The Latest Achievements in the Design of Permanent Fillings for Conservative Dentistry Based on Indenoquinoxaline Derivatives as Photoinitiators of Visible-Light Polymerization: Mass and Colour Stability
Abstract
1. Introduction
2. Results
2.1. Spectroscopic Properties
2.2. Creating a Triplet State
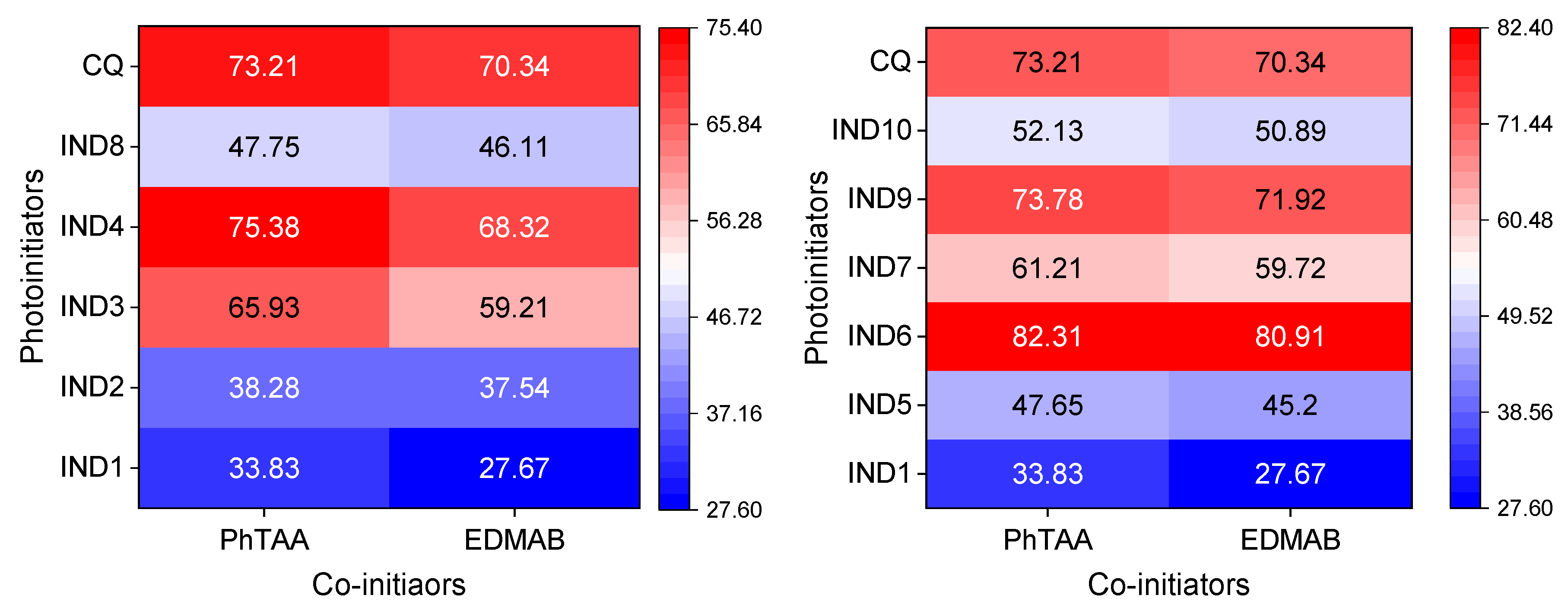
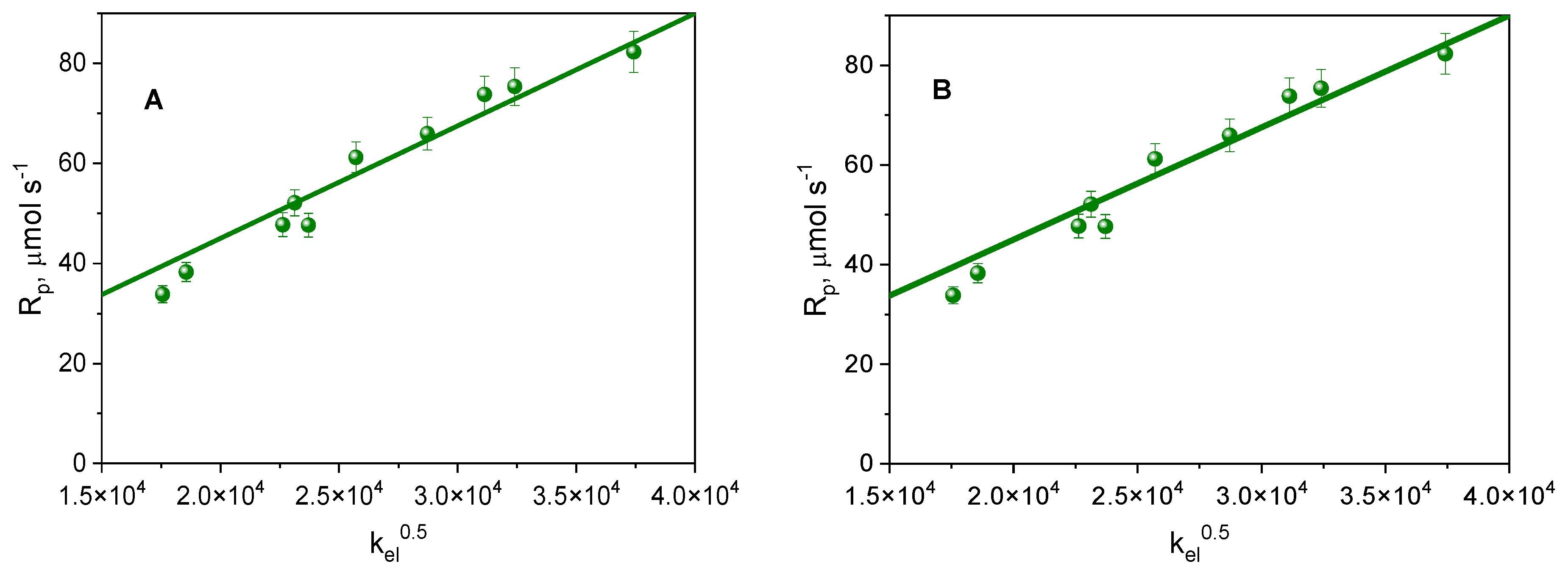
2.3. Photoinitiation of Free Radical Polymerization of Triacrylates
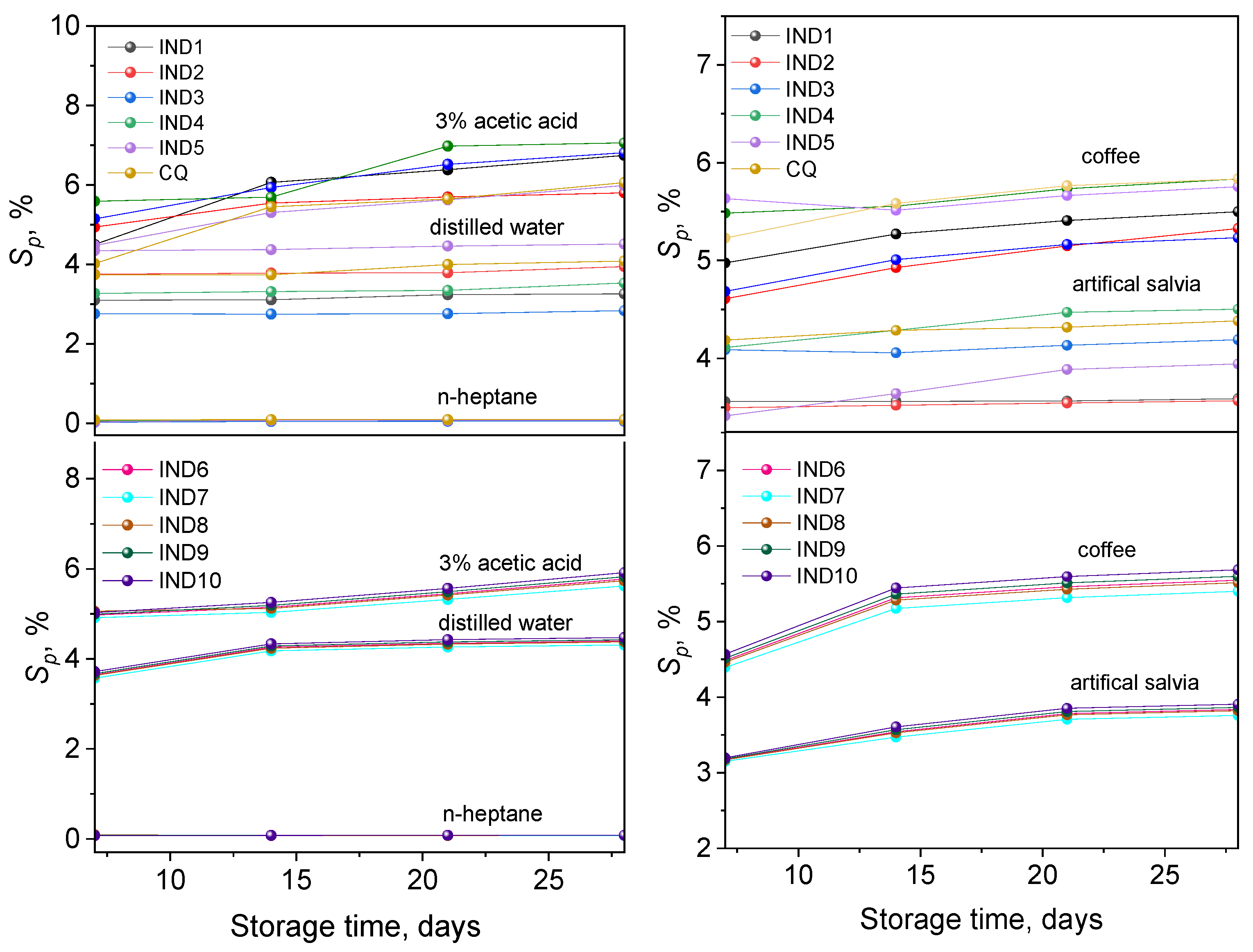
2.4. Mass Stability
2.4.1. Sorption
2.4.2. Solubility
2.4.3. Change in Mass
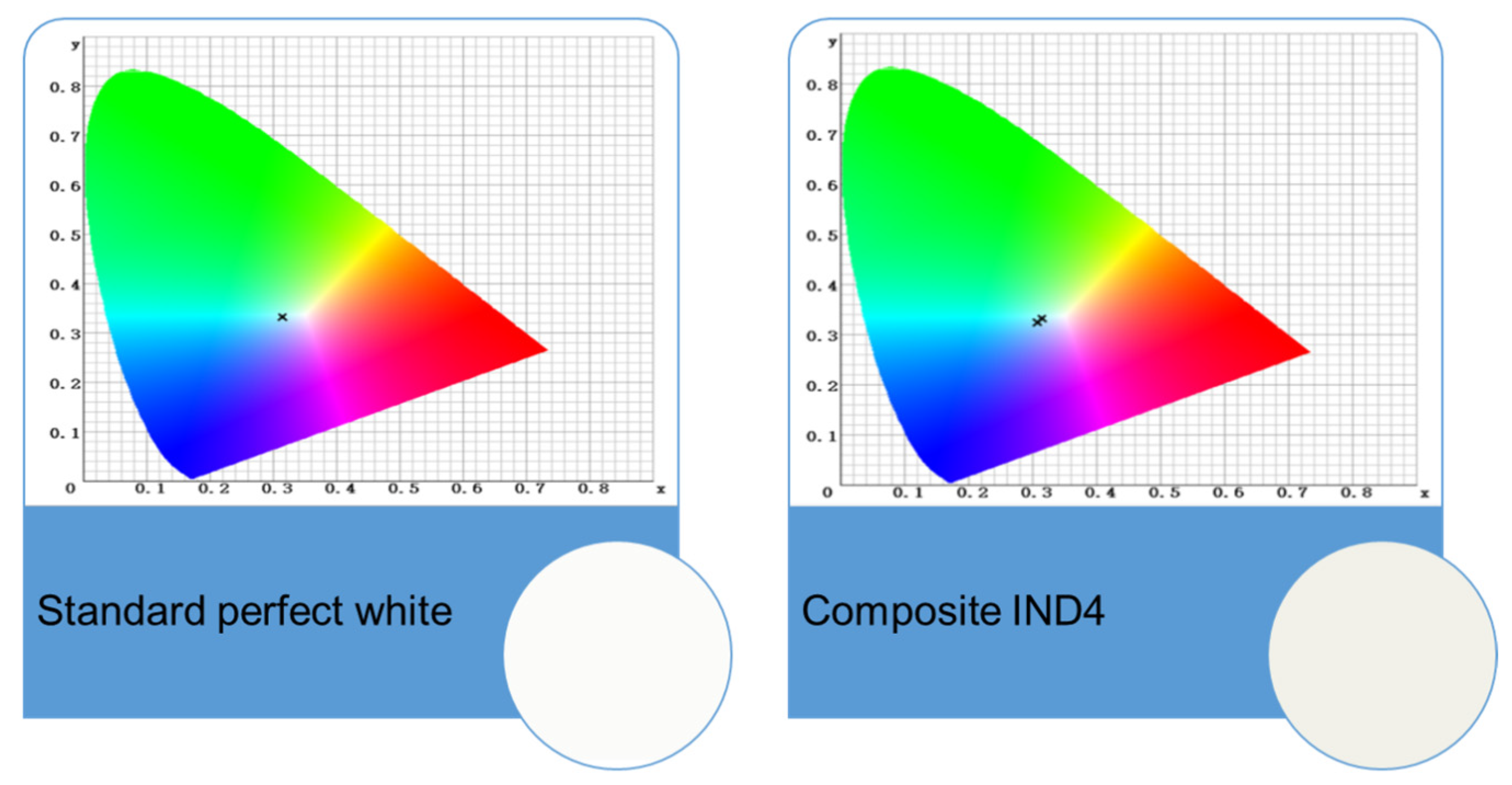
2.5. Colour Stability
3. Materials and Methods
3.1. Reagents
3.2. Methods
3.2.1. Nuclear Magnetic Resonance
3.2.2. HPLC Analyses
3.2.3. Electronic Absorption Spectra
3.2.4. Photopolymerization
3.2.5. Lifetime of an Excited Triplet State
3.2.6. Quantum Yield of Triplet-State Formation
3.2.7. The Rate Constant of Quenching of the Excited Triplet State
3.2.8. Mass Stability Test
3.2.9. Colour Stability Test
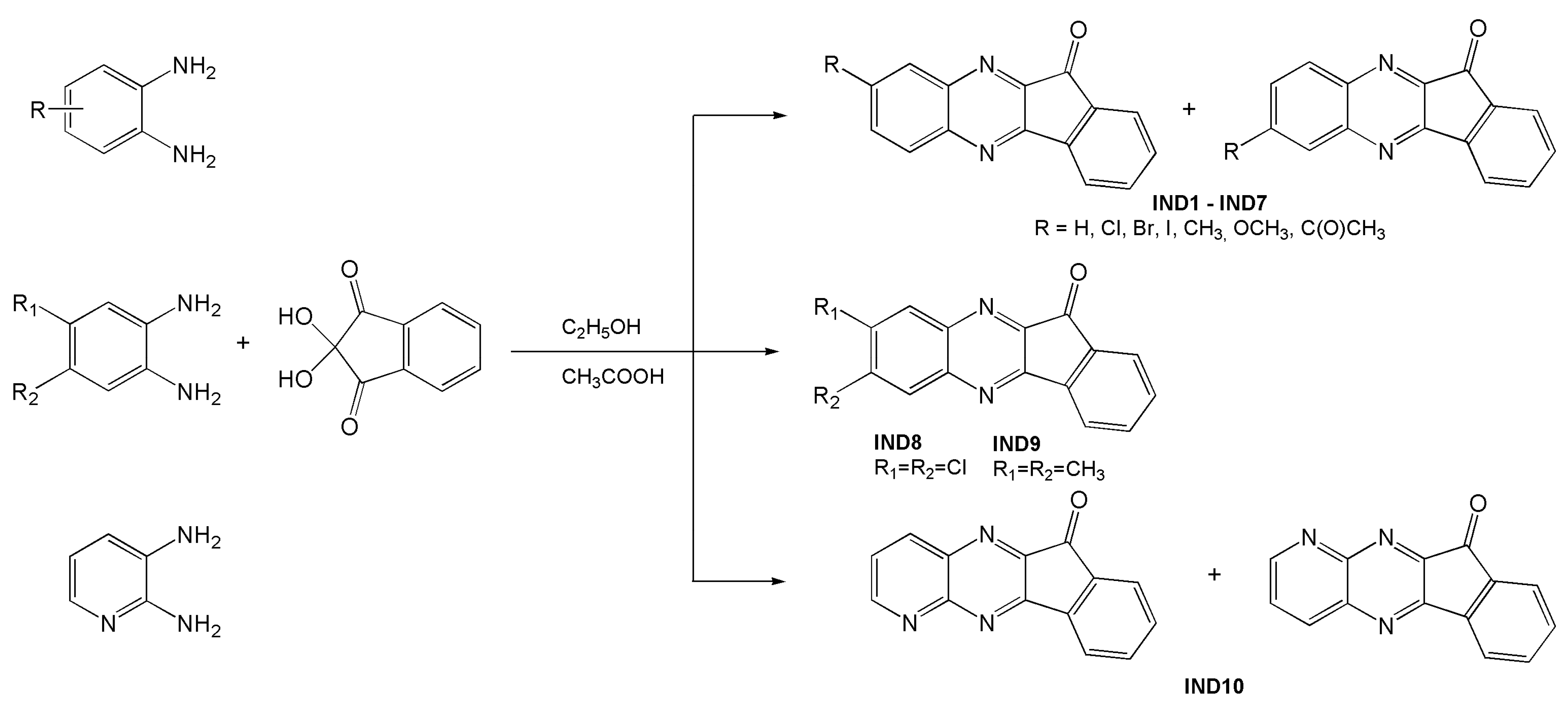
3.3. Design of the Photoinitiator Structure
Synthesis—General Procedure for Obtaining Indenoquinoxalines
3.4. Preparation of Dental Composites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aminoroaya, A.; Neisiany, R.E.; Khorasani, S.N.; Panahi, P.; Das, O.; Madry, H.; Cucchiarini, M.; Ramakrishna, S. A Review of Dental Composites: Challenges, Chemistry Aspects, Filler Influences, and Future Insights. Compos. Part B Eng. 2021, 216, 108852. [Google Scholar] [CrossRef]
- Zhang, Y.; Gong, H.; Feng, D.; Fang, T.; Cao, D.; Shi, Z.; Zhu, S.; Cui, Z. New Strategy for Overcoming Microleakage: An Elastic Layer for Dental Caries Restoration. J. Mater. Chem. B 2015, 3, 4401–4405. [Google Scholar] [CrossRef]
- Yadav, R.; Kumar, M. Dental Restorative Composite Materials: A Review. J. Oral Biosci. 2019, 61, 78–83. [Google Scholar] [CrossRef]
- Szczesio-Wlodarczyk, A.; Domarecka, M.; Kopacz, K.; Sokolowski, J.; Bociong, K. An Evaluation of the Properties of Urethane Dimethacrylate-Based Dental Resins. Materials 2021, 14, 2727. [Google Scholar] [CrossRef] [PubMed]
- Karabela, M.M.; Sideridou, I.D. Synthesis and Study of Properties of Dental Resin Composites with Different Nanosilica Particles Size. Dent. Mater. 2011, 27, 825–835. [Google Scholar] [CrossRef]
- Mount, G.J.; Hume, W.R. A New Cavity Classification. Aust. Dent. J. 1998, 43, 153–159. [Google Scholar] [CrossRef]
- Durner, J.; Obermaier, J.; Draenert, M.; Ilie, N. Correlation of the Degree of Conversion with the Amount of Elutable Substances in Nano-Hybrid Dental Composites. Dent. Mater. 2012, 28, 1146–1153. [Google Scholar] [CrossRef]
- De Nys, S.; Duca, R.C.; Vervliet, P.; Covaci, A.; Boonen, I.; Elskens, M.; Vanoirbeek, J.; Godderis, L.; Van Meerbeek, B.; Van Landuyt, K.L. Bisphenol A Release from Short-Term Degraded Resin-Based Dental Materials. J. Dent. 2022, 116, 103894. [Google Scholar] [CrossRef]
- Rogalewicz, R.; Batko, K.; Voelkel, A. Identification of Organic Extractables from Commercial Resin-Modified Glass-Ionomers Using HPLC-MS. J. Environ. Monit. 2006, 8, 750–758. [Google Scholar] [CrossRef]
- Gates, T. The Physical and Chemical Ageing of Polymeric Composites. In Ageing of Composites; Martin, R., Ed.; Woodhead Publishing: Cambridge, UK, 2008; pp. 3–33. ISBN 978-1-84569-352-7. [Google Scholar]
- Szczesio-Wlodarczyk, A.; Sokolowski, J.; Kleczewska, J.; Bociong, K. Ageing of Dental Composites Based on Methacrylate Resins—A Critical Review of the Causes and Method of Assessment. Polymers 2020, 12, 882. [Google Scholar] [CrossRef]
- Fugolin, A.P.P.; Pfeifer, C.S. New Resins for Dental Composites. J. Dent. Res. 2017, 96, 1085–1091. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, D.C.R.S.; Rocha, M.G.; Gatti, A.; Correr, A.B.; Ferracane, J.L.; Sinhoret, M.A.C. Effect of Different Photoinitiators and Reducing Agents on Cure Efficiency and Colour Stability of Resin-Based Composites Using Different LED Wavelengths. J. Dent. 2015, 43, 1565–1572. [Google Scholar] [CrossRef] [PubMed]
- Ilie, N.; Hickel, R. Investigations on a Methacrylate-Based Flowable Composite Based on the SDRTM Technology. Dent. Mater. 2011, 27, 348–355. [Google Scholar] [CrossRef]
- Ding, Y.; Li, B.; Wang, M.; Liu, F.; He, J. Bis-GMA Free Dental Materials Based on UDMA/SR833s Dental Resin System. Adv. Polym. Technol. 2016, 35, 396–401. [Google Scholar] [CrossRef]
- Rahim, T.N.A.T.; Mohamad, D.; Md Akil, H.; Ab Rahman, I. Water Sorption Characteristics of Restorative Dental Composites Immersed in Acidic Drinks. Dent. Mater. 2012, 28, e63–e70. [Google Scholar] [CrossRef] [PubMed]
- Atai, M.; Nekoomanesh, M.; Hashemi, S.A.; Amani, S. Physical and Mechanical Properties of an Experimental Dental Composite Based on a New Monomer. Dent. Mater. 2004, 20, 663–668. [Google Scholar] [CrossRef]
- Wei, Y.; Silikas, N.; Zhang, Z.; Watts, D.C. Diffusion and Concurrent Solubility of Self-Adhering and New Resin–Matrix Composites during Water Sorption/Desorption Cycles. Dent. Mater. 2011, 27, 197–205. [Google Scholar] [CrossRef]
- Ferracane, J.L. Hygroscopic and Hydrolytic Effects in Dental Polymer Networks. Dent. Mater. 2006, 22, 211–222. [Google Scholar] [CrossRef]
- Sideridou, I.; Achilias, D.S.; Spyroudi, C.; Karabela, M. Water Sorption Characteristics of Light-Cured Dental Resins and Composites Based on Bis-EMA/PCDMA. Biomaterials 2004, 25, 367–376. [Google Scholar] [CrossRef]
- Alsulaimani, A.F.; Alfehaid, K.W.; Alhabash, K.M.; AlShehri, M.A. Effect of Herbal Medication and Supplements on Oral Health. J. Healthc. Sci. 2024, 4, 637–643. [Google Scholar] [CrossRef]
- Santerre, J.P.; Shajii, L.; Leung, B.W. Relation of Dental Composite Formulations To Their Degradation and the Release of Hydrolyzed Polymeric-Resin-Derived Products. Crit. Rev. Oral Biol. Med. 2001, 12, 136–151. [Google Scholar] [CrossRef] [PubMed]
- Ferracane, J.L. Current Trends in Dental Composites. Crit. Rev. Oral Biol. Med. 1995, 6, 302–318. [Google Scholar] [CrossRef] [PubMed]
- Ferracane, J.L. Resin Composite—State of the Art. Dent. Mater. 2011, 27, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.-H. Update on Dental Nanocomposites. J. Dent. Res. 2010, 89, 549–560. [Google Scholar] [CrossRef]
- Stansbury, J.W. Curing Dental Resins and Composites by Photopolymerization. J. Esthet. Restor. Dent. 2000, 12, 300–308. [Google Scholar] [CrossRef]
- Nie, J.; Andrzejewska, E.; Rabek, J.F.; Lindén, L.Å.; Fouassier, J.P.; Paczkowski, J.; Scigalski, F.; Wrzyszczynski, A. Effect of Peroxides and Hydroperoxides on the Camphorquinone-Initiated Photopolymerization. Macromol. Chem. Phys. 1999, 200, 1692–1701. [Google Scholar] [CrossRef]
- Nie, J.; Lindén, L.Å.; Rabek, J.F.; Ekstrand, J. Photocuring Kinetic Studies of New Dental Restorative Resins Based on Poly(Ethylene Glycol) Diacrylate and Tris[2-(Acryloyloxy)-Ethyl]Isocyanurate. Angew. Makromol. Chem. 1998, 257, 47–52. [Google Scholar] [CrossRef]
- Cook, W.D. Photopolymerization Kinetics of Dimethacrylates Using the Camphorquinone/Amine Initiator System. Polymer 1992, 33, 600–609. [Google Scholar] [CrossRef]
- Stern, O.; Volmer, M. Über Die Abklingungszeit Der Fluoreszenz. Phys. Z. 1919, 20, 183–188. [Google Scholar]
- Dahmani, M. Organotin (IV) Derivative of Piperic Acid and Phenylthioacetic Acid: Synthesis, Crystal Structure, Spectroscopic Characterizations and Biological Activities. Moroc. J. Chem. 2020, 8, 244–263. [Google Scholar] [CrossRef]
- Velmurugan, V.; Nandini Asha, R.; Ravindran Durai Nayagam, B.; Kumaresan, S.; Bhuvanesh, N. Synthesis, Characterization and Biological Activity of (Phenylthio)Acetic Acid:Theophylline Cocrystal. J. Chem. Crystallogr. 2021, 51, 225–234. [Google Scholar] [CrossRef]
- Pączkowski, J.; Neckers, D.C. Photoinduced Electron Transfer Initiating Systems for Free-Radical Polymerization. In Electron Transfer in Chemistry; Wiley: Hoboken, NJ, USA, 2008; Volume 5. [Google Scholar]
- Allen, N.S. Photochemistry and Photophysics of Polymer Materials; Wiley: Hoboken, NJ, USA, 2010. [Google Scholar]
- Pyszka, I.; Kucybała, Z.; Pączkowski, J. Reinvestigation of the Mechanism of the Free Radical Polymerization Photoinitiation Process by Camphorquinone–Coinitiator Systems: New Results. Macromol. Chem. Phys. 2004, 205, 2371–2375. [Google Scholar] [CrossRef]
- Braden, M.; Causton, E.E.; Clarke, R.L. Diffusion of Water in Composite Filling Materials. J. Dent. Res. 1976, 55, 730–732. [Google Scholar] [CrossRef] [PubMed]
- Miettinen, V.M.; Narva, K.K.; Vallittu, P.K. Water Sorption, Solubility and Effect of Post-Curing of Glass Fibre Reinforced Polymers. Biomaterials 1999, 20, 1187–1194. [Google Scholar] [CrossRef]
- Okulus, Z.; Héberger, K.; Voelkel, A. Sorption, Solubility, and Mass Changes of Hydroxyapatite-Containing Composites in Artificial Saliva, Food Simulating Solutions, Tea, and Coffee. J. Appl. Polym. Sci. 2014, 131, 39856. [Google Scholar] [CrossRef]
- Skrtic, D.; Antonucci, J.M. Dental Composites Based on Amorphous Calcium Phosphate—Resin Composition/Physicochemical Properties Study. J. Biomater. Appl. 2007, 21, 375–393. [Google Scholar] [CrossRef]
- Tuna, S.H.; Keyf, F.; Gumus, H.O.; Uzun, C. The Evaluation of Water Sorption/Solubility on Various Acrylic Resins. Eur. J. Dent. 2019, 02, 191–197. [Google Scholar] [CrossRef]
- Cramer, N.B.; Stansbury, J.W.; Bowman, C.N. Recent Advances and Developments in Composite Dental Restorative Materials. J. Dent. Res. 2011, 90, 402–416. [Google Scholar] [CrossRef]
- Sideridou, I.D.; Karabela, M.M. Sorption of Water, Ethanol or Ethanol/Water Solutions by Light-Cured Dental Dimethacrylate Resins. Dent. Mater. 2011, 27, 1003–1010. [Google Scholar] [CrossRef]
- Zimmerli, B.; Strub, M.; Stadler, O.; Jeger, F.; Lussi, A. Composite Materials: Composition, Properties and Clinical Applications. A Literature Review. Schweiz. Monatsschr. Zahnmed. 2010, 120, 972–986. [Google Scholar]
- Musanje, L.; Shu, M.; Darvell, B.W. Water Sorption and Mechanical Behaviour of Cosmetic Direct Restorative Materials in Artificial Saliva. Dent. Mater. 2001, 17, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Vichi, A.; Ferrari, M.; Davidson, C.L. Colour and Opacity Variations in Three Different Resin-Based Composite Products after Water Aging. Dent. Mater. 2004, 20, 530–534. [Google Scholar] [CrossRef] [PubMed]
- He, W.-H.; Park, C.J.; Byun, S.; Tan, D.; Lin, C.Y.; Chee, W. Evaluating the Relationship between Tooth Colour and Enamel Thickness, Using Twin Flash Photography, Cross-Polarization Photography, and Spectrophotometer. J. Esthet. Restor. Dent. 2020, 32, 91–101. [Google Scholar] [CrossRef]
- Pyszka, I.; Jędrzejewska, B. Acenaphthoquinoxaline Derivatives as Dental Photoinitiators of Acrylates Polymerization. Materials 2021, 14, 4881. [Google Scholar] [CrossRef]
- Pyszka, I.; Kucybała, Z.; Jędrzejewska, B. Effective Singlet Oxygen Sensitizers Based on the Phenazine Skeleton as Efficient Light Absorbers in Dye Photoinitiating Systems for Radical Polymerization of Acrylates. Materials 2021, 14, 3085. [Google Scholar] [CrossRef]
- Pyszka, I.; Skowroński, Ł.; Jędrzejewska, B. Study on New Dental Materials Containing Quinoxaline-Based Photoinitiators in Terms of Exothermicity of the Photopolymerization Process. Int. J. Mol. Sci. 2023, 24, 2752. [Google Scholar] [CrossRef] [PubMed]
- Pyszka, I.; Jędrzejewska, B. Design of Dyes Based on the Quinoline or Quinoxaline Skeleton towards Visible Light Photoinitiators. Int. J. Mol. Sci. 2024, 25, 4289. [Google Scholar] [CrossRef]
- Lament, B.; Karpiuk, J.; Waluk, J. Determination of Triplet Formation Efficiency from Kinetic Profiles of the Ground State Recovery. Photochem. Photobiol. Sci. 2003, 2, 267–272. [Google Scholar] [CrossRef]
- Gonulol, N.; Ozer, S.; Sen Tunc, E. Water Sorption, Solubility, and Colour Stability of Giomer Restoratives. J. Esthet. Restor. Dent. 2015, 27, 300–306. [Google Scholar] [CrossRef]
- KOKSAL, T.; DIKBAS, I. Colour Stability of Different Denture Teeth Materials against Various Staining Agents. Dent. Mater. J. 2008, 27, 139–144. [Google Scholar] [CrossRef]
- Ertas, E.; Güler, A.U.; Yücel, A.Ç.; Köprülü, H.; Güler, E. Colour Stability of Resin Composites after Immersion in Different Drinks. Dent. Mater. J. 2006, 25, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Uchimura, J.Y.T.; Sato, F.; Bianchi, G.; Baesso, M.L.; Santana, R.G.; Pascotto, R.C. Colour Stability Over Time of Three Resin-Based Restorative Materials Stored Dry and in Artificial Saliva. J. Esthet. Restor. Dent. 2014, 26, 279–287. [Google Scholar] [CrossRef]
- Haselton, D.R.; Diaz-Arnold, A.M.; Dawson, D.V. Colour Stability of Provisional Crown and Fixed Partial Denture Resins. J. Prosthet. Dent. 2005, 93, 70–75. [Google Scholar] [CrossRef] [PubMed]
- PN-EN ISO 4049:2019; Dentistry—Polymer-Based Restorative Materials. ISO: Geneve, Switzerland, 2019.
- Örtengren, U.; Wellendorf, H.; Karlsson, S.; Ruyter, I.E. Water Sorption and Solubility of Dental Composites and Identification of Monomers Released in an Aqueous Environment. J. Oral Rehabil. 2001, 28, 1106–1115. [Google Scholar] [CrossRef]
- Domingo, C.; Arcís, R.W.; Osorio, E.; Osorio, R.; Fanovich, M.A.; Rodríguez-Clemente, R.; Toledano, M. Hydrolytic Stability of Experimental Hydroxyapatite-Filled Dental Composite Materials. Dent. Mater. 2003, 19, 478–486. [Google Scholar] [CrossRef]
- Johnston, W.M. Colour Measurement in Dentistry. J. Dent. 2009, 37, e2–e6. [Google Scholar] [CrossRef]
- Chen, J.; Chen, Y.; Wu, Y.; Wang, X.; Yu, Z.; Xiao, L.; Liu, Y.; Tian, H.; Yao, J.; Fu, H. Modulated Emission from Dark Triplet Excitons in Aza-Acene Compounds: Fluorescence versus Phosphorescence. New J. Chem. 2017, 41, 1864–1871. [Google Scholar] [CrossRef]
- Pyszka, I.; Jędrzejewska, B. Photoinitiation Abilities of Indeno- and Indoloquinoxaline Derivatives and Mechanical Properties of Dental Fillings Based on Multifunctional Acrylic Monomers and Glass Ionomer. Polymer 2023, 266, 125625. [Google Scholar] [CrossRef]
- Zhang, C.; Xu, Z.; Zhang, L.; Jiao, N. Et3N-Catalyzed Oxidative Dehydrogenative Coupling of α-Unsubstituted Aldehydes and Ketones with Aryl Diamines Leading to Quinoxalines Using Molecular Oxygen as Oxidant. Tetrahedron 2012, 68, 5258–5262. [Google Scholar] [CrossRef]
- Yadavalli, V.D.N.; Katla, R. An Overview of Quinoxaline Synthesis by Green Methods: Recent Reports. Phys. Sci. Rev. 2023, 8, 3323–3392. [Google Scholar] [CrossRef]
- Maghsoodlou, M.T.; Habibi-Khorassani, S.M.; Moradi, A.; Hazeri, N.; Davodi, A.; Sajadikhah, S.S. One-Pot Three-Component Synthesis of Functionalized Spirolactones by Means of Reaction between Aromatic Ketones, Dimethyl Acetylenedicarboxylate, and N-Heterocycles. Tetrahedron 2011, 67, 8492–8495. [Google Scholar] [CrossRef]
- Tseng, C.-H.; Tzeng, C.-C.; Chiu, C.-C.; Yang, C.-L.; Lu, P.-J.; Chou, C.-K.; Liu, C.-Y.; Chen, Y.-L. Synthesis and Antiproliferative Evaluation of 9-Methoxy-6-(Piperazin-1-Yl)-11H-Indeno[1,2-c]Quinoline-11-One Derivatives. Part 4. MedChemComm 2014, 5, 937–948. [Google Scholar] [CrossRef]
- Tseng, C.-H.; Chen, Y.-L.; Yang, C.-L.; Cheng, C.-M.; Han, C.-H.; Tzeng, C.-C. Synthesis of 6-Substituted 9-Methoxy-11H-Indeno[1,2-c]Quinoline-11-One Derivatives as Potential Anticancer Agents. Bioorg. Med. Chem. 2012, 20, 4397–4404. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.-H.; Tzeng, C.-C.; Chung, K.-Y.; Kao, C.-L.; Hsu, C.-Y.; Cheng, C.-M.; Huang, K.-S.; Chen, Y.-L. Synthesis and Antiproliferative Evaluation of 6-Aryl-11-Iminoindeno[1,2-c]Quinoline Derivatives. Bioorg. Med. Chem. 2011, 19, 7653–7663. [Google Scholar] [CrossRef]
- Song, J.H.; Bae, S.M.; Shin, H.Y.; Jung, D.I.; Cho, J.H. Synthesis of Spirohydantoins and Schiff Bases of Indenoquinoxalinones and Indenopyridopyrazinones. Asian J. Chem. 2020, 32, 1925–1930. [Google Scholar] [CrossRef]
- Ivashchenko, A.V.; Drushlyak, A.G.; Titov, V.V. Reaction of O-Phenylenediamine with Isatins. Chem. Heterocycl. Compd. 1984, 20, 537–542. [Google Scholar] [CrossRef]
- Kanhed, A.M.; Patel, D.V.; Patel, N.R.; Sinha, A.; Thakor, P.S.; Patel, K.B.; Prajapati, N.K.; Patel, K.V.; Yadav, M.R. Indoloquinoxaline Derivatives as Promising Multi-Functional Anti-Alzheimer Agents. J. Biomol. Struct. Dyn. 2022, 40, 2498–2515. [Google Scholar] [CrossRef] [PubMed]
- Edayadulla, N.; Lee, Y.R. Cerium Oxide Nanoparticle-Catalyzed Three-Component Protocol for the Synthesis of Highly Substituted Novel Quinoxalin-2-Amine Derivatives and 3,4-Dihydroquinoxalin-2-Amines in Water. RSC Adv. 2014, 4, 11459–11468. [Google Scholar] [CrossRef]
- Mishra, A.; Singh, S.; Quraishi, M.A.; Srivastava, V. A Catalyst-Free Expeditious Green Synthesis of Quinoxaline, Oxazine, Thiazine, and Dioxin Derivatives in Water under Ultrasound Irradiation. Org. Prep. Proced. Int. 2019, 51, 345–356. [Google Scholar] [CrossRef]
- Junek, H.; Fischer-Colbrie, H.; Sterk, H. Synthesen Mit Nitrilen, XLV. 2-(1,3-Dioxo-2-Indanyliden)Benzimidazolin—Ein Indigo-Isomeres. Chem. Ber. 1977, 110, 2276–2282. [Google Scholar] [CrossRef]
- Zhang, C.; Li, S.; Ji, L.; Liu, S.; Li, Z.; Li, S.; Meng, X. Design, Synthesis and Antitumor Activity of Non-Camptothecin Topoisomerase I Inhibitors. Bioorg. Med. Chem. Lett. 2015, 25, 4693–4696. [Google Scholar] [CrossRef] [PubMed]
- Kucybala, Z.; Kosobucka, A.; Paczkowski, J. Development of New Dyeing Photoinitiators for Free Radical Polymerization Based on 3-Methyl-1-Phenyl-1H-Pentaazacyclopenta[b]Naphthalene Skeleton III. J. Photochem. Photobiol. Chem. 2000, 136, 227–234. [Google Scholar] [CrossRef]
- Kucybała, Z.; Pyszka, I.; Pączkowski, J. Development of New Dyeing Photoinitiators for Free Radical Polymerization Based on the 1H-Pyrazolo[3,4-b]Quinoxaline Skeleton. Part 2. J. Chem. Soc. Perkin Trans. 2000, 2, 1559–1567. [Google Scholar] [CrossRef]
- Bowen, R.L.; Marjenhoff, W.A. Dental Composites/Glass Ionomers: The Materials. Adv. Dent. Res. 1992, 6, 44–49. [Google Scholar] [CrossRef]
- Moszner, N.; Salz, U. New Developments of Polymeric Dental Composites. Prog. Polym. Sci. 2001, 26, 535–576. [Google Scholar] [CrossRef]
- Peutzfeldt, A. Resin Composites in Dentistry: The Monomer Systems. Eur. J. Oral Sci. 1997, 105, 97–116. [Google Scholar] [CrossRef]
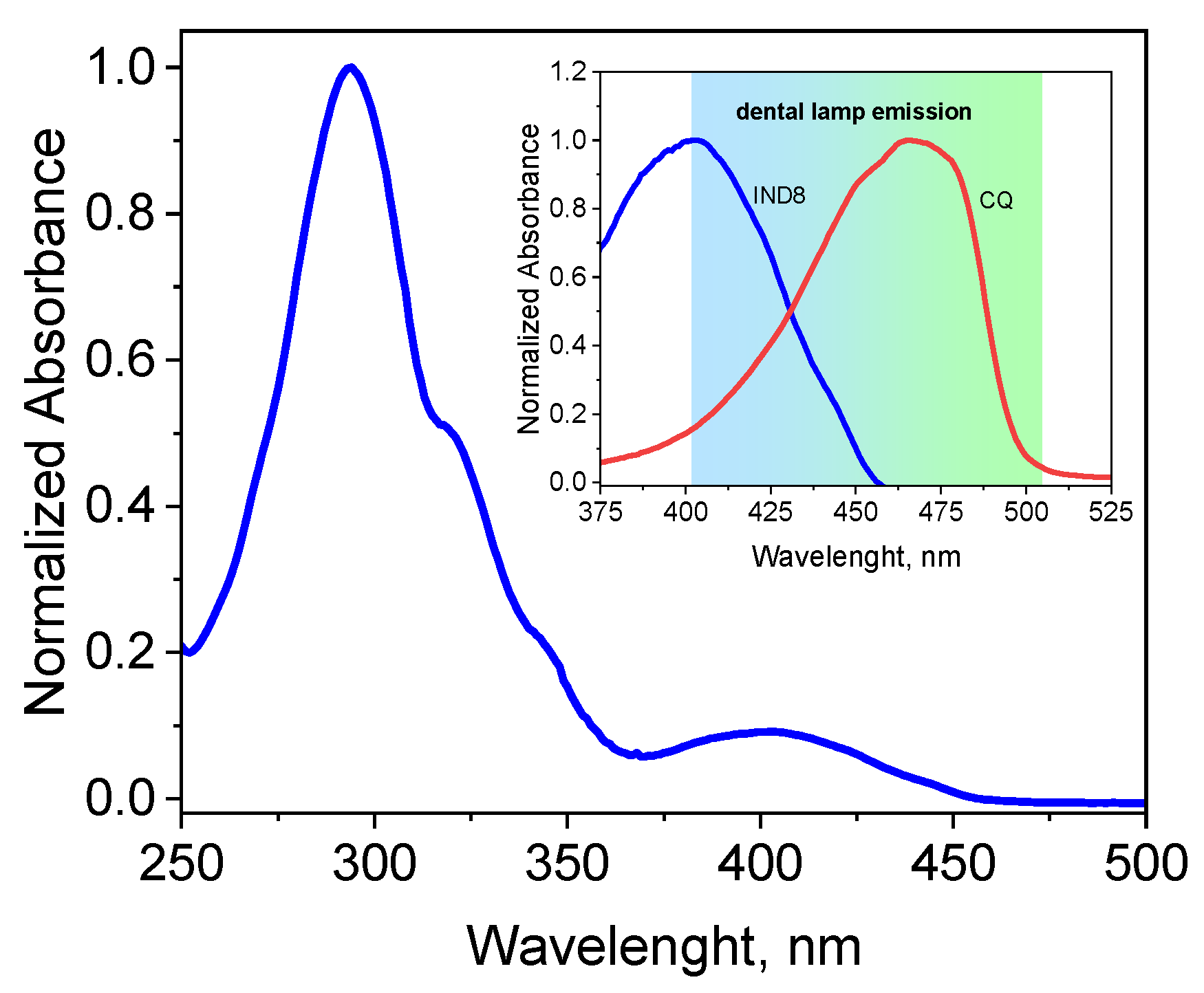
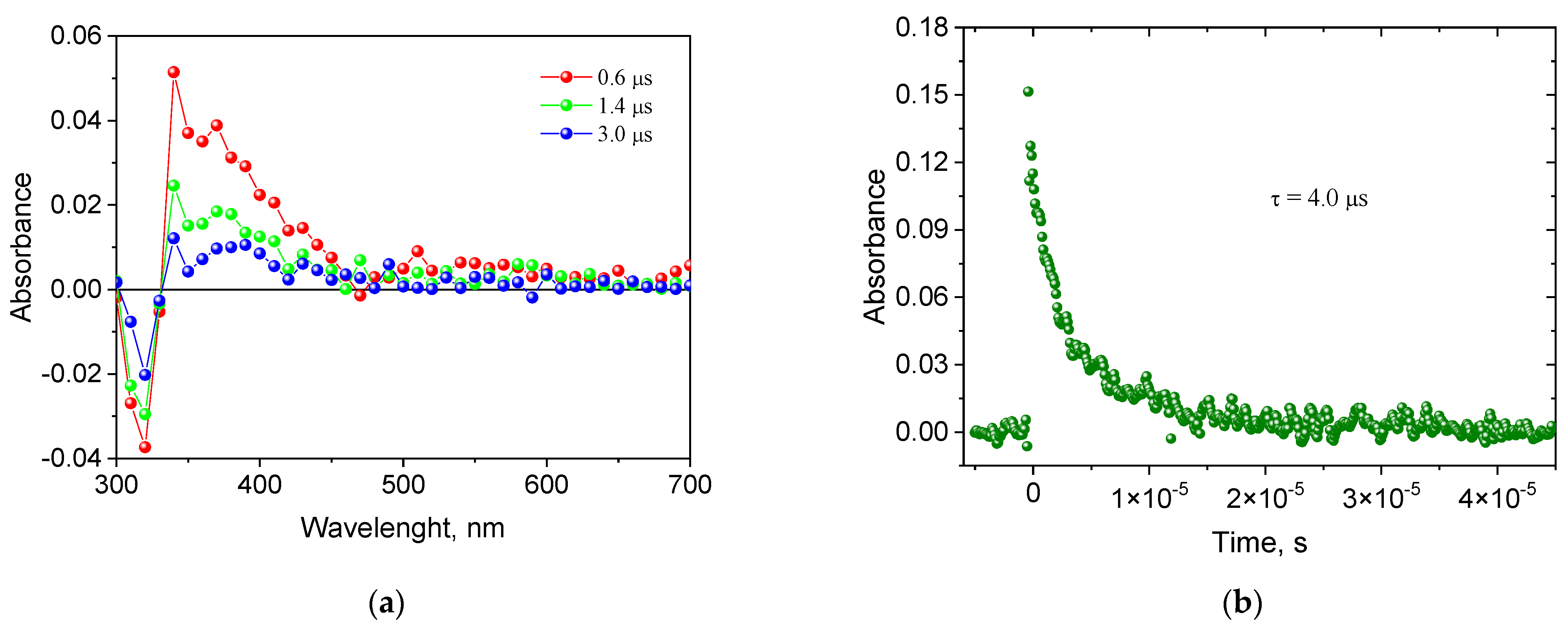
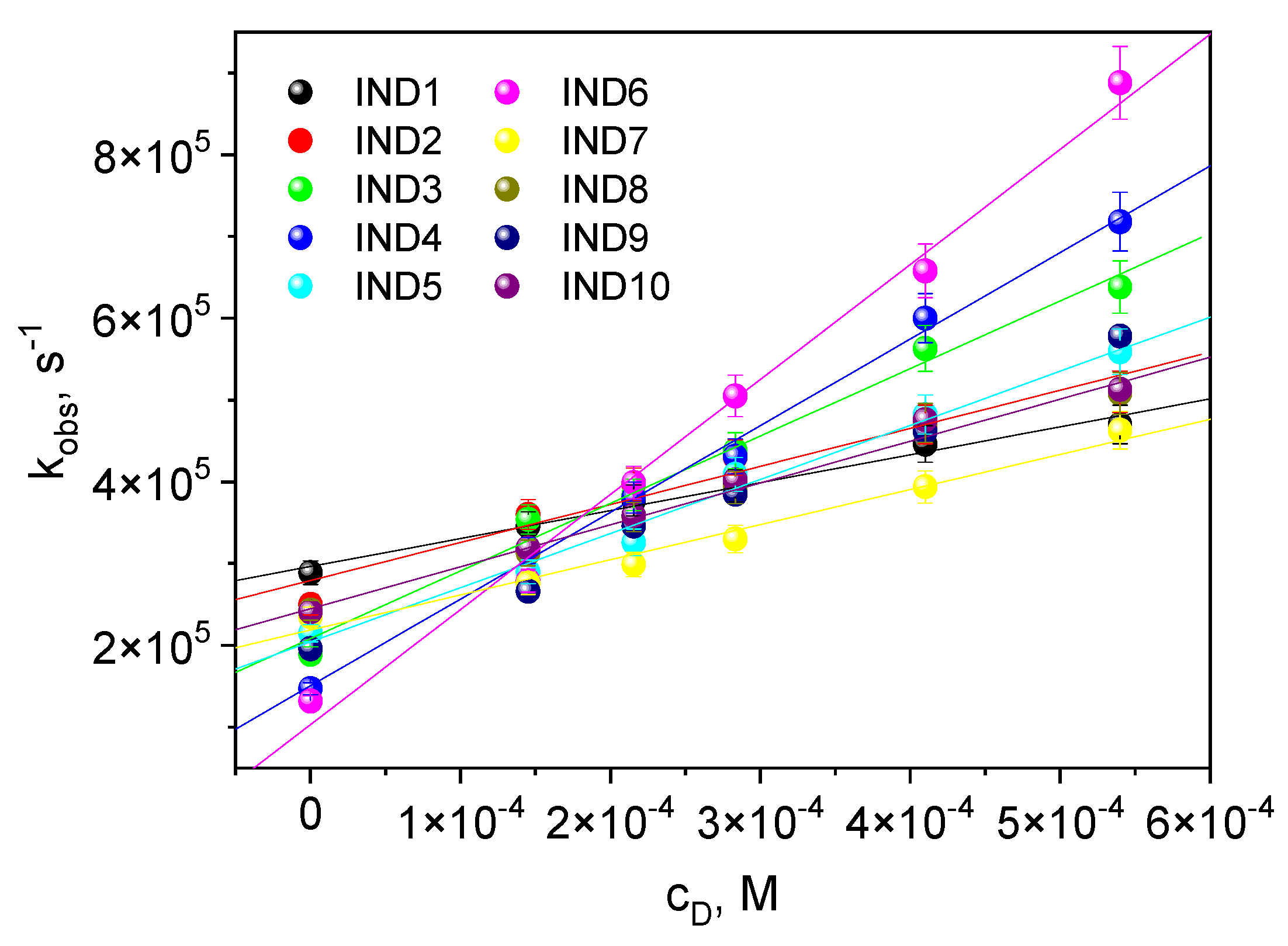



| Dye | , nm | εmax, M−1·cm−1 | Dye | , nm | εmax, M−1·cm−1 |
|---|---|---|---|---|---|
| IND1 | 287 381 | 34,600 3500 | IND6 | 299 398 | 37,400 4560 |
| IND2 | 275 382 | 33,100 3200 | IND7 | 277 374 | 32,500 4400 |
| IND3 | 279 383 | 34,200 3350 | IND8 | 294 403 | 34,200 3660 |
| IND4 | 293 384 | 35,100 3700 | IND9 | 305 398 | 36,200 3880 |
| IND5 | 294 384 | 35,500 3820 | IND10 | 301 395 | 35,900 3650 |
| Dye | Rp, μmol s−1 | ΦT | τT, μs | kq *, M−1s−1 | |
|---|---|---|---|---|---|
| PhTAA | EDMAB | ||||
| IND1 | 33.83 | 27.67 | 0.08 | 3.46 | 3.09 × 108 |
| IND2 | 38.28 | 37.54 | 0.1 | 4.00 | 3.45 × 108 |
| IND3 | 65.93 | 59.21 | 0.24 | 5.29 | 8.25 × 108 |
| IND4 | 75.38 | 68.32 | 0.31 | 6.68 | 1.05 × 109 |
| IND5 | 47.65 | 45.20 | 0.16 | 4.65 | 5.62 × 108 |
| IND6 | 82.31 | 80.91 | 0.48 | 7.57 | 1.40 × 109 |
| IND7 | 61.21 | 59.72 | 0.18 | 4.25 | 6.61 × 108 |
| IND8 | 47.75 | 46.11 | 0.11 | 4.10 | 5.12 × 108 |
| IND9 | 73.78 | 71.92 | 0.28 | 5.10 | 9.69 × 108 |
| IND10 | 52.13 | 50.89 | 0.13 | 4.15 | 5.35 × 108 |
| Dye | Storage Time, Days | |||||
|---|---|---|---|---|---|---|
| 7 | 28 | 7 | 28 | 7 | 28 | |
| Distilled Water | Artificial Saliva | 3% Acetic Acid | ||||
| IND1 | 13.42 ± 0.44 | 13.02 ± 0.21 | 13.65 ± 0.62 | 13.36 ± 0.56 | 13.35 ± 0.26 | 11.10 ± 0.96 |
| IND2 | 13.02 ± 0.021 | 11.42 ± 0.27 | 12.73 ± 0.26 | 12.72 ± 0.48 | 12.54 ± 0.31 | 9.83 ± 0.11 |
| IND3 | 10.20 ± 0.25 | 9.99 ± 0.31 | 10.28 ± 0.31 | 10.17 ± 0.73 | 10.33 ± 0.45 | 7.72 ± 0.49 |
| IND4 | 7.82 ± 0.32 | 6.09 ± 0.42 | 7.91 ± 0.98 | 7.85 ± 0.51 | 7.45 ± 0.24 | 3.07 ± 0.24 |
| IND5 | 14.87 ± 0.45 | 14.63 ± 0.27 | 14.99 ± 0.24 | 14.27 ± 0.32 | 14.79 ± 0.38 | 11.03 ± 0.91 |
| IND6 | 15.83 ± 0.39 | 15.00 ± 0.24 | 15.22 ± 0.19 | 15.05 ± 0.68 | 15.44 ± 0.25 | 11.25 ± 0.74 |
| IND7 | 11.33 ± 0.25 | 10.58 ± 0.38 | 11.25 ± 0.52 | 11.01 ± 0.46 | 11.13 ± 0.82 | 5.18 ± 0.31 |
| IND8 | 10.59 ± 0.49 | 9.95 ± 0.52 | 10.99 ± 0.57 | 10.41 ± 0.31 | 10.13 ± 0.27 | 7.17 ± 0.87 |
| IND9 | 12.88 ± 0.35 | 11.71 ± 0.17 | 12.89 ± 0.49 | 12.48 ± 0.49 | 12.75 ± 0.41 | 13.82 ± 0.75 |
| IND10 | 16.97 ± 0.28 | 16.05 ± 0.42 | 16.63 ± 0.56 | 15.99 ± 0.56 | 16.63 ± 0.38 | 16.48 ± 0.58 |
| CQ | 9.90 ± 0.45 | 8.48 ± 0.27 | 9.43 ± 0.78 | 8.82 ± 0.32 | 9.43 ± 0.28 | 6.72 ± 0.14 |
| n-heptane | coffee | |||||
| IND1 | 13.22 ± 0.40 | 13.20 ± 0.21 | 13.22 ± 0.32 | 5.11 ± 0.48 | ||
| IND2 | 12.53 ± 0.72 | 12.50 ± 0.32 | 12.53 ± 0.24 | 13.59 ± 0.21 | ||
| IND3 | 10.63 ± 0.57 | 10.58 ± 0.58 | 10.63 ± 0.58 | 11.26 ± 0.57 | ||
| IND4 | 7.83 ± 0.63 | 7.63 ± 0.31 | 7.83 ± 0.62 | 8.91 ± 0.77 | ||
| IND5 | 14.52 ± 0.28 | 14.48 ± 0.74 | 14.52 ± 0.34 | 15.92 ± 0.21 | ||
| IND6 | 15.02 ± 0.55 | 14.96 ± 0.82 | 15.02 ± 0.27 | 17.28 ± 0.66 | ||
| IND7 | 11.31 ± 0.79 | 11.02 ± 0.62 | 11.31 ± 0.44 | 12.59 ± 0.49 | ||
| IND8 | 10.59 ± 0.82 | 10.29 ± 0.31 | 10.59 ± 0.51 | 12.63 ± 0.45 | ||
| IND9 | 12.62 ± 0.91 | 12.55 ± 0.29 | 12.62 ± 0.72 | 14.98 ± 0.69 | ||
| IND10 | 16.03 ± 0.32 | 15.98 ± 0.11 | 16.03 ± 0.23 | 18.23 ± 0.45 | ||
| CQ | 9.37 ± 0.27 | 9.07 ± 0.25 | 9.37 ± 0.11 | 11.54 ± 0.82 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pyszka, I.; Szczepańska, O.; Jędrzejewska, B. The Latest Achievements in the Design of Permanent Fillings for Conservative Dentistry Based on Indenoquinoxaline Derivatives as Photoinitiators of Visible-Light Polymerization: Mass and Colour Stability. Int. J. Mol. Sci. 2025, 26, 5424. https://doi.org/10.3390/ijms26115424
Pyszka I, Szczepańska O, Jędrzejewska B. The Latest Achievements in the Design of Permanent Fillings for Conservative Dentistry Based on Indenoquinoxaline Derivatives as Photoinitiators of Visible-Light Polymerization: Mass and Colour Stability. International Journal of Molecular Sciences. 2025; 26(11):5424. https://doi.org/10.3390/ijms26115424
Chicago/Turabian StylePyszka, Ilona, Oliwia Szczepańska, and Beata Jędrzejewska. 2025. "The Latest Achievements in the Design of Permanent Fillings for Conservative Dentistry Based on Indenoquinoxaline Derivatives as Photoinitiators of Visible-Light Polymerization: Mass and Colour Stability" International Journal of Molecular Sciences 26, no. 11: 5424. https://doi.org/10.3390/ijms26115424
APA StylePyszka, I., Szczepańska, O., & Jędrzejewska, B. (2025). The Latest Achievements in the Design of Permanent Fillings for Conservative Dentistry Based on Indenoquinoxaline Derivatives as Photoinitiators of Visible-Light Polymerization: Mass and Colour Stability. International Journal of Molecular Sciences, 26(11), 5424. https://doi.org/10.3390/ijms26115424





