Macrophage-Derived Factors with the Potential to Contribute to the Pathogenicity of HIV-1 and HIV-2: Roles of M-CSF and CXCL7
Abstract
1. Introduction
2. Results
2.1. HIV-2 Isolates Use Various Co-Receptors for Infection
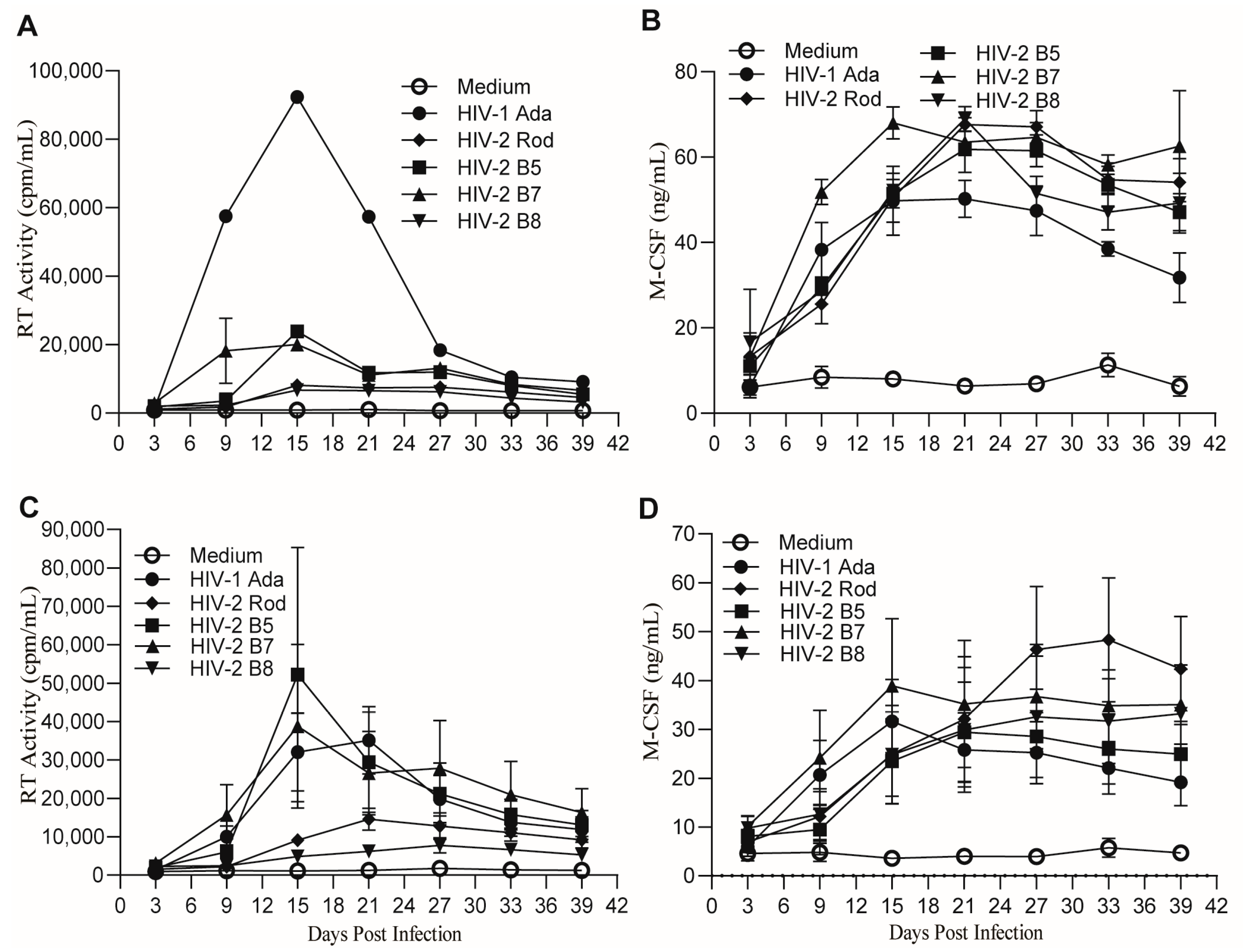
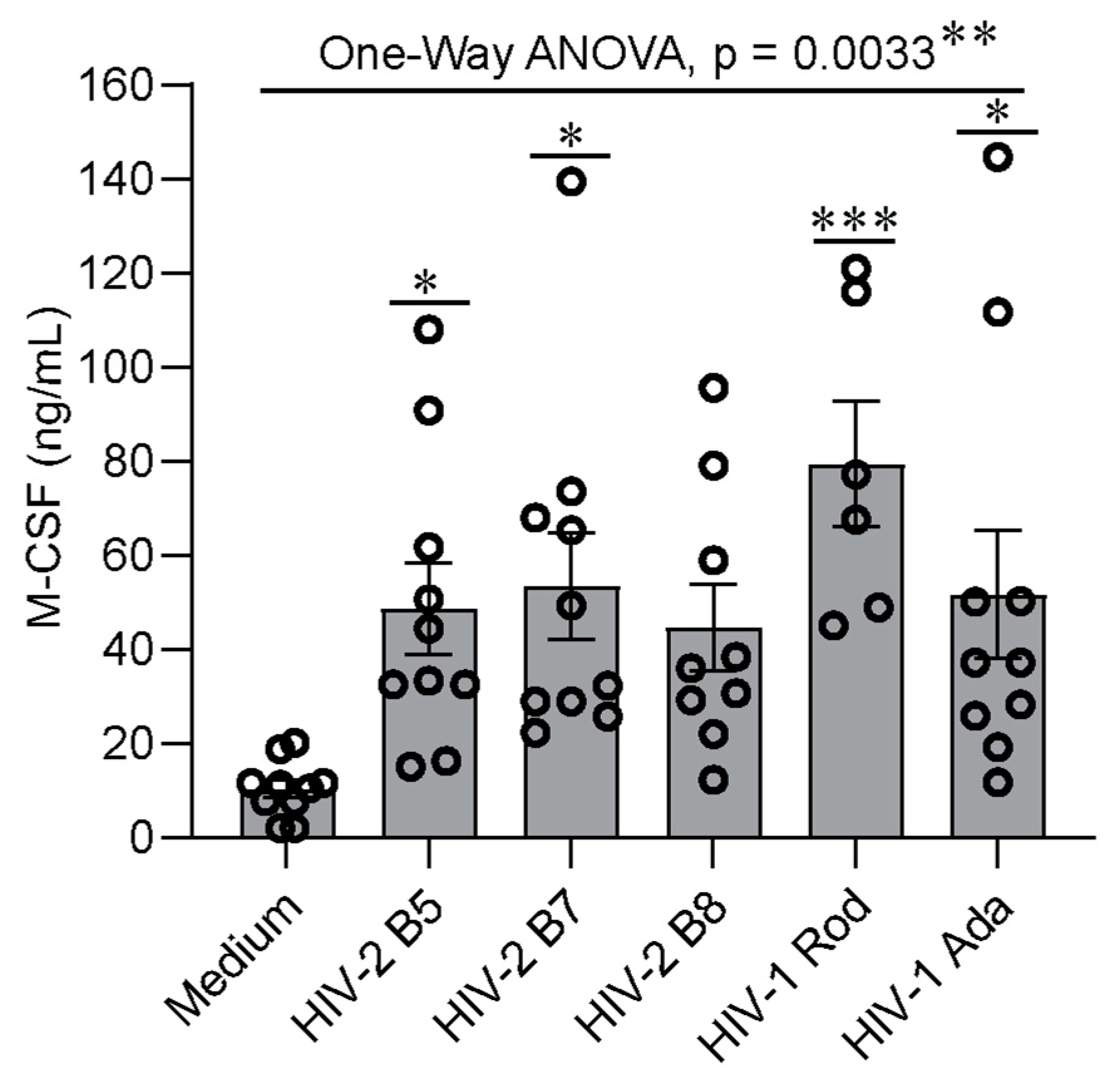
2.2. MDMs Infected with HIV-1 and HIV-2 Isolates Produce Similar Levels of M-CSF
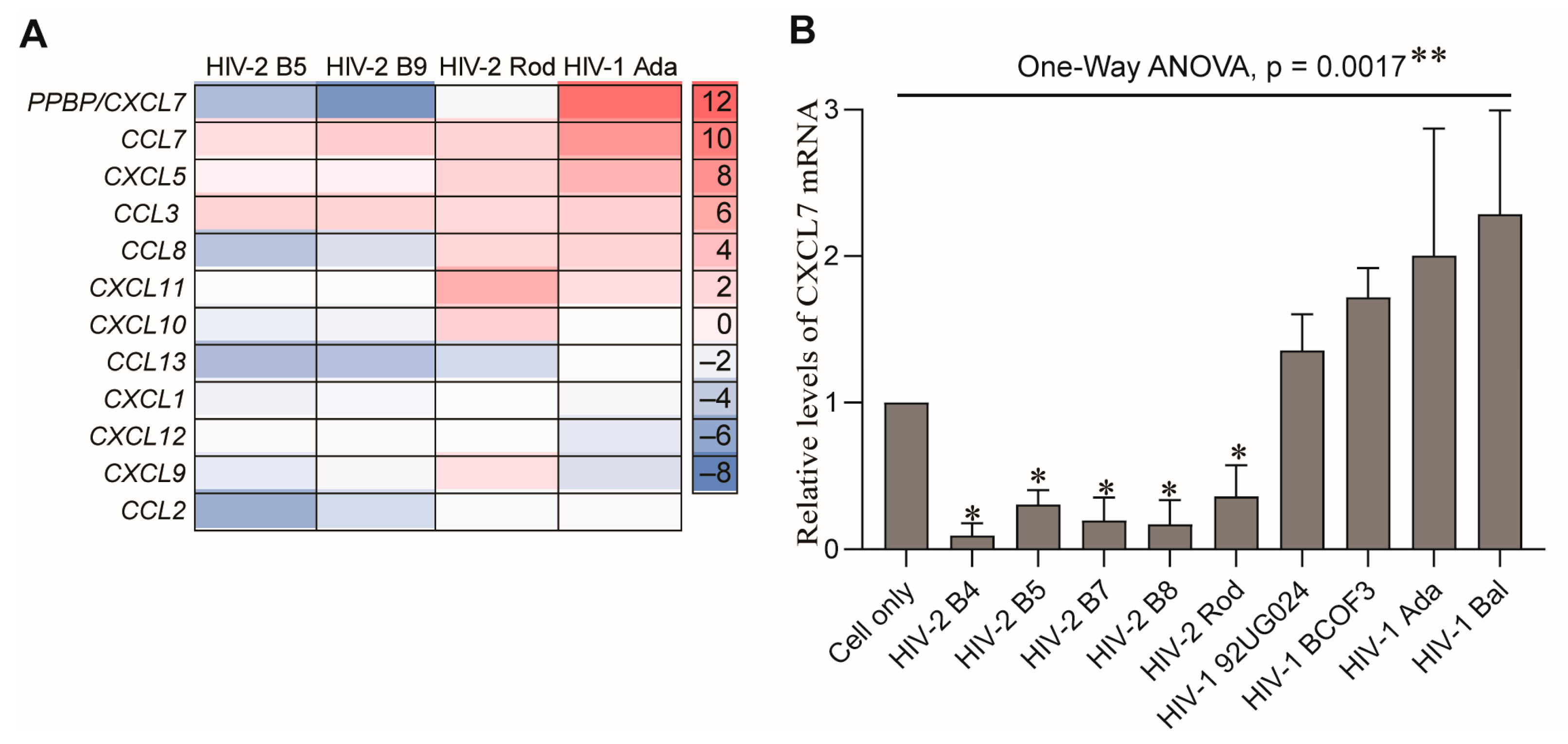
2.3. MDMs Infected with HIV-1 and HIV-2 Isolates Produce Different Levels of CXCL7
3. Discussion
4. Materials and Methods
4.1. Monocyte Isolation and Culture
4.2. HIV Infection of Human Monocyte-Derived Macrophages
4.3. GHOST Cell Assay for Determination of HIV Co-Receptor Usage
4.4. Reverse Transcriptase Assay
4.5. M-CSF Bioassay
4.6. CXCL7 ELISA
4.7. Quantitative PCR
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kedzierska, K.; Crowe, S.M. The role of monocytes and macrophages in the pathogenesis of HIV-1 infection. Curr. Med. Chem. 2002, 9, 1893–1903. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.S.; Hellmann, N.; Levy, J.A.; DeCock, K.; Lange, J. The spread, treatment, and prevention of HIV-1: Evolution of a global pandemic. J. Clin. Investig. 2008, 118, 1244–1254. [Google Scholar] [CrossRef] [PubMed]
- Reeves, J.D.; Doms, R.W. Human immunodeficiency virus type 2. J. Gen. Virol. 2002, 83 Pt 6, 1253–1265. [Google Scholar] [CrossRef] [PubMed]
- de Silva, T.I.; Cotten, M.; Rowland-Jones, S.L. HIV-2: The forgotten AIDS virus. Trends Microbiol. 2008, 16, 588–595. [Google Scholar] [CrossRef]
- Campbell-Yesufu, O.T.; Gandhi, R.T. Update on human immunodeficiency virus (HIV)-2 infection. Clin. Infect. Dis. 2011, 52, 780–787. [Google Scholar] [CrossRef]
- Acchioni, C.; Sandini, S.; Acchioni, M.; Sgarbanti, M. Co-Infections and Superinfections between HIV-1 and Other Human Viruses at the Cellular Level. Pathogens 2024, 13, 349. [Google Scholar] [CrossRef]
- Williams, A.; Menon, S.; Crowe, M.; Agarwal, N.; Biccler, J.; Bbosa, N.; Ssemwanga, D.; Adungo, F.; Moecklinghoff, C.; Macartney, M.; et al. Geographic and Population Distributions of Human Immunodeficiency Virus (HIV)-1 and HIV-2 Circulating Subtypes: A Systematic Literature Review and Meta-analysis (2010–2021). J. Infect. Dis. 2023, 228, 1583–1591. [Google Scholar] [CrossRef]
- Esbjörnsson, J.; Jansson, M.; Jespersen, S.; Månsson, F.; Hønge, B.L.; Lindman, J.; Medina, C.; da Silva, Z.J.; Norrgren, H.; Medstrand, P.; et al. HIV-2 as a model to identify a functional HIV cure. AIDS Res. Ther. 2019, 16, 24. [Google Scholar] [CrossRef]
- Pandrea, I.; Sodora, D.L.; Silvestri, G.; Apetrei, C. Into the wild: Simian immunodeficiency virus (SIV) infection in natural hosts. Trends Immunol. 2008, 29, 419–428. [Google Scholar] [CrossRef]
- Sauter, D.; Kirchhoff, F. Key Viral Adaptations Preceding the AIDS Pandemic. Cell Host Microbe 2019, 25, 27–38. [Google Scholar] [CrossRef]
- Leligdowicz, A.; Rowland-Jones, S. Tenets of protection from progression to AIDS: Lessons from the immune responses to HIV-2 infection. Expert Rev. Vaccines 2008, 7, 319–331. [Google Scholar] [CrossRef] [PubMed]
- Esbjörnsson, J.; Månsson, F.; Kvist, A.; Isberg, P.E.; Biague, A.J.; da Silva, Z.J.; Jansson, M.; Fenyö, E.M.; Norrgren, H.; Medstrand, P. Increased survival among HIV-1 and HIV-2 dual-infected individuals compared to HIV-1 single-infected individuals. AIDS 2014, 28, 949–957. [Google Scholar] [CrossRef] [PubMed]
- Esbjörnsson, J.; Månsson, F.; Kvist, A.; Isberg, P.E.; Nowroozalizadeh, S.; Biague, A.J.; da Silva, Z.J.; Jansson, M.; Fenyö, E.M.; Norrgren, H.; et al. Inhibition of HIV-1 disease progression by contemporaneous HIV-2 infection. N. Engl. J. Med. 2012, 367, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Prince, P.D.; Matser, A.; van Tienen, C.; Whittle, H.C.; Schim van der Loeff, M.F. Mortality rates in people dually infected with HIV-1/2 and those infected with either HIV-1 or HIV-2: A systematic review and meta-analysis. AIDS 2014, 28, 549–558. [Google Scholar] [CrossRef]
- Ter Schiphorst, E.; Hansen, K.C.; Holm, M.; Hønge, B.L. Mother-to-child HIV-2 transmission: Comparison with HIV-1 and evaluation of factors influencing the rate of transmission. A systematic review. Trans. R. Soc. Trop. Med. Hyg. 2022, 116, 399–408. [Google Scholar] [CrossRef]
- Visseaux, B.; Le Hingrat, Q.; Damond, F.; Charpentier, C.; Descamps, D. Physiopathology of HIV-2 infection. Virologie 2019, 23, 277–291. [Google Scholar] [CrossRef]
- McMichael, A.J.; Borrow, P.; Tomaras, G.D.; Goonetilleke, N.; Haynes, B.F. The immune response during acute HIV-1 infection: Clues for vaccine development. Nat. Rev. Immunol. 2010, 10, 11–23. [Google Scholar] [CrossRef]
- Azevedo-Pereira, J.M.; Santos-Costa, Q. HIV Interaction With Human Host: HIV-2 As a Model of a Less Virulent Infection. AIDS Rev. 2016, 18, 44–53. [Google Scholar]
- Vidya Vijayan, K.K.; Karthigeyan, K.P.; Tripathi, S.P.; Hanna, L.E. Pathophysiology of CD4+ T-Cell Depletion in HIV-1 and HIV-2 Infections. Front. Immunol. 2017, 8, 580. [Google Scholar] [CrossRef]
- Melchjorsen, J.; Sørensen, L.N.; Paludan, S.R. Expression and function of chemokines during viral infections: From molecular mechanisms to in vivo function. J. Leukoc. Biol. 2003, 74, 331–343. [Google Scholar] [CrossRef]
- Berger, E.A.; Murphy, P.M.; Farber, J.M. Chemokine receptors as HIV-1 coreceptors: Roles in viral entry, tropism, and disease. Annu. Rev. Immunol. 1999, 17, 657–700. [Google Scholar] [CrossRef] [PubMed]
- Salazar-Gonzalez, J.F.; Salazar, M.G.; Keele, B.F.; Learn, G.H.; Giorgi, E.E.; Li, H.; Decker, J.M.; Wang, S.; Baalwa, J.; Kraus, M.H.; et al. Genetic identity, biological phenotype, and evolutionary pathways of transmitted/founder viruses in acute and early HIV-1 infection. J. Exp. Med. 2009, 206, 1273–1289. [Google Scholar] [CrossRef]
- Wodarz, D.; Lloyd, A.L.; Jansen, V.A.; Nowak, M.A. Dynamics of macrophage and T cell infection by HIV. J. Theor. Biol. 1999, 196, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Kedzierska, K.; Crowe, S.M.; Turville, S.; Cunningham, A.L. The influence of cytokines, chemokines and their receptors on HIV-1 replication in monocytes and macrophages. Rev. Med. Virol. 2003, 13, 39–56. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Abbas, W.; Herbein, G. HIV-1 latency in monocytes/macrophages. Viruses 2014, 6, 1837–1860. [Google Scholar] [CrossRef]
- Brown, D.; Mattapallil, J.J. Gastrointestinal tract and the mucosal macrophage reservoir in HIV infection. Clin. Vaccine Immunol. 2014, 21, 1469–1473. [Google Scholar] [CrossRef]
- Wallet, C.; De Rovere, M.; Van Assche, J.; Daouad, F.; De Wit, S.; Gautier, V.; Mallon, P.W.G.; Marcello, A.; Van Lint, C.; Rohr, O.; et al. Microglial Cells: The Main HIV-1 Reservoir in the Brain. Front. Cell Infect. Microbiol. 2019, 9, 362. [Google Scholar] [CrossRef]
- Joseph, J.; Daley, W.; Lawrence, D.; Lorenzo, E.; Perrin, P.; Rao, V.R.; Tsai, S.Y.; Varthakavi, V. Role of macrophages in HIV pathogenesis and cure: NIH perspectives. J. Leukoc. Biol. 2022, 112, 1233–1243. [Google Scholar] [CrossRef]
- Tang, Y.; Chaillon, A.; Gianella, S.; Wong, L.M.; Li, D.; Simermeyer, T.L.; Porrachia, M.; Ignacio, C.; Woodworth, B.; Zhong, D.; et al. Brain microglia serve as a persistent HIV reservoir despite durable antiretroviral therapy. J. Clin. Investig. 2023, 133, e167417. [Google Scholar] [CrossRef]
- Siliciano, R.F. Latency and reservoirs for HIV-1. AIDS 1999, 13, S49–S58. [Google Scholar]
- Cohen, D.E.; Walker, B.D. Human immunodeficiency virus pathogenesis and prospects for immune control in patients with established infection. Clin. Infect. Dis. 2001, 32, 1756–1768. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Balestra, E.; Perno, C.F.; Aquaro, S.; Panti, S.; Bertoli, A.; Piacentini, M.; Forbici, F.; D’Arrigo, R.; Calio, R.; Garaci, E. Macrophages: A crucial reservoir for human immunodeficiency virus in the body. J. Biol. Regul. Homeost. Agents 2001, 15, 272–276. [Google Scholar] [PubMed]
- Tan, J.; Sattentau, Q.J. The HIV-1-containing macrophage compartment: A perfect cellular niche? Trends Microbiol. 2013, 21, 405–412. [Google Scholar] [CrossRef]
- Machado Andrade, V.; Stevenson, M. Host and Viral Factors Influencing Interplay between the Macrophage and HIV-1. J. Neuroimmune Pharmacol. 2019, 14, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Hendricks, C.M.; Cordeiro, T.; Gomes, A.P.; Stevenson, M. The Interplay of HIV-1 and Macrophages in Viral Persistence. Front. Microbiol. 2021, 12, 646447. [Google Scholar] [CrossRef]
- Abbas, W.; Tariq, M.; Iqbal, M.; Kumar, A.; Herbein, G. Eradication of HIV-1 from the macrophage reservoir: An uncertain goal? Viruses 2015, 7, 1578–1598. [Google Scholar] [CrossRef]
- Khan, T.; Mayuresh Patkar, M.; Momin, M.; Omri, A. Macrophage targeted nanocarrier delivery systems in HIV therapeutics. Expert Opin. Drug Deliv. 2020, 17, 903–918. [Google Scholar] [CrossRef]
- Teer, E.; Joseph, D.E.; Glashoff, R.H.; Faadiel Essop, M. Monocyte/Macrophage-Mediated Innate Immunity in HIV-1 Infection: From Early Response to Late Dysregulation and Links to Cardiovascular Diseases Onset. Virol. Sin. 2021, 36, 565–576. [Google Scholar] [CrossRef]
- Herskovitz, J.; Gendelman, H.E. HIV and the Macrophage: From Cell Reservoirs to Drug Delivery to Viral Eradication. J. Neuroimmune Pharmacol. 2019, 14, 52–67. [Google Scholar] [CrossRef]
- Valentin, A.; Albert, J.; Fenyö, E.M.; Asjö, B. Dual tropism for macrophages and lymphocytes is a common feature of primary human immunodeficiency virus type 1 and 2 isolates. J. Virol. 1994, 68, 6684–6689. [Google Scholar] [CrossRef]
- Gao, C.; Ouyang, W.; Kutza, J.; Grimm, T.A.; Fields, K.; Lankford, C.S.R.; Schwartzkopff, F.; Paciga, M.; Stantchev, T.; Tiffany, L.; et al. Macrophage-Derived Factors with the Potential to Contribute to Pathogenicity of HIV-1 and HIV-2: Role of CCL-2/MCP-1. Viruses 2023, 15, 2160. [Google Scholar] [CrossRef]
- Edinger, A.L.; Clements, J.E.; Doms, R.W. Chemokine and orphan receptors in HIV-2 and SIV tropism and pathogenesis. Virology 1999, 260, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Morner, A.; Bjorndal, A.; Leandersson, A.C.; Albert, J.; Bjorling, E.; Jansson, M. CCR5 or CXCR4 is required for efficient infection of peripheral blood mononuclear cells by promiscuous human immunodeficiency virus type 2 primary isolates. AIDS Res. Hum. Retroviruses 2002, 18, 193–200. [Google Scholar] [CrossRef]
- Azevedo-Pereira, J.M.; Santos-Costa, Q.; Moniz-Pereira, J. HIV-2 infection and chemokine receptors usage—Clues to reduced virulence of HIV-2. Curr. HIV Res. 2005, 3, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Stacey, A.R.; Norris, P.J.; Qin, L.; Haygreen, E.A.; Taylor, E.; Heitman, J.; Lebedeva, M.; DeCamp, A.; Li, D.; Grove, D.; et al. Induction of a striking systemic cytokine cascade prior to peak viremia in acute human immunodeficiency virus type 1 infection, in contrast to more modest and delayed responses in acute hepatitis B and C virus infections. J. Virol. 2009, 83, 3719–3733. [Google Scholar] [CrossRef] [PubMed]
- Gruber, M.F.; Weih, K.A.; Boone, E.J.; Smith, P.D.; Clouse, K.A. Endogenous macrophage CSF production is associated with viral replication in HIV-1-infected human monocyte-derived macrophages. J. Immunol. 1995, 154, 5528–5535. [Google Scholar] [CrossRef]
- Gendelman, H.E.; Orenstein, J.M.; Martin, M.A.; Ferrua, C.; Mitra, R.; Phipps, T.; Wahl, L.A.; Lane, H.C.; Fauci, A.S.; Burke, D.S.; et al. Efficient isolation and propagation of human immunodeficiency virus on recombinant colony-stimulating factor 1-treated monocytes. J. Exp. Med. 1988, 167, 1428–1441. [Google Scholar] [CrossRef]
- Kalter, D.C.; Nakamura, M.; Turpin, J.A.; Baca, L.M.; Hoover, D.L.; Dieffenbach, C.; Ralph, P.; Gendelman, H.E.; Meltzer, M.S. Enhanced HIV replication in macrophage colony-stimulating factor-treated monocytes. J. Immunol. 1991, 146, 298–306. [Google Scholar] [CrossRef]
- Bergamini, A.; Perno, C.F.; Dini, L.; Capozzi, M.; Pesce, C.D.; Ventura, L.; Cappannoli, L.; Falasca, L.; Milanese, G.; Caliò, R.; et al. Macrophage colony-stimulating factor enhances the susceptibility of macrophages to infection by human immunodeficiency virus and reduces the activity of compounds that inhibit virus binding. Blood 1994, 84, 3405–3412. [Google Scholar] [CrossRef]
- Hattori, N.; Michaels, F.; Fargnoli, K.; Marcon, L.; Gallo, R.C.; Franchini, G. The human immunodeficiency virus type 2 vpr gene is essential for productive infection of human macrophages. Proc. Natl. Acad. Sci. USA 1990, 87, 8080–8084. [Google Scholar] [CrossRef]
- Marchant, D.; Neil, S.J.; McKnight, A. Human immunodeficiency virus types 1 and 2 have different replication kinetics in human primary macrophage culture. J. Gen. Virol. 2006, 87 Pt 2, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.E.; Jaworowski, A.; Hearps, A.C. The HIV Reservoir in Monocytes and Macrophages. Front. Immunol. 2019, 10, 1435. [Google Scholar] [CrossRef] [PubMed]
- Khanal, S.; Schank, M.; El Gazzar, M.; Moorman, J.P.; Yao, Z.Q. HIV-1 Latency and Viral Reservoirs: Existing Reversal Approaches and Potential Technologies, Targets, and Pathways Involved in HIV Latency Studies. Cells 2021, 10, 475. [Google Scholar] [CrossRef] [PubMed]
- Vellas, C.; Martres, D.; Requena, M.; Nayrac, M.; Collercandy, N.; Latour, J.; Barange, K.; Alric, L.; Martin-Blondel, G.; Izopet, J.; et al. Compartmentalized HIV-1 reservoir in intestinal monocytes/macrophages on antiretroviral therapy. J. Infect. Dis. 2025, 231, 611–621. [Google Scholar] [CrossRef]
- Kutza, J.; Crim, L.; Feldman, S.; Hayes, M.P.; Gruber, M.; Beeler, J.; Clouse, K.A. Macrophage colony-stimulating factor antagonists inhibit replication of HIV-1 in human macrophages. J. Immunol. 2000, 164, 4955–4960. [Google Scholar] [CrossRef]
- Heredia, A.; Vallejo, A.; Soriano, V.; Epstein, J.S.; Hewlett, I.K. Chemokine receptors and HIV-2. AIDS 1997, 11, 1198–1199. [Google Scholar] [CrossRef]
- Deng, H.; Liu, R.; Ellmeier, W.; Choe, S.; Unutmaz, D.; Burkhart, M.; Di Marzio, P.; Marmon, S.; Sutton, R.E.; Hill, C.M.; et al. Identification of a major co-receptor for primary isolates of HIV-1. Nature 1996, 381, 661–666. [Google Scholar] [CrossRef]
- Mörner, A.; Björndal, A.; Albert, J.; Kewalramani, V.N.; Littman, D.R.; Inoue, R.; Thorstensson, R.; Fenyö, E.M.; Björling, E. Primary human immunodeficiency virus type 2 (HIV-2) isolates, like HIV-1 isolates, frequently use CCR5 but show promiscuity in coreceptor usage. J. Virol. 1999, 73, 2343–2349. [Google Scholar] [CrossRef]
- Schenk, B.I.; Petersen, F.; Flad, H.D.; Brandt, E. Platelet-derived chemokines CXC chemokine ligand (CXCL)7, connective tissue-activating peptide III, and CXCL4 differentially affect and cross-regulate neutrophil adhesion and transendothelial migration. J. Immunol. 2002, 169, 2602–2610. [Google Scholar] [CrossRef]
- Wu, Q.; Tu, H.; Li, J. Multifaceted Roles of Chemokine C-X-C Motif Ligand 7 in Inflammatory Diseases and Cancer. Front. Pharmacol. 2022, 13, 914730. [Google Scholar] [CrossRef]
- Haine, V.; Fischer-Smith, T.; Rappaport, J. Macrophage colony-stimulating factor in the pathogenesis of HIV infection: Potential target for therapeutic intervention. J. Neuroimmune Pharmacol. 2006, 1, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Preisser, L.; Miot, C.; Le Guillou-Guillemette, H.; Beaumont, E.; Foucher, E.D.; Garo, E.; Blanchard, S.; Frémaux, I.; Croué, A.; Fouchard, I.; et al. IL-34 and macrophage colony-stimulating factor are overexpressed in hepatitis C virus fibrosis and induce profibrotic macrophages that promote collagen synthesis by hepatic stellate cells. Hepatology 2014, 60, 1879–1890. [Google Scholar] [CrossRef] [PubMed]
- Shim, A.H.; Chang, R.A.; Chen, X.; Longnecker, R.; He, X. Multipronged attenuation of macrophage-colony stimulating factor signaling by Epstein-Barr virus BARF1. Proc. Natl. Acad. Sci. USA 2012, 109, 12962–12967. [Google Scholar] [CrossRef] [PubMed]
- Gilardini Montani, M.S.; Falcinelli, L.; Santarelli, R.; Romeo, M.A.; Granato, M.; Faggioni, A.; Cirone, M. Kaposi Sarcoma Herpes Virus (KSHV) infection inhibits macrophage formation and survival by counteracting Macrophage Colony-Stimulating Factor (M-CSF)-induced increase of Reactive Oxygen Species (ROS), c-Jun N-terminal kinase (JNK) phosphorylation and autophagy. Int. J. Biochem. Cell Biol. 2019, 114, 105560. [Google Scholar]
- Osman, A.; Bhuyan, F.; Hashimoto, M.; Nasser, H.; Maekawa, T.; Suzu, S. M-CSF inhibits anti-HIV-1 activity of IL-32, but they enhance M2-like phenotypes of macrophages. J. Immunol. 2014, 192, 5083–5089. [Google Scholar] [CrossRef]
- Robinson, T.O.; Zhang, M.; Ochsenbauer, C.; Smythies, L.E.; Cron, R.Q. CD4 regulatory T cells augment HIV-1 expression of polarized M1 and M2 monocyte derived macrophages. Virology 2017, 504, 79–87. [Google Scholar] [CrossRef]
- Mangino, G.; Percario, Z.A.; Fiorucci, G.; Vaccari, G.; Acconcia, F.; Chiarabelli, C.; Leone, S.; Noto, A.; Horenkamp, F.A.; Manrique, S.; et al. HIV-1 Nef induces proinflammatory state in macrophages through its acidic cluster domain: Involvement of TNF alpha receptor associated factor 2. PLoS ONE 2011, 6, e22982. [Google Scholar] [CrossRef]
- Rossi, F.W.; Prevete, N.; Rivellese, F.; Lobasso, A.; Napolitano, F.; Granata, F.; Selleri, C.; de Paulis, A. HIV-1 Nef promotes migration and chemokine synthesis of human basophils and mast cells through the interaction with CXCR4. Clin. Mol. Allergy 2016, 14, 15. [Google Scholar] [CrossRef][Green Version]
- Aiello, A.; Giannessi, F.; Percario, Z.A.; Fecchi, K.; Arenaccio, C.; Leone, S.; Carollo, M.; D’Aversa, E.; Chaperot, L.; Gambari, R.; et al. HIV-1 Nef Protein Affects Cytokine and Extracellular Vesicles Production in the GEN2.2 Plasmacytoid Dendritic Cell Line. Viruses 2021, 14, 74. [Google Scholar] [CrossRef]
- Suzu, S.; Harada, H.; Matsumoto, T.; Okada, S. HIV-1 Nef interferes with M-CSF receptor signaling through Hck activation and inhibits M-CSF bioactivities. Blood 2005, 105, 3230–3237. [Google Scholar] [CrossRef]
- Oravecz, T.; Pall, M.; Roderiquez, G.; Gorrell, M.D.; Ditto, M.; Nguyen, N.Y.; Boykins, R.; Unsworth, E.; Norcross, M.A. Regulation of the receptor specificity and function of the chemokine RANTES (regulated on activation, normal T cell expressed and secreted) by dipeptidyl peptidase IV (CD26)-mediated cleavage. J. Exp. Med. 1997, 186, 1865–1872. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Sharron, M.; Montaner, L.J.; Weissman, D.; Doms, R.W. Quantification of CD4, CCR5, and CXCR4 levels on lymphocyte subsets, dendritic cells, and differentially conditioned monocyte-derived macrophages. Proc. Natl. Acad. Sci. USA 1999, 96, 5215–5220. [Google Scholar] [CrossRef]
- Schwartzkopff, F.; Grimm, T.A.; Lankford, C.S.; Fields, K.; Wang, J.; Brandt, E.; Clouse, K.A. Platelet factor 4 (CXCL4) facilitates human macrophage infection with HIV-1 and potentiates virus replication. Innate Immun. 2009, 15, 368–379. [Google Scholar] [CrossRef] [PubMed]
- Amzazi, S.; Ylisastigui, L.; Bakri, Y.; Rabehi, L.; Gattegno, L.; Parmentier, M.; Gluckman, J.C.; Benjouad, A. The inhibitory effect of RANTES on the infection of primary macrophages by R5 human immunodeficiency virus type-1 depends on the macrophage activation state. Virology 1998, 252, 96–105. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Blanco, A.; Coronado, R.A.; Arun, N.; Ma, K.; Dar, R.D.; Kieffer, C. Monocyte to macrophage differentiation and changes in cellular redox homeostasis promote cell type-specific HIV latency reactivation. Proc. Natl. Acad. Sci. USA 2024, 121, e2313823121. [Google Scholar] [CrossRef]
- Sattentau, Q.J.; Stevenson, M. Macrophages and HIV-1: An Unhealthy Constellation. Cell Host Microbe 2016, 19, 304–310. [Google Scholar] [CrossRef]
- Fantuzzi, L.; Canini, I.; Belardelli, F.; Gessani, S. HIV-1 gp120 stimulates the production of beta-chemokines in human peripheral blood monocytes through a CD4-independent mechanism. J. Immunol. 2001, 166, 5381–5387. [Google Scholar] [CrossRef]
- Fantuzzi, L.; Conti, L.; Gauzzi, M.C.; Eid, P.; Del Cornò, M.; Varano, B.; Canini, I.; Belardelli, F.; Gessani, S. Regulation of chemokine/cytokine network during in vitro differentiation and HIV-1 infection of human monocytes: Possible importance in the pathogenesis of AIDS. J. Leukoc. Biol. 2000, 68, 391–399. [Google Scholar] [CrossRef]
- Ansari, A.W.; Bhatnagar, N.; Dittrich-Breiholz, O.; Kracht, M.; Schmidt, R.E.; Heiken, H. Host chemokine (C-C motif) ligand-2 (CCL2) is differentially regulated in HIV type 1 (HIV-1)-infected individuals. Int. Immunol. 2006, 18, 1443–1451. [Google Scholar] [CrossRef]
- Yan, J.; Zhao, Q.; Wang, J.; Tian, X.; Wang, J.; Xia, X.; Ott, M.; Rao, G.; Heimberger, A.B.; Li, S. FGL2-wired macrophages secrete CXCL7 to regulate the stem-like functionality of glioma cells. Cancer Lett. 2021, 506, 83–94. [Google Scholar] [CrossRef]
- Wang, Y.H.; Shen, C.Y.; Lin, S.C.; Kuo, W.H.; Kuo, Y.T.; Hsu, Y.L.; Wang, W.C.; Lin, K.T.; Wang, L.H. Monocytes secrete CXCL7 to promote breast cancer progression. Cell Death Dis. 2021, 12, 1090. [Google Scholar] [CrossRef] [PubMed]
- Smith, N.L.; Bromley, M.J.; Denning, D.W.; Simpson, A.; Bowyer, P. Elevated levels of the neutrophil chemoattractant pro-platelet basic protein in macrophages from individuals with chronic and allergic aspergillosis. J. Infect. Dis. 2015, 211, 651–660. [Google Scholar] [CrossRef] [PubMed]
- Brandt, E.; Petersen, F.; Ludwig, A.; Ehlert, J.E.; Bock, L.; Flad, H.D. The beta-thromboglobulins and platelet factor 4: Blood platelet-derived CXC chemokines with divergent roles in early neutrophil regulation. J. Leukoc. Biol. 2000, 67, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Heusinger, E.; Kirchhoff, F. Primate Lentiviruses Modulate NF-κB Activity by Multiple Mechanisms to Fine-Tune Viral and Cellular Gene Expression. Front. Microbiol. 2017, 8, 198. [Google Scholar] [CrossRef]
- Unver, N.; Esendagli, G.; Yilmaz, G.; Guc, D. CXCL7-induced macrophage infiltration in lung tumor is independent of CXCR2 expression: CXCL7-induced macrophage chemotaxis in LLC tumors. Cytokine 2015, 75, 330–337. [Google Scholar] [CrossRef]
- Zhou, C.; Gao, Y.; Ding, P.; Wu, T.; Ji, G. The role of CXCL family members in different diseases. Cell Death Discov. 2023, 9, 212. [Google Scholar] [CrossRef]
- Landrø, L.; Ueland, T.; Otterdal, K.; Frøland, S.S.; Aukrust, P. Persistently raised plasma levels of platelet-derived inflammatory mediators in HIV-infected patients during highly active anti-retroviral therapy. J. Thromb. Haemost. 2011, 9, 1075–1077. [Google Scholar] [CrossRef]
- Jacobs, E.S.; Keating, S.M.; Abdel-Mohsen, M.; Gibb, S.L.; Heitman, J.W.; Inglis, H.C.; Martin, J.N.; Zhang, J.; Kaidarova, Z.; Deng, X.; et al. Cytokines Elevated in HIV Elite Controllers Reduce HIV Replication In Vitro and Modulate HIV Restriction Factor Expression. J. Virol. 2017, 91, e02051. [Google Scholar] [CrossRef]
- Hao, Y.; Bai, G.; Wang, J.; Zhao, L.; Sutherland, K.; Cai, J.; Cao, C. Identifiable biomarker and treatment development using HIV-1 long term non-progressor sera. BMC Immunol. 2015, 16, 25. [Google Scholar] [CrossRef]
- Gendelman, H.E.; Orenstein, J.M.; Baca, L.M.; Weiser, B.; Burger, H.; Kalter, D.C.; Meltzer, M.S. The macrophage in the persistence and pathogenesis of HIV infection. AIDS 1989, 3, 475–495. [Google Scholar] [CrossRef]
- Clavel, F.; Guetard, D.; Brun-Vezinet, F.; Chamaret, S.; Rey, M.A.; Santos-Ferreira, M.O.; Laurent, A.G.; Dauguet, C.; Katlama, C.; Rouzioux, C.; et al. Isolation of a new human retrovirus from West African patients with AIDS. Science 1986, 233, 343–346. [Google Scholar] [CrossRef] [PubMed]
- Landau, N.R.; Littman, D.R. Packaging system for rapid production of murine leukemia virus vectors with variable tropism. J. Virol. 1992, 66, 5110–5113. [Google Scholar] [CrossRef] [PubMed]
- Devadas, K.; Biswas, S.; Haleyurgirisetty, M.; Ragupathy, V.; Wang, X.; Lee, S.; Hewlett, I. Identification of Host Micro RNAs That Differentiate HIV-1 and HIV-2 Infection Using Genome Expression Profiling Techniques. Viruses 2016, 8, 121. [Google Scholar] [CrossRef] [PubMed]
- Nakoinz, I.; Lee, M.T.; Weaver, J.F.; Ralph, P. Differentiation of the IL-3-dependent NFS-60 cell line and adaption to growth in macrophage colony-stimulating factor. J. Immunol. 1990, 145, 860–864. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]



| Isolate | Origin | Co-Receptor 1 |
|---|---|---|
| HIV-2 B2 | Portugal | CXCR4, CCR5, CCR1, CCR2B |
| HIV-2 B3 | Portugal | CCR5 |
| HIV-2 B4 | Portugal | CCR5 |
| HIV-2 B5 | Portugal | CXCR4, CCR5, CCR1, CCR2B |
| HIV-2 B7 | Portugal | CCR5 |
| HIV-2 B8 | Portugal | CCR5 |
| HIV-2 B9 | Portugal | CCR5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, C.; Kutza, J.; Ouyang, W.; Grimm, T.A.; Fields, K.; Lankford, C.S.R.; Schwartzkopff, F.; Paciga, M.; Machuca, A.; Tiffany, L.; et al. Macrophage-Derived Factors with the Potential to Contribute to the Pathogenicity of HIV-1 and HIV-2: Roles of M-CSF and CXCL7. Int. J. Mol. Sci. 2025, 26, 5028. https://doi.org/10.3390/ijms26115028
Gao C, Kutza J, Ouyang W, Grimm TA, Fields K, Lankford CSR, Schwartzkopff F, Paciga M, Machuca A, Tiffany L, et al. Macrophage-Derived Factors with the Potential to Contribute to the Pathogenicity of HIV-1 and HIV-2: Roles of M-CSF and CXCL7. International Journal of Molecular Sciences. 2025; 26(11):5028. https://doi.org/10.3390/ijms26115028
Chicago/Turabian StyleGao, Chunling, Joseph Kutza, Weiming Ouyang, Tobias A. Grimm, Karen Fields, Carla S. R. Lankford, Franziska Schwartzkopff, Mark Paciga, Ana Machuca, Linda Tiffany, and et al. 2025. "Macrophage-Derived Factors with the Potential to Contribute to the Pathogenicity of HIV-1 and HIV-2: Roles of M-CSF and CXCL7" International Journal of Molecular Sciences 26, no. 11: 5028. https://doi.org/10.3390/ijms26115028
APA StyleGao, C., Kutza, J., Ouyang, W., Grimm, T. A., Fields, K., Lankford, C. S. R., Schwartzkopff, F., Paciga, M., Machuca, A., Tiffany, L., Stantchev, T., & Clouse, K. A. (2025). Macrophage-Derived Factors with the Potential to Contribute to the Pathogenicity of HIV-1 and HIV-2: Roles of M-CSF and CXCL7. International Journal of Molecular Sciences, 26(11), 5028. https://doi.org/10.3390/ijms26115028






