CliniMACS Prodigy Manufacturing of Switchable, AND-Gate CAR T Cells
Abstract
1. Introduction
2. Results
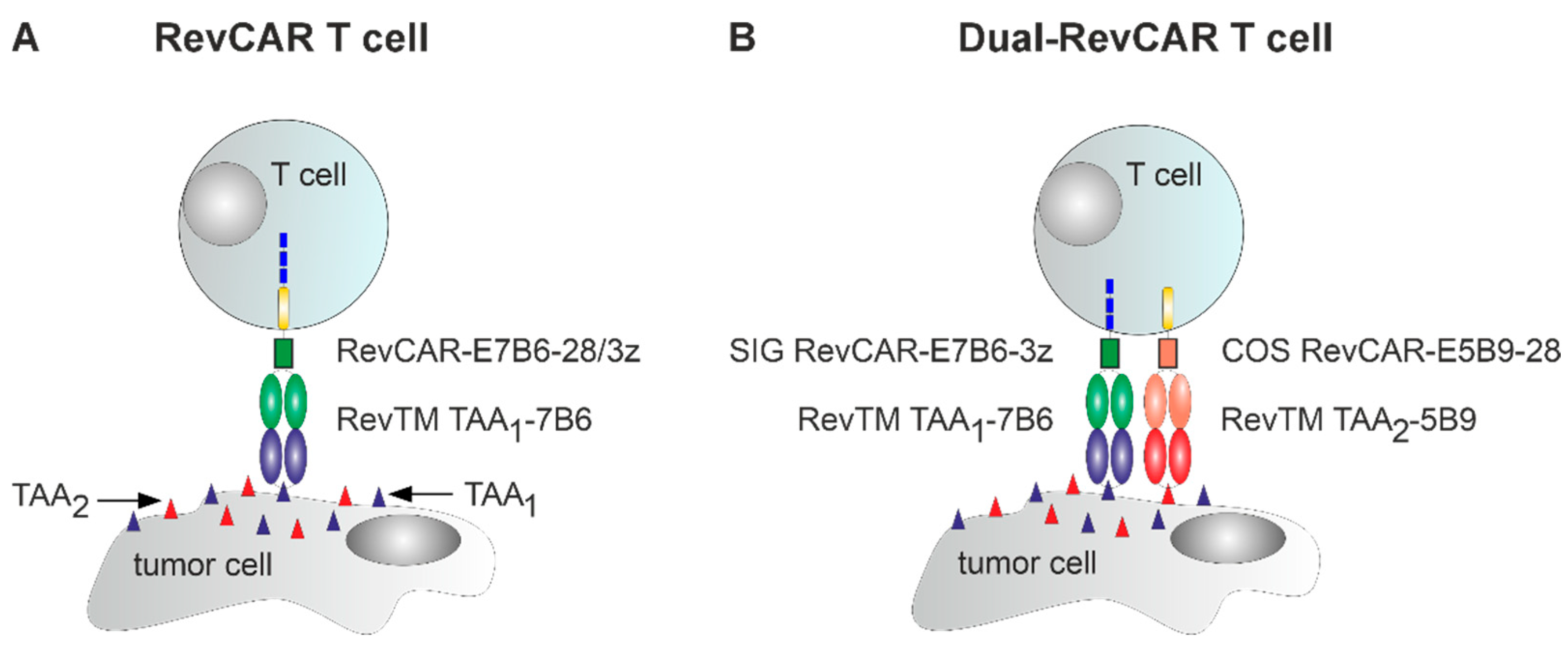
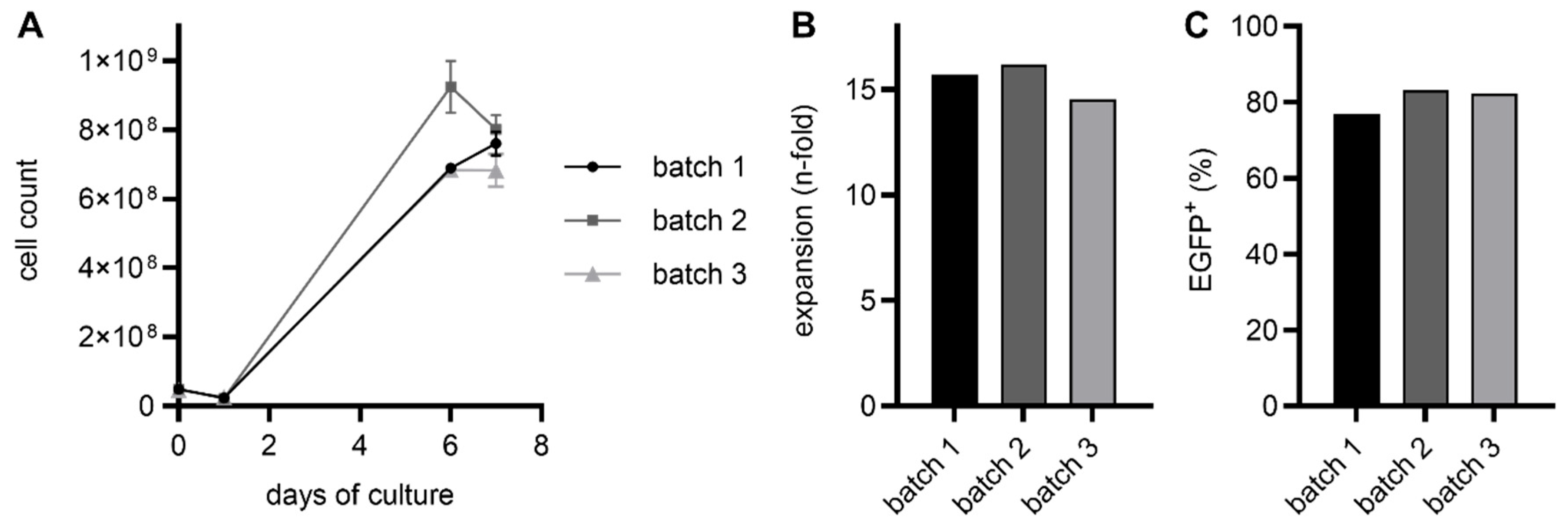
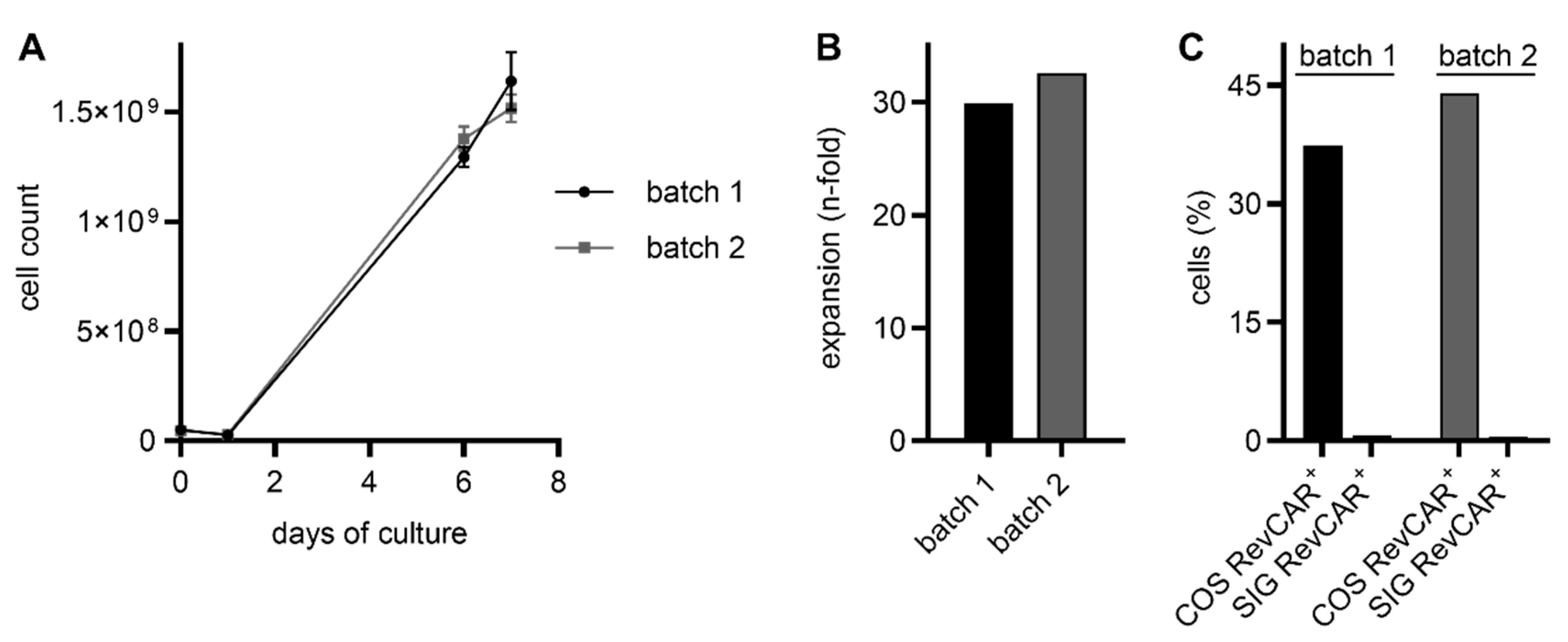
2.1. RevCAR and Dual-RevCAR T Cells Were Manufactured via the CliniMACS Prodigy with High Transduction Efficiencies and Expansion Rates
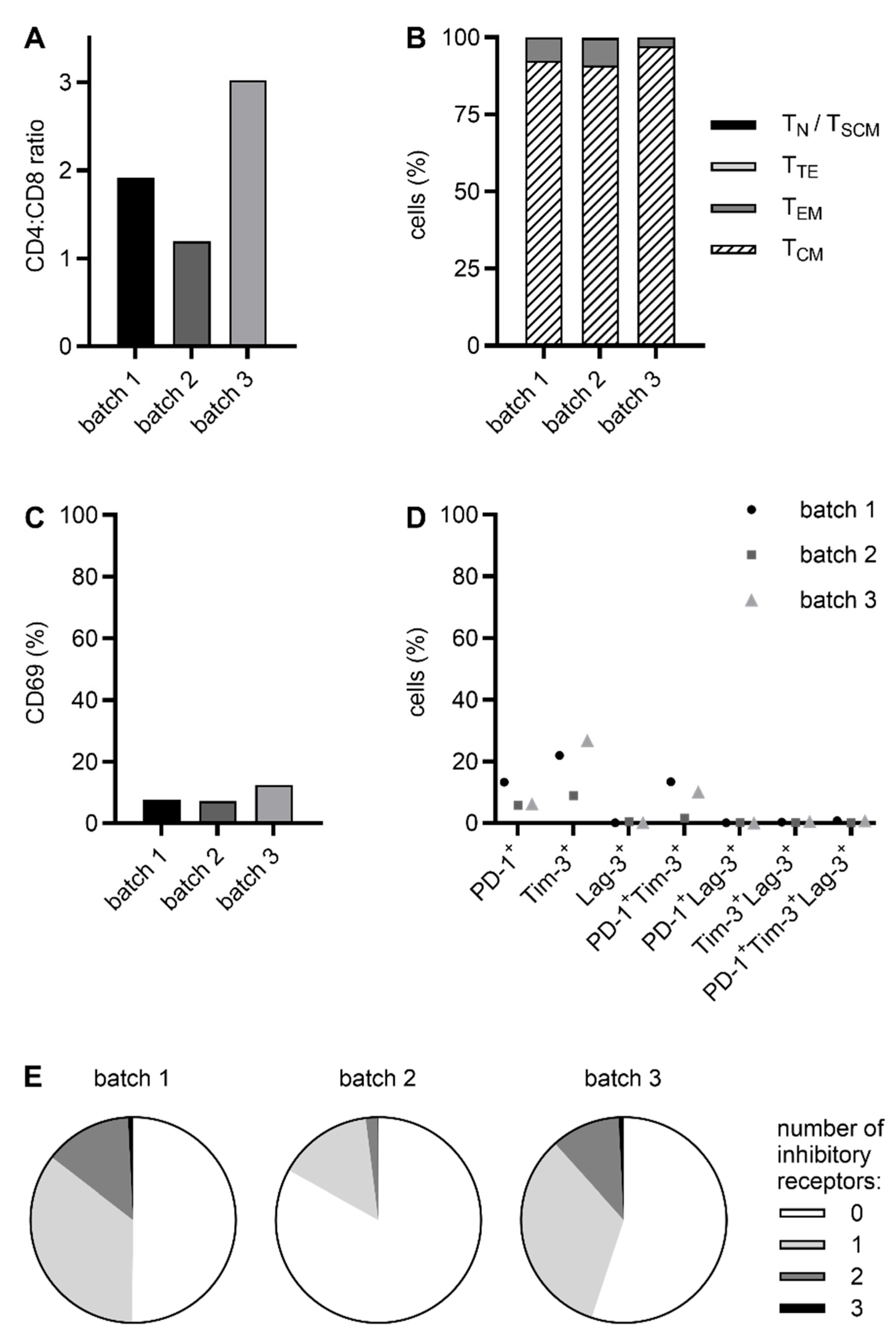
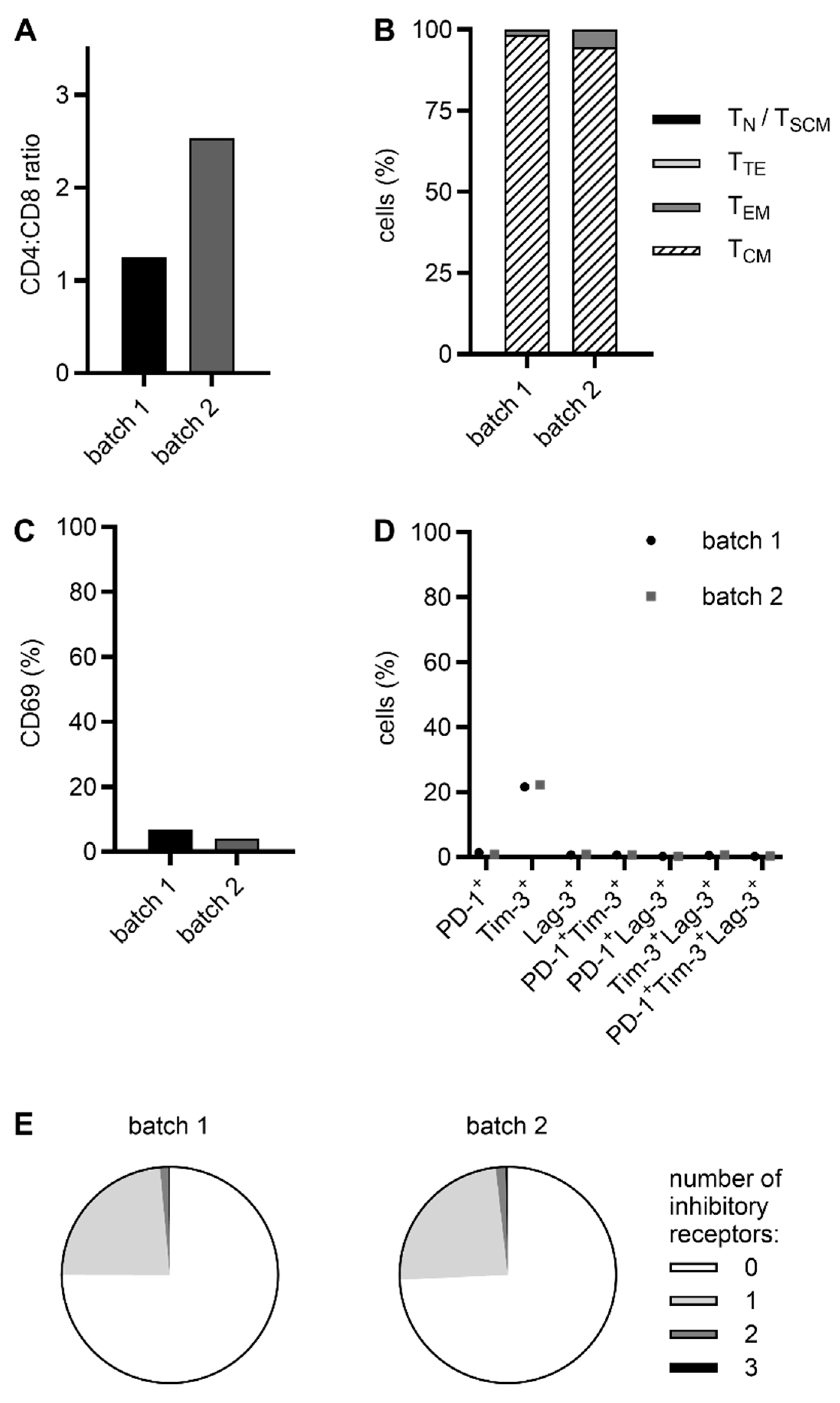
2.2. CliniMACS Prodigy-Manufactured RevCAR and Dual-RevCAR T Cells Possess a Favorable Phenotype for Clinical Application
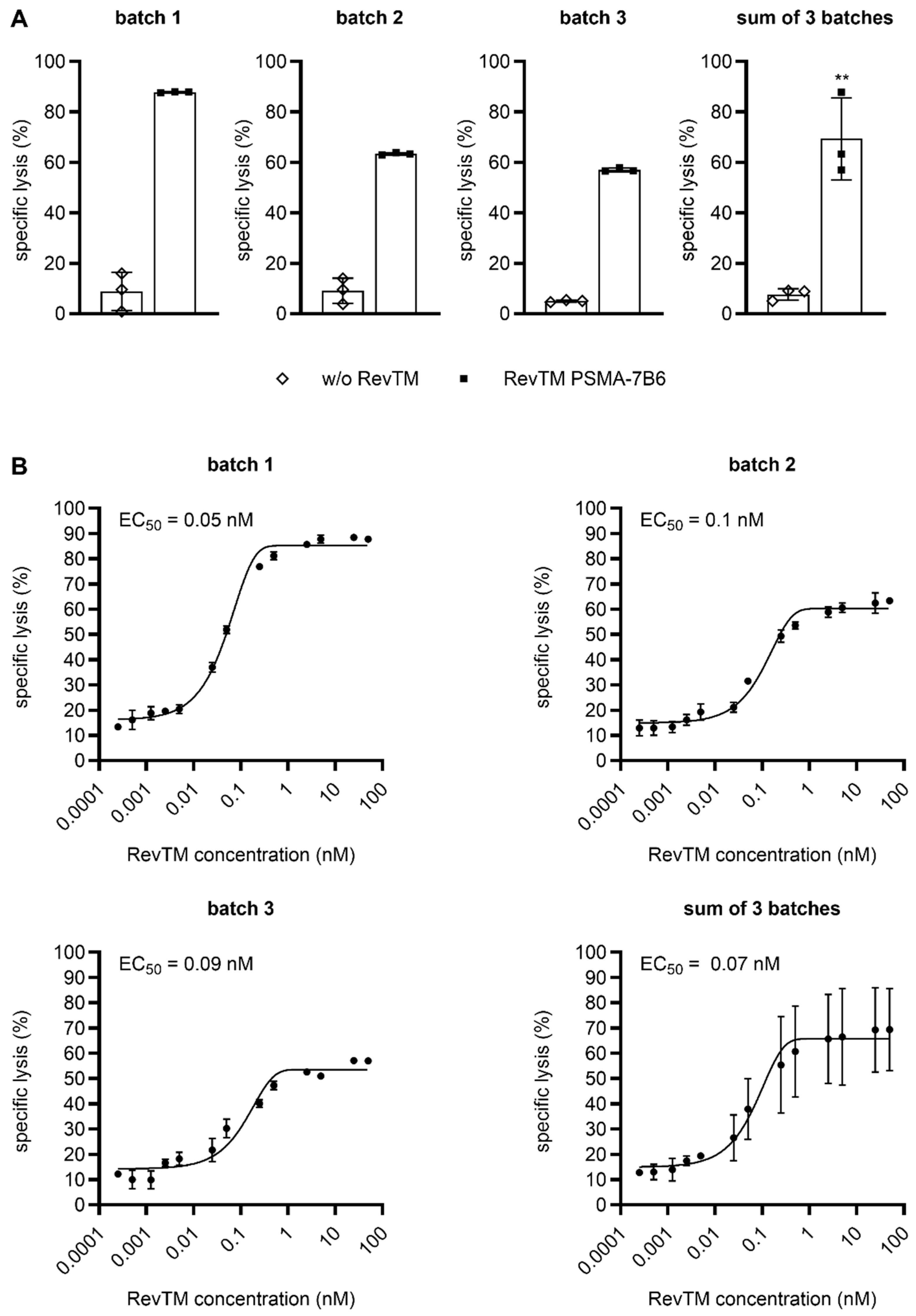
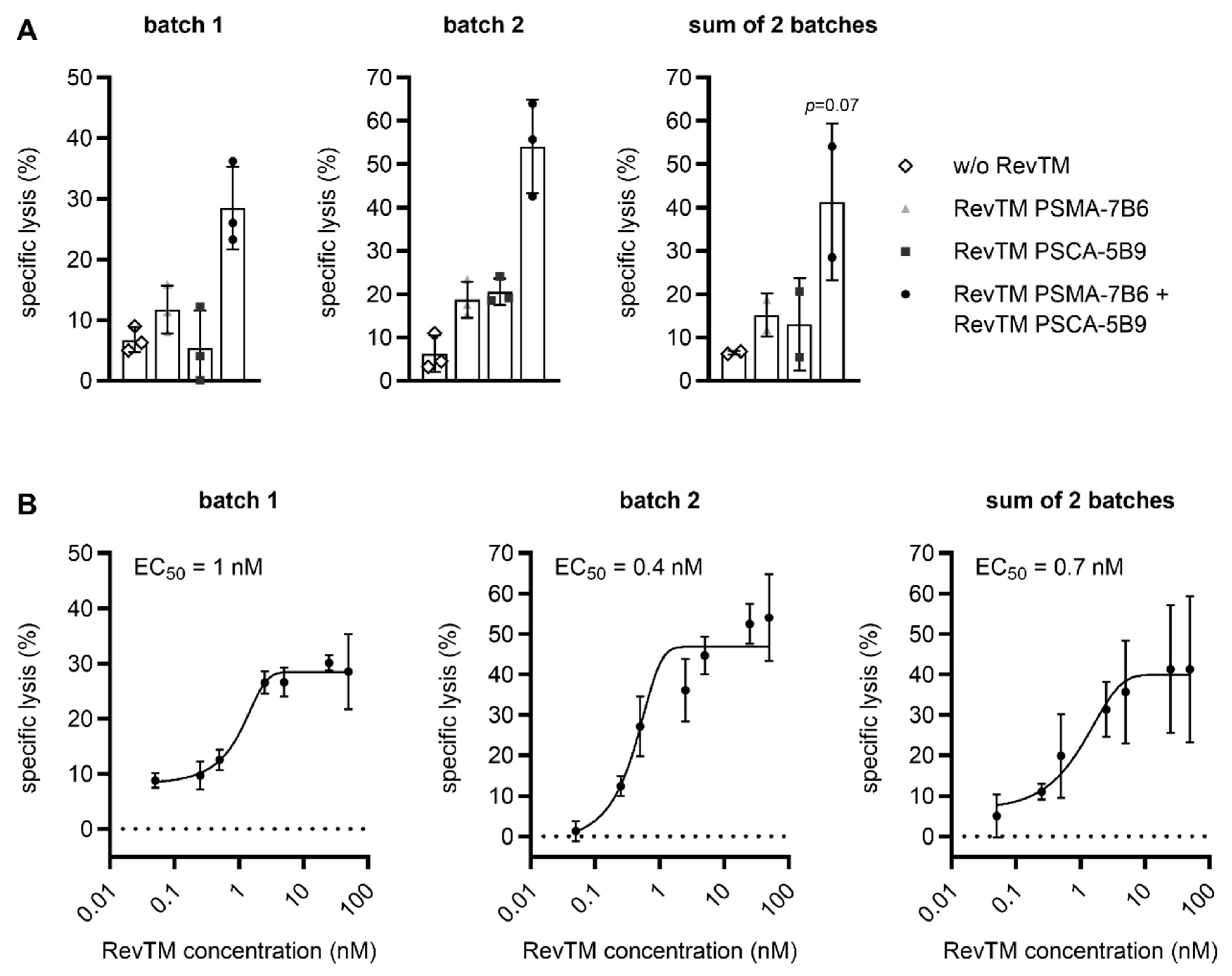
2.3. CliniMACS Prodigy-Manufactured RevCAR and Dual-RevCAR T Cells Potently Kill Tumor Cells and Efficiently Secrete Cytokines
3. Discussion
4. Material and Methods
4.1. Cell Lines
4.2. TM Expression and Purification
4.3. PBMC Isolation
4.4. Manufacturing of RevCAR and Dual-RevCAR T Cells Using the CliniMACS Prodigy
4.5. Flow Cytometry
4.6. Luminescence-Based Cytotoxicity Assay
4.7. Enzyme-Linked Immunosorbent Assay
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maude, S.L.; Laetsch, T.W.; Buechner, J.; Rives, S.; Boyer, M.; Bittencourt, H.; Bader, P.; Verneris, M.R.; Stefanski, H.E.; Myers, G.D.; et al. Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. N. Engl. J. Med. 2018, 378, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Neelapu, S.S.; Locke, F.L.; Bartlett, N.L.; Lekakis, L.J.; Miklos, D.B.; Jacobson, C.A.; Braunschweig, I.; Oluwole, O.O.; Siddiqi, T.; Lin, Y.; et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N. Engl. J. Med. 2017, 377, 2531–2544. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.; Latchford, T. Associated Toxicities: Assessment and Management Related to CAR T-Cell Therapy. Clin. J. Oncol. Nurs. 2019, 23, 13–19. [Google Scholar]
- Cho, J.H.; Collins, J.J.; Wong, W.W. Universal Chimeric Antigen Receptors for Multiplexed and Logical Control of T Cell Responses. Cell 2018, 173, 1426–1438.e1411. [Google Scholar] [CrossRef]
- Koristka, S.; Cartellieri, M.; Feldmann, A.; Arndt, C.; Loff, S.; Michalk, I.; Aliperta, R.; von Bonin, M.; Bornhäuser, M.; Ehninger, A.; et al. Flexible Antigen-Specific Redirection of Human Regulatory T Cells Via a Novel Universal Chimeric Antigen Receptor System. Blood 2014, 124, 3494. [Google Scholar] [CrossRef]
- Minutolo, N.G.; Sharma, P.; Poussin, M.; Shaw, L.C.; Brown, D.P.; Hollander, E.E.; Smole, A.; Rodriguez-Garcia, A.; Hui, J.Z.; Zappala, F.; et al. Quantitative Control of Gene-Engineered T-Cell Activity through the Covalent Attachment of Targeting Ligands to a Universal Immune Receptor. J. Am. Chem. Soc. 2020, 142, 6554–6568. [Google Scholar] [CrossRef]
- Ruffo, E.; Butchy, A.A.; Tivon, Y.; So, V.; Kvorjak, M.; Parikh, A.; Adams, E.L.; Miskov-Zivanov, N.; Finn, O.J.; Deiters, A.; et al. Post-translational covalent assembly of CAR and synNotch receptors for programmable antigen targeting. Nat. Commun. 2023, 14, 2463. [Google Scholar] [CrossRef]
- Schlegel, L.S.; Werbrouck, C.; Boettcher, M.; Schlegel, P. Universal CAR 2.0 to overcome current limitations in CAR therapy. Front. Immunol. 2024, 15, 1383894. [Google Scholar] [CrossRef]
- Tamada, K.; Geng, D.; Sakoda, Y.; Bansal, N.; Srivastava, R.; Li, Z.; Davila, E. Redirecting Gene-Modified T Cells toward Various Cancer Types Using Tagged Antibodies. Clin. Cancer Res. 2012, 18, 6436–6445. [Google Scholar] [CrossRef]
- Albert, S.; Arndt, C.; Feldmann, A.; Bergmann, R.; Bachmann, D.; Koristka, S.; Ludwig, F.; Ziller-Walter, P.; Kegler, A.; Gärtner, S.; et al. A novel nanobody-based target module for retargeting of T lymphocytes to EGFR-expressing cancer cells via the modular UniCAR platform. Oncoimmunology 2017, 6, e1287246. [Google Scholar] [CrossRef]
- Cartellieri, M.; Feldmann, A.; Koristka, S.; Arndt, C.; Loff, S.; Ehninger, A.V.; Von Bonin, M.; Bejestani, E.P.; Ehninger, G.; Bachmann, M.P. Switching CAR T cells on and off: A novel modular platform for retargeting of T cells to AML blasts. Blood Cancer J. 2016, 6, e458. [Google Scholar] [CrossRef] [PubMed]
- Kegler, A.; Drewitz, L.; Arndt, C.; Daglar, C.; Loureiro, L.R.; Mitwasi, N.; Neuber, C.; Soto, K.E.G.; Bartsch, T.; Baraban, L.; et al. A novel ACE2 decoy for both neutralization of SARS-CoV-2 variants and killing of infected cells. Front. Immunol. 2023, 14, 1204543. [Google Scholar] [CrossRef]
- Kegler, A.; Koristka, S.; Bergmann, R.; Berndt, N.; Arndt, C.; Feldmann, A.; Hoffmann, A.; Bornhäuser, M.; Schmitz, M.; Bachmann, M.P. T cells engrafted with a UniCAR 28/z outperform UniCAR BB/z-transduced T cells in the face of regulatory T cell-mediated immunosuppression. Oncoimmunology 2019, 8, e1621676. [Google Scholar] [CrossRef]
- Koristka, S.; Kegler, A.; Bergmann, R.; Arndt, C.; Feldmann, A.; Albert, S.; Cartellieri, M.; Ehninger, A.; Ehninger, G.; Middeke, J.M.; et al. Engrafting human regulatory T cells with a flexible modular chimeric antigen receptor technology. J. Autoimmun. 2018, 90, 116–131. [Google Scholar] [CrossRef]
- Mitwasi, N.; Feldmann, A.; Bergmann, R.; Berndt, N.; Arndt, C.; Koristka, S.; Kegler, A.; Jureczek, J.; Hoffmann, A.; Ehninger, A.; et al. Development of novel target modules for retargeting of UniCAR T cells to GD2 positive tumor cells. Oncotarget 2017, 8, 108584–108603. [Google Scholar] [CrossRef]
- Feldmann, A.; Hoffmann, A.; Bergmann, R.; Koristka, S.; Berndt, N.; Arndt, C.; Loureiro, L.R.; Kittel-Boselli, E.; Mitwasi, N.; Kegler, A.; et al. Versatile chimeric antigen receptor platform for controllable and combinatorial T cell therapy. Oncoimmunology 2020, 9, 1785608. [Google Scholar] [CrossRef]
- Kittel-Boselli, E.; Soto, K.E.G.; Loureiro, L.R.; Hoffmann, A.; Bergmann, R.; Arndt, C.; Koristka, S.; Mitwasi, N.; Kegler, A.; Bartsch, T.; et al. Targeting Acute Myeloid Leukemia Using the RevCAR Platform: A Programmable, Switchable and Combinatorial Strategy. Cancers 2021, 13, 4785. [Google Scholar] [CrossRef]
- Saleh, H.A.; Mitwasi, N.; Ullrich, M.; Kubeil, M.; Toussaint, M.; Deuther-Conrad, W.; Neuber, C.; Arndt, C.; Loureiro, L.R.; Kegler, A.; et al. Specific and safe targeting of glioblastoma using switchable and logic-gated RevCAR T cells. Front. Immunol. 2023, 14, 1166169. [Google Scholar] [CrossRef]
- Soto, K.E.G.; Loureiro, L.R.; Bartsch, T.; Arndt, C.; Kegler, A.; Mitwasi, N.; Drewitz, L.; Hoffmann, L.; Saleh, H.A.; Crespo, E.; et al. Targeting colorectal cancer cells using AND-gated adaptor RevCAR T-cells. Front. Immunol. 2023, 14, 1302354. [Google Scholar] [CrossRef]
- Wermke, M.; Kraus, S.; Ehninger, A.; Bargou, R.C.; Goebeler, M.-E.; Middeke, J.M.; Kreissig, C.; von Bonin, M.; Koedam, J.; Pehl, M.; et al. Proof of concept for a rapidly switchable universal CAR-T platform with UniCAR-T-CD123 in relapsed/refractory AML. Blood 2021, 137, 3145–3148. [Google Scholar] [CrossRef]
- Wermke, M.; Metzelder, S.; Kraus, S.; Sala, E.; Vucinic, V.; Fiedler, W.; Wetzko, K.; Schäfer, J.; Goebeler, M.-E.; Koedam, J.; et al. Updated Results from a Phase I Dose Escalation Study of the Rapidly-Switchable Universal CAR-T Therapy UniCAR-T-CD123 in Relapsed/Refractory AML. Blood 2023, 142, 3465. [Google Scholar] [CrossRef]
- Carmo-Fonseca, M.; Pfeifer, K.; Schröder, H.C.; Vaz, M.; Fonseca, J.; Müller, W.E.; Bachmann, M. Identification of La ribonucleoproteins as a component of interchromatin granules. Exp. Cell Res. 1989, 185, 73–85. [Google Scholar] [CrossRef] [PubMed]
- E Yiannaki, E.; Tzioufas, A.G.; Bachmann, M.; Hantoumi, J.; Tsikaris, V.; Sakarellos-Daitsiotis, M.; Sakarellos, C.; Moutsopoulos, H.M. The value of synthetic linear epitope analogues of La/SSB for the detection of autoantibodies to La/SSB; specificity, sensitivity and comparison of methods. Clin. Exp. Immunol. 1998, 112, 152–158. [Google Scholar] [CrossRef]
- Morgan, R.A.; Yang, J.C.; Kitano, M.; Dudley, M.E.; Laurencot, C.M.; Rosenberg, S.A. Case Report of a Serious Adverse Event Following the Administration of T Cells Transduced with a Chimeric Antigen Receptor Recognizing ERBB2. Mol. Ther. 2010, 18, 843–851. [Google Scholar] [CrossRef]
- Newick, K.; O’Brien, S.; Moon, E.; Albelda, S.M. CAR T Cell Therapy for Solid Tumors. Annu. Rev. Med. 2017, 68, 139–152. [Google Scholar] [CrossRef]
- Castella, M.; Caballero-Baños, M.; Ortiz-Maldonado, V.; González-Navarro, E.A.; Suñé, G.; Antoñana-Vidósola, A.; Boronat, A.; Marzal, B.; Millán, L.; Martín-Antonio, B.; et al. Point-Of-Care CAR T-Cell Production (ARI-0001) Using a Closed Semi-automatic Bioreactor: Experience From an Academic Phase I Clinical Trial. Front. Immunol. 2020, 11, 482. [Google Scholar] [CrossRef]
- Jackson, Z.; Roe, A.; Sharma, A.A.; Lopes, F.B.T.P.; Talla, A.; Kleinsorge-Block, S.; Zamborsky, K.; Schiavone, J.; Manjappa, S.; Schauner, R.; et al. Automated Manufacture of Autologous CD19 CAR-T Cells for Treatment of Non-hodgkin Lymphoma. Front. Immunol. 2020, 11, 1941. [Google Scholar] [CrossRef]
- Malakhova, E.; Pershin, D.; Kulakovskaya, E.; Vedmedskaia, V.; Fadeeva, M.; Lodoeva, O.; Sozonova, T.; Muzalevskii, Y.; Kazachenok, A.; Belchikov, V.; et al. Extended characterization of anti-CD19 CAR T cell products manufactured at the point of care using the CliniMACS Prodigy system: Comparison of donor sources and process duration. Cytotherapy 2024, 26, 567–578. [Google Scholar] [CrossRef]
- Mock, U.; Nickolay, L.; Philip, B.; Cheung, G.W.-K.; Zhan, H.; Johnston, I.C.D.; Kaiser, A.D.; Peggs, K.; Pule, M.; Thrasher, A.; et al. Automated manufacturing of chimeric antigen receptor T cells for adoptive immunotherapy using CliniMACS Prodigy. Cytotherapy 2016, 18, 1002–1011. [Google Scholar] [CrossRef]
- Zhang, W.; Jordan, K.R.; Schulte, B.; Purev, E. Characterization of clinical grade CD19 chimeric antigen receptor T cells produced using automated CliniMACS prodigy system. Drug Des. Dev. Ther. 2018, 12, 3343–3356. [Google Scholar] [CrossRef]
- Thommen, D.S.; Schreiner, J.; Müller, P.; Herzig, P.; Roller, A.; Belousov, A.; Umana, P.; Pisa, P.; Klein, C.; Bacac, M.; et al. Progression of Lung Cancer Is Associated with Increased Dysfunction of T Cells Defined by Coexpression of Multiple Inhibitory Receptors. Cancer Immunol. Res. 2015, 3, 1344–1355. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Aguilar, B.; Starr, R.; Alizadeh, D.; Brito, A.; Sarkissian, A.; Ostberg, J.R.; Forman, S.J.; Brown, C.E. Glioblastoma-targeted CD4+ CAR T cells mediate superior antitumor activity. JCI Insight 2018, 3, e99048. [Google Scholar] [CrossRef]
- Deng, Q.; Han, G.; Puebla-Osorio, N.; Ma, M.C.J.; Strati, P.; Chasen, B.; Dai, E.; Dang, M.; Jain, N.; Yang, H.; et al. Characteristics of anti-CD19 CAR T cell infusion products associated with efficacy and toxicity in patients with large B cell lymphomas. Nat. Med. 2020, 26, 1878–1887. [Google Scholar] [CrossRef]
- Fraietta, J.A.; Lacey, S.F.; Orlando, E.J.; Pruteanu-Malinici, I.; Gohil, M.; Lundh, S.; Boesteanu, A.C.; Wang, Y.; O’connor, R.S.; Hwang, W.-T.; et al. Determinants of response and resistance to CD19 chimeric antigen receptor (CAR) T cell therapy of chronic lymphocytic leukemia. Nat. Med. 2018, 24, 563–571. [Google Scholar] [CrossRef]
- Guo, Y.; Feng, K.-C.; Liu, Y.; Wu, Z.; Dai, H.; Yang, Q.-M.; Wang, Y.; Jia, H.; Han, W. Phase I Study of Chimeric Antigen Receptor–Modified T Cells in Patients with EGFR-Positive Advanced Biliary Tract Cancers. Clin. Cancer Res. 2018, 24, 1277–1286. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, Y.; Wu, Z.; Feng, K.; Tong, C.; Wang, Y.; Dai, H.; Shi, F.; Yang, Q.; Han, W. Anti-EGFR chimeric antigen receptor-modified T cells in metastatic pancreatic carcinoma: A phase I clinical trial. Cytotherapy 2020, 22, 573–580. [Google Scholar] [CrossRef]
- Louis, C.U.; Savoldo, B.; Dotti, G.; Pule, M.; Yvon, E.; Myers, G.D.; Rossig, C.; Russell, H.V.; Diouf, O.; Liu, E.; et al. Antitumor activity and long-term fate of chimeric antigen receptor–positive T cells in patients with neuroblastoma. Blood 2011, 118, 6050–6056. [Google Scholar] [CrossRef]
- Monfrini, C.; Stella, F.; Aragona, V.; Magni, M.; Ljevar, S.; Vella, C.; Fardella, E.; Chiappella, A.; Nanetti, F.; Pennisi, M.; et al. Phenotypic Composition of Commercial Anti-CD19 CAR T Cells Affects In Vivo Expansion and Disease Response in Patients with Large B-cell Lymphoma. Clin. Cancer Res. 2022, 28, 3378–3386. [Google Scholar] [CrossRef]
- Alnabhan, R.; Gaballa, A.; Mörk, L.-M.; Mattsson, J.; Uhlin, M.; Magalhaes, I. Media evaluation for production and expansion of anti-CD19 chimeric antigen receptor T cells. Cytotherapy 2018, 20, 941–951. [Google Scholar] [CrossRef]
- Sommermeyer, D.; Hudecek, M.; Kosasih, P.L.; Gogishvili, T.; Maloney, D.G.; Turtle, C.J.; Riddell, S.R. Chimeric antigen receptor-modified T cells derived from defined CD8+ and CD4+ subsets confer superior antitumor reactivity in vivo. Leukemia 2016, 30, 492–500. [Google Scholar] [CrossRef]
- Berger, C.; Jensen, M.C.; Lansdorp, P.M.; Gough, M.; Elliott, C.; Riddell, S.R. Adoptive transfer of effector CD8+ T cells derived from central memory cells establishes persistent T cell memory in primates. J. Clin. Investig. 2008, 118, 294–305. [Google Scholar] [CrossRef] [PubMed]
- Arndt, C.; Bergmann, R.; Striese, F.; Merkel, K.; Máthé, D.; Loureiro, L.R.; Mitwasi, N.; Kegler, A.; Fasslrinner, F.; González Soto, K.E.; et al. Development and Functional Characterization of a Versatile Radio-/Immunotheranostic Tool for Prostate Cancer Management. Cancers 2022, 14, 1996. [Google Scholar] [CrossRef] [PubMed]
- Feldmann, A.; Stamova, S.; Bippes, C.C.; Bartsch, H.; Wehner, R.; Schmitz, M.; Temme, A.; Cartellieri, M.; Bachmann, M. Retargeting of T cells to prostate stem cell antigen expressing tumor cells: Comparison of different antibody formats. Prostate 2011, 71, 998–1011. [Google Scholar] [CrossRef] [PubMed]
- Morgenroth, A.; Cartellieri, M.; Schmitz, M.; Günes, S.; Weigle, B.; Bachmann, M.; Abken, H.; Rieber, E.P.; Temme, A. Targeting of tumor cells expressing the prostate stem cell antigen (PSCA) using genetically engineered T-cells. Prostate 2007, 67, 1121–1131. [Google Scholar] [CrossRef]
- Bachmann, M.P.; Bartsch, T.; Bippes, C.C.; Bachmann, D.; Puentes-Cala, E.; Bachmann, J.; Bartsch, H.; Arndt, C.; Koristka, S.; Loureiro, L.R.; et al. T Cell Mediated Conversion of a Non-Anti-La Reactive B Cell to an Autoreactive Anti-La B Cell by Somatic Hypermutation. Int. J. Mol. Sci. 2021, 22, 1198. [Google Scholar] [CrossRef]
- Bippes, C.C.; Feldmann, A.; Stamova, S.; Cartellieri, M.; Schwarzer, A.; Wehner, R.; Schmitz, M.; Rieber, E.P.; Zhao, S.; Schäkel, K.; et al. A Novel Modular Antigen Delivery System for Immuno Targeting of Human 6-sulfo LacNAc-Positive Blood Dendritic Cells (SlanDCs). PLoS ONE 2011, 6, e16315. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
von Jutrzenka-Trzebiatowski, A.; Gupte, R.; Daglar, C.; Berndt, N.; Arndt, C.; Bachmann, M.; Feldmann, A. CliniMACS Prodigy Manufacturing of Switchable, AND-Gate CAR T Cells. Int. J. Mol. Sci. 2025, 26, 5024. https://doi.org/10.3390/ijms26115024
von Jutrzenka-Trzebiatowski A, Gupte R, Daglar C, Berndt N, Arndt C, Bachmann M, Feldmann A. CliniMACS Prodigy Manufacturing of Switchable, AND-Gate CAR T Cells. International Journal of Molecular Sciences. 2025; 26(11):5024. https://doi.org/10.3390/ijms26115024
Chicago/Turabian Stylevon Jutrzenka-Trzebiatowski, Alexandra, Rutuja Gupte, Cansu Daglar, Nicole Berndt, Claudia Arndt, Michael Bachmann, and Anja Feldmann. 2025. "CliniMACS Prodigy Manufacturing of Switchable, AND-Gate CAR T Cells" International Journal of Molecular Sciences 26, no. 11: 5024. https://doi.org/10.3390/ijms26115024
APA Stylevon Jutrzenka-Trzebiatowski, A., Gupte, R., Daglar, C., Berndt, N., Arndt, C., Bachmann, M., & Feldmann, A. (2025). CliniMACS Prodigy Manufacturing of Switchable, AND-Gate CAR T Cells. International Journal of Molecular Sciences, 26(11), 5024. https://doi.org/10.3390/ijms26115024







