Microbiome Engineering for Biotherapeutic in Alzheimer’s Disease Through the Gut–Brain Axis: Potentials and Limitations
Abstract
1. Introduction
2. Alzheimer’s Diseases, Gut–Brain Axis, and Microbiome
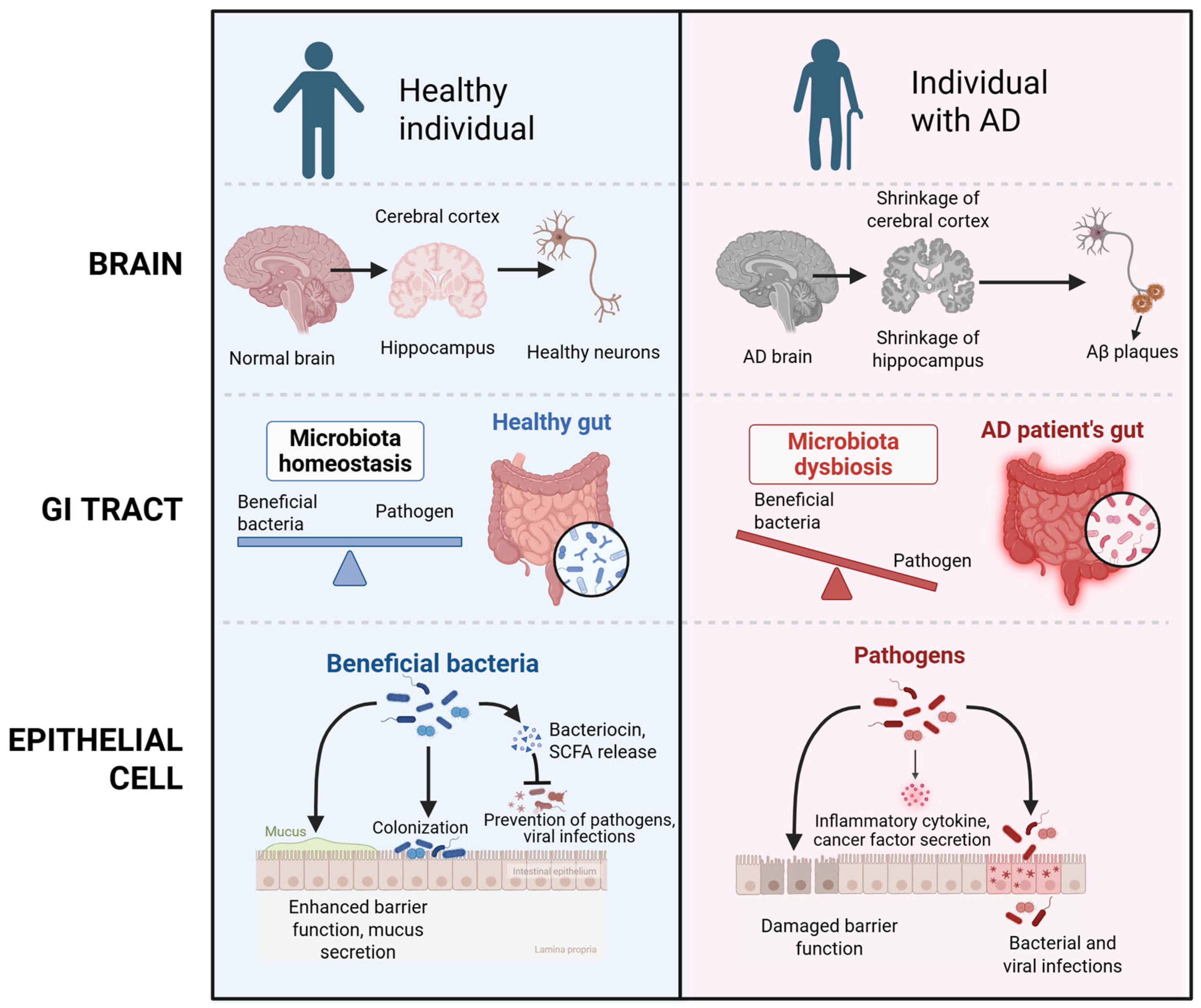
2.1. Pathophysiology of Alzheimer’s Diseases
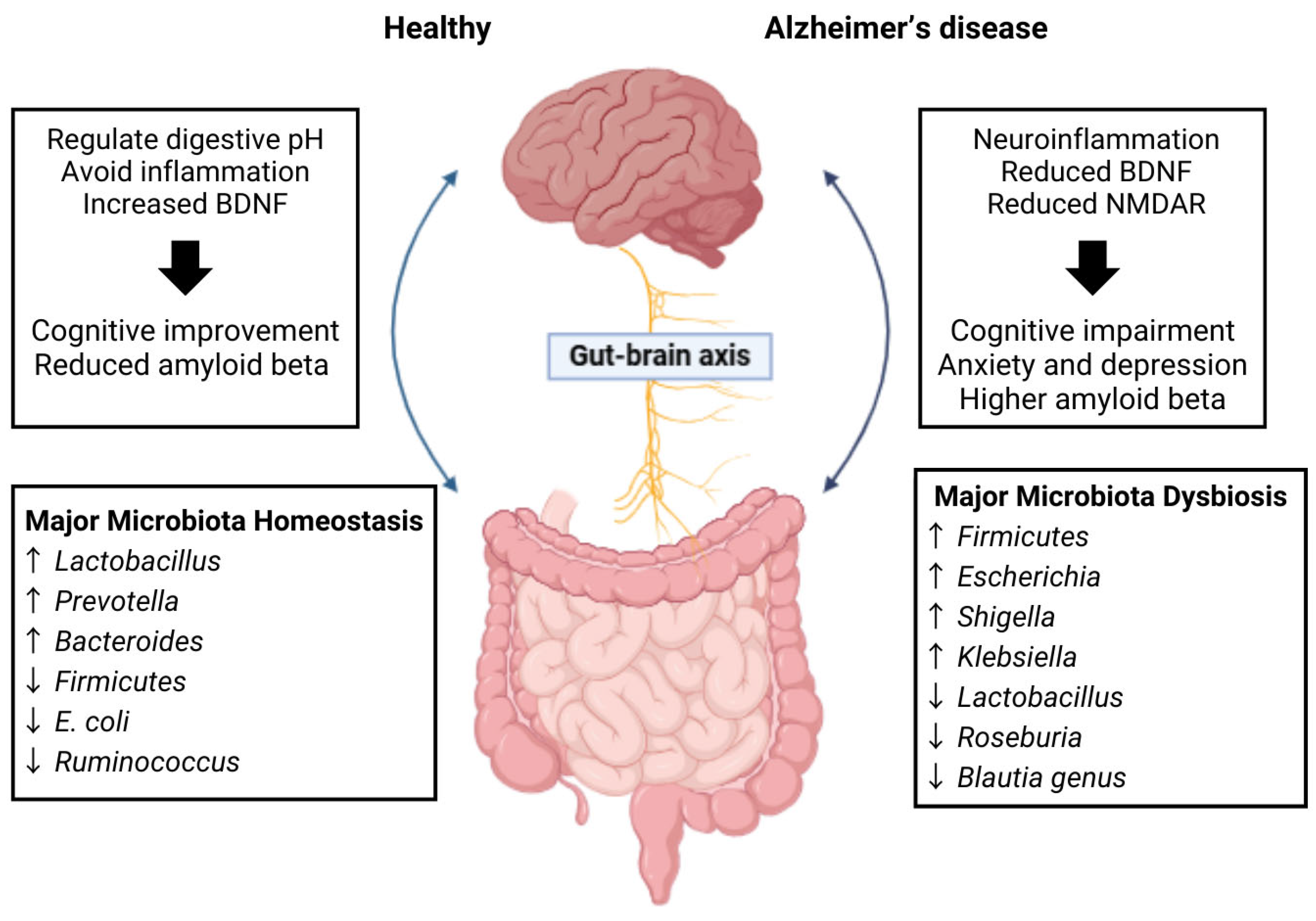
2.2. Gut–Brain Axis
2.3. Microbiome Alterations in Alzheimer’s Disease
3. Current Treatment of Alzheimer’s Disease and Its Limitations
3.1. Current Treatment of Alzheimer’s Disease
3.2. Limitations of Current Treatment
4. Mechanisms of AD via the Gut–Brain Axis
4.1. Gut Microbiota Imbalance (Dysbiosis)
4.2. Inflammation Modulation and the Immune System Activation
4.3. Impact on Metabolites Production
4.3.1. Short-Chain Fatty Acids (SCFAs)
4.3.2. Tryptophan
4.4. Impact on Neurotransmitters
4.4.1. Serotonin
4.4.2. Gamma-Aminobutyric Acid (GABA)
4.4.3. Dopamine
4.4.4. Acetylcholine
4.5. Direct Vagus Nerve Communication
4.6. Neuroprotection Enhancement
4.7. Gut-Derived Antioxidants and Mitochondrial Protection
5. Biotherapeutic Strategies for Alzheimer’s Disease
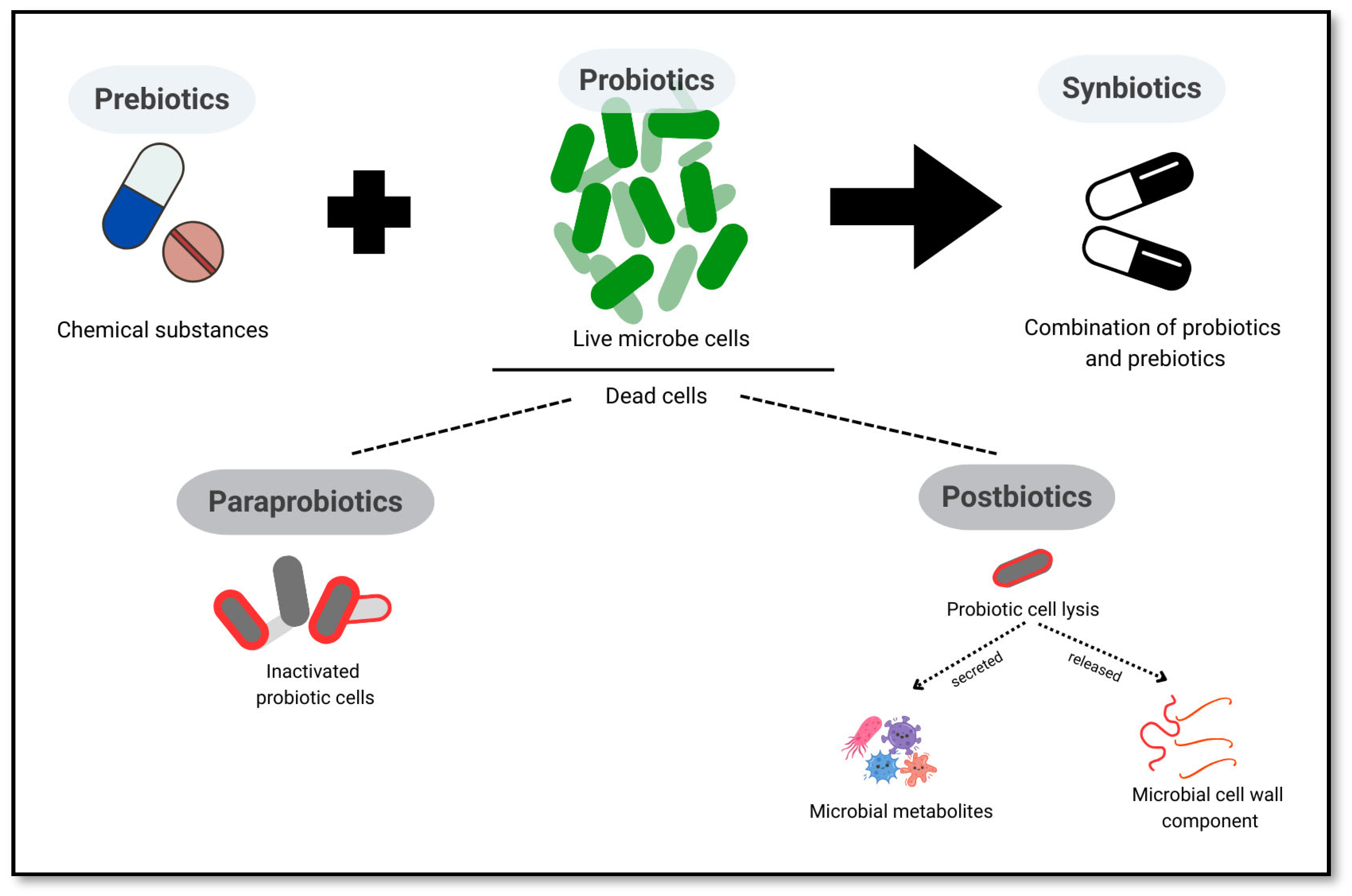
5.1. Probiotics, Prebiotics, Synbiotics, Postbiotics, and Paraprobiotics
5.2. Evidence from Animal Studies
5.2.1. Probiotics
5.2.2. Prebiotics
5.2.3. Synbiotics
5.2.4. Postbiotics
| Agents | Model Investigated | Treatments | Administration | Main Observations | Reference |
|---|---|---|---|---|---|
| Probiotics | Two-month-old C57BL/6J mice | B. breve MCC1274 1 × 109 CFU/6.25 mg/200 μL saline/mouse/day | Oral gavage for four months |
| [98] |
| Probiotics | 2–3 months male and female C57BL/6J and AppNL-G-F mice | VSL#3® 4 × 109 CFU/day/25 g mice (L. plantarum, L. delbrueckii subsp. Bulgaricus, L. paracasei, L. acidophilus, B. breve, B. longum, B. infantis, and S. salivarius subsp. Thermophilus) | Drinking water for 8 weeks. |
| [107] |
| Probiotics | 6-month-old senescence-accelerated-mouse-prone 8 (SAMP8) and senescence-accelerated-mouse-resistant 1 (SAMR1) | Probiotic-2 (P2, a probiotic mixture of Bifidobacterium lactis and Lactobacillus rhamnosus) and probiotic-3 (P3, a probiotic mixture of Bifidobacterium lactis, Lactobacillus acidophilus, and Lactobacillus rhamnosus) 1 × 109 CFU/mouse/day | Drinking water for 8 weeks |
| [99] |
| Probiotics | Male 9-month-old SAMP8 and SAMR1 mice | ProBiotic-4, a probiotic preparation composed of B. lactis (50%), L. casei (25%), B. bifidum (12.5%), and L. acidophilus (12.5%) | Drinking water for 12 weeks |
| [100] |
| Probiotics | 3xTg-AD mice | Lab4P probiotic consortium, composed of Lactobacillus acidophilus CUL21 (NCIMB 30156), Lactobacillus acidophilus CUL60 (NCIMB 30157), Lactobacillus plantarum CUL66 (NCIMB 30280), Bifidobacterium bifidum CUL20 (NCIMB 30153) and Bifidobacterium animalis subsp. lactis CUL34 (NCIMB 30172) delivering a daily dose of ~5 × 108 CFU/mouse/day (human equivalent dose of ~5 × 1010 CFU/day) | Lyophilized preparation (mixed with feed) for 12 weeks and 24 weeks |
| [126] |
| Probiotics | Male and female TH-CRE rats expressing pseudophosphorylated tau | ProBiotic-4, a probiotic preparation composed of B. lactis (50%), L. casei (25%), B. bifidum (12.5%), and L. acidophilus (12.5%) 3 × 109 CFU/mouse/day | Drinking water for 3 months |
| [127] |
| Prebiotic | 6–8 months old 5×FAD male mice | Pseudostellaria heterophylla polysaccharide (PH-PS) 100 mg/kg/day | Oral gavage for 33 days |
| [78] |
| Prebiotics | Male 5×FAD-transgenic mice | MOS (0.12% w/v in the drinking water, with a purity ~85%; Yuansen Biological Technology Ltd., Xi’an, China) or SCFAs mixture (acetate 67.5 mM, propionate 40 mM, butyrate 25 mM; Yuanye Biological Technology Ltd., Shanghai, China) | Drinking water added MOS or SCFAs for 8 weeks. |
| [110] |
| Prebiotic | 4-month-old APOE4 mice | Prebiotic inulin diet contained 8% fiber from inulin. | Fed prebiotic inulin or control diet at four months for 16 weeks |
| [104] |
| Synbiotic | Six-month-old male APPSWE/PS1ΔE9 (APP/PS1) double-transgenic mice | Nicotinamide mononucleotide (NMN) synbiotics, a combination of NMN, Lactiplantibacillus plantarum CGMCC 1.16089, and lactulose (NMN: 300 mg/kg/day, tz-3647; L. plantarum: 108 CFU/mL; lactulose: 200 mg/kg/day) | Daily gavage supplementation for three months |
| [115] |
| Synbiotic | 7-Month-old male 5 × FAD mice | Clostridium sporogenes ((ATCC15579, 1010 CFU/day via gavage)) and xylan (1% w/w) | Drinking water for 30 days |
| [119] |
| Synbiotic | Male APPswe/PS1ΔE9 double transgenic mice, aged two months | NMN (300 mg/kg/day), Lactobacillus plantarum (108 CFU/mL), and lactulose (200 mg/kg/day) | Gavage for three months |
| [114] |
| Synbiotic | 8-week old 3xTg-AD mice | Red lentils and coated with a probiotic carrier (dark chocolate) containing SLAB51 (Streptococcus thermophilus DSM 32245, Bifidobacterium lactis DSM 32246, Bifidobacterium lactis DSM 32247, Lactobacillus acidophilus DSM 32241, Lactobacillus helveticus DSM 32242, Lactobacillus paracasei DSM 32243, Lactobacillus plantarum DSM 32244, Lactobacillus brevis DSM 27961) 2 × 1011 bacteria/kg/day of SLAB51. | Drinking water for 4 months. |
| [128] |
| Postbiotic | Male APP/PS1 transgenic mice (B6C3-Tg (APPswe, PSEN1dE9)85Dbo/Mmjax; APP/PS1TG) | FRAMELIM® contains tyndallized Bifidobacterium longum and Lactobacillus acidophiluslysates, in addition vitamins A, B1, B3, B6, B9, B12 and omega 3 fatty acids in cod liver oil. | FRAMELIM® was administered five times weekly (120 mg/day) for 20 weeks with rodent chow (SDS/VRF1(P)). |
| [122] |
5.3. Evidence from Human Studies
| Subjects | Treatments | Doses | Study Design | N | Duration | Effects Compared to Placebo | Reference |
|---|---|---|---|---|---|---|---|
| Healthy elders ≥ 65 years | Bifidobacterium bifidum BGN4 and Bifidobacterium longum BORI | 1 × 109 CFU/day | Randomized, double-blind, placebo-controlled, multicenter clinical trial | 63 | 12 weeks |
| [138] |
| Patients with mild cognitive impairment (MCI) aged from 65 to 88 years old | Bifidobacterium breve MCC1274 (A1) | 2 × 1010 CFU/day | Randomized, double-blind, placebo-controlled trial | 130 | 24 weeks |
| [139] |
| AD patients aged between 50 and 90 years | Bifidobacterium longum subsp. infantis BLI-02, B. breve Bv-889, B. animalis subsp. lactis CP-9, B. bifidum VDD088, and Lactobacillus plantarum PL-02 | 5 × 107 CFU/capsule | Randomized, double-blind active-controlled trial | 40 | 12 weeks |
| [140] |
| Healthy older adults without cognitive impairment | Bifidobacterium longum BB68S (BB68S) | 5 × 1010 CFU/sachet | Randomized, double-blind, placebo-controlled trial | 60 | 8 weeks |
| [131] |
| AD patients aged 50–90 years | Lacticaseibacillus rhamnosus HA-114 or Bifidobacterium longum R0175 | 7.5 × 109 twice daily | Randomized, double-blind, placebo-controlled trial | 90 | 12 weeks |
| [141] |
| Aged between 55 and 80 years | Streptococcus thermophilus GH, Streptococcus salivarius GH NEXARS, Lactobacilus plantarum GH, and Pediococcus pentosaceus GH | 106 CFU/day | Randomized, double-blind, placebo-controlled trial with a cross-over design | 91 | 12 weeks |
| [142] |
| Older adults aged 50–79 years | Bifidobacterium breve A1 | 2 × 1010 CFU/day | Double-blind, randomized placebo-controlled trial | 80 | 16 weeks |
| [143] |
6. Safety and Efficacy
7. Current Limitations and Future Directions
7.1. Current Limitations
7.2. Future Implication
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Knopman, D.S.; Amieva, H.; Petersen, R.C.; Chételat, G.; Holtzman, D.M.; Hyman, B.T.; Nixon, R.A.; Jones, D.T. Alzheimer disease. Nat. Rev. Dis. Primer 2021, 7, 33. [Google Scholar] [CrossRef] [PubMed]
- Reitz, C.; Brayne, C.; Mayeux, R. Epidemiology of Alzheimer disease. Nat. Rev. Neurol. 2011, 7, 137–152. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.N.; Lu, L.; Chew, L.Y.; Mu, Y. Alzheimer’s Disease—A Panorama Glimpse. Int. J. Mol. Sci. 2014, 15, 12631–12650. [Google Scholar] [CrossRef] [PubMed]
- Atri, A. Current and Future Treatments in Alzheimer’s Disease. Semin. Neurol. 2019, 39, 227–240. [Google Scholar] [CrossRef]
- Ghezzi, L.; Cantoni, C.; Rotondo, E.; Galimberti, D. The Gut Microbiome-Brain Crosstalk in Neurodegenerative Diseases. Biomedicines 2022, 10, 1486. [Google Scholar] [CrossRef]
- Zheng, Y.; Bonfili, L.; Wei, T.; Eleuteri, A.M. Understanding the Gut-Brain Axis and Its Therapeutic Implications for Neurodegenerative Disorders. Nutrients 2023, 15, 4631. [Google Scholar] [CrossRef]
- Krishaa, L.; Ng, T.K.S.; Wee, H.N.; Ching, J. Gut-brain axis through the lens of gut microbiota and their relationships with Alzheimer’s disease pathology: Review and recommendations. Mech. Ageing Dev. 2023, 211, 111787. [Google Scholar] [CrossRef]
- Cerdó, T.; Ruíz, A.; Suárez, A.; Campoy, C. Probiotic, Prebiotic, and Brain Development. Nutrients 2017, 9, 1247. [Google Scholar] [CrossRef]
- Swanson, K.S.; Gibson, G.R.; Hutkins, R.; Reimer, R.A.; Reid, G.; Verbeke, K.; Scott, K.P.; Holscher, H.D.; Azad, M.B.; Delzenne, N.M.; et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 687–701. [Google Scholar] [CrossRef]
- Żółkiewicz, J.; Marzec, A.; Ruszczyński, M.; Feleszko, W. Postbiotics—A Step Beyond Pre- and Probiotics. Nutrients 2020, 12, 2189. [Google Scholar] [CrossRef]
- Surolia, R.; Tyagi, M.; Singh, A. A Holistic Approach: Exploring Pre, Pro, Syn, Post and Paraprobiotics in Sustainable Diets. In Sustainable Food Systems (Volume I): SFS: Framework, Sustainable Diets, Traditional Food Culture & Food Production; Thakur, M., Ed.; Springer Nature: Cham, Switzerland, 2024; pp. 177–190. [Google Scholar] [CrossRef]
- Manassi, C.F.; De Souza, S.S.; Hassemer, G.D.S.; Sartor, S.; Lima, C.M.G.; Miotto, M.; De Dea Lindner, J.; Rezzadori, K.; Pimentel, T.C.; Ramos, G.L.D.P.A.; et al. Functional meat products: Trends in pro-, pre-, syn-, para- and post-biotic use. Food Res. Int. 2022, 154, 111035. [Google Scholar] [CrossRef] [PubMed]
- Haaksma, M.L.; Vilela, L.R.; Marengoni, A.; Calderón-Larrañaga, A.; Leoutsakos, J.-M.S.; Olde Rikkert, M.G.M.; Melis, R.J.F. Comorbidity and progression of late onset Alzheimer’s disease: A systematic review. PLoS ONE 2017, 12, e0177044. [Google Scholar] [CrossRef]
- Honjo, Y.; Kawasaki, I.; Nagai, K.; Harada, S.; Ogawa, N. Families of patients with Alzheimer’s disease dementia notice progression from symptoms of disorientation and visual memory disturbance. Psychogeriatr. Off. J. Jpn. Psychogeriatr. Soc. 2023, 23, 747–751. [Google Scholar] [CrossRef] [PubMed]
- Giri, M.; Zhang, M.; Lü, Y. Genes associated with Alzheimer’s disease: An overview and current status. Clin. Interv. Aging 2016, 11, 665–681. [Google Scholar] [CrossRef]
- Megur, A.; Baltriukienė, D.; Bukelskienė, V.; Burokas, A. The Microbiota–Gut–Brain Axis and Alzheimer’s Disease: Neuroinflammation Is to Blame? Nutrients 2020, 13, 37. [Google Scholar] [CrossRef]
- Tang, Y.; Zhang, D.; Gong, X.; Zheng, J. A mechanistic survey of Alzheimer’s disease. Biophys. Chem. 2022, 281, 106735. [Google Scholar] [CrossRef]
- von Bernhardi, R.; Eugenín, J. Alzheimer’s disease: Redox dysregulation as a common denominator for diverse pathogenic mechanisms. Antioxid. Redox Signal. 2012, 16, 974–1031. [Google Scholar] [CrossRef]
- Tönnies, E.; Trushina, E. Oxidative Stress, Synaptic Dysfunction, and Alzheimer’s Disease. J. Alzheimers Dis. 2017, 57, 1105–1121. [Google Scholar] [CrossRef]
- Sharma, C.; Kim, S.; Nam, Y.; Jung, U.J.; Kim, S.R. Mitochondrial Dysfunction as a Driver of Cognitive Impairment in Alzheimer’s Disease. Int. J. Mol. Sci. 2021, 22, 4850. [Google Scholar] [CrossRef]
- Wang, W.; Zhao, F.; Ma, X.; Perry, G.; Zhu, X. Mitochondria dysfunction in the pathogenesis of Alzheimer’s disease: Recent advances. Mol. Neurodegener. 2020, 15, 30. [Google Scholar] [CrossRef]
- Desler, C.; Lillenes, M.S.; Tønjum, T.; Rasmussen, L.J. The Role of Mitochondrial Dysfunction in the Progression of Alzheimer’s Disease. Curr. Med. Chem. 2018, 25, 5578–5587. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Lu, L.; Pember, E.; Li, X.; Zhang, B.; Zhu, Z. New Insights into Neuroinflammation Involved in Pathogenic Mechanism of Alzheimer’s Disease and Its Potential for Therapeutic Intervention. Cells 2022, 11, 1925. [Google Scholar] [CrossRef] [PubMed]
- Al-Ghraiybah, N.F.; Wang, J.; Alkhalifa, A.E.; Roberts, A.B.; Raj, R.; Yang, E.; Kaddoumi, A. Glial Cell-Mediated Neuroinflammation in Alzheimer’s Disease. Int. J. Mol. Sci. 2022, 23, 10572. [Google Scholar] [CrossRef]
- Andronie-Cioara, F.L.; Ardelean, A.I.; Nistor-Cseppento, C.D.; Jurcau, A.; Jurcau, M.C.; Pascalau, N.; Marcu, F. Molecular Mechanisms of Neuroinflammation in Aging and Alzheimer’s Disease Progression. Int. J. Mol. Sci. 2023, 24, 1869. [Google Scholar] [CrossRef]
- Doifode, T.; Giridharan, V.V.; Generoso, J.S.; Bhatti, G.; Collodel, A.; Schulz, P.E.; Forlenza, O.V.; Barichello, T. The impact of the microbiota-gut-brain axis on Alzheimer’s disease pathophysiology. Pharmacol. Res. 2021, 164, 105314. [Google Scholar] [CrossRef]
- Bauer, K.C.; Huus, K.E.; Finlay, B.B. Microbes and the mind: Emerging hallmarks of the gut microbiota-brain axis. Cell. Microbiol. 2016, 18, 632–644. [Google Scholar] [CrossRef]
- Nandwana, V.; Nandwana, N.K.; Das, Y.; Saito, M.; Panda, T.; Das, S.; Almaguel, F.; Hosmane, N.S.; Das, B.C. The Role of Microbiome in Brain Development and Neurodegenerative Diseases. Molecules 2022, 27, 3402. [Google Scholar] [CrossRef]
- Sherwin, E.; Dinan, T.G.; Cryan, J.F. Recent developments in understanding the role of the gut microbiota in brain health and disease. Ann. N. Y. Acad. Sci. 2018, 1420, 5–25. [Google Scholar] [CrossRef]
- de la Fuente-Nunez, C.; Meneguetti, B.T.; Franco, O.L.; Lu, T.K. Neuromicrobiology: How Microbes Influence the Brain. ACS Chem. Neurosci. 2018, 9, 141–150. [Google Scholar] [CrossRef]
- Zhuang, Z.-Q.; Shen, L.-L.; Li, W.-W.; Fu, X.; Zeng, F.; Gui, L.; Lü, Y.; Cai, M.; Zhu, C.; Tan, Y.-L.; et al. Gut Microbiota is Altered in Patients with Alzheimer’s Disease. J. Alzheimers Dis. JAD 2018, 63, 1337–1346. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Y.; Xiayu, X.; Shi, C.; Chen, W.; Song, N.; Fu, X.; Zhou, R.; Xu, Y.-F.; Huang, L.; et al. Altered Gut Microbiota in a Mouse Model of Alzheimer’s Disease. J. Alzheimers Dis. JAD 2017, 60, 1241–1257. [Google Scholar] [CrossRef]
- Wang, X.; Sun, G.; Feng, T.; Zhang, J.; Huang, X.; Wang, T.; Xie, Z.; Chu, X.; Yang, J.; Wang, H.; et al. Sodium oligomannate therapeutically remodels gut microbiota and suppresses gut bacterial amino acids-shaped neuroinflammation to inhibit Alzheimer’s disease progression. Cell Res. 2019, 29, 787–803. [Google Scholar] [CrossRef]
- Köhler, C.A.; Maes, M.; Slyepchenko, A.; Berk, M.; Solmi, M.; Lanctôt, K.L.; Carvalho, A.F. The Gut-Brain Axis, Including the Microbiome, Leaky Gut and Bacterial Translocation: Mechanisms and Pathophysiological Role in Alzheimer’s Disease. Curr. Pharm. Des. 2016, 22, 6152–6166. [Google Scholar] [CrossRef]
- Yiannopoulou, K.G.; Papageorgiou, S.G. Current and future treatments for Alzheimer’s disease. Ther. Adv. Neurol. Disord. 2013, 6, 19–33. [Google Scholar] [CrossRef]
- Pardo-Moreno, T.; González-Acedo, A.; Rivas-Domínguez, A.; García-Morales, V.; García-Cozar, F.J.; Ramos-Rodríguez, J.J.; Melguizo-Rodríguez, L. Therapeutic Approach to Alzheimer’s Disease: Current Treatments and New Perspectives. Pharmaceutics 2022, 14, 1117. [Google Scholar] [CrossRef]
- Dyer, O. Donanemab: FDA experts recommend approval of Alzheimer’s drug. BMJ 2024, 385, q1327. [Google Scholar] [CrossRef]
- Vaz, M.; Silvestre, S. Alzheimer’s disease: Recent treatment strategies. Eur. J. Pharmacol. 2020, 887, 173554. [Google Scholar] [CrossRef]
- Passeri, E.; Elkhoury, K.; Morsink, M.; Broersen, K.; Linder, M.; Tamayol, A.; Malaplate, C.; Yen, F.T.; Arab-Tehrany, E. Alzheimer’s Disease: Treatment Strategies and Their Limitations. Int. J. Mol. Sci. 2022, 23, 13954. [Google Scholar] [CrossRef]
- Pathan, A. Limitations of Alzheimer’s Disease Medications. NeuroPharmac J. 2023, 8, 11–17. [Google Scholar] [CrossRef]
- Miculas, D.C.; Negru, P.A.; Bungau, S.G.; Behl, T.; Hassan, S.S.U.; Tit, D.M. Pharmacotherapy Evolution in Alzheimer’s Disease: Current Framework and Relevant Directions. Cells 2022, 12, 131. [Google Scholar] [CrossRef]
- Giovannini, M.G.; Lana, D.; Traini, C.; Vannucchi, M.G. The Microbiota–Gut–Brain Axis and Alzheimer Disease. From Dysbiosis to Neurodegeneration: Focus on the Central Nervous System Glial Cells. J. Clin. Med. 2021, 10, 2358. [Google Scholar] [CrossRef]
- Shukla, P.K.; Delotterie, D.F.; Xiao, J.; Pierre, J.F.; Rao, R.; McDonald, M.P.; Khan, M.M. Alterations in the Gut-Microbial-Inflammasome-Brain Axis in a Mouse Model of Alzheimer’s Disease. Cells 2021, 10, 779. [Google Scholar] [CrossRef]
- Lin, C.; Zhao, S.; Zhu, Y.; Fan, Z.; Wang, J.; Zhang, B.; Chen, Y. Microbiota-gut-brain axis and toll-like receptors in Alzheimer’s disease. Comput. Struct. Biotechnol. J. 2019, 17, 1309–1317. [Google Scholar] [CrossRef]
- Xie, J.; Van Hoecke, L.; Vandenbroucke, R.E. The Impact of Systemic Inflammation on Alzheimer’s Disease Pathology. Front. Immunol. 2022, 12, 796867. [Google Scholar] [CrossRef]
- Leblhuber, F.; Ehrlich, D.; Steiner, K.; Geisler, S.; Fuchs, D.; Lanser, L.; Kurz, K. The Immunopathogenesis of Alzheimer’s Disease Is Related to the Composition of Gut Microbiota. Nutrients 2021, 13, 361. [Google Scholar] [CrossRef]
- Princiotta Cariddi, L.; Mauri, M.; Cosentino, M.; Versino, M.; Marino, F. Alzheimer’s Disease: From Immune Homeostasis to Neuroinflammatory Condition. Int. J. Mol. Sci. 2022, 23, 13008. [Google Scholar] [CrossRef]
- Griciuc, A.; Tanzi, R.E. The role of innate immune genes in Alzheimer’s disease. Curr. Opin. Neurol. 2021, 34, 228. [Google Scholar] [CrossRef]
- Dionisio-Santos, D.A.; Olschowka, J.A.; O’Banion, M.K. Exploiting microglial and peripheral immune cell crosstalk to treat Alzheimer’s disease. J. Neuroinflamm. 2019, 16, 74. [Google Scholar] [CrossRef]
- Chunchai, T.; Thunapong, W.; Yasom, S.; Wanchai, K.; Eaimworawuthikul, S.; Metzler, G.; Lungkaphin, A.; Pongchaidecha, A.; Sirilun, S.; Chaiyasut, C.; et al. Decreased microglial activation through gut-brain axis by prebiotics, probiotics, or synbiotics effectively restored cognitive function in obese-insulin resistant rats. J. Neuroinflamm. 2018, 15, 11. [Google Scholar] [CrossRef]
- Pasinetti, G. Synbiotic-Derived Metabolites Reduce Neuroinflammatory Symptoms of Alzheimer’s Disease. Curr. Dev. Nutr. 2022, 6, 804. [Google Scholar] [CrossRef]
- Liu, X.; Cao, S.; Zhang, X. Modulation of Gut Microbiota-Brain Axis by Probiotics, Prebiotics, and Diet. J. Agric. Food Chem. 2015, 63, 7885–7895. [Google Scholar] [CrossRef]
- Ho, L.; Ono, K.; Tsuji, M.; Mazzola, P.; Singh, R.; Pasinetti, G.M. Protective roles of intestinal microbiota derived short chain fatty acids in Alzheimer’s disease-type beta-amyloid neuropathological mechanisms. Expert Rev. Neurother. 2018, 18, 83–90. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, H.; Zhang, X.; Wang, W.; Chen, Y.; Cai, Z.; Wang, Q.; Wang, J.; Shi, Y. Promotion of astrocyte-neuron glutamate-glutamine shuttle by SCFA contributes to the alleviation of Alzheimer’s disease. Redox Biol. 2023, 62, 102690. [Google Scholar] [CrossRef]
- Gao, L.; Zhang, Y.; Sterling, K.; Song, W. Brain-derived neurotrophic factor in Alzheimer’s disease and its pharmaceutical potential. Transl. Neurodegener. 2022, 11, 4. [Google Scholar] [CrossRef]
- Hestad, K.; Alexander, J.; Rootwelt, H.; Aaseth, J.O. The Role of Tryptophan Dysmetabolism and Quinolinic Acid in Depressive and Neurodegenerative Diseases. Biomolecules 2022, 12, 998. [Google Scholar] [CrossRef]
- Fathi, M.; Vakili, K.; Yaghoobpoor, S.; Tavasol, A.; Jazi, K.; Hajibeygi, R.; Shool, S.; Sodeifian, F.; Klegeris, A.; McElhinney, A.; et al. Dynamic changes in metabolites of the kynurenine pathway in Alzheimer’s disease, Parkinson’s disease, and Huntington’s disease: A systematic Review and meta-analysis. Front. Immunol. 2022, 13, 997240. [Google Scholar] [CrossRef]
- Willette, A.A.; Pappas, C.; Hoth, N.; Wang, Q.; Klinedinst, B.; Willette, S.A.; Larsen, B.; Pollpeter, A.; Li, T.; Le, S.; et al. Inflammation, negative affect, and amyloid burden in Alzheimer’s disease: Insights from the kynurenine pathway. Brain. Behav. Immun. 2021, 95, 216–225. [Google Scholar] [CrossRef]
- Stolero, N.; Frenkel, D. The dialog between neurons and microglia in Alzheimer’s disease: The neurotransmitters view. J. Neurochem. 2021, 158, 1412–1424. [Google Scholar] [CrossRef]
- Jorfi, M.; Maaser-Hecker, A.; Tanzi, R.E. The neuroimmune axis of Alzheimer’s disease. Genome Med. 2023, 15, 6. [Google Scholar] [CrossRef]
- Hodo, T.W.; de Aquino, M.T.P.; Shimamoto, A.; Shanker, A. Critical Neurotransmitters in the Neuroimmune Network. Front. Immunol. 2020, 11, 1869. [Google Scholar] [CrossRef]
- Layunta, E.; Buey, B.; Mesonero, J.E.; Latorre, E. Crosstalk Between Intestinal Serotonergic System and Pattern Recognition Receptors on the Microbiota–Gut–Brain Axis. Front. Endocrinol. 2021, 12, 748254. [Google Scholar] [CrossRef] [PubMed]
- Aaldijk, E.; Vermeiren, Y. The role of serotonin within the microbiota-gut-brain axis in the development of Alzheimer’s disease: A narrative review. Ageing Res. Rev. 2022, 75, 101556. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Han, D.; Hao, Y.; Song, Z.; Sun, Z.; Dai, Z. Linking serotonin homeostasis to gut function: Nutrition, gut microbiota and beyond. Crit. Rev. Food Sci. Nutr. 2024, 64, 7291–7310. [Google Scholar] [CrossRef]
- Hata, T.; Asano, Y.; Yoshihara, K.; Kimura-Todani, T.; Miyata, N.; Zhang, X.-T.; Takakura, S.; Aiba, Y.; Koga, Y.; Sudo, N. Regulation of gut luminal serotonin by commensal microbiota in mice. PLoS ONE 2017, 12, e0180745. [Google Scholar] [CrossRef]
- Conn, K.A.; Borsom, E.M.; Cope, E.K. Implications of microbe-derived ɣ-aminobutyric acid (GABA) in gut and brain barrier integrity and GABAergic signaling in Alzheimer’s disease. Gut Microbes 2024, 16, 2371950. [Google Scholar] [CrossRef]
- Duranti, S.; Ruiz, L.; Lugli, G.A.; Tames, H.; Milani, C.; Mancabelli, L.; Mancino, W.; Longhi, G.; Carnevali, L.; Sgoifo, A.; et al. Bifidobacterium adolescentis as a key member of the human gut microbiota in the production of GABA. Sci. Rep. 2020, 10, 14112. [Google Scholar] [CrossRef]
- Braga, J.D.; Thongngam, M.; Kumrungsee, T. Gamma-aminobutyric acid as a potential postbiotic mediator in the gut–brain axis. Npj Sci. Food 2024, 8, 16. [Google Scholar] [CrossRef]
- Villageliú, D.; Lyte, M. Dopamine production in Enterococcus faecium: A microbial endocrinology-based mechanism for the selection of probiotics based on neurochemical-producing potential. PLoS ONE 2018, 13, e0207038. [Google Scholar] [CrossRef]
- Hamamah, S.; Aghazarian, A.; Nazaryan, A.; Hajnal, A.; Covasa, M. Role of Microbiota-Gut-Brain Axis in Regulating Dopaminergic Signaling. Biomedicines 2022, 10, 436. [Google Scholar] [CrossRef]
- Luck, B.; Horvath, T.D.; Engevik, K.A.; Ruan, W.; Haidacher, S.J.; Hoch, K.M.; Oezguen, N.; Spinler, J.K.; Haag, A.M.; Versalovic, J.; et al. Neurotransmitter Profiles Are Altered in the Gut and Brain of Mice Mono-Associated with Bifidobacterium dentium. Biomolecules 2021, 11, 1091. [Google Scholar] [CrossRef]
- D’Alessandro, G.; Lauro, C.; Quaglio, D.; Ghirga, F.; Botta, B.; Trettel, F.; Limatola, C. Neuro-Signals from Gut Microbiota: Perspectives for Brain Glioma. Cancers 2021, 13, 2810. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-Vieira, T.H.; Guimaraes, I.M.; Silva, F.R.; Ribeiro, F.M. Alzheimer’s disease: Targeting the Cholinergic System. Curr. Neuropharmacol. 2016, 14, 101–115. [Google Scholar] [CrossRef]
- Lombardo, S.; Maskos, U. Role of the nicotinic acetylcholine receptor in Alzheimer’s disease pathology and treatment. Neuropharmacology 2015, 96, 255–262. [Google Scholar] [CrossRef]
- Vallés, A.S.; Borroni, M.V.; Barrantes, F.J. Targeting Brain α7 Nicotinic Acetylcholine Receptors in Alzheimer’s Disease: Rationale and Current Status. CNS Drugs 2014, 28, 975–987. [Google Scholar] [CrossRef] [PubMed]
- Peterson, C.T. Dysfunction of the Microbiota-Gut-Brain Axis in Neurodegenerative Disease: The Promise of Therapeutic Modulation with Prebiotics, Medicinal Herbs, Probiotics, and Synbiotics. J. Evid.-Based Integr. Med. 2020, 25, 2515690X20957225. [Google Scholar] [CrossRef]
- Kaczmarczyk, R.; Tejera, D.; Simon, B.J.; Heneka, M.T. Microglia modulation through external vagus nerve stimulation in a murine model of Alzheimer’s disease. J. Neurochem. 2017, 21, 76–85. [Google Scholar] [CrossRef]
- He, C.; Jiang, J.; Liu, J.; Zhou, L.; Ge, Y.; Yang, Z. Pseudostellaria heterophylla polysaccharide mitigates Alzheimer’s-like pathology via regulating the microbiota-gut-brain axis in 5 × FAD mice. Int. J. Biol. Macromol. 2024, 270, 132372. [Google Scholar] [CrossRef]
- Petrella, C.; Strimpakos, G.; Torcinaro, A.; Middei, S.; Ricci, V.; Gargari, G.; Mora, D.; De Santa, F.; Farioli-Vecchioli, S. Corrigendum to “Proneurogenic and neuroprotective effect of a multi strain probiotic mixture in a mouse model of acute inflammation: Involvement of the gut-brain axis”. Pharmacol. Res. 2021, 173, 105925. [Google Scholar] [CrossRef]
- Liu, X.; Du, Z.R.; Wang, X.; Sun, X.R.; Zhao, Q.; Zhao, F.; Wong, W.T.; Wong, K.H.; Dong, X.-L. Polymannuronic acid prebiotic plus Lacticaseibacillus rhamnosus GG probiotic as a novel synbiotic promoted their separate neuroprotection against Parkinson’s disease. Food Res. Int. Ott. Ont 2022, 155, 111067. [Google Scholar] [CrossRef]
- Aso, E.; Ferrer, I. Cannabinoids for treatment of Alzheimer’s disease: Moving toward the clinic. Front. Pharmacol. 2014, 5, 37. [Google Scholar] [CrossRef]
- Kanwal, H.; Sangineto, M.; Ciarnelli, M.; Castaldo, P.; Villani, R.; Romano, A.D.; Serviddio, G.; Cassano, T. Potential Therapeutic Targets to Modulate the Endocannabinoid System in Alzheimer’s Disease. Int. J. Mol. Sci. 2024, 25, 4050. [Google Scholar] [CrossRef]
- Talarico, G.; Trebbastoni, A.; Bruno, G.; de Lena, C. Modulation of the Cannabinoid System: A New Perspective for the Treatment of the Alzheimer’s Disease. Curr. Neuropharmacol. 2019, 17, 176–183. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, A. A review on mitochondrial restorative mechanism of antioxidants in Alzheimer’s disease and other neurological conditions. Front. Pharmacol. 2015, 6, 206. [Google Scholar] [CrossRef] [PubMed]
- Di Domenico, F.; Barone, E.; Perluigi, M.; Butterfield, D.A. Strategy to reduce free radical species in Alzheimer’s disease: An update of selected antioxidants. Expert Rev. Neurother. 2015, 15, 19–40. [Google Scholar] [CrossRef] [PubMed]
- Pappolla, M.A.; Perry, G.; Fang, X.; Zagorski, M.; Sambamurti, K.; Poeggeler, B. Indoles as essential mediators in the gut-brain axis. Their role in Alzheimer’s disease. Neurobiol. Dis. 2021, 156, 105403. [Google Scholar] [CrossRef]
- Bonfili, L.; Cecarini, V.; Cuccioloni, M.; Angeletti, M.; Berardi, S.; Scarpona, S.; Rossi, G.; Eleuteri, A.M. SLAB51 Probiotic Formulation Activates SIRT1 Pathway Promoting Antioxidant and Neuroprotective Effects in an AD Mouse Model. Mol. Neurobiol. 2018, 55, 7987–8000. [Google Scholar] [CrossRef]
- Markowiak, P.; Śliżewska, K. Effects of Probiotics, Prebiotics, and Synbiotics on Human Health. Nutrients 2017, 9, 1021. [Google Scholar] [CrossRef]
- Zhang, P.; Wu, X.; Liang, S.; Shao, X.; Wang, Q.; Chen, R.; Zhu, W.; Shao, C.; Jin, F.; Jia, C. A dynamic mouse peptidome landscape reveals probiotic modulation of the gut-brain axis. Sci. Signal. 2020, 13, eabb0443. [Google Scholar] [CrossRef]
- Gomez Quintero, D.F.; Kok, C.R.; Hutkins, R. The Future of Synbiotics: Rational Formulation and Design. Front. Microbiol. 2022, 13, 919725. [Google Scholar] [CrossRef]
- Shafi, A.; Farooq, U.; Akram, K.; Hayat, Z.; Murtaza, M.A. Prevention and Control of Diseases by Use of Pro- and Prebiotics (Synbiotics). Food Rev. Int. 2014, 30, 291–316. [Google Scholar] [CrossRef]
- Pandey, K.R.; Naik, S.R.; Vakil, B.V. Probiotics, prebiotics and synbiotics- a review. J. Food Sci. Technol. 2015, 52, 7577. [Google Scholar] [CrossRef] [PubMed]
- Yadav, M.K.; Kumari, I.; Singh, B.; Sharma, K.K.; Tiwari, S.K. Probiotics, prebiotics and synbiotics: Safe options for next-generation therapeutics. Appl. Microbiol. Biotechnol. 2022, 106, 505–521. [Google Scholar] [CrossRef] [PubMed]
- Yan, R.; Zeng, X.; Shen, J.; Wu, Z.; Guo, Y.; Du, Q.; Tu, M.; Pan, D. New clues for postbiotics to improve host health: A review from the perspective of function and mechanisms. J. Sci. Food Agric. 2024, 104, 6376–6387. [Google Scholar] [CrossRef]
- Feizi, H.; Plotnikov, A.; Rezaee, M.A.; Ganbarov, K.; Kamounah, F.S.; Nikitin, S.; Kadkhoda, H.; Gholizadeh, P.; Pagliano, P.; Kafil, H.S. Postbiotics versus probiotics in early-onset colorectal cancer. Crit. Rev. Food Sci. Nutr. 2024, 64, 3573–3582. [Google Scholar] [CrossRef] [PubMed]
- Cuevas-González, P.F.; Liceaga, A.M.; Aguilar-Toalá, J.E. Postbiotics and paraprobiotics: From concepts to applications. Food Res. Int. 2020, 136, 109502. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, Z.; Zhao, L.; Zhao, Y.; Yang, G.; Wang, C.; Gao, L.; Niu, C.; Li, S. Lactobacillus plantarum DP189 Reduces α-SYN Aggravation in MPTP-Induced Parkinson’s Disease Mice via Regulating Oxidative Damage, Inflammation, and Gut Microbiota Disorder. J. Agric. Food Chem. 2022, 70, 1163–1173. [Google Scholar] [CrossRef]
- Abdelhamid, M.; Zhou, C.; Jung, C.-G.; Michikawa, M. Probiotic Bifidobacterium breve MCC1274 Mitigates Alzheimer’s Disease-Related Pathologies in Wild-Type Mice. Nutrients 2022, 14, 2543. [Google Scholar] [CrossRef]
- Qian, X.-H.; Chen, S.-Y.; Tang, H.-D. Multi-strain probiotics ameliorate Alzheimer’s-like cognitive impairment and pathological changes through the AKT/GSK-3β pathway in senescence-accelerated mouse prone 8 mice. Brain. Behav. Immun. 2024, 119, 14–27. [Google Scholar] [CrossRef]
- Yang, X.; Yu, D.; Xue, L.; Li, H.; Du, J. Probiotics modulate the microbiota–gut–brain axis and improve memory deficits in aged SAMP8 mice. Acta Pharm. Sin. B 2020, 10, 475–487. [Google Scholar] [CrossRef]
- Zhu, J.; Liu, S.; Zhang, H.; Zhao, W.; Ding, J.; Dai, R.; Xu, K.; He, C.; Liu, J.; Yang, L.; et al. Dynamic distribution of gut microbiota during Alzheimer’s disease progression in a mice model. APMIS 2023, 131, 480–490. [Google Scholar] [CrossRef]
- de Rijke, T.J.; Doting, M.H.E.; van Hemert, S.; De Deyn, P.P.; van Munster, B.C.; Harmsen, H.J.M.; Sommer, I.E.C. A Systematic Review on the Effects of Different Types of Probiotics in Animal Alzheimer’s Disease Studies. Front. Psychiatry 2022, 13, 879491. [Google Scholar] [CrossRef] [PubMed]
- Bi, M.; Liu, C.; Wang, Y.; Liu, S.-J. Therapeutic Prospect of New Probiotics in Neurodegenerative Diseases. Microorganisms 2023, 11, 1527. [Google Scholar] [CrossRef] [PubMed]
- Kwon, O.Y.; Lee, S.H. Animal Models of Cognitive Deficits for Probiotic Treatment. Food Sci. Anim. Resour. 2022, 42, 981–995. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.-J.; Chen, J.-L.; Liao, J.-F.; Chen, Y.-H.; Chieu, M.-W.; Ke, Y.-Y.; Hsu, C.-C.; Tsai, Y.-C.; Hsieh-Li, H.M. Lactobacillus plantarum PS128 prevents cognitive dysfunction in Alzheimer’s disease mice by modulating propionic acid levels, glycogen synthase kinase 3 beta activity, and gliosis. BMC Complement. Med. Ther. 2021, 21, 259. [Google Scholar] [CrossRef]
- Beck, L.C.; Masi, A.C.; Young, G.R.; Vatanen, T.; Lamb, C.A.; Smith, R.; Coxhead, J.; Butler, A.; Marsland, B.J.; Embleton, N.D.; et al. Strain-specific impacts of probiotics are a significant driver of gut microbiome development in very preterm infants. Nat. Microbiol. 2022, 7, 1525–1535. [Google Scholar] [CrossRef]
- Kaur, H.; Nookala, S.; Singh, S.; Mukundan, S.; Nagamoto-Combs, K.; Combs, C.K. Sex-Dependent Effects of Intestinal Microbiome Manipulation in a Mouse Model of Alzheimer’s Disease. Cells 2021, 10, 2370. [Google Scholar] [CrossRef]
- Cantu-Jungles, T.M.; Rasmussen, H.E.; Hamaker, B.R. Potential of Prebiotic Butyrogenic Fibers in Parkinson’s Disease. Front. Neurol. 2019, 10, 663. [Google Scholar] [CrossRef]
- Kang, J.W.; Zivkovic, A.M. The Potential Utility of Prebiotics to Modulate Alzheimer’s Disease: A Review of the Evidence. Microorganisms 2021, 9, 2310. [Google Scholar] [CrossRef]
- Liu, Q.; Xi, Y.; Wang, Q.; Liu, J.; Li, P.; Meng, X.; Liu, K.; Chen, W.; Liu, X.; Liu, Z. Mannan oligosaccharide attenuates cognitive and behavioral disorders in the 5xFAD Alzheimer’s disease mouse model via regulating the gut microbiota-brain axis. Brain. Behav. Immun. 2021, 95, 330–343. [Google Scholar] [CrossRef]
- Zhang, S.; Lv, S.; Li, Y.; Wei, D.; Zhou, X.; Niu, X.; Yang, Z.; Song, W.; Zhang, Z.; Peng, D. Prebiotics modulate the microbiota–gut–brain axis and ameliorate cognitive impairment in APP/PS1 mice. Eur. J. Nutr. 2023, 62, 2991–3007. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, J.; Jiang, C.; Wang, S.; Que, R.; An, L. Up-regulation of neprilysin mediates the protection of fructo-oligosaccharides against Alzheimer’s disease. Food Funct. 2020, 11, 6565–6572. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-H.; Yanckello, L.M.; Chlipala, G.E.; Green, S.J.; Aware, C.; Runge, A.; Xing, X.; Chen, A.; Wenger, K.; Flemister, A.; et al. Prebiotic inulin enhances gut microbial metabolism and anti-inflammation in apolipoprotein E4 mice with sex-specific implications. Sci. Rep. 2023, 13, 15116. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, X.; Xu, H.; Liu, X.; He, Y.; Tan, X.; Gu, J. NMN synbiotics intervention modulates gut microbiota and metabolism in APP/PS1 Alzheimer’s disease mouse models. Biochem. Biophys. Res. Commun. 2024, 726, 150274. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhao, X.; Xu, H.; Liu, X.; He, Y.; Gu, J. NMN Synbiotics: A Multifaceted Therapeutic Approach for Alzheimer’s Disease. Neurochem. Res. 2024, 49, 2888–2896. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Hai, W.; Chen, S.; Zhang, M.; Jiang, X.; Tang, H. Multi-omics data reveals aberrant gut microbiota-host glycerophospholipid metabolism in association with neuroinflammation in APP/PS1 mice. Gut Microbes 2023, 15, 2282790. [Google Scholar] [CrossRef]
- Smith, C. Synbiotic-derived metabolites reduce neuroinflammatory symptoms of Alzheimer’s disease. Alzheimers Dement. 2020, 16, e046288. [Google Scholar] [CrossRef]
- Deng, S.-M.; Chen, C.-J.; Lin, H.-L.; Cheng, I.H. The beneficial effect of synbiotics consumption on Alzheimer’s disease mouse model via reducing local and systemic inflammation. IUBMB Life 2022, 74, 748–753. [Google Scholar] [CrossRef]
- Li, L.; Yang, C.; Jia, M.; Wang, Y.; Zhao, Y.; Li, Q.; Gong, J.; He, Y.; Xu, K.; Liu, X.; et al. Synbiotic therapy with Clostridium sporogenes and xylan promotes gut-derived indole-3-propionic acid and improves cognitive impairments in an Alzheimer’s disease mouse model. Food Funct. 2024, 15, 7865–7882. [Google Scholar] [CrossRef]
- Arora, K.; Green, M.; Prakash, S. The Microbiome and Alzheimer’s Disease: Potential and Limitations of Prebiotic, Synbiotic, and Probiotic Formulations. Front. Bioeng. Biotechnol. 2020, 8, 537847. [Google Scholar] [CrossRef]
- Bashir, B.; Alam, S.; Khandale, N.; Birla, D.; Vishwas, S.; Pandey, N.K.; Gupta, G.; Paudel, K.R.; Dureja, H.; Kumar, P.; et al. Opening avenues for treatment of neurodegenerative disease using post-biotics: Breakthroughs and bottlenecks in clinical translation. Ageing Res. Rev. 2024, 95, 102236. [Google Scholar] [CrossRef]
- Kolonics, A.; Bori, Z.; Torma, F.; Abraham, D.; Fehér, J.; Radak, Z. Exercise combined with postbiotics treatment results in synergistic improvement of mitochondrial function in the brain of male transgenic mice for Alzheimer’s disease. BMC Neurosci. 2023, 24, 68. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, J.D.; Seidler, Y.; Bailey, H.R.; Whitacre, L.; Bargo, F.; Lüersen, K.; Rimbach, G.; Pighetti, G.M.; Ipharraguerre, I.R.; Ríus, A.G. A postbiotic from Aspergillus oryzae attenuates the impact of heat stress in ectothermic and endothermic organisms. Sci. Rep. 2021, 11, 6407. [Google Scholar] [CrossRef]
- Aran, K.R.; Porel, P.; Hunjan, G.; Singh, S.; Gupta, G.D.; Rohit. Postbiotics as a therapeutic tool in Alzheimer’s disease: Insights into molecular pathways and neuroprotective effects. Ageing Res. Rev. 2025, 106, 102685. [Google Scholar] [CrossRef]
- Bashir, B.; Gulati, M.; Vishwas, S.; Gupta, G.; Dhanasekaran, M.; Paudel, K.R.; Chellappan, D.K.; Anand, K.; Negi, P.; Singh, P.K.; et al. Bridging gap in the treatment of Alzheimer’s disease via postbiotics: Current practices and future prospects. Ageing Res. Rev. 2025, 105, 102689. [Google Scholar] [CrossRef]
- Webberley, T.S.; Bevan, R.J.; Kerry-Smith, J.; Dally, J.; Michael, D.R.; Thomas, S.; Rees, M.; Morgan, J.E.; Marchesi, J.R.; Good, M.A.; et al. Assessment of Lab4P Probiotic Effects on Cognition in 3xTg-AD Alzheimer’s Disease Model Mice and the SH-SY5Y Neuronal Cell Line. Int. J. Mol. Sci. 2023, 24, 4683. [Google Scholar] [CrossRef]
- Flynn, C.M.; Omoluabi, T.; Janes, A.M.; Rodgers, E.J.; Torraville, S.E.; Negandhi, B.L.; Nobel, T.E.; Mayengbam, S.; Yuan, Q. Targeting early tau pathology: Probiotic diet enhances cognitive function and reduces inflammation in a preclinical Alzheimer’s model. Alzheimers Res. Ther. 2025, 17, 24. [Google Scholar] [CrossRef] [PubMed]
- Bonfili, L.; Grasselli, F.M.; Cuccioloni, M.; Cecarini, V.; Lufrano, D.; Vittadini, E.; Galosi, L.; Sonsini, G.; Ubaldi, M.; Turck, J.L.; et al. A red lentils-based synbiotic cookie exerts neuroprotective effects in a mouse model of Alzheimer’s disease. J. Nutr. Biochem. 2025, 141, 109904. [Google Scholar] [CrossRef]
- Ojha, S.; Patil, N.; Jain, M.; Kole, C.; Kaushik, P. Probiotics for Neurodegenerative Diseases: A Systemic Review. Microorganisms 2023, 11, 1083. [Google Scholar] [CrossRef]
- Xiang, S.; Ji, J.-L.; Li, S.; Cao, X.-P.; Xu, W.; Tan, L.; Tan, C.-C. Efficacy and Safety of Probiotics for the Treatment of Alzheimer’s Disease, Mild Cognitive Impairment, and Parkinson’s Disease: A Systematic Review and Meta-Analysis. Front. Aging Neurosci. 2022, 14, 730036. [Google Scholar] [CrossRef]
- Shi, S.; Zhang, Q.; Sang, Y.; Ge, S.; Wang, Q.; Wang, R.; He, J. Probiotic Bifidobacterium longum BB68S Improves Cognitive Functions in Healthy Older Adults: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients 2023, 15, 51. [Google Scholar] [CrossRef]
- Mak, C.C.H.; Meng, H.Y.H.; Mak, J.W.Y.; Ko, O.H.; Tao, Z.; Chan, F.K.L. IDDF2020-ABS-0203 Investigating the evidence of prebiotic supplementation in the attenuation of age-related neurodegeneration in in vivo studies: A systematic review and meta-analysis with bayesian inference. Gut 2020, 69, A64–A65. [Google Scholar] [CrossRef]
- Hall, D.A.; Voigt, R.M.; Cantu-Jungles, T.M.; Hamaker, B.; Engen, P.A.; Shaikh, M.; Raeisi, S.; Green, S.J.; Naqib, A.; Forsyth, C.B.; et al. An open label, non-randomized study assessing a prebiotic fiber intervention in a small cohort of Parkinson’s disease participants. Nat. Commun. 2023, 14, 926. [Google Scholar] [CrossRef] [PubMed]
- Krüger, J.F.; Hillesheim, E.; Pereira, A.C.S.N.; Camargo, C.Q.; Rabito, E.I. Probiotics for dementia: A systematic review and meta-analysis of randomized controlled trials. Nutr. Rev. 2021, 79, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Głowacka, P.; Oszajca, K.; Pudlarz, A.; Szemraj, J.; Witusik-Perkowska, M. Postbiotics as Molecules Targeting Cellular Events of Aging Brain—The Role in Pathogenesis, Prophylaxis and Treatment of Neurodegenerative Diseases. Nutrients 2024, 16, 2244. [Google Scholar] [CrossRef]
- Zhang, T.; Gao, G.; Kwok, L.-Y.; Sun, Z. Gut microbiome-targeted therapies for Alzheimer’s disease. Gut Microbes 2023, 15, 2271613. [Google Scholar] [CrossRef]
- Lista, S.; Munafò, A.; Caraci, F.; Imbimbo, C.; Emanuele, E.; Minoretti, P.; Pinto-Fraga, J.; Merino-País, M.; Crespo-Escobar, P.; López-Ortiz, S.; et al. Gut microbiota in Alzheimer’s disease: Understanding molecular pathways and potential therapeutic perspectives. Ageing Res. Rev. 2025, 104, 102659. [Google Scholar] [CrossRef]
- Kim, C.-S.; Cha, J.; Sim, M.; Jung, S.; Chun, W.Y.; Baik, H.W.; Shin, D.-M. Probiotic Supplementation Improves Cognitive Function and Mood with Changes in Gut Microbiota in Community-Dwelling Older Adults: A Randomized, Double-Blind, Placebo-Controlled, Multicenter Trial. J. Gerontol. Ser. A 2021, 76, 32–40. [Google Scholar] [CrossRef]
- Asaoka, D.; Xiao, J.; Takeda, T.; Yanagisawa, N.; Yamazaki, T.; Matsubara, Y.; Sugiyama, H.; Endo, N.; Higa, M.; Kasanuki, K.; et al. Effect of Probiotic Bifidobacterium breve in Improving Cognitive Function and Preventing Brain Atrophy in Older Patients with Suspected Mild Cognitive Impairment: Results of a 24-Week Randomized, Double-Blind, Placebo-Controlled Trial. J. Alzheimers Dis. 2022, 88, 75–95. [Google Scholar] [CrossRef]
- Hsu, Y.-C.; Huang, Y.-Y.; Tsai, S.-Y.; Kuo, Y.-W.; Lin, J.-H.; Ho, H.-H.; Chen, J.-F.; Hsia, K.-C.; Sun, Y. Efficacy of Probiotic Supplements on Brain-Derived Neurotrophic Factor, Inflammatory Biomarkers, Oxidative Stress and Cognitive Function in Patients with Alzheimer’s Dementia: A 12-Week Randomized, Double-Blind Active-Controlled Study. Nutrients 2024, 16, 16. [Google Scholar] [CrossRef]
- Akhgarjand, C.; Vahabi, Z.; Shab-Bidar, S.; Anoushirvani, A.; Djafarian, K. The effects of probiotic supplements on oxidative stress and inflammation in subjects with mild and moderate Alzheimer’s disease: A randomized, double-blind, placebo-controlled study. Inflammopharmacology 2024, 32, 1413–1420. [Google Scholar] [CrossRef]
- Bartos, A.; Weinerova, J.; Diondet, S. Effects of human probiotics on memory and psychological and physical measures in community-dwelling older adults with normal and mildly impaired cognition: Results of a bi-center, double-blind, randomized, and placebo-controlled clinical trial (CleverAge biota). Front. Aging Neurosci. 2023, 15, 1163727. [Google Scholar] [CrossRef]
- Xiao, J.; Katsumata, N.; Bernier, F.; Ohno, K.; Yamauchi, Y.; Odamaki, T.; Yoshikawa, K.; Ito, K.; Kaneko, T. Probiotic Bifidobacterium breve in Improving Cognitive Functions of Older Adults with Suspected Mild Cognitive Impairment: A Randomized, Double-Blind, Placebo-Controlled Trial. J. Alzheimers Dis. 2020, 77, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Zhao, J.; Qin, Y.; Wang, Y.; Zhang, Y.; Sun, S. Probiotics, Prebiotics, and Synbiotics Improve Uremic, Inflammatory, and Gastrointestinal Symptoms in End-Stage Renal Disease with Dialysis: A Network Meta-Analysis of Randomized Controlled Trials. Front. Nutr. 2022, 9, 850425. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Xu, X.; Pang, B.; Yang, R.; Sun, H.; Jiang, C.; Shao, D.; Shi, J. Probiotic and prebiotic interventions for non-alcoholic fatty liver disease: A systematic review and network meta-analysis. Benef. Microbes 2021, 12, 517–529. [Google Scholar] [CrossRef]
- Wang, X.; Yang, J.; Qiu, X.; Wen, Q.; Liu, M.; Zhou, D.; Chen, Q. Probiotics, Pre-biotics and Synbiotics in the Treatment of Pre-diabetes: A Systematic Review of Randomized Controlled Trials. Front. Public Health 2021, 9, 645035. [Google Scholar] [CrossRef]
- Espín, J.C.; González-Sarrías, A.; Tomás-Barberán, F.A. The gut microbiota: A key factor in the therapeutic effects of (poly)phenols. Biochem. Pharmacol. 2017, 139, 82–93. [Google Scholar] [CrossRef]
- Sheyholislami, H.; Connor, K.L. Are Probiotics and Prebiotics Safe for Use during Pregnancy and Lactation? A Systematic Review and Meta-Analysis. Nutrients 2021, 13, 2382. [Google Scholar] [CrossRef]
- Van den Nieuwboer, M.; Brummer, R.J.; Guarner, F.; Morelli, L.; Cabana, M.; Claasen, E. The administration of probiotics and synbiotics in immune compromised adults: Is it safe? Benef. Microbes 2015, 6, 3–17. [Google Scholar] [CrossRef]
- Rouhani, M.H.; Hadi, A.; Ghaedi, E.; Salehi, M.; Mahdavi, A.; Mohammadi, H. Do probiotics, prebiotics and synbiotics affect adiponectin and leptin in adults? A systematic review and meta-analysis of clinical trials. Clin. Nutr. Edinb. Scotl. 2019, 38, 2031–2037. [Google Scholar] [CrossRef]
- Liu, S.; Gao, J.; Zhu, M.; Liu, K.; Zhang, H.-L. Gut Microbiota and Dysbiosis in Alzheimer’s Disease: Implications for Pathogenesis and Treatment. Mol. Neurobiol. 2020, 57, 5026–5043. [Google Scholar] [CrossRef]
- Calero, C.D.Q.; Rincón, E.O.; Marqueta, P.M. Probiotics, prebiotics and synbiotics: Useful for athletes and active individuals? A systematic review. Benef. Microbes 2020, 11, 135–149. [Google Scholar] [CrossRef] [PubMed]
- Ohshima, T.; Kojima, Y.; Seneviratne, C.J.; Maeda, N. Therapeutic Application of Synbiotics, a Fusion of Probiotics and Prebiotics, and Biogenics as a New Concept for Oral Candida Infections: A Mini Review. Front. Microbiol. 2016, 7, 10. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.R.; Tajmim, A.; Ali, M.; Sharif, M. Overview and Current Status of Alzheimer’s Disease in Bangladesh. J. Alzheimers Dis. Rep. 2017, 1, 27–42. [Google Scholar] [CrossRef] [PubMed]

| No | Pharmacological Therapy | Dosage | Mechanism(s) of Action | Side Effect/Limitation | References |
|---|---|---|---|---|---|
| 1. | Monoclonal antibody
| The initial three doses should be administered at a dosage of 700 mg, followed by a subsequent dosage of 1400 mg, delivered intravenously every four weeks. | A humanized monoclonal antibody of the immunoglobulin gamma 1 (IgG1) class that targets insoluble N-truncated pyroglutamate amyloid beta. | Amyloid-related imaging abnormalities or ARIA, headache, reactions associated with infusions. | [37] |
| 2. | Anticholinesterase (AChE) inhibitors
| The starting dosage is 5 mg administered once daily, which may be increased to 10 mg once daily, the highest recommended dosage, after a period of 4 to 6 weeks. | Elevating acetylcholine concentrations in the brain is crucial, as this molecule facilitates the communication of information among specific neurons and is integral to memory processes. Furthermore, acetylcholinesterase (AChE) inhibitors promote cholinergic neurotransmission by obstructing the hydrolysis of acetylcholine, thereby leading to an increase in its synaptic availability. | The likelihood of experiencing secondary adverse effects is heightened, while the effectiveness of the medication is diminished. | [38] |
| 3. | Anti-glutaminergics
| The starting dosage is 5 mg administered once daily, with a maximum recommended dosage of 20 mg to be considered after a duration of 4 weeks. | Modulate glutamate concentrations by employing a noncompetitive antagonist action on NMDA receptors. The NMDA receptor blockade inhibits the entry of intracellular calcium (Ca2+), thereby mitigating excitotoxicity that leads to neuronal degeneration in AD. | The effectiveness of interventions is, at best, limited and transient, addressing only the outcomes of Alzheimer’s disease rather than its underlying causes. | [39] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Renesteen, E.; Boyajian, J.L.; Islam, P.; Kassab, A.; Abosalha, A.; Makhlouf, S.; Santos, M.; Chen, H.; Shum-Tim, C.; Prakash, S. Microbiome Engineering for Biotherapeutic in Alzheimer’s Disease Through the Gut–Brain Axis: Potentials and Limitations. Int. J. Mol. Sci. 2025, 26, 5351. https://doi.org/10.3390/ijms26115351
Renesteen E, Boyajian JL, Islam P, Kassab A, Abosalha A, Makhlouf S, Santos M, Chen H, Shum-Tim C, Prakash S. Microbiome Engineering for Biotherapeutic in Alzheimer’s Disease Through the Gut–Brain Axis: Potentials and Limitations. International Journal of Molecular Sciences. 2025; 26(11):5351. https://doi.org/10.3390/ijms26115351
Chicago/Turabian StyleRenesteen, Editha, Jacqueline L. Boyajian, Paromita Islam, Amal Kassab, Ahmed Abosalha, Stephanie Makhlouf, Madison Santos, Hongmei Chen, Cedrique Shum-Tim, and Satya Prakash. 2025. "Microbiome Engineering for Biotherapeutic in Alzheimer’s Disease Through the Gut–Brain Axis: Potentials and Limitations" International Journal of Molecular Sciences 26, no. 11: 5351. https://doi.org/10.3390/ijms26115351
APA StyleRenesteen, E., Boyajian, J. L., Islam, P., Kassab, A., Abosalha, A., Makhlouf, S., Santos, M., Chen, H., Shum-Tim, C., & Prakash, S. (2025). Microbiome Engineering for Biotherapeutic in Alzheimer’s Disease Through the Gut–Brain Axis: Potentials and Limitations. International Journal of Molecular Sciences, 26(11), 5351. https://doi.org/10.3390/ijms26115351









