Multisystem Symptoms in Myotonic Dystrophy Type 1: A Management and Therapeutic Perspective
Abstract
1. Introduction
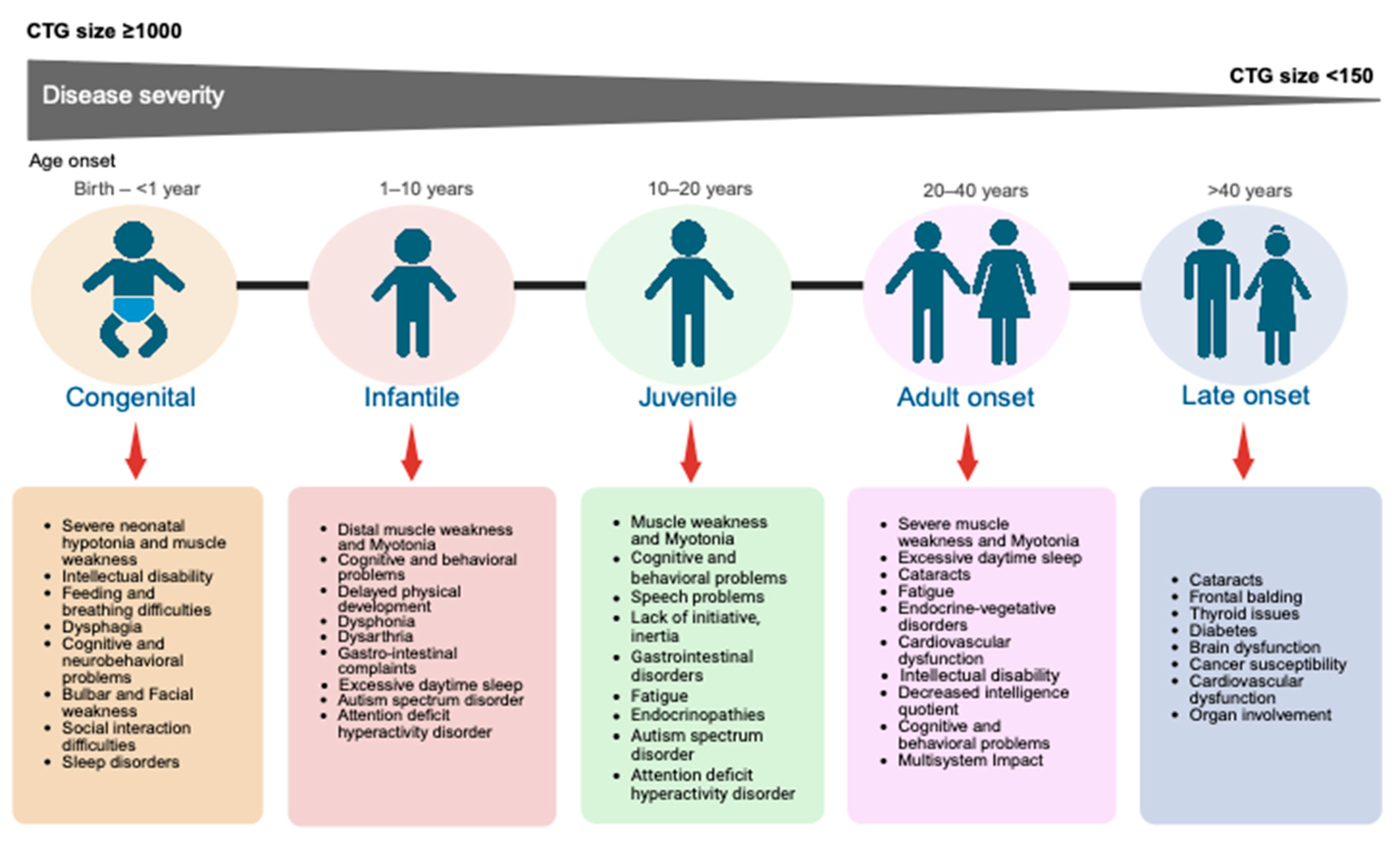
2. Myotonic Dystrophy Type 1 Classification
3. Myotonic Dystrophy Type 1 Molecular Pathophysiology
4. Multisystem Symptoms Management and Overview of the Current Clinical Treatments
4.1. Musculoskeletal System
4.2. Respiratory System
4.3. Cardiovascular System
4.4. Gastrointestinal System
4.5. Central Nervous System
4.6. Visual System
4.7. Endocrine System and Metabolism
4.8. Reproductive System
4.9. Integumentary System
5. Towards the Development of Promising Therapeutic Interventions for DM1

5.1. Small Molecules
| Class | Drug Candidate (Compound) | Clinical Trials Registry Identifier; Clinical Trial Phase | Mode of Action (MoA) | Study Design | Body Systems/Outcomes Improved | References |
|---|---|---|---|---|---|---|
| Repurposed small molecule | Tideglusib (AMO-02) | NCT02858908; Phase II | Inhibition of GSK3β activity |
|
| [249,258] |
| NCT03692312; Phase II/III | Inhibition of GSK3β activity |
|
| [259] | ||
| NCT05004129; Phase II/III | Inhibition of GSK3β activity |
|
| [260,261] | ||
| Erythromycin (MYD-0124) | JPRN-jRCT2051190069; Phase II | Reduction of RNA toxicity |
|
| [251] | |
| Metformin | 2013-001732-21; Phase II | AMPK activation |
|
| [262] | |
| 2018-000692-32; Phase III | AMPK Activation |
|
| [262] | ||
| NCT05532813; Phase III | AMPK Activation |
|
| [263] | ||
| Mexiletine | NCT01406873; Phase II | Na+ channel blocker |
|
| [110] | |
| NCT04624750; Phase III | Na+ channel blocker |
|
| [264,265] | ||
| NCT04622553; Not Applicable | Na+ channel blocker |
|
| [266] | ||
| NCT04616807; Not Applicable | Na+ channel blocker |
|
| [267] | ||
| NCT04700046; Phase III (Withdrawn) | Na+ channel blocker |
|
| [110,268] | ||
| Flumazenil (ERX-963) | NCT03959189; Phase I | GABA receptor antagonist |
|
| [269] | |
| Ranolazine | NCT02251457; Phase I | Inhibition of late sodium current |
|
| [270,271] | |
| Pitolisant | NCT04886518; Phase II | H3 receptor antagonist |
|
| [272] | |
| Repurposed small molecule (Natural compounds) | Theobromine and caffeine (MYODM™) | NCT04634682; Not Applicable | Reduction of toxic DMPK RNA |
|
| [273] |
| Antibody fragment conjugated antisense oligonucleotide (ASO) | DYNE-101 (TfR1-targeted antibody (Fab) linked to ASO) | NCT05481879; Phase II/III | Reduction of toxic DMPK RNA |
|
| [274,275] |
| IONIS-DMPKRx (Baliforsen; ISIS 598769) | NCT02312011; Phase I/IIa | Reduction of toxic DMPK RNA |
|
| [276] | |
| Anti- microRNA oligonucleotide | ARTHEx’s ATX-01 | NCT06300307; Phase I/IIa | Anti-microRNA, MBNL upregulation |
|
| [277] |
| Antibody oligonucleotide conjugated | MARINA AOC 1001 | NCT05027269; Phase I/II | TfR1-targeted antibody (mAb) conjugated to siRNA targeting DMPK |
|
| [278,279] |
| AOC 1001 | NCT05479981; Phase II | TfR1-targeted antibody (mAb) conjugated to siRNA targeting DMPK |
|
| [280] | |
| Del-desiran (Delpacibart Etedesiran), previously AOC 1001 | NCT06411288; Phase III | TfR1-targeted antibody (mAb) conjugated to siRNA targeting DMPK |
|
| [281,282] | |
| Peptide conjugated antisense oligonucleotide (ASO) | PGN-EDODM1 | NCT06204809; Phase I | RNase H-mediated degradation of target RNA |
|
| [283,284] |
| Small interfering RNAs (siRNA) conjugates | ARO-DM1 | NCT06138743; Phase I/IIa | RNA interference (RNAi) technology targets specific mRNA molecules |
|
| [285] |
| Peptide conjugated oligonucleotide | VX-670 | NCT06185764; Phase I/II | Degrades the toxic DMPK |
|
| [286] |
5.2. Nucleic Acid-Based Therapies
5.3. Antisense Oligonucleotides (ASOs)
5.4. SiRNA-Based Therapies
5.5. RNAI and Phosphorodiamidate Morpholino Oligomers (PMO) Therapeutics
5.6. Genome/Transcriptome Engineering Approaches
6. Challenges and Future Directions
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, H. Myotonic Dystrophy Type 1. In Atlas of Genetic Diagnosis and Counseling; Chen, H., Ed.; Springer: New York, NY, USA, 2014; pp. 1–13. [Google Scholar] [CrossRef]
- Brazil, D.P.; Yang, Z.-Z.; Hemmings, B.A. Advances in protein kinase B signalling: AKTion on multiple fronts. Trends Biochem. Sci. 2004, 29, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Brook, J.D.; McCurrach, M.E.; Harley, H.G.; Buckler, A.J.; Church, D.; Aburatani, H.; Hunter, K.; Stanton, V.P.; Thirion, J.-P.; Hudson, T. Molecular basis of myotonic dystrophy: Expansion of a trinucleotide (CTG) repeat at the 3′ end of a transcript encoding a protein kinase family member. Cell 1992, 68, 799–808. [Google Scholar] [CrossRef] [PubMed]
- Mahadevan, M.; Tsilfidis, C.; Sabourin, L.; Shutler, G.; Amemiya, C.; Jansen, G.; Neville, C.; Narang, M.; Barceló, J.; O’Hoy, K. Myotonic dystrophy mutation: An unstable CTG repeat in the 3′ untranslated region of the gene. Science 1992, 255, 1253–1255. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, R.; Hernández-Hernández, O.; Magaña, J.J.; González-Ramírez, R.; García-López, E.; Cisneros, B. Altered nuclear structure in myotonic dystrophy type 1-derived fibroblasts. Mol. Biol. Rep. 2015, 42, 479–488. [Google Scholar] [CrossRef]
- De Antonio, M.; Dogan, C.; Daidj, F.; Eymard, B.; Puymirat, J.; Mathieu, J.; Gagnon, C.; Katsahian, S.; Arne Bes, M.C.; Attarian, S.; et al. The DM-scope registry: A rare disease innovative framework bridging the gap between research and medical care. Orphanet J. Rare Dis. 2019, 14, 122. [Google Scholar] [CrossRef]
- Kroksmark, A.-K.; Ekström, A.-B.; Björck, E.; Tulinius, M. Myotonic dystrophy: Muscle involvement in relation to disease type and size of expanded CTG-repeat sequence. Dev. Med. Child Neurol. 2005, 47, 478–485. [Google Scholar] [CrossRef]
- Jaradeh, S. Muscle disorders affecting oral and pharyngeal swallowing. GI Motil. Online 2006. Available online: https://www.nature.com/gimo/contents/pt1/full/gimo35.html (accessed on 28 May 2025).
- Hilbert, J.E.; Ashizawa, T.; Day, J.W.; Luebbe, E.A.; Martens, W.B.; McDermott, M.P.; Tawil, R.; Thornton, C.A.; Moxley, R.T. Diagnostic odyssey of patients with myotonic dystrophy. J. Neurol. 2013, 260, 2497–2504. [Google Scholar] [CrossRef]
- Johnson, N.E.; Butterfield, R.J.; Mayne, K.; Newcomb, T.; Imburgia, C.; Dunn, D.; Duval, B.; Feldkamp, M.L.; Weiss, R.B. Population-Based Prevalence of Myotonic Dystrophy Type 1 Using Genetic Analysis of Statewide Blood Screening Program. Neurology 2021, 96, e1045–e1053. [Google Scholar] [CrossRef]
- Liao, Q.; Zhang, Y.; He, J.; Huang, K. Global prevalence of myotonic dystrophy: An updated systematic review and meta-analysis. Neuroepidemiology 2022, 56, 163–173. [Google Scholar] [CrossRef]
- Furlong, P.; Dugar, A.; White, M. Patient engagement in clinical trial design for rare neuromuscular disorders: Impact on the DELIVER and ACHIEVE clinical trials. Res. Involv. Engagem. 2024, 10, 1. [Google Scholar] [CrossRef] [PubMed]
- Hermans, M.C.E.; Faber, C.G.; Vanhoutte, E.K.; Bakkers, M.; De Baets, M.H.; de Die-Smulders, C.E.M.; Merkies, I.S.J. Peripheral neuropathy in myotonic dystrophy type 1. J. Peripher. Nerv. Syst. 2011, 16, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Solbakken, G.; Løseth, S.; Frich, J.C.; Dietrichs, E.; Ørstavik, K. Small and large fiber neuropathy in adults with myotonic dystrophy type 1. Front. Neurol. 2024, 15, 1375218. [Google Scholar] [CrossRef]
- Bassez, G.; Lazarus, A.; Desguerre, I.; Varin, J.; Laforêt, P.; Bécane, H.M.; Meune, C.; Arne-Bes, M.C.; Ounnoughene, Z.; Radvanyi, H.; et al. Severe cardiac arrhythmias in young patients with myotonic dystrophy type 1. Neurology 2004, 63, 1939–1941. [Google Scholar] [CrossRef]
- Bhakta, D.; Lowe, M.R.; Groh, W.J. Prevalence of structural cardiac abnormalities in patients with myotonic dystrophy type I. Am. Heart J. 2004, 147, 224–227. [Google Scholar] [CrossRef]
- Brunet Garcia, L.; Hajra, A.; Field, E.; Wacher, J.; Walsh, H.; Norrish, G.; Manzur, A.; Muntoni, F.; Munot, P.; Robb, S.; et al. Cardiac Manifestations of Myotonic Dystrophy in a Pediatric Cohort. Front. Pediatr. 2022, 10, 910660. [Google Scholar] [CrossRef] [PubMed]
- Arsenault, M.-E.; Prévost, C.; Lescault, A.; Laberge, C.; Puymirat, J.; Mathieu, J. Clinical characteristics of myotonic dystrophy type 1 patients with small CTG expansions. Neurology 2006, 66, 1248–1250. [Google Scholar] [CrossRef]
- Cheminelle, M.; Nougues, M.-C.; Isapof, A.; Aubertin, G.; Corvol, H.; Beydon, N.; Taytard, J. Respiratory function and sleep in children with myotonic dystrophy type 1. Neuromuscul. Disord. 2023, 33, 263–269. [Google Scholar] [CrossRef]
- Giubilei, F.; Antonini, G.; Bastianello, S.; Morino, S.; Paolillo, A.; Fiorelli, M.; Ferretti, C.; Fieschi, C. Excessive daytime sleepiness in myotonic dystrophy. J. Neurol. Sci. 1999, 164, 60–63. [Google Scholar] [CrossRef]
- Souayah, N.; Tick Chong, P.S.; Dreyer, M.; Cros, D.; Schmahmann, J.D. Myotonic Dystrophy Type 1 Presenting With Ventilatory Failure. J. Clin. Neuromuscul. Dis. 2007, 9, 252–255. [Google Scholar] [CrossRef]
- Kuntawala, D.H.; Martins, F.; Vitorino, R.; Rebelo, S. Automatic Text-Mining Approach to Identify Molecular Target Candidates Associated with Metabolic Processes for Myotonic Dystrophy Type 1. Int. J. Environ. Res. Public Health 2023, 20, 2283. [Google Scholar] [CrossRef] [PubMed]
- Lagrue, E.; Dogan, C.; De Antonio, M.; Audic, F.; Bach, N.; Barnerias, C.; Bellance, R.; Cances, C.; Chabrol, B.; Cuisset, J.-M. A large multicenter study of pediatric myotonic dystrophy type 1 for evidence-based management. Neurology 2019, 92, e852–e865. [Google Scholar] [CrossRef]
- Meola, G.; Sansone, V. Cerebral involvement in myotonic dystrophies. Muscle Nerve Off. J. Am. Assoc. Electrodiagn. Med. 2007, 36, 294–306. [Google Scholar] [CrossRef]
- Ricci, F.S.; Vacchetti, M.; Brusa, C.; D’Alessandro, R.; La Rosa, P.; Martone, G.; Davico, C.; Vitiello, B.; Mongini, T.E. Cognitive, neuropsychological and emotional-behavioural functioning in a sample of children with myotonic dystrophy type 1. Eur. J. Paediatr. Neurol. 2022, 39, 59–64. [Google Scholar] [CrossRef]
- Rosado Bartolomé, A.; Puertas Martín, V.; Domínguez González, C.; Ramos Miranda, M. Alteración cognitiva en la distrofia miotónica tipo 1 (enfermedad de Steinert). Med. Fam. SEMERGEN 2022, 48, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Alsaggaf, R.; St. George, D.M.M.; Zhan, M.; Pfeiffer, R.M.; Wang, Y.; Anderson, L.A.; Liu, Z.; Koshiol, J.; Bauer, A.J.; Wagner, K.R.; et al. Benign tumors in myotonic dystrophy type I target disease-related cancer sites. Ann. Clin. Transl. Neurol. 2019, 6, 1510–1518. [Google Scholar] [CrossRef]
- D’Ambrosio, E.S.; Gonzalez-Perez, P. Cancer and Myotonic Dystrophy. J. Clin. Med. 2023, 12, 1939. [Google Scholar] [CrossRef]
- Fernández-Torrón, R.; García-Puga, M.; Emparanza, J.-I.; Maneiro, M.; Cobo, A.-M.; Poza, J.-J.; Espinal, J.-B.; Zulaica, M.; Ruiz, I.; Martorell, L.; et al. Cancer risk in DM1 is sex-related and linked to miRNA-200/141 downregulation. Neurology 2016, 87, 1250–1257. [Google Scholar] [CrossRef] [PubMed]
- Seo, I.; Park, J.-M. Myotonic dystrophy type 1 in South Korea: A comprehensive analysis of cancer and comorbidity risks. Neurol. Sci. 2024, 45, 4573–4581. [Google Scholar] [CrossRef]
- Morimoto, Y.; Yoshimatsu, A.; Yoshimura, M. Anesthetic management for a patient with myotonic dystrophy with remimazolam. JA Clin. Rep. 2021, 7, 10. [Google Scholar] [CrossRef]
- Kumar, A.; Agarwal, S.; Pradhan, S. Assessment of Premutation in Myotonic Dystrophy Type 1 Affected Family Members by TP-PCR and Genetic Counseling. Case Rep. Med. 2014, 2014, 289643. [Google Scholar] [CrossRef][Green Version]
- Martorell, L.; Monckton, D.G.; Sanchez, A.; Lopez de Munain, A.; Baiget, M. Frequency and stability of the myotonic dystrophy type 1 premutation. Neurology 2001, 56, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Dogan, C.; De Antonio, M.; Hamroun, D.; Varet, H.; Fabbro, M.; Rougier, F.; Amarof, K.; Arne Bes, M.-C.; Bedat-Millet, A.-L.; Behin, A. Gender as a modifying factor influencing myotonic dystrophy type 1 phenotype severity and mortality: A nationwide multiple databases cross-sectional observational study. PLoS ONE 2016, 11, e0148264. [Google Scholar] [CrossRef]
- Mateus, T.; Martins, F.; Nunes, A.; Herdeiro, M.T.; Rebelo, S. Metabolic Alterations in Myotonic Dystrophy Type 1 and Their Correlation with Lipin. Int. J. Environ. Res. Public Health 2021, 18, 1794. [Google Scholar] [CrossRef]
- De Antonio, M.; Dogan, C.; Hamroun, D.; Mati, M.; Zerrouki, S.; Eymard, B.; Katsahian, S.; Bassez, G.; Network, F.M.D.C. Unravelling the myotonic dystrophy type 1 clinical spectrum: A systematic registry-based study with implications for disease classification. Rev. Neurol. 2016, 172, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Ho, G.; Cardamone, M.; Farrar, M. Congenital and childhood myotonic dystrophy: Current aspects of disease and future directions. World J. Clin. Pediatr. 2015, 4, 66–80. [Google Scholar] [CrossRef]
- Johnson, N.E. Myotonic muscular dystrophies. Contin. Lifelong Learn. Neurol. 2019, 25, 1682–1695. [Google Scholar] [CrossRef] [PubMed]
- Ostojić, S.; Kovačević, G.; Meola, G.; Pešović, J.; Savić-Pavićević, D.; Brkušanin, M.; Kravljanac, R.; Perić, M.; Martić, J.; Pejić, K.; et al. Main features and disease outcome of congenital myotonic dystrophy—Experience from a single tertiary center. Neuromuscul. Disord. 2024, 40, 16–23. [Google Scholar] [CrossRef]
- Campbell, C.; Levin, S.; Siu, V.M.; Venance, S.; Jacob, P. Congenital myotonic dystrophy: Canadian population-based surveillance study. J. Pediatr. 2013, 163, 120–125. [Google Scholar] [CrossRef]
- Douniol, M.; Jacquette, A.; Cohen, D.; Bodeau, N.; Rachidi, L.; Angeard, N.; Cuisset, J.-M.; Vallée, L.; Eymard, B.; Plaza, M.; et al. Psychiatric and cognitive phenotype of childhood myotonic dystrophy type 1. Dev. Med. Child Neurol. 2012, 54, 905–911. [Google Scholar] [CrossRef]
- Echenne, B.; Bassez, G. Chapter 144—Congenital and infantile myotonic dystrophy. In Handbook of Clinical Neurology; Dulac, O., Lassonde, M., Sarnat, H.B., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; Volume 113, pp. 1387–1393. Available online: https://www.sciencedirect.com/science/article/pii/B9780444595652000095 (accessed on 7 January 2025).
- Peric, S.; Pesovic, J.; Savic-Pavicevic, D.; Rakocevic Stojanovic, V.; Meola, G. Molecular and Clinical Implications of Variant Repeats in Myotonic Dystrophy Type 1. Int. J. Mol. Sci. 2022, 23, 354. [Google Scholar] [CrossRef] [PubMed]
- Johnson, N.E.; Heatwole, C.R. Myotonic Dystrophy: From Bench to Bedside; Thieme Medical Publishers: New York, NY, USA, 2012; Volume 32, pp. 246–254. [Google Scholar]
- Stokes, M.; Varughese, N.; Iannaccone, S.; Castro, D. Clinical and genetic characteristics of childhood-onset myotonic dystrophy. Muscle Nerve 2019, 60, 732–738. [Google Scholar] [CrossRef]
- Bird, T.D. Myotonic dystrophy type 1. In GeneReviews; University of Washington: Seattle, WA, USA, 2021. [Google Scholar]
- Hasuike, Y.; Mochizuki, H.; Nakamori, M. Cellular Senescence and Aging in Myotonic Dystrophy. Int. J. Mol. Sci. 2022, 23, 2339. [Google Scholar] [CrossRef]
- Mateos-Aierdi, A.J.; Goicoechea, M.; Aiastui, A.; Fernández-Torrón, R.; Garcia-Puga, M.; Matheu, A.; López de Munain, A. Muscle wasting in myotonic dystrophies: A model of premature aging. Front. Aging Neurosci. 2015, 7, 125. [Google Scholar] [CrossRef] [PubMed]
- Winblad, S.; Eliasdottir, O.; Nordström, S.; Lindberg, C. Neurocognitive disorder in Myotonic dystrophy type 1. Heliyon 2024, 10, e30875. [Google Scholar] [CrossRef]
- Yum, K.; Wang, E.T.; Kalsotra, A. Myotonic dystrophy: Disease repeat range, penetrance, age of onset, and relationship between repeat size and phenotypes. Curr. Opin. Genet. Dev. 2017, 44, 30–37. [Google Scholar] [CrossRef]
- Cascais, I.; Garrido, C.; Morais, L.; Amorim, R.; Lima, R.; Mansilha, H.F.; Correia, T.; Oliveira, A.; Santos, M. Myotonic dystrophy type 1 (Steinert disease): 29 years of experience at a tertiary pediatric hospital. Eur. J. Paediatr. Neurol. 2024, 48, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Ho, G.; Carey, K.A.; Cardamone, M.; Farrar, M.A. Myotonic dystrophy type 1: Clinical manifestations in children and adolescents. Arch. Dis. Child. 2019, 104, 48–52. [Google Scholar] [CrossRef]
- Ionova, S.A.; Murtazina, A.F.; Marakhonov, A.A.; Shchagina, O.A.; Ryadninskaya, N.V.; Tebieva, I.S.; Kadyshev, V.V.; Borovikov, A.O.; Ginter, E.K.; Kutsev, S.I.; et al. The Study of the Inheritance Mechanisms of Myotonic Dystrophy Type 1 (DM1) in Families from the Republic of North Ossetia-Alania. Int. J. Mol. Sci. 2024, 25, 9734. [Google Scholar] [CrossRef]
- Joosten, I.B.T.; Hellebrekers, D.M.E.I.; de Greef, B.T.A.; Smeets, H.J.M.; de Die-Smulders, C.E.M.; Faber, C.G.; Gerrits, M.M. Parental repeat length instability in myotonic dystrophy type 1 pre- and protomutations. Eur. J. Hum. Genet. 2020, 28, 956–962. [Google Scholar] [CrossRef]
- Karnebeek, I.E.A.; Boon, H.T.M.; Huis, A.M.P.; Cup, E.H.C.; Eggink, C.A.; Schouten, M.I.; van der Looij, H.J.; van Engelen, B.G.M.; Smulders, F.H.P.; Voermans, N.C. Having an eye for myotonic dystrophy: A qualitative study on experiences and support needs in myotonic dystrophy type 1 patients with a diagnostic delay after early-onset cataract. Neuromuscul. Disord. 2022, 32, 829–835. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.; Berggren, K.N.; Hung, M.; Bates, K.; Dixon, M.M.; Bax, K.; Adams, H.; Butterfield, R.J.; Campbell, C.; Johnson, N.E. Neurobehavioral Phenotype of Children With Congenital Myotonic Dystrophy. Neurology 2024, 102, e208115. [Google Scholar] [CrossRef]
- Soltanzadeh, P. Myotonic Dystrophies: A Genetic Overview. Genes 2022, 13, 367. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wei, Q.; Lin, J.; Shang, H.; Ou, R. Cognitive impairment, neuroimaging abnormalities, and their correlations in myotonic dystrophy: A comprehensive review. Front. Cell. Neurosci. 2024, 18, 1369332. [Google Scholar] [CrossRef] [PubMed]
- Gudde, A.E.E.G.; van Heeringen, S.J.; de Oude, A.I.; van Kessel, I.D.G.; Estabrook, J.; Wang, E.T.; Wieringa, B.; Wansink, D.G. Antisense transcription of the myotonic dystrophy locus yields low-abundant RNAs with and without (CAG)n repeat. RNA Biol. 2017, 14, 1374–1388. [Google Scholar] [CrossRef]
- Izzo, M.; Battistini, J.; Provenzano, C.; Martelli, F.; Cardinali, B.; Falcone, G. Molecular Therapies for Myotonic Dystrophy Type 1: From Small Drugs to Gene Editing. Int. J. Mol. Sci. 2022, 23, 4622. [Google Scholar] [CrossRef]
- Michel, L.; Huguet-Lachon, A.; Gourdon, G. Sense and antisense DMPK RNA foci accumulate in DM1 tissues during development. PLoS ONE 2015, 10, e0137620. [Google Scholar] [CrossRef]
- Goodwin, M.; Mohan, A.; Batra, R.; Lee, K.-Y.; Charizanis, K.; Gómez, F.J.F.; Eddarkaoui, S.; Sergeant, N.; Buée, L.; Kimura, T.; et al. MBNL Sequestration by Toxic RNAs and RNA Misprocessing in the Myotonic Dystrophy Brain. Cell Rep. 2015, 12, 1159–1168. [Google Scholar] [CrossRef]
- Konieczny, P.; Stepniak-Konieczna, E.; Sobczak, K. MBNL proteins and their target RNAs, interaction and splicing regulation. Nucleic Acids Res. 2014, 42, 10873–10887. [Google Scholar] [CrossRef]
- Childs-Disney, J.L.; Stepniak-Konieczna, E.; Tran, T.; Yildirim, I.; Park, H.; Chen, C.Z.; Hoskins, J.; Southall, N.; Marugan, J.J.; Patnaik, S.; et al. Induction and reversal of myotonic dystrophy type 1 pre-mRNA splicing defects by small molecules. Nat. Commun. 2013, 4, 2044. [Google Scholar] [CrossRef]
- López-Martínez, A.; Soblechero-Martín, P.; de-la-Puente-Ovejero, L.; Nogales-Gadea, G.; Arechavala-Gomeza, V. An Overview of Alternative Splicing Defects Implicated in Myotonic Dystrophy Type I. Genes 2020, 11, 1109. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Mankodi, A.; Swanson, M.S.; Moxley, R.T.; Thornton, C.A. Myotonic dystrophy type 1 is associated with nuclear foci of mutant RNA, sequestration of muscleblind proteins and deregulated alternative splicing in neurons. Hum. Mol. Genet. 2004, 13, 3079–3088. [Google Scholar] [CrossRef] [PubMed]
- Gramegna, L.L.; Giannoccaro, M.P.; Manners, D.N.; Testa, C.; Zanigni, S.; Evangelisti, S.; Bianchini, C.; Oppi, F.; Poda, R.; Avoni, P.; et al. Mitochondrial dysfunction in myotonic dystrophy type 1. Neuromuscul. Disord. 2018, 28, 144–149. [Google Scholar] [CrossRef]
- García-Puga, M.; Saenz-Antoñanzas, A.; Fernández-Torrón, R.; de Munain, A.L.; Matheu, A. Myotonic Dystrophy type 1 cells display impaired metabolism and mitochondrial dysfunction that are reversed by metformin. Aging 2020, 12, 6260–6275. [Google Scholar] [CrossRef]
- Kajdasz, A.; Niewiadomska, D.; Sekrecki, M.; Sobczak, K. Distribution of alternative untranslated regions within the mRNA of the CELF1 splicing factor affects its expression. Sci. Rep. 2022, 12, 190. [Google Scholar] [CrossRef]
- Timchenko, L. Correction of RNA-Binding Protein CUGBP1 and GSK3β Signaling as Therapeutic Approach for Congenital and Adult Myotonic Dystrophy Type 1. Int. J. Mol. Sci. 2020, 21, 94. [Google Scholar] [CrossRef]
- Wojciechowska, M.; Taylor, K.; Sobczak, K.; Napierala, M.; Krzyzosiak, W.J. Small molecule kinase inhibitors alleviate different molecular features of myotonic dystrophy type 1. RNA Biol. 2014, 11, 742–754. [Google Scholar] [CrossRef]
- Kuyumcu-Martinez, N.M.; Wang, G.-S.; Cooper, T.A. Increased Steady-State Levels of CUGBP1 in Myotonic Dystrophy 1 Are Due to PKC-Mediated Hyperphosphorylation. Mol. Cell 2007, 28, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.T.; Ward, A.J.; Cherone, J.M.; Giudice, J.; Wang, T.T.; Treacy, D.J.; Lambert, N.J.; Freese, P.; Saxena, T.; Cooper, T.A. Antagonistic regulation of mRNA expression and splicing by CELF and MBNL proteins. Genome Res. 2015, 25, 858–871. [Google Scholar] [CrossRef]
- Kino, Y.; Washizu, C.; Oma, Y.; Onishi, H.; Nezu, Y.; Sasagawa, N.; Nukina, N.; Ishiura, S. MBNL and CELF proteins regulate alternative splicing of the skeletal muscle chloride channel CLCN1. Nucleic Acids Res. 2009, 37, 6477–6490. [Google Scholar] [CrossRef]
- Tahraoui-Bories, J.; Mérien, A.; González-Barriga, A.; Lainé, J.; Leteur, C.; Polvèche, H.; Carteron, A.; De Lamotte, J.D.; Nicoleau, C.; Polentes, J.; et al. MBNL-dependent impaired development within the neuromuscular system in myotonic dystrophy type 1. Neuropathol. Appl. Neurobiol. 2023, 49, e12876. [Google Scholar] [CrossRef]
- Ebralidze, A.; Wang, Y.; Petkova, V.; Ebralidse, K.; Junghans, R.P. RNA Leaching of Transcription Factors Disrupts Transcription in Myotonic Dystrophy. Science 2004, 303, 383–387. [Google Scholar] [CrossRef]
- Imbriano, C.; Molinari, S. Alternative Splicing of Transcription Factors Genes in Muscle Physiology and Pathology. Genes 2018, 9, 107. [Google Scholar] [CrossRef] [PubMed]
- Osborne, R.J.; Lin, X.; Welle, S.; Sobczak, K.; O’Rourke, J.R.; Swanson, M.S.; Thornton, C.A. Transcriptional and post-transcriptional impact of toxic RNA in myotonic dystrophy. Hum. Mol. Genet. 2009, 18, 1471–1481. [Google Scholar] [CrossRef] [PubMed]
- Magaña, J.J.; Suárez-Sánchez, R.; Leyva-García, N.; Cisneros, B.; Hernández-Hernández, O. Myotonic Dystrophy Protein Kinase: Structure, Function and Its Possible Role in the Pathogenesis of Myotonic Dystrophy Type 1. Adv. Protein Kinases 2012, 213–242. [Google Scholar] [CrossRef][Green Version]
- Perbellini, R.; Greco, S.; Sarra-Ferraris, G.; Cardani, R.; Capogrossi, M.C.; Meola, G.; Martelli, F. Dysregulation and cellular mislocalization of specific miRNAs in myotonic dystrophy type 1. Neuromuscul. Disord. 2011, 21, 81–88. [Google Scholar] [CrossRef]
- Costa, A.; Cruz, A.C.; Martins, F.; Rebelo, S. Protein Phosphorylation Alterations in Myotonic Dystrophy Type 1: A Systematic Review. Int. J. Mol. Sci. 2023, 24, 3091. [Google Scholar] [CrossRef]
- Sahoo, S.K.; Shaikh, S.A.; Sopariwala, D.H.; Bal, N.C.; Periasamy, M. Sarcolipin Protein Interaction with Sarco(endo)plasmic Reticulum Ca2+ATPase (SERCA) Is Distinct from Phospholamban Protein, and Only Sarcolipin Can Promote Uncoupling of the SERCA Pump. J. Biol. Chem. 2013, 288, 6881–6889. [Google Scholar] [CrossRef]
- Shaikh, S.A.; Sahoo, S.K.; Periasamy, M. Phospholamban and sarcolipin: Are they functionally redundant or distinct regulators of the Sarco(Endo)Plasmic Reticulum Calcium ATPase? J. Mol. Cell. Cardiol. 2016, 91, 81–91. [Google Scholar] [CrossRef]
- Haeusler, A.R.; Donnelly, C.J.; Periz, G.; Simko, E.A.; Shaw, P.G.; Kim, M.-S.; Maragakis, N.J.; Troncoso, J.C.; Pandey, A.; Sattler, R. C9orf72 nucleotide repeat structures initiate molecular cascades of disease. Nature 2014, 507, 195–200. [Google Scholar] [CrossRef]
- Swinnen, B.; Robberecht, W.; Van Den Bosch, L. RNA toxicity in non-coding repeat expansion disorders. EMBO J. 2020, 39, e101112. [Google Scholar] [CrossRef] [PubMed]
- Zu, T.; Gibbens, B.; Doty, N.S.; Gomes-Pereira, M.; Huguet, A.; Stone, M.D.; Margolis, J.; Peterson, M.; Markowski, T.W.; Ingram, M.A. Non-ATG–initiated translation directed by microsatellite expansions. Proc. Natl. Acad. Sci. USA 2011, 108, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Banez-Coronel, M.; Ranum, L.P. Repeat-associated non-AUG (RAN) translation: Insights from pathology. Lab. Investig. 2019, 99, 929–942. [Google Scholar] [CrossRef]
- Koehorst, E.; Núñez-Manchón, J.; Ballester-López, A.; Almendrote, M.; Lucente, G.; Arbex, A.; Chojnacki, J.; Vázquez-Manrique, R.P.; Gómez-Escribano, A.P.; Pintos-Morell, G. Characterization of RAN translation and antisense transcription in primary cell cultures of patients with myotonic dystrophy type 1. J. Clin. Med. 2021, 10, 5520. [Google Scholar] [CrossRef]
- Kalsotra, A.; Singh, R.K.; Gurha, P.; Ward, A.J.; Creighton, C.J.; Cooper, T.A. The Mef2 Transcription Network Is Disrupted in Myotonic Dystrophy Heart Tissue, Dramatically Altering miRNA and mRNA Expression. Cell Rep. 2014, 6, 336–345. [Google Scholar] [CrossRef]
- Burks, T.N.; Cohn, R.D. Role of TGF-β signaling in inherited and acquired myopathies. Skelet. Muscle 2011, 1, 19. [Google Scholar] [CrossRef] [PubMed]
- Boyer, J.G.; Prasad, V.; Song, T.; Lee, D.; Fu, X.; Grimes, K.M.; Sargent, M.A.; Sadayappan, S.; Molkentin, J.D. ERK1/2 signaling induces skeletal muscle slow fiber-type switching and reduces muscular dystrophy disease severity. JCI Insight 2019, 4, e127356. [Google Scholar] [CrossRef]
- Beffy, P.; Del Carratore, R.; Masini, M.; Furling, D.; Puymirat, J.; Masiello, P.; Simili, M. Altered signal transduction pathways and induction of autophagy in human myotonic dystrophy type 1 myoblasts. Int. J. Biochem. Cell Biol. 2010, 42, 1973–1983. [Google Scholar] [CrossRef]
- Brockhoff, M.; Rion, N.; Chojnowska, K.; Wiktorowicz, T.; Eickhorst, C.; Erne, B.; Frank, S.; Angelini, C.; Furling, D.; Rüegg, M.A. Targeting deregulated AMPK/mTORC1 pathways improves muscle function in myotonic dystrophy type I. J. Clin. Investig. 2017, 127, 549–563. [Google Scholar] [CrossRef]
- Braun, M.; Shoshani, S.; Tabach, Y. Transcriptome changes in DM1 patients’ tissues are governed by the RNA interference pathway. Front. Mol. Biosci. 2022, 9, 955753. [Google Scholar] [CrossRef]
- Todorow, V.; Hintze, S.; Schoser, B.; Meinke, P. Nuclear envelope transmembrane proteins involved in genome organization are misregulated in myotonic dystrophy type 1 muscle. Front. Cell Dev. Biol. 2023, 10, 1007331. [Google Scholar] [CrossRef] [PubMed]
- Meinke, P.; Hintze, S.; Limmer, S.; Schoser, B. Myotonic Dystrophy—A Progeroid Disease? Front. Neurol. 2018, 9, 601. [Google Scholar] [CrossRef]
- Takeshima, K.; Ariyasu, H.; Ishibashi, T.; Kawai, S.; Uraki, S.; Koh, J.; Ito, H.; Akamizu, T. Myotonic dystrophy type 1 with diabetes mellitus, mixed hypogonadism and adrenal insufficiency. Endocrinol. Diabetes Metab. Case Rep. 2018, 2018, 17-0143. [Google Scholar] [CrossRef]
- Viegas, D.; Pereira, C.D.; Martins, F.; Mateus, T.; da Cruz e Silva, O.A.B.; Herdeiro, M.T.; Rebelo, S. Nuclear Envelope Alterations in Myotonic Dystrophy Type 1 Patient-Derived Fibroblasts. Int. J. Mol. Sci. 2022, 23, 522. [Google Scholar] [CrossRef] [PubMed]
- Harmon, E.B.; Harmon, M.L.; Larsen, T.D.; Yang, J.; Glasford, J.W.; Perryman, M.B. Myotonic Dystrophy Protein Kinase Is Critical for Nuclear Envelope Integrity. J. Biol. Chem. 2011, 286, 40296–40306. [Google Scholar] [CrossRef]
- Meinke, P.; Schirmer, E.C. The increasing relevance of nuclear envelope myopathies. Curr. Opin. Neurol. 2016, 29, 651–661. [Google Scholar] [CrossRef] [PubMed]
- Bitetto, G.; Di Fonzo, A. Nucleo–cytoplasmic transport defects and protein aggregates in neurodegeneration. Transl. Neurodegener. 2020, 9, 25. [Google Scholar] [CrossRef]
- Bonnet, A.; Palancade, B. Regulation of mRNA Trafficking by Nuclear Pore Complexes. Genes 2014, 5, 767–791. [Google Scholar] [CrossRef]
- Holt, I.; Mittal, S.; Furling, D.; Butler-Browne, G.S.; David Brook, J.; Morris, G.E. Defective mRNA in myotonic dystrophy accumulates at the periphery of nuclear splicing speckles. Genes Cells 2007, 12, 1035–1048. [Google Scholar] [CrossRef]
- Bhatti, J.S.; Bhatti, G.K.; Reddy, P.H. Mitochondrial dysfunction and oxidative stress in metabolic disorders—A step towards mitochondria based therapeutic strategies. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2017, 1863, 1066–1077. [Google Scholar] [CrossRef]
- Heher, P.; Ganassi, M.; Weidinger, A.; Engquist, E.N.; Pruller, J.; Nguyen, T.H.; Tassin, A.; Declèves, A.-E.; Mamchaoui, K.; Banerji, C.R.S.; et al. Interplay between mitochondrial reactive oxygen species, oxidative stress and hypoxic adaptation in facioscapulohumeral muscular dystrophy: Metabolic stress as potential therapeutic target. Redox Biol. 2022, 51, 102251. [Google Scholar] [CrossRef] [PubMed]
- Toscano, A.; Messina, S.; Campo, G.M.; Leo, R.D.; Musumeci, O.; Rodolico, C.; Aguennouz, M.; Annesi, G.; Messina, C.; Vita, G. Oxidative stress in myotonic dystrophy type 1. Free Radic. Res. 2005, 39, 771–776. [Google Scholar] [CrossRef]
- Ricker, K.; Grimm, T.; Koch, M.; Schneider, C.; Kress, W.; Reimers, C.; Schulte-Mattler, W.; Mueller-Myhsok, B.; Toyka, K.; Mueller, C. Linkage of proximal myotonic myopathy to chromosome 3q. Neurology 1999, 52, 170. [Google Scholar] [CrossRef]
- Fujino, H.; Saito, T.; Takahashi, M.P.; Takada, H.; Nakayama, T.; Imura, O.; Matsumura, T. Quality of life and subjective symptom impact in Japanese patients with myotonic dystrophy type 1. BMC Neurol. 2022, 22, 55. [Google Scholar] [CrossRef]
- D’Mello, S.; Shum, L. A review of the use of mexiletine in patients with myotonic dystrophy and non-dystrophic myotonia. Eur. J. Hosp. Pharm. 2016, 23, 359–363. [Google Scholar] [CrossRef] [PubMed]
- Heatwole, C.; Luebbe, E.; Rosero, S.; Eichinger, K.; Martens, W.; Hilbert, J.; Dekdebrun, J.; Dilek, N.; Zizzi, C.; Johnson, N.; et al. Mexiletine in Myotonic Dystrophy Type 1. Neurology 2021, 96, e228–e240. [Google Scholar] [CrossRef]
- Logigian, E.L.; Martens, W.B.; Moxley, R.T.; McDermott, M.P.; Dilek, N.; Wiegner, A.W.; Pearson, A.T.; Barbieri, C.A.; Annis, C.L.; Thornton, C.A.; et al. Mexiletine is an effective antimyotonia treatment in myotonic dystrophy type 1. Neurology 2010, 74, 1441–1448. [Google Scholar] [CrossRef] [PubMed]
- Ashizawa, T.; Gagnon, C.; Groh, W.J.; Gutmann, L.; Johnson, N.E.; Meola, G.; Moxley III, R.; Pandya, S.; Rogers, M.T.; Simpson, E. Consensus-based care recommendations for adults with myotonic dystrophy type 1. Neurol. Clin. Pract. 2018, 8, 507–520. [Google Scholar] [CrossRef]
- McBride, D.; Deshmukh, A.; Shore, S.; Elafros, M.A.; Liang, J.J. Cardiac Involvement and Arrhythmias Associated with Myotonic Dystrophy. RCM 2022, 23, 126. [Google Scholar] [CrossRef]
- Frank, A.O.; De Souza, L.H. Clinical features of children and adults with a muscular dystrophy using powered indoor/outdoor wheelchairs: Disease features, comorbidities and complications of disability. Disabil. Rehabil. 2018, 40, 1007–1013. [Google Scholar] [CrossRef]
- Tripathi, A.; Miliken, A. Myotonic Dystrophy Type 1: A Comprehensive Literary Review. J. Stud. Res. 2023, 12. [Google Scholar] [CrossRef]
- Roussel, M.-P.; Morin, M.; Gagnon, C.; Duchesne, E. What is known about the effects of exercise or training to reduce skeletal muscle impairments of patients with myotonic dystrophy type 1? A scoping review. BMC Musculoskelet. Disord. 2019, 20, 101. [Google Scholar] [CrossRef]
- Heatwole, C.R.; Eichinger, K.J.; Friedman, D.I.; Hilbert, J.E.; Jackson, C.E.; Logigian, E.L.; Martens, W.B.; McDermott, M.P.; Pandya, S.K.; Quinn, C. Open-label trial of recombinant human insulin-like growth factor 1/recombinant human insulin-like growth factor binding protein 3 in myotonic dystrophy type 1. Arch. Neurol. 2011, 68, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Pénisson-Besnier, I.; Devillers, M.; Porcher, R.; Orlikowski, D.; Doppler, V.; Desnuelle, C.; Ferrer, X.; Bes, M.-C.; Bouhour, F.; Tranchant, C. Dehydroepiandrosterone for myotonic dystrophy type 1. Neurology 2008, 71, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Tarnopolsky, M.; Mahoney, D.; Thompson, T.; Naylor, H.; Doherty, T.J. Creatine monohydrate supplementation does not increase muscle strength, lean body mass, or muscle phosphocreatine in patients with myotonic dystrophy type 1. Muscle Nerve 2004, 29, 51–58. [Google Scholar] [CrossRef]
- Walter, M.C.; Reilich, P.; Lochmüller, H.; Kohnen, R.; Schlotter, B.; Hautmann, H.; Dunkl, E.; Pongratz, D.; Müller-Felber, W. Creatine monohydrate in myotonic dystrophy: A double-blind, placebo-controlled clinical study. J. Neurol. 2002, 249, 1717–1722. [Google Scholar] [CrossRef] [PubMed]
- Ahsan, M.; Garneau, L.; Aguer, C. The bidirectional relationship between AMPK pathway activation and myokine secretion in skeletal muscle: How it affects energy metabolism. Front. Physiol. 2022, 13, 1040809. [Google Scholar] [CrossRef]
- Ravel-Chapuis, A.; Jasmin, B.J. Combinatorial therapies for rescuing myotonic dystrophy type 1 skeletal muscle defects. Trends Mol. Med. 2022, 28, 439–442. [Google Scholar] [CrossRef]
- Silva, S.F.M.; de Magalhães, H.L.; de Deus, F.A.; Andrade, K.K.S.; Lima, V.P.; Gaiad, T.P. Rehabilitation interventions targeting the activity and participation of patient with neuromuscular diseases: What do we know? A systematic review. Arq. Neuropsiquiatr. 2024, 82, s00441779295. [Google Scholar] [CrossRef]
- Mackenzie, S.J.; Hamel, J.; Thornton, C.A. Benefits of aerobic exercise in myotonic dystrophy type 1. J. Clin. Investig. 2022, 132, e160229. [Google Scholar] [CrossRef]
- Mikhail, A.I.; Nagy, P.L.; Manta, K.; Rouse, N.; Manta, A.; Ng, S.Y.; Nagy, M.F.; Smith, P.; Lu, J.-Q.; Nederveen, J.P. Aerobic exercise elicits clinical adaptations in myotonic dystrophy type 1 patients independently of pathophysiological changes. J. Clin. Investig. 2022, 132, e156125. [Google Scholar] [CrossRef] [PubMed]
- Whittaker, R.G.; Ferenczi, E.; Hilton-Jones, D. Myotonic dystrophy: Practical issues relating to assessment of strength. J. Neurol. Neurosurg. Psychiatry 2006, 77, 1282–1283. [Google Scholar] [CrossRef]
- Mateus, T.; Costa, A.; Viegas, D.; Marques, A.; Herdeiro, M.T.; Rebelo, S. Outcome measures frequently used to assess muscle strength in patients with myotonic dystrophy type 1: A systematic review. Neuromuscul. Disord. 2022, 32, 99–115. [Google Scholar] [CrossRef] [PubMed]
- Hartog, L.; Zhao, J.; Reynolds, J.; Brokamp, G.; Vilson, F.; Arnold, W.D.; LoRusso, S. Factors Influencing the Severity and Progression of Respiratory Muscle Dysfunction in Myotonic Dystrophy Type 1. Front. Neurol. 2021, 12, 658532. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, J.; Allard, P.; Potvin, L.; Prevost, C.; Begin, P. A 10-year study of mortality in a cohort of patients with myotonic dystrophy. Neurology 1999, 52, 1658. [Google Scholar] [CrossRef]
- Poussel, M.; Kaminsky, P.; Renaud, P.; Laroppe, J.; Pruna, L.; Chenuel, B. Supine changes in lung function correlate with chronic respiratory failure in myotonic dystrophy patients. Respir. Physiol. Neurobiol. 2014, 193, 43–51. [Google Scholar] [CrossRef]
- Sansone, V.; Gagnon, C. 207th ENMC Workshop on chronic respiratory insufficiency in myotonic dystrophies: Management and implications for research, 27–29 June 2014, Naarden, The Netherlands. Neuromuscul. Disord. 2015, 25, 432–442. [Google Scholar] [CrossRef]
- Pfeffer, G.; Povitz, M.; Gibson, G.J.; Chinnery, P.F. Diagnosis of muscle diseases presenting with early respiratory failure. J. Neurol. 2015, 262, 1101–1114. [Google Scholar] [CrossRef]
- West, S.D.; Lochmüller, H.; Hughes, J.; Atalaia, A.; Marini-Bettolo, C.; Baudouin, S.V.; Anderson, K.N. Sleepiness and sleep-related breathing disorders in myotonic dystrophy and responses to treatment: A prospective cohort study. J. Neuromuscul. Dis. 2016, 3, 529–537. [Google Scholar] [CrossRef]
- Dhand, U.K.; Dhand, R. Sleep disorders in neuromuscular diseases. Curr. Opin. Pulm. Med. 2006, 12, 402–408. [Google Scholar] [CrossRef]
- Seshagiri, D.V.; Huddar, A.; Nashi, S.; Ray, S.; Ramaswamy, P.; Oommen, A.T.; Chawla, T.; Yadav, S.; Annapureddy, J.; Jankar, R.; et al. Altered REM sleep architecture in patients with Myotonic dystrophy type 1: Is related to sleep apnea? Sleep Med. 2021, 79, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Drager, L.F.; McEvoy, R.D.; Barbe, F.; Lorenzi-Filho, G.; Redline, S. Sleep apnea and cardiovascular disease: Lessons from recent trials and need for team science. Circulation 2017, 136, 1840–1850. [Google Scholar] [CrossRef] [PubMed]
- Londoño-Tobón, L.; García, C.A.O. Pneumonia and de novo atrial fibrillation in a patient with myotonic dystrophy type 1: A case report. Medicine 2022, 101, e30518. [Google Scholar] [CrossRef]
- Erokhina, E.K.; Melnik, E.A.; Lebedeva, D.D.; Shamtieva, K.V.; Peters, T.V.; Pavlikova, E.P.; Gepard, V.V.; Vlodavets, D.V. Sleep Disorders and Fatigue in Patients with Different Forms of Myotonic Dystrophy Type 1. Neurosci. Behav. Physiol. 2024, 54, 35–40. [Google Scholar] [CrossRef]
- Zhang, Y.; Ren, R.; Yang, L.; Jin, H.; Nie, Y.; Zhang, H.; Shi, Y.; Sanford, L.D.; Vitiello, M.V.; Tang, X. Polysomnographic findings of myotonic dystrophy type 1/type 2: Evidence from case–control studies. Sleep 2024, 47, zsad280. [Google Scholar] [CrossRef]
- Vosse, B.A.; Seijger, C.; Cobben, N.; van Engelen, B.; van Kuijk, S.M.; Faber, C.; Wijkstra, P. Noninvasive home mechanical ventilation in adult myotonic dystrophy type 1: A systematic review. Respiration 2021, 100, 816–825. [Google Scholar] [CrossRef]
- Vosse, B.A.H.; Horlings, C.G.C.; Joosten, I.B.T.; Cobben, N.A.M.; van Kuijk, S.M.J.; Wijkstra, P.J.; Faber, C.G. Role of respiratory characteristics in treatment adherence with noninvasive home mechanical ventilation in myotonic dystrophy type 1, a retrospective study. Neuromuscul. Disord. 2023, 33, 57–62. [Google Scholar] [CrossRef]
- Boentert, M.; Cao, M.; Mass, D.; De Mattia, E.; Falcier, E.; Goncalves, M.; Holland, V.; Katz, S.L.; Orlikowski, D.; Sannicolò, G. Consensus-based care recommendations for pulmonologists treating adults with myotonic dystrophy type 1. Respiration 2020, 99, 360–368. [Google Scholar] [CrossRef]
- Subramony, S.H.; Wymer, J.P.; Pinto, B.S.; Wang, E.T. Sleep disorders in myotonic dystrophies. Muscle Nerve 2020, 62, 309–320. [Google Scholar] [CrossRef]
- Assessing the Effect of the MYODM Food Supplement on Quality of Life, Fatigue and Hypersomnia in Patients with Myotonic Dystrophy Type 1. 2020. Available online: https://clinicaltrials.gov/study/NCT04634682 (accessed on 9 November 2020).
- Puymirat, J.; Bouchard, J.-P.; Mathieu, J. Efficacy and tolerability of a 20-mg dose of methylphenidate for the treatment of daytime sleepiness in adult patients with myotonic dystrophy type 1: A 2-center, randomized, double-blind, placebo-controlled, 3-week crossover trial. Clin. Ther. 2012, 34, 1103–1111. [Google Scholar] [CrossRef]
- Pelargonio, G.; Russo, A.D.; Sanna, T.; De Martino, G.; Bellocci, F. Myotonic dystrophy and the heart. Heart 2002, 88, 665–670. [Google Scholar] [CrossRef] [PubMed]
- Russo, V.; Antonini, G.; Massa, R.; Casali, C.; Mauriello, A.; Martino, A.M.; Marconi, R.; Garibaldi, M.; Franciosa, P.; Zecchin, M.; et al. Comprehensive Cardiovascular Management of Myotonic Dystrophy Type 1 Patients: A Report from the Italian Neuro-Cardiology Network. J. Cardiovasc. Dev. Dis. 2024, 11, 63. [Google Scholar] [CrossRef] [PubMed]
- Petri, H.; Vissing, J.; Witting, N.; Bundgaard, H.; Køber, L. Cardiac manifestations of myotonic dystrophy type 1. Int. J. Cardiol. 2012, 160, 82–88. [Google Scholar] [CrossRef]
- Russo, V.; Capolongo, A.; Bottino, R.; Carbone, A.; Palladino, A.; Liccardo, B.; Nigro, G.; Marchel, M.; Golino, P.; D’Andrea, A. Echocardiographic Features of Cardiac Involvement in Myotonic Dystrophy 1: Prevalence and Prognostic Value. J. Clin. Med. 2023, 12, 1947. [Google Scholar] [CrossRef]
- Russo, V.; Papa, A.A.; Lioncino, M.; Rago, A.; Di Fraia, F.; Palladino, A.; Politano, L.; Golino, P.; Nigro, G. Prevalence of atrial fibrillation in myotonic dystrophy type 1: A systematic review. Neuromuscul. Disord. 2021, 31, 281–290. [Google Scholar] [CrossRef]
- Russo, V.; Papa, A.A.; Williams, E.A.; Rago, A.; Palladino, A.; Politano, L.; Nigro, G. ACE inhibition to slow progression of myocardial fibrosis in muscular dystrophies. Trends Cardiovasc. Med. 2018, 28, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Russo, V.; Papa, A.A.; Rago, A.; Ciardiello, C.; Martino, A.M.; Stazi, A.; Golino, P.; Calò, L.; Nigro, G. Arrhythmic CArdiac DEath in MYotonic dystrophy type 1 patients (ACADEMY 1) study: The predictive role of programmed ventricular stimulation. EP Eur. 2022, 24, 1148–1155. [Google Scholar] [CrossRef]
- Petri, H.; Witting, N.; Ersbøll, M.K.; Sajadieh, A.; Dunø, M.; Helweg-Larsen, S.; Vissing, J.; Køber, L.; Bundgaard, H. High prevalence of cardiac involvement in patients with myotonic dystrophy type 1: A cross-sectional study. Int. J. Cardiol. 2014, 174, 31–36. [Google Scholar] [CrossRef]
- Petri, H.; Mohammad, B.J.Y.; Kristensen, A.T.; Thune, J.J.; Vissing, J.; Køber, L.; Witting, N.; Bundgaard, H.; Christensen, A.H. Natural history of cardiac involvement in myotonic dystrophy type 1—Emphasis on the need for lifelong follow-up. Int. J. Cardiol. 2024, 406, 132070. [Google Scholar] [CrossRef]
- McNally, E.M.; Mann, D.L.; Pinto, Y.; Bhakta, D.; Tomaselli, G.; Nazarian, S.; Groh, W.J.; Tamura, T.; Duboc, D.; Itoh, H.; et al. Clinical Care Recommendations for Cardiologists Treating Adults with Myotonic Dystrophy. J. Am. Heart Assoc. 2020, 9, e014006. [Google Scholar] [CrossRef]
- Blaszczyk, E.; Lim, C.; Kellman, P.; Schmacht, L.; Gröschel, J.; Spuler, S.; Schulz-Menger, J. Progressive myocardial injury in myotonic dystrophy type II and facioscapulohumeral muscular dystrophy 1: A cardiovascular magnetic resonance follow-up study. J. Cardiovasc. Magn. Reson. 2021, 23, 130. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, S.A.; Ward, C.C.; Al-Khatib, S.M. The use of implantable cardioverter-defibrillators in the prevention of sudden cardiac death: A focus on congenital heart disease and inherited arrhythmia syndromes. J. Innov. Card. Rhythm Manag. 2018, 9, 2996–3005. [Google Scholar] [CrossRef] [PubMed]
- Leong, A.M.; Arnold, A.D.; Whinnett, Z.I. Implantable Cardioverter Defibrillator Tachycardia Therapies: Past, Present and Future Directions. J. Cardiovasc. Dev. Dis. 2024, 11, 92. [Google Scholar] [CrossRef]
- Russo, V.; Papa, A.A.; Rago, A.; Ciardiello, C.; Nigro, G. Effect of dual-chamber minimal ventricular pacing on paroxysmal atrial fibrillation incidence in myotonic dystrophy type 1 patients: A prospective, randomized, single-blind, crossover study. Heart Rhythm. 2018, 15, 962–968. [Google Scholar] [CrossRef]
- Frommeyer, G.; Garthmann, J.; Ellermann, C.; Dechering, D.G.; Kochhäuser, S.; Reinke, F.; Köbe, J.; Wasmer, K.; Eckardt, L. Broad antiarrhythmic effect of mexiletine in different arrhythmia models. EP Eur. 2018, 20, 1375–1381. [Google Scholar] [CrossRef]
- Wahbi, K.; Bassez, G.; Duchateau, J.; Salort-Campana, E.; Vicart, S.; Desaphy, J.-F.; Labombarda, F.; Sellal, J.-M.; Deharo, J.-C. Expert opinion on mexiletine treatment in adult patients with myotonic dystrophy. Arch. Cardiovasc. Dis. 2024, 117, 450–456. [Google Scholar] [CrossRef]
- Tracy, C.M.; Epstein, A.E.; Darbar, D.; DiMarco, J.P.; Dunbar, S.B.; Estes, N.A.M.; Ferguson, T.B.; Hammill, S.C.; Karasik, P.E.; Link, M.S.; et al. 2012 ACCF/AHA/HRS Focused Update Incorporated into the ACCF/AHA/HRS 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J. Am. Coll. Cardiol. 2013, 61, e6–e75. [Google Scholar]
- Wahbi, K.; Meune, C.; Porcher, R.; Bécane, H.M.; Lazarus, A.; Laforêt, P.; Stojkovic, T.; Béhin, A.; Radvanyi-Hoffmann, H.; Eymard, B. Electrophysiological study with prophylactic pacing and survival in adults with myotonic dystrophy and conduction system disease. JAMA 2012, 307, 1292–1301. [Google Scholar] [CrossRef]
- Mahdavi, M.; Prévost, K.; Balthazar, P.; Hus, I.F.-P.; Duchesne, É.; Dumont, N.; Gagné-Ouellet, V.; Gagnon, C.; Laforest-Lapointe, I.; Massé, E. Disturbance of the human gut microbiota in patients with Myotonic Dystrophy type 1. Comput. Struct. Biotechnol. J. 2024, 23, 2097–2108. [Google Scholar] [CrossRef]
- Peterson, J.A.M.; Cooper, T.A. Clinical and Molecular Insights into Gastrointestinal Dysfunction in Myotonic Dystrophy Types 1 & 2. Int. J. Mol. Sci. 2022, 23, 14779. [Google Scholar]
- Hilbert, J.E.; Barohn, R.J.; Clemens, P.R.; Luebbe, E.A.; Martens, W.B.; McDermott, M.P.; Parkhill, A.L.; Tawil, R.; Thornton, C.A.; Moxley III, R.T. High frequency of gastrointestinal manifestations in myotonic dystrophy type 1 and type 2. Neurology 2017, 89, 1348–1354. [Google Scholar] [CrossRef] [PubMed]
- Ercolin, B.; Sassi, F.C.; Mangilli, L.D.; Mendonça, L.I.Z.; Limongi, S.C.O.; de Andrade, C.R.F. Oral Motor Movements and Swallowing in Patients with Myotonic Dystrophy Type 1. Dysphagia 2013, 28, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Fisette-Paulhus, I.; Gagnon, C.; Girard-Côté, L.; Morin, M. Genitourinary and lower gastrointestinal conditions in patients with myotonic dystrophy type 1: A systematic review of evidence and implications for clinical practice. Neuromuscul. Disord. 2022, 32, 361–376. [Google Scholar] [CrossRef]
- Tieleman, A.A.; van Vliet, J.; Jansen, J.B.M.J.; van der Kooi, A.J.; Borm, G.F.; van Engelen, B.G.M. Gastrointestinal involvement is frequent in Myotonic Dystrophy type 2. Neuromuscul. Disord. 2008, 18, 646–649. [Google Scholar] [CrossRef]
- Marcon, M.; Briani, C.; Ermani, M.; Menegazzo, E.; Iurilli, V.; Feltrin, G.; Novelli, G.; Gennarelli, M.; Angelini, C. Positive correlation of CTG expansion and pharyngoesophageal alterations in myotonic dystrophy patients. Ital. J. Neurol. Sci. 1998, 19, 75–80. [Google Scholar] [CrossRef]
- Wu, J.; Wang, K.; Wang, X.; Pang, Y.; Jiang, C. The role of the gut microbiome and its metabolites in metabolic diseases. Protein Cell 2021, 12, 360–373. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Zogg, H.; Wei, L.; Bartlett, A.; Ghoshal, U.C.; Rajender, S.; Ro, S. Gut Microbial Dysbiosis in the Pathogenesis of Gastrointestinal Dysmotility and Metabolic Disorders. J. Neurogastroenterol. Motil. 2021, 27, 19–34. [Google Scholar] [CrossRef]
- Argov, Z.; de Visser, M. Dysphagia in adult myopathies. Neuromuscul. Disord. 2021, 31, 5–20. [Google Scholar] [CrossRef]
- Jones, K.; Pitceathly, R.; Rose, M.; McGowan, S.; Hill, M.; Badrising, U.; Hughes, T. Interventions for dysphagia in long-term, progressive muscle disease. Cochrane Database Syst. Rev. 2016, 2016, CD004303. [Google Scholar] [CrossRef]
- Pilz, W.; Baijens, L.W.; Kremer, B. Oropharyngeal dysphagia in myotonic dystrophy type 1: A systematic review. Dysphagia 2014, 29, 319–331. [Google Scholar] [CrossRef]
- Annunziata, A.; Valente, T.; Cauteruccio, R.; Fiorentino, G. Silent dysphagia in two patients with Steinert disease and recurrent respiratory exacerbations. Acta Myol. 2020, 39, 141–143. [Google Scholar] [PubMed]
- Dipasquale, V.; Morello, R.; Romano, C. Gastrointestinal and nutritional care in pediatric neuromuscular disorders. World J. Clin. Pediatr. 2023, 12, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Bellini, M.; Biagi, S.; Stasi, C.; Costa, F.; Mumolo, M.G.; Ricchiuti, A.; Marchi, S. Gastrointestinal manifestations in myotonic muscular dystrophy. World J. Gastroenterol. 2006, 12, 1821–1828. [Google Scholar] [CrossRef] [PubMed]
- Maagdenberg, S.J.M.; Klinkenberg, S.; Sophie van den Berg, J.; Altena-Rensen, S.; Vrijens, D.; Janssen, E.J.M.; Gierenz, N.; de Wall, L.L.; Braakman, H.M.H. Impact of gastrointestinal and urological symptoms in children with myotonic dystrophy type 1. Neuromuscul. Disord. 2024, 35, 1–7. [Google Scholar] [CrossRef]
- Rossi, S.; Silvestri, G. Fluid Biomarkers of Central Nervous System (CNS) Involvement in Myotonic Dystrophy Type 1 (DM1). Int. J. Mol. Sci. 2023, 24, 2204. [Google Scholar] [CrossRef]
- Dhaenens, C.-M.; Tran, H.; Frandemiche, M.-L.; Carpentier, C.; Schraen-Maschke, S.; Sistiaga, A.; Goicoechea, M.; Eddarkaoui, S.; Van Brussels, E.; Obriot, H. Mis-splicing of Tau exon 10 in myotonic dystrophy type 1 is reproduced by overexpression of CELF2 but not by MBNL1 silencing. Biochim. Biophys. Acta BBA-Mol. Basis Dis. 2011, 1812, 732–742. [Google Scholar] [CrossRef]
- Morin, A.; Funkiewiez, A.; Routier, A.; Le Bouc, R.; Borderies, N.; Galanaud, D.; Levy, R.; Pessiglione, M.; Dubois, B.; Eymard, B.; et al. Unravelling the impact of frontal lobe impairment for social dysfunction in myotonic dystrophy type 1. Brain Commun. 2022, 4, fcac111. [Google Scholar] [CrossRef]
- Simoncini, C.; Spadoni, G.; Lai, E.; Santoni, L.; Angelini, C.; Ricci, G.; Siciliano, G. Central Nervous System Involvement as Outcome Measure for Clinical Trials Efficacy in Myotonic Dystrophy Type 1. Front. Neurol. 2020, 11, 624. [Google Scholar] [CrossRef]
- Hermans, M.C.; Merkies, I.S.; Laberge, L.; Blom, E.W.; Tennant, A.; Faber, C.G. Fatigue and daytime sleepiness scale in myotonic dystrophy type 1. Muscle Nerve 2013, 47, 89–95. [Google Scholar] [CrossRef]
- Okkersen, K.; Jimenez-Moreno, C.; Wenninger, S.; Daidj, F.; Glennon, J.; Cumming, S.; Littleford, R.; Monckton, D.G.; Lochmüller, H.; Catt, M.; et al. Cognitive behavioural therapy with optional graded exercise therapy in patients with severe fatigue with myotonic dystrophy type 1: A multicentre, single-blind, randomised trial. Lancet Neurol. 2018, 17, 671–680. [Google Scholar] [CrossRef]
- Moshirfar, M.; Webster, C.R.; Seitz, T.S.; Ronquillo, Y.C.; Hoopes, P.C. Ocular features and clinical approach to cataract and corneal refractive surgery in patients with myotonic dystrophy. Clin. Ophthalmol. Auckl. NZ 2022, 16, 2837–2842. [Google Scholar] [CrossRef]
- Kaup, S.; Pandey, S.K. Cataract surgery in patients with Fuchs’ endothelial corneal dystrophy. Community Eye Health 2019, 31, 86–87. [Google Scholar] [PubMed]
- Mootha, V.V.; Hansen, B.; Rong, Z.; Mammen, P.P.; Zhou, Z.; Xing, C.; Gong, X. Fuchs’ Endothelial Corneal Dystrophy and RNA Foci in Patients With Myotonic Dystrophy. Investig. Ophthalmol. Vis. Sci. 2017, 58, 4579–4585. [Google Scholar] [CrossRef]
- Pagoulatos, D.; Kapsala, Z.; Makri, O.E.; Georgakopoulos, C.D. Christmas tree cataract and myotonic dystrophy type 1. Eye 2018, 32, 1794–1795. [Google Scholar] [CrossRef]
- Nizami, A.A.; Gurnani, B.; Gulani, A.C.; Redmond, S.B. Cataract (Nursing). In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Kersten, H.M.; Roxburgh, R.H.; Child, N.; Polkinghorne, P.J.; Frampton, C.; Danesh-Meyer, H.V. Epiretinal membrane: A treatable cause of visual disability in myotonic dystrophy type 1. J. Neurol. 2014, 261, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Takada, H.; Matsumura, T.; Shimamura, H.; Matsui, M.; Kon, S.; Fukumoto, A.; Kubota, T.; Yoshida, K.; Iwahashi, H.; Takahashi, M.P. Investigation of Glucose Metabolism by Continuous Glucose Monitoring and Validation of Dipeptidyl Peptidase 4 Inhibitor Use in Patients with Myotonic Dystrophy Type 1. J. Clin. Med. 2024, 13, 5252. [Google Scholar] [CrossRef]
- Vantyghem, M.-C.; Dobbelaere, D.; Mention, K.; Wemeau, J.-L.; Saudubray, J.-M.; Douillard, C. Endocrine manifestations related to inherited metabolic diseases in adults. Orphanet J. Rare Dis. 2012, 7, 11. [Google Scholar] [CrossRef] [PubMed]
- Spaziani, M.; Semeraro, A.; Bucci, E.; Rossi, F.; Garibaldi, M.; Papassifachis, M.A.; Pozza, C.; Anzuini, A.; Lenzi, A.; Antonini, G.; et al. Hormonal and metabolic gender differences in a cohort of myotonic dystrophy type 1 subjects: A retrospective, case–control study. J. Endocrinol. Investig. 2020, 43, 663–675. [Google Scholar] [CrossRef]
- Vaidya, R.A.; Desai, S.; Moitra, P.; Salis, S.; Agashe, S.; Battalwar, R.; Mehta, A.; Madan, J.; Kalita, S.; Udipi, S.A.; et al. Hyperinsulinemia: An early biomarker of metabolic dysfunction. Front. Clin. Diabetes Healthc. 2023, 4, 1159664. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2022. Diabetes Care 2021, 45 (Suppl. 1), S17–S38. [Google Scholar] [CrossRef]
- García-Puga, M.; Saenz-Antoñanzas, A.; Matheu, A.; López de Munain, A. Targeting Myotonic Dystrophy Type 1 with Metformin. Int. J. Mol. Sci. 2022, 23, 2901. [Google Scholar] [CrossRef] [PubMed]
- He, L. Metformin and Systemic Metabolism. Trends Pharmacol. Sci. 2020, 41, 868–881. [Google Scholar] [CrossRef] [PubMed]
- Chong, Z.Z.; Menkes, D.L.; Souayah, N. Targeting neuroinflammation in distal symmetrical polyneuropathy in diabetes. Drug Discov. Today 2024, 29, 104087. [Google Scholar] [CrossRef]
- Passeri, E.; Bugiardini, E.; Sansone, V.; Valaperta, R.; Costa, E.; Ambrosi, B.; Meola, G.; Corbetta, S. Vitamin D, parathyroid hormone and muscle impairment in myotonic dystrophies. J. Neurol. Sci. 2013, 331, 132–135. [Google Scholar] [CrossRef]
- Wenninger, S.; Montagnese, F.; Schoser, B. Core Clinical Phenotypes in Myotonic Dystrophies. Front. Neurol. 2018, 9, 303. [Google Scholar] [CrossRef]
- Galaviz, K.I.; Narayan, K.M.V.; Lobelo, F.; Weber, M.B. Lifestyle and the Prevention of Type 2 Diabetes: A Status Report. Am. J. Lifestyle Med. 2018, 12, 4–20. [Google Scholar] [CrossRef] [PubMed]
- Mateus, T.; Almeida, I.; Costa, A.; Viegas, D.; Magalhães, S.; Martins, F.; Herdeiro, M.T.; da Cruz e Silva, O.A.B.; Fraga, C.; Alves, I.; et al. Fourier-Transform Infrared Spectroscopy as a Discriminatory Tool for Myotonic Dystrophy Type 1 Metabolism: A Pilot Study. Int. J. Environ. Res. Public Health 2021, 18, 3800. [Google Scholar] [CrossRef]
- Kim, W.B.; Jeong, J.Y.; Doo, S.W.; Yang, W.J.; Song, Y.S.; Lee, S.R.; Park, J.W.; Kim, D.W. Myotonic Dystrophy Type 1 Presenting as Male Infertility. Korean J. Urol. 2012, 53, 134–136. [Google Scholar] [CrossRef]
- Dechanet, C.; Castelli, C.; Reyftmann, L.; Coubes, C.; Hamamah, S.; Hedon, B.; Dechaud, H.; Anahory, T. Myotonic dystrophy type 1 and PGD: Ovarian stimulation response and correlation analysis between ovarian reserve and genotype. Reprod. Biomed. Online 2010, 20, 610–618. [Google Scholar] [CrossRef]
- Sahu, B.; Ozturk, O.; Deo, N.; Fordham, K.; Ranierri, M.; Serhal, P. Response to controlled ovarian stimulation and oocyte quality in women with myotonic dystrophy type I. J. Assist. Reprod. Genet. 2008, 25, 1–5. [Google Scholar] [CrossRef][Green Version]
- Srebnik, N.; Margalioth, E.J.; Rabinowitz, R.; Varshaver, I.; Altarescu, G.; Renbaum, P.; Levi-Lahad, E.; Weintraub, A.; Eldar-Geva, T. Ovarian reserve and PGD treatment outcome in women with myotonic dystrophy. Reprod. Biomed. Online 2014, 29, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Feyereisen, E.; Amar, A.; Kerbrat, V.; Steffann, J.; Munnich, A.; Vekemans, M.; Frydman, R.; Frydman, N. Myotonic dystrophy: Does it affect ovarian follicular status and responsiveness to controlled ovarian stimulation? Hum. Reprod. 2006, 21, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Ijuin, A.; Hayama, T.; Yamamoto, M.; Ueno, H.; Hamada, H.; Miyakoshi, A.; Nishi, M.; Saito, M.; Tochihara, S.; Takeshima, T.; et al. High Mitochondrial Dna Replication In Embryos Derived From Myotonic Dystrophy 1 Female. Fertil. Steril. 2021, 116, e225. [Google Scholar] [CrossRef]
- Smith, C.A.; Gutmann, L. Myotonic dystrophy type 1 management and therapeutics. Curr. Treat. Options Neurol. 2016, 18, 52. [Google Scholar] [CrossRef] [PubMed]
- Awater, C.; Zerres, K.; Rudnik-Schöneborn, S. Pregnancy course and outcome in women with hereditary neuromuscular disorders: Comparison of obstetric risks in 178 patients. Eur. J. Obstet. Gynecol. Reprod. Biol. 2012, 162, 153–159. [Google Scholar] [CrossRef]
- Hopkins, A.N.; Alshaeri, T.; Akst, S.A.; Berger, J.S. Neurologic disease with pregnancy and considerations for the obstetric anesthesiologist. Spec. Top. Obstet. Anesth. 2014, 38, 359–369. [Google Scholar] [CrossRef]
- Johnson, N.E.; Hung, M.; Nasser, E.; Hagerman, K.A.; Chen, W.; Ciafaloni, E.; Heatwole, C.R. The Impact of Pregnancy on Myotonic Dystrophy: A Registry-Based Study. J. Neuromuscul. Dis. 2015, 2, 447–452. [Google Scholar] [CrossRef]
- Verpoest, W.; Seneca, S.; De Rademaeker, M.; Sermon, K.; De Rycke, M.; De Vos, M.; Haentjens, P.; Devroey, P.; Liebaers, I. The reproductive outcome of female patients with myotonic dystrophy type 1 (DM1) undergoing PGD is not affected by the size of the expanded CTG repeat tract. J. Assist. Reprod. Genet. 2010, 27, 327–333. [Google Scholar] [CrossRef]
- Graham, M.E.; Jelin, A.; Hoon Jr, A.H.; Wilms Floet, A.M.; Levey, E.; Graham, E.M. Assisted reproductive technology: Short- and long-term outcomes. Dev. Med. Child Neurol. 2023, 65, 38–49. [Google Scholar] [CrossRef]
- Morton, A. Myotonic disorders and pregnancy. Obstet. Med. 2020, 13, 14–19. [Google Scholar] [CrossRef]
- Zaki, M.; Boyd, P.A.; Impey, L.; Roberts, A.; Chamberlain, P. Congenital myotonic dystrophy: Prenatal ultrasound findings and pregnancy outcome. Ultrasound Obstet. Gynecol. 2007, 29, 284–288. [Google Scholar] [CrossRef]
- Hamza, A.H.D.; Solomayer, E.F.; Meyberg-Solomayer, G. Polyhydramnios: Causes, Diagnosis and Therapy. Geburtshilfe Frauenheilkd. 2013, 73, 1241–1246. [Google Scholar] [CrossRef]
- Huri, M.; Di Tommaso, M.; Seravalli, V. Amniotic Fluid Disorders: From Prenatal Management to Neonatal Outcomes. Children 2023, 10, 561. [Google Scholar] [CrossRef]
- Campanati, A.; Giannoni, M.; Buratti, L.; Cagnetti, C.; Giuliodori, K.; Ganzetti, G.; Silvestrini, M.; Provinciali, L.; Offidani, A. Skin features in myotonic dystrophy type 1: An observational study. Neuromuscul. Disord. 2015, 25, 409–413. [Google Scholar] [CrossRef]
- Campione, E.; Botta, A.; Di Prete, M.; Rastelli, E.; Gibellini, M.; Petrucci, A.; Bernardini, S.; Novelli, G.; Bianchi, L.; Orlandi, A.; et al. Cutaneous features of myotonic dystrophy types 1 and 2: Implication of premature aging and vitamin D homeostasis. Neuromuscul. Disord. 2017, 27, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Geh, J.L.C.; Moss, A.L.H. Multiple pilomatrixomata and myotonic dystrophy: A familial association. Br. J. Plast. Surg. 1999, 52, 143–145. [Google Scholar] [CrossRef] [PubMed]
- Saponaro, A.E.; Marini, M.A.; Rossi, G.C.; Casas, J.G. Multiple basal cell carcinomas in a patient with myotonic dystrophy type 1. Int. J. Dermatol. 2006, 45, 87–88. [Google Scholar] [CrossRef] [PubMed]
- Rübben, A.; Wahl, R.U.; Eggermann, T.; Dahl, E.; Ortiz-Brüchle, N.; Cacchi, C. Mutation analysis of multiple pilomatricomas in a patient with myotonic dystrophy type 1 suggests a DM1-associated hypermutation phenotype. PLoS ONE 2020, 15, e0230003. [Google Scholar] [CrossRef]
- Pascual-Gilabert, M.; Artero, R.; López-Castel, A. The myotonic dystrophy type 1 drug development pipeline: 2022 edition. Drug Discov. Today 2023, 28, 103489. [Google Scholar] [CrossRef]
- Zambon, A.A.; Falzone, Y.M.; Bolino, A.; Previtali, S.C. Molecular mechanisms and therapeutic strategies for neuromuscular diseases. Cell. Mol. Life Sci. 2024, 81, 198. [Google Scholar] [CrossRef]
- Kandi, V.; Vadakedath, S. Clinical Trials and Clinical Research: A Comprehensive Review. Cureus 2023, 15, e35077. [Google Scholar] [CrossRef]
- Bérenger-Currias, N.; Martinat, C.; Baghdoyan, S. Pluripotent Stem Cells in Disease Modeling and Drug Discovery for Myotonic Dystrophy Type 1. Cells 2023, 12, 571. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Cooper, T.A. Antisense Oligonucleotides: Rising Stars in Eliminating RNA Toxicity in Myotonic Dystrophy. Hum. Gene Ther. 2013, 24, 499–507. [Google Scholar] [CrossRef]
- Stepniak-Konieczna, E.; Konieczny, P.; Cywoniuk, P.; Dluzewska, J.; Sobczak, K. AON-induced splice-switching and DMPK pre-mRNA degradation as potential therapeutic approaches for Myotonic Dystrophy type 1. Nucleic Acids Res. 2020, 48, 2531–2543. [Google Scholar] [CrossRef] [PubMed]
- Tanner, M.K.; Tang, Z.; Thornton, C.A. Targeted splice sequencing reveals RNA toxicity and therapeutic response in myotonic dystrophy. Nucleic Acids Res. 2021, 49, 2240–2254. [Google Scholar] [CrossRef]
- Piasecka, A.; Szcześniak, M.W.; Sekrecki, M.; Kajdasz, A.; Sznajder, Ł.J.; Baud, A.; Sobczak, K. MBNL splicing factors regulate the microtranscriptome of skeletal muscles. Nucleic Acids Res. 2024, 52, 12055–12073. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Xu, J.; Pan, L. Functions of the Muscleblind-like protein family and their role in disease. Cell Commun. Signal. 2025, 23, 97. [Google Scholar] [CrossRef]
- Lee, J.E.; Cooper, T.A. Pathogenic mechanisms of myotonic dystrophy. Biochem. Soc. Trans. 2009, 37, 1281–1286. [Google Scholar] [CrossRef]
- Wei, C.; Jones, K.; Timchenko, N.A.; Timchenko, L. GSK3β is a new therapeutic target for myotonic dystrophy type 1. Rare Dis. 2013, 1, e26555. [Google Scholar] [CrossRef]
- Savkur, R.S.; Philips, A.V.; Cooper, T.A. Aberrant regulation of insulin receptor alternative splicing is associated with insulin resistance in myotonic dystrophy. Nat. Genet. 2001, 29, 40–47. [Google Scholar] [CrossRef]
- Nieuwenhuis, S.; Okkersen, K.; Widomska, J.; Blom, P.; ’t Hoen, P.A.C.; van Engelen, B.; Glennon, J.C. Insulin Signaling as a Key Moderator in Myotonic Dystrophy Type 1. Front. Neurol. 2019, 10, 1229. [Google Scholar] [CrossRef]
- Thornton, C.A. Myotonic Dystrophy. Myopathies 2014, 32, 705–719. [Google Scholar] [CrossRef] [PubMed]
- Day, J.W.; Ricker, K.; Jacobsen, J.F.; Rasmussen, L.J.; Dick, K.A.; Kress, W.; Schneider, C.; Koch, M.C.; Beilman, G.J.; Harrison, A.R.; et al. Myotonic dystrophy type 2. Neurology 2003, 60, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Laustriat, D.; Gide, J.; Barrault, L.; Chautard, E.; Benoit, C.; Auboeuf, D.; Boland, A.; Battail, C.; Artiguenave, F.; Deleuze, J.-F.; et al. In Vitro and In Vivo Modulation of Alternative Splicing by the Biguanide Metformin. Mol. Ther. Nucleic Acids 2015, 4, e262. [Google Scholar] [CrossRef] [PubMed]
- Overby, S.J.; Cerro-Herreros, E.; Llamusi, B.; Artero, R. RNA-mediated therapies in myotonic dystrophy. Drug Discov. Today 2018, 23, 2013–2022. [Google Scholar] [CrossRef]
- Stoodley, J.; Vallejo-Bedia, F.; Seone-Miraz, D.; Debasa-Mouce, M.; Wood, M.J.A.; Varela, M.A. Application of Antisense Conjugates for the Treatment of Myotonic Dystrophy Type 1. Int. J. Mol. Sci. 2023, 24, 2697. [Google Scholar] [CrossRef]
- Timchenko, L. Development of Therapeutic Approaches for Myotonic Dystrophies Type 1 and Type 2. Int. J. Mol. Sci. 2022, 23, 10491. [Google Scholar] [CrossRef]
- López-Morató, M.; Brook, J.D.; Wojciechowska, M. Small Molecules Which Improve Pathogenesis of Myotonic Dystrophy Type 1. Front. Neurol. 2018, 9, 349. [Google Scholar] [CrossRef]
- Wang, M.; Weng, W.-C.; Stock, L.; Lindquist, D.; Martinez, A.; Gourdon, G.; Timchenko, N.; Snape, M.; Timchenko, L. Correction of Glycogen Synthase Kinase 3β in Myotonic Dystrophy 1 Reduces the Mutant RNA and Improves Postnatal Survival of DMSXL Mice. Mol. Cell. Biol. 2019, 39, e00155-19. [Google Scholar] [CrossRef]
- Jones, K.; Wei, C.; Iakova, P.; Bugiardini, E.; Schneider-Gold, C.; Meola, G.; Woodgett, J.; Killian, J.; Timchenko, N.A.; Timchenko, L.T. GSK3β mediates muscle pathology in myotonic dystrophy. J. Clin. Investig. 2012, 122, 4461–4472. [Google Scholar] [CrossRef]
- Liu, J.; Guo, Z.-N.; Yan, X.-L.; Yang, Y.; Huang, S. Brain Pathogenesis and Potential Therapeutic Strategies in Myotonic Dystrophy Type 1. Front. Aging Neurosci. 2021, 13, 755392. [Google Scholar] [CrossRef] [PubMed]
- Lutz, M.; Levanti, M.; Karns, R.; Gourdon, G.; Lindquist, D.; Timchenko, N.A.; Timchenko, L. Therapeutic Targeting of the GSK3β-CUGBP1 Pathway in Myotonic Dystrophy. Int. J. Mol. Sci. 2023, 24, 10650. [Google Scholar] [CrossRef] [PubMed]
- Horrigan, J.; Gomes, T.B.; Snape, M.; Nikolenko, N.; McMorn, A.; Evans, S.; Yaroshinsky, A.; Della Pasqua, O.; Oosterholt, S.; Lochmüller, H. A phase 2 study of AMO-02 (Tideglusib) in congenital and childhood-onset myotonic dystrophy type 1 (DM1). Pediatr. Neurol. 2020, 112, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Hicks, S.M.; Frias, J.A.; Mishra, S.K.; Scotti, M.; Muscato, D.R.; Valero, M.C.; Adams, L.M.; Cleary, J.D.; Nakamori, M.; Wang, E.; et al. Alternative splicing dysregulation across tissue and therapeutic approaches in a mouse model of myotonic dystrophy type 1. Mol. Ther.-Nucleic Acids 2024, 35, 102338. [Google Scholar] [CrossRef]
- Nakamori, M.; Nakatani, D.; Sato, T.; Hasuike, Y.; Kon, S.; Saito, T.; Nakamura, H.; Takahashi, M.P.; Hida, E.; Komaki, H.; et al. Erythromycin for myotonic dystrophy type 1: A multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. eClinicalMedicine 2024, 67, 102390. [Google Scholar] [CrossRef]
- Nakamori, M.; Taylor, K.; Mochizuki, H.; Sobczak, K.; Takahashi, M.P. Oral administration of erythromycin decreases RNA toxicity in myotonic dystrophy. Ann. Clin. Transl. Neurol. 2016, 3, 42–54. [Google Scholar] [CrossRef]
- Platon, V.-M.; Dragoi, B.; Marin, L. Erythromycin Formulations—A Journey to Advanced Drug Delivery. Pharmaceutics 2022, 14, 2180. [Google Scholar] [CrossRef]
- Sanjurjo-Rodríguez, C.; Castro-Viñuelas, R.; Piñeiro-Ramil, M.; Rodríguez-Fernández, S.; Fuentes-Boquete, I.; Blanco, F.J.; Díaz-Prado, S. Versatility of Induced Pluripotent Stem Cells (iPSCs) for Improving the Knowledge on Musculoskeletal Diseases. Int. J. Mol. Sci. 2020, 21, 6124. [Google Scholar] [CrossRef]
- Talbot, R.; Nimmo, J.; Julian, D.; Clark, R.; Neilson, J.; Prescott, L. Treatment of ventricular arrhythmias with mexiletine (kö1173). Lancet 1973, 302, 399–404. [Google Scholar] [CrossRef]
- Double-Blind, Placebo-Controlled, Dose-Range-Finding, Crossover Trial of Single Day Administration of ERX-963 in Adults with Myotonic Dystrophy Type 1. 2019. Available online: https://clinicaltrials.gov/study/NCT03959189 (accessed on 17 June 2019).
- Conte, T.C.; Duran-Bishop, G.; Orfi, Z.; Mokhtari, I.; Deprez, A.; Côté, I.; Molina, T.; Kim, T.-Y.; Tellier, L.; Roussel, M.-P.; et al. Clearance of defective muscle stem cells by senolytics restores myogenesis in myotonic dystrophy type 1. Nat. Commun. 2023, 14, 4033. [Google Scholar] [CrossRef]
- A Single-Blind, Phase 2 Study to Evaluate The Safety and Efficacy of Tideglusib 400mg or 1000mg for the Treatment of Adolescent and Adult Congenital and Juvenile-Onset Myotonic Dystrophy . 2016. Available online: https://clinicaltrials.gov/study/NCT02858908 (accessed on 20 July 2016).
- Horrigan, J.; Snape, M.; Fantelli, E. P49 The efficacy and safety of Tideglusib in a randomized, placebo-controlled, double blind study in children and adolescents with congenital myotonic dystrophy (REACH CDM study). Neuromuscul. Disord. 2023, 33, S70–S71. [Google Scholar] [CrossRef]
- Davion, J.-B.; Tard, C.; Kuchcinski, G.; Fragoso, L.; Wilu-Wilu, A.; Maurage, P.; Nguyen The Tich, S.; Defebvre, L.; D’Hondt, F.; Delbeuck, X. Characterization of theory of mind performance in patients with myotonic dystrophy type 1. Cortex 2023, 168, 181–192. [Google Scholar] [CrossRef]
- Yamada, T.; Fukano, N.; Kai, K.; Kuribayashi, Y.; Jikumaru, M.; Eto, S.; Kawano, Y. Undiagnosed myotonic dystrophy: A case report and literature review. Med. Int. 2023, 3, 46. [Google Scholar] [CrossRef] [PubMed]
- Bassez, G.; Audureau, E.; Hogrel, J.-Y.; Arrouasse, R.; Baghdoyan, S.; Bhugaloo, H.; Gourlay-Chu, M.-L.; Le Corvoisier, P.; Peschanski, M. Improved mobility with metformin in patients with myotonic dystrophy type 1: A randomized controlled trial. Brain 2018, 141, 2855–2865. [Google Scholar] [CrossRef]
- Evaluation of the Efficacy and Safety of Metformin in the Myotonic Dystrophy Type 1 (Steinert’s Disease). A Phase III, Prospective, Multicentre, Randomized, Double-Blind Controlled Study. 2022. Available online: https://clinicaltrials.gov/study/NCT05532813 (accessed on 1 March 2024).
- An Open-label, Non-Comparative Study to Evaluate the Steady-State Pharmacokinetics, Safety, and Efficacy of Mexiletine in Adolescents and Children with Myotonic Disorders. 2020. Available online: https://clinicaltrials.gov/study/NCT04624750 (accessed on 3 September 2021).
- Mousele, C.; Matthews, E.; Pitceathly, R.D.S.; Hanna, M.G.; MacDonald, S.; Savvatis, K.; Carr, A.; Turner, C. Long-term Safety and Efficacy of Mexiletine in Myotonic Dystrophy Types 1 and 2. Neurol. Clin. Pract. 2021, 11, e682–e685. [Google Scholar] [CrossRef] [PubMed]
- Open-label Extension Study to Evaluate the Long-term Safety and Efficacy of Mexiletine in Paediatric Patients with Myotonic Disorders Who Have Completed the MEX-NM-301 Study. 2020. Available online: https://clinicaltrials.gov/study/NCT04622553 (accessed on 5 November 2021).
- An Observational Study to Describe the Long-Term Safety and Effectiveness of Namuscla in the Symptomatic Management of Myotonia in Adult Patients with Non-dystrophic Myotonic Disorders. 2020. Available online: https://clinicaltrials.gov/study/NCT04616807 (accessed on 17 December 2020).
- A Randomized, Double-Blind, Placebo-Controlled, Multi-Center Study to Investigate the Efficacy and Safety of Mexiletine During 26 Weeks of Treatment in Patients with Myotonic Dystrophy Type 1 and Type 2 [The MIND Study]. 2021. Available online: https://clinicaltrials.gov/study/NCT04700046 (accessed on 3 September 2021).
- Sampson, J.; Wang, E.; Day, J.; Gutmann, L.; Mezerhane, E.; Seto, A.; Ehrich, E. Results of Double-blind, Placebo-controlled, Dose Range Finding, Crossover Study of Single Day Administration of ERX-963 (IV Flumazenil) in Adults with Myotonic Dystrophy Type 1 (2834). Neurology 2021, 96 (Suppl. 15), 2834. [Google Scholar] [CrossRef]
- Lawless, M.; Arnold, W.; Agriesti, J.; Moravec, T.; Moravec, T.; Moravec, T. Investigation of Ranolazine as an Anti-myotonia Treatment in Myotonic Dystrophy Type 1 (P5.443). Neurology, 2018; 90, (Suppl. 15), P5.443. [Google Scholar]
- Lorusso, S.; Kline, D.; Bartlett, A.; Freimer, M.; Agriesti, J.; Hawash, A.A.; Rich, M.M.; Kissel, J.T.; David Arnold, W. Open-label trial of ranolazine for the treatment of paramyotonia congenita. Muscle Nerve 2019, 59, 240–243. [Google Scholar] [CrossRef]
- A Randomized, Double-Blind, Placebo-Controlled Study to Evaluate the Safety and Efficacy of Pitolisant on Excessive Daytime Sleepiness and Other Non-Muscular Symptoms in Patients with Myotonic Dystrophy Type 1, Followed by an Open-Label Extension. 2021. Available online: https://clinicaltrials.gov/study/NCT04886518 (accessed on 28 June 2021).
- Pascual-Gilabert, M.; López-Castel, A.; Artero, R. Myotonic dystrophy type 1 drug development: A pipeline toward the market. Drug Discov. Today 2021, 26, 1765–1772. [Google Scholar] [CrossRef]
- Dyne Therapeutics Announces New Clinical Data from ACHIEVE Trial of DYNE-101 in DM1 and DELIVER Trial of DYNE-251 in DMD Demonstrating Compelling Impact on Key Disease Biomarkers and Improvement in Multiple Functional Endpoints. Press Release Presented at 20 May 2024. Available online: https://investors.dyne-tx.com/news-releases/news-release-details/dyne-therapeutics-announces-new-clinical-data-achieve-trial-dyne (accessed on 25 August 2024).
- Wolf, D.; Mix, C.; Han, B.; Dugar, A.; Farwell, W. P50 A phase 1/2 randomized, placebo-controlled, multiple ascending dose study (ACHIEVE) of DYNE-101 in individuals with myotonic dystrophy type 1 (DM1). Neuromuscul. Disord. 2023, 33, S71. [Google Scholar] [CrossRef]
- Thornton, C.A.; Moxley, R.T., III; Eichinger, K.; Heatwole, C.; Mignon, L.; Arnold, W.D.; Ashizawa, T.; Day, J.W.; Dent, G.; Tanner, M.K.; et al. Antisense oligonucleotide targeting DMPK in patients with myotonic dystrophy type 1: A multicentre, randomised, dose-escalation, placebo-controlled, phase 1/2a trial. Lancet Neurol. 2023, 22, 218–228. [Google Scholar] [CrossRef]
- A Phase 1/2a Double-Blind, Placebo-Controlled, Single- and Multiple Ascending Dose Study to Assess the Safety, Tolerability, PK, PD and Efficacy of IV Administration of ATX-01 in Male and Female Participants Aged 18 to 64 with Classic DM1. 2024. Available online: https://clinicaltrials.gov/study/NCT06300307 (accessed on 24 May 2024).
- A Randomized, Double-Blind, Placebo-Controlled, Phase 1/2 Study to Evaluate the Safety, Tolerability, Pharmacokinetics and Pharmacodynamics of Single and Multiple-Doses of AOC 1001 Administered Intravenously to Adult Myotonic Dystrophy Type 1 (DM1) Patients . 2021. Available online: https://clinicaltrials.gov/study/NCT05027269 (accessed on 28 October 2021).
- Johnson, N.; Day, J.; Hamel, J.; Thornton, C.; Subramony, S.; Soltanzadeh, P.; Statland, J.; Arnold, W.; Wicklund, M.; Ditrapani, K.; et al. Preliminary Assessment of the Phase 1/2 Clinical Trial Evaluating the Safety, Tolerability, Pharmacokinetics and Pharmacodynamics of AOC 1001 Administered Intravenously to Adult Patients with Myotonic Dystrophy Type 1 (DM1) (MARINA) (S48.002). Neurology 2023, 100 (Suppl. 2), 2405. [Google Scholar] [CrossRef]
- A Phase 2 Extension Study to Evaluate the Long-Term Safety, Tolerability, Efficacy, Pharmacokinetics, and Pharmacodynamics of AOC 1001 Administered Intravenously to Adult Myotonic Dystrophy Type 1 (DM1) Patients. 2022. Available online: https://clinicaltrials.gov/study/NCT05479981 (accessed on 4 August 2022).
- A Phase 3 Randomized, Double-Blind, Placebo-Controlled, Global Study to Evaluate the Efficacy and Safety of Intravenous AOC 1001 for the Treatment of Myotonic Dystrophy Type 1. 2024. Available online: https://clinicaltrials.gov/study/NCT06411288 (accessed on 30 May 2024).
- Fowler, M.; Johnson, N.; Thornton, C.; Day, J.; Sansone, V.; McEvoy, B.; Tai, L.; Knisely, B.; Brandt, T.; Gallagher, K.; et al. 226P Phase 3, randomized, global study assessing efficacy and safety of del-desiran for the treatment of myotonic dystrophy type 1: HARBOR trial design. Neuromuscul. Disord. 2024, 43, 104441.68. [Google Scholar] [CrossRef]
- Gilbert, J.; Klein, A.; Lonkar, P.; Gutnick, A.; Foy, J.; Yu, S.; Reid, T.; Sarkar, K.; Cleary, J.; Berglund, J.; et al. 440P Nonclinical data for PGN-EDODM1 demonstrated nuclear delivery, mechanistic and meaningful activity for the potential treatment of DM1. Neuromuscul. Disord. 2024, 43, 104441.515. [Google Scholar] [CrossRef]
- Larkindale, J.; Shoskes, J.; Garg, B.; Song, G.; Lonkar, P.; Babcock, S.; Vacca, S.; Yu, S.; Mellion, M. 461P Evaluation of PGN-EDODM1: FREEDOM-DM1 and FREEDOM2-DM1 clinical trials in myotonic dystrophy type 1. Neuromuscul. Disord. 2024, 43, 104441.536. [Google Scholar] [CrossRef]
- Dyke, J.V.; Ai, T.; Li, X. Silencing DMPK Gene by ARO-DM1, an siRNA Therapeutic, for Type 1 Myotonic Dystrophy. 2024. Available online: https://www.mdaconference.org/abstract-library/silencing-dmpk-gene-by-aro-dm1-an-sirna-therapeutic-for-type-1-myotonic-dystrophy/ (accessed on 24 August 2024).
- Leckie, J.; Yokota, T. Potential of Cell-Penetrating Peptide-Conjugated Antisense Oligonucleotides for the Treatment of SMA. Molecules 2024, 29, 2658. [Google Scholar] [CrossRef]
- Aoki, Y.; Wood, M.J.A. Emerging Oligonucleotide Therapeutics for Rare Neuromuscular Diseases. J. Neuromuscul. Dis. 2021, 8, 869–884. [Google Scholar] [CrossRef]
- Belgrad, J.; Fakih, H.H.; Khvorova, A. Nucleic Acid Therapeutics: Successes, Milestones, and Upcoming Innovation. Nucleic Acid Ther. 2024, 34, 52–72. [Google Scholar] [CrossRef]
- Liang, X.; Nichols, J.G.; De Hoyos, C.L.; Crooke, S.T. Some ASOs that bind in the coding region of mRNAs and induce RNase H1 cleavage can cause increases in the pre-mRNAs that may blunt total activity. Nucleic Acids Res. 2020, 48, 9840–9858. [Google Scholar] [CrossRef]
- Weeden, T.; Picariello, T.; Quinn, B.; Spring, S.; Shen, P.-Y.; Qiu, Q.; Vieira, B.F.; Schlaefke, L.; Russo, R.J.; Chang, Y.-A.; et al. FORCE platform overcomes barriers of oligonucleotide delivery to muscle and corrects myotonic dystrophy features in preclinical models. Commun. Med. 2025, 5, 22. [Google Scholar] [CrossRef]
- Cerro-Herreros, E.; Sabater-Arcis, M.; Fernandez-Costa, J.M.; Moreno, N.; Perez-Alonso, M.; Llamusi, B.; Artero, R. miR-23b and miR-218 silencing increase Muscleblind-like expression and alleviate myotonic dystrophy phenotypes in mammalian models. Nat. Commun. 2018, 9, 2482. [Google Scholar] [CrossRef]
- Núñez-Manchón, J.; Capó, J.; Martínez-Piñeiro, A.; Juanola, E.; Pesovic, J.; Mosqueira-Martín, L.; González-Imaz, K.; Maestre-Mora, P.; Odria, R.; Savic-Pavicevic, D.; et al. Immortalized human myotonic dystrophy type 1 muscle cell lines to address patient heterogeneity. iScience 2024, 27, 109930. [Google Scholar] [CrossRef] [PubMed]
- Pierre, M.; Jauvin, D.; Puymirat, J.; Boutjdir, M.; Chahine, M. Generation of three myotonic dystrophy type 1 patient iPSC lines (CBRCULi018-A, CBRCULi019-A, CBRCULi020-A) derived from lymphoblastoid cell lines for disease modelling and therapeutic research. Stem Cell Res. 2024, 76, 103375. [Google Scholar] [CrossRef] [PubMed]
- De Serres-Bérard, T.; Ait Benichou, S.; Jauvin, D.; Boutjdir, M.; Puymirat, J.; Chahine, M. Recent Progress and Challenges in the Development of Antisense Therapies for Myotonic Dystrophy Type 1. Int. J. Mol. Sci. 2022, 23, 13359. [Google Scholar] [CrossRef]
- Klein, A.F.; Varela, M.A.; Arandel, L.; Holland, A.; Naouar, N.; Arzumanov, A.; Seoane, D.; Revillod, L.; Bassez, G.; Ferry, A. Peptide-conjugated oligonucleotides evoke long-lasting myotonic dystrophy correction in patient-derived cells and mice. J. Clin. Investig. 2019, 129, 4739–4744. [Google Scholar] [CrossRef]
- PepGen Reports Second Quarter 2024 Financial Results and Recent Corporate Highlights. PepGen Inc. August 2024. Available online: https://investors.pepgen.com/news-releases/news-release-details/pepgen-reports-second-quarter-2024-financial-results-and-recent (accessed on 24 August 2024).
- Maksudov, F.; Kliuchnikov, E.; Pierson, D.; Ujwal, M.L.; Marx, K.A.; Chanda, A.; Barsegov, V. Therapeutic phosphorodiamidate morpholino oligonucleotides: Physical properties, solution structures, and folding thermodynamics. Mol. Ther.-Nucleic Acids 2023, 31, 631–647. [Google Scholar] [CrossRef]
- Li, X.; Kheirabadi, M.; Dougherty, P.G.; Kamer, K.J.; Shen, X.; Estrella, N.L.; Peddigari, S.; Pathak, A.; Blake, S.L.; Sizensky, E.; et al. The endosomal escape vehicle platform enhances delivery of oligonucleotides in preclinical models of neuromuscular disorders. Mol. Ther.-Nucleic Acids 2023, 33, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Almeida, C.F.; Robriquet, F.; Vetter, T.A.; Huang, N.; Neinast, R.; Hernandez-Rosario, L.; Rajakumar, D.; Arnold, W.D.; McBride, K.L.; Flanigan, K.M.; et al. Promising AAV.U7snRNAs vectors targeting DMPK improve DM1 hallmarks in patient-derived cell lines. Front. Cell Dev. Biol. 2023, 11, 1181040. [Google Scholar] [CrossRef]
- Ponomarev, A.S.; Chulpanova, D.S.; Yanygina, L.M.; Solovyeva, V.V.; Rizvanov, A.A. Emerging Gene Therapy Approaches in the Management of Spinal Muscular Atrophy (SMA): An Overview of Clinical Trials and Patent Landscape. Int. J. Mol. Sci. 2023, 24, 13743. [Google Scholar] [CrossRef]
- Developing AAV-Based Genetic Medicines for Rare Neuromuscular Diseases. July 2023. Available online: https://www.astellasgenetherapies.com/our-pipeline (accessed on 26 August 2024).
- Cardinali, B.; Provenzano, C.; Izzo, M.; Voellenkle, C.; Battistini, J.; Strimpakos, G.; Golini, E.; Mandillo, S.; Scavizzi, F.; Raspa, M.; et al. Time-controlled and muscle-specific CRISPR/Cas9-mediated deletion of CTG-repeat expansion in the DMPK gene. Mol. Ther.-Nucleic Acids 2022, 27, 184–199. [Google Scholar] [CrossRef]
- Asmamaw Mengstie, M. Viral Vectors for the in Vivo Delivery of CRISPR Components: Advances and Challenges. Front. Bioeng. Biotechnol. 2022, 10, 895713. [Google Scholar] [CrossRef]
- Scrudato, M.L.; Poulard, K.; Sourd, C.; Tomé, S.; Klein, A.F.; Corre, G.; Huguet, A.; Furling, D.; Gourdon, G.; Buj-Bello, A. Genome editing of expanded CTG repeats within the human DMPK gene reduces nuclear RNA foci in the muscle of DM1 mice. Mol. Ther. 2019, 27, 1372–1388. [Google Scholar] [CrossRef] [PubMed]
- Batra, R.; Nelles, D.A.; Roth, D.M.; Krach, F.; Nutter, C.A.; Tadokoro, T.; Thomas, J.D.; Sznajder, Ł.J.; Blue, S.M.; Gutierrez, H.L.; et al. The sustained expression of Cas9 targeting toxic RNAs reverses disease phenotypes in mouse models of myotonic dystrophy type 1. Nat. Biomed. Eng. 2021, 5, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Arandel, L.; Matloka, M.; Klein, A.F.; Rau, F.; Sureau, A.; Ney, M.; Cordier, A.; Kondili, M.; Polay-Espinoza, M.; Naouar, N. Reversal of RNA toxicity in myotonic dystrophy via a decoy RNA-binding protein with high affinity for expanded CUG repeats. Nat. Biomed. Eng. 2022, 6, 207–220. [Google Scholar] [CrossRef] [PubMed]
- Rogalska, Z.; Sobczak, K. Sustainable recovery of MBNL activity in autoregulatory feedback loop in myotonic dystrophy. Mol. Ther.-Nucleic Acids 2022, 30, 438–448. [Google Scholar] [CrossRef]
- Rapti, K.; Grimm, D. Adeno-Associated Viruses (AAV) and Host Immunity—A Race Between the Hare and the Hedgehog. Front. Immunol. 2021, 12, 753467. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-H.; Gessler, D.J.; Zhan, W.; Gallagher, T.L.; Gao, G. Adeno-associated virus as a delivery vector for gene therapy of human diseases. Signal Transduct. Target. Ther. 2024, 9, 78. [Google Scholar] [CrossRef]
- Dastidar, S.; Ardui, S.; Singh, K.; Majumdar, D.; Nair, N.; Fu, Y.; Reyon, D.; Samara, E.; Gerli, M.F.M.; Klein, A.F.; et al. Efficient CRISPR/Cas9-mediated editing of trinucleotide repeat expansion in myotonic dystrophy patient-derived iPS and myogenic cells. Nucleic Acids Res. 2018, 46, 8275–8298. [Google Scholar] [CrossRef]
- Zhang, M.-L.; Li, H.-B.; Jin, Y. Application and perspective of CRISPR/Cas9 genome editing technology in human diseases modeling and gene therapy. Front. Genet. 2024, 15, 1364742. [Google Scholar] [CrossRef]
- Porquet, F.; Weidong, L.; Jehasse, K.; Gazon, H.; Kondili, M.; Blacher, S.; Massotte, L.; Di Valentin, E.; Furling, D.; Gillet, N.A.; et al. Specific DMPK-promoter targeting by CRISPRi reverses myotonic dystrophy type 1-associated defects in patient muscle cells. Mol. Ther.-Nucleic Acids 2023, 32, 857–871. [Google Scholar] [CrossRef]
- Button, K.S.; Ioannidis, J.P.A.; Mokrysz, C.; Nosek, B.A.; Flint, J.; Robinson, E.S.J.; Munafò, M.R. Power failure: Why small sample size undermines the reliability of neuroscience. Nat. Rev. Neurosci. 2013, 14, 365–376. [Google Scholar] [CrossRef]
- Kaczmarek, J.C.; Kowalski, P.S.; Anderson, D.G. Advances in the delivery of RNA therapeutics: From concept to clinical reality. Genome Med. 2017, 9, 60. [Google Scholar] [CrossRef] [PubMed]
- Morelli, K.H.; Smargon, A.A.; Yeo, G.W. Programmable macromolecule-based RNA-targeting therapies to treat human neurological disorders. RNA 2023, 29, 489–497. [Google Scholar] [CrossRef]
- Ezike, T.C.; Okpala, U.S.; Onoja, U.L.; Nwike, C.P.; Ezeako, E.C.; Okpara, O.J.; Okoroafor, C.C.; Eze, S.C.; Kalu, O.L.; Odoh, E.C.; et al. Advances in drug delivery systems, challenges and future directions. Heliyon 2023, 9, e17488. [Google Scholar] [CrossRef]
- Yetisgin, A.A.; Cetinel, S.; Zuvin, M.; Kosar, A.; Kutlu, O. Therapeutic Nanoparticles and Their Targeted Delivery Applications. Molecules 2020, 25, 2193. [Google Scholar] [CrossRef]
- Zhao, Z.; Ukidve, A.; Kim, J.; Mitragotri, S. Targeting Strategies for Tissue-Specific Drug Delivery. Cell 2020, 181, 151–167. [Google Scholar] [CrossRef] [PubMed]
- Pizzamiglio, C.; Vernon, H.J.; Hanna, M.G.; Pitceathly, R.D.S. Designing clinical trials for rare diseases: Unique challenges and opportunities. Nat. Rev. Methods Primer 2022, 2, 13. [Google Scholar] [CrossRef] [PubMed]
- Savić Pavićević, D.; Miladinović, J.; Brkušanin, M.; Šviković, S.; Djurica, S.; Brajušković, G.; Romac, S. Molecular Genetics and Genetic Testing in Myotonic Dystrophy Type 1. BioMed Res. Int. 2013, 2013, 391821. [Google Scholar] [CrossRef]
- Baldanzi, S.; Bevilacqua, F.; Lorio, R.; Volpi, L.; Simoncini, C.; Petrucci, A.; Cosottini, M.; Massimetti, G.; Tognoni, G.; Ricci, G.; et al. Disease awareness in myotonic dystrophy type 1: An observational cross-sectional study. Orphanet J. Rare Dis. 2016, 11, 34. [Google Scholar] [CrossRef]
- Churruca, K.; Pomare, C.; Ellis, L.A.; Long, J.C.; Henderson, S.B.; Murphy, L.E.D.; Leahy, C.J.; Braithwaite, J. Patient-reported outcome measures (PROMs): A review of generic and condition-specific measures and a discussion of trends and issues. Health Expect. 2021, 24, 1015–1024. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services FDA Center for Drug Evaluation and Research; U.S. Department of Health and Human Services FDA Center for Biologics Evaluation and Research; U.S. Department of Health and Human Services FDA Center for Devices and Radiological Health. Guidance for industry: Patient-reported outcome measures: Use in medical product development to support labeling claims: Draft guidance. Health Qual. Life Outcomes 2006, 4, 79. [Google Scholar] [CrossRef]
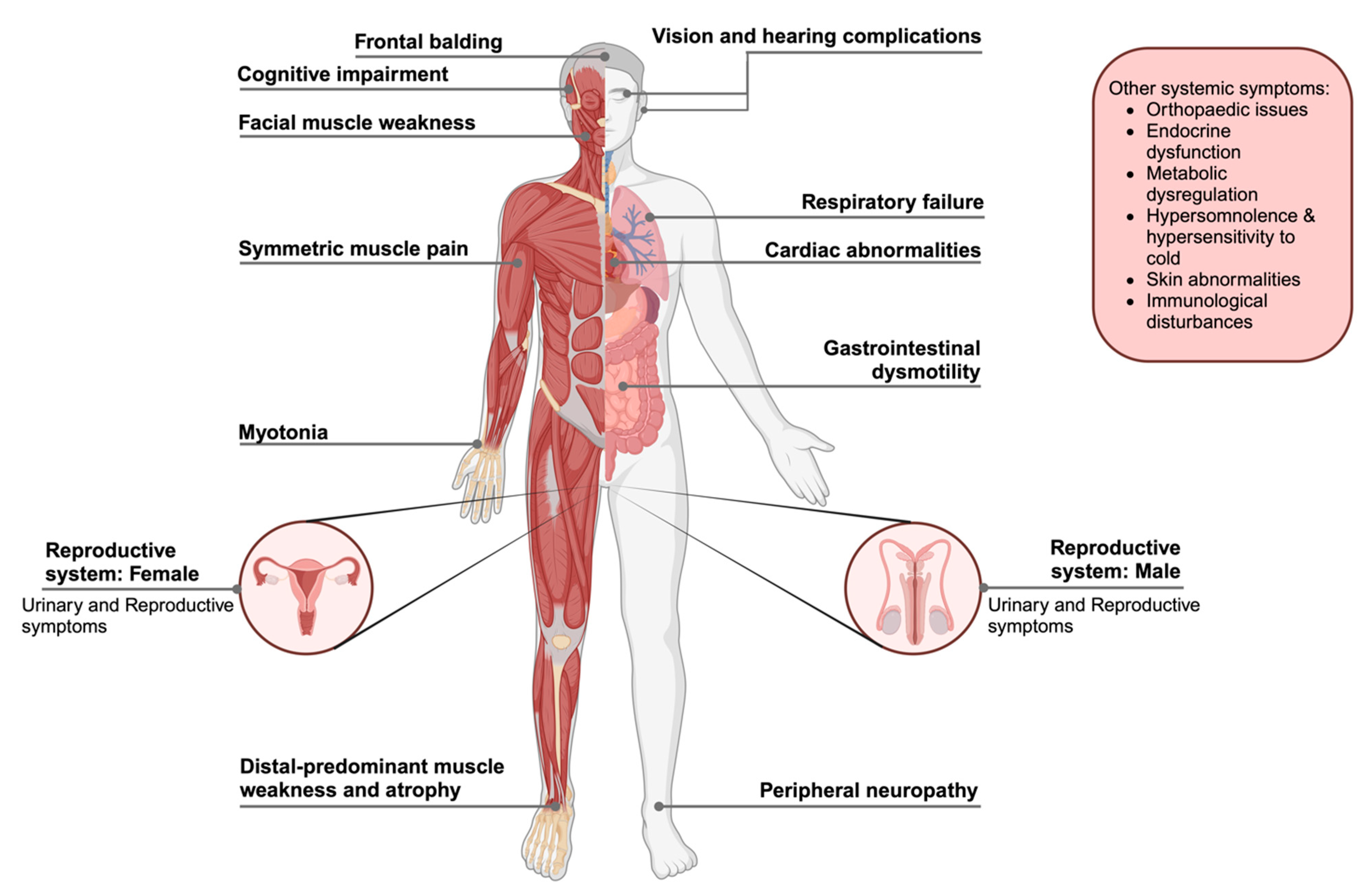
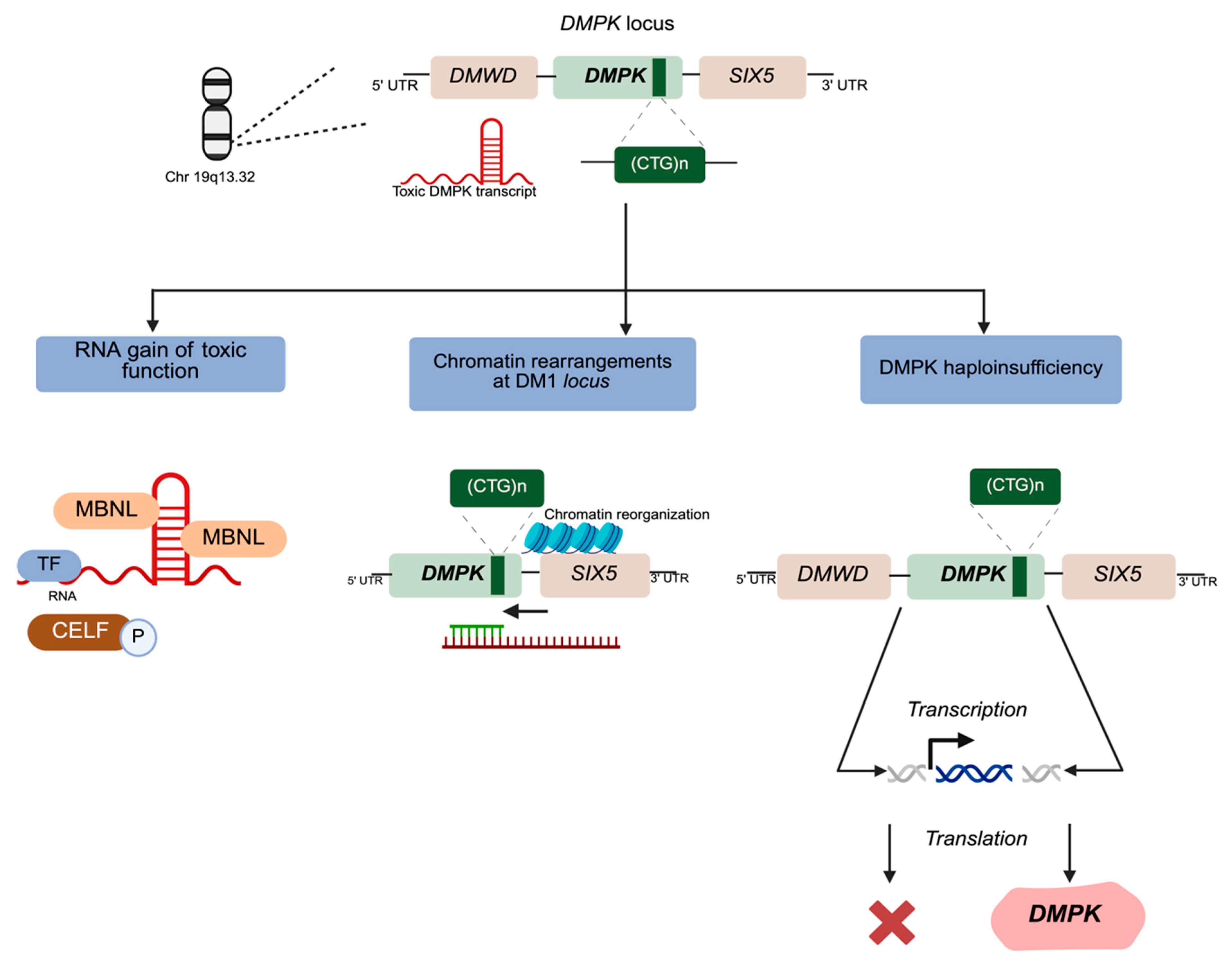
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuntawala, D.H.; Vitorino, R.; Cruz, A.C.; Martins, F.; Rebelo, S. Multisystem Symptoms in Myotonic Dystrophy Type 1: A Management and Therapeutic Perspective. Int. J. Mol. Sci. 2025, 26, 5350. https://doi.org/10.3390/ijms26115350
Kuntawala DH, Vitorino R, Cruz AC, Martins F, Rebelo S. Multisystem Symptoms in Myotonic Dystrophy Type 1: A Management and Therapeutic Perspective. International Journal of Molecular Sciences. 2025; 26(11):5350. https://doi.org/10.3390/ijms26115350
Chicago/Turabian StyleKuntawala, Dhvani H., Rui Vitorino, Ana C. Cruz, Filipa Martins, and Sandra Rebelo. 2025. "Multisystem Symptoms in Myotonic Dystrophy Type 1: A Management and Therapeutic Perspective" International Journal of Molecular Sciences 26, no. 11: 5350. https://doi.org/10.3390/ijms26115350
APA StyleKuntawala, D. H., Vitorino, R., Cruz, A. C., Martins, F., & Rebelo, S. (2025). Multisystem Symptoms in Myotonic Dystrophy Type 1: A Management and Therapeutic Perspective. International Journal of Molecular Sciences, 26(11), 5350. https://doi.org/10.3390/ijms26115350








