Brief Weekly Magnetic Field Exposure Enhances Avian Oxidative Muscle Character During Embryonic Development
Abstract
1. Introduction
2. Results
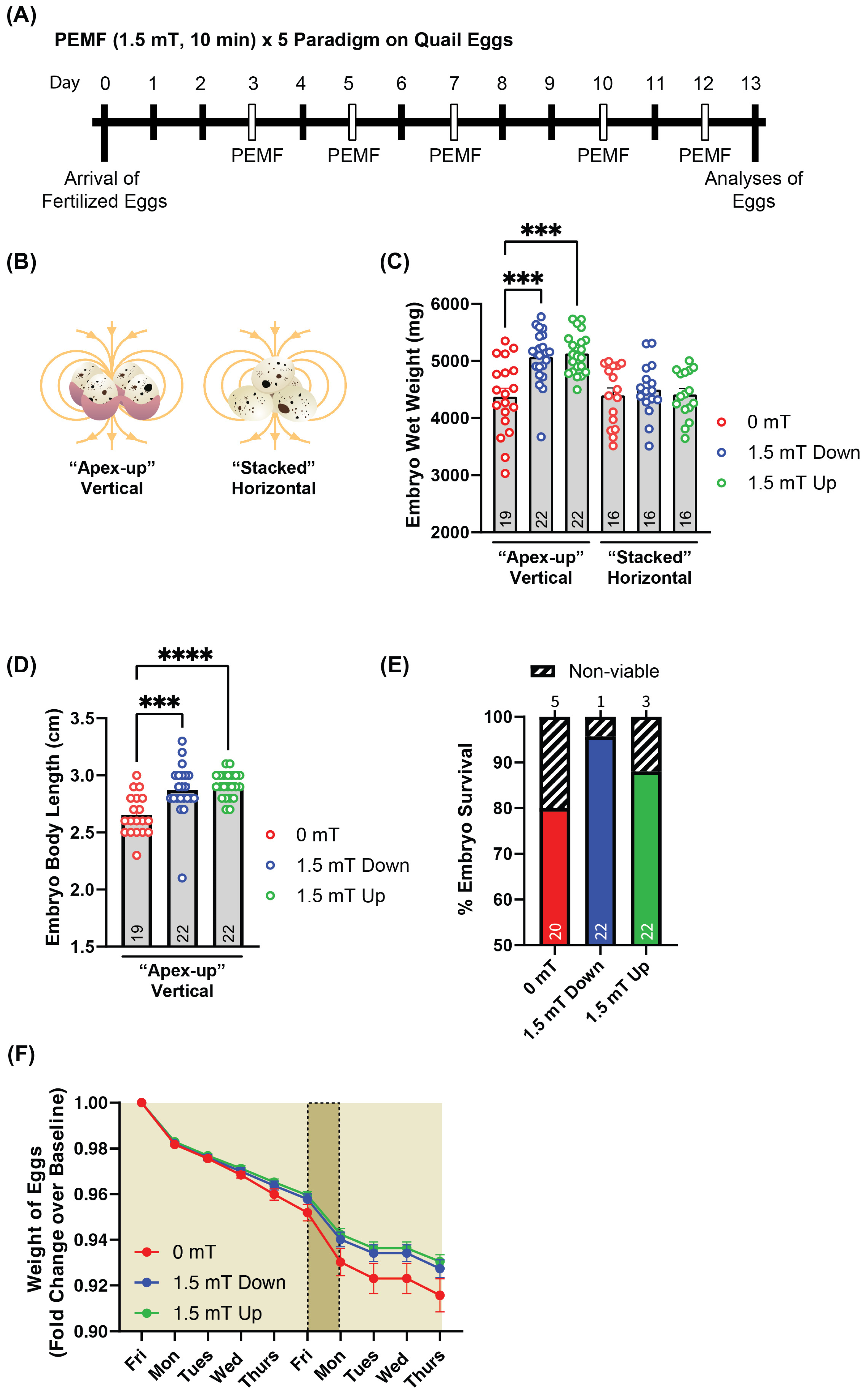
2.1. Upward-Directed PEMFs Yield the Greatest Response in Quail Embryo Development
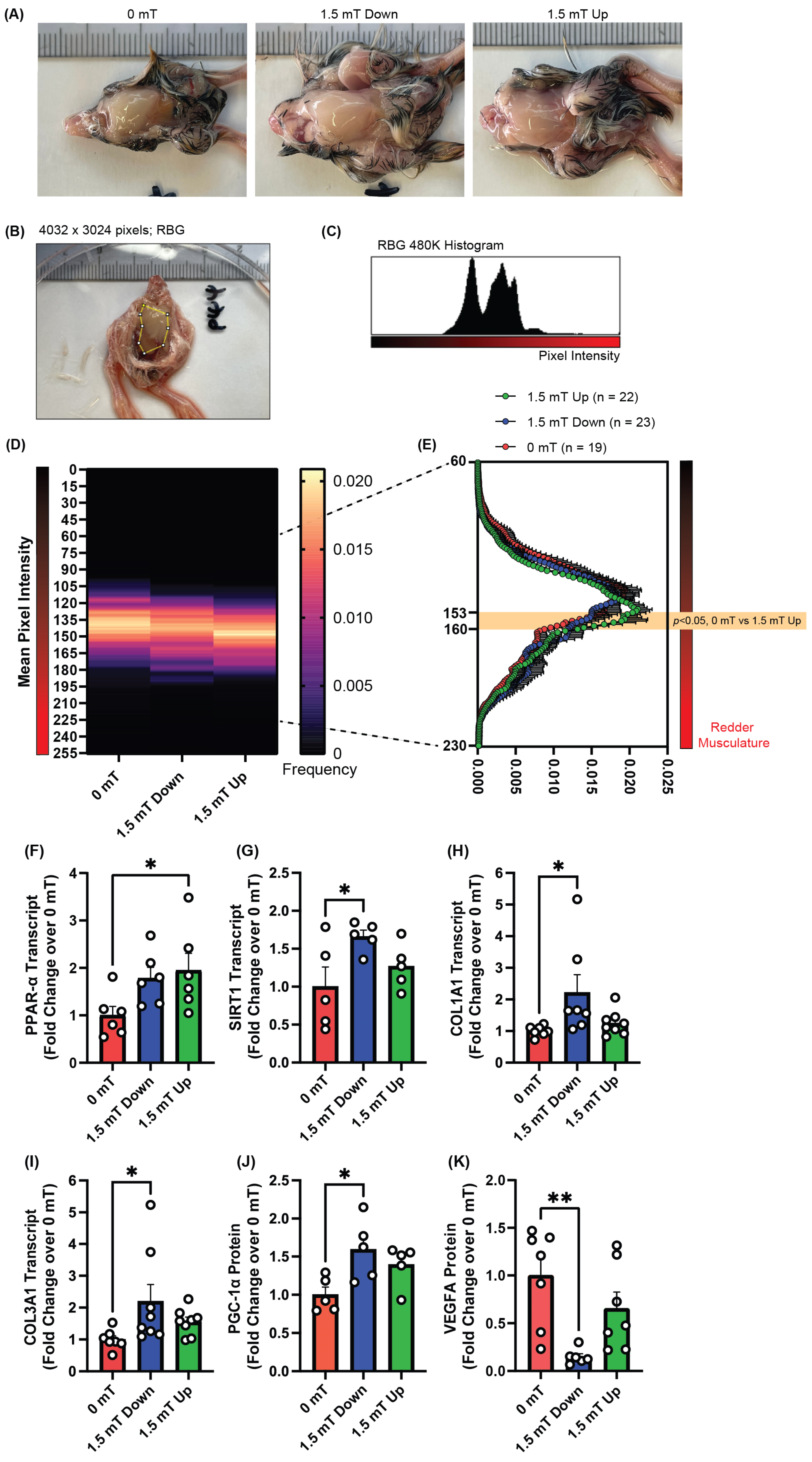
2.2. PEMF Exposure Promotes Oxidative Red Muscle
3. Discussion
4. Materials and Methods
4.1. Quail Eggs and the Incubation Conditions
4.2. Pulsed Electromagnetic Fields (PEMF) Exposure
4.3. Breast Tissue Musculature and Color Analysis
4.4. Quantitative RT-PCR
4.5. Western Blot
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PEMFs | Pulsed electromagnetic fields |
| PGC-1α | Peroxisome proliferator-activated receptor gamma coactivator 1 alpha |
| PPAR-α | Peroxisome proliferator-activated receptor alpha |
| AMPK | AMP-activated protein kinase |
| SIRT1 | Sirtuin 1 |
| HIF-1α | Hypoxia-inducible factor 1α |
| VEGFA | Vascular endothelial growth factor A |
| TRPC1 | Canonical transient receptor potential channel 1 |
References
- Karstoft, K.; Pedersen, B.K. Skeletal muscle as a gene regulatory endocrine organ. Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Severinsen, M.C.K.; Pedersen, B.K. Muscle-Organ Crosstalk: The Emerging Roles of Myokines. Endocr. Rev. 2020, 41, 594–609. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.C.Q.; Stead, C.A.; Burniston, J.G.; Phillips, S.M. Exercise-specific adaptations in human skeletal muscle: Molecular mechanisms of making muscles fit and mighty. Free Radic. Biol. Med. 2024, 223, 341–356. [Google Scholar] [CrossRef]
- Plotkin, D.L.; Roberts, M.D.; Haun, C.T.; Schoenfeld, B.J. Muscle Fiber Type Transitions with Exercise Training: Shifting Perspectives. Sports 2021, 9, 127. [Google Scholar] [CrossRef]
- Fulco, M.; Sartorelli, V. Comparing and contrasting the roles of AMPK and SIRT1 in metabolic tissues. Cell Cycle 2008, 7, 3669–3679. [Google Scholar] [CrossRef] [PubMed]
- Franco-Obregon, A.; Tai, Y.K.; Wu, K.Y.; Iversen, J.N.; Wong, C.J.K. The Developmental Implications of Muscle-Targeted Magnetic Mitohormesis: A Human Health and Longevity Perspective. Bioengineering 2023, 10, 956. [Google Scholar] [CrossRef]
- McCurdy, C.E.; Schenk, S.; Hetrick, B.; Houck, J.; Drew, B.G.; Kaye, S.; Lashbrook, M.; Bergman, B.C.; Takahashi, D.L.; Dean, T.A.; et al. Maternal obesity reduces oxidative capacity in fetal skeletal muscle of Japanese macaques. JCI Insight 2016, 1, e86612. [Google Scholar] [CrossRef]
- Lin, J.; Wu, H.; Tarr, P.T.; Zhang, C.Y.; Wu, Z.; Boss, O.; Michael, L.F.; Puigserver, P.; Isotani, E.; Olson, E.N.; et al. Transcriptional co-activator PGC-1 alpha drives the formation of slow-twitch muscle fibres. Nature 2002, 418, 797–801. [Google Scholar] [CrossRef]
- Svedenhag, J.; Henriksson, J.; Juhlin-Dannfelt, A. Beta-adrenergic blockade and training in human subjects: Effects on muscle metabolic capacity. Am. J. Physiol.-Endocrinol. Metab. 1984, 247, E305–E311. [Google Scholar] [CrossRef]
- Cheng, C.-F.; Ku, H.-C.; Lin, H. PGC-1α as a Pivotal Factor in Lipid and Metabolic Regulation. Int. J. Mol. Sci. 2018, 19, 3447. [Google Scholar] [CrossRef]
- Winder, W.W.; Thomson, D.M. Cellular energy sensing and signaling by AMP-activated protein kinase. Cell Biochem. Biophys. 2007, 47, 332–347. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Marcos, P.J.; Auwerx, J. Regulation of PGC-1α, a nodal regulator of mitochondrial biogenesis. Am. J. Clin. Nutr. 2011, 93, 884S–890S. [Google Scholar] [CrossRef] [PubMed]
- Park, D.R.; Kim, J.S.; Kim, C.K. The effect of SIRT1 protein knock down on PGC-1α acetylation during skeletal muscle contraction. J. Exerc. Nutr. Biochem. 2014, 18, 1–7. [Google Scholar] [CrossRef]
- Nalbandian, M.; Takeda, M. Lactate as a Signaling Molecule That Regulates Exercise-Induced Adaptations. Biology 2016, 5, 38. [Google Scholar] [CrossRef]
- Ohno, H.; Shirato, K.; Sakurai, T.; Ogasawara, J.; Sumitani, Y.; Sato, S.; Imaizumi, K.; Ishida, H.; Kizaki, T. Effect of exercise on HIF-1 and VEGF signaling. J. Phys. Fit. Sports Med. 2012, 1, 5–16. [Google Scholar] [CrossRef]
- Abe, T.; Kitaoka, Y.; Kikuchi, D.M.; Takeda, K.; Numata, O.; Takemasa, T. High-intensity interval training-induced metabolic adaptation coupled with an increase in Hif-1α and glycolytic protein expression. J. Appl. Physiol. 2015, 119, 1297–1302. [Google Scholar] [CrossRef] [PubMed]
- Yap, J.L.Y.; Tai, Y.K.; Fröhlich, J.; Fong, C.H.H.; Yin, J.N.; Foo, Z.L.; Ramanan, S.; Beyer, C.; Toh, S.J.; Casarosa, M.; et al. Ambient and supplemental magnetic fields promote myogenesis via a TRPC1-mitochondrial axis: Evidence of a magnetic mitohormetic mechanism. FASEB J. 2019, 33, 12853–12872. [Google Scholar] [CrossRef]
- Tai, Y.K.; Ng, C.; Purnamawati, K.; Yap, J.L.Y.; Yin, J.N.; Wong, C.; Patel, B.K.; Soong, P.L.; Pelczar, P.; Fröhlich, J.; et al. Magnetic fields modulate metabolism and gut microbiome in correlation with Pgc-1α expression: Follow-up to an in vitro magnetic mitohormetic study. FASEB J. 2020, 34, 11143–11167. [Google Scholar] [CrossRef]
- Wong, C.J.K.; Tai, Y.K.; Yap, J.L.Y.; Fong, C.H.H.; Loo, L.S.W.; Kukumberg, M.; Fröhlich, J.; Zhang, S.; Li, J.Z.; Wang, J.-W.; et al. Brief exposure to directionally-specific pulsed electromagnetic fields stimulates extracellular vesicle release and is antagonized by streptomycin: A potential regenerative medicine and food industry paradigm. Biomaterials 2022, 287, 121658. [Google Scholar] [CrossRef]
- Tai, Y.K.; Iversen, J.N.; Chan, K.K.W.; Fong, C.H.H.; Abdul Razar, R.B.; Ramanan, S.; Yap, L.Y.J.; Yin, J.N.; Toh, S.J.; Wong, C.J.K.; et al. Secretome from Magnetically Stimulated Muscle Exhibits Anticancer Potency: Novel Preconditioning Methodology Highlighting HTRA1 Action. Cells 2024, 13, 460. [Google Scholar] [CrossRef]
- Franco-Obregon, A. Harmonizing Magnetic Mitohormetic Regenerative Strategies: Developmental Implications of a Calcium-Mitochondrial Axis Invoked by Magnetic Field Exposure. Bioengineering 2023, 10, 1176. [Google Scholar] [CrossRef] [PubMed]
- Venugobal, S.; Tai, Y.K.; Goh, J.; Teh, S.; Wong, C.; Goh, I.; Maier, A.B.; Kennedy, B.K.; Franco-Obregon, A. Brief, weekly magnetic muscle therapy improves mobility and lean body mass in older adults: A Southeast Asia community case study. Aging 2023, 15, 1768–1790. [Google Scholar] [CrossRef] [PubMed]
- Morabito, C.; Rovetta, F.; Bizzarri, M.; Mazzoleni, G.; Fanò, G.; Mariggiò, M.A. Modulation of redox status and calcium handling by extremely low frequency electromagnetic fields in C2C12 muscle cells: A real-time, single-cell approach. Free Radic. Biol. Med. 2010, 48, 579–589. [Google Scholar] [CrossRef] [PubMed]
- Morabito, C.; Di Sinno, N.; Mariggiò, M.A.; Guarnieri, S. Impact of Extremely Low-Frequency Electromagnetic Fields on Skeletal Muscle of Sedentary Adult Mice: A Pilot Study. Int. J. Mol. Sci. 2024, 25, 9857. [Google Scholar] [CrossRef]
- Adler, D.; Shapira, Z.; Weiss, S.; Shainberg, A.; Katz, A. Weak Electromagnetic Fields Accelerate Fusion of Myoblasts. Int. J. Mol. Sci. 2021, 22, 4407. [Google Scholar] [CrossRef]
- Torretta, E.; Moriggi, M.; Capitanio, D.; Orfei, C.P.; Raffo, V.; Setti, S.; Cadossi, R.; de Girolamo, L.; Gelfi, C. Effects of Pulsed Electromagnetic Field Treatment on Skeletal Muscle Tissue Recovery in a Rat Model of Collagenase-Induced Tendinopathy: Results from a Proteome Analysis. Int. J. Mol. Sci. 2024, 25, 8852. [Google Scholar] [CrossRef]
- Maiullari, S.; Cicirelli, A.; Picerno, A.; Giannuzzi, F.; Gesualdo, L.; Notarnicola, A.; Sallustio, F.; Moretti, B. Pulsed Electromagnetic Fields Induce Skeletal Muscle Cell Repair by Sustaining the Expression of Proteins Involved in the Response to Cellular Damage and Oxidative Stress. Int. J. Mol. Sci. 2023, 24, 16631. [Google Scholar] [CrossRef]
- Muoio, D.M.; MacLean, P.S.; Lang, D.B.; Li, S.; Houmard, J.A.; Way, J.M.; Winegar, D.A.; Corton, J.C.; Dohm, G.L.; Kraus, W.E. Fatty acid homeostasis and induction of lipid regulatory genes in skeletal muscles of peroxisome proliferator-activated receptor (PPAR) alpha knock-out mice. Evidence for compensatory regulation by PPAR delta. J. Biol. Chem. 2002, 277, 26089–26097. [Google Scholar] [CrossRef]
- Russell, A.P.; Feilchenfeldt, J.; Schreiber, S.; Praz, M.; Crettenand, A.; Gobelet, C.; Meier, C.A.; Bell, D.R.; Kralli, A.; Giacobino, J.P.; et al. Endurance training in humans leads to fiber type-specific increases in levels of peroxisome proliferator-activated receptor-gamma coactivator-1 and peroxisome proliferator-activated receptor-alpha in skeletal muscle. Diabetes 2003, 52, 2874–2881. [Google Scholar] [CrossRef]
- Lim, J.H.; Lee, Y.M.; Chun, Y.S.; Chen, J.; Kim, J.E.; Park, J.W. Sirtuin 1 modulates cellular responses to hypoxia by deacetylating hypoxia-inducible factor 1alpha. Mol. Cell 2010, 38, 864–878. [Google Scholar] [CrossRef]
- Chen, C.; Zhou, M.; Ge, Y.; Wang, X. SIRT1 and aging related signaling pathways. Mech. Ageing Dev. 2020, 187, 111215. [Google Scholar] [CrossRef] [PubMed]
- Qaisar, R.; Bhaskaran, S.; Van Remmen, H. Muscle fiber type diversification during exercise and regeneration. Free Radic. Biol. Med. 2016, 98, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, M. Vascular Endothelial Growth Factor (VEGF) and Its Receptor (VEGFR) Signaling in Angiogenesis: A Crucial Target for Anti- and Pro-Angiogenic Therapies. Genes Cancer 2011, 2, 1097–1105. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, M.C.; Krishna, L.; Pannir Selvan, R.M.; Tai, Y.K.; Kit Wong, C.J.; Yin, J.N.; Toh, S.J.; Torta, F.; Triebl, A.; Fröhlich, J.; et al. Magnetic field therapy enhances muscle mitochondrial bioenergetics and attenuates systemic ceramide levels following ACL reconstruction: Southeast Asian randomized-controlled pilot trial. J. Orthop. Transl. 2022, 35, 99–112. [Google Scholar] [CrossRef]
- Lee, W.J.; Kim, M.; Park, H.-S.; Kim, H.S.; Jeon, M.J.; Oh, K.S.; Koh, E.H.; Won, J.C.; Kim, M.-S.; Oh, G.T.; et al. AMPK activation increases fatty acid oxidation in skeletal muscle by activating PPARα and PGC-1. Biochem. Biophys. Res. Commun. 2006, 340, 291–295. [Google Scholar] [CrossRef]
- Lira, V.A.; Benton, C.R.; Yan, Z.; Bonen, A. PGC-1α regulation by exercise training and its influences on muscle function and insulin sensitivity. Am. J. Physiol.-Endocrinol. Metab. 2010, 299, E145–E161. [Google Scholar] [CrossRef]
- Zanou, N.; Schakman, O.; Louis, P.; Ruegg, U.T.; Dietrich, A.; Birnbaumer, L.; Gailly, P. Trpc1 Ion Channel Modulates Phosphatidylinositol 3-Kinase/Akt Pathway during Myoblast Differentiation and Muscle Regeneration*. J. Biol. Chem. 2012, 287, 14524–14534. [Google Scholar] [CrossRef]
- Zanou, N.; Shapovalov, G.; Louis, M.; Tajeddine, N.; Gallo, C.; Van Schoor, M.; Anguish, I.; Cao, M.L.; Schakman, O.; Dietrich, A.; et al. Role of TRPC1 channel in skeletal muscle function. Am. J. Physiol.-Cell Physiol. 2010, 298, C149–C162. [Google Scholar] [CrossRef]
- Xia, L.; Cheung, K.-K.; Yeung, S.S.; Yeung, E.W. The involvement of transient receptor potential canonical type 1 in skeletal muscle regrowth after unloading-induced atrophy. J. Physiol. 2016, 594, 3111–3126. [Google Scholar] [CrossRef]
- Kiselyov, K.; Patterson, R.L. The integrative function of TRPC channels. Front. Biosci. 2009, 14, 45–58. [Google Scholar] [CrossRef]
- Fraysse, B.; Desaphy, J.F.; Pierno, S.; De Luca, A.; Liantonio, A.; Mitolo, C.I.; Camerino, D.C. Decrease in resting calcium and calcium entry associated with slow-to-fast transition in unloaded rat soleus muscle. FASEB J. 2003, 17, 1916–1918. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, J.T.; Lerin, C.; Haas, W.; Gygi, S.P.; Spiegelman, B.M.; Puigserver, P. Nutrient control of glucose homeostasis through a complex of PGC-1α and SIRT1. Nature 2005, 434, 113–118. [Google Scholar] [CrossRef]
- Arany, Z.; Foo, S.-Y.; Ma, Y.; Ruas, J.L.; Bommi-Reddy, A.; Girnun, G.; Cooper, M.; Laznik, D.; Chinsomboon, J.; Rangwala, S.M.; et al. HIF-independent regulation of VEGF and angiogenesis by the transcriptional coactivator PGC-1α. Nature 2008, 451, 1008–1012. [Google Scholar] [CrossRef]
- Rodriguez-Miguelez, P.; Lima-Cabello, E.; Martinez-Florez, S.; Almar, M.; Cuevas, M.J.; Gonzalez-Gallego, J. Hypoxia-inducible factor-1 modulates the expression of vascular endothelial growth factor and endothelial nitric oxide synthase induced by eccentric exercise. J. Appl. Physiol. 2015, 118, 1075–1083. [Google Scholar] [CrossRef]
- Richardson, R.S.; Wagner, H.; Mudaliar, S.R.; Saucedo, E.; Henry, R.; Wagner, P.D. Exercise adaptation attenuates VEGF gene expression in human skeletal muscle. Am. J. Physiol. Heart Circ. Physiol. 2000, 279, H772–H778. [Google Scholar] [CrossRef]
- Keane, A.J.; Sanz-Nogués, C.; Jayasooriya, D.; Creane, M.; Chen, X.; Lyons, C.J.; Sikri, I.; Goljanek-Whysall, K.; O’Brien, T. miR-1, miR-133a, miR-29b and skeletal muscle fibrosis in chronic limb-threatening ischaemia. Sci. Rep. 2024, 14, 29393. [Google Scholar] [CrossRef]
- Li, X.; Yang, X.; Shan, B.; Shi, J.; Xia, D.; Wegner, J.; Zhao, R. Meat quality is associated with muscle metabolic status but not contractile myofiber type composition in premature pigs. Meat Sci. 2009, 81, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Chen, J.; Jia, C.; Zhao, R. Gene expression of calpain 3 and PGC-1α is correlated with meat tenderness in the longissimus dorsi muscle of Sutai pigs. Livest. Sci. 2012, 147, 119–125. [Google Scholar] [CrossRef]
- Hou, Y.; Su, L.; Su, R.; Luo, Y.; Wang, B.; Yao, D.; Zhao, L.; Jin, Y. Effect of feeding regimen on meat quality, MyHC isoforms, AMPK, and PGC-1α genes expression in the biceps femoris muscle of Mongolia sheep. Food Sci. Nutr. 2020, 8, 2262–2270. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, B.; Yu, J.; Zheng, P.; Huang, Z.; Luo, Y.; Luo, J.; Mao, X.; Yan, H.; He, J.; et al. Butyrate promotes slow-twitch myofiber formation and mitochondrial biogenesis in finishing pigs via inducing specific microRNAs and PGC-1α expression1. J. Anim. Sci. 2019, 97, 3180–3192. [Google Scholar] [CrossRef]
- Yang, L.-Q.; Li, J.; Wang, C.; Wu, Q.-Y.; Chen, X.-Y.; Lai, S.-J.; Song, T.-Z.; Zhang, M. Expression patterns of PPARγ2, PGC-1α, and MEF2C and their association with intramuscular fat content and skeletal muscle tenderness of crossbred Simmental bulls. Can. J. Anim. Sci. 2019, 99, 367–376. [Google Scholar] [CrossRef]
- Iversen, J.N.; Tai, Y.K.; Wu, K.Y.; Wong, C.J.K.; Lim, H.Y.; Franco-Obregon, A. Magnetically Stimulated Myogenesis Recruits a CRY2-TRPC1 Photosensitive Signaling Axis. Cells 2025, 14, 231. [Google Scholar] [CrossRef] [PubMed]
- Usselman, R.J.; Chavarriaga, C.; Castello, P.R.; Procopio, M.; Ritz, T.; Dratz, E.A.; Singel, D.J.; Martino, C.F. The Quantum Biology of Reactive Oxygen Species Partitioning Impacts Cellular Bioenergetics. Sci. Rep. 2016, 6, 38543. [Google Scholar] [CrossRef]
- Polk, C. Physical mechanisms by which low-frequency magnetic fields can affect the distribution of counterions on cylindrical biological cell surfaces. J. Biol. Phys. 1986, 14, 3–8. [Google Scholar] [CrossRef]
| Antibody Name | Dilution Factor | Cat. No. | Manufacturer |
|---|---|---|---|
| PGC-1α | 1:1000 | 66369-1-lg | Proteintech (Rosemont, IL, USA) |
| VEGFA | 1:1000 | PA1-16948 | Thermo Fisher Scientific (Waltham, MA, USA) |
| GAPDH | 1:10,000 | 60004-1-lg | Proteintech (Rosemont, IL, USA) |
| α-Tubulin | 1:10,000 | 66031-1-lg | Proteintech (Rosemont, IL, USA) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yap, J.L.Y.; Wu, K.Y.; Tai, Y.K.; Fong, C.H.H.; Manazir, N.; Paul, A.P.; Yeo, O.; Franco-Obregón, A. Brief Weekly Magnetic Field Exposure Enhances Avian Oxidative Muscle Character During Embryonic Development. Int. J. Mol. Sci. 2025, 26, 5423. https://doi.org/10.3390/ijms26115423
Yap JLY, Wu KY, Tai YK, Fong CHH, Manazir N, Paul AP, Yeo O, Franco-Obregón A. Brief Weekly Magnetic Field Exposure Enhances Avian Oxidative Muscle Character During Embryonic Development. International Journal of Molecular Sciences. 2025; 26(11):5423. https://doi.org/10.3390/ijms26115423
Chicago/Turabian StyleYap, Jasmine Lye Yee, Kwan Yu Wu, Yee Kit Tai, Charlene Hui Hua Fong, Neha Manazir, Anisha Praiselin Paul, Olivia Yeo, and Alfredo Franco-Obregón. 2025. "Brief Weekly Magnetic Field Exposure Enhances Avian Oxidative Muscle Character During Embryonic Development" International Journal of Molecular Sciences 26, no. 11: 5423. https://doi.org/10.3390/ijms26115423
APA StyleYap, J. L. Y., Wu, K. Y., Tai, Y. K., Fong, C. H. H., Manazir, N., Paul, A. P., Yeo, O., & Franco-Obregón, A. (2025). Brief Weekly Magnetic Field Exposure Enhances Avian Oxidative Muscle Character During Embryonic Development. International Journal of Molecular Sciences, 26(11), 5423. https://doi.org/10.3390/ijms26115423







