Medicinal Mushrooms in Colon Cancer Therapy: Mechanisms of Action of Bioactive Compounds and Therapeutic Potential
Abstract
1. Introduction
2. Edible and Medicinal Mushrooms with Anti-Colon Cancer Effects
2.1. Definition of Medicinal and Edible Mushrooms
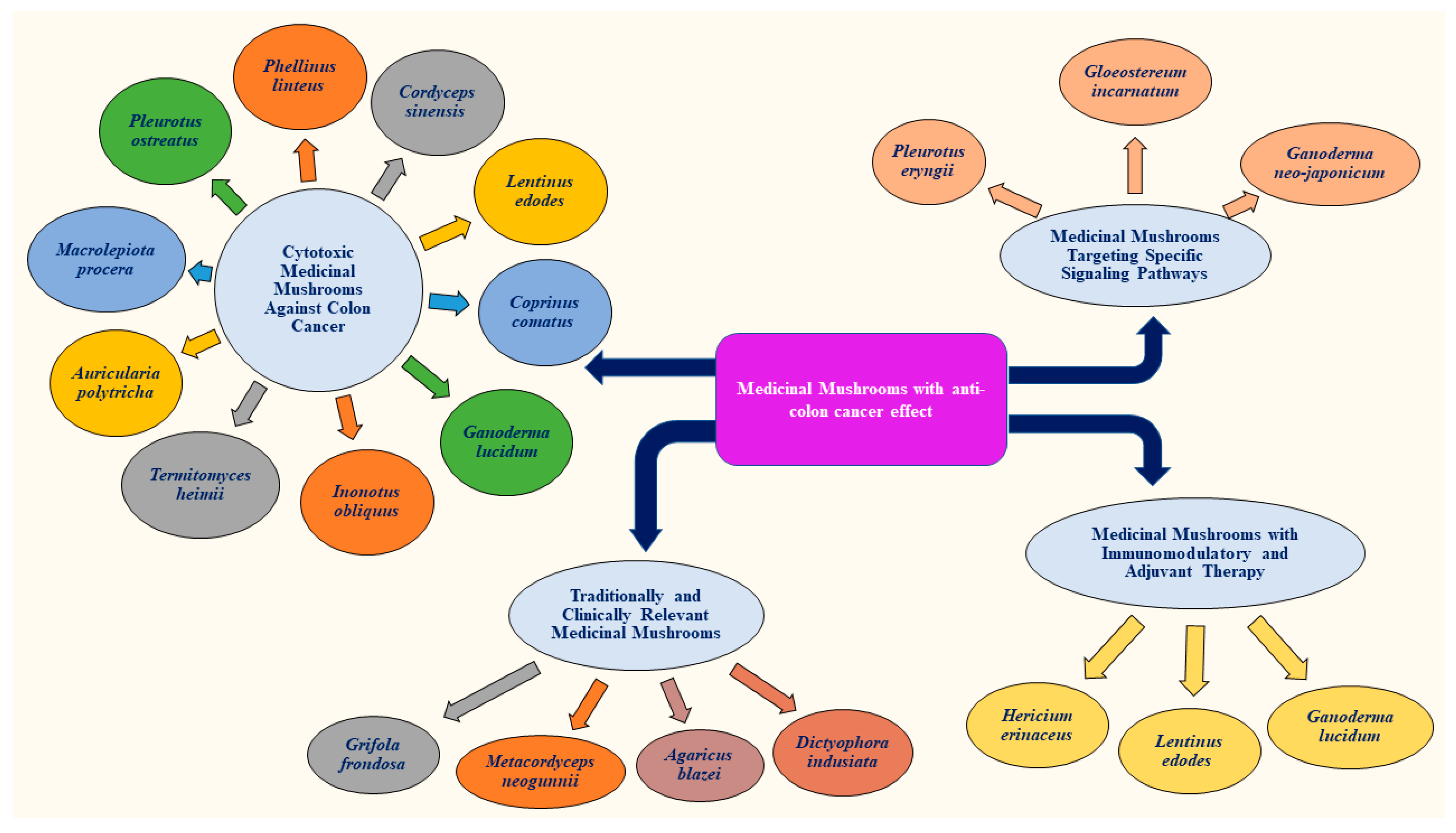
2.2. Types of Edible and Medicinal Mushrooms with Anti-Colon Cancer Properties
2.2.1. Potent Cytotoxic Medicinal Mushrooms Against Colon Cancer
2.2.2. Medicinal Mushrooms with Immunomodulatory Effects and Adjuvant Therapy
2.2.3. Medicinal Mushrooms Targeting Specific Signaling Pathways
2.2.4. Traditionally and Clinically Relevant Medicinal Mushrooms
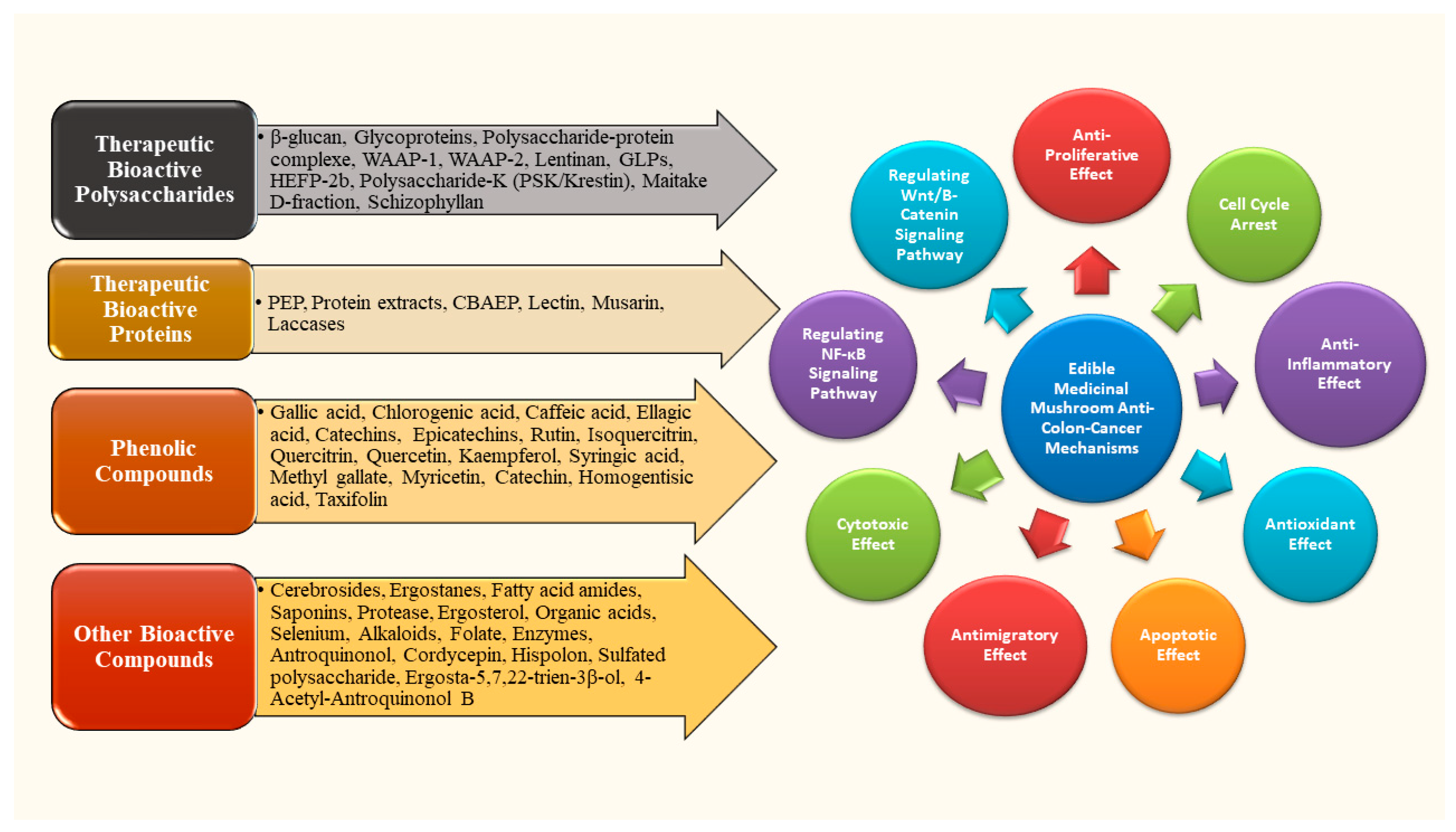
2.3. Chemical Compositions of Mushrooms with Anti-Colon Cancer Properties
2.4. Bioactive Compounds in Edible and Medicinal Mushrooms with Anti-Colon Cancer Effects
2.4.1. Polysaccharides as Bioactive Compounds in Edible and Medicinal Mushrooms
Anti-Colon Cancer Mechanisms of Medicinal Mushroom Polysaccharides
Structural Diversity and Bioactivity of Polysaccharides in Medicinal Mushrooms
Future Directions, Limitations, and Suggestions Concerning the Use of Polysaccharides in Medicinal Mushrooms
2.4.2. Proteins as Bioactive Compounds in Edible and Medicinal Mushrooms
2.4.3. Phenolic Compounds as Bioactive Compounds in Edible and Medicinal Mushrooms
Unique Attributes of Phenolic Compounds in Medicinal Mushrooms
Anti-Colon Cancer Potential of Medicinal Mushroom-Derived Phenolics
2.4.4. Other Bioactive Compounds in Edible and Medicinal Mushrooms
3. Mechanisms of Action Against Colon Cancer
3.1. Anti-Colon Cancer Mechanism Through Anti-Proliferative Effects
3.1.1. Introduction to Anti-Proliferative Activity of Medicinal Mushrooms
3.1.2. In Vitro and In Vivo Studies of the Anti-Proliferative Activity of Medicinal Mushrooms
3.1.3. Limitations and Future Directions of In Vitro and In Vivo Studies on the Anti-Proliferative Activity of Medicinal Mushrooms
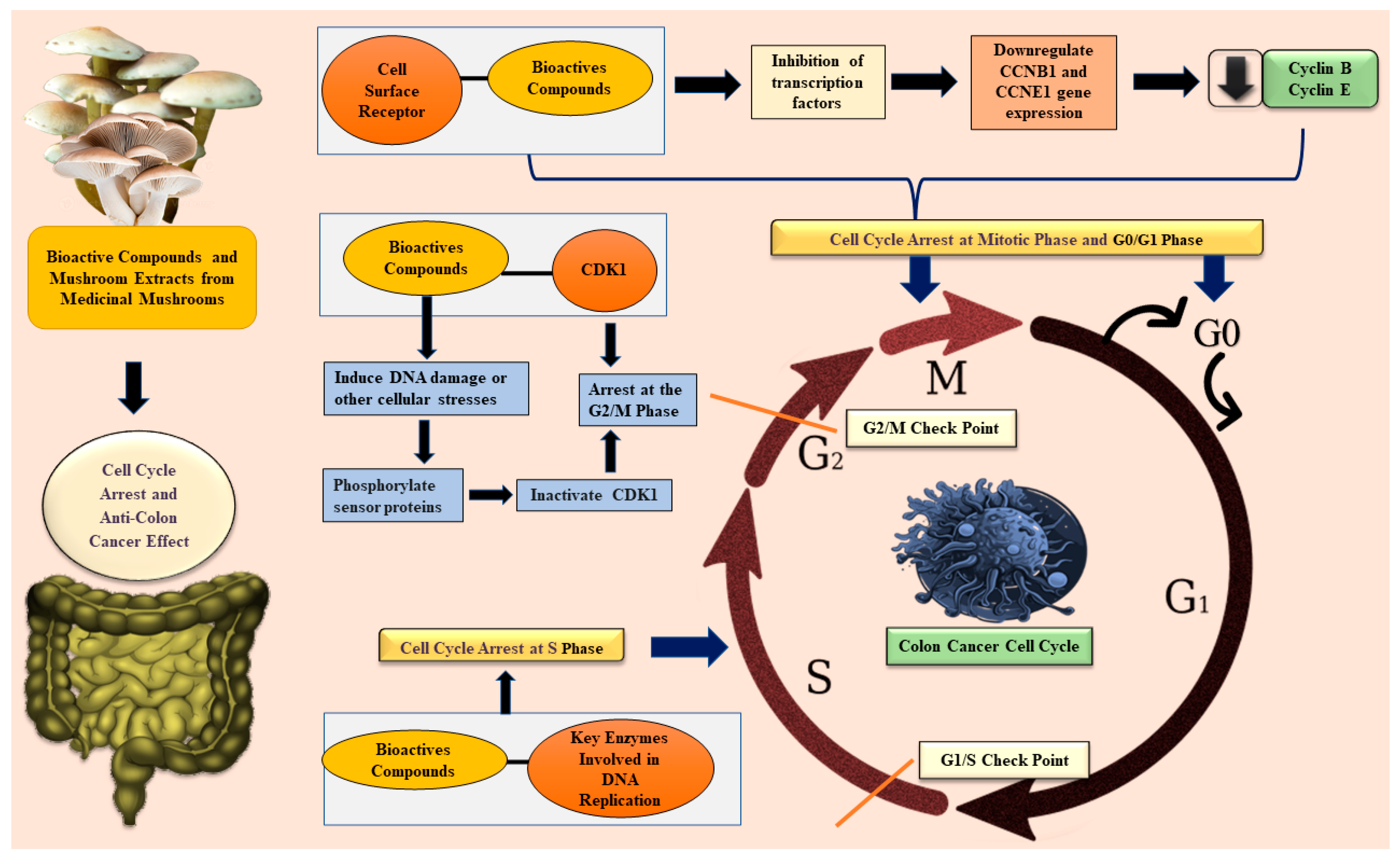
3.2. Anti-Colon Cancer Mechanism Through Cell Cycle Arrest
3.2.1. Introduction to Anti-Colon Cancer Mechanism Through Cell Cycle Arrest by Medicinal Mushrooms
3.2.2. In Vitro and In Vivo Studies on Cell Cycle Arrest by Medicinal Mushrooms
3.3. Anti-Colon Cancer Mechanism Through Anti-Inflammatory Effects
3.4. Anti-Colon Cancer Mechanism Through Antioxidant Effects
3.5. Anti-Colon Cancer Mechanism Through the NF-κB Signaling Pathway
3.6. Anti-Colon Cancer Mechanism Through the Wnt/β-Catenin Signaling Pathway
3.7. Anti-Colon Cancer Mechanism Through Apoptotic Effects
3.7.1. Initiation of Apoptotic Effects Through Intrinsic and Extrinsic Pathways
3.7.2. Apoptotic Effects of Inonotus obliquus, Pleurotus ostreatus, and Agaricus bisporus
3.7.3. Apoptotic Effects of Pleurotus eryngii and Ganoderma lucidum
3.7.4. Apoptotic Effects of Lentinus edodes as a Promising Medicinal Mushroom
3.7.5. Apoptotic Effects of Other Promising Medicinal Mushrooms in Anti-Colon Cancer Therapy
3.8. Anti-Colon Cancer Mechanism Through Antimigratory Effects
3.9. Anti-Colon Cancer Mechanism Through Cytotoxic Effecst
3.10. Anti-Colon Cancer Mechanism Through Gene Modulation
3.10.1. Anti-Colon Cancer Mechanism Through BIRC5 (Survivin) Modulation
3.10.2. Anti-Colon Cancer Mechanism Through Telomerase Reverse Transcriptase Modulation
3.10.3. Anti-Colon Cancer Mechanism Through Hypoxia-Inducible Factor 1 Alpha Modulation
3.10.4. Anti-Colon Cancer Mechanism Through Multidrug Resistance Protein 1 Modulation
4. Challenges and Limitations
5. Future Directions and Research Opportunities
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ABC | ATP-binding cassette |
| Andosan™ | Water extracts of the mycelia of Agaricus blazei (82.4%), Hericeum erinaceus (14.7%), and Grifola frondosa (2.9%) |
| BAX | Bcl-2-associated x, apoptosis regulator |
| Bcl-2 | B-cell lymphoma 2 |
| BIRC5 | Baculoviral IAP repeat-containing 5 |
| BSGLWE | Sporoderm-broken spores of G. lucidum water extract |
| Caco-2 | Human colorectal adenocarcinoma cell line |
| CBAEP | Cibacron blue affinity eluted protein |
| CCNB1 | Cyclin B1 |
| CCNE1 | Cyclin E1 |
| CDK1 | Cyclin-dependent kinase 1 |
| COLO-205 | Human colorectal adenocarcinoma cell line 205 |
| COX-2 | Cyclooxygenase-2 |
| CRC | Colorectal cancer |
| CRISPR | Clustered regularly interspaced short palindromic repeats |
| DNA | Deoxyribonucleic acid |
| ERK | Extracellular signal-regulated kinase |
| FIP | Fungal immunomodulatory protein |
| GIPS | Polysaccharides purified from Gloeostereum incarnatum |
| GLE | Ganoderma Lucidum extract |
| GLP | Ganoderma lucidum polysaccharides |
| GLSF | Ganoderma lucidum extract containg spores and fruiting bodies (30:8 ratio) |
| HCT-116 | Human colorectal carcinoma cell line 116 |
| HIF-1α or HIF1A | Hypoxia-inducible factor-1 alpha |
| HT-29 | Human colorectal adenocarcinoma cell line 29 |
| hTERT | Human telomerase reverse transcriptase |
| IkappaB-α | NF-kappa-B inhibitor alpha |
| IL | Interleukin |
| LAP | Lentinus edodes alcohol precipitate |
| LEM | Lentinus edodes mycelium extracts |
| MDR1 | Multidrug resistance protein 1 |
| MEK | Mitogen-activated protein kinase |
| MM | Medicinal mushrooms |
| MMP-2 | Matrix metallopeptidase-2 |
| MRP1 | Multidrug resistance-associated protein 1 |
| NAG-1 | Nonsteroidal anti-inflammatory drug-activated gene-1 |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| NK cells | Natural killer cells |
| Nrf2 | Nuclear factor erythroid 2 |
| p21/Cip1 or CDKN1A | Cyclin-dependent kinase inhibitor 1 |
| p27/Kip1 or CDKN1B | Cyclin-dependent kinase inhibitor 1B |
| p53 | Tumor protein p53 |
| PARP | Poly (ADP-ribose) polymerase |
| PSC-hex | n-hexane fraction of Pleurotus sajor-caju |
| RAF-1 | RAF proto-oncogene serine/threonine-protein kinase |
| ROS | Reactive oxygen species |
| SLNT | Water-extracted polysaccharide from Lentinus edodes |
| SW-480 | Surg pathol-derived colorectal cancer cell line 480 |
| SW620/Ad300 | Surg pathol-derived colorectal cancer cell line 620 |
| TGF-β | Transforming growth factor beta |
| THP-1 | Tohoku Hospital Pediatrics-1 human monocytic leukemia cell line |
| TNF-α | Tumor necrosis factor alpha |
| WAAP | A homogeneous neutral polysaccharide |
References
- Chaturvedi, V.K.; Agarwal, S.; Gupta, K.K.; Ramteke, P.W.; Singh, M.P. Medicinal mushroom: Boon for therapeutic applications. 3 Biotech. 2018, 8, 334. [Google Scholar] [CrossRef] [PubMed]
- Valverde, M.E.; Hernández-Pérez, T.; Paredes-López, O. Edible mushrooms: Improving human health and promoting quality life. Int. J. Microbiol. 2015, 2015, 376387. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.T.; Wasser, S.P. The role of culinary-medicinal mushrooms on human welfare with a pyramid model for human health. Int. J. Med. Mushrooms. 2012, 14, 95–134. [Google Scholar] [CrossRef] [PubMed]
- Finimundy, T.C.; Abreu, R.M.V.; Bonetto, N.; Scariot, F.J.; Dillon, A.J.P.; Echeverrigaray, S.; Barros, L.; Ferreira, I.C.F.R.; Henriques, J.A.P.; Roesch-Ely, M. Apoptosis induction by Pleurotus sajor-caju (Fr.) Singer extracts on colorectal cancer cell lines. Food Chem. Toxicol. 2018, 112, 383–392. [Google Scholar] [CrossRef]
- Hu, D.D.; Zhang, R.Y.; Zhang, G.Q.; Wang, H.X.; Ng, T.B. A laccase with antiproliferative activity against tumor cells from an edible mushroom, white common Agrocybe cylindracea. Phytomedicine 2011, 18, 374–379. [Google Scholar] [CrossRef]
- Lee, S.H.; Hwang, H.S.; Yun, J.W. Antitumor activity of water extract of a mushroom, Inonotus obliquus, against HT-29 human colon cancer cells. Phytother. Res. 2009, 23, 1784–1789. [Google Scholar] [CrossRef]
- Torre, L.A.; Siegel, R.L.; Ward, E.M.; Jemal, A. Global cancer incidence and mortality rates and trends--an update. Cancer Epidemiol. Biomark. Prev. 2016, 25, 16–27. [Google Scholar] [CrossRef]
- Rawla, P.; Sunkara, T.; Barsouk, A. Epidemiology of colorectal cancer: Incidence, mortality, survival, and risk factors. Przeglad Gastroenterologiczny. 2019, 14, 89–103. [Google Scholar] [CrossRef]
- Singha, K.; Hor, P.K.; Soren, J.P.; Mondal, J.; Mondal, K.C.; Pati, B.R.; Mohapatra, P.K.D. Exploration of bioactive prospects of a polysaccharide fraction from Termitomyces heimii against colorectal cancer and broad-spectrum bacteria. Bioact. Carbohydr. Diet. Fibre 2021, 25, 100255. [Google Scholar] [CrossRef]
- Abdel-Azeem, A.M.; Abdel-Azeem, M.A.; Khalil, W.F. Endophytic fungi as a new source of antirheumatoid metabolites. In Bioactive Food as Dietary Interventions for Arthritis and Related Inflammatory Diseases, 2nd ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 355–384. [Google Scholar] [CrossRef]
- Hamza, A.; Mylarapu, A.; Krishna, K.V.; Kumar, D.S. An insight into the nutritional and medicinal value of edible mushrooms: A natural treasury for human health. J. Biotechnol. 2024, 381, 86–99. [Google Scholar] [CrossRef]
- Frljak, J.; Mulabećirović, A.H.; Isaković, S.; Karahmet, E.; Toroman, A.; Frljak, J.; Mulabećirović, A.H.; Isaković, S.; Karahmet, E.; Toroman, A. Biological active components of selected medical fungi. Open J. Prev. Med. 2021, 11, 9–22. [Google Scholar] [CrossRef]
- Kumar, K.; Mehra, R.; Guiné, R.P.F.; Lima, M.J.; Kumar, N.; Kaushik, R.; Ahmed, N.; Yadav, A.N.; Kumar, H. Edible mushrooms: A comprehensive review on bioactive compounds with health benefits and processing aspects. Foods 2021, 10, 2996. [Google Scholar] [CrossRef] [PubMed]
- Khatun, S.; Islam, A.; Cakilcioglu, U.; Chatterjee, N.C. Research on mushroom as a potential source of nutraceuticals: A review on Indian perspective. J. Exp. Agric Int. 2012, 2, 47–73. [Google Scholar] [CrossRef]
- He, Z.; Lin, J.; He, Y.; Liu, S. Polysaccharide-peptide from Trametes versicolor: The potential medicine for colorectal cancer treatment. Biomedicines 2022, 10, 2841. [Google Scholar] [CrossRef]
- Sadowska, A.; Zapora, E.; Sawicka, D.; Niemirowicz-Laskowska, K.; Suraży ński, A.; Sułkowska-Ziaja, K.; Kała, K.; Stocki, M.; Wołkowycki, M.; Bakier, S.; et al. Heterobasidion annosum induces apoptosis in DLD-1 cells and decreases colon cancer growth in in vivo model. Int. J. Mol. Sci. 2020, 21, 3447. [Google Scholar] [CrossRef]
- Zhang, N.; Liu, Y.; Tang, F.Y.; Yang, L.Y.; Wang, J.H. Structural characterization and in vitro anti-colon cancer activity of a homogeneous polysaccharide from Agaricus bisporus. Int. J. Biol. Macromol. 2023, 251, 126410. [Google Scholar] [CrossRef]
- Šeklić, D.S.; Stanković, M.S.; Milutinović, M.G.; Topuzović, M.D.; Štajn, A.Š.; Marković, S.D. Cytotoxic, antimigratory, pro-and antioxidative activities of extracts from medicinal mushrooms on colon cancer cell lines. Arch. Biol. Sci. 2016, 68, 93–105. [Google Scholar] [CrossRef]
- Arora, S.; Goyal, S.; Balani, J.; Tandon, S. Enhanced antiproliferative effects of aqueous extracts of some medicinal mushrooms on colon cancer cells. Int. J. Med. Mushrooms 2013, 15, 301–314. [Google Scholar] [CrossRef]
- Fekry, T.; Salem, M.F.; Abd-Elaziz, A.A.; Muawia, S.; Naguib, Y.M.; Khalil, H. Anticancer properties of selenium-enriched oyster culinary-medicinal mushroom, Pleurotus ostreatus (Agaricomycetes), in colon cancer in vitro. Int. J. Med. Mushrooms 2022, 24, 1–20. [Google Scholar] [CrossRef]
- Wu, C.H.; Wu, C.C.; Ho, Y.S. Antitumor activity of combination treatment of Lentinus edodes mycelium extracts with 5-Fluorouracil against human colon cancer cells xenografted in nude mice. J. Cancer Mol. 2007, 3, 15–22. [Google Scholar] [CrossRef]
- Liu, N.; Zou, S.; Xie, C.; Meng, Y.; Xu, X. Effect of the β-glucan from Lentinus edodes on colitis-associated colorectal cancer and gut microbiota. Carbohydr. Polym. 2023, 316, 121069. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.M.; Liu, T.; Yeung, S.; Wang, Z.; Andresen, B.; Parsa, C.; Orlando, R.; Zhou, B.; Wu, W.; Li, X.; et al. Inhibitory activity of medicinal mushroom Ganoderma lucidum on colorectal cancer by attenuating inflammation. Precis. Clin. Med. 2021, 4, 231–245. [Google Scholar] [CrossRef] [PubMed]
- Wachtel-Galor, S.; Yuen, J.; Buswell, J.A.; Benzie, I.F.F. Ganoderma lucidum (Lingzhi or Reishi). In Herbal Medicine: Biomolecular and Clinical Aspects, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2011; pp. 175–199. Available online: https://www.ncbi.nlm.nih.gov/books/NBK92757/ (accessed on 15th March 2025).
- Liu, J.Y.; Hou, X.X.; Li, Z.Y.; Shan, S.H.; Chang, M.C.; Feng, C.P.; Wei, Y. Isolation and structural characterization of a novel polysaccharide from Hericium erinaceus fruiting bodies and its arrest of the cell cycle at S-phage in colon cancer cells. Int. J. Biol. Macromol. 2020, 157, 288–295. [Google Scholar] [CrossRef]
- He, J.; Yang, A.; Zhao, X.; Liu, Y.; Liu, S.; Wang, D. Anti-colon cancer activity of water-soluble polysaccharides extracted from Gloeostereum incarnatum via Wnt/β-catenin signaling pathway. Food Sci. Hum. Well. 2021, 10, 460–470. [Google Scholar] [CrossRef]
- Lau, M.F.; Chua, K.H.; Sabaratnam, V.; Kuppusamy, U.R. In vitro and in silico anticancer evaluation of a medicinal mushroom, Ganoderma neo-japonicum Imazeki, against human colonic carcinoma cells. Biotech. Appl. Biochem. 2021, 68, 902–917. [Google Scholar] [CrossRef]
- Hu, Q.; Yuan, B.; Xiao, H.; Zhao, L.; Wu, X.; Rakariyatham, K.; Zhong, L.; Han, Y.; Muinde Kimatu, B.; Yang, W. Polyphenols-rich extract from Pleurotus eryngii with growth inhibitory of HCT116 colon cancer cells and anti-inflammatory function in RAW264.7 cells. Food Funct. 2018, 9, 1601–1611. [Google Scholar] [CrossRef]
- Peng, H.; Shahidi, F. Bioactive compounds and bioactive properties of Chaga (Inonotus obliquus) mushroom: A review. J. Food Bioact. 2020, 12, 9–75. [Google Scholar] [CrossRef]
- Daba, G.M.; Elkhateeb, W.A.; El-Dein, A.N.; Ahmed, E.F.; El Hagrassi, A.M.; Fayad, W.; Wen, T.C. Therapeutic potentials of n-hexane extracts of the three medicinal mushrooms regarding their anti-colon cancer, antioxidant, and hypocholesterolemic capabilities. Biodiversitas 2020, 21, 2437–2445. [Google Scholar] [CrossRef]
- Na, K.; Li, K.; Sang, T.; Wu, K.; Wang, Y.; Wang, X. Anticarcinogenic effects of water extract of sporoderm-broken spores of Ganoderma lucidum on colorectal cancer in vitro and in vivo. Int. J. Oncol. 2017, 50, 1541–1554. [Google Scholar] [CrossRef]
- Wang, J.; Li, W.; Huang, X.; Liu, Y.; Li, Q.; Zheng, Z.; Wang, K. A polysaccharide from Lentinus edodes inhibits human colon cancer cell proliferation and suppresses tumor growth in athymic nude mice. Oncotarget 2017, 8, 610–623. [Google Scholar] [CrossRef]
- Bisen, P.S.; Baghel, R.K.; Sanodiya, B.S.; Thakur, G.S.; Prasad, G.B.K.S. Lentinus edodes: A macrofungus with pharmacological activities. Curr. Med. Chem. 2010, 17, 2419–2430. [Google Scholar] [CrossRef] [PubMed]
- Firenzuoli, F.; Gori, L.; Lombardo, G. The medicinal mushroom Agaricus blazei Murrill: Review of literature and pharmaco-toxicological problems. Evid.-Based Complement. Altern. Med. 2007, 5, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Hetland, G.; Eide, D.M.; Tangen, J.M.; Haugen, M.H.; Mirlashari, M.R.; Paulsen, J.E. The Agaricus blaze-based mushroom extract, AndosanTM, protects against intestinal tumorigenesis in the A/J Min/+ mouse. PLoS ONE 2016, 11, e0167754. [Google Scholar] [CrossRef] [PubMed]
- Wasser, S.P. Current findings, future trends, and unsolved problems in studies of medicinal mushrooms. Appl. Microbiol. Biotechnol. 2011, 89, 1323–1332. [Google Scholar] [CrossRef]
- Macharia, J.M.; Zhang, L.; Mwangi, R.W.; Rozmann, N.; Kaposztas, Z.; Varjas, T.; Sugár, M.; Alfatafta, H.; Pintér, M.; Bence, R.L. Are chemical compounds in medical mushrooms potent against colorectal cancer carcinogenesis and antimicrobial growth? Cancer Cell Int. 2022, 22, 379. [Google Scholar] [CrossRef]
- Liu, J.; Jia, L.; Kan, J.; Jin, C.H. In vitro and in vivo antioxidant activity of ethanolic extract of white button mushroom (Agaricus bisporus). Food Chem. Toxicol. 2013, 51, 310–316. [Google Scholar] [CrossRef]
- Mwangi, R.W.; Macharia, J.M.; Wagara, I.N.; Bence, R.L. The antioxidant potential of different edible and medicinal mushrooms. Biomed. Pharmacother. 2022, 147, 112621. [Google Scholar] [CrossRef]
- El-Deeb, N.M.; Ibrahim, O.M.; Mohamed, M.A.; Farag, M.M.S.; Farrag, A.A.; El-Aassar, M.R. Alginate/κ-carrageenan oral microcapsules loaded with Agaricus bisporus polysaccharides MH751906 for natural killer cells mediated colon cancer immunotherapy. Int. J. Biol. Macromol. 2022, 205, 385–395. [Google Scholar] [CrossRef]
- Xu, Y.; Xu, T.; Huang, C.; Liu, L.; Kwame, A.W.; Zhu, Y.; Ren, J. Preventive intervention with Agaricus blazei murill polysaccharide exerts anti-tumor immune effect on intraperitoneal metastasis colorectal cancer. Int. J. Biol. Macromol. 2024, 282 Pt 3, 136810. [Google Scholar] [CrossRef]
- Dong, K.; Wang, J.; Tang, F.; Liu, Y.; Gao, L. A polysaccharide with a triple helix structure from Agaricus bisporus: Characterization and anti-colon cancer activity. Int. J. Biol. Macromol. 2024, 281 Pt 4, 136521. [Google Scholar] [CrossRef]
- He, Y.Y.; Liu, S.; Newburg, D.S. Musarin, a novel protein with tyrosine kinase inhibitory activity from Trametes versicolor, inhibits colorectal cancer stem cell growth. Biomed. Pharmacother. 2021, 144, 112339. [Google Scholar] [CrossRef] [PubMed]
- Lavi, I.; Friesem, D.; Geresh, S.; Hadar, Y.; Schwartz, B. An aqueous polysaccharide extract from the edible mushroom Pleurotus ostreatus induces anti-proliferative and pro-apoptotic effects on HT-29 colon cancer cells. Cancer Lett. 2006, 244, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Chaitanya, N.S.; Devi, A.; Sahu, S.; Alugoju, P. Molecular mechanisms of action of Trehalose in cancer: A comprehensive review. Life Sci. 2021, 269, 118968. [Google Scholar] [CrossRef]
- Quero, J.; Paesa, M.; Morales, C.; Mendoza, G.; Osada, J.; Teixeira, J.A.; Ferreira-Santos, P.; Rodríguez-Yoldi, M.J. Biological properties of Boletus edulis extract on Caco-2 cells: Antioxidant, anticancer, and anti-inflammatory effects. Antioxidants 2024, 13, 908. [Google Scholar] [CrossRef]
- Park, G.S.; Shin, J.; Hong, S.; Gopal, J.; Oh, J.W. Anticarcinogenic potential of the mushroom polysaccharide lentinan on gastric and colon cancer cells: Antiproliferative, antitumorigenic, Mu-2-related death-inducing gene, MUDENG ramifications. J. Ind. Eng. Chem. 2024, 135, 122–130. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, Y.; Zhang, L.; Tian, Q. Mushroom polysaccharide lentinan for treating different types of cancers: A review of 12 years clinical studies in China. Prog. Mol. Biol. Translat. Sci. 2019, 163, 297–328. [Google Scholar] [CrossRef]
- Fritz, H.; Kennedy, D.A.; Ishii, M.; Fergusson, D.; Fernandes, R.; Cooley, K.; Seely, D. Polysaccharide K and Coriolus versicolor extracts for lung cancer: A systematic review. Integr. Cancer Ther. 2015, 14, 201–211. [Google Scholar] [CrossRef]
- Lu, H.; Yang, Y.; Gad, E.; Wenner, C.A.; Chang, A.; Larson, E.R.; Dang, Y.; Martzen, M.; Standish, L.J.; Disis, M.L. Polysaccharide Krestin is a novel TLR2 agonist that mediates inhibition of tumor growth via stimulation of CD8 T cells and NK cells. Clin. Cancer Res. 2010, 17, 67. [Google Scholar] [CrossRef]
- Rosendahl, A.H.; Sun, C.; Wu, D.Q.; Andersson, R. Polysaccharide-K (PSK) increases p21(WAF/Cip1) and promotes apoptosis in pancreatic cancer cells. Pancreatology 2012, 12, 467–474. [Google Scholar] [CrossRef]
- Camilleri, E.; Blundell, R.; Baral, B.; Karpiński, T.M.; Aruci, E.; Atrooz, O.M. Unveiling the full spectrum of maitake mushrooms: A comprehensive review of their medicinal, therapeutic, nutraceutical, and cosmetic potential. Heliyon 2024, 10, e30254. [Google Scholar] [CrossRef]
- Fontes Vieira, P.A.; Gontijo, D.C.; Vieira, B.C.; Fontes, E.A.F.; de Assunção, L.S.; Leite, J.P.V.; Oliveira, M.G.D.A.; Kasuya, M.C.M. Antioxidant activities, total phenolics and metal contents in Pleurotus ostreatus mushrooms enriched with iron, zinc or lithium. LWT 2013, 54, 421–425. [Google Scholar] [CrossRef]
- Kodama, N.; Komuta, K.; Sakai, N.; Nanba, H. Effects of D-Fraction, a polysaccharide from Grifola frondosa on tumor growth involve activation of NK cells. Biol. Pharm. Bull. 2002, 25, 1647–1650. [Google Scholar] [CrossRef]
- Zhao, F.; Guo, Z.; Ma, Z.R.; Ma, L.L.; Zhao, J. Antitumor activities of Grifola frondosa (Maitake) polysaccharide: A meta-analysis based on preclinical evidence and quality assessment. J. Ethnopharmacol. 2021, 280, 114395. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.D.; Gordon, S. Immune recognition. A new receptor for beta-glucans. Nature 2001, 413, 36–37. [Google Scholar] [CrossRef] [PubMed]
- Lemieszek, M.; Rzeski, W. Anticancer properties of polysaccharides isolated from fungi of the Basidiomycetes class. Contemp. Oncol. 2012, 16, 285. [Google Scholar] [CrossRef]
- Yuan, B.; Ma, N.; Zhao, L.; Zhao, E.; Gao, Z.; Wang, W.; Song, M.; Zhang, G.; Hu, Q.; Xiao, H. In vitro and in vivo inhibitory effects of a Pleurotus eryngii protein on colon cancer cells. Food Funct. 2017, 8, 3553–3562. [Google Scholar] [CrossRef]
- Yuan, B.; Zhao, L.; Rakariyatham, K.; Han, Y.; Gao, Z.; Muinde Kimatu, B.; Hu, Q.; Xiao, H. Isolation of a novel bioactive protein from an edible mushroom Pleurotus eryngii and its anti-inflammatory potential. Food Funct. 2017, 8, 2175–2183. [Google Scholar] [CrossRef]
- Chen, C.H.; Wu, J.Y.; Chen, C.H.; Chang, W.H.; Chung, K.T.; Liu, Y.W.; Lu, F.J. Anti-cancer effects of protein extracts from Calvatia lilacina, Pleurotus ostreatus and Volvariella volvacea. Evid. Based Complement. Altern. Med. 2011, 2011, 982368. [Google Scholar] [CrossRef]
- Rezvani, V.; Pourianfar, H.R.; Mohammadnejad, S.; Madjid Ansari, A.; Farahmand, L. Anticancer potentiality and mode of action of low-carbohydrate proteins and peptides from mushrooms. Appl. Microbiol. Biotechnol. 2020, 104, 6855–6871. [Google Scholar] [CrossRef]
- Maiti, S.; Bhutia, S.K.; Mallick, S.K.; Kumar, A.; Khadgi, N.; Maiti, T.K. Antiproliferative and immunostimulatory protein fraction from edible mushrooms. Environ. Toxicol. Pharm. 2008, 26, 187–191. [Google Scholar] [CrossRef]
- Evans, R.; Rhodes, J.; Kinsella, A. Mushroom lectin inhibits invasion of a human colon cancer cell line into collagen gels. Clin. Sci. 1995, 88, 12P. [Google Scholar] [CrossRef]
- Evans, R.C.; Fear, S.; Ashby, D.; Williams, E.; Van der Vliet, M.; Rhodes, J.M.; Dunstan, F.D.J. Diet and colorectal cancer: An investigation of the lectin/galactose hypothesis. Gastroenterology 2002, 122, 1784–1792. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.G.; Fernig, D.G.; White, M.R.H.; Spiller, D.G.; Appleton, P.; Evans, R.C.; Grierson, I.; Smith, J.A.; Davies, H.; Gerasimenko, O.V.; et al. Edible mushroom (Agaricus bisporus) lectin, which reversibly inhibits epithelial cell proliferation, blocks nuclear localization sequence-dependent nuclear protein import. J. Biol. Chem. 1999, 274, 4890–4899. [Google Scholar] [CrossRef]
- Zhou, R.; Liu, Z.K.; Zhang, Y.N.; Wong, J.H.; Ng, T.B.; Liu, F. Research progress of bioactive proteins from the edible and medicinal mushrooms. Curr. Prot. Peptide Sci. 2018, 20, 196–219. [Google Scholar] [CrossRef]
- Singh, S.S.; Wang, H.; Chan, Y.S.; Pan, W.; Dan, X.; Yin, C.M.; Akkouh, O.; Ng, T.B. Lectins from edible mushrooms. Molecules 2014, 20, 446. [Google Scholar] [CrossRef]
- Camilleri, E.; Blundell, R.; Baral, B.; Karpiński, T.M.; Aruci, E.; Atrooz, O.M. A comprehensive review of the health benefits, phytochemicals, and enzymatic constituents for potential therapeutic and industrial applications of turkey tail mushrooms. Discov. Appl. Sci. 2024, 6, 257. [Google Scholar] [CrossRef]
- Sun, J.; Chen, Q.J.; Zhu, M.J.; Wang, H.X.; Zhang, G.Q. An extracellular laccase with antiproliferative activity from the Sanghuang mushroom Inonotus baumii. J. Mol. Catal. B Enzym. 2014, 99, 20–25. [Google Scholar] [CrossRef]
- Yuzugullu Karakus, Y.; Isik, S.; Kale Bakir, E.; Turkmenoglu, A.; Deveci Ozkan, A. Characterization of the three-phase partitioned laccase from Trametes versicolor strain with antiproliferative activity against breast cancer cells. Int. J. Biol. Macromol. 2025, 286, 138504. [Google Scholar] [CrossRef]
- Kim, M.Y.; Seguin, P.; Ahn, J.K.; Kim, J.J.; Chun, S.C.; Kim, E.H.; Seo, S.H.; Kang, E.Y.; Kim, S.L.; Park, Y.J.; et al. Phenolic compound concentration and antioxidant activities of edible and medicinal mushrooms from Korea. J. Agric. Food Chem. 2008, 56, 7265–7270. [Google Scholar] [CrossRef]
- Paloi, S.; Kumla, J.; Paloi, B.P.; Srinuanpan, S.; Hoijang, S.; Karunarathna, S.C.; Acharya, K.; Suwannarach, N.; Lumyong, S. Termite mushrooms (Termitomyces), a potential source of nutrients and bioactive compounds exhibiting human health benefits: A review. J. Fungi 2023, 9, 112. [Google Scholar] [CrossRef]
- Silva, M.; Lageiro, M.; Ramos, A.C.; Reboredo, F.H.; Gonçalves, E.M. Cultivated mushrooms: A comparative study of antioxidant activity and phenolic content. Biol. Life Sci. Forum 2024, 40, 13. [Google Scholar] [CrossRef]
- Chu, M.; Khan, R.D.; Zhou, Y.; Agar, O.T.; Barrow, C.J.; Dunshea, F.R.; Suleria, H.A.R. LC-ESI-QTOF-MS/MS characterization of phenolic compounds in common commercial mushrooms and their potential antioxidant activities. Processes 2023, 11, 1711. [Google Scholar] [CrossRef]
- Durgo, K.; Koncar, M.; Komes, D.; Belscak-Cvitanovic, A.; Franekic, J.; Jakopovich, I.; Jakopovich, N.; Jakopovich, B. Cytotoxicity of blended versus single medicinal mushroom extracts on human cancer cell lines: Contribution of polyphenol and polysaccharide content. Int. J. Med. Mushrooms 2013, 15, 435–448. [Google Scholar] [CrossRef]
- Palacios, I.; Lozano, M.; Moro, C.; D’Arrigo, M.; Rostagno, M.A.; Martínez, J.A.; García-Lafuente, A.; Guillamón, E.; Villares, A. Antioxidant properties of phenolic compounds occurring in edible mushrooms. Food Chem. 2011, 128, 674–678. [Google Scholar] [CrossRef]
- Lin, S.; Ching, L.T.; Chen, J.; Cheung, P.C.K. Antioxidant and anti-angiogenic effects of mushroom phenolics-rich fractions. J. Funct. Foods 2015, 17, 802–815. [Google Scholar] [CrossRef]
- Park, H.J. Current uses of mushrooms in cancer treatment and their anticancer mechanisms. Int. J. Mol. Sci. 2022, 23, 10502. [Google Scholar] [CrossRef]
- Chang, T.C.; Yeh, C.T.; Adebayo, B.O.; Lin, Y.C.; Deng, L.; Rao, Y.K.; Huang, C.C.; Lee, W.H.; Wu, A.T.H.; Hsiao, M.; et al. 4-Acetylantroquinonol B inhibits colorectal cancer tumorigenesis and suppresses cancer stem-like phenotype. Toxicol. Appl. Pharmacol. 2015, 288, 258–268. [Google Scholar] [CrossRef]
- Chu, Y.C.; Tsai, T.-Y.; Yadav, V.K.; Deng, L.; Huang, C.-C.; Tzeng, Y.-M.; Yeh, C.-T.; Chen, M.-Y. 4-Acetyl-Antroquinonol B improves the sensitization of cetuximab on both Kras mutant and wild type colorectal cancer by modulating the expression of Ras/Raf/miR-193a-3p signaling axis. Int. J. Mol. Sci. 2021, 22, 7508. [Google Scholar] [CrossRef]
- Ayeka, P.A. Potential of mushroom compounds as immunomodulators in cancer immunotherapy: A review. Evid. Based Complement. Altern. Med. 2018, 2018, 7271509. [Google Scholar] [CrossRef]
- Dan, A.; Swain, R.; Belonce, S.; Jacobs, R.J. Therapeutic effects of medicinal mushrooms on gastric, breast, and colorectal cancer: A scoping review. Cureus 2023, 15, e37574. [Google Scholar] [CrossRef]
- De Silva, D.D.; Rapior, S.; Fons, F.; Bahkali, A.H.; Hyde, K.D. Medicinal mushrooms in supportive cancer therapies: An approach to anti-cancer effects and putative mechanisms of action. Fungal Divers. 2012, 55, 1–35. [Google Scholar] [CrossRef]
- Figueiredo, L.; Régis, W.C.B. Medicinal mushrooms in adjuvant cancer therapies: An approach to anticancer effects and presumed mechanisms of action. Nutrire 2017, 42, 28. [Google Scholar] [CrossRef]
- Subramanian, R.R.; Yamakawa, A. Combination therapy targeting Raf-1 and MEK causes apoptosis of HCT116 colon cancer cells. Int. J. Oncol. 2012, 41, 1855–1862. [Google Scholar] [CrossRef]
- Lavi, I.; Nimri, L.; Levinson, D.; Peri, I.; Hadar, Y.; Schwartz, B. Glucans from the edible mushroom Pleurotus pulmonarius inhibit colitis-associated colon carcinogenesis in mice. J. Gastroenterol. 2012, 47, 504–518. [Google Scholar] [CrossRef]
- Zaila, C.S.; Zuraina, M.F.; Norfazlina, M.N.; Mun, L.L.; Nurshahirah, N.; Florinsiah, L.; Rajab, N.F. Antiproliferative effect of Lignosus rhinocerotis, the Tiger Milk mushroom on HCT 116 human colorectal cancer cells. Open Conf. Proc. J. 2013, 4, 65–70. [Google Scholar] [CrossRef]
- Aakif, M.; Balfe, P.; Elfaedy, O.; Awan, F.N.; Pretorius, F.; Silvio, L.; Castinera, C.; Mustafa, H. Study on colorectal cancer presentation, treatment and follow-up. Int. J. Color. Dis. 2016, 31, 1361–1363. [Google Scholar] [CrossRef]
- Li, J.; Huang, L.; Zhao, H.; Yan, Y.; Lu, J. The role of interleukins in colorectal cancer. Int. J. Biol. Sci. 2020, 16, 2323. [Google Scholar] [CrossRef]
- Bergman, M.; Levin, G.S.; Bessler, H.; Djaldetti, M.; Salman, H. Resveratrol affects the cross talk between immune and colon cancer cells. Biomed. Pharmacother. 2013, 67, 43–47. [Google Scholar] [CrossRef]
- Pastille, E.; Wasmer, M.H.; Adamczyk, A.; Vu, V.P.; Mager, L.F.; Phuong, N.N.T.; Palmieri, V.; Simillion, C.; Hansen, W.; Kasper, S.; et al. The IL-33/ST2 pathway shapes the regulatory T cell phenotype to promote intestinal cancer. Mucosal Immunol. 2019, 12, 990. [Google Scholar] [CrossRef]
- Al Obeed, O.A.; Alkhayal, K.A.; Al Sheikh, A.; Zubaidi, A.M.; Vaali-Mohammed, M.A.; Boushey, R.; Mckerrow, J.H.; Abdulla, M.H. Increased expression of tumor necrosis factor-α is associated with advanced colorectal cancer stages. World J. Gastroenterol. WJG 2014, 20, 18390. [Google Scholar] [CrossRef]
- Zhao, P.; Zhang, Z. TNF-α promotes colon cancer cell migration and invasion by upregulating TROP-2. Oncol. Lett. 2018, 15, 3820–3827. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, Y.; Sun, X.; Muftuoglu, Y.; Wang, B.; Yu, T.; Hu, Y.; Ma, L.; Xiang, M.; Guo, G.; et al. Powerful anti-colon cancer effect of modified nanoparticle-mediated IL-15 immunogene therapy through activation of the host immune system. Theranostics 2018, 8, 3490–3503. [Google Scholar] [CrossRef] [PubMed]
- Waldmann, T.A. The biology of interleukin-2 and interleukin-15: Implications for cancer therapy and vaccine design. Nat. Rev. Immunol. 2006, 6, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Malik, A.; Guy, C.S.; Karki, R.; Vogel, P.; Kanneganti, T.D. Pyrin inflammasome regulates tight junction integrity to restrict colitis and tumorigenesis. Gastroenterology 2017, 154, 948. [Google Scholar] [CrossRef]
- Muszyńska, B.; Grzywacz, A.; Kała, K.; Gdula-Argasińska, J. Anti-inflammatory potential of in vitro cultures of the white button mushroom, Agaricus bisporus (Agaricomycetes), in Caco-2 Cells. Int. J. Med. Mushrooms 2018, 20, 129–139. [Google Scholar] [CrossRef]
- Chaudhary, P.; Janmeda, P.; Docea, A.O.; Yeskaliyeva, B.; Abdull Razis, A.F.; Modu, B.; Calina, D.; Sharifi-Rad, J. Oxidative stress, free radicals and antioxidants: Potential crosstalk in the pathophysiology of human diseases. Front. Chem. 2023, 11, 1158198. [Google Scholar] [CrossRef]
- Muchtaridi, M.; Az-Zahra, F.; Wongso, H.; Setyawati, L.U.; Novitasari, D.; Ikram, E.H.K. Molecular mechanism of natural food antioxidants to regulate ROS in treating cancer: A review. Antioxidants 2024, 13, 207. [Google Scholar] [CrossRef]
- Pham-Huy, L.A.; He, H.; Pham-Huy, C. Free radicals, antioxidants in disease and health. Int. J. Biomed. Sci. 2008, 4, 89. [Google Scholar] [CrossRef]
- Baeuerle, P.A.; Henkel, T. Function and activation of NF-KappaB in the immune system. Annu. Rev. Immunol. 1994, 12, 141–179. [Google Scholar] [CrossRef]
- Barkett, M.; Gilmore, T.D. Control of apoptosis by Rel/NF-κB transcription factors. Oncogene 1999, 18, 6910–6924. [Google Scholar] [CrossRef]
- Dolcet, X.; Llobet, D.; Pallares, J.; Matias-Guiu, X. NF-kB in development and progression of human cancer. Virchows Arch. 2005, 446, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Wan, F.; Lenardo, M.J. Specification of DNA binding activity of NF- B proteins. Cold Spring Harb. Perspect. Biol. 2009, 1, a000067. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, M.; Deng, K. Blocking the Wnt/β-catenin signaling pathway to treat colorectal cancer: Strategies to improve current therapies (Review). Int. J. Oncol. 2022, 62, 24. [Google Scholar] [CrossRef]
- He, K.; Gan, W.J. Wnt/β-Catenin signaling pathway in the development and progression of colorectal cancer. Cancer Manag. Res. 2023, 15, 435. [Google Scholar] [CrossRef]
- Heslin, M.J.; Yan, J.; Johnson, M.R.; Weiss, H.; Diasio, R.B.; Urist, M.M. Role of matrix metalloproteinases in colorectal carcinogenesis. Ann. Surg. 2001, 233, 786. [Google Scholar] [CrossRef]
- Said, A.H.; Raufman, J.P.; Xie, G. The role of matrix metalloproteinases in colorectal cancer. Cancers 2014, 6, 366. [Google Scholar] [CrossRef]
- Pfeffer, C.M.; Singh, A.T.K. Apoptosis: A target for anticancer therapy. Int. J. Mol. Sci. 2018, 19, 448. [Google Scholar] [CrossRef]
- Xu, J.; Shen, R.; Jiao, Z.; Chen, W.; Peng, D.; Wang, L.; Yu, N.; Peng, C.; Cai, B.; Song, H.; et al. Current advancements in antitumor properties and mechanisms of medicinal components in edible mushrooms. Nutrients 2022, 14, 2622. [Google Scholar] [CrossRef]
- Ramesh, P.; Medema, J.P. BCL-2 family deregulation in colorectal cancer: Potential for BH3 mimetics in therapy. Apoptosis 2020, 25, 305. [Google Scholar] [CrossRef]
- Güllülü, Ö.; Hehlgans, S.; Rödel, C.; Fokas, E.; Rödel, F. Tumor suppressor protein p53 and inhibitor of apoptosis proteins in colorectal cancer—A promising signaling network for therapeutic interventions. Cancers 2021, 13, 624. [Google Scholar] [CrossRef]
- Zhou, M.; Liu, X.; Li, Z.; Huang, Q.; Li, F.; Li, C.Y. Caspase-3 regulates the migration, invasion, and metastasis of colon cancer cells. Int. J. Cancer 2018, 143, 921–930. [Google Scholar] [CrossRef] [PubMed]
- Baek, S.J.; Kim, J.S.; Jackson, F.R.; Eling, T.E.; McEntee, M.F.; Lee, S.H. Epicatechin gallate-induced expression of NAG-1 is associated with growth inhibition and apoptosis in colon cancer cells. Carcinogenesis 2004, 25, 2425–2432. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.Y.; Park, S.Y.; Kang, Y.; Thapa, D.; Choi, H.G.; Kim, J.A. Role of nonsteroidal anti-inflammatory drug-activated gene-1 in docetaxel-induced cell death of human colorectal cancer cells with different p53 status. Arch. Pharm. Res. 2011, 34, 323–330. [Google Scholar] [CrossRef]
- Krieg, A.; Werner, T.A.; Verde, P.E.; Stoecklein, N.H.; Knoefel, W.T. Prognostic and clinicopathological significance of survivin in colorectal cancer: A meta-analysis. PLoS ONE 2013, 8, e65338. [Google Scholar] [CrossRef]
- Li, G.; Wang, Y.; Wang, W.; Lv, G.; Li, X.; Wang, J.; Liu, X.; Yuan, D.; Deng, S.; You, D. BIRC5 as a prognostic and diagnostic biomarker in pan-cancer: An integrated analysis of expression, immune subtypes, and functional networks. Front. Genet. 2024, 15, 1509342. [Google Scholar] [CrossRef]
- Gariboldi, M.B.; Marras, E.; Ferrario, N.; Vivona, V.; Prini, P.; Vignati, F.; Perletti, G. Anti-cancer potential of edible/medicinal mushrooms in breast cancer. Int. J. Mol. Sci. 2023, 24, 10120. [Google Scholar] [CrossRef]
- Jakopovic, B.; Oršolić, N.; Jakopovich, I. Proteomic research on the antitumor properties of medicinal mushrooms. Molecules 2021, 26, 6708. [Google Scholar] [CrossRef]
- Randeni, N.; Xu, B. New insights into signaling pathways of cancer prevention effects of polysaccharides from edible and medicinal mushrooms. Phytomedicine 2024, 132, 155875. [Google Scholar] [CrossRef]
- Jakopovic, B.; Horvatić, A.; Klobučar, M.; Gelemanović, A.; Grbčić, P.; Oršolić, N.; Jakopovich, I.; Pavelić, S.K. Treatment with medicinal mushroom extract mixture inhibits translation and reprograms metabolism in advanced colorectal cancer animal model as evidenced by tandem mass Tags proteomics analysis. Front. Pharmacol. 2020, 11, 1202. [Google Scholar] [CrossRef]
- Jiang, Y.A.; Luo, H.S.; Zhang, Y.Y.; Fan, L.F.; Jiang, C.Q.; Chen, W.J. Telomerase activity and cell apoptosis in colon cancer cell by human telomerase reverse transcriptase gene antisense oligodeoxynucleotide. World J. Gastroenterol. 2003, 9, 1981–1984. [Google Scholar] [CrossRef]
- Simsek, B.C.; Pehlivan, S.; Karaoglu, A. Human telomerase reverse transcriptase expression in colorectal tumors: Correlations with immunohistochemical expression and clinicopathologic features. Ann. Diagn. Pathol. 2010, 14, 413–417. [Google Scholar] [CrossRef] [PubMed]
- Huo, J.; Nie, K.; Yang, T.; Zhang, S.; Zhu, Z.; Peng, X.; Zhang, Y. Network pharmacology combined with transcriptomics reveals that Ganoderma lucidum spore and Sanghuangporus vaninii compound extract exerts anti-colorectal cancer effects via CYP24A1-mediated VDR pathway and TERT-mediated Wnt signaling pathway. J. Ethnopharmacol. 2025, 348, 119820. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.; Hsiao, Y.; Hsu, C.; Lin, M.; Wang, J.C.; Huang, Y.; Ko, J. Transcriptionally mediated inhibition of telomerase of fungal immunomodulatory protein from Ganoderma tsugae in A549 human lung adenocarcinoma cell line. Mol. Carcinog. 2006, 45, 220–229. [Google Scholar] [CrossRef]
- Dang, D.T.; Chen, F.; Gardner, L.B.; Cummins, J.M.; Rago, C.; Bunz, F.; Kantsevoy, S.V.; Dang, L.H. Hypoxia-inducible factor-1alpha promotes nonhypoxia-mediated proliferation in colon cancer cells and xenografts. Cancer Res. 2006, 66, 1684–1936. [Google Scholar] [CrossRef]
- Ioannou, M.; Paraskeva, E.; Baxevanidou, K.; Simos, G.; Papamichali, R.; Papacharalambous, C.; Samara, M.; Koukoulis, G. HIF-1α in colorectal carcinoma: Review of the literature. J. Buon. 2015, 20, 680–689. Available online: https://pubmed.ncbi.nlm.nih.gov/26214618 (accessed on 17th March 2025).
- Kirdeeva, Y.; Fedorova, O.; Daks, A.; Barlev, N.; Shuvalov, O. How should the worldwide knowledge of traditional cancer healing be integrated with herbs and mushrooms into modern molecular pharmacology? Pharmaceuticals 2022, 15, 868. [Google Scholar] [CrossRef]
- Maithani, R.; Tulsawani, R. Lingzhi or Reishi medicinal mushroom (agaricomycetes) Ganoderma lucidum aqueous extract reverses hypoxia induced redox imbalance and inflammatory response in rat thymocytes. Int. J. Med. Mushrooms 2025, 27, 51–63. [Google Scholar] [CrossRef]
- Cao, D.; Qin, S.; Mu, Y.; Zhong, M. The role of MRP1 in the multidrug resistance of colorectal cancer. Oncol. Lett. 2017, 13, 2471–2476. [Google Scholar] [CrossRef]
- Jiang, Z.; Jin, T.; Gao, F.; Liu, J.; Zhong, J.; Zhao, H. Effects of ganoderic acid Me on inhibiting multidrug resistance and inducing apoptosis in multidrug resistant colon cancer cells. Process Biochem. 2011, 46, 1307–1314. [Google Scholar] [CrossRef]
- Chang, S.T.; Wasser, S.P. Current and future research trends in agricultural and biomedical applications of medicinal mushrooms and mushroom products (Review). Int. J. Med. Mushrooms 2018, 20, 1121–1133. [Google Scholar] [CrossRef]
- Wasser, S.P. Medicinal mushroom science: History, current status, future trends, and unsolved problems. Int. J. Med. Mushrooms 2010, 12, 1–16. [Google Scholar] [CrossRef]
- Jeitler, M.; Michalsen, A.; Frings, D.; Hübner, M.; Fischer, M.; Koppold-Liebscher, D.A.; Murthy, V.; Kessler, C.S. Significance of medicinal mushrooms in integrative oncology: A narrative review. Fron. Pharmacol. 2020, 11, 580656. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.R.; Akash, S.; Rahman, M.M.; Nowrin, F.T.; Akter, T.; Shohag, S.; Rauf, A.; Aljohani, A.S.M.; Simal-Gandara, J. Colon cancer and colorectal cancer: Prevention and treatment by potential natural products. Chem. Biol. Inter. 2022, 368, 110170. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Goyal, A. Recent developments in mushrooms as anti-cancer therapeutics: A review. 3 Biotech 2012, 2, 1–15. [Google Scholar] [CrossRef]
- Paul, D.; Sanap, G.; Shenoy, S.; Kalyane, D.; Kalia, K.; Tekade, R.K. Artificial intelligence in drug discovery and development. Drug Discov. Today 2021, 26, 80–93. [Google Scholar] [CrossRef]
- Leal, K.; Rojas, E.; Madariaga, D.; Contreras, M.J.; Nuñez-Montero, K.; Barrientos, L.; Goméz-Espinoza, O.; Iturrieta-González, I. Unlocking fungal potential: The CRISPR-Cas system as a strategy for secondary metabolite discovery. J. Fungi 2024, 10, 748. [Google Scholar] [CrossRef]
- Kwong, K.W.; Xin, Y.; Lai, N.C.; Sung, J.C.; Wu, K.C.; Hamied, Y.K.; Sze, E.T.; Lam, D.M. Oral Vaccines: A Better Future of Immunization. Vaccines 2023, 11, 1232. [Google Scholar] [CrossRef]
- Pérez-Martínez, A.S.; Acevedo-Padilla, S.A.; Bibbins-Martínez, M.; Galván-Alonso, J.; Rosales-Mendoza, S. A perspective on the use of Pleurotus for the development of convenient fungi-made oral subunit vaccines. Vaccine 2015, 33, 25–33. [Google Scholar] [CrossRef]
- Uddin, M.S.; Kaldis, A.; Menassa, R.; Ortiz Guluarte, J.; Barreda, D.R.; Guan, L.L.; Alexander, T.W. Mucosal immunization with spore-based vaccines against Mannheimia haemolytica enhances antigen-specific immunity. Vaccines 2024, 12, 375. [Google Scholar] [CrossRef]


| Scientific Name | Common Name | Active Component/Fraction | Extraction Method | Experimental Model | Mechanism | References |
|---|---|---|---|---|---|---|
| Agaricus bisporus | White button mushroom | WAAP-1, WAAP-2 | Polysaccharide extraction | HT-29 human colon cancer cells | Upregulation of Caspase-3, BAX, E-cadherin; downregulation of Bcl-2, Vimentin | [17,42] |
| Microencapsulated polysaccharide extract | Polysaccharide extraction | Caco-2 human colon cancer cells | Downregulation of Bcl-2, TGF-β; upregulation of IkappaB-α; inhibits NF-κB signaling pathway | [40] | ||
| Lentinus edodes | Shiitake mushroom | LAP | Mycelium extract | COLO 205 human colon cancer cells | Upregulation of p53, p21/Cip1, p27/Kip1; downregulation of cyclins B and D3; induces apoptosis | [21] |
| SLNT | Water extraction | HT-29 human colon cancer cells | Activation of mitochondrial apoptotic pathways, caspase-3 and caspase-8 activation, cytochrome c release, MMP loss | [32] | ||
| Pleurotus eryngii | King oyster mushroom | Polyphenol-rich extract, PEP (Protein) | Polyphenol extraction | HCT-116 human colon cancer cells | Upregulation of caspase-3, caspase-8 p53, c-PARP; cleaves caspase-3; induces cell death | [28,58,59] |
| Pleurotus ostreatus | Oyster mushroom | α-Glucan | Polysaccharide extraction | HT-29 human colon cancer cells | Upregulation of BAX, cytochrome c; induces apoptosis | [44] |
| Protein extract | Protein extraction | SW480 human colon cancer cells | Increased ROS production, glutathione depletion, loss of mitochondrial membrane potential | [60] | ||
| Pleurotus pulmonarius | Indian oyster mushroom | Polysaccharides | Fruiting body/mycelium extract | HT-29 human colon cancer cells | Regulation of Bcl-2, BAX, cytochrome C; Induces apoptosis | [86] |
| Volvariella volvacea | Straw mushroom | Protein extract | Protein extraction | SW480 human colon cancer cells | Increased SubG1 phase cells, loss of mitochondrial membrane potential | [60] |
| Agaricus blazei, Hericeum erinaceus, Grifola frondosa | - | Andosan™ | Water extraction | Caco-2 human colon cancer cells | Induces early and late apoptosis | [35] |
| Ganoderma neo-japonicum | Reishi mushroom | Hexane and chloroform fractions | Fraction extraction | Colonic carcinoma cells | Induction of apoptosis | [27] |
| Ganoderma lucidum | BSGLWE | Water extraction | HCT-116 human colon cancer cells | Upregulation of NAG-1; downregulation of Bcl-2, pro-caspase-3, pro-caspase-9; cleaves PARP; caspase-8 activation | [31] | |
| Pleurotus sajor-caju | - | PSC-hex | n-hexane extraction | - | ROS generation; p53, BAX, caspase-3 upregulation; Bcl-2 downregulation | [4] |
| Scientific Name | Common Name | Active Compound/Fraction | Experimental Model | Mechanism of Action | References |
|---|---|---|---|---|---|
| Phellinus linteus | N/A | Methanolic extract | HCT-116 and SW-480 colon cancer cell lines | Significant antimigratory effects against both cell lines; correlated with increased superoxide anion radical production and reduced β-catenin expression | [18] |
| Cordyceps sinensis | N/A | Methanolic extract | HCT-116 colon cancer cell line | Significant antimigratory effect; correlated with increased superoxide anion radical production and reduced β-catenin expression | [18] |
| Lentinus edodes | Shiitake mushroom | Methanolic extract | HCT-116 colon cancer cell line | Significant antimigratory effect; correlated with increased superoxide anion radical production and reduced β-catenin expression | [18] |
| Coprinus comatus | Shaggymane | Methanolic extract | HCT-116 colon cancer cell line | Significant antimigratory effect; correlated with increased superoxide anion radical production and reduced β-catenin expression | [18] |
| Ganoderma lucidum | Reishi mushroom | Methanolic extract | HCT-116 colon cancer cell line | Significant antimigratory effect; correlated with increased superoxide anion radical production and reduced β-catenin expression | [18] |
| Macrolepiota procera | Parasol mushroom | Ethanolic extract | COLO-205 human colon cancer cell line | Significant cytotoxic effect | [19] |
| Pleurotus ostreatus | Oyster mushroom | Aqueous extract | COLO-205 human colon cancer cell line | Significant cytotoxic effect | [19] |
| Hericium erinaceus | Lion’s mane mushroom | n-hexane extract | HCT-116 human colon carcinoma cell line | Lower cytotoxicity level | [30] |
| Metacordyceps neogunnii | N/A | n-hexane extract | HCT-116 human colon carcinoma cell line | Highest anti-colon cancer effect (68.6 ± 3.6% cytotoxicity) | [30] |
| Dictyophora indusiata | Bamboo mushroom | n-hexane extract | HCT-116 human colon carcinoma cell line | Lower cytotoxicity level | [30] |
| Agaricus bisporus | Button mushroom | Microcapsulated polysaccharide extract | Caco-2 human colon cancer cell line | Increased CD16+CD56+ NK cell population; 74.09% cytotoxic activity; G0/G1 phase cell cycle arrest | [40] |
| Calvatia lilacina | N/A | Protein extract | SW480 and THP-1 cells | Concentration-dependent cytotoxicity; significant decrease in cell viability at high concentrations | [60] |
| Pleurotus ostreatus | Oyster mushroom | Protein extract | SW480 cell line | Concentration-dependent cytotoxicity; significant decrease in cell viability at high concentrations | [60] |
| Volvariella volvacea | Straw mushroom | Protein extract | SW480 cell line | Concentration-dependent cytotoxicity; significant decrease in cell viability at high concentrations | [60] |
| Ganoderma neo-japonicum | N/A | Hexane and chloroform fractions | Colonic carcinoma cells | Cytotoxic effect | [27] |
| Lentinus edodes | Shiitake mushroom | Alcohol precipitate | Colon epithelial cells | Selective cytotoxicity in cancerous cells | [21] |
| PSC-hex | N/A | Hexane extract | Colorectal cancer cells | Strong cytotoxicity (IC50 = 0.05 mg/mL) | [4] |
| Agaricus blazei, Hericeum erinaceus, Grifola frondosa | - | Andosan™ | Caco-2 human colon cancer cell line | Concentration-dependent cytotoxic effects; cell viability reduction correlated with concentration | [35] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bentharavithana, J.; Islam, T.; Xu, B. Medicinal Mushrooms in Colon Cancer Therapy: Mechanisms of Action of Bioactive Compounds and Therapeutic Potential. Int. J. Mol. Sci. 2025, 26, 5304. https://doi.org/10.3390/ijms26115304
Bentharavithana J, Islam T, Xu B. Medicinal Mushrooms in Colon Cancer Therapy: Mechanisms of Action of Bioactive Compounds and Therapeutic Potential. International Journal of Molecular Sciences. 2025; 26(11):5304. https://doi.org/10.3390/ijms26115304
Chicago/Turabian StyleBentharavithana, Jinangi, Tahidul Islam, and Baojun Xu. 2025. "Medicinal Mushrooms in Colon Cancer Therapy: Mechanisms of Action of Bioactive Compounds and Therapeutic Potential" International Journal of Molecular Sciences 26, no. 11: 5304. https://doi.org/10.3390/ijms26115304
APA StyleBentharavithana, J., Islam, T., & Xu, B. (2025). Medicinal Mushrooms in Colon Cancer Therapy: Mechanisms of Action of Bioactive Compounds and Therapeutic Potential. International Journal of Molecular Sciences, 26(11), 5304. https://doi.org/10.3390/ijms26115304









