Abstract
Skin aging is a complex biological process influenced by both intrinsic factors such as hormonal changes, genetic programming, and immunosenescence and extrinsic stressors including ultraviolet (UV) radiation (particularly UV-A and UV-B), pollution, and lifestyle habits. One of the most prominent manifestations of skin aging is wrinkle formation, which arises from the progressive degradation of key extracellular matrix (ECM) components like collagen and elastin. Emerging evidence highlights the skin microbiome as a critical, yet underappreciated, modulator of these structural changes. This review summarizes current understanding of how aging alters skin structure and microbial composition, and how these changes contribute to wrinkle development. Age-associated skin is characterized by reduced hydration, sebum production, and barrier integrity, accompanied by a shift in microbial communities. These microbial shifts promote local inflammation, matrix metalloproteinase (MMP) activation, and oxidative stress, all of which accelerate ECM degradation. We further discuss how commensal microbes and their bioactive products such as probiotics and postbiotics can counteract wrinkle formation. Clinical studies support the efficacy of strains such as Lactobacillus plantarum HY7714 and Bifidobacterium breve in improving skin elasticity and reducing wrinkle depth. Additionally, this review highlights the emerging role of microbiome-based interventions in skincare, including oral supplements, topical formulations, and postbiotic-enriched products. Overall, we emphasized the therapeutic potential of microbiome modulation as a novel strategy for maintaining skin health and preventing wrinkle formation during aging.
1. Introduction
The human skin, encompassing approximately 1.8 square meters, is not only the body’s largest organ but also a complex ecological interface between the host and the environment. Structurally composed of the epidermis, dermis, and subcutaneous tissue, the skin is populated by a diverse community of microorganisms collectively known as the skin microbiome that includes bacteria, fungi, viruses, and microscopic mites [1,2,3]. These microbes inhabit specialized microenvironments across sebaceous, moist, and dry skin regions, with microbial densities reaching up to ten million organisms per square centimeter in some areas [4,5]. The dominant bacterial phyla Actinomycetes, Firmicutes, Proteobacteria, and Bacteroidetes form stable yet site-specific communities that contribute to key physiological functions, including immune regulation, barrier maintenance, and pathogen defense [6,7]. Thus, immune–microbiome crosstalk plays an important role in regulation aging and associated diseases [8].
With aging, the skin undergoes a range of intrinsic and extrinsic changes that alter its structural integrity and function. Intrinsically, aged skin exhibits reduced fibroblast activity, slower cell turnover, decreased hydration, and a thinning dermal matrix. Extrinsically, chronic exposure to ultraviolet (UV) radiation, especially UV-A and UV-B radiation, and environmental pollutants exacerbates oxidative stress and inflammation, accelerating these degenerative changes [9,10,11,12]. Simultaneously, the composition of the skin microbiome shifts with aging and the relative abundance of beneficial strains of Cutibacterium acnes decline, while potentially pathogenic taxa such as Corynebacterium and Staphylococcus increases [13,14]. It is important to note that Corynebacterium acnes is a heterogenous species, meaning while some strains offer distinct benefits, others may contribute to increased inflammation [15]. These changes in microbial composition are influenced by reduced sebum secretion, loss of barrier function, and immunosenescence, all of which contribute to microbial dysbiosis and altered host–microbe interactions [16,17].
Emerging evidence suggests that age-related microbial imbalances are not merely reflective of skin aging but may actively participate in the process. The decline in lipophilic commensals and rise in opportunistic bacteria create a pro-inflammatory environment that compromises epidermal homeostasis. This microbial dysregulation has been linked to oxidative stress, matrix metalloproteinase (MMP) activation, and barrier disruption factors that collectively accelerate the onset of visible signs of aging, most notably wrinkle formation [18,19].
Wrinkles represent one of the most recognizable features of aged skin and arise from both intrinsic and extrinsic aging mechanisms. The degradation of collagen and elastin, increased production of MMPs, and the accumulation of advanced glycation end-products (AGEs) contribute to the thinning and weakening of the dermis, leading to sagging and furrowed skin [20,21,22]. Notably, recent studies have identified correlations between microbiome composition and wrinkle severity.
These findings underscore the potential role of the skin microbiome in mitigating age-related dermatological changes. Probiotics such as Lactobacillus plantarum and Bifidobacterium breve can reduce inflammation, promote ceramide synthesis, and enhance hydration, thereby improving skin elasticity and minimizing wrinkle depth [23,24]. Similarly, postbiotic metabolites like short-chain fatty acids and bacterial lysates can upregulate collagen gene expression, protect against oxidative damage, and restore barrier integrity [25,26]. The role of postbiotics in mitigating inflammation and associated changes in the gut has been noted [27]. Further, clinical evidence further supports that both oral and topical administration of these agents contributes to wrinkle reduction and improved skin texture in older adults. This review addresses the interaction between skin microbiota and host aging mechanisms, highlighting their contribution to wrinkle development. We discuss about how microbial dysbiosis leads to the destruction of collagen and elastin, the extracellular matrix, the barrier, and the process of inflammaging. Mechanistic insights into oxidative stress, i.e., matrix metalloproteinase alteration, are identified as significant contributors to skin aging. Finally, we elaborated the therapeutic strategies, including probiotics, postbiotics, and microbiome-based skincare products, aimed at restoring microbial balance, strengthening the extracellular matrix, and improving skin resilience.
2. Microbiome–Skin Interaction During Aging
The structure and functions of the skin change with age due to internal factors such as cellular metabolism, the immune system, and hormonal changes, and external factors such as UV/UV-A/UV-B radiation [10,11,12]. As such, the microbiota can change over the lifetime [28] due to one’s lifestyle and living conditions. [29].
As one ages, the skin experiences a decrease in sebum and hydration levels. Further, immune dysfunction may occur, which results in significant alterations in skin physiology [9]. As these changes occur, the cutaneous ecology shifts, causing an imbalance in the cutaneous microbiota [16]. Consequently, the skin microbiome differs between younger and older individuals [14]. A report has demonstrated that, in puberty, the density of lipophilic Cutibacterium acnes increases along with sebum levels, whereas it is much lower in elderly skin [30]. Furthermore, another report indicated a reduction in Actinomycetes abundance in aged skin [13]. Further, Figure 1 represents the mechanistic insights into the role of the skin microbiome in wrinkle formation and aging.
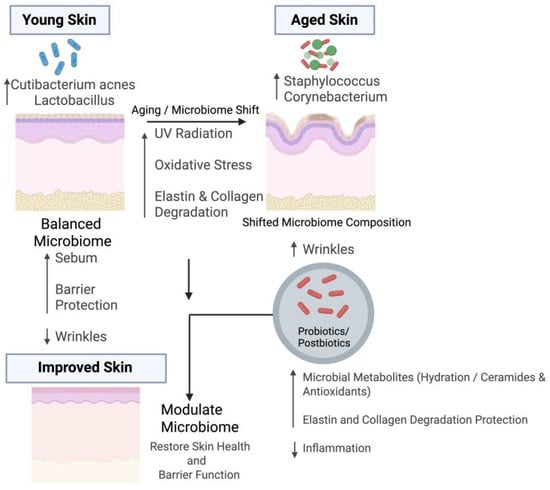
Figure 1.
Impact of the Skin Microbiome on Wrinkle Formation and Potential Modulation During Aging. The upper left side represents microbial features of young and healthy skin, which is characterized by higher abundance of Cutibacterium acnes and Lactobacillus species. These microbes support skin health by producing short-chain fatty acids, maintaining sebum production, and strengthening barrier integrity, which collectively reduce wrinkle formation. In contrast, the upper right side shows aged skin, where an increased abundance of Staphylococcus and Corynebacterium species contributes to inflammation, oxidative stress, and extracellular matrix (ECM) degradation. These changes are driven by aging and environmental factors such as UV exposure. Probiotics and postbiotics can help modulate the aged microbiome by producing beneficial metabolites, enhancing skin hydration, increasing ceramide levels, and suppressing inflammation, ultimately restoring skin health and barrier function. Figure created with BioRender.com.
Therefore, there is a reduction in bacterial diversity during aging. This reduced microbial diversity influence skin physiology by altering lipid metabolism and fostering pro-inflammatory cytokines, thereby contributing to age-related skin dryness and barrier dysfunction [31,32]. It is reported that the diversification and compositional shifts of the skin microbiome in older individuals are closely linked to chronological and physiological aging, driven by impaired barrier integrity, reduced sebum secretion, diminished hydration, and immune senescence [31]. These changes create an unfavorable niche for commensal bacteria while facilitating colonization by opportunistic or pro-inflammatory species [31]. Further, studies have reported that aging skin typically shows a decline in Cutibacterium acnes, predominant in youthful sebaceous regions, alongside an increased prevalence of Corynebacterium and Staphylococcus species, including S. epidermidis and the potentially pathogenic S. aureus [17,33].
Moreover, systemic aging processes, including immunosenescence and inflammaging, may extend beyond the skin to influence microbial ecosystems at distant sites, such as the gut and oral cavity [19,34]. These interconnected microbiomes—gut, oral, and skin—exhibit correlated aging signatures. In fact, recent machine-learning models have demonstrated that among these, the skin microbiome offers the highest predictive accuracy for chronological age, with deviations within four years on average [35]. This suggests that while microbiome changes may serve as robust biomarkers of aging, their causal role remains debated.
Further research on the skin microbiome has increasingly focused on strategies to restore and maintain its diverse microbial communities. Such approaches may also provide insights into how modulation of the gut microbiota in older individuals could prevent or mitigate age-related skin dysfunction [33]. For example, Dimarzio et al. demostrate that the stratum corneum can experience enhanced ceramide levels when Streptococcus thermophilus is topically applied on the skin [36]. Recently, oral and topical probiotics have been proposed as a therapy to restore microbiota balance, support skin barrier function, and protect against environmental factors, especially UV-B induced skin damage [37,38]. Huang et al. implicate that UV-A-induced photoaging impairs autophagic degradation in dermal fibroblasts by reducing lysosomal acidification and cathepsin activity, heavily influencing skin photoaging [39]. Interestingly, probiotics may help restore the balance between free radical removal and production, which may slow aging [40]. Oral and topical compounds are being explored for their potential to modulate the skin microbiome [41]. Orobanche rapum extract promotes skin rejuvenation and protects the cutaneous microbiota, promoting healthier skin [42]. Recently, the term photobiomics has been introduced to describe the use of low levels of visible or near-infrared light to influence the gut microbiome through photo-biomodulation [43].
Overall, aging-driven changes in skin physiology disrupt the microbial balance, reducing commensals and favoring opportunistic species. Probiotic and postbiotic interventions may reveal a promising approach to restore skin homeostasis. These insights implicate understanding how microbiome alterations directly contribute to wrinkle formation (Table 1).

Table 1.
Role of Microbiome on Aging Skin.
3. Microbiome Associated with Wrinkles
The relationship between skin microbiome composition and skin aging is complex and multifaceted. A diverse and balanced microbiome is generally associated with healthy and young skin [18]. Skin microbial communities influence skin aging by regulating immune responses, protection against UV damage, maintenance of skin barrier function, production of beneficial metabolites, and modulation of skin pH. Research has identified several microbial pathways that may be particularly relevant to skin aging, such as the biosynthesis of antibiotics and other metabolites that can influence skin health [13,18]. Specific commensal bacteria produce AMPs and other products that inhibit the growth of pathogens. This protects skin barrier function and reduces inflammation, which indirectly slows wrinkle formation.
Certain bacterial species may be associated with increased or decreased wrinkle formation. Bacteria associated with reduced wrinkle formation include certain species of Cutibacterium acnes and Lactobacillus. Cutibacterium acnes is typically dominant in younger skin and has been linked to reduced signs of aging. This species is known to play a role in maintaining skin health by producing short-chain fatty acids and other metabolites that help maintain skin barrier function [18,19]. Its abundance tends to decrease with age, particularly in individuals over 55–60 years old. While not typically abundant on the skin, some Lactobacillus species have been associated with anti-aging effects. In contrast, some bacteria such as Corynebacterium and Staphylococcus species have been associated with increased wrinkle formation. In this context, Blaise et al. demonstrate that the increased abundance of Corynebacterium species is responsible for producing keratolytic effect [48]. This shift may be related to changes in skin physiology that occur with age. While some Staphylococcus species are common skin commensals, an overabundance of certain strains has been associated with skin aging and inflammation. The balance of different Staphylococcus species may play a role in skin health and overall appearance [18,19].
In the therapeutic perspective by Lee et al., it was demonstrated that the oral administration of Lactobacillus strains, such as L. plantarum HY7714, may improve skin hydration and reduce wrinkle depth [23]. Similarly, Bouilly-Gauthier et al. demonstrate that oral consumption of a synbiotic formulation comprising Lactobacillus johnsonii and carotenoids improves skin resistance to high UV-A exposure and natural sunlight [49]. Furthermore, Rong et al. implicate that Lactobacillus helveticus supernatant increases resistance to UV-B-induced oxidative stress and hyperpigmentation [50]. As our understanding of the skin microbiome’s role in aging continues to evolve, there is a growing interest in developing microbiome-based interventions to promote skin health and reduce signs of aging (See Table 2).

Table 2.
Studies evaluating skin microbiome and wrinkle-associated outcomes.
4. Understanding of Microbiome on Wrinkle Formation During Aging
The skin microbiome plays a crucial role in wrinkle formation by modulating extracellular matrix integrity through immune and oxidative pathways. Beneficial microbes help maintain collagen and elastin stability by reducing inflammation and oxidative stress, whereas dysbiosis promotes MMP activation, collagen degradation, and accelerated wrinkle development (shown in Figure 2).
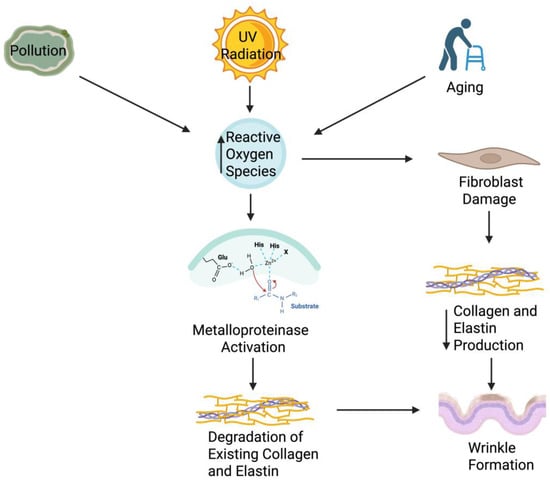
Figure 2.
Mechanisms of Wrinkle Formation via Extracellular Matrix Degradation. Extrinsic stressors like pollution and UV radiation, together with intrinsic aging, elevate reactive oxygen species (ROS). ROS activate MMPs, which degrade dermal collagen and elastin, while fibroblast damage limits ECM synthesis. These changes weaken skin structure and promote wrinkle formation. The figure highlights MMP activation, underscoring oxidative stress as a key driver of ECM breakdown. Figure created with BioRender.com.
4.1. Mechanistic Overview of Wrinkle Formation in Aging Skin
As people age, their skin undergoes physiological changes that affect its structure and composition. It has been demonstrated that reduced elastin and collagen synthesis is one of the factors responsible for wrinkle formation [51]. As a result, the dermis thins, leading to sagging and deeper lines. In addition, a report has shown that chronic exposure to UV-B further accelerates wrinkle development [52,53]. Evidence indicates that wound healing capacity declines with age as well, largely due to reduced fibroblast and keratinocyte activity, along with a diminished inflammatory response [53,54]. Moreover, Lähteenvuo et al. implicate that a reduction in angiogenesis in older individuals leads to reduced amounts of oxygen and nutrients available for the tissues, hindering the healing process as well [55]. Another study, Berard et al., shows that aging correlates with a reduction in immunological responsiveness, leading to a diminished cutaneous reactivity to allergens [56]. At the same time, deterioration of the skin barrier permits greater penetration of irritants and pathogens, thereby increasing susceptibility to infections and further highlighting the detrimental effects of aging on skin health [56]. Therefore, there is a clear need to further explore this area to better understand the mechanisms underlying age-related skin vulnerability and to develop strategies that can strengthen barrier function and enhance cutaneous immunity.
Skin aging is a complex, multifactorial process involving both intrinsic (genetic) and extrinsic (environmental) factors. Intrinsic factors include turnover, diminished moisture content, and thinning of the dermis. Extrinsic aging, primarily driven by UV radiation (photoaging), pollution, and lifestyle factors like smoking, exacerbates these changes [57].
Alkawar et al. have shown that UV-B exposure induces p53 target genes p21 and DNA polymerase eta (pol η) in keratinocytes and skin explants, but this response was diminished when IGF-1 signaling was inhibited [58]. Since p21 and pol η help prevent mutagenic DNA replication, reduced IGF-1 activity in aged skin may impair p53 function, thereby increasing susceptibility to nonmelanoma skin cancers in older individuals [58].
Wrinkles, both fine lines and deeper furrows, form because of these changes. They are most observed in sun-exposed areas, reflecting the chronic impact of environmental stressors. At the molecular level, chronic UV-B exposure stimulates the overproduction of ROS, which in turn activate MMPs [59]. MMPs degrade critical structural proteins such as collagen, elastin, and fibronectin, leading to reduced dermal integrity and elasticity [60]. Additionally, repeated facial expressions contribute to dynamic wrinkle formation, while intrinsic aging leads to static wrinkles due to progressive collagen loss and fragmentation (Figure 2). Together, these processes highlight that wrinkles are not merely superficial cosmetic concerns but rather a visible outcome of complex biological events that compromise the structural integrity of the skin.
4.2. Elastin Degradation and Microbiome Interactions
Elastin is a highly resilient protein, forming a network in the skin’s dermis that allows tissues to stretch and recoil. It is produced by fibroblasts during early development and remains relatively stable throughout life, but its synthesis declines after maturity [20]. With aging, elastin fibers become fragmented and cross-linked due to enzymatic degradation (mediated by enzymes like elastase) and the accumulation of AGEs. This leads to a loss of skin elasticity, contributing to the appearance of sagging and wrinkles [61]. Solar elastosis, a hallmark of photoaged skin, occurs when prolonged UV exposure damages elastin fibers. The result is the accumulation of abnormal, disorganized elastin in the skin. Intrinsically, as we age, the skin undergoes structural and functional changes, including thinning of the dermis, which further weakens the skin’s elasticity and increases the prominence of wrinkles [62].
Emerging research indicates that the skin microbiome also influences elastin homeostasis. As indicated by Cheung et al., certain pro-inflammatory strains of Cutibacterium acnes and Staphylococcus epidermidis produce enzymes like proteases and elastases that can degrade the components of the extracellular matrix [63]. Conversely, Shirzad et al. explain commensals like Lactobacillus species may help to preserve the elastin integrity by inhibiting elastase activity [64]. An imbalance in the skin microbiome can thus accelerate elastin degradation, contributing to wrinkle formation. Moreover, UV radiation promotes the production of ROS, which upregulate MMPs and elastases, accelerating elastin degradation. This not only replaces healthy elastin fibers but also disrupts the surrounding ECM, further diminishing skin elasticity and contributing to coarse wrinkling and uneven skin texture [22]. UV-A radiation is a well-known inducer of ROS that can alter elastic fiber structure, thereby reducing skin elasticity and contributing to wrinkle formation and aging [11,12]. At the molecular level, the degradation of elastin reflects the interplay between intrinsic genetic programming and extrinsic environmental insults. Aging skin also experiences reduced fibroblast activity and dermal thinning, which together limit the skin’s capacity to repair and regenerate its elastic fiber network [65]. Clinical and experimental evidence indicates that wrinkle formation is significantly associated with UV-B-induced reduction in skin elasticity and structural modifications of elastic fibers. UV-B activates keratinocyte-derived cytokines that increase fibroblast elastase activity, which breaks down elastic fibers [53]. Elastase inhibition is crucial in preventing wrinkle formation, highlighting fibroblast elastase as a key mediator in the mechanism of UVB-induced wrinkle formation [53].
Therefore, elastin serves as a cornerstone of dermal structure and function. Its gradual degradation through enzymatic activity, oxidative stress, and glycation, compounded by external factors such as UV-A/UV-B exposure, is central to the pathophysiology of skin aging [66]. Understanding these mechanisms highlights why strategies targeting oxidative stress, glycation, and enzymatic degradation are key to preventing and reducing wrinkles and maintaining youthful skin integrity.
4.3. Role of MMP-1 and MMP-9 in Wrinkle Formation
MMPs, particularly MMP-1 and MMP-9, are key enzymes involved in the degradation of extracellular matrix components during skin aging and can lead to increased wrinkle formation. MMP-1 breaks down fibrillar collagen types I and III [67]. On the other hand, MMP-9 contributes to gelatin and elastin degradation [68]. These enzymes are upregulated in response to ultraviolet radiation, oxidative stress, and chronic inflammation. This is achieved largely through the activation of the AP-1 and NF- κB signaling pathways [69]. This enzymatic activity leads to collagen fragmentation, dermal thinning, and wrinkle formation. In UV-B-induced photoaging, elevated MMP-1 and MMP-9 expressions are correlated with collagen degradation and visible wrinkling. Further, the skin microbiome has been identified as a key environmental modulator of MMP activity [70]. Further, Tirka et al. demonstrate that elevated serum MMP-1 levels are significantly associated with increased severity of facial wrinkles in photoaging [71]. A moderate positive correlation was observed between MMP-1 levels and wrinkle scores, with nearly half of the variance in wrinkle severity explained by MMP-1 expression [71]. Moreover, Hong et al. show that lipoteichoic acid (pLTA) from Lactobacillus plantarum inhibits UV-induced MMP-1 expression, suppresses MAPK/AP-1 and NF-κB activation, reduces ROS generation, and enhances type I procollagen synthesis in human dermal fibroblasts [72]. These findings suggest that pLTA may serve as a promising agent for the prevention and treatment of skin photoaging [72]. Oral administration of Lactobacillus plantarum HY7714 significantly suppressed MMP expression in experimental models, thus preserving collagen levels and reducing wrinkle depth [73]. These results were supported in a clinical trial where Lactobacillus plantarum HY7714 supplementation improved both skin elasticity and reduced trans epidermal water loss in human subjects [23]. Both probiotics and postbiotics have been shown to inhibit MMP activity indirectly. The suppression of MMPs by these probiotic strains is believed to occur through the downregulation of oxidative stress and pro-inflammatory cytokines that are upstream activators of MMP expression. This highlights the microbiome’s potential as a regulator of host signaling pathways such as NF-κB and AP-1, both of which are involved in MMP-9 upregulation [74]. This suggests that targeted microbial interventions may help suppress MMP-mediated matrix degradation and slow wrinkle formation.
4.4. Collagen and ECM Breakdown in Skin Aging: Microbial and Molecular Modulation
In addition to elastin, collagen is a critical structural protein in the dermis that provides tensile strength to the skin [75]. Type I and III collagens are predominant in youthful skin, forming tightly packed fibers that maintain the skin’s integrity. Type I collagen is the most abundant and primarily contributes to resistance to mechanical forces, while Type III collagen supports skin elasticity and is more prevalent during early wound healing [76]. During aging, collagen production decreases while its degradation increases [77]. This is primarily due to the upregulation of MMPs triggered by prolonged UV exposure, oxidative stress, and inflammation. An in vitro study shows that UV exposure increases MMP-1 expression in dermal fibroblasts, which degrades type I collagen [69]. It has been demonstrated that UV-B exposure in specific increased MMP-1 and MMP-9 expression, leading to the degradation of type I collagen and dermal thinning [23]. As the collagen breaks down, the thickness of the skin is consequently reduced, causing the formation of wrinkles.
In this context, emerging evidence indicates that the skin microbiome can also modulate collagen turnover, positioning it as a potential upstream regulator of age-related changes in dermal structure [78].
The skin harbors a complex ecosystem of commensal and pathogenic microbes that interact closely with host immune and structural systems. Specific commensals like Lactobacillus plantarum and Streptococcus thermophiles have been shown to preserve collagen integrity by reducing inflammation and oxidative stress, which leads to decreased metalloproteinase activity [79]. These beneficial strains may also influence host gene expression and barrier function, further stabilizing the extracellular matrix. Conversely, the overgrowth of pro-inflammatory strains of Cutibacterium acnes or loss of microbial diversity can exacerbate collagen degradation through the production of collagenolytic enzymes [80]. This dysbiosis may act synergistically with external stressors like UV exposure, accelerating the aging process by weakening the skin’s structural integrity.
Other extracellular matrix components such as fibronectin and hyaluronic acid play essential roles in maintaining dermal structure and hydration. Fibronectin is involved in both wound healing and cell adhesion. Its reduction contributes to decreased skin regeneration [81]. Hyaluronic acid, on the other hand, retains water and helps keep the skin plump and elastic. As one ages, hyaluronic acid production declines, contributing to skin dryness and fine lines [82]. The deterioration of these extracellular matrix elements contributes to the loss of dermal volume and elasticity, increasing wrinkle depth and skin looseness (Table 3).
A recent study indicates that microbial imbalances contribute to the breakdown of the extracellular matrix by promoting inflammation and oxidative stress [83]. Another study implicates blocking TNFα with etanercept reduced UV-B-induced recruitment of inflammatory cells and inhibited MMP13 expression. However, it also decreased mature collagen, increased collagen fragmentation, and lowered procollagen levels. These results suggest that while TNFα blockade limits inflammation, it may impair collagen synthesis and matrix integrity in UV-B-irradiated skin [84]. Importantly, UV-A irradiation accelerates photoaging by inducing oxidative stress, DNA damage, collagen degradation, and cellular senescence in dermal fibroblasts [85]. This study demonstrates that rapamycin counteracts these effects by enhancing autophagy, reducing p53 and phosphorylated HSP27 levels, limiting oxidative and genotoxic stress, and preserving collagen via activation of the TGF-β/Smad and MAPK/AP-1 pathways [85]. These findings underscore the potential of probiotic and postbiotic intervention in restoring the microbial balance and mitigating age-related structural damage.

Table 3.
Mechanism of Wrinkles and the Role of the Microbiome.
Table 3.
Mechanism of Wrinkles and the Role of the Microbiome.
| Wrinkle Formation Contributor | Mechanism of Action | Microbiome Influence |
|---|---|---|
| Collagen Degradation | Matrix metalloproteinases break down collagen, and are activated by factors such as UV radiation, reactive oxygen factors, and inflammation. | The microbiome modulates inflammation. Good microbiota help suppress inflammation, keeping matrix metalloproteinases levels low. Certain microbes also produce antioxidants that neutralize oxidants [86] |
| Oxidative Stress | Reactive oxygen species cause wrinkles by damaging the dermal fibroblasts, activating matrix metalloproteinases, and leading to a reduction in skin elasticity. | Certain skin bacteria produce antioxidant enzymes that neutralize ROS [87] |
| Inflammation | Inflammation activates matrix metalloproteinases, damage the fibroblasts that produce collagen, and increase overall oxidative stress. | Healthy skin microbiota help suppress inflammation by inhibiting immune pathways and creating anti-inflammatory cytokines [88] |
| Loss of Skin Hydration | The stratum corneum contains high levels of water, maintaining the elasticity of the skin. | Commensal bacteria promote tight junction integrity and lipid production, which leads to less trans epidermal water loss and better moisture retention, keeping the skin elastic [89] |
5. Therapeutic Approach
5.1. Probiotics/Postbiotics in Reducing Wrinkles
The role of probiotics and postbiotics in reducing wrinkles has become increasingly popular, highlighting the importance of the gut-skin axis. Probiotics are live microorganisms, while postbiotics are the byproducts or metabolites produced by these microbes. As the skin ages, there is less production of elastin and collagen, as well as diminished hydration and increased oxidative stress [90]. All these factors can contribute to the formation of wrinkles. Probiotics can influence these processes in multiple ways. For instance, probiotic strains like Lactobacillus and Bifidobacterium species have been shown to reduce markers of inflammation and suppress the production of inflammatory cytokines, thereby limiting collagen breakdown and preserving the integrity of the skin [91]. Topical applications directly interact with the epidermal layer, which promotes resistance to the effects of aging on skin. Postbiotics like short-chain fatty acids and lipoteichoic acids have antioxidant effects, relieving the stress caused my environmental stressors such as UV radiation and pollution [92]. Postbiotics have also been found to increase skin hydration by stimulating the production of ceramides and natural moisturizing factors, which decreases the presence of fine lines on the skin [93]. Some fermented skincare products, which contain postbiotics, can upregulate the expression of genes involved in collagen synthesis, supporting the dermal integrity and minimizing the production of wrinkles as well [94]. Probiotics may also reinforce the skin barrier and prevent transdermal water loss, keeping the skin hydrated and healthy (Figure 3).
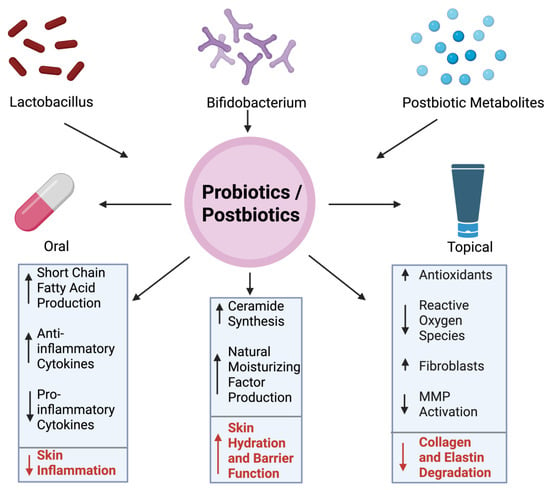
Figure 3.
Mechanism of Wrinkle Reduction via Probiotics/Postbiotics. The central circle represents probiotic and postbiotic interventions, which can be administered either orally or topically. Benefits of these two include increased production of short-chain fatty acids, modulation of cytokine profiles to reduce inflammation, promotion of ceramide synthesis and natural moisturizing factor production, antioxidant generation, suppression of ROS, reduced MMP activation, and preservation of fibroblasts. Together, these mechanisms help protect collagen and elastin from degradation and contribute to healthier, more youthful skin. Figure created with BioRender.com.
Figure 3 represents the effect of probiotics and postbiotics on skin.
Probiotics, particularly strains such as Lactobacillus and Bifidobacterium, are recognized for their ability to modulate skin health through both the gut-skin and skin-skin axes. These microbes can be administered orally or applied topically, with each route offering distinct mechanisms for improving skin condition. Probiotics that are taken orally help to restore the gut microbiome, which can reduce systemic inflammation and oxidative stress, both of which contribute to dermal aging [95]. As highlighted in a study, strains like L. plantarum and B. breve have demonstrated the ability to boost anti-inflammatory cytokines such as IL-10 while downregulating pro-inflammatory markers like IL-6, leading to lower skin inflammation and improved elasticity [73]. Topical probiotics enhance the skin barrier and microbial diversity by promoting the colonization of beneficial microbes. This acts to outcompete harmful strains associated with aging [96].
Probiotics can combat wrinkles by influencing processes such as skin hydration and collagen stability [23]. Furthermore, probiotics have been linked to increased production of ceramides and NMFs, both of which help prevent water loss in the skin. This helps the skin remain moisturized and plump. In addition, probiotics indirectly inhibit MMPs enzymes that degrade collagen and elastin by reducing inflammation and oxidative stress [24]. By preserving the extracellular matrix’s integrity, the formation and appearance of fine lines and wrinkles is reduced. Probiotics also support fibroblast viability, which helps maintain dermal structure and repair UV-induced damage [97]. Mitochondrial dysfunction in aging fibroblasts leads to excess ROS production, which triggers DNA damage responses and promotes cellular senescence. As a result, collagen synthesis is reduced while the secretion of matrix-degrading enzymes is increased, leading to an accelerated rate of dermal aging [98].
Postbiotics, which include short-chain fatty acids (SCFAs), lipoteichoic acids, and bacterial peptides, are the products of probiotics [25]. SCFAs such as butyrate exhibit anti-inflammatory and antioxidant effects, which help to neutralize ROS that accumulate from stressors such as UV radiation or pollution. These metabolites also signal the upregulation of genes involved in ceramide synthesis and skin barrier repair. Moreover, fermented skin care products rich in probiotics have been found to directly stimulate collagen gene expression, improving dermal density and smoothness [26] (Figure 3). Apart from probiotics and postbiotics, natural products like fish oils rich in docosahexaenoic acid (DHA) show effectiveness against aging-induced skin oxidation.
DHA is readily incorporated into fibroblast membrane phospholipids, and UVA irradiation promotes the generation of oxidized DHA-derived lipids that activate Nrf2 signaling [99]. In DHA-supplemented cells, Nrf2 enhances protective gene expression, whereas Nrf2-deficient fibroblasts show increased TNFα and MMP13 induction along with higher levels of oxidized phospholipids. These findings suggest that an intact Nrf2 pathway is essential to counteract DHA-induced inflammation and matrix degradation under UV stress [99]. Further, Alchemilla and Chamomile represent rich sources of bioactive compounds with well-documented dermatological benefits, ranging from anti-inflammatory, antioxidant, and antimicrobial effects to anti-aging and regenerative activities [100,101]. Their traditional use is now substantiated by modern research, positioning them as promising candidates for cosmetic and therapeutic applications, while future studies should emphasize clinical validation and optimized formulations.
5.2. Clinical Evidence and Preventive Potential of Probiotics and Postbiotics in Skin Aging
Clinical trials have yielded results that indicate the potential probiotics and postbiotics have in preventing wrinkles. Oral supplementation of Lactobacillus plantarum HY7714 was associated with improved skin elasticity and reduced wrinkle depth after several weeks of use [23]. Similarly, postbiotic-containing creams have been shown to reduce wrinkle severity and increase the dermal density in both human and animal subjects [93]. All these studies indicate that an effective strategy for preserving the skin can be to address the skin’s microbiome. Each probiotic and postbiotic can have variable effects and address different aspects of the skin, highlighting the importance of continued research on the skin microbiome. In total, probiotics and postbiotics have demonstrated the capability to reduce the appearance of wrinkles through various pathways, such as enhancing skin barrier function of the skin, reducing inflammation, increasing hydration, or stimulating the production of certain useful proteins. Further, there are some marketed products highlighted in Table 4.

Table 4.
Microbiome- and Synbiotic-Based Products for Skin Health and Anti-Photoaging Applications.
6. Diagnostic Potential of Skin Microbiome Profiling
Recent technological advances have led to the development of non-invasive diagnostic approaches using skin microbiome signatures to assess current and future skin conditions. Multi-omics and machine learning models have demonstrated that the skin microbiome can serve as a reliable biomarker for chronological and biological aging [35]. Similarly, large-scale metagenomic and imaging-based surveys have enabled the formulation of indices such as the Facial Aging Index, which integrates skin physio-optical parameters with microbial composition to assess aging-related changes [45]. Moreover, 16S rRNA and shotgun metagenomic analyses are increasingly being utilized in dermatological diagnostics to detect microbial dysbiosis linked to conditions such as acne, eczema, rosacea, and atopic dermatitis—conditions that often precede or accelerate skin aging [44,46].
7. Conclusions
The skin microbiome plays an essential role in the aging process and maintenance of skin health. Age-related shifts disrupt barrier integrity, create more inflammation, and accelerate the process of collagen and elastin breakdown, all of which contribute to the formation of wrinkles. Probiotics containing Bifidobacterium and lactobacillus and their lysate as a postbiotic can restore microbial balance and prevent inflammation, keeping the skin well-kept. Early clinical trials demonstrate that improvements in skin elasticity, hydration, and wrinkle depth, highlighting the importance of microbiome-targeted approaches. Further clinical trials and mechanistic studies will be essential in translating these findings into anti-aging solutions.
Author Contributions
V.C., S.K.P., S.G., D.Y. and L.L. conducted the literature review and wrote the manuscript. H.Y., generated this idea and revised the manuscript and formatting. S.J. critically reviewed the manuscript and suggested revisions. All authors have read and agreed to the published version of the manuscript.
Funding
No funding from public, private, or nonprofit organizations was received for this review article.
Acknowledgments
The authors acknowledge the continued support provided to Yadav’s lab by the Florida Department of Health’s Ed and Ethel Moore Alzheimer’s Disease Research Program (Grant 22A17), the National Institutes of Health, and the National Institute on Aging through grants RF1AG071762, R21AG072379, U01AG076928, U54HL160273, and R21AG085881. For resources provided by the Center for Microbiome Research, the Microbiomes Institute, the Center for Excellence in Aging and Brain Repair, the Department of Neurosurgery, Brain and Spine, and the USF Morsani College of Medicine, the authors also thank the University of South Florida.
Conflicts of Interest
Hariom Yadav is the Chief Scientific Officer and co-founder of Postbiotics Inc. and BiomAge Inc. He is also the co-founder of MusB LLC, MusB Research LLC, and MeraBiome Inc. with Shalini Jain. The authors, however, say that there are no conflicts of interest related to the content or findings of this review article.
References
- Fredricks, D.N. Microbial ecology of human skin in health and disease. J. Investig. Dermatol. Symp. Proc. 2001, 6, 167–169. [Google Scholar] [CrossRef] [PubMed]
- Chiller, K.; Selkin, B.A.; Murakawa, G.J. Skin microflora and bacterial infections of the skin. J. Investig. Dermatol. Symp. Proc. 2001, 6, 170–174. [Google Scholar] [CrossRef]
- Prajapati, S.K.; Lekkala, L.; Yadav, D.; Jain, S.; Yadav, H. Microbiome and Postbiotics in Skin Health. Biomedicines 2025, 13, 791. [Google Scholar] [CrossRef]
- Costello, E.K.; Lauber, C.L.; Hamady, M.; Fierer, N.; Gordon, J.I.; Knight, R. Bacterial community variation in human body habitats across space and time. Science 2009, 326, 1694–1697. [Google Scholar] [CrossRef]
- Grice, E.A.; Kong, H.H.; Conlan, S.; Deming, C.B.; Davis, J.; Young, A.C.; NISC Comparative Sequencing Program; Bouffard, G.G.; Blakesley, R.W.; Murray, P.R.; et al. Topographical and temporal diversity of the human skin microbiome. Science 2009, 324, 1190–1192. [Google Scholar] [CrossRef]
- Grice, E.A.; Segre, J.A. The skin microbiome. Nat. Rev. Microbiol. 2011, 9, 244–253. [Google Scholar] [CrossRef]
- Sanford, J.A.; Gallo, R.L. Functions of the skin microbiota in health and disease. Semin. Immunol. 2013, 25, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Prajapati, S.K.; Jain, S.; Yadav, H. Age-Related Cognitive Decline and Dementia: Interface of Microbiome-Immune-Neuronal Interactions. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2025, 80, glaf038. [Google Scholar] [CrossRef] [PubMed]
- Tobin, D.J. Introduction to skin aging. J. Tissue Viability 2017, 26, 37–46. [Google Scholar] [CrossRef]
- Hussein, R.S.; Dayel, S.B.; Abahussein, O.; El-Sherbiny, A.A. Influences on Skin and Intrinsic Aging: Biological, Environmental, and Therapeutic Insights. J. Cosmet. Dermatol. 2024, 24, e16688. [Google Scholar] [CrossRef]
- Amaro-Ortiz, A.; Yan, B.; D’Orazio, J.A. Ultraviolet radiation, aging and the skin: Prevention of damage by topical cAMP manipulation. Molecules 2014, 19, 6202–6219. [Google Scholar] [CrossRef] [PubMed]
- Bossi, O.; Gartsbein, M.; Leitges, M.; Kuroki, T.; Grossman, S.; Tennenbaum, T. UV irradiation increases ROS production via PKCδ signaling in primary murine fibroblasts. J. Cell. Biochem. 2008, 105, 194–207. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Bai, X.; Peng, T.; Yi, X.; Luo, L.; Yang, J.; Liu, J.; Wang, Y.; He, T.; Wang, X. New insights into the skin microbial communities and skin aging. Front. Microbiol. 2020, 11, 565549. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-J.; Kim, J.J.; Myeong, N.R.; Kim, T.; Kim, D.; An, S.; Kim, H.; Park, T.; Jang, S.I.; Yeon, J.H.; et al. Segregation of age-related skin microbiome characteristics by functionality. Sci. Rep. 2019, 9, 16748. [Google Scholar] [CrossRef]
- Cros, M.P.; Mir-Pedrol, J.; Toloza, L.; Knödlseder, N.; Maruotti, J.; Zouboulis, C.C.; Güell, M.; Fábrega, M.-J. New insights into the role of Cutibacterium acnes-derived extracellular vesicles in inflammatory skin disorders. Sci. Rep. 2023, 13, 16058, Corrected in Sci. Rep. 2024, 14, 3027. [Google Scholar]
- Szabó, K.; Erdei, L.; Bolla, B.S.; Tax, G.; Bíró, T.; Kemény, L. Factors shaping the composition of the cutaneous microbiota. Br. J. Dermatol. 2017, 176, 344–351. [Google Scholar] [CrossRef]
- Shibagaki, N.; Suda, W.; Clavaud, C.; Bastien, P.; Takayasu, L.; Iioka, E.; Kurokawa, R.; Yamashita, N.; Hattori, Y.; Shindo, C.; et al. Aging-related changes in the diversity of women’s skin microbiomes associated with oral bacteria. Sci. Rep. 2017, 7, 10567. [Google Scholar] [CrossRef]
- Myers, T.; Bouslimani, A.; Huang, S.; Hansen, S.T.; Clavaud, C.; Azouaoui, A.; Ott, A.; Gueniche, A.; Bouez, C.; Zheng, Q.; et al. A multi-study analysis enables identification of potential microbial features associated with skin aging signs. Front. Aging 2024, 4, 1304705. [Google Scholar] [CrossRef]
- Ratanapokasatit, Y.; Laisuan, W.; Rattananukrom, T.; Petchlorlian, A.; Thaipisuttikul, I.; Sompornrattanaphan, M. How microbiomes affect skin aging: The updated evidence and current perspectives. Life 2022, 12, 936. [Google Scholar] [CrossRef]
- Baumann, L.; Bernstein, E.F.; Weiss, A.S.; Bates, D.; Humphrey, S.; Silberberg, M.; Daniels, R. Clinical relevance of elastin in the structure and function of skin. Aesthetic Surg. J. Open Forum 2021, 3, ojab019. [Google Scholar] [CrossRef]
- Ryu, H.S.; Joo, Y.H.; Kim, S.O.; Park, K.C.; Youn, S.W. Influence of age and regional differences on skin elasticity as measured by the Cutometer®. Skin Res. Technol. 2008, 14, 354–358. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.-Y. Solar Elastosis. 2022. Available online: https://dermnetnz.org/topics/solar-elastosis (accessed on 22 July 2025).
- Lee, D.E.; Huh, C.-S.; Ra, J.; Choi, I.-D.; Jeong, J.-W.; Kim, S.-H.; Ryu, J.H.; Seo, Y.K.; Koh, J.S.; Lee, J.-H.; et al. Clinical evidence of effects of Lactobacillus plantarum HY7714 on skin aging: A randomized, double blind, placebo-controlled study. J. Microbiol. Biotechnol. 2015, 25, 2160–2168. [Google Scholar] [CrossRef]
- Teng, Y.; Huang, Y.; Danfeng, X.; Tao, X.; Fan, Y. The role of probiotics in skin photoaging and related mechanisms: A review. Clin. Cosmet. Investig. Dermatol. 2022, 15, 2455–2464. [Google Scholar] [CrossRef]
- Scott, E.; De Paepe, K.; Van de Wiele, T. Postbiotics and their health modulatory biomolecules. Biomolecules 2022, 12, 1640. [Google Scholar] [CrossRef]
- Huuskonen, L.; Anglenius, H.; Ahonen, I.; Tiihonen, K. Effects of bacterial lysates and metabolites on collagen homeostasis in TNF-α-challenged human dermal fibroblasts. Microorganisms 2023, 11, 1465. [Google Scholar] [CrossRef]
- Prajapati, S.K.; Yadav, D.; Katiyar, S.; Jain, S.; Yadav, H. Postbiotics as Mitochondrial Modulators in Inflammatory Bowel Disease: Mechanistic Insights and Therapeutic Potential. Biomolecules 2025, 15, 954. [Google Scholar] [CrossRef] [PubMed]
- O’Toole, P.W.; Claesson, M.J. Gut microbiota: Changes throughout the lifespan from infancy to elderly. Int. Dairy J. 2010, 20, 281–291. [Google Scholar] [CrossRef]
- Gottlieb, M.G.V.; Closs, V.E.; Junges, V.M.; Schwanke, C.H.A. Impact of human aging and modern lifestyle on gut microbiota. Crit. Rev. Food Sci. Nutr. 2018, 58, 1557–1564. [Google Scholar] [CrossRef]
- Thakur, R.; Batheja, P.; Kaushik, D.; Michniak, B. Structural and biochemical changes in aging skin and their impact on skin permeability barrier. Ski. Aging Handb. 2009, 55–90. [Google Scholar] [CrossRef]
- Pagac, M.P.; Davient, B.; Plado, L.A.; Lam, H.Y.I.; Lee, S.M.; Ravikrishnan, A.; Chua, W.L.E.; Muralidharan, S.; Sridharan, A.; Irudayaswamy, A.S.; et al. Life stage impact on the human skin ecosystem: Lipids and the microbial community. npj Biofilms Microbiomes 2025, 11, 13. [Google Scholar] [CrossRef]
- Kreouzi, M.; Theodorakis, N.; Nikolaou, M.; Feretzakis, G.; Anastasiou, A.; Kalodanis, K.; Sakagianni, A. Skin microbiota: Mediator of interactions between metabolic disorders and cutaneous health and disease. Microorganisms 2025, 13, 161. [Google Scholar] [CrossRef]
- Ling, Z.; Liu, X.; Cheng, Y.; Yan, X.; Wu, S. Gut microbiota and aging. Crit. Rev. Food Sci. Nutr. 2022, 62, 3509–3534. [Google Scholar] [CrossRef]
- Pająk, J.; Nowicka, D.; Szepietowski, J.C. Inflammaging and immunosenescence as part of skin aging—A narrative review. Int. J. Mol. Sci. 2023, 24, 7784. [Google Scholar] [CrossRef]
- Huang, S.; Haiminen, N.; Carrieri, A.-P.; Hu, R.; Jiang, L.; Parida, L.; Russell, B.; Allaband, C.; Zarrinpar, A.; Vázquez-Baeza, Y.; et al. Human skin, oral, and gut microbiomes predict chronological age. Msystems 2020, 5, e00630-19. [Google Scholar] [CrossRef]
- Dimarzio, L.; Cinque, B.; Cupelli, F.; De Simone, C.; Cifone, M.; Giuliani, M. Increase of skin-ceramide levels in aged subjects following a short-term topical application of bacterial sphingomyelinase from Streptococcus thermophilus. Int. J. Immunopathol. Pharmacol. 2008, 21, 137–143. [Google Scholar] [CrossRef]
- Souak, D.; Barreau, M.; Courtois, A.; André, V.; Duclairoir Poc, C.D.; Feuilloley, M.G.; Gault, M. Challenging cosmetic innovation: The skin microbiota and probiotics protect the skin from UV-induced damage. Microorganisms 2021, 9, 936. [Google Scholar] [CrossRef] [PubMed]
- D’Orazio, J.; Jarrett, S.; Amaro-Ortiz, A.; Scott, T. UV radiation and the skin. Int. J. Mol. Sci. 2013, 14, 12222–12248. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Li, Y.; Qu, Y.; Zheng, Y.; Ouyang, M.; Zhang, Y.; Lai, W.; Xu, Q. UVA-induced photoaging inhibits autophagic degradation by impairing lysosomal function in dermal fibroblasts. Biochem. Biophys. Res. Commun. 2019, 518, 611–618. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, Y.; Wang, Y.; Xu, H.; Mei, X.; Yu, D.; Wang, Y.; Li, W. Antioxidant properties of probiotic bacteria. Nutrients 2017, 9, 521. [Google Scholar] [CrossRef] [PubMed]
- Vollmer, D.L.; West, V.A.; Lephart, E.D. Enhancing skin health: By oral administration of natural compounds and minerals with implications to the dermal microbiome. Int. J. Mol. Sci. 2018, 19, 3059. [Google Scholar] [CrossRef]
- Meunier, M.; Scandolera, A.; Chapuis, E.; Lambert, C.; Jarrin, C.; Robe, P.; Chajra, H.; Auriol, D.; Reynaud, R. From stem cells protection to skin microbiota balance: Orobanche rapum extract, a new natural strategy. J. Cosmet. Dermatol. 2019, 18, 1140–1154. [Google Scholar] [CrossRef]
- Liebert, A.; Bicknell, B.; Johnstone, D.M.; Gordon, L.C.; Kiat, H.; Hamblin, M.R. “Photobiomics”: Can light, including photobiomodulation, alter the microbiome? Photobiomodulation Photomed. Laser Surg. 2019, 37, 681–693. [Google Scholar] [CrossRef] [PubMed]
- Woolery-Lloyd, H.; Andriessen, A.; Day, D.; Gonzalez, N.; Green, L.; Grice, E.; Henry, M. Review of the microbiome in skin aging and the effect of a topical prebiotic containing thermal spring water. J. Cosmet. Dermatol. 2023, 22, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Hu, G.; Yi, L.; Ge, W.; Yang, Q.; Yang, X.; He, Y.; Liu, Z.; Chen, W.-H. Integrated analysis of facial microbiome and skin physio-optical properties unveils cutotype-dependent aging effects. Microbiome 2024, 12, 163. [Google Scholar] [CrossRef] [PubMed]
- Howard, B.; Bascom, C.C.; Hu, P.; Binder, R.L.; Fadayel, G.; Huggins, T.G.; Jarrold, B.B.; Osborne, R.; Rocchetta, H.L.; Swift, D.; et al. Aging-associated changes in the adult human skin microbiome and the host factors that affect skin microbiome composition. J. Investig. Dermatol. 2022, 142, 1934–1946.e21. [Google Scholar] [CrossRef] [PubMed]
- Garlet, A.; Andre-Frei, V.; Del Bene, N.; Cameron, H.J.; Samuga, A.; Rawat, V.; Ternes, P.; Leoty-Okombi, S. Facial skin microbiome composition and functional shift with aging. Microorganisms 2024, 12, 1021. [Google Scholar] [CrossRef]
- Blaise, G.; Nikkels, A.F.; Hermanns-Lê, T.; Nikkels-Tassoudji, N.; Piérard, G.E. Corynebacterium-associated skin infections. Int. J. Dermatol. 2008, 47, 884–890. [Google Scholar] [CrossRef]
- Bouilly-Gauthier, D.; Jeannes, C.; Maubert, Y.; Duteil, L.; Queille-Roussel, C.; Piccardi, N.; Montastier, C.; Manissier, P.; Pierard, G.; Ortonne, J.P. Clinical evidence of benefits of a dietary supplement containing probiotic and carotenoids on ultraviolet-induced skin damage. Br. J. Dermatol. 2010, 163, 536–543. [Google Scholar] [CrossRef]
- Rong, J.; Shan, C.; Liu, S.; Zheng, H.; Liu, C.; Liu, M.; Jin, F.; Wang, L. Skin resistance to UVB-induced oxidative stress and hyperpigmentation by the topical use of Lactobacillus helveticus NS8-fermented milk supernatant. J. Appl. Microbiol. 2017, 123, 511–523. [Google Scholar] [CrossRef]
- Moloney, S.J.; Edmonds, S.H.; Giddens, L.D.; Learn, D.B. The hairless mouse model of photoaging: Evaluation of the relationship between dermal elastin, collagen, skin thickness and wrinkles. Photochem. Photobiol. 1992, 56, 505–511. [Google Scholar] [CrossRef]
- Passeron, T.; Krutmann, J.; Andersen, M.; Katta, R.; Zouboulis, C. Clinical and biological impact of the exposome on the skin. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 4–25. [Google Scholar] [CrossRef] [PubMed]
- Imokawa, G. Mechanism of UVB-induced wrinkling of the skin: Paracrine cytokine linkage between keratinocytes and fibroblasts leading to the stimulation of elastase. J. Investig. Dermatol. Symp. Proc. 2009, 14, 36–43. [Google Scholar] [CrossRef]
- Ashcroft, G.S.; Mills, S.J.; Ashworth, J.J. Ageing and wound healing. Biogerontology 2002, 3, 337–345. [Google Scholar] [CrossRef]
- Lähteenvuo, J.; Rosenzweig, A. Effects of aging on angiogenesis. Circ. Res. 2012, 110, 1252–1264. [Google Scholar] [CrossRef] [PubMed]
- Berard, F.; Marty, J.-P.; Nicolas, J.-F. Allergen penetration through the skin. Eur. J. Dermatol. 2003, 13, 324–330. [Google Scholar]
- Shin, S.H.; Lee, Y.H.; Rho, N.-K.; Park, K.Y. Skin aging from mechanisms to interventions: Focusing on dermal aging. Front. Physiol. 2023, 14, 1195272. [Google Scholar] [CrossRef] [PubMed]
- Alkawar, A.M.; Castellanos, A.J.; Carpenter, M.A.; Hutcherson, R.J.; Madkhali, M.A.; Johnson, R.M.; Bottomley, M.; Kemp, M.G. Insulin-like Growth Factor-1 Impacts p53 Target Gene Induction in UVB-irradiated Keratinocytes and Human Skin. Photochem. Photobiol. 2020, 96, 1332–1341. [Google Scholar] [CrossRef]
- Panich, U.; Sittithumcharee, G.; Rathviboon, N.; Jirawatnotai, S. Ultraviolet radiation-induced skin aging: The role of DNA damage and oxidative stress in epidermal stem cell damage mediated skin aging. Stem Cells Int. 2016, 2016, 7370642. [Google Scholar] [CrossRef]
- Fisher, G.J.; Quan, T.; Purohit, T.; Shao, Y.; Cho, M.K.; He, T.; Varani, J.; Kang, S.; Voorhees, J.J. Collagen fragmentation promotes oxidative stress and elevates matrix metalloproteinase-1 in fibroblasts in aged human skin. Am. J. Pathol. 2009, 174, 101–114. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Zhang, J.-Q.; Li, L.; Guo, M.-M.; He, Y.-F.; Dong, Y.-M.; Meng, H.; Yi, F. Advanced glycation end products in the skin: Molecular mechanisms, methods of measurement, and inhibitory pathways. Front. Med. 2022, 9, 837222. [Google Scholar] [CrossRef] [PubMed]
- Sellheyer, K. Pathogenesis of solar elastosis: Synthesis or degradation? J. Cutan. Cutan. Pathol. 2003, 30, 123–127. [Google Scholar] [CrossRef]
- Cheung, C.T.; Lancien, U.; Corvec, S.; Mengeaud, V.; Mias, C.; Véziers, J.; Khammari, A.; Dréno, B. Pro-inflammatory activity of Cutibacterium acnes phylotype IA1 and extracellular vesicles: An in vitro study. Exp. Dermatol. 2024, 33, e15150. [Google Scholar] [CrossRef] [PubMed]
- Shirzad, M.; Hamedi, J.; Motevaseli, E.; Modarressi, M.H. Anti-elastase and anti-collagenase potential of Lactobacilli exopolysaccharides on human fibroblast. Artif. Cells Nanomed. Biotechnol. 2018, 46 (Suppl. S1), 1051–1061. [Google Scholar] [CrossRef] [PubMed]
- Zorina, A.; Zorin, V.; Kudlay, D.; Kopnin, P. Age-related changes in the fibroblastic differon of the dermis: Role in skin aging. Int. J. Mol. Sci. 2022, 23, 6135. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Li, H.; Go, Y.; Chan, X.H.; Huang, Q.; Wu, J. Research advances on the damage mechanism of skin glycation and related inhibitors. Nutrients 2022, 14, 4588. [Google Scholar] [CrossRef]
- Singh, D.; Rai, V.; Agrawal, D.K. Regulation of collagen I and collagen III in tissue injury and regeneration. Cardiol. Cardiovasc. Med. 2023, 7, 5. [Google Scholar] [CrossRef]
- Van Doren, S.R. Matrix metalloproteinase interactions with collagen and elastin. Matrix Biol. 2015, 44, 224–231. [Google Scholar] [CrossRef]
- Quan, T.; Qin, Z.; Xia, W.; Shao, Y.; Voorhees, J.J.; Fisher, G.J. Matrix-degrading metalloproteinases in photoaging. J. Investig. Dermatol. Symp. Proc. 2009, 14, 20–24. [Google Scholar] [CrossRef]
- Li, Y.; Chen, H.; Xie, X.; Pang, R.; Huang, S.; Ying, H.; Chen, M.; Xue, L.; Zhang, J.; Ding, Y.; et al. Skin microbiome profiling reveals the crucial role of microbial metabolites in anti-photoaging. Photodermatol. Photoimmunol. Photomed. 2024, 40, e12987. [Google Scholar] [CrossRef]
- Tirka, P.S.W.; Praharsini, I.; IGAAD, R.L.K.; Suryawati, N.; Vibriyantikarna, N. High serum level of matrix metalloproteinase-1 (MMP-1) correlates positively with The severity of photoaging facial wrinkles. Int. J. Sci. Adv. 2023, 4, 122–129. [Google Scholar] [CrossRef]
- Hong, Y.-F.; Lee, H.Y.; Jung, B.J.; Jang, S.; Chung, D.K.; Kim, H. Lipoteichoic acid isolated from Lactobacillus plantarum down-regulates UV-induced MMP-1 expression and up-regulates type I procollagen through the inhibition of reactive oxygen species generation. Mol. Immunol. 2015, 67, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.M.; Lee, D.E.; Park, S.D.; Kim, Y.-T.; Kim, Y.J.; Jeong, J.W.; Jang, S.S.; Ahn, Y.-T.; Sim, J.-H.; Huh, C.-S.; et al. Oral administration of Lactobacillus plantarum HY7714 protects hairless mouse against ultraviolet B-induced photoaging. J. Microbiol. Biotechnol. 2014, 24, 1583–1591. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, Y.; Zhao, J.; Mo, Q.; Wang, C.; Wang, D.; Li, M. Weizmannia coagulans extracellular proteins reduce skin acne by inhibiting pathogenic bacteria and regulating TLR2/TRAF6-mediated NF-κB and MAPKs signaling pathways. Probiotics Antimicrob. Proteins 2025, 17, 705–720. [Google Scholar] [CrossRef]
- Daly, C.H. Biomechanical properties of dermis. J. Investig. Dermatol. 1982, 79, 17–20. [Google Scholar] [CrossRef]
- Ciornei, B.; Vaduva, A.; David, V.L.; Popescu, D.; Vulcanescu, D.D.; Adam, O.; Avram, C.R.; Pacurari, A.C.; Boia, E.S. Comparison of type I and type III collagen concentration between Oreochromis mossambicus and Oreochromis niloticus in relation to skin scaffolding. Medicina 2023, 59, 1002. [Google Scholar] [CrossRef]
- Varani, J.; Dame, M.K.; Rittie, L.; Fligiel, S.E.; Kang, S.; Fisher, G.J.; Voorhees, J.J. Decreased collagen production in chronologically aged skin: Roles of age-dependent alteration in fibroblast function and defective mechanical stimulation. Am. J. Pathol. 2006, 168, 1861–1868. [Google Scholar] [CrossRef]
- Russo, E.; Di Gloria, L.; Cerboneschi, M.; Smeazzetto, S.; Baruzzi, G.P.; Romano, F.; Ramazzotti, M.; Amedei, A. Facial skin microbiome: Aging-related changes and exploratory functional associations with host genetic factors, a pilot study. Biomedicines 2023, 11, 684. [Google Scholar] [CrossRef]
- Park, J.-Y.; Lee, J.Y.; Kim, Y.; Kang, C.-H. Lactic acid bacteria improve the photoprotective effect via MAPK/AP-1/MMP signaling pathway on skin fibroblasts. Microorganisms 2022, 10, 2481. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Cho, S.; Chung, J.H.; Hammerberg, C.; Fisher, G.J.; Voorhees, J.J. Inflammation and extracellular matrix degradation mediated by activated transcription factors nuclear factor-κB and activator protein-1 in inflammatory acne lesions in vivo. Am. J. Pathol. 2005, 166, 1691–1699. [Google Scholar] [CrossRef]
- Grinnell, F. Fibronectin and wound healing. J. Cell. Biochem. 1984, 26, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Chylińska, N.; Maciejczyk, M. Hyaluronic Acid and Skin: Its Role in Aging and Wound-Healing Processes. Gels 2025, 11, 281. [Google Scholar] [CrossRef] [PubMed]
- Ni, Q.; Zhang, P.; Li, Q.; Han, Z. Oxidative stress and gut microbiome in inflammatory skin diseases. Front. Cell Dev. Biol. 2022, 10, 849985. [Google Scholar] [CrossRef]
- Sharma, M.R.; Mitrani, R.; Werth, V.P. Effect of TNFα blockade on UVB-induced inflammatory cell migration and collagen loss in mice. J. Photochem. Photobiol. B Biol. 2020, 213, 112072. [Google Scholar]
- Bai, G.-L.; Wang, P.; Huang, X.; Wang, Z.-Y.; Cao, D.; Liu, C.; Liu, Y.-Y.; Li, R.-L.; Chen, A.-J. Rapamycin protects skin fibroblasts from UVA-induced photoaging by inhibition of p53 and phosphorylated HSP27. Front. Cell Dev. Biol. 2021, 9, 633331. [Google Scholar] [CrossRef]
- Etherington, D.J. Collagen degradation. Ann. Rheum. Dis. 1977, 36 (Suppl. S2), 14. [Google Scholar] [CrossRef]
- Wenk, J.; Brenneisen, P.; Meewes, C.; Wlaschek, M.; Peters, T.; Blaudschun, R.; Ma, W.; Kuhr, L.; Schneider, L.; Scharffetter-Kochanek, K. UV-induced oxidative stress and photoaging. Curr. Probl. Dermatol. 2001, 29, 83–94. [Google Scholar]
- Pessa, J.E.; Nguyen, H.; John, G.B.; Scherer, P.E. The anatomical basis for wrinkles. Aesthetic Surg. J. 2014, 34, 227–234. [Google Scholar] [CrossRef]
- Choi, J.W.; Kwon, S.H.; Huh, C.H.; Park, K.C.; Youn, S.W. The influences of skin visco-elasticity, hydration level and aging on the formation of wrinkles: A comprehensive and objective approach. Ski. Res. Technol. 2013, 19, e349–e355. [Google Scholar]
- Papaccio, F.; Caputo, S.; Bellei, B. Focus on the contribution of oxidative stress in skin aging. Antioxidants 2022, 11, 1121. [Google Scholar] [CrossRef]
- Tarapatzi, G.; Filidou, E.; Kandilogiannakis, L.; Spathakis, M.; Gaitanidou, M.; Arvanitidis, K.; Drygiannakis, I.; Valatas, V.; Kotzampassi, K.; Manolopoulos, V.G.; et al. The Probiotic Strains Bifidοbacterium lactis, Lactobacillus acidophilus, Lactiplantibacillus plantarum and Saccharomyces boulardii Regulate Wound Healing and Chemokine Responses in Human Intestinal Subepithelial Myofibroblasts. Pharmaceuticals 2022, 15, 1293. [Google Scholar] [CrossRef] [PubMed]
- Blazheva, D.; Mihaylova, D.; Averina, O.; Slavchev, A.; Brazkova, M.; Poluektova, E.; Danilenko, V.; Krastanov, A. Antioxidant potential of probiotics and postbiotics: A biotechnological approach to improving their stability. Russ. J. Genet. 2022, 58, 1036–1050. [Google Scholar] [CrossRef]
- Theodorou, I.M.; Kapoukranidou, D.; Theodorou, M.; Tsetis, J.K.; Menni, A.E.; Tzikos, G.; Bareka, S.; Shrewsbury, A.; Stavrou, G.; Kotzampassi, K. Cosmeceuticals: A review of clinical studies claiming to contain specific, well-characterized strains of probiotics or postbiotics. Nutrients 2024, 16, 2526. [Google Scholar] [CrossRef] [PubMed]
- Pu, C.; Shi, D.; Ingrassia, M.; Gedeon, H.; Chu, T.; Zhang, J.; Wang, C. Skincare Benefits of a Postbiotic Ferment Produced Through Djon Djon Mushroom Fermentation by Saccharomyces. J. Cosmet. Dermatol. 2025, 24, e70067. [Google Scholar] [CrossRef] [PubMed]
- Colletti, A.; Pellizzato, M.; Cicero, A.F. The possible role of probiotic supplementation in inflammation: A narrative review. Microorganisms 2023, 11, 2160. [Google Scholar] [CrossRef] [PubMed]
- França, K. Topical probiotics in dermatological therapy and skincare: A concise review. Dermatol. Ther. 2021, 11, 71–77. [Google Scholar] [CrossRef]
- Zhao, J.; Fu, H.; Zhang, Y.; Li, M.; Wang, D.; Zhao, D.; Zhang, J.; Wang, C. Protective effects of Lactobacillus reuteri SJ-47 strain exopolysaccharides on human skin fibroblasts damaged by UVA radiation. Bioresour. Bioprocess. 2022, 9, 127. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, H.; Man, M.Q.; Hu, L. Aging in the dermis: Fibroblast senescence and its significance. Aging Cell 2024, 23, e14054. [Google Scholar] [CrossRef]
- Gruber, F.; Ornelas, C.M.; Karner, S.; Narzt, M.-S.; Nagelreiter, I.M.; Gschwandtner, M.; Bochkov, V.; Tschachler, E. Nrf2 deficiency causes lipid oxidation, inflammation, and matrix-protease expression in DHA-supplemented and UVA-irradiated skin fibroblasts. Free Radic. Biol. Med. 2015, 88, 439–451. [Google Scholar] [CrossRef]
- Kanak, S.; Krzemińska, B. The Active compounds in plants of the genus Alchemilla with proven skin care and therapeutic formulations in the treatment of skin diseases. Prospect. Pharm. Sci. 2024, 22, 188–198. [Google Scholar] [CrossRef]
- Melnyk, N.; Nyczka, A.; Piwowarski, J.P.; Granica, S. Traditional Use of Chamomile Flowers (Matricariae flos) in Inflammatory-Associated Skin Disorders. Prospect. Pharm. Sci. 2024, 22, 59–73. [Google Scholar] [CrossRef]
- Mao, Z. Frontiers in Skin Rejuvenation: Recent Advances in Anti-Aging Skincare Technologies Based on Proteins, Peptides, and Peptide Derivatives. Mod. Health Sci. 2025, 8, 69. [Google Scholar] [CrossRef]
- Zhang, H.; Duan, Y.; Cai, F.; Cao, D.; Wang, L.; Qiao, Z.; Hong, Q.; Li, N.; Zheng, Y.; Su, M.; et al. Next-generation probiotics: Microflora intervention to human diseases. BioMed Res. Int. 2022, 2022, 5633403. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).