Abstract
Curcumin, a polyphenolic compound derived from Curcuma longa, has drawn significant attention for its pleiotropic pharmacological activities, including anti-inflammatory and anticancer effects. Pyroptosis, an inflammatory form of programmed cell death mediated by inflammasome activation and gasdermin cleavage, has emerged as a critical target in both chronic inflammatory diseases and cancer therapy. This review comprehensively explores the dual roles of curcumin in the regulation of NLRP3 inflammasome-mediated pyroptosis. Curcumin exerts inhibitory effects by suppressing NF-κB signaling, attenuating mitochondrial reactive oxygen species (ROS) and ER stress, preventing potassium efflux, and disrupting inflammasome complex assembly. Conversely, in certain cancer contexts, curcumin promotes pyroptosis by stabilizing NLRP3 through the inhibition of Smurf2-mediated ubiquitination. Molecular docking studies support curcumin’s direct binding to several pyroptosis-associated proteins, including NLRP3, AMPK, caspase-1, and Smurf2. These context-dependent regulatory effects underscore the therapeutic potential of curcumin as both an inflammasome suppressor in inflammatory diseases and a pyroptosis inducer in cancer.
1. Introduction
Pyroptosis is a lytic and highly inflammatory form of programmed cell death that plays a pivotal role in both innate immunity and disease progression [1]. Unlike apoptosis, which is generally non-inflammatory and immunologically silent, pyroptosis is characterized by cell swelling, membrane rupture, and the release of intracellular contents including damage-associated molecular patterns (DAMPs) and pro-inflammatory cytokines such as interleukin (IL)-1β and IL-18 [2,3]. This process is predominantly executed by gasdermin family proteins (e.g., GSDMD, GSDME), which form membrane pores upon cleavage by activated caspases such as caspase-1 in the canonical inflammasome pathway [4], and caspase-4/5/11 in the non-canonical pathway [5]. Additional pathways involve granzyme-mediated cleavage in immune cells, highlighting the complexity and context-dependent regulation of pyroptotic death.
In the context of cancer, pyroptosis exhibits a paradoxical duality, functioning as both a tumor-suppressive and tumor-promoting mechanism depending on the cellular environment, the specific gasdermin involved, and the intensity and duration of activation [6,7,8,9]. On one hand, chronic low-grade pyroptosis can facilitate tumor development by maintaining a persistent inflammatory microenvironment. This condition supports angiogenesis, tumor cell proliferation, and immune evasion. For example, GSDME-mediated pyroptosis has been shown to promote the development of colitis-associated colorectal cancer by releasing HMGB1, a prototypical DAMP, which in turn activates the ERK1/2 signaling pathway and upregulates proliferating cell nuclear antigen (PCNA), contributing to tumor proliferation [10]. Similarly, asbestos exposure-induced pyroptosis is implicated in the pathogenesis of malignant mesothelioma, highlighting a link between persistent inflammation and tumorigenesis [11].
On the other hand, acute and robust induction of pyroptosis within tumor cells has emerged as a promising anticancer strategy. When activated explosively, pyroptosis results in immunogenic cell death, releasing a surge of DAMPs and pro-inflammatory cytokines that recruit and activate components of the immune system. IL-1β and IL-18 released during pyroptosis enhance the activation and maturation of dendritic cells (DCs), promote CD8+ cytotoxic T lymphocyte responses, and recruit natural killer (NK) cells. These immune events contribute to a shift from an immunosuppressive to an immunostimulatory tumor microenvironment, ultimately enhancing antitumor immunity [12,13,14]. Therefore, selectively suppressing chronic inflammation-driven pyroptosis while promoting acute pyroptosis in tumor cells offers a dual-pronged therapeutic strategy that targets both the tumor and its immunological niche.
Among the natural compounds investigated for their ability to modulate pyroptosis, curcumin, a bioactive polyphenol derived from Curcuma longa, has gained substantial attention due to its pleiotropic biological activities and excellent safety profile [15,16,17]. Curcumin has been shown to suppress chronic inflammation-related pyroptosis by downregulating the NLRP3 inflammasome, reducing ROS production, and inhibiting the release of IL-1β and IL-18 in various cell types and disease models [18,19]. These anti-inflammatory effects are particularly relevant in preventing the tumor-promoting consequences of prolonged pyroptotic signaling. For instance, in macrophages and endothelial cells, curcumin inhibits NLRP3 activation and reduces pro-inflammatory cytokine secretion, thereby attenuating chronic inflammation in the tumor microenvironment.
In parallel, curcumin can also function as an inducer of pyroptotic cell death in tumor cells. It has been reported to increase the expression and activation of caspase-1 and GSDME in certain cancer cell lines, leading to membrane pore formation, release of immunostimulatory factors, and enhanced recruitment of immune effector cells [20,21]. These effects culminate in both direct tumor cell killing and stimulation of antitumor immune responses. Moreover, curcumin has been found to sensitize tumor cells to other pyroptosis-inducing agents, suggesting its potential use in combination therapy to enhance cancer immunogenicity.
Taken together, these findings suggest that curcumin holds great promise as a context-dependent modulator of pyroptosis. By simultaneously suppressing chronic inflammation-associated pyroptosis and promoting acute immunogenic pyroptosis in cancer cells, curcumin may serve as a valuable therapeutic tool in cancer treatment strategies that aim to leverage the immune system while minimizing inflammation-driven tumor progression.
This review aims to provide a comprehensive synthesis of recent advances in understanding how curcumin modulates pyroptosis in the tumor microenvironment, with a particular focus on its dual role in attenuating chronic inflammatory pyroptosis and promoting acute cancer cell pyroptosis as a potential strategy for anticancer therapy.
2. Pyroptosis Signaling Pathways
Pyroptosis is mediated through four distinct but interrelated signaling pathways. These include the canonical inflammasome pathway, which activates caspase-1 in response to pathogen- or damage-associated signals; the non-canonical pathway, where caspase-4/5 (in humans) or caspase-11 (in mice) directly sense cytosolic lipopolysaccharide (LPS); the apoptotic caspase-mediated pathway, in which apoptotic caspases like caspase-3, -8, and -9 cleave gasdermin proteins to switch apoptosis to pyroptosis; and the granzymes-mediated pathway, where immune cells such as NK cells and CTLs deliver granzymes that directly cleave gasdermins in target cells. These pathways collectively illustrate the complexity and flexibility of pyroptosis in host defense and disease regulation.
2.1. Canonical Inflammasome Pathway
The canonical inflammasome pathway, first identified among inflammasome mechanisms, is a defense system that detects both pathogenic elements (PAMPs) and endogenous stress signals (DAMPs). It involves the assembly of large protein complexes composed of cytosolic pattern recognition receptors (PRRs), the adaptor protein ASC (apoptosis-associated speck-like protein containing a CARD), and inflammatory caspases such as caspase-1 [22].
Common PRRs that participate in this process include several members of the nucleotide-binding oligomerization domain-like receptor (NLR) family, namely NLRP1, NLRP3, and NLRC4, as well as AIM2 and pyrin [23,24]. Each of these PRRs has a unique domain architecture and sensing mechanism. For example, NLRP1 features an N-terminal pyrin domain (PYD), a nucleotide-binding domain (NOD), a leucine-rich repeat (LRR) region, and a C-terminal caspase recruitment domain (CARD) [25]. The PYD is essential for interaction with ASC, while the NOD contributes to ATP-dependent oligomerization. The LRR acts in autoinhibition and ligand detection, and the CARD domain is involved in the direct recruitment of pro-caspase-1. Activators of NLRP1 include anthrax lethal toxin, muramyl dipeptide, and Toxoplasma gondii components [26].
NLRP3, another key PRR, has a similar PYD-NOD-LRR structure but lacks a CARD domain. It can be activated by a wide variety of stimuli such as microbial infections (bacteria, viruses, fungi), danger signals like ATP, uric acid, and reactive oxygen species (ROS), and other host-derived stressors [27]. For instance, extracellular ATP binds to P2X7 receptors, leading to potassium efflux and triggering the assembly of the NLRP3 inflammasome, ultimately activating caspase-1 and promoting IL-1β secretion [28].
In contrast, NLRC4 carries an N-terminal CARD domain, a central nucleotide-binding domain (NBD), and a C-terminal LRR. It recognizes intracellular bacterial components like flagellin and proteins secreted via the bacterial type III secretion system [29].
AIM2, a non-NLR sensor, comprises a PYD and a HIN-200 domain that binds to double-stranded DNA from microbial origins such as viruses or bacteria [30]. Pyrin, another inflammasome sensor, contains a PYD, two B-boxes, and a SPRY/PRY domain. It is activated upon detection of bacterial-mediated modifications of host Rho GTPases [24].
Once activated, these PRRs recruit pro-caspase-1 either directly via their own CARD domains or indirectly through ASC, forming an inflammasome complex. Within this complex, pro-caspase-1 undergoes autocatalytic activation, which leads to two major downstream events: (1) the maturation of pro-inflammatory cytokines IL-1β and IL-18 and (2) the cleavage of gasdermin D (GSDMD). The N-terminal domain of GSDMD (GSDMD-NT) then translocates to the plasma membrane to form pores, triggering pyroptotic cell death [31].
2.2. Non-Canonical Inflammasome Pathway
The non-canonical inflammasome pathway functions independently of the classical inflammasome complex and is mainly activated by Gram-negative bacteria. Extracellular LPS promotes the production of type I interferons, which activate their receptors and induce caspase-11 expression [32,33].
Vacuolar Gram-negative bacteria can release LPS into the cytosol after vacuole rupture, a process mediated by guanylate-binding proteins. Cytosolic LPS directly binds to caspase-11, activating it. Activated caspase-11 cleaves gasdermin D (GSDMD), and the released N-terminal fragment forms membrane pores, leading to pyroptosis [34,35]. In humans, caspase-4 and -5 are activated similarly by intracellular LPS. While these caspases do not process pro-IL-1β or pro-IL-18 directly, GSDMD-mediated K+ efflux activates the NLRP3 inflammasome and caspase-1, enabling cytokine maturation [36]. Caspase-11 activation also leads to pannexin-1 cleavage and ATP release. ATP then stimulates P2X7 receptors, enhancing K+ efflux and further promoting NLRP3 and caspase-1 activation in bone marrow-derived macrophages [37]. Thus, caspase-11 plays a central role in linking cytosolic LPS sensing to both pyroptosis and cytokine release in the non-canonical inflammasome pathway.
2.3. Apoptotic Caspases-Mediated Pathway
Pyroptosis can also be initiated by apoptotic caspases, in addition to inflammatory caspases like caspase-1/4/5/11. Caspase-3, typically involved in apoptosis, can cleave GSDME in GSDME-expressing cells, converting apoptosis into pyroptosis. Chemotherapy agents such as cisplatin utilize this mechanism to induce pyroptotic cell death [38]. Caspase-8 also contributes by cleaving GSDMD, particularly during Yersinia infection [39,40]. Inhibition of TAK1 by Yersinia effector YopJ activates a signaling complex involving FADD, RIPK1, and caspase-8 on the lysosomal Rag-Ragulator platform, promoting pyroptosis [41]. Additionally, caspase-8 can cleave GSDMC to trigger pyroptosis in cancer cells [42], while apoptotic caspases-3, -6, and -7 can cleave GSDMB, releasing its pore-forming N-terminal domain and inducing pyroptosis [43].
2.4. Granzymes-Mediated Pathway
Cytotoxic immune cells such as natural killer (NK) cells, cytotoxic T lymphocytes (CTLs), and CAR T cells can induce pyroptosis in cancer cells by delivering granzymes through perforin. These granzymes cleave gasdermin family proteins, triggering membrane pore formation and pyroptotic cell death.
Granzyme A (GZMA), although abundant, shows weak cytotoxicity in vitro unless at high concentrations [44,45].
However, it has been linked to inflammatory responses, including the release of pro-inflammatory cytokines [46,47]. GZMA from CTLs can cleave GSDMB, causing pyroptosis in GSDMB-positive cancer cells [48], though GSDMB is not expressed in all human tissues and is absent in mice.
Granzyme B (GZMB), released by NK cells, cleaves GSDME at the same site as caspase-3, releasing its active N-terminal domain to induce pyroptosis [49]. GZMB acts both directly on GSDME and indirectly via caspase-3, offering an alternative pyroptotic pathway in caspase-resistant, GSDME-expressing cancer cells. Granzymes-mediated pyroptosis can amplify inflammation in the tumor microenvironment, enhancing immune cell recruitment and antitumor immunity. The key molecules involved in pyroptosis and their functional characteristics are summarized in Table 1, while the mechanisms underlying pyroptosis induction are illustrated in Figure 1.

Table 1.
Key molecules involved in pyroptosis and their functional characteristics.
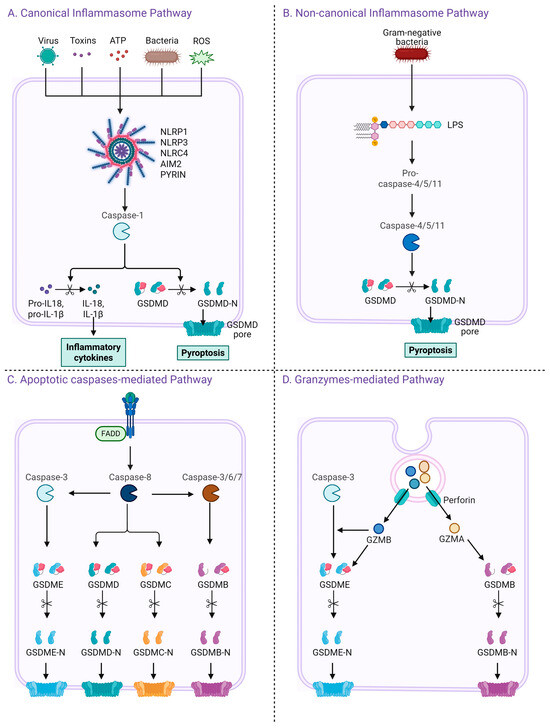
Figure 1.
Four distinct pathways leading to pyroptosis and gasdermin activation. (A) The canonical inflammasome pathway is triggered by various danger signals including viruses, toxins, ATP, bacteria, and ROS. These stimuli activate cytosolic pattern recognition receptors such as NLRP1, NLRP3, NLRC4, AIM2, and PYRIN, which subsequently activate caspase-1. Activated caspase-1 cleaves GSDMD and pro-inflammatory cytokines pro-IL-1β and pro-IL-18 into their mature forms, leading to pyroptosis and cytokine release. (B) The non-canonical inflammasome pathway is activated by intracellular lipopolysaccharide (LPS) from Gram-negative bacteria, which directly activates caspase-4, -5 (human), or caspase-11 (mouse), leading to GSDMD cleavage and pyroptosis. (C) In the apoptotic caspase-mediated pathway, caspase-8 or caspase-3/6/7 cleaves several members of the gasdermin family including GSDMD, GSDME, GSDMC, and GSDMB, resulting in the release of their N-terminal domains and formation of membrane pores. (D) In the granzyme-mediated pathway, cytotoxic lymphocytes deliver granzymes (GZMA or GZMB) and perforin into target cells. GZMB activates caspase-3, leading to GSDME cleavage, while GZMA directly cleaves GSDMB. Both mechanisms result in pore formation and pyroptotic cell death.
3. Pyroptosis as a Therapeutic Strategy in Cancer: Mechanisms, Targets, and Context-Dependent Roles Across Tumor Types
Pyroptosis is emerging as a crucial form of regulated cell death with potential implications in various cancers, especially where traditional therapies are limited by resistance mechanisms. In lung cancer, particularly in non-small-cell lung cancer (NSCLC), conventional chemotherapy shows limited efficacy due to the cancer cells’ ability to evade apoptosis [50,51]. Studies show that agents like simvastatin and polyphyllin VI (PPVI) induce caspase-1-dependent pyroptosis through ROS and inflammasome activation, inhibiting NSCLC progression [52,53]. Additionally, natural compounds such as cucurbitacin B and dasatinib have been shown to activate GSDMD or GSDME, resulting in enhanced pyroptotic and apoptotic death of cancer cells, sometimes involving mitochondrial ROS, STAT3, and NF-κB pathways [54,55]. CD8+ T cells also depend on GSDMD for effective immune response against NSCLC [56]. Metformin and piperlongumine analogues act via the AMP-activated protein kinase (AMPK)/SIRT1/NF-κB/caspase-3/GSDME axis to induce pyroptosis [57,58]. Other molecules like EF24 derivatives modulate pyroptosis–apoptosis switching through NF-κB signaling [59], and APE1 inhibition promotes both pyroptosis and apoptosis via p53 upregulation [60,61]. Inhibition of maternal embryonic leucine zipper kinase (MELK) using OTSSP167 also promotes pyroptotic and apoptotic death through FOXM1 and Akt suppression [62,63].
In gastric cancer (GC), the activation of pyroptosis-related genes (PRGs) such as GSDMD and GSDME is linked to favorable prognosis, while agents like famotidine, simvastatin, icariin, and diosbulbin-B trigger NLRP3 or caspase-3-mediated pyroptosis [64,65,66,67].
In hepatocellular carcinoma (HCC), pyroptosis serves as a therapeutic mechanism to counteract drug resistance [68]. PRGs, GSDMD, and GSDME act as potential diagnostic and therapeutic markers [69,70]. Knockdown of NIMA-related kinase 7 (NEK7) activates the NLRP3/caspase-1/GSDMD axis to inhibit tumor progression [71]. Compounds such as euxanthone, alpinumisoflavone, miltirone, and cannabidiol trigger pyroptosis via inflammasome activation and ROS accumulation [72,73,74,75]. Estradiol similarly promotes antitumor effects via NLRP3 activation, FOXO3 signaling, and IL-6/STAT3 suppression [76]. Berberine exerts antitumor effects through both caspase-1-dependent pyroptosis and NF-κB/AMPK-mediated apoptosis [77].
In breast cancer (BC), pyroptosis-related genes correlate with prognosis and therapeutic sensitivity [78]. Compounds such as polydatin, cisplatin, dihydroartemisinin, and Nobiletin induce pyroptosis by targeting JAK/STAT, NLRP3/caspase-1, AIM2/caspase-3, and NF-κB pathways [79,80,81,82]. Other agents like tetraarsenic hexoxide and triclabendazole promote GSDME-mediated pyroptosis to suppress tumor growth [83,84].
Colorectal cancer (CRC) is also responsive to pyroptosis-inducing strategies. Arsenic trioxide and ascorbic acid co-treatment activates inflammasome formation and caspase-1-mediated pyroptosis [85]. Decitabine enhances inflammasome expression and triggers apoptosis via miR-133b induction [86,87]. Together, these findings support pyroptosis as a valuable mechanism across multiple cancer types, highlighting its therapeutic potential and the need for further research to optimize its application.
4. Pharmacological Potential and Structural Features of Curcumin
Curcumin (CUR), a lipophilic polyphenol derived from the rhizome of Curcuma longa (family Zingiberaceae), represents the principal active component among curcuminoids, which also include demethoxycurcumin and bisdemethoxycurcumin [88]. Historically, curcumin has been employed in traditional Ayurvedic practices for managing ailments such as gastrointestinal inflammation, wounds, sinusitis, and indigestion [89].
Recent research highlights curcumin’s broad pharmacological activities, including anti-inflammatory, pro-oxidant and antioxidant, anticancer, and antibacterial effects [90,91,92]. These effects are mediated through the modulation of multiple intracellular signaling pathways such as NF-κB, MAPK, JAK/STAT, WNT/β-catenin, Hippo, NOTCH, and Akt/mTOR [93,94,95,96,97,98]. Additionally, curcumin’s potential as a photosensitizer in photodynamic therapy (PDT) against cancer and infections has been proposed [89].
Structurally, curcumin offers rich opportunities for chemical optimization. Its multiple functional groups, including α,β-unsaturated carbonyls capable of Michael addition with thiol-containing proteins [99], phenolic hydroxyl groups contributing to radical scavenging, and a 1,3-diketone system that chelates metal ions, all play key roles in its biological activity [100]. This structure–activity relationship (SAR) highlights curcumin’s versatility as a pharmacophore, making it a compelling candidate for further derivatization and medicinal chemistry development. The structural features of curcumin are illustrated in Figure 2.
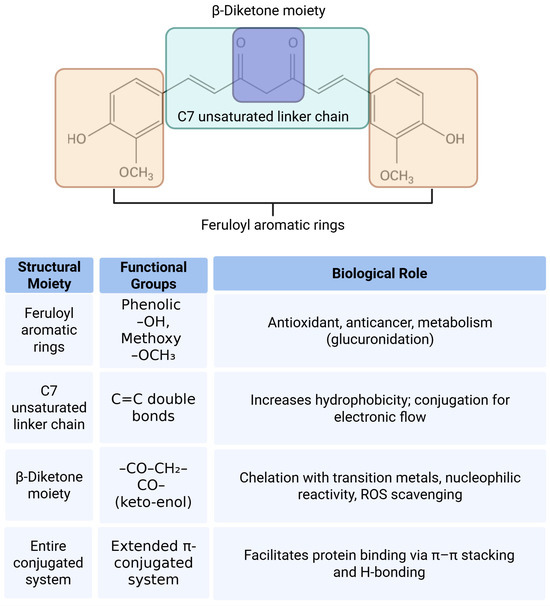
Figure 2.
Structural moieties and functional groups of curcumin and their associated biological roles. The diagram illustrates the major structural components of the curcumin molecule including the feruloyl aromatic rings, C7 unsaturated linker chain, and β-diketone moiety. Each region contains specific functional groups such as phenolic hydroxyls, methoxy groups, α,β-unsaturated carbonyls, and a conjugated pi system. These structural features contribute to various biological activities of curcumin including antioxidant, anticancer, metal-chelating, and protein-binding functions. The accompanying table summarizes the relationship between each moiety, its chemical groups, and their respective biological roles.
Despite its promising pharmacological profile, curcumin suffers from poor pharmacokinetic properties, including low aqueous solubility, limited gastrointestinal absorption, rapid metabolism, and systemic elimination, which significantly restrict its clinical efficacy. To overcome these limitations, several strategies have been developed. Nanoparticle-based delivery systems, such as liposomes, polymeric micelles, and PLGA-based nanocarriers, have been shown to enhance curcumin’s solubility, stability, and bioavailability. Curcumin analogues, including EF24 and GO-Y030, have been synthetically modified to improve metabolic stability and potency. Additionally, chemical conjugation approaches—such as PEGylation, phospholipid complexation, and peptide coupling—have demonstrated improved pharmacokinetic profiles and targeted delivery. These advancements aim to maximize curcumin’s therapeutic potential and facilitate its translation into clinical applications.
5. Inhibition of Inflammatory Pyroptosis by Curcumin: Molecular Mechanisms and Cellular Targets
Curcumin has been widely investigated for its anti-inflammatory potential across various disease models. A significant portion of its therapeutic efficacy is attributed to its ability to suppress inflammasome activation, particularly the NLRP3 inflammasome, which is a key driver of innate immune responses and chronic inflammation. Recent studies have revealed that curcumin exerts its inhibitory effects on inflammasomes through multiple interconnected molecular mechanisms that act both at the transcriptional and post-translational levels.
One of the central actions of curcumin involves the attenuation of mitochondrial reactive oxygen species, which are known to trigger the activation of the NLRP3 inflammasome [101]. In LPS-primed murine macrophages, such as J774A.1 cells and primary peritoneal macrophages, curcumin scavenges mitochondrial ROS [19]. This prevents the dissociation of thioredoxin from thioredoxin-interacting protein and subsequently inhibits the interaction between TXNIP and NLRP3, a critical event in inflammasome assembly. Additionally, in these macrophages, curcumin suppresses nuclear factor-kappa B (NF-κB) signaling by inhibiting the phosphorylation and degradation of IκBα and preventing the nuclear translocation of the p65 subunit [101,102]. This leads to downregulation of pro-inflammatory genes including NLRP3, pro-IL-1β, and pro-IL-18, effectively blocking the priming stage of inflammasome activation.
Curcumin also interferes with inflammasome complex formation by inhibiting the oligomerization of the Apoptosis-associated speck like protein containing a caspase recruitment domain CARD (ASC), which is essential for pro-caspase-1 recruitment [103,104]. Immunofluorescence studies in LPS-primed J774A.1 macrophages revealed a marked reduction in ASC speck formation following curcumin treatment, indicating impaired inflammasome assembly.
Moreover, in macrophages, renal tubular epithelial cells, and colonic epithelial cells, curcumin modulates upstream events necessary for inflammasome activation, such as potassium ion efflux and lysosomal membrane destabilization. It prevents potassium efflux, which is necessary for NLRP3 activation, and stabilizes lysosomes to block cathepsin B release, thereby further impairing inflammasome activation [105,106].
In renal tubular epithelial cells from potassium oxonate-induced hyperuricemic mouse models, curcumin suppressed NLRP3 inflammasome activation and reduced serum uric acid, creatinine, and BUN levels, with therapeutic effects comparable to those of allopurinol [105]. These effects were associated with decreased mRNA expression of NLRP3, ASC, caspase-1, and IL-1β.
In hepatic cell lines such as HepG2 and BRL-3A cells, curcumin inhibited the TXNIP–NLRP3 axis under fructose-induced metabolic stress [107]. This effect was accompanied by the upregulation of miR-200a, a known inhibitor of TXNIP, resulting in reduced hepatic steatosis, improved lipid metabolism, and suppressed infiltration of inflammatory cells in the liver tissue of fructose-fed rats.
In neuroinflammatory conditions, including models of epilepsy, depression, and ischemic stroke, curcumin showed protective effects in neuronal cell lines such as SH-SY5Y cells, as well as in hippocampal neurons and glial cells including microglia and astrocytes. Curcumin suppressed NLRP3 inflammasome activation and reduced expression of IL-1β, caspase-1, and TXNIP. These effects were further linked to the inhibition of endoplasmic reticulum stress, the maintenance of mitochondrial membrane potential, and the modulation of signaling pathways such as AMPK, JAK2/STAT3, and NF-κB [108,109].
In WI38VA13 lung epithelial cells subjected to paraquat-induced acute lung injury, curcumin attenuated oxidative stress and inflammatory responses by reducing TXNIP and NLRP3 expression, restoring Bcl-2/Bax balance, and downregulating Notch1 signaling [110].
Taken together, curcumin inhibits inflammasome activation through a combination of antioxidant, ion-regulating, transcription-modulating, and inflammasome-disrupting mechanisms. These effects have been consistently demonstrated across diverse cell types, including macrophages, epithelial cells (renal, hepatic, pulmonary, colonic), neurons, and glial cells. This underscores curcumin’s broad therapeutic potential in conditions involving aberrant inflammasome activation. Table 2 summarizes key studies that have reported the inhibitory effects of curcumin on NLRP3 inflammasome activation.

Table 2.
Key studies demonstrating curcumin’s inhibitory effects on NLRP3 inflammasome activation.
6. Curcumin-Induced Pyroptosis: Molecular Mechanisms and Therapeutic Strategies in Cancer
While curcumin has demonstrated robust anti-inflammatory activity by suppressing inflammasome activation in various non-cancerous disease contexts, emerging evidence suggests that in cancer cells, curcumin can paradoxically induce pyroptosis—a form of inflammatory cell death—through selective activation of inflammasome-related pathways. This dual role highlights its context-dependent functionality and therapeutic versatility in both inflammatory and neoplastic diseases.
A key mechanism through which curcumin induces pyroptosis is via the activation of inflammasome complexes. In non-small cell lung cancer (NSCLC), curcumin stabilizes and activates the NLRP3 inflammasome by suppressing Smurf2, an E3 ubiquitin ligase that normally facilitates NLRP3 degradation. This stabilization promotes ASC and pro-caspase-1 recruitment, culminating in GSDMD cleavage and pyroptosis [111].
In models of acute myeloid leukemia (AML), curcumin appears to operate through a non-canonical inflammasome pathway by upregulating interferon-stimulated gene ISG3, thereby activating NLRC4, AIM2, and IFI16 inflammasome sensors. This triggers caspase-1 activation and GSDMD-mediated pyroptosis. Restoration of GSDMD expression in resistant leukemia cells re-established curcumin-induced pyroptosis, highlighting its dependency on gasdermin-mediated execution [112].
Nanoplatforms have further enhanced curcumin’s pyroptotic potential. One example is “pharm-dots,” a PLGA-based nanoformulation encapsulating non-emissive curcumin that generates singlet oxygen upon photoactivation. This photodynamically triggered system initiates caspase-3/GSDME signaling and results in the controlled pyroptotic death of cancer cells, enabling precise spatial and temporal targeting in tumor therapy [113]. To clarify the distinction between apoptosis and pyroptosis in the context of GSDME-mediated cell death, recent studies have demonstrated that caspase-3 can cleave GSDME to generate its N-terminal fragment, which forms membrane pores and triggers a pyroptosis-like lytic cell death. Wang et al. provided key experimental evidence including LDH release, membrane rupture, and dependency on GSDME expression, supporting the classification of this process as pyroptosis rather than classical apoptosis [38].
Curcumin has also been integrated into calcium-based nanocarriers such as CUR@CaCO3-PArg@HA, which exploit the acidic tumor microenvironment for localized release. Curcumin in this system inhibits Ca2+ efflux, amplifies ROS levels, and contributes to mitochondrial dysfunction. Simultaneously, poly-L-arginine induces NO release and endoplasmic reticulum-derived Ca2+ mobilization, collectively activating caspase-1 and GSDMD to induce pyroptosis [20].
Another calcium-based nanoinducer, CaZCH, incorporates curcumin, calcium ions, and H2O2 to induce caspase-3/GSDME-dependent pyroptosis through mitochondrial Ca2+ overload and oxidative stress. This system not only eliminates tumor cells but also reprograms tumor-associated macrophages (TAMs) from the immunosuppressive M2 to the pro-inflammatory M1 phenotype, enhancing dendritic cell maturation and CD8+ T cell-mediated antitumor immunity [114].
In hepatocellular carcinoma (HepG2) cells, curcumin has been shown to directly trigger pyroptosis through ROS accumulation. This activates caspase-3, leading to GSDME cleavage and pore formation. The involvement of ROS was further confirmed through the use of ROS scavengers, which attenuated the pyroptotic response [21].
Curcumin’s pyroptosis-modulating effects are not limited to cancer. In inflammatory joint disease models, such as IL-1β-treated chondrocytes, curcumin inhibited TRPM2 and NLRP3 activation, thereby suppressing caspase-1-driven pyroptosis and reducing cartilage inflammation—suggesting potential in the treatment of osteoarthritis [115].
Innovative nanostructures have further leveraged curcumin’s functionality. For example, a Ca2+-based nanoplatform co-loaded with curcumin and H2O2 was shown to initiate GSDME cleavage and mitochondrial stress-induced pyroptosis. Beyond tumor killing, this strategy also promoted immunogenic cell death (ICD), enhanced antigen presentation, and boosted T cell-mediated immune responses [116].
In liver cancer, curcumin-loaded PLGA microbubbles have been utilized in sonodynamic therapy. Upon ultrasound and photonic stimulation, these microbubbles generate ROS, concurrently activating apoptosis and pyroptosis in HepG2 cells, showcasing curcumin’s utility in multimodal oncologic strategies [117].
In colorectal cancer (CRC) models, curcumin has consistently demonstrated apoptotic activity. However, its ability to trigger NLRP3 inflammasome-dependent pyroptosis appears to be cell line-specific. While evident in SW480 and HCT116 cells, no such effect was observed in LoVo or HT29, indicating the need to explore alternative inflammasome pathways in CRC [118].
Finally, chemical modification of curcumin has yielded analogues with improved pharmacokinetic and anticancer properties. One such analogue, compound B2, exhibited enhanced stability and potency in lung cancer models by increasing ROS production, inducing ER stress, and activating both apoptosis and pyroptosis pathways [119].
Collectively, these findings highlight curcumin’s dualistic and context-sensitive role in regulating pyroptosis. While it can suppress inappropriate inflammation in degenerative diseases, under tumor-specific stimuli or advanced delivery platforms, curcumin potently activates pyroptosis, contributing to both direct tumor cell death and stimulation of antitumor immunity. This versatility underscores its promise as a precision adjunct in cancer therapeutics and warrants further investigation into optimized delivery strategies and target-specific modulation. Table 3 provides an overview of the mechanisms and therapeutic implications of curcumin-induced pyroptosis in cancer. Figure 3 illustrates the dual roles of curcumin in modulating NLRP3 inflammasome-mediated pyroptosis.

Table 3.
Mechanisms and therapeutic implications of curcumin-induced pyroptosis in cancer.
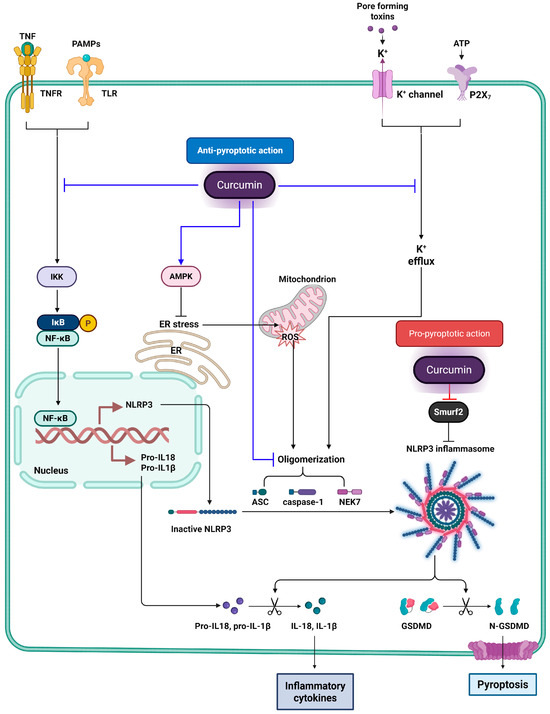
Figure 3.
Dual regulatory effects of curcumin on NLRP3 inflammasome-mediated pyroptosis. This figure illustrates the dual modulatory effects of curcumin on NLRP3 inflammasome-mediated pyroptosis. Blue lines represent the anti-pyroptotic actions of curcumin, with flat-headed lines (⊣) indicating inhibition, and arrow-headed lines (→) indicating activation. Curcumin inhibits upstream signaling pathways such as NF-κB by blocking IKK activity, thereby reducing the transcription of NLRP3 and pro-inflammatory cytokines. It also activates AMPK, which alleviates endoplasmic reticulum (ER) stress and decreases mitochondrial ROS production—both of which are known triggers of NLRP3 activation. Additionally, curcumin blocks potassium efflux through inhibition of P2X7 receptor and K+ channels and disrupts inflammasome assembly by interfering with the oligomerization of ASC and caspase-1. Red lines represent the pro-pyroptotic effects of curcumin. In particular, curcumin inhibits Smurf2, an E3 ubiquitin ligase that promotes NLRP3 degradation. By targeting Smurf2, curcumin stabilizes NLRP3 protein levels, facilitating inflammasome assembly, caspase-1 activation, and GSDMD cleavage, ultimately leading to pyroptotic cell death.
However, as pyroptosis is inherently inflammatory, its therapeutic application in tumors must be carefully balanced against potential risks such as systemic cytokine release or local immune overactivation. Future studies should assess optimal dosing, delivery platforms, and combination strategies to maximize antitumor efficacy while minimizing inflammatory toxicity.
7. Molecular Docking Evidence for Curcumin Binding to Pyroptosis-Associated Targets
Molecular docking approaches have provided critical insights into the direct interactions between curcumin and key proteins involved in pyroptosis regulation. These simulations offer structural support for curcumin’s modulatory roles beyond its indirect effects via oxidative stress or transcriptional regulation.
Curcumin was predicted to bind directly to the NACHT domain of NLRP3, a critical ATPase-containing region responsible for inflammasome oligomerization and activation. In docking simulations using the human NLRP3 crystal structure (PDB ID: 7ALV), curcumin occupied the same ATP-binding pocket targeted by the selective NLRP3 inhibitor NP3-146, with a reported binding energy of −6.02 kcal/mol [120]. Within this pocket, curcumin formed hydrogen bonds with Glu369 and Ala228, suggesting a potential to interfere with ATP binding. Interestingly, demethoxycurcumin (DMC), a natural curcumin analog, showed a stronger binding affinity (−6.44 kcal/mol) and formed an additional hydrogen bond with Thr439. These subtle structural differences are consistent with DMC’s slightly enhanced efficacy in suppressing NLRP3 inflammasome activation, implying that curcumin may act by blocking ATPase activity and preventing NLRP3 oligomerization.
In parallel, docking studies have shown that curcumin exhibits strong binding affinity toward caspase-1, a critical executioner protease in the pyroptotic pathway. Using the crystal structure of human caspase-1, curcumin was predicted to bind its catalytic domain with a binding energy of approximately −970.7 kJ/mol [121]. It formed hydrogen bonds with key active site residues, including Cys285, His237, and Ser339. These interactions suggest a potential for curcumin to inhibit caspase-1 enzymatic activity by stabilizing its inactive conformation or obstructing substrate access. Moreover, curcumin was also shown to bind moderately to other inflammasome proteins such as NLRP3 (−850.2 kJ/mol) and ASC (−770.2 kJ/mol). Protein–protein docking models indicated that curcumin disrupts inflammasome assembly by weakening interactions between NLRP3 and caspase-1, thereby interfering with complex formation and downstream IL-1β maturation.
Further docking studies support curcumin’s interaction with AMP-activated protein kinase (AMPK), a metabolic sensor that modulates autophagy and inflammation. Using the crystal structure of AMPKα2 (PDB ID: 4CFH), curcumin was found to stably occupy the nucleotide-binding cleft, with a binding energy of −10.3 kcal/mol [122]. This pose involved hydrogen bonds with Glu100, Arg83, and Val24, consistent with potential allosteric activation. These structural predictions align with experimental data showing that curcumin enhances AMPK phosphorylation and activates downstream autophagy in endothelial cells, indicating that curcumin may engage AMPK both indirectly through redox modulation and directly via molecular interaction.
Finally, in the context of cancer, curcumin has been shown to induce NLRP3-dependent pyroptosis by targeting Smurf2, an E3 ubiquitin ligase that mediates NLRP3 degradation. Molecular docking simulations demonstrated that curcumin binds with high affinity to a hydrophobic pocket within the HECT domain of Smurf2 [111]. Although exact binding energy and residues were not specified, curcumin was predicted to form hydrogen bonds and hydrophobic contacts that impair Smurf2’s ubiquitin ligase function. This inhibition stabilizes NLRP3 protein levels, facilitating inflammasome activation, caspase-1 cleavage, and GSDMD-mediated pyroptosis. These mechanistic insights highlight curcumin’s role in modulating post-translational regulators of pyroptosis in cancer cells. The molecular docking of curcumin with pyroptosis-associated proteins is presented in Figure 4.
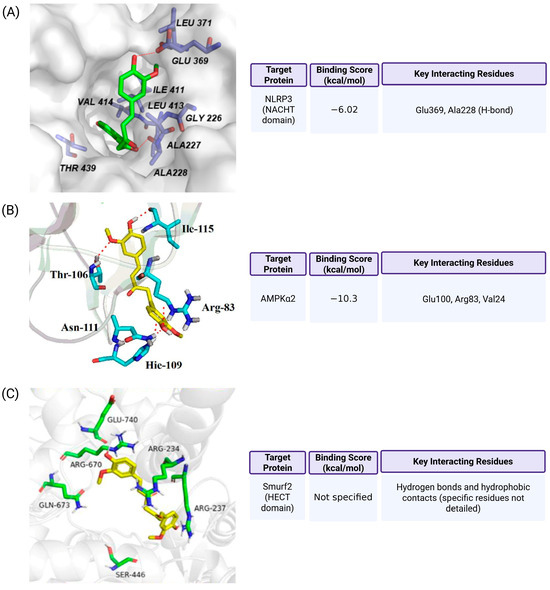
Figure 4.
Molecular docking of curcumin with pyroptosis-associated proteins. (A) Curcumin binds to the NACHT domain of NLRP3 (PDB ID: 7ALV), overlapping with the binding site of the selective inhibitor NP3-146. It forms hydrogen bonds with Glu369 and Ala228, with a docking score of −6.02 kcal/mol, suggesting moderate to strong affinity (Ref. [120]). (B) Curcumin interacts with the nucleotide-binding pocket of AMPKα2 (PDB ID: 4CFH), forming hydrogen bonds with Glu100, Arg83, and Val24. The binding energy is −10.3 kcal/mol, supporting a potential allosteric regulatory role (Ref. [122]). (C) Curcumin is predicted to occupy a hydrophobic pocket in the HECT domain of Smurf2, engaging in hydrogen bonding and hydrophobic interactions. Although specific residues and binding score were not provided, this interaction is proposed to inhibit Smurf2’s ubiquitin ligase activity and stabilize NLRP3 (Ref. [111]). Methodological details, including the docking software, receptor preparation, and validation strategies, are available in the cited references (Refs. [111,120,122]).
8. Dual Regulatory Roles of Curcumin in Pyroptosis: A Context-Dependent Mechanistic Perspective
Curcumin functions as a multifaceted modulator of pyroptosis, exhibiting both inhibitory and promotive effects depending on the specific cellular environment and pathological condition. These seemingly opposing actions are attributed to its differential targeting of key molecular pathways involved in inflammasome regulation.
In many inflammatory disease models, curcumin has been shown to suppress NLRP3 inflammasome activation and pyroptotic cell death. This inhibitory effect is mediated through several mechanisms. Curcumin reduces upstream stress signals such as mitochondrial reactive oxygen species and endoplasmic reticulum stress. It also inhibits NF-κB activation, thereby downregulating the transcription of inflammasome-related genes including NLRP3, pro-IL-1β, and pro-IL-18. Additionally, curcumin blocks potassium efflux, a critical ionic signal for NLRP3 activation, and interferes with the assembly of inflammasome components by preventing the oligomerization of ASC and caspase-1. Together, these effects contribute to the attenuation of inflammatory cytokine release and cellular pyroptosis.
In contrast, under certain conditions such as in cancer cells, curcumin has been reported to promote pyroptosis. This pro-pyroptotic activity involves the inhibition of Smurf2, an E3 ubiquitin ligase that facilitates the degradation of NLRP3. Curcumin binding leads to the stabilization of NLRP3, which enhances inflammasome assembly, caspase-1 activation, and the cleavage of gasdermin D. This process ultimately induces pyroptotic cell death and may contribute to curcumin’s anticancer effects by enhancing immunogenic cell clearance.
Overall, the regulatory role of curcumin in pyroptosis depends on the biological context and molecular target. It acts as a suppressor of pyroptosis in inflammatory conditions while functioning as an inducer in cancer settings. Understanding these dual actions provides valuable insight into curcumin’s therapeutic potential and highlights the importance of context-specific targeting in its application.
9. Conclusions
Curcumin exhibits dual and context-dependent roles in regulating NLRP3 inflammasome-mediated pyroptosis. Its inhibitory effects are well-documented in chronic inflammatory and metabolic disorders, where it mitigates inflammasome activation by targeting key upstream signals such as ROS, ER stress, and NF-κB. At the same time, curcumin has been shown to enhance pyroptotic cell death in cancer by stabilizing NLRP3 via inhibition of Smurf2, thereby activating the inflammasome complex. Molecular docking analyses provide structural insights into these mechanisms, revealing curcumin’s capacity to directly bind and modulate various inflammasome-related proteins. These findings highlight the versatile therapeutic potential of curcumin and warrant further investigation into its application as a context-specific modulator of pyroptosis in both inflammatory and cancerous conditions. Although numerous studies have reported the therapeutic effects of curcumin in regulating pyroptosis and related disease pathways, it is important to acknowledge that many of these findings are based on in vitro or preclinical models. Therefore, further validation through well-designed clinical studies is essential to confirm the translational relevance of curcumin in pyroptosis modulation. Future in vivo studies using dual models that recapitulate both inflammatory and tumor microenvironments are needed to validate the context-specific effects of curcumin-induced pyroptosis and assess its therapeutic safety and efficacy.
Funding
This research was supported by Daegu University Research Grant, 2025 (2025-0168).
Conflicts of Interest
The author declares no conflicts of interest.
References
- Yu, P.; Zhang, X.; Liu, N.; Tang, L.; Peng, C.; Chen, X. Pyroptosis: Mechanisms and diseases. Signal Transduct. Target. Ther. 2021, 6, 128. [Google Scholar] [CrossRef]
- Shi, J.; Gao, W.; Shao, F. Pyroptosis: Gasdermin-Mediated Programmed Necrotic Cell Death. Trends Biochem. Sci. 2017, 42, 245–254. [Google Scholar] [CrossRef]
- Wei, X.; Xie, F.; Zhou, X.; Wu, Y.; Yan, H.; Liu, T.; Huang, J.; Wang, F.; Zhou, F.; Zhang, L. Role of pyroptosis in inflammation and cancer. Cell. Mol. Immunol. 2022, 19, 971–992. [Google Scholar] [CrossRef] [PubMed]
- Burdette, B.E.; Esparza, A.N.; Zhu, H.; Wang, S. Gasdermin D in pyroptosis. Acta Pharm. Sin. B 2021, 11, 2768–2782. [Google Scholar] [CrossRef]
- Sharma, A.K.; Ismail, N. Non-Canonical Inflammasome Pathway: The Role of Cell Death and Inflammation in Ehrlichiosis. Cells 2023, 12, 2597. [Google Scholar] [CrossRef]
- Rao, Z.; Zhu, Y.; Yang, P.; Chen, Z.; Xia, Y.; Qiao, C.; Liu, W.; Deng, H.; Li, J.; Ning, P.; et al. Pyroptosis in inflammatory diseases and cancer. Theranostics 2022, 12, 4310–4329. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Tian, S.; Pan, Y.; Li, W.; Wang, Q.; Tang, Y.; Yu, T.; Wu, X.; Shi, Y.; Ma, P.; et al. Pyroptosis: A new frontier in cancer. Biomed. Pharmacother. 2020, 121, 109595. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wang, J.W.; Huang, J.; Tang, L.; Xu, Y.H.; Sun, H.; Tang, J.; Wang, G. Pyroptosis, a target for cancer treatment? Apoptosis 2022, 27, 1–13. [Google Scholar] [CrossRef]
- Lu, X.; Guo, T.; Zhang, X. Pyroptosis in Cancer: Friend or Foe? Cancers 2021, 13, 3620. [Google Scholar] [CrossRef]
- Tan, G.; Huang, C.; Chen, J.; Zhi, F. HMGB1 released from GSDME-mediated pyroptotic epithelial cells participates in the tumorigenesis of colitis-associated colorectal cancer through the ERK1/2 pathway. J. Hematol. Oncol. 2020, 13, 149. [Google Scholar] [CrossRef]
- Zolondick, A.A.; Gaudino, G.; Xue, J.; Pass, H.I.; Carbone, M.; Yang, H. Asbestos-induced chronic inflammation in malignant pleural mesothelioma and related therapeutic approaches-a narrative review. Precis. Cancer Med. 2021, 4, 27. [Google Scholar] [CrossRef]
- Du, T.; Gao, J.; Li, P.; Wang, Y.; Qi, Q.; Liu, X.; Li, J.; Wang, C.; Du, L. Pyroptosis, metabolism, and tumor immune microenvironment. Clin. Transl. Med. 2021, 11, e492. [Google Scholar] [CrossRef]
- Ben-Sasson, S.Z.; Hogg, A.; Hu-Li, J.; Wingfield, P.; Chen, X.; Crank, M.; Caucheteux, S.; Ratner-Hurevich, M.; Berzofsky, J.A.; Nir-Paz, R.; et al. IL-1 enhances expansion, effector function, tissue localization, and memory response of antigen-specific CD8 T cells. J. Exp. Med. 2013, 210, 491–502. [Google Scholar] [CrossRef]
- Dinarello, C.A. Overview of the IL-1 family in innate inflammation and acquired immunity. Immunol. Rev. 2018, 281, 8–27. [Google Scholar] [CrossRef]
- Pancholi, V.; Smina, T.P.; Kunnumakkara, A.B.; Maliakel, B.; Krishnakumar, I.M. Safety assessment of a highly bioavailable curcumin-galactomannoside complex (CurQfen) in healthy volunteers, with a special reference to the recent hepatotoxic reports of curcumin supplements: A 90-days prospective study. Toxicol. Rep. 2021, 8, 1255–1264. [Google Scholar] [CrossRef]
- Zeng, L.; Yang, T.; Yang, K.; Yu, G.; Li, J.; Xiang, W.; Chen, H. Efficacy and Safety of Curcumin and Curcuma longa Extract in the Treatment of Arthritis: A Systematic Review and Meta-Analysis of Randomized Controlled Trial. Front. Immunol. 2022, 13, 891822. [Google Scholar] [CrossRef]
- Liu, S.; Liu, J.; He, L.; Liu, L.; Cheng, B.; Zhou, F.; Cao, D.; He, Y. A Comprehensive Review on the Benefits and Problems of Curcumin with Respect to Human Health. Molecules 2022, 27, 4400. [Google Scholar] [CrossRef]
- Benameur, T.; Frota Gaban, S.V.; Giacomucci, G.; Filannino, F.M.; Trotta, T.; Polito, R.; Messina, G.; Porro, C.; Panaro, M.A. The Effects of Curcumin on Inflammasome: Latest Update. Molecules 2023, 28, 742. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.; Zhou, J.; Li, H.; Gao, Y.; Xu, C.; Zhao, S.; Chen, Y.; Cai, W.; Wu, J. Curcumin suppresses NLRP3 inflammasome activation and protects against LPS-induced septic shock. Mol. Nutr. Food Res. 2015, 59, 2132–2142. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Xing, Z.; Wang, J.; Guo, Y.; Wu, X.; Ma, Y.; Xu, Z.; Kuang, Y.; Liao, T.; Li, C. Hyaluronic acid-mediated targeted nano-modulators for activation of pyroptosis for cancer therapy through multichannel regulation of Ca2+ overload. Int. J. Biol. Macromol. 2025, 299, 140116. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.F.; Gong, Y.X.; Li, H.F.; Sun, F.L.; Li, W.L.; Chen, D.Q.; Xie, D.P.; Ren, C.X.; Guo, X.Y.; Wang, Z.Y.; et al. Curcumin Activates ROS Signaling to Promote Pyroptosis in Hepatocellular Carcinoma HepG2 Cells. In Vivo 2021, 35, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Liwinski, T.; Elinav, E. Inflammasome activation and regulation: Toward a better understanding of complex mechanisms. Cell Discov. 2020, 6, 36. [Google Scholar] [CrossRef]
- Wu, J.; Fernandes-Alnemri, T.; Alnemri, E.S. Involvement of the AIM2, NLRC4, and NLRP3 inflammasomes in caspase-1 activation by Listeria monocytogenes. J. Clin. Immunol. 2010, 30, 693–702. [Google Scholar] [CrossRef]
- Xu, H.; Yang, J.; Gao, W.; Li, L.; Li, P.; Zhang, L.; Gong, Y.N.; Peng, X.; Xi, J.J.; Chen, S.; et al. Innate immune sensing of bacterial modifications of Rho GTPases by the Pyrin inflammasome. Nature 2014, 513, 237–241. [Google Scholar] [CrossRef]
- Ting, J.P.; Lovering, R.C.; Alnemri, E.S.; Bertin, J.; Boss, J.M.; Davis, B.K.; Flavell, R.A.; Girardin, S.E.; Godzik, A.; Harton, J.A.; et al. The NLR gene family: A standard nomenclature. Immunity 2008, 28, 285–287. [Google Scholar] [CrossRef]
- Mitchell, P.S.; Sandstrom, A.; Vance, R.E. The NLRP1 inflammasome: New mechanistic insights and unresolved mysteries. Curr. Opin. Immunol. 2019, 60, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Elliott, E.I.; Sutterwala, F.S. Initiation and perpetuation of NLRP3 inflammasome activation and assembly. Immunol. Rev. 2015, 265, 35–52. [Google Scholar] [CrossRef] [PubMed]
- Kahlenberg, J.M.; Dubyak, G.R. Mechanisms of caspase-1 activation by P2X7 receptor-mediated K+ release. Am. J. Physiol. Cell Physiol. 2004, 286, C1100–C1108. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yang, J.; Shi, J.; Gong, Y.N.; Lu, Q.; Xu, H.; Liu, L.; Shao, F. The NLRC4 inflammasome receptors for bacterial flagellin and type III secretion apparatus. Nature 2011, 477, 596–600. [Google Scholar] [CrossRef]
- Hornung, V.; Ablasser, A.; Charrel-Dennis, M.; Bauernfeind, F.; Horvath, G.; Caffrey, D.R.; Latz, E.; Fitzgerald, K.A. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature 2009, 458, 514–518. [Google Scholar] [CrossRef]
- Miao, E.A.; Leaf, I.A.; Treuting, P.M.; Mao, D.P.; Dors, M.; Sarkar, A.; Warren, S.E.; Wewers, M.D.; Aderem, A. Caspase-1-induced pyroptosis is an innate immune effector mechanism against intracellular bacteria. Nat. Immunol. 2010, 11, 1136–1142. [Google Scholar] [CrossRef]
- Kayagaki, N.; Warming, S.; Lamkanfi, M.; Vande Walle, L.; Louie, S.; Dong, J.; Newton, K.; Qu, Y.; Liu, J.; Heldens, S.; et al. Non-canonical inflammasome activation targets caspase-11. Nature 2011, 479, 117–121. [Google Scholar] [CrossRef]
- Man, S.M.; Kanneganti, T.D. Regulation of inflammasome activation. Immunol. Rev. 2015, 265, 6–21. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Zhao, Y.; Wang, Y.; Gao, W.; Ding, J.; Li, P.; Hu, L.; Shao, F. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature 2014, 514, 187–192. [Google Scholar] [CrossRef]
- Kayagaki, N.; Stowe, I.B.; Lee, B.L.; O’Rourke, K.; Anderson, K.; Warming, S.; Cuellar, T.; Haley, B.; Roose-Girma, M.; Phung, Q.T.; et al. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature 2015, 526, 666–671. [Google Scholar] [CrossRef] [PubMed]
- Rühl, S.; Broz, P. Caspase-11 activates a canonical NLRP3 inflammasome by promoting K(+) efflux. Eur. J. Immunol. 2015, 45, 2927–2936. [Google Scholar] [CrossRef]
- Yang, D.; He, Y.; Muñoz-Planillo, R.; Liu, Q.; Núñez, G. Caspase-11 Requires the Pannexin-1 Channel and the Purinergic P2X7 Pore to Mediate Pyroptosis and Endotoxic Shock. Immunity 2015, 43, 923–932. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, W.; Shi, X.; Ding, J.; Liu, W.; He, H.; Wang, K.; Shao, F. Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. Nature 2017, 547, 99–103. [Google Scholar] [CrossRef]
- Sarhan, J.; Liu, B.C.; Muendlein, H.I.; Li, P.; Nilson, R.; Tang, A.Y.; Rongvaux, A.; Bunnell, S.C.; Shao, F.; Green, D.R.; et al. Caspase-8 induces cleavage of gasdermin D to elicit pyroptosis during Yersinia infection. Proc. Natl. Acad. Sci. USA 2018, 115, E10888–E10897. [Google Scholar] [CrossRef] [PubMed]
- Orning, P.; Weng, D.; Starheim, K.; Ratner, D.; Best, Z.; Lee, B.; Brooks, A.; Xia, S.; Wu, H.; Kelliher, M.A.; et al. Pathogen blockade of TAK1 triggers caspase-8-dependent cleavage of gasdermin D and cell death. Science 2018, 362, 1064–1069. [Google Scholar] [CrossRef]
- Zheng, Z.; Deng, W.; Bai, Y.; Miao, R.; Mei, S.; Zhang, Z.; Pan, Y.; Wang, Y.; Min, R.; Deng, F.; et al. The Lysosomal Rag-Ragulator Complex Licenses RIPK1 and Caspase-8-mediated Pyroptosis by Yersinia. Science 2021, 372, eabg0269. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Zhao, R.; Xia, W.; Chang, C.W.; You, Y.; Hsu, J.M.; Nie, L.; Chen, Y.; Wang, Y.C.; Liu, C.; et al. PD-L1-mediated gasdermin C expression switches apoptosis to pyroptosis in cancer cells and facilitates tumour necrosis. Nat. Cell Biol. 2020, 22, 1264–1275. [Google Scholar] [CrossRef]
- Chao, K.L.; Kulakova, L.; Herzberg, O. Gene polymorphism linked to increased asthma and IBD risk alters gasdermin-B structure, a sulfatide and phosphoinositide binding protein. Proc. Natl. Acad. Sci. USA 2017, 114, E1128–E1137. [Google Scholar] [CrossRef]
- Chowdhury, D.; Lieberman, J. Death by a thousand cuts: Granzyme pathways of programmed cell death. Annu. Rev. Immunol. 2008, 26, 389–420. [Google Scholar] [CrossRef]
- Joeckel, L.T.; Bird, P.I. Are all granzymes cytotoxic in vivo? Biol. Chem. 2014, 395, 181–202. [Google Scholar] [CrossRef]
- Wensink, A.C.; Hack, C.E.; Bovenschen, N. Granzymes regulate proinflammatory cytokine responses. J. Immunol. 2015, 194, 491–497. [Google Scholar] [CrossRef]
- van Daalen, K.R.; Reijneveld, J.F.; Bovenschen, N. Modulation of Inflammation by Extracellular Granzyme A. Front. Immunol. 2020, 11, 931. [Google Scholar] [CrossRef]
- Zhou, Z.; He, H.; Wang, K.; Shi, X.; Wang, Y.; Su, Y.; Wang, Y.; Li, D.; Liu, W.; Zhang, Y.; et al. Granzyme A from cytotoxic lymphocytes cleaves GSDMB to trigger pyroptosis in target cells. Science 2020, 368, eaaz7548. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, Y.; Xia, S.; Kong, Q.; Li, S.; Liu, X.; Junqueira, C.; Meza-Sosa, K.F.; Mok, T.M.Y.; Ansara, J.; et al. Gasdermin E suppresses tumour growth by activating anti-tumour immunity. Nature 2020, 579, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Fan, T.W.M.; Zhang, X.; Wang, C.; Yang, Y.; Kang, W.Y.; Arnold, S.; Higashi, R.M.; Liu, J.; Lane, A.N. Exosomal lipids for classifying early and late stage non-small cell lung cancer. Anal. Chim. Acta 2018, 1037, 256–264. [Google Scholar] [CrossRef]
- Schwartz, A.G.; Cote, M.L. Epidemiology of Lung Cancer. Adv. Exp. Med. Biol. 2016, 893, 21–41. [Google Scholar] [PubMed]
- Teng, J.F.; Mei, Q.B.; Zhou, X.G.; Tang, Y.; Xiong, R.; Qiu, W.Q.; Pan, R.; Law, B.Y.; Wong, V.K.; Yu, C.L.; et al. Polyphyllin VI Induces Caspase-1-Mediated Pyroptosis via the Induction of ROS/NF-κB/NLRP3/GSDMD Signal Axis in Non-Small Cell Lung Cancer. Cancers 2020, 12, 193. [Google Scholar] [CrossRef]
- Wang, F.; Liu, W.; Ning, J.; Wang, J.; Lang, Y.; Jin, X.; Zhu, K.; Wang, X.; Li, X.; Yang, F.; et al. Simvastatin Suppresses Proliferation and Migration in Non-small Cell Lung Cancer via Pyroptosis. Int. J. Biol. Sci. 2018, 14, 406–417. [Google Scholar] [CrossRef]
- Yuan, R.; Zhao, W.; Wang, Q.Q.; He, J.; Han, S.; Gao, H.; Feng, Y.; Yang, S. Cucurbitacin B inhibits non-small cell lung cancer in vivo and in vitro by triggering TLR4/NLRP3/GSDMD-dependent pyroptosis. Pharmacol. Res. 2021, 170, 105748. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, Y.; He, Q. Distinct characteristics of dasatinib-induced pyroptosis in gasdermin E-expressing human lung cancer A549 cells and neuroblastoma SH-SY5Y cells. Oncol. Lett. 2020, 20, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Xi, G.; Gao, J.; Wan, B.; Zhan, P.; Xu, W.; Lv, T.; Song, Y. GSDMD is required for effector CD8(+) T cell responses to lung cancer cells. Int. Immunopharmacol. 2019, 74, 105713. [Google Scholar] [CrossRef]
- Zheng, Z.; Bian, Y.; Zhang, Y.; Ren, G.; Li, G. Metformin activates AMPK/SIRT1/NF-κB pathway and induces mitochondrial dysfunction to drive caspase3/GSDME-mediated cancer cell pyroptosis. Cell Cycle 2020, 19, 1089–1104. [Google Scholar] [CrossRef]
- Li, Q.; Chen, L.; Dong, Z.; Zhao, Y.; Deng, H.; Wu, J.; Wu, X.; Li, W. Piperlongumine analogue L50377 induces pyroptosis via ROS mediated NF-κB suppression in non-small-cell lung cancer. Chem. Biol. Interact. 2019, 313, 108820. [Google Scholar] [CrossRef]
- Chen, L.; Li, Q.; Zheng, Z.; Xie, J.; Lin, X.; Jiang, C.; Xu, H.; Wu, X.; Wu, J.; Zhang, H. Design and optimize N-substituted EF24 as effective and low toxicity NF-κB inhibitor for lung cancer therapy via apoptosis-to-pyroptosis switch. Chem. Biol. Drug Des. 2019, 94, 1368–1377. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, C.; Qing, Y.; Cheng, Y.; Jiang, X.; Li, M.; Yang, Z.; Wang, D. Genistein induces apoptosis by stabilizing intracellular p53 protein through an APE1-mediated pathway. Free Radic. Biol. Med. 2015, 86, 209–218. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, W.; Lin, Z.; Li, C.; Wang, Y.; Yang, L.; Liu, G. Reduced apurinic/apyrimidinic endonuclease activity enhances the antitumor activity of oxymatrine in lung cancer cells. Int. J. Oncol. 2016, 49, 2331–2340. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Li, W.; Zheng, X.; Ren, L.; Liu, J.; Li, S.; Wang, J.; Du, G. MELK is an oncogenic kinase essential for metastasis, mitotic progression, and programmed death in lung carcinoma. Signal Transduct. Target. Ther. 2020, 5, 279. [Google Scholar] [CrossRef]
- Inoue, H.; Kato, T.; Olugbile, S.; Tamura, K.; Chung, S.; Miyamoto, T.; Matsuo, Y.; Salgia, R.; Nakamura, Y.; Park, J.H. Effective growth-suppressive activity of maternal embryonic leucine-zipper kinase (MELK) inhibitor against small cell lung cancer. Oncotarget 2016, 7, 13621–13633. [Google Scholar] [CrossRef]
- Huang, J.; Fan, P.; Liu, M.; Weng, C.; Fan, G.; Zhang, T.; Duan, X.; Wu, Y.; Tang, L.; Yang, G.; et al. Famotidine promotes inflammation by triggering cell pyroptosis in gastric cancer cells. BMC Pharmacol. Toxicol. 2021, 22, 62. [Google Scholar] [CrossRef]
- Xia, Y.; Jin, Y.; Cui, D.; Wu, X.; Song, C.; Jin, W.; Huang, H. Antitumor Effect of Simvastatin in Combination With DNA Methyltransferase Inhibitor on Gastric Cancer via GSDME-Mediated Pyroptosis. Front. Pharmacol. 2022, 13, 860546. [Google Scholar] [CrossRef]
- Zhang, F.; Yin, Y.; Xu, W.; Song, Y.; Zhou, Z.; Sun, X.; Li, P. Icariin inhibits gastric cancer cell growth by regulating the hsa_circ_0003159/miR-223-3p/NLRP3 signaling axis. Hum. Exp. Toxicol. 2022, 41, 9603271221097363. [Google Scholar] [CrossRef]
- Li, C.; Qiu, J.; Xue, Y. Low-dose Diosbulbin-B (DB) activates tumor-intrinsic PD-L1/NLRP3 signaling pathway mediated pyroptotic cell death to increase cisplatin-sensitivity in gastric cancer (GC). Cell Biosci. 2021, 11, 38. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.Y.; Zhu, M.X.; Zhang, P.F.; Huang, X.Y.; Wan, J.K.; Yao, X.Z.; Hu, Z.T.; Chai, X.Q.; Peng, R.; Yang, X.; et al. PKCα/ZFP64/CSF1 axis resets the tumor microenvironment and fuels anti-PD1 resistance in hepatocellular carcinoma. J. Hepatol. 2022, 77, 163–176. [Google Scholar] [CrossRef]
- Hu, K.; Xu, Z.; Yao, L.; Yan, Y.; Zhou, L.; Li, J. Integrated analysis of expression, prognostic value and immune infiltration of GSDMs in hepatocellular carcinoma. Aging 2021, 13, 24117–24135. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.; Hu, Y.; Dong, S. Pan-cancer analysis reveals the expression, genetic alteration and prognosis of pyroptosis key gene GSDMD. Int. Immunopharmacol. 2021, 101 Pt A, 108270. [Google Scholar] [CrossRef]
- Yan, Z.; Da, Q.; Li, Z.; Lin, Q.; Yi, J.; Su, Y.; Yu, G.; Ren, Q.; Liu, X.; Lin, Z.; et al. Inhibition of NEK7 Suppressed Hepatocellular Carcinoma Progression by Mediating Cancer Cell Pyroptosis. Front. Oncol. 2022, 12, 812655. [Google Scholar] [CrossRef]
- Chen, Y.F.; Qi, H.Y.; Wu, F.L. Euxanthone exhibits anti-proliferative and anti-invasive activities in hepatocellular carcinoma by inducing pyroptosis: Preliminary results. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 8186–8196. [Google Scholar]
- Zhang, Y.; Yang, H.; Sun, M.; He, T.; Liu, Y.; Yang, X.; Shi, X.; Liu, X. Alpinumisoflavone suppresses hepatocellular carcinoma cell growth and metastasis via NLRP3 inflammasome-mediated pyroptosis. Pharmacol. Rep. 2020, 72, 1370–1382. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, P.; An, L.; Sun, N.; Peng, L.; Tang, W.; Ma, D.; Chen, J. Miltirone induces cell death in hepatocellular carcinoma cell through GSDME-dependent pyroptosis. Acta Pharm. Sin. B 2020, 10, 1397–1413. [Google Scholar] [CrossRef]
- Shangguan, F.; Zhou, H.; Ma, N.; Wu, S.; Huang, H.; Jin, G.; Wu, S.; Hong, W.; Zhuang, W.; Xia, H.; et al. A Novel Mechanism of Cannabidiol in Suppressing Hepatocellular Carcinoma by Inducing GSDME Dependent Pyroptosis. Front. Cell Dev. Biol. 2021, 9, 697832. [Google Scholar] [CrossRef]
- Wei, Q.; Zhu, R.; Zhu, J.; Zhao, R.; Li, M. E2-Induced Activation of the NLRP3 Inflammasome Triggers Pyroptosis and Inhibits Autophagy in HCC Cells. Oncol. Res. 2019, 27, 827–834. [Google Scholar] [CrossRef]
- Chu, Q.; Jiang, Y.; Zhang, W.; Xu, C.; Du, W.; Tuguzbaeva, G.; Qin, Y.; Li, A.; Zhang, L.; Sun, G.; et al. Pyroptosis is involved in the pathogenesis of human hepatocellular carcinoma. Oncotarget 2016, 7, 84658–84665. [Google Scholar] [CrossRef]
- Luo, J.; Lai, J. Pyroptosis-related molecular classification and immune microenvironment infiltration in breast cancer: A novel therapeutic target. J. Cell. Mol. Med. 2022, 26, 2259–2272. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Li, Y.; Kong, B.; Zhang, G.; Zhang, Q. Polydatin down-regulates the phosphorylation level of STAT3 and induces pyroptosis in triple-negative breast cancer mice with a high-fat diet. Ann. Transl. Med. 2022, 10, 173. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Luo, B.; Wu, X.; Guan, F.; Yu, X.; Zhao, L.; Ke, X.; Wu, J.; Yuan, J. Cisplatin Induces Pyroptosis via Activation of MEG3/NLRP3/caspase-1/GSDMD Pathway in Triple-Negative Breast Cancer. Int. J. Biol. Sci. 2021, 17, 2606–2621. [Google Scholar] [CrossRef]
- Li, Y.; Wang, W.; Li, A.; Huang, W.; Chen, S.; Han, F.; Wang, L. Dihydroartemisinin induces pyroptosis by promoting the AIM2/caspase-3/DFNA5 axis in breast cancer cells. Chem. Biol. Interact. 2021, 340, 109434. [Google Scholar] [CrossRef]
- Wang, J.G.; Jian, W.J.; Li, Y.; Zhang, J. Nobiletin promotes the pyroptosis of breast cancer via regulation of miR-200b/JAZF1 axis. Kaohsiung J. Med. Sci. 2021, 37, 572–582. [Google Scholar] [CrossRef]
- An, H.; Heo, J.S.; Kim, P.; Lian, Z.; Lee, S.; Park, J.; Hong, E.; Pang, K.; Park, Y.; Ooshima, A.; et al. Tetraarsenic hexoxide enhances generation of mitochondrial ROS to promote pyroptosis by inducing the activation of caspase-3/GSDME in triple-negative breast cancer cells. Cell Death Dis. 2021, 12, 159. [Google Scholar] [CrossRef]
- Yan, L.; Liu, Y.; Ma, X.F.; Hou, D.; Zhang, Y.H.; Sun, Y.; Shi, S.S.; Forouzanfar, T.; Lin, H.Y.; Fan, J.; et al. Triclabendazole Induces Pyroptosis by Activating Caspase-3 to Cleave GSDME in Breast Cancer Cells. Front. Pharmacol. 2021, 12, 670081. [Google Scholar] [CrossRef]
- Tian, W.; Wang, Z.; Tang, N.N.; Li, J.T.; Liu, Y.; Chu, W.F.; Yang, B.F. Ascorbic Acid Sensitizes Colorectal Carcinoma to the Cytotoxicity of Arsenic Trioxide via Promoting Reactive Oxygen Species-Dependent Apoptosis and Pyroptosis. Front. Pharmacol. 2020, 11, 123. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Wang, B.; Sun, J.; Na, H.; Chen, Z.; Zhu, Z.; Yan, L.; Ren, S.; Zuo, Y. DAC can restore expression of NALP1 to suppress tumor growth in colon cancer. Cell Death Dis. 2015, 6, e1602. [Google Scholar] [CrossRef][Green Version]
- Lv, L.V.; Zhou, J.; Lin, C.; Hu, G.; Yi, L.U.; Du, J.; Gao, K.; Li, X. DNA methylation is involved in the aberrant expression of miR-133b in colorectal cancer cells. Oncol. Lett. 2015, 10, 907–912. [Google Scholar] [CrossRef] [PubMed]
- Amalraj, A.; Pius, A.; Gopi, S.; Gopi, S. Biological activities of curcuminoids, other biomolecules from turmeric and their derivatives—A review. J. Tradit. Complement. Med. 2017, 7, 205–233. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Ji, X.; Zhang, Q.; Wei, Y. Curcumin combined with photodynamic therapy, promising therapies for the treatment of cancer. Biomed. Pharmacother. 2022, 146, 112567. [Google Scholar] [CrossRef]
- Sandur, S.K.; Ichikawa, H.; Pandey, M.K.; Kunnumakkara, A.B.; Sung, B.; Sethi, G.; Aggarwal, B.B. Role of pro-oxidants and antioxidants in the anti-inflammatory and apoptotic effects of curcumin (diferuloylmethane). Free Radic. Biol. Med. 2007, 43, 568–580. [Google Scholar] [CrossRef]
- Doello, K.; Ortiz, R.; Alvarez, P.J.; Melguizo, C.; Cabeza, L.; Prados, J. Latest in Vitro and in Vivo Assay, Clinical Trials and Patents in Cancer Treatment using Curcumin: A Literature Review. Nutr. Cancer 2018, 70, 569–578. [Google Scholar] [CrossRef]
- Xue, B.; Huang, J.; Zhang, H.; Li, B.; Xu, M.; Zhang, Y.; Xie, M.; Li, X. Micronized curcumin fabricated by supercritical CO2 to improve antibacterial activity against Pseudomonas aeruginosa. Artif. Cells Nanomed. Biotechnol. 2020, 48, 1135–1143. [Google Scholar] [CrossRef]
- Kamat, A.M.; Sethi, G.; Aggarwal, B.B. Curcumin potentiates the apoptotic effects of chemotherapeutic agents and cytokines through down-regulation of nuclear factor-kappaB and nuclear factor-kappaB-regulated gene products in IFN-alpha-sensitive and IFN-alpha-resistant human bladder cancer cells. Mol. Cancer Ther. 2007, 6, 1022–1030. [Google Scholar] [CrossRef]
- Zhu, G.H.; Dai, H.P.; Shen, Q.; Ji, O.; Zhang, Q.; Zhai, Y.L. Curcumin induces apoptosis and suppresses invasion through MAPK and MMP signaling in human monocytic leukemia SHI-1 cells. Pharm. Biol. 2016, 54, 1303–1311. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Sun, W.; Han, N.; Zou, Y.; Yin, D. Curcumin inhibits proliferation, migration, invasion and promotes apoptosis of retinoblastoma cell lines through modulation of miR-99a and JAK/STAT pathway. BMC Cancer 2018, 18, 1230. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Yang, X.; Tan, J.; Tian, R.; Shen, P.; Cai, W.; Liao, H. Curcumin suppresses the stemness of non-small cell lung cancer cells via promoting the nuclear-cytoplasm translocation of TAZ. Environ. Toxicol. 2021, 36, 1135–1142. [Google Scholar] [CrossRef] [PubMed]
- Sha, J.; Li, J.; Wang, W.; Pan, L.; Cheng, J.; Li, L.; Zhao, H.; Lin, W. Curcumin induces G0/G1 arrest and apoptosis in hormone independent prostate cancer DU-145 cells by down regulating Notch signaling. Biomed. Pharmacother. 2016, 84, 177–184. [Google Scholar] [CrossRef]
- Borges, G.A.; Elias, S.T.; Amorim, B.; de Lima, C.L.; Coletta, R.D.; Castilho, R.M.; Squarize, C.H.; Guerra, E.N.S. Curcumin downregulates the PI3K-AKT-mTOR pathway and inhibits growth and progression in head and neck cancer cells. Phytother. Res. 2020, 34, 3311–3324. [Google Scholar] [CrossRef]
- Priyadarsini, K.I. Chemical and structural features influencing the biological activity of curcumin. Curr. Pharm. Des. 2013, 19, 2093–2100. [Google Scholar]
- Nelson, K.M.; Dahlin, J.L.; Bisson, J.; Graham, J.; Pauli, G.F.; Walters, M.A. The Essential Medicinal Chemistry of Curcumin. J. Med. Chem. 2017, 60, 1620–1637. [Google Scholar] [CrossRef]
- Chen, B.; Li, H.; Ou, G.; Ren, L.; Yang, X.; Zeng, M. Curcumin attenuates MSU crystal-induced inflammation by inhibiting the degradation of IκBα and blocking mitochondrial damage. Arthritis Res. Ther. 2019, 21, 193. [Google Scholar] [CrossRef] [PubMed]
- Ran, Y.; Su, W.; Gao, F.; Ding, Z.; Yang, S.; Ye, L.; Chen, X.; Tian, G.; Xi, J.; Liu, Z. Curcumin Ameliorates White Matter Injury after Ischemic Stroke by Inhibiting Microglia/Macrophage Pyroptosis through NF-κB Suppression and NLRP3 Inflammasome Inhibition. Oxidative Med. Cell. Longev. 2021, 2021, 1552127. [Google Scholar] [CrossRef]
- Yin, H.; Guo, Q.; Li, X.; Tang, T.; Li, C.; Wang, H.; Sun, Y.; Feng, Q.; Ma, C.; Gao, C.; et al. Curcumin Suppresses IL-1β Secretion and Prevents Inflammation through Inhibition of the NLRP3 Inflammasome. J. Immunol. 2018, 200, 2835–2846. [Google Scholar] [CrossRef]
- Fan, Z.; Jing, H.; Yao, J.; Li, Y.; Hu, X.; Shao, H.; Shen, G.; Pan, J.; Luo, F.; Tian, X. The protective effects of curcumin on experimental acute liver lesion induced by intestinal ischemia-reperfusion through inhibiting the pathway of NF-κB in a rat model. Oxidative Med. Cell. Longev. 2014, 2014, 191624. [Google Scholar] [CrossRef]
- Chen, Y.; Li, C.; Duan, S.; Yuan, X.; Liang, J.; Hou, S. Curcumin attenuates potassium oxonate-induced hyperuricemia and kidney inflammation in mice. Biomed. Pharmacother. 2019, 118, 109195. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.; Zhao, S.; Zhou, J.; Yan, J.; Wang, L.; Du, X.; Li, H.; Chen, Y.; Cai, W.; Wu, J. Curcumin alleviates DSS-induced colitis via inhibiting NLRP3 inflammsome activation and IL-1β production. Mol. Immunol. 2018, 104, 11–19. [Google Scholar] [CrossRef]
- Ding, X.Q.; Wu, W.Y.; Jiao, R.Q.; Gu, T.T.; Xu, Q.; Pan, Y.; Kong, L.D. Curcumin and allopurinol ameliorate fructose-induced hepatic inflammation in rats via miR-200a-mediated TXNIP/NLRP3 inflammasome inhibition. Pharmacol. Res. 2018, 137, 64–75. [Google Scholar] [CrossRef]
- Li, Y.; Li, J.; Li, S.; Li, Y.; Wang, X.; Liu, B.; Fu, Q.; Ma, S. Curcumin attenuates glutamate neurotoxicity in the hippocampus by suppression of ER stress-associated TXNIP/NLRP3 inflammasome activation in a manner dependent on AMPK. Toxicol. Appl. Pharmacol. 2015, 286, 53–63. [Google Scholar] [CrossRef]
- Liu, S.; Li, Q.; Zhang, M.T.; Mao-Ying, Q.L.; Hu, L.Y.; Wu, G.C.; Mi, W.L.; Wang, Y.Q. Curcumin ameliorates neuropathic pain by down-regulating spinal IL-1β via suppressing astroglial NALP1 inflammasome and JAK2-STAT3 signalling. Sci. Rep. 2016, 6, 28956. [Google Scholar] [CrossRef]
- Ren, Y.; Yang, Z.; Sun, Z.; Zhang, W.; Chen, X.; Nie, S. Curcumin relieves paraquat-induced lung injury through inhibiting the thioredoxin interacting protein/NLR pyrin domain containing 3-mediated inflammatory pathway. Mol. Med. Rep. 2019, 20, 5032–5040. [Google Scholar] [CrossRef] [PubMed]
- Xi, Y.; Zeng, S.; Tan, X.; Deng, X. Curcumin inhibits the activity of ubiquitin ligase Smurf2 to promote NLRP3-dependent pyroptosis in non-small cell lung cancer cells. Int. J. Oncol. 2025, 66, 21. [Google Scholar] [CrossRef]
- Zhou, Y.; Kong, Y.; Jiang, M.; Kuang, L.; Wan, J.; Liu, S.; Zhang, Q.; Yu, K.; Li, N.; Le, A.; et al. Curcumin activates NLRC4, AIM2, and IFI16 inflammasomes and induces pyroptosis by up-regulated ISG3 transcript factor in acute myeloid leukemia cell lines. Cancer Biol. Ther. 2022, 23, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Wang, R.; Zhao, M.; Li, Y.; Sun, C.; Xie, J.; Chen, Y.; Jing, Q.; Mi, D.; Shi, S. PLGA confers upon conventional nonfluorescent molecules luminescent properties to trigger 1O2-induced pyroptosis and immune response in tumors. J. Nanobiotechnology 2025, 23, 35. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; He, L.; Wang, J.; Lai, L.; Ma, L.; Qu, K.; Yang, Z.; Wang, X.; Zhao, R.; Weng, L.; et al. Synergistic immunotherapy with a calcium-based nanoinducer: Evoking pyroptosis and remodeling tumor-associated macrophages for enhanced antitumor immune response. Nanoscale 2024, 16, 18570–18583. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Bi, J.; Zhang, Q.; Yang, Y.; Li, J.; Liang, Y. Mechanism of action of curcumin targeting TRPM2/NLRP3 signaling axis to mediate cell death in the treatment of knee osteoarthritis. Hum. Exp. Toxicol. 2024, 43, 9603271241308798. [Google Scholar] [CrossRef]
- Zheng, P.; Ding, B.; Zhu, G.; Li, C.; Lin, J. Biodegradable Ca2+ Nanomodulators Activate Pyroptosis through Mitochondrial Ca2+ Overload for Cancer Immunotherapy. Angew. Chem. Int. Ed. Engl. 2022, 61, e202204904. [Google Scholar] [CrossRef]
- Zhu, J.X.; Zhu, W.T.; Hu, J.H.; Yang, W.; Liu, P.; Liu, Q.H.; Bai, Y.X.; Xie, R. Curcumin-Loaded Poly(L-lactide-co-glycolide) Microbubble-Mediated Sono-photodynamic Therapy in Liver Cancer Cells. Ultrasound Med. Biol. 2020, 46, 2030–2043. [Google Scholar] [CrossRef]
- Dal, Z.; Aru, B. The role of curcumin on apoptosis and NLRP3 inflammasome-dependent pyroptosis on colorectal cancer in vitro. Turk. J. Med. Sci. 2023, 53, 883–893. [Google Scholar] [CrossRef]
- Wei, T.; Zheng, Z.; Wei, X.; Liu, Y.; Li, W.; Fang, B.; Yun, D.; Dong, Z.; Yi, B.; Li, W.; et al. Rational design, synthesis, and pharmacological characterisation of dicarbonyl curcuminoid analogues with improved stability against lung cancer via ROS and ER stress mediated cell apoptosis and pyroptosis. J. Enzym. Inhib. Med. Chem. 2022, 37, 2357–2369. [Google Scholar] [CrossRef]
- Nam, Y.J.; Choi, J.; Lee, J.S.; Seo, C.; Lee, G.; Lee, Y.; Kim, J.K.; Kim, P.; Lim, J.J.; Choi, H.S.; et al. Curcuma phaeocaulis Inhibits NLRP3 Inflammasome in Macrophages and Ameliorates Nanoparticle-Induced Airway Inflammation in Mice. Molecules 2022, 27, 2101. [Google Scholar] [CrossRef]
- Jena, A.B.; Dash, U.C.; Duttaroy, A.K. An in silico investigation on the interactions of curcumin and epigallocatechin-3-gallate with NLRP3 Inflammasome complex. Biomed. Pharmacother. 2022, 156, 113890. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Cui, C.; Xu, P.; Dang, R.; Cai, H.; Liao, D.; Yang, M.; Feng, Q.; Yan, X.; Jiang, P. Curcumin Activates AMPK Pathway and Regulates Lipid Metabolism in Rats Following Prolonged Clozapine Exposure. Front. Neurosci. 2017, 11, 558. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).