Nutrients and Bioactive Compounds from Cannabis sativa Seeds: A Review Focused on Omics-Based Investigations
Abstract
1. Introduction
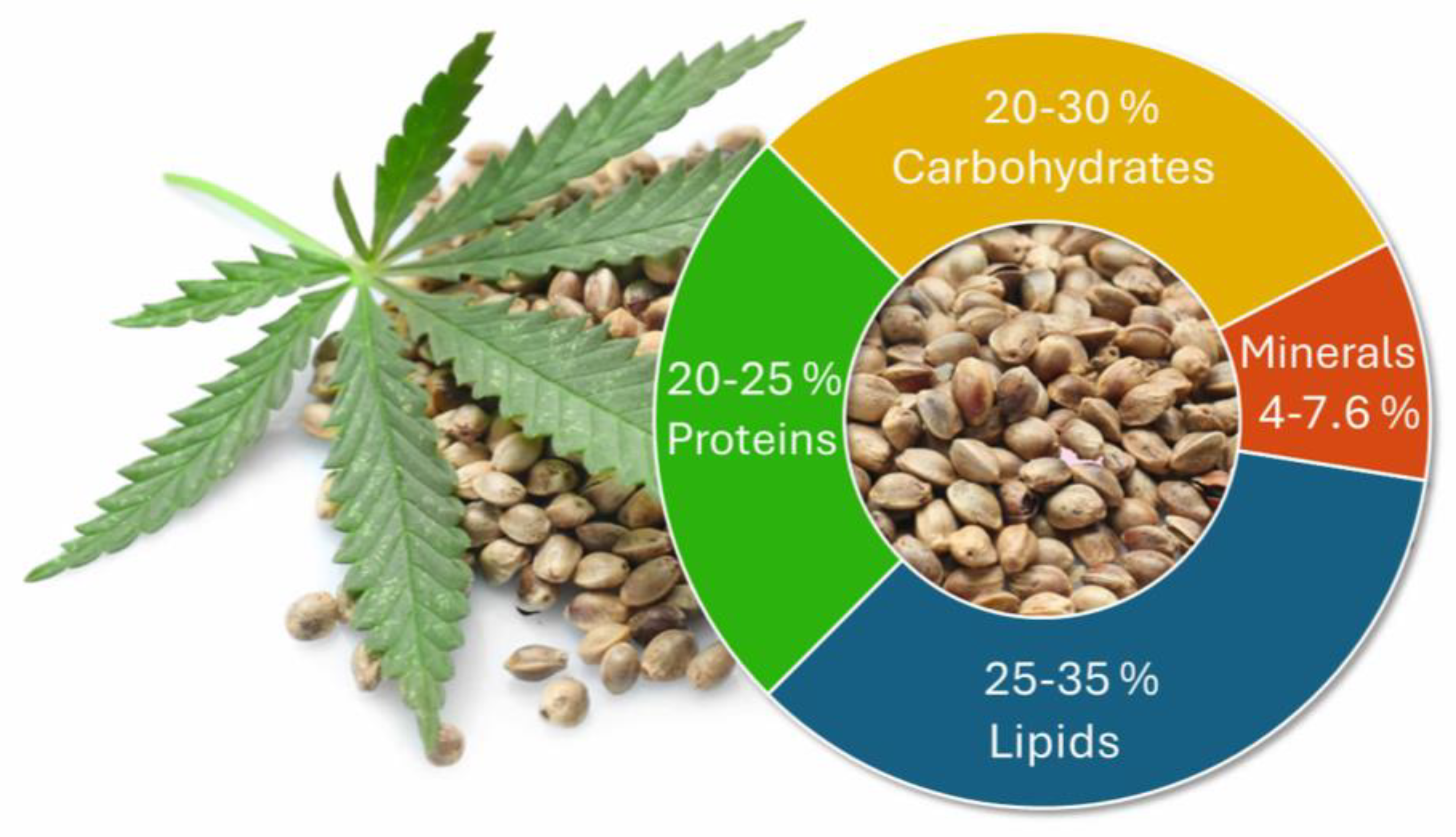
2. Hemp Seed Nutrients and Bioactive Compounds
2.1. Proteins
2.2. Lipids
2.3. Carbohydrates
2.4. Vitamins and Minerals
| Component | Concentration | Biological Effects and Properties | References |
|---|---|---|---|
| Total Oil | ~25–35% | [14,39] | |
| Unsaturated fatty acids | ~90% of hemp seed total oil | Protective effects against cardiovascular diseases, obesity, diabetes mellitus, and anti-inflammatory disorders | [14,39] |
| PUFAs | ~70–80% of unsaturated FAs | [10,14] | |
| Linoleic acid (LA) | ~55.1–63.7% of oil | Omega-6/omega-3 ratio, optimal value between 3:1 and 5:1 (EFSA) for reduction of chronic disease risk and mortality | [10,14,39,75] |
| α-linolenic acid (ALA) | ~15.2–26.2% of oil | Neuroprotection, vasodilation of brain arteries, and neuroplasticity action | [14,39,75,92] |
| γ-linoleic acid (GLA) | ~0.6–6.2% of oil | Anti-inflammatory action, reduction of deficit/hyperactivity disorder, cancer, dry eye syndrome, osteoporosis, diabetic neuropathy, ulcerative colitis, rheumatoid arthritis, and atopic dermatitis | [10,77,93,94] |
| Stearidonic acid (SDA) | ~0.2–1.5% of oil | Sustainable omega-3 source, anti-inflammatory action | [10] |
| Polysaccharides | ~20–30% | Prebiotic compounds, protection of intestinal epithelial cells from hydrogen peroxide-induced oxidative stress, and reduction of appetite and total LDL in hypercholesterolemia. Insulin sensitivity | [9,10,14,95,96] |
| Water-soluble polysaccharides | ~5.5% of polysaccharides | Antimicrobial activity | [11,97] |
| Insoluble polysaccharides | ~22% of polysaccharides | Decrease in obesity and diabetes mellitus | [11,14,97] |
| Proteins | ~20–25% of total content | Antioxidant, antihypertensive, and hypo-allergenic agents | [25,39,41,57,66] |
| Edestin | ~60–75% of total storage protein content | Vasodilatation and human blood circulation improvement; antioxidant and antihypertensive properties | [9,14,64,67] |
| Albumin | ~25–30% of total storage protein content | High radical scavenging activity | [9,14,42,43] |
| Vicilin | ~5% of total storage protein content | Solubility and foaming/emulsifying properties; radical scavenging activity | [9,14,42,43,46] |
| Vitamin E (Tocopherols) | ~562.8–929.67 mg/kg | Mitigation of oxidative stress and prevention of degenerative diseases | [14,21,81,83] |
| γ -tocopherols | ~92.5–93.3% of total tocopherols | Antioxidant, anti-inflammatory, and anticancer properties | [14,21,81,83,98] |
| δ-tocopherols | ~1.9–3.5% of total tocopherols | Reduction of lipid accumulation in lipid storage disorders. Antiangiogenic effects | [14,21,81,83,99,100] |
| α-tocopherols | ~3.8–6.2% of total tocopherols | Reduction of cardiovascular diseases, and iskemic stroke | [14,21,81,83,101] |
| Vitamin A | ~78 mg/kg | Essential for healthy skin development Anti-inflammatory mechanism | [14,81,102] |
| Minerals | ~4–7.6% of total content | Essential for human physiological and structural functions | [7,10,13,14,40,91] |
| Phosphorous (P) | ~890–1.170 mg/100 g | Support for bone augmentation and maintenance | [10,103] |
| Potassium (K) | ~250–2.821 mg/100 g | Blood pressure reduction and positive influence on the risk of stroke and coronary heart disease | [10,104] |
| Magnesium (Mg) | ~237–694 mg/100 g | Support for nerve transmission, cardiac excitability, neuromuscular conduction, blood pressure, and glucose metabolism | [10,105] |
2.5. Bioactive Compounds
| Bioactive Compound | Concentration Values | Hemp Seed Part | Biological Effects and Properties | References |
|---|---|---|---|---|
| Phenolics | Antioxidant, anti-microbial, anti-inflammatory, anti-neuroinflammatory, neuroprotective, and anti-cancer action | [10,16,40,106,113] | ||
| Total phenolic content (TPC) | ~100–300 mg GAE/100 g | Whole hemp seeds | [10,106,117,118] | |
| Total lignanamides | ~20–100 µg/g DW | Whole hemp seeds | Properties similar to the medicines used for the treatment of mild-to-moderate Alzheimer’s disease, such as galanthamine | [6,110,111] |
| Total hydroxycinnamic acid amides (HCAAs) | ~22 CTE/g | Defatted hemp seeds | Antioxidant action | [106] |
| Phytosterols | Property against cardiovascular diseases, reduction of cholesterol absorption, antiviral, antifungal, and anti-inflammatory properties | [40,115] | ||
| Total phytosterol content (TPC) | ~230 mg/100 g ~650 mg/100 g | Whole hemp seeds Oil | [119] | |
| β-sitosterol | ~140–160 mg/100 g ~390–455 mg/100 g | Whole hemp seeds Oil | Attenuation of epidermal hyperplasia and immune cell infiltration in the psoriasis-like mouse model | [83,119,120] |
| Terpenes | Anti-inflammatory, anti-cancer, and antioxidant functions | [109] | ||
| β-myrcene | ~3170 ng/g ~1180 ng/g | Raw hemp seeds Roasted hemp seeds | Muscle relaxant and sedating effects | [109,121,122] |
| D-limonene | ~1347 ng/g ~470 ng/g | Raw hemp seeds Roasted hemp seeds | Immunomodulatory properties, including antitumor effects | [121,123] |
| Carotenoids | Antioxidant activity | [87,124] | ||
| Lutein | ~1.4–3.4 mg/100 g | Whole hemp seeds | Beneficial effects on eye health | [53,125] |
| β-carotene | ~0.2–0.8 mg/100 g | Whole hemp seeds | Protection against skin damage | [53,126] |
| Zeaxanthin | ~0.2–0.5 mg/100 g | Whole hemp seeds | Prevention of the progression of eye diseases and antioxidant protection of heart and skin | [53,127] |
| Cannabinoids | ~2.3–234 mg/kg | Oil | Pharmacological benefits of CBD enhanced by terpenes, through the ‘entourage effect’ | [85,109] |
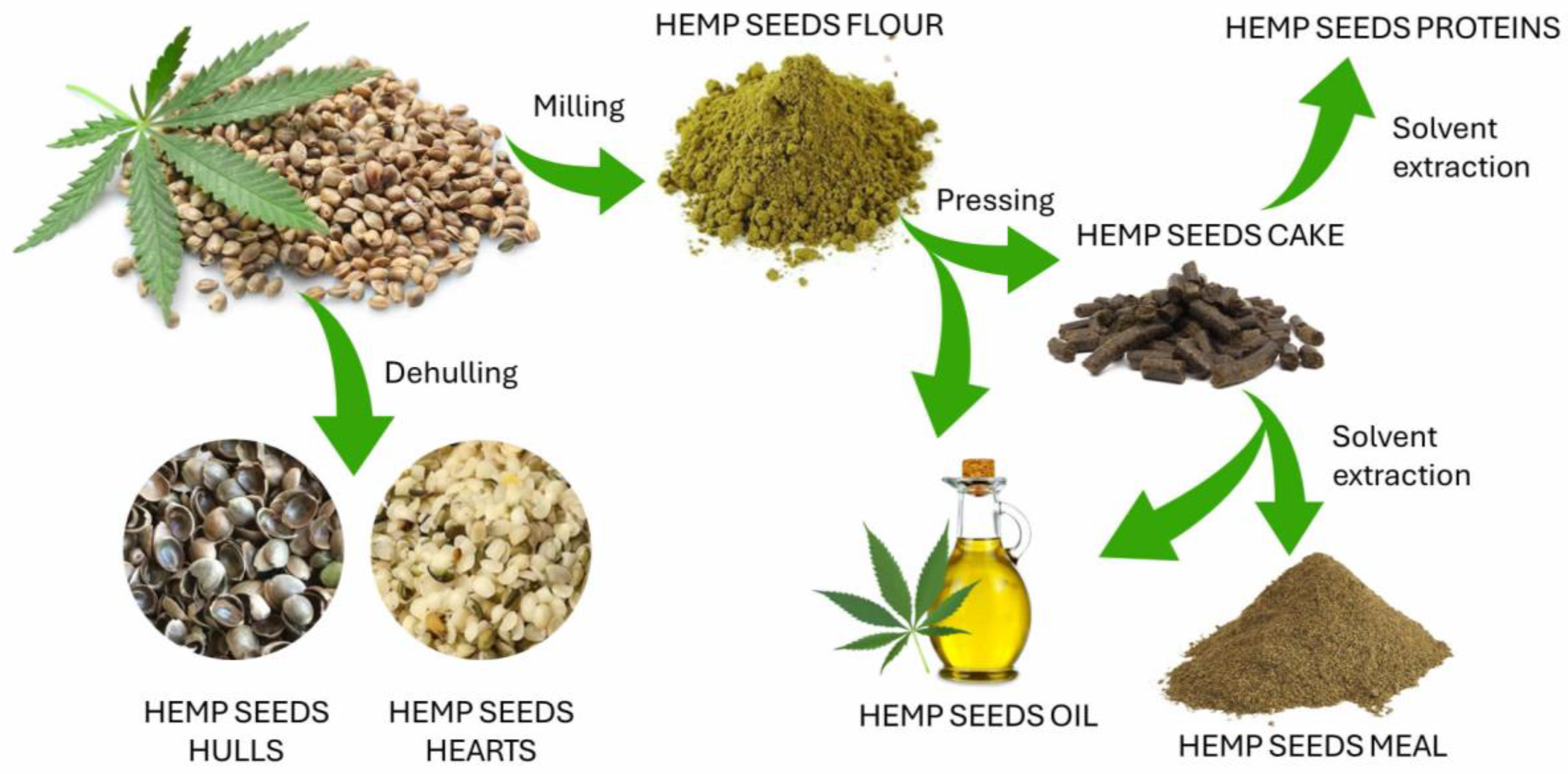
3. Hemp Seed By-Products
4. Omics in Hemp Seeds
4.1. Genomics in Hemp Seeds
4.2. Transcriptomics in Hemp Seeds
4.3. Metabolomics in Hemp Seeds
4.4. Lipidomics in Hemp Seeds
4.5. Ionomics in Hemp Seeds
4.6. Proteomics in Hemp Seeds
5. Multi-Omics in Hemp Seeds
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CBD | Cannabidiol |
| DAD | Diode array detection |
| EFAs | Essential fatty acids |
| ESI | Electro-spray ionization |
| GC | Gas chromatography |
| GC-FID | Gas chromatography–flame ionization detection |
| GC-MS/O | Gas chromatography–mass spectrometry–olfactometry |
| GWAS | Genome-wide association study |
| HPC | Hemp protein concentrates |
| HPI | Hemp protein isolates |
| HPLC | High-performance liquid chromatography |
| HPP | High-pressure processing |
| HRGC | High-resolution gas chromatography |
| ICP-OES | Inductively coupled plasma coupled to optical emission spectrometry |
| MALDI-TOF | Matrix-assisted laser desorption ionization time-of-flight |
| MALS | Multi-angle light scattering |
| nLC-ESI-MS/MS | Nanoflow liquid chromatography electrospray-ionization tandem mass spectrometry |
| NMR | Nuclear magnetic resonance |
| PUFAs | Polyunsaturated fatty acids |
| qRT-PCR | Quantitative reverse transcription polymerase chain reaction |
| Q-TOF-MS | Quadrupole time-of-flight-mass spectrometry |
| RI | Refractive index |
| RNA-Seq | RNA sequencing |
| SEC | Size exclusion chromatography |
| SDS-PAGE | Sodium dodecyl sulphate-polyacrylamide gel electrophoresis |
| SPME | Solid-phase microextraction |
| THC | Δ9-tetrahydrocannabinol |
| UAE | Ultrasound-assisted extraction |
| UHPLC-QQQ-MS/MS | Ultra-high performance liquid chromatography coupled with triple quadrupole mass spectrometry |
| UV | Ultraviolet |
References
- Andre, C.M.; Hausman, J.; Guerriero, G. Cannabis sativa: The plant of the thousand and one molecules. Front. Plant Sci. 2016, 7, 19. [Google Scholar] [CrossRef]
- Clarke, R.C.; Merlin, M.D. Cannabis domestication, breeding history, present day genetic diversity, and future prospects. Crit.Rev. Plant Sci. 2016, 35, 293–327. [Google Scholar] [CrossRef]
- Morales, P.; Hurst, D.P.; Reggio, P.H. Molecular targets of the phytocannabinoids: A complex picture. Prog. Chem. Org. Nat. Prod. 2017, 103, 103–131. [Google Scholar]
- Kovalchuk, I.; Pellino, M.; Rigault, P.; van Velzen, R.; Ebersbach, J.; Ashnest, J.R.; Mau, M.; Schranz, M.; Alcorn, J.; Laprairie, R. The genomics of cannabis and its close relatives. Annu. Rev. Plant Biol. 2020, 71, 713–739. [Google Scholar] [CrossRef]
- Schluttenhofer, C.; Yuan, L. Challenges towards revitalizing hemp: A multifaceted crop. Trends Plant Sci. 2017, 22, 917–929. [Google Scholar] [CrossRef]
- Callaway, J.C. Hempseed as a nutritional resource: An overview. Euphytica 2004, 140, 65–72. [Google Scholar] [CrossRef]
- Tănase Apetroaei, V.; Pricop, E.M.; Istrati, D.I.; Vizireanu, C. Hemp Seeds (Cannabis sativa L.) as a valuable source of natural ingredients for functional foods—A review. Molecules 2024, 29, 2097. [Google Scholar] [CrossRef]
- House, J.D.; Neufeld, J.; Leson, G. Evaluating the quality of protein from hemp seed (Cannabis sativa L.) products through the use of the protein digestibility-corrected amino acid score method. J. Agric. Food Chem. 2010, 58, 11801–11807. [Google Scholar] [CrossRef]
- Sun, X.; Sun, Y.; Li, Y.; Wu, Q.; Wang, L. Identification and characterization of the seed storage proteins and related genes of Cannabis sativa L. Front Nutr. 2021, 8, 678421. [Google Scholar] [CrossRef]
- Farinon, B.; Molinari, R.; Costantini, L.; Merendino, N. The seed of industrial hemp (Cannabis sativa L.): Nutritional quality and potential functionality for human health and nutrition. Nutrients 2020, 12, 1935. [Google Scholar] [CrossRef]
- Julakanti, S.; Raja Charles, A.P.; Syed, R.; Bullock, F.; Wu, Y. Hempseed polysaccharide (Cannabis sativa L.): Physicochemical characterization and comparison with flaxseed polysaccharide. Food Hydrocoll. 2023, 143, 108900. [Google Scholar] [CrossRef]
- Ning, K.; Hou, C.; Wei, X.; Zhou, Y.; Zhang, S.; Chen, Y.; Yu, H.; Dong, L.; Chen, S. Metabolomics analysis revealed the characteristicm of hemp seeds varieties and metabolites responsible for antioxidant properties. Front. Plant Sci. 2022, 13, 904163. [Google Scholar] [CrossRef]
- Crini, G.; Lichtfouse, E.; Chanet, G.; Morin-Crini, N. Applications of hemp in textiles, paper industry, insulation and building materials, horticulture, animal nutrition, food and beverages, nutraceuticals, cosmetics and hygiene, medicine, agrochemistry, energy production and environment: A review. Environ. Chem. Lett. 2020, 18, 1451–1476. [Google Scholar] [CrossRef]
- Montero, L.; Fernando, A. Hemp seeds: Nutritional value, associated bioactivities and the potential food applications in the Colombian context. Front. Nutr. 2023, 9, 1039180. [Google Scholar] [CrossRef]
- Simopoulos, A.P. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp. Biol. Med. 2008, 233, 674–688. [Google Scholar] [CrossRef]
- Chen, T.; He, J.; Zhang, J.; Li, X.; Zhang, H.; Hao, J.; Li, L. The isolation and identification of two compounds with predominant radical scavenging activity in hempseed (seed of Cannabis sativa L.). Food Chem. 2012, 134, 1030–1037. [Google Scholar] [CrossRef]
- Rashid, A.; Ali, V.; Khajuria, M.; Faiz, S.; Gairola, S.; Vyas, D. GC–MS based metabolomic approach to understand nutraceutical potential of Cannabis seeds from two different environments. Food Chem. 2021, 339, 128076. [Google Scholar] [CrossRef]
- Abrahamsen, F.; Reddy, G.; Abebe, W.; Gurung, N. Effect of varying levels of hempseed meal supplementation on humoral and cell-mediated immune responses of goats. Animals 2021, 11, 2764. [Google Scholar] [CrossRef]
- Majewski, M.; Jurgoński, A. The effect of hemp (Cannabis sativa L.) seeds and hemp seed oil on vascular dysfunction in obese male zucker rats. Nutrients 2021, 13, 2575. [Google Scholar] [CrossRef]
- Gulcin, İ.; Ozden, E.M.; Mutlu, M.; Mirzaee, Z.; Bingol, Z.; Köksal, E.; Alwasel, S.; Goren, A.C. Exploring of biological activity and diverse metabolites in hemp (Cannabis sativa) seed oil by GC/MS, GC–FID, and LC–HRMS chromatographies. Futur. J. Pharm. Sci. 2024, 10, 130. [Google Scholar] [CrossRef]
- Rupasinghe, H.P.V.; Davis, A.; Kumar, S.K.; Murray, B.; Zheljazkov, V.D. Industrial hemp (Cannabis sativa subsp. sativa) as an emerging source for value-added functional food ingredients and nutraceuticals. Molecules 2020, 25, 4078. [Google Scholar] [CrossRef] [PubMed]
- Buzzanca, C.; Di Stefano, V. Hemp flour, from waste to nutritional and nutraceuticals reuse. In Nutraceutical and Functional Foods; Wildman, R.E.C., Ed.; CRC Press: Boca Raton, FL, USA, 2023. [Google Scholar]
- Occhiuto, C.; Aliberto, G.; Ingegneri, M.; Trombetta, D.; Circosta, C.; Smeriglio, A. Comparative evaluation of the nutrients, phytochemicals, and antioxidant activity of two hempseed oils and their byproducts after cold pressing. Molecules 2022, 27, 3431. [Google Scholar] [CrossRef] [PubMed]
- Burton, R.A.; Andres, M.; Cole, M.; Cowely, J.M.; Augustin, M.A. Industrial hemp seed: From the field to value-added food ingredients. J. Cannabis Res. 2022, 4, 45. [Google Scholar] [CrossRef]
- Gumus, Z.P.; Ustun Argon, Z.; Celenk, V.U.; Ertas, H. Bioactive Phytochemicals from Hemp (Cannabis sativa) Seed Oil Processing By-products. In Bioactive Phytochemicals from Vegetable Oil and Oilseed Processing By-products. Reference Series in Phytochemistry; Ramadan Hassanien, M.F., Ed.; Springer: Cham, Switzerland, 2022. [Google Scholar]
- Kumar, S.; Kushwaha, R.; Verma, M.L. Recovery and utilization of bioactives from food processing waste. In Biotechnological Production of Bioactive Compounds, 1st ed.; Verma, M.L., Chandel, A.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 37–68. [Google Scholar]
- Nevara, G.A.; Giwa Ibrahim, S.; Syed Muhammad, S.K.; Zawawi, N.; Mustapha, N.A.; Karim, R. Oilseed meals into foods: An approach for the valorization of oilseed by-products. Crit. Rev. Food Sci. Nutr. 2022, 63, 6330–6343. [Google Scholar] [CrossRef]
- Sirangelo, T.M.; Ludlow, R.A.; Chenet, T.; Pasti, L.; Spadafora, N.D. Multi-Omics and Genome Editing Studies on Plant Cell Walls to Improve Biomass Quality. Agriculture 2023, 13, 752. [Google Scholar] [CrossRef]
- Otles, S.; Despoudi, S.; Bucatariu, C.; Kartal, C. Food waste management, valorization, and sustainability in the food industry. In Food Waste Recovery, 1st ed.; Galanakis, C.M., Ed.; Elsevier Inc.: London, UK, 2015; pp. 3–23. [Google Scholar]
- Borrello, M.; Caracciolo, F.; Lombardi, A.; Pascucci, S.; Cembalo, L. Consumers’ perspective on circular economy strategy for reducing food waste. Sustainability 2017, 9, 141. [Google Scholar] [CrossRef]
- Esposito, B.; Sessa, M.R.; Sica, D.; Malandrino, O. Towards Circular economy in the agri-food sector. A systematic literature review. Sustainability 2020, 12, 7401. [Google Scholar] [CrossRef]
- Ancuța, P.; Sonia, A. Oil press-cakes and meals valorization through circular economy approaches: A review. Appl. Sci. 2020, 10, 7432. [Google Scholar] [CrossRef]
- Aliferis, K.A.; Bernard-Perron, D. Cannabinomics: Application of metabolomics in Cannabis (Cannabis sativa L.) research and development. Front. Plant Sci. 2020, 11, 554. [Google Scholar] [CrossRef]
- Cerrato, A.; Citti, C.; Cannazza, G.; Capriotti, A.L.; Cavaliere, C.; Grassi, G.; Marini, F.; Montone, C.M.; Paris, R.; Piovesana, S.; et al. Phytocannabinomics: Untargeted metabolomics as a tool for cannabis chemovar differentiation. Talanta 2021, 230, 122313. [Google Scholar] [CrossRef]
- Hazekamp, A.; Tejkalová, K.; Papadimitriou, S. Cannabis: From cultivar to chemovar II—A metabolomics approach to cannabis classification. Cannabis Cannabinoid Res. 2016, 1, 202–215. [Google Scholar] [CrossRef]
- Sirangelo, T.M.; Ludlow, R.A.; Spadafora, N.D. Multi-Omics approaches to study molecular mechanisms in Cannabis sativa. Plants 2022, 11, 2182. [Google Scholar] [CrossRef]
- Braich, S.; Baillie, R.C.; Jewell, L.S.; Spangenberg, G.C.; Cogan, N.O.I. Generation of a comprehensive transcriptome atlas and transcriptome dynamics in medicinal cannabis. Sci. Rep. 2019, 9, 16583. [Google Scholar] [CrossRef] [PubMed]
- Guerriero, G.; Behr, M.; Legay, S.; Mangeot-Peter, L.; Zorzan, S.; Ghoniem, M.; Hausman, J.F. Transcriptomic profiling of hemp bast fibers at different developmental stages. Sci. Rep. 2017, 7, 4961. [Google Scholar] [CrossRef]
- Vonapartis, E.; Aubin, M.P.; Seguin, P.; Mustafa, A.F.; Charron, J.B. Seed composition of ten industrial hemp cultivars approved for production in Canada. J. Food Compos. Anal. 2015, 39, 8–12. [Google Scholar] [CrossRef]
- Siano, F.; Moccia, S.; Picariello, G.; Russo, G.L.; Sorrentino, G.; Di Stasio, M.; La Cara, F.; Volpe, M.G. Comparative study of chemical, biochemical characteristic and ATR-FTIR analysis of seeds, oil and four of the edible Fedora cultivar hemp (Cannabis sativa L.). Molecules 2019, 24, 83. [Google Scholar] [CrossRef]
- Aiello, G.; Fasoli, E.; Boschin, G.; Lammi, C.; Zanoni, C.; Citterio, A.; Arnoldi, A. Proteomic characterization of hempseed (Cannabis sativa L.). J. Proteomics 2016, 147, 187–196. [Google Scholar] [CrossRef]
- Cattaneo, C.; Givonetti, A.; Leoni, V.; Guerrieri, N.; Manfredi, M.; Giorgi, A.; Cavaletto, M. Biochemical aspects of seeds from Cannabis sativa L. plants grown in a mountain environment. Sci. Rep. 2021, 11, 3927. [Google Scholar] [CrossRef]
- Cattaneo, C.; Givonetti, A.; Cavaletto, M. Protein mass fingerprinting and antioxidant power of hemp seeds in relation to plant cultivar and environment. Plants 2023, 12, 782. [Google Scholar] [CrossRef]
- Sciacca, F.; Virzì, N.; Pecchioni, N.; Melilli, M.G.; Buzzanca, C.; Bonacci, S.; Di Stefano, V. Functional end-use of hemp seed waste: Technological, qualitative, nutritional, and sensorial characterization of fortified bread. Sustainability 2023, 15, 12899. [Google Scholar] [CrossRef]
- Cabral, E.M.; Zhu, X.; Garcia-Vaquero, M.; Pérez-Vila, S.; Tang, J.; Gómez-Mascaraque, L.G.; Poojary, M.M.; Curtin, J.; Tiwari, B.K. Recovery of protein from industrial hemp waste (Cannabis sativa, L.) using high-pressure processing and ultrasound technologies. Foods 2023, 12, 2883. [Google Scholar] [CrossRef] [PubMed]
- Bárta, J.; Roudnický, P.; Jarošová, M.; Zdráhal, Z.; Stupková, A.; Bártová, V.; Krejčová, Z.; Kyselka, J.; Filip, V.; Říha, V.; et al. Proteomic profiles of whole seeds, hulls, and dehulled seeds of two industrial hemp (Cannabis sativa L.) cultivars. Plants 2024, 13, 111. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Chen, Y.; Deng, J.; Cai, G.; Fu, W.; Shentu, C.; Xu, Y.; Liu, J.; Zhou, Y.; Luo, Y.; et al. Integrated metabolomics and proteomics analyses to reveal anticancer mechanism of hemp oil extract in colorectal cancer. J. Pharm. Biomed. Anal. 2024, 249, 116379. [Google Scholar] [CrossRef]
- Xu, J.; Bai, M.; Song, H.; Yang, L.; Zhu, D.; Liu, H. Hemp (Cannabis sativa subsp. sativa) Chemical composition and the application of hempseeds in food formulations. Plant Foods Hum. Nutr. 2022, 77, 504–513. [Google Scholar] [CrossRef]
- Yano, H.; Fu, W. Hemp: A sustainable plant with high industrial value in food processing. Foods 2023, 12, 651. [Google Scholar] [CrossRef]
- Chen, Z.; Hao, S.; He, Z.; Liu, J.; Zhao, J.; Chen, C.; Jia, G.; Chen, H. Widely targeted metabolomics analysis reveals the major metabolites in the hemp seeds from the longevity village of Bama, China. Ind. Crops Prod. 2023, 206, 117661. [Google Scholar] [CrossRef]
- Naim-Feil, E.; Elkins, A.C.; Malmberg, M.M.; Ram, D.; Tran, J.; Spangenberg, G.C.; Rochfort, S.J.; Cogan, N.O.I. The Cannabis Plant as a Complex System: Interrelationships between Cannabinoid Compositions, Morphological, Physiological and Phenological Traits. Plants 2023, 12, 493. [Google Scholar] [CrossRef]
- Porto, C.D.; Decorti, D.; Natolino, A. Potential Oil Yield, Fatty acid composition, and oxidation stability of the hempseed oil from four Cannabis sativa L. cultivars. J. Diet. Suppl. 2015, 12, 1–10. [Google Scholar] [CrossRef]
- Irakli, M.; Tsaliki, E.; Kalivas, A.; Kleisiaris, F.; Sarrou, E.; Cook, C.M. Effect of genotype and growing year on the nutritional, phytochemical, and antioxidant properties of industrial hemp (Cannabis sativa L.) seeds. Antioxidants 2019, 8, 491. [Google Scholar] [CrossRef]
- Lančaričová, A.; Kuzmiaková, B.; Porvaz, P.; Havrlentová, M.; Nemeček, P.; Kraic, J. Nutritional quality of hemp seeds (Cannabis sativa L.) in different environments. J. Cent. Eur. Agric. 2021, 22, 748–761. [Google Scholar] [CrossRef]
- Shen, P.; Gao, Z.; Xu, M.; Ohm, J.B.; Rao, J.; Chen, B. The impact of hempseed dehulling on chemical composition, structure properties and aromatic profile of hemp protein isolate. Food Hydrocoll. 2020, 106, 105889. [Google Scholar] [CrossRef]
- Mattila, P.; Mäkinen, S.; Eurola, M.; Jalava, T.; Pihlava, J.M.; Hellström, J.; Pihlanto, A. Nutritional value of commercial protein-rich plant products. Plant Foods Hum. Nutr. 2018, 73, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Aluko, R.E. Hemp seed (Cannabis sativa L.) proteins: Composition, structure, enzymatic modification, and functional or bioactive properties. In Sustainable Protein Sources; Academic Press: Cambridge, MA, USA, 2017; pp. 121–132. [Google Scholar]
- Wang, X.-S.; Tang, C.-H.; Yang, X.-Q.; Gao, W.-R. Characterization, amino acid composition and in vitro digestibility of hemp (Cannabis sativa L.) proteins. Food Chem. 2008, 107, 11–18. [Google Scholar] [CrossRef]
- El-Sohaimy, S.A.; Androsova, N.V.; Toshev, A.D.; El-Enshasy, H.A. Nutritional quality, chemical, and functional characteristics of hemp (Cannabis sativa ssp. sativa) protein isolate. Plants 2022, 11, 2825. [Google Scholar] [PubMed]
- Wu, G.; Bazer, F.W.; Davis, T.A.; Kim, S.W.; Li, P.; Rhoads, J.M.; Satterfield, M.C.; Smith, S.B.; Spencer, T.E.; Yin, Y. Arginine metabolism and nutrition in growth, health and disease. Amino Acids 2009, 37, 153–168. [Google Scholar] [CrossRef]
- Lin, Y.; Pangloli, P.; Meng, X.; Dia, V.P. Effect of heating on the digestibility of isolated hempseed (Cannabis sativa L.) protein and bioactivity of its pepsin-pancreatin digests. Food Chem. 2020, 314, 126198. [Google Scholar] [CrossRef]
- Hu, J.X.; Liu, M.; Jing, X.; Na, D.Q. Study on toxicological safety of hemp seed proteins. Chin. J. Health Lab. Technol. 2007, 17, 2185–2190. [Google Scholar]
- Marcone, M.F. Biochemical and biophysical properties of plant storage proteins: A current understanding with emphasis on 11S seed globulins. Food Res. Int. 1999, 32, 79–92. [Google Scholar] [CrossRef]
- Ponzoni, E.; Brambilla, I.M.; Galasso, I. Genome-wide identification and organization of seed storage protein genes of Cannabis sativa. Biol. Plant. 2018, 62, 1–10. [Google Scholar] [CrossRef]
- Shen, P.; Gao, Z.; Fang, B.; Rao, J.; Chen, B. Ferreting out the secrets of industrial hemp protein as emerging functional food ingredients. Trends Food Sci. Technol. 2021, 112, 1–15. [Google Scholar] [CrossRef]
- Park, S.; Seo, J.; Lee, M. Proteomic profiling of hempseed proteins from Cheungsam. Biochimica Et Biophysica Acta (BBA). Proteins Proteom. 2012, 1824, 374–382. [Google Scholar] [CrossRef] [PubMed]
- Docimo, T.; Caruso, I.; Ponzoni, E.; Mattana, M.; Galasso, I. Molecular characterization of edestin gene family in Cannabis sativa L. Plant Physiol. Biochem. 2014, 84, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Zając, M.; Guzik, P.; Kulawik, P.; Tkaczewska, J.; Florkiewicz, A.; Migdał, W. The quality of pork loaves with the addition of hemp seeds, de-hulled hemp seeds, hemp protein and hemp flour. LWT 2019, 105, 190–199. [Google Scholar] [CrossRef]
- Pojić, M.; Hadnađev, T.D.; Hadnađev, M.; Rakita, S.; Brlek, T. Bread supplementation with hemp seed cake: A by-product of hemp oil processing. J. Food Qual. 2015, 38, 431–440. [Google Scholar] [CrossRef]
- Grasso, N.; Alonso-Miravalles, L.; O’Mahony, J.A. Composition, physicochemical and sensorial properties of commercial plant-based yogurts. Foods 2020, 9, 252. [Google Scholar] [CrossRef]
- Norajit, K.; Gu, B.-J.; Ryu, G.-H. Effects of the addition of hemp powder on the physicochemical properties and energy bar qualities of extruded rice. Food Chem. 2011, 129, 1919–1925. [Google Scholar] [CrossRef]
- Aiello, G.; Lammi, C.; Boschin, G.; Zanoni, C.; Arnoldi, A. Exploration of potentially bioactive peptides generated from the enzymatic hydrolysis of hempseed proteins. J. Agric. Food Chem. 2017, 65, 10174–10184. [Google Scholar] [CrossRef] [PubMed]
- Lammi, C.; Aiello, G.; Vistoli, G.; Zanoni, C.; Arnoldi, A.; Sambuy, Y.; Ferruzza, S.; Ranaldi, G. A multidisciplinary investigation on the bioavailability and activity of peptides from lupin protein. J. Funct. Foods 2016, 24, 297–306. [Google Scholar] [CrossRef]
- Kwaśnica, A.; Teleszko, M.; Marcinkowski, D.; Kmiecik, D.; Grygier, A.; Golimowski, W. Analysis of changes in the amount of phytosterols after the bleaching process of hemp oils. Molecules 2022, 27, 7196. [Google Scholar] [CrossRef]
- Sala-Vila, A.; Fleming, J.; Kris-Etherton, P.; Ros, E. Impact of α-linolenic acid, the vegetable ω-3 fatty acid, on cardiovascular disease and cognition. Adv. Nutr. 2022, 13, 1584–1602. [Google Scholar] [CrossRef]
- Nie, J.; Ma, W.; Ma, X.; Zhu, D.; Li, X.; Wang, C.; Xu, G.; Chen, C.; Luo, D.; Xie, S.; et al. Integrated transcriptomic and metabolomic analysis reveal the dynamic process of Bama hemp seed development and the accumulation mechanism of α-linolenic acid and linoleic acid. Agric. Food Chem. 2024, 72, 10862–10878. [Google Scholar] [CrossRef]
- Sergeant, S.; Rahbar, E.; Chilton, F.H. Gamma-linolenic acid, dihommo-gamma linolenic, eicosanoids and inflammatory processes. Eur. J. Pharmacol. 2016, 785, 77–86. [Google Scholar] [CrossRef]
- Lu, H.; Li, L.; Zou, Z.; Han, B.; Gong, M. The therapeutic potential of hemp seed oil in d-galactose-induced aging rat model was determined through the combined assessment of 1H NMR metabolomics and 16S rRNA gene sequencing. Metabolites 2024, 14, 304. [Google Scholar] [CrossRef] [PubMed]
- Lattimer, J.M.; Haub, M.D. Effects of dietary fiber and its components on metabolic health. Nutrients 2010, 2, 1266–1289. [Google Scholar] [CrossRef]
- Rizzo, G.; Storz, M.A.; Calapai, G. The role of hemp (Cannabis sativa L.) as a functional food in vegetarian nutrition. Foods 2023, 12, 3505. [Google Scholar] [CrossRef]
- Frassinetti, S.; Moccia, E.; Caltavuturo, L.; Gabriele, M.; Longo, V.; Bellani, L.; Giorgi, G.; Giorgetti, L. Nutraceutical potential of hemp (Cannabis sativa L.) seeds and sprouts. Food Chem. 2018, 262, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Rezvankhah, A.; Emam-Djomeh, Z.; Safari, M.; Askari, G.; Salami, M. Microwave-assisted extraction of hempseed oil: Studying and comparing of fatty acid composition, antioxidant activity, physiochemical and thermal properties with Soxhlet extraction. J. Food Sci. Tech. 2019, 56, 4198–4210. [Google Scholar] [CrossRef] [PubMed]
- Blasi, F.; Tringaniello, C.; Verducci, G.; Cossignani, L. Bioactive minor components of Italian and Extra-European hemp seed oils. LWT 2022, 158, 113167. [Google Scholar] [CrossRef]
- Crescente, G.; Piccolella, S.; Esposito, A.; Scognamiglio, M.; Fiorentino, A.; Pacifico, S. Chemical composition and nutraceutical properties of hempseed: An ancient food with actual functional value. Phytochem. Rev. 2018, 17, 733–749. [Google Scholar] [CrossRef]
- Izzo, L.; Pacifico, S.; Piccolella, S.; Castaldo, L.; Narváez, A.; Grosso, M.; Ritieni, A. Chemical analysis of minor bioactive components and cannabidiolic acid in commercial hemp seed oil. Molecules 2020, 25, 3710. [Google Scholar] [CrossRef]
- Aiello, A.; Pizzolongo, F.; Scognamiglio, G.; Romano, A.; Masi, P.; Romano, R. Effects of supercritical and liquid carbon dioxide extraction on hemp (Cannabis sativa L.) seed oil. Int. J. Food Sci. Technol. 2020, 55, 2472–2480. [Google Scholar] [CrossRef]
- Liang, J.; Appukuttan Aachary, A.; Thiyam-Holländer, U. Hemp seed oil: Minor components and oil quality. Lipid Technol. 2015, 27, 231–233. [Google Scholar] [CrossRef]
- Debier, C.; Larondelle, Y. Vitamins A and E: Metabolism, roles and transfer to offspring. Br. J. Nutr. 2005, 93, 153–174. [Google Scholar] [CrossRef] [PubMed]
- Rbah, Y.; Taaifi, Y.; Allay, A.; Belhaj, K.; Melhaoui, R.; Houmy, N.; Moumen, A.B.; Azeroual, E.; Addi, M.; Mansouri, F.; et al. A comprehensive exploration of the fatty acids profile, cholesterol, and tocopherols levels in liver from laying hens fed diets containing nonindustrial hemp seed. Hindawi Sci. 2024, 2024, 8848436. [Google Scholar] [CrossRef]
- Warren, T.; McAllister, R.; Morgan, A.; Rai, T.S.; McGilligan, V.; Ennis, M.; Page, C.; Kelly, C.; Peace, A.; Corfe, B.M.; et al. The interdependency and co-regulation of the vitamin D and cholesterol metabolism. Cells 2021, 10, 2007. [Google Scholar] [CrossRef] [PubMed]
- Rusu, I.E.; Marc Vlaic, R.A.; Mureşan, C.C.; Mureşan, A.E.; Filip, M.R.; Onica, B.M.; Csaba, K.B.; Alexa, E.; Szanto, L.; Muste, S. Advanced characterization of hemp flour (Cannabis sativa L.) from Dacia Secuieni and Zenit varieties, compared to wheat flour. Plants 2021, 10, 1237. [Google Scholar] [CrossRef] [PubMed]
- Blondeau, N.; Lipsky, R.H.; Bourourou, M.; Duncan, M.W.; Gorelick, P.B.; Marini, A.M. Alpha-linolenic acid: An omega-3 fatty acid with neuroprotective properties-ready for use in the stroke clinic? Biomed. Res. Int. 2015, 2015, 519830. [Google Scholar] [CrossRef]
- Fan, Y.Y.; Chapkin, R.S. Importance of dietary gamma-linolenic acid in human health and nutrition. J. Nutr. 1998, 128, 1411–1414. [Google Scholar] [CrossRef]
- Das, U.N. Gamma-linolenic acid therapy of human glioma—A review of in vitro, in vivo, and clinical studies. Med. Sci. Monit. 2007, 13, RA119–RA131. [Google Scholar]
- Cerino, P.; Buonerba, C.; Cannazza, G.; D’Auria, J.; Ottoni, E.; Fulgione, A.; Di Stasio, A.; Pierri, B.; Gallo, A. A review of hemp as food and nutritional supplement. Cannabis Cannabinoid Res. 2020, 6, 19–27. [Google Scholar] [CrossRef]
- Wen, Z.-S.; Xue, R.; Du, M.; Tang, Z.; Xiang, X.-W.; Zheng, B.; Qu, Y.-L. Hemp seed polysaccharides protect intestinal epithelial cells from hydrogen peroxide-induced oxidative stress. Int. J. Biol. Macromol. 2019, 135, 203–211. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, X.; Chen, T.; Chen, X. A review of the antibacterial activity and mechanisms of plant polysaccharides. Trends Food Sci. Technol. 2022, 123, 264–280. [Google Scholar] [CrossRef]
- Es-Sai, B.; Wahnou, H.; Benayad, S.; Rabbaa, S.; Laaziouez, Y.; El Kebbaj, R.; Limami, Y.; Duval, R.E. Gamma-Tocopherol: A comprehensive review of its antioxidant, anti-Inflammatory, and anticancer properties. Molecules 2025, 30, 653. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Liu, K.; Swaroop, M.; Porter, F.D.; Sidhu, R.; Firnkes, S.; Ory, D.S.; Marugan, J.J.; Xiao, J.; Southall, N.; et al. delta-Tocopherol reduces lipid accumulation in Niemann-Pick type C1 and Wolman cholesterol storage disorders. J. Biol. Chem. 2012, 287, 39349–39360. [Google Scholar] [CrossRef]
- Shibata, A.; Nakagawa, K.; Tsuduki, T.; Miyazawa, T. Alpha-tocopherol suppresses antiangiogenic effect of delta-tocotrienol in human umbilical veinendothelial cells. J. Nutr. Biochem. 2015, 26, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Mathur, P.; Ding, Z.; Saldeen, T.; Mehta, J.L. Tocopherols in the prevention and treatment of atherosclerosis and related cardiovascular disease. Clin. Cardiol. 2015, 38, 511–576. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Chen, R.; Chen, J.; Yang, N.; Li, K.; Zhang, Z.; Zhang, R. Study of the Anti-Inflammatory Mechanism of β-Carotene Based on Network Pharmacology. Molecules 2023, 28, 7540. [Google Scholar] [CrossRef]
- Heaney, R.P. Phosphorus nutrition and the treatment of osteoporosis. Mayo Clin. Proc. 2004, 79, 91–97. [Google Scholar] [CrossRef]
- Weaver, C.M. Potassium and health. Adv Nutr. 2013, 4, 368S–377S. [Google Scholar] [CrossRef]
- Volpe, S.L. Magnesium in disease prevention and overall health. Adv. Nutr. 2013, 4, 378S–383S. [Google Scholar] [CrossRef]
- Benkirane, C.; Ben, M.A.; Fauconnier, M.L.; Belhaj, K.; Abid, M.; Caid, H.S.; Elamrani, A.; Mansouri, F. Bioactive compounds from hemp (Cannabis sativa L.) seeds: Optimization of phenolic antioxidant extraction using simplex lattice mixture design and HPLC-DAD/ESI-MS2 analysis. RSC Adv. 2022, 12, 25764–25777. [Google Scholar] [CrossRef]
- Sirangelo, T.M.; Ludlow, R.A.; Spadafora, N.D. Molecular mechanisms underlying potential pathogen resistance in Cannabis sativa. Plants 2023, 12, 2764. [Google Scholar] [CrossRef]
- Chen, C.; Pan, Z. Cannabidiol and terpenes from hemp–ingredients for future foods and processing technologies. J. Future Foods 2021, 1, 113–127. [Google Scholar] [CrossRef]
- Nahler, G.; Jones, T.M.; Russo, E.B. Cannabidiol and contributions of major hemp phytocompounds to the “entourage effect”; possible mechanisms. J. Altern. Complementary Integr. Med. 2019, 5, 66. [Google Scholar] [CrossRef] [PubMed]
- Leonard, W.; Zhang, P.; Ying, D.; Xiong, Y.; Fang, Z. Extrusion improves the phenolic profile and biological activities of hempseed (Cannabis sativa L.). Food Chem. 2021, 346, 128606. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Tang, J.; dos Santos Passos, C.; Nurisso, A.; Simões-Pires, C.A.; Ji, M.; Lou, H.; Fan, P. Characterization of lignanamides from hemp (Cannabis sativa L.) seed and their antioxidant and acetylcholinesterase inhibitory activities. J. Agric. Food Chem. 2015, 63, 10611–10619. [Google Scholar] [CrossRef]
- Bourjot, M.; Zedet, A.; Demange, B.; Pudlo, M.; Girard-Thernier, C. In vitro mammalian arginase inhibitory and antioxidant effects of amide derivatives isolated from thehempseed cakes (Cannabis sativa). Planta Med. Int. Open 2016, 3, e64–e67. [Google Scholar]
- Frazzini, S.; Torresani, M.C.; Roda, G.; Dell’Anno, M.; Ruffo, G.; Rossi, L. Chemical and functional characterization of the main bioactive molecules contained in hulled Cannabis sativa L. seeds for use as functional ingredients. J. Agric. Food Res. 2024, 16, 101084. [Google Scholar] [CrossRef]
- Dias, M.C.; Pinto, D.C.G.A.; Silva, A.M.S. Plant flavonoids: Chemical characteristics and biological activity. Molecules 2021, 26, 5377. [Google Scholar] [CrossRef]
- Augustyńska-Prejsnar, A.; Topczewska, J.; Ormian, M.; Sokołowicz, Z. Quality of poultry roast enriched with hemp seeds, hemp oil, and hemp flour. Foods 2022, 11, 3907. [Google Scholar] [CrossRef]
- Yang, Y.; Lewis, M.M.; Bello, A.M.; Wasilewski, E.; Clarke, H.A.; Kotra, L.P. Cannabis sativa (hemp) seeds, Δ9-tetrahydrocannabinol, and potential overdose. Cannabis Cannabinoid Res. 2017, 2, 274–281. [Google Scholar] [CrossRef]
- Singh, D.; Singh Raghuvanshi, R.; Dutta, A.; Kumar, A. Nutritional qualities of hemp seed (Cannabis sativa L.): An underutilized source of protein and fat. Pharma Innov. J. 2022, 11, 518–521. [Google Scholar]
- Przybylska-Balcerek, A.; Frankowski, J.; Graczyk, M.; Niedziela, G.; Sieracka, D.; Wacławek, S.; Sázavská, T.H.; Buśko, M.; Szwajkowska-Michałek, L.; Stuper-Szablewska, K. Profile of polyphenols, fatty acids, and terpenes in Henola hemp seeds depending on the method of fertilization. Molecules 2024, 29, 4178. [Google Scholar] [CrossRef]
- Frankowski, J.; Przybylska-Balcerek, A.; Graczyk, M.; Niedziela, G.; Sieracka, D.; Stuper-Szablewska, K. The Effect of mineral fertilization on the content of bioactive compounds in hemp seeds and oil. Molecules 2023, 28, 4870. [Google Scholar] [CrossRef] [PubMed]
- Chang, Z.-Y.; Chen, C.-W.; Tsai, M.-J.; Chen, C.-C.; Alshetaili, A.; Hsiao, Y.-T.; Fang, J.-Y. The elucidation of structure–activity and structure-permeation relationships for the cutaneous delivery of phytosterols to attenuate psoriasiform inflammation. Int. Immunopharmacol. 2023, 119, 110202. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, F.; Allay, A.; Moumen, A.B.; Benkirane, C.; Taaifi, Y.; Belhaj, K.; Addi, M.; Hano, C.; Fauconnier, M.L.; Caid, H.S.; et al. Laboratory-scale optimization of hemp seed roasting temperature and time for producing a high-quality pressed oil. J. Food Process. Preserv. 2023, 1, 8261279. [Google Scholar] [CrossRef]
- Vale, T.G.; Matos, F.J.A.; de Lima, T.C.M.; Viana, G.S.B. Behavioral effects of essential oils from Lippia alba (Mill.) N.E. brown chemotypes. J. Ethnopharmacol. 1999, 67, 127–133. [Google Scholar] [CrossRef]
- Yu, L.; Yan, J.; Sun, Z. D-limonene exhibits anti-inflammatory and antioxidant properties in an ulcerative colitis rat model via regulation of iNOS, COX-2, PGE2 and ERK signaling pathways. Mol. Med. Rep. 2017, 15, 2339–2346. [Google Scholar] [CrossRef]
- Yu, L.L.; Zhou, K.K.; Parry, J. Antioxidant properties of cold-pressed black caraway, carrot, cranberry, and hemp seed oils. Food Chem. 2005, 91, 723–729. [Google Scholar] [CrossRef]
- Buscemi, S.; Corleo, D.; Di Pace, F.; Petroni, M.L.; Satriano, A.; Marchesini, G. The Effect of Lutein on Eye and Extra-Eye Health. Nutrients 2018, 10, 1321. [Google Scholar] [CrossRef]
- Stahl, W.; Sies, H. β-Carotene and other carotenoids in protection from sunlight. Am. J. Clin. Nutr. 2012, 96, 1179S–1184S. [Google Scholar] [CrossRef] [PubMed]
- Murillo, A.G.; Hu, S.; Fernandez, M.L. Zeaxanthin: Metabolism, properties, and antioxidant protection of eyes, heart, liver, and skin. Antioxidants 2019, 8, 390. [Google Scholar] [CrossRef] [PubMed]
- Devi, V.; Khanam, S. Comparative study of different extraction processes for hemp (Cannabis sativa) seed oil considering physical, chemical and industrial-scale economic aspects. J. Clean. Prod. 2019, 207, 645–657. [Google Scholar] [CrossRef]
- Mandrioli, M.; Tura, M.; Valli, E.; Toschi, T.G. Composition of cold-pressed hemp seed oils: Key elements of quality and authenticity. La Riv. Ital. Delle Sostanze Grasse 2023, 100, 5–17. [Google Scholar]
- Santos-Zea, L.; Villela-Castrejón, J.; Gutiérrez-Uribe, J.A. Bound Phenolics in Foods. In Reference Series in Phytochemistry; Mérillon, J.M., Ramawat, K., Eds.; Springer: Cham, Switzerland, 2018. [Google Scholar]
- Laverty, K.U.; Stout, J.M.; Sullivan, M.J.; Shah, H.; Gill, N.; Holbrook, L.; Deikus, G.; Sebra, R.; Hughes, T.R.; Page, J.E. A physical and genetic map of Cannabis sativa identifies extensive rearrangements at the THC/CBD acid synthase loci. Genome Res. 2019, 29, 146–156. [Google Scholar] [CrossRef]
- Van Bakel, H.; Stout, J.; Cote, A.; Tallon, C.; Sharpe, A.; Hughes, T.; Page, J. The draft genome and transcriptome of Cannabis sativa. Genome Biol. 2011, 12, 102. [Google Scholar] [CrossRef]
- Grassa, C.J.; Weiblen, G.D.; Wenger, J.P.; Dabney, C.; Poplawski, S.G.; Motley, S.T.; Michael, T.P.; Schwartz, C.J. A new Cannabis genome assembly associates elevated cannabidiol (CBD) with hemp introgressed into marijuana. New Phytol. 2021, 230, 1665–1679. [Google Scholar] [CrossRef]
- Hurgobin, B.; Tamiru-Oli, M.; Welling, M.T.; Doblin, M.S.; Bacic, A.; Whelan, J.; Lewsey, M.G. Recent advances in Cannabis sativa genomics research. New Phytol. 2021, 230, 73–89. [Google Scholar] [CrossRef]
- Gao, S.; Wang, B.; Xie, S.; Xu, X.; Zhang, J.; Pei, L.; Yu, Y.; Yang, W.; Zhang, Y. A high quality reference genome of wild Cannabis sativa. Hortic. Res. 2020, 7, 73. [Google Scholar] [CrossRef]
- Wei, H.; Yang, Z.; Niyitanga, S.; Tao, A.; Xu, J.; Fang, P.; Lin, L.; Zhang, L.; Qi, J.; Ming, R.; et al. The reference genome of seed hemp (Cannabis sativa) provides new insights into fatty acid and vitamin E synthesis. Plant Commun. 2024, 5, 100718. [Google Scholar] [CrossRef]
- Woods, P.; Campbell, B.J.; Nicodemus, T.J.; Cahoon, E.B.; Mullen, J.L.; McKay, J.K. Quantitative trait loci controlling agronomic and biochemical traits in Cannabis sativa. Genetics 2021, 219, iyab099. [Google Scholar] [CrossRef] [PubMed]
- Petit, J.; Salentijn, E.M.J.; Paulo, M.-J.; Denneboom, C.; Trindade, L.M. Genetic architecture of flowering time and sex determination in hemp (Cannabis sativa L.): A genome-wide association study. Front. Plant Sci. 2020, 11, 569958. [Google Scholar] [CrossRef]
- Yuan, Y.; Wang, X.; Wang, L.; Xing, H.; Wang, Q.; Saeed, M.; Tao, J.; Feng, W.; Zhang, G.; Song, X.-L.; et al. Genome-wide association study identifies candidate genes related to seed oil composition and protein content in Gossypium Hirsutum L. Front. Plant Sci. 2018, 9, 1359. [Google Scholar] [CrossRef]
- Uhdre, R.; Coyne, C.J.; Bourland, B.; Piaskowski, J.; Zheng, P.; Ganjyal, G.M.; Zhang, Z.; McGee, R.J.; Main, D.; Bandillo, N.; et al. Association study of crude seed protein and fat concentration in a USDA pea diversity panel. Plant Genome 2025, 18, e20485. [Google Scholar] [CrossRef]
- Galasso, I.; Russo, R.; Mapelli, S.; Ponzoni, E.; Brambilla, I.M.; Battelli, G.; Reggiani, R. Variability in seed traits in a collection of Cannabis sativa L. genotypes. Front. Plant Sci. 2016, 7, 688. [Google Scholar] [CrossRef]
- Crossa, J.; Pérez-Rodríguez, P.; Cuevas, J.; Montesinos-López, O.; Jarquín, D.; de los Campos, G.; Burgueño, J.; González-Camacho, J.M.; Pérez-Elizalde, S.; Beyene, Y.; et al. Genomic selection in plant breeding: Methods, models, and perspectives. Trends Plant Sci. 2017, 22, 961–975. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, R. Upgrading a maize breeding program via two-cycle genome wide selection: Same cost, same or less time, and larger gains. Crop Sci. 2021, 61, 2444–2455. [Google Scholar] [CrossRef]
- Woods, P.; Price, N.; Matthews, P.; McKay, J.K. Genome-wide polymorphism and genic selection in feral and domesticated lineages of Cannabis sativa. G3 Genes|Genomes|Genet. 2023, 13, jkac209. [Google Scholar] [CrossRef]
- Yoosefzadeh Najafabadi, M.; Torkamaneh, D. Machine learning-enhanced multi-trait genomic prediction for optimizing cannabinoid profiles in cannabis. Plant J. 2025, 121, e17164. [Google Scholar] [CrossRef]
- Deguchi, M.; Shriya, K.; Potlakayala, S.; George, H.; Proano, R.; Sheri, V.; Curtis, W.R.; Rudrabhatla, S. Metabolic engineering strategies of industrial hemp (Cannabis sativa L.): A brief review of the sdvances and challenges. Front. Plant Sci. 2020, 11, 580621. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, G.; Cheng, C.; Lei, L.; Sun, J.; Xu, Y.; Deng, C.; Dai, Z.; Yang, Z.; Chen, X.; et al. Establishment of an Agrobacterium-mediated genetic transformation and CRISPR/Cas9-mediated targeted mutagenesis in hemp (Cannabis Sativa L.). Plant Biotechnol. J. 2021, 19, 1979–1987. [Google Scholar] [CrossRef] [PubMed]
- Shiels, D.; Prestwich, B.D.; Koo, O.; Kanchiswamy, C.N.; O’Halloran, R.; Badmi, R. Hemp genome editing—Challenges and opportunities. Front. Genome Ed. 2022, 4, 823486. [Google Scholar] [CrossRef]
- Turner, T.R.; James, E.K.; Poole, P.S. The plant microbiome. Genome Biol. 2013, 14, 209. [Google Scholar] [CrossRef] [PubMed]
- Koberl, M.; Schmidt, R.; Ramadan, E.M.; Bauer, R.; Berg, G. The microbiome of medicinal plants: Diversity and importance for plant growth, quality, and health. Front. Microbiol. 2013, 4, 400. [Google Scholar] [CrossRef] [PubMed]
- Duan, M.; Rao, M.J.; Li, Q.; Zhao, F.; Fan, H.; Li, B.; He, D.; Han, S.; Zhang, J.; Wang, L. Metabolomic and transcriptomic analyses provide new insights into health-promoting metabolites from Cannabis seeds growing in the Bama region of China. Agronomy 2024, 14, 787. [Google Scholar] [CrossRef]
- Markley, J.L.; Brüschweiler, R.; Edison, A.S.; Eghbalnia, H.R.; Powers, R.; Raftery, D.; Wishart, D.S. The future of NMR-based metabolomics. Curr. Opin. Biotechnol. 2017, 43, 34–40. [Google Scholar] [CrossRef]
- Siudem, P.; Zielińska, Z.; Kowalska, V.; Paradowska, K. 1H NMR and chemometric methods in verification of hemp-seed oil quality. J. Pharm. Biomed. Anal. 2022, 212, 114650. [Google Scholar] [CrossRef]
- Rosso, E.; Armone, R.; Costale, A.; Meineri, G.; Chiofalo, B. Hemp seed (Cannabis sativa L.) varieties: Lipids profile and antioxidant capacity for monogastric nutrition. Animals 2024, 14, 2699. [Google Scholar] [CrossRef]
- Schultz, C.J.; Lim, W.L.; Khor, S.F.; Neumann, K.A.; Schulz, J.M.; Ansari, O.; Skewes, M.A.; Burton, R.A. Consumer and health-related traits of seed from selected commercial and breeding lines of industrial hemp, Cannabis sativa L. J. Agric. Food Res. 2020, 2, 100025. [Google Scholar] [CrossRef]
- Padilla-González, G.F.; Rosselli, A.; Sadgrove, N.J.; Cui, M.; Simmonds, M.S.J. Mining the chemical diversity of the hemp seed (Cannabis sativa L.) metabolome: Discovery of a new molecular family widely distributed across hemp. Front. Plant Sci. 2023, 14, 1114398. [Google Scholar] [CrossRef]
- Jeong, H.; Yoon, S.; Jo, S.M.; Hong, S.J.; Ban, Y.; Park, H.; Youn, M.Y.; Shin, E.C. Chemosensory of hemp seed oil extracted with hemp seed (Cannabis sativa L.) roasted under various conditions using electronic sensors and GC–MS/Olfactometry. Food Chem. 2024, 21, 101226. [Google Scholar]
- Citti, C.; Linciano, P.; Panseri, S.; Vezzalini, F.; Forni, F.; Vandelli, M.A.; Cannazza, G. Cannabinoid profiling of hemp seed oil by liquid chromatography coupled to high-resolution mass spectrometry. Front. Plant Sci. 2019, 10, 120. [Google Scholar] [PubMed]
- Han, X.L.; Gross, R.W. Electrospray-ionization mass spectroscopic analysis of human erythrocyte plasma-membrane phospholipids. Proc. Natl. Acad. Sci. USA 1994, 91, 10635–10639. [Google Scholar] [CrossRef]
- Han, X.L.; Gross, R.W. Global analyses of cellular lipidomes directly from crude extracts of biological samples by ESI mass spectrometry: A bridge to lipidomics. J. Lipid Res. 2003, 44, 1071–1079. [Google Scholar] [CrossRef] [PubMed]
- Buré, C.; Solgadi, A.; Yen-Nicolay, S.; Bardeau, T.; Libong, D.; Abreu, S.; Chaminade, P.; Subra-Paternault, P.; Cansell, M. Electrospray mass spectrometry as a tool to characterize phospholipid composition of plant cakes. Eur. J. Lipid Sci. Technol. 2016, 118, 1282–1292. [Google Scholar] [CrossRef]
- Cerrato, A.; Aita, S.A.; Cannazza, G.; Cavaliere, C.; Cavazzini, A.; Citti, C.; Montone, C.M.; Taglioni, E.; Laganà, A. One-phase extraction coupled with photochemical reaction allows the in-depth lipid characterization of hempseeds by untargeted lipidomics. Talanta 2024, 271, 125686. [Google Scholar] [CrossRef] [PubMed]
- Rustam, Y.H.; Reid, G.E. Analytical challenges and recent advances in mass spectrometry based lipidomics. Anal Chem. 2018, 90, 374–397. [Google Scholar] [CrossRef]
- Han, X.L.; Gross, R.W. Shotgun lipidomics: Electrospray ionization mass spectrometric analysis and quantitation of cellular lipidomes directly from crude extracts of biological samples. Mass Spectrom. Rev. 2005, 24, 367–412. [Google Scholar] [CrossRef]
- Cerrato, A.; Capriotti, A.L.; Montone, C.M.; Aita, S.E.; Cannazza, G.; Citti, C.; Piovesana, S.; Aldo, L. Analytical Methodologies for Lipidomics in Hemp Plant. In Mass Spectrometry-Based Lipidomics: Methods and Protocols; Springer: New York, NY, USA, 2021; Volume 2306, pp. 257–273. [Google Scholar]
- Bakhytkyzy, I.; Hewelt-Belka, W.; Kot-Wasik, A. A comprehensive lipidomic analysis of oilseeds using LC-Q-TOF-MS and dispersive micro-solid phase (D-μ-SPE) extraction techniques. J. Food Compos. Anal. 2023, 116, 105037. [Google Scholar]
- Kozub, A.; Nikolaichuk, H.; Przykaza, K.; Tomaszewska-Gras, J.; Fornal, E. Lipidomic characteristics of three edible cold-pressed oils by LC/Q-TOF for simple quality and authenticity assurance. Food Chem. 2023, 415, 135761. [Google Scholar] [CrossRef]
- Lopez, C.; Novales, B.; Rabesona, H.; Weber, M.; Chardot, T.; Anton, M. Deciphering the properties of hemp seed oil bodies for food applications: Lipid composition, microstructure, surface properties and physical stability. Food Res. Int. 2021, 150, 110759. [Google Scholar] [CrossRef] [PubMed]
- Küllenberg, D.; Taylor, L.A.; Schneider, M.; Massing, U. Health effects of dietary phospholipids. Lipids Health. Dis. 2012, 11, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Salt, D.E.; Baxter, I.; Lahner, B. Ionomics and the study of the plant ionome. Annu Rev Plant Biol. 2008, 59, 709–733. [Google Scholar] [CrossRef] [PubMed]
- Esteban, J.I.A.; Torija-Isasa, M.E.; Sánchez-Mata, M.C. Mineral elements and related antinutrients, in whole and hulled hemp (Cannabis sativa L.) seeds. J. Food Compos. Anal. 2022, 109, 104516. [Google Scholar] [CrossRef]
- Skoog, D.A.; Holler, F.J.; Crouch, S.R. Principal of Instrumental Analysis, 7th ed.; Sunder College Publisher: New York, NY, USA, 2017. [Google Scholar]
- Lan, Y.; Zha, F.; Peckrul, A.; Hanson, B.; Johnson, B.; Rao, J.; Chen, B. Genotype x environmental effects on yielding ability and seed chemical composition of industrial hemp (Cannabis sativa L) varieties grown in North Dakota, USA. J. Am. Oil Chem. Soc. 2019, 96, 1417–1425. [Google Scholar] [CrossRef]
- Ramos-Sanchez, R.; Hayward, N.J.; Henderson, D.; Duncan, G.J.; Russell, W.R.; Duncan, S.H.; Neacsu, M. Hemp seed-based foods and processing by-products are sustainable rich sources of nutrients and plant metabolites supporting dietary biodiversity, health, and nutritional needs. Foods 2025, 14, 875. [Google Scholar] [CrossRef]
- Righetti, P.G.; Fasoli, E.; Boschetti, E. Combinatorial peptide ligand libraries: The conquest of the ‘hidden proteome’ advances at great strides. Electrophoresis 2011, 32, 960–966. [Google Scholar] [CrossRef]
- Mamone, G.; Picariello, G.; Ramondo, A.; Nicolai, M.A.; Ferranti, P. Production, digestibility and allergenicity of hemp (Cannabis sativa L.) protein isolates. Food Res. Int. 2019, 115, 562–571. [Google Scholar]
- Dong, X.; Rawiwan, P.; Middleditch, M.; Guo, G.; Woo, M.W.; Quek, S.Y. Effects of protein variations by different extraction and dehydration approaches on hempseed protein isolate: Protein pattern, amino acid profiling and label-free proteomics. Food Chem. 2024, 460, 140426. [Google Scholar] [CrossRef]
- Cabral, E.M.; Poojary, M.M.; Lund, M.N.; Curtin, J.; Fenelon, M.; Tiwari, B.K. Effect of solvent composition on the extraction of proteins from hemp oil processing stream. J. Sci. Food Agric. 2022, 102, 6293–6298. [Google Scholar] [CrossRef]
- Zha, F.; Dong, S.; Rao, J.; Chen, B. The structural modification of pea protein concentrate with gum Arabic by controlled Maillard reaction enhances its functional properties and flavor attributes. Food Hydrocoll. 2019, 92, 30–40. [Google Scholar] [CrossRef]
- Dupree, E.J.; Jayathirtha, M.; Yorkey, H.; Mihasan, M.; Petre, B.A.; Darie, C.C. A critical review of bottom-up proteomics: The good, the bad, and the future of this field. Proteomes 2020, 8, 14. [Google Scholar] [CrossRef] [PubMed]
- Malomo, S.A.; Aluko, R.E. A comparative study of the structural and functional properties of isolated hemp seed (Cannabis sativa L.) albumin and globulin fractions. Food Hydrocoll. 2015, 43, 743–752. [Google Scholar] [CrossRef]
- Decuyper, I.I.; Van Gasse, A.L.; Cop, N.; Sabato, V.; Faber, M.A.; Mertens, C.; Bridts, C.H.; Hagendorens, M.M.; De Clerck, L.; Rihs, H.P.; et al. Cannabis sativa allergy: Looking through the fog. Allergy 2017, 72, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Julakanti, S.; Charles, A.P.R.; Zhao, J.; Bullock, F.; Syed, R.; Myles, Y.; Wu, Y. Hempseed protein (Cannabis sativa L.): Influence of extraction pH and ball milling on physicochemical and functional properties. Food Hydrocoll. 2023, 143, 108835. [Google Scholar] [CrossRef]
- Suchintita, D.R.; Tiwari, B.K.; Chemat, F.; Garcia-Vaquero, M. Impact of ultrasound processing on alternative protein systems: Protein extraction, nutritional effects and associated challenges. Ultrason. Sonochem. 2022, 91, 106234. [Google Scholar] [CrossRef]
- Pandita, D.; Pandita, A.; Wani, S.H.; Abdelmohsen, S.A.M.; Alyousef, H.A.; Abdelbacki, A.M.M.; Al-Yafrasi, M.A.; Al-Mana, F.A.; Elansary, H.O. Crosstalk of multi-omics platforms with plants of therapeutic importance. Cells 2021, 10, 1296. [Google Scholar] [CrossRef]
- Huo, Q.; Song, R.; Ma, Z. Recent advances in exploring transcriptional regulatory landscape of crops. Front. Plant Sci. 2024, 15, 1421503. [Google Scholar] [CrossRef]
- Depuydt, T.; De Rybel, B.; Vandepoele, K. Charting plant gene functions in the multi-omics and single-cell era. Trends Plant Sci. 2023, 28, 283–296. [Google Scholar] [CrossRef]
- Amer, B.; Baidoo, E.E.K. Omics-Driven Biotechnology for Industrial Applications. Front. Bioeng. Biotechnol. 2021, 9, 613307. [Google Scholar] [CrossRef]
- Gong, M.; Lu, H.; Li, L.; Feng, M.; Zou, Z. Integration of transcriptomics and metabonomics revealed the protective effects of hemp seed oil against methionine-choline-deficient diet-induced non-alcoholic steatohepatitis in mice. Food Funct. 2023, 14, 2096–2111. [Google Scholar] [CrossRef] [PubMed]
| Nutrients and Bioactive Compounds | Omics Approach | Hemp Variety | Main Methods | Main Results | References |
|---|---|---|---|---|---|
| PUFAs, tocopherol crude proteins, and phenolics | Metabolomics | CanMa, Anka, Jutta and Yvonne, Delores, CFX-1, CFX-2, and CRS-1, and Finola | GC and GC-FID |
| [39] |
| FAs, phenolics, amino acids, and cannabinoids | Untargeted metabolomics | Two accessions cultivated in Madhya Pradesh and in Himachal Pradesh | GC-MS and HPLC |
| [17] |
| Lipids, lignans, flavanonoids, EAAs, saccha-rides, vitamins, and cannabinoids | Targeted metabolomics | Seven Chinese hemp varieties | UHPLC-QQQ-MS/MS |
| [12] |
| FAs phenolics, crude proteins, and fibers | Targeted metabolomics | Carmaenecta, Enectaliana, and Enectarol | GC-FID and HPLC-MS |
| [154] |
| FAs (LA, ALA, and γ-linolenic acid) | Metabolomics | Felina 32, Chamaeleon, Uso31, and Finola | GC-FID |
| [52] |
| FAs (LA, ALA cholesterol, and tocopherol | Metabolomics | _ | GC-FID | Diet including hemp seeds:
| [89] |
| Carbohydrates, proteins, lipids, phytate, and lignin | Metabolomics | 20 hemp cultivars and advanced breeding lines | HPLC and GC-FID |
| [155] |
| Polysaccharides (soluble) | Metabolomics | Futura 75 | HPLC |
| [11] |
| Terpenes (α-pinene, β-pinene, and linalool) | Metabolomics | _ | GC–MS and GC-O |
| [157] |
| Oils, terpenes (β-myrcene, and D-limonene) | Metabolomics | _ | GC-FID and SPME |
| [121] |
| FAs, Lignanamides, and HCAAs | Untargeted metabolomics | Kongo Hanf, Spanish accession, French accession, and Italian Eletta Campana | LC-MS |
| [156] |
| Phenylpropanoids, HCAAs, lignanamides, and cannabinoids | Metabolomics | _ | HPLC-DAD/ESI-MS/MS |
| [106] |
| Lignanamides and HCAAs | Metabolomics | CRS1 variety | HPLC-ESI-Q-TOF-MS/MS. HPLC-DAD |
| [110] |
| Polyphenols, Flavonoids, crude lipids, and fiber | Metabolomics | _ | HPLC-Q-TOF-MS/MS |
| [113] |
| Lipids, flavonoids, amino acids, lignans coumarins, and terpenoids | Targeted metabolomics | Hemp seeds of seven villages in Bama County | UPLC-MS/MS |
| [50] |
| Phytosterol (β-sitosterol, and campesterol) | Metabolomics | Italian (IT) and Extra-European (EE) varieties | HRGC and HPLC-GC-FID |
| [83] |
| FAs (ALA, LA), polyphenols, proteins, polysaccarides, and bioactive compounds | Metabolomics | Santhica 27, Fedora 32, Felina 32, Futura 75, Tygra, Bialobrzeskie, and Finola | HPLC-DAD |
| [53] |
| Oils, carotenoids, phenols, and tocopherols | Metabolomics | Thirteen different commercial hemp varieties | UHPLC, HPLC-UV, and HPLC |
| [85] |
| Phytocannabinoids | Metabolomics | _ | UHPLC-HRGC/MS |
| [158] |
| PL, ALA, and LA | Lipidomics | C. sativa | Shotgun and RPLC associated to MRM-NPLC |
| [161] |
| FA, PL, and minor lipid classes | Lipidomics | Nine hemp varieties | HRMS |
| [162] |
| DAGs and TAGs | Lipidomics | C. sativa | LC-Q-TOF-MS |
| [167] |
| LPC, LPE, PC, DG, and TG | Lipidomics | C. sativa | HRMS |
| [166] |
| Proteins (11S edestin, 2S al-bumin, and 7S vicilin-like) | Proteomics | Futura 75 and Finola | SDS-PAGE, 2D-gel electrophoresis, and MS |
| [42] |
| Proteins (11S edestin, 2S al-bumin, 7S vicilin-like, etc.) | Proteomics | Futura | SDS-PAGE separation and nLC-ESI-MS/MS identification |
| [41] |
| Proteins (11S edestin, 2S al-bumin, 7S vicilin-like, etc.) | Proteomics | Cheungsam | 2D-gel electrophoresis and MS |
| [66] |
| Minerals (Fe, Zn, Mn, Cu, Mo, Ni, and Co), FAs, phytosterols, and phenols | Metabolomics and ionomics | Hemp genotype Fedora | GC-FID, ICP-OES, and RP-HPLC-DAD |
| [40] |
| Minerals (P, K, Mg, Zn, Ca, Mn, and Cu) and antinutrients (phytates) | Metabolomics and ionomics | Bialobrzeskie, Carmagnola, Fedora 17, Felina 32, KC Dora, Kompolti, Santhica 27, and Tiborszallasi | UV/visible spectroscopy |
| [171] |
| Minerals (Ca, Na, K, Fe, Mn, P, and Mg), crude proteins, and FAs | Metabolomics and ionomics | Industrial hemps | GC-FID and ICP-OES |
| [173] |
| Lipids (ALA and LA) | Metabolomics and transcriptomics | Bama county hemp seeds varieties | MS and qRT-PCR |
| [76] |
| Proteins (11S edestin, 2S al-bumin, and 7S vicilin-like) | Genomics and transcriptomics | Futura | GWAS and qRT-PCR |
| [64] |
| Proteins (11S edestin) | Genomics, transcriptomics, and proteomics | Carmagnola | qRT-PCR and SDS PAGE |
| [67] |
| Health-promoting metabolites (cannflavins (A, B, and C), trigonelline, citric acid, vitexin, choline alfoscerate, and choline) | Targeted metabolomics and transcriptomics | Bama hemp seeds varieties | UHPLC–ESI–MS/MS and RNA-Seq |
| [151] |
| Nutrients and Bioactive Compounds | Omics Approach | Variety of Hemp | Main Methods | Main Results | Properties of Hemp Seed by-Products | Reference |
|---|---|---|---|---|---|---|
| 11S edestin, 2S albumin, and 7S vicilin-like In whole, dehulled seed, and hull | Metabolomics and proteomics | Santhica 27 and Uso-31 | LC-MS/MS and SDS-PAGE |
|
| [46] |
| Phenolics, oils, and proteins In whole and dehulled seed, and hull | Metabolomics | Bama and Yunma | LH-20 gel chromatography and HPLC methods |
|
| [16] |
| HPI In flour | Proteomics | Carmagnola | LC-ESI-MS/MS, and SDS PAGE |
|
| [176] |
| FAs, polyphenols, and amino acids In flour | Metabolomics | _ | GC-MS, HPLC-FLD, HPLC-FLD, and GC-FID |
|
| [44] |
| HCAAs, caffeic acid, cannaflin C, FAs, EAAs, gliadin, and glutenin-free proteins In flour | Metabolomics | Cannabis sativa | UHPLC- ESI/QTOF-MS |
|
| [22] |
| HPI In meal | Proteomics | _ | SDS-PAGE |
|
| [178] |
| HPI In meal | Proteomics | - | SDS-PAGE |
|
| [45] |
| HPI In meal | Metabolomics and proteomics | Different industrial hempseeds cultivars from crop year 2017 | SDS-PAGE, SEC-HPLC-UV/RI/MALS, CD spectroscopy and DSC, and HS-SPME-GC–MS |
|
| [55] |
| HPC In meal | Proteomics | Futura 75 | SDS PAGE |
|
| [183] |
| HPI In meal | Metabolomics | _ | HPLC |
|
| [181] |
| Phenols, tocopherols, FAs, proteins, and crude fibers In meal | Metabolomics | USO 31 and Futura 75 | LC-DAD-ESI-MS |
|
| [23] |
| Hemp seed oil extract (HOE) | Metabolomics, proteomics, and transcriptomics | _ | UPLC-MS, GC-MS, and RNA-Seq |
|
| [47] |
| Minerals (K, Mg, P, Mn, and Fe), proteins, fibers, FAs, and bioactive compounds (p-coumaric acid and syringaresinol) In oil, dehulled seeds, cake, and flour | Metabolomics and proteomics | _ | ICP-MS |
|
| [174] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sirangelo, T.M.; Diretto, G.; Fiore, A.; Felletti, S.; Chenet, T.; Catani, M.; Spadafora, N.D. Nutrients and Bioactive Compounds from Cannabis sativa Seeds: A Review Focused on Omics-Based Investigations. Int. J. Mol. Sci. 2025, 26, 5219. https://doi.org/10.3390/ijms26115219
Sirangelo TM, Diretto G, Fiore A, Felletti S, Chenet T, Catani M, Spadafora ND. Nutrients and Bioactive Compounds from Cannabis sativa Seeds: A Review Focused on Omics-Based Investigations. International Journal of Molecular Sciences. 2025; 26(11):5219. https://doi.org/10.3390/ijms26115219
Chicago/Turabian StyleSirangelo, Tiziana M., Gianfranco Diretto, Alessia Fiore, Simona Felletti, Tatiana Chenet, Martina Catani, and Natasha Damiana Spadafora. 2025. "Nutrients and Bioactive Compounds from Cannabis sativa Seeds: A Review Focused on Omics-Based Investigations" International Journal of Molecular Sciences 26, no. 11: 5219. https://doi.org/10.3390/ijms26115219
APA StyleSirangelo, T. M., Diretto, G., Fiore, A., Felletti, S., Chenet, T., Catani, M., & Spadafora, N. D. (2025). Nutrients and Bioactive Compounds from Cannabis sativa Seeds: A Review Focused on Omics-Based Investigations. International Journal of Molecular Sciences, 26(11), 5219. https://doi.org/10.3390/ijms26115219







