Neutrophil Extracellular Trap Formation Model Induced by Monosodium Urate and Phorbol Myristate Acetate: Involvement in MAPK Signaling Pathways
Abstract
1. Introduction
2. Results
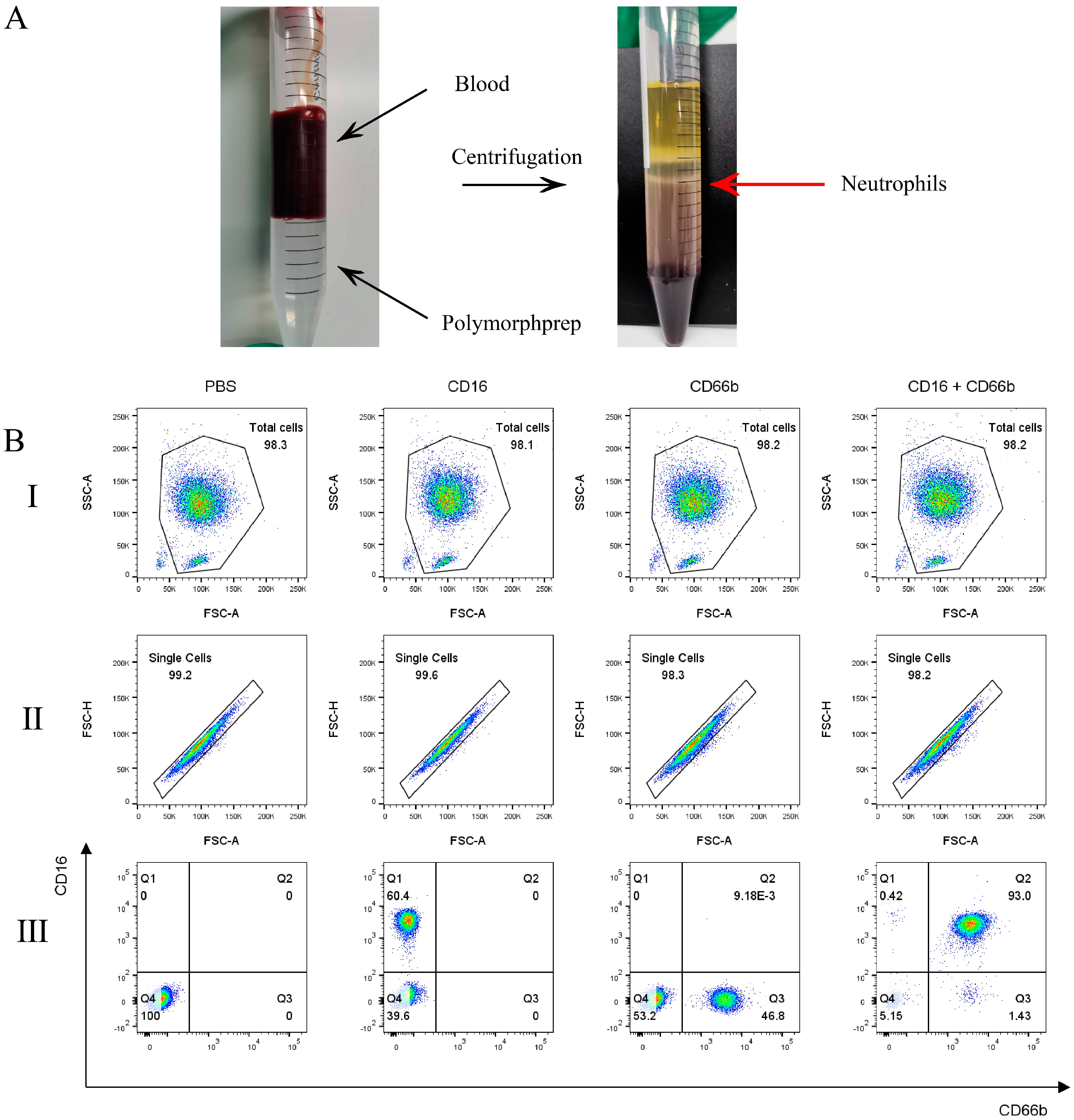
2.1. Neutrophils Purity
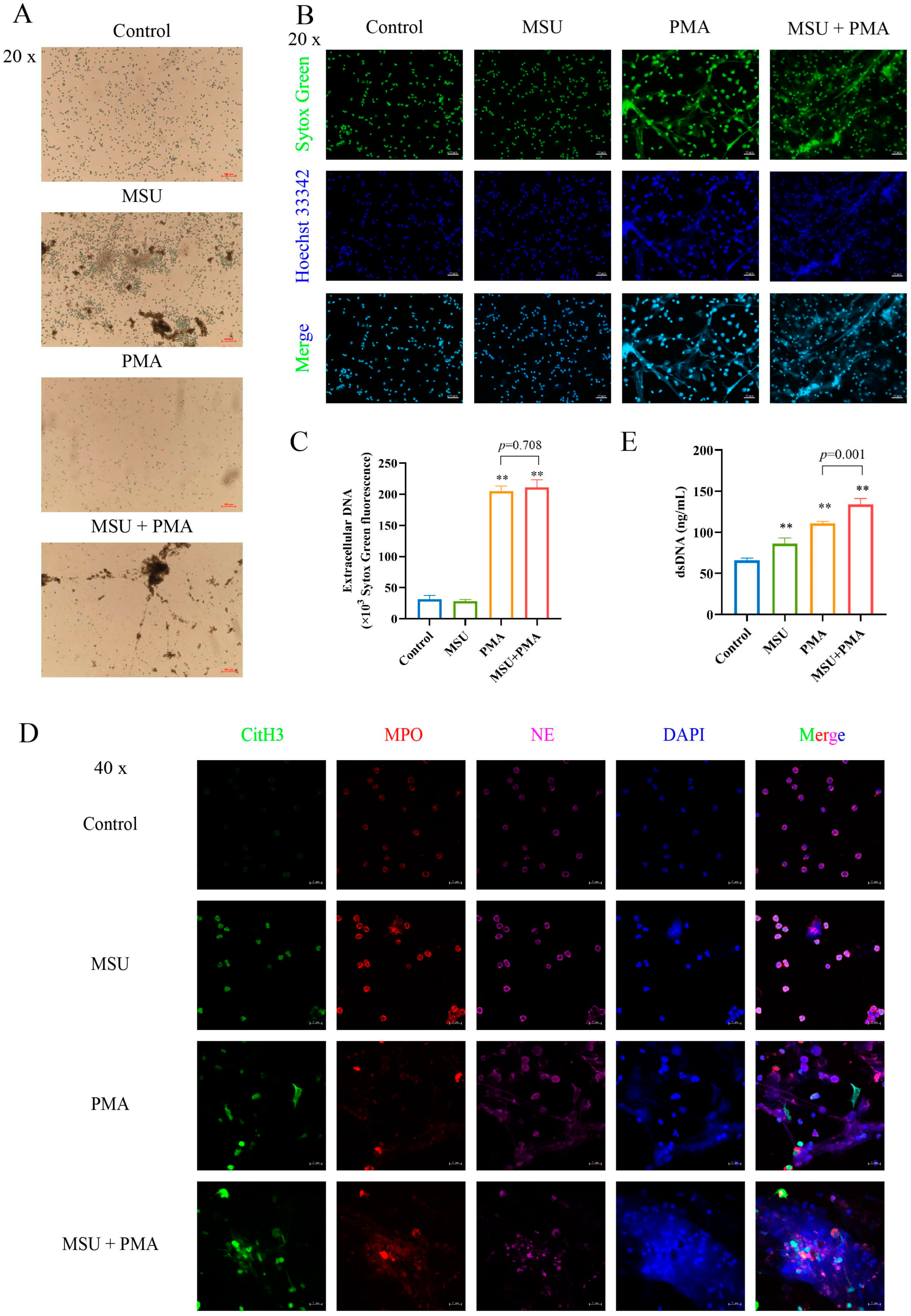
2.2. NETs Are Formed by MSU and PMA-Induced Neutrophil
2.3. MSU and PMA Promote the Release of the Intracellular Components of Neutrophils
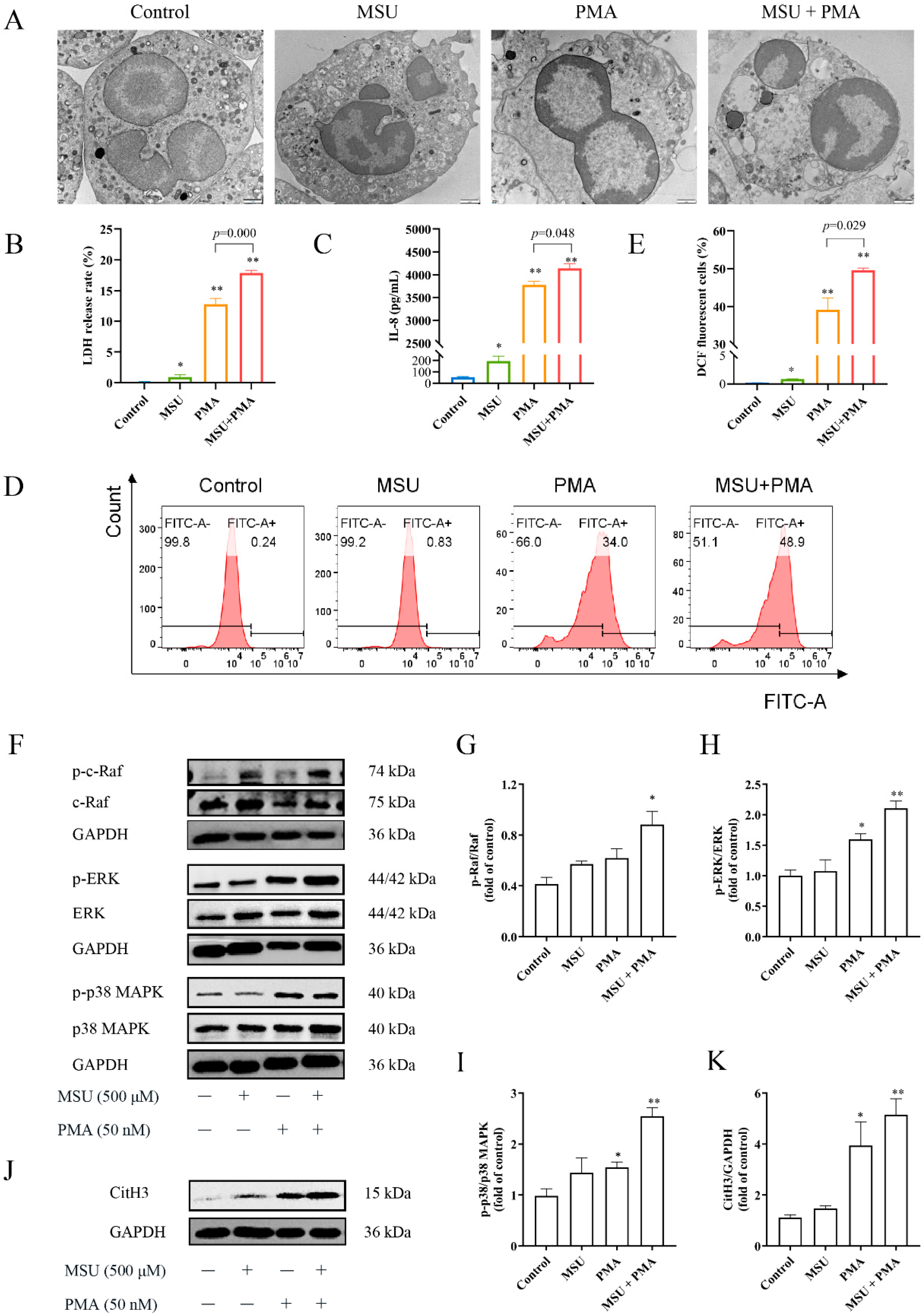
2.4. The Structure of Neutrophils Was Changed by MSU and PMA
2.5. MSU and PMA Induce Neutrophils to Release Chemokines and Produce ROS
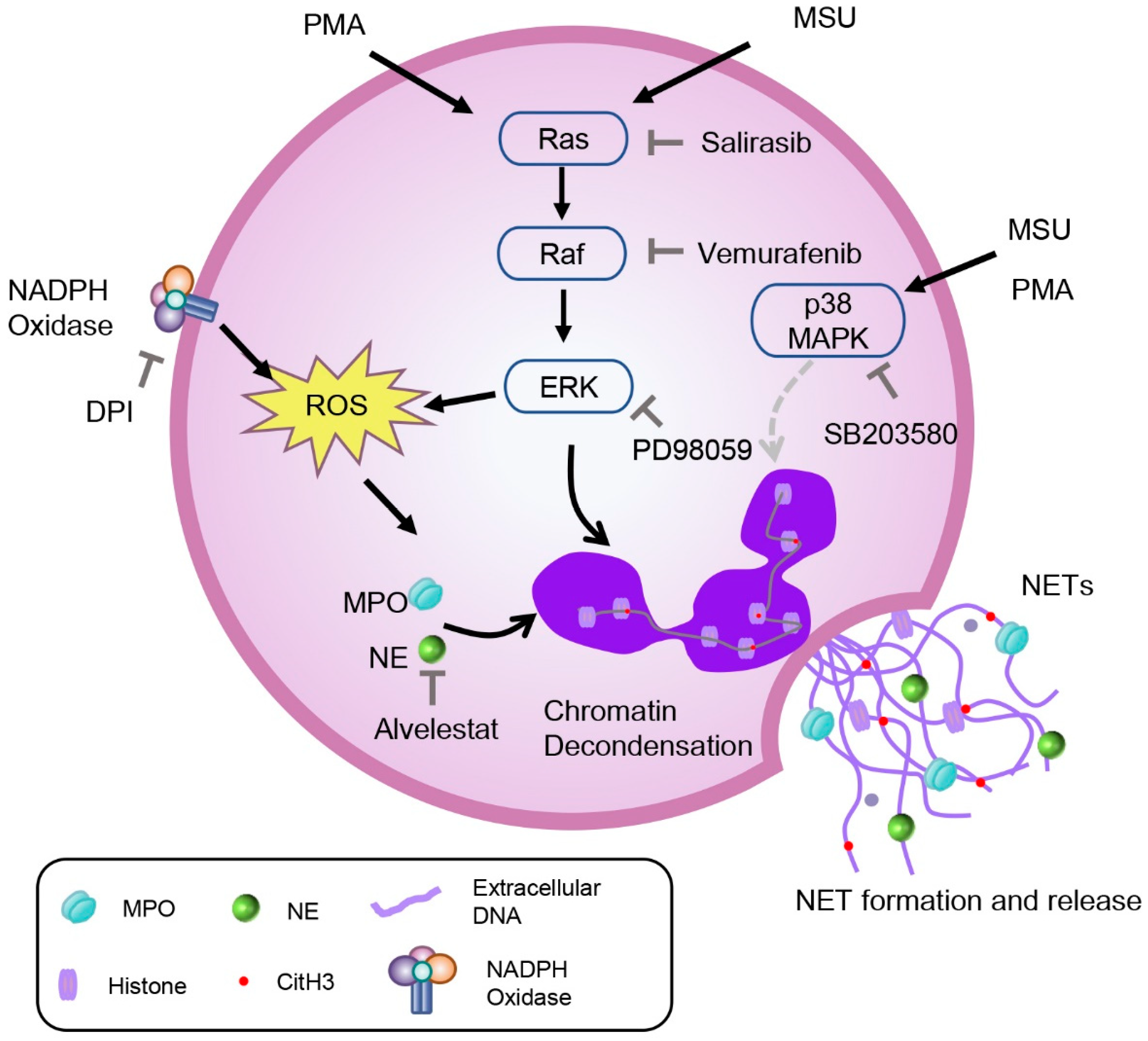
2.6. The NETs Produced by MSU and PMA Is Related to MAPK Signaling Pathway
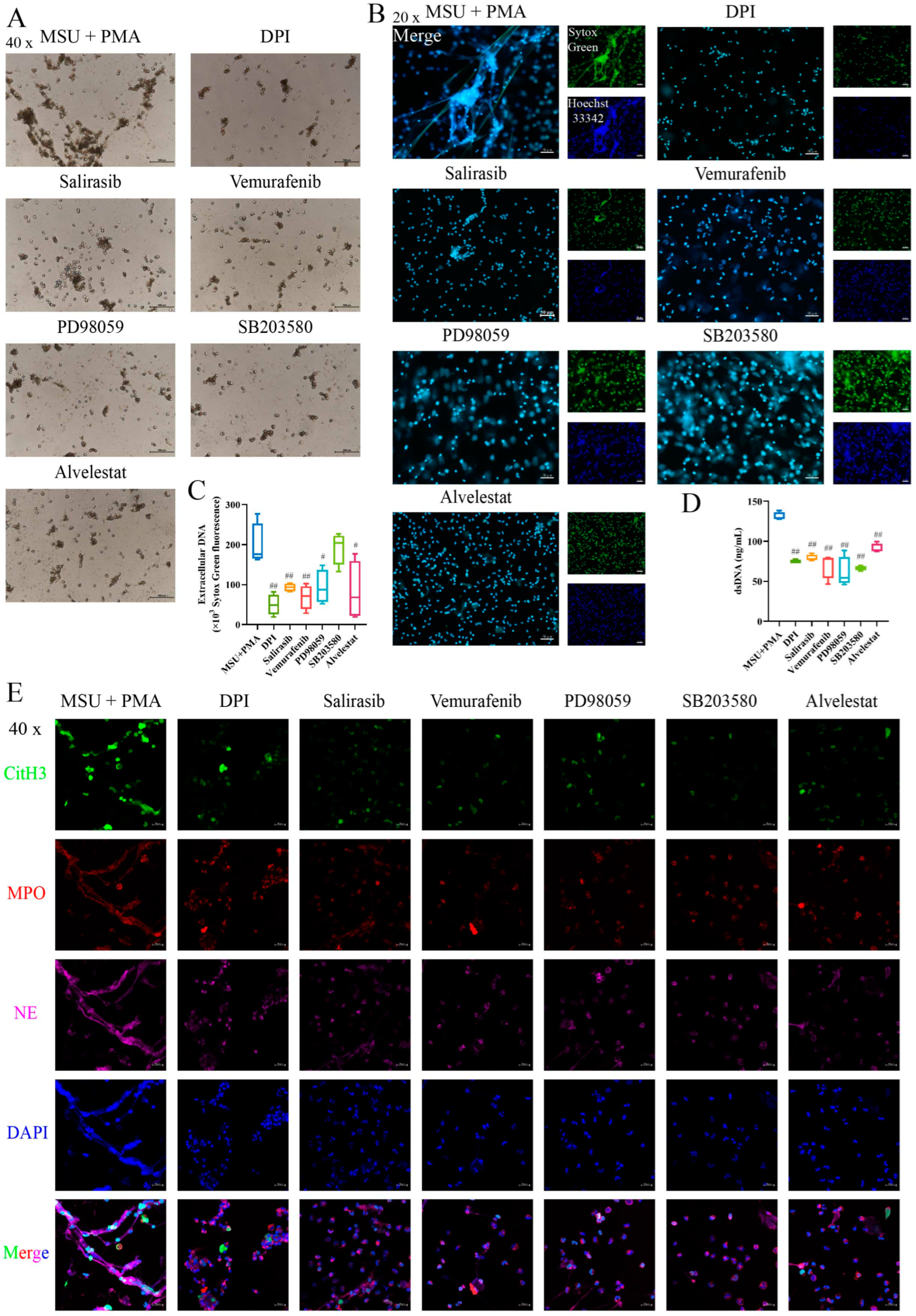
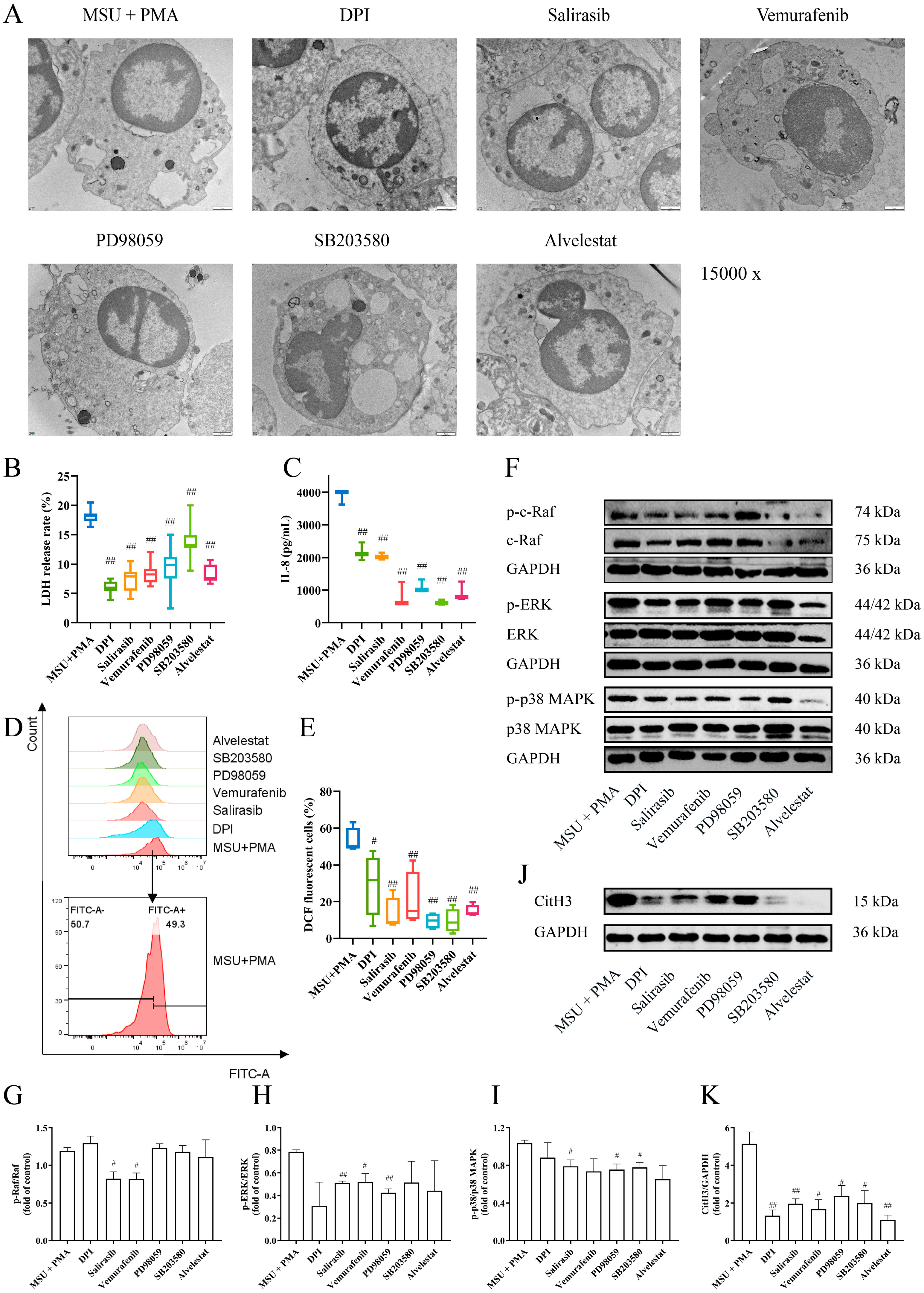
2.7. The Formation of NETs Can Be Prevented by Inhibitors of the MAPK Signaling Pathway
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Experimental Design
4.3. Extraction and Isolation of Neutrophils
4.4. Detection of Neutrophils Purity
4.5. Observation of NETs Generation
4.6. Quantification of NETs
4.7. Detection of LDH Release
4.8. Determination of IL-8 and dsDNA
4.9. Detection of ROS Generation
4.10. Observation of NETs Construction of by Laser Confocal Microscopy
4.11. Observation of NETs Microstructure by Transmission Electron Microscopy (TEM)
4.12. Western Blotting
4.13. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Ma, Y.; Wei, J.; He, W.; Ren, J. Neutrophil extracellular traps in cancer. MedComm 2024, 5, e647. [Google Scholar] [CrossRef]
- Smolarz, M.; Zawrotniak, M.; Satala, D.; Rapala-Kozik, M. Extracellular nucleic acids present in the candida albicans biofilm trigger the release of neutrophil extracellular traps. Front. Cell. Infect. Microbiol. 2021, 11, 681030. [Google Scholar] [CrossRef] [PubMed]
- Galkina, S.I.; Fedorova, N.V.; Golenkina, E.A.; Stadnichuk, V.I.; Sud’ina, G.F. Cytonemes versus neutrophil extracellular traps in the fight of neutrophils with microbes. Int. J. Mol. Sci. 2020, 21, 586. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhang, Y.; Ding, W.; Feng, C.; Wang, Y.; Wei, X.; Qu, Z.; Wang, H.; Liu, X.; Wang, H.; et al. Neutrophil extracellular traps induced by interleukin 8 via CXCR1/2 promote the progression of gastric carcinoma through transcription factor IIB-related factor 1 and cyclin. Genes Dis. 2024, 11, 575–578. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil extracellular traps kill bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef]
- Dalbeth, N.; Merriman, T.R.; Stamp, L.K. Gout. Lancet 2016, 388, 2039–2052. [Google Scholar] [CrossRef]
- Brostjan, C.; Oehler, R. The role of neutrophil death in chronic inflammation and cancer. Cell Death Discov. 2020, 6, 26. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhou, X.; Yin, Y.; Mai, Y.; Wang, D.; Zhang, X. Hyperglycemia induces neutrophil extracellular traps formation through an nadph oxidase-dependent pathway in diabetic retinopathy. Front. Immunol. 2018, 9, 3076. [Google Scholar] [CrossRef] [PubMed]
- Desai, J.; Kumar, S.V.; Mulay, S.R.; Konrad, L.; Romoli, S.; Schauer, C.; Herrmann, M.; Bilyy, R.; Müller, S.; Popper, B.; et al. PMA and crystal-induced neutrophil extracellular trap formation involves RIPK1-RIPK3-MLKL signaling. Eur. J. Immunol. 2016, 46, 223–229. [Google Scholar] [CrossRef]
- Hidalgo, A.; Libby, P.; Soehnlein, O.; Aramburu, I.V.; Papayannopoulos, V.; Silvestre-Roig, C. Neutrophil extracellular traps: From physiology to pathology. Cardiovasc. Res. 2022, 118, 2737–2753. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chandra, V.; Riquelme Sanchez, E.; Dutta, P.; Quesada, P.R.; Rakoski, A.; Zoltan, M.; Arora, N.; Baydogan, S.; Horne, W.; et al. Interleukin-17-induced neutrophil extracellular traps mediate resistance to checkpoint blockade in pancreatic cancer. J. Exp. Med. 2020, 217, e20190354. [Google Scholar] [CrossRef]
- Munir, H.; Jones, J.O.; Janowitz, T.; Hoffmann, M.; Euler, M.; Martins, C.P.; Welsh, S.J.; Shields, J.D. Stromal-driven and Amyloid β-dependent induction of neutrophil extracellular traps modulates tumor growth. Nat. Commun. 2021, 12, 683. [Google Scholar] [CrossRef] [PubMed]
- Azzouz, D.; Palaniyar, N. ROS and DNA repair in spontaneous versus agonist-induced NETosis: Context matters. Front. Immunol. 2022, 13, 1033815. [Google Scholar] [CrossRef] [PubMed]
- Azzouz, D.; Palaniyar, N. How Do ROS Induce NETosis? Oxidative DNA damage, DNA repair, and chromatin decondensation. Biomolecules 2024, 14, 1307. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.H.; Griffiths, H.R. The dual role of Reactive Oxygen Species in autoimmune and inflammatory diseases: Evidence from preclinical models. Free Radic. Biol. Med. 2018, 125, 62–71. [Google Scholar] [CrossRef]
- Ansari, J.; Vital, S.A.; Yadav, S.; Gavins, F.N.E. Regulating neutrophil PAD4/NOX-dependent cerebrovasular thromboinflammation. Int. J. Biol. Sci. 2023, 19, 852–864. [Google Scholar] [CrossRef]
- Li, M.; Gao, Y.; Wang, Z.; Wu, B.; Zhang, J.; Xu, Y.; Han, X.; Phouthapane, V.; Miao, J. Taurine inhibits Streptococcus uberis-induced NADPH oxidase-dependent neutrophil extracellular traps via TAK1/MAPK signaling pathways. Front. Immunol. 2022, 13, 927215. [Google Scholar] [CrossRef]
- Schorn, C.; Janko, C.; Krenn, V.; Zhao, Y.; Munoz, L.E.; Schett, G.; Herrmann, M. Bonding the foe-NETting neutrophils immobilize the pro-inflammatory monosodium urate crystals. Front. Immunol. 2012, 3, 376. [Google Scholar] [CrossRef]
- Papayannopoulos, V. Neutrophil extracellular traps in immunity and disease. Nat. Rev. Immunol. 2018, 18, 134–147. [Google Scholar] [CrossRef]
- Ravindran, M.; Khan, M.A.; Palaniyar, N. Neutrophil Extracellular Trap Formation: Physiology, Pathology, and Pharmacology. Biomolecules 2019, 9, 365. [Google Scholar] [CrossRef]
- Fonseca, Z.; Díaz-Godínez, C.; Mora, N.; Alemán, O.R.; Uribe-Querol, E.; Carrero, J.C.; Rosales, C. Entamoeba histolytica induce signaling via Raf/MEK/ERK for neutrophil extracellular trap (NET) formation. Front. Cell. Infect. Microbiol. 2018, 8, 226. [Google Scholar] [CrossRef]
- Khan, M.A.; Philip, L.M.; Cheung, G.; Vadakepeedika, S.; Grasemann, H.; Sweezey, N.; Palaniyar, N. Regulating NETosis: Increasing pH promotes NADPH oxidase-dependent NETosis. Front. Med. 2018, 5, 19. [Google Scholar] [CrossRef]
- Awasthi, D.; Nagarkoti, S.; Kumar, A.; Dubey, M.; Singh, A.K.; Pathak, P.; Chandra, T.; Barthwal, M.K.; Dikshit, M. Oxidized LDL induced extracellular trap formation in human neutrophils via TLR-PKC-IRAK-MAPK and NADPH-oxidase activation. Free Radic. Biol. Med. 2016, 93, 190–203. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Yu, P.; Chu, C.; Wang, Z.; Xu, W.; Cheng, F.; Zhao, H.; Qiu, Z. Magnesium hydride attenuates intestinal barrier injury during hemorrhage shock by regulating neutrophil extracellular trap formation via the ROS/MAPK/PAD4 pathway. Int. Immunopharmacol. 2024, 130, 111688. [Google Scholar] [CrossRef] [PubMed]
- Keller, S.F.; Mandell, B.F. Management and Cure of Gouty Arthritis. Med. Clin. N. Am. 2021, 105, 297–310. [Google Scholar] [CrossRef]
- Cumpelik, A.; Ankli, B.; Zecher, D.; Schifferli, J.A. Neutrophil microvesicles resolve gout by inhibiting C5a-mediated priming of the inflammasome. Ann. Rheum. Dis. 2016, 75, 1236–1245. [Google Scholar] [CrossRef]
- Desai, J.; Steiger, S.; Anders, H.-J. Molecular Pathophysiology of Gout. Trends Mol. Med. 2017, 23, 756–768. [Google Scholar] [CrossRef] [PubMed]
- Chatfield, S.M.; Grebe, K.; Whitehead, L.W.; Rogers, K.L.; Nebl, T.; Murphy, J.M.; Wicks, I.P. Monosodium urate crystals generate nuclease-resistant neutrophil extracellular traps via a distinct molecular pathway. J. Immunol. 2018, 200, 1802–1816. [Google Scholar] [CrossRef]
- Mitroulis, I.; Kambas, K.; Chrysanthopoulou, A.; Skendros, P.; Apostolidou, E.; Kourtzelis, I.; Drosos, G.I.; Boumpas, D.T.; Ritis, K. Neutrophil extracellular trap formation is associated with IL-1β and autophagy-related signaling in gout. PLoS ONE 2011, 6, e29318. [Google Scholar] [CrossRef]
- Tatsiy, O.; Mayer, T.Z.; de Carvalho Oliveira, V.; Sylvain-Prévost, S.; Isabel, M.; Dubois, C.M.; McDonald, P.P. Cytokine production and NET formation by monosodium urate-activated human neutrophils involves early and late events, and requires upstream TAK1 and Syk. Front. Immunol. 2019, 10, 2996. [Google Scholar] [CrossRef]
- Garcia-Gonzalez, E.; Gamberucci, A.; Lucherini, O.-M.; Alì, A.; Simpatico, A.; Lorenzini, S.; Lazzerini, P.-E.; Tripodi, S.; Frediani, B.; Selvi, E. Neutrophil extracellular traps release in gout and pseudogout depends on the number of crystals regardless of leukocyte count. Rheumatology 2021, 60, 4920–4928. [Google Scholar] [CrossRef] [PubMed]
- Zeng, D.; Yin, C.; Wei, H.; Li, Y.; Yang, Y.; Nie, H.; Pan, Y.; Xu, R.; Tai, Y.; Du, J.; et al. Activation of Nrf2 antioxidant signaling alleviates gout arthritis pain and inflammation. Biomed. Pharmacother. 2024, 170, 115957. [Google Scholar] [CrossRef]
- Yin, C.; Lyu, Q.; Dong, Z.; Liu, B.; Zhang, K.; Liu, Z.; Yu, Q.; Li, P.; Wei, Z.; Tai, Y.; et al. Well-defined alginate oligosaccharides ameliorate joint pain and inflammation in a mouse model of gouty arthritis. Theranostics 2024, 14, 3082–3103. [Google Scholar] [CrossRef] [PubMed]
- Hoppenbrouwers, T.; Autar, A.S.A.; Sultan, A.R.; Abraham, T.E.; van Cappellen, W.A.; Houtsmuller, A.B.; van Wamel, W.J.B.; van Beusekom, H.M.M.; van Neck, J.W.; de Maat, M.P.M. In vitro induction of NETosis: Comprehensive live imaging comparison and systematic review. PLoS ONE 2017, 12, e0176472. [Google Scholar] [CrossRef] [PubMed]
- Björnsdottir, H.; Welin, A.; Michaëlsson, E.; Osla, V.; Berg, S.; Christenson, K.; Sundqvist, M.; Dahlgren, C.; Karlsson, A.; Bylund, J. Neutrophil NET formation is regulated from the inside by myeloperoxidase-processed reactive oxygen species. Free Radic. Biol. Med. 2015, 89, 1024–1035. [Google Scholar] [CrossRef]
- Schorn, C.; Janko, C.; Latzko, M.; Chaurio, R.; Schett, G.; Herrmann, M. Monosodium urate crystals induce extracellular DNA traps in neutrophils, eosinophils, and basophils but not in mononuclear cells. Front. Immunol. 2012, 3, 277. [Google Scholar] [CrossRef]
- Wen, S.; Arakawa, H.; Tamai, I. Uric acid in health and disease: From physiological functions to pathogenic mechanisms. Pharmacol. Ther. 2024, 256, 108615. [Google Scholar] [CrossRef] [PubMed]
- Schauer, C.; Janko, C.; Munoz, L.E.; Zhao, Y.; Kienhöfer, D.; Frey, B.; Lell, M.; Manger, B.; Rech, J.; Naschberger, E.; et al. Aggregated neutrophil extracellular traps limit inflammation by degrading cytokines and chemokines. Nat. Med. 2014, 20, 511–517. [Google Scholar] [CrossRef]
- Jeong, J.H.; Choi, S.J.; Ahn, S.M.; Oh, J.S.; Kim, Y.-G.; Lee, C.-K.; Yoo, B.; Hong, S. Neutrophil extracellular trap clearance by synovial macrophages in gout. Arthritis Res. Ther. 2021, 23, 88. [Google Scholar] [CrossRef]
- Davidsson, L.; Dahlstrand Rudin, A.; Sanchez Klose, F.P.; Buck, A.; Björkman, L.; Christenson, K.; Bylund, J. In vivo transmigrated human neutrophils are highly primed for intracellular radical production induced by monosodium urate crystals. Int. J. Mol. Sci. 2020, 21, 3750. [Google Scholar] [CrossRef]
- Wu, C.; Li, F.; Zhang, X.; Xu, W.; Wang, Y.; Yao, Y.; Han, Z.; Xia, D. (-)-Epicatechin ameliorates monosodium urate-induced acute gouty arthritis through inhibiting NLRP3 inflammasome and the NF-κB signaling pathway. Front. Pharmacol. 2022, 13, 799552. [Google Scholar] [CrossRef]
- Xu, W.; Li, F.; Zhang, X.; Wu, C.; Wang, Y.; Yao, Y.; Xia, D. The protective effects of neoastilbin on monosodium urate stimulated THP-1-derived macrophages and gouty arthritis in mice through NF-κB and NLRP3 inflammasome pathways. Molecules 2022, 27, 3477. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Steiger, S. Neutrophils and extracellular traps in crystal-associated diseases. Trends Mol. Med. 2024, 30, 809–823. [Google Scholar] [CrossRef]
- Wang, H.; Kim, S.J.; Lei, Y.; Wang, S.; Wang, H.; Huang, H.; Zhang, H.; Tsung, A. Neutrophil extracellular traps in homeostasis and disease. Signal Transduct. Target. Ther. 2024, 9, 235. [Google Scholar] [CrossRef]
- Li, C.; Wu, C.; Li, F.; Xu, W.; Zhang, X.; Huang, Y.; Xia, D. Targeting Neutrophil extracellular traps in gouty arthritis: Insights into pathogenesis and therapeutic potential. J. Inflamm. Res. 2024, 17, 1735–1763. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Kronbichler, A.; Park, D.D.-Y.; Park, Y.; Moon, H.; Kim, H.; Choi, J.H.; Choi, Y.; Shim, S.; Lyu, I.S.; et al. Neutrophil extracellular traps (NETs) in autoimmune diseases: A comprehensive review. Autoimmun. Rev. 2017, 16, 1160–1173. [Google Scholar] [CrossRef] [PubMed]
- Boon, K.; Vanalken, N.; Szpakowska, M.; Chevigné, A.; Schols, D.; Van Loy, T. Systematic assessment of chemokine ligand bias at the human chemokine receptor CXCR2 indicates G protein bias over β-arrestin recruitment and receptor internalization. Cell Commun. Signal. 2024, 22, 43. [Google Scholar] [CrossRef]
- Van Avondt, K.; van der Linden, M.; Naccache, P.H.; Egan, D.A.; Meyaard, L. Signal inhibitory receptor on leukocytes-1 limits the formation of neutrophil extracellular traps, but preserves intracellular bacterial killing. J. Immunol. 2016, 196, 3686–3694. [Google Scholar] [CrossRef] [PubMed]
- van der Linden, M.; Westerlaken, G.H.A.; van der Vlist, M.; van Montfrans, J.; Meyaard, L. Differential signalling and kinetics of neutrophil extracellular trap release revealed by quantitative live imaging. Sci. Rep. 2017, 7, 6529. [Google Scholar] [CrossRef]
- Popa-Nita, O.; Rollet-Labelle, E.; Thibault, N.; Gilbert, C.; Bourgoin, S.G.; Naccache, P.H. Crystal-induced neutrophil activation. IX. Syk-dependent activation of class Ia phosphatidylinositol 3-kinase. J. Leukoc. Biol. 2007, 82, 763–773. [Google Scholar] [CrossRef] [PubMed]
- Moreau, R.; Périanin, A.; Arroyo, V. Review of defective NADPH oxidase activity and myeloperoxidase release in neutrophils from patients with cirrhosis. Front. Immunol. 2019, 10, 1044. [Google Scholar] [CrossRef] [PubMed]
- Parker, H.A.; Jones, H.M.; Kaldor, C.D.; Hampton, M.B.; Winterbourn, C.C. Neutrophil NET formation with microbial stimuli requires late stage NADPH oxidase Activity. Antioxidants 2021, 10, 1791. [Google Scholar] [CrossRef]
- Remijsen, Q.; Vanden Berghe, T.; Wirawan, E.; Asselbergh, B.; Parthoens, E.; De Rycke, R.; Noppen, S.; Delforge, M.; Willems, J.; Vandenabeele, P. Neutrophil extracellular trap cell death requires both autophagy and superoxide generation. Cell Res. 2011, 21, 290–304. [Google Scholar] [CrossRef]
- Papayannopoulos, V.; Metzler, K.D.; Hakkim, A.; Zychlinsky, A. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J. Cell Biol. 2010, 191, 677–691. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhou, X.; Tan, H.; Hu, Y.; Zhang, L.; Liu, S.; Dai, M.; Li, Y.; Li, Q.; Mao, Z.; et al. Neutrophil extracellular traps contribute to the pathogenesis of acid-aspiration-induced ALI/ARDS. Oncotarget 2018, 9, 1772–1784. [Google Scholar] [CrossRef]
- Chen, M.-S.; Lin, W.-C.; Yeh, H.-T.; Hu, C.-L.; Sheu, S.-M. Propofol specifically reduces PMA-induced neutrophil extracellular trap formation through inhibition of p-ERK and HOCl. Life Sci. 2019, 221, 178–186. [Google Scholar] [CrossRef]
- Stoiber, W.; Obermayer, A.; Steinbacher, P.; Krautgartner, W.-D. The role of reactive oxygen species (ROS) in the formation of extracellular traps (ETs) in Humans. Biomolecules 2015, 5, 702–723. [Google Scholar] [CrossRef] [PubMed]
- Dahlgren, C.; Karlsson, A.; Bylund, J. Intracellular neutrophil oxidants: From laboratory curiosity to clinical reality. J. Immunol. 2019, 202, 3127–3134. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-J.; Wei, Z.-K.; Han, Z.; Liu, Z.-Y.; Zhang, Y.; Zhu, X.-Y.; Li, X.-W.; Wang, K.; Yang, Z.-T. Sodium fluoride exposure triggered the formation of neutrophil extracellular traps. Environ. Pollut. 2020, 257, 113583. [Google Scholar] [CrossRef]
- Keshari, R.S.; Verma, A.; Barthwal, M.K.; Dikshit, M. Reactive oxygen species-induced activation of ERK and p38 MAPK mediates PMA-induced NETs release from human neutrophils. J. Cell. Biochem. 2013, 114, 532–540. [Google Scholar] [CrossRef]
- Wang, S.; Zheng, S.; Zhang, Q.; Yang, Z.; Yin, K.; Xu, S. Atrazine hinders PMA-induced neutrophil extracellular traps in carp via the promotion of apoptosis and inhibition of ROS burst, autophagy and glycolysis. Environ. Pollut. 2018, 243 Pt A, 282–291. [Google Scholar] [CrossRef]
- Chen, F.; Chu, C.; Wang, X.; Yang, C.; Deng, Y.; Duan, Z.; Wang, K.; Liu, B.; Ji, W.; Ding, W. Hesperetin attenuates sepsis-induced intestinal barrier injury by regulating neutrophil extracellular trap formation via the ROS/autophagy signaling pathway. Food Funct. 2023, 14, 4213–4227. [Google Scholar] [CrossRef] [PubMed]
- Meher, A.K.; Spinosa, M.; Davis, J.P.; Pope, N.; Laubach, V.E.; Su, G.; Serbulea, V.; Leitinger, N.; Ailawadi, G.; Upchurch, G.R. Novel Role of IL (Interleukin)-1β in neutrophil extracellular trap formation and abdominal aortic aneurysms. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 843–853. [Google Scholar] [CrossRef]
- Vaidya, K.; Tucker, B.; Kurup, R.; Khandkar, C.; Pandzic, E.; Barraclough, J.; Machet, J.; Misra, A.; Kavurma, M.; Martinez, G.; et al. Colchicine inhibits neutrophil extracellular trap formation in patients with acute coronary syndrome after percutaneous coronary intervention. J. Am. Heart Assoc. 2021, 10, e018993. [Google Scholar] [CrossRef] [PubMed]
- Björnsdottir, H.; Dahlstrand Rudin, A.; Klose, F.P.; Elmwall, J.; Welin, A.; Stylianou, M.; Christenson, K.; Urban, C.F.; Forsman, H.; Dahlgren, C.; et al. Phenol-soluble modulin α peptide toxins from aggressive staphylococcus aureus induce rapid formation of neutrophil extracellular traps through a reactive oxygen species-independent pathway. Front. Immunol. 2017, 8, 257. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, C.; Xu, X.; Shi, Y.; Li, F.; Zhang, X.; Huang, Y.; Xia, D. Neutrophil Extracellular Trap Formation Model Induced by Monosodium Urate and Phorbol Myristate Acetate: Involvement in MAPK Signaling Pathways. Int. J. Mol. Sci. 2025, 26, 143. https://doi.org/10.3390/ijms26010143
Wu C, Xu X, Shi Y, Li F, Zhang X, Huang Y, Xia D. Neutrophil Extracellular Trap Formation Model Induced by Monosodium Urate and Phorbol Myristate Acetate: Involvement in MAPK Signaling Pathways. International Journal of Molecular Sciences. 2025; 26(1):143. https://doi.org/10.3390/ijms26010143
Chicago/Turabian StyleWu, Chenxi, Xinru Xu, Yueyue Shi, Fenfen Li, Xiaoxi Zhang, Yan Huang, and Daozong Xia. 2025. "Neutrophil Extracellular Trap Formation Model Induced by Monosodium Urate and Phorbol Myristate Acetate: Involvement in MAPK Signaling Pathways" International Journal of Molecular Sciences 26, no. 1: 143. https://doi.org/10.3390/ijms26010143
APA StyleWu, C., Xu, X., Shi, Y., Li, F., Zhang, X., Huang, Y., & Xia, D. (2025). Neutrophil Extracellular Trap Formation Model Induced by Monosodium Urate and Phorbol Myristate Acetate: Involvement in MAPK Signaling Pathways. International Journal of Molecular Sciences, 26(1), 143. https://doi.org/10.3390/ijms26010143





