Resilience of Maize to Environmental Stress: Insights into Drought and Heat Tolerance
Abstract
1. Introduction
2. Major Challenges Posed by Abiotic Stress in Maize
3. Significance of Understanding and Improving Maize Adaptability to Drought and Heat
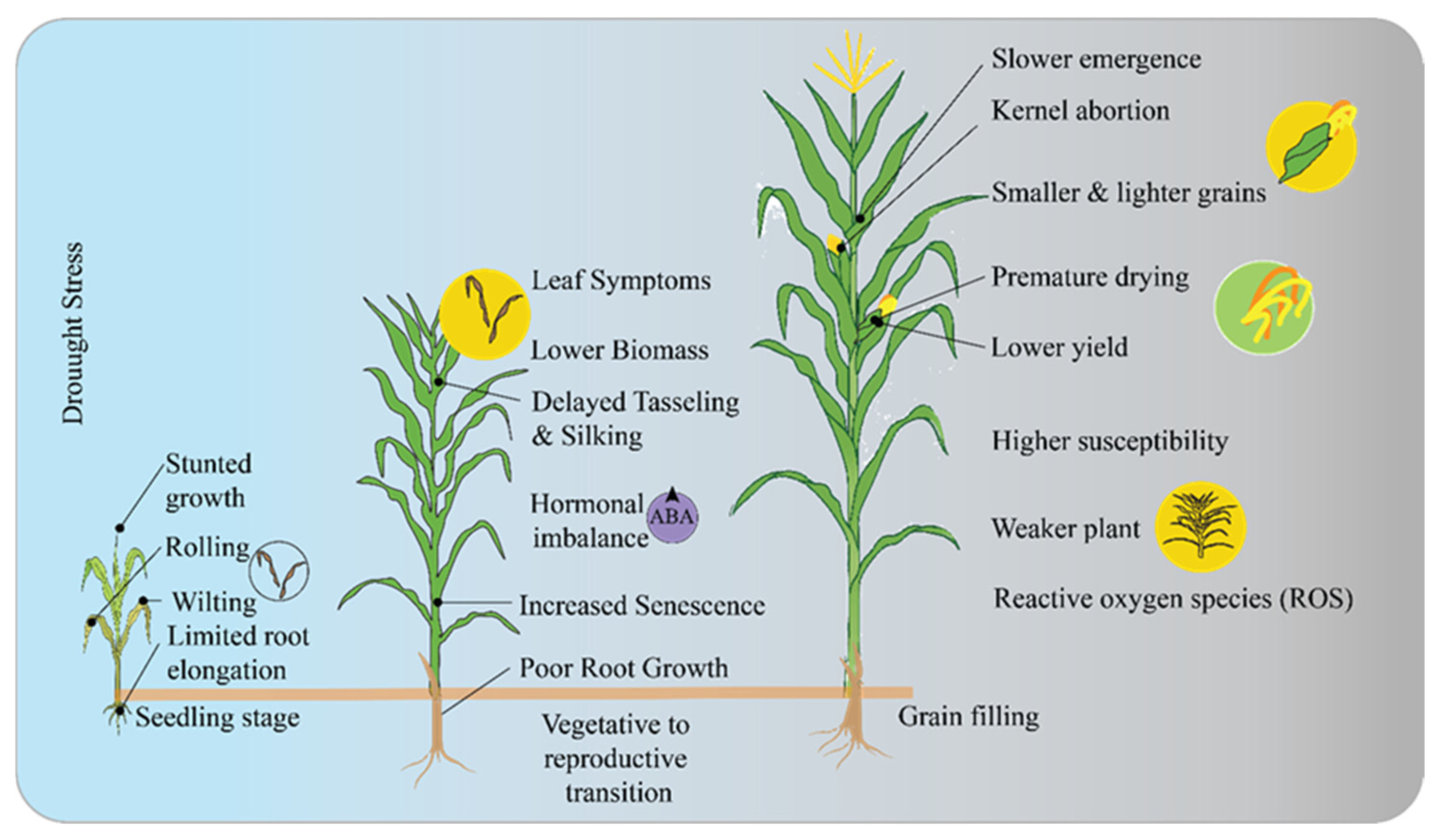
4. Drought Resistance in Maize
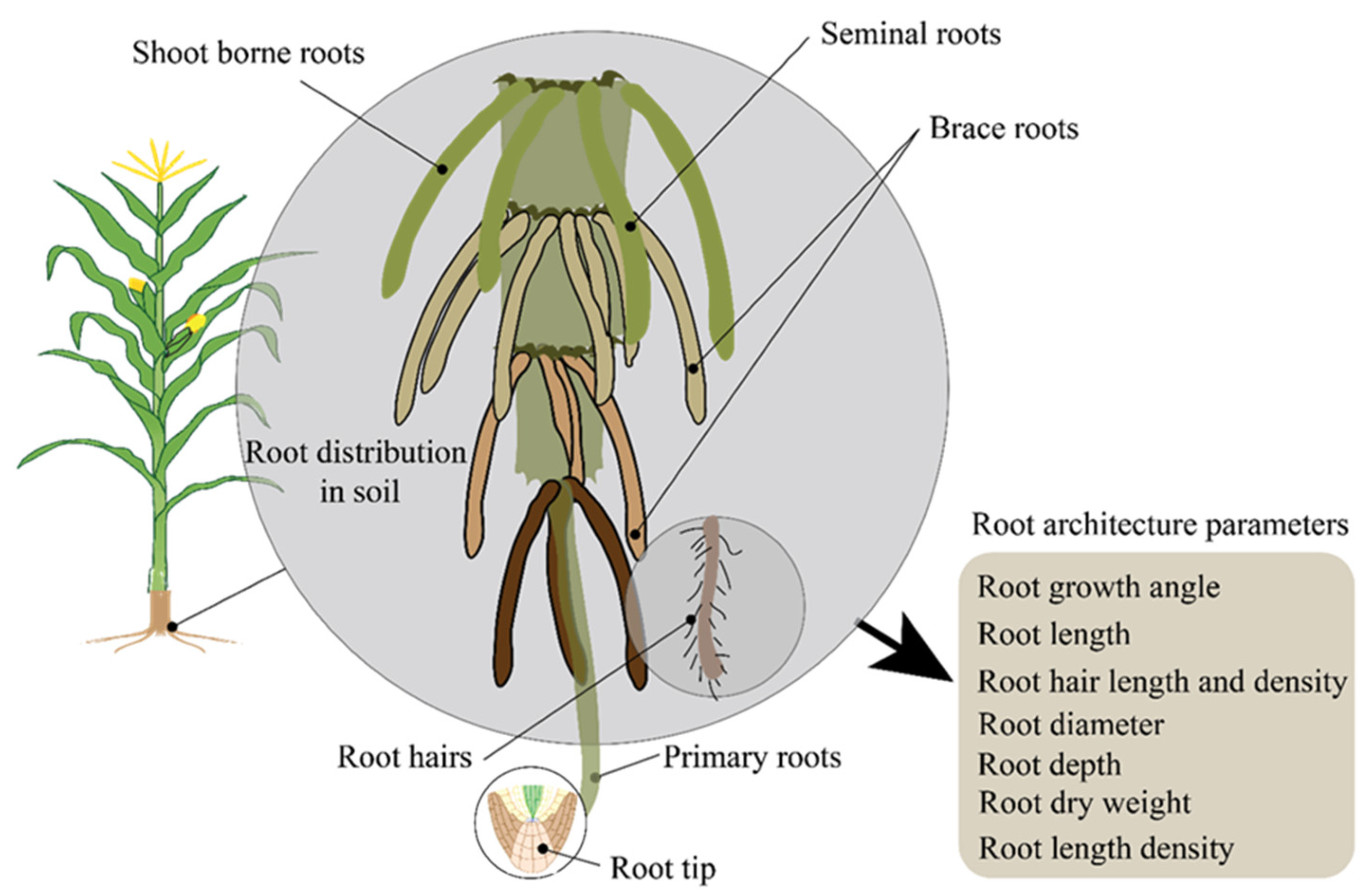
4.1. Root Architecture and Water Uptake Efficiency
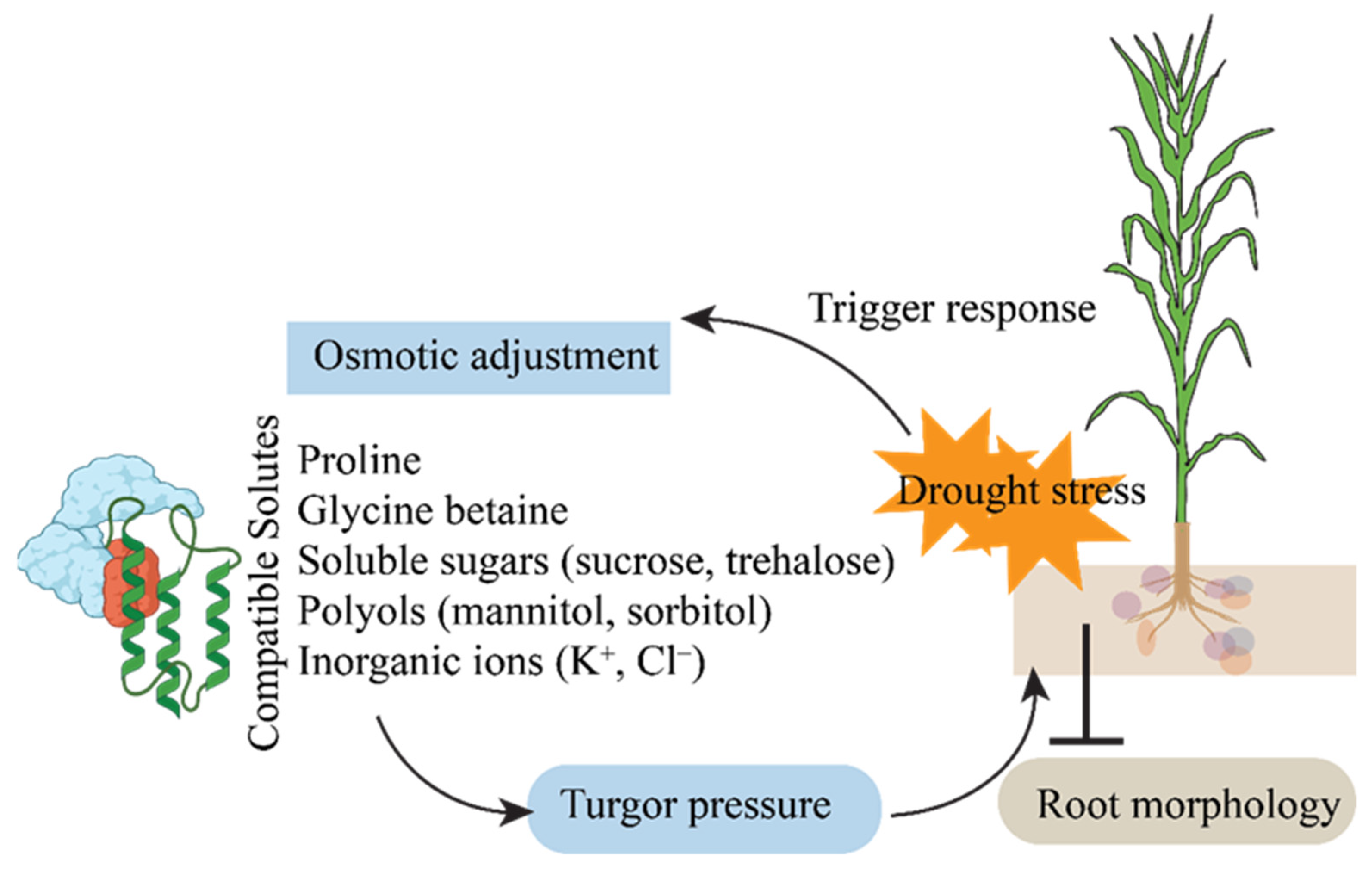
4.2. Osmotic Adjustment and Accumulation of Compatible Solutes
4.3. Role of ABA (Abscisic Acid) in Stomatal Regulation
4.4. Molecular and Genetic Approaches for Drought Tolerance
| S. No | Gene | Details | Function/s | Reference |
|---|---|---|---|---|
| 1 | ZmHB53 | Homeodomain-leucine zipper I (HD-Zip I) transcription factors (TFs) | ABA receptor ZmPYL4 | [85] |
| 2 | ZmPHR1 | Transcription factor | phosphorus homeostasis | [86] |
| 3 | ZmTIP2;3 | Tonoplast intrinsic protein | arbuscular mycorrhiza fungi symbiosis | [87] |
| 4 | ZmSCE1a | E3 SUMO ligase | enhancing the stability of ZmGCN5 | [88] |
| 5 | ZmNAC55 | Trnacription factor | negatively regulate drought stress via increasing ZmHOP3 expression in maize | [89] |
| 6 | ZmMIK2-ZmC2DP1 | Kinase 2 proteins | negative regulatory module in maize drought- and salt-stress responses | [90] |
| 7 | ZmCML3 | Calmodulin-like proteins (CMLs) | through increasing proline (Pro) content | [78] |
| 8 | ZmGA20ox3 | Loss-of-function mutations of GA biosynthesis enzyme | significantly increased ABA, JA, and DIMBOA levels in mutants | [91] |
| 9 | ZmEULD1b | Euonymus europaeus (EUL) related lectin family, | stomatal development and promotes water-use efficiency | [92] |
| 10 | ZmMYB39 | Transcription factor | stomatal development and promotes water-use efficiency | [93] |
| 11 | ZmGLYI-8 | Glyoxalase I (GLYI) | Overexpressed in model plants | [94] |
| 12 | ZmbHLH47-ZmSnRK2.9 | Transcription factor | ABA response and drought tolerance | [95] |
| 13 | ZmAPX2 | Ascorbate peroxidase 2 | reducing ROS content | [96] |
| 14 | ZmSK1 | Glycogen synthase kinase 3 (GSK3)-like kinases | reduces drought tolerance in maize | [97] |
| 15 | ZmDST44 | Drought and salinity tolerance (DST) gene | positive regulator of drought tolerance (ZmmiR139 regulates ZmDST44 by cleaving its mRNA) | [98] |
| 16 | ZmPL1 | Phylloplanin-like | negatively regulates drought tolerance in maize (CRISPER-cas9) | [81] |
| 17 | ZmC2H2-149 | Cys(2)/His(2) zinc-finger-proteins (C2H2-ZFPs) | repressing ZmHSD1 in maize (negative regulator) | [99] |
| 18 | ZmPRX1 | Peroxidase genes | promoting root development and lignification | [100] |
| 19 | ZmSUS1 | Sucrose synthase (SUS) | regulating sucrose metabolism and increasing soluble sugar content | [101] |
| 20 | ZmGRAS15 | GRAS transcription factor | regulating primary root length at the seedling stage | [102] |
| 21 | ZmCYB5-1 | Cytochrome b5 proteins (CYB5s) | negative regulator of drought stress | [103] |
| 22 | ZmHsf28 | Transcription factors | ZmSnRK2.2-ZmHsf28-ZmJAZ14/17 module is identified to regulate drought tolerance through coordinating ABA and JA signaling | [104] |
| 23 | miR166e/ZmATHB14 | Micro RNAs | miR166e-ZmATHB14 module regulates drought tolerance | [105] |
| 24 | ZmSNAC06 | NAC transcription factor family | hypersensitivity to abscisic acid (ABA)-positive regulator | [106] |
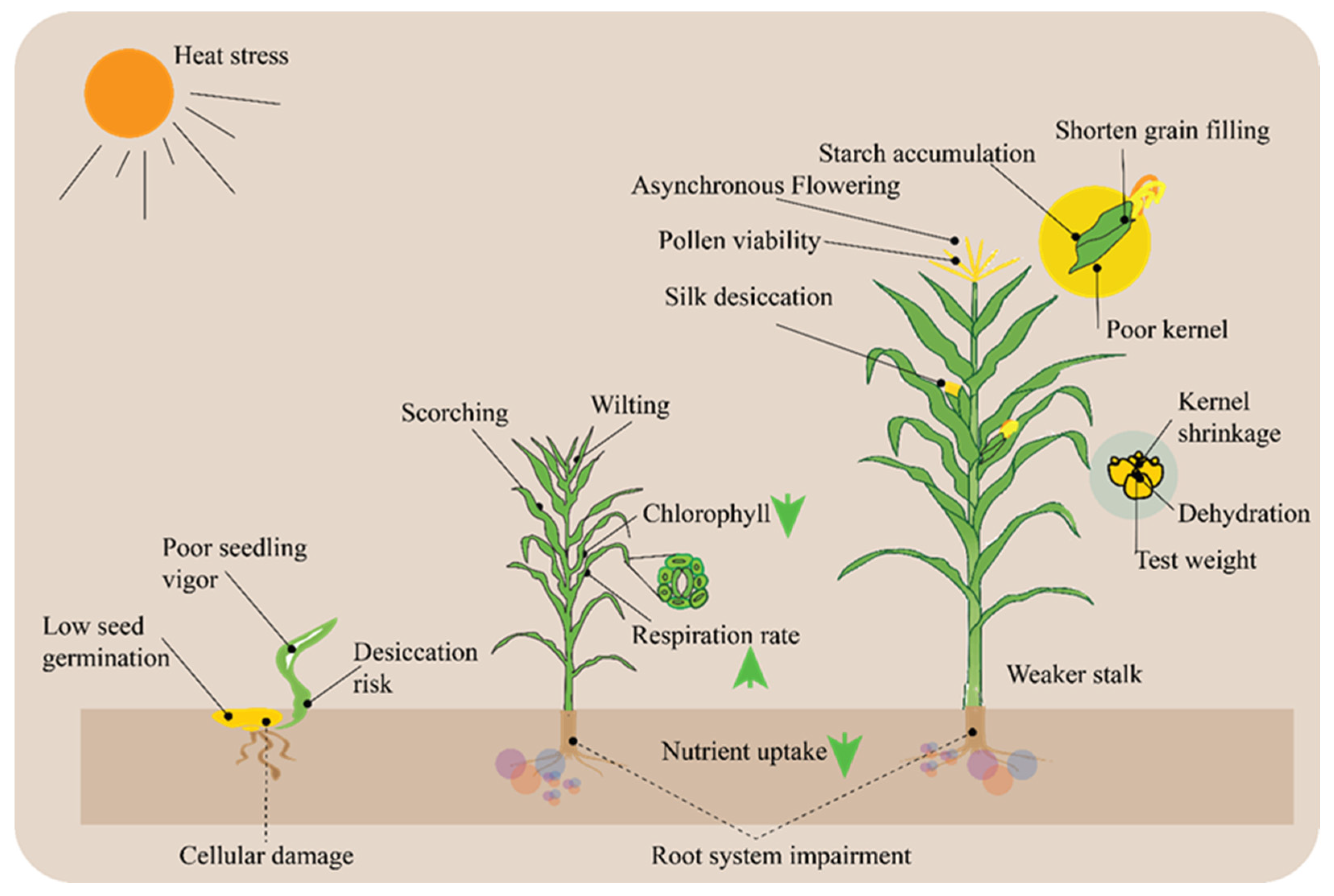
5. Heat Resistance in Maize: Effects and Mechanisms
5.1. Impact of High Temperatures on Maize Physiology
5.2. Heat Tolerance Mechanism
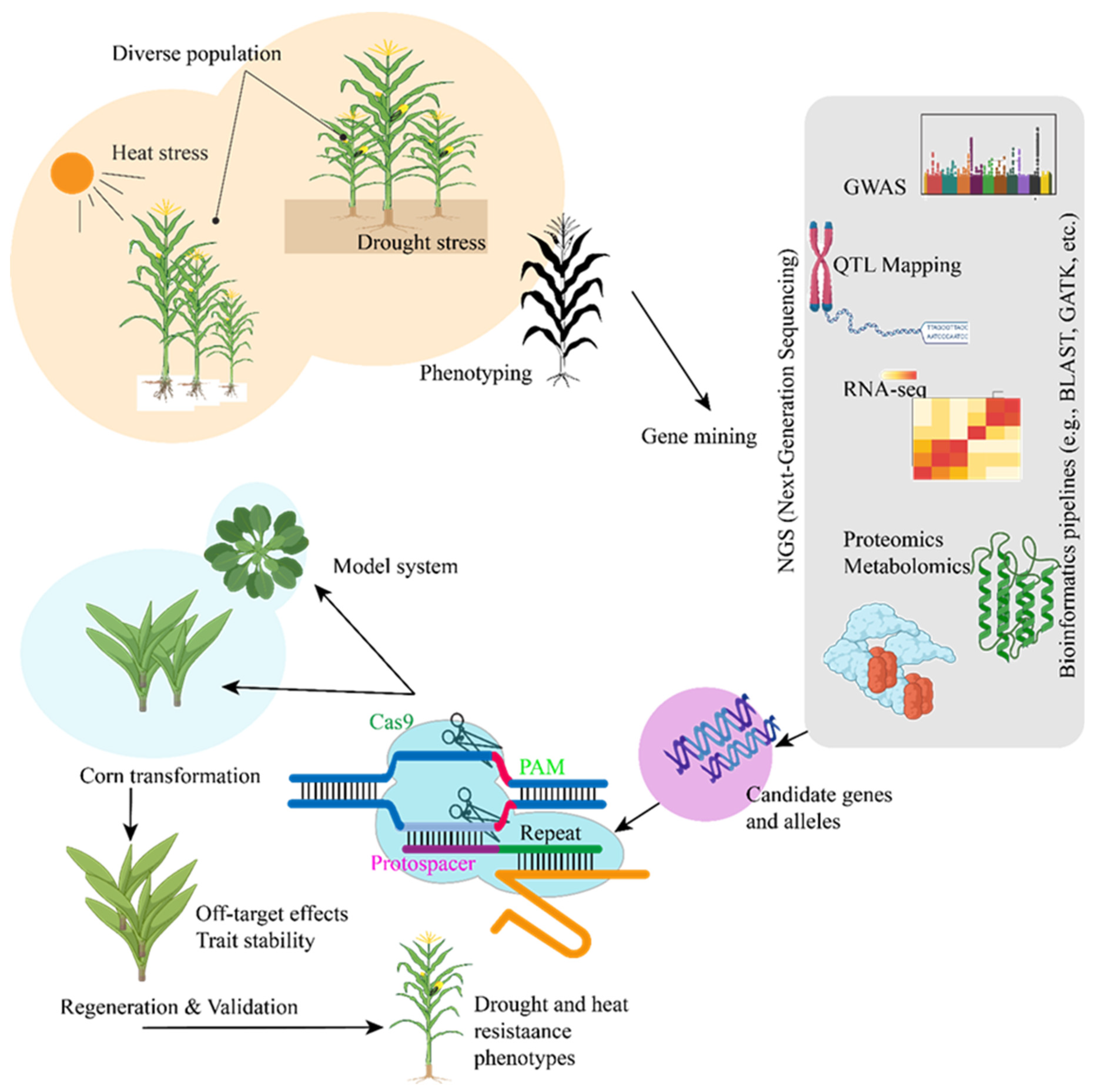
6. Emerging Technologies for Enhancing Drought and Heat Resistance in Maize: From a CRISPR Perspective
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Farooq, M.; Bramley, H.; Palta, J.A.; Siddique, K.H. Heat Stress in Wheat during Reproductive and Grain-Filling Phases. Crit. Rev. Plant Sci. 2011, 30, 491–507. [Google Scholar] [CrossRef]
- Molla, M.S.H.; Nakasathien, S.; Ali, M.A.; Khan, A.; Alam, M.R.; Hossain, A.; Farooq, M.; El Sabagh, A. Influence of nitrogen application on dry biomass allocation and translocation in two maize varieties under short pre-anthesis and prolonged bracketing flowering periods of drought. Arch. Agron. Soil Sci. 2019, 65, 928–944. [Google Scholar] [CrossRef]
- Rojas-Downing, M.M.; Nejadhashemi, A.P.; Harrigan, T.; Woznicki, S.A. Climate change and livestock: Impacts, adaptation, and mitigation. Clim. Risk Manag. 2017, 16, 145–163. [Google Scholar] [CrossRef]
- Vogel, E.; Donat, M.G.; Alexander, L.V.; Meinshausen, M.; Ray, D.K.; Karoly, D.; Meinshausen, N.; Frieler, K. The effects of climate extremes on global agricultural yields. Environ. Res. Lett. 2019, 14, 54010. [Google Scholar] [CrossRef]
- Shiferaw, B.; Prasanna, B.M.; Hellin, J.; Bänziger, M. Crops that feed the world 6. Past successes and future challenges to the role played by maize in global food security. Food Secur. 2011, 3, 307–327. [Google Scholar] [CrossRef]
- Khaliq, A.; Iqbal, M.A.; Zafar, M.; Gulzar, A. Appraising economic dimension of maize production under coherent fertilization in Azad Kashmir, Pakistan. Custos E Agronegocio 2019, 15, 243–253. [Google Scholar]
- El Sabagh, A.; Barutcular, C.; Islam, M.S. Relatıonshıps between stomatal conductance and yıeld under defıcıt ırrıgatıon ın maıze (Zea mays L.). J. Exp. Biol. Agric. Sci. 2017, 5, 14–21. [Google Scholar] [CrossRef]
- Rezaei, E.E.; Webber, H.; Asseng, S.; Boote, K.; Durand, J.L.; Ewert, F.; Martre, P.; MacCarthy, D.S. Climate change impacts on crop yields. Nat. Rev. Earth Environ. 2023, 4, 831–846. [Google Scholar] [CrossRef]
- El-Sappah, A.H.; Rather, S.A.; Wani, S.H.; Elrys, A.S.; Bilal, M.; Huang, Q.; Dar, Z.A.; Elashtokhy, M.M.A.; Soaud, N.; Koul, M.; et al. Heat Stress-Mediated Constraints in Maize (Zea mays) Production: Challenges and Solutions. Front. Plant Sci. 2022, 13, 879366. [Google Scholar] [CrossRef]
- Li, T.; Zhang, X.-p.; Liu, Q.; Liu, J.; Chen, Y.-q.; Sui, P. Yield penalty of maize (Zea mays L.) under heat stress in different growth stages: A review. J. Integr. Agric. 2022, 21, 2465–2476. [Google Scholar] [CrossRef]
- Cairns, J.E.; Sonder, K.; Zaidi, P.H.; Verhulst, N.; Mahuku, G.; Babu, R.; Nair, S.K.; Das, B.; Govaerts, B.; Vinayan, M.T.; et al. Chapter one—Maize Production in a Changing Climate: Impacts, Adaptation, and Mitigation Strategies. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: Cambridge, MA, USA, 2012; Volume 114, pp. 1–58. [Google Scholar]
- Iqbal, M.A.; Iqbal, M.A.; Aslam, Z.; Maqsood, M.; Ahmad, Z.; Akbar, N.; Khan, H.Z.; Abbas, R.; Khan, R.; Abbas, G.; et al. Boosting forage yield and quality of maize (Zea mays L.) with multi-species bacterial inoculation in Pakistan. Phyton 2017, 86, 84. [Google Scholar]
- Zhao, F.; Zhang, D.; Zhao, Y.; Wang, W.; Yang, H.; Tai, F.; Li, C.; Hu, X. The Difference of Physiological and Proteomic Changes in Maize Leaves Adaptation to Drought, Heat, and Combined Both Stresses. Front. Plant Sci. 2016, 7, 1471. [Google Scholar] [CrossRef] [PubMed]
- Cairns, J.E.; Hellin, J.; Sonder, K.; Araus, J.L.; MacRobert, J.F.; Thierfelder, C.; Prasanna, B.M. Adapting maize production to climate change in sub-Saharan Africa. Food Secur. 2013, 5, 345–360. [Google Scholar] [CrossRef]
- Djalovic, I.; Kundu, S.; Bahuguna, R.N.; Pareek, A.; Raza, A.; Singla-Pareek, S.L.; Prasad, P.V.V.; Varshney, R.K. Maize and heat stress: Physiological, genetic, and molecular insights. Plant Genome 2024, 17, e20378. [Google Scholar] [CrossRef]
- Nuss, E.T.; Tanumihardjo, S.A. Maize: A Paramount Staple Crop in the Context of Global Nutrition. Compr. Rev. Food Sci. Food Saf. 2010, 9, 417–436. [Google Scholar] [CrossRef]
- Chhabra, G.; Kaur, G.; Singh, K.; Kaur, J.; Praba, U.P.; Singh, R.; Karnatam, K.S.; Garg, T.; Ranjan, R.; Vikal, Y. Biofortification of Maize: A Promising Approach for Better Nutrition. In Harnessing Crop Biofortification for Sustainable Agriculture; Tiwari, S., Singh, B., Eds.; Springer Nature: Singapore, 2024; pp. 145–178. [Google Scholar]
- Goredema-Matongera, N.; Ndhlela, T.; Magorokosho, C.; Kamutando, C.N.; van Biljon, A.; Labuschagne, M. Multinutrient Biofortification of Maize (Zea mays L.) in Africa: Current Status, Opportunities and Limitations. Nutrients 2021, 13, 1039. [Google Scholar] [CrossRef]
- Hatfield, J.L.; Prueger, J.H. Temperature extremes: Effect on plant growth and development. Weather Clim. Extrem. 2015, 10, 4–10. [Google Scholar] [CrossRef]
- Schlenker, W.; Roberts, M.J. Nonlinear temperature effects indicate severe damages to U.S. crop yields under climate change. Proc. Natl. Acad. Sci. USA 2009, 106, 15594–15598. [Google Scholar] [CrossRef]
- Lobell, D.B.; Schlenker, W.; Costa-Roberts, J. Climate Trends and Global Crop Production Since 1980. Science 2011, 333, 616–620. [Google Scholar] [CrossRef]
- Bellard, C.; Bertelsmeier, C.; Leadley, P.; Thuiller, W.; Courchamp, F. Impacts of climate change on the future of biodiversity. Ecol. Lett. 2012, 15, 365–377. [Google Scholar] [CrossRef]
- Rosenzweig, C.; Elliott, J.; Deryng, D.; Ruane, A.C.; Müller, C.; Arneth, A.; Boote, K.J.; Folberth, C.; Glotter, M.; Khabarov, N.; et al. Assessing agricultural risks of climate change in the 21st century in a global gridded crop model intercomparison. Proc. Natl. Acad. Sci. USA 2014, 111, 3268–3273. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, T.; von Braun, J. Climate Change Impacts on Global Food Security. Science 2013, 341, 508–513. [Google Scholar] [CrossRef] [PubMed]
- Sulmon, C.; van Baaren, J.; Cabello-Hurtado, F.; Gouesbet, G.; Hennion, F.; Mony, C.; Renault, D.; Bormans, M.; El Amrani, A.; Wiegand, C.; et al. Abiotic stressors and stress responses: What commonalities appear between species across biological organization levels? Environ. Pollut. 2015, 202, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Rivero, R.M.; Shulaev, V.; Blumwald, E.; Mittler, R. Abiotic and biotic stress combinations. New Phytol. 2014, 203, 32–43. [Google Scholar] [CrossRef]
- Yoshida, T.; Christmann, A.; Yamaguchi-Shinozaki, K.; Grill, E.; Fernie, A.R. Revisiting the Basal Role of ABA—Roles Outside of Stress. Trends Plant Sci. 2019, 24, 625–635. [Google Scholar] [CrossRef]
- Des Marais, D.L.; Hernandez, K.M.; Juenger, T.E. Genotype-by-Environment Interaction and Plasticity: Exploring Genomic Responses of Plants to the Abiotic Environment. Annu. Rev. Ecol. Evol. Syst. 2013, 44, 5–29. [Google Scholar] [CrossRef]
- Liu, H.-J.; Liu, J.; Zhai, Z.; Dai, M.; Tian, F.; Wu, Y.; Tang, J.; Lu, Y.; Wang, H.; Jackson, D.; et al. Maize2035: A decadal vision for intelligent maize breeding. Mol. Plant 2025, 18, 313–332. [Google Scholar] [CrossRef]
- Aliniaeifard, S.; Rezayian, M.; Mousavi, S.H. Drought Stress: Involvement of Plant Hormones in Perception, Signaling, and Response. In Plant Hormones and Climate Change; Ahammed, G.J., Yu, J., Eds.; Springer Nature: Singapore, 2023; pp. 227–250. [Google Scholar]
- Khalili, M.; Naghavi, M.R.; Aboughadareh, A.P.; Rad, H.N. Effects of Drought Stress on Yield and Yield Components in Maize cultivars (Zea mays L.). Int. J. Agron. Plant Prod. 2013, 4, 809–812. [Google Scholar]
- Fahad, S.; Bajwa, A.A.; Nazir, U.; Anjum, S.A.; Farooq, A.; Zohaib, A.; Sadia, S.; Nasim, W.; Adkins, S.; Saud, S.; et al. Crop Production under Drought and Heat Stress: Plant Responses and Management Options. Front. Plant Sci. 2017, 8, 1147. [Google Scholar] [CrossRef]
- Daryanto, S.; Wang, L.; Jacinthe, P.-A. Global Synthesis of Drought Effects on Maize and Wheat Production. PLoS ONE 2016, 11, e0156362. [Google Scholar] [CrossRef]
- Sánchez, B.; Rasmussen, A.; Porter, J.R. Temperatures and the growth and development of maize and rice: A review. Glob. Change Biol. 2014, 20, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Calleja-Cabrera, J.; Boter, M.; Oñate-Sánchez, L.; Pernas, M. Root Growth Adaptation to Climate Change in Crops. Front. Plant Sci. 2020, 11, 544. [Google Scholar] [CrossRef] [PubMed]
- Gobezie, A.; Ademe, D.; Sharma, L.K. CERES-Maize (DSSAT) Model Applications for Maize Nutrient Management Across Agroecological Zones: A Systematic Review. Plants 2025, 14, 661. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Jin, J. Improving CERES-Maize for simulating maize growth and yield under water stress conditions. Eur. J. Agron. 2020, 117, 126072. [Google Scholar] [CrossRef]
- Lobell, D.B.; Hammer, G.L.; McLean, G.; Messina, C.; Roberts, M.J.; Schlenker, W. The critical role of extreme heat for maize production in the United States. Nat. Clim. Change 2013, 3, 497–501. [Google Scholar] [CrossRef]
- Wahid, A.; Gelani, S.; Ashraf, M.; Foolad, M.R. Heat tolerance in plants: An overview. Environ. Exp. Bot. 2007, 61, 199–223. [Google Scholar] [CrossRef]
- Duveiller, E.; Singh, R.P.; Nicol, J.M. The challenges of maintaining wheat productivity: Pests, diseases, and potential epidemics. Euphytica 2007, 157, 417–430. [Google Scholar] [CrossRef]
- Schussler, J.R.; Westgate, M.E. Maize Kernel Set at Low Water Potential: I. Sensitivity to Reduced Assimilates during Early Kernel Growth. Crop Sci. 1991, 31, 1189–1195. [Google Scholar] [CrossRef]
- Abrecht, D.G.; Carberry, P.S. The influence of water deficit prior to tassel initiation on maize growth, development and yield. Field Crops Res. 1993, 31, 55–69. [Google Scholar] [CrossRef]
- Bolaños, J.; Edmeades, G.O. Eight cycles of selection for drought tolerance in lowland tropical maize. II. Responses in reproductive behavior. Field Crops Res. 1993, 31, 253–268. [Google Scholar] [CrossRef]
- Ribaut, J.M.; Hoisington, D.A.; Deutsch, J.A.; Jiang, C.; Gonzalez-de-Leon, D. Identification of quantitative trait loci under drought conditions in tropical maize. 1. Flowering parameters and the anthesis-silking interval. Theor. Appl. Genet. 1996, 92, 905–914. [Google Scholar] [CrossRef] [PubMed]
- Maqbool, S.; Hassan, M.A.; Xia, X.; York, L.M.; Rasheed, A.; He, Z. Root system architecture in cereals: Progress, challenges and perspective. Plant J. 2022, 110, 23–42. [Google Scholar] [CrossRef] [PubMed]
- Robinson, D. Optimal relations between root length and nutrient inflow rate in plant root systems. J. Theor. Biol. 1988, 135, 359–370. [Google Scholar] [CrossRef]
- Robinson, D. The responses of plants to non-uniform supplies of nutrients. New Phytol. 1994, 127, 635–674. [Google Scholar] [CrossRef]
- Robinson, D.A.; Campbell, C.S.; Hopmans, J.W.; Hornbuckle, B.K.; Jones, S.B.; Knight, R.; Ogden, F.; Selker, J.; Wendroth, O. Soil Moisture Measurement for Ecological and Hydrological Watershed-Scale Observatories: A Review. Vadose Zone J. 2008, 7, 358–389. [Google Scholar] [CrossRef]
- Ding, H.; Li, X.; Zhuge, S.; Du, J.; Wu, M.; Li, W.; Li, Y.; Ma, H.; Zhang, P.; Wang, X.; et al. Genome-Wide Identification and Functional Analysis of the Genes of the ATL Family in Maize during High-Temperature Stress in Maize. Genes 2024, 15, 1106. [Google Scholar] [CrossRef]
- Ranjan, A.; Sinha, R.; Singla-Pareek, S.L.; Pareek, A.; Singh, A.K. Shaping the root system architecture in plants for adaptation to drought stress. Physiol. Plant. 2022, 174, e13651. [Google Scholar] [CrossRef]
- Lynch, J. Root architecture and plant productivity. Plant Physiol. 1995, 109, 7–13. [Google Scholar] [CrossRef]
- Fitter, A.H. Functional significance of root morphology and root system architecture. Spec. Publ. Br. Ecol. Soc. 1985, 87–106. [Google Scholar]
- Fitter, A.H. Architectural approach to the comparative ecology of plant root systems. New Phytol. 1987, 106, 61–77. [Google Scholar] [CrossRef]
- Jackson, R.B.; Caldwell, M.M. Integrating Resource Heterogeneity and Plant Plasticity: Modelling Nitrate and Phosphate Uptake in a Patchy Soil Environment. J. Ecol. 1996, 84, 891–903. [Google Scholar] [CrossRef]
- Qiao, M.; Hong, C.; Jiao, Y.; Hou, S.; Gao, H. Impacts of Drought on Photosynthesis in Major Food Crops and the Related Mechanisms of Plant Responses to Drought. Plants 2024, 13, 1808. [Google Scholar] [CrossRef] [PubMed]
- Voothuluru, P.; Wu, Y.; Sharp, R.E. Not so hidden anymore: Advances and challenges in understanding root growth under water deficits. Plant Cell 2024, 36, 1377–1409. [Google Scholar] [CrossRef]
- Li, J.; Wang, T.; Wang, C.; Cui, R.; Zhang, X.; Feng, Q.; Liu, S.; Xie, Z. Effects of drought stress on tassel growth and characteristics physiological of maize varieties. Appl. Ecol. Environ. Res. 2022, 20, 685–697. [Google Scholar] [CrossRef]
- Wood, J.M.; Bremer, E.; Csonka, L.N.; Kraemer, R.; Poolman, B.; van der Heide, T.; Smith, L.T. Osmosensing and osmoregulatory compatible solute accumulation by bacteria. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2001, 130, 437–460. [Google Scholar] [CrossRef]
- Bohnert, H.J.; Shen, B. Transformation and compatible solutes. Sci. Hortic. 1998, 78, 237–260. [Google Scholar] [CrossRef]
- Yancey, P.H. Organic osmolytes as compatible, metabolic and counteracting cytoprotectants in high osmolarity and other stresses. J. Exp. Biol. 2005, 208, 2819–2830. [Google Scholar] [CrossRef]
- Gagneul, D.; Aïnouche, A.; Duhazé, C.; Lugan, R.; Larher, F.R.; Bouchereau, A. A reassessment of the function of the so-called compatible solutes in the halophytic plumbaginaceae Limonium latifolium. Plant Physiol. 2007, 144, 1598–1611. [Google Scholar] [CrossRef]
- Ashraf, M.; Harris, P.J.C. Potential biochemical indicators of salinity tolerance in plants. Plant Sci. 2004, 166, 3–16. [Google Scholar] [CrossRef]
- Guei, R.G.; Wassom, C.E. Genetics of osmotic adjustment in breeding maize for drought tolerance. Heredity 1993, 71, 436–441. [Google Scholar] [CrossRef]
- Lemcoff, J.H.; Chimenti, C.A.; Davezac, T.A.E. Osmotic adjustment in maize (Zea mays L.): Changes with ontogeny and its relationship with phenotypic stability. J. Agron. Crop Sci. 1998, 180, 241–247. [Google Scholar] [CrossRef]
- Ashwini, S.; Chandrakala, N.; Ravikumar, R.L. Genetic variability for osmotic adjustment in pollen grains and its association with field tolerance to moisture stress in maize inbred lines. Curr. Sci. 2019, 116, 279–285. [Google Scholar] [CrossRef]
- Studer, C.; Hu, Y.; Schmidhalter, U. Evaluation of the differential osmotic adjustments between roots and leaves of maize seedlings with single or combined NPK-nutrient supply. Funct. Plant Biol. 2007, 34, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, C.A.M.; Bergonci, J.I.; Bergamaschi, H.; Dalmago, G.A. Osmotic adjustment in maize cultivated in different soil tillage systems and water availability. Pesqui. Agropecu. Bras. 2005, 40, 645–651. [Google Scholar] [CrossRef]
- Finkelstein, R. Abscisic Acid synthesis and response. Arab. Book 2013, 11, e0166. [Google Scholar] [CrossRef]
- Sirko, A.; Wawrzyńska, A.; Brzywczy, J.; Sieńko, M. Control of ABA Signaling and Crosstalk with Other Hormones by the Selective Degradation of Pathway Components. Int. J. Mol. Sci. 2021, 22, 4638. [Google Scholar] [CrossRef]
- Ma, Y.; Szostkiewicz, I.; Korte, A.; Moes, D.; Yang, Y.; Christmann, A.; Grill, E. Regulators of PP2C Phosphatase Activity Function as Abscisic Acid Sensors. Science 2009, 324, 1064–1068. [Google Scholar] [CrossRef]
- Park, S.Y.; Fung, P.; Nishimura, N.; Jensen, D.R.; Fujii, H.; Zhao, Y.; Lumba, S.; Santiago, J.; Rodrigues, A.; Chow, T.F.F.; et al. Abscisic Acid Inhibits Type 2C Protein Phosphatases via the PYR/PYL Family of START Proteins. Science 2009, 324, 1068–1071. [Google Scholar] [CrossRef]
- Bharath, P.; Gahir, S.; Raghavendra, A.S. Abscisic Acid-Induced Stomatal Closure: An Important Component of Plant Defense Against Abiotic and Biotic Stress. Front. Plant Sci. 2021, 12, 615114. [Google Scholar] [CrossRef]
- Sussmilch, F.C.; Schultz, J.; Hedrich, R.; Roelfsema, M.R.G. Acquiring Control: The Evolution of Stomatal Signalling Pathways. Trends Plant Sci. 2019, 24, 342–351. [Google Scholar] [CrossRef]
- Hsu, P.-K.; Takahashi, Y.; Munemasa, S.; Merilo, E.; Laanemets, K.; Waadt, R.; Pater, D.; Kollist, H.; Schroeder, J.I. Abscisic acid-independent stomatal CO2 signal transduction pathway and convergence of CO2 and ABA signaling downstream of OST1 kinase. Proc. Natl. Acad. Sci. USA 2018, 115, E9971–E9980. [Google Scholar] [CrossRef] [PubMed]
- Keller, B.U.; Hedrich, R.; Raschke, K. Voltage-dependent anion channels in the plasma membrane of guard cells. Nature 1989, 341, 450–453. [Google Scholar] [CrossRef]
- Schroeder, J.I.; Hagiwara, S. Cytosolic calcium regulates ion channels in the plasma membrane of Vicia faba guard cells. Nature 1989, 338, 427–430. [Google Scholar] [CrossRef]
- Jiang, Z.; Li, Y.P.; Gai, P.Z.; Gao, J.; Xu, L. Exogenously applied ABA alleviates dysplasia of maize (Zea mays L.) ear under drought stress by altering photosynthesis and sucrose transport. Plant Signal. Behav. 2025, 20, 2462497. [Google Scholar] [CrossRef]
- Li, D.; Wang, H.; Luo, F.; Li, M.; Wu, Z.; Liu, M.; Wang, Z.; Zang, Z.; Jiang, L. A Maize Calmodulin-like 3 Gene Positively Regulates Drought Tolerance in Maize and Arabidopsis. Int. J. Mol. Sci. 2025, 26, 1329. [Google Scholar] [CrossRef]
- Liu, L.; Gallagher, J.; Arevalo, E.D.; Chen, R.; Skopelitis, T.; Wu, Q.; Bartlett, M.; Jackson, D. Enhancing grain-yield-related traits by CRISPR-Cas9 promoter editing of maize CLE genes. Nat. Plants 2021, 7, 287–294. [Google Scholar] [CrossRef]
- Filyushin, M.A.; Kochieva, E.Z.; Shchennikova, A.V. ZmDREB2.9 Gene in Maize (Zea mays L.): Genome-Wide Identification, Characterization, Expression, and Stress Response. Plants 2022, 11, 3060. [Google Scholar] [CrossRef]
- Liu, S.; Wang, X.; Wang, H.; Xin, H.; Yang, X.; Yan, J.; Li, J.; Tran, L.-S.P.; Shinozaki, K.; Yamaguchi-Shinozaki, K.; et al. Genome-Wide Analysis of ZmDREB Genes and Their Association with Natural Variation in Drought Tolerance at Seedling Stage of Zea mays L. PLoS Genet. 2013, 9, e1003790. [Google Scholar] [CrossRef]
- Qin, F.; Kakimoto, M.; Sakuma, Y.; Maruyama, K.; Osakabe, Y.; Tran, L.S.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Regulation and functional analysis of ZmDREB2A in response to drought and heat stresses in Zea mays L. Plant J. 2007, 50, 54–69. [Google Scholar] [CrossRef]
- Sun, Y.; Mu, C.; Zheng, H.; Lu, S.; Zhang, H.; Zhang, X.; Liu, X. Exogenous Pi supplementation improved the salt tolerance of maize (Zea mays L.) by promoting Na+ exclusion. Sci. Rep. 2018, 8, 16203. [Google Scholar] [CrossRef]
- Zhao, L.; Yan, J.; Xiang, Y.; Sun, Y.; Zhang, A. ZmWRKY104 Transcription Factor Phosphorylated by ZmMPK6 Functioning in ABA-Induced Antioxidant Defense and Enhance Drought Tolerance in Maize. Biology 2021, 10, 893. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Yan, X.; Wang, N.; Zenda, T.; Dong, A.; Zhai, X.; Yang, Q.; Duan, H. ZmHB53, a Maize Homeodomain-Leucine Zipper I Transcription Factor Family Gene, Contributes to Abscisic Acid Sensitivity and Confers Seedling Drought Tolerance by Promoting the Activity of ZmPYL4. Plant Cell Environ. 2025, 48, 3829–3843. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.-Z.; Wang, H.-F.; Tian, Y.; Hao, J.; Guo, H.-L.; Chen, L.-M.; Wei, Y.-K.; Zhan, S.-H.; Yu, H.-T.; Chen, Y.-F. ZmPHR1 contributes to drought resistance by modulating phosphate homeostasis in maize. Plant Biotechnol. J. 2024, 22, 3085–3098. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Ni, Y.; Xie, K.; Li, Y.; Wu, W.; Shan, H.; Cheng, B.; Li, X. Aquaporin ZmTIP2;3 Promotes Drought Resistance of Maize through Symbiosis with Arbuscular Mycorrhizal Fungi. Int. J. Mol. Sci. 2024, 25, 4205. [Google Scholar] [CrossRef]
- Feng, T.; Wang, Y.; Zhang, M.; Zhuang, J.; Zhou, Y.; Duan, L. ZmSCE1a positively regulates drought tolerance by enhancing the stability of ZmGCN5. Plant J. 2024, 120, 2101–2112. [Google Scholar] [CrossRef]
- Mao, H.; Yu, L.; Han, R.; Li, Z.; Liu, H. ZmNAC55, a maize stress-responsive NAC transcription factor, confers drought resistance in transgenic Arabidopsis. Plant Physiol. Biochem. 2016, 105, 55–66. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, C.; Zhu, T.; He, J.; Wang, Y.; Yang, S.; Liu, Y.; Zhao, B.; Zhu, C.; Ye, S.; et al. An LRR-RLK protein modulates drought- and salt-stress responses in maize. J. Genet. Genom. 2025, 52, 388–399. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Z.; Zhang, C.; Guo, J.; Liu, Q.; Yin, Y.; Hu, Y.; Xia, H.; Li, B.; Sun, X.; et al. Gene editing of ZmGA20ox3 improves plant architecture and drought tolerance in maize. Plant Cell Rep. 2024, 43, 18. [Google Scholar] [CrossRef]
- Lan, Q.; He, G.; Wang, D.; Li, S.; Jiang, Y.; Guan, H.; Li, Y.; Liu, X.; Wang, T.; Li, Y.; et al. Overexpression of ZmEULD1b enhances maize seminal root elongation and drought tolerance. Plant Sci. 2025, 352, 112355. [Google Scholar] [CrossRef]
- Ren, Z.; Zhang, P.; Su, H.; Xie, X.; Shao, J.; Ku, L.; Tian, Z.; Deng, D.; Wei, L. Regulatory mechanisms used by ZmMYB39 to enhance drought tolerance in maize (Zea mays) seedlings. Plant Physiol. Biochem. 2024, 211, 108696. [Google Scholar] [CrossRef]
- Yu, T.; Dong, W.; Hou, X.; Sun, A.; Li, X.; Yu, S.; Zhang, J. The Maize Gene ZmGLYI-8 Confers Salt and Drought Tolerance in Transgenic Arabidopsis Plants. Int. J. Mol. Sci. 2024, 25, 10937. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Zhang, F.; Mu, C.; Ma, C.; Yao, G.; Sun, Y.; Hou, J.; Leng, B.; Liu, X. The ZmbHLH47-ZmSnRK2.9 Module Promotes Drought Tolerance in Maize. Int. J. Mol. Sci. 2024, 25, 4957. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; Wang, Y.; Qu, J.; Miao, M.; Zhao, Y.; Liu, S.; Guan, S.; Ma, Y. The overexpression of ascorbate peroxidase 2 (APX2) gene improves drought tolerance in maize. Mol. Breed. 2025, 45, 27. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Liu, W.; Niu, Y.; Li, Q.; Zhao, C.; Pan, Y.; Li, G.; Bian, X.; Miao, Y.; Zhang, A. The maize GSK3-like kinase ZmSK1 negatively regulates drought tolerance by phosphorylating the transcription factor ZmCPP2. Plant Cell 2025, 37, koaf032. [Google Scholar] [CrossRef]
- Chai, W.; Li, H.; Xu, H.; Zhu, Q.; Li, S.; Yuan, C.; Ji, W.; Wang, J.; Sheng, L. ZmDST44 Gene is a Positive Regulator in Plant Drought Stress Tolerance. Biology 2024, 13, 552. [Google Scholar] [CrossRef]
- Wang, C.; Zhou, Y.; Wang, Y.; Jiao, P.; Liu, S.; Guan, S.; Ma, Y. CRISPR-Cas9-mediated editing of ZmPL1 gene improves tolerance to drought stress in maize. GM Crops Food 2025, 16, 1–16. [Google Scholar] [CrossRef]
- Liu, H.; Wu, Z.; Bao, M.; Gao, F.; Yang, W.; Abou-Elwafa, S.F.; Liu, Z.; Ren, Z.; Zhu, Y.; Ku, L.; et al. ZmC2H2-149 negatively regulates drought tolerance by repressing ZmHSD1 in maize. Plant Cell Environ. 2024, 47, 885–899. [Google Scholar] [CrossRef]
- Zhai, X.; Yan, X.; Zenda, T.; Wang, N.; Dong, A.; Yang, Q.; Zhong, Y.; Xing, Y.; Duan, H. Overexpression of the peroxidase gene ZmPRX1 increases maize seedling drought tolerance by promoting root development and lignification. Crop J. 2024, 12, 753–765. [Google Scholar] [CrossRef]
- Xiao, N.; Ma, H.; Wang, W.; Sun, Z.; Li, P.; Xia, T. Overexpression of ZmSUS1 increased drought resistance of maize (Zea mays L.) by regulating sucrose metabolism and soluble sugar content. Planta 2024, 259, 43. [Google Scholar] [CrossRef]
- Wang, D.; Liu, X.; He, G.; Wang, K.; Li, Y.; Guan, H.; Wang, T.; Zhang, D.; Li, C.; Li, Y. GWAS and transcriptome analyses unravel ZmGRAS15 regulates drought tolerance and root elongation in maize. BMC Genom. 2025, 26, 246. [Google Scholar] [CrossRef]
- Che, R.; Tan, X.; Meng, X.; Li, H. ZmCYB5-1, a cytochrome b5 Gene, negatively regulates drought stress tolerance in maize. Gene 2025, 954, 149422. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Tang, C.; Zhang, Y.; Sha, X.; Tian, S.; Luo, Z.; Wei, G.; Zhu, L.; Li, Y.; Fu, J.; et al. The SnRK2.2-ZmHsf28-JAZ14/17 module regulates drought tolerance in maize. New Phytol. 2025, 245, 1985–2003. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Wang, C.; Wang, Y.; Zhao, Y.; Ma, Y.; Liu, S.; Guan, S.; Jiao, P. miR166e/ZmATHB14 module contributes to drought tolerance in maize root. Int. J. Biol. Macromol. 2025, 297, 139707. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Chen, Y.; Yang, R.; Luo, P.; Wang, H.; Zhang, R.; Li, W.; Yang, K.; Xu, X.; Hao, Z.; et al. Identification of ZmSNAC06, a Maize NAC Family Transcription Factor with Multiple Transcripts Conferring Drought Tolerance in Arabidopsis. Plants 2025, 14, 12. [Google Scholar] [CrossRef]
- Waqas, M.A.; Wang, X.; Zafar, S.A.; Noor, M.A.; Hussain, H.A.; Azher Nawaz, M.; Farooq, M. Thermal Stresses in Maize: Effects and Management Strategies. Plants 2021, 10, 293. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, X.; Lin, Z.; Wang, J.; Liu, H.; Zhou, L.; Zhong, S.; Li, Y.; Zhu, C.; Lai, J.; et al. A Large Transposon Insertion in the stiff1 Promoter Increases Stalk Strength in Maize. Plant Cell 2020, 32, 152–165. [Google Scholar] [CrossRef]
- Li, Y.; Huang, Y.; Sun, H.; Wang, T.; Ru, W.; Pan, L.; Zhao, X.; Dong, Z.; Huang, W.; Jin, W. Heat shock protein 101 contributes to the thermotolerance of male meiosis in maize. Plant Cell 2022, 34, 3702–3717. [Google Scholar] [CrossRef]
- Shao, R.X.; Yu, K.K.; Li, H.W.; Jia, S.J.; Yang, Q.H.; Zhao, X.; Zhao, Y.L.; Liu, T.X. The effect of elevating temperature on the growth and development of reproductive organs and yield of summer maize. J. Integr. Agric. 2021, 20, 1783–1795. [Google Scholar] [CrossRef]
- Xue, M.; You, Y.; Zhang, L.; Cao, J.; Xu, M.; Chen, S. ZmHsp18 screened from the ZmHsp20 gene family confers thermotolerance in maize. BMC Plant Biol. 2024, 24, 1048. [Google Scholar] [CrossRef]
- Cao, L.; Fahim, A.M.; Liang, X.; Fan, S.; Song, Y.; Liu, H.; Ye, F.; Ma, C.; Zhang, D.; Lu, X. Melatonin Enhances Heat Tolerance via Increasing Antioxidant Enzyme Activities and Osmotic Regulatory Substances by Upregulating zmeno1 Expression in Maize (Zea mays L.). Antioxidants 2024, 13, 1144. [Google Scholar] [CrossRef]
- Li, G.; Chen, Z.; Guo, X.; Tian, D.; Li, C.; Lin, M.; Hu, C.; Yan, J. Genome-Wide Identification and Analysis of Maize DnaJ Family Genes in Response to Salt, Heat, and Cold at the Seedling Stage. Plants 2024, 13, 2488. [Google Scholar] [CrossRef] [PubMed]
- Dong, A.; Wang, N.; Zenda, T.; Zhai, X.; Zhong, Y.; Yang, Q.; Xing, Y.; Duan, H.; Yan, X. ZmDnaJ-ZmNCED6 module positively regulates drought tolerance via modulating stomatal closure in maize. Plant Physiol. Biochem. 2025, 218, 109286. [Google Scholar] [CrossRef] [PubMed]
- Duan, P.; Kan, Y.; Zhao, D.; Yang, S.; Chen, J. Genome-Wide Identification and Characterization of CHROMO Domain Family Genes Reveal Roles of the Maize Genes in Heat Stress Response. Biol. Bull. 2023, 50 (Suppl. S3), S289–S297. [Google Scholar] [CrossRef]
- Cao, L.; Wang, G.; Fahim, A.M.; Pang, Y.; Zhang, Q.; Zhang, X.; Wang, Z.; Lu, X. Comprehensive Analysis of the DnaJ/HSP40 Gene Family in Maize (Zea mays L.) Reveals that ZmDnaJ96 Enhances Abiotic Stress Tolerance. J. Plant Growth Regul. 2024, 43, 1548–1569. [Google Scholar] [CrossRef]
- Hunter, C.T. CRISPR/Cas9 Targeted Mutagenesis for Functional Genetics in Maize. Plants 2021, 10, 723. [Google Scholar] [CrossRef]
- Hassan, F.U.; Nawaz, A.; Rehman, M.S.; Ali, M.A.; Dilshad, S.M.R.; Yang, C. Prospects of HSP70 as a genetic marker for thermo-tolerance and immuno-modulation in animals under climate change scenario. Anim. Nutr. 2019, 5, 340–350. [Google Scholar] [CrossRef]
- Jiang, L.; Hu, W.; Qian, Y.; Ren, Q.; Zhang, J. Genome-wide identification, classification and expression analysis of the Hsf and Hsp70 gene families in maize. Gene 2021, 770, 145348. [Google Scholar] [CrossRef]
- Jiang, Y.; An, X.; Li, Z.; Yan, T.; Zhu, T.; Xie, K.; Liu, S.; Hou, Q.; Zhao, L.; Wu, S.; et al. CRISPR/Cas9-based discovery of maize transcription factors regulating male sterility and their functional conservation in plants. Plant Biotechnol. J. 2021, 19, 1769–1784. [Google Scholar] [CrossRef]
- Rai, M.N.; Rhodes, B.; Jinga, S.; Kanchupati, P.; Ross, E.; Carlson, S.R.; Moose, S.P. Efficient mutagenesis and genotyping of maize inbreds using biolistics, multiplex CRISPR/Cas9 editing, and Indel-Selective PCR. Plant Methods 2025, 21, 43. [Google Scholar] [CrossRef]
- Liu, X.; Zhai, S.; Zhao, Y.; Sun, B.; Liu, C.; Yang, A.; Zhang, J. Overexpression of the phosphatidylinositol synthase gene (ZmPIS) conferring drought stress tolerance by altering membrane lipid composition and increasing ABA synthesis in maize. Plant Cell Environ. 2013, 36, 1037–1055. [Google Scholar] [CrossRef]
- Moustafa, K. CRISPR: Beyond the Excitement. J. Bioethical Inq. 2024, 21, 7–9. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.; Chen, Z.; Huang, J.; Ye, H.; Lu, T.; Lu, M.; Rao, Y. Application of CRISPR-Cas9 gene editing technology in crop breeding. Sheng Wu Gong Cheng Xue Bao = Chin. J. Biotechnol. 2023, 39, 399–424. [Google Scholar]
- Ebrahimi, V.; Hashemi, A. CRISPR-based gene editing in plants: Focus on reagents and their delivery tools. Bioimpacts 2025, 15, 30019. [Google Scholar] [CrossRef] [PubMed]
- Molla, K.A.; Sretenovic, S.; Bansal, K.C.; Qi, Y. Precise plant genome editing using base editors and prime editors. Nat. Plants 2021, 7, 1166–1187. [Google Scholar] [CrossRef]
- Zong, Y.; Song, Q.; Li, C.; Jin, S.; Zhang, D.; Wang, Y.; Qiu, J.-L.; Gao, C. Efficient C-to-T base editing in plants using a fusion of nCas9 and human APOBEC3A. Nat. Biotechnol. 2018, 36, 950–953. [Google Scholar] [CrossRef]
| Gene | Details | Mechanisms/Method | References |
|---|---|---|---|
| ZmHsp18 | ZmHsp20 gene family | Gene family-based perfection | [111] |
| ZmENO1 | Enolase (ENO, 2-phospho-D-glycerate hydrolyase) | Antioxidant enzyme activities and osmotic regulation | [112] |
| ZmDnaJ genes | HSP40s | Correlation between heat stress tolerance and the regulation of genes | [113] |
| ZmDnaJ-ZmNCED6 | Heat shock protein | Involved in ABA signal transduction pathways | [114] |
| cpSRP43 | CMT2 and cpSRP43 | CHROMO domain family genes | [115] |
| ZmDnaJ96 | DnaJ/HSP40 gene family | Increased antioxidant enzyme activity | [116] |
| ZmATL10 and AtATL27 | ATL family | [49] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, H.; Zhang, L.; Xie, X.; Wang, Y.; Wang, T.; Liu, C. Resilience of Maize to Environmental Stress: Insights into Drought and Heat Tolerance. Int. J. Mol. Sci. 2025, 26, 5274. https://doi.org/10.3390/ijms26115274
Tang H, Zhang L, Xie X, Wang Y, Wang T, Liu C. Resilience of Maize to Environmental Stress: Insights into Drought and Heat Tolerance. International Journal of Molecular Sciences. 2025; 26(11):5274. https://doi.org/10.3390/ijms26115274
Chicago/Turabian StyleTang, Huaijun, Lei Zhang, Xiaoqing Xie, Yejian Wang, Tianyu Wang, and Cheng Liu. 2025. "Resilience of Maize to Environmental Stress: Insights into Drought and Heat Tolerance" International Journal of Molecular Sciences 26, no. 11: 5274. https://doi.org/10.3390/ijms26115274
APA StyleTang, H., Zhang, L., Xie, X., Wang, Y., Wang, T., & Liu, C. (2025). Resilience of Maize to Environmental Stress: Insights into Drought and Heat Tolerance. International Journal of Molecular Sciences, 26(11), 5274. https://doi.org/10.3390/ijms26115274






