Adaptations of Bacterial Extracellular Vesicles in Response to Antibiotic Pressure
Abstract
1. Introduction: Duality of EVs in Gram-Negative and Gram-Positive Bacteria
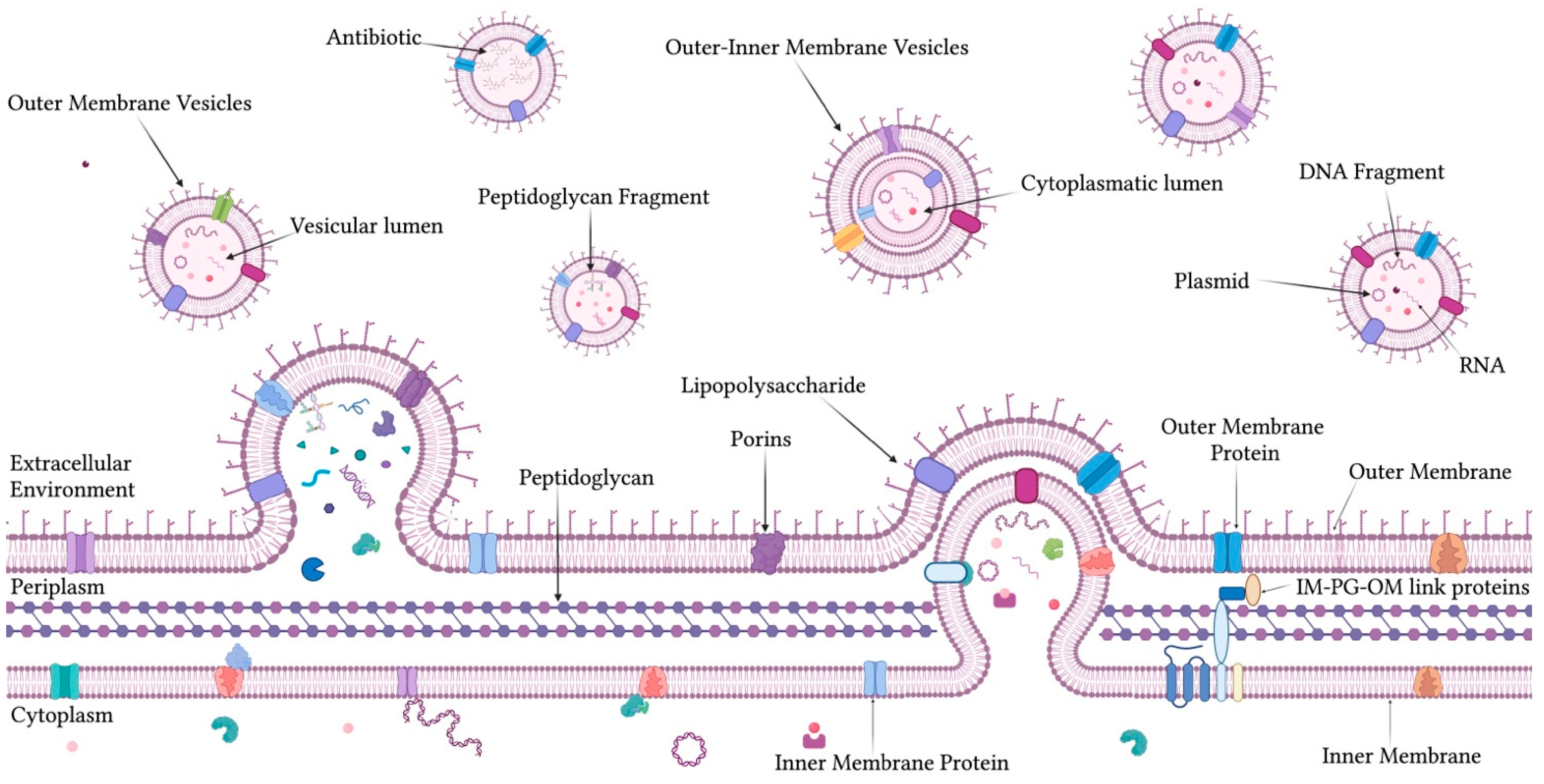
1.1. OMVs: Composition, Biogenesis, and Functional Roles
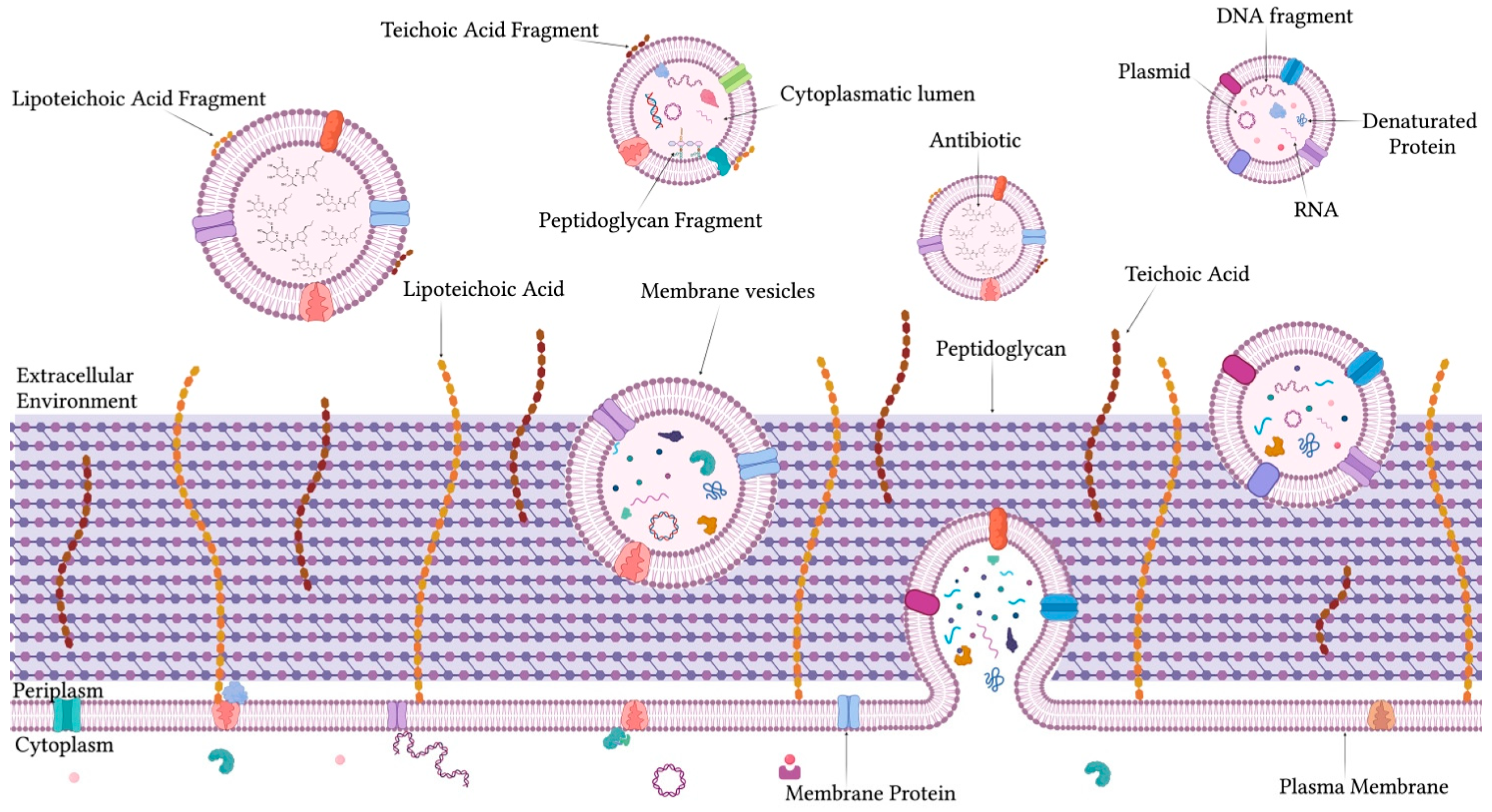
1.2. Structure, Biogenesis, and Roles of MVs in Gram-Positive Bacteria
2. Modulation of Bacterial Vesicles in Response to Antibiotics
2.1. Antibiotic-Induced Changes in OMVs Production in Gram-Negative Bacteria
2.1.1. Secretion of P. aeruginosa-OMVs Under Antibiotic Stress
2.1.2. Antibiotic-Induced Modulation of Escherichia coli-OMVs
2.1.3. OMVs Response to Antibiotics in Other Gram-Negative Bacteria
2.2. Antibiotic-Induced Modulation of MVs in Gram-Positive Bacteria
2.3. Major Enzymes and Protein Classes Involved in OMV-Mediated Antibiotic Resistance
2.3.1. β-Lactamases and Carbapenemases
2.3.2. Efflux Pumps
2.3.3. OMVs Proteome Remodeling
- -
- Cationic antimicrobial peptide (CAMP) resistance proteins, for example, 4-amino-4-deoxy-L-arabinose transferase and PhoPQ two-component kinase that allows lipid A and OM modifications and are required for resistance to polymyxin and AMPs. Other related enzymes are undecaprenyl phosphate-alpha-L-Ara4N transferase, copper homeostasis protein, and thiol: disulfide interchange protein;
- -
- OM and envelope remodeling, such as proteins for LPS biosynthesis and O-antigen modification (e.g., KdsA, GmhA, and HldE). The OmpA and others (e.g., OmpX and asmA) showed significant reduction in polymyxin-treated susceptible OMVs;
- -
- Two-component systems (TCS) CpxA, a protein involved in envelope stress response, is downregulated following the treatment;
- -
- Protein export and translocation systems, significant changes in Sec and Tat pathways are observed (e.g., the downregulation of SecA, YajC, and YidC), and the downregulation of SurA, a key chaperone for OM protein folding;
- -
- RNA processing and repair: altered abundance of RNA degradosome proteins (e.g., RNase E, RhlB, and GroEL) and nucleotide excision repair proteins were observed upon polymyxin treatment.
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Guo, J.; Huang, Z.; Wang, Q.; Wang, M.; Ming, Y.; Chen, W.; Huang, Y.; Tang, Z.; Huang, M.; Liu, H.; et al. Opportunities and Challenges of Bacterial Extracellular Vesicles in Regenerative Medicine. J. Nanobiotechnol. 2025, 23, 4. [Google Scholar] [CrossRef] [PubMed]
- Ñahui Palomino, R.A.; Vanpouille, C.; Costantini, P.E.; Margolis, L. Microbiota-Host Communications: Bacterial Extracellular Vesicles as a Common Language. PLoS Pathog. 2021, 17, e1009508. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Yazdani, N.; Moran, C.S.; Salomon, C.; Seneviratne, C.J.; Ivanovski, S.; Han, P. Unveiling Clinical Applications of Bacterial Extracellular Vesicles as Natural Nanomaterials in Disease Diagnosis and Therapeutics. Acta Biomater. 2024, 180, 18–45. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.A.; Baba, S.K.; Sadida, H.Q.; Marzooqi, S.A.; Jerobin, J.; Altemani, F.H.; Algehainy, N.; Alanazi, M.A.; Abou-Samra, A.-B.; Kumar, R.; et al. Extracellular Vesicles as Tools and Targets in Therapy for Diseases. Signal Transduct. Target. Ther. 2024, 9, 27. [Google Scholar] [CrossRef]
- Rafieezadeh, D.; Rafieezadeh, A. Extracellular Vesicles and Their Therapeutic Applications: A Review Article (Part1). Int. J. Physiol. Pathophysiol. Pharmacol. 2024, 16, 1–9. [Google Scholar] [CrossRef]
- Ahmed, A.A.Q.; McKay, T.J.M. Environmental and Ecological Importance of Bacterial Extracellular Vesicles (BEVs). Sci. Total Environ. 2024, 907, 168098. [Google Scholar] [CrossRef]
- Barber, M.F.; Fitzgerald, J.R. Mechanisms of Host Adaptation by Bacterial Pathogens. FEMS Microbiol. Rev. 2024, 48, fuae019. [Google Scholar] [CrossRef]
- Magaña, G.; Harvey, C.; Taggart, C.C.; Rodgers, A.M. Bacterial Outer Membrane Vesicles: Role in Pathogenesis and Host-Cell Interactions. Antibiotics 2023, 13, 32. [Google Scholar] [CrossRef]
- Toyofuku, M.; Schild, S.; Kaparakis-Liaskos, M.; Eberl, L. Composition and Functions of Bacterial Membrane Vesicles. Nat. Rev. Microbiol. 2023, 21, 415–430. [Google Scholar] [CrossRef]
- Briaud, P.; Carroll, R.K. Extracellular Vesicle Biogenesis and Functions in Gram-Positive Bacteria. Infect. Immun. 2020, 88, e00433-20. [Google Scholar] [CrossRef]
- Kim, J.Y.; Suh, J.W.; Kang, J.S.; Kim, S.B.; Yoon, Y.K.; Sohn, J.W. Gram-Negative Bacteria’s Outer Membrane Vesicles. Infect. Chemother. 2023, 55, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Dell’Annunziata, F.; Ilisso, C.P.; Dell’Aversana, C.; Greco, G.; Coppola, A.; Martora, F.; Dal Piaz, F.; Donadio, G.; Falanga, A.; Galdiero, M.; et al. Outer Membrane Vesicles Derived from Klebsiella pneumoniae Influence the miRNA Expression Profile in Human Bronchial Epithelial BEAS-2B Cells. Microorganisms 2020, 8, 1985. [Google Scholar] [CrossRef] [PubMed]
- Sartorio, M.G.; Pardue, E.J.; Feldman, M.F.; Haurat, M.F. Bacterial Outer Membrane Vesicles: From Discovery to Applications. Annu. Rev. Microbiol. 2021, 75, 609–630. [Google Scholar] [CrossRef]
- Ghadami, S.; Dellinger, K. The Lipid Composition of Extracellular Vesicles: Applications in Diagnostics and Therapeutic Delivery. Front. Mol. Biosci. 2023, 10, 1198044. [Google Scholar] [CrossRef]
- Bose, S.; Aggarwal, S.; Singh, D.V.; Acharya, N. Extracellular Vesicles: An Emerging Platform in Gram-Positive Bacteria. Microb. Cell 2020, 7, 312–322. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Xie, C.; Liu, Y.; Qin, X.; Liu, J. An Update on Our Understanding of Gram-Positive Bacterial Membrane Vesicles: Discovery, Functions, and Applications. Front. Cell Infect. Microbiol. 2023, 13, 1273813. [Google Scholar] [CrossRef]
- Gan, Y.; Zhao, G.; Wang, Z.; Zhang, X.; Wu, M.X.; Lu, M. Bacterial Membrane Vesicles: Physiological Roles, Infection Immunology, and Applications. Adv. Sci. 2023, 10, e2301357. [Google Scholar] [CrossRef]
- Kumar, S.D.; Park, J.; Radhakrishnan, N.K.; Aryal, Y.P.; Jeong, G.H.; Pyo, I.H.; Ganbaatar, B.; Lee, C.W.; Yang, S.; Shin, Y.; et al. Novel Leech Antimicrobial Peptides, Hirunipins: Real-Time 3D Monitoring of Antimicrobial and Antibiofilm Mechanisms Using Optical Diffraction Tomography. Adv. Sci. 2025, 12, 2409803. [Google Scholar] [CrossRef]
- Dell’Annunziata, F.; Ciaglia, E.; Folliero, V.; Lopardo, V.; Maciag, A.; Galdiero, M.; Puca, A.A.; Franci, G. Klebsiella pneumoniae-OMVs Activate Death-Signaling Pathways in Human Bronchial Epithelial Host Cells (BEAS-2B). Heliyon 2024, 10, e29017. [Google Scholar] [CrossRef]
- Jeong, D.; Kim, M.J.; Park, Y.; Chung, J.; Kweon, H.-S.; Kang, N.-G.; Hwang, S.J.; Youn, S.H.; Hwang, B.K.; Kim, D. Visualizing Extracellular Vesicle Biogenesis in Gram-Positive Bacteria Using Super-Resolution Microscopy. BMC Biol. 2022, 20, 270. [Google Scholar] [CrossRef]
- Aloi, N.; Drago, G.; Ruggieri, S.; Cibella, F.; Colombo, P.; Longo, V. Extracellular Vesicles and Immunity: At the Crossroads of Cell Communication. Int. J. Mol. Sci. 2024, 25, 1205. [Google Scholar] [CrossRef] [PubMed]
- Schemiko Almeida, K.; Rossi, S.A.; Alves, L.R. RNA-Containing Extracellular Vesicles in Infection. RNA Biol. 2024, 21, 37–51. [Google Scholar] [CrossRef] [PubMed]
- Berumen Sánchez, G.; Bunn, K.E.; Pua, H.H.; Rafat, M. Extracellular Vesicles: Mediators of Intercellular Communication in Tissue Injury and Disease. Cell Commun. Signal 2021, 19, 104. [Google Scholar] [CrossRef] [PubMed]
- Weaver, B.P.; Haselwandter, C.A.; Boedicker, J.Q. Stochastic Effects in Bacterial Communication Mediated by Extracellular Vesicles. Phys. Rev. E 2023, 107, 024409. [Google Scholar] [CrossRef]
- Zhao, A.; Sun, J.; Liu, Y. Understanding Bacterial Biofilms: From Definition to Treatment Strategies. Front. Cell Infect. Microbiol. 2023, 13, 1137947. [Google Scholar] [CrossRef]
- Dhital, S.; Deo, P.; Stuart, I.; Naderer, T. Bacterial Outer Membrane Vesicles and Host Cell Death Signaling. Trends Microbiol. 2021, 29, 1106–1116. [Google Scholar] [CrossRef]
- Potapova, A.; Garvey, W.; Dahl, P.; Guo, S.; Chang, Y.; Schwechheimer, C.; Trebino, M.A.; Floyd, K.A.; Phinney, B.S.; Liu, J.; et al. Outer Membrane Vesicles and the Outer Membrane Protein OmpU Govern Vibrio Cholerae Biofilm Matrix Assembly. mBio 2024, 15, e0330423. [Google Scholar] [CrossRef]
- Lei, E.K.; Azmat, A.; Henry, K.A.; Hussack, G. Outer Membrane Vesicles as a Platform for the Discovery of Antibodies to Bacterial Pathogens. Appl. Microbiol. Biotechnol. 2024, 108, 232. [Google Scholar] [CrossRef]
- Prajapati, J.D.; Kleinekathöfer, U.; Winterhalter, M. How to Enter a Bacterium: Bacterial Porins and the Permeation of Antibiotics. Chem. Rev. 2021, 121, 5158–5192. [Google Scholar] [CrossRef]
- Skerniškytė, J.; Karazijaitė, E.; Lučiūnaitė, A.; Sužiedėlienė, E. OmpA Protein-Deficient Acinetobacter baumannii Outer Membrane Vesicles Trigger Reduced Inflammatory Response. Pathogens 2021, 10, 407. [Google Scholar] [CrossRef]
- Furuyama, N.; Sircili, M.P. Outer Membrane Vesicles (OMVs) Produced by Gram-Negative Bacteria: Structure, Functions, Biogenesis, and Vaccine Application. Biomed Res. Int. 2021, 2021, 1490732. [Google Scholar] [CrossRef] [PubMed]
- Płaczkiewicz, J.; Gieczewska, K.; Musiałowski, M.; Adamczyk-Popławska, M.; Bącal, P.; Kwiatek, A. Availability of Iron Ions Impacts Physicochemical Properties and Proteome of Outer Membrane Vesicles Released by Neisseria gonorrhoeae. Sci. Rep. 2023, 13, 18733. [Google Scholar] [CrossRef] [PubMed]
- Juodeikis, R.; Carding, S.R. Outer Membrane Vesicles: Biogenesis, Functions, and Issues. Microbiol. Mol. Biol. Rev. 2022, 86, e0003222. [Google Scholar] [CrossRef]
- Zhao, X.; Wei, Y.; Bu, Y.; Ren, X.; Dong, Z. Review on Bacterial Outer Membrane Vesicles: Structure, Vesicle Formation, Separation and Biotechnological Applications. Microb. Cell Fact. 2025, 24, 27. [Google Scholar] [CrossRef]
- Rueter, C.; Bielaszewska, M. Secretion and Delivery of Intestinal Pathogenic Escherichia coli Virulence Factors via Outer Membrane Vesicles. Front. Cell Infect. Microbiol. 2020, 10, 91. [Google Scholar] [CrossRef]
- Da Costa, R.M.; Rooke, J.L.; Wells, T.J.; Cunningham, A.F.; Henderson, I.R. Type 5 Secretion System Antigens as Vaccines against Gram-Negative Bacterial Infections. NPJ Vaccines 2024, 9, 159. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, F.; Duda, K.A.; Lanzetta, R.; Silipo, A.; De Castro, C.; Molinaro, A. A Journey from Structure to Function of Bacterial Lipopolysaccharides. Chem. Rev. 2022, 122, 15767–15821. [Google Scholar] [CrossRef]
- Lima, S.; Matinha-Cardoso, J.; Tamagnini, P.; Oliveira, P. Extracellular Vesicles: An Overlooked Secretion System in Cyanobacteria. Life 2020, 10, 129. [Google Scholar] [CrossRef]
- Dowhan, W.; Bogdanov, M.; Eugene, P. Kennedy’s Legacy: Defining Bacterial Phospholipid Pathways and Function. Front. Mol. Biosci. 2021, 8, 666203. [Google Scholar] [CrossRef]
- Fuentes, J.M.; Morcillo, P. The Role of Cardiolipin in Mitochondrial Function and Neurodegenerative Diseases. Cells 2024, 13, 609. [Google Scholar] [CrossRef]
- Page, M.J.; Kell, D.B.; Pretorius, E. The Role of Lipopolysaccharide-Induced Cell Signalling in Chronic Inflammation. Chronic Stress 2022, 6, 24705470221076390. [Google Scholar] [CrossRef] [PubMed]
- Gilmore, W.J.; Johnston, E.L.; Zavan, L.; Bitto, N.J.; Kaparakis-Liaskos, M. Immunomodulatory Roles and Novel Applications of Bacterial Membrane Vesicles. Mol. Immunol. 2021, 134, 72–85. [Google Scholar] [CrossRef] [PubMed]
- Okamura, H.; Hirota, K.; Yoshida, K.; Weng, Y.; He, Y.; Shiotsu, N.; Ikegame, M.; Uchida-Fukuhara, Y.; Tanai, A.; Guo, J. Outer Membrane Vesicles of Porphyromonas gingivalis: Novel Communication Tool and Strategy. Jpn. Dent. Sci. Rev. 2021, 57, 138–146. [Google Scholar] [CrossRef] [PubMed]
- De Chiara, S.; De Simone Carone, L.; Cirella, R.; Andretta, E.; Silipo, A.; Molinaro, A.; Mercogliano, M.; Di Lorenzo, F. Beyond the Toll-Like Receptor 4. Structure-Dependent Lipopolysaccharide Recognition Systems: How Far Are We? ChemMedChem 2025, 20, e202400780. [Google Scholar] [CrossRef]
- Ryu, S.; Ni, K.; Wang, C.; Sivanantham, A.; Carnino, J.M.; Ji, H.-L.; Jin, Y. Bacterial Outer Membrane Vesicles Promote Lung Inflammatory Responses and Macrophage Activation via Multi-Signaling Pathways. Biomedicines 2023, 11, 568. [Google Scholar] [CrossRef]
- Dell’Annunziata, F.; Folliero, V.; Giugliano, R.; De Filippis, A.; Santarcangelo, C.; Izzo, V.; Daglia, M.; Galdiero, M.; Arciola, C.R.; Franci, G. Gene Transfer Potential of Outer Membrane Vesicles of Gram-Negative Bacteria. Int. J. Mol. Sci. 2021, 22, 5985. [Google Scholar] [CrossRef]
- Ajam-Hosseini, M.; Akhoondi, F.; Parvini, F.; Fahimi, H. Gram-Negative Bacterial sRNAs Encapsulated in OMVs: An Emerging Class of Therapeutic Targets in Diseases. Front. Cell Infect. Microbiol. 2023, 13, 1305510. [Google Scholar] [CrossRef]
- Xie, Z.; Wang, X.; Huang, Y.; Chen, S.; Liu, M.; Zhang, F.; Li, M.; Wang, X.; Gu, Y.; Yang, Y.; et al. Pseudomonas aeruginosa Outer Membrane Vesicle-Packed sRNAs Can Enter Host Cells and Regulate Innate Immune Responses. Microb. Pathog. 2024, 188, 106562. [Google Scholar] [CrossRef]
- Stanton, B.A. Extracellular Vesicles and Host-Pathogen Interactions: A Review of Inter-Kingdom Signaling by Small Noncoding RNA. Genes 2021, 12, 1010. [Google Scholar] [CrossRef]
- Layton, E.; Fairhurst, A.-M.; Griffiths-Jones, S.; Grencis, R.K.; Roberts, I.S. Regulatory RNAs: A Universal Language for Inter-Domain Communication. Int. J. Mol. Sci. 2020, 21, 8919. [Google Scholar] [CrossRef]
- Tao, S.; Chen, H.; Li, N.; Wang, T.; Liang, W. The Spread of Antibiotic Resistance Genes In Vivo Model. Can. J. Infect. Dis. Med. Microbiol. 2022, 2022, 3348695. [Google Scholar] [CrossRef] [PubMed]
- Suri, K.; D’Souza, A.; Huang, D.; Bhavsar, A.; Amiji, M. Bacterial Extracellular Vesicle Applications in Cancer Immunotherapy. Bioact. Mater. 2023, 22, 551–566. [Google Scholar] [CrossRef]
- Mozaheb, N.; Mingeot-Leclercq, M.-P. Membrane Vesicle Production as a Bacterial Defense Against Stress. Front. Microbiol. 2020, 11, 600221. [Google Scholar] [CrossRef] [PubMed]
- Muteeb, G.; Rehman, M.T.; Shahwan, M.; Aatif, M. Origin of Antibiotics and Antibiotic Resistance, and Their Impacts on Drug Development: A Narrative Review. Pharmaceuticals 2023, 16, 1615. [Google Scholar] [CrossRef] [PubMed]
- Tajer, L.; Paillart, J.-C.; Dib, H.; Sabatier, J.-M.; Fajloun, Z.; Abi Khattar, Z. Molecular Mechanisms of Bacterial Resistance to Antimicrobial Peptides in the Modern Era: An Updated Review. Microorganisms 2024, 12, 1259. [Google Scholar] [CrossRef]
- Schwechheimer, C.; Kuehn, M.J. Outer-Membrane Vesicles from Gram-Negative Bacteria: Biogenesis and Functions. Nat. Rev. Microbiol. 2015, 13, 605–619. [Google Scholar] [CrossRef]
- Maerz, J.K.; Steimle, A.; Lange, A.; Bender, A.; Fehrenbacher, B.; Frick, J.-S. Outer Membrane Vesicles Blebbing Contributes to B. Vulgatus Mpk-Mediated Immune Response Silencing. Gut Microbes 2018, 9, 1–12. [Google Scholar] [CrossRef]
- Cai, W.; Kesavan, D.K.; Wan, J.; Abdelaziz, M.H.; Su, Z.; Xu, H. Bacterial Outer Membrane Vesicles, a Potential Vaccine Candidate in Interactions with Host Cells Based. Diagn. Pathol. 2018, 13, 95. [Google Scholar] [CrossRef]
- Gill, S.; Catchpole, R.; Forterre, P. Extracellular Membrane Vesicles in the Three Domains of Life and Beyond. FEMS Microbiol. Rev. 2019, 43, 273–303. [Google Scholar] [CrossRef]
- Li, M.; Xing, X.; Yuan, J.; Zeng, Z. Research Progress on the Regulatory Role of Cell Membrane Surface Tension in Cell Behavior. Heliyon 2024, 10, e29923. [Google Scholar] [CrossRef]
- Mandal, P.K.; Ballerin, G.; Nolan, L.M.; Petty, N.K.; Whitchurch, C.B. Bacteriophage Infection of Escherichia coli Leads to the Formation of Membrane Vesicles via Both Explosive Cell Lysis and Membrane Blebbing. Microbiology 2021, 167, 001021. [Google Scholar] [CrossRef] [PubMed]
- Turnbull, L.; Toyofuku, M.; Hynen, A.L.; Kurosawa, M.; Pessi, G.; Petty, N.K.; Osvath, S.R.; Cárcamo-Oyarce, G.; Gloag, E.S.; Shimoni, R.; et al. Explosive Cell Lysis as a Mechanism for the Biogenesis of Bacterial Membrane Vesicles and Biofilms. Nat. Commun. 2016, 7, 11220. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, Q.; Wang, S.; Weng, W.; Jing, Y.; Su, J. Bacterial Extracellular Vesicles as Bioactive Nanocarriers for Drug Delivery: Advances and Perspectives. Bioact. Mater. 2022, 14, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Rutherford, S.T.; Silhavy, T.J.; Huang, K.C. Physical Properties of the Bacterial Outer Membrane. Nat. Rev. Microbiol. 2022, 20, 236–248. [Google Scholar] [CrossRef]
- Cail, R.C.; Drubin, D.G. Membrane Curvature as a Signal to Ensure Robustness of Diverse Cellular Processes. Trends Cell Biol. 2023, 33, 427–441. [Google Scholar] [CrossRef]
- Zhou, G.; Wang, Q.; Wang, Y.; Wen, X.; Peng, H.; Peng, R.; Shi, Q.; Xie, X.; Li, L. Outer Membrane Porins Contribute to Antimicrobial Resistance in Gram-Negative Bacteria. Microorganisms 2023, 11, 1690. [Google Scholar] [CrossRef]
- Wang, X.; Nyenhuis, S.B.; Bernstein, H.D. The Translocation Assembly Module (TAM) Catalyzes the Assembly of Bacterial Outer Membrane Proteins in Vitro. Nat. Commun. 2024, 15, 7246. [Google Scholar] [CrossRef]
- Manganelli, V.; Capozzi, A.; Recalchi, S.; Riitano, G.; Mattei, V.; Longo, A.; Misasi, R.; Garofalo, T.; Sorice, M. The Role of Cardiolipin as a Scaffold Mitochondrial Phospholipid in Autophagosome Formation: In Vitro Evidence. Biomolecules 2021, 11, 222. [Google Scholar] [CrossRef]
- Mitchison-Field, L.M.; Belin, B.J. Bacterial Lipid Biophysics and Membrane Organization. Curr. Opin. Microbiol. 2023, 74, 102315. [Google Scholar] [CrossRef]
- Zheng, K.; Feng, Y.; Li, L.; Kong, F.; Gao, J.; Kong, X. Engineered Bacterial Outer Membrane Vesicles: A Versatile Bacteria-Based Weapon against Gastrointestinal Tumors. Theranostics 2024, 14, 761–787. [Google Scholar] [CrossRef]
- Nevermann, J.; Silva, A.; Otero, C.; Oyarzún, D.P.; Barrera, B.; Gil, F.; Calderón, I.L.; Fuentes, J.A. Identification of Genes Involved in Biogenesis of Outer Membrane Vesicles (OMVs) in Salmonella enterica Serovar Typhi. Front. Microbiol. 2019, 10, 104. [Google Scholar] [CrossRef]
- Aktar, S.; Okamoto, Y.; Ueno, S.; Tahara, Y.O.; Imaizumi, M.; Shintani, M.; Miyata, M.; Futamata, H.; Nojiri, H.; Tashiro, Y. Incorporation of Plasmid DNA Into Bacterial Membrane Vesicles by Peptidoglycan Defects in Escherichia coli. Front. Microbiol. 2021, 12, 747606. [Google Scholar] [CrossRef] [PubMed]
- McMillan, H.M.; Kuehn, M.J. The Extracellular Vesicle Generation Paradox: A Bacterial Point of View. EMBO J. 2021, 40, e108174. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhu, L.; Wang, Y.; Zhou, X.; Li, Y. Bacterial Outer Membrane Vesicles in Cancer: Biogenesis, Pathogenesis, and Clinical Application. Biomed. Pharmacother. 2023, 165, 115120. [Google Scholar] [CrossRef]
- Tikhomirova, A.; McNabb, E.R.; Petterlin, L.; Bellamy, G.L.; Lin, K.H.; Santoso, C.A.; Daye, E.S.; Alhaddad, F.M.; Lee, K.P.; Roujeinikova, A. Campylobacter Jejuni Virulence Factors: Update on Emerging Issues and Trends. J. Biomed. Sci. 2024, 31, 45. [Google Scholar] [CrossRef] [PubMed]
- Charpentier, L.A.; Dolben, E.F.; Hendricks, M.R.; Hogan, D.A.; Bomberger, J.M.; Stanton, B.A. Bacterial Outer Membrane Vesicles and Immune Modulation of the Host. Membranes 2023, 13, 752. [Google Scholar] [CrossRef]
- Mazgaeen, L.; Gurung, P. Recent Advances in Lipopolysaccharide Recognition Systems. Int. J. Mol. Sci. 2020, 21, 379. [Google Scholar] [CrossRef]
- Romerio, A.; Peri, F. Increasing the Chemical Variety of Small-Molecule-Based TLR4 Modulators: An Overview. Front. Immunol. 2020, 11, 1210. [Google Scholar] [CrossRef]
- Kim, H.-J.; Kim, H.; Lee, J.-H.; Hwangbo, C. Toll-like Receptor 4 (TLR4): New Insight Immune and Aging. Immun. Ageing 2023, 20, 67. [Google Scholar] [CrossRef]
- Jeong, G.-J.; Khan, F.; Tabassum, N.; Cho, K.-J.; Kim, Y.-M. Bacterial Extracellular Vesicles: Modulation of Biofilm and Virulence Properties. Acta Biomater. 2024, 178, 13–23. [Google Scholar] [CrossRef]
- Collins, S.M.; Brown, A.C. Bacterial Outer Membrane Vesicles as Antibiotic Delivery Vehicles. Front. Immunol. 2021, 12, 733064. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Pereira, T.; Groleau, M.-C.; Déziel, E. Surface Growth of Pseudomonas aeruginosa Reveals a Regulatory Effect of 3-Oxo-C12-Homoserine Lactone in the Absence of Its Cognate Receptor, LasR. mBio 2023, 14, e0092223. [Google Scholar] [CrossRef]
- Tuon, F.F.; Dantas, L.R.; Suss, P.H.; Tasca Ribeiro, V.S. Pathogenesis of the Pseudomonas aeruginosa Biofilm: A Review. Pathogens 2022, 11, 300. [Google Scholar] [CrossRef] [PubMed]
- Juszczuk-Kubiak, E. Molecular Aspects of the Functioning of Pathogenic Bacteria Biofilm Based on Quorum Sensing (QS) Signal-Response System and Innovative Non-Antibiotic Strategies for Their Elimination. Int. J. Mol. Sci. 2024, 25, 2655. [Google Scholar] [CrossRef]
- Dell’Annunziata, F.; Dell’Aversana, C.; Doti, N.; Donadio, G.; Dal Piaz, F.; Izzo, V.; De Filippis, A.; Galdiero, M.; Altucci, L.; Boccia, G.; et al. Outer Membrane Vesicles Derived from Klebsiella pneumoniae Are a Driving Force for Horizontal Gene Transfer. Int. J. Mol. Sci. 2021, 22, 8732. [Google Scholar] [CrossRef]
- Marinacci, B.; Krzyżek, P.; Pellegrini, B.; Turacchio, G.; Grande, R. Latest Update on Outer Membrane Vesicles and Their Role in Horizontal Gene Transfer: A Mini-Review. Membranes 2023, 13, 860. [Google Scholar] [CrossRef]
- Abe, K.; Nomura, N.; Suzuki, S. Biofilms: Hot Spots of Horizontal Gene Transfer (HGT) in Aquatic Environments, with a Focus on a New HGT Mechanism. FEMS Microbiol. Ecol. 2020, 96, fiaa031. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Yang, B.; Wang, Z.; Liu, Y. Augmented Dissemination of Antibiotic Resistance Elicited by Non-Antibiotic Factors. Ecotoxicol. Environ. Saf. 2023, 262, 115124. [Google Scholar] [CrossRef]
- Barut, D.; Enuh, B.M.; Derkuş, B.; Güler, Ü.; Salih, B.; Aytar Çelik, P. The Relationship between Bacterial Outer Membrane Vesicles and Halophilic Adaptation. Mol. Omics 2023, 19, 174–181. [Google Scholar] [CrossRef]
- Gurunathan, S.; Thangaraj, P.; Das, J.; Kim, J.-H. Antibacterial and Antibiofilm Effects of Pseudomonas aeruginosa Derived Outer Membrane Vesicles against Streptococcus Mutans. Heliyon 2023, 9, e22606. [Google Scholar] [CrossRef]
- Wawrzeniak, K.; Gaur, G.; Sapi, E.; Senejani, A.G. Effect of Borrelia Burgdorferi Outer Membrane Vesicles on Host Oxidative Stress Response. Antibiotics 2020, 9, 275. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.-J.; Wang, X.-H.; Fan, G.-C. Versatile Effects of Bacterium-Released Membrane Vesicles on Mammalian Cells and Infectious/Inflammatory Diseases. Acta Pharmacol. Sin. 2018, 39, 514–533. [Google Scholar] [CrossRef] [PubMed]
- Rudnicka, M.; Noszczyńska, M.; Malicka, M.; Kasperkiewicz, K.; Pawlik, M.; Piotrowska-Seget, Z. Outer Membrane Vesicles as Mediators of Plant-Bacterial Interactions. Front. Microbiol. 2022, 13, 902181. [Google Scholar] [CrossRef]
- Huang, Y.; Nieh, M.-P.; Chen, W.; Lei, Y. Outer Membrane Vesicles (OMVs) Enabled Bio-Applications: A Critical Review. Biotechnol. Bioeng. 2022, 119, 34–47. [Google Scholar] [CrossRef]
- Aytar Çelik, P.; Erdogan-Gover, K.; Barut, D.; Enuh, B.M.; Amasya, G.; Sengel-Türk, C.T.; Derkus, B.; Çabuk, A. Bacterial Membrane Vesicles as Smart Drug Delivery and Carrier Systems: A New Nanosystems Tool for Current Anticancer and Antimicrobial Therapy. Pharmaceutics 2023, 15, 1052. [Google Scholar] [CrossRef]
- Naskar, A.; Cho, H.; Lee, S.; Kim, K.-S. Biomimetic Nanoparticles Coated with Bacterial Outer Membrane Vesicles as a New-Generation Platform for Biomedical Applications. Pharmaceutics 2021, 13, 1887. [Google Scholar] [CrossRef]
- Luo, Z.; Cheng, X.; Feng, B.; Fan, D.; Liu, X.; Xie, R.; Luo, T.; Wegner, S.V.; Ma, D.; Chen, F.; et al. Engineering Versatile Bacteria-Derived Outer Membrane Vesicles: An Adaptable Platform for Advancing Cancer Immunotherapy. Adv. Sci. 2024, 11, e2400049. [Google Scholar] [CrossRef] [PubMed]
- Rohde, M. The Gram-Positive Bacterial Cell Wall. Microbiol. Spectr. 2019, 7. [Google Scholar] [CrossRef]
- Strach, M.; Koch, F.; Fiedler, S.; Liebeton, K.; Graumann, P.L. Protein Secretion Zones during Overexpression of Amylase within the Gram-Positive Cell Wall. BMC Biol. 2023, 21, 206. [Google Scholar] [CrossRef]
- Ramírez-Larrota, J.S.; Eckhard, U. An Introduction to Bacterial Biofilms and Their Proteases, and Their Roles in Host Infection and Immune Evasion. Biomolecules 2022, 12, 306. [Google Scholar] [CrossRef]
- Xiu, L.; Wu, Y.; Lin, G.; Zhang, Y.; Huang, L. Bacterial Membrane Vesicles: Orchestrators of Interkingdom Interactions in Microbial Communities for Environmental Adaptation and Pathogenic Dynamics. Front. Immunol. 2024, 15, 1371317. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Feng, S.; Tong, Y.; Zhang, H.; Yang, H. Adaptive Defensive Mechanism of Bioleaching Microorganisms under Extremely Environmental Acid Stress: Advances and Perspectives. Biotechnol. Adv. 2020, 42, 107580. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zeng, J.; Ren, L.; Yan, Q.; Wu, Q.L. Enhanced Metabolic Potentials and Functional Gene Interactions of Microbial Stress Responses to a 4,100-m Elevational Increase in Freshwater Lakes. Front. Microbiol. 2020, 11, 595967. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, B.V.; Kuehn, M.J. Staphylococcus Aureus Secretes Immunomodulatory RNA and DNA via Membrane Vesicles. Sci. Rep. 2020, 10, 18293. [Google Scholar] [CrossRef]
- Payandeh, Z.; Tangruksa, B.; Synnergren, J.; Heydarkhan-Hagvall, S.; Nordin, J.Z.; Andaloussi, S.E.; Borén, J.; Wiseman, J.; Bohlooly-Y, M.; Lindfors, L.; et al. Extracellular Vesicles Transport RNA between Cells: Unraveling Their Dual Role in Diagnostics and Therapeutics. Mol. Aspects Med. 2024, 99, 101302. [Google Scholar] [CrossRef]
- Toyofuku, M.; Nomura, N.; Eberl, L. Types and Origins of Bacterial Membrane Vesicles. Nat. Rev. Microbiol. 2019, 17, 13–24. [Google Scholar] [CrossRef]
- Skotland, T.; Llorente, A.; Sandvig, K. Lipids in Extracellular Vesicles: What Can Be Learned about Membrane Structure and Function? Cold Spring Harb. Perspect. Biol. 2023, 15, a041415. [Google Scholar] [CrossRef]
- Luchini, A.; Cavasso, D.; Radulescu, A.; D’Errico, G.; Paduano, L.; Vitiello, G. Structural Organization of Cardiolipin-Containing Vesicles as Models of the Bacterial Cytoplasmic Membrane. Langmuir 2021, 37, 8508–8516. [Google Scholar] [CrossRef]
- Joyce, L.R.; Doran, K.S. Gram-Positive Bacterial Membrane Lipids at the Host-Pathogen Interface. PLoS Pathog. 2023, 19, e1011026. [Google Scholar] [CrossRef]
- Prince, A.; Tiwari, A.; Mandal, T.; Koiri, D.; Meher, G.; Sinha, D.K.; Saleem, M. Lipid Specificity of the Fusion of Bacterial Extracellular Vesicles with the Host Membrane. J. Phys. Chem. B 2024, 128, 8116–8130. [Google Scholar] [CrossRef]
- Schiera, G.; Di Liegro, C.M.; Di Liegro, I. Extracellular Membrane Vesicles as Vehicles for Brain Cell-to-Cell Interactions in Physiological as Well as Pathological Conditions. Biomed. Res. Int. 2015, 2015, 152926. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; He, S.; Wen, R.; Li, C.; Chen, X.; Lin, X.; Wang, H.; Tang, Y. Membrane Vesicles Derived from Enterococcus faecalis Promote the Co-Transfer of Important Antibiotic Resistance Genes Located on Both Plasmids and Chromosomes. J. Antimicrob. Chemother. 2024, 79, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Beltrán, J.; DelaFuente, J.; León-Sampedro, R.; MacLean, R.C.; San Millán, Á. Beyond Horizontal Gene Transfer: The Role of Plasmids in Bacterial Evolution. Nat. Rev. Microbiol. 2021, 19, 347–359. [Google Scholar] [CrossRef]
- Zwanzig, M. The Ecology of Plasmid-Coded Antibiotic Resistance: A Basic Framework for Experimental Research and Modeling. Comput. Struct. Biotechnol. J. 2021, 19, 586–599. [Google Scholar] [CrossRef]
- Agashe, D.; Sane, M.; Singhal, S. Revisiting the Role of Genetic Variation in Adaptation. Am. Nat. 2023, 202, 486–502. [Google Scholar] [CrossRef]
- Stange, M.; Barrett, R.D.H.; Hendry, A.P. The Importance of Genomic Variation for Biodiversity, Ecosystems and People. Nat. Rev. Genet. 2021, 22, 89–105. [Google Scholar] [CrossRef]
- Pita, T.; Feliciano, J.R.; Leitão, J.H. Extracellular RNAs in Bacterial Infections: From Emerging Key Players on Host-Pathogen Interactions to Exploitable Biomarkers and Therapeutic Targets. Int. J. Mol. Sci. 2020, 21, 9634. [Google Scholar] [CrossRef]
- Luo, R.; Chang, Y.; Liang, H.; Zhang, W.; Song, Y.; Li, G.; Yang, C. Interactions between Extracellular Vesicles and Microbiome in Human Diseases: New Therapeutic Opportunities. Imeta 2023, 2, e86. [Google Scholar] [CrossRef] [PubMed]
- Amabebe, E.; Kumar, A.; Tatiparthy, M.; Kammala, A.K.; Taylor, B.D.; Menon, R. Cargo Exchange between Human and Bacterial Extracellular Vesicles in Gestational Tissues: A New Paradigm in Communication and Immune Development. Extracell. Vesicles Circ. Nucl. Acids 2024, 5, 297–328. [Google Scholar] [CrossRef]
- Condinho, M.; Carvalho, B.; Cruz, A.; Pinto, S.N.; Arraiano, C.M.; Pobre, V. The Role of RNA Regulators, Quorum Sensing and c-Di-GMP in Bacterial Biofilm Formation. FEBS Open Bio 2023, 13, 975–991. [Google Scholar] [CrossRef]
- Nagakubo, T.; Nomura, N.; Toyofuku, M. Cracking Open Bacterial Membrane Vesicles. Front. Microbiol. 2019, 10, 3026. [Google Scholar] [CrossRef] [PubMed]
- Srivatsav, A.T.; Kapoor, S. The Emerging World of Membrane Vesicles: Functional Relevance, Theranostic Avenues and Tools for Investigating Membrane Function. Front. Mol. Biosci. 2021, 8, 640355. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xiao, J.; Wang, S.; Zhou, J.; Qin, J.; Jia, Z.; Wang, Y.; Wang, Z.; Zhang, Y.; Hao, H. Research Progress on Bacterial Membrane Vesicles and Antibiotic Resistance. Int. J. Mol. Sci. 2022, 23, 11553. [Google Scholar] [CrossRef] [PubMed]
- Vermassen, A.; Leroy, S.; Talon, R.; Provot, C.; Popowska, M.; Desvaux, M. Cell Wall Hydrolases in Bacteria: Insight on the Diversity of Cell Wall Amidases, Glycosidases and Peptidases Toward Peptidoglycan. Front. Microbiol. 2019, 10, 331. [Google Scholar] [CrossRef]
- Richter, M.; Vader, P.; Fuhrmann, G. Approaches to Surface Engineering of Extracellular Vesicles. Adv. Drug Deliv. Rev. 2021, 173, 416–426. [Google Scholar] [CrossRef]
- Torres, M.; Parets, S.; Fernández-Díaz, J.; Beteta-Göbel, R.; Rodríguez-Lorca, R.; Román, R.; Lladó, V.; Rosselló, C.A.; Fernández-García, P.; Escribá, P.V. Lipids in Pathophysiology and Development of the Membrane Lipid Therapy: New Bioactive Lipids. Membranes 2021, 11, 919. [Google Scholar] [CrossRef]
- Mondal, S.; Baumgart, T. Membrane Reshaping by Protein Condensates. Biochim. Biophys. Acta Biomembr. 2023, 1865, 184121. [Google Scholar] [CrossRef]
- Volgers, C.; Savelkoul, P.H.M.; Stassen, F.R.M. Gram-Negative Bacterial Membrane Vesicle Release in Response to the Host-Environment: Different Threats, Same Trick? Crit. Rev. Microbiol. 2018, 44, 258–273. [Google Scholar] [CrossRef]
- Tran, F.; Gangan, M.S.; Weaver, B.P.; Boedicker, J.Q. Membrane-Binding Biomolecules Influence the Rate of Vesicle Exchange between Bacteria. Appl. Environ. Microbiol. 2022, 88, e0134622. [Google Scholar] [CrossRef]
- Zhao, G.; Jones, M.K. Role of Bacterial Extracellular Vesicles in Manipulating Infection. Infect. Immun. 2023, 91, e0043922. [Google Scholar] [CrossRef]
- Tian, C.-M.; Yang, M.-F.; Xu, H.-M.; Zhu, M.-Z.; Zhang, Y.; Yao, J.; Wang, L.-S.; Liang, Y.-J.; Li, D.-F. Emerging Role of Bacterial Outer Membrane Vesicle in Gastrointestinal Tract. Gut Pathog. 2023, 15, 20. [Google Scholar] [CrossRef] [PubMed]
- György, B.; Szabó, T.G.; Pásztói, M.; Pál, Z.; Misják, P.; Aradi, B.; László, V.; Pállinger, E.; Pap, E.; Kittel, A.; et al. Membrane Vesicles, Current State-of-the-Art: Emerging Role of Extracellular Vesicles. Cell Mol. Life Sci. 2011, 68, 2667–2688. [Google Scholar] [CrossRef] [PubMed]
- Su, W.-C.; Ho, J.C.S.; Gettel, D.L.; Rowland, A.T.; Keating, C.D.; Parikh, A.N. Kinetic Control of Shape Deformations and Membrane Phase Separation inside Giant Vesicles. Nat. Chem. 2024, 16, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Amalia, L.; Tsai, S.-L. Functionalization of OMVs for Biocatalytic Applications. Membranes 2023, 13, 459. [Google Scholar] [CrossRef]
- Walker, E.; van Niekerk, S.; Hanning, K.; Kelton, W.; Hicks, J. Mechanisms of Host Manipulation by Neisseria gonorrhoeae. Front. Microbiol. 2023, 14, 1119834. [Google Scholar] [CrossRef]
- Goh, K.G.K.; Desai, D.; Thapa, R.; Prince, D.; Acharya, D.; Sullivan, M.J.; Ulett, G.C. An Opportunistic Pathogen under Stress: How Group B Streptococcus Responds to Cytotoxic Reactive Species and Conditions of Metal Ion Imbalance to Survive. FEMS Microbiol. Rev. 2024, 48, fuae009. [Google Scholar] [CrossRef]
- Macion, A.; Wyszyńska, A.; Godlewska, R. Delivery of Toxins and Effectors by Bacterial Membrane Vesicles. Toxins 2021, 13, 845. [Google Scholar] [CrossRef]
- Sangiorgio, G.; Nicitra, E.; Bivona, D.; Bonomo, C.; Bonacci, P.; Santagati, M.; Musso, N.; Bongiorno, D.; Stefani, S. Interactions of Gram-Positive Bacterial Membrane Vesicles and Hosts: Updates and Future Directions. Int. J. Mol. Sci. 2024, 25, 2904. [Google Scholar] [CrossRef]
- Kengmo Tchoupa, A.; Peschel, A. Staphylococcus Aureus Releases Proinflammatory Membrane Vesicles To Resist Antimicrobial Fatty Acids. mSphere 2020, 5, e00804-20. [Google Scholar] [CrossRef]
- Kannan, S.; Balakrishnan, J.; Govindasamy, A. Listeria Monocytogens—Amended Understanding of Its Pathogenesis with a Complete Picture of Its Membrane Vesicles, Quorum Sensing, Biofilm and Invasion. Microb. Pathog. 2020, 149, 104575. [Google Scholar] [CrossRef]
- Xuan, S.; Xuan, G. Bacterial Membrane Vesicles: Formation, Functions, and Roles in Bacterial-Phage Interactions. World J. Microbiol. Biotechnol. 2024, 40, 329. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Li, Y.; Liang, X.; Qin, K.; Zhang, Y.; Wang, J.; Wu, Q.; Gupta, T.B.; Ding, Y. Bacterial Extracellular Vesicle: A Non-Negligible Component in Biofilm Life Cycle and Challenges in Biofilm Treatments. Biofilm 2024, 8, 100216. [Google Scholar] [CrossRef] [PubMed]
- Marchant, P.; Vivanco, E.; Silva, A.; Nevermann, J.; Fuentes, I.; Barrera, B.; Otero, C.; Calderón, I.L.; Gil, F.; Fuentes, J.A. β-Lactam-Induced OMV Release Promotes Polymyxin Tolerance in Salmonella enterica Sv. Typhi. Front. Microbiol. 2024, 15, 1389663. [Google Scholar] [CrossRef] [PubMed]
- Peregrino, E.S.; Castañeda-Casimiro, J.; Vázquez-Flores, L.; Estrada-Parra, S.; Wong-Baeza, C.; Serafín-López, J.; Wong-Baeza, I. The Role of Bacterial Extracellular Vesicles in the Immune Response to Pathogens, and Therapeutic Opportunities. Int. J. Mol. Sci. 2024, 25, 6210. [Google Scholar] [CrossRef]
- Moura de Sousa, J.; Lourenço, M.; Gordo, I. Horizontal Gene Transfer among Host-Associated Microbes. Cell Host Microbe 2023, 31, 513–527. [Google Scholar] [CrossRef]
- Emamalipour, M.; Seidi, K.; Zununi Vahed, S.; Jahanban-Esfahlan, A.; Jaymand, M.; Majdi, H.; Amoozgar, Z.; Chitkushev, L.T.; Javaheri, T.; Jahanban-Esfahlan, R.; et al. Horizontal Gene Transfer: From Evolutionary Flexibility to Disease Progression. Front. Cell Dev. Biol. 2020, 8, 229. [Google Scholar] [CrossRef]
- Kadurugamuwa, J.L.; Beveridge, T.J. Virulence Factors Are Released from Pseudomonas aeruginosa in Association with Membrane Vesicles during Normal Growth and Exposure to Gentamicin: A Novel Mechanism of Enzyme Secretion. J. Bacteriol. 1995, 177, 3998–4008. [Google Scholar] [CrossRef]
- Maredia, R.; Devineni, N.; Lentz, P.; Dallo, S.F.; Yu, J.; Guentzel, N.; Chambers, J.; Arulanandam, B.; Haskins, W.E.; Weitao, T. Vesiculation from Pseudomonas aeruginosa under SOS. Sci. World J. 2012, 2012, 402919. [Google Scholar] [CrossRef]
- Siqueira, V.L.D.; Cardoso, R.F.; Caleffi-Ferracioli, K.R.; de Lima Scodro, R.B.; Fernandez, M.A.; Fiorini, A.; Ueda-Nakamura, T.; Dias-Filho, B.P.; Nakamura, C.V. Structural Changes and Differentially Expressed Genes in Pseudomonas aeruginosa Exposed to Meropenem-Ciprofloxacin Combination. Antimicrob. Agents Chemother. 2014, 58, 3957–3967. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, Y.; Jiang, L.; Zhang, C.; Qian, X.; Gu, J.; Song, Z. Bacterial Outer Membrane Vesicles Increase Polymyxin Resistance in Pseudomonas aeruginosa While Inhibiting Its Quorum Sensing. J. Hazard. Mater. 2024, 478, 135588. [Google Scholar] [CrossRef]
- Chan, K.W.; Shone, C.; Hesp, J.R. Antibiotics and Iron-Limiting Conditions and Their Effect on the Production and Composition of Outer Membrane Vesicles Secreted from Clinical Isolates of Extraintestinal Pathogenic E coli. Proteomics Clin. Appl. 2017, 11. [Google Scholar] [CrossRef] [PubMed]
- Bauwens, A.; Kunsmann, L.; Karch, H.; Mellmann, A.; Bielaszewska, M. Antibiotic-Mediated Modulations of Outer Membrane Vesicles in Enterohemorrhagic Escherichia coli O104:H4 and O157:H7. Antimicrob. Agents Chemother. 2017, 61, e00937-17. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Li, J.; He, T.; Ji, X.; Wei, R.; Yu, M.; Wang, R. Sub-MIC Antibiotics Modulate Productions of Outer Membrane Vesicles in Tigecycline-Resistant Escherichia coli. Antibiotics 2024, 13, 276. [Google Scholar] [CrossRef]
- Yun, S.H.; Park, E.C.; Lee, S.-Y.; Lee, H.; Choi, C.-W.; Yi, Y.-S.; Ro, H.-J.; Lee, J.C.; Jun, S.; Kim, H.-Y.; et al. Antibiotic Treatment Modulates Protein Components of Cytotoxic Outer Membrane Vesicles of Multidrug-Resistant Clinical Strain, Acinetobacter baumannii DU202. Clin. Proteom. 2018, 15, 28. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Iida, K.; Takade, A.; Meno, Y.; Nair, G.B.; Yoshida, S. Release of Shiga Toxin by Membrane Vesicles in Shigella Dysenteriae Serotype 1 Strains and in Vitro Effects of Antimicrobials on Toxin Production and Release. Microbiol. Immunol. 2004, 48, 965–969. [Google Scholar] [CrossRef]
- Lucena, A.C.R.; Ferrarini, M.G.; de Oliveira, W.K.; Marcon, B.H.; Morello, L.G.; Alves, L.R.; Faoro, H. Modulation of Klebsiella pneumoniae Outer Membrane Vesicle Protein Cargo under Antibiotic Treatment. Biomedicines 2023, 11, 1515. [Google Scholar] [CrossRef]
- Murray, B.O.; Dawson, R.A.; Alsharaf, L.M.; Anne Winter, J. Protective Effects of Helicobacter pylori Membrane Vesicles against Stress and Antimicrobial Agents. Microbiology 2020, 166, 751–758. [Google Scholar] [CrossRef]
- Devos, S.; Van Oudenhove, L.; Stremersch, S.; Van Putte, W.; De Rycke, R.; Van Driessche, G.; Vitse, J.; Raemdonck, K.; Devreese, B. The Effect of Imipenem and Diffusible Signaling Factors on the Secretion of Outer Membrane Vesicles and Associated Ax21 Proteins in Stenotrophomonas maltophilia. Front. Microbiol. 2015, 6, 298. [Google Scholar] [CrossRef]
- Fang, Y.; Yang, G.; Wu, X.; Qin, B.; Xie, Y.; Zhuang, L. Sub-MIC Antibiotics Affect Microbial Ferrihydrite Reduction by Extracellular Membrane Vesicles. J. Hazard. Mater. 2023, 458, 131876. [Google Scholar] [CrossRef]
- Kim, M.H.; Kim, S.Y.; Son, J.H.; Kim, S.I.; Lee, H.; Kim, S.; Shin, M.; Lee, J.C. Production of Membrane Vesicles by Enterococcus Faecium Cultured With or Without Subinhibitory Concentrations of Antibiotics and Their Pathological Effects on Epithelial Cells. Front. Cell Infect. Microbiol. 2019, 9, 295. [Google Scholar] [CrossRef]
- Andreoni, F.; Toyofuku, M.; Menzi, C.; Kalawong, R.; Mairpady Shambat, S.; François, P.; Zinkernagel, A.S.; Eberl, L. Antibiotics Stimulate Formation of Vesicles in Staphylococcus Aureus in Both Phage-Dependent and -Independent Fashions and via Different Routes. Antimicrob. Agents Chemother. 2019, 63, e01439-18. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W.; Seo, J.-S.; Park, S.B.; Lee, A.R.; Lee, J.S.; Jung, J.W.; Chun, J.H.; Lazarte, J.M.S.; Kim, J.; Kim, J.-H.; et al. Significant Increase in the Secretion of Extracellular Vesicles and Antibiotics Resistance from Methicillin-Resistant Staphylococcus Aureus Induced by Ampicillin Stress. Sci. Rep. 2020, 10, 21066. [Google Scholar] [CrossRef]
- Shapiro, J.T.; Zorea, A.; Kav, A.B.; Ontiveros, V.J.; Mizrahi, I.; Pilosof, S. Multilayer Networks of Plasmid Genetic Similarity Reveal Potential Pathways of Gene Transmission. ISME J. 2023, 17, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Vrancianu, C.O.; Gheorghe, I.; Dobre, E.-G.; Barbu, I.C.; Cristian, R.E.; Popa, M.; Lee, S.H.; Limban, C.; Vlad, I.M.; Chifiriuc, M.C. Emerging Strategies to Combat β-Lactamase Producing ESKAPE Pathogens. Int. J. Mol. Sci. 2020, 21, 8527. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Wei, B.; Wang, Y.; Xu, B.; Yang, M.; Chen, X.; Chen, F. A Critical Role of Outer Membrane Vesicles in Antibiotic Resistance in Carbapenem-Resistant Klebsiella pneumoniae. Ann. Clin. Microbiol. Antimicrob. 2023, 22, 95. [Google Scholar] [CrossRef]
- Zhang, X.; Qian, C.; Tang, M.; Zeng, W.; Kong, J.; Fu, C.; Xu, C.; Ye, J.; Zhou, T. Carbapenemase-Loaded Outer Membrane Vesicles Protect Pseudomonas aeruginosa by Degrading Imipenem and Promoting Mutation of Antimicrobial Resistance Gene. Drug Resist. Updat. 2023, 68, 100952. [Google Scholar] [CrossRef]
- Martínez, M.M.B.; Bonomo, R.A.; Vila, A.J.; Maffía, P.C.; González, L.J. On the Offensive: The Role of Outer Membrane Vesicles in the Successful Dissemination of New Delhi Metallo-β-Lactamase (NDM-1). mBio 2021, 12, e0183621. [Google Scholar] [CrossRef]
- Toppi, V.; Scattini, G.; Musa, L.; Stefanetti, V.; Pascucci, L.; Chiaradia, E.; Tognoloni, A.; Giovagnoli, S.; Franciosini, M.P.; Branciari, R.; et al. Evaluation of β-Lactamase Enzyme Activity in Outer Membrane Vesicles (OMVs) Isolated from Extended Spectrum β-Lactamase (ESBL) Salmonella Infantis Strains. Antibiotics 2023, 12, 744. [Google Scholar] [CrossRef]
- Dhital, S.; Deo, P.; Bharathwaj, M.; Horan, K.; Nickson, J.; Azad, M.; Stuart, I.; Chow, S.H.; Gunasinghe, S.D.; Bamert, R.; et al. Neisseria Gonorrhoeae-Derived Outer Membrane Vesicles Package β-Lactamases to Promote Antibiotic Resistance. Microlife 2022, 3, uqac013. [Google Scholar] [CrossRef]
- Capodimonte, L.; Meireles, F.T.P.; Bahr, G.; Bonomo, R.A.; Dal Peraro, M.; López, C.; Vila, A.J. OXA β-Lactamases from Acinetobacter Spp. Are Membrane Bound and Secreted into Outer Membrane Vesicles. mBio 2025, 16, e0334324. [Google Scholar] [CrossRef]
- Xu, J.; Mei, C.; Zhi, Y.; Liang, Z.-X.; Zhang, X.; Wang, H.-J. Comparative Genomics Analysis and Outer Membrane Vesicle-Mediated Horizontal Antibiotic-Resistance Gene Transfer in Avibacterium paragallinarum. Microbiol. Spectr. 2022, 10, e0137922. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Li, Z.; Liu, X.; Li, Z.; Lei, Z.; Zhao, J.; Zhang, Y.; Wu, Y.; Yang, X.; Lu, B. In-Host Intra- and Inter-Species Transfer of blaKPC-2 and blaNDM-1 in Serratia marcescens and Its Local and Global Epidemiology. Int. J. Antimicrob. Agents 2024, 64, 107327. [Google Scholar] [CrossRef]
- Vahhabi, A.; Hasani, A.; Rezaee, M.A.; Baradaran, B.; Hasani, A.; Samadi Kafil, H.; Abbaszadeh, F.; Dehghani, L. A Plethora of Carbapenem Resistance in Acinetobacter baumannii: No End to a Long Insidious Genetic Journey. J. Chemother. 2021, 33, 137–155. [Google Scholar] [CrossRef]
- Kesavan, D.; Vasudevan, A.; Wu, L.; Chen, J.; Su, Z.; Wang, S.; Xu, H. Integrative Analysis of Outer Membrane Vesicles Proteomics and Whole-Cell Transcriptome Analysis of Eravacycline Induced Acinetobacter baumannii Strains. BMC Microbiol. 2020, 20, 31. [Google Scholar] [CrossRef] [PubMed]
- Balhuizen, M.D.; van Dijk, A.; Jansen, J.W.A.; van de Lest, C.H.A.; Veldhuizen, E.J.A.; Haagsman, H.P. Outer Membrane Vesicles Protect Gram-Negative Bacteria against Host Defense Peptides. mSphere 2021, 6, e0052321. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, Y.; Pei, F.; Liu, Y.; Zheng, Y. Loss of OprD Function Is Sufficient for Carbapenem-Resistance-Only but Insufficient for Multidrug Resistance in Pseudomonas aeruginosa. BMC Microbiol. 2025, 25, 218. [Google Scholar] [CrossRef]
- Juliano, S.A.; Serafim, L.F.; Duay, S.S.; Heredia Chavez, M.; Sharma, G.; Rooney, M.; Comert, F.; Pierce, S.; Radulescu, A.; Cotten, M.L.; et al. A Potent Host Defense Peptide Triggers DNA Damage and Is Active against Multidrug-Resistant Gram-Negative Pathogens. ACS Infect. Dis. 2020, 6, 1250–1263. [Google Scholar] [CrossRef]
- Li, Q.; Ou, Z.; Lin, J.; Tang, D.; He, B.; Wu, Y.; Huang, X.; Huang, X.; Ru, B.; Wang, Q.; et al. Specific Labeling of Outer Membrane Vesicles with Antibiotic-Conjugated Probe Reveals Early Bacterial Infections in Blood. Nat Commun 2025, 16, 3535. [Google Scholar] [CrossRef] [PubMed]
- Costa, W.D.; Rocha, J.E.; de Oliveira, V.M.; de Lima, L.F.; de Freitas, T.S.; de Souza, M.A.; Silva Pereira, R.L.; Marinho, M.M.; Maria Lima Dias, J.; Guedes, J.M.; et al. Evaluation of the Intrinsic Antibacterial and Antibiotic Potentiating Activity against Antibiotic Resistance in Staphylococcus Aureus and Inhibition of the NorA and MepA Efflux Pumps by a Hydrazone Derivative of Isoniazid. Microb. Pathog. 2025, 204, 107588. [Google Scholar] [CrossRef]
- Sakalauskienė, G.V.; Radzevičienė, A. Antimicrobial Resistance: What Lies Beneath This Complex Phenomenon? Diagnostics 2024, 14, 2319. [Google Scholar] [CrossRef]
- Lee, C.-R.; Lee, J.H.; Park, M.; Park, K.S.; Bae, I.K.; Kim, Y.B.; Cha, C.-J.; Jeong, B.C.; Lee, S.H. Biology of Acinetobacter baumannii: Pathogenesis, Antibiotic Resistance Mechanisms, and Prospective Treatment Options. Front. Cell Infect. Microbiol. 2017, 7, 55. [Google Scholar] [CrossRef] [PubMed]
- Hussein, M.; Jasim, R.; Gocol, H.; Baker, M.; Thombare, V.J.; Ziogas, J.; Purohit, A.; Rao, G.G.; Li, J.; Velkov, T. Comparative Proteomics of Outer Membrane Vesicles from Polymyxin-Susceptible and Extremely Drug-Resistant Klebsiella pneumoniae. mSphere 2023, 8, e0053722. [Google Scholar] [CrossRef] [PubMed]
- Zou, C.; Zhang, Y.; Liu, H.; Wu, Y.; Zhou, X. Extracellular Vesicles: Recent Insights Into the Interaction Between Host and Pathogenic Bacteria. Front. Immunol. 2022, 13, 840550. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Lai, Y.; Xiao, W.; Zhong, T.; Liu, F.; Gong, J.; Huang, J. Microbial Extracellular Vesicles Contribute to Antimicrobial Resistance. PLoS Pathog. 2024, 20, e1012143. [Google Scholar] [CrossRef]
- Barathan, M.; Ng, S.-L.; Lokanathan, Y.; Ng, M.H.; Law, J.X. Unseen Weapons: Bacterial Extracellular Vesicles and the Spread of Antibiotic Resistance in Aquatic Environments. Int. J. Mol. Sci. 2024, 25, 3080. [Google Scholar] [CrossRef]
- Martora, F.; Pinto, F.; Folliero, V.; Cammarota, M.; Dell’Annunziata, F.; Squillaci, G.; Galdiero, M.; Morana, A.; Schiraldi, C.; Giovane, A.; et al. Isolation, Characterization and Analysis of pro-Inflammatory Potential of Klebsiella pneumoniae Outer Membrane Vesicles. Microb. Pathog. 2019, 136, 103719. [Google Scholar] [CrossRef]
- Nguyen, M.-T.; Matsuo, M.; Niemann, S.; Herrmann, M.; Götz, F. Lipoproteins in Gram-Positive Bacteria: Abundance, Function, Fitness. Front. Microbiol. 2020, 11, 582582. [Google Scholar] [CrossRef]
- Rajput, P.; Nahar, K.S.; Rahman, K.M. Evaluation of Antibiotic Resistance Mechanisms in Gram-Positive Bacteria. Antibiotics 2024, 13, 1197. [Google Scholar] [CrossRef]
- Chen, J.; Lin, J.-J.; Wang, W.; Huang, H.; Pan, Z.; Ye, G.; Dong, S.; Lin, Y.; Lin, C.; Huang, Q. EV-COMM: A Database of Interspecies and Intercellular Interactions Mediated by Extracellular Vesicles. J. Extracell. Vesicles 2024, 13, e12442. [Google Scholar] [CrossRef]
- Yamin, D.; Uskoković, V.; Wakil, A.M.; Goni, M.D.; Shamsuddin, S.H.; Mustafa, F.H.; Alfouzan, W.A.; Alissa, M.; Alshengeti, A.; Almaghrabi, R.H.; et al. Current and Future Technologies for the Detection of Antibiotic-Resistant Bacteria. Diagnostics 2023, 13, 3246. [Google Scholar] [CrossRef]
- Qu, S.; Zhang, Y.; Weng, L.; Shan, X.; Cheng, P.; Li, Q.; Li, L. The Role of Bacterial Extracellular Vesicles in Promoting Antibiotic Resistance. Crit. Rev. Microbiol. 2024, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Rädler, J.; Gupta, D.; Zickler, A.; Andaloussi, S.E. Exploiting the Biogenesis of Extracellular Vesicles for Bioengineering and Therapeutic Cargo Loading. Mol. Ther. 2023, 31, 1231–1250. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Sim, W.K.; Shen, T.-L.; Lim, S.K. Engineered EVs with Pathogen Proteins: Promising Vaccine Alternatives to LNP-mRNA Vaccines. J. Biomed. Sci. 2024, 31, 9. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.I.; Park, J.; Zhu, Y.; Wang, X.; Han, Y.; Zhang, D. Recent Advances in Extracellular Vesicles for Therapeutic Cargo Delivery. Exp. Mol. Med. 2024, 56, 836–849. [Google Scholar] [CrossRef]
| Bacteria | Antibiotic | Evidence | Reference |
|---|---|---|---|
| P. aeruginosa | Gentamicin | Gentamicin significantly increased the production of OMVs in P. aeruginosa by destabilizing the OM, enriching it with proteins, enzymes, and DNA, suggesting a role for OMVs in the transport of virulence factors during infection. | [147] |
| P. aeruginosa | Ciprofloxacin | Ciprofloxacin induces a significant increase in OMV production in P. aeruginosa through the activation of the SOS response, regulated by LexA, enriching the vesicles with proteins involved in antibiotic resistance and virulence, increasing the cytotoxicity and pathogenicity of the bacterium. | [148] |
| P. aeruginosa | Meropenem Ciprofloxacin | Combination therapy with meropenem and ciprofloxacin in MDR P. aeruginosa significantly reduces OMV production, attenuates bacterial virulence, and modulates the expression of genes associated with resistance and cellular repair, suggesting a potential benefit in the combined use of these antibiotics. | [149] |
| P. aeruginosa | Polymyxin B | P. aeruginosa OMVs regulate intercellular communication by influencing QS and biofilm formation, modulating polymyxin B resistance. | [150] |
| E. coli | Gentamicin Amoxicillin/ clavulanate | Exposure to gentamicin and amoxicillin/clavulanate modulates the production and protein composition of OMVs secreted by extraintestinal pathogenic E. coli strains. | [151] |
| E. coli | Ciprofloxacin Mitomycin C Fosfomycin Meropenem Polymixin B Rifaximin Tigecycline Azithromycin | Ciprofloxacin and mitomycin C increased OMVs in E. coli EHEC, with an increase in Stx2a toxin and cytotoxicity, whereas fosfomycin, meropenem, and polymyxin B stimulated only OMVs production. Rifaximin, tigecycline, and azithromycin reduced Stx2a and cytotoxicity. | [152] |
| E. coli | Ciprofloxacin Mitomycin C Ceftazidime Tigecycline Meropenem Gentamicin Rifaximin | Ciprofloxacin, mitomycin C, ceftazidime, tigecycline, meropenem, gentamicin, and rifaximin stimulated the production of OMVs in tigecycline-resistant E. coli, modifying their size and composition and promoting the spread of the tet (X4) resistance gene. | [153] |
| A. baumanni | Imipenem | Imipenem treatment reduced phage proteins but increased β-lactamase OXA-23, enriching A. baumanni OMVs with proteases and membrane proteins (OmpA, OmpW) associated with virulence, biofilm, and antibiotic resistance. | [154] |
| S. dysenteriae | Mitomycin C Ciprofloxacin, Norfloxacin Fosfomycin Nalidixic acid | Mitomycin C increased Shiga toxin production in OMVs in S. dysenteriae type 1, whereas ciprofloxacin, norfloxacin, fosfomycin, and nalidixic acid showed no significant effects. OMVs treated with mitomycin C were more numerous, larger (20–150 nm), and highly dense. | [155] |
| K. pneumoniae | Meropenem Polymyxin B | In K. pneumoniae KpHCD1, meropenem and polymyxin B increased OMV production, while amikacin decreased it. OMVs contained resistance genes and virulence factors, with protein expression modulated by antibiotics. | [156] |
| H. pylori | Clarithromycin Amoxicillin Metronidazole. | OMVs protected H. pylori bacteria from antimicrobial peptides and some antibiotics. OMVs sequestered clarithromycin, reducing its efficacy, but did not protect against amoxicillin or metronidazole. | [157] |
| S. maltophilia | Imipenem | Imipenem and QS molecules (DSF) increased OMVs production in S. maltophilia, enriching them with β-lactamases L1 and L2 to counteract antibiotic stress. | [158] |
| G. sulfurreducens | Ampicillin Ciprofloxacin | Ampicillin and ciprofloxacin increased the production of OMVs in G. sulfurreducens, altering their morphology and functionality. Ciprofloxacin induced OMVs through explosive cell lysis and phage activation, while ampicillin stimulated the formation of classical OMVs. | [159] |
| Bacteria | Antibiotic | Evidence | Reference |
|---|---|---|---|
| E. faecium | Vancomycin Linezolid | Vancomycin and linezolid increased MV production in E. faecium and altered their protein composition. MVs produced with linezolid showed greater cytotoxicity, while those produced with vancomycin induced a stronger inflammatory response. | [160] |
| S. aureus | Mitomycin C Ciprofloxacin Flucloxacillin Ceftaroline Daptomycin | Mitomycin C and ciprofloxacin stimulated MV production in S. aureus through a phage-dependent SOS response mechanism, whereas flucloxacillin and ceftaroline acted independently of phages, damaging the cell wall. The produced MVs protected from daptomycin treatment. | [161] |
| S. aureus | Ampicillin | Ampicillin increased the production of MVs derived from S. aureus MRSA, enriching them with β-lactamases and resistance-related proteins, allowing the degradation of the antibiotic and protecting S. aureus without transmitting genetic resistance. | [162] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Federica, D.; Cosimato, I.; Salzano, F.; Mensitieri, F.; Andretta, V.; Santoro, E.; Boccia, G.; Folliero, V.; Franci, G. Adaptations of Bacterial Extracellular Vesicles in Response to Antibiotic Pressure. Int. J. Mol. Sci. 2025, 26, 5025. https://doi.org/10.3390/ijms26115025
Federica D, Cosimato I, Salzano F, Mensitieri F, Andretta V, Santoro E, Boccia G, Folliero V, Franci G. Adaptations of Bacterial Extracellular Vesicles in Response to Antibiotic Pressure. International Journal of Molecular Sciences. 2025; 26(11):5025. https://doi.org/10.3390/ijms26115025
Chicago/Turabian StyleFederica, Dell’Annunziata, Ilaria Cosimato, Flora Salzano, Francesca Mensitieri, Vincenzo Andretta, Emanuela Santoro, Giovanni Boccia, Veronica Folliero, and Gianluigi Franci. 2025. "Adaptations of Bacterial Extracellular Vesicles in Response to Antibiotic Pressure" International Journal of Molecular Sciences 26, no. 11: 5025. https://doi.org/10.3390/ijms26115025
APA StyleFederica, D., Cosimato, I., Salzano, F., Mensitieri, F., Andretta, V., Santoro, E., Boccia, G., Folliero, V., & Franci, G. (2025). Adaptations of Bacterial Extracellular Vesicles in Response to Antibiotic Pressure. International Journal of Molecular Sciences, 26(11), 5025. https://doi.org/10.3390/ijms26115025







