Purification of Prudu6 from Almond and Its Cross-Reactivity with Glym6 from Soybean
Abstract
1. Introduction
2. Results
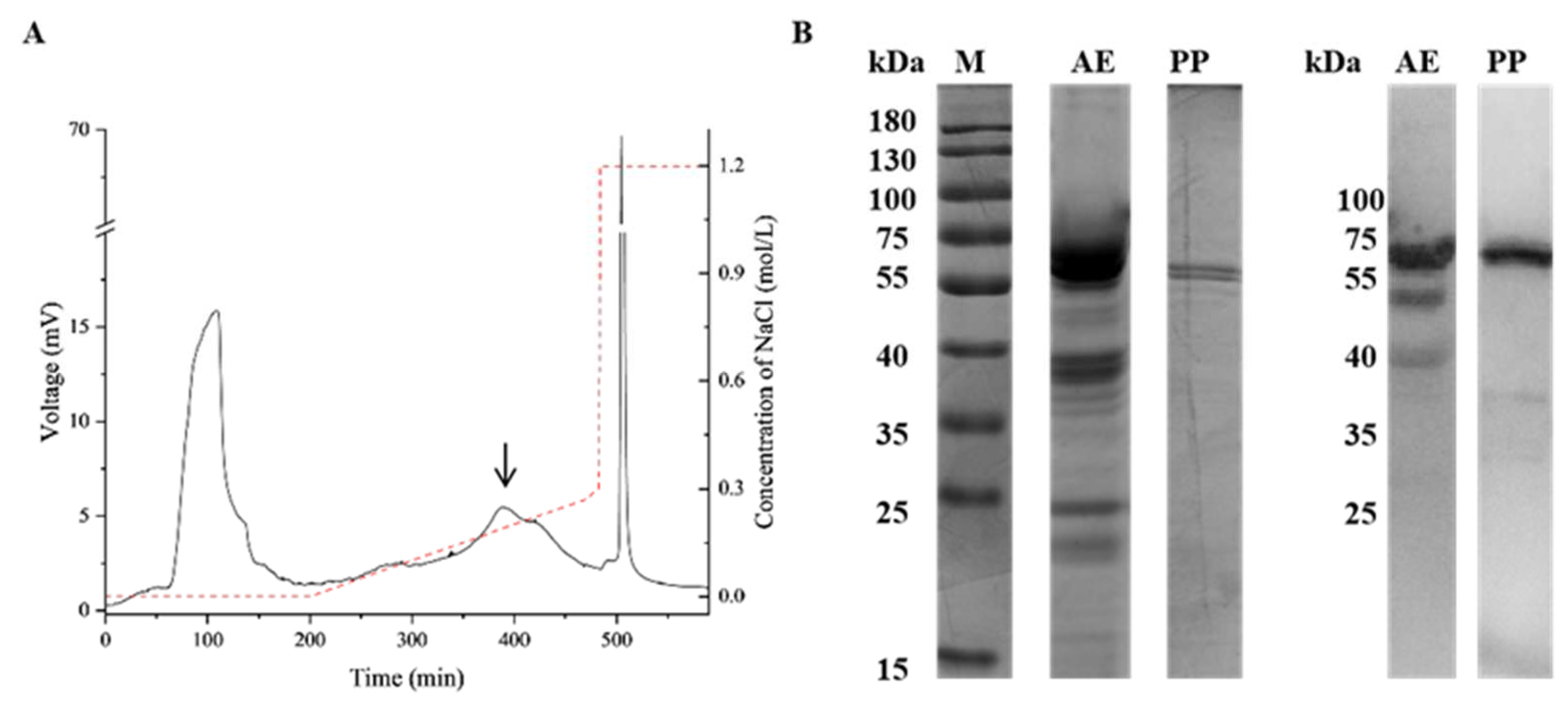
2.1. Purification and Identification of Prudu6
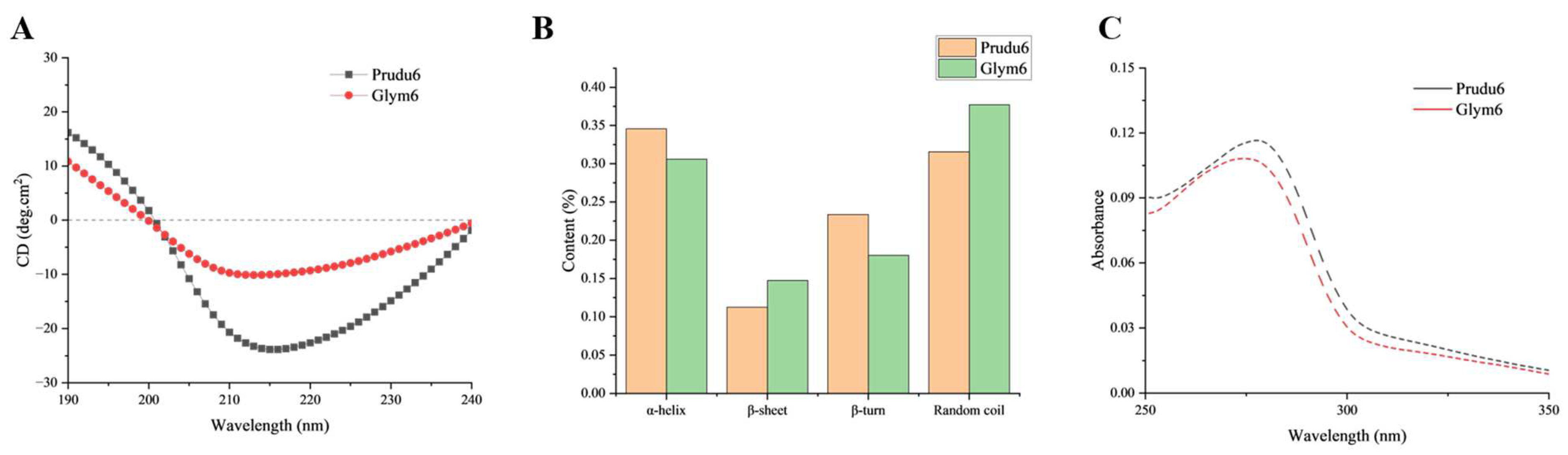
2.2. Characterization of Prudu6 and Glym6
2.3. Cross-Reactivity Between Prudu6 and Glym6
2.4. Epitope Analysis of Prudu6 and Glym6
3. Discussion
4. Materials and Methods
4.1. Human Sera
4.2. Almond and Soybean Protein Extracts
4.3. Purification of Prudu6 and Glym6
4.4. SDS-PAGE
4.5. IgE-Banding Capacity
4.6. Identification of Purified Protein
4.7. ELISA Inhibition Assays
4.8. Structure Characterization and Epitope Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| WB | Western blot |
| ELISA | enzyme-linked immunosorbent assay |
| PBS | phosphate-buffered saline |
| CD | circular dichroism |
| PVDF | polyvinylidene fluoride |
| BSA | bovine sera albumin |
| UV | ultraviolet |
| M | mol/L |
References
- Alfredo, B.; Paola, M.; Milena, M.; Cosimo, P.; Sonia, P. Metabolomics and antioxidant activity of the leaves of Prunus dulcis Mill. (Italian cvs. Toritto and Avola). J. Pharm. Biomed. Anal. 2018, 158, 54–65. [Google Scholar]
- Griel, A.E.; Kris-Etherton, P.M. Tree nuts and the lipid profile: A review of clinical studies. Br. J. Nutr. 2006, 96, S68–S78. [Google Scholar] [CrossRef] [PubMed]
- Mandalari, G.; Tomaino, A.; Arcoraci, T.; Martorana, M.; Wickham, M.S.J. Characterization of polyphenols, lipids and dietary fibre from almond skins (Amygdalus communis L.). J. Food Compos. Anal. 2010, 23, 166–174. [Google Scholar] [CrossRef]
- Jenkins, D.J.A.; Hu, F.B.; Tapsell, L.C.; Josse, A.R.; Kendall, C.W.C. Possible benefit of nuts in type 2 diabetes. J. Nutr. 2008, 138, 1752S–1756S. [Google Scholar] [CrossRef]
- Kozłowska, A.; Szostak-Węgierek, D. Flavonoids--food sources and health benefits. Rocz. Państwowego Zakładu Hig. 2014, 65, 79–85. [Google Scholar]
- Berryman, C.E.; Preston, A.G.; Karmally, W.; Deckelbaum, R.J.; Kris Etherton, P.M. Effects of almond consumption on the reduction of LDL-cholesterol: A discussion of potential mechanisms and future research directions. Nutr. Rev. 2011, 69, 171–185. [Google Scholar] [CrossRef]
- Tian, H.; Zhang, H.; Zhan, P.; Tian, F.W. Composition and antioxidant and antimicrobial activities of white apricot almond (Amygdalus communis L.) oil. Eur. J. Lipid Sci. Technol. 2011, 113, 1138–1144. [Google Scholar] [CrossRef]
- He, W.S.; Wang, Q.; Zhao, L.; Zhao, L.Y.; Li, J.; Li, J.J.; Wei, N.; Chen, G. Nutritional composition, health-promoting effects, bioavailability, and encapsulation of tree peony seed oil: A review. Food Funct. 2023, 14, 21. [Google Scholar] [CrossRef]
- Garcia-Perez, P.; Xiao, J.; Munekata, P.E.S.; Lorenzo, J.M.; Simal-Gandara, J. Revalorization of almond by-products for the design of novel functional foods: An updated review. Foods 2021, 10, 1823. [Google Scholar] [CrossRef]
- Geiselhart, S.; Hoffmann-Sommergruber, K.; Bublin, M. Tree nut allergens. Mol. Immunol. 2018, 100, 71–81. [Google Scholar] [CrossRef]
- Segura, L.T.R.; Pérez, E.F.; Wegrzyn, A.N.; Siepmann, T.; Linnemann, D.L. Food allergen sensitization patterns in a large allergic population in Mexico. Allergol. Immunopathol. 2020, 48, 553–559. [Google Scholar] [CrossRef]
- Rentzos, G.; Johanson, L.; Goksör, E.; Telemo, E.; Lundbäck, B.; Ekerljung, L. Prevalence of food hypersensitivity in relation to IgE sensitisation to common food allergens among the general adult population in West Sweden. Clin. Transl. Allergy 2019, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Tamar, W.; Scott, S. Current perspectives on tree nut allergy: A review. J. Asthma Allergy 2018, 11, 41–51. [Google Scholar]
- Cox, A.L.; Eigenmann, P.A.; Sicherer, S.H. Clinical Relevance of Cross-Reactivity in Food Allergy. J. Allergy Clin. Immunol. Pract. 2021, 9, 82–99. [Google Scholar] [CrossRef]
- Sinson, E.; Ocampo, C.; Liao, C.; Nguyen, S.; Dinh, L.; Rodems, K.; Whitters, E.; Hamilton, R.G. Cross-reactive carbohydrate determinant interference in cellulose-based IgE allergy tests utilizing recombinant allergen components. PLoS ONE 2020, 15, e0231344. [Google Scholar] [CrossRef]
- Egger, M.; Hauser, M.; Mari, A.; Ferreira, F.; Gadermaier, G. The role of lipid transfer proteins in allergic diseases. Curr. Allergy Asthma Rep. 2010, 10, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Sung-Ho, L.; Benmoussa, M.; Sathe, S.K.; Roux, K.H.; Teuber, S.S.; Hamaker, B.R. A 50 kDa Maize γ-Zein Has Marked Cross-Reactivity with the Almond Major Protein. J. Agric. Food Chem. 2005, 53, 7965–7970. [Google Scholar]
- De Leon, M.P.; Drew, A.C.; Glaspole, I.N.; Suphioglu, C.; O’Hehir, R.E.; Rolland, J.M. IgE cross-reactivity between the major peanut allergen Ara h 2 and tree nut allergens. Mol. Immunol. 2007, 44, 463–471. [Google Scholar] [CrossRef]
- Breiteneder, H.; Radauer, C. A classification of plant food allergens. J. Allergy Clin. Immunol. 2004, 113, 821–830. [Google Scholar] [CrossRef]
- Garcia-Mas, J.; Messeguer, R.; Arús, P.; Puigdomènech, P. Molecular characterization of cDNAs corresponding to genes expressed during almond (Prunus amygdalus Batsch) seed development. Plant Mol. Biol. 1995, 27, 205–210. [Google Scholar] [CrossRef]
- Jin, T.; Albillos, S.M.; Guo, F.; Howard, A.; Fu, T.J.; Kothary, M.H.; Zhang, Y.Z. Crystal structure of prunin-1, a major component of the almond (Prunus dulcis) allergen amandin. J. Agric. Food Chem. 2009, 57, 8643–8651. [Google Scholar] [CrossRef] [PubMed]
- Sathe, S.K.; Wolf, W.J.; Roux, K.H.; Teuber, S.S.; Sze-Tao, K.W.C. Biochemical Characterization of Amandin, the Major Storage Protein in Almond (Prunus dulcis L.). J. Agric. Food Chem. 2002, 50, 4333–4341. [Google Scholar] [CrossRef]
- Shewry, P.R.; Jenkins, J.A.; Beaudoin, F.; Mills, E.N.C. The Classification, Functions and Evolutionary Relationships of Plant Proteins in Relation to Food Allergies. In Plant Food Allergens 2007, 2, 24–41. [Google Scholar]
- Ewan, P.W. Clinical study of peanut and nut allergy in 62 consecutive patients: New features and associations. BMJ 1996, 312, 1074–1078. [Google Scholar] [CrossRef]
- Sicherer, S.H.; Sampson, H.A.; Munoz, F.A. Prevalence of peanut and tree nut allergy in the United States determined by means of a random digit dial telephone survey: A 5-year follow-up study. J. Allergy Clin. Immunol. 2003, 112, 1203–1207. [Google Scholar] [CrossRef]
- Costa, J.; Mafra, I.; Carrapatoso, I.; Oliveira, M.B.P. Almond Allergens: Molecular Characterization, Detection, and Clinical Relevance. J. Agric. Food Chem. 2012, 60, 1337–1349. [Google Scholar] [CrossRef]
- Roux, K.H.; Teuber, S.S.; Robotham, J.M.; Sathe, S.K. Detection and stability of the major almond allergen in foods. J. Agric. Food Chem. 2001, 49, 2131. [Google Scholar] [CrossRef] [PubMed]
- Venkatachalam, M.; Teuber, S.S.; Roux, K.H.; Sathe, S.K. Effects of roasting, blanching, autoclaving, and microwave heating on antigenicity of almond (Prunus dulcis L.) proteins. J. Agric. Food Chem. 2002, 50, 3544–3548. [Google Scholar] [CrossRef]
- Boateng, I.D.; Yang, X.M. Do non-thermal pretreatments followed by intermediate-wave infrared drying affect toxicity, allergenicity, bioactives, functional groups, and flavor components of Ginkgo biloba seed? A case study. Ind. Crops Prod. 2021, 165, 113421. [Google Scholar] [CrossRef]
- Holzhauser, T.; Wackermann, O.; Ballmer-Weber, B.K.; Bindslev-Jensen, C.; Scibilia, J.; Perono-Garoffo, L.; Utsumi, S.; Poulsen, L.K.; Vieths, S. Soybean (Glycine max) allergy in Europe: Gly m 5 (β-conglycinin) and Gly m 6 (glycinin) are potential diagnostic markers for severe allergic reactions to soy. J. Allergy Clin. Immunol. 2009, 123, 452–458. [Google Scholar] [CrossRef]
- Ebisawa, M.; Brostedt, P.; Sjölander, S.; Sato, S.; Borres, M.P.; Ito, K. Gly m 2S albumin is a major allergen with a high diagnostic value in soybean-allergic children. J. Allergy Clin. Immunol. 2013, 132, 976–978. [Google Scholar] [CrossRef] [PubMed]
- Mills, E.N.C.; Madsen, C.; Shewry, P.R.; Wichers, H.J. Food allergens of plant origin—Their molecular and evolutionary relationships. Trends Food Sci. Technol. 2003, 14, 145–156. [Google Scholar] [CrossRef]
- Xia, X.; Tang, P.; Bai, J.; Zhou, Y.L.; Shen, T.T.; Wu, Z.H.; Li, X.; Tong, P.; Chen, H.B.; Yang, A.S. Anti-food allergic activity of soymilk fermented by Lactobacillus in vitro. J. Food Sci. Technol. 2024, 61, 1–12. [Google Scholar] [CrossRef]
- Hu, F.; Ye, Z.; Dong, K.; Zhang, W.M.; Fang, D.; Cao, J. Divergent structures and functions of the Cupin proteins in plants. Int. J. Biol. Macromol. 2023, 242, 124791. [Google Scholar] [CrossRef] [PubMed]
- Westernberg, L.; Schulten, V.; Greenbaum, J.A.; Mann, M. T-cell epitope conservation across allergen species is a major determinant of immunogenicity. J. Allergy Clin. Immunol. 2016, 138, 571–578. [Google Scholar] [CrossRef]
- Bublin, M.; Breiteneder, H. Cross-reactivities of non-homologous allergens. Allergy 2020, 75, 1019–1022. [Google Scholar] [CrossRef]
- Bueno-Díaz, C.; Martín-Pedraza, L.; Parrón, J. Characterization of Relevant Biomarkers for the Diagnosis of Food Allergies: An Overview of the 2S Albumin Family. Foods 2021, 10, 1235. [Google Scholar] [CrossRef]
- Dreskin, S.C.; Koppelman, S.J.; Andorf, S.; Nadeau, K.C.; Kalra, A.; Braun, W.; Negi, S.S.; Chen, X.; Schein, C.H. The importance of the 2S albumins for allergenicity and cross-reactivity of peanuts, tree nuts, and sesame seeds. J. Allergy Clin. Immunol. 2021, 147, 1154–1163. [Google Scholar] [CrossRef]
- Carugo, O. How root-mean-square distance (r.m.s.d.) values depend on the resolution of protein structures that are compared. J. Appl. Crystallogr. 2003, 36, 125–128. [Google Scholar] [CrossRef]
- Sawsan, K.; Bakker, F.T.; Dunwell, J.M. Phylogeny, Function, and Evolution of the Cupins, a Structurally Conserved, Functionally Diverse Superfamily of Proteins. Mol. Biol. Evol. 2001, 4, 593–605. [Google Scholar]
- Peng, C.; Tang, X.; Shu, Y.; He, M.C.; Xia, X.D.; Zhang, Y.; Cao, C.M.; Li, Y.; Feng, S.B.; Wang, X.C. Effects of 7S and 11S on the intestine of weaned piglets after injection and oral administration of soybean antigen protein. Anim. Sci. J. 2019, 90, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Sirison, J.; Ishii, T.; Matsumiya, K.; Samoto, M.; Matsumura, Y. Comparison of surface and foaming properties of soy lipophilic protein with those of glycinin and β-conglycinin. Food Hydrocoll. 2021, 112, 106345. [Google Scholar] [CrossRef]
- Willison, L.A.N.; Tripathi, P.; Sharma, G.; Teuber, S.S.; Sathe, S.K.; Roux, K.H. Cloning, Expression and Patient IgE Reactivity of Recombinant Prudu6, an 11S Globulin from Almond. Int. Arch. Allergy Immunol. 2011, 156, 267–281. [Google Scholar] [CrossRef]
- Coutsias, E.A.; Wester, M.J. RMSD and Symmetry. Journal of computational chemistry. 2019, 40, 1496–1508. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.N.; Lee, K.E.; Hong, J.Y.; Kim, K.W.; Kim, K.E.; Sohn, M.H.; Park, J.W. IgE cross-reactivity of peanut with walnut and soybean in children with food allergy. Allergol. Immunopathol. 2016, 44, 524–530. [Google Scholar] [CrossRef]
- Saeed, H.; Gagnon, C.; Cober, E.; Gleddie, S. Using patient serum to epitope map soybean glycinins reveals common epitopes shared with many legumes and tree nuts. Mol. Immunol. 2016, 70, 125–133. [Google Scholar] [CrossRef]
- Thanh, V.H.; Shibasaki, K. Major proteins of soybean seeds. A straightforward fractionation and their characterization. J. Agric. Food Chem. 1976, 24, 1117. [Google Scholar] [CrossRef]
- Wisniewski, J.R.; Zougman, A.; Nagaraj, N.; Mann, M. Universal sample preparation method for proteome analysis. Nat. Methods 2009, 6, 359–362. [Google Scholar] [CrossRef]



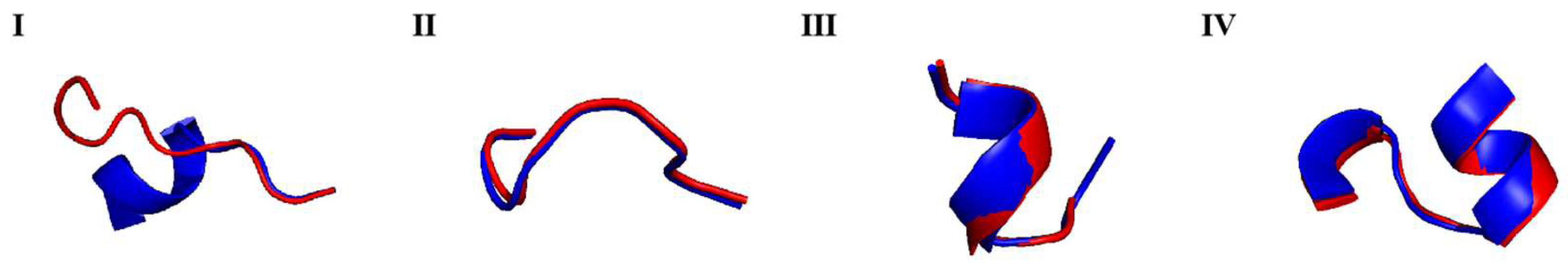
| Prudu6 | Glym6 | Sequence Similarity | RMSD | |
|---|---|---|---|---|
| I | 113EESQQSSQQG122 | 112EEPQQPQQRG121 | 40% | 3.740 |
| II | 232HNQLDQNP239 | 172ENQLDQMP179 | 75% | 0.087 |
| III | 288GNNVFSGF295 | 217GGSILSGF224 | 62.5% | 0.218 |
| IV | 516VLANAYQISREQ527 | 459VIQHTFNLKSQQ470 | 58.33% | 0.199 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, C.; Luo, Q.; Zhou, L.; Zhu, W.; Gao, K.; Geng, Q.; Li, X.; Yang, A.; Tong, P.; Wu, Z.; et al. Purification of Prudu6 from Almond and Its Cross-Reactivity with Glym6 from Soybean. Int. J. Mol. Sci. 2025, 26, 5425. https://doi.org/10.3390/ijms26115425
Hu C, Luo Q, Zhou L, Zhu W, Gao K, Geng Q, Li X, Yang A, Tong P, Wu Z, et al. Purification of Prudu6 from Almond and Its Cross-Reactivity with Glym6 from Soybean. International Journal of Molecular Sciences. 2025; 26(11):5425. https://doi.org/10.3390/ijms26115425
Chicago/Turabian StyleHu, Changbao, Qishu Luo, Lihua Zhou, Weichao Zhu, Kuan Gao, Qin Geng, Xin Li, Anshu Yang, Ping Tong, Zhihua Wu, and et al. 2025. "Purification of Prudu6 from Almond and Its Cross-Reactivity with Glym6 from Soybean" International Journal of Molecular Sciences 26, no. 11: 5425. https://doi.org/10.3390/ijms26115425
APA StyleHu, C., Luo, Q., Zhou, L., Zhu, W., Gao, K., Geng, Q., Li, X., Yang, A., Tong, P., Wu, Z., & Chen, H. (2025). Purification of Prudu6 from Almond and Its Cross-Reactivity with Glym6 from Soybean. International Journal of Molecular Sciences, 26(11), 5425. https://doi.org/10.3390/ijms26115425






