Establishing a Dual Murine Model to Explore the Interactions Between Diabetes and Periodontitis in Mice
Abstract
1. Introduction
2. Results
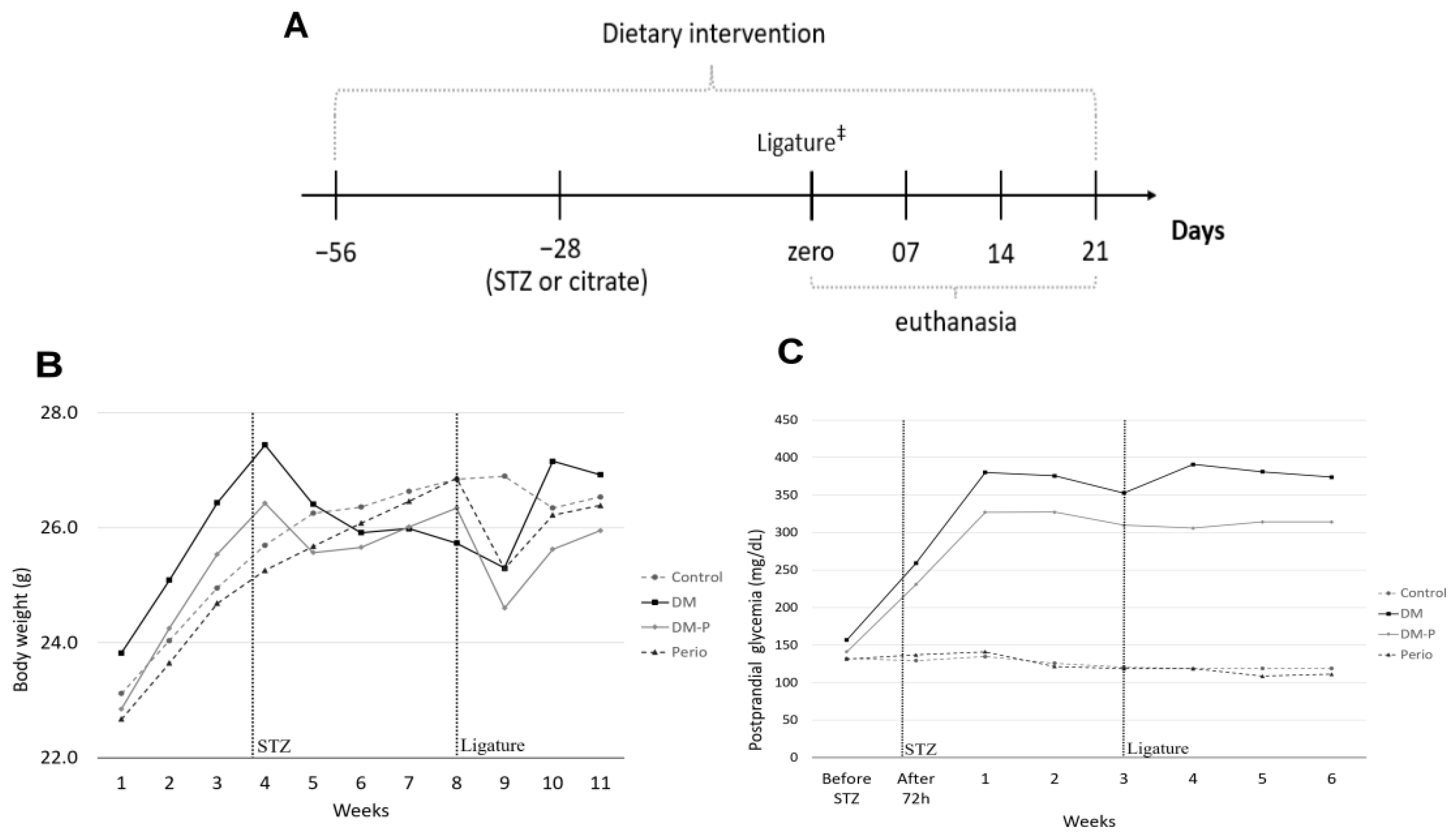
2.1. Animal Monitoring
2.2. Lipid and Carbohydrate Marker Levels
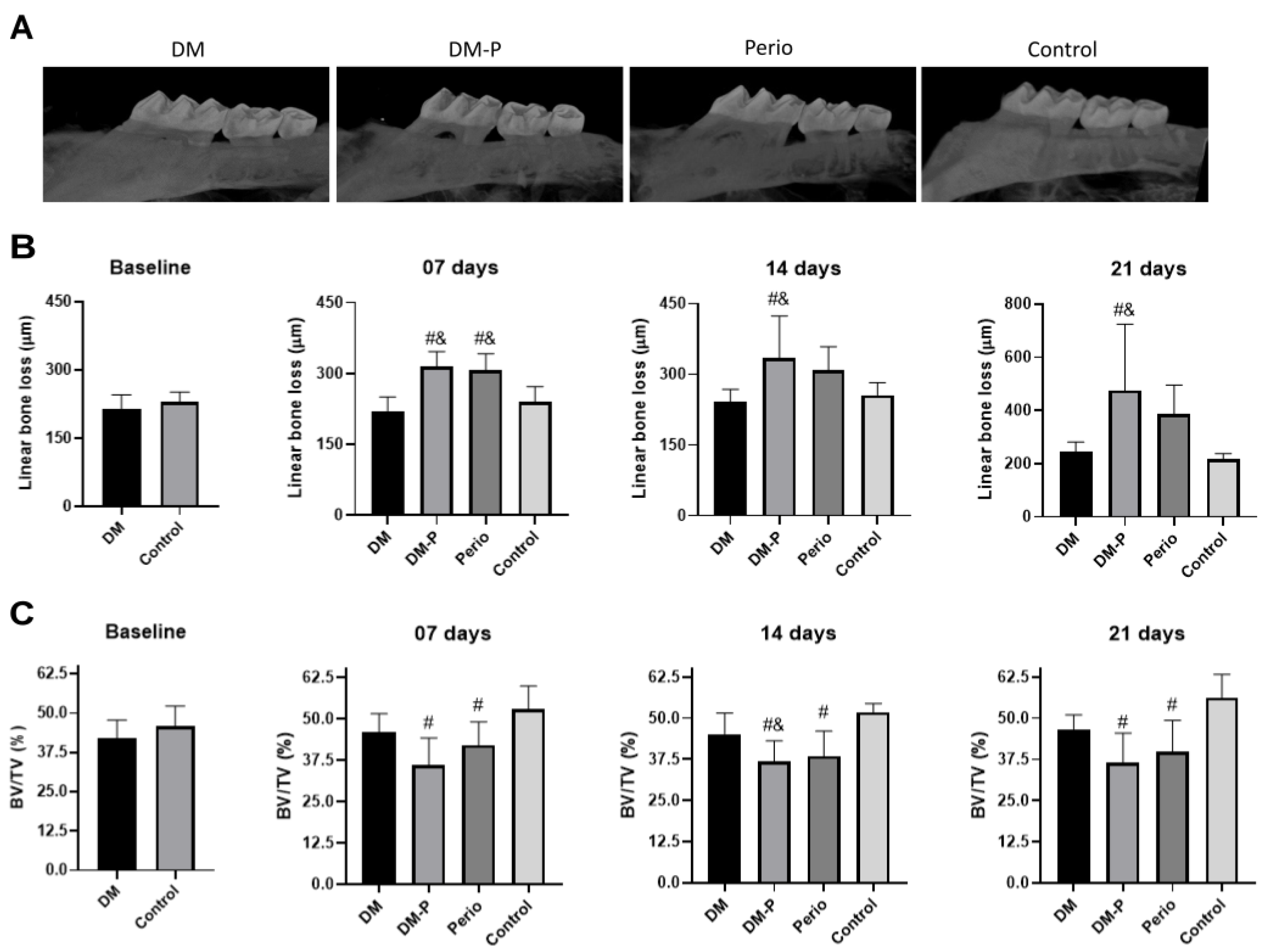
2.3. Alveolar Bone Loss
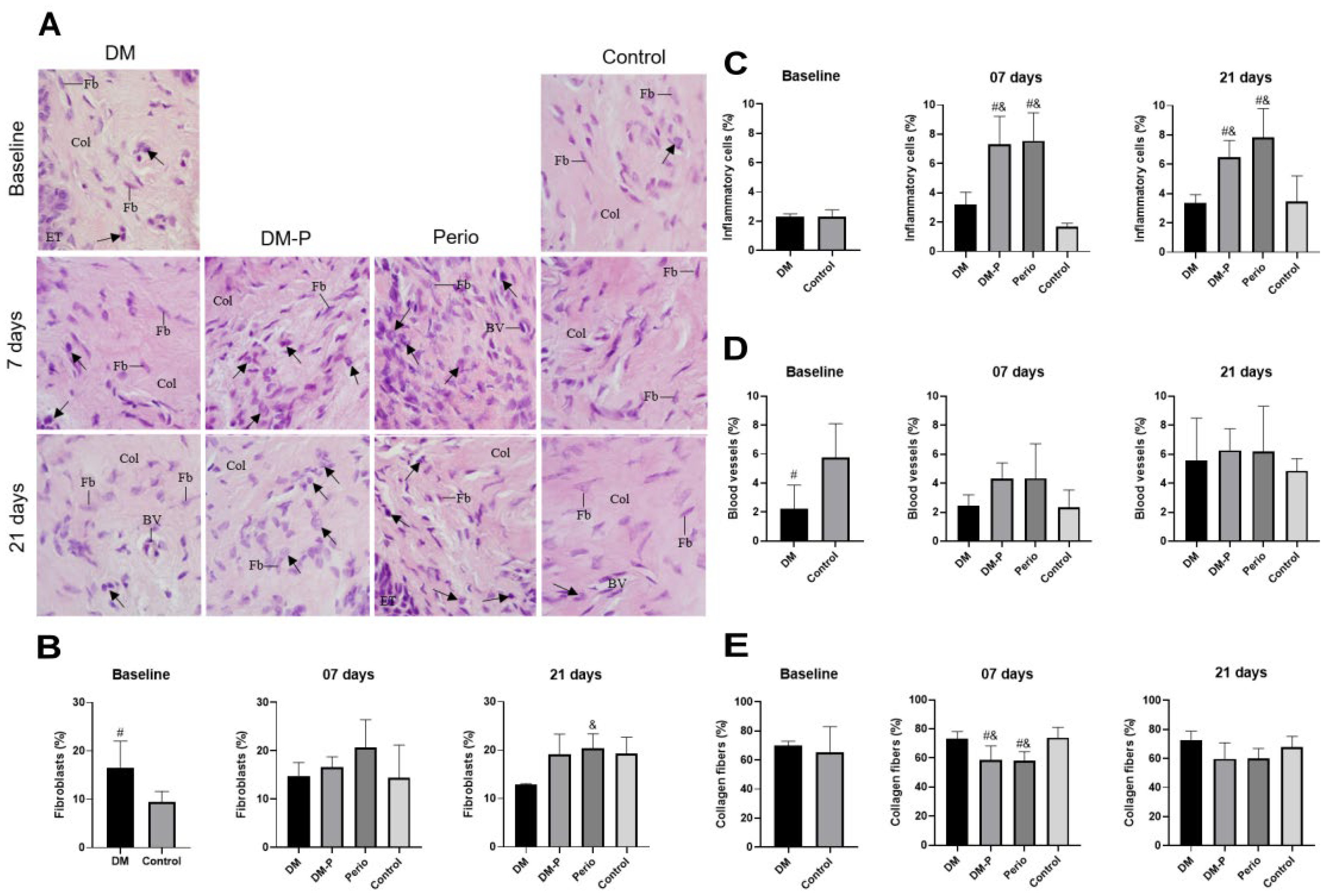
2.4. Inflammatory Process of Gingival Tissues
3. Discussion
4. Materials and Methods
4.1. Animals and Experimental Design
4.2. Experimental DM Model
4.3. Experimental Periodontitis Model
4.3.1. Cultivation of P. gingivalis
4.3.2. Ligature Placement
4.4. Analysis of Lipid and Carbohydrate Metabolism Markers
4.5. Microcomputed Tomography Analysis (Micro-CT)
4.6. Morphometric Analysis of Gingival Tissue
4.7. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Global Oral Health Status Report: Towards Universal Health Coverage for Oral Health by 2030; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Chen, M.X.; Zhong, Y.J.; Dong, Q.Q.; Wong, H.M.; Wen, Y.F. Global, regional, and national burden of severe periodontitis, 1990–2019: An analysis of the Global Burden of Disease Study 2019. J. Clin. Periodontol. 2021, 48, 1165–1188. [Google Scholar] [CrossRef] [PubMed]
- Van Dyke, T.E.; Bartold, P.M.; Reynolds, E.C. The Nexus Between Periodontal Inflammation and Dysbiosis. Front. Immunol. 2020, 11, 511. [Google Scholar] [CrossRef]
- Trindade, D.; Carvalho, R.; Machado, V.; Chambrone, L.; Mendes, J.J.; Botelho, J. Prevalence of periodontitis in dentate people between 2011 and 2020: A systematic review and meta-analysis of epidemiological studies. J. Clin. Periodontol. 2023, 50, 604–626. [Google Scholar] [CrossRef]
- Albandar, J.M.; Susin, C.; Hughes, F.J. Manifestations of systemic diseases and conditions that affect the periodontal attachment apparatus: Case definitions and diagnostic considerations. J. Periodontol. 2018, 89 (Suppl. S1), S183–S203. [Google Scholar] [CrossRef]
- Papapanou, P.N.; Sanz, M.; Buduneli, N.; Dietrich, T.; Feres, M.; Fine, D.H.; Flemmig, T.F.; Garcia, R.; Giannobile, W.V.; Graziani, F.; et al. Periodontitis: Consensus report of workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Clin. Periodontol. 2018, 45 (Suppl. S20), S162–S170. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee. 2. Diagnosis and Classification of Diabetes: Standards of Care in Diabetes-2024. Diabetes Care 2024, 47 (Suppl. S1), S20–S42. [Google Scholar] [CrossRef]
- Atlas, D. IDF Diabetes Atlas, 8th ed.; International Diabetes Federation: Brussels, Belgium, 2017. [Google Scholar]
- Ahmad, E.; Lim, S.; Lamptey, R.; Webb, D.R.; Davies, M.J. Type 2 diabetes. Lancet 2022, 400, 1803–1820. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee. 8. Obesity and Weight Management for the Prevention and Treatment of Type 2 Diabetes: Standards of Care in Diabetes-2024. Diabetes Care 2024, 47 (Suppl. S1), S145–S157. [Google Scholar] [CrossRef]
- Ruze, R.; Liu, T.; Zou, X.; Song, J.; Chen, Y.; Xu, R.; Yin, X.; Xu, Q. Obesity and type 2 diabetes mellitus: Connections in epidemiology, pathogenesis, and treatments. Front. Endocrinol. 2023, 14, 1161521. [Google Scholar] [CrossRef]
- Graves, D.T.; Ding, Z.; Yang, Y. The impact of diabetes on periodontal diseases. Periodontol. 2000 2020, 82, 214–224. [Google Scholar] [CrossRef]
- Su, Y.; Ye, L.; Hu, C.; Zhang, Y.; Liu, J.; Shao, L. Periodontitis as a promoting factor of T2D: Current evidence and mechanisms. Int. J. Oral Sci. 2023, 15, 25. [Google Scholar] [CrossRef] [PubMed]
- Stohr, J.; Barbaresko, J.; Neuenschwander, M.; Schlesinger, S. Bidirectional association between periodontal disease and diabetes mellitus: A systematic review and meta-analysis of cohort studies. Sci. Rep. 2021, 11, 13686. [Google Scholar] [CrossRef] [PubMed]
- Hajishengallis, G.; Chavakis, T. Local and systemic mechanisms linking periodontal disease and inflammatory comorbidities. Nat. Rev. Immunol. 2021, 21, 426–440. [Google Scholar] [CrossRef] [PubMed]
- Graves, D.T.; Kang, J.; Andriankaja, O.; Wada, K.; Rossa, C., Jr. Animal models to study host-bacteria interactions involved in periodontitis. Front. Oral Biol. 2012, 15, 117–132. [Google Scholar] [CrossRef]
- Bodet, C.; Chandad, F.; Grenier, D. Pathogenic potential of Porphyromonas gingivalis, Treponema denticola and Tannerella forsythia, the red bacterial complex associated with periodontitis. Pathol. Biol. 2007, 55, 154–162. [Google Scholar] [CrossRef]
- Acqua, Y.D.; Hernandez, C.; Fogacci, M.; Barbirato, D.; Palioto, D. Local and systemic effects produced in different models of experimental periodontitis in mice: A systematic review. Arch. Oral Biol. 2022, 143, 105528. [Google Scholar] [CrossRef]
- Mysak, J.; Podzimek, S.; Sommerova, P.; Lyuya-Mi, Y.; Bartova, J.; Janatova, T.; Prochazkova, J.; Duskova, J. Porphyromonas gingivalis: Major periodontopathic pathogen overview. J. Immunol. Res. 2014, 2014, 476068. [Google Scholar] [CrossRef]
- Liu, F.; Zhu, B.; An, Y.; Zhou, Z.; Xiong, P.; Li, X.; Mi, Y.; He, T.; Chen, F.; Wu, B. Gingipain from Porphyromonas gingivalis causes insulin resistance by degrading insulin receptors through direct proteolytic effects. Int. J. Oral Sci. 2024, 16, 53. [Google Scholar] [CrossRef]
- Furman, B.L. Streptozotocin-Induced Diabetic Models in Mice and Rats. Curr. Protoc. 2021, 1, e78. [Google Scholar] [CrossRef]
- Wang, B.; Chandrasekera, P.C.; Pippin, J.J. Leptin- and leptin receptor-deficient rodent models: Relevance for human type 2 diabetes. Curr. Diabetes Rev. 2014, 10, 131–145. [Google Scholar] [CrossRef]
- King, A.J. The use of animal models in diabetes research. Br. J. Pharmacol. 2012, 166, 877–894. [Google Scholar] [CrossRef] [PubMed]
- Skovso, S. Modeling type 2 diabetes in rats using high fat diet and streptozotocin. J. Diabetes Investig. 2014, 5, 349–358. [Google Scholar] [CrossRef] [PubMed]
- de Molon, R.S.; de Avila, E.D.; Boas Nogueira, A.V.; Chaves de Souza, J.A.; Avila-Campos, M.J.; de Andrade, C.R.; Cirelli, J.A. Evaluation of the host response in various models of induced periodontal disease in mice. J. Periodontol. 2014, 85, 465–477. [Google Scholar] [CrossRef] [PubMed]
- de Molon, R.S.; Mascarenhas, V.I.; de Avila, E.D.; Finoti, L.S.; Toffoli, G.B.; Spolidorio, D.M.; Scarel-Caminaga, R.M.; Tetradis, S.; Cirelli, J.A. Long-term evaluation of oral gavage with periodontopathogens or ligature induction of experimental periodontal disease in mice. Clin. Oral Investig. 2016, 20, 1203–1216. [Google Scholar] [CrossRef]
- Lou, J.; Zhang, B.; Cai, J.; Zhang, L.; Zhao, Y.; Zhao, Z. Diabetes exacerbates periodontitis by disrupting IL-33-mediated interaction between periodontal ligament fibroblasts and macrophages. Int. Immunopharmacol. 2025, 147, 113896. [Google Scholar] [CrossRef]
- Fu, X.; Liu, B.; Sun, J.; Zhang, X.; Zhu, Z.; Wang, H.; Xiao, A.; Gan, X. Perturbation of mitochondrial dynamics links to the aggravation of periodontitis by diabetes. J. Histotechnol. 2023, 46, 139–150. [Google Scholar] [CrossRef]
- Cavagni, J.; de Macedo, I.C.; Gaio, E.J.; Souza, A.; de Molon, R.S.; Cirelli, J.A.; Hoefel, A.L.; Kucharski, L.C.; Torres, I.L.; Rosing, C.K. Obesity and Hyperlipidemia Modulate Alveolar Bone Loss in Wistar Rats. J. Periodontol. 2016, 87, e9–e17. [Google Scholar] [CrossRef]
- Ranbhise, J.S.; Ju, S.; Singh, M.K.; Han, S.; Akter, S.; Ha, J.; Choe, W.; Kim, S.S.; Kang, I. Chronic Inflammation and Glycemic Control: Exploring the Bidirectional Link Between Periodontitis and Diabetes. Dent. J. 2025, 13, 100. [Google Scholar] [CrossRef]
- Chen, X.; He, Y.; Zhou, Y.; Gong, H.; Zhang, J.; Qiu, G.; Shen, Y.; Qin, W. Modulatory role of exogenous arachidonic acid in periodontitis with type 2 diabetes mellitus mice. BMC Oral Health 2025, 25, 264. [Google Scholar] [CrossRef]
- Li, Y.; Lu, Z.; Zhang, L.; Kirkwood, C.L.; Kirkwood, K.L.; Lopes-Virella, M.F.; Huang, Y. Inhibition of acid sphingomyelinase by imipramine abolishes the synergy between metabolic syndrome and periodontitis on alveolar bone loss. J. Periodontal Res. 2022, 57, 173–185. [Google Scholar] [CrossRef]
- Li, L.; Qin, W.; Ye, T.; Wang, C.; Qin, Z.; Ma, Y.; Mu, Z.; Jiao, K.; Tay, F.R.; Niu, W.; et al. Bioactive Zn-V-Si-Ca Glass Nanoparticle Hydrogel Microneedles with Antimicrobial and Antioxidant Properties for Bone Regeneration in Diabetic Periodontitis. ACS Nano 2025, 19, 7981–7995. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Wang, J.; Ye, C.; Lin, J.; Ran, J.; Jia, Z.; Gong, J.; Zhang, Y.; Xiang, J.; Lu, X.; et al. Polyphenol-mediated redox-active hydrogel with H2S gaseous-bioelectric coupling for periodontal bone healing in diabetes. Nat. Commun. 2024, 15, 9071. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Golub, L.M.; Lee, H.M.; Walker, S.G. Diabetes, periodontal disease, and novel therapeutic approaches- host modulation therapy. Front. Clin. Diabetes Healthc. 2025, 6, 1529086. [Google Scholar] [CrossRef] [PubMed]
- Heydemann, A. An Overview of Murine High Fat Diet as a Model for Type 2 Diabetes Mellitus. J. Diabetes Res. 2016, 2016, 2902351. [Google Scholar] [CrossRef]
- Racine, K.C.; Iglesias-Carres, L.; Herring, J.A.; Wieland, K.L.; Ellsworth, P.N.; Tessem, J.S.; Ferruzzi, M.G.; Kay, C.D.; Neilson, A.P. The high-fat diet and low-dose streptozotocin type-2 diabetes model induces hyperinsulinemia and insulin resistance in male but not female C57BL/6J mice. Nutr. Res. 2024, 131, 135–146. [Google Scholar] [CrossRef]
- Winzell, M.S.; Ahren, B. The high-fat diet-fed mouse: A model for studying mechanisms and treatment of impaired glucose tolerance and type 2 diabetes. Diabetes 2004, 53 (Suppl. S3), S215–S219. [Google Scholar] [CrossRef]
- Costa, M.C.; Lima, T.F.O.; Arcaro, C.A.; Inacio, M.D.; Batista-Duharte, A.; Carlos, I.Z.; Spolidorio, L.C.; Assis, R.P.; Brunetti, I.L.; Baviera, A.M. Trigonelline and curcumin alone, but not in combination, counteract oxidative stress and inflammation and increase glycation product detoxification in the liver and kidney of mice with high-fat diet-induced obesity. J. Nutr. Biochem. 2020, 76, 108303. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, P.; Aprecio, R.; Zhang, D.; Li, H.; Ji, N.; Mohamed, O.; Zhang, W.; Li, Y.; Ding, Y. Comparison of Experimental Diabetic Periodontitis Induced by Porphyromonas gingivalis in Mice. J. Diabetes Res. 2016, 2016, 4840203. [Google Scholar] [CrossRef]
- Huang, K.C.; Chuang, P.Y.; Yang, T.Y.; Tsai, Y.H.; Li, Y.Y.; Chang, S.F. Diabetic Rats Induced Using a High-Fat Diet and Low-Dose Streptozotocin Treatment Exhibit Gut Microbiota Dysbiosis and Osteoporotic Bone Pathologies. Nutrients 2024, 16, 1220. [Google Scholar] [CrossRef]
- Xepapadaki, E.; Nikdima, I.; Sagiadinou, E.C.; Zvintzou, E.; Kypreos, K.E. HDL and type 2 diabetes: The chicken or the egg? Diabetologia 2021, 64, 1917–1926. [Google Scholar] [CrossRef]
- Hahn, M.; van Krieken, P.P.; Nord, C.; Alanentalo, T.; Morini, F.; Xiong, Y.; Eriksson, M.; Mayer, J.; Kostromina, E.; Ruas, J.L.; et al. Topologically selective islet vulnerability and self-sustained downregulation of markers for beta-cell maturity in streptozotocin-induced diabetes. Commun. Biol. 2020, 3, 541. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Fan, C.; Cai, Y.; Fang, S.; Zeng, Y.; Zhang, Y.; Lin, X.; Zhang, H.; Xue, Y.; Guan, M. Transplantation of brown adipose tissue up-regulates miR-99a to ameliorate liver metabolic disorders in diabetic mice by targeting NOX4. Adipocyte 2020, 9, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Chao, P.C.; Li, Y.; Chang, C.H.; Shieh, J.P.; Cheng, J.T.; Cheng, K.C. Investigation of insulin resistance in the popularly used four rat models of type-2 diabetes. Biomed. Pharmacother. 2018, 101, 155–161. [Google Scholar] [CrossRef]
- Martinez-Garcia, M.; Hernandez-Lemus, E. Periodontal Inflammation and Systemic Diseases: An Overview. Front. Physiol. 2021, 12, 709438. [Google Scholar] [CrossRef]
- Palioto, D.B.; Finoti, L.S.; Kinane, D.F.; Benakanakere, M. Epigenetic and inflammatory events in experimental periodontitis following systemic microbial challenge. J. Clin. Periodontol. 2019, 46, 819–829. [Google Scholar] [CrossRef]
- Murugaiyan, V.; Utreja, S.; Hovey, K.M.; Sun, Y.; LaMonte, M.J.; Wactawski-Wende, J.; Diaz, P.I.; Buck, M.J. Defining Porphyromonas gingivalis strains associated with periodontal disease. Sci. Rep. 2024, 14, 6222. [Google Scholar] [CrossRef]
- Haugsten, H.R.; Kristoffersen, A.K.; Haug, T.M.; Soland, T.M.; Ovstebo, R.; Aass, H.C.D.; Enersen, M.; Galtung, H.K. Isolation, characterization, and fibroblast uptake of bacterial extracellular vesicles from Porphyromonas gingivalis strains. Microbiologyopen 2023, 12, e1388. [Google Scholar] [CrossRef]
- Blasco-Baque, V.; Garidou, L.; Pomie, C.; Escoula, Q.; Loubieres, P.; Le Gall-David, S.; Lemaitre, M.; Nicolas, S.; Klopp, P.; Waget, A.; et al. Periodontitis induced by Porphyromonas gingivalis drives periodontal microbiota dysbiosis and insulin resistance via an impaired adaptive immune response. Gut 2017, 66, 872–885. [Google Scholar] [CrossRef]
- Tian, J.; Liu, C.; Zheng, X.; Jia, X.; Peng, X.; Yang, R.; Zhou, X.; Xu, X. Porphyromonas gingivalis Induces Insulin Resistance by Increasing BCAA Levels in Mice. J. Dent. Res. 2020, 99, 839–846. [Google Scholar] [CrossRef]
- Cai, Z.; Du, S.; Zhao, N.; Huang, N.; Yang, K.; Qi, L. Periodontitis promotes the progression of diabetes mellitus by enhancing autophagy. Heliyon 2024, 10, e24366. [Google Scholar] [CrossRef]
- Zhang, B.; Yang, Y.; Yi, J.; Zhao, Z.; Ye, R. Hyperglycemia modulates M1/M2 macrophage polarization via reactive oxygen species overproduction in ligature-induced periodontitis. J. Periodontal Res. 2021, 56, 991–1005. [Google Scholar] [CrossRef] [PubMed]
- Xiao, E.; Mattos, M.; Vieira, G.H.A.; Chen, S.; Correa, J.D.; Wu, Y.; Albiero, M.L.; Bittinger, K.; Graves, D.T. Diabetes Enhances IL-17 Expression and Alters the Oral Microbiome to Increase Its Pathogenicity. Cell Host Microbe 2017, 22, 120–128.e124. [Google Scholar] [CrossRef] [PubMed]
- Canadian Council on Animal Care. Guide to the Care and Use of Experimental Animals, 2nd ed.; Canadian Council on Animal Care: Ottawa, ON, Canada, 1993; Volume 1, p. 201. [Google Scholar]
- Reeves, P.G.; Nielsen, F.H.; Fahey, G.C., Jr. AIN-93 purified diets for laboratory rodents: Final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J. Nutr. 1993, 123, 1939–1951. [Google Scholar] [CrossRef]
- De Avila, E.D.; Castro, A.G.; Tagit, O.; Krom, B.P.; Löwik, D.; Van Well, A.A.; Bannenberg, L.J.; Vergani, C.E.; Van den Beucken, J.J. Anti-bacterial efficacy via drug-delivery system from layer-by-layer coating for percutaneous dental implant components. Appl. Surf. Sci. 2019, 488, 194–204. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, X.; Lin, J.; Hu, Y.; Zhao, Q.; Kawai, T.; Taubman, M.A.; Han, X. B10 Cells Alleviate Periodontal Bone Loss in Experimental Periodontitis. Infect. Immun. 2017, 85, e00335-17. [Google Scholar] [CrossRef]
- Lee, S.; Muniyappa, R.; Yan, X.; Chen, H.; Yue, L.Q.; Hong, E.G.; Kim, J.K.; Quon, M.J. Comparison between surrogate indexes of insulin sensitivity and resistance and hyperinsulinemic euglycemic clamp estimates in mice. Am. J. Physiol. Endocrinol. Metab. 2008, 294, E261–E270. [Google Scholar] [CrossRef]
- Nogueira, A.V.; de Molon, R.S.; Nokhbehsaim, M.; Deschner, J.; Cirelli, J.A. Contribution of biomechanical forces to inflammation-induced bone resorption. J. Clin. Periodontol. 2017, 44, 31–41. [Google Scholar] [CrossRef]
- Longhini, R.; Aparecida de Oliveira, P.; Sasso-Cerri, E.; Cerri, P.S. Cimetidine reduces alveolar bone loss in induced periodontitis in rat molars. J. Periodontol. 2014, 85, 1115–1125. [Google Scholar] [CrossRef]
- Silva, R.C.L.; Sasso-Cerri, E.; Cerri, P.S. Diacerein-induced interleukin-1beta deficiency reduces the inflammatory infiltrate and immunoexpression of matrix metalloproteinase-8 in periodontitis in rat molars. J. Periodontol. 2022, 93, 1540–1552. [Google Scholar] [CrossRef]
- Fernandes, N.A.R.; Camilli, A.C.; Maldonado, L.A.G.; Pacheco, C.G.P.; Silva, A.F.; Molon, R.S.; Spolidorio, L.C.; Ribeiro de Assis, L.; Regasini, L.O.; Rossa Junior, C.; et al. Chalcone T4, a novel chalconic compound, inhibits inflammatory bone resorption in vivo and suppresses osteoclastogenesis in vitro. J. Periodontal Res. 2021, 56, 569–578. [Google Scholar] [CrossRef]
- Souza, J.A.C.; Magalhaes, F.A.C.; Oliveira, G.; Molon, R.S.; Zuanon, J.A.; Souza, P.P.C. Pam2CSK4 (TLR2 agonist) induces periodontal destruction in mice. Braz. Oral Res. 2020, 34, e012. [Google Scholar] [CrossRef]
| Time Point | DM | DM-P | Perio | Control | |
|---|---|---|---|---|---|
| Physical parameter | |||||
| Body weight (g) | baseline | 23.5 (±2.9) b | - | - | 27.5 (±1.2) a |
| 7 days | 27.0 (±2.1) a | 25.6 (±1.6) a | 25.2 (±2.6) a | 25.2 (±1.7) a | |
| 14 days | 27.9 (±2.4) a | 25.4 (±2.1) a | 26.0 (±2.0) a | 26.4 (±2.2) a | |
| 21 days | 26.0 (±1.8) a | 27.0 (±1.8) a | 27.1 (±2.0) a | 26.7 (±1.7) a | |
| Plasma markers | |||||
| Fasting blood glucose (mg/dL) | baseline | 278.5 (±57.8) a | - | - | 92.7 (±31.2) b |
| 7 days | 238.0 (±99.4) a | 284.3 (±74.6) a | 91.1 (±24.0) b | 78.9 (±27.8) b | |
| 14 days | 281.1 (±92.1) a | 228.7 (±81.1) a | 111.8 (±28.2) b | 105.1 (±21.9) b | |
| 21 days | 235.3 (±106.7) a | 233.8 (±68.7) a | 98.7 (±23.0) b | 60.0 (±20.0) b | |
| Insulin (pg/mL) | baseline | 75.4 (±11.1) a | - | - | 84.2 (±15.9) a |
| 7 days | 80.5 (±7.9) a | 92.9 (±10.4) a | 89.0 (±12.8) a | 90.3 (±9.6) a | |
| 14 days | 98.7 (±14.8) a | 96.0 (±15.9) a | 97.2 (±9.1) a | 94.3 (±18.1) a | |
| 21 days | 99.9 (±14.4) a | 111.0 (±13.1) a | 103.9 (±19.6) a | 99.2 (±37.3) a | |
| Triglycerides (mg/dL) | baseline | 33.7 (±9.4) a | - | - | 34.6 (±5.7) a |
| 7 days | 39.0 (±12.3) a | 32.7 (±5.2) a | 34.6 (±10.9) a | 30.9 (±16.0) a | |
| 14 days | 43.6 (±18.4) a | 32.0 (±7.5) a | 35.8 (±7.0) a | 37.5 (±4.7) a | |
| 21 days | 35.3 (±12.0) a | 22.0 (±7.0) a | 40.0 (±12.9) a | 34.7 (±21.4) a | |
| HDL-cholesterol (mg/dL) | baseline | 38.4 (±13.5) a | - | - | 21.1 (±5.7) b |
| 7 days | 31.4 (±11.8) a | 32.0 (±5.7) a | 22.6 (±6.4) a | 19.7 (±8.3) a | |
| 14 days | 35.8 (±14.7) a | 29.3 (±5.1) a,b | 24.4 (±7.1) a,b | 21.8 (±6.5) b | |
| 21 days | 31.7 (±12.5) a | 30.7 (±9.6) a | 24.3 (±7.1) a | 20.0 (±8.2) a | |
| Total cholesterol (mg/dL) | baseline | 87.0 (±40.6) a | - | - | 39.8 (±11.4) b |
| 7 days | 61.6 (±25.2) a | 61.1 (±10.2) a | 43.6 (±12.0) a,b | 35.1 (±15.3) b | |
| 14 days | 65.7 (±28.0) a | 59.7 (±6.4) a,b | 47.0 (±12.1) a,b | 41.1 (±8.5) b | |
| 21 days | 61.7 (±21.4) a | 59.2 (±22.9) a | 43.0 (±12.8) a | 37.0 (±17.4) a | |
| Marker of insulin resistance | |||||
| HOMA-IR | baseline | 1.4 (±0.6) a | - | - | 0.5 (±0.2) b |
| 7 days | 1.2 (±0.5) a | 1.6 (±0.5) a | 0.5 (±0.2) b | 0.4(±0.2) b | |
| 14 days | 1.7 (±0.7) a | 1.4 (±0.6) a | 0.7 (±0.2) b | 0.6 (±0.2) b | |
| 21 days | 1.5 (±0.8) a | 1.6 (±0.4) a | 0.6 (±0.2) b | 0.4 (±0.2) b |
| Diet | ||
|---|---|---|
| Ingredients (g/100 g) | High-Fat | Control |
| Corn starch | 14.95 | 42.75 |
| Casein | 20.00 | 20.00 |
| Dextrinized corn starch | 10.00 | 13.20 |
| Sucrose | 10.00 | 10.00 |
| Soybean oil | 4.00 | 4.00 |
| Cellulose | 5.00 | 5.00 |
| Mineral mix (AIN 93G) * | 3.50 | 3.50 |
| Vitamin mix (AIN 93) * | 1.00 | 1.00 |
| L-cystine | 0.30 | 0.30 |
| Choline bitartrate | 0.25 | 0.25 |
| Lard | 31.00 | - |
| Total | 100.00 | 100.00 |
| Energy (kcal/100 g) | 540 | 385 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, B.R.; Hidalgo, M.A.R.; Silva, R.C.L.; de Avila, E.D.; Fuentes, D.L.P.; Carlos, I.Z.; Figueiredo, I.D.; Cerri, E.S.; Cerri, P.S.; Baviera, A.M.; et al. Establishing a Dual Murine Model to Explore the Interactions Between Diabetes and Periodontitis in Mice. Int. J. Mol. Sci. 2025, 26, 5611. https://doi.org/10.3390/ijms26125611
Silva BR, Hidalgo MAR, Silva RCL, de Avila ED, Fuentes DLP, Carlos IZ, Figueiredo ID, Cerri ES, Cerri PS, Baviera AM, et al. Establishing a Dual Murine Model to Explore the Interactions Between Diabetes and Periodontitis in Mice. International Journal of Molecular Sciences. 2025; 26(12):5611. https://doi.org/10.3390/ijms26125611
Chicago/Turabian StyleSilva, Bárbara R., Marco A. R. Hidalgo, Renata C. L. Silva, Erica D. de Avila, Deivys L. P. Fuentes, Iracilda Z. Carlos, Ingrid D. Figueiredo, Estela S. Cerri, Paulo S. Cerri, Amanda M. Baviera, and et al. 2025. "Establishing a Dual Murine Model to Explore the Interactions Between Diabetes and Periodontitis in Mice" International Journal of Molecular Sciences 26, no. 12: 5611. https://doi.org/10.3390/ijms26125611
APA StyleSilva, B. R., Hidalgo, M. A. R., Silva, R. C. L., de Avila, E. D., Fuentes, D. L. P., Carlos, I. Z., Figueiredo, I. D., Cerri, E. S., Cerri, P. S., Baviera, A. M., de Molon, R. S., & Scarel-Caminaga, R. M. (2025). Establishing a Dual Murine Model to Explore the Interactions Between Diabetes and Periodontitis in Mice. International Journal of Molecular Sciences, 26(12), 5611. https://doi.org/10.3390/ijms26125611





