Phase Separation in Chromatin Organization and Human Diseases
Abstract
1. Introduction
2. Organization of Multi-Level Chromatin Structure via Phase Separation
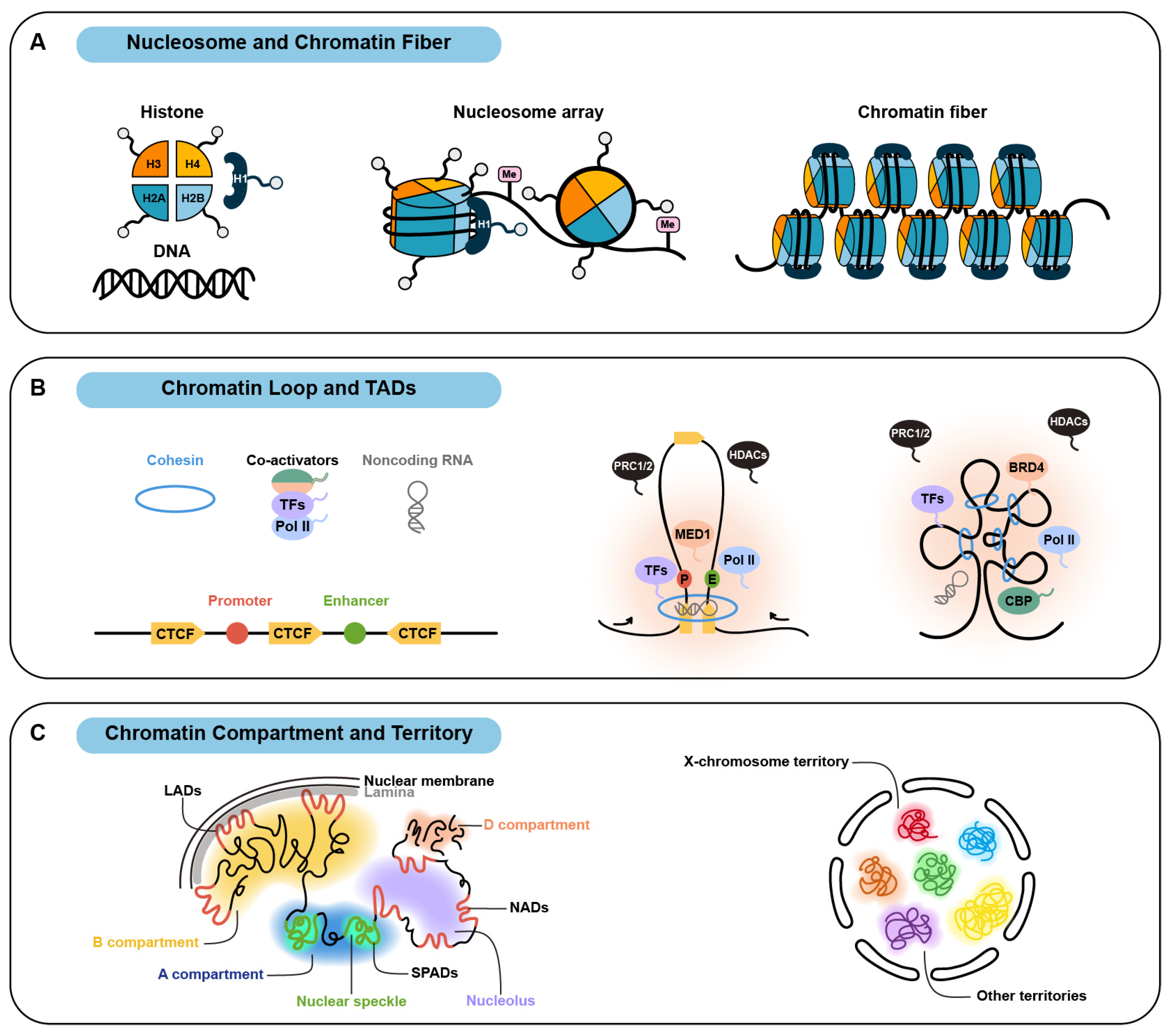
2.1. Nucleosome and Chromatin Fiber
2.2. Chromatin Loop and Topologically Associating Domain
2.3. Chromatin Compartment and Chromosome Territory
3. Dysregulation of Chromatin Structure Mediated by Phase Separation in Human Diseases
3.1. Cancers
| Genes | Pathological Functions Through Chromatin Organization Associated with Phase Separation | Refs. |
|---|---|---|
| NUP98 fusions with HOXA9, KDM5A, LNP1, PRRX1, NSD1 | NUP98 fusion proteins form phase-separated condensates and promote transcriptionally active chromatin loops at proto-oncogenic locus, further assembling super-enhancers to amplify oncogene activation in human hematological malignancies. | [146,147,148,149,150] |
| FET family fusions (FUS, EWS, TAF15) | FET family fusion proteins condensate at specifically silenced locus and attract RNA polymerase II and chromatin remodeling complexes BAFs to form transcriptionally active chromatin hubs, contributing to oncogenic transformation in sarcomas and leukemia. | [154,155,156] |
| YAP-MAMLD1, C11ORF95-YAP | YAP fusion proteins nuclear condensates concentrate transcription factors and coactivators (TEAD, BRD4, MED1) and exclude polycomb repressive complex PRC2, inducing transcriptionally active chromatin loops that promote ependymoma tumorigenesis. | [153] |
| BRD4-NUT | BRD4-NUT recognizes acetylated chromatin and binds acetyltransferase p300 to form condensates, inducing histone hyperacetylation and chromatin subcompartment that sustain aberrant anti-differentiation genes transcription and perpetual tumor cell growth in midline carcinomas. | [157,158,159] |
| SS18-SSX1 | SS18-SSX1 condensates at H2AK119ub-marked oncogenic locus, recruits BAFs complexes and histone acetyltransferase CBP/p300 while excludes HDAC1/2 deacetylase complexes to assemble transcriptionally active chromatin loops/TADs that elevate H3K27ac level and sustain oncogene overexpression in synovial sarcoma. | [160,161,162] |
| UTX | UTX condensates demethylate H3K27me3 and recruit histone lysine methyltransferase MLL4 and p300 to establish transcriptionally active chromatin loops that activate immune-related genes while suppressing cell division-related genes. The pancreatic cancer and myeloid leukemia-associated mutations in UTX impair these condensates. | [173] |
| FOXA1 | FOXA1 condensates unpack heterochromatin and activate tumor suppressor genes. Mutations in DNA-binding domain of FOXA1 abrogate its tumor-suppressive function driven by heterochromatin targeting and condensate formation in breast and prostate cancers. | [174,175,176,177,178,179] |
| ZHX2 | ZHX2 condensates, in response to hypoxic tumor microenvironment, recruit CTCF, BRD4, and MED1, reshaping chromatin loops to activate oncogenes and promote metastasis | [182] |
| ARID1A, HDAC6, FOXM1 | Hyperactivated ARID1A/phospho-HDAC6/FOXM1 forms similar condensates in Ewing’s sarcoma or breast cancer recruiting BAFs complexes, Pol II, and coactivators to remodel chromatin structure that drive oncogenic transcription and tumor progression. | [168,183,184] |
| TERRA | TERRA, an overexpressed lncRNA in telomerase-negative cancers, collaborates with histone lysine demethylase LSD1 and RNA-binding protein HNRNPA1 to form telomeric condensates which elongate telomeres and stabilize telomeric heterochromatin for immortalization. | [186,187,188] |
3.2. Developmental Disorders
| Genes | Pathological Functions Through Chromatin Organization Associated with Phase Separation | Refs. |
|---|---|---|
| MECP2 | MECP2 recognizes and binds methylated DNA, condensing chromatin fibers to form heterochromatin compartments. Rett syndrome-associated MECP2 mutations impair these compartments, leading to related heterochromatin dysregulation and pathogenic genes activation. | [189,190,191] |
| MLL4 | MLL4 condensates methylate H3K4 and recruit MED1/BRD4 while excluding PRC1/2. Kabuki syndrome-associated mutations in MLL4 impair these condensates and disrupt the balance between chromatin compartments, resulting in transcriptional dysregulation. | [192] |
| HOXD13, HOXA13, RUNX2 | HOXD13/HOXA13/RUNX2 condensates with expanded polyalanine mutation fail to recruit coactivators and disrupt the formation of transcriptionally active TADs, causing synpolydactyly, hand-foot genital syndrome, or cleidocranial dysplasia. | [203] |
| HMGB1 | HMGB1 with arginine-rich basic tail mutation fails to organize the chromatin loops and forms aberrant condensates that invade the nucleolus, disrupting its function and leading to brachyphalangy, polydactyly, and tibial aplasia/hypoplasia syndrome. | [204,208] |
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bonev, B.; Cavalli, G. Organization and function of the 3D genome. Nat. Rev. Genet. 2016, 17, 661–678. [Google Scholar] [CrossRef] [PubMed]
- Rowley, M.J.; Corces, V.G. Organizational principles of 3D genome architecture. Nat. Rev. Genet. 2018, 19, 789–800. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Su, J.H.; Beliveau, B.J.; Bintu, B.; Moffitt, J.R.; Wu, C.T.; Zhuang, X. Spatial organization of chromatin domains and compartments in single chromosomes. Science 2016, 353, 598–602. [Google Scholar] [CrossRef]
- Ou, H.D.; Phan, S.; Deerinck, T.J.; Thor, A.; Ellisman, M.H.; O’Shea, C.C. ChromEMT: Visualizing 3D chromatin structure and compaction in interphase and mitotic cells. Science 2017, 357, eaag0025. [Google Scholar] [CrossRef] [PubMed]
- Lieberman-Aiden, E.; van Berkum, N.L.; Williams, L.; Imakaev, M.; Ragoczy, T.; Telling, A.; Amit, I.; Lajoie, B.R.; Sabo, P.J.; Dorschner, M.O.; et al. Comprehensive mapping of long-range interactions reveals folding principles of the human genome. Science 2009, 326, 289–293. [Google Scholar] [CrossRef]
- Dixon, J.R.; Selvaraj, S.; Yue, F.; Kim, A.; Li, Y.; Shen, Y.; Hu, M.; Liu, J.S.; Ren, B. Topological domains in mammalian genomes identified by analysis of chromatin interactions. Nature 2012, 485, 376–380. [Google Scholar] [CrossRef]
- Szabo, Q.; Bantignies, F.; Cavalli, G. Principles of genome folding into topologically associating domains. Sci. Adv. 2019, 5, eaaw1668. [Google Scholar] [CrossRef]
- Spielmann, M.; Lupianez, D.G.; Mundlos, S. Structural variation in the 3D genome. Nat. Rev. Genet. 2018, 19, 453–467. [Google Scholar] [CrossRef]
- Zheng, H.; Xie, W. The role of 3D genome organization in development and cell differentiation. Nat. Rev. Mol. Cell Biol. 2019, 20, 535–550. [Google Scholar] [CrossRef]
- Fudenberg, G.; Imakaev, M.; Lu, C.; Goloborodko, A.; Abdennur, N.; Mirny, L.A. Formation of Chromosomal Domains by Loop Extrusion. Cell Rep. 2016, 15, 2038–2049. [Google Scholar] [CrossRef]
- Strom, A.R.; Emelyanov, A.V.; Mir, M.; Fyodorov, D.V.; Darzacq, X.; Karpen, G.H. Phase separation drives heterochromatin domain formation. Nature 2017, 547, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Misteli, T. The Self-Organizing Genome: Principles of Genome Architecture and Function. Cell 2020, 183, 28–45. [Google Scholar] [CrossRef] [PubMed]
- Dolgin, E. What lava lamps and vinaigrette can teach us about cell biology. Nature 2018, 555, 300–302. [Google Scholar] [CrossRef] [PubMed]
- Brangwynne, C.P.; Eckmann, C.R.; Courson, D.S.; Rybarska, A.; Hoege, C.; Gharakhani, J.; Julicher, F.; Hyman, A.A. Germline P granules are liquid droplets that localize by controlled dissolution/condensation. Science 2009, 324, 1729–1732. [Google Scholar] [CrossRef]
- Yang, P.; Mathieu, C.; Kolaitis, R.M.; Zhang, P.; Messing, J.; Yurtsever, U.; Yang, Z.; Wu, J.; Li, Y.; Pan, Q.; et al. G3BP1 Is a Tunable Switch that Triggers Phase Separation to Assemble Stress Granules. Cell 2020, 181, 325–345.e28. [Google Scholar] [CrossRef]
- Brangwynne, C.P.; Mitchison, T.J.; Hyman, A.A. Active liquid-like behavior of nucleoli determines their size and shape in Xenopus laevis oocytes. Proc. Natl. Acad. Sci. USA 2011, 108, 4334–4339. [Google Scholar] [CrossRef]
- Banani, S.F.; Lee, H.O.; Hyman, A.A.; Rosen, M.K. Biomolecular condensates: Organizers of cellular biochemistry. Nat. Rev. Mol. Cell Biol. 2017, 18, 285–298. [Google Scholar] [CrossRef]
- Erdel, F.; Rippe, K. Formation of Chromatin Subcompartments by Phase Separation. Biophys. J. 2018, 114, 2262–2270. [Google Scholar] [CrossRef]
- Hildebrand, E.M.; Dekker, J. Mechanisms and Functions of Chromosome Compartmentalization. Trends Biochem. Sci. 2020, 45, 385–396. [Google Scholar] [CrossRef]
- Mittag, T.; Pappu, R.V. A conceptual framework for understanding phase separation and addressing open questions and challenges. Mol. Cell 2022, 82, 2201–2214. [Google Scholar] [CrossRef]
- Olins, D.E.; Olins, A.L. Chromatin history: Our view from the bridge. Nat. Rev. Mol. Cell Biol. 2003, 4, 809–814. [Google Scholar] [CrossRef] [PubMed]
- Song, F.; Chen, P.; Sun, D.; Wang, M.; Dong, L.; Liang, D.; Xu, R.M.; Zhu, P.; Li, G. Cryo-EM study of the chromatin fiber reveals a double helix twisted by tetranucleosomal units. Science 2014, 344, 376–380. [Google Scholar] [CrossRef] [PubMed]
- Dubochet, J.; Adrian, M.; Schultz, P.; Oudet, P. Cryo-electron microscopy of vitrified SV40 minichromosomes: The liquid drop model. EMBO J. 1986, 5, 519–528. [Google Scholar] [CrossRef]
- Maeshima, K.; Rogge, R.; Tamura, S.; Joti, Y.; Hikima, T.; Szerlong, H.; Krause, C.; Herman, J.; Seidel, E.; DeLuca, J.; et al. Nucleosomal arrays self-assemble into supramolecular globular structures lacking 30-nm fibers. EMBO J. 2016, 35, 1115–1132. [Google Scholar] [CrossRef]
- Maeshima, K.; Ide, S.; Hibino, K.; Sasai, M. Liquid-like behavior of chromatin. Curr. Opin. Genet. Dev. 2016, 37, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, M.; Giuliani, A.; Yoshikawa, K. Cell-Fate Determination from Embryo to Cancer Development: Genomic Mechanism Elucidated. Int. J. Mol. Sci. 2020, 21, 4581. [Google Scholar] [CrossRef]
- Tsuchiya, M.; Giuliani, A.; Hashimoto, M.; Erenpreisa, J.; Yoshikawa, K. Self-Organizing Global Gene Expression Regulated through Criticality: Mechanism of the Cell-Fate Change. PLoS ONE 2016, 11, e0167912. [Google Scholar] [CrossRef]
- Gordon, F.; Luger, K.; Hansen, J.C. The core histone N-terminal tail domains function independently and additively during salt-dependent oligomerization of nucleosomal arrays. J. Biol. Chem. 2005, 280, 33701–33706. [Google Scholar] [CrossRef]
- Hammonds, E.F.; Harwig, M.C.; Paintsil, E.A.; Tillison, E.A.; Hill, R.B.; Morrison, E.A. Histone H3 and H4 tails play an important role in nucleosome phase separation. Biophys. Chem. 2022, 283, 106767. [Google Scholar] [CrossRef]
- Gibson, B.A.; Doolittle, L.K.; Schneider, M.W.G.; Jensen, L.E.; Gamarra, N.; Henry, L.; Gerlich, D.W.; Redding, S.; Rosen, M.K. Organization of Chromatin by Intrinsic and Regulated Phase Separation. Cell 2019, 179, 470–484.e21. [Google Scholar] [CrossRef]
- Shakya, A.; Park, S.; Rana, N.; King, J.T. Liquid-Liquid Phase Separation of Histone Proteins in Cells: Role in Chromatin Organization. Biophys. J. 2020, 118, 753–764. [Google Scholar] [CrossRef] [PubMed]
- Gallego, L.D.; Schneider, M.; Mittal, C.; Romanauska, A.; Gudino Carrillo, R.M.; Schubert, T.; Pugh, B.F.; Kohler, A. Phase separation directs ubiquitination of gene-body nucleosomes. Nature 2020, 579, 592–597. [Google Scholar] [CrossRef] [PubMed]
- Farr, S.E.; Woods, E.J.; Joseph, J.A.; Garaizar, A.; Collepardo-Guevara, R. Nucleosome plasticity is a critical element of chromatin liquid-liquid phase separation and multivalent nucleosome interactions. Nat. Commun. 2021, 12, 2883. [Google Scholar] [CrossRef]
- Leicher, R.; Osunsade, A.; Chua, G.N.L.; Faulkner, S.C.; Latham, A.P.; Watters, J.W.; Nguyen, T.; Beckwitt, E.C.; Christodoulou-Rubalcava, S.; Young, P.G.; et al. Single-stranded nucleic acid binding and coacervation by linker histone H1. Nat. Struct. Mol. Biol. 2022, 29, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Turner, A.L.; Watson, M.; Wilkins, O.G.; Cato, L.; Travers, A.; Thomas, J.O.; Stott, K. Highly disordered histone H1-DNA model complexes and their condensates. Proc. Natl. Acad. Sci. USA 2018, 115, 11964–11969. [Google Scholar] [CrossRef]
- Dekker, C.; Haering, C.H.; Peters, J.M.; Rowland, B.D. How do molecular motors fold the genome? Science 2023, 382, 646–648. [Google Scholar] [CrossRef]
- Schwarzer, W.; Abdennur, N.; Goloborodko, A.; Pekowska, A.; Fudenberg, G.; Loe-Mie, Y.; Fonseca, N.A.; Huber, W.; Haering, C.H.; Mirny, L.; et al. Two independent modes of chromatin organization revealed by cohesin removal. Nature 2017, 551, 51–56. [Google Scholar] [CrossRef]
- Petela, N.J.; Gligoris, T.G.; Metson, J.; Lee, B.G.; Voulgaris, M.; Hu, B.; Kikuchi, S.; Chapard, C.; Chen, W.; Rajendra, E.; et al. Scc2 Is a Potent Activator of Cohesin’s ATPase that Promotes Loading by Binding Scc1 without Pds5. Mol. Cell 2018, 70, 1134–1148.e7. [Google Scholar] [CrossRef]
- Rao, S.S.; Huntley, M.H.; Durand, N.C.; Stamenova, E.K.; Bochkov, I.D.; Robinson, J.T.; Sanborn, A.L.; Machol, I.; Omer, A.D.; Lander, E.S.; et al. A 3D map of the human genome at kilobase resolution reveals principles of chromatin looping. Cell 2014, 159, 1665–1680. [Google Scholar] [CrossRef]
- Rao, S.S.P.; Huang, S.C.; Glenn St Hilaire, B.; Engreitz, J.M.; Perez, E.M.; Kieffer-Kwon, K.R.; Sanborn, A.L.; Johnstone, S.E.; Bascom, G.D.; Bochkov, I.D.; et al. Cohesin Loss Eliminates All Loop Domains. Cell 2017, 171, 305–320.e24. [Google Scholar] [CrossRef]
- Haarhuis, J.H.I.; van der Weide, R.H.; Blomen, V.A.; Yanez-Cuna, J.O.; Amendola, M.; van Ruiten, M.S.; Krijger, P.H.L.; Teunissen, H.; Medema, R.H.; van Steensel, B.; et al. The Cohesin Release Factor WAPL Restricts Chromatin Loop Extension. Cell 2017, 169, 693–707.e14. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.Q.; Maresca, M.; van den Brand, T.; Braccioli, L.; Schijns, M.; Teunissen, H.; Bruneau, B.G.; Nora, E.P.; de Wit, E. WAPL maintains a cohesin loading cycle to preserve cell-type-specific distal gene regulation. Nat. Genet. 2021, 53, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Hafner, A.; Park, M.; Berger, S.E.; Murphy, S.E.; Nora, E.P.; Boettiger, A.N. Loop stacking organizes genome folding from TADs to chromosomes. Mol. Cell 2023, 83, 1377–1392.e6. [Google Scholar] [CrossRef]
- Sexton, T.; Yaffe, E.; Kenigsberg, E.; Bantignies, F.; Leblanc, B.; Hoichman, M.; Parrinello, H.; Tanay, A.; Cavalli, G. Three-dimensional folding and functional organization principles of the Drosophila genome. Cell 2012, 148, 458–472. [Google Scholar] [CrossRef]
- Nora, E.P.; Lajoie, B.R.; Schulz, E.G.; Giorgetti, L.; Okamoto, I.; Servant, N.; Piolot, T.; van Berkum, N.L.; Meisig, J.; Sedat, J.; et al. Spatial partitioning of the regulatory landscape of the X-inactivation centre. Nature 2012, 485, 381–385. [Google Scholar] [CrossRef]
- Gabriele, M.; Brandao, H.B.; Grosse-Holz, S.; Jha, A.; Dailey, G.M.; Cattoglio, C.; Hsieh, T.S.; Mirny, L.; Zechner, C.; Hansen, A.S. Dynamics of CTCF- and cohesin-mediated chromatin looping revealed by live-cell imaging. Science 2022, 376, 496–501. [Google Scholar] [CrossRef]
- Thiecke, M.J.; Wutz, G.; Muhar, M.; Tang, W.; Bevan, S.; Malysheva, V.; Stocsits, R.; Neumann, T.; Zuber, J.; Fraser, P.; et al. Cohesin-Dependent and -Independent Mechanisms Mediate Chromosomal Contacts between Promoters and Enhancers. Cell Rep. 2020, 32, 107929. [Google Scholar] [CrossRef]
- Aljahani, A.; Hua, P.; Karpinska, M.A.; Quililan, K.; Davies, J.O.J.; Oudelaar, A.M. Analysis of sub-kilobase chromatin topology reveals nano-scale regulatory interactions with variable dependence on cohesin and CTCF. Nat. Commun. 2022, 13, 2139. [Google Scholar] [CrossRef] [PubMed]
- Karpinska, M.A.; Oudelaar, A.M. The role of loop extrusion in enhancer-mediated gene activation. Curr. Opin. Genet. Dev. 2023, 79, 102022. [Google Scholar] [CrossRef]
- Arnould, C.; Rocher, V.; Finoux, A.L.; Clouaire, T.; Li, K.; Zhou, F.; Caron, P.; Mangeot, P.E.; Ricci, E.P.; Mourad, R.; et al. Loop extrusion as a mechanism for formation of DNA damage repair foci. Nature 2021, 590, 660–665. [Google Scholar] [CrossRef]
- Piazza, A.; Bordelet, H.; Dumont, A.; Thierry, A.; Savocco, J.; Girard, F.; Koszul, R. Cohesin regulates homology search during recombinational DNA repair. Nat. Cell Biol. 2021, 23, 1176–1186. [Google Scholar] [CrossRef] [PubMed]
- Chiolo, I.; Altmeyer, M.; Legube, G.; Mekhail, K. Nuclear and genome dynamics underlying DNA double-strand break repair. Nat. Rev. Mol. Cell Biol. 2025. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Panchakshari, R.A.; Zhang, T.; Zhang, Y.; Hu, J.; Volpi, S.A.; Meyers, R.M.; Ho, Y.J.; Du, Z.; Robbiani, D.F.; et al. Orientation-specific joining of AID-initiated DNA breaks promotes antibody class switching. Nature 2015, 525, 134–139. [Google Scholar] [CrossRef]
- Jain, S.; Ba, Z.; Zhang, Y.; Dai, H.Q.; Alt, F.W. CTCF-Binding Elements Mediate Accessibility of RAG Substrates During Chromatin Scanning. Cell 2018, 174, 102–116.e14. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, X.; Dai, H.Q.; Hu, H.; Alt, F.W. The role of chromatin loop extrusion in antibody diversification. Nat. Rev. Immunol. 2022, 22, 550–566. [Google Scholar] [CrossRef]
- Ulianov, S.V.; Velichko, A.K.; Magnitov, M.D.; Luzhin, A.V.; Golov, A.K.; Ovsyannikova, N.; Kireev, I.I.; Gavrikov, A.S.; Mishin, A.S.; Garaev, A.K.; et al. Suppression of liquid-liquid phase separation by 1,6-hexanediol partially compromises the 3D genome organization in living cells. Nucleic Acids Res. 2021, 49, 10524–10541. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Jiang, S.; Ma, L.; Qu, J.; Zhao, L.; Zhu, X.; Ding, J. Time-dependent effect of 1,6-hexanediol on biomolecular condensates and 3D chromatin organization. Genome Biol. 2021, 22, 230. [Google Scholar] [CrossRef]
- Gamliel, A.; Meluzzi, D.; Oh, S.; Jiang, N.; Destici, E.; Rosenfeld, M.G.; Nair, S.J. Long-distance association of topological boundaries through nuclear condensates. Proc. Natl. Acad. Sci. USA 2022, 119, e2206216119. [Google Scholar] [CrossRef]
- Sabari, B.R.; Dall’Agnese, A.; Boija, A.; Klein, I.A.; Coffey, E.L.; Shrinivas, K.; Abraham, B.J.; Hannett, N.M.; Zamudio, A.V.; Manteiga, J.C.; et al. Coactivator condensation at super-enhancers links phase separation and gene control. Science 2018, 361, eaar3958. [Google Scholar] [CrossRef]
- Cho, W.K.; Spille, J.H.; Hecht, M.; Lee, C.; Li, C.; Grube, V.; Cisse, I.I. Mediator and RNA polymerase II clusters associate in transcription-dependent condensates. Science 2018, 361, 412–415. [Google Scholar] [CrossRef]
- Boija, A.; Klein, I.A.; Sabari, B.R.; Dall’Agnese, A.; Coffey, E.L.; Zamudio, A.V.; Li, C.H.; Shrinivas, K.; Manteiga, J.C.; Hannett, N.M.; et al. Transcription Factors Activate Genes through the Phase-Separation Capacity of Their Activation Domains. Cell 2018, 175, 1842–1855.e16. [Google Scholar] [CrossRef]
- Wang, W.; Qiao, S.; Li, G.; Cheng, J.; Yang, C.; Zhong, C.; Stovall, D.B.; Shi, J.; Teng, C.; Li, D.; et al. A histidine cluster determines YY1-compartmentalized coactivators and chromatin elements in phase-separated enhancer clusters. Nucleic Acids Res. 2022, 50, 4917–4937. [Google Scholar] [CrossRef] [PubMed]
- Goel, V.Y.; Huseyin, M.K.; Hansen, A.S. Region Capture Micro-C reveals coalescence of enhancers and promoters into nested microcompartments. Nat. Genet. 2023, 55, 1048–1056. [Google Scholar] [CrossRef]
- Lu, H.; Yu, D.; Hansen, A.S.; Ganguly, S.; Liu, R.; Heckert, A.; Darzacq, X.; Zhou, Q. Phase-separation mechanism for C-terminal hyperphosphorylation of RNA polymerase II. Nature 2018, 558, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.E.; Manteiga, J.C.; Henninger, J.E.; Sabari, B.R.; Dall’Agnese, A.; Hannett, N.M.; Spille, J.H.; Afeyan, L.K.; Zamudio, A.V.; Shrinivas, K.; et al. Pol II phosphorylation regulates a switch between transcriptional and splicing condensates. Nature 2019, 572, 543–548. [Google Scholar] [CrossRef]
- Wang, J.; Yu, H.; Ma, Q.; Zeng, P.; Wu, D.; Hou, Y.; Liu, X.; Jia, L.; Sun, J.; Chen, Y.; et al. Phase separation of OCT4 controls TAD reorganization to promote cell fate transitions. Cell Stem Cell 2021, 28, 1868–1883.e11. [Google Scholar] [CrossRef] [PubMed]
- Khanna, N.; Zhang, Y.; Lucas, J.S.; Dudko, O.K.; Murre, C. Chromosome dynamics near the sol-gel phase transition dictate the timing of remote genomic interactions. Nat. Commun. 2019, 10, 2771. [Google Scholar] [CrossRef]
- Denholtz, M.; Bonora, G.; Chronis, C.; Splinter, E.; de Laat, W.; Ernst, J.; Pellegrini, M.; Plath, K. Long-range chromatin contacts in embryonic stem cells reveal a role for pluripotency factors and polycomb proteins in genome organization. Cell Stem Cell 2013, 13, 602–616. [Google Scholar] [CrossRef]
- Schoenfelder, S.; Sugar, R.; Dimond, A.; Javierre, B.M.; Armstrong, H.; Mifsud, B.; Dimitrova, E.; Matheson, L.; Tavares-Cadete, F.; Furlan-Magaril, M.; et al. Polycomb repressive complex PRC1 spatially constrains the mouse embryonic stem cell genome. Nat. Genet. 2015, 47, 1179–1186. [Google Scholar] [CrossRef]
- Ngan, C.Y.; Wong, C.H.; Tjong, H.; Wang, W.; Goldfeder, R.L.; Choi, C.; He, H.; Gong, L.; Lin, J.; Urban, B.; et al. Chromatin interaction analyses elucidate the roles of PRC2-bound silencers in mouse development. Nat. Genet. 2020, 52, 264–272. [Google Scholar] [CrossRef]
- Hsieh, T.S.; Cattoglio, C.; Slobodyanyuk, E.; Hansen, A.S.; Darzacq, X.; Tjian, R. Enhancer-promoter interactions and transcription are largely maintained upon acute loss of CTCF, cohesin, WAPL or YY1. Nat. Genet. 2022, 54, 1919–1932. [Google Scholar] [CrossRef] [PubMed]
- Kundu, S.; Ji, F.; Sunwoo, H.; Jain, G.; Lee, J.T.; Sadreyev, R.I.; Dekker, J.; Kingston, R.E. Polycomb Repressive Complex 1 Generates Discrete Compacted Domains that Change during Differentiation. Mol. Cell 2017, 65, 432–446.e5. [Google Scholar] [CrossRef] [PubMed]
- Ogiyama, Y.; Schuettengruber, B.; Papadopoulos, G.L.; Chang, J.M.; Cavalli, G. Polycomb-Dependent Chromatin Looping Contributes to Gene Silencing during Drosophila Development. Mol. Cell 2018, 71, 73–88.e5. [Google Scholar] [CrossRef]
- Du, Z.; Zheng, H.; Kawamura, Y.K.; Zhang, K.; Gassler, J.; Powell, S.; Xu, Q.; Lin, Z.; Xu, K.; Zhou, Q.; et al. Polycomb Group Proteins Regulate Chromatin Architecture in Mouse Oocytes and Early Embryos. Mol. Cell 2020, 77, 825–839.e7. [Google Scholar] [CrossRef]
- Boyle, S.; Flyamer, I.M.; Williamson, I.; Sengupta, D.; Bickmore, W.A.; Illingworth, R.S. A central role for canonical PRC1 in shaping the 3D nuclear landscape. Genes Dev. 2020, 34, 931–949. [Google Scholar] [CrossRef]
- Tatavosian, R.; Kent, S.; Brown, K.; Yao, T.; Duc, H.N.; Huynh, T.N.; Zhen, C.Y.; Ma, B.; Wang, H.; Ren, X. Nuclear condensates of the Polycomb protein chromobox 2 (CBX2) assemble through phase separation. J. Biol. Chem. 2019, 294, 1451–1463. [Google Scholar] [CrossRef]
- Plys, A.J.; Davis, C.P.; Kim, J.; Rizki, G.; Keenen, M.M.; Marr, S.K.; Kingston, R.E. Phase separation of Polycomb-repressive complex 1 is governed by a charged disordered region of CBX2. Genes Dev. 2019, 33, 799–813. [Google Scholar] [CrossRef]
- Kent, S.; Brown, K.; Yang, C.H.; Alsaihati, N.; Tian, C.; Wang, H.; Ren, X. Phase-Separated Transcriptional Condensates Accelerate Target-Search Process Revealed by Live-Cell Single-Molecule Imaging. Cell Rep. 2020, 33, 108248. [Google Scholar] [CrossRef] [PubMed]
- Seif, E.; Kang, J.J.; Sasseville, C.; Senkovich, O.; Kaltashov, A.; Boulier, E.L.; Kapur, I.; Kim, C.A.; Francis, N.J. Phase separation by the polyhomeotic sterile alpha motif compartmentalizes Polycomb Group proteins and enhances their activity. Nat. Commun. 2020, 11, 5609. [Google Scholar] [CrossRef]
- Niekamp, S.; Marr, S.K.; Oei, T.A.; Subramanian, R.; Kingston, R.E. Modularity of PRC1 composition and chromatin interaction define condensate properties. Mol. Cell 2024, 84, 1651–1666.e12. [Google Scholar] [CrossRef]
- Guo, Y.; Zhao, S.; Wang, G.G. Polycomb Gene Silencing Mechanisms: PRC2 Chromatin Targeting, H3K27me3 ‘Readout’, and Phase Separation-Based Compaction. Trends Genet. 2021, 37, 547–565. [Google Scholar] [CrossRef] [PubMed]
- Hansen, A.S.; Hsieh, T.S.; Cattoglio, C.; Pustova, I.; Saldana-Meyer, R.; Reinberg, D.; Darzacq, X.; Tjian, R. Distinct Classes of Chromatin Loops Revealed by Deletion of an RNA-Binding Region in CTCF. Mol. Cell 2019, 76, 395–411.e13. [Google Scholar] [CrossRef] [PubMed]
- Saldana-Meyer, R.; Rodriguez-Hernaez, J.; Escobar, T.; Nishana, M.; Jacome-Lopez, K.; Nora, E.P.; Bruneau, B.G.; Tsirigos, A.; Furlan-Magaril, M.; Skok, J.; et al. RNA Interactions Are Essential for CTCF-Mediated Genome Organization. Mol. Cell 2019, 76, 412–422.e5. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Zhu, G.; Eshelman, M.A.; Fung, T.K.; Lai, Q.; Wang, F.; Zeisig, B.B.; Lesperance, J.; Ma, X.; Chen, S.; et al. HOTTIP-dependent R-loop formation regulates CTCF boundary activity and TAD integrity in leukemia. Mol. Cell 2022, 82, 833–851.e11. [Google Scholar] [CrossRef]
- Islam, Z.; Saravanan, B.; Walavalkar, K.; Farooq, U.; Singh, A.K.; Radhakrishnan, S.; Thakur, J.; Pandit, A.; Henikoff, S.; Notani, D. Active enhancers strengthen insulation by RNA-mediated CTCF binding at chromatin domain boundaries. Genome Res. 2023, 33, 1–17. [Google Scholar] [CrossRef]
- Schertzer, M.D.; Braceros, K.C.A.; Starmer, J.; Cherney, R.E.; Lee, D.M.; Salazar, G.; Justice, M.; Bischoff, S.R.; Cowley, D.O.; Ariel, P.; et al. lncRNA-Induced Spread of Polycomb Controlled by Genome Architecture, RNA Abundance, and CpG Island DNA. Mol. Cell 2019, 75, 523–537.e10. [Google Scholar] [CrossRef]
- Quinodoz, S.A.; Jachowicz, J.W.; Bhat, P.; Ollikainen, N.; Banerjee, A.K.; Goronzy, I.N.; Blanco, M.R.; Chovanec, P.; Chow, A.; Markaki, Y.; et al. RNA promotes the formation of spatial compartments in the nucleus. Cell 2021, 184, 5775–5790.e30. [Google Scholar] [CrossRef]
- Tomita, S.; Abdalla, M.O.A.; Fujiwara, S.; Matsumori, H.; Maehara, K.; Ohkawa, Y.; Iwase, H.; Saitoh, N.; Nakao, M. A cluster of noncoding RNAs activates the ESR1 locus during breast cancer adaptation. Nat. Commun. 2015, 6, 6966. [Google Scholar] [CrossRef]
- Nair, S.J.; Yang, L.; Meluzzi, D.; Oh, S.; Yang, F.; Friedman, M.J.; Wang, S.; Suter, T.; Alshareedah, I.; Gamliel, A.; et al. Phase separation of ligand-activated enhancers licenses cooperative chromosomal enhancer assembly. Nat. Struct. Mol. Biol. 2019, 26, 193–203. [Google Scholar] [CrossRef]
- Garcia-Jove Navarro, M.; Kashida, S.; Chouaib, R.; Souquere, S.; Pierron, G.; Weil, D.; Gueroui, Z. RNA is a critical element for the sizing and the composition of phase-separated RNA-protein condensates. Nat. Commun. 2019, 10, 3230. [Google Scholar] [CrossRef]
- Roden, C.; Gladfelter, A.S. RNA contributions to the form and function of biomolecular condensates. Nat. Rev. Mol. Cell Biol. 2021, 22, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Henninger, J.E.; Oksuz, O.; Shrinivas, K.; Sagi, I.; LeRoy, G.; Zheng, M.M.; Andrews, J.O.; Zamudio, A.V.; Lazaris, C.; Hannett, N.M.; et al. RNA-Mediated Feedback Control of Transcriptional Condensates. Cell 2021, 184, 207–225.e24. [Google Scholar] [CrossRef] [PubMed]
- Dekker, J.; Mirny, L. The 3D Genome as Moderator of Chromosomal Communication. Cell 2016, 164, 1110–1121. [Google Scholar] [CrossRef] [PubMed]
- Zimatore, G.; Tsuchiya, M.; Hashimoto, M.; Kasperski, A.; Giuliani, A. Self-organization of whole-gene expression through coordinated chromatin structural transition. Biophys. Rev. 2021, 2, 31303. [Google Scholar] [CrossRef]
- Erenpreisa, J.; Giuliani, A. Resolution of Complex Issues in Genome Regulation and Cancer Requires Non-Linear and Network-Based Thermodynamics. Int. J. Mol. Sci. 2019, 21, 240. [Google Scholar] [CrossRef]
- Bickmore, W.A.; van Steensel, B. Genome architecture: Domain organization of interphase chromosomes. Cell 2013, 152, 1270–1284. [Google Scholar] [CrossRef]
- van Steensel, B.; Belmont, A.S. Lamina-Associated Domains: Links with Chromosome Architecture, Heterochromatin, and Gene Repression. Cell 2017, 169, 780–791. [Google Scholar] [CrossRef]
- Falk, M.; Feodorova, Y.; Naumova, N.; Imakaev, M.; Lajoie, B.R.; Leonhardt, H.; Joffe, B.; Dekker, J.; Fudenberg, G.; Solovei, I.; et al. Heterochromatin drives compartmentalization of inverted and conventional nuclei. Nature 2019, 570, 395–399. [Google Scholar] [CrossRef]
- Takizawa, T.; Meaburn, K.J.; Misteli, T. The meaning of gene positioning. Cell 2008, 135, 9–13. [Google Scholar] [CrossRef]
- Cremer, T.; Cremer, M. Chromosome territories. Cold Spring Harb. Perspect. Biol. 2010, 2, a003889. [Google Scholar] [CrossRef]
- Shin, Y.; Brangwynne, C.P. Liquid phase condensation in cell physiology and disease. Science 2017, 357, eaaf4382. [Google Scholar] [CrossRef] [PubMed]
- Nuebler, J.; Fudenberg, G.; Imakaev, M.; Abdennur, N.; Mirny, L.A. Chromatin organization by an interplay of loop extrusion and compartmental segregation. Proc. Natl. Acad. Sci. USA 2018, 115, E6697–E6706. [Google Scholar] [CrossRef] [PubMed]
- Larson, A.G.; Elnatan, D.; Keenen, M.M.; Trnka, M.J.; Johnston, J.B.; Burlingame, A.L.; Agard, D.A.; Redding, S.; Narlikar, G.J. Liquid droplet formation by HP1alpha suggests a role for phase separation in heterochromatin. Nature 2017, 547, 236–240. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Gao, Y.; Zheng, X.; Liu, C.; Dong, S.; Li, R.; Zhang, G.; Wei, Y.; Qu, H.; Li, Y.; et al. Histone Modifications Regulate Chromatin Compartmentalization by Contributing to a Phase Separation Mechanism. Mol. Cell 2019, 76, 646–659.e6. [Google Scholar] [CrossRef]
- Sanulli, S.; Trnka, M.J.; Dharmarajan, V.; Tibble, R.W.; Pascal, B.D.; Burlingame, A.L.; Griffin, P.R.; Gross, J.D.; Narlikar, G.J. HP1 reshapes nucleosome core to promote phase separation of heterochromatin. Nature 2019, 575, 390–394. [Google Scholar] [CrossRef]
- Grunstein, M. Histone acetylation in chromatin structure and transcription. Nature 1997, 389, 349–352. [Google Scholar] [CrossRef]
- Shogren-Knaak, M.; Ishii, H.; Sun, J.M.; Pazin, M.J.; Davie, J.R.; Peterson, C.L. Histone H4-K16 acetylation controls chromatin structure and protein interactions. Science 2006, 311, 844–847. [Google Scholar] [CrossRef]
- Wei, C.; Jia, L.; Huang, X.; Tan, J.; Wang, M.; Niu, J.; Hou, Y.; Sun, J.; Zeng, P.; Wang, J.; et al. CTCF organizes inter-A compartment interactions through RYBP-dependent phase separation. Cell Res. 2022, 32, 744–760. [Google Scholar] [CrossRef]
- He, S.; Yu, Y.; Wang, L.; Zhang, J.; Bai, Z.; Li, G.; Li, P.; Feng, X. Linker histone H1 drives heterochromatin condensation via phase separation in Arabidopsis. Plant Cell 2024, 36, 1829–1843. [Google Scholar] [CrossRef]
- Shin, Y.; Chang, Y.C.; Lee, D.S.W.; Berry, J.; Sanders, D.W.; Ronceray, P.; Wingreen, N.S.; Haataja, M.; Brangwynne, C.P. Liquid Nuclear Condensates Mechanically Sense and Restructure the Genome. Cell 2018, 175, 1481–1491.e13. [Google Scholar] [CrossRef]
- Cabianca, D.S.; Munoz-Jimenez, C.; Kalck, V.; Gaidatzis, D.; Padeken, J.; Seeber, A.; Askjaer, P.; Gasser, S.M. Active chromatin marks drive spatial sequestration of heterochromatin in C. elegans nuclei. Nature 2019, 569, 734–739. [Google Scholar] [CrossRef] [PubMed]
- Bian, Q.; Anderson, E.C.; Yang, Q.; Meyer, B.J. Histone H3K9 methylation promotes formation of genome compartments in Caenorhabditis elegans via chromosome compaction and perinuclear anchoring. Proc. Natl. Acad. Sci. USA 2020, 117, 11459–11470. [Google Scholar] [CrossRef] [PubMed]
- Lerra, L.; Panatta, M.; Bar, D.; Zanini, I.; Tan, J.Y.; Pisano, A.; Mungo, C.; Baroux, C.; Panse, V.G.; Marques, A.C.; et al. An RNA-dependent and phase-separated active subnuclear compartment safeguards repressive chromatin domains. Mol. Cell 2024, 84, 1667–1683.e10. [Google Scholar] [CrossRef]
- McCord, R.P.; Xu, Y.; Li, H.; Das, P.; San Martin, R. SnapShot: Chromosome organization. Mol. Cell 2022, 82, 2350.e1. [Google Scholar] [CrossRef]
- Guelen, L.; Pagie, L.; Brasset, E.; Meuleman, W.; Faza, M.B.; Talhout, W.; Eussen, B.H.; de Klein, A.; Wessels, L.; de Laat, W.; et al. Domain organization of human chromosomes revealed by mapping of nuclear lamina interactions. Nature 2008, 453, 948–951. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, A.; Conesa, A.; Santoyo-Lopez, J.; Medina, I.; Montaner, D.; Peterfia, B.; Solovei, I.; Cremer, T.; Dopazo, J.; Langst, G. Initial genomics of the human nucleolus. PLoS Genet. 2010, 6, e1000889. [Google Scholar] [CrossRef]
- van Koningsbruggen, S.; Gierlinski, M.; Schofield, P.; Martin, D.; Barton, G.J.; Ariyurek, Y.; den Dunnen, J.T.; Lamond, A.I. High-resolution whole-genome sequencing reveals that specific chromatin domains from most human chromosomes associate with nucleoli. Mol. Biol. Cell 2010, 21, 3735–3748. [Google Scholar] [CrossRef]
- Jack, A.; Kim, Y.; Strom, A.R.; Lee, D.S.W.; Williams, B.; Schaub, J.M.; Kellogg, E.H.; Finkelstein, I.J.; Ferro, L.S.; Yildiz, A.; et al. Compartmentalization of telomeres through DNA-scaffolded phase separation. Dev. Cell 2022, 57, 277–290.e9. [Google Scholar] [CrossRef]
- Feric, M.; Vaidya, N.; Harmon, T.S.; Mitrea, D.M.; Zhu, L.; Richardson, T.M.; Kriwacki, R.W.; Pappu, R.V.; Brangwynne, C.P. Coexisting Liquid Phases Underlie Nucleolar Subcompartments. Cell 2016, 165, 1686–1697. [Google Scholar] [CrossRef]
- Mitrea, D.M.; Cika, J.A.; Guy, C.S.; Ban, D.; Banerjee, P.R.; Stanley, C.B.; Nourse, A.; Deniz, A.A.; Kriwacki, R.W. Nucleophosmin integrates within the nucleolus via multi-modal interactions with proteins displaying R-rich linear motifs and rRNA. eLife 2016, 5, e13571. [Google Scholar] [CrossRef]
- Shi, X.; Li, Y.; Zhou, H.; Hou, X.; Yang, J.; Malik, V.; Faiola, F.; Ding, J.; Bao, X.; Modic, M.; et al. DDX18 coordinates nucleolus phase separation and nuclear organization to control the pluripotency of human embryonic stem cells. Nat. Commun. 2024, 15, 10803. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.M.; Green, J.; das Neves, R.P.; Wallace, H.A.; Smith, A.J.; Hughes, J.; Gray, N.; Taylor, S.; Wood, W.G.; Higgs, D.R.; et al. Association between active genes occurs at nuclear speckles and is modulated by chromatin environment. J. Cell Biol. 2008, 182, 1083–1097. [Google Scholar] [CrossRef]
- Quinodoz, S.A.; Ollikainen, N.; Tabak, B.; Palla, A.; Schmidt, J.M.; Detmar, E.; Lai, M.M.; Shishkin, A.A.; Bhat, P.; Takei, Y.; et al. Higher-Order Inter-chromosomal Hubs Shape 3D Genome Organization in the Nucleus. Cell 2018, 174, 744–757.e24. [Google Scholar] [CrossRef]
- Greig, J.A.; Nguyen, T.A.; Lee, M.; Holehouse, A.S.; Posey, A.E.; Pappu, R.V.; Jedd, G. Arginine-Enriched Mixed-Charge Domains Provide Cohesion for Nuclear Speckle Condensation. Mol. Cell 2020, 77, 1237–1250.e4. [Google Scholar] [CrossRef]
- Dion, W.; Ballance, H.; Lee, J.; Pan, Y.; Irfan, S.; Edwards, C.; Sun, M.; Zhang, J.; Zhang, X.; Liu, S.; et al. Four-dimensional nuclear speckle phase separation dynamics regulate proteostasis. Sci. Adv. 2022, 8, eabl4150. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Gu, Z.; Guo, S.; Sun, Y.; Ma, S.; Yang, S.; Guo, J.; Fang, C.; Shu, L.; Ge, Y.; et al. SRRM2 phase separation drives assembly of nuclear speckle subcompartments. Cell Rep. 2024, 43, 113827. [Google Scholar] [CrossRef]
- Kang, M.; Zhang, T.; Ning, C.; Bao, Y.; Liu, Z.; Gao, L.; Luan, L.; Wang, C.; Liu, J.; Ke, Y. Step-wise organization of genomic nuclear speckle-associated domains (SPADs) during mammalian embryonic development. Protein Cell 2025, pwaf015. [Google Scholar] [CrossRef] [PubMed]
- Iacovoni, J.S.; Caron, P.; Lassadi, I.; Nicolas, E.; Massip, L.; Trouche, D.; Legube, G. High-resolution profiling of gammaH2AX around DNA double strand breaks in the mammalian genome. EMBO J. 2010, 29, 1446–1457. [Google Scholar] [CrossRef]
- Kilic, S.; Lezaja, A.; Gatti, M.; Bianco, E.; Michelena, J.; Imhof, R.; Altmeyer, M. Phase separation of 53BP1 determines liquid-like behavior of DNA repair compartments. EMBO J. 2019, 38, e101379. [Google Scholar] [CrossRef]
- Arnould, C.; Rocher, V.; Saur, F.; Bader, A.S.; Muzzopappa, F.; Collins, S.; Lesage, E.; Le Bozec, B.; Puget, N.; Clouaire, T.; et al. Chromatin compartmentalization regulates the response to DNA damage. Nature 2023, 623, 183–192. [Google Scholar] [CrossRef]
- Galupa, R.; Heard, E. X-Chromosome Inactivation: A Crossroads Between Chromosome Architecture and Gene Regulation. Annu. Rev. Genet. 2018, 52, 535–566. [Google Scholar] [CrossRef] [PubMed]
- Pandya-Jones, A.; Markaki, Y.; Serizay, J.; Chitiashvili, T.; Mancia Leon, W.R.; Damianov, A.; Chronis, C.; Papp, B.; Chen, C.K.; McKee, R.; et al. A protein assembly mediates Xist localization and gene silencing. Nature 2020, 587, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Markaki, Y.; Gan Chong, J.; Wang, Y.; Jacobson, E.C.; Luong, C.; Tan, S.Y.X.; Jachowicz, J.W.; Strehle, M.; Maestrini, D.; Banerjee, A.K.; et al. Xist nucleates local protein gradients to propagate silencing across the X chromosome. Cell 2021, 184, 6174–6192.e32. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.; Wang, D.; Chen, H.; Kesner, B.; Grimm, N.B.; Weissbein, U.; Lappala, A.; Jiang, J.; Rivera, C.; Lou, J.; et al. A biophysical basis for the spreading behavior and limited diffusion of Xist. Cell 2025, 188, 978–997.e25. [Google Scholar] [CrossRef]
- Schneider, M.W.G.; Gibson, B.A.; Otsuka, S.; Spicer, M.F.D.; Petrovic, M.; Blaukopf, C.; Langer, C.C.H.; Batty, P.; Nagaraju, T.; Doolittle, L.K.; et al. A mitotic chromatin phase transition prevents perforation by microtubules. Nature 2022, 609, 183–190. [Google Scholar] [CrossRef]
- Zhao, S.; Allis, C.D.; Wang, G.G. The language of chromatin modification in human cancers. Nat. Rev. Cancer 2021, 21, 413–430. [Google Scholar] [CrossRef]
- Mehta, S.; Zhang, J. Liquid-liquid phase separation drives cellular function and dysfunction in cancer. Nat. Rev. Cancer 2022, 22, 239–252. [Google Scholar] [CrossRef]
- Pei, G.; Lyons, H.; Li, P.; Sabari, B.R. Transcription regulation by biomolecular condensates. Nat. Rev. Mol. Cell Biol. 2025, 26, 213–236. [Google Scholar] [CrossRef]
- Gao, Q.; Liang, W.W.; Foltz, S.M.; Mutharasu, G.; Jayasinghe, R.G.; Cao, S.; Liao, W.W.; Reynolds, S.M.; Wyczalkowski, M.A.; Yao, L.; et al. Driver Fusions and Their Implications in the Development and Treatment of Human Cancers. Cell Rep. 2018, 23, 227–238.e3. [Google Scholar] [CrossRef]
- Brien, G.L.; Stegmaier, K.; Armstrong, S.A. Targeting chromatin complexes in fusion protein-driven malignancies. Nat. Rev. Cancer 2019, 19, 255–269. [Google Scholar] [CrossRef]
- Tripathi, S.; Shirnekhi, H.K.; Gorman, S.D.; Chandra, B.; Baggett, D.W.; Park, C.G.; Somjee, R.; Lang, B.; Hosseini, S.M.H.; Pioso, B.J.; et al. Defining the condensate landscape of fusion oncoproteins. Nat. Commun. 2023, 14, 6008. [Google Scholar] [CrossRef]
- Quiroga, I.Y.; Ahn, J.H.; Wang, G.G.; Phanstiel, D. Oncogenic fusion proteins and their role in three-dimensional chromatin structure, phase separation, and cancer. Curr. Opin. Genet. Dev. 2022, 74, 101901. [Google Scholar] [CrossRef]
- Xu, C.; Kim, A.; Corbin, J.M.; Wang, G.G. Onco-condensates: Formation, multi-component organization, and biological functions. Trends Cancer 2023, 9, 738–751. [Google Scholar] [CrossRef]
- Gough, S.M.; Slape, C.I.; Aplan, P.D. NUP98 gene fusions and hematopoietic malignancies: Common themes and new biologic insights. Blood 2011, 118, 6247–6257. [Google Scholar] [CrossRef]
- Michmerhuizen, N.L.; Klco, J.M.; Mullighan, C.G. Mechanistic insights and potential therapeutic approaches for NUP98-rearranged hematologic malignancies. Blood 2020, 136, 2275–2289. [Google Scholar] [CrossRef]
- Ahn, J.H.; Davis, E.S.; Daugird, T.A.; Zhao, S.; Quiroga, I.Y.; Uryu, H.; Li, J.; Storey, A.J.; Tsai, Y.H.; Keeley, D.P.; et al. Phase separation drives aberrant chromatin looping and cancer development. Nature 2021, 595, 591–595. [Google Scholar] [CrossRef]
- Terlecki-Zaniewicz, S.; Humer, T.; Eder, T.; Schmoellerl, J.; Heyes, E.; Manhart, G.; Kuchynka, N.; Parapatics, K.; Liberante, F.G.; Muller, A.C.; et al. Biomolecular condensation of NUP98 fusion proteins drives leukemogenic gene expression. Nat. Struct. Mol. Biol. 2021, 28, 190–201. [Google Scholar] [CrossRef]
- Chandra, B.; Michmerhuizen, N.L.; Shirnekhi, H.K.; Tripathi, S.; Pioso, B.J.; Baggett, D.W.; Mitrea, D.M.; Iacobucci, I.; White, M.R.; Chen, J.; et al. Phase Separation Mediates NUP98 Fusion Oncoprotein Leukemic Transformation. Cancer Discov. 2022, 12, 1152–1169. [Google Scholar] [CrossRef]
- Jevtic, Z.; Matafora, V.; Casagrande, F.; Santoro, F.; Minucci, S.; Garre, M.; Rasouli, M.; Heidenreich, O.; Musco, G.; Schwaller, J.; et al. SMARCA5 interacts with NUP98-NSD1 oncofusion protein and sustains hematopoietic cells transformation. J. Exp. Clin. Cancer Res. 2022, 41, 34. [Google Scholar] [CrossRef]
- Oka, M.; Otani, M.; Miyamoto, Y.; Oshima, R.; Adachi, J.; Tomonaga, T.; Asally, M.; Nagaoka, Y.; Tanaka, K.; Toyoda, A.; et al. Phase-separated nuclear bodies of nucleoporin fusions promote condensation of MLL1/CRM1 and rearrangement of 3D genome structure. Cell Rep. 2023, 42, 112884. [Google Scholar] [CrossRef]
- Boulay, G.; Sandoval, G.J.; Riggi, N.; Iyer, S.; Buisson, R.; Naigles, B.; Awad, M.E.; Rengarajan, S.; Volorio, A.; McBride, M.J.; et al. Cancer-Specific Retargeting of BAF Complexes by a Prion-like Domain. Cell 2017, 171, 163–178.e19. [Google Scholar] [CrossRef]
- Alekseyenko, A.A.; Walsh, E.M.; Wang, X.; Grayson, A.R.; Hsi, P.T.; Kharchenko, P.V.; Kuroda, M.I.; French, C.A. The oncogenic BRD4-NUT chromatin regulator drives aberrant transcription within large topological domains. Genes Dev. 2015, 29, 1507–1523. [Google Scholar] [CrossRef]
- Hu, X.; Wu, X.; Berry, K.; Zhao, C.; Xin, D.; Ogurek, S.; Liu, X.; Zhang, L.; Luo, Z.; Sakabe, M.; et al. Nuclear condensates of YAP fusion proteins alter transcription to drive ependymoma tumourigenesis. Nat. Cell Biol. 2023, 25, 323–336. [Google Scholar] [CrossRef]
- Wei, M.T.; Chang, Y.C.; Shimobayashi, S.F.; Shin, Y.; Strom, A.R.; Brangwynne, C.P. Nucleated transcriptional condensates amplify gene expression. Nat. Cell Biol. 2020, 22, 1187–1196. [Google Scholar] [CrossRef]
- Zuo, L.; Zhang, G.; Massett, M.; Cheng, J.; Guo, Z.; Wang, L.; Gao, Y.; Li, R.; Huang, X.; Li, P.; et al. Loci-specific phase separation of FET fusion oncoproteins promotes gene transcription. Nat. Commun. 2021, 12, 1491. [Google Scholar] [CrossRef]
- Davis, R.B.; Kaur, T.; Moosa, M.M.; Banerjee, P.R. FUS oncofusion protein condensates recruit mSWI/SNF chromatin remodeler via heterotypic interactions between prion-like domains. Protein Sci. 2021, 30, 1454–1466. [Google Scholar] [CrossRef]
- Rosencrance, C.D.; Ammouri, H.N.; Yu, Q.; Ge, T.; Rendleman, E.J.; Marshall, S.A.; Eagen, K.P. Chromatin Hyperacetylation Impacts Chromosome Folding by Forming a Nuclear Subcompartment. Mol. Cell 2020, 78, 112–126.e12. [Google Scholar] [CrossRef]
- Yu, D.; Liang, Y.; Kim, C.; Jaganathan, A.; Ji, D.; Han, X.; Yang, X.; Jia, Y.; Gu, R.; Wang, C.; et al. Structural mechanism of BRD4-NUT and p300 bipartite interaction in propagating aberrant gene transcription in chromatin in NUT carcinoma. Nat. Commun. 2023, 14, 378. [Google Scholar] [CrossRef]
- Kosno, M.; Currie, S.L.; Kumar, A.; Xing, C.; Rosen, M.K. Molecular features driving condensate formation and gene expression by the BRD4-NUT fusion oncoprotein are overlapping but distinct. Sci. Rep. 2023, 13, 11907. [Google Scholar] [CrossRef]
- Cheng, Y.; Shen, Z.; Gao, Y.; Chen, F.; Xu, H.; Mo, Q.; Chu, X.; Peng, C.L.; McKenzie, T.T.; Palacios, B.E.; et al. Phase transition and remodeling complex assembly are important for SS18-SSX oncogenic activity in synovial sarcomas. Nat. Commun. 2022, 13, 2724. [Google Scholar] [CrossRef]
- Kuang, J.; Li, P.; Zhai, Z.; Fan, Y.; Xu, H.; Zhao, C.; Li, W.; Li, X.; Liang, Z.; Huang, T.; et al. Exclusion of HDAC1/2 complexes by oncogenic nuclear condensates. Mol. Cancer 2024, 23, 85. [Google Scholar] [CrossRef]
- Li, P.; Zhai, Z.; Fan, Y.; Li, W.; Ke, M.; Li, X.; Gao, H.; Fu, Y.; Ma, Z.; Zhang, W.; et al. Condensate remodeling reorganizes innate SS18 in synovial sarcomagenesis. Oncogenesis 2024, 13, 38. [Google Scholar] [CrossRef]
- Kadoch, C.; Hargreaves, D.C.; Hodges, C.; Elias, L.; Ho, L.; Ranish, J.; Crabtree, G.R. Proteomic and bioinformatic analysis of mammalian SWI/SNF complexes identifies extensive roles in human malignancy. Nat. Genet. 2013, 45, 592–601. [Google Scholar] [CrossRef]
- Mashtalir, N.; Suzuki, H.; Farrell, D.P.; Sankar, A.; Luo, J.; Filipovski, M.; D’Avino, A.R.; St Pierre, R.; Valencia, A.M.; Onikubo, T.; et al. A Structural Model of the Endogenous Human BAF Complex Informs Disease Mechanisms. Cell 2020, 183, 802–817.e24. [Google Scholar] [CrossRef]
- Mashtalir, N.; D’Avino, A.R.; Michel, B.C.; Luo, J.; Pan, J.; Otto, J.E.; Zullow, H.J.; McKenzie, Z.M.; Kubiak, R.L.; St Pierre, R.; et al. Modular Organization and Assembly of SWI/SNF Family Chromatin Remodeling Complexes. Cell 2018, 175, 1272–1288.e20. [Google Scholar] [CrossRef]
- Kuang, J.; Zhai, Z.; Li, P.; Shi, R.; Guo, W.; Yao, Y.; Guo, J.; Zhao, G.; He, J.; Xu, S.; et al. SS18 regulates pluripotent-somatic transition through phase separation. Nat. Commun. 2021, 12, 4090. [Google Scholar] [CrossRef]
- Patil, A.; Strom, A.R.; Paulo, J.A.; Collings, C.K.; Ruff, K.M.; Shinn, M.K.; Sankar, A.; Cervantes, K.S.; Wauer, T.; St Laurent, J.D.; et al. A disordered region controls cBAF activity via condensation and partner recruitment. Cell 2023, 186, 4936–4955.e26. [Google Scholar] [CrossRef]
- Kim, Y.R.; Joo, J.; Lee, H.J.; Kim, C.; Park, J.C.; Yu, Y.S.; Kim, C.R.; Lee, D.H.; Cha, J.; Kwon, H.; et al. Prion-like domain mediated phase separation of ARID1A promotes oncogenic potential of Ewing’s sarcoma. Nat. Commun. 2024, 15, 6569. [Google Scholar] [CrossRef]
- Lin, R.; Zhai, Z.; Kuang, J.; Wu, C.; Yao, Y.; Shi, R.; He, J.; Xu, S.; Li, P.; Fan, Y.; et al. H3K27ac mediated SS18/BAFs relocation regulates JUN induced pluripotent-somatic transition. Cell Biosci. 2022, 12, 89. [Google Scholar] [CrossRef]
- Andricovich, J.; Perkail, S.; Kai, Y.; Casasanta, N.; Peng, W.; Tzatsos, A. Loss of KDM6A Activates Super-Enhancers to Induce Gender-Specific Squamous-like Pancreatic Cancer and Confers Sensitivity to BET Inhibitors. Cancer Cell 2018, 33, 512–526.e8. [Google Scholar] [CrossRef]
- Gozdecka, M.; Meduri, E.; Mazan, M.; Tzelepis, K.; Dudek, M.; Knights, A.J.; Pardo, M.; Yu, L.; Choudhary, J.S.; Metzakopian, E.; et al. UTX-mediated enhancer and chromatin remodeling suppresses myeloid leukemogenesis through noncatalytic inverse regulation of ETS and GATA programs. Nat. Genet. 2018, 50, 883–894. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Shilatifard, A. UTX Mutations in Human Cancer. Cancer Cell 2019, 35, 168–176. [Google Scholar] [CrossRef]
- Shi, B.; Li, W.; Song, Y.; Wang, Z.; Ju, R.; Ulman, A.; Hu, J.; Palomba, F.; Zhao, Y.; Le, J.P.; et al. UTX condensation underlies its tumour-suppressive activity. Nature 2021, 597, 726–731. [Google Scholar] [CrossRef]
- Quail, T.; Golfier, S.; Elsner, M.; Ishihara, K.; Murugesan, V.; Renger, R.; Julicher, F.; Brugués, J. Force generation by protein-DNA co-condensation. Nat. Phys. 2021, 17, 1007–1012. [Google Scholar] [CrossRef]
- Thorat, M.A.; Marchio, C.; Morimiya, A.; Savage, K.; Nakshatri, H.; Reis-Filho, J.S.; Badve, S. Forkhead box A1 expression in breast cancer is associated with luminal subtype and good prognosis. J. Clin. Pathol. 2008, 61, 327–332. [Google Scholar] [CrossRef]
- Adams, E.J.; Karthaus, W.R.; Hoover, E.; Liu, D.; Gruet, A.; Zhang, Z.; Cho, H.; DiLoreto, R.; Chhangawala, S.; Liu, Y.; et al. FOXA1 mutations alter pioneering activity, differentiation and prostate cancer phenotypes. Nature 2019, 571, 408–412. [Google Scholar] [CrossRef]
- Parolia, A.; Cieslik, M.; Chu, S.C.; Xiao, L.; Ouchi, T.; Zhang, Y.; Wang, X.; Vats, P.; Cao, X.; Pitchiaya, S.; et al. Distinct structural classes of activating FOXA1 alterations in advanced prostate cancer. Nature 2019, 571, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Tang, L. Measuring condensation forces. Nat. Methods 2021, 18, 986. [Google Scholar] [CrossRef]
- Ji, D.; Shao, C.; Yu, J.; Hou, Y.; Gao, X.; Wu, Y.; Wang, L.; Chen, P. FOXA1 forms biomolecular condensates that unpack condensed chromatin to function as a pioneer factor. Mol. Cell 2024, 84, 244–260.e7. [Google Scholar] [CrossRef]
- Harris, A.L. Hypoxia—A key regulatory factor in tumour growth. Nat. Rev. Cancer 2002, 2, 38–47. [Google Scholar] [CrossRef]
- Rankin, E.B.; Giaccia, A.J. Hypoxic control of metastasis. Science 2016, 352, 175–180. [Google Scholar] [CrossRef]
- Gao, C.; Gao, A.; Jiang, Y.; Gao, R.; Guo, Y.; Peng, Z.; Jiang, W.; Zhang, M.; Zhou, Z.; Yan, C.; et al. Hypoxia-induced phase separation of ZHX2 alters chromatin looping to drive cancer metastasis. Mol. Cell 2025, 85, 1525–1542.e10. [Google Scholar] [CrossRef]
- Lu, B.; Qiu, R.; Wei, J.; Wang, L.; Zhang, Q.; Li, M.; Zhan, X.; Chen, J.; Hsieh, I.Y.; Yang, C.; et al. Phase separation of phospho-HDAC6 drives aberrant chromatin architecture in triple-negative breast cancer. Nat. Cancer 2024, 5, 1622–1640. [Google Scholar] [CrossRef]
- Xie, F.; Zhou, X.; Ran, Y.; Li, R.; Zou, J.; Wan, S.; Su, P.; Meng, X.; Yan, H.; Lu, H.; et al. Targeting FOXM1 condensates reduces breast tumour growth and metastasis. Nature 2025, 638, 1112–1121. [Google Scholar] [CrossRef]
- Shay, J.W.; Reddel, R.R.; Wright, W.E. Cancer. Cancer and telomeres—An ALTernative to telomerase. Science 2012, 336, 1388–1390. [Google Scholar] [CrossRef]
- Xu, M.; Senanayaka, D.; Zhao, R.; Chigumira, T.; Tripathi, A.; Tones, J.; Lackner, R.M.; Wondisford, A.R.; Moneysmith, L.N.; Hirschi, A.; et al. TERRA-LSD1 phase separation promotes R-loop formation for telomere maintenance in ALT cancer cells. Nat. Commun. 2024, 15, 2165. [Google Scholar] [CrossRef]
- Xu, Z.; Liu, Y.; Li, F.; Yang, Y.; Zhang, H.; Meng, F.; Liu, X.; Xie, X.; Chen, X.; Shi, Y.; et al. Phase separation of hnRNPA1 and TERRA regulates telomeric stability. J. Mol. Cell Biol. 2025, 16, mjae037. [Google Scholar] [CrossRef]
- Yu, X.; Zhang, H. Biomolecular Condensates in Telomere Maintenance of ALT Cancer Cells. J. Mol. Biol. 2025, 437, 168951. [Google Scholar] [CrossRef]
- Wang, L.; Hu, M.; Zuo, M.Q.; Zhao, J.; Wu, D.; Huang, L.; Wen, Y.; Li, Y.; Chen, P.; Bao, X.; et al. Rett syndrome-causing mutations compromise MeCP2-mediated liquid-liquid phase separation of chromatin. Cell Res. 2020, 30, 393–407. [Google Scholar] [CrossRef]
- Fan, C.; Zhang, H.; Fu, L.; Li, Y.; Du, Y.; Qiu, Z.; Lu, F. Rett mutations attenuate phase separation of MeCP2. Cell Discov. 2020, 6, 38. [Google Scholar] [CrossRef]
- Li, C.H.; Coffey, E.L.; Dall’Agnese, A.; Hannett, N.M.; Tang, X.; Henninger, J.E.; Platt, J.M.; Oksuz, O.; Zamudio, A.V.; Afeyan, L.K.; et al. MeCP2 links heterochromatin condensates and neurodevelopmental disease. Nature 2020, 586, 440–444. [Google Scholar] [CrossRef] [PubMed]
- Fasciani, A.; D’Annunzio, S.; Poli, V.; Fagnocchi, L.; Beyes, S.; Michelatti, D.; Corazza, F.; Antonelli, L.; Gregoretti, F.; Oliva, G.; et al. MLL4-associated condensates counterbalance Polycomb-mediated nuclear mechanical stress in Kabuki syndrome. Nat. Genet. 2020, 52, 1397–1411. [Google Scholar] [CrossRef]
- Tsang, B.; Pritisanac, I.; Scherer, S.W.; Moses, A.M.; Forman-Kay, J.D. Phase Separation as a Missing Mechanism for Interpretation of Disease Mutations. Cell 2020, 183, 1742–1756. [Google Scholar] [CrossRef]
- Pohodich, A.E.; Zoghbi, H.Y. Rett syndrome: Disruption of epigenetic control of postnatal neurological functions. Hum. Mol. Genet. 2015, 24, R10–R16. [Google Scholar] [CrossRef]
- Townend, G.S.; Bartolotta, T.E.; Urbanowicz, A.; Wandin, H.; Curfs, L.M.G. Development of consensus-based guidelines for managing communication of individuals with Rett syndrome. Augment. Altern. Commun. 2020, 36, 71–81. [Google Scholar] [CrossRef]
- Amir, R.E.; Van den Veyver, I.B.; Wan, M.; Tran, C.Q.; Francke, U.; Zoghbi, H.Y. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat. Genet. 1999, 23, 185–188. [Google Scholar] [CrossRef] [PubMed]
- Moretti, P.; Zoghbi, H.Y. MeCP2 dysfunction in Rett syndrome and related disorders. Curr. Opin. Genet. Dev. 2006, 16, 276–281. [Google Scholar] [CrossRef]
- Sandweiss, A.J.; Brandt, V.L.; Zoghbi, H.Y. Advances in understanding of Rett syndrome and MECP2 duplication syndrome: Prospects for future therapies. Lancet Neurol. 2020, 19, 689–698. [Google Scholar] [CrossRef]
- Xiang, Y.; Tanaka, Y.; Patterson, B.; Hwang, S.M.; Hysolli, E.; Cakir, B.; Kim, K.Y.; Wang, W.; Kang, Y.J.; Clement, E.M.; et al. Dysregulation of BRD4 Function Underlies the Functional Abnormalities of MeCP2 Mutant Neurons. Mol. Cell 2020, 79, 84–98.e9. [Google Scholar] [CrossRef]
- Ng, S.B.; Bigham, A.W.; Buckingham, K.J.; Hannibal, M.C.; McMillin, M.J.; Gildersleeve, H.I.; Beck, A.E.; Tabor, H.K.; Cooper, G.M.; Mefford, H.C.; et al. Exome sequencing identifies MLL2 mutations as a cause of Kabuki syndrome. Nat. Genet. 2010, 42, 790–793. [Google Scholar] [CrossRef]
- Schuettengruber, B.; Bourbon, H.M.; Di Croce, L.; Cavalli, G. Genome Regulation by Polycomb and Trithorax: 70 Years and Counting. Cell 2017, 171, 34–57. [Google Scholar] [CrossRef] [PubMed]
- Valencia, A.M.; Sankar, A.; van der Sluijs, P.J.; Satterstrom, F.K.; Fu, J.; Talkowski, M.E.; Vergano, S.A.S.; Santen, G.W.E.; Kadoch, C. Landscape of mSWI/SNF chromatin remodeling complex perturbations in neurodevelopmental disorders. Nat. Genet. 2023, 55, 1400–1412. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Mackowiak, S.D.; Niskanen, H.; Knezevic, D.; Asimi, V.; Grosswendt, S.; Geertsema, H.; Ali, S.; Jerkovic, I.; Ewers, H.; et al. Unblending of Transcriptional Condensates in Human Repeat Expansion Disease. Cell 2020, 181, 1062–1079.e30. [Google Scholar] [CrossRef] [PubMed]
- Mensah, M.A.; Niskanen, H.; Magalhaes, A.P.; Basu, S.; Kircher, M.; Sczakiel, H.L.; Reiter, A.M.V.; Elsner, J.; Meinecke, P.; Biskup, S.; et al. Aberrant phase separation and nucleolar dysfunction in rare genetic diseases. Nature 2023, 614, 564–571. [Google Scholar] [CrossRef]
- Muragaki, Y.; Mundlos, S.; Upton, J.; Olsen, B.R. Altered growth and branching patterns in synpolydactyly caused by mutations in HOXD13. Science 1996, 272, 548–551. [Google Scholar] [CrossRef]
- Goodman, F.R.; Bacchelli, C.; Brady, A.F.; Brueton, L.A.; Fryns, J.P.; Mortlock, D.P.; Innis, J.W.; Holmes, L.B.; Donnenfeld, A.E.; Feingold, M.; et al. Novel HOXA13 mutations and the phenotypic spectrum of hand-foot-genital syndrome. Am. J. Hum. Genet. 2000, 67, 197–202. [Google Scholar] [CrossRef]
- Shibata, A.; Machida, J.; Yamaguchi, S.; Kimura, M.; Tatematsu, T.; Miyachi, H.; Matsushita, M.; Kitoh, H.; Ishiguro, N.; Nakayama, A.; et al. Characterisation of novel RUNX2 mutation with alanine tract expansion from Japanese cleidocranial dysplasia patient. Mutagenesis 2016, 31, 61–67. [Google Scholar] [CrossRef]
- Sofiadis, K.; Josipovic, N.; Nikolic, M.; Kargapolova, Y.; Ubelmesser, N.; Varamogianni-Mamatsi, V.; Zirkel, A.; Papadionysiou, I.; Loughran, G.; Keane, J.; et al. HMGB1 coordinates SASP-related chromatin folding and RNA homeostasis on the path to senescence. Mol. Syst. Biol. 2021, 17, e9760. [Google Scholar] [CrossRef]
- Banani, S.F.; Afeyan, L.K.; Hawken, S.W.; Henninger, J.E.; Dall’Agnese, A.; Clark, V.E.; Platt, J.M.; Oksuz, O.; Hannett, N.M.; Sagi, I.; et al. Genetic variation associated with condensate dysregulation in disease. Dev. Cell 2022, 57, 1776–1788.e8. [Google Scholar] [CrossRef]
- McSwiggen, D.T.; Mir, M.; Darzacq, X.; Tjian, R. Evaluating phase separation in live cells: Diagnosis, caveats, and functional consequences. Genes Dev. 2019, 33, 1619–1634. [Google Scholar] [CrossRef]
- Leslie, M. Separation anxiety. Science 2021, 371, 336–338. [Google Scholar] [CrossRef] [PubMed]
- Erdel, F.; Rademacher, A.; Vlijm, R.; Tunnermann, J.; Frank, L.; Weinmann, R.; Schweigert, E.; Yserentant, K.; Hummert, J.; Bauer, C.; et al. Mouse Heterochromatin Adopts Digital Compaction States without Showing Hallmarks of HP1-Driven Liquid-Liquid Phase Separation. Mol. Cell 2020, 78, 236–249.e7. [Google Scholar] [CrossRef] [PubMed]
- Muzzopappa, F.; Hummert, J.; Anfossi, M.; Tashev, S.A.; Herten, D.P.; Erdel, F. Detecting and quantifying liquid-liquid phase separation in living cells by model-free calibrated half-bleaching. Nat. Commun. 2022, 13, 7787. [Google Scholar] [CrossRef] [PubMed]
- Chong, S.; Graham, T.G.W.; Dugast-Darzacq, C.; Dailey, G.M.; Darzacq, X.; Tjian, R. Tuning levels of low-complexity domain interactions to modulate endogenous oncogenic transcription. Mol. Cell 2022, 82, 2084–2097.e5. [Google Scholar] [CrossRef]
- Trojanowski, J.; Frank, L.; Rademacher, A.; Mucke, N.; Grigaitis, P.; Rippe, K. Transcription activation is enhanced by multivalent interactions independent of phase separation. Mol. Cell 2022, 82, 1878–1893.e10. [Google Scholar] [CrossRef]
- Klein, I.A.; Boija, A.; Afeyan, L.K.; Hawken, S.W.; Fan, M.; Dall’Agnese, A.; Oksuz, O.; Henninger, J.E.; Shrinivas, K.; Sabari, B.R.; et al. Partitioning of cancer therapeutics in nuclear condensates. Science 2020, 368, 1386–1392. [Google Scholar] [CrossRef]
- Wu, Y.; Zhou, L.; Zou, Y.; Zhang, Y.; Zhang, M.; Xu, L.; Zheng, L.; He, W.; Yu, K.; Li, T.; et al. Disrupting the phase separation of KAT8-IRF1 diminishes PD-L1 expression and promotes antitumor immunity. Nat. Cancer 2023, 4, 382–400. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhai, Z.; Meng, F.; Kuang, J.; Pei, D. Phase Separation in Chromatin Organization and Human Diseases. Int. J. Mol. Sci. 2025, 26, 5156. https://doi.org/10.3390/ijms26115156
Zhai Z, Meng F, Kuang J, Pei D. Phase Separation in Chromatin Organization and Human Diseases. International Journal of Molecular Sciences. 2025; 26(11):5156. https://doi.org/10.3390/ijms26115156
Chicago/Turabian StyleZhai, Ziwei, Fei Meng, Junqi Kuang, and Duanqing Pei. 2025. "Phase Separation in Chromatin Organization and Human Diseases" International Journal of Molecular Sciences 26, no. 11: 5156. https://doi.org/10.3390/ijms26115156
APA StyleZhai, Z., Meng, F., Kuang, J., & Pei, D. (2025). Phase Separation in Chromatin Organization and Human Diseases. International Journal of Molecular Sciences, 26(11), 5156. https://doi.org/10.3390/ijms26115156






