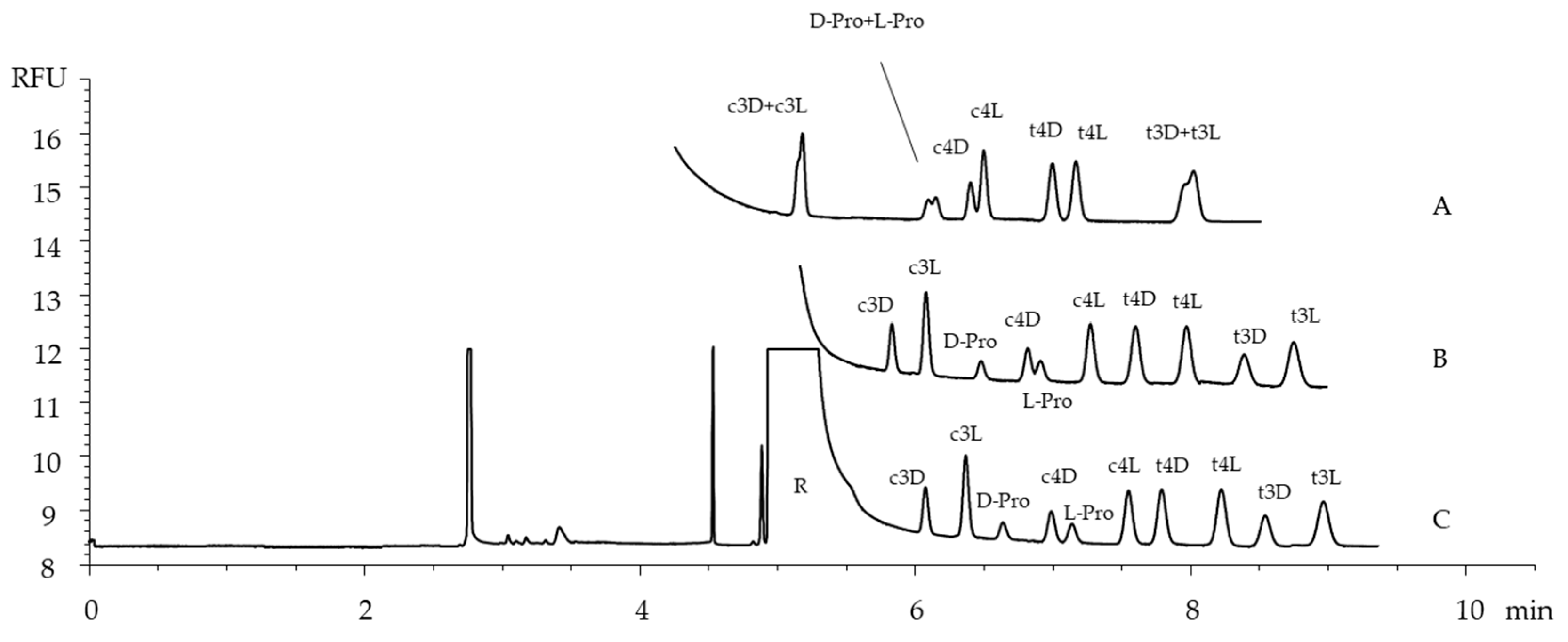
2.1. The Optimization of the Separation Conditions
2.2. The Optimization of the Derivatization Conditions
2.3. Method Validation
2.4. Application to Chicken Collagen Hydrolysates
3. Materials and Methods
3.1. Materials
3.2. Solutions
3.3. Gas-Phase Hydrolysis of Collagen Samples
3.4. Derivatization Reactions
3.5. CE Instrumentation and Analysis
3.6. CE Method Validation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CE | Capillary Electrophoresis |
| DM-βCD | Heptakis(2,6-di-O-methyl)-β-cyclodextrin |
| L-Pro | L-Proline |
| D-Pro | D-Proline |
| t4L | trans-4-hydroxy-L-proline |
| t3L | trans-3-hydroxy-L-proline |
| t4D | trans-4-hydroxy-D-proline |
| t3D | trans-3-hydroxy-D-proline |
| c4L | cis-4-hydroxy-L-proline |
| c3L | cis-3-hydroxy-L-proline |
| c4D | cis-4-hydroxy-D-proline |
| c3D | cis-3-hydroxy-D-proline |
| HPLC | High-Performance Liquid Chromatography |
| MS | Mass Spectrometry |
| GC-MS | Gas Chromatography Mass Spectrometry |
| BGE | Background Electrolyte |
| CD | Cyclodextrin |
| MEKC | Micellar Electrokinetic Chromatography |
| CZE | Capillary Zone Electrophoresis |
| CD-CZE | Cyclodextrin-modified Capillary Zone Electrophoresis |
| FMOC-Cl | 9-fluorenylmethylchloroformate |
| (R)-NCS | (R)-(-)-4-(3-Isothiocyanatopyrrolidin-yl)-7-nitro-2,1,3-benzoxadiazole |
| LEDIF | light-emitting diode-induced fluorescence |
| β-CD | β-cyclodextrin |
| EOF | Electroosmotic flow |
| γCD | γ-cyclodextrin |
| HP-βCD | (2-hydroxypropyl)-β-cyclodextrin |
| TM-βCD | heptakis(2,3,6-tri-O-methyl)-β-cyclodextrin |
| OPA | ortho-phthalaldheyde |
| ME | 2-mercaptoethanol |
| TEA | Triethylamine |
| ACN | Acetonitrile |
| RFU | Relative Fluorescence Unit |
| LOD | Limit of Detection |
| LOQ | Limit of Quantitation |
| SM | Spaghetti Meat myopathy |
| WB | Wooden Breast myopathy |
References
- Sowbhagya, R.; Muktha, H.; Ramakrishnaiah, T.N.; Surendra, A.S.; Sushma, S.M.; Tejaswini, C.; Roopini, K.; Rajashekara, S. Collagen as the extracellular matrix biomaterials in the arena of medical sciences. Tissue Cell 2024, 90, 102497. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; He, W.; Wu, G. Hydroxyproline in animal metabolism, nutrition, and cell signaling. Amino Acids 2022, 54, 513–528. [Google Scholar] [CrossRef] [PubMed]
- Rappu, P.; Salo, A.M.; Myllyharju, J.; Heino, J. Role of prolyl hydroxylation in the molecular interactions of collagens. Essays Biochem. 2019, 63, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Shoulders, M.D.; Raines, R.T. Collagen structure and stability. Annu. Rev. Biochem. 2009, 78, 929–958. [Google Scholar] [CrossRef]
- Polly Chan, S.W.; Greaves, J.; Da Silva, N.A.; Wang, S.-W. Assaying proline hydroxylation in recombinant collagen variants by liquid chromatography-mass spectrometry. BMC Biotechnol. 2012, 12, 51. [Google Scholar] [CrossRef]
- Gjaltema, R.A.F.; Bank, R.A. Molecular insights into prolyl and lysyl hydroxylation of fibrillar collagens in health and disease. Crit. Rev. Biochem. Mol. Biol. 2016, 52, 74–95. [Google Scholar] [CrossRef]
- Dziewiatkowski, D.D.; Hascall, V.C.; Riolo, R.L. Epimerization of trans-4-hydroxy-Lproline to cis-4-hydroxy-D-proline during acid hydrolysis of collagen. Anal. Biochem. 1972, 49, 550–558. [Google Scholar] [CrossRef]
- Langrock, T.; García-Villar, N.; Hoffmann, R. Analysis of hydroxyproline isomers and hydroxylysine by reversed-phase HPLC and mass spectrometry. J. Chromatogr. B 2007, 847, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Lioi, M.; Tengattini, S.; Gotti, R.; Bagatin, F.; Galliani, S.; Massolini, G.; Daly, S.; Temporini, C. Chromatographic separation by RPLC-ESI-MS of all hydroxyproline isomers for the characterization of collagens from different sources. J. Chromatogr. A 2024, 1720, 464771. [Google Scholar] [CrossRef]
- Lüpke, M.; Brückner, H. Gas chromatographic evaluation of amino acid epimerisation in the course of gelatin manufacturing and processing. Z. Leb. Unters Forsch. A 1998, 206, 323–328. [Google Scholar] [CrossRef]
- Opekar, S.; Zahradníčková, H.; Vodrážka, P.; Řimnáčová, L.; Šimek, P.; Moos, M. A chiral GC–MS method for analysis of secondary amino acids after heptafluorobutyl chloroformate & methylamine derivatization. Amino Acids 2021, 53, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Bernardo-Bermejo, S.; Sánchez-López, E.; Castro-Puyana, M.; Marina, M.L. Chiral capillary electrophoresis. TrAC Trends Anal. Chem. 2020, 124, 115807. [Google Scholar] [CrossRef]
- Chankvetadze, B.; Scriba, G.K.E. Cyclodextrins as chiral selectors in capillary electrophoresis: Recent trends in mechanistic studies. TrAC Trends Anal. Chem. 2023, 160, 116987. [Google Scholar] [CrossRef]
- Peluso, P.; Chankvetadze, B. Native and substituted cyclodextrins as chiral selectors for capillary electrophoresis enantioseparations: Structures, features, application, and molecular modeling. Electrophoresis 2021, 42, 1676–1708. [Google Scholar] [CrossRef]
- Orlandini, S.; Hancu, G.; Szabó, Z.I.; Modroiu, A.; Papp, L.A.; Gotti, R.; Furlanetto, S. New trends in the quality control of enantiomeric drugs: Quality by design-compliant development of chiral capillary electrophoresis methods. Molecules 2022, 27, 7058. [Google Scholar] [CrossRef]
- Yi, G.; Ji, B.; Du, J.; Zhou, J.; Chen, Z.; Mao, Y.; Wei, Y.; Xia, Z.; Fu, Q. Enhanced enantioseparation performance in cyclodextrin-electrokinetic chromatography using quinine modified polydopamine coated capillary column. Microchem. J. 2021, 167, 106315. [Google Scholar] [CrossRef]
- Chu, Q.; Evans, B.T.; Zeece, M.G. Quantitative separation of 4-hydroxyproline from skeletal muscle collagen by micellar electrokinetic capillary electrophoresis. J. Chromatogr. B 1997, 692, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Bernardo-Bermejo, S.; Adámez-Rodríguez, S.; Sánchez-López, E.; Castro-Puyana, M.; Marina, M.L. Stereoselective separation of 4-hydroxyproline by electrokinetic chromatography. Microchem. J. 2023, 185, 108279. [Google Scholar] [CrossRef]
- Toyo’oka, T.; Liu, Y.-M. Development of Optically Active Fluorescent Edman-type Reagents. Analyst 1995, 120, 385–390. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, W.; Shi, Q.; Toyo’oka, T.; Min, J.Z. Determination of D,L-Amino Acids in Collagen from Pig and Cod Skins by UPLC Using Pre-column Fluorescent Derivatization. Food Anal. Meth. 2018, 11, 3130–3137. [Google Scholar] [CrossRef]
- Gotti, R.; Pasquini, B.; Orlandini, S.; Furlanetto, S. Recent applications of the derivatization techniques in capillary electrophoresis. J. Pharm. Biomed. Anal. Open 2023, 1, 100003. [Google Scholar] [CrossRef]
- Molnar-Perl, I.; Vasanits, A. Stability and characteristics of the o-phthaldialdehyde/3-mercaptopropionic acid and o-phthaldialdehyde/N-acetyl-L-cysteine reagents and their amino acid derivatives measured by high-performance liquid chromatography. J. Chromatogr. A 1999, 835, 73–91. [Google Scholar] [CrossRef]
- Hanczko, R.; Kőrös, Á.; Tóth, F.; Molnár-Perl, I. Behavior and characteristics of biogenic amines, ornithine and lysine derivatized with the o-phthalaldehyde–ethanethiol–fluorenylmethyl chloroformate reagent. J. Chromatogr. A 2005, 1087, 210–222. [Google Scholar] [CrossRef]
- ICH Harmonised Tripartite Guideline. Validation of Analytical Procedures Q2(R2); International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use: Geneva, Switzerland, 2022. [Google Scholar]
- Ishimoto, S.; Goto, I.; Kuroiwa, Y. Early morphological changes in the striated muscles in normal and dystrophic chickens. J. Comp. Path. 1988, 98, 69–79. [Google Scholar] [CrossRef]
- Pizzey, J.A.; Barnard, E.A. Structural change in muscles of the dystrophic chicken. II Progression of the histopathology in the pectorals muscles. Neuropathol. Appl. Neurobiol. 1983, 9, 149–164. [Google Scholar] [CrossRef]
- DeMichele, S.J.; Glenn Brown, R.; Krasin, B.W.; Sweeny, P.R. Connective tissue metabolism in muscular dystrophy. Amino acid composition of native types I, III, IV an V collagen isolated from the gastrocnemius muscle of embryonic chickens with genetic muscular dystrophy. Comp. Biochem. Physiol. 1985, 81B, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Mazzoni, M.; Soglia, F.; Petracci, M.; Sirri, F.; Lattanzio, G.; Clavenzani, P. Fiber metabolism, procollagen and collagen type III immunoreactivity in broiler pectoralis major affected by muscles abnormalities. Animals 2020, 10, 1081. [Google Scholar] [CrossRef]
- Soglia, F.; Petracci, M.; Davoli, R.; Zappaterra, M. A critical review of the mechanisms involved in the occurrence of growth related abnormalities affecting broiler chicken breast muscles. Poult. Sci. 2021, 100, 101180. [Google Scholar] [CrossRef]
- Liu, X.; Wu, H.; Byrne, M.; Krane, S.; Jaenisch, R. Type III collagen is crucial for collagen I fibrillogenesis and for normal cardiovascular development. Proc. Natl. Acad. Sci. USA 1997, 94, 1852–1856. [Google Scholar] [CrossRef]
- Gorres, K.L.; Raines, R.T. Prolyl 4-hydroxylase. Crit. Rev. Biochem. Mol. Biol. 2010, 45, 106–124. [Google Scholar] [CrossRef]
- Krane, S.K. The importance of proline residues in the structure, stability and susceptibility to proteolytic degradation of collagens. Amino Acids 2008, 35, 703–710. [Google Scholar] [CrossRef] [PubMed]
- Zahradníčková, H.; Opekar, S.; Řimnáčová, L.; Šimek, P.; Moos, M. Chiral secondary amino acids, their importance, and methods of analysis. Amino Acids 2022, 54, 687–719. [Google Scholar] [CrossRef] [PubMed]
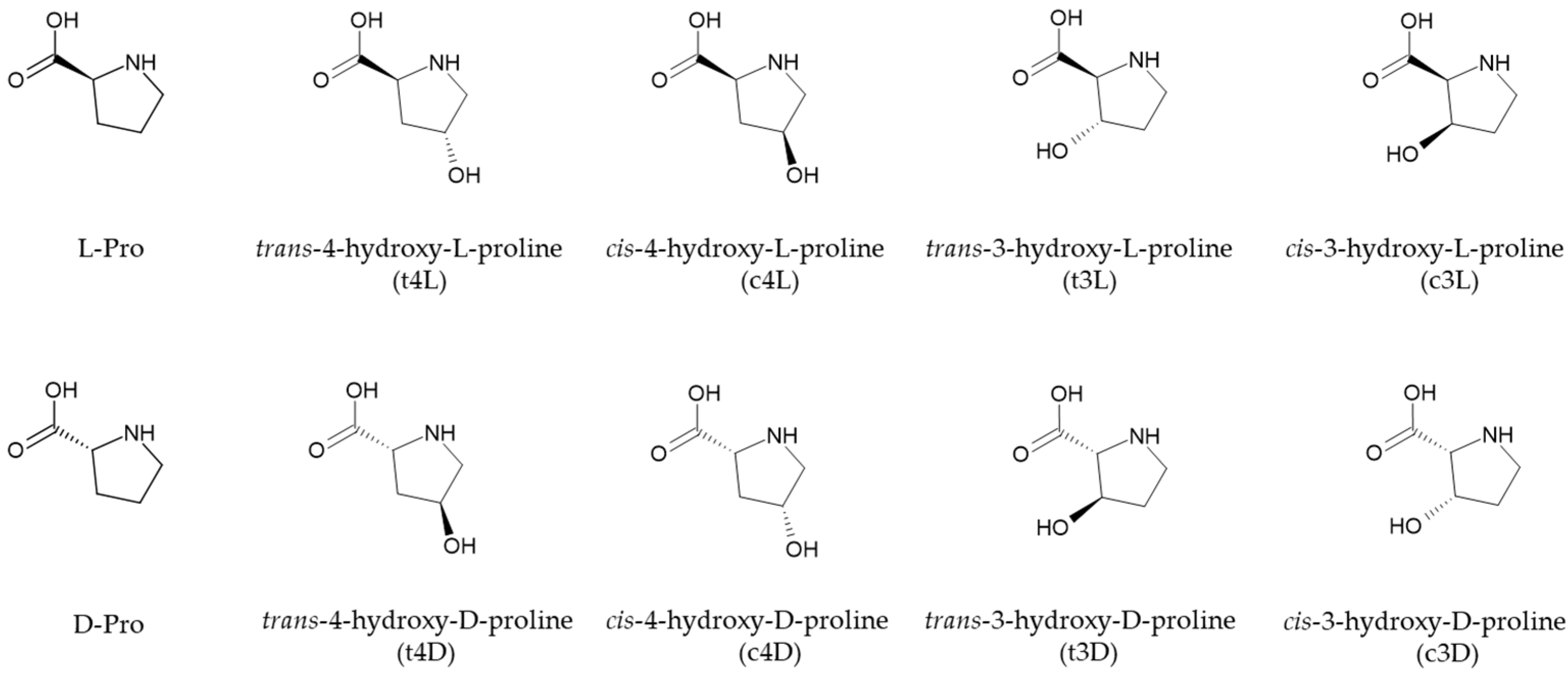




| Amino Acid (2) | Response 1 ± sd (3) | Response 2 (4) ± sd (3) |
|---|---|---|
| c3D | 0.509 ± 0.02 | 0.548 ± 0.02 |
| c3L | 1.00 ± 0.04 | 0.937 ± 0.02 |
| D-Pro | 0.221 ± 0.01 | 0.194 ± 0.01 |
| c4D | 0.639 ± 0.03 | 0.678 ± 0.02 |
| L-Pro | 0.232 ± 0.01 | 0.208 ± 0.01 |
| c4L | 0.956 ± 0.03 | 0.940 ± 0.03 |
| t4D | 1.02 ± 0.04 | 0.928 ± 0.04 |
| t4L | 1.05 ± 0.03 | 1.12 ± 0.03 |
| t3D | 0.658 ± 0.03 | 0.738 ± 0.04 |
| t3L | 0.995 ± 0.04 | 1.08 ± 0.04 |
| Amino Acid | Range, μM | Equation (1) | r2 | LOD | LOQ (2) |
|---|---|---|---|---|---|
| c3D | 1–100 | y = 0.3240x − 0.0147 | 0.999 | 0.08 | 0.20 |
| c3L | 1–100 | y = 0.2051x − 0.0361 | 0.998 | 0.08 | 0.20 |
| D-Pro | 1–100 | y = 0.0643x + 0.0168 | 0.998 | 0.20 | 0.50 |
| c4D | 1−100 | y = 0.2264x + 0.0513 | 0.999 | 0.08 | 0.20 |
| L-Pro | 20–200 | y = 0.0416x − 0.0506 | 0.999 | 0.20 | 0.50 |
| c4L | 1–100 | y = 0.1727x − 0.0949 | 0.998 | 0.10 | 0.30 |
| t4D | 1–100 | y = 0.1900x − 0.0316 | 0.999 | 0.10 | 0.30 |
| t4L | 20–200 | y = 0.2033x − 0.0251 | 0.999 | 0.10 | 0.30 |
| t3D | 1–100 | y = 0.1938x + 0.0185 | 0.998 | 0.15 | 0.40 |
| t3L | 1−100 | y = 0.3119x + 0.0327 | 0.999 | 0.15 | 0.40 |
| Amino Acid | Content (1) ± sd | Spike (1) | Recovery% (RSD%) |
|---|---|---|---|
| D-Pro | 2.51 ± 0.12 | 3.0 | 105.4 (5.5) |
| c4D | 2.56 ± 0.17 | 3.0 | 92.1 (6.7) |
| L-Pro | 66.6 ± 2.1 | 80.0 | 94.6 (5.6) |
| t4L | 89.2 ± 2.8 | 80.0 | 93.5 (4.2) |
| t3L | 0.80 ± 0.07 | 1.0 | 112.0 (7.6) |
| Amino Acid | SM | WB | N |
|---|---|---|---|
| D-Pro | 1.21 ± 0.23 | 0.98 ± 0.37 | 0.68 ± 0.35 |
| c4D | 1.66 ± 0.22 | 1.25 ± 0.26 | 1.08 ± 0.19 |
| L-Pro | 39.6 ± 2.2 | 40.5 ± 2.54 | 38.7 ± 2.6 |
| t4L | 55.8 ± 2.2 | 56.6 ± 2.3 | 59.3 ± 1.9 |
| t3L | 0.95 ± 0.11 | 0.59 ± 0.19 | 0.65 ± 0.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vodova, M.; Babini, E.; Soglia, F.; Bordini, M.; Lioi, M.; Tengattini, S.; Temporini, C.; Gotti, R. Cyclodextrin-Modified Capillary Zone Electrophoresis for the Chiral Analysis of Proline and Hydroxyproline Stereoisomers in Chicken Collagen Hydrolysates. Int. J. Mol. Sci. 2025, 26, 5832. https://doi.org/10.3390/ijms26125832
Vodova M, Babini E, Soglia F, Bordini M, Lioi M, Tengattini S, Temporini C, Gotti R. Cyclodextrin-Modified Capillary Zone Electrophoresis for the Chiral Analysis of Proline and Hydroxyproline Stereoisomers in Chicken Collagen Hydrolysates. International Journal of Molecular Sciences. 2025; 26(12):5832. https://doi.org/10.3390/ijms26125832
Chicago/Turabian StyleVodova, Milada, Elena Babini, Francesca Soglia, Martina Bordini, Martina Lioi, Sara Tengattini, Caterina Temporini, and Roberto Gotti. 2025. "Cyclodextrin-Modified Capillary Zone Electrophoresis for the Chiral Analysis of Proline and Hydroxyproline Stereoisomers in Chicken Collagen Hydrolysates" International Journal of Molecular Sciences 26, no. 12: 5832. https://doi.org/10.3390/ijms26125832
APA StyleVodova, M., Babini, E., Soglia, F., Bordini, M., Lioi, M., Tengattini, S., Temporini, C., & Gotti, R. (2025). Cyclodextrin-Modified Capillary Zone Electrophoresis for the Chiral Analysis of Proline and Hydroxyproline Stereoisomers in Chicken Collagen Hydrolysates. International Journal of Molecular Sciences, 26(12), 5832. https://doi.org/10.3390/ijms26125832








