Anti-Inflammatory Actions of Plant-Derived Compounds and Prevention of Chronic Diseases: From Molecular Mechanisms to Applications
Abstract
1. Introduction
2. Materials and Methods
3. Chronic Inflammation and Disease Relationships
3.1. Mechanisms of Chronic Inflammation
3.1.1. Overproduction of Inflammatory Cytokines
3.1.2. Oxidative Stress and ROS Generation
3.1.3. Activation of Inflammatory Signaling Pathways
3.1.4. Immune Cell Hyperactivation and Tissue Infiltration
3.1.5. Polyphenol-Mediated Regulation of Antioxidant Enzymes: Focus on Catalase
3.2. Role of Inflammation in Chronic Disease
3.2.1. CVDs
3.2.2. Metabolic Syndromes and Type 2 Diabetes
3.2.3. Neurodegenerative Diseases
3.2.4. Cancer
3.2.5. Chronic Obesity
3.2.6. Dermatological Diseases
4. Major Plant-Derived Compounds and Their Anti-Inflammatory Effects
4.1. Polyphenols
4.2. Carotenoids
4.3. Curcumin and Gingerol
5. Molecular Mechanisms
5.1. Inhibition of the NF-κB Pathway
5.2. Inhibition of the Inflammasome
5.3. Control of ROS Generation
6. Challenges to Practical Application
6.1. Increased Bioavailability
6.2. Evaluation of Long-Term Safety
6.3. Prospects for Practical Application
6.4. Integration into Personalized Nutrition
6.5. Plant-Derived Dietary Fiber Resources and Their Effective Utilization
7. Conclusions
8. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CVD | cardiovascular disease |
| IL-1β | interleukin-1β |
| IL-6 | interleukin-6 |
| TNF-α | tumor necrosis factor-α |
| ROS | reactive oxygen species |
| NSAIDs | nonsteroidal anti-inflammatory drugs |
| NF-κB | nuclear factor-κB |
| MAPK | mitogen-activated protein kinase |
| AD | Alzheimer’s disease |
| PD | Parkinson’s disease |
| CNS | central nervous system |
| SOD | superoxide dismutase |
References
- Papikinou, M.A.; Pavlidis, K.; Cholidis, P.; Kranas, D.; Adamantidi, T.; Anastasiadou, C.; Tsoupras, A. Marine Fungi Bioactives with Anti-Inflammatory, Antithrombotic and Antioxidant Health-Promoting Properties Against Inflammation-Related Chronic Diseases. Mar. Drugs 2024, 22, 520. [Google Scholar] [CrossRef]
- Pan, X.; Lv, J.; Liu, M.; Li, Y.; Zhang, Y.; Zhang, R.; Liu, J.; Sun, C.; Guo, H. Chronic systemic inflammation predicts long-term mortality among patients with fatty liver disease: Data from the National Health and Nutrition Examination Survey 2007–2018. PLoS ONE 2024, 19, e0312877. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xu, Y.; Jiang, M.; Wang, M.; Ji, M.; Xie, X.; Sheng, H. Chronic stress induces depression-like behavior in rats through affecting brain mitochondrial function and inflammation. Psychoneuroendocrinology 2024, 172, 107261. [Google Scholar] [CrossRef]
- Martinez Nieto, M.; De Leon Rodriguez, M.L.; Anaya Macias, R.D.C.; Lomeli Martinez, S.M. Periodontitis and chronic kidney disease: A bidirectional relationship based on inflammation and oxidative stress. World J. Clin. Cases 2024, 12, 6775–6781. [Google Scholar] [CrossRef] [PubMed]
- Hertis Petek, T.; Marcun Varda, N. Childhood Cardiovascular Health, Obesity, and Some Related Disorders: Insights into Chronic Inflammation and Oxidative Stress. Int. J. Mol. Sci. 2024, 25, 9706. [Google Scholar] [CrossRef] [PubMed]
- Brzozowski, T.; Konturek, P.; Konturek, S.J.; Kwiecien, S.; Sliwowski, Z.; Pajdo, R.; Duda, A.; Ptak, A.; Hahn, E.G. Implications of reactive oxygen species and cytokines in gastroprotection against stress-induced gastric damage by nitric oxide releasing aspirin. Int. J. Color. Dis. 2003, 18, 320–329. [Google Scholar] [CrossRef]
- MohanKumar, S.M.J.; Murugan, A.; Palaniyappan, A.; MohanKumar, P.S. Role of cytokines and reactive oxygen species in brain aging. Mech. Ageing Dev. 2023, 214, 111855. [Google Scholar] [CrossRef]
- Llanos, P.; Palomero, J. Reactive Oxygen and Nitrogen Species (RONS) and Cytokines-Myokines Involved in Glucose Uptake and Insulin Resistance in Skeletal Muscle. Cells 2022, 11, 4008. [Google Scholar] [CrossRef]
- Gonzalez, A.L.; Dungan, M.M.; Smart, C.D.; Madhur, M.S.; Doran, A.C. Inflammation Resolution in the Cardiovascular System: Arterial Hypertension, Atherosclerosis, and Ischemic Heart Disease. Antioxid. Redox Signal 2024, 40, 292–316. [Google Scholar] [CrossRef]
- Shimabukuro, M. IGF-1 and Cardiovascular and Non-Cardiovascular Mortality Risk in Patients with Chronic Kidney Disease: A Model of “Malnutrition-Inflammation-Atherosclerosis Syndrome”. J. Atheroscler. Thromb. 2022, 29, 1138–1139. [Google Scholar] [CrossRef]
- Hong, S.; Dimitrov, S.; Cheng, T.; Redwine, L.; Pruitt, C.; Mills, P.J.; Ziegler, M.G.; Green, J.M.; Shaikh, F.; Wilson, K. Beta-adrenergic receptor mediated inflammation control by monocytes is associated with blood pressure and risk factors for cardiovascular disease. Brain Behav. Immun. 2015, 50, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Conen, D. Inflammation, blood pressure and cardiovascular disease: Heading east. J. Hum. Hypertens 2013, 27, 71. [Google Scholar] [CrossRef]
- Curro, D.; Vergani, E.; Bruno, C.; Comi, S.; D’Abate, C.; Mancini, A. Plasmatic lipocalin-2 levels in chronic low-grade inflammation syndromes: Comparison between metabolic syndrome, total and partial adult growth hormone deficiency. Biofactors 2020, 46, 629–636. [Google Scholar] [CrossRef]
- Rumyantsev, K.A.; Polyakova, V.V.; Sorokina, I.V.; Feoktistova, P.S.; Khatkov, I.E.; Bodunova, N.A.; Zhukova, L.G. The Gut Microbiota Impacts Gastrointestinal Cancers through Obesity, Diabetes, and Chronic Inflammation. Life 2024, 14, 1219. [Google Scholar] [CrossRef]
- Zhai, W.; Zhao, M.; Wen, C.; Meng, L.; Zhao, A.; Zhang, Y.; Cui, X.; Xu, Y.; Sun, L. Obesity-induced chronic low-grade inflammation in adipose tissue: A pathway to Alzheimer’s disease. Ageing Res. Rev. 2024, 99, 102402. [Google Scholar] [CrossRef]
- Subeta, P.; Lana, A.J.; Schlachetzki, J.C.M. Chronic peripheral inflammation: A possible contributor to neurodegenerative diseases. Neural Regen. Res. 2021, 16, 1711–1714. [Google Scholar] [CrossRef]
- Saeed, B.A.; Faisal, A.J.; Mahmood, B.S.; Thanoon, A.H. Chronic Inflammation Induced by Escherichia coli Blood Infections as a Risk Factor for Pancreatic Cancer Progression. Asian Pac. J. Cancer Prev. 2024, 25, 4407–4414. [Google Scholar] [CrossRef]
- Rogovskii, V. Cancer and Autoimmune Diseases as Two Sides of Chronic Inflammation and the Method of Therapy. Curr. Cancer Drug Targets 2024, 24, 1089–1103. [Google Scholar] [CrossRef] [PubMed]
- Zadori, Z.S.; Kiraly, K.; Al-Khrasani, M.; Gyires, K. Interactions between NSAIDs, opioids and the gut microbiota—Future perspectives in the management of inflammation and pain. Pharmacol. Ther. 2023, 241, 108327. [Google Scholar] [CrossRef]
- Han, Y.; Lu, Y.; Li, X.; Niu, X.; Chang, A.K.; Yang, Z.; Li, X.; He, X.; Bi, X. Novel organoselenides (NSAIDs-Se derivatives) protect against LPS-induced inflammation in microglia by targeting the NOX2/NLRP3 signaling pathway. Int. Immunopharmacol. 2021, 101, 108377. [Google Scholar] [CrossRef]
- Martin, C.S.; Crastin, A.; Sagmeister, M.S.; Kalirai, M.S.; Turner, J.D.; MacDonald, L.; Kurowska-Stolarska, M.; Scheel-Toellner, D.; Taylor, A.E.; Gilligan, L.C.; et al. Inflammation dynamically regulates steroid hormone metabolism and action within macrophages in rheumatoid arthritis. J. Autoimmun. 2024, 147, 103263. [Google Scholar] [CrossRef] [PubMed]
- Ardelean, M.; Buzas, R.; Ardelean, O.; Preda, M.; Morariu, S.I.; Levai, C.M.; Rosca, C.I.; Lighezan, D.F.; Kundnani, N.R. Clinical and Biochemical Differences in Patients Having Non-Variceal Upper Gastrointestinal Bleeding on NSAIDs, Oral Anticoagulants, and Antiplatelet Therapy. J. Clin. Med. 2024, 13, 5622. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, X.; Zhang, R.; Tian, Y.; Ma, X.; Wang, X.; Jiang, Y.; Man, C. Stress-Induced Immunosuppression Inhibits Regional Immune Responses in Chicken Adipose Tissue Partially through Suppressing T Cells by Up-Regulating Steroid Metabolism. Animals 2024, 14, 225. [Google Scholar] [CrossRef]
- Baron, G.; Ferrario, G.; Marinello, C.; Carini, M.; Morazzoni, P.; Aldini, G. Effect of Extraction Solvent and Temperature on Polyphenol Profiles, Antioxidant and Anti-Inflammatory Effects of Red Grape Skin By-Product. Molecules 2021, 26, 5454. [Google Scholar] [CrossRef]
- Wu, H.; Pang, H.; Chen, Y.; Huang, L.; Liu, H.; Zheng, Y.; Sun, C.; Zhang, G.; Wang, G. Anti-Inflammatory Effect of a Polyphenol-Enriched Fraction from Acalypha wilkesiana on Lipopolysaccharide-Stimulated RAW 264.7 Macrophages and Acetaminophen-Induced Liver Injury in Mice. Oxid. Med. Cell Longev. 2018, 2018, 7858094. [Google Scholar] [CrossRef] [PubMed]
- Cirmi, S.; Randazzo, B.; Russo, C.; Musumeci, L.; Maugeri, A.; Montalbano, G.; Guerrera, M.C.; Lombardo, G.E.; Levanti, M. Anti-inflammatory effect of a flavonoid-rich extract of orange juice in adult zebrafish subjected to Vibrio anguillarum-induced enteritis. Nat. Prod. Res. 2021, 35, 5350–5353. [Google Scholar] [CrossRef]
- Unal, N.G.; Kozak, A.; Karakaya, S.; Oruc, N.; Barutcuoglu, B.; Aktan, C.; Sezak, M.; Ozutemiz, A.O. Anti-Inflammatory Effect of Crude Momordica charantia L. Extract on 2,4,6-Trinitrobenzene Sulfonic Acid-Induced Colitis Model in Rat and the Bioaccessibility of its Carotenoid Content. J. Med. Food 2020, 23, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, C.A.; Ali, K.M.; Sha, A.M.; Gul, S.S. Effect of Curcumin gel on inflammatory and anti-inflammatory biomarkers in experimental induced periodontitis in rats: A biochemical and immunological study. Front. Microbiol. 2023, 14, 1274189. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Dang, M.; Kumar, M. Anti-inflammatory and renal protective effect of gingerol in high-fat diet/streptozotocin-induced diabetic rats via inflammatory mechanism. Inflammopharmacology 2019, 27, 1243–1254. [Google Scholar] [CrossRef]
- Li, Z.; Liu, T.; Xie, W.; Wang, Z.; Gong, B.; Yang, M.; He, Y.; Bai, X.; Liu, K.; Xie, Z.; et al. Protopanaxadiol derivative: A plant origin of novel selective glucocorticoid receptor modulator with anti-inflammatory effect. Eur. J. Pharmacol. 2024, 983, 176901. [Google Scholar] [CrossRef]
- Faubel, N.; Makran, M.; Barbera, R.; Garcia-Llatas, G.; Giardina, I.C.; Tesoriere, L.; Attanzio, A.; Cilla, A. Anti-inflammatory activity of plant sterols in a co-culture model of intestinal inflammation: Focus on food-matrix effect. Food Funct. 2024, 15, 6502–6511. [Google Scholar] [CrossRef] [PubMed]
- Ma, A.; Zhou, J.; Zou, H.; Yuan, L.; Zhong, R.; Zhu, Y.; Gao, C. Anti-inflammatory effect of nestorone in a lipopolysaccharide-induced acute lung injury model through regulation of the TLR-4/Myd88/NF-kappaB signaling pathway. Inflammopharmacology 2024, 33, 1473–1489. [Google Scholar] [CrossRef] [PubMed]
- Hao, R.; Liu, Q.; Wang, L.; Jian, W.; Cheng, Y.; Zhang, Q.; Hayer, K.; Kamarudin Raja Idris, R.; Zhang, Y.; Lu, H.; et al. Anti-inflammatory effect of Lactiplantibacillus plantarum T1 cell-free supernatants through suppression of oxidative stress and NF-kappaB- and MAPK-signaling pathways. Appl. Environ. Microbiol. 2023, 89, e0060823. [Google Scholar] [CrossRef]
- Taher, I.; El-Masry, E.; Abouelkheir, M.; Taha, A.E. Anti-inflammatory effect of metformin against an experimental model of LPS-induced cytokine storm. Exp. Ther. Med. 2023, 26, 415. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.A.; Jang, S.E.; Hong, S.W.; Hana, M.J.; Kim, D.H. The role of intestinal microflora in anti-inflammatory effect of baicalin in mice. Biomol. Ther. 2012, 20, 36–42. [Google Scholar] [CrossRef]
- Mu, W.; Patankar, V.; Kitchen, S.; Zhen, A. Examining Chronic Inflammation, Immune Metabolism, and T Cell Dysfunction in HIV Infection. Viruses 2024, 16, 219. [Google Scholar] [CrossRef]
- Winston, C.N.; Noel, A.; Neustadtl, A.; Parsadanian, M.; Barton, D.J.; Chellappa, D.; Wilkins, T.E.; Alikhani, A.D.; Zapple, D.N.; Villapol, S.; et al. Dendritic Spine Loss and Chronic White Matter Inflammation in a Mouse Model of Highly Repetitive Head Trauma. Am. J. Pathol. 2016, 186, 552–567. [Google Scholar] [CrossRef]
- Corrado, A.; Guadagni, I.; Picarelli, G.; Variola, A. Obesity and Chronic Inflammation: Implications for Rheumatoid Arthritis, Spondyloarthritis, and Ulcerative Colitis. Immun. Inflamm. Dis. 2025, 13, e70080. [Google Scholar] [CrossRef]
- Kurochkina, N.S.; Orlova, M.A.; Vigovskiy, M.A.; Zgoda, V.G.; Vepkhvadze, T.F.; Vavilov, N.E.; Makhnovskii, P.A.; Grigorieva, O.A.; Boroday, Y.R.; Philippov, V.V.; et al. Age-related changes in human skeletal muscle transcriptome and proteome are more affected by chronic inflammation and physical inactivity than primary aging. Aging Cell 2024, 23, e14098. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, H.; Wang, X.; Chen, L.; Liu, W.; Cai, D.; Deng, S.; Chu, H.; Liu, Y.; Feng, X.; et al. Long-term environmental levels of microcystin-LR exposure induces colorectal chronic inflammation, fibrosis and barrier disruption via CSF1R/Rap1b signaling pathway. J. Hazard. Mater. 2022, 440, 129793, Corrigendum in J. Hazard. Mater. 2023, 451, 131110. [Google Scholar] [CrossRef]
- Berger, M.; Maqua, H.; Lysaja, K.; Mause, S.F.; Hindle, M.S.; Naseem, K.; Dahl, E.; Speer, T.; Marx, N.; Schutt, K. Platelets from patients with chronic inflammation have a phenotype of chronic IL-1beta release. Res. Pract. Thromb. Haemost. 2024, 8, 102261. [Google Scholar] [CrossRef] [PubMed]
- Sater, M.S.; AlDehaini, D.M.B.; Malalla, Z.H.A.; Ali, M.E.; Giha, H.A. Plasma IL-6, TREM1, uPAR, and IL6/IL8 biomarkers increment further witnessing the chronic inflammation in type 2 diabetes. Horm. Mol. Biol. Clin. Investig. 2023, 44, 259–269. [Google Scholar] [CrossRef]
- Joveini, S.; Yarmohammadi, F.; Iranshahi, M.; Nikpoor, A.R.; Askari, V.R.; Attaranzadeh, A.; Etemad, L.; Taherzadeh, Z. Distinct therapeutic effects of auraptene and umbelliprenin on TNF-alpha and IL-17 levels in a murine model of chronic inflammation. Heliyon 2024, 10, e40731. [Google Scholar] [CrossRef] [PubMed]
- Perez-Figueroa, E.; Alvarez-Carrasco, P.; Ortega, E. Crosslinking of membrane CD13 in human neutrophils mediates phagocytosis and production of reactive oxygen species, neutrophil extracellular traps and proinflammatory cytokines. Front. Immunol. 2022, 13, 994496. [Google Scholar] [CrossRef] [PubMed]
- Dong, P.; Qiu, H.; Wen, R.; Zou, X.; Sun, X.; Yu, L.; Zhang, S.; Wu, Y.; Lan, F. Reactive Oxygen and Nitrogen Species—“Nanosweeper” for Rheumatoid Arthritis Theranostics by Macrophage Reprogramming. ACS Appl. Mater. Interfaces 2024, 16, 70322–70338. [Google Scholar] [CrossRef]
- Ma, E.; Sasazuki, S.; Shimazu, T.; Sawada, N.; Yamaji, T.; Iwasaki, M.; Inoue, M.; Tsugane, S. Reactive oxygen species and gastric cancer risk: A large nested case-control study in Japan. Eur. J. Epidemiol. 2015, 30, 589–594. [Google Scholar] [CrossRef]
- Wang, Z.; Li, M.; Chen, J.; Zhang, S.; Wang, B.; Wang, J. Immunomodulatory Hydrogel for Electrostatically Capturing Pro-inflammatory Factors and Chemically Scavenging Reactive Oxygen Species in Chronic Diabetic Wound Remodeling. Adv. Heal. Mater. 2024, 13, e2402080. [Google Scholar] [CrossRef]
- Dughbaj, M.A.; Jayne, J.G.; Park, A.Y.J.; Bensman, T.J.; Algorri, M.; Ouellette, A.J.; Selsted, M.E.; Beringer, P.M. Anti-Inflammatory Effects of RTD-1 in a Murine Model of Chronic Pseudomonas aeruginosa Lung Infection: Inhibition of NF-kappaB, Inflammasome Gene Expression, and Pro-IL-1beta Biosynthesis. Antibiotics 2021, 10, 1043. [Google Scholar] [CrossRef]
- Nerland, D.; Ash, A.; Garman, A.; Foltz, J.; Berenbeim, G.; Wilke, B.; Winter, L.; Christian, D.T.; Duric, V. Chronic Inflammatory Pain Alters Expression of Limbic MAPK Phosphatases. Chronic Pain. Manag. 2024, 8, 155. [Google Scholar] [CrossRef]
- Suikkila, A.; Lyly, A.; Savinko, T.; Vento, S.I.; Saarinen, R.; Hafren, L. Inflammatory Cytokines in Middle Ear Effusion of Patients With Asthma, Chronic Rhinosinusitis With Nasal Polyps With or Without NSAID Intolerance. Otol. Neurotol. 2024, 45, 765–772. [Google Scholar] [CrossRef]
- Ajrawat, P.; Yang, Y.; Wasilewski, E.; Leroux, T.; Ladha, K.S.; Bhatia, A.; Singh, M.; Thaker, S.; Kapoor, M.; Furlan, A.D.; et al. Medical Cannabis Use and Inflammatory Cytokines and Chemokines Among Adult Chronic Pain Patients. Cannabis Cannabinoid Res. 2024, 9, 267–281. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, C.; Baumeister, B.; Kipnowski, J.; Schiermeyer-Dunkhase, B.; Vetter, H. Alteration of prostaglandin E2 and leukotriene B4 synthesis in chronic inflammatory bowel disease. Hepatogastroenterology 1996, 43, 1508–1512. [Google Scholar]
- Kokkosis, A.G.; Madeira, M.M.; Hage, Z.; Valais, K.; Koliatsis, D.; Resutov, E.; Tsirka, S.E. Chronic psychosocial stress triggers microglial-/macrophage-induced inflammatory responses leading to neuronal dysfunction and depressive-related behavior. Glia 2024, 72, 111–132. [Google Scholar] [CrossRef]
- Huriyati, E.; Darwin, E.; Yanwirasti, Y.; Wahid, I. Differences in Expression of Inflammatory Mediator in Mucosal and Polyp Tissue between Chronic Rhinosinusitis and Recurrent Chronic Rhinosinusitis. Open Access Maced. J. Med. Sci. 2019, 7, 1733–1738. [Google Scholar] [CrossRef]
- Chai, B.; Qiao, Y.; Wang, H.; Zhang, X.; Wang, J.; Wang, C.; Zhou, P.; Chen, X. Identification of YfiH and the Catalase CatA As Polyphenol Oxidases of Aeromonas media and CatA as a Regulator of Pigmentation by Its Peroxyl Radical Scavenging Capacity. Front. Microbiol. 2017, 8, 1939. [Google Scholar] [CrossRef] [PubMed]
- Garcia-de Los Santos, A.; Lopez, E.; Cubillas, C.A.; Noel, K.D.; Brom, S.; Romero, D. Requirement of a plasmid-encoded catalase for survival of Rhizobium etli CFN42 in a polyphenol-rich environment. Appl. Environ. Microbiol. 2008, 74, 2398–2403. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Choi, J.Y.; Lee, H.J.; Byun, C.J.; Park, J.H.; Park, J.H.; Cho, H.S.; Cho, S.J.; Jo, S.A.; Jo, I. The Green Tea Component (-)-Epigallocatechin-3-Gallate Sensitizes Primary Endothelial Cells to Arsenite-Induced Apoptosis by Decreasing c-Jun N-Terminal Kinase-Mediated Catalase Activity. PLoS ONE 2015, 10, e0138590. [Google Scholar] [CrossRef]
- Chen, B.; He, Q.; Chen, C.; Lin, Y.; Xiao, J.; Pan, Z.; Li, M.; Li, S.; Yang, J.; Wang, F.; et al. Combination of curcumin and catalase protects against chondrocyte injury and knee osteoarthritis progression by suppressing oxidative stress. Biomed. Pharmacother. 2023, 168, 115751. [Google Scholar] [CrossRef]
- Qi, X.; Meng, J.; Li, C.; Cheng, W.; Fan, A.; Huang, J.; Lin, W. Praelolide alleviates collagen-induced arthritis through increasing catalase activity and activating Nrf2 pathway. Phytomedicine 2024, 135, 156040. [Google Scholar] [CrossRef]
- Oyedemi, S.O.; Nwaogu, G.; Chukwuma, C.I.; Adeyemi, O.T.; Matsabisa, M.G.; Swain, S.S.; Aiyegoro, O.A. Quercetin modulates hyperglycemia by improving the pancreatic antioxidant status and enzymes activities linked with glucose metabolism in type 2 diabetes model of rats: In silico studies of molecular interaction of quercetin with hexokinase and catalase. J. Food Biochem. 2020, 44, e13127. [Google Scholar] [CrossRef]
- Bensenor, I.M.; Goulart, A.C.; Pereira, A.C.; Brunoni, A.R.; Alencar, A.; Santos, R.D.; Bittencourt, M.S.; Telles, R.W.; Machado, L.A.C.; Barreto, S.M.; et al. Chronic inflammatory diseases, subclinical atherosclerosis, and cardiovascular diseases: Design, objectives, and baseline characteristics of a prospective case-cohort study—ELSA-Brasil. Clinics 2022, 77, 100013. [Google Scholar] [CrossRef] [PubMed]
- Hansen, P.R. Chronic Inflammatory Diseases and Atherosclerotic Cardiovascular Disease: Innocent Bystanders or Partners in Crime? Curr. Pharm. Des. 2018, 24, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.W.; Guo, Y.L.; Wu, N.Q.; Zhu, C.G.; Dong, Q.; Sun, J.; Dou, K.F.; Li, J.J. Low-density lipoprotein triglyceride predicts outcomes in patients with chronic coronary syndrome following percutaneous coronary intervention according to inflammatory status. Clin. Chim. Acta 2023, 551, 117631. [Google Scholar] [CrossRef] [PubMed]
- Weissman, S.; Sinh, P.; Mehta, T.I.; Thaker, R.K.; Derman, A.; Heiberger, C.; Qureshi, N.; Amrutiya, V.; Atoot, A.; Dave, M.; et al. Atherosclerotic cardiovascular disease in inflammatory bowel disease: The role of chronic inflammation. World J. Gastrointest. Pathophysiol. 2020, 11, 104–113. [Google Scholar] [CrossRef]
- Straub, R.H. Insulin resistance, selfish brain, and selfish immune system: An evolutionarily positively selected program used in chronic inflammatory diseases. Arthritis Res. Ther. 2014, 16 (Suppl. S2), S4. [Google Scholar] [CrossRef]
- Li, D.; Wu, Y.; Tian, P.; Zhang, X.; Wang, H.; Wang, T.; Ying, B.; Wang, L.; Shen, Y.; Wen, F. Adipokine CTRP-5 as a Potential Novel Inflammatory Biomarker in Chronic Obstructive Pulmonary Disease. Medicine 2015, 94, e1503. [Google Scholar] [CrossRef]
- Liu, W.S.; Zhang, Y.R.; Ge, Y.J.; Wang, H.F.; Cheng, W.; Yu, J.T. Inflammation and Brain Structure in Alzheimer’s Disease and Other Neurodegenerative Disorders: A Mendelian Randomization Study. Mol. Neurobiol. 2024, 61, 1593–1604. [Google Scholar] [CrossRef]
- Simpson, D.S.A.; Oliver, P.L. ROS Generation in Microglia: Understanding Oxidative Stress and Inflammation in Neurodegenerative Disease. Antioxidants 2020, 9, 743. [Google Scholar] [CrossRef]
- Mella, C.; Tsarouhas, P.; Brockwell, M.; Ball, H.C. The Role of Chronic Inflammation in Pediatric Cancer. Cancers 2025, 17, 154. [Google Scholar] [CrossRef]
- Guadagni, F.; Ferroni, P.; Palmirotta, R.; Portarena, I.; Formica, V.; Roselli, M. Review. TNF/VEGF cross-talk in chronic inflammation-related cancer initiation and progression: An early target in anticancer therapeutic strategy. In Vivo 2007, 21, 147–161. [Google Scholar]
- Lee, H.T.; Lin, C.S.; Liu, C.Y.; Chen, P.; Tsai, C.Y.; Wei, Y.H. Mitochondrial Plasticity and Glucose Metabolic Alterations in Human Cancer under Oxidative Stress-From Viewpoints of Chronic Inflammation and Neutrophil Extracellular Traps (NETs). Int. J. Mol. Sci. 2024, 25, 9458. [Google Scholar] [CrossRef]
- Bartsch, H.; Nair, J. Chronic inflammation and oxidative stress in the genesis and perpetuation of cancer: Role of lipid peroxidation, DNA damage, and repair. Langenbecks Arch. Surg. 2006, 391, 499–510. [Google Scholar] [CrossRef]
- Sorrentino, C.; Di Gisi, M.; Gentile, G.; Licitra, F.; D’Angiolo, R.; Giovannelli, P.; Migliaccio, A.; Castoria, G.; Di Donato, M. Agri-Food By-Products in Cancer: New Targets and Strategies. Cancers 2022, 14, 5517. [Google Scholar] [CrossRef]
- Yarden, Y.; Pines, G. The ERBB network: At last, cancer therapy meets systems biology. Nat. Rev. Cancer 2012, 12, 553–563. [Google Scholar] [CrossRef] [PubMed]
- Mohiuddin, M.; Kasahara, K. Cisplatin Activates the Growth Inhibitory Signaling Pathways by Enhancing the Production of Reactive Oxygen Species in Non-small Cell Lung Cancer Carrying an EGFR Exon 19 Deletion. Cancer Genom. Proteom. 2021, 18, 471–486. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, M.; Ray, S.K. Direct transfection of miR-137 mimics is more effective than DNA demethylation of miR-137 promoter to augment anti-tumor mechanisms of delphinidin in human glioblastoma U87MG and LN18 cells. Gene 2015, 573, 141–152. [Google Scholar] [CrossRef]
- Zatterale, F.; Longo, M.; Naderi, J.; Raciti, G.A.; Desiderio, A.; Miele, C.; Beguinot, F. Chronic Adipose Tissue Inflammation Linking Obesity to Insulin Resistance and Type 2 Diabetes. Front. Physiol. 2019, 10, 1607. [Google Scholar] [CrossRef] [PubMed]
- Rakotoarivelo, V.; Lacraz, G.; Mayhue, M.; Brown, C.; Rottembourg, D.; Fradette, J.; Ilangumaran, S.; Menendez, A.; Langlois, M.F.; Ramanathan, S. Inflammatory Cytokine Profiles in Visceral and Subcutaneous Adipose Tissues of Obese Patients Undergoing Bariatric Surgery Reveal Lack of Correlation With Obesity or Diabetes. EBioMedicine 2018, 30, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Tian, X.; Ren, Z.; Liu, Z.; Sun, C. Mechanistic insights into the role of USP14 in adipose tissue macrophage recruitment and insulin resistance in obesity. Int. J. Biol. Macromol. 2024, 267, 131645. [Google Scholar] [CrossRef]
- Nakadate, K.; Kawakami, K.; Yamazaki, N. Combined Ingestion of Tea Catechin and Citrus beta-Cryptoxanthin Improves Liver Function via Adipokines in Chronic Obesity. Nutrients 2023, 15, 3345. [Google Scholar] [CrossRef]
- Nakadate, K.; Kawakami, K.; Yamazaki, N. Anti-Obesity and Anti-Inflammatory Synergistic Effects of Green Tea Catechins and Citrus β-Cryptoxanthin Ingestion in Obese Mice. Int. J. Mol. Sci. 2023, 24, 7054. [Google Scholar] [CrossRef] [PubMed]
- Nakadate, K.; Kawakami, K.; Yamazaki, N. Synergistic Effect of beta-Cryptoxanthin and Epigallocatechin Gallate on Obesity Reduction. Nutrients 2024, 16, 2344. [Google Scholar] [CrossRef]
- Saitoh, H.; Sakaguchi, M.; Miruno, F.; Muramatsu, N.; Ito, N.; Tadokoro, K.; Kawakami, K.; Nakadate, K. Histopathological Analysis of Lipopolysaccharide-Induced Liver Inflammation and Thrombus Formation in Mice: The Protective Effects of Aspirin. Curr. Issues Mol. Biol. 2024, 46, 14291–14303. [Google Scholar] [CrossRef] [PubMed]
- Dousdampanis, P.; Aggeletopoulou, I.; Mouzaki, A. The role of M1/M2 macrophage polarization in the pathogenesis of obesity-related kidney disease and related pathologies. Front. Immunol. 2024, 15, 1534823. [Google Scholar] [CrossRef]
- Lee, C.H.; Kim, H.J.; Lee, Y.S.; Kang, G.M.; Lim, H.S.; Lee, S.H.; Song, D.K.; Kwon, O.; Hwang, I.; Son, M.; et al. Hypothalamic Macrophage Inducible Nitric Oxide Synthase Mediates Obesity-Associated Hypothalamic Inflammation. Cell Rep. 2018, 25, 934–946.e935. [Google Scholar] [CrossRef]
- Cao, X.; Gao, T.; Lv, F.; Wang, Y.; Li, B.; Wang, X. ROS-triggered and macrophage-targeted micelles modulate mitochondria function and polarization in obesity. Nanotechnology 2024, 35, 475707. [Google Scholar] [CrossRef]
- Wu, Z.; Yu, W.; Ni, W.; Teng, C.; Ye, W.; Yu, C.; Zeng, Y. Improvement of obesity by Liupao tea is through the IRS-1/PI3K/AKT/GLUT4 signaling pathway according to network pharmacology and experimental verification. Phytomedicine 2023, 110, 154633. [Google Scholar] [CrossRef]
- Feng, M.; Liu, F.; Xing, J.; Zhong, Y.; Zhou, X. Anemarrhena saponins attenuate insulin resistance in rats with high-fat diet-induced obesity via the IRS-1/PI3K/AKT pathway. J. Ethnopharmacol. 2021, 277, 114251. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Paerhati, G.; Wu, Y.; Cheng, L.F. Modulation of gut microbiota, up-regulation of ZO-1, and promotion of metabolism as therapeutic mechanisms of indole-3-carbinol against obesity in mice. Front. Pharmacol. 2024, 15, 1499142. [Google Scholar] [CrossRef]
- Keirns, B.H.; Medlin, A.R.; Maki, K.A.; McClanahan, K.; Fruit, S.E.; Sciarrillo, C.M.; Hart, S.M.; Joyce, J.; Lucas, E.A.; Emerson, S.R. Biomarkers of intestinal permeability are associated with inflammation in metabolically healthy obesity but not normal-weight obesity. Am. J. Physiol. Heart Circ. Physiol. 2024, 327, H1135–H1145. [Google Scholar] [CrossRef]
- Wu, J.; Liu, Y.; Dou, Z.; Wu, T.; Liu, R.; Sui, W.; Jin, Y.; Zhang, M. Black garlic melanoidins prevent obesity, reduce serum LPS levels and modulate the gut microbiota composition in high-fat diet-induced obese C57BL/6J mice. Food Funct. 2020, 11, 9585–9598. [Google Scholar] [CrossRef] [PubMed]
- Kopczynska, J.; Kowalczyk, M. The potential of short-chain fatty acid epigenetic regulation in chronic low-grade inflammation and obesity. Front. Immunol. 2024, 15, 1380476. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Gautam, D.N.S.; Radu, A.F.; Behl, T.; Bungau, S.G.; Vesa, C.M. Reviewing the Traditional/Modern Uses, Phytochemistry, Essential Oils/Extracts and Pharmacology of Embelia ribes Burm. Antioxidants 2022, 11, 1359. [Google Scholar] [CrossRef]
- Khuntia, A.; Martorell, M.; Ilango, K.; Bungau, S.G.; Radu, A.F.; Behl, T.; Sharifi-Rad, J. Theoretical evaluation of Cleome species’ bioactive compounds and therapeutic potential: A literature review. Biomed. Pharmacother. 2022, 151, 113161. [Google Scholar] [CrossRef]
- Yang, S.C.; Chang, Z.Y.; Hsiao, C.Y.; Alshetaili, A.; Wei, S.H.; Hsiao, Y.T.; Fang, J.Y. Topical Anti-Inflammatory Effects of Quercetin Glycosides on Atopic Dermatitis-Like Lesions: Influence of the Glycone Type on Efficacy and Skin Absorption. Inflammation 2025. [Google Scholar] [CrossRef] [PubMed]
- Beken, B.; Serttas, R.; Yazicioglu, M.; Turkekul, K.; Erdogan, S. Quercetin Improves Inflammation, Oxidative Stress, and Impaired Wound Healing in Atopic Dermatitis Model of Human Keratinocytes. Pediatr. Allergy Immunol. Pulmonol. 2020, 33, 69–79. [Google Scholar] [CrossRef]
- Nolan, J.M.; Power, R.; Howard, A.N.; Bergin, P.; Roche, W.; Prado-Cabrero, A.; Pope, G.; Cooke, J.; Power, T.; Mulcahy, R. Supplementation with Carotenoids, Omega-3 Fatty Acids, and Vitamin E Has a Positive Effect on the Symptoms and Progression of Alzheimer’s Disease. J. Alzheimers Dis. 2022, 90, 233–249. [Google Scholar] [CrossRef]
- Yang, Q.N.; Deng, W.; Wu, D.T.; Li, J.; Liu, H.Y.; Yan, H.L.; Du, K.; Hu, Y.C.; Zou, L.; Huang, J.W. Characterization, Antioxidant Capacity, and Anti-Inflammatory Activity of Polyphenol-Enriched Extracts Obtained from Unripe, Mature, and Overripe Fruits of Red-Fleshed Kiwifruit Cultivars. Foods 2024, 13, 2860. [Google Scholar] [CrossRef]
- Tong, Q.; Yi, Z.; Ma, L.; Tan, Y.; Liu, D.; Cao, X.; Ma, X.; Li, X. Microenvironment-Responsive Antibacterial, Anti-Inflammatory, and Antioxidant Pickering Emulsion Stabilized by Curcumin-Loaded Tea Polyphenol Particles for Accelerating Infected Wound Healing. ACS Appl. Mater. Interfaces 2024, 16, 44467–44484. [Google Scholar] [CrossRef]
- Kurtz, J.A.; Feresin, R.G.; Grazer, J.; Otis, J.; Wilson, K.E.; Doyle, J.A.; Zwetsloot, K.A. Effects of Quercetin and Citrulline on Nitric Oxide Metabolites and Antioxidant Biomarkers in Trained Cyclists. Nutrients 2025, 17, 224. [Google Scholar] [CrossRef]
- Xia, W.; Chen, K.; Zhu, Y.Z.; Zhang, C.J.; Chen, Y.L.; Wang, F.; Xie, Y.Y.; Hider, R.C.; Zhou, T. Antioxidant and anti-tyrosinase activity of a novel stilbene analogue as an anti-browning agent. J. Sci. Food Agric. 2022, 102, 3817–3825. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, X.; Yao, Y.; Huang, X.; Li, C.; Deng, P.; Jiang, G.; Dai, Q. The effect of epigallocatechin gallate on laying performance, egg quality, immune status, antioxidant capacity, and hepatic metabolome of laying ducks reared in high temperature condition. Vet. Q. 2023, 43, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Wang, D.; Yang, Z.; Wang, T. Pharmacological Effects of Polyphenol Phytochemicals on the Intestinal Inflammation via Targeting TLR4/NF-kappaB Signaling Pathway. Int. J. Mol. Sci. 2022, 23, 6939. [Google Scholar] [CrossRef]
- Kuriya, K.; Itoh, S.; Isoda, A.; Tanaka, S.; Nishio, M.; Umekawa, H. Green tea polyphenol EGCg induces cell fusion via reactive oxygen species. Biochem. Biophys. Rep. 2023, 35, 101536. [Google Scholar] [CrossRef]
- Ellis, L.Z.; Liu, W.; Luo, Y.; Okamoto, M.; Qu, D.; Dunn, J.H.; Fujita, M. Green tea polyphenol epigallocatechin-3-gallate suppresses melanoma growth by inhibiting inflammasome and IL-1beta secretion. Biochem. Biophys. Res. Commun. 2011, 414, 551–556. [Google Scholar] [CrossRef]
- Elmadhun, N.Y.; Sabe, A.A.; Robich, M.P.; Chu, L.M.; Lassaletta, A.D.; Sellke, F.W. The pig as a valuable model for testing the effect of resveratrol to prevent cardiovascular disease. Ann. N. Y Acad. Sci. 2013, 1290, 130–135. [Google Scholar] [CrossRef]
- Lyu, Y.L.; Zhou, H.F.; Yang, J.; Wang, F.X.; Sun, F.; Li, J.Y. Biological Activities Underlying the Therapeutic Effect of Quercetin on Inflammatory Bowel Disease. Mediat. Inflamm. 2022, 2022, 5665778. [Google Scholar] [CrossRef]
- Ojo, A.B.; Adanlawo, I.G. Antioxidant, antidiabetic, and anti-inflammatory activities of flavonoid-rich fractions of Solanum anguivi Lam. fruit: In vitro and ex vivo studies. Heliyon 2024, 10, e31895. [Google Scholar] [CrossRef]
- She, R.; Zhang, Z.; Han, M.; Zhao, D.; Li, X.; Zhou, J.; Chang, Y.; Zhang, X.; Li, X. Luteolin Exhibits Anxiolytic and Antidepressant Potential in Parkinson’s Disease Rat: Antioxidant and Anti-Inflammatory Effects. Rejuvenation Res. 2025, 28, 67–82. [Google Scholar] [CrossRef]
- Kuru Bektasoglu, P.; Demir, D.; Koyuncuoglu, T.; Yuksel, M.; Peker Eyuboglu, I.; Karagoz Koroglu, A.; Akakin, D.; Yildirim, A.; Celikoglu, E.; Gurer, B. Possible anti-inflammatory, antioxidant, and neuroprotective effects of apigenin in the setting of mild traumatic brain injury: An investigation. Immunopharmacol. Immunotoxicol. 2023, 45, 185–196. [Google Scholar] [CrossRef]
- Khalil, H.E.; Ibrahim, H.M.; Ahmed, E.A.; Emeka, P.M.; Alhaider, I.A. Orientin, a Bio-Flavonoid from Trigonella hamosa L., Regulates COX-2/PGE-2 in A549 Cell Lines via miR-26b and miR-146a. Pharmaceuticals 2022, 15, 154. [Google Scholar] [CrossRef] [PubMed]
- Monmai, C.; Kim, J.S.; Chin, J.H.; Lee, S.; Baek, S.H. Inhibitory Effects of Polyphenol- and Flavonoid-Enriched Rice Seed Extract on Melanogenesis in Melan-a Cells via MAPK Signaling-Mediated MITF Downregulation. Int. J. Mol. Sci. 2023, 24, 11841. [Google Scholar] [CrossRef]
- Ren, Q.; Guo, F.; Tao, S.; Huang, R.; Ma, L.; Fu, P. Flavonoid fisetin alleviates kidney inflammation and apoptosis via inhibiting Src-mediated NF-kappaB p65 and MAPK signaling pathways in septic AKI mice. Biomed. Pharmacother. 2020, 122, 109772. [Google Scholar] [CrossRef]
- Che, D.N.; Cho, B.O.; Kim, J.S.; Shin, J.Y.; Kang, H.J.; Jang, S.I. Luteolin and Apigenin Attenuate LPS-Induced Astrocyte Activation and Cytokine Production by Targeting MAPK, STAT3, and NF-kappaB Signaling Pathways. Inflammation 2020, 43, 1716–1728. [Google Scholar] [CrossRef]
- Kasahara, K.; Kerby, R.L.; Cross, T.L.; Everhart, J.; Kay, C.; Bolling, B.W.; Backhed, F.; Rey, F.E. Gut microbiota and diet matrix modulate the effects of the flavonoid quercetin on atherosclerosis. Res. Sq. 2023. [Google Scholar] [CrossRef]
- Chen, S.Y.; Zhou, Q.Y.; Chen, L.; Liao, X.; Li, R.; Xie, T. The Aurantii Fructus Immaturus flavonoid extract alleviates inflammation and modulate gut microbiota in DSS-induced colitis mice. Front. Nutr. 2022, 9, 1013899. [Google Scholar] [CrossRef] [PubMed]
- Tratensek, A.; Locatelli, I.; Grabnar, I.; Drobne, D.; Vovk, T. Oxidative stress-related biomarkers as promising indicators of inflammatory bowel disease activity: A systematic review and meta-analysis. Redox Biol. 2024, 77, 103380. [Google Scholar] [CrossRef]
- Inoue, H.; Shimamoto, S.; Takahashi, H.; Kawashima, Y.; Wataru, S.; Ijiri, D.; Ohtsuka, A. Effects of astaxanthin-rich dried cell powder from Paracoccus carotinifaciens on carotenoid composition and lipid peroxidation in skeletal muscle of broiler chickens under thermo-neutral or realistic high temperature conditions. Anim. Sci. J. 2019, 90, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Berretta, M.; Quagliariello, V.; Bignucolo, A.; Facchini, S.; Maurea, N.; Di Francia, R.; Fiorica, F.; Sharifi, S.; Bressan, S.; Richter, S.N.; et al. The Multiple Effects of Vitamin D against Chronic Diseases: From Reduction of Lipid Peroxidation to Updated Evidence from Clinical Studies. Antioxidants 2022, 11, 1090. [Google Scholar] [CrossRef]
- Lee, Y.R.; Lee, W.H.; Lee, S.Y.; Lee, J.; Kim, M.S.; Moon, M.; Park, G.W.; Kim, H.S.; Kim, J.I.; Lee, J.S.; et al. Regulation of Reactive Oxygen Species Promotes Growth and Carotenoid Production Under Autotrophic Conditions in Rhodobacter sphaeroides. Front. Microbiol. 2022, 13, 847757. [Google Scholar] [CrossRef]
- Crawford, A.C.; Francki, M.G. Chromosomal location of wheat genes of the carotenoid biosynthetic pathway and evidence for a catalase gene on chromosome 7A functionally associated with flour b* colour variation. Mol. Genet. Genom. 2013, 288, 483–493. [Google Scholar] [CrossRef]
- Wu, H.; Li, S.; Wang, L.; Liang, J.; Yan, L.; Dong, J. Fucoxanthin, a Marine Carotenoid, Suppresses Mycoplasma pneumoniae-Triggered Inflammatory Cytokine Production and Promotes Bacterial Clearance in a Murine Model. Evid. Based Complement. Altern. Med. 2022, 2022, 6238162. [Google Scholar] [CrossRef] [PubMed]
- Meurer, M.C.; Mees, M.; Mariano, L.N.B.; Boeing, T.; Somensi, L.B.; Mariott, M.; da Silva, R.; Dos Santos, A.C.; Longo, B.; Santos Franca, T.C.; et al. Hydroalcoholic extract of Tagetes erecta L. flowers, rich in the carotenoid lutein, attenuates inflammatory cytokine secretion and improves the oxidative stress in an animal model of ulcerative colitis. Nutr. Res. 2019, 66, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Baz, L.; Algarni, S.; Al-Thepyani, M.; Aldairi, A.; Gashlan, H. Lycopene Improves Metabolic Disorders and Liver Injury Induced by a Hight-Fat Diet in Obese Rats. Molecules 2022, 27, 7736. [Google Scholar] [CrossRef]
- Yang, X.; Li, L.; Shang, G.; Zhuo, M.; Zhu, H.; Xu, S.; Zhao, J.; Hou, X.; Shi, Y. Oral Curcumin through Mesoporous Silica Nanomaterials with Distinct Morphologies: Synthesis, Characterization, Biosafety Evaluation, and Antioxidant Activity In Vivo. Langmuir 2024, 40, 27216–27227. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, H.; Wang, J.; Chen, Y.; Lin, J.; Wang, Q.; Wu, C.; Chen, H.; Lin, Y. Curcumin attenuates ulcerative colitis via regulation of Sphingosine kinases 1/NF-kappaB signaling pathway. Biofactors 2025, 51, e70001. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, E.; Kotiya, A.; Bhuyan, R.; Raza, S.T.; Misra, A.; Ahmad, R.; Mahdi, A.A. Curcumin chemo-sensitizes intrinsic apoptosis through ROS-mediated mitochondrial hyperpolarization and DNA damage in breast cancer cells. Cell Signal 2025, 128, 111637. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, R.; Zou, M.; Jiang, L.; Kong, L.; Zhao, S.; Zhang, X.; Wang, W.; Xu, B. Combined ROS Sensitive Folate Receptor Targeted Micellar Formulations of Curcumin Effective Against Rheumatoid Arthritis in Rat Model. Int. J. Nanomed. 2024, 19, 4217–4234. [Google Scholar] [CrossRef]
- Alsahli, M.A.; Almatroodi, S.A.; Almatroudi, A.; Khan, A.A.; Anwar, S.; Almutary, A.G.; Alrumaihi, F.; Rahmani, A.H. 6-Gingerol, a Major Ingredient of Ginger Attenuates Diethylnitrosamine-Induced Liver Injury in Rats through the Modulation of Oxidative Stress and Anti-Inflammatory Activity. Mediat. Inflamm. 2021, 2021, 6661937. [Google Scholar] [CrossRef]
- Isa, Y.; Miyakawa, Y.; Yanagisawa, M.; Goto, T.; Kang, M.S.; Kawada, T.; Morimitsu, Y.; Kubota, K.; Tsuda, T. 6-Shogaol and 6-gingerol, the pungent of ginger, inhibit TNF-alpha mediated downregulation of adiponectin expression via different mechanisms in 3T3-L1 adipocytes. Biochem. Biophys. Res. Commun. 2008, 373, 429–434. [Google Scholar] [CrossRef]
- Lee, T.Y.; Lee, K.C.; Chen, S.Y.; Chang, H.H. 6-Gingerol inhibits ROS and iNOS through the suppression of PKC-alpha and NF-kappaB pathways in lipopolysaccharide-stimulated mouse macrophages. Biochem. Biophys. Res. Commun. 2009, 382, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Siddique, R.; Muhammad, F.; Faisal, M.N.; Akhtar, B.; Saleem, A.; Kousar, S.; Sharif, A.; Saeed, M.; Muhammad, S. Gingerol nanoparticles attenuate complete Freund adjuvant-induced arthritis in rats via targeting the RANKL/OPG signaling pathway. Inflammopharmacology 2024, 32, 3311–3326. [Google Scholar] [CrossRef]
- Sahler, J.; Bernard, J.J.; Spinelli, S.L.; Blumberg, N.; Phipps, R.P. The Feverfew plant-derived compound, parthenolide enhances platelet production and attenuates platelet activation through NF-kappaB inhibition. Thromb. Res. 2011, 127, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Najera-Martinez, M.; Lara-Vega, I.; Avilez-Alvarado, J.; Pagadala, N.S.; Dzul-Caamal, R.; Dominguez-Lopez, M.L.; Tuszynski, J.; Vega-Lopez, A. The Generation of ROS by Exposure to Trihalomethanes Promotes the IkappaBalpha/NF-kappaB/p65 Complex Dissociation in Human Lung Fibroblast. Biomedicines 2024, 12, 2399. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.; Han, D.; Deng, K.; Zhou, H.; Wu, M. Quercetin Boosts Pulsatile Gonadotropin-Releasing Hormone Release to Improve Luteal Function via Inhibiting NF-kappaB/NLRP3-Mediated Neuron Pyroptosis. Mol. Nutr. Food Res. 2024, 68, e2400649. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Wang, W.; Ou, H.; Ning, J.; Zhou, Y.; Ke, J.; Hou, A.; Chen, L.; Li, P.; Ma, Y.; et al. Identification of chalcone analogues as anti-inflammatory agents through the regulation of NF-kappaB and JNK activation. RSC Med. Chem. 2024, 15, 2002–2017. [Google Scholar] [CrossRef]
- Ma, Y.; Ma, Z.; Zhang, Y.; Luo, C.; Huang, P.; Tong, J.; Ding, H.; Liu, H. Apigenin and baicalein ameliorate thoracic aortic structural deterioration and cognitive deficit via inhibiting AGEs/RAGE/NF-kappaB pathway in D-galactose-induced aging rats. Eur. J. Pharmacol. 2024, 976, 176660. [Google Scholar] [CrossRef]
- Hossen, I.; Kaiqi, Z.; Hua, W.; Junsong, X.; Mingquan, H.; Yanping, C. Epigallocatechin gallate (EGCG) inhibits lipopolysaccharide-induced inflammation in RAW 264.7 macrophage cells via modulating nuclear factor kappa-light-chain enhancer of activated B cells (NF-kappaB) signaling pathway. Food Sci. Nutr. 2023, 11, 4634–4650. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, B.; Shi, M.; Ye, T.; Li, H. NLRP3 Inflammasome Activation Is Involved in Geniposide-Induced Hepatotoxicity. Mediat. Inflamm. 2025, 2025, 4112856. [Google Scholar] [CrossRef]
- Zu, R.; Lu, H.; Liu, W.; Shao, S.; Zheng, J.; Ying, X.; Zhou, Y.; Li, Z.; Wang, W.; Li, D.; et al. Research Progress in the Molecular Mechanism of NLRP3 Inflammasome in Alzheimer’s Disease and Regulation by Natural Plant Products. Mol. Neurobiol. 2025, 62, 7296–7312. [Google Scholar] [CrossRef]
- Yang, K.; Zeng, L.; He, Q.; Wang, S.; Xu, H.; Ge, J. Advancements in research on the immune-inflammatory mechanisms mediated by NLRP3 inflammasome in ischemic stroke and the regulatory role of natural plant products. Front. Pharmacol. 2024, 15, 1250918. [Google Scholar] [CrossRef] [PubMed]
- Santos-Garcia, I.; Bascunana, P.; Brackhan, M.; Villa, M.; Eiriz, I.; Bruning, T.; Pahnke, J. The ABC transporter A7 modulates neuroinflammation via NLRP3 inflammasome in Alzheimer’s disease mice. Alzheimers Res. Ther. 2025, 17, 30. [Google Scholar] [CrossRef]
- Sharma, P.; Chouhan, R.; Bakshi, P.; Gandhi, S.G.; Kaur, R.; Sharma, A.; Bhardwaj, R. Amelioration of Chromium-Induced Oxidative Stress by Combined Treatment of Selected Plant-Growth-Promoting Rhizobacteria and Earthworms via Modulating the Expression of Genes Related to Reactive Oxygen Species Metabolism in Brassica juncea. Front. Microbiol. 2022, 13, 802512. [Google Scholar] [CrossRef] [PubMed]
- Farhan, M. Cytotoxic Activity of the Red Grape Polyphenol Resveratrol against Human Prostate Cancer Cells: A Molecular Mechanism Mediated by Mobilization of Nuclear Copper and Generation of Reactive Oxygen Species. Life 2024, 14, 611. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Jiao, Y.; Chen, K.; Chen, T.; Wu, X.; Jiang, X.; Bu, W.; Liu, C.; Qu, X. Redox-active polyphenol nanoparticles deprive endogenous glutathione of electrons for ROS generation and tumor chemodynamic therapy. Acta Biomater. 2023, 172, 423–440. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, K.D.; Pittaway, J.K.; Ball, M.J. Effects of olive oil and tomato lycopene combination on serum lycopene, lipid profile, and lipid oxidation. Nutrition 2006, 22, 259–265. [Google Scholar] [CrossRef]
- Andrade, P.; Santamarina, A.B.; de Freitas, J.A.; Marum, A.; Pessoa, A.F.M. Personalized nutrition and precision medicine in perimenopausal women: A minireview of genetic polymorphisms COMT, FUT2, and MTHFR. Clinics 2025, 80, 100549. [Google Scholar] [CrossRef]
- Gardner, D.S.L.; Saboo, B.; Kesavadev, J.; Mustafa, N.; Villa, M.; Mahoney, E.; Bajpai, S. Digital Health Technology in Diabetes Management in the Asia-Pacific Region: A Narrative Review of the Current Scenario and Future Outlook. Diabetes Ther. 2025, 16, 349–369. [Google Scholar] [CrossRef]
- Giri, N.A.; Gaikwad, P.; Gaikwad, N.N.; Manjunatha, N.; Krishnakumar, T.; Kad, V.; Raigond, P.; Suryavanshi, S.; Marathe, R.A. Development of fiber-enriched muffins using pomegranate peel powder and its effect on physico-chemical properties and shelf life of the muffins. J. Sci. Food Agric. 2024, 104, 2346–2358. [Google Scholar] [CrossRef]
- Ben-Othman, S.; Joudu, I.; Bhat, R. Bioactives From Agri-Food Wastes: Present Insights and Future Challenges. Molecules 2020, 25, 510. [Google Scholar] [CrossRef]
- Nunez-Gomez, V.; Gonzalez-Barrio, R.; Baenas, N.; Moreno, D.A.; Periago, M.J. Dietary-Fibre-Rich Fractions Isolated from Broccoli Stalks as a Potential Functional Ingredient with Phenolic Compounds and Glucosinolates. Int. J. Mol. Sci. 2022, 23, 13309. [Google Scholar] [CrossRef] [PubMed]
- Slavin, J.L. Dietary fiber and body weight. Nutrition 2005, 21, 411–418. [Google Scholar] [CrossRef] [PubMed]
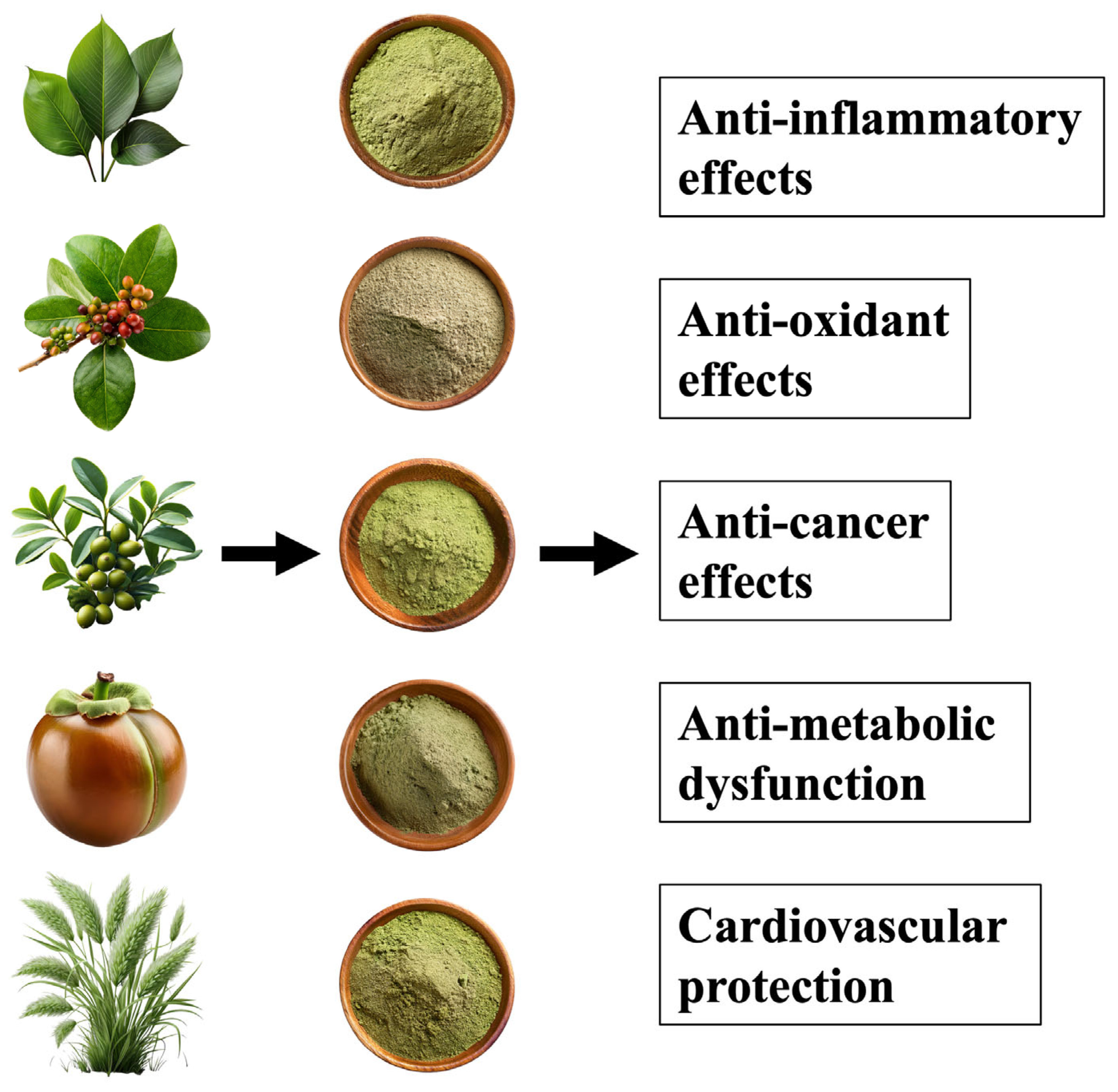
| High Content Plants | Principal Effect | |
|---|---|---|
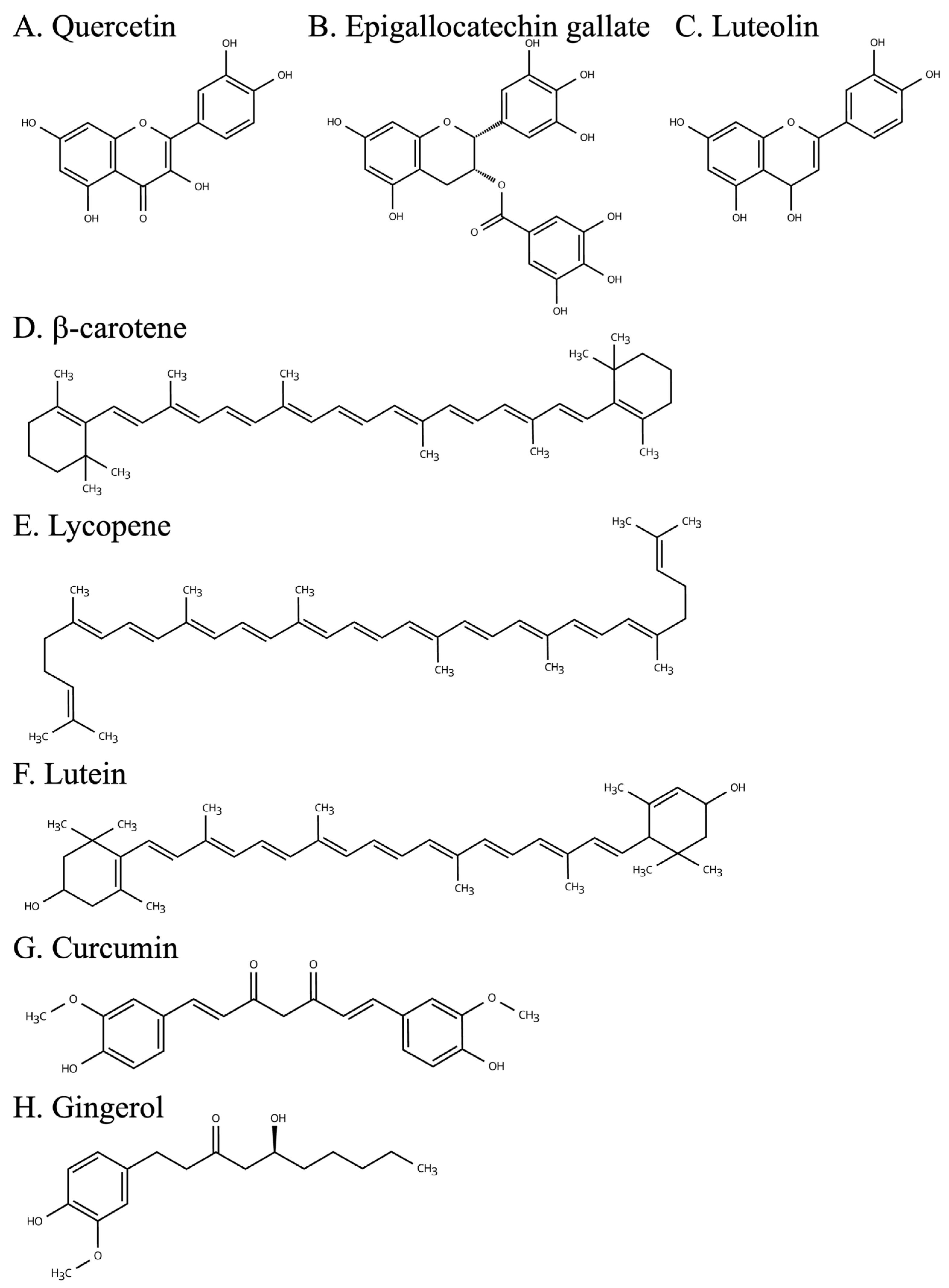
| Quercetin | Apples, onion | Antioxidant, anti-inflammatory effect |
| Epigallocatechin gallate | Green tea leaves | Antioxidant, anti-inflammatory effect |
| Luteolin | Celery, bell peppers | Antioxidant, anti-inflammatory effect |
| β-carotene | Carrots, squash | Antioxidant, anti-inflammatory effect |
| Lycopene | Tomatoes, watermelon | Anti-inflammatory, and reduction the risk of obesity-related diseases effect |
| Lutein | Spinach, kale | Anti-inflammatory effect |
| Curcumin | Curcuma longa | Antioxidant, anti-inflammatory effect |
| Gingerol | Zingiber officinale | Anti-inflammatory effect |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakadate, K.; Ito, N.; Kawakami, K.; Yamazaki, N. Anti-Inflammatory Actions of Plant-Derived Compounds and Prevention of Chronic Diseases: From Molecular Mechanisms to Applications. Int. J. Mol. Sci. 2025, 26, 5206. https://doi.org/10.3390/ijms26115206
Nakadate K, Ito N, Kawakami K, Yamazaki N. Anti-Inflammatory Actions of Plant-Derived Compounds and Prevention of Chronic Diseases: From Molecular Mechanisms to Applications. International Journal of Molecular Sciences. 2025; 26(11):5206. https://doi.org/10.3390/ijms26115206
Chicago/Turabian StyleNakadate, Kazuhiko, Nozomi Ito, Kiyoharu Kawakami, and Noriko Yamazaki. 2025. "Anti-Inflammatory Actions of Plant-Derived Compounds and Prevention of Chronic Diseases: From Molecular Mechanisms to Applications" International Journal of Molecular Sciences 26, no. 11: 5206. https://doi.org/10.3390/ijms26115206
APA StyleNakadate, K., Ito, N., Kawakami, K., & Yamazaki, N. (2025). Anti-Inflammatory Actions of Plant-Derived Compounds and Prevention of Chronic Diseases: From Molecular Mechanisms to Applications. International Journal of Molecular Sciences, 26(11), 5206. https://doi.org/10.3390/ijms26115206






