Abstract
T lymphocytes infiltrate the CNS in response to murine cytomegalovirus (MCMV) infection and form a pool of long-lived brain tissue-resident memory T-cells (bTRMs), which display markers of residency (i.e., CD103, CD69, CD49a). However, the functional role of these bTRMs is still unknown. By 30 days postinfection, a latent viral brain infection was established, as indicated by absence of viral transcripts (IE1, E1, and gB) produced during productive infection. Following intracerebroventricular injection of either depleting α-CD8 Ab (clone YTS169.4) or α-CD103-sap (clone IT50) into the brain, 90–95% T-cell depletion was achieved. Using luciferase-expressing mice, we observed recommenced imaging signals indicative of de novo MCMV IE promoter activity in depleted animals. Surprisingly, using an explant assay, we efficiently recovered reactivatable, infectious virus from untreated, latent animals, but not from those depleted of bTRMs (viral recovery in explants was reduced from 100% to 50% by day 21). We identified Lgals3 (galectin 3), Gpnmb (glycoprotein nonmetastatic melanoma protein B) and Hmox1 (heme oxygenase 1) as genes that were most upregulated in bTRM-depleted groups. When bTRMs were depleted, there was transient expression of viral IE genes which resulted in antiviral microglia with a phagocytic, disease-associated (DAM) or neurodegenerative (MGnD) phenotype. These data provide new insights into the role of bTRMs in controlling both CNS reactivation and driving microglial phenotypes.
1. Introduction
Previous work from our laboratory has demonstrated that neural stem cells (NSCs) in the sub-ventricular zone (SVZ) and hippocampus are primary targets for murine cytomegalovirus (MCMV, a β-herpesvirus) during acute viral brain infection [1,2,3]. However, following the acute phase, a subset of these infected NSCs survive and subsequently develop into mature neurons which harbor latent MCMV genomes. Subsequent studies using explant cultures of brain tissue have shown that reactivated virus can then be grown out of this neuronal reservoir months after primary infection [4]. The resistance of neurons to cytotoxic T-cell (CTL) activity makes them a hospitable host cell for harboring long-term, latent infections. But without reactivation, latent infections likely would not cause problems. However, repeated cycles of reactivation from latent central nervous system (CNS) reservoirs, along with corresponding de novo viral protein synthesis, is a likely source of foreign antigen within the brain, which may drive chronic neuroinflammation.
Infections of the brain elicit neuroimmune responses that are necessary to limit viral spread, and data from our laboratory have shown that CD8+ T-cells infiltrate the brain in response to primary viral infection and, subsequently, form a pool of long-lived, brain-resident memory T-cells (bTRMs) which display markers of tissue residency (e.g., CD103, CD69, CD49a) [5,6]. Although the functional role of these brain-resident T-cells is unknown, an appreciation of the deleterious consequences of viral reactivation-driven inflammation on chronic neurodegenerative disease is beginning to emerge.
Because bona fide bTRMs do not express tissue residency markers within the CNS until after acute, productive infection has been resolved i.e., 14–21 days post infection (d p.i.), these findings suggest that bTRMs function during the later stages of brain infection [6]. A previous study by Brizic et al., showed that adoptive transfer of virus-specific T-cells protected newborn mice against MCMV infection, and that depletion of bTRMs resulted in virus reactivation and enhanced neuroinflammation [7]. So, the current study was undertaken to further understand the role of bTRMs in controlling reactivation of MCMV in our brain infection model. To this end, we employed FVB transgenic mice which express luciferase under the RosA26 promoter as well as explant assay, the gold standard to assess production of infectious, reactivated virus.
Microglial cell phenotypes are constantly being shaped by their particular brain microenvironment, such as those induced by bTRMs or viral infection. Although over a dozen alternative microglial activation phenotypes have been described, a prototypic microglial alternate activation phenotypic signature (i.e., disease-associated microglia [DAM]) has been identified in a variety of common neurodegenerative diseases [8]. The DAM signature is elicited by the triggering receptor on the myeloid cells 2 (TREM2) pathway [9], which activates neurodegenerative markers (e.g., GPNMB, LGALS3) and also promotes phagocytosis [10], while at the same time suppressing markers of the homeostatic phenotype (e.g., the purinergic receptor P2RY12). In response to specific brain microenvironments, microglia first mature into stage 1 DAM and upregulate TREM2. Subsequent full differentiation into stage 2 DAM is TREM2-dependent. These DAM cells are generally believed to be protective because they are highly phagocytic. So, we went on to investigate glial cell gene expression during bTRM depletion and their involvement in inducing phagocytic, antiviral microglia.
2. Results
2.1. Viral Expression and Establishment of Latency Following MCMV Infection
BALB/c mice were infected with MCMV via the intracerebroventricular (icv) route and analyzed for the presence of viral promoter activity, DNA, or RNA at 7 d and 30 d post infection (p.i.). We first detected X-gal staining indicative of β-galactosidase expressing virus in the infected brain at 5 d p.i. (Figure 1A). This observation was confirmed using RNAscope at 5 d p.i., where we detected RNA specific for MCMV IE-1 (Figure 1B). Using similar techniques, we also observed MCMV nucleic acid at 30 d p.i. (Figure 1C). Furthermore, we carried out qRT-PCR experiments using brain tissue harvested at both 7 d (acute phase) and 30 d p.i. (latent phase). Expression of all classes of viral genes (IE-1, E-1, and gB) was observed at 7 d, but not at 30 d p.i. (Figure 1D).
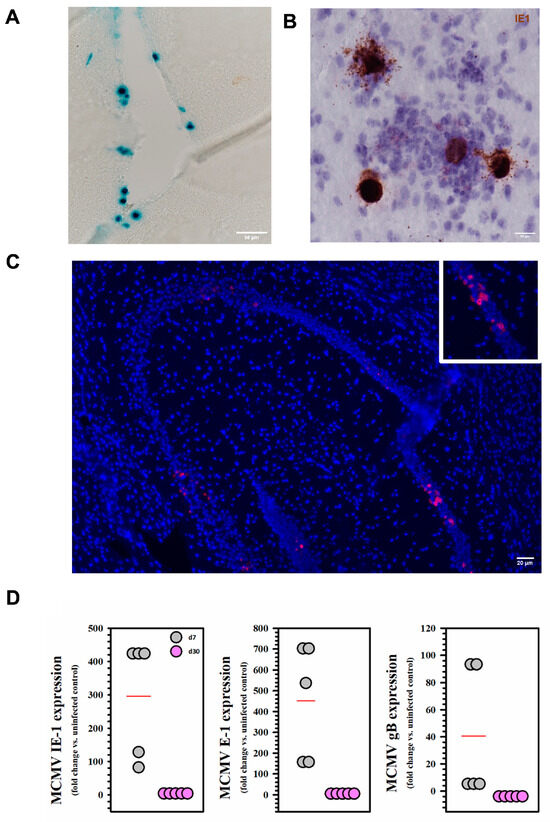
Figure 1.
Viral expression and establishment of latency following MCMV infection. Mice were infected intracerebroventricularly with 1 × 105 TCID50 units (in 10 μL) of MCMV. At 5 d p.i., brain tissues from infected animals were harvested. (A) X-gal staining of β-gal-expressing virus (MCMV RM461) in the infected brain. (B) Staining for IE1 viral transcripts using RNAscope (brown). (C) Positive RNAscope staining for viral nucleic acid at D30. (D) Expression of IE1, E1, and gB transcripts was assessed during both acute (d7) and latent infection (d30) using real-time RT-PCR. Each dot represents RNA extracted from one animal (5 mice/timepoint).
2.2. Co-Localization of CD8+ T-Cells and MCMV E1-Retaining Cells
We went on to assess the localization of CD8+ T-cells near latently infected cells. Previous studies using neonatal mice reported that the MCMV early gene E1 (m112–113) product has a tendency to be retained in at least some neurons following MCMV-infection [11], and its presence can be detected for a prolonged time [12]. We previously obtained this monoclonal α-E1 antibody (Ab), (kindly provided by Dr. I. Kosugi, Hamamatsu University, Japan), and used it to successfully stain MCMV infected cells at extended times (i.e., 30 d) following resolution of acute infection [4]. Here, this Ab was used to co-localize CD8+ bTRMs with MCMV E1-retaining cells (Figure 2). These findings suggest that bTRM neuronal interactions may affect viral reactivation and recurrence.
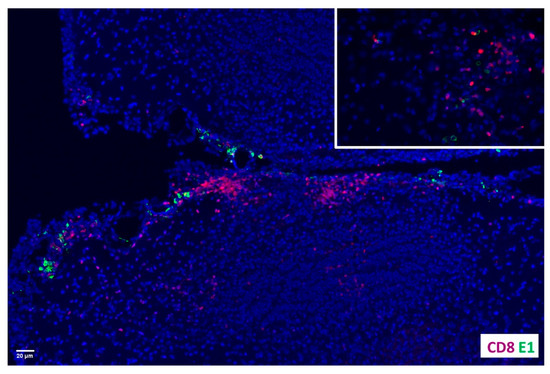
Figure 2.
Co-localization of CD8+ T-cells and MCMV E1-retaining cells. Brain sections of mice infected with MCMV for 30 d were double-stained for MCMV E1 and CD8 and viewed using fluorescent microscopy. Red indicates α-CD8 staining while green displays α-E1.
2.3. Depletion of CD8+ and CD8+CD103+ T-Cells from the Brains of MCMV-Infected Animals
To assess the role of bTRMs (CD8+CD103+) in controlling latent viral reactivation, we went on to deplete these cells from the brains of latently infected mice. Mice latently infected with MCMV were injected with either α-CD8 depleting antibody or α-CD103-sap. At 3 d post-injection, mice were euthanized and brain tissues were harvested to isolate brain mononuclear cells (BMNCs) which were then analyzed for the presence of CD8 + T-cells or CD8+CD103+ T-cells by flow cytometry. We observed only 5.1% of cells to be CD8+ following depletion, as compared to 71.0% in the non-depleted group (Figure 3A,B). Similarly, we observed 10.2% of CD8+CD103+ T-cells in animals injected with α-CD103-sap as compared to the control (non-depleted and IgG-sap groups) animals (Figure 3C,D).
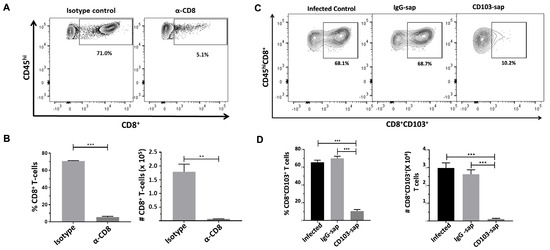
Figure 3.
Depletion of CD8+ and CD8+CD103+ T-cells from the brains of MCMV-infected animals. Mice were infected with 1 × 105 TCID50 units (in 10 μL) via icv injection. At 30 d p.i., mice were injected with either α-CD8 depleting antibody [YTS-169.4 (65 µg)] or its isotype control [LTF-2 (65 µg)], α-CD103-sap [2 µg, IT-50], or its isotype control, IgG-sap [2 µg, IT-17]. At 3 d post-injection, mice were euthanized and brain tissues were harvested to isolate brain mononuclear cells (BMNCs), which were labeled with Abs specific for anti-CD45-PE-Cy7, anti-CD8-BV510, and anti-CD103-FITC for analysis by flow cytometry. (A) Representative contour plots show the percentages of CD8+ T lymphocytes within depleted (α-CD8 treated) and non-depleted groups (isotype control). (B) Frequency and the number of CD8+ T-cells belonging to the indicated groups. (C) Representative contour plots show the percentages of CD8+CD103+ T lymphocytes within infected control, IgG-sap treated, and α-CD103-sap treated animals. (D) Frequency and number of CD8+CD103+ T-cells belonging to the indicated group. Pooled data present absolute numbers (mean ± SE) of CD8+ and CD8+CD103+ T-cells from two independent experiments using 4–6 animals per group. ** p < 0.01, *** p < 0.001.
2.4. Establishment of Latent cre-MCMV Infection in FVB Transgenic Mice Using Bioluminescent Imaging
To longitudinally examine CNS viral reactivation, we established a latent MCMV infection in FVB transgenic mice containing a luciferase reporter gene but flanked by stop signals (Figure 4A). For these experiments, we used a Cre recombinase-expressing recombinant MCMV virus, where cre is inserted into the MCMV IE2 locus to infect the FVB transgenic mice (Figure 4A). Mice infected with cre-MCMV exhibited a strong signal both quantitatively and qualitatively on imaging, as observed in Figure 4B,C, while mice infected with MCMV RM461 failed to exhibit any signal. Further, we expanded the experiment using 10 mice and found infection at day 5 p.i. in all the mice as observed by a strong signal. Importantly, upon imaging these mice at day 30 p.i., we did not observe any signal (Figure 4D); which indicated the establishment of latent infection.
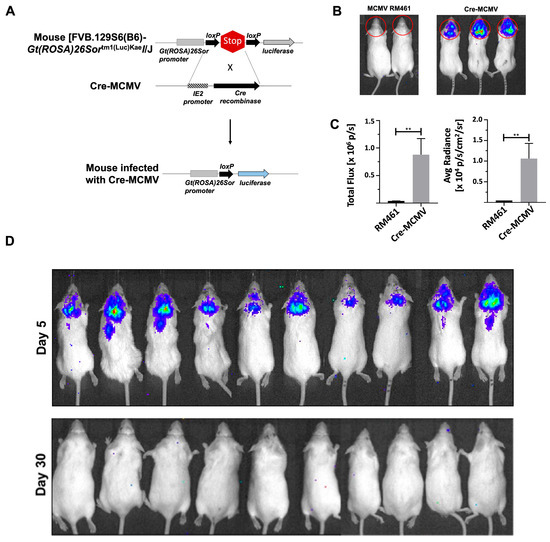
Figure 4.
Bioluminescent imaging of cre-MCMV-infected FVB transgenic mice and establishment of latent cre-MCMV infection. (A) Schematic outline. FVB transgenic mice that express luciferase under the ROSA26 promoter, but have a stop signal flanked by LoxP sites, were infected with cre-MCMV, a virus which expresses Cre recombinase under control of the IE2 promoter (i.e., during productive infection). (B) Infected animals were imaged at day 5 p.i. using an IVIS 100 after injecting 150 µg of D-luciferin (i.p.). Animals were imaged 5 min after D-luciferin administration and data were acquired using a 5-min exposure window. Bioluminescence imaging was conducted using cre-MCMV-infected as well as β-gal expressing MCMV-infected FVB mice. Red circle is the area from which the signal is quantified. (C) Data were analyzed using Living Image software. (https://www.revvity.com/category/in-vivo-imaging-software, (accessed on 25 May 2025) Revvity, San Diego, CA, USA). (D) Luciferase-expressing FVB transgenic mice were infected with cre-MCMV and imaged at days 5 and 30 p.i. The figure shows pooled data from two independent experiments using five animals each. ** p < 0.01.
2.5. Role of CD8 or CD103 in Controlling Viral Reactivation Within the Brain
To further assess the role of CD8+ and CD103+ T-cells in controlling viral reactivation, we then established latency in FVB transgenic mice (infected with cre-MCMV), as demonstrated in Figure 4. These latently infected mice were then depleted of CD8 and CD103 by injecting α-CD8 and α-CD103-sap and monitored longitudinally for luciferase expression by bioluminescent imaging. We observed luciferase expression in the latently infected transgenic mice following 10 d of CD8+ T-cells, as well as following CD103+ T-cell depletion (Figure 5A,B).
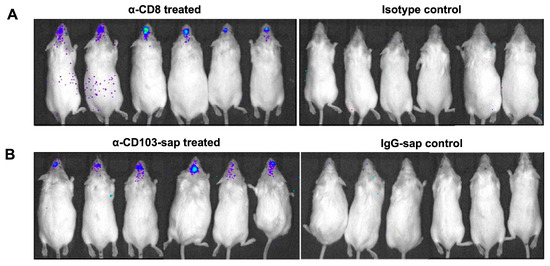
Figure 5.
Role of CD8 or CD103 in controlling viral recrudescence within the brain. (A) Latently-infected FVB transgenic mice were injected with either α-CD8 or its isotype control 30 d following infection with cre-MCMV. Animals were subsequently imaged longitudinally at various times post-antibody injection; day 10 post-injection is shown. (B) After 30 d of cre-MCMV infection, FVB mice were injected with either α-CD103-sap or IgG-sap control. Animals were imaged at day 10 post-T-cell depletion. Pooled data from two independent experiments using three animals each are shown.
2.6. Recovery of Reactivated Infectious Virus from the Brains of Latently-Infected Animals Depleted of bTRM
To further analyze the role of bTRM in controlling viral recrudescence, we employed a classic explant assay. For this, we infected BALB/c animals and allowed the virus to go latent. A group of these animals was then treated with α-CD8 alone or injected with α-CD8 intraperitoneally followed by icv injection of either IgG-sap or CD103-sap. Three days post-depletion, the brains were collected and chopped into fine pieces. Some pieces of brain were used to study the expression of viral genes using qRT-PCR while the rest were co-cultured with mixed glial cells and analyzed by X-gal staining at day 7, 14, and 21 post-explant. In these experiments, we observed significant MCMV IE expression in animals depleted of bTRMs (Figure 6A). However, we did not observe reactivated virus by explant culture at 7 d post-bTRM depletion (0% as compared to 20–30% in control groups). Similar results were obtained at later time points. Surprisingly, although a higher level of IE gene expression was observed in the bTRM-depleted animals (Figure 6A), the recovery of reactivated, infectious virus was paradoxically less in animals following bTRM depletion at 14 d (i.e., in 20% of animals as compared to 60–100% in the three control groups), (Figure 6B,C). At 21 d post-explant, viral reactivation was observed in 50% of bTRM-depleted animals compared to 100% of the animals in each of the control groups.
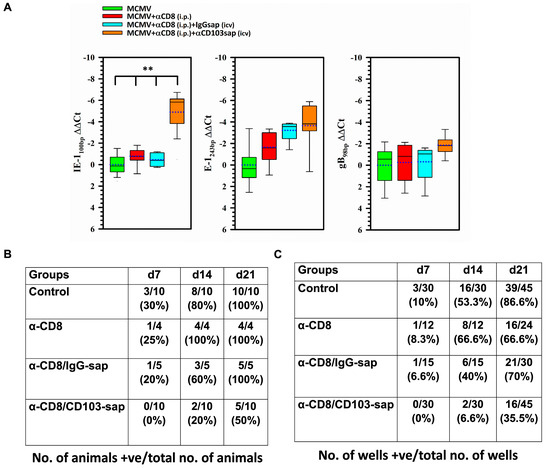
Figure 6.
Viral gene expression and recovery of reactivated, infectious virus from the brains of latently-infected animals following bTRM depletion. Periventricular brain tissue obtained from latently-infected animals (30 d p.i.) was cut into 1 mm pieces and placed onto primary murine glial cell cultures. (A) At 3 d post-explant, these cultures were processed for real-time PCR for MCMV IE1, E1, and gB transcripts. (B,C) At 7, 14, and 21 d post-explant, these cultures were monitored for reactivated virus by cytopathic effect and β-gal expression. ** p < 0.01.
2.7. Profiling Brain Microenvironments
To investigate the effects of bTRM depletion on brain-resident cells, we carried out nCounter Glial Cell Profiling using Nanostring technology, and the resulting data were analyzed using ROSALIND. We compared RNA extracted from the brains of latently infected animals to those of latently infected animals following in vivo depletion of CD103+ bTRMs. Taken together, we observed a total of 84 genes that were differentially expressed between the depleted and undepleted groups, 30 of which belong to the DAM, or neurodegenerative microglia (MGnD), core theme annotation, including Lgals3, Gpnmb, Csf-1 (colony stimulating factor 1), and Trem2 (triggering receptor on myeloid cells 2) in the bTRM-depleted groups (Figure 7A,B).
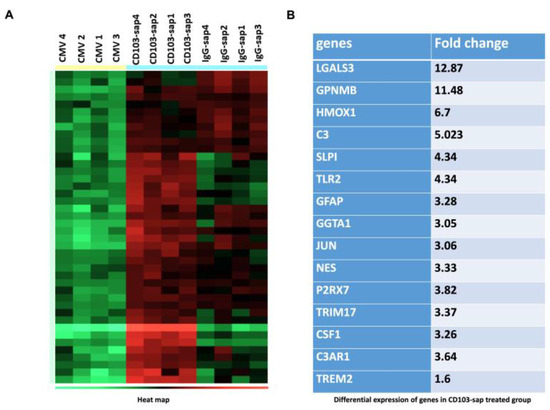
Figure 7.
Profiling brain microenvironments. RNA was extracted using Qiagen RNeasy Lipid Tissue extraction kit from the brains of mice latently infected (>30 d p.i.) with MCMV either treated with CD103-depleting Ab (CD103-sap) or IgG control (IgG-sap) or left untreated (CMV) 3 d post-depletion. RNA was analyzed using the NanoString nCounter Glial Cell panel and Rosalind Bioinformatics Online Platform Software (https://www.rosalind.bio/). (A) Heat map depicting glial cell profiling using the nCounter panel. (B) List of the most differentially expressed genes in CD103-depleted group.
3. Discussion
We first demonstrate that acute MCMV brain infection is followed by establishment of latency within the CNS. Herpesvirus gene expression can be classified as immediate early (alpha), early (beta), or late (gamma) according to their temporal order of expression. Upon initial infection, we saw expression of all classes of viral genes (i.e., IE1, E1, and gB) at 7 d (acute phase), but not 30 d p.i. When RNA extracts of brain homogenates were examined at this later time point, it was apparent that there was a true latent infection within the brain, as opposed to a chronic productive infection, because transcription of none of these gene classes was detected. Despite the absence of detectable viral IE1, E1, or gB transcripts using real-time RT-PCR, the latent viral nucleic acid was still detectable within neurons, as indicated by positive RNAscope staining. Using this same animal model, we have previously shown the presence of CD8+ T-cells within the brain, and further determined that they form a pool of brain-resident memory T-cells which persist for a lifetime [5,6].
Previous studies, using neonatal mice, reported that the MCMV early gene E1 (m112–113) product has a tendency to be retained in at least some neurons following MCMV-infection [11], and its presence can be detected for a prolonged time [12]. We previously obtained this monoclonal α-E1 Ab (kindly provided by Dr. I. Kosugi, Hamamatsu University, Japan), and used it to successfully stain MCMV infected cells at extended times following resolution of acute infection [4]. In this study, we show that CD8+ T-cells residing within the brain at latent time points co-exist with MCMV E1-retaining cells using fluorescent microscopy. These findings suggest that bTRM—neuronal interactions may affect viral control. To test this hypothesis, we went on to deplete CD8+ and CD103+ T-cells in the brain through the injection of depleting anti-CD8 and anti-CD103-saporin Abs, respectively. Depletion was achieved via ICV injection of either α-CD8 Ab (clone YTS169.4) or α-CD103-sap (clone IT50), which is an Ab linked to the saporin toxin (Advanced Targeting Systems, Carlsbad, CA, USA), into the latently-infected brain. Both of these methods achieved 90–95% T-cell depletion. This depletion was then used to analyze the role of these cells in viral reactivation, through imaging experiments and explant assays.
To longitudinally examine real-time viral reactivation in the brains of infected animals, groups of LucRep reporter gene-containing transgenic mice were infected with the cre-MCMV virus (Figure 4). A similar approach has previously been shown to be useful in studies of herpes simplex virus-1 [13] and murine gammaherpesvirus 68 (MHV-68), [14]. Using the Cre recombinase expressing virus in imaging experiments, we successfully demonstrated acute infection in transgenic FVB mice where the STOP signal was excised by virus-expressed Cre recombinase. Importantly, the infection was found to go latent as indicated by the absence of imaging signal at day 30. These data indicate that luciferase-expressing cells during acute infection were cleared following acute infection.
Latent Cre-expressing MCMV was found to be reactivated following bTRM depletion. When the signal from the acute phase was no longer detectable via bioluminescent imaging, we longitudinally imaged the animals every other day for two weeks to assess viral reactivation and determine optimal time points for further studies. It should be noted that the imaging signal observed could be due to the presence of only IE promoter activity without further viral gene expression (i.e., indicative of an abortive full productive infection). Evidence exists to support the idea that limited expression of MCMV T-cell epitope-encoding genes during reactivation events can lead to recognition of the reactivating target cells by T lymphocytes even before the productive cycle is completed and infectious virions are produced [15,16,17]. In two of the anti-CD8-treated animals, it appears that the luciferase activity may have spread beyond the CNS (Figure 5A). This signal was initially dismissed as background, but it is possible that reactivated virus spread from the CNS following depletion of the CD8+ T-cells.
We examined this possibility using an explant assay. We found that explant reactivation of brain tissue obtained from animals following bTRM depletion resulted in de novo expression of IE1 transcripts, as determined using real-time RT-PCR. The detection of IE gene expression, but not the later classes of viral genes, in the explant assay following CD103+ T-cell depletion could suggest that viral reactivation was blocked or aborted prior to the full production of infectious particles (Figure 6). While imaging and transcriptional analysis provide valuable information regarding viral reactivation, actually isolating infectious virus from latent brain tissue using explant cultures is the “gold standard” for assessing reactivation of infectious virus. Through comparison of viral explant reactivation in groups of animals with and without T-cell depletion, the role of bTRMs in confining productive reactivation following brain explant was investigated. Latent virus can be grown out of the brains of undepleted control animals (100% by 21 d, Figure 6C) using explant culture alone. Paradoxically, in animals depleted of bTRMs, we found viral reactivation kinetics to be delayed when compared to undepleted controls. We observed no CPE indicative of productive virus in any of the 10 animals examined at 7 d post-explant. However, given sufficient time (i.e., 21 d) following explant, virus was recovered from 50% of the bTRM-depleted animals (vs. 100% in each control group).
It is possible that neurons expressing IE proteins (i.e., those produced first upon viral reactivation) are quelled by viral-specific brain-resident memory CD8+ T cells and thereby prevent subsequent gene expression and viral reactivation. Unexpectedly, it appears that the signal observed during imaging experiments did not translate into a productive infection as indicated by our explant study. So, we hypothesize that when we depleted bTRMs, there was transient viral expression of at least IE genes, which was sufficient to activate surveying microglia and induce phagocytic glial cell phenotypes resulting in a tissue-wide anti-viral state. Although over a dozen alternative microglial activation phenotypes have been described, disease-associated microglia (DAM), which were first identified in Alzheimer’s disease, are observed in neurodegenerative models [8]. But, in addition to slowly progressive neurodegenerative diseases, recent studies have shown that cells of the DAM phenotype also develop following a number of viral brain infections (e.g., ZIKV, WNV, HIV-1, and HSV-1) [18,19,20]. Microglia of the DAM phenotype are generally believed to be protective because they are highly phagocytic, but it is likely that overzealous or aberrant DAM-mediated synaptic elimination is harmful for cognitive function. One caveat is that the NanoString data obtained may not be exclusive to microglial cell populations since RNA was extracted from whole brain. These data were generated using nCounter Glial Cell Profiling, which also includes prototypical astrocyte genes.
Although still controversial, neurodegenerative diseases following the reactivation of latent herpesvirus infections from neuronal reservoirs, and induced by the ensuing neuroinflammatory responses, have been associated with a number of human herpesviruses, including cytomegalovirus (CMV) and human herpesvirus (HHV)-6, in addition to herpes simplex virus (HSV)-1 [21,22,23,24,25,26,27,28,29,30]. Although extrapolation regarding the role of TRMs in human neurodegenerative diseases in the absence of direct evidence needs to be tempered, upon reactivation of quiescent neurotropic viruses, bTRMs likely respond to de novo-produced viral Ag through rapid release of interferon (IFN)-γ [31]. Through this mechanism, a small number of adaptive bTRMs may amplify responses to viral proteins produced during reactivation, leading to an organ-wide innate protective state [32,33,34]. In the absence of bTRMs, the replication cycle is allowed to proceed and brain-resident glia likely respond by phagocytosing the increased levels of foreign viral protein. Over time, this protective phagocytic immune activation may have cumulative neurotoxic and neurocognitive consequences [35].
4. Materials and Methods
4.1. Ethical Statement
This study was carried out strictly in accordance with recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Institutional Animal Care and Use Committee (Protocol Number: 2211-40536A, approval date: 1 October 2023) of the University of Minnesota. All animals were routinely cared for according to the guidelines of Research Animal Resources (RAR), University of Minnesota. All surgery was performed under Ketamine/Xylazine anesthesia and Bupivacaine administration and all efforts were made to ameliorate animal suffering. Animals were sacrificed after isoflurane inhalation, whenever required.
4.2. Experimental Animals
BALB/c (8 weeks old) mice were infected with β-gal-expressing MCMV, while FVB transgenic mice (LucRep) were infected with cre-expressing MCMV. Pathogen-free BALB/c (stock #028), and FBV transgenic (FVB.129S6(B6)-Gt (ROSA)26Sortm1(Luc)Kael/J, stock #005125) mice were purchased from Charles River Laboratories (Wilmington, MA, USA) and The Jackson Laboratories (Bar Harbor, ME, USA), respectively. Animals (4–5 per cage) were housed on a rack (7 columns × 8 rows) in the animal room under a 12-h light-dark cycle (6 a.m.–6 p.m. light) with free access to standard chow and water. Each rack had 2 cages of sentinel mice for periodic pathogen screening. Animals were checked daily, and cages were changed every week by RAR staff. Mice were acclimated for a minimum of one week prior to research use and weighed approximately 21 g at 8 weeks old.
4.3. Virus and Growth Conditions
RM461, a recombinant MCMV expressing E. coli β-galactosidase under the control of the human ie1/ie2 promoter/enhancer, was kindly provided by Edward S. Mocarski. cre-MCMV, a Cre-recombinase expressing recombinant virus in which cre is inserted into the MCMV IE2 locus, was a generous gift from Luka Cicin-sain (Helmholtz Center for Infection Research, Braunschweig, Germany) [36]. Viral stocks were passaged in salivary glands of weanling Balb/c mice to retain their virulence. Virus isolated from the salivary glands was then passaged twice on NIH 3T3 fibroblasts to minimize any carry-over of salivary gland tissue. Infected 3T3 cultures were harvested at 80% to 100% cytopathic effect and subjected to three freeze–thaw cycles. Cellular debris was removed by centrifugation (1000× g) at 4 °C, and the virus was pelleted through a 35% sucrose cushion (in Tris-buffered saline [50 mM Tris–HCl, 150 mM NaCl, pH 7.4]) at 23,000× g for 2 h at 4 °C. The pellet was suspended in Tris buffered saline containing 10% heat-inactivated fetal bovine serum (FBS). Viral stock titers were determined on 3T3 cells as 50% tissue culture infective doses (TCID50) per milliliter. This sucrose gradient-purified RM461 and cre-MCMV were used for ICV infections of mice.
4.4. Intracerebroventricular (icv) Infection of Mice
Infection of BALB/c mice and FVB transgenic mice with MCMV RM461 and cre-MCMV, respectively, was performed as previously described. Briefly, female mice (8 weeks old) were anesthetized using a combination of Ketamine (100 mg/kg body weight; Akorn Inc., Lake Forest, IL, USA) and Xylazine (10 mg/kg body weight; Bimeda Inc., Le Sueur, MN, USA) and immobilized on a small animal stereotactic instrument equipped with a Cunningham mouse adapter (Stoelting Co., Wood Dale, IL, USA). Skin was sterilized using a Betadine solution (Stamford, CT, USA) and subcutaneous injection of the analgesic bupivacaine (Hospira Inc., Lake Forest, IL, USA); [1–2 mg/kg (0.4–0.8 mL/kg of a 0.25% solution)] was administered in the head area prior to incision to ameliorate pain. The skin and underlying connective tissue were reflected to expose reference sutures (sagittal and coronal) on the skull. The sagittal plane was attuned so that bregma and lambda were positioned at the same coordinates on the vertical plane. A burr hole was drilled at pre-determined co-ordinates (AP = 0.9 mm, ML = 0.5 mm from bregma, and DV = 3.0 from skull surface) to access the right ventricle. Animals were injected with virulent, salivary gland-passaged MCMV RM461 (1.0 × 105 TCID50 units in 10 μL) or cre-MCMV (2.5 × 104 TCID50 units in 10 μL) into the ventricles using a 10 µL Hamilton syringe fitted to a 27 G needle over a period of 5 min. The opening in the skull was sealed with sterile bone wax (Guaynabo, Puerto Rico) and the skin was closed using 4–0 silk sutures with a FS-2 needle (Ethicon, Somerville, NJ, USA).
4.5. Isolation of Brain Leukocytes and Flow Cytometric Analysis
Brain mononuclear cells were isolated from MCMV-infected BALB/c mice using a previously described procedure with minor modifications [37,38,39]. In brief, whole brain tissues were harvested, (n = 4–6 animals/group/experiment), and minced finely using a scalpel in RPMI 1640 (2 g/L D-glucose and 10 mM HEPES) and digested in 0.0625% trypsin (in Ca/Mg-free HBSS) at room temperature for 20 min. Single cell preparations of infected brains were suspended in 30% Percoll and banded on a 70% Percoll cushion at 900× g for 10 min at 15 °C. Brain leukocytes obtained from the 30–70% Percoll interface were collected. Following preparation of single cell suspensions, cells were treated with Fc block (anti-CD32/CD16 in the form of 2.4G2 hybridoma culture supernatant with 2% normal rat and 2% normal mouse serum) to inhibit nonspecific Ab binding. Cells were then counted using the trypan blue dye exclusion method, and 1 × 106 cells were subsequently stained with anti-mouse immune cell surface markers for 15 min at 4 °C (anti-CD45-PE-Cy7, anti-CD8-FITC, anti-CD103-PE (eBioscience, San Diego, CA, USA), anti-MHCII-BV510 (BioLegend, San Diego, CA, USA). A total of 105 cells were acquired per sample by using a FACS Fortessa flow-cytometer by employing FACS DIVA software v8.0 (BD Biosciences, San Jose, CA, USA). Data were analyzed using FlowJo software v10.10.0 (Ashland, OR, USA).
4.6. Bioluminescence Imaging
Expression of firefly luciferase was monitored by imaging live animals using an IVIS100 (Xenogen; now PerkinElmer, Waltham, MA, USA) equipped with a charge-coupled camera device. Briefly, 150 µg of D-luciferin (Gold Biotechnology, St. Louis, MO, USA) was injected in mice intraperitoneally 5 min before imaging. Data were acquired using a 5-min exposure window.
4.7. Semi-Quantitative RT-PCR
Total RNA from infected brain tissue was extracted using a RNeasy Lipid Tissue Mini Kit (Qiagen, Valencia, CA, USA) according to the manufacturer’s recommendations. The cDNA was synthesized from DNase-treated total RNA (1 μg) using Superscript III reverse transcriptase, RNase inhibitors (Invitrogen, Carlsbad, CA), and oligo d(T)12–18 primers (Gene Link, Hawthorne, NY). PCR was performed with the SYBR Advantage qPCR premix (Takara Bio USA, Mountain View, CA). The qPCR conditions were as follows: 1 denaturation cycle at 95 °C for 10 s; 40 amplification cycles of 95 °C for 10 s, 60 °C annealing for 10 s, and elongation at 72 °C for 10 s, followed by 1 dissociation cycle (Bio-Rad CFX96 qPCR System, Hercules, CA, USA). The relative expression levels were quantified using the 2−∆∆Ct method [40] and were normalized to the housekeeping gene hypoxanthine phosphoribosyl transferase (HPRT). The primer sequences were 5′-ATCTGAAACAGCCGTATATCATCTTG-3′ sense and 5′-TCAGCCATCAACTCTGCTACCAAC-3′ antisense for IE-1; 5′-GTAAGCACGCAAGCAAGCACT-3′ sense and 5′-CAGAGGGGGACCAGGGATAATA-3′ antisense for E-1 and 5′-GCTGTTTTAACGCGCGGAGTATCA-3′ sense and 5′-TGACGATTCGGGTAAGGCGTGGACTA-3′ antisense for gB.
4.8. Immunohistochemistry
Brains were harvested from MCMV-infected animals that were perfused with phosphate-buffered saline (PBS), 2% sodium nitrate to remove contaminating blood cells, and prefixed with 4% paraformaldehyde. Murine brains were subsequently submerged in 4% paraformaldehyde for 24 h and transferred to 25% sucrose solution for 2 d prior to sectioning. After blocking (10% normal goat serum and 0.3% Triton X-100 in PBS) for 1 h at room temperature (RT), brain sections (30 µm) were incubated overnight at 4 °C with the following primary antibodies: monoclonal anti-E1 antibody (MAb kindly provided by Dr. I Kosugi, Hamamatsu University, Hamamatsu, Japan) and monoclonal anti-CD8 antibody. Brain sections were washed three times with PBS and then incubated with fluorescein (FITC)–conjugated anti rat antibody (1:200; Vector Laboratories, Burlingame, CA), or Cy3-conjugated streptavidin (1:400; Jackson ImmunoResearch Laboratories) for 1 h at RT.
4.9. RNAscope™ ISH
Commercially available MCMV target probes and reagents were purchased from Advanced Cell Diagnostics (ACD Bio, now Biotechne). Parafilm embedded sections were heated, dewaxed, and dehydrated. RNAscope Pretreat 1 reagent (endogenous peroxidase block) was used to incubate slides for 10 min at room temperature, followed by boiling slides in RNAscope Pretreat 2 buffer (citrate buffer [10 nmol/L, pH 6]) for 30 min; they were then washed, dehydrated, and air dried. Slides were incubated in diluted RNAscope pretreat 3 reagent (protease digestion solution; 2.5 ug/mL) for 20 to 25 min at 40 °C in a HybEZ hybridization oven. After rinsing with water, the slides were incubated with pre-warmed target probes (20 nmol/L of each oligo probe) in hybridization buffer A (6X SSC [1XSSC is 0.15 mol/L NaCl, 0.015 mol/L Na-citrate], 25% formamide, 0.2% lithium dodecyl sulfate, blocking reagents) for 2 h at 40 °C. After washing with buffer (0.1X or 0.05X SSC, 0.03% lithium dodecyl sulfate), slides were incubated with amplification reagents, as described in the RNAscope 2.0 HD detection protocol. Since Amplification 6 contained alkaline phosphatase, chromogenic detection was performed using FastRed as a substrate to generate a red signal and DAB (ImmPACT™ DAB, Vector Laboratories) to generate a brown signal. Slides were counterstained with haematoxylin, mounted in Permount, scanned, and photographed under the microscope.
4.10. Explant Assay
Brain tissues were isolated from latently infected mice with or without bTRM depletion. One mm coronal sections were cut using a precision brain matrix (Braintree Scientific, Braintree, MA, USA). Approximately 25 pieces were collected from each brain that represented tissue adjoining lateral and central ventricles. Three to four pieces were then inoculated onto previously prepared primary mouse mixed brain cultures in a 96-well plate. The cultures were maintained in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum and antibiotics. Cytopathic effect (CPE) was assessed and the cultures were stained for reactivated viral expression using X-gal.
4.11. Nanostring Analysis
Total RNA was extracted from the brains of the BALB/c animals latently infected with MCMV using a Qiagen RNeasy Lipid Tissue Extraction kit. The nCounter single molecule counting and digital quantification was then performed at the University of Minnesota Genomics Center. This technology is based on gene-specific probe pairs that are hybridized in a single multiplexed reaction to the sample in combination with automated imaging and detection; this eliminates enzymatic reactions that might bias the results. Data generated using the nCounter were then analyzed by our laboratory using the nSolver (version 4) and Rosalind online platform software tools.
4.12. Statistical Analysis
One-way analysis of variance (ANOVA) with Tukey’s multiple comparison test was used for graphical analysis. Differences were considered significant when p < 0.05. For statistical analysis and generation of graphs, Prism 5 software (Version 10; GraphPad Software Inc., San Diego, CA, USA) was used.
Author Contributions
Conceptualization, P.C., W.S.S., S.H. and J.R.L.; methodology, P.C., W.S.S., S.H. and S.P.; software, P.C.; validation, P.C., W.S.S. and S.H.; formal analysis, P.C.; writing—original draft preparation, P.C.; writing—review and editing, J.R.L.; supervision, J.R.L.; project administration, J.R.L.; funding acquisition, J.R.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by grant support from the University of Minnesota Foundation.
Institutional Review Board Statement
This study was carried out strictly in accordance with recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Institutional Animal Care and Use Committee (Protocol Number: 2211-40536A) of the University of Minnesota.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author(s).
Acknowledgments
The University Imaging Centers (UIC) and the University Flow Cytometry Resource (UFCR) supported in vivo small animal imaging and flow cytometry, respectively.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| MCMV | Murine cytomegalovirus |
| bTRMs | Brain tissue-resident memory T-cells |
| LgalS3 | Galectin 3 |
| gpnmb | Glycoprotein nonmetastatic melanoma protein B |
| Hmox1 | Heme oxygenase I |
| TREM2 | Triggering receptor on myeloid cells 2 |
| DAM | Disease-associated Microglia |
| MGnD | Neurodegenerative Microglia |
| NSC | Neural Stem cells |
| SVZ | Sub-ventricular zone |
| CTL | Cytotoxic T-cell |
| CNS | Central Nervous system |
| d.p.i | Days post infection |
| ICV | Intracerebroventricular |
| Ab | Antibody |
| Ag | Antigen |
| BMNCs | Brain mononuclear cells |
| HSV | Herpes Simplex virus |
| IFN | Interferon |
| FBS | Fetal bovine serum |
| TCID50 | 50% Tissue culture infective doses |
| CPE | Cytopathic effect |
| HPRT | Hypoxanthine phosphoribosyl transferase |
| PBS | Phosphate-buffered saline |
| RT | Room temperature |
References
- Hu, S.; Rotschafer, J.H.; Lokensgard, J.R.; Cheeran, M.C. Activated CD8+ T lymphocytes inhibit neural stem/progenitor cell proliferation: Role of interferon-gamma. PLoS ONE 2014, 9, e105219. [Google Scholar] [CrossRef] [PubMed]
- Cheeran, M.C.; Jiang, Z.; Hu, S.; Ni, H.T.; Palmquist, J.M.; Lokensgard, J.R. Cytomegalovirus infection and interferon-gamma modulate major histocompatibility complex class I expression on neural stem cells. J. Neurovirol. 2008, 14, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Mutnal, M.B.; Cheeran, M.C.; Hu, S.; Lokensgard, J.R. Murine cytomegalovirus infection of neural stem cells alters neurogenesis in the developing brain. PLoS ONE 2011, 6, e16211. [Google Scholar] [CrossRef] [PubMed]
- Mutnal, M.B.; Hu, S.; Lokensgard, J.R. Persistent humoral immune responses in the CNS limit recovery of reactivated murine cytomegalovirus. PLoS ONE 2012, 7, e33143. [Google Scholar] [CrossRef]
- Prasad, S.; Hu, S.; Sheng, W.S.; Singh, A.; Lokensgard, J.R. Tregs Modulate Lymphocyte Proliferation, Activation, and Resident-Memory T-Cell Accumulation within the Brain during MCMV Infection. PLoS ONE 2015, 10, e0145457. [Google Scholar] [CrossRef]
- Prasad, S.; Hu, S.; Sheng, W.S.; Chauhan, P.; Lokensgard, J.R. Reactive glia promote development of CD103(+) CD69(+) CD8(+) T-cells through programmed cell death-ligand 1 (PD-L1). Immun. Inflamm. Dis. 2018, 6, 332–344. [Google Scholar] [CrossRef]
- Brizic, I.; Susak, B.; Arapovic, M.; Huszthy, P.C.; Hirsl, L.; Kvestak, D.; Juranic Lisnic, V.; Golemac, M.; Pernjak Pugel, E.; Tomac, J.; et al. Brain-resident memory CD8(+) T cells induced by congenital CMV infection prevent brain pathology and virus reactivation. Eur. J. Immunol. 2018, 48, 950–964. [Google Scholar] [CrossRef]
- Yeh, H.; Ikezu, T. Transcriptional and Epigenetic Regulation of Microglia in Health and Disease. Trends Mol. Med. 2019, 25, 96–111. [Google Scholar] [CrossRef]
- Krasemann, S.; Madore, C.; Cialic, R.; Baufeld, C.; Calcagno, N.; El Fatimy, R.; Beckers, L.; O’Loughlin, E.; Xu, Y.; Fanek, Z.; et al. The TREM2-APOE Pathway Drives the Transcriptional Phenotype of Dysfunctional Microglia in Neurodegenerative Diseases. Immunity 2017, 47, 566–581.e9. [Google Scholar] [CrossRef]
- Afridi, R.; Lee, W.H.; Suk, K. Microglia Gone Awry: Linking Immunometabolism to Neurodegeneration. Front. Cell. Neurosci. 2020, 14, 246. [Google Scholar] [CrossRef]
- Shinmura, Y.; Aiba-Masago, S.; Kosugi, I.; Li, R.Y.; Baba, S.; Tsutsui, Y. Differential expression of the immediate-early and early antigens in neuronal and glial cells of developing mouse brains infected with murine cytomegalovirus. Am. J. Pathol. 1997, 151, 1331–1340. [Google Scholar] [PubMed]
- Tsutsui, Y.; Kashiwai, A.; Kawamura, N.; Aiba-Masago, S.; Kosugi, I. Prolonged infection of mouse brain neurons with murine cytomegalovirus after pre- and perinatal infection. Arch. Virol. 1995, 140, 1725–1736. [Google Scholar] [CrossRef] [PubMed]
- Proenca, J.T.; Coleman, H.M.; Connor, V.; Winton, D.J.; Efstathiou, S. A historical analysis of herpes simplex virus promoter activation in vivo reveals distinct populations of latently infected neurones. J. Gen. Virol. 2008, 89, 2965–2974. [Google Scholar] [CrossRef] [PubMed]
- Dutia, B.M.; Reid, S.J.; Drummond, D.D.; Ligertwood, Y.; Bennet, I.; Rietberg, W.; Silvia, O.; Jarvis, M.A.; Nash, A.A. A novel Cre recombinase imaging system for tracking lymphotropic virus infection in vivo. PLoS ONE 2009, 4, e6492. [Google Scholar] [CrossRef]
- Simon, C.O.; Holtappels, R.; Tervo, H.M.; Bohm, V.; Daubner, T.; Oehrlein-Karpi, S.A.; Kuhnapfel, B.; Renzaho, A.; Strand, D.; Podlech, J.; et al. CD8 T cells control cytomegalovirus latency by epitope-specific sensing of transcriptional reactivation. J. Virol. 2006, 80, 10436–10456. [Google Scholar] [CrossRef]
- Reddehase, M.J.; Simon, C.O.; Seckert, C.K.; Lemmermann, N.; Grzimek, N.K. Murine model of cytomegalovirus latency and reactivation. Curr. Top. Microbiol. Immunol. 2008, 325, 315–331. [Google Scholar]
- Seckert, C.K.; Griessl, M.; Buttner, J.K.; Scheller, S.; Simon, C.O.; Kropp, K.A.; Renzaho, A.; Kuhnapfel, B.; Grzimek, N.K.; Reddehase, M.J. Viral latency drives ‘memory inflation’: A unifying hypothesis linking two hallmarks of cytomegalovirus infection. Med. Microbiol. Immunol. 2012, 201, 551–566. [Google Scholar] [CrossRef]
- Di Liberto, G.; Egervari, K.; Kreutzfeldt, M.; Schurch, C.M.; Hewer, E.; Wagner, I.; Du Pasquier, R.; Merkler, D. Neurodegenerative phagocytes mediate synaptic stripping in Neuro-HIV. Brain 2022, 145, 2730–2741. [Google Scholar] [CrossRef]
- Thammahakin, P.; Maezono, K.; Maekawa, N.; Kariwa, H.; Kobayashi, S. Detection of disease-associated microglia among various microglia phenotypes induced by West Nile virus infection in mice. J. Neurovirol. 2023, 29, 367–375. [Google Scholar] [CrossRef]
- Manet, C.; Mansuroglu, Z.; Conquet, L.; Bortolin, V.; Comptdaer, T.; Segrt, H.; Bourdon, M.; Menidjel, R.; Stadler, N.; Tian, G.; et al. Zika virus infection of mature neurons from immunocompetent mice generates a disease-associated microglia and a tauopathy-like phenotype in link with a delayed interferon beta response. J. Neuroinflamm. 2022, 19, 307. [Google Scholar] [CrossRef]
- Lee, K.H.; Kwon, D.E.; Do Han, K.; La, Y.; Han, S.H. Association between cytomegalovirus end-organ diseases and moderate-to-severe dementia: A population-based cohort study. BMC Neurol. 2020, 20, 216. [Google Scholar] [CrossRef] [PubMed]
- De Chiara, G.; Piacentini, R.; Fabiani, M.; Mastrodonato, A.; Marcocci, M.E.; Limongi, D.; Napoletani, G.; Protto, V.; Coluccio, P.; Celestino, I.; et al. Recurrent herpes simplex virus-1 infection induces hallmarks of neurodegeneration and cognitive deficits in mice. PLoS Pathog. 2019, 15, e1007617. [Google Scholar] [CrossRef]
- Marcocci, M.E.; Napoletani, G.; Protto, V.; Kolesova, O.; Piacentini, R.; Li Puma, D.D.; Lomonte, P.; Grassi, C.; Palamara, A.T.; De Chiara, G. Herpes Simplex Virus-1 in the Brain: The Dark Side of a Sneaky Infection. Trends Microbiol. 2020, 28, 808–820. [Google Scholar] [CrossRef] [PubMed]
- Readhead, B.; Haure-Mirande, J.V.; Funk, C.C.; Richards, M.A.; Shannon, P.; Haroutunian, V.; Sano, M.; Liang, W.S.; Beckmann, N.D.; Price, N.D.; et al. Multiscale Analysis of Independent Alzheimer’s Cohorts Finds Disruption of Molecular, Genetic, and Clinical Networks by Human Herpesvirus. Neuron 2018, 99, 64–82.e7. [Google Scholar] [CrossRef] [PubMed]
- Harris, S.A.; Harris, E.A. Molecular Mechanisms for Herpes Simplex Virus Type 1 Pathogenesis in Alzheimer’s Disease. Front. Aging Neurosci. 2018, 10, 48. [Google Scholar] [CrossRef]
- Rizzo, R. Controversial role of herpesviruses in Alzheimer’s disease. PLoS Pathog. 2020, 16, e1008575. [Google Scholar] [CrossRef]
- Romeo, M.A.; Gilardini Montani, M.S.; Gaeta, A.; D’Orazi, G.; Faggioni, A.; Cirone, M. HHV-6A infection dysregulates autophagy/UPR interplay increasing beta amyloid production and tau phosphorylation in astrocytoma cells as well as in primary neurons, possible molecular mechanisms linking viral infection to Alzheimer’s disease. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165647. [Google Scholar] [CrossRef]
- Romeo, M.A.; Faggioni, A.; Cirone, M. Could autophagy dysregulation link neurotropic viruses to Alzheimer’s disease? Neural Regen. Res. 2019, 14, 1503–1506. [Google Scholar]
- Barnes, L.L.; Capuano, A.W.; Aiello, A.E.; Turner, A.D.; Yolken, R.H.; Torrey, E.F.; Bennett, D.A. Cytomegalovirus infection and risk of Alzheimer disease in older black and white individuals. J. Infect. Dis. 2015, 211, 230–237. [Google Scholar] [CrossRef]
- Lin, W.R.; Wozniak, M.A.; Wilcock, G.K.; Itzhaki, R.F. Cytomegalovirus is present in a very high proportion of brains from vascular dementia patients. Neurobiol. Dis. 2002, 9, 82–87. [Google Scholar] [CrossRef]
- McMaster, S.R.; Wilson, J.J.; Wang, H.; Kohlmeier, J.E. Airway-Resident Memory CD8 T Cells Provide Antigen-Specific Protection against Respiratory Virus Challenge through Rapid IFN-gamma Production. J. Immunol. 2015, 195, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Ariotti, S.; Hogenbirk, M.A.; Dijkgraaf, F.E.; Visser, L.L.; Hoekstra, M.E.; Song, J.Y.; Jacobs, H.; Haanen, J.B.; Schumacher, T.N. T cell memory. Skin-resident memory CD8(+) T cells trigger a state of tissue-wide pathogen alert. Science 2014, 346, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; Hu, S.; Sheng, W.S.; Chauhan, P.; Lokensgard, J.R. Recall Responses from Brain-Resident Memory CD8(+) T Cells (bT(RM)) Induce Reactive Gliosis. iScience 2019, 20, 512–526. [Google Scholar] [CrossRef] [PubMed]
- Schenkel, J.M.; Fraser, K.A.; Beura, L.K.; Pauken, K.E.; Vezys, V.; Masopust, D. T cell memory. Resident memory CD8 T cells trigger protective innate and adaptive immune responses. Science 2014, 346, 98–101. [Google Scholar] [CrossRef]
- Kempuraj, D.; Thangavel, R.; Natteru, P.A.; Selvakumar, G.P.; Saeed, D.; Zahoor, H.; Zaheer, S.; Iyer, S.S.; Zaheer, A. Neuroinflammation Induces Neurodegeneration. J. Neurol. Neurosurg. Spine 2016, 1, 1003. [Google Scholar]
- Cicin-Sain, L.; Podlech, J.; Messerle, M.; Reddehase, M.J.; Koszinowski, U.H. Frequent coinfection of cells explains functional in vivo complementation between cytomegalovirus variants in the multiply infected host. J. Virol. 2005, 79, 9492–9502. [Google Scholar] [CrossRef]
- Cheeran, M.C.; Hu, S.; Palmquist, J.M.; Bakken, T.; Gekker, G.; Lokensgard, J.R. Dysregulated interferon-gamma responses during lethal cytomegalovirus brain infection of IL-10-deficient mice. Virus Res. 2007, 130, 96–102. [Google Scholar] [CrossRef]
- Ford, A.L.; Goodsall, A.L.; Hickey, W.F.; Sedgwick, J.D. Normal adult ramified microglia separated from other central nervous system macrophages by flow cytometric sorting. Phenotypic differences defined and direct ex vivo antigen presentation to myelin basic protein-reactive CD4+ T cells compared. J. Immunol. 1995, 154, 4309–4321. [Google Scholar] [CrossRef]
- Marten, N.W.; Stohlman, S.A.; Zhou, J.; Bergmann, C.C. Kinetics of virus-specific CD8+ -T-cell expansion and trafficking following central nervous system infection. J. Virol. 2003, 77, 2775–2778. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).