Exploring the Biological Impact of β-TCP Surface Polarization on Osteoblast and Osteoclast Activity
Abstract
1. Introduction
2. Results
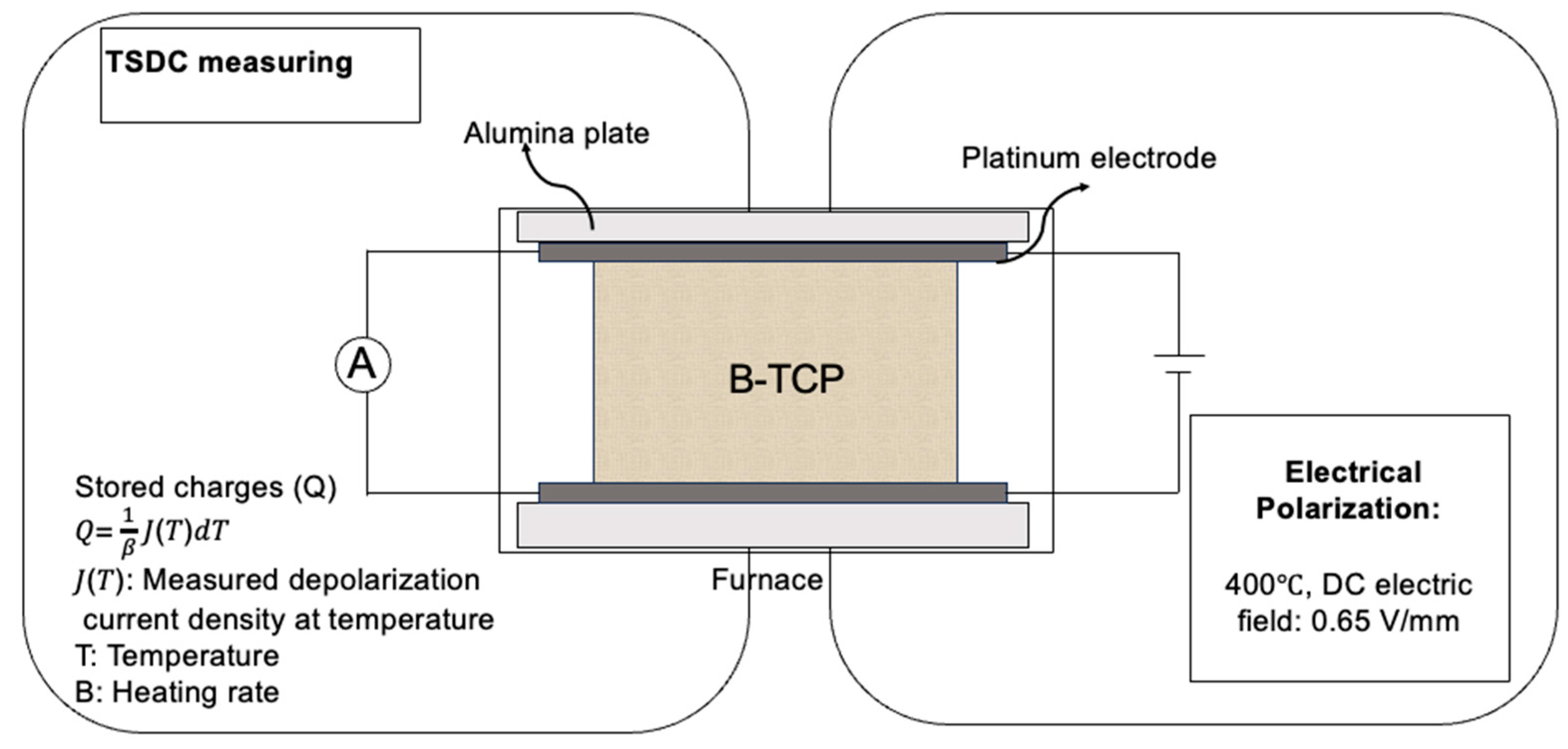
2.1. Electrical Polarization of β-TCP Samples
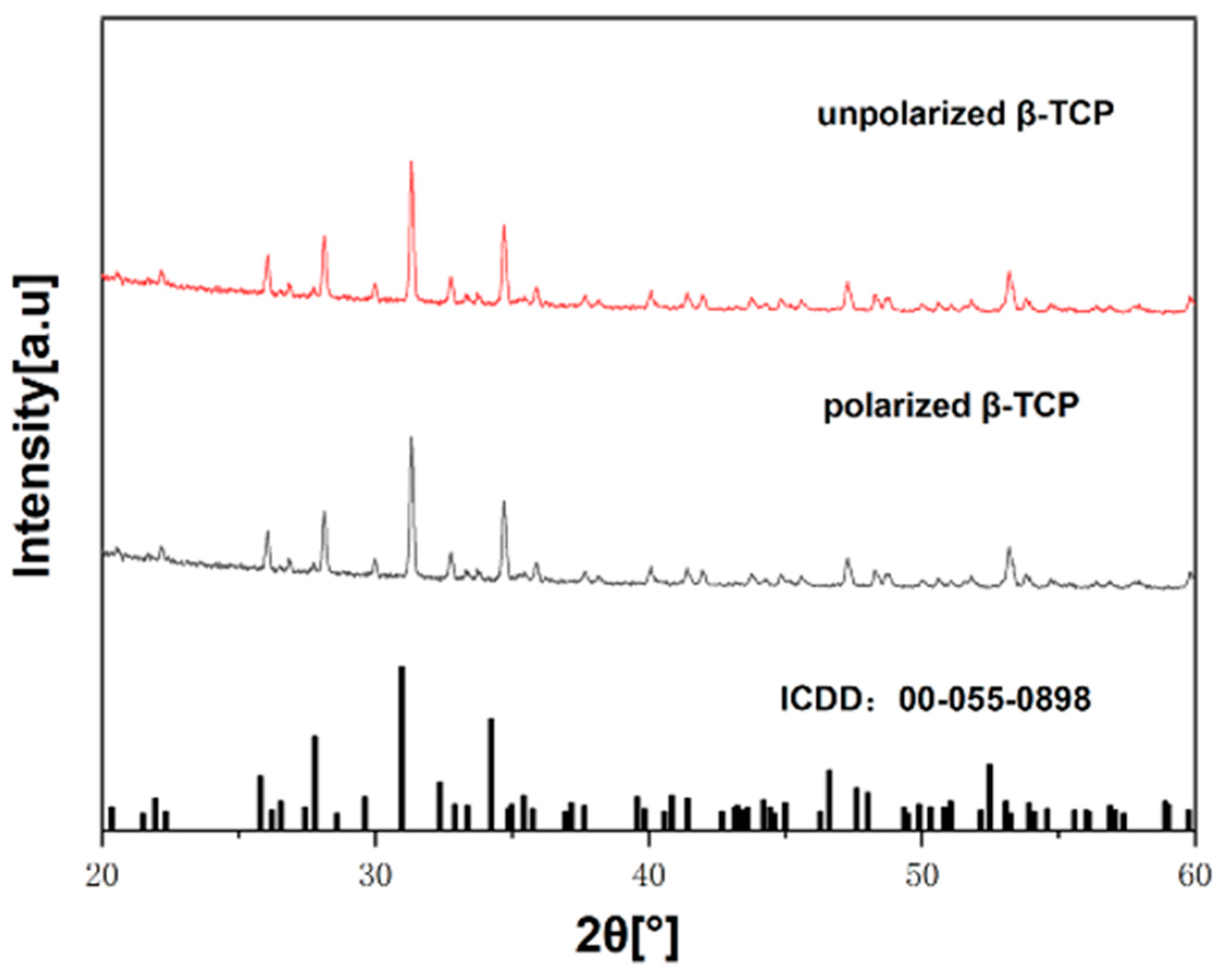
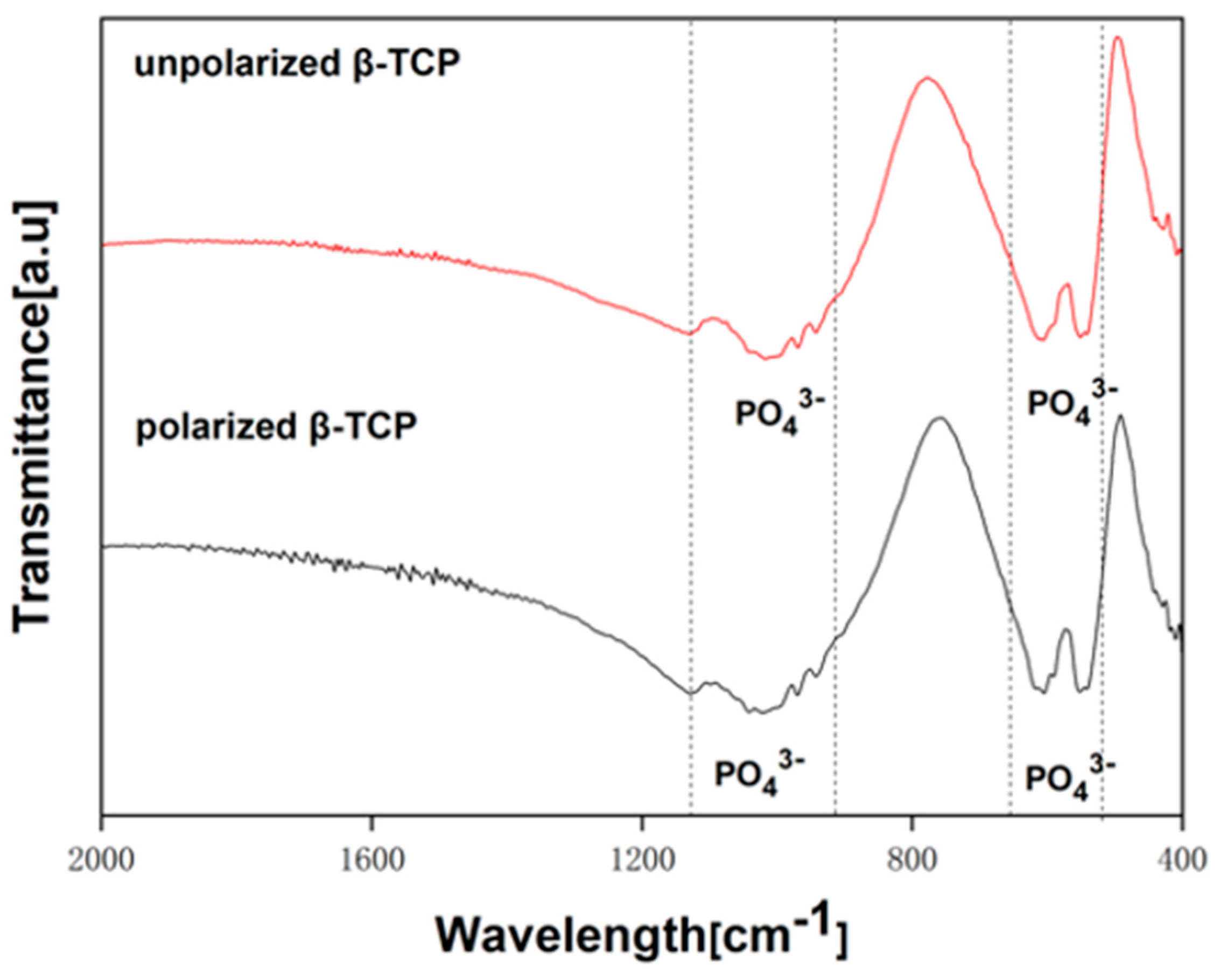
2.2. Characterization of Polarized β-TCP Samples
2.3. Osteoblast Proliferation Assay
2.4. Osteoblast Differentiation Assay
2.5. TRAP Staining and TRAP Activities
2.6. β-TCP Resorption Capacity
3. Discussion
4. Materials and Methods
4.1. Preparation of β-TCP Samples
4.2. Electrical Polarization and TSDC Measurement of β-TCP
4.3. Surface Characteristics
4.4. Cell Culture
4.5. Osteoblast-like Cell Proliferation
4.6. Osteoblast-like Cell Differentiation
4.7. Tartrate-Resistant Acid Phosphatase (TRAP) Activity Assay
4.8. Osteoclast Formation Assay
4.9. Visualization and Measurement of Resorption Pit
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schmidt, A.H. Autologous bone graft: Is it still the gold standard? Injury 2021, 52, S18–S22. [Google Scholar] [CrossRef]
- Long, J.; Teng, B.; Zhang, W.; Li, L.; Zhang, M.; Chen, Y.; Yao, Z.; Meng, X.; Wang, X.; Qin, L. Preclinical evaluation of acute systemic toxicity of magnesium incorporated poly (lactic-co-glycolic acid) porous scaffolds by three-dimensional printing. Biomater. Transl. 2021, 2, 272. [Google Scholar]
- Sohn, H.S.; Oh, J.K. Review of bone graft and bone substitutes with an emphasis on fracture surgeries. Biomater. Res. 2019, 23, 9. [Google Scholar] [CrossRef]
- Lewis, J.W.; Frost, K.; Neag, G.; Wahid, M.; Finlay, M.; Northall, E.H.; Abudu, O.; Kemble, S.; Davis, E.T.; Powell, E. Therapeutic avenues in bone repair: Harnessing an anabolic osteopeptide, PEPITEM, to boost bone growth and prevent bone loss. Cell Rep. Med. 2024, 5, 101574. [Google Scholar] [CrossRef]
- Jitaru, S.; Hodisan, I.; Timis, L.; Lucian, A.; Bud, M. The use of bioceramics in endodontics-literature review. Clujul Med. 2016, 89, 470. [Google Scholar] [CrossRef]
- Sharma, A.K.; Gupta, S. Microwave processing of biomaterials for orthopedic implants: Challenges and possibilities. JOM 2020, 72, 1211–1228. [Google Scholar] [CrossRef]
- Jeong, J.; Kim, J.H.; Shim, J.H.; Hwang, N.S.; Heo, C.Y. Bioactive calcium phosphate materials and applications in bone regeneration. Biomater. Res. 2019, 23, 4. [Google Scholar] [CrossRef]
- Huang, J.; Li, X.; Guo, Z. Biomechanical and biochemical compatibility in innovative biomaterials. In Biocompatibility and Performance of Medical Devices; Elsevier: Amsterdam, The Netherlands, 2020; pp. 23–46. [Google Scholar]
- Kumar, R.; Pattanayak, I.; Dash, P.A.; Mohanty, S. Bioceramics: A review on design concepts toward tailor-made (multi)-functional materials for tissue engineering applications. J. Mater. Sci. 2023, 58, 3460–3484. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, H.; Zhang, Y.; Liu, Z.; He, D.; Xu, W.; Li, S.; Zhang, C.; Zhang, Z. Li–Mg–Si bioceramics provide a dynamic immuno-modulatory and repair-supportive microenvironment for peripheral nerve regeneration. Bioact. Mater. 2023, 28, 227–242. [Google Scholar] [CrossRef] [PubMed]
- Rafikova, G.; Piatnitskaia, S.; Shapovalova, E.; Chugunov, S.; Kireev, V.; Ialiukhova, D.; Bilyalov, A.; Pavlov, V.; Kzhyshkowska, J. Interaction of ceramic implant materials with immune system. Int. J. Mol. Sci. 2023, 24, 4200. [Google Scholar] [CrossRef] [PubMed]
- Casarrubios, L.; Gómez-Cerezo, N.; Sánchez-Salcedo, S.; Feito, M.; Serrano, M.; Saiz-Pardo, M.; Ortega, L.; De Pablo, D.; Díaz-Güemes, I.; Fernández-Tomé, B. Silicon substituted hydroxyapatite/VEGF scaffolds stimulate bone regeneration in osteoporotic sheep. Acta Biomater. 2020, 101, 544–553. [Google Scholar] [CrossRef] [PubMed]
- Khajuria, D.K.; Disha, C.; Vasireddi, R.; Razdan, R.; Mahapatra, D.R. Risedronate/zinc-hydroxyapatite based nanomedicine for osteoporosis. Mater. Sci. Eng. C 2016, 63, 78–87. [Google Scholar] [CrossRef]
- Khajuria, D.K.; Kumar, V.B.; Gigi, D.; Gedanken, A.; Karasik, D. Accelerated bone regeneration by nitrogen-doped carbon dots functionalized with hydroxyapatite nanoparticles. ACS Appl. Mater. Interfaces 2018, 10, 19373–19385. [Google Scholar] [CrossRef]
- Khajuria, D.K.; Vasireddi, R.; Trebbin, M.; Karasik, D.; Razdan, R. Novel therapeutic intervention for osteoporosis prepared with strontium hydroxyapatite and zoledronic acid: In vitro and pharmacodynamic evaluation. Mater. Sci. Eng. C 2017, 71, 698–708. [Google Scholar] [CrossRef]
- Khajuria, D.K.; Razdan, R.; Mahapatra, D.R. Development, in vitro and in vivo characterization of zoledronic acid functionalized hydroxyapatite nanoparticle based formulation for treatment of osteoporosis in animal model. Eur. J. Pharm. Sci. 2015, 66, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, M.; Chen, F.; Wang, Y.; Wang, M.; Chen, X.; Xiao, Y.; Zhang, X. Design of hydroxyapatite bioceramics with micro-/nano-topographies to regulate the osteogenic activities of bone morphogenetic protein-2 and bone marrow stromal cells. Nanoscale 2020, 12, 7284–7300. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Decaris, M.L.; Leach, J.K. Bioceramic-mediated trophic factor secretion by mesenchymal stem cells enhances in vitro endothelial cell persistence and in vivo angiogenesis. Tissue Eng. Part A 2012, 18, 1520–1528. [Google Scholar] [CrossRef]
- Long, S.; Zhu, J.; Jing, Y.; He, S.; Cheng, L.; Shi, Z. A comprehensive review of surface modification techniques for enhancing the biocompatibility of 3D-printed titanium implants. Coatings 2023, 13, 1917. [Google Scholar] [CrossRef]
- Gillman, C.E.; Jayasuriya, A.C. FDA-approved bone grafts and bone graft substitute devices in bone regeneration. Mater. Sci. Eng. C 2021, 130, 112466. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Cai, Q.; Lian, P.; Fang, Z.; Duan, S.; Yang, X.; Deng, X.; Ryu, S. β-Tricalcium phosphate nanoparticles adhered carbon nanofibrous membrane for human osteoblasts cell culture. Mater. Lett. 2010, 64, 725–728. [Google Scholar] [CrossRef]
- Kamitakahara, M.; Ohtsuki, C.; Miyazaki, T. Review paper: Behavior of ceramic biomaterials derived from tricalcium phosphate in physiological condition. J. Biomater. Appl. 2008, 23, 197–212. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.; Kim, S.; Chung, S.H. Clinical outcome of beta-tricalcium phosphate use for bone defects after operative treatment of benign tumors. Clin. Orthop. Surg. 2019, 11, 233. [Google Scholar] [CrossRef] [PubMed]
- Hernigou, P.; Dubory, A.; Pariat, J.; Potage, D.; Roubineau, F.; Jammal, S.; Lachaniette, C.F. Beta-tricalcium phosphate for orthopedic reconstructions as an alternative to autogenous bone graft. Morphologie 2017, 101, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Yu, H.; Chen, C. Biological properties of calcium phosphate biomaterials for bone repair: A review. RSC Adv. 2018, 8, 2015–2033. [Google Scholar] [CrossRef]
- Chappard, D.; Guillaume, B.; Mallet, R.; Pascaretti-Grizon, F.; Baslé, M.F.; Libouban, H. Sinus lift augmentation and beta-TCP: A microCT and histologic analysis on human bone biopsies. Micron 2010, 41, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Nozaki, K.; Nagai, A.; Endo, T.; Hashimoto, K.; Yamashita, K. Electrical polarization and ionic conduction properties of β-tricalcium phosphate bioceramics with controlled vacancies by sodium ion substitution. Ceram. Int. 2022, 48, 15791–15799. [Google Scholar] [CrossRef]
- Brett, C.M.A.; Brett, A.M.O. Principles, methods, and applications. Electrochemistry 1993, 67, 444. [Google Scholar]
- Wang, W.; Itoh, S.; Yamamoto, N.; Okawa, A.; Nagai, A.; Yamashita, K. Enhancement of nerve regeneration along a chitosan nanofiber mesh tube on which electrically polarized β-tricalcium phosphate particles are immobilized. Acta Biomater. 2010, 6, 4027–4033. [Google Scholar] [CrossRef]
- Tarafder, S.; Bodhak, S.; Bandyopadhyay, A.; Bose, S. Effect of electrical polarization and composition of biphasic calcium phosphates on early stage osteoblast interactions. J. Biomed. Mater. Res. Part. B Appl. Biomater. 2011, 97, 306–314. [Google Scholar] [CrossRef]
- Bandyopadhyay, A.; Shivaram, A.; Mitra, I.; Bose, S. Electrically polarized TiO2 nanotubes on Ti implants to enhance early-stage osseointegration. Acta Biomater. 2019, 96, 686–693. [Google Scholar] [CrossRef] [PubMed]
- Horiuchi, N.; Nakamura, M.; Nagai, A.; Katayama, K.; Yamashita, K. Proton conduction related electrical dipole and space charge polarization in hydroxyapatite. J. Appl. Phys. 2012, 112, 074901. [Google Scholar] [CrossRef]
- Nakamura, M.; Hori, N.; Ando, H.; Namba, S.; Toyama, T.; Nishimiya, N.; Yamashita, K. Surface free energy predominates in cell adhesion to hydroxyapatite through wettability. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 62, 283–292. [Google Scholar] [CrossRef]
- Bodhak, S.; Bose, S.; Bandyopadhyay, A. Role of surface charge and wettability on early stage mineralization and bone cell–materials interactions of polarized hydroxyapatite. Acta Biomater. 2009, 5, 2178–2188. [Google Scholar] [CrossRef] [PubMed]
- Tofail, S.A.M.; Gandhi, A. Electrical modifications of biomaterials’ surfaces: Beyond hydrophobicity and hydrophilicity. In Biological Interactions with Surface Charge Biomaterials; Royal Society of Chemistry: London, UK, 2011; pp. 3–14. [Google Scholar]
- Wang, W.; Itoh, S.; Yamamoto, N.; Okawa, A.; Nagai, A.; Yamashita, K. Electrical polarization of β-tricalcium phosphate ceramics. J. Am. Ceram. Soc. 2010, 93, 2175–2177. [Google Scholar] [CrossRef]
- Nohara, K.; Itoh, S.; Akizuki, T.; Nakamura, M.; Fukuba, S.; Matsuura, T.; Okada, M.; Izumi, Y.; Iwata, T.; Yamashita, K. Enhanced new bone formation in canine maxilla by a graft of electrically polarized β-tricalcium phosphate. J. Biomed. Mater. Res. Part. B Appl. Biomater. 2020, 108, 2820–2826. [Google Scholar] [CrossRef]
- Grigg, A.T.; Mee, M.; Mallinson, P.M.; Fong, S.K.; Gan, Z.; Dupree, R.; Holland, D. Cation substitution in β-tricalcium phosphate investigated using multi-nuclear, solid-state NMR. J. Solid. State Chem. 2014, 212, 227–236. [Google Scholar] [CrossRef]
- Holzapfel, B.M.; Reichert, J.C.; Schantz, J.-T.; Gbureck, U.; Rackwitz, L.; Nöth, U.; Jakob, F.; Rudert, M.; Groll, J.; Hutmacher, D.W. How smart do biomaterials need to be? A translational science and clinical point of view. Adv. Drug Deliv. Rev. 2013, 65, 581–603. [Google Scholar] [CrossRef] [PubMed]
- Welle, A.; Kröger, M.; Döring, M.; Niederer, K.; Pindel, E.; Chronakis, I.S. Electrospun aliphatic polycarbonates as tailored tissue scaffold materials. Biomater. 2007, 28, 2211–2219. [Google Scholar] [CrossRef]
- Pasche, S.; Vörös, J.; Griesser, H.J.; Spencer, N.D.; Textor, M. Effects of ionic strength and surface charge on protein adsorption at PEGylated surfaces. J. Phys. Chem. B 2005, 109, 17545–17552. [Google Scholar] [CrossRef]
- Nagai, A.; Tanaka, K.; Tanaka, Y.; Nakamura, M.; Hashimoto, K.; Yamashita, K. Electric polarization and mechanism of B-type carbonated apatite ceramics. J. Biomed. Mater. Res. Part. A 2011, 99, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Tofail, S.A.; Bauer, J. Electrically polarized biomaterials. Adv. Mater. 2016, 28, 5470–5484. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho, A.B.G.; Rahimnejad, M.; Oliveira, R.L.; Sikder, P.; Saavedra, G.S.; Bhaduri, S.B.; Gawlitta, D.; Malda, J.; Kaigler, D.; Trichês, E.S. Personalized bioceramic grafts for craniomaxillofacial bone regeneration. Int. J. Oral. Sci. 2024, 16, 62. [Google Scholar] [CrossRef]
- Bhumiratana, S.; Vunjak-Novakovic, G. Concise review: Personalized human bone grafts for reconstructing head and face. Stem cells Transl. Med. 2012, 1, 64–69. [Google Scholar] [CrossRef]
- Nakamura, M.; Nagai, A.; Hentunen, T.; Salonen, J.; Sekijima, Y.; Okura, T.; Hashimoto, K.; Toda, Y.; Monma, H.; Yamashita, K. Surface electric fields increase osteoblast adhesion through improved wettability on hydroxyapatite electret. ACS Appl. Mater. Interf. 2009, 1, 2181–2189. [Google Scholar] [CrossRef]
- Green, D.; Walsh, D.; Mann, S.; Oreffo, R.O. The potential of biomimesis in bone tissue engineering: Lessons from the design and synthesis of invertebrate skeletons. Bone 2002, 30, 810–815. [Google Scholar] [CrossRef] [PubMed]
- Gai, T.; Zhang, Y.; Li, G.; Zhou, F.; He, C.; Wang, X.; Su, J. Engineered hydrogel microspheres for spheroids and organoids construction. Chem. Eng. J. 2024, 498, 155131. [Google Scholar] [CrossRef]
- O’brien, F.J. Biomaterials & scaffolds for tissue engineering. Mater. Today 2011, 14, 88–95. [Google Scholar]
- Gregory, C.A.; Gunn, W.G.; Peister, A.; Prockop, D.J. An Alizarin red-based assay of mineralization by adherent cells in culture: Comparison with cetylpyridinium chloride extraction. Anal. Biochem. 2004, 329, 77–84. [Google Scholar] [CrossRef]
- Yamashita, K.; Oikawa, N.; Umegaki, T. Acceleration and deceleration of bone-like crystal growth on ceramic hydroxyapatite by electric poling. Chem. Mater. 1996, 8, 2697–2700. [Google Scholar] [CrossRef]
- Bodhak, S.; Bose, S.; Bandyopadhyay, A. Electrically polarized HAp-coated Ti: In vitro bone cell–material interactions. Acta Biomater. 2010, 6, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Kizuki, T.; Ohgaki, M.; Katsura, M.; Nakamura, S.; Hashimoto, K.; Toda, Y.; Udagawa, S.; Yamashita, K. Effect of bone-like layer growth from culture medium on adherence of osteoblast-like cells. Biomater. 2003, 24, 941–947. [Google Scholar] [CrossRef]
- Nakamura, M.; Nagai, A.; Tanaka, Y.; Sekijima, Y.; Yamashita, K. Polarized hydroxyapatite promotes spread and motility of osteoblastic cells. J. Biomed. Mater. Res. Part A 2010, 92, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Gittings, J.; Turner, I.G.; Bowen, C.; Bastida-Hidalgo, A.; Cartmell, S. Polarization of hydroxyapatite: Influence on osteoblast cell proliferation. Acta Biomater. 2010, 6, 1549–1554. [Google Scholar] [CrossRef]
- Agarwal, R.; García, A.J. Biomaterial strategies for engineering implants for enhanced osseointegration and bone repair. Adv. Drug Deliv. Rev. 2015, 94, 53–62. [Google Scholar] [CrossRef]
- Elgali, I.; Omar, O.; Dahlin, C.; Thomsen, P. Guided bone regeneration: Materials and biological mechanisms revisited. Eur. J. Oral. Sci. 2017, 125, 315–337. [Google Scholar] [CrossRef]
- Dirckx, N.; Moorer, M.C.; Clemens, T.L.; Riddle, R.C. The role of osteoblasts in energy homeostasis. Nat. Rev. Endocrinol. 2019, 15, 651–665. [Google Scholar] [CrossRef]
- Lin, X.; Patil, S.; Gao, Y.G.; Qian, A. The bone extracellular matrix in bone formation and regeneration. Front. Pharmacol. 2020, 11, 757. [Google Scholar] [CrossRef]
- Liang, Q.; Cheng, Z.; Qin, L. Advanced nanoparticles in osteoarthritis treatment. Biomater. Transl. 2024, 5, 95. [Google Scholar]
- Luo, J.; Yang, Z.; Ma, Y.; Yue, Z.; Lin, H.; Qu, G.; Huang, J.; Dai, W.; Li, C.; Zheng, C. LGR4 is a receptor for RANKL and negatively regulates osteoclast differentiation and bone resorption. Nat. Med. 2016, 22, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Salhotra, A.; Shah, H.N.; Levi, B.; Longaker, M.T. Mechanisms of bone development and repair. Nat. Rev. Mol. Cell Biol. 2020, 21, 696–711. [Google Scholar] [CrossRef]
- Li, M.; Yu, B.; Wang, S.; Zhou, F.; Cui, J.; Su, J. Microenvironment-responsive nanocarriers for targeted bone disease therapy. Nano Today 2023, 50, 101838. [Google Scholar] [CrossRef]
- Arnett, T.R.; Orriss, I.R. Metabolic properties of the osteoclast. Bone 2018, 115, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Bergara-Muguruza, L.; Mäkelä, K.; Yrjälä, T.; Salonen, J.; Yamashita, K.; Nakamura, M. Surface electric fields increase human osteoclast resorption through improved wettability on carbonate-incorporated apatite. ACS Appl. Mater. Interf. 2021, 13, 58270–58278. [Google Scholar] [CrossRef]
- Kartsogiannis, V.; Ng, K.W. Cell lines and primary cell cultures in the study of bone cell biology. Mol. Cell Endocrinol. 2004, 228, 79–102. [Google Scholar] [CrossRef]
- Sheikh, Z.; Abdallah, M.-N.; Hanafi, A.A.; Misbahuddin, S.; Rashid, H.; Glogauer, M. Mechanisms of in vivo degradation and resorption of calcium phosphate based biomaterials. Mater 2015, 8, 7913–7925. [Google Scholar] [CrossRef] [PubMed]
- Pu’ad, N.M.; Haq, R.A.; Noh, H.M.; Abdullah, H.; Idris, M.; Lee, T. Synthesis method of hydroxyapatite: A review. Mater. Today Proc. 2020, 29, 233–239. [Google Scholar]
- Arbez, B.; Manero, F.; Mabilleau, G.; Libouban, H.; Chappard, D. Human macrophages and osteoclasts resorb β-tricalcium phosphate in vitro but not mouse macrophages. Micron 2019, 125, 102730. [Google Scholar] [CrossRef] [PubMed]
- Pascaretti-Grizon, F.; Guillaume, B.; Terranova, L.; Arbez, B.; Libouban, H.; Chappard, D. Maxillary sinus lift with beta-tricalcium phosphate (β-TCP) in edentulous patients: A nanotomographic and Raman study. Calcif. Tissue Int. 2017, 101, 280–290. [Google Scholar] [CrossRef]
- Nyangoga, H.; Aguado, E.; Goyenvalle, E.; Baslé, M.F.; Chappard, D. A non-steroidal anti-inflammatory drug (ketoprofen) does not delay β-TCP bone graft healing. Acta Biomater. 2010, 6, 3310–3317. [Google Scholar] [CrossRef]
- Murray, D.; Rushton, N. Macrophages stimulate bone resorption when they phagocytose particles. J. Bone Jt. Surg. Br. Vol. 1990, 72, 988–992. [Google Scholar] [CrossRef]
- Matsumoto, N.; Yoshida, K.; Hashimoto, K.; Toda, Y. Dissolution mechanisms of β-tricalcium phosphate doped with monovalent metal ions. J. Ceram. Soc. Jpn. 2010, 118, 451–457. [Google Scholar] [CrossRef]
- Nozaki, K.; Koizumi, H.; Horiuchi, N.; Nakamura, M.; Okura, T.; Yamashita, K.; Nagai, A. Suppression effects of dental glass-ceramics with polarization-induced highly dense surface charges against bacterial adhesion. Dent. Mater. J. 2015, 34, 671–678. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, J.; Nozaki, K.; Hashimoto, K.; Yamashita, K.; Wakabayashi, N. Exploring the Biological Impact of β-TCP Surface Polarization on Osteoblast and Osteoclast Activity. Int. J. Mol. Sci. 2025, 26, 141. https://doi.org/10.3390/ijms26010141
Zheng J, Nozaki K, Hashimoto K, Yamashita K, Wakabayashi N. Exploring the Biological Impact of β-TCP Surface Polarization on Osteoblast and Osteoclast Activity. International Journal of Molecular Sciences. 2025; 26(1):141. https://doi.org/10.3390/ijms26010141
Chicago/Turabian StyleZheng, Jingpu, Kosuke Nozaki, Kazuaki Hashimoto, Kimihiro Yamashita, and Noriyuki Wakabayashi. 2025. "Exploring the Biological Impact of β-TCP Surface Polarization on Osteoblast and Osteoclast Activity" International Journal of Molecular Sciences 26, no. 1: 141. https://doi.org/10.3390/ijms26010141
APA StyleZheng, J., Nozaki, K., Hashimoto, K., Yamashita, K., & Wakabayashi, N. (2025). Exploring the Biological Impact of β-TCP Surface Polarization on Osteoblast and Osteoclast Activity. International Journal of Molecular Sciences, 26(1), 141. https://doi.org/10.3390/ijms26010141





