Cardiac Damage in Hypertension: From Molecular Mechanisms to Novel Therapeutic Approaches
Abstract
1. Introduction
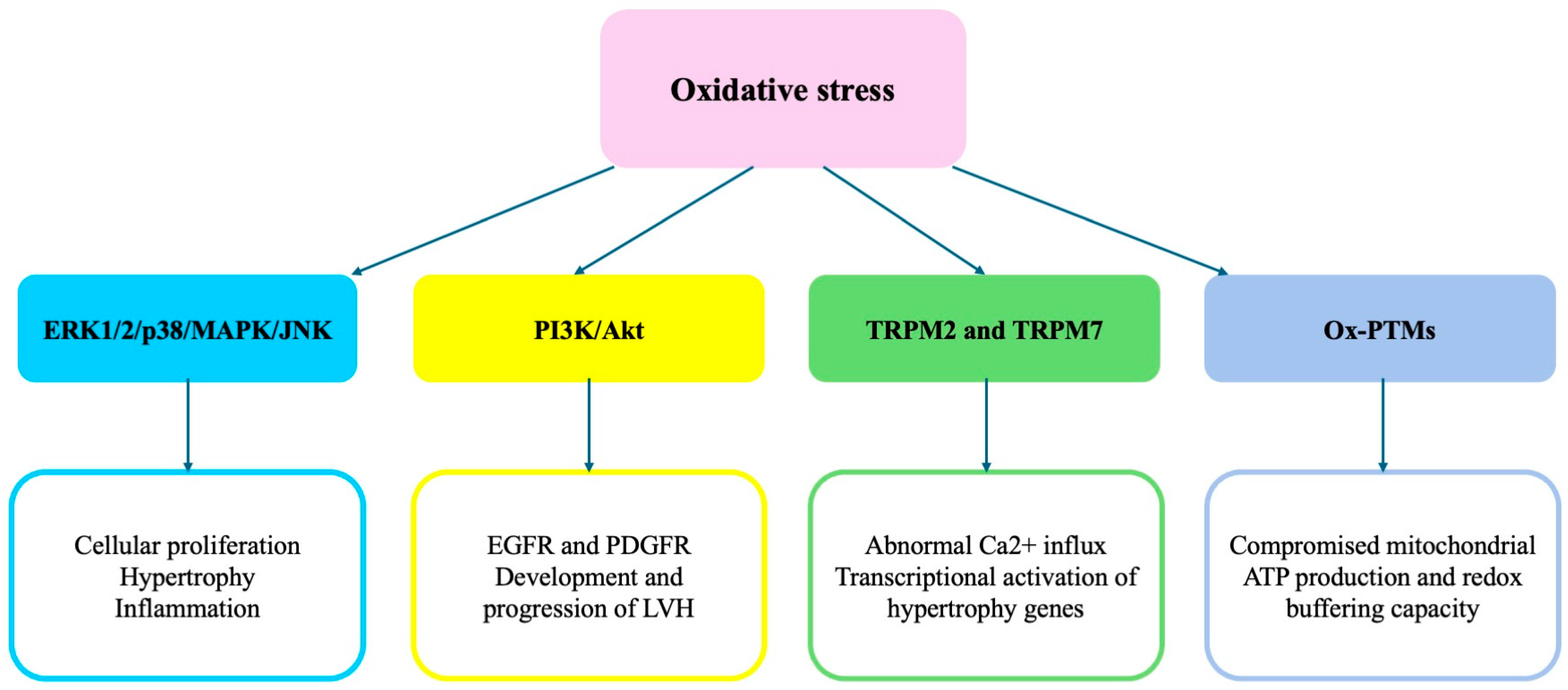
2. Signaling Pathways Underlying ROS-Mediated Development of HMOD
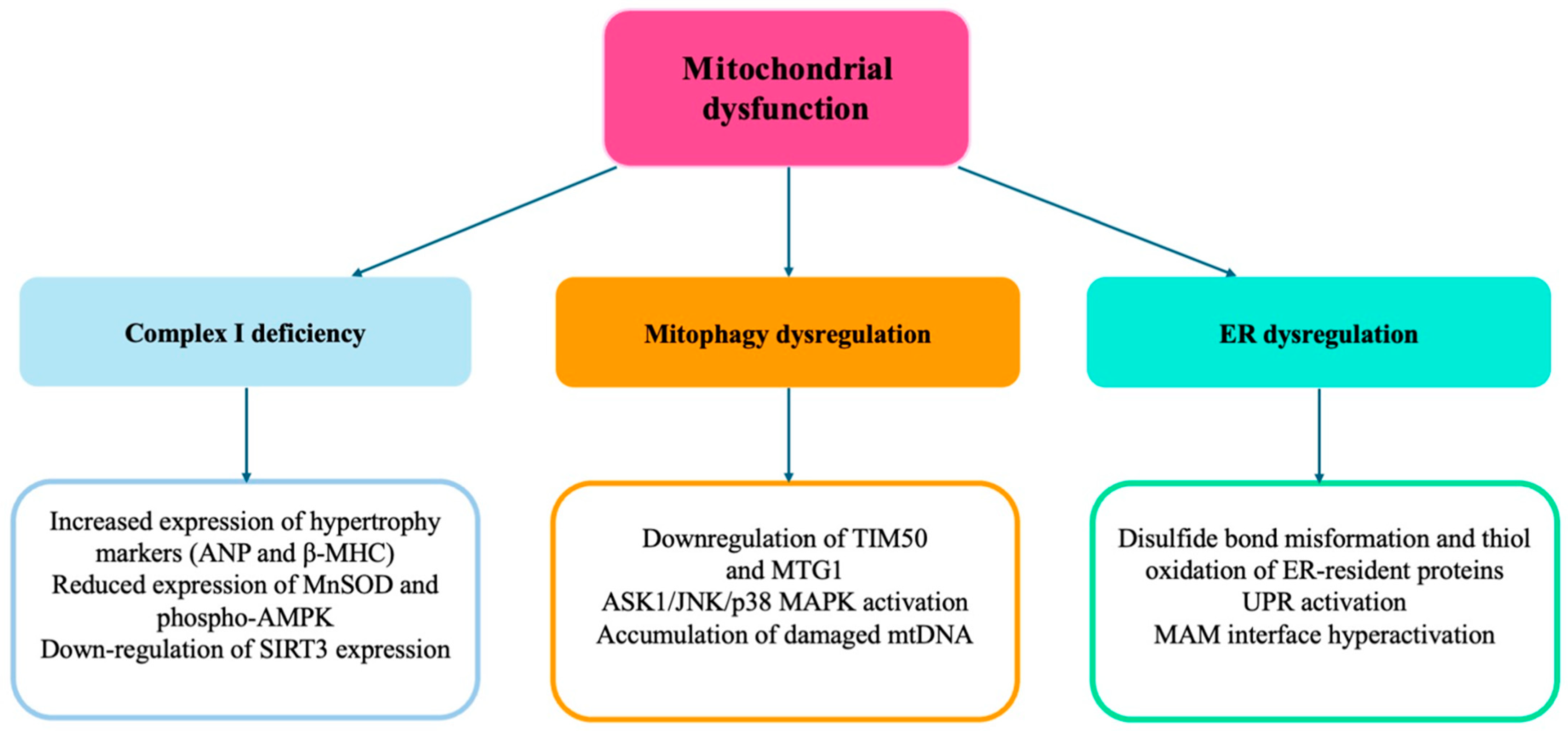
3. Role of Mitochondrial Dysfunction
4. Epigenetic Modulation
5. Role of Inflammation
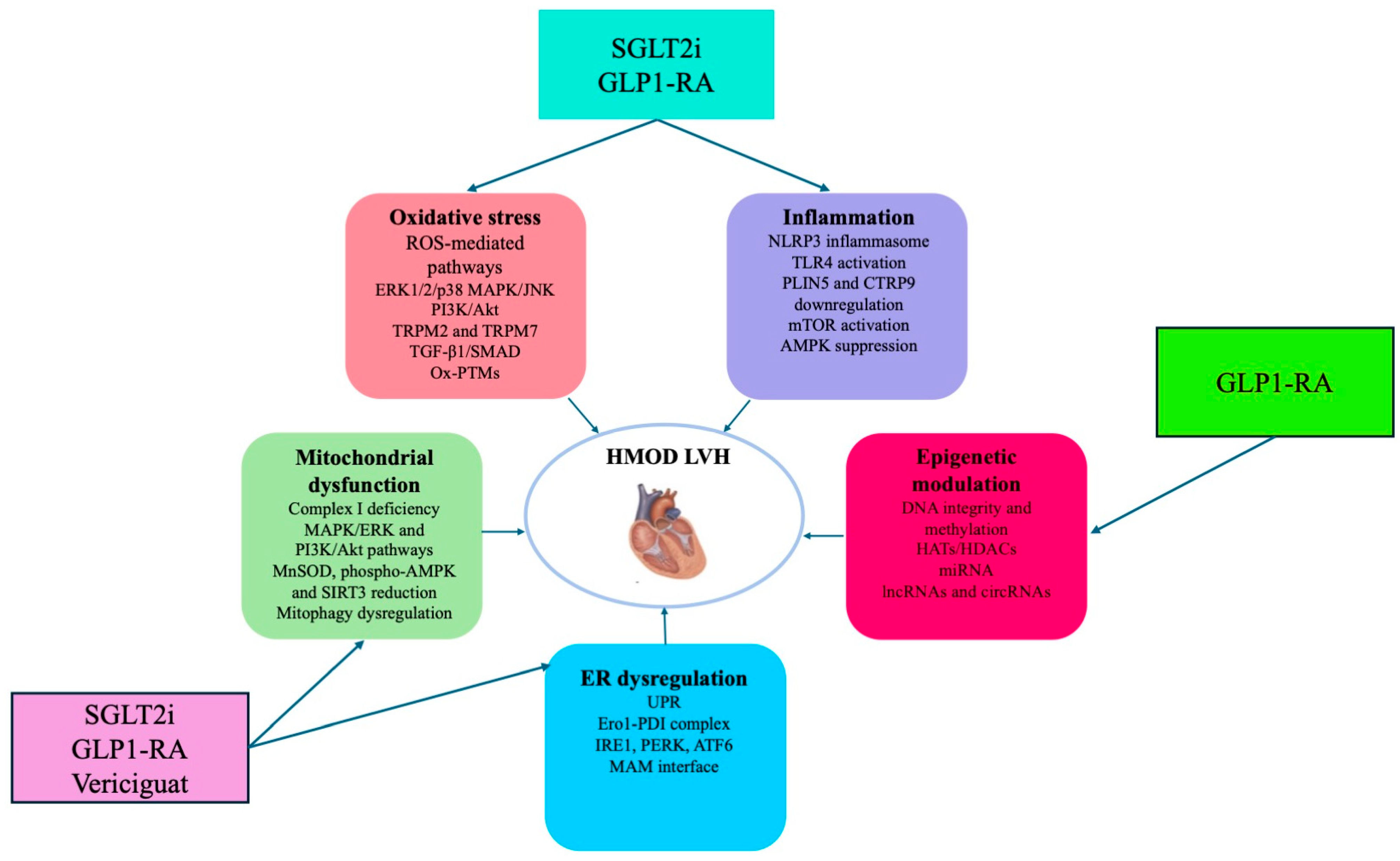
6. Therapeutic Perspectives
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fuchs, F.D.; Whelton, P.K. High Blood Pressure and Cardiovascular Disease. Hypertension 2020, 75, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Vasan, R.S.; Song, R.J.; Xanthakis, V.; Beiser, A.; DeCarli, C.; Mitchell, G.F.; Seshadri, S. Hypertension-Mediated Organ Damage: Prevalence, Correlates, and Prognosis in the Community. Hypertension 2022, 79, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Nadar, S.K.; Lip, G.Y.H. The Heart in Hypertension. J. Hum. Hypertens. 2021, 35, 383–386. [Google Scholar] [CrossRef]
- Chen, S.; Li, Q.; Shi, H.; Li, F.; Duan, Y.; Guo, Q. New insights into the role of mitochondrial dynamics in oxidative stress-induced diseases. Biomed. Pharmacother. 2024, 178, 117084. [Google Scholar] [CrossRef]
- Ma, J.; Li, Y.; Yang, X.; Liu, K.; Zhang, X.; Zuo, X.; Ye, R.; Wang, Z.; Shi, R.; Meng, Q.; et al. Signaling Pathways in Vascular Function and Hypertension: Molecular Mechanisms and Therapeutic Interventions. Signal Transduct. Target. Ther. 2023, 8, 168. [Google Scholar] [PubMed]
- Griendling, K.K.; Camargo, L.L.; Rios, F.J.; Alves-Lopes, R.; Montezano, A.C.; Touyz, R.M. Oxidative Stress and Hypertension. Circ. Res. 2021, 128, 993–1020. [Google Scholar] [CrossRef]
- Touyz, R.M.; Rios, F.J.; Alves-Lopes, R.; Neves, K.B.; Camargo, L.L.; Montezano, A.C. Oxidative Stress: A Unifying Paradigm in Hypertension. Can. J. Cardiol. 2020, 36, 659–670. [Google Scholar] [CrossRef]
- McMaster, W.G.; Kirabo, A.; Madhur, M.S.; Harrison, D.G. Inflammation, immunity, and hypertensive end-organ damage. Circ. Res. 2015, 116, 1022–1033. [Google Scholar] [CrossRef]
- Moris, D.; Spartalis, M.; Spartalis, E.; Karachaliou, G.S.; Karaolanis, G.I.; Tsourouflis, G.; Tsilimigras, D.I.; Tzatzaki, E.; Theocharis, S. The Role of Reactive Oxygen Species in the Pathophysiology of Cardiovascular Diseases and the Clinical Significance of Myocardial Redox. Ann. Transl. Med. 2017, 5, 326. [Google Scholar] [CrossRef]
- Gallo, G.; Volpe, M.; Savoia, C. Endothelial Dysfunction in Hypertension: Current Concepts and Clinical Implications. Front. Med. 2022, 8, 798958. [Google Scholar] [CrossRef]
- Liu, R.; Molkentin, J.D. Regulation of Cardiac Hypertrophy and Remodeling through the Dual-Specificity MAPK Phosphatases (DUSPs). J. Mol. Cell. Cardiol. 2016, 101, 44–49. [Google Scholar] [CrossRef]
- Liu, F.; Fan, L.M.; Geng, L.; Li, J.M. p47phox-Dependent Oxidant Signalling through ASK1, MKK3/6 and MAPKs in Angiotensin II-Induced Cardiac Hypertrophy and Apoptosis. Antioxidants 2021, 10, 1363. [Google Scholar] [CrossRef]
- Li, J.; Wang, C.Q.; Xiao, W.C.; Chen, Y.; Tu, J.; Wan, F.; Deng, K.Q.; Li, H.P. TRAF Family Member 4 Promotes Cardiac Hypertrophy through the Activation of the AKT Pathway. J. Am. Heart Assoc. 2023, 12, e028185. [Google Scholar] [CrossRef]
- De Nicolo, B.; Cataldi-Stagetti, E.; Diquigiovanni, C.; Bonora, E. Calcium and Reactive Oxygen Species Signaling Interplays in Cardiac Physiology and Pathologies. Antioxidants 2023, 12, 353. [Google Scholar] [CrossRef] [PubMed]
- Alves-Lopes, R.; Neves, K.B.; Anagnostopoulou, A.; Rios, F.J.; Lacchini, S.; Montezano, A.C.; Touyz, R.M. Crosstalk Between Vascular Redox and Calcium Signaling in Hypertension Involves TRPM2 (Transient Receptor Potential Melastatin 2) Cation Channel. Hypertension 2020, 75, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Li, S.; Liu, X.; Si, D.; Chen, W.; Yang, F.; Sun, H.; Yang, P. RyR2 Stabilizer Attenuates Cardiac Hypertrophy by Downregulating TNF-α/NF-κB/NLRP3 Signaling Pathway through Inhibiting Calcineurin. J. Cardiovasc. Transl. Res. 2024, 17, 481–495. [Google Scholar] [CrossRef]
- Anderson, M.E.; Brown, J.H.; Bers, D.M. CaMKII in Myocardial Hypertrophy and Heart Failure. J. Mol. Cell. Cardiol. 2011, 51, 468–473. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.C.Y.; Williams, A.L.; Wang, L.; Xie, G.; Jia, W.; Fujimoto, A.; Gerschenson, M.; Shohet, R.V. PKM2 Regulates Metabolic Flux and Oxidative Stress in the Murine Heart. Physiol. Rep. 2024, 12, e70040. [Google Scholar] [CrossRef]
- Rusciano, M.R.; Sommariva, E.; Douin-Echinard, V.; Ciccarelli, M.; Poggio, P.; Maione, A.S. CaMKII Activity in the Inflammatory Response of Cardiac Diseases. Int. J. Mol. Sci. 2019, 20, 4374. [Google Scholar] [CrossRef]
- Guo, Q.; Jin, Y.; Chen, X.; Ye, X.; Shen, X.; Lin, M.; Zeng, C.; Zhou, T.; Zhang, J. NF-κB in Biology and Targeted Therapy: New Insights and Translational Implications. Signal Transduct. Target. Ther. 2024, 9, 53. [Google Scholar] [CrossRef]
- Huc, L.; Lemarié, C.A.; Tedgui, A.; Boulanger, C.M.; Blanc-Brude, O. Angiotensin II-Induced Hypertension and Oxidative Stress Reduce DNA Methylation of the AT1a Receptor Gene in the Mouse Aorta. J. Hypertens. 2011, 29, 117–125. [Google Scholar]
- Dai, D.-F.; Chen, T.; Szeto, H.; Nieves-Cintrón, M.; Kutyavin, V.; Santana, L.F.; Rabinovitch, P.S. Mitochondrial Targeted Antioxidant Peptide Ameliorates Hypertensive Cardiomyopathy. J. Am. Coll. Cardiol. 2011, 58, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Nahapetian, R.N.; Jaffer, F.A.; Creager, M.A. Pathophysiology of Hypertension: Interactions Among Arteries, the Heart, and the Kidneys. Circ. Res. 2021, 128, 847–865. [Google Scholar]
- Yu, Y.; Dong, Y.; Yang, M.; He, W.; Xie, D.; Jiang, Y.; Li, Z.; Zhu, Y.; He, B.; Liu, X. Mitophagy in Hypertension: Role, Mechanism, and Therapeutic Potential. Front. Cell Dev. Biol. 2023, 11, 1169735. [Google Scholar]
- Gibb, A.A.; Hill, B.G. Metabolic Coordination of Physiological and Pathological Cardiac Remodeling. Circ. Res. 2018, 123, 107–128. [Google Scholar] [CrossRef] [PubMed]
- Nickel, A.; Löffler, J.R.; Maack, C. Myocardial Energetics in Heart Failure. Basic Res. Cardiol. 2022, 117, 12. [Google Scholar]
- Frisk, M.; Koivumäki, J.T.; Norseng, P.A.; Maleckar, M.M.; Sejersted, O.M.; Louch, W.E. Variable T-Tubule Organization and Ca2+ Homeostasis Across the Atrial Wall. Am. J. Physiol. Heart Circ. Physiol. 2014, 307, H609–H620. [Google Scholar] [CrossRef]
- Krishnan, K.J.; Bender, A.; Taylor, R.W.; Turnbull, D.M. A Multiplex Real-Time PCR Method to Detect and Quantify Mitochondrial DNA Deletions in Individual Cells. Anal. Biochem. 2007, 370, 127–129. [Google Scholar] [CrossRef]
- Dai, D.F.; Santana, L.F.; Vermulst, M.; Tomazela, D.M.; Emond, M.J.; MacCoss, M.J.; Gollahon, K.; Martin, G.M.; Loeb, L.A.; Ladiges, W.C.; et al. Overexpression of Catalase Targeted to Mitochondria Attenuates Murine Cardiac Aging. Circulation 2009, 119, 2789–2797. [Google Scholar] [CrossRef]
- Sabouny, R.; Shutt, T.E. Reciprocal Regulation of Mitochondrial Fission and Fusion. Trends Biochem. Sci. 2020, 45, 564–577. [Google Scholar] [CrossRef]
- Cho, Y.; Shi, R.; Kim, M.S.; Mukherjee, R.; Jang, Y.C. Mitochondrial Network Remodeling and Mitochondrial-Derived Vesicle Biogenesis in Hypertensive Cardiomyopathy. Antioxidants 2023, 12, 253. [Google Scholar]
- Dai, D.F.; Karunadharma, P.P.; Chiao, Y.A.; Basisty, N.; Crispin, D.; Hsieh, E.J.; Chen, T.; Gu, H.; Djukovic, D.; Raftery, D.; et al. Altered Proteome Turnover and Remodeling by Short-Term Caloric Restriction or Rapamycin Rejuvenate the Aging Heart. Aging Cell 2014, 13, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, X.; Yue, T.; Yang, L.; Guo, J.; Yuan, H. SIRT3 in Cardiovascular Diseases: Emerging Roles and Therapeutic Potential. Int. J. Mol. Sci. 2020, 21, 6693. [Google Scholar]
- Cheng, Y.; Zhang, Q.; Zhao, L.; Zhou, C.; Chen, X.; Jiang, H. SIRT3 Inhibits Angiotensin II-Induced Myocardial Hypertrophy Through Promotion of AMPK-Dependent Autophagy. Front. Cardiovasc. Med. 2021, 8, 754256. [Google Scholar]
- Chen, Y.; Yang, Y.; Wang, F.; Guo, Z.; Wang, X.; Yu, S.; Shi, Q.; Yan, C.; Yang, S.; Zhang, S.; et al. Mitochondrial Ca2+ Uptake Regulates Cardiac Remodeling Through Sirtuin 3-Mediated Deacetylation of GSK-3β and Modulation of β-Catenin Signaling. EBioMedicine 2022, 83, 104203. [Google Scholar]
- Corbi, G.; Conti, V.; Davinelli, S.; Scapagnini, G.; Filippelli, A.; Ferrara, N. Dietary Phytochemicals in Neuroimmunoaging: A New Therapeutic Possibility for Humans? Front. Pharmacol. 2016, 7, 364. [Google Scholar] [CrossRef]
- Budzynski, M.A.; Purol, M.; Drozdz-Afelt, K.; Rygula, K.; Szyndler, J.; Tarnowski, M.; Budzynska, B. The Role of Sirtuins in Hypertension and Vascular Aging. Cells 2023, 12, 340. [Google Scholar]
- Palomer, X.; Román-Anguiano, N.G.; Pizarro-Delgado, J.; Planavila, A.; Villarroya, F.; Vazquez-Carrera, M. SIRT1 Regulates the UCP3 Gene Expression Through PPARα and PGC-1α in Brown Adipocytes. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2014, 1841, 482–490. [Google Scholar]
- Xu, Y.; Wang, N.; Xu, H.; Kong, B.; Zhang, L.; Zhao, Y. A Review of Sirtuins in Metabolic Regulation and Hypertension. Front. Med. 2021, 8, 664898. [Google Scholar]
- Bagul, P.K.; Dinda, A.K.; Banerjee, S.K. Effect of Resveratrol on Sirtuins Expression and Cardiac Complications in Diabetes. Biochem. Biophys. Res. Commun. 2015, 468, 221–227. [Google Scholar] [CrossRef]
- Fagiani, F.; di Eusanio, M.; Bolego, C.; Trevisi, L.; Sitia, L. The Role of SIRT6 in the Cardiovascular System. Front. Physiol. 2021, 12, 729266. [Google Scholar]
- Wu, J.; Liu, S.; Hu, L.; Ran, Q.; Lin, Z.; Zhu, L.; Yang, J.; Zheng, Y. SIRT6 Represses Cardiac Hypertrophy and Inflammation Through Targeting MKP-1-Dependent Inhibition of p38 MAPK Pathway. Cell Death Discov. 2021, 7, 313. [Google Scholar]
- Sorriento, D.; Valeria Pascale, A.; Finelli, R.; Cipolletta, E.; Monaco, S.; Trimarco, B.; Iaccarino, G. Targeting Mitochondrial Dynamics in Hypertension: An Emerging Role for Mitochondrial Fusion and Fission. Eur. Heart J. Cardiovasc. Pharmacother. 2023, 9, 80–89. [Google Scholar]
- Wang, L.; Zhang, J.; Duan, Y.; Zhang, H.; Liu, Y.; Liu, B.; Li, W.; Zhang, L.; Zhang, Y.; Hu, X.; et al. Mitochondrial Remodeling via the PGC-1α Pathway in Hypertensive Heart Disease: The Role of Aerobic Exercise. Front. Cardiovasc. Med. 2021, 8, 722445. [Google Scholar]
- Zhai, M.; Li, B.; Duan, W.; Jing, L.; Zhang, B.; Zhang, M.; Yu, L.; Liu, D.; Li, C.; Ren, K.; et al. Melatonin Ameliorates Myocardial Infarction-Induced Mitochondrial Dysfunction via AMPK-PGC1α-SIRT3 Pathway. J. Pineal Res. 2017, 63, e12438. [Google Scholar]
- Wu, N.N.; Zhang, Y.; Ren, J. Mitophagy, Mitochondrial Dynamics, and Homeostasis in Cardiovascular Aging. Oxid. Med. Cell. Longev. 2019, 2019, 9825061. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Pan, C.S.; Liu, Y.Y.; Zhang, Y.Y.; Liu, H.T.; Han, J.Y.; Wang, C.S.; Wei, X.H.; Fan, J.Y.; Han, J.Q.; et al. Melatonin Attenuates Hypertension-Induced Retinopathy by Activating the Nrf2 Pathway. Cell Death Dis. 2022, 13, 395. [Google Scholar]
- Garcia, J.A.; Volt, H.; Venegas, C.; Doerrier, C.; Escames, G.; López, L.C.; Acuña-Castroviejo, D. Disruption of the NF-κB/NLRP3 Connection by Melatonin Requires Retinoid-Related Orphan Receptor-α and Blocks the Septin Inflammasome. FASEB J. 2019, 33, 12316–12328. [Google Scholar]
- Acuña-Castroviejo, D.; Escames, G.; Venegas, C.; Díaz-Casado, M.E.; Lima-Cabello, E.; López, L.C.; Rosales-Corral, S.; Tan, D.X.; Reiter, R.J. Extrapineal Melatonin: Sources, Regulation, and Potential Functions. Cell. Mol. Life Sci. 2014, 71, 2997–3025. [Google Scholar] [CrossRef]
- Liu, R.; Fu, A.; Hoffman, N.E.; Ballinger, S.W.; Van Houten, B.; Yan, L.J. Mitochondrial Dysfunction and Oxidative Stress in Aging and Disease. Curr. Opin. Genet. Dev. 2023, 78, 102127. [Google Scholar]
- Zhang, Q.; Wu, J.; Cao, Q.; Sun, J.; Liu, C.; Pei, H.; Zeng, H.; Wang, L.; Chen, Y.; Zhou, Z. Oxidative Stress Induces Inflammation and Cell Death Through the PGC-1α Signaling Pathway in Hypoxic Pulmonary Hypertension. Oxid. Med. Cell. Longev. 2022, 2022, 9509460. [Google Scholar]
- Zhou, H.; Wang, J.; Zhu, P.; Zhu, H.; Toan, S.; Hu, S.; Chen, Y. NR4A1 Aggravates the Cardiac Microvascular Ischemia Reperfusion Injury Through Inhibiting FUNDC1-Mediated Mitophagy via DNA-PKcs Signaling. Nat. Commun. 2021, 12, 4877. [Google Scholar]
- Tan, X.; Wang, M.; Yu, W.; Xia, M. Oxidative Stress Mediates Uric Acid-Induced Aortic Inflammation by Activating the SIRT1/NF-κB Signaling Pathway in Rats with Metabolic Syndrome. Mol. Nutr. Food Res. 2016, 60, 830–841. [Google Scholar]
- Yang, H.C.; Deleon-Pennell, K.Y.; Trinh, T.H.; Ma, Y.; Bajona, P.; Jin, Y.F.; Lange, R.A.; Lindsey, M.L. TNF-α Promotes Activation of MMP-13 During Cardiac Remodeling After Myocardial Infarction. Am. J. Physiol. Heart Circ. Physiol. 2016, 311, H862–H873. [Google Scholar]
- Yu, L.; Wang, L.; Chen, S. Endogenous Toll-like Receptor Ligands and Their Biological Significance. J. Cell. Mol. Med. 2010, 14, 2592–2603. [Google Scholar] [CrossRef]
- Mastrocola, R.; Penna, C.; Tullio, F.; Femminò, S.; Alloatti, G.; Chiazza, F.; Collino, M. Pharmacological Inhibition of Mitochondrial Fission Protects the Heart from Hypertension-Induced Cardiovascular Damage. J. Hypertens. 2021, 39, 892–904. [Google Scholar]
- Gallo, G.; Rubattu, S.; Volpe, M. Mitochondrial Dysfunction in Heart Failure: From Pathophysiological Mechanisms to Therapeutic Opportunities. Int. J. Mol. Sci. 2024, 25, 2667. [Google Scholar] [CrossRef]
- Raffa, S.; Forte, M.; Gallo, G.; Ranieri, D.; Marchitti, S.; Magrì, D.; Testa, M.; Stanzione, R.; Bianchi, F.; Cotugno, M.; et al. Atrial natriuretic peptide stimulates autophagy/mitophagy and improves mitochondrial function in chronic heart failure. Cell Mol. Life Sci. 2023, 80, 13. [Google Scholar] [CrossRef]
- Li, J.; Qu, X.; Ricardo, S.D.; Bertram, J.F.; Nikolic-Paterson, D.J. Resveratrol Inhibits Renal Fibrosis in the Oblate Nephrectomy Model Through Modulation of Oxidative Stress. Kidney Int. 2006, 70, 1184–1191. [Google Scholar]
- Lin, C.-J.; Wu, V.; Wu, P.-C.; Wu, C.-J. Meta-Analysis: The Effects of Resveratrol on Cardiometabolic Risk Factors and Liver Enzymes. Eur. J. Clin. Investig. 2021, 51, e13442. [Google Scholar]
- Planavila, A.; Iglesias, R.; Giralt, M.; Villarroya, F. SIRT1 Acts in Association with PPARα to Protect the Heart from Hypertrophy, Metabolic Dysregulation, and Inflammation. Cardiovasc. Res. 2011, 90, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Singh, C.K.; George, J.; Ahmad, N. Resveratrol-Based Combinatorial Strategies for Cancer Management. Ann. N. Y. Acad. Sci. 2013, 1290, 113–121. [Google Scholar] [CrossRef]
- Xu, S.; Zhou, L.; Ponnusamy, M.; Zhao, Y.; Wang, Y.; Wang, G.; Li, P. Control of the Epigenome by Mitochondrial Metabolism: Emerging Roles in Tumorigenesis. Nat. Rev. Mol. Cell Biol. 2020, 21, 55–70. [Google Scholar]
- Schneider, A.; Steinbicker, A.U. Hypoxia, Hypoxia-Inducible Factors (HIF), and Iron Homeostasis: A Complicated Alliance. J. Clin. Med. 2021, 10, 284. [Google Scholar]
- Lizcano, F.; Bustamante, L. Molecular perspectives in hypertrophic heart disease: An epigenetic approach from chromatin modification. Front. Cell Dev. Biol. 2022, 10, 1070338. [Google Scholar] [CrossRef]
- Tsai, Y.C.; Chuang, Y.H.; Huang, P.H.; Chen, J.W.; Lin, S.J. Resveratrol Regulates Inflammatory Cytokines via Modulation of Oxidative Stress in Hypoxia-Induced Pulmonary Arterial Hypertension. Biochem. Biophys. Res. Commun. 2018, 508, 1021–1027. [Google Scholar]
- Hoppstädter, J.; Kessler, S.M.; Bruscoli, S.; Huwer, H.; Riccardi, C.; Kiemer, A.K. Glucocorticoid-Induced Leucine Zipper: A Limiting Factor for NLRP3 Inflammasome Activation. Front. Immunol. 2022, 13, 866011. [Google Scholar]
- Yang, W.; Tao, Y.; Wu, Y.; Zhao, X.; Ye, W.; Zhao, D.; Zheng, Q.; Wei, Y.; Zhu, L.; Wang, L.; et al. Metformin Inhibits NLRP3 Inflammasome Activation by Promoting Autophagy in Human Vascular Endothelial Cells. BioMed Res. Int. 2019, 2019, 8307137. [Google Scholar]
- Li, J.; Zhang, D.; Brash, D.E. UVA-Induced Oxidative Stress Represses NF-κB Activity by Inhibiting p65 Acetylation via SIRT1 in Human Keratinocytes. Free Radic. Biol. Med. 2013, 65, 1033–1041. [Google Scholar]
- Gu, X.; Han, L.; Zhang, J.; Zhang, Y.; Fu, M.; Zhao, W.; Zhang, X.; Xu, Y.; Zheng, Y. SIRT1-Mediated Inhibition of Vascular Inflammation by Activating KLF2 Improves Vascular Dysfunction in Diabetic Mice. Clin. Sci. 2019, 133, 659–674. [Google Scholar]
- Ma, Y.; Zhang, Y.; Wang, X.; Wu, Y.; Wu, B.; Li, J.; Zhao, Y.; Sun, H.; Zhang, C.; Xia, M.; et al. SIRT1 Activation by Resveratrol Alleviates Cardiac Dysfunction via Mitochondrial Regulation in Diabetic Cardiomyopathy Mice. Oxid. Med. Cell. Longev. 2017, 2017, 4602715. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Zeng, H.; Chen, J.X. Emerging Role of SIRT3 in Endothelial Metabolism, Angiogenesis, and Cardiovascular Disease. J. Cell. Physiol. 2019, 234, 2252–2265. [Google Scholar] [CrossRef]
- Li, H.; Guan, Y.; Liang, B.; Ding, P.; Hou, X.; Wei, W.; Ma, Y. Therapeutic potential of MCC950, a specific inhibitor of NLRP3 inflammasome. Eur. J. Pharmacol. 2022, 928, 175091. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Dittenhafer-Reed, K.E.; Denu, J.M. SIRT3 Protein Deacetylates Isocitrate Dehydrogenase 2 (IDH2) and Regulates Mitochondrial Redox Status. J. Biol. Chem. 2012, 287, 14078–14086. [Google Scholar] [CrossRef]
- Pillai, V.B.; Sundaresan, N.R.; Jeevanandam, V.; Gupta, M.P. Mitochondrial SIRT3 and Heart Disease. Cardiovasc. Res. 2010, 88, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Jiang, Y.; Wu, H.; Sun, F.; Li, Y.; Li, Y.; Zhou, H.; Han, X.; Zhang, C.; Xu, C.; et al. SIRT3-Mediated Deacetylation of Cyclophilin D Attenuates Mitochondrial Dysfunction in Diabetic Hearts. J. Cell. Mol. Med. 2021, 25, 3779–3790. [Google Scholar]
- Tsutsui, H.; Ishibashi, Y.; Takahashi, M.; Namba, T.; Tagawa, H.; Imanaka-Yoshida, K.; Takeshita, A. Chronic colchicine administration attenuates cardiac hypertrophy in spontaneously hypertensive rats. J. Mol. Cell Cardiol. 1999, 31, 1203–1213. [Google Scholar] [CrossRef]
- Scopacasa, B.S.; Teixeira, V.P.; Franchini, K.G. Colchicine attenuates left ventricular hypertrophy but preserves cardiac function of aortic-constricted rats. J. Appl. Physiol. 2003, 94, 1627–1633. [Google Scholar] [CrossRef][Green Version]
- Tseng, A.H.H.; Shieh, S.S.; Wang, D.L. SIRT3 Deacetylates FOXO3 to Protect Mitochondria Against Oxidative Damage. Free Radic. Biol. Med. 2013, 63, 222–234. [Google Scholar] [CrossRef]
- Sundaresan, N.R.; Samant, S.A.; Pillai, V.B.; Rajamohan, S.B.; Gupta, M.P. SIRT3 Is a Stress-Responsive Deacetylase in Cardiomyocytes that Protects Cells from Stress-Mediated Cell Death by Deacetylation of Ku70. Mol. Cell. Biol. 2008, 28, 6384–6401. [Google Scholar] [CrossRef]
- Liu, B.; Che, W.; Xu, H.; Zhu, H.; Yang, C.; Zhao, M.; Li, C.; Li, H.; Liu, M.; Liu, H.; et al. SIRT3 Overexpression Alleviates Angiotensin II-Induced Cardiac Fibrosis by Blocking the TGF-β1/Smad2/3 Pathway via the Upregulation of Manganese Superoxide Dismutase. Free Radic. Biol. Med. 2020, 161, 212–224. [Google Scholar]
- Fan, X.; Zhang, X.; He, J.; Xiao, P.; Zhao, B. Resveratrol Reduces Mitochondrial Damage by Increasing SIRT3 Expression and Inhibiting the TGF-β1/Smad3 Pathway in High Glucose-Treated Renal Tubular Epithelial Cells. Biochem. Biophys. Res. Commun. 2020, 529, 467–473. [Google Scholar]
- Liu, M.; Xu, H.; Liu, B.; Sun, X.; Zhao, M.; Yang, C.; Zhu, H.; Liu, H.; Li, C.; Wang, C.; et al. SIRT3 Promotes Angiogenesis in Hypertensive Mice Post-Myocardial Infarction by Enhancing Glycolysis in Endothelial Cells via HIF-1α/VEGF/Notch1 Pathway. J. Mol. Cell. Cardiol. 2022, 167, 1–12. [Google Scholar]
- Fang, E.F.; Lautrup, S.; Hou, Y.; Demarest, T.G.; Croteau, D.L.; Mattson, M.P.; Bohr, V.A. NAD+ in Aging: Molecular Mechanisms and Translational Implications. Trends Mol. Med. 2017, 23, 899–916. [Google Scholar] [CrossRef]
- Liu, X.; Yang, M.; Lip, G.Y.H.; McDowell, G. Plasma Biomarkers for Hypertension-Mediated Organ Damage Detection: A Narrative Review. Biomedicines 2024, 12, 1071. [Google Scholar] [CrossRef]
- Katsyuba, E.; Romani, M.; Hofer, D.; Auwerx, J. NAD+ Homeostasis in Health and Disease. Nat. Metab. 2020, 2, 9–31. [Google Scholar] [CrossRef]
- Zhang, H.; Ryu, D.; Wu, Y.; Gariani, K.; Wang, X.; Luan, P.; D’Amico, D.; Ropelle, E.R.; Lutolf, M.P.; Aebersold, R.; et al. NAD+ Repletion Improves Mitochondrial and Stem Cell Function and Enhances Life Span in Mice. Science 2016, 352, 1436–1443. [Google Scholar] [CrossRef] [PubMed]
- Lautrup, S.; Sinclair, D.A.; Mattson, M.P.; Fang, E.F. NAD+ in Brain Aging and Neurodegenerative Disorders. Cell Metab. 2019, 30, 630–655. [Google Scholar] [CrossRef]
- Cameron, A.M.; Castoldi, A.; Sanin, D.E.; Flachsmann, L.J.; Field, C.S.; Puleston, D.J.; Kyle, R.L.; Patterson, A.E.; Hässler, F.; Buescher, J.M.; et al. Inflammatory Macrophage Dependence on NAD+ Salvage Is a Consequence of Reactive Oxygen Species–Mediated DNA Damage. Nat. Immunol. 2019, 20, 420–432. [Google Scholar] [CrossRef]
- Bhatt, D.P.; Puig, K.L.; Gencay, M.M.; Bhattacharyya, A.; Magrane, J.; Rebeck, G.W.; Mahley, R.W.; LaDu, M.J. A Human Apolipoprotein E4 Transgene Impairs Mitochondrial Morphology and Function in a Mouse Model of Alzheimer’s Disease. J. Neurosci. 2019, 39, 4172–4181. [Google Scholar]
- Gariani, K.; Menzies, K.J.; Ryu, D.; Wegner, C.J.; Wang, X.; Ropelle, E.R.; Moullan, N.; Zhang, H.; Perino, A.; Lemos, V.; et al. Eliciting the Mitochondrial Unfolded Protein Response by Nicotinamide Adenine Dinucleotide Repletion Reverses Fatty Liver Disease in Mice. Hepatology 2016, 63, 1190–1204. [Google Scholar] [CrossRef] [PubMed]
- Traba, J.; Han, K.; Ferré, P.; Hans, C.P.; Lang, J.; Kozlov, A.V.; Shirihai, O.; Sack, M.N. Prolonged Fasting Suppresses Mitochondrial Respiration through SIRT3-Mediated Deacetylation of PDH Complex. Cell Metab. 2021, 33, 2290–2303.e6. [Google Scholar]
- Okabe, K.; Matsumoto, T.; Akiyoshi, M.; Kohama, Y.; Murakami, M.; Ikutomo, M.; Tsunoda, S.; Kataoka, T.R.; Tanaka, Y.; Oike, Y. Hypoxia-Inducible Factor 1–Mediated Regulation of the NAD+ Biosynthetic Pathway Enhances Resistance to Photodynamic Therapy in Tumors. Cancer Res. 2019, 79, 4103–4114. [Google Scholar]
- McDonnell, E.; Peterson, B.S.; Bomze, H.M.; Hirschey, M.D. SIRT3 Regulates Progression and Development of Diseases of Aging. Trends Endocrinol. Metab. 2015, 26, 486–492. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, Y.; Yang, X.; Shao, Z.; Ma, H.; He, P.; Huang, X.; Zhang, S.; Han, X. SIRT3 Impairs Angiotensin II-Induced Cardiomyocyte Hypertrophy by Targeting Mitochondrial Dynamics and Functions. Biochim. Biophys. Acta Mol. Basis Dis. 2021, 1867, 166260. [Google Scholar] [CrossRef] [PubMed]
- Llorens-Cebrià, C.; Molina-Van den Bosch, M.; Vergara, A.; Jacobs-Cachá, C.; Soler, M.J. Antioxidant Roles of SGLT2 Inhibitors in the Kidney. Biomolecules 2022, 12, 143. [Google Scholar] [CrossRef]
- Takagi, S.; Li, J.; Takagaki, Y.; Kitada, M.; Nitta, K.; Takasu, T.; Kanasaki, K.; Koya, D. Ipragliflozin improves mitochondrial abnormalities in renal tubules induced by a high-fat diet. J. Diabetes Investig. 2018, 9, 1025–1032. [Google Scholar] [CrossRef]
- Zhou, B.; Wang, D.D.; Qian, H.; Guo, H.; Zhao, Y.; Zhang, L.; Ding, G.; Tan, Y.; Liu, B.; Ma, L.; et al. Mitochondrial Dysfunction Promotes Angiotensin II-Induced Vascular Remodeling and Hypertension in Mice. J. Hypertens. 2022, 40, 1804–1814. [Google Scholar]
- Qiu, X.; Brown, K.; Hirschey, M.D.; Verdin, E.; Chen, D. Calorie Restriction Reduces Oxidative Stress by SIRT3-Mediated SOD2 Activation. Cell Metab. 2010, 12, 662–667. [Google Scholar] [CrossRef]
- Mohanty, J.G.; Nagababu, E.; Rifkind, J.M. Red Blood Cell Oxidative Stress Impairs Oxygen Delivery and Induces Red Blood Cell Aging. Front. Physiol. 2014, 5, 84. [Google Scholar] [CrossRef]
- Vasan, R.S. Cardiac Function and Obesity. Heart 2003, 89, 1127–1129. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, Y.; Tang, D.; Wang, J.; Ding, X.; Zhou, W.; Tang, X.; Wang, M.; Song, J.; Xu, Y. Berberine Attenuates Vascular Inflammation by Inhibiting the TLR4/NF-κB Pathway in Atherosclerosis. Front. Pharmacol. 2020, 11, 594025. [Google Scholar]
- Dai, D.F.; Rabinovitch, P.S.; Ungvari, Z. Mitochondria and Cardiovascular Aging. Circ. Res. 2012, 110, 1109–1124. [Google Scholar] [CrossRef] [PubMed]
- Paneni, F.; Costantino, S.; Cosentino, F. Epigenetic Signatures and Vascular Risk in Type 2 Diabetes: A Clinical Perspective. Atherosclerosis 2018, 271, 156–162. [Google Scholar] [CrossRef]
- Findeisen, H.M.; Pearson, K.J.; Gizard, F.; Zhao, Y.; Qing, H.; Jones, K.L.; Cavanaugh, J.E.; Bruemmer, D. Oxidative Stress Accumulates in Adipose Tissue during Aging and Inhibits Adipogenesis. PLoS ONE 2011, 6, e18532. [Google Scholar] [CrossRef]
- Tormos, K.V.; Anso, E.; Hamanaka, R.B.; Eisenbart, J.; Joseph, J.; Kalyanaraman, B.; Chandel, N.S. Mitochondrial Complex III ROS Regulate Adipocyte Differentiation. Cell Metab. 2011, 14, 537–544. [Google Scholar] [CrossRef]
- Green, D.R.; Galluzzi, L.; Kroemer, G. Mitochondria and the Autophagy–Inflammation–Cell Death Axis in Organismal Aging. Science 2011, 333, 1109–1112. [Google Scholar] [CrossRef]
- Finkel, T.; Holbrook, N.J. Oxidants, Oxidative Stress and the Biology of Ageing. Nature 2000, 408, 239–247. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gallo, G.; Rubattu, S. Cardiac Damage in Hypertension: From Molecular Mechanisms to Novel Therapeutic Approaches. Int. J. Mol. Sci. 2025, 26, 5610. https://doi.org/10.3390/ijms26125610
Gallo G, Rubattu S. Cardiac Damage in Hypertension: From Molecular Mechanisms to Novel Therapeutic Approaches. International Journal of Molecular Sciences. 2025; 26(12):5610. https://doi.org/10.3390/ijms26125610
Chicago/Turabian StyleGallo, Giovanna, and Speranza Rubattu. 2025. "Cardiac Damage in Hypertension: From Molecular Mechanisms to Novel Therapeutic Approaches" International Journal of Molecular Sciences 26, no. 12: 5610. https://doi.org/10.3390/ijms26125610
APA StyleGallo, G., & Rubattu, S. (2025). Cardiac Damage in Hypertension: From Molecular Mechanisms to Novel Therapeutic Approaches. International Journal of Molecular Sciences, 26(12), 5610. https://doi.org/10.3390/ijms26125610





