Coffee and Its Major Polyphenols in the Prevention and Management of Type 2 Diabetes: A Comprehensive Review
Abstract
1. Introduction
2. Coffee and T2DM
2.1. Evidence from Epidemiological and Clinical Studies
2.2. Experimental Evidence from Animal Studies
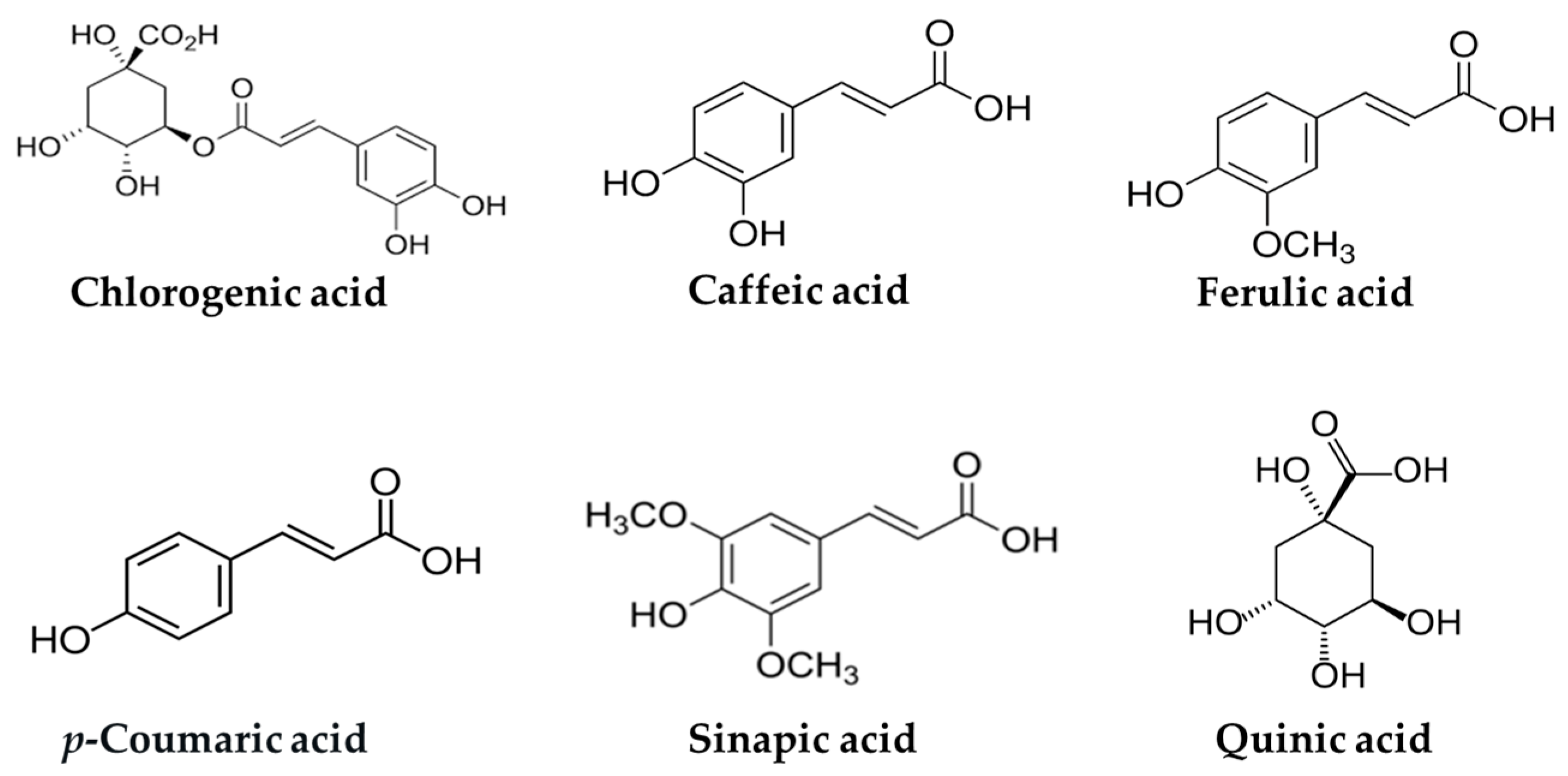
3. Potential Role of Chlorogenic Acid and Related Hydroxycinnamic Acids in T2DM
| Compound | Content in Coffee | Reference |
|---|---|---|
| Chlorogenic acid | 76–84% of total chlorogenic acids; approx. 100 mg/g dry weight (green coffee beans) | [46] |
| Caffeic acid | 63.1–96.0 mg/100 mL brewed coffee | [47] |
| Ferulic acid | 9.1–14.3 mg/100 g coffee | [44,48,49] |
| p-Coumaric acid | Approx. 5 mg/200 mL brewed coffee (1 cup) | [50] |
| Sinapic acid | 350–1750 mg/L coffee beverage (70–350 mg/200 mL serving); 10.34 and 10.89 mg/100 g (green/light roast beans) | [51,52] |
3.1. Potential Role of Chlorogenic Acid in T2DM
3.1.1. In Vitro Studies
3.1.2. Animal Studies
3.1.3. Human Studies
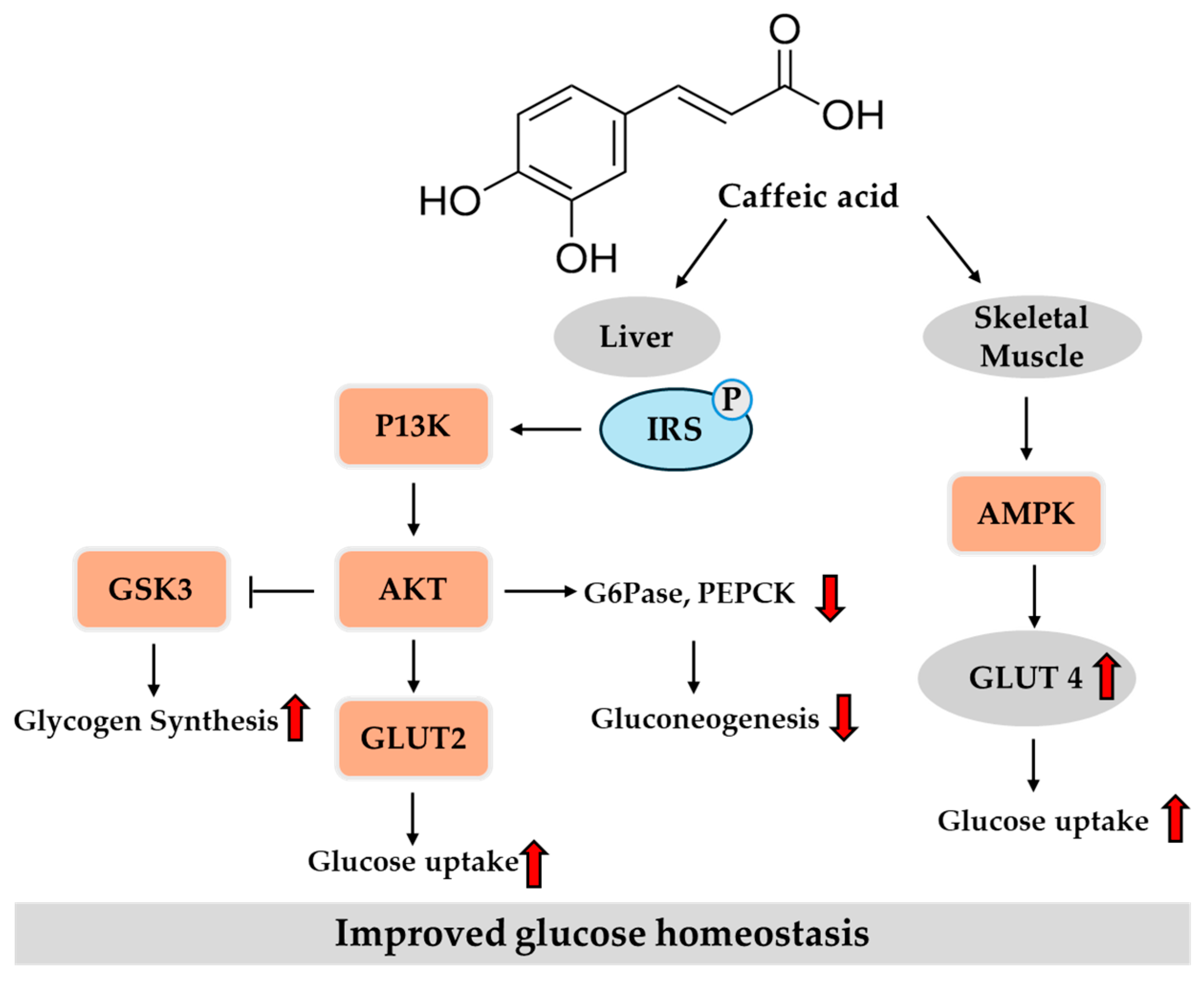
3.2. Potential Role of Caffeic Acid in T2DM
3.2.1. In Vitro Studies
3.2.2. In Vivo Studies
3.3. Potential Role of Ferulic Acid in T2DM
3.3.1. In Vitro Studies
3.3.2. Animal Studies
3.3.3. Human Studies
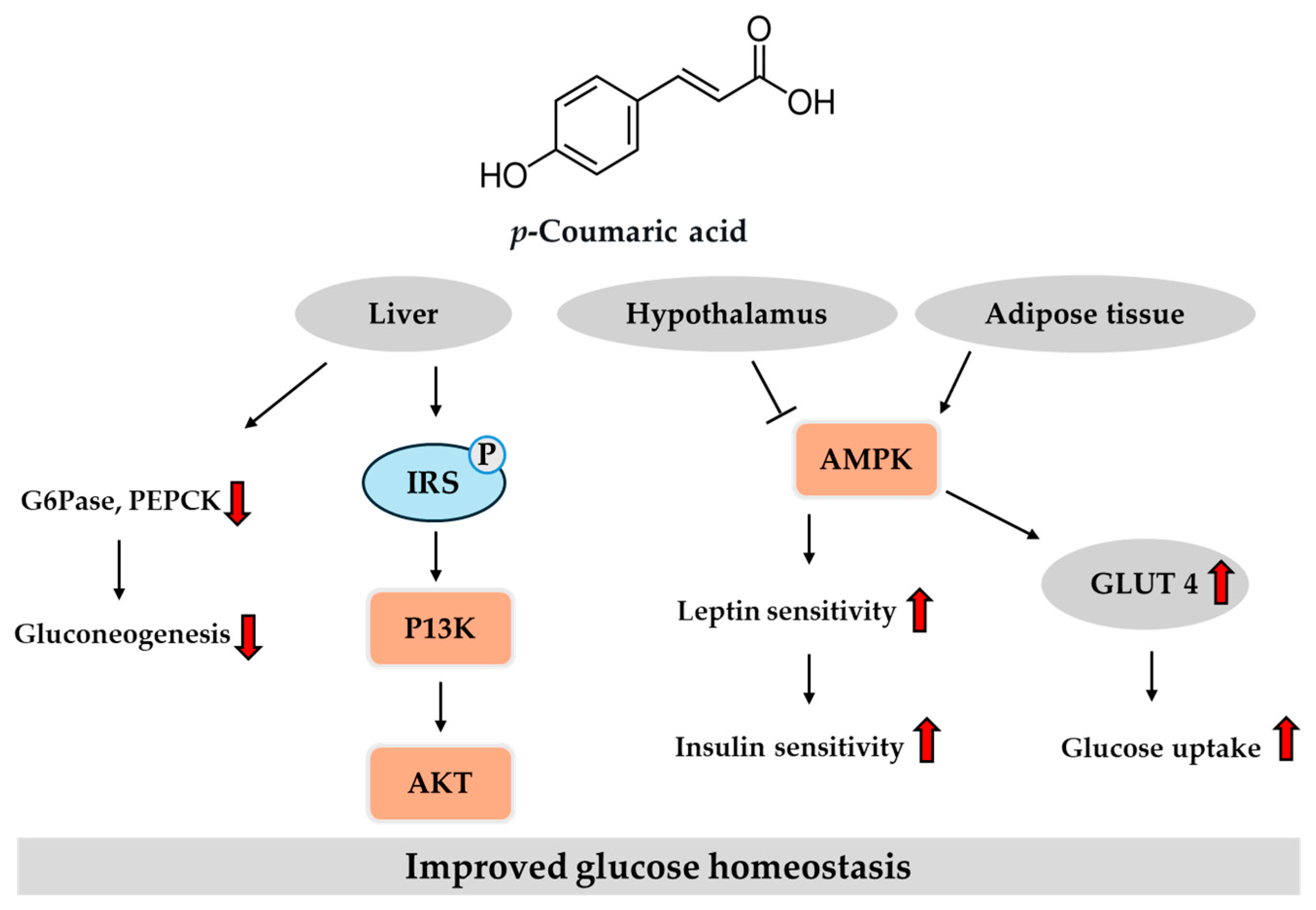
3.4. Potential Role of p-Coumaric Acid in T2DM
3.4.1. In Vitro Studies
3.4.2. In Vivo Studies
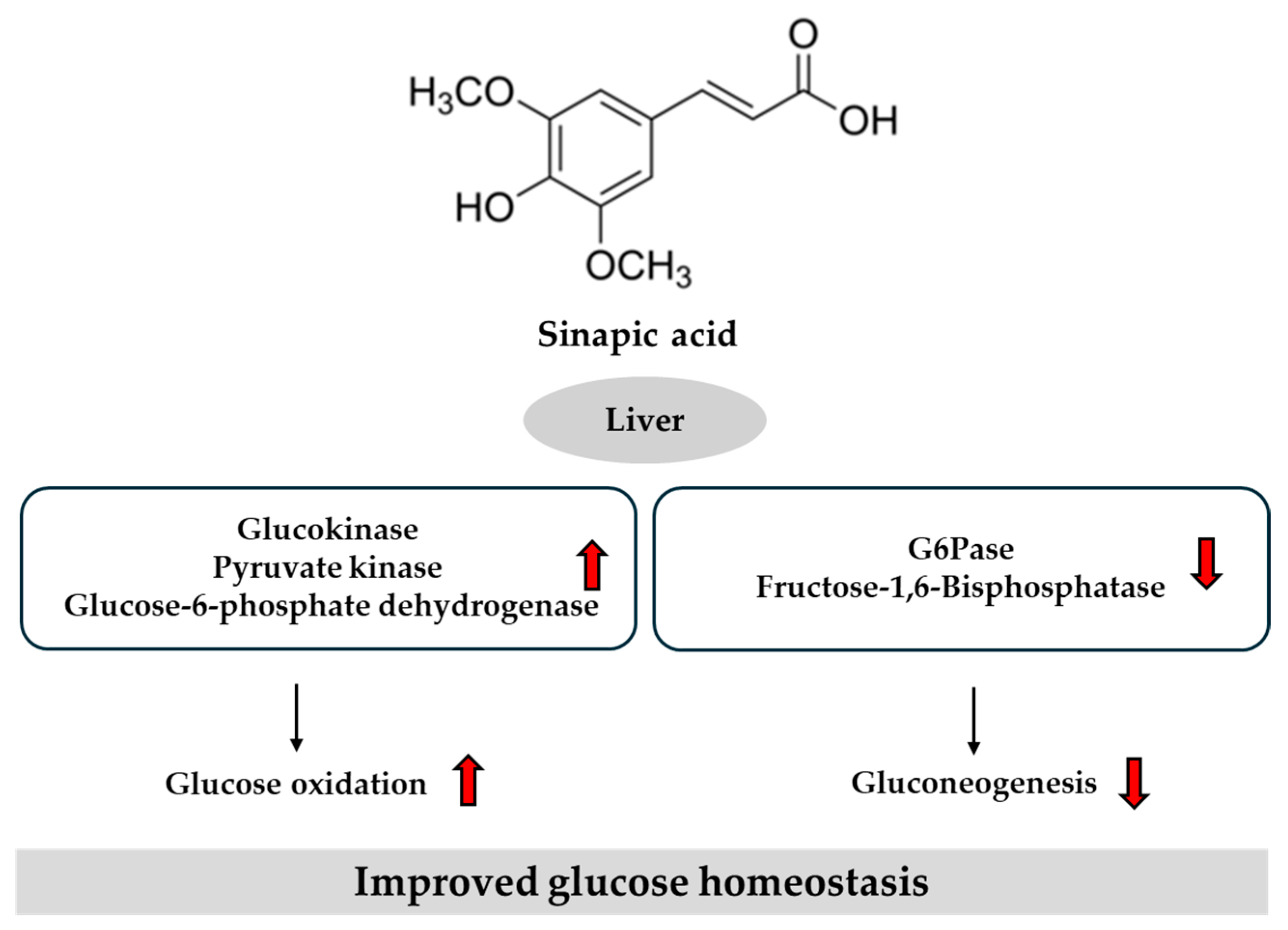
3.5. Potential Role of Sinapic Acid in T2DM
4. Limitations and Future Directions
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AGEs | Advanced glycation end products |
| AMPK | Adenosine monophosphate-activated protein kinase |
| FOXO1 | Forkhead box protein O1 |
| G6Pase | Glucose-6-phosphatase |
| GLP-1 | Glucagon-like peptide-1 |
| GLUT2 | Glucose transporter 2 |
| HbA1c | Hemoglobin A1c |
| HDL | High-density lipoprotein |
| HFD | High-fat diet |
| HNF | Hepatocyte nuclear factor |
| HPLC | High-performance liquid chromatography |
| IL | Interleukin |
| LDL | Low-density lipoprotein |
| MGAT1 | Monoacylglycerol acyltransferase 1 |
| NF-κB | Nuclear factor kappa B |
| Nrf | Nuclear factor erythroid 2-related factor |
| PEPCK | Phosphoenolpyruvate carboxykinase |
| PI3K | Phosphatidylinositol 3-kinase |
| PPA | Pancreatic α-amylase |
| PPAR | Peroxisome proliferator-activated receptor |
| RCTs | Randomized controlled trials |
| ROS | Reactive oxygen species |
| SGLT2 | Sodium glucose cotransporter 2 |
| SOD | Superoxide dismutase |
| SREBP1c | Sterol regulatory element-binding protein 1c |
| STZ | Streptozotocin |
| TNF-α | Tumor necrosis factor-α |
| T2DM | Type 2 diabetes mellitus |
References
- World Health Organization. Diabetes. Available online: https://www.who.int/news-room/fact-sheets/detail/diabetes (accessed on 7 May 2025).
- International Diabetes Federation. IDF Diabetes Atlas, 10th ed.; International Diabetes Federation: Brussels, Belgium, 2021; Available online: https://diabetesatlas.org/atlas/tenth-edition/ (accessed on 7 May 2025).
- Galicia-Garcia, U.; Benito-Vicente, A.; Jebari, S.; Larrea-Sebal, A.; Siddiqi, H.; Uribe, K.B.; Ostolaza, H.; Martín, C. Pathophysiology of Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2020, 21, 6275. [Google Scholar] [CrossRef] [PubMed]
- Yeh, Y.K.; Yen, F.S.; Hwu, C.M. Diet and exercise are a fundamental part of comprehensive care for type 2 diabetes. J. Diabetes Investig. 2023, 14, 936–939. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.J.; Aroda, V.R.; Collins, B.S.; Gabbay, R.A.; Green, J.; Maruthur, N.M.; Rosas, S.E.; Del Prato, S.; Mathieu, C.; Mingrone, G.; et al. Management of Hyperglycemia in Type 2 Diabetes, 2022. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2022, 45, 2753–2786. [Google Scholar] [CrossRef] [PubMed]
- Tamel Selvan, K.; Goon, J.A.; Makpol, S.; Tan, J.K. Effects of microalgae on metabolic syndrome. Antioxidants 2023, 12, 449. [Google Scholar] [CrossRef]
- Sánchez-Estrada, M.L.; Aguirre-Becerra, H.; Feregrino-Pérez, A.A. Bioactive compounds and biological activity in edible insects: A review. Heliyon 2024, 10, e24045. [Google Scholar] [CrossRef]
- Rahman, M.M.; Dhar, P.S.; Anika, F.; Ahmed, L.; Islam, M.R.; Sultana, N.A.; Cavalu, S.; Pop, O.; Rauf, A. Exploring the plant-derived bioactive substances as antidiabetic agent: An extensive review. Biomed. Pharmacother. 2022, 152, 113217. [Google Scholar] [CrossRef]
- Lavecchia, T.; Rea, G.; Antonacci, A.; Giardi, M.T. Healthy and adverse effects of plant-derived functional metabolites: The need of revealing their content and bioactivity in a complex food matrix. Crit. Rev. Food Sci. Nutr. 2013, 53, 198–213. [Google Scholar] [CrossRef]
- García-Conesa, M.; Larrosa, M. Polyphenol-rich foods for human health and disease. Nutrients 2020, 12, 400. [Google Scholar] [CrossRef]
- Micek, A.; Jurek, J.; Owczarek, M.; Guerrera, I.; Torrisi, S.A.; Castellano, S.; Grosso, G.; Alshatwi, A.A.; Godos, J. Polyphenol-rich beverages and mental health outcomes. Antioxidants 2023, 12, 272. [Google Scholar] [CrossRef]
- Kolb, H.; Martin, S.; Kempf, K. Coffee and Lower Risk of Type 2 Diabetes: Arguments for a Causal Relationship. Nutrients 2021, 13, 1144. [Google Scholar] [CrossRef]
- Mohamed, A.I.; Erukainure, O.L.; Salau, V.F.; Islam, M.S. Impact of coffee and its bioactive compounds on the risks of type 2 diabetes and its complications: A comprehensive review. Diabetes Metab. Syndr. 2024, 18, 103075. [Google Scholar] [CrossRef] [PubMed]
- International Coffee Organization. Coffee Report and Outlook April. 2023. Available online: https://icocoffee.org/documents/cy2023-24/Coffee_Report_and_Outlook_December_2023_ICO.pdf (accessed on 7 May 2025).
- Huxley, R.; Lee, C.M.; Barzi, F.; Timmermeister, L.; Czernichow, S.; Perkovic, V.; Grobbee, D.E.; Batty, D.; Woodward, M. Coffee, decaffeinated coffee, and tea consumption in relation to incident type 2 diabetes mellitus: A systematic review with meta-analysis. Arch. Intern. Med. 2009, 169, 2053–2063. [Google Scholar] [CrossRef] [PubMed]
- Bhupathiraju, S.N.; Pan, A.; Manson, J.E.; Willett, W.C.; van Dam, R.M.; Hu, F.B. Changes in coffee intake and subsequent risk of type 2 diabetes: Three large cohorts of US men and women. Diabetologia 2014, 57, 1346–1354. [Google Scholar] [CrossRef]
- Iso, H.; Date, C.; Wakai, K.; Fukui, M.; Tamakoshi, A. The relationship between green tea and total caffeine intake and risk for self-reported type 2 diabetes among Japanese adults. Ann. Intern. Med. 2006, 144, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Muley, A.; Muley, P.; Shah, M. Coffee to reduce risk of type 2 diabetes?: A systematic review. Curr. Diabetes Rev. 2012, 8, 162–168. [Google Scholar] [CrossRef]
- Ding, M.; Bhupathiraju, S.N.; Chen, M.; van Dam, R.M.; Hu, F.B. Caffeinated and decaffeinated coffee consumption and risk of type 2 diabetes: A systematic review and a dose-response meta-analysis. Diabetes Care 2014, 37, 569–586. [Google Scholar] [CrossRef]
- Jiang, X.; Zhang, D.; Jiang, W. Coffee and caffeine intake and incidence of type 2 diabetes mellitus: A meta-analysis of prospective studies. Eur. J. Nutr. 2014, 53, 25–38. [Google Scholar] [CrossRef]
- Wachamo, H. Review on health benefit and risk of coffee consumption. Med. Aromat. Plants 2017, 6, 301. [Google Scholar] [CrossRef]
- Greer, F.; Hudson, R.; Ross, R.; Graham, T. Caffeine ingestion decreases glucose disposal during a hyperinsulinemic-euglycemic clamp in sedentary humans. Diabetes 2001, 50, 2349–2354. [Google Scholar] [CrossRef]
- Keijzers, G.B.; De Galan, B.E.; Tack, C.J.; Smits, P. Caffeine can decrease insulin sensitivity in humans. Diabetes Care 2002, 25, 364–369. [Google Scholar] [CrossRef]
- Cheraskin, E.; Ringsdorf, W.M., Jr.; Setyaadmadja, A.T.; Barrett, R.A. Effect of caffeine versus placebo supplementation on blood-glucose concentration. Lancet 1967, 1, 1299–1300. [Google Scholar] [CrossRef] [PubMed]
- Lane, J.D.; Barkauskas, C.E.; Surwit, R.S.; Feinglos, M.N. Caffeine impairs glucose metabolism in type 2 diabetes. Diabetes Care 2004, 27, 2047–2048. [Google Scholar] [CrossRef] [PubMed][Green Version]
- van Dam, R.M.; Pasman, W.J.; Verhoef, P. Effects of coffee consumption on fasting blood glucose and insulin concentrations: Randomized controlled trials in healthy volunteers. Diabetes Care 2004, 27, 2990–2992. [Google Scholar] [CrossRef]
- Wedick, N.M.; Brennan, A.M.; Sun, Q.; Hu, F.B.; Mantzoros, C.S.; van Dam, R.M. Effects of caffeinated and decaffeinated coffee on biological risk factors for type 2 diabetes: A randomized controlled trial. Nutr. J. 2011, 10, 93. [Google Scholar] [CrossRef]
- Li, S.; Shin, H.J.; Ding, E.L.; van Dam, R.M. Adiponectin levels and risk of type 2 diabetes: A systematic review and meta-analysis. JAMA 2009, 302, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Mathews, S.T.; Chellam, N.; Srinivas, P.R.; Cintron, V.J.; Leon, M.A.; Goustin, A.S.; Grunberger, G. Alpha2-HSG, a specific inhibitor of insulin receptor autophosphorylation, interacts with the insulin receptor. Mol. Cell Endocrinol. 2000, 164, 87–98. [Google Scholar] [CrossRef]
- Kempf, K.; Herder, C.; Erlund, I.; Kolb, H.; Martin, S.; Carstensen, M.; Koenig, W.; Sundvall, J.; Bidel, S.; Kuha, S.; et al. Effects of coffee consumption on subclinical inflammation and other risk factors for type 2 diabetes: A clinical trial. Am. J. Clin. Nutr. 2010, 91, 950–957. [Google Scholar] [CrossRef]
- Thom, E. The effect of chlorogenic acid enriched coffee on glucose absorption in healthy volunteers and its effect on body mass when used long-term in overweight and obese people. J. Int. Med. Res. 2007, 35, 900–908. [Google Scholar] [CrossRef]
- Ohnaka, K.; Ikeda, M.; Maki, T.; Okada, T.; Shimazoe, T.; Adachi, M.; Nomura, M.; Takayanagi, R.; Kono, S. Effects of 16-week consumption of caffeinated and decaffeinated instant coffee on glucose metabolism in a randomized controlled trial. J. Nutr. Metab. 2012, 2012, 207426. [Google Scholar] [CrossRef]
- Fernández-Cardero, Á.; Sierra-Cinos, J.L.; Bravo, L.; Sarriá, B. Consumption of a Coffee Rich in Phenolic Compounds May Improve the Body Composition of People with Overweight or Obesity: Preliminary Insights from a Randomized, Controlled and Blind Crossover Study. Nutrients 2024, 16, 2848. [Google Scholar] [CrossRef]
- Radosinska, D.; Jasenovec, T.; Golianova, A.; Szadvari, I.; Vazan, R.; Kovacicova, I.; Snurikova, D.; Vrbjar, N.; Radosinska, J. Controlled coffee intake enhances erythrocyte deformability, Na,K-ATPase activity, and GSH/GSSG ratio in healthy young adults. Biomedicines 2024, 12, 2570. [Google Scholar] [CrossRef] [PubMed]
- Rustenbeck, I.; Lier-Glaubitz, V.; Willenborg, M.; Eggert, F.; Engelhardt, U.; Jörns, A. Effect of chronic coffee consumption on weight gain and glycaemia in a mouse model of obesity and type 2 diabetes. Nutr. Diabetes 2014, 4, e123. [Google Scholar] [CrossRef] [PubMed]
- Frost-Meyer, N.J.; Logomarsino, J.V. Impact of coffee components on inflammatory markers: A review. J. Funct. Foods 2012, 4, 819–830. [Google Scholar] [CrossRef]
- Yamauchi, R.; Kobayashi, M.; Matsuda, Y.; Ojika, M.; Shigeoka, S.; Yamamoto, Y.; Tou, Y.; Inoue, T.; Katagiri, T.; Murai, A.; et al. Coffee and caffeine ameliorate hyperglycemia, fatty liver, and inflammatory adipocytokine expression in spontaneously diabetic KK-Ay mice. J. Agric. Food Chem. 2010, 58, 5597–5603. [Google Scholar] [CrossRef]
- Martins, B.C.; Soares, A.C.; Martins, F.F.; Resende, A.C.; Inada, K.O.P.; Souza-Mello, V.; Nunes, N.M.; Daleprane, J.B. Coffee consumption prevents obesity-related comorbidities and attenuates brown adipose tissue whitening in high-fat diet-fed mice. J. Nutr. Biochem. 2023, 117, 109336. [Google Scholar] [CrossRef]
- Gamboa-Gómez, C.I.; Barragán-Zúñiga, L.J.; Guerrero-Romero, F.; Martínez-Aguilar, G.; Gónzalez, J.L.; Valenzuela-Ramírez, A.A.; Rojas-Contreras, J.A.; Anese, M.; Flores, M.C.; Alongi, M. Effects of coffee with different roasting degrees on obesity and related metabolic disorders. J. Funct. Foods 2023, 111, 105889. [Google Scholar] [CrossRef]
- Lim, S.; Kim, J.W.; Targher, G. Links between metabolic syndrome and metabolic dysfunction-associated fatty liver disease. Trends Endocrinol. Metab. 2021, 32, 500–514. [Google Scholar] [CrossRef]
- Belayneh, A.; Molla, F. The Effect of Coffee on Pharmacokinetic Properties of Drugs: A Review. BioMed Res. Int. 2020, 2020, 7909703. [Google Scholar] [CrossRef]
- Clifford, M.N.; Johnston, K.L.; Knight, S.; Kuhnert, N. Hierarchical scheme for LC-MSn identification of chlorogenic acids. J. Agric. Food Chem. 2003, 51, 2900–2911. [Google Scholar] [CrossRef]
- Zuo, J.; Tang, W.; Xu, Y. Chapter 68—Anti-Hepatitis B Virus Activity of Chlorogenic Acid and Its Related Compounds. In Coffee in Health and Disease Prevention; Preedy, V.R., Ed.; Academic Press: San Diego, CA, USA, 2015; pp. 607–613. [Google Scholar]
- Mattila, P.; Hellstrom, J.; Torronen, R. Phenolic acids in berries, fruits, and beverages. J. Agric. Food Chem. 2006, 54, 7193–7199. [Google Scholar] [CrossRef]
- Moeenfard, M.; Rocha, L.; Alves, A. Quantification of caffeoylquinic acids in coffee brews by HPLC-DAD. J. Anal. Methods Chem. 2014, 2014, 965353. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Tian, Z.; Cui, Y.; Liu, Z.; Ma, X. Chlorogenic acid: A comprehensive review of the dietary sources, processing effects, bioavailability, beneficial properties, mechanisms of action, and future directions. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3130–3158. [Google Scholar] [CrossRef] [PubMed]
- Mattila, P.; Kumpulainen, J. Determination of free and total phenolic acids in plant-derived foods by HPLC with diode-array detection. J. Agric. Food Chem. 2002, 50, 3660–3667. [Google Scholar] [CrossRef]
- Nardini, M.; Cirillo, E.; Natella, F.; Scaccini, C. Absorption of phenolic acids in humans after coffee consumption. J. Agric. Food Chem. 2002, 50, 5735–5741. [Google Scholar] [CrossRef]
- Zhao, Z.; Moghadasian, M.H. Chemistry, natural sources, dietary intake and pharmacokinetic properties of ferulic acid: A review. Food Chem. 2008, 109, 691–702. [Google Scholar] [CrossRef] [PubMed]
- Natella, F.; Nardini, M.; Belelli, F.; Scaccini, C. Coffee drinking induces incorporation of phenolic acids into LDL and increases the resistance of LDL to ex vivo oxidation in humans. Am. J. Clin. Nutr. 2007, 86, 604–609. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef]
- Somporn, C.; Kamtuo, A.; Theerakulpisut, P.; Siriamornpun, S. Effects of roasting degree on radical scavenging activity, phenolics and volatile compounds of Arabica coffee beans (Coffea arabica L. cv. Catimor). Int. J. Food Sci. Technol. 2011, 46, 2287–2296. [Google Scholar] [CrossRef]
- Rojas-González, A.; Figueroa-Hernández, C.Y.; González-Rios, O.; Suárez-Quiroz, M.L.; González-Amaro, R.M.; Hernández-Estrada, Z.J.; Rayas-Duarte, P. Coffee Chlorogenic Acids Incorporation for Bioactivity Enhancement of Foods: A Review. Molecules 2022, 27, 3400. [Google Scholar] [CrossRef]
- Jeszka-Skowron, M.; Sentkowska, A.; Pyrzyńska, K.; De Peña, M.P. Chlorogenic acids, caffeine content and antioxidant properties of green coffee extracts: Influence of green coffee bean preparation. Eur. Food Res. Technol. 2016, 242, 1403–1409. [Google Scholar] [CrossRef]
- Miao, M.; Xiang, L. Pharmacological action and potential targets of chlorogenic acid. Adv. Pharmacol. 2020, 87, 71–88. [Google Scholar] [CrossRef] [PubMed]
- Ong, K.W.; Hsu, A.; Tan, B.K. Anti-diabetic and anti-lipidemic effects of chlorogenic acid are mediated by ampk activation. Biochem. Pharmacol. 2013, 85, 1341–1351. [Google Scholar] [CrossRef]
- Jin, S.; Chang, C.; Zhang, L.; Liu, Y.; Huang, X.; Chen, Z. Chlorogenic acid improves late diabetes through adiponectin receptor signaling pathways in db/db mice. PLoS ONE 2015, 10, e0120842. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Gao, M.; Liu, D. Chlorogenic acid improves high fat diet-induced hepatic steatosis and insulin resistance in mice. Pharm. Res. 2015, 32, 1200–1209. [Google Scholar] [CrossRef]
- Nyambe-Silavwe, H.; Williamson, G. Chlorogenic and phenolic acids are only very weak inhibitors of human salivary α-amylase and rat intestinal maltase activities. Food Res. Int. 2018, 113, 452–455. [Google Scholar] [CrossRef] [PubMed]
- Narita, Y.; Inouye, K. Kinetic analysis and mechanism on the inhibition of chlorogenic acid and its components against porcine pancreas alpha-amylase isozymes I and II. J. Agric. Food Chem. 2009, 57, 9218–9225. [Google Scholar] [CrossRef]
- Welsch, C.A.; Lachance, P.A.; Wasserman, B.P. Dietary phenolic compounds: Inhibition of Na+-dependent D-glucose uptake in rat intestinal brush border membrane vesicles. J. Nutr. 1989, 119, 1698–1704. [Google Scholar] [CrossRef]
- Alonso-Castro, A.J.; Miranda-Torres, A.C.; González-Chávez, M.M.; Salazar-Olivo, L.A. Cecropia obtusifolia Bertol and its active compound, chlorogenic acid, stimulate 2-NBDglucose uptake in both insulin-sensitive and insulin-resistant 3T3 adipocytes. J. Ethnopharmacol. 2008, 120, 458–464. [Google Scholar] [CrossRef]
- Tousch, D.; Lajoix, A.D.; Hosy, E.; Azay-Milhau, J.; Ferrare, K.; Jahannault, C.; Cros, G.; Petit, P. Chicoric acid, a new compound able to enhance insulin release and glucose uptake. Biochem. Biophys. Res. Commun. 2008, 377, 131–135. [Google Scholar] [CrossRef]
- Gao, J.; He, X.; Ma, Y.; Zhao, X.; Hou, X.; Hao, E.; Deng, J.; Bai, G. Chlorogenic acid targeting of the AKT PH domain activates AKT/GSK3β/FOXO1 signaling and improves glucose metabolism. Nutrients 2018, 10, 1366. [Google Scholar] [CrossRef]
- Ong, K.W.; Hsu, A.; Tan, B.K. Chlorogenic acid stimulates glucose transport in skeletal muscle via AMPK activation: A contributor to the beneficial effects of coffee on diabetes. PLoS ONE 2012, 7, e32718. [Google Scholar] [CrossRef]
- Arion, W.J.; Canfield, W.K.; Ramos, F.C.; Schindler, P.W.; Burger, H.J.; Hemmerle, H.; Schubert, G.; Below, P.; Herling, A.W. Chlorogenic acid and hydroxynitrobenzaldehyde: New inhibitors of hepatic glucose 6-phosphatase. Arch. Biochem. Biophys. 1997, 339, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Bassoli, B.K.; Cassolla, P.; Borba-Murad, G.R.; Constantin, J.; Salgueiro-Pagadigorria, C.L.; Bazotte, R.B.; da Silva, R.S.; de Souza, H.M. Chlorogenic acid reduces the plasma glucose peak in the oral glucose tolerance test: Effects on hepatic glucose release and glycaemia. Cell Biochem. Funct. 2008, 26, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Hall, A.M.; Soufi, N.; Chambers, K.T.; Chen, Z.; Schweitzer, G.G.; McCommis, K.S.; Erion, D.M.; Graham, M.J.; Su, X.; Finck, B.N. Abrogating monoacylglycerol acyltransferase activity in liver improves glucose tolerance and hepatic insulin signaling in obese mice. Diabetes 2014, 63, 2284–2296. [Google Scholar] [CrossRef]
- Johnston, K.L.; Clifford, M.N.; Morgan, L.M. Coffee acutely modifies gastrointestinal hormone secretion and glucose tolerance in humans: Glycemic effects of chlorogenic acid and caffeine. Am. J. Clin. Nutr. 2003, 78, 728–733. [Google Scholar] [CrossRef] [PubMed]
- van Dijk, A.E.; Olthof, M.R.; Meeuse, J.C.; Seebus, E.; Heine, R.J.; van Dam, R.M. Acute effects of decaffeinated coffee and the major coffee components chlorogenic acid and trigonelline on glucose tolerance. Diabetes Care 2009, 32, 1023–1025. [Google Scholar] [CrossRef]
- Zuñiga, L.Y.; Aceves-de la Mora, M.C.A.; González-Ortiz, M.; Ramos-Núñez, J.L.; Martínez-Abundis, E. Effect of Chlorogenic Acid Administration on Glycemic Control, Insulin Secretion, and Insulin Sensitivity in Patients with Impaired Glucose Tolerance. J. Med. Food 2018, 21, 469–473. [Google Scholar] [CrossRef]
- Mansour, A.; Mohajeri-Tehrani, M.R.; Samadi, M.; Qorbani, M.; Merat, S.; Adibi, H.; Poustchi, H.; Hekmatdoost, A. Effects of supplementation with main coffee components including caffeine and/or chlorogenic acid on hepatic, metabolic, and inflammatory indices in patients with non-alcoholic fatty liver disease and type 2 diabetes: A randomized, double-blind, placebo-controlled, clinical trial. Nutr. J. 2021, 20, 35. [Google Scholar] [CrossRef]
- McCarty, M.F. A chlorogenic acid-induced increase in GLP-1 production may mediate the impact of heavy coffee consumption on diabetes risk. Med. Hypotheses 2005, 64, 848–853. [Google Scholar] [CrossRef]
- Ebrahim, N.; Shakirova, K.; Dashinimaev, E. PDX1 is the cornerstone of pancreatic β-cell functions and identity. Front. Mol. Biosci. 2022, 9, 1091757. [Google Scholar] [CrossRef]
- Olthof, M.R.; Hollman, P.C.; Katan, M.B. Chlorogenic acid and caffeic acid are absorbed in humans. J. Nutr. 2001, 131, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, R.; Singh, S.V.; Jaiswal, K.; Kumar, R.; Pandey, A.K. Modulatory effect of caffeic acid in alleviating diabetes and associated complications. World J. Diabetes 2023, 14, 62–75. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.W.; Shen, S.C.; Wu, J.S. Effects of caffeic acid and cinnamic acid on glucose uptake in insulin-resistant mouse hepatocytes. J. Agric. Food Chem. 2009, 57, 7687–7692. [Google Scholar] [CrossRef]
- Tian, Y.; Ou, Z.; Xiong, W.; Fan, W.; Yang, W.; Zhang, B.; Pan, L.; Ren, H. Extraction and optimization of polyphenols from Morchella spp. using ultrasound-assisted deep eutectic solvents: Potential intervention for type 2 diabetes mellitus. J. Food Sci. 2025, 90, e70145. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Oksbjerg, N.; Young, J.F.; Jeppesen, P.B. Caffeic acid, naringenin and quercetin enhance glucose-stimulated insulin secretion and glucose sensitivity in INS-1E cells. Diabetes Obes. Metab. 2014, 16, 602–612. [Google Scholar] [CrossRef]
- Oboh, G.; Agunloye, O.M.; Adefegha, S.A.; Akinyemi, A.J.; Ademiluyi, A.O. Caffeic and chlorogenic acids inhibit key enzymes linked to type 2 diabetes (in vitro): A comparative study. J. Basic. Clin. Physiol. Pharmacol. 2015, 26, 165–170. [Google Scholar] [CrossRef]
- Ontawong, A.; Duangjai, A.; Srimaroeng, C. Coffea arabica bean extract inhibits glucose transport and disaccharidase activity in Caco-2 cells. Biomed. Rep. 2021, 15, 73. [Google Scholar] [CrossRef]
- Mukundh, S.T.; Veeraraghavan, V.P.; Ponnusamy, B.; Jayaraman, S. Phytochemical Screening and Antidiabetic Activity of Aqueous Extract of Evolvulus Alsinoides Leaves: An In Vitro and In Silico Study. J. Pharm. Bioallied Sci. 2024, 16, S1246–S1248. [Google Scholar] [CrossRef] [PubMed]
- McMillan, J.; Bester, M.J.; Apostolides, Z. In silico docking and ADMET studies on clinical targets for type 2 diabetes correlated to in vitro inhibition of pancreatic alpha-amylase and alpha-glucosidase by rutin, caffeic acid, p-coumaric acid, and vanillin. Silico Pharmacol. 2025, 13, 42. [Google Scholar] [CrossRef]
- Matowane, G.R.; Ramorobi, L.M.; Mashele, S.S.; Bonnet, S.L.; Noreljaleel, A.E.M.; Swain, S.S.; Makhafola, T.J.; Chukwuma, C.I. Novel Caffeic Acid—Zinc Acetate Complex: Studies on Promising Antidiabetic and Antioxidative Synergism Through Complexation. Med. Chem. 2023, 19, 147–162. [Google Scholar] [CrossRef]
- Huang, D.-W.; Shen, S.-C. Caffeic acid and cinnamic acid ameliorate glucose metabolism via modulating glycogenesis and gluconeogenesis in insulin-resistant mouse hepatocytes. J. Funct. Foods 2012, 4, 358–366. [Google Scholar] [CrossRef]
- Jung, U.J.; Lee, M.K.; Park, Y.B.; Jeon, S.M.; Choi, M.S. Antihyperglycemic and antioxidant properties of caffeic acid in db/db mice. J. Pharmacol. Exp. Ther. 2006, 318, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Hsu, F.L.; Chen, Y.C.; Cheng, J.T. Caffeic acid as active principle from the fruit of Xanthium strumarium to lower plasma glucose in diabetic rats. Planta Med. 2000, 66, 228–230. [Google Scholar] [CrossRef]
- Tsuda, S.; Egawa, T.; Ma, X.; Oshima, R.; Kurogi, E.; Hayashi, T. Coffee polyphenol caffeic acid but not chlorogenic acid increases 5′AMP-activated protein kinase and insulin-independent glucose transport in rat skeletal muscle. J. Nutr. Biochem. 2012, 23, 1403–1409. [Google Scholar] [CrossRef]
- Chang, W.-C.; Kuo, P.-L.; Chen, C.-W.; Wu, J.S.-B.; Shen, S.-C. Caffeic acid improves memory impairment and brain glucose metabolism via ameliorating cerebral insulin and leptin signaling pathways in high-fat diet-induced hyperinsulinemic rats. Food Res. Int. 2015, 77, 24–33. [Google Scholar] [CrossRef]
- Salau, V.F.; Erukainure, O.L.; Ijomone, O.M.; Islam, M.S. Caffeic acid regulates glucose homeostasis and inhibits purinergic and cholinergic activities while abating oxidative stress and dyslipidaemia in fructose-streptozotocin-induced diabetic rats. J. Pharm. Pharmacol. 2022, 74, 973–984. [Google Scholar] [CrossRef]
- Matowane, G.R.; Mashele, S.S.; Makhafola, T.J.; Chukwuma, C.I. The ameliorative effect of zinc acetate with caffeic acid in the animal model of type 2 diabetes. Biomed. Pharmacother. 2023, 163, 114779. [Google Scholar] [CrossRef]
- Cao, X.; Xia, Y.; Zeng, M.; Wang, W.; He, Y.; Liu, J. Caffeic Acid Inhibits the Formation of Advanced Glycation End Products (AGEs) and Mitigates the AGEs-Induced Oxidative Stress and Inflammation Reaction in Human Umbilical Vein Endothelial Cells (HUVECs). Chem. Biodivers. 2019, 16, e1900174. [Google Scholar] [CrossRef] [PubMed]
- Fratantonio, D.; Speciale, A.; Canali, R.; Natarelli, L.; Ferrari, D.; Saija, A.; Virgili, F.; Cimino, F. Low nanomolar caffeic acid attenuates high glucose-induced endothelial dysfunction in primary human umbilical-vein endothelial cells by affecting NF-κB and Nrf2 pathways. Biofactors 2017, 43, 54–62. [Google Scholar] [CrossRef]
- Toma, L.; Sanda, G.M.; Niculescu, L.S.; Deleanu, M.; Stancu, C.S.; Sima, A.V. Caffeic acid attenuates the inflammatory stress induced by glycated LDL in human endothelial cells by mechanisms involving inhibition of AGE-receptor, oxidative, and endoplasmic reticulum stress. Biofactors 2017, 43, 685–697. [Google Scholar] [CrossRef]
- Natarelli, L.; Ranaldi, G.; Leoni, G.; Roselli, M.; Guantario, B.; Comitato, R.; Ambra, R.; Cimino, F.; Speciale, A.; Virgili, F.; et al. Nanomolar Caffeic Acid Decreases Glucose Uptake and the Effects of High Glucose in Endothelial Cells. PLoS ONE 2015, 10, e0142421. [Google Scholar] [CrossRef] [PubMed]
- Beecher, G.R. Nutrient content of tomatoes and tomato products. Proc. Soc. Exp. Biol. Med. 1998, 218, 98–100. [Google Scholar] [CrossRef]
- Adam, A.; Crespy, V.; Levrat-Verny, M.A.; Leenhardt, F.; Leuillet, M.; Demigné, C.; Rémésy, C. The bioavailability of ferulic acid is governed primarily by the food matrix rather than its metabolism in intestine and liver in rats. J. Nutr. 2002, 132, 1962–1968. [Google Scholar] [CrossRef]
- McCarty, M.F.; Assanga, S.B.I. Ferulic acid may target MyD88-mediated pro-inflammatory signaling—Implications for the health protection afforded by whole grains, anthocyanins, and coffee. Med. Hypotheses 2018, 118, 114–120. [Google Scholar] [CrossRef]
- Khatun, M.M.; Bhuia, M.S.; Chowdhury, R.; Sheikh, S.; Ajmee, A.; Mollah, F.; Al Hasan, M.S.; Coutinho, H.D.M.; Islam, M.T. Potential utilization of ferulic acid and its derivatives in the management of metabolic diseases and disorders: An insight into mechanisms. Cell. Signal. 2024, 121, 111291. [Google Scholar] [CrossRef] [PubMed]
- Choi, R.; Kim, B.H.; Naowaboot, J.; Lee, M.Y.; Hyun, M.R.; Cho, E.J.; Lee, E.S.; Lee, E.Y.; Yang, Y.C.; Chung, C.H. Effects of ferulic acid on diabetic nephropathy in a rat model of type 2 diabetes. Exp. Mol. Med. 2011, 43, 676–683. [Google Scholar] [CrossRef] [PubMed]
- Salau, V.F.; Erukainure, O.L.; Olofinsan, K.O.; Bharuth, V.; Ijomone, O.M.; Islam, M.S. Ferulic acid improves glucose homeostasis by modulation of key diabetogenic activities and restoration of pancreatic architecture in diabetic rats. Fundam. Clin. Pharmacol. 2023, 37, 324–339. [Google Scholar] [CrossRef]
- Ruamyod, K.; Watanapa, W.B.; Kakhai, C.; Nambundit, P.; Treewaree, S.; Wongsanupa, P. Ferulic acid enhances insulin secretion by potentiating L-type Ca(2+) channel activation. J. Integr. Med. 2023, 21, 99–105. [Google Scholar] [CrossRef]
- Nomura, E.; Kashiwada, A.; Hosoda, A.; Nakamura, K.; Morishita, H.; Tsuno, T.; Taniguchi, H. Synthesis of amide compounds of ferulic acid, and their stimulatory effects on insulin secretion in vitro. Bioorg Med. Chem. 2003, 11, 3807–3813. [Google Scholar] [CrossRef]
- Matowane, G.R.; Ramorobi, L.M.; Mashele, S.S.; Bonnet, S.L.; Noreljaleel, A.E.M.; Swain, S.S.; Makhafola, T.J.; Chukwuma, C.I. Complexation potentiated promising anti-diabetic and anti-oxidative synergism between ZN(ii) and ferulic acid: A multimode study. Diabet. Med. 2022, 39, e14905. [Google Scholar] [CrossRef]
- Gogoi, B.; Chatterjee, P.; Mukherjee, S.; Buragohain, A.K.; Bhattacharya, S.; Dasgupta, S. A polyphenol rescues lipid induced insulin resistance in skeletal muscle cells and adipocytes. Biochem. Biophys. Res. Commun. 2014, 452, 382–388. [Google Scholar] [CrossRef]
- Naowaboot, J.; Piyabhan, P.; Tingpej, P.; Munkong, N.; Parklak, W.; Pannangpetch, P. Anti-insulin resistant effect of ferulic acid on high fat diet-induced obese mice. Asian Pac. J. Trop. Biomed. 2018, 8, 604–608. [Google Scholar] [CrossRef]
- Zheng, Y.; Tian, J.; Yang, W.; Chen, S.; Liu, D.; Fang, H.; Zhang, H.; Ye, X. Inhibition mechanism of ferulic acid against α-amylase and α-glucosidase. Food Chem. 2020, 317, 126346. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Zou, W.; Cao, X.; Xu, W.; Lu, Z.; Zhu, Y.; Hu, X.; Hu, J.; Zhu, Q. Ferulic acid attenuates high glucose-induced apoptosis in retinal pigment epithelium cells and protects retina in db/db mice. PeerJ 2022, 10, e13375. [Google Scholar] [CrossRef] [PubMed]
- Sompong, W.; Cheng, H.; Adisakwattana, S. Protective Effects of Ferulic Acid on High Glucose-Induced Protein Glycation, Lipid Peroxidation, and Membrane Ion Pump Activity in Human Erythrocytes. PLoS ONE 2015, 10, e0129495. [Google Scholar] [CrossRef]
- Li, D.; Rui, Y.X.; Guo, S.D.; Luan, F.; Liu, R.; Zeng, N. Ferulic acid: A review of its pharmacology, pharmacokinetics and derivatives. Life Sci. 2021, 284, 119921. [Google Scholar] [CrossRef]
- Balasubashini, M.S.; Rukkumani, R.; Viswanathan, P.; Menon, V.P. Ferulic acid alleviates lipid peroxidation in diabetic rats. Phytother. Res. 2004, 18, 310–314. [Google Scholar] [CrossRef]
- Ohnishi, M.; Matuo, T.; Tsuno, T.; Hosoda, A.; Nomura, E.; Taniguchi, H.; Sasaki, H.; Morishita, H. Antioxidant activity and hypoglycemic effect of ferulic acid in STZ-induced diabetic mice and KK-Ay mice. Biofactors 2004, 21, 315–319. [Google Scholar] [CrossRef]
- Naowaboot, J.; Piyabhan, P.; Munkong, N.; Parklak, W.; Pannangpetch, P. Ferulic acid improves lipid and glucose homeostasis in high-fat diet-induced obese mice. Clin. Exp. Pharmacol. Physiol. 2016, 43, 242–250. [Google Scholar] [CrossRef]
- Jung, E.H.; Kim, S.R.; Hwang, I.K.; Ha, T.Y. Hypoglycemic effects of a phenolic acid fraction of rice bran and ferulic acid in C57BL/KsJ-db/db mice. J. Agric. Food Chem. 2007, 55, 9800–9804. [Google Scholar] [CrossRef]
- Song, Y.; Wu, T.; Yang, Q.; Chen, X.; Wang, M.; Wang, Y.; Peng, X.; Ou, S. Ferulic acid alleviates the symptoms of diabetes in obese rats. J. Funct. Foods 2014, 9, 141–147. [Google Scholar] [CrossRef]
- Narasimhan, A.; Chinnaiyan, M.; Karundevi, B. Ferulic acid exerts its antidiabetic effect by modulating insulin-signalling molecules in the liver of high-fat diet and fructose-induced type-2 diabetic adult male rat. Appl. Physiol. Nutr. Metab. 2015, 40, 769–781. [Google Scholar] [CrossRef] [PubMed]
- Narasimhan, A.; Chinnaiyan, M.; Karundevi, B. Ferulic acid regulates hepatic GLUT2 gene expression in high fat and fructose-induced type-2 diabetic adult male rat. Eur. J. Pharmacol. 2015, 761, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Vellai, R.D.; Chandiran, S.; Pillai, S.S. GTF-231, a Mixture of Gymnemic Acid, Trigonelline and Ferulic Acid Significantly Ameliorates Oxidative Stress in Experimental Type 2 Diabetes in Rats. Can. J. Diabetes 2018, 42, 237–244. [Google Scholar] [CrossRef]
- Huang, J.; Wang, X.; Tao, G.; Song, Y.; Ho, C.; Zheng, J.; Ou, S. Feruloylated oligosaccharides from maize bran alleviate the symptoms of diabetes in streptozotocin-induced type 2 diabetic rats. Food Funct. 2018, 9, 1779–1789. [Google Scholar] [CrossRef]
- El-Gammal, H.L.; Omar, F.; Hyder, A. Ferulic acid protects rat offspring from maternal high-fat, high-fructose diet-induced toxicity and developmental retardation through a direct effect on pancreatic islets. Food Chem. Toxicol. 2025, 197, 115265. [Google Scholar] [CrossRef]
- Song, Y.; Wu, M.S.; Tao, G.; Lu, M.W.; Lin, J.; Huang, J.Q. Feruloylated oligosaccharides and ferulic acid alter gut microbiome to alleviate diabetic syndrome. Food Res. Int. 2020, 137, 109410. [Google Scholar] [CrossRef]
- Nankar, R.; Prabhakar, P.K.; Doble, M. Hybrid drug combination: Combination of ferulic acid and metformin as anti-diabetic therapy. Phytomedicine 2017, 37, 10–13. [Google Scholar] [CrossRef]
- Prabhakar, P.K.; Prasad, R.; Ali, S.; Doble, M. Synergistic interaction of ferulic acid with commercial hypoglycemic drugs in streptozotocin induced diabetic rats. Phytomedicine 2013, 20, 488–494. [Google Scholar] [CrossRef]
- Agudelo-Ochoa, G.M.; Pulgarín-Zapata, I.C.; Velásquez-Rodriguez, C.M.; Duque-Ramírez, M.; Naranjo-Cano, M.; Quintero-Ortiz, M.M.; Lara-Guzmán, O.J.; Muñoz-Durango, K. Coffee Consumption Increases the Antioxidant Capacity of Plasma and Has No Effect on the Lipid Profile or Vascular Function in Healthy Adults in a Randomized Controlled Trial. J. Nutr. 2016, 146, 524–531. [Google Scholar] [CrossRef]
- Costabile, G.; Vitale, M.; Della Pepa, G.; Cipriano, P.; Vetrani, C.; Testa, R.; Mena, P.; Bresciani, L.; Tassotti, M.; Calani, L.; et al. A wheat aleurone-rich diet improves oxidative stress but does not influence glucose metabolism in overweight/obese individuals: Results from a randomized controlled trial. Nutr. Metab. Cardiovasc. Dis. 2022, 32, 715–726. [Google Scholar] [CrossRef] [PubMed]
- Clifford, M.N.; Marks, S.; Knight, S.; Kuhnert, N. Characterization by LC-MS(n) of four new classes of p-coumaric acid-containing diacyl chlorogenic acids in green coffee beans. J. Agric. Food Chem. 2006, 54, 4095–4101. [Google Scholar] [CrossRef] [PubMed]
- Kashtoh, H.; Baek, K.H. New Insights into the Latest Advancement in α-Amylase Inhibitors of Plant Origin with Anti-Diabetic Effects. Plants 2023, 12, 2944. [Google Scholar] [CrossRef]
- Khan, M.S.; Alokail, M.S.; Alenad, A.M.H.; Altwaijry, N.; Alafaleq, N.O.; Alamri, A.M.; Zawba, M.A. Binding Studies of Caffeic and p-Coumaric Acid with α-Amylase: Multispectroscopic and Computational Approaches Deciphering the Effect on Advanced Glycation End Products (AGEs). Molecules 2022, 27, 3992. [Google Scholar] [CrossRef] [PubMed]
- Khalid, M.; Petroianu, G.; Adem, A. Advanced Glycation End Products and Diabetes Mellitus: Mechanisms and Perspectives. Biomolecules 2022, 12, 542. [Google Scholar] [CrossRef]
- Yoon, S.A.; Kang, S.I.; Shin, H.S.; Kang, S.W.; Kim, J.H.; Ko, H.C.; Kim, S.J. p-Coumaric acid modulates glucose and lipid metabolism via AMP-activated protein kinase in L6 skeletal muscle cells. Biochem. Biophys. Res. Commun. 2013, 432, 553–557. [Google Scholar] [CrossRef]
- Kang, S.W.; Kang, S.I.; Shin, H.S.; Yoon, S.A.; Kim, J.H.; Ko, H.C.; Kim, S.J. Sasa quelpaertensis Nakai extract and its constituent p-coumaric acid inhibit adipogenesis in 3T3-L1 cells through activation of the AMPK pathway. Food Chem. Toxicol. 2013, 59, 380–385. [Google Scholar] [CrossRef]
- Kim, J.H.; Kang, S.I.; Shin, H.S.; Yoon, S.A.; Kang, S.W.; Ko, H.C.; Kim, S.J. Sasa quelpaertensis and p-coumaric acid attenuate oleic acid-induced lipid accumulation in HepG2 cells. Biosci. Biotechnol. Biochem. 2013, 77, 1595–1598. [Google Scholar] [CrossRef]
- Dludla, P.V.; Mabhida, S.E.; Ziqubu, K.; Nkambule, B.B.; Mazibuko-Mbeje, S.E.; Hanser, S.; Basson, A.K.; Pheiffer, C.; Kengne, A.P. Pancreatic β-cell dysfunction in type 2 diabetes: Implications of inflammation and oxidative stress. World J. Diabetes 2023, 14, 130–146. [Google Scholar] [CrossRef]
- Amalan, V.; Vijayakumar, N.; Indumathi, D.; Ramakrishnan, A. Antidiabetic and antihyperlipidemic activity of p-coumaric acid in diabetic rats, role of pancreatic GLUT 2: In vivo approach. Biomed. Pharmacother. 2016, 84, 230–236. [Google Scholar] [CrossRef]
- Amalan, V.; Natesan, V.; Arumugam, R. P-Coumaric acid regulates blood glucose and antioxidant levels in streptozotocin induced diabetic rats. J. Chem. Pharm. Res. 2015, 7, 831–839. [Google Scholar]
- Mani, A.; Kushwaha, K.; Khurana, N.; Gupta, J. p-Coumaric acid attenuates high-fat diet-induced oxidative stress and nephropathy in diabetic rats. J. Anim. Physiol. Anim. Nutr. 2022, 106, 872–880. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.J.; Jung, U.J. p-Coumaric acid alleviates metabolic dysregulation in high-fructose diet-fed hamsters. Nutr. Res. Pract. 2025, 19, 200–214. [Google Scholar] [CrossRef] [PubMed]
- Yoon, D.S.; Cho, S.Y.; Yoon, H.J.; Kim, S.R.; Jung, U.J. Protective effects of p-coumaric acid against high-fat diet-induced metabolic dysregulation in mice. Biomed. Pharmacother. 2021, 142, 111969. [Google Scholar] [CrossRef]
- Liu, Z.; Yang, Y.; Xu, Y.; Zhang, Z.; Tang, R.; Liu, J.; Jiang, H.; Zhao, R. Procyanidin B1 and p-coumaric acid from whole highland barley ameliorated HFD-induced impaired glucose tolerance via small intestinal barrier and hepatic glucose metabolism. Food Funct. 2024, 15, 9272–9283. [Google Scholar] [CrossRef]
- Nguyen, L.V.; Nguyen, K.D.A.; Ma, C.T.; Nguyen, Q.T.; Nguyen, H.T.H.; Yang, D.J.; Tran, T.L.; Kim, K.W.; Doan, K.V. p-Coumaric acid enhances hypothalamic leptin signaling and glucose homeostasis in mice via differential effects on AMPK activation. Int. J. Mol. Sci. 2021, 22, 1431. [Google Scholar] [CrossRef]
- Han, X.; Guo, J.; You, Y.; Zhan, J.; Huang, W. p-Coumaric acid prevents obesity via activating thermogenesis in brown adipose tissue mediated by mTORC1-RPS6. FASEB J. 2020, 34, 7810–7824. [Google Scholar] [CrossRef]
- Abdel-Moneim, A.; El-Twab, S.M.A.; Yousef, A.I.; Reheim, E.S.A.; Ashour, M.B. Modulation of hyperglycemia and dyslipidemia in experimental type 2 diabetes by gallic acid and p-coumaric acid: The role of adipocytokines and PPARγ. Biomed. Pharmacother. 2018, 105, 1091–1097. [Google Scholar] [CrossRef]
- Nićiforović, N.; Abramovič, H. Sinapic Acid and Its Derivatives: Natural Sources and Bioactivity. Compr. Rev. Food Sci. Food Saf. 2014, 13, 34–51. [Google Scholar] [CrossRef]
- Cherng, Y.G.; Tsai, C.C.; Chung, H.H.; Lai, Y.W.; Kuo, S.C.; Cheng, J.T. Antihyperglycemic action of sinapic acid in diabetic rats. J. Agric. Food Chem. 2013, 61, 12053–12059. [Google Scholar] [CrossRef]
- Sorimuthu Pillai, S. Sinapic Acid Regulates Glucose Homeostasis by Modulating the Activities of Carbohydrate Metabolizing Enzymes in High Fat Diet Fed-Low Dose STZ Induced Experimental Type 2 Diabetes in Rats. Glob. J. Obes. Diabetes Metab. Syndr. 2017, 4, 054–061. [Google Scholar] [CrossRef]
- Nithya, R.; Subramanian, S.P. Sinapic Acid, a Naturally Occurring Carboxylic Acid Derivative Ameliorates Hyperglycemia in High Fat Diet-Low Dose STZ Induced Experimental Diabetic Rats. Int. J. Sci. Eng. Technol. Res. 2015, 4, 5746–5750. [Google Scholar]
- Zych, M.; Wojnar, W.; Borymski, S.; Szałabska, K.; Bramora, P.; Kaczmarczyk-Sedlak, I. Effect of Rosmarinic Acid and Sinapic Acid on Oxidative Stress Parameters in the Cardiac Tissue and Serum of Type 2 Diabetic Female Rats. Antioxidants 2019, 8, 579. [Google Scholar] [CrossRef] [PubMed]
- Alaofi, A.L. Sinapic Acid Ameliorates the Progression of Streptozotocin (STZ)-Induced Diabetic Nephropathy in Rats via NRF2/HO-1 Mediated Pathways. Front. Pharmacol. 2020, 11, 1119. [Google Scholar] [CrossRef]
- Meesa, M.; Yellu, N.R. Impact of Sinapic acid on Ertugliflozin Pharmacokinetics and Pharmacodynamics in Type-2 Diabetic Rats. J. Young Pharm. 2023, 15, 485–490. [Google Scholar] [CrossRef]



| Study | Population/Model | Key Findings |
|---|---|---|
| Huxley et al. [15] | 457,000+ participants (18 prospective cohorts; median follow-up: 2–20 yrs) | 7% ↓ risk per cup/day (dose-dependent) |
| Bhupathiraju et al. [16] | 120,000+ participants (3 U.S. cohorts; follow-up: 4 yr diabetes risk assessed after 4 yr coffee intake) | 12% ↓ risk with >1 cup/day |
| Iso et al. [17] | Japanese adults (follow-up: 5 yrs) | 42% ↓ risk with ≥3 cups/day |
| Muley et al. [18] | 1,200,000+ participants (13 cohorts; varied follow-up: 5–18 yrs | ↓ risk with 4–6 or ≥6–7 cups/day (filtered and decaf. favored) |
| Ding et al. [19] | 1,100,000+ participants (28 prospective cohorts; follow-up: 10 mos~18 yrs) | 33% ↓ risk with 6 cups/day (dose-dependent) |
| Jiang et al. [20] | 1,000,000+ participants (meta-analysis; varied follow-up: 2.6–24 yrs) | 21–30% ↓ risk in highest vs. lowest intake (coffee, decaf., caf.) |
| van Dam et al. [26] | Healthy adults (4 wk intervention) | ↑ fasting insulin (caf. coffee)/no change in glucose |
| Wedick et al. [27] | Overweight adults (8 wk intervention) | ↑ adiponectin (caf.), ↓ fetuin-A (decaf.) |
| Kempf et al. [30] | Nondiabetic adults < 65 y at elevated risk of T2DM (8 wk intervention) | ↑ CGA/CA metabolites, ↓ IL-18, ↑ adiponectin, HDL C with 8 cups/day |
| Thom [31] | Healthy adults/Overweight adults (12 wk intervention) | 6.9% ↓ glucose absorption in healthy adults/5.4 kg ↓ body weight in overweight adults (CGA-enriched coffee) |
| Ohnaka et al. [32] | Overweight men with IFG (16 wk intervention) | ↓ post-load glucose and waist (caf.); slight ↓ glucose (decaf.) |
| Fernández-Cardero et al. [33] | Overweight adults (12 wk intervention) | ↓ fat % (LRC > RC); ↑ muscle %; no weight or MetS change |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.; Kim, S.R.; Jung, U.J. Coffee and Its Major Polyphenols in the Prevention and Management of Type 2 Diabetes: A Comprehensive Review. Int. J. Mol. Sci. 2025, 26, 5544. https://doi.org/10.3390/ijms26125544
Kim H, Kim SR, Jung UJ. Coffee and Its Major Polyphenols in the Prevention and Management of Type 2 Diabetes: A Comprehensive Review. International Journal of Molecular Sciences. 2025; 26(12):5544. https://doi.org/10.3390/ijms26125544
Chicago/Turabian StyleKim, HwiCheol, Sang Ryong Kim, and Un Ju Jung. 2025. "Coffee and Its Major Polyphenols in the Prevention and Management of Type 2 Diabetes: A Comprehensive Review" International Journal of Molecular Sciences 26, no. 12: 5544. https://doi.org/10.3390/ijms26125544
APA StyleKim, H., Kim, S. R., & Jung, U. J. (2025). Coffee and Its Major Polyphenols in the Prevention and Management of Type 2 Diabetes: A Comprehensive Review. International Journal of Molecular Sciences, 26(12), 5544. https://doi.org/10.3390/ijms26125544







