The Predictive Impact of HPV Genotypes, Tumor Suppressors and Local Immune Response in the Regression of Cervical Intraepithelial Neoplasia 2-3: A Prospective Population-Based Cohort Study
Abstract
1. Introduction
2. Results
2.1. Genotypes and Regression Rate
2.2. Levels of Biomarkers and CIN Lesion Length in Relation to Regression Rate
2.3. Levels of Biomarkers, CIN Lesion Lengths, and Regression Rates Relative to HPV Genotypes
3. Discussion
4. Materials and Methods
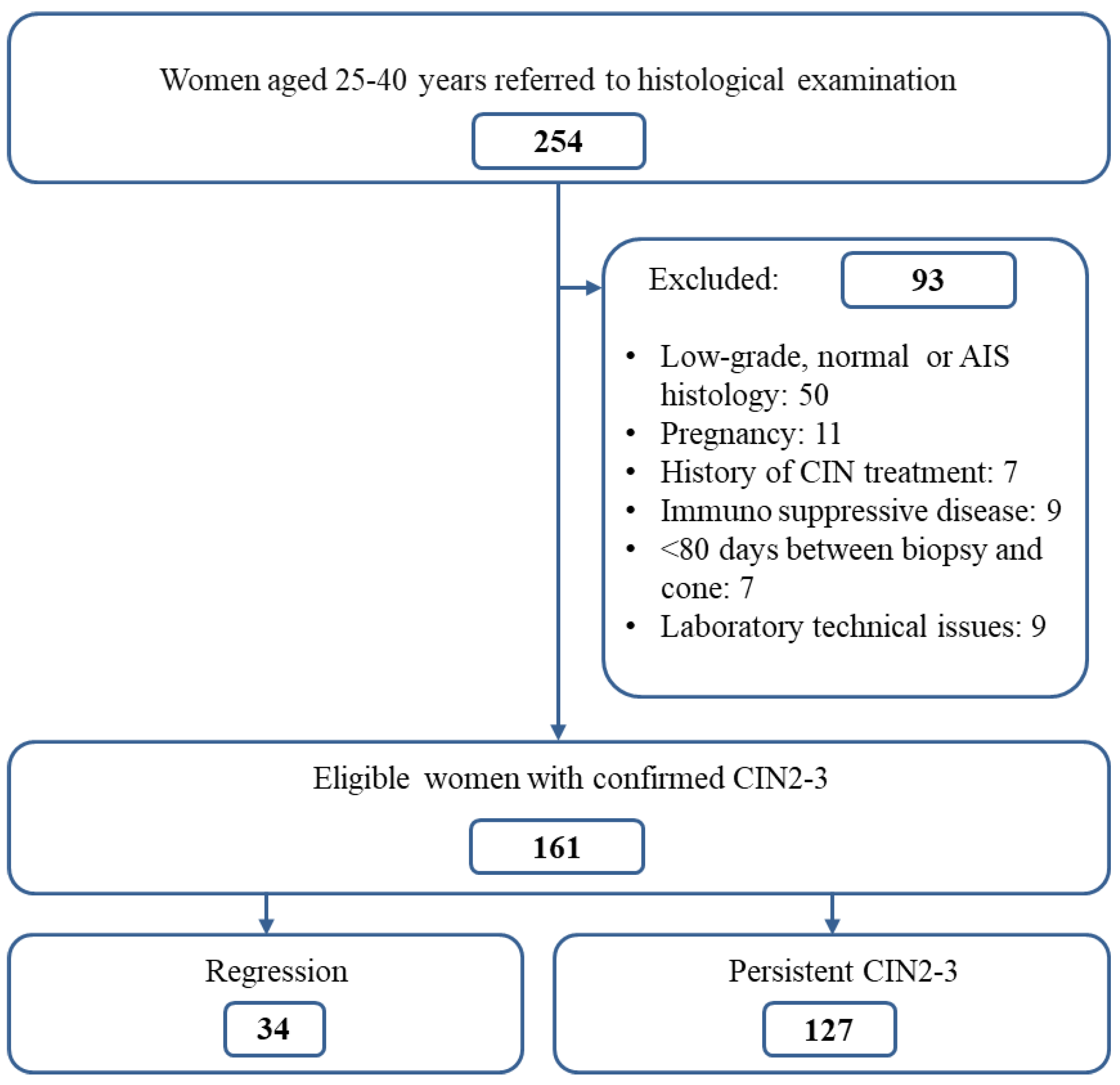
4.1. Study Population
4.2. Gynecologic Methods
4.3. Pathology
4.4. Semi-Quantitative Scoring of Immunohistochemical Staining and CIN Length Measurements
4.5. HPV Analyses
4.6. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HPV | Human papilloma virus |
| hr-HPV | High-risk human papilloma virus |
| CIN | Cervical intraepithelial neoplasia |
| LSIL | Low-grade squamous intraepithelial lesion |
| ASCUS | Atypical squamous cells of undetermined significance |
| ASC-H | Atypical squamous cells cannot rule out a high-grade lesion |
| HSIL | High-grade squamous intraepithelial lesion |
| AGUS | Atypical glandular cells |
| AIS | Adenocarcinoma in situ |
| ADC | Adenocarcinoma |
| SCC | Squamous cell carcinoma |
| Th | T-helper cell |
| DNA | Deoxyribonucleic acid |
| mRNA | Messenger ribonucleic acid |
| HR | Hazard ratio |
| RR | Relative risk |
| ROC | Receiver operating characteristic curve |
| CIs | Confidence intervals |
| AUC | Area under the curve |
| HES | Hematoxylin–eosin–saffron |
| LEEP | Loop electrosurgical excision procedure |
References
- Coutlée, F.; Ratnam, S.; Ramanakumar, A.V.; Insinga, R.R.; Bentley, J.; Escott, N.; Ghatage, P.; Koushik, A.; Ferenczy, A.; Franco, E.L. Distribution of human papillomavirus genotypes in cervical intraepithelial neoplasia and invasive cervical cancer in Canada. J. Med. Virol. 2011, 83, 1034–1041. [Google Scholar] [CrossRef]
- Walboomers, J.M.; Jacobs, M.V.; Manos, M.M.; Bosch, F.X.; Kummer, J.A.; Shah, K.V.; Snijders, P.J.; Peto, J.; Meijer, C.J.; Muñoz, N. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J. Pathol. 1999, 189, 12–19. [Google Scholar] [CrossRef]
- Shin, C.H.; Schorge, J.O.; Lee, K.R.; Sheets, E.E. Conservative management of adenocarcinoma in situ of the cervix. Gynecol. Oncol. 2000, 79, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Madeleine, M.M.; Daling, J.R.; Schwartz, S.M.; Shera, K.; McKnight, B.; Carter, J.J.; Wipf, G.C.; Critchlow, C.W.; McDougall, J.K.; Porter, P.; et al. Human papillomavirus and long-term oral contraceptive use increase the risk of adenocarcinoma in situ of the cervix. Cancer Epidemiol. Biomark. Prev. 2001, 10, 171–177. [Google Scholar] [PubMed]
- Lee, K.R.; Flynn, C.E. Early invasive adenocarcinoma of the cervix. Cancer 2000, 89, 1048–1055. [Google Scholar] [CrossRef]
- Watson, M.; Soman, A.; Flagg, E.W.; Unger, E.; Deapen, D.; Chen, V.W.; Peres, L.C.; Copeland, G.; Tucker, T.C.; Garnett, E.; et al. Surveillance of high-grade cervical cancer precursors (CIN III/AIS) in four population-based cancer registries, United States, 2009–2012. Prev. Med. 2017, 103, 60–65. [Google Scholar] [CrossRef]
- Bruno, M.T.; Valenti, G.; Cassaro, N.; Palermo, I.; Incognito, G.G.; Cavallaro, A.G.; Sgalambro, F.; Panella, M.M.; Mereu, L. The Coexistence of Cervical Intraepithelial Neoplasia (CIN3) and Adenocarcinoma In Situ (AIS) in LEEP Excisions Performed for CIN3. Cancers 2024, 16, 847. [Google Scholar] [CrossRef]
- Trottier, H.; Franco, E.L. The epidemiology of genital human papillomavirus infection. Vaccine 2006, 24 (Suppl. 1), S1–S15. [Google Scholar] [CrossRef]
- Cuschieri, K.S.; Cubie, H.A.; Whitley, M.W.; Gilkison, G.; Arends, M.J.; Graham, C.; McGoogan, E. Persistent high risk HPV infection associated with development of cervical neoplasia in a prospective population study. J. Clin. Pathol. 2005, 58, 946–950. [Google Scholar] [CrossRef]
- McCredie, M.R.; Sharples, K.J.; Paul, C.; Baranyai, J.; Medley, G.; Jones, R.W.; Skegg, D.C. Natural history of cervical neoplasia and risk of invasive cancer in women with cervical intraepithelial neoplasia 3: A retrospective cohort study. Lancet Oncol. 2008, 9, 425–434. [Google Scholar] [CrossRef]
- Ostor, A.G. Natural history of cervical intraepithelial neoplasia: A critical review. Int. J. Gynecol. Pathol. 1993, 12, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Munk, A.C.; Gudlaugsson, E.; Ovestad, I.T.; Lovslett, K.; Fiane, B.; Hidle Bv Kruse, A.J.; Skaland, I.; Janssen, E.A.; Baak, J.P. Interaction of epithelial biomarkers, local immune response and condom use in cervical intraepithelial neoplasia 2-3 regression. Gynecol. Oncol. 2012, 127, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Ovestad, I.T.; Gudlaugsson, E.; Skaland, I.; Malpica, A.; Munk, A.C.; Janssen, E.A.; Baak, J.P. The impact of epithelial biomarkers, local immune response and human papillomavirus genotype in the regression of cervical intraepithelial neoplasia grades 2–3. J. Clin. Pathol. 2011, 64, 303–307. [Google Scholar] [CrossRef]
- Trimble, C.L.; Piantadosi, S.; Gravitt, P.; Ronnett, B.; Pizer, E.; Elko, A.; Wilgus, B.; Yutzy, W.; Daniel, R.; Shah, K.; et al. Spontaneous regression of high-grade cervical dysplasia: Effects of human papillomavirus type and HLA phenotype. Clin. Cancer Res. 2005, 11, 4717–4723. [Google Scholar] [CrossRef] [PubMed]
- Skorstengaard, M.; Lynge, E.; Suhr, J.; Napolitano, G. Conservative management of women with cervical intraepithelial neoplasia grade 2 in Denmark: A cohort study. BJOG 2020, 127, 729–736. [Google Scholar] [CrossRef]
- Perkins, R.B.; Guido, R.S.; Castle, P.E.; Chelmow, D.; Einstein, M.H.; Garcia, F.; Huh, W.K.; Kim, J.J.; Moscicki, A.B.; Nayar, R.; et al. 2019 ASCCP Risk-Based Management Consensus Guidelines for Abnormal Cervical Cancer Screening Tests and Cancer Precursors. J. Low. Genit. Tract Dis. 2020, 24, 102–131. [Google Scholar] [CrossRef]
- NHS England. Colposcopic Diagnosis, Treatment and Follow Up; NHS England: London, UK, 2023. [Google Scholar]
- Kyrgiou, M.; Athanasiou, A.; Paraskevaidi, M.; Mitra, A.; Kalliala, I.; Martin-Hirsch, P.; Arbyn, M.; Bennett, P.; Paraskevaidis, E. Adverse obstetric outcomes after local treatment for cervical preinvasive and early invasive disease according to cone depth: Systematic review and meta-analysis. BMJ 2016, 354, i3633. [Google Scholar] [CrossRef]
- Kjær, S.K.; Frederiksen, K.; Munk, C.; Iftner, T. Long-term absolute risk of cervical intraepithelial neoplasia grade 3 or worse following human papillomavirus infection: Role of persistence. J. Natl. Cancer Inst. 2010, 102, 1478–1488. [Google Scholar] [CrossRef]
- Thomsen, L.T.; Frederiksen, K.; Munk, C.; Junge, J.; Iftner, T.; Kjaer, S.K. Long-term risk of cervical intraepithelial neoplasia grade 3 or worse according to high-risk human papillomavirus genotype and semi-quantitative viral load among 33,288 women with normal cervical cytology. Int. J. Cancer 2015, 137, 193–203. [Google Scholar] [CrossRef]
- Cosper, P.F.; Bradley, S.; Luo, L.; Kimple, R.J. Biology of HPV Mediated Carcinogenesis and Tumor Progression. Semin. Radiat. Oncol. 2021, 31, 265–273. [Google Scholar] [CrossRef]
- Muntinga, C.L.P.; de Vos van Steenwijk, P.J.; Bekkers, R.L.M.; van Esch, E.M.G. Importance of the Immune Microenvironment in the Spontaneous Regression of Cervical Squamous Intraepithelial Lesions (cSIL) and Implications for Immunotherapy. J. Clin. Med. 2022, 11, 1432. [Google Scholar] [CrossRef] [PubMed]
- Insinga, R.P.; Dasbach, E.J.; Elbasha, E.H.; Liaw, K.L.; Barr, E. Progression and regression of incident cervical HPV 6, 11, 16 and 18 infections in young women. Infect. Agent. Cancer 2007, 2, 15. [Google Scholar] [CrossRef]
- Graham, S.V. The human papillomavirus replication cycle, and its links to cancer progression: A comprehensive review. Clin. Sci. 2017, 131, 2201–2221. [Google Scholar] [CrossRef]
- Scarth, J.A.; Patterson, M.R.; Morgan, E.L.; Macdonald, A. The human papillomavirus oncoproteins: A review of the host pathways targeted on the road to transformation. J. Gen. Virol. 2021, 102, 001540. [Google Scholar] [CrossRef]
- Castellsague, X.; Munoz, N. Chapter 3: Cofactors in human papillomavirus carcinogenesis–role of parity, oral contraceptives, and tobacco smoking. JNCI Monogr. 2003, 2003, 20–28. [Google Scholar] [CrossRef]
- Gadducci, A.; Barsotti, C.; Cosio, S.; Domenici, L.; Riccardo Genazzani, A. Smoking habit, immune suppression, oral contraceptive use, and hormone replacement therapy use and cervical carcinogenesis: A review of the literature. Gynecol. Endocrinol. 2011, 27, 597–604. [Google Scholar] [CrossRef]
- Matsumoto, K.; Oki, A.; Furuta, R.; Maeda, H.; Yasugi, T.; Takatsuka, N.; Hirai, Y.; Mitsuhashi, A.; Fujii, T.; Iwasaka, T.; et al. Tobacco smoking and regression of low-grade cervical abnormalities. Cancer Sci. 2010, 101, 2065–2073. [Google Scholar] [CrossRef] [PubMed]
- Leo, P.J.; Madeleine, M.M.; Wang, S.; Schwartz, S.M.; Newell, F.; Pettersson-Kymmer, U.; Hemminki, K.; Hallmans, G.; Tiews, S.; Steinberg, W.; et al. Defining the genetic susceptibility to cervical neoplasia—A genome-wide association study. PLoS Genet. 2017, 13, e1006866. [Google Scholar] [CrossRef]
- Bicskei, B.; Lillesand, M.; Rewcastle, E.; Ovestad, I.T.; Motlu, P.; Christiansen, I.K.; Gudlaugsson, E.G.; Janssen, E.A.; Mohr Berland, J. A comparison of the Linear Array and the Automated INNO-LiPA HPV Genotyping Systems on Pathological Specimens. J. Cancer Sci. Clin. Ther. 2020, 4, 349–364. [Google Scholar] [CrossRef]
- Smith, J.S.; Lindsay, L.; Hoots, B.; Keys, J.; Franceschi, S.; Winer, R.; Clifford, G.M. Human papillomavirus type distribution in invasive cervical cancer and high-grade cervical lesions: A meta-analysis update. Int. J. Cancer 2007, 121, 621–632. [Google Scholar] [CrossRef]
- de Sanjose, S.; Quint, W.G.; Alemany, L.; Geraets, D.T.; Klaustermeier, J.E.; Lloveras, B.; Tous, S.; Felix, A.; Bravo, L.E.; Shin, H.R.; et al. Human papillomavirus genotype attribution in invasive cervical cancer: A retrospective cross-sectional worldwide study. Lancet Oncol. 2010, 11, 1048–1056. [Google Scholar] [CrossRef] [PubMed]
- Cogliano, V.; Baan, R.; Straif, K.; Grosse, Y.; Secretan, B.; El Ghissassi, F. Carcinogenicity of human papillomaviruses. Lancet Oncol. 2005, 6, 204. [Google Scholar] [CrossRef]
- Cai, S.; Han, K. Research on expression and importance of p53, p16 and VEGF-C in cervical cancer. J. Gynecol. Obstet. Biol. Reprod. 2015, 44, 639–645. [Google Scholar] [CrossRef]
- Sand, F.L.; Munk, C.; Frederiksen, K.; Junge, J.; Iftner, T.; Dehlendorff, C.; Kjaer, S.K. Risk of CIN3 or worse with persistence of 13 individual oncogenic HPV types. Int. J. Cancer 2019, 144, 1975–1982. [Google Scholar] [CrossRef] [PubMed]
- Clifford, G.; Franceschi, S. Members of the human papillomavirus type 18 family (alpha-7 species) share a common association with adenocarcinoma of the cervix. Int. J. Cancer 2008, 122, 1684–1685. [Google Scholar] [CrossRef]
- Pinheiro, M.; Gage, J.C.; Clifford, G.M.; Demarco, M.; Cheung, L.C.; Chen, Z.; Yeager, M.; Cullen, M.; Boland, J.F.; Chen, X.; et al. Association of HPV35 with cervical carcinogenesis among women of African ancestry: Evidence of viral-host interaction with implications for disease intervention. Int. J. Cancer 2020, 147, 2677–2686. [Google Scholar] [CrossRef]
- Wei, F.; Georges, D.; Man, I.; Baussano, I.; Clifford, G.M. Causal attribution of human papillomavirus genotypes to invasive cervical cancer worldwide: A systematic analysis of the global literature. Lancet 2024, 404, 435–444. [Google Scholar] [CrossRef]
- Kombe Kombe, A.J.; Li, B.; Zahid, A.; Mengist, H.M.; Bounda, G.A.; Zhou, Y.; Jin, T. Epidemiology and Burden of Human Papillomavirus and Related Diseases, Molecular Pathogenesis, and Vaccine Evaluation. Front. Public Health 2020, 8, 552028. [Google Scholar] [CrossRef]
- Dovey de la Cour, C.; Guleria, S.; Nygård, M.; Trygvadóttir, L.; Sigurdsson, K.; Liaw, K.L.; Hortlund, M.; Lagheden, C.; Hansen, B.T.; Munk, C.; et al. Human papillomavirus types in cervical high-grade lesions or cancer among Nordic women-Potential for prevention. Cancer Med. 2019, 8, 839–849. [Google Scholar] [CrossRef]
- Castellsagué, X.; Díaz, M.; de Sanjosé, S.; Muñoz, N.; Herrero, R.; Franceschi, S.; Peeling, R.W.; Ashley, R.; Smith, J.S.; Snijders, P.J.; et al. Worldwide human papillomavirus etiology of cervical adenocarcinoma and its cofactors: Implications for screening and prevention. J. Natl. Cancer Inst. 2006, 98, 303–315. [Google Scholar] [CrossRef]
- Holl, K.; Nowakowski, A.M.; Powell, N.; McCluggage, W.G.; Pirog, E.C.; Collas De Souza, S.; Tjalma, W.A.; Rosenlund, M.; Fiander, A.; Castro Sánchez, M.; et al. Human papillomavirus prevalence and type-distribution in cervical glandular neoplasias: Results from a European multinational epidemiological study. Int. J. Cancer 2015, 137, 2858–2868. [Google Scholar] [CrossRef] [PubMed]
- Ho, G.Y.; Bierman, R.; Beardsley, L.; Chang, C.J.; Burk, R.D. Natural history of cervicovaginal papillomavirus infection in young women. N. Engl. J. Med. 1998, 338, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sampath, A.; Raychaudhuri, P.; Bagchi, S. Both Rb and E7 are regulated by the ubiquitin proteasome pathway in HPV-containing cervical tumor cells. Oncogene 2001, 20, 4740–4749. [Google Scholar] [CrossRef]
- Baak, J.P.; Kruse, A.J.; Garland, S.M.; Skaland, I.; Janssen, E.A.; Tabrizi, S.; Fagerheim, S.; Robboy, S.; Nilsen, S.T. Combined p53 and retinoblastoma protein detection identifies persistent and regressive cervical high-grade squamous intraepithelial lesions. Am. J. Surg. Pathol. 2005, 29, 1062–1066. [Google Scholar] [CrossRef]
- Kruse, A.J.; Skaland, I.; Janssen, E.A.; Buhr-Wildhagen, S.; Klos, J.; Arends, M.J.; Baak, J.P. Quantitative molecular parameters to identify low-risk and high-risk early CIN lesions: Role of markers of proliferative activity and differentiation and Rb availability. Int. J. Gynecol. Pathol. 2004, 23, 100–109. [Google Scholar] [CrossRef]
- Chang, L.; Goldman, R.D. Intermediate filaments mediate cytoskeletal crosstalk. Nat. Rev. Mol. Cell Biol. 2004, 5, 601–613. [Google Scholar] [CrossRef] [PubMed]
- Kadish, A.S.; Timmins, P.; Wang, Y.; Ho, G.Y.; Burk, R.D.; Ketz, J.; He, W.; Romney, S.L.; Johnson, A.; Angeletti, R.; et al. Regression of cervical intraepithelial neoplasia and loss of human papillomavirus (HPV) infection is associated with cell-mediated immune responses to an HPV type 16 E7 peptide. Cancer Epidemiol. Biomark. Prev. 2002, 11, 483–488. [Google Scholar]
- Garima Pandey, S.; Pandey, L.K.; Saxena, A.K.; Patel, N. The Role of p53 Gene in Cervical Carcinogenesis. J. Obstet. Gynaecol. India 2016, 66 (Suppl. 1), 383–388. [Google Scholar] [CrossRef][Green Version]
- Furber, S.E.; Weisberg, E.; Simpson, J.M. Progression and regression of low-grade epithelial abnormalities of the cervix. Aust. N. Z. J. Obstet. Gynaecol. 1997, 37, 107–112. [Google Scholar] [CrossRef]
- Spinillo, A.; Gardella, B.; Iacobone, A.D.; Cesari, S.; Alberizzi, P.; Silini, E.M. Multiple Papillomavirus Infection and Size of Colposcopic Lesions Among Women with Cervical Intraepithelial Neoplasia. J. Low. Genit. Tract Dis. 2016, 20, 22–25. [Google Scholar] [CrossRef]
- Jeronimo, J.; Bansil, P.; Valdez, M.; Kang, L.N.; Zhao, F.H.; Qiao, Y.L.; Chen, W.; Zhang, X.; Paul, P.; Bai, P.; et al. The Influence of Human Papillomavirus Genotypes on Visual Screening and Diagnosis of Cervical Precancer and Cancer. J. Low. Genit. Tract Dis. 2015, 19, 220–223. [Google Scholar] [CrossRef]
- Chen, Q.; Du, H.; Pretorius, R.G.; Wang, C.; Yang, B.; Wang, G.; Tang, J.; Belinson, J.L.; Wu, R. High-Grade Cervical Intraepithelial Neoplasia Detected by Colposcopy-Directed or Random Biopsy Relative to Age, Cytology, Human Papillomavirus 16, and Lesion Size. J. Low. Genit. Tract Dis. 2016, 20, 207–212. [Google Scholar] [CrossRef]
- Wentzensen, N.; Walker, J.; Schiffman, M.; Yang, H.P.; Zuna, R.E.; Dunn, S.T.; Allen, R.A.; Zhang, R.; Sherman, M.; Gold, M.A.; et al. Heterogeneity of high-grade cervical intraepithelial neoplasia related to HPV16: Implications for natural history and management. Int. J. Cancer 2013, 132, 148–154. [Google Scholar] [CrossRef]
- Øvestad, I.T.; Gudlaugsson, E.; Skaland, I.; Malpica, A.; Kruse, A.J.; Janssen, E.A.; Baak, J.P. Local immune response in the microenvironment of CIN2-3 with and without spontaneous regression. Mod. Pathol. 2010, 23, 1231–1240. [Google Scholar] [CrossRef]
- Patel, S.; Chiplunkar, S. Host immune responses to cervical cancer. Curr. Opin. Obstet. Gynecol. 2009, 21, 54–59. [Google Scholar] [CrossRef]
- Cosmi, L.; Maggi, L.; Santarlasci, V.; Liotta, F.; Annunziato, F. T helper cells plasticity in inflammation. Cytom. A 2014, 85, 36–42. [Google Scholar] [CrossRef]
- Luckheeram, R.V.; Zhou, R.; Verma, A.D.; Xia, B. CD4(+)T cells: Differentiation and functions. Clin. Dev. Immunol. 2012, 2012, 925135. [Google Scholar] [CrossRef]
- Litwin, T.R.; Irvin, S.R.; Chornock, R.L.; Sahasrabuddhe, V.V.; Stanley, M.; Wentzensen, N. Infiltrating T-cell markers in cervical carcinogenesis: A systematic review and meta-analysis. Br. J. Cancer 2021, 124, 831–841. [Google Scholar] [CrossRef]
- Raeber, M.E.; Rosalia, R.A.; Schmid, D.; Karakus, U.; Boyman, O. Interleukin-2 signals converge in a lymphoid-dendritic cell pathway that promotes anticancer immunity. Sci. Transl. Med. 2020, 12, eaba5464. [Google Scholar] [CrossRef]
- Zhang, L.; Mao, Z.; Lai, Y.; Wan, T.; Zhang, K.; Zhou, B. A review of the research progress in T-lymphocyte immunity and cervical cancer. Transl. Cancer Res. 2020, 9, 2026–2036. [Google Scholar] [CrossRef]
- Brusko, T.M.; Wasserfall, C.H.; Hulme, M.A.; Cabrera, R.; Schatz, D.; Atkinson, M.A. Influence of membrane CD25 stability on T lymphocyte activity: Implications for immunoregulation. PLoS ONE 2009, 4, e7980. [Google Scholar] [CrossRef]
- Karlsen, F.; Kalantari, M.; Chitemerere, M.; Johansson, B.; Hagmar, B. Modifications of human and viral deoxyribonucleic acid by formaldehyde fixation. Lab. Investig. 1994, 71, 604–611. [Google Scholar]
- Biedermann, K.; Dandachi, N.; Trattner, M.; Vogl, G.; Doppelmayr, H.; Moré, E.; Staudach, A.; Dietze, O.; Hauser-Kronberger, C. Comparison of real-time PCR signal-amplified in situ hybridization and conventional PCR for detection and quantification of human papillomavirus in archival cervical cancer tissue. J. Clin. Microbiol. 2004, 42, 3758–3765. [Google Scholar] [CrossRef]
- van Oortmarssen, G.J.; Habbema, J.D. Epidemiological evidence for age-dependent regression of pre-invasive cervical cancer. Br. J. Cancer 1991, 64, 559–565. [Google Scholar] [CrossRef]
- Holowaty, P.; Miller, A.B.; Rohan, T.; To, T. Natural history of dysplasia of the uterine cervix. J. Natl. Cancer Inst. 1999, 91, 252–258. [Google Scholar] [CrossRef]
- Halle, M.K.; Munk, A.C.; Engesæter, B.; Akbari, S.; Frafjord, A.; Hoivik, E.A.; Forsse, D.; Fasmer, K.E.; Woie, K.; Haldorsen, I.S.; et al. A Gene Signature Identifying CIN3 Regression and Cervical Cancer Survival. Cancers 2021, 13, 5737. [Google Scholar] [CrossRef]
- Vink, F.J.; Meijer, C.J.L.M.; Clifford, G.M.; Poljak, M.; Oštrbenk, A.; Petry, K.U.; Rothe, B.; Bonde, J.; Pedersen, H.; de Sanjosé, S.; et al. FAM19A4/miR124-2 methylation in invasive cervical cancer: A retrospective cross-sectional worldwide study. Int. J. Cancer 2020, 147, 1215–1221. [Google Scholar] [CrossRef]
- De Strooper, L.M.A.; Berkhof, J.; Steenbergen, R.D.M.; Lissenberg-Witte, B.I.; Snijders, P.J.F.; Meijer, C.J.L.M.; Heideman, D.A.M. Cervical cancer risk in HPV-positive women after a negative FAM19A4/mir124-2 methylation test: A post hoc analysis in the POBASCAM trial with 14 year follow-up. Int. J. Cancer 2018, 143, 1541–1548. [Google Scholar] [CrossRef]
- Kremer, W.W.; Dick, S.; Heideman, D.A.M.; Steenbergen, R.D.M.; Bleeker, M.C.G.; Verhoeve, H.R.; van Baal, W.M.; van Trommel, N.; Kenter, G.G.; Meijer, C.J.L.M.; et al. Clinical Regression of High-Grade Cervical Intraepithelial Neoplasia Is Associated with Absence of FAM19A4/miR124-2 DNA Methylation (CONCERVE Study). J. Clin. Oncol. 2022, 40, 3037–3046. [Google Scholar] [CrossRef]
- Vintermyr, O.K.; Skar, R.; Iversen, O.E.; Haugland, H.K. Usefulness of HPV test on cell sample from the cervix. Tidsskr. Nor. Laegeforen 2008, 128, 171–173. [Google Scholar]
- Kruse, A.J.; Baak, J.P.; de Bruin, P.C.; Jiwa, M.; Snijders, W.P.; Boodt, P.J.; Fons, G.; Houben, P.W.; The, H.S. Ki-67 immunoquantitation in cervical intraepithelial neoplasia (CIN): A sensitive marker for grading. J. Pathol. 2001, 193, 48–54. [Google Scholar] [CrossRef]
- Soreide, K. Receiver-operating characteristic curve analysis in diagnostic, prognostic and predictive biomarker research. J. Clin. Pathol. 2009, 62, 1–5. [Google Scholar] [CrossRef]
| HPV Genotypes (Yes/No) | Regression | Regression Rate per 1000 | HR (95% CI) |
|---|---|---|---|
| HPV16 | |||
| No | 26/76 | 2.26 (1.50–3.02) | 1.00 |
| Yes | 8/51 | 1.20 (0.42–1.97) | 0.53 (0.26–1.10) |
| HPV31 | |||
| No | 31/102 | 2.10 (1.42–2.70) | 1.00 |
| Yes | 3/25 | 0.96 (0.00–1.99) | 0.46 (0.15–1.43) |
| HPV52 | |||
| No | 32/116 | 1.92 (1.33–2.51) | 1.00 |
| Yes | 2/11 | 1.31 (0.00–3.03) | 0.69 (0.18–2.60) |
| HPV58 | |||
| No | 34/119 | - | - |
| Yes | 0/8 | - | - |
| HPV18 | |||
| No | 29/118 | 1.74 (1.17–2.31) | 1.00 |
| Yes | 5/9 | 3.35 (1.05–5.64) | 1.93 (0.90–4.13) |
| HPV33 | |||
| No | 28/114 | 1.74 (1.16–2.32) | 1.00 |
| Yes | 6/13 | 2.89 (0.93–4.85) | 1.66 (0.78–3.54) |
| HPV35 | |||
| No | 28/120 | 1.67 (1.11–2.24) | 1.00 |
| Yes | 6/7 | 4.15 (1.74–6.57) | 2.48 (1.27–4.86) |
| HPV39 | |||
| No | 31/120 | 1.81 (1.24–2.39) | 1.00 |
| Yes | 3/7 | 2.72 (1.91–5.25) | 1.50 (0.56–4.00) |
| HPV45 | |||
| No | 31/119 | 1.83 (1.25–2.41) | 1.00 |
| Yes | 3/8 | 2.45 (0.06–4.85) | 1.34 (0.48–3.74) |
| HPV Genotypes (Yes/No) | Regression | Regression Rate per 1000 | HR (95% CI) |
|---|---|---|---|
| Low-regression group 1 | |||
| No | 21/43 | 2.92 (1.89–3.95) | 1.00 |
| Yes | 13/84 | 1.18 (0.58–1.78) | 0.54 (0.22–0.75) |
| High-regression group 2 | |||
| No | 13/88 | 1.11 (0.55–1.68) | 1.00 |
| Yes | 21/39 | 3.20 (2.09–4.30) | 2.85 (1.54–5.28) |
| Total | High-Regression Group 1 | Low-Regression Group 2 | ||||
|---|---|---|---|---|---|---|
| Variables | Regression (yes/no) | HR 3 (95% CI) | Regression (yes/no) | HR 3 (95% CI) | Regression (yes/no) | HR 3 (95% CI) |
| pRb+ in lower half of epithelium | ||||||
| ≤30% | 4/58 | 0.21 (0.07–0.61) | 2/16 | 0.21 (0.04–1.14) | 1/45 | 0.09 (0.01–0.72) |
| >30% | 30/68 | 1.00 | 19/23 | 1.00 | 12/38 | 1.00 |
| Length of CIN lesion | ||||||
| ≤2.5 mm | 22/40 | 1.00 | 13/10 | 1.00 | 8/26 | 1.00 |
| >2.5 mm | 12/87 | 0.39 (0.19–0.80) | 8/29 | 0.48 (0.18–1.26) | 5/58 | 0.31 (0.91–1.04) |
| p53 LH+ in lower half of epithelium | ||||||
| ≤10 | 23/58 | 1.00 | 13/18 | 1.00 | 9/41 | 1.00 |
| >10 | 11/68 | 0.32 (0.15–0.71) | 8/21 | 0.84 (0.35–1.99) | 4/42 | 0.20 (0.04–0.88) |
| CD4/CD25 in stroma | ||||||
| ≤9.75 | 26/115 | 1.00 | 17/35 | 1.00 | 10/72 | 1.00 |
| >9.75 | 7/11 | 4.96 (2.46–9.99) | 3/4 | 4.27 (1.22–14.87) | 3/11 | 6.17 (1.29–29.57) |
| CD4/CD8 in stroma | ||||||
| ≤0.67 | 27/81 | 1.00 | 17/21 | 1.00 | 10/50 | 1.00 |
| >0.67 | 6/46 | 0.43 (0.21–0.91) | 3/14 | 0.34 (0.11–1.05) | 3/34 | 0.36 (0.11–1.17) |
| Harrell’s C 4 | 78% | 71% | 82% | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sustova, P.; Engesæter, B.; Øvestad, I.T.; Gudlaugsson, E.G.; Ghiasvand, R.; Skaland, I.; Baak, J.P.A.; Tropé, A.; Janssen, E.A.M.; Munk, A.C. The Predictive Impact of HPV Genotypes, Tumor Suppressors and Local Immune Response in the Regression of Cervical Intraepithelial Neoplasia 2-3: A Prospective Population-Based Cohort Study. Int. J. Mol. Sci. 2025, 26, 5205. https://doi.org/10.3390/ijms26115205
Sustova P, Engesæter B, Øvestad IT, Gudlaugsson EG, Ghiasvand R, Skaland I, Baak JPA, Tropé A, Janssen EAM, Munk AC. The Predictive Impact of HPV Genotypes, Tumor Suppressors and Local Immune Response in the Regression of Cervical Intraepithelial Neoplasia 2-3: A Prospective Population-Based Cohort Study. International Journal of Molecular Sciences. 2025; 26(11):5205. https://doi.org/10.3390/ijms26115205
Chicago/Turabian StyleSustova, Pavla, Birgit Engesæter, Irene Tveiterås Øvestad, Einar G. Gudlaugsson, Reza Ghiasvand, Ivar Skaland, Jan P. A. Baak, Ameli Tropé, Emiel A. M. Janssen, and Ane Cecilie Munk. 2025. "The Predictive Impact of HPV Genotypes, Tumor Suppressors and Local Immune Response in the Regression of Cervical Intraepithelial Neoplasia 2-3: A Prospective Population-Based Cohort Study" International Journal of Molecular Sciences 26, no. 11: 5205. https://doi.org/10.3390/ijms26115205
APA StyleSustova, P., Engesæter, B., Øvestad, I. T., Gudlaugsson, E. G., Ghiasvand, R., Skaland, I., Baak, J. P. A., Tropé, A., Janssen, E. A. M., & Munk, A. C. (2025). The Predictive Impact of HPV Genotypes, Tumor Suppressors and Local Immune Response in the Regression of Cervical Intraepithelial Neoplasia 2-3: A Prospective Population-Based Cohort Study. International Journal of Molecular Sciences, 26(11), 5205. https://doi.org/10.3390/ijms26115205






