Resveratrol as a Promising Nutraceutical: Implications in Gut Microbiota Modulation, Inflammatory Disorders, and Colorectal Cancer
Abstract
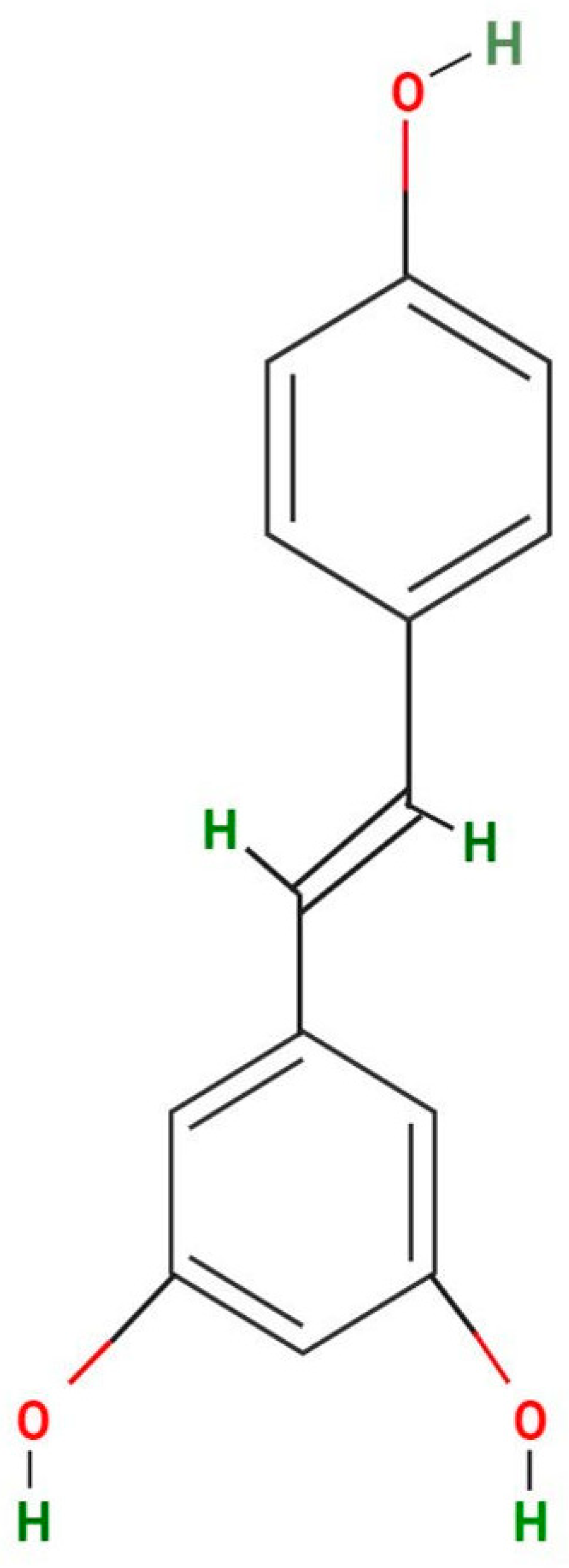
1. Introduction
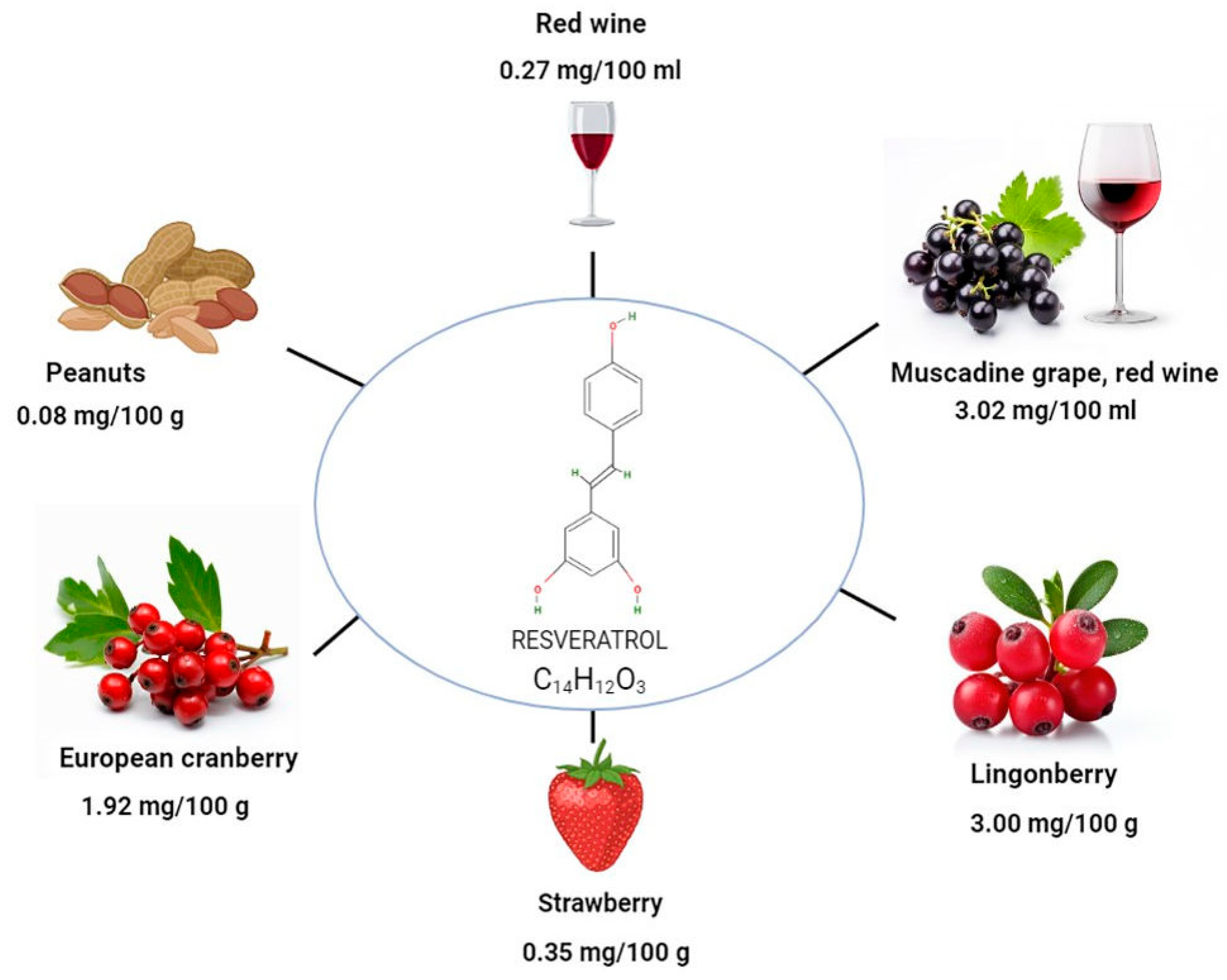
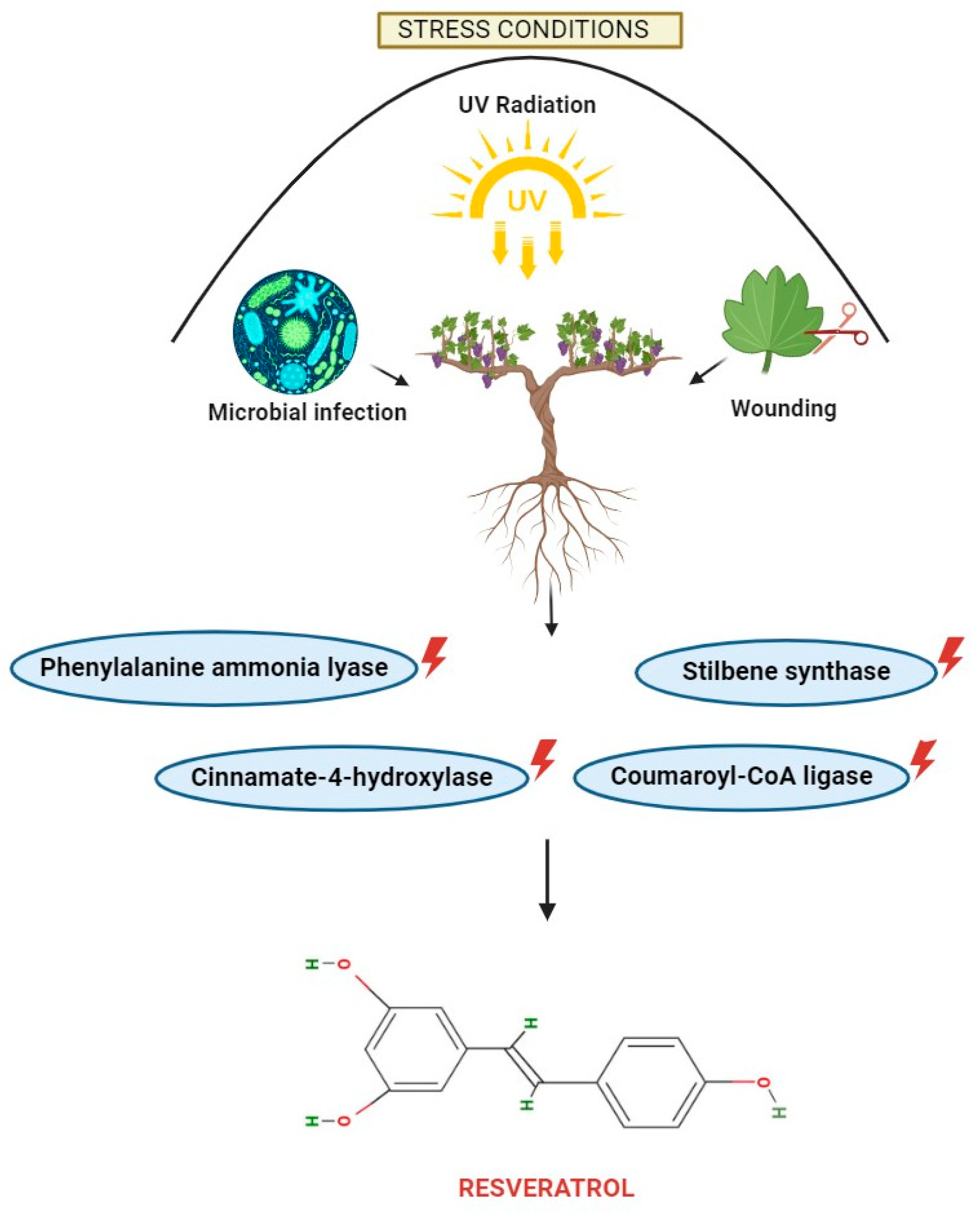
2. Different Sources of Resveratrol
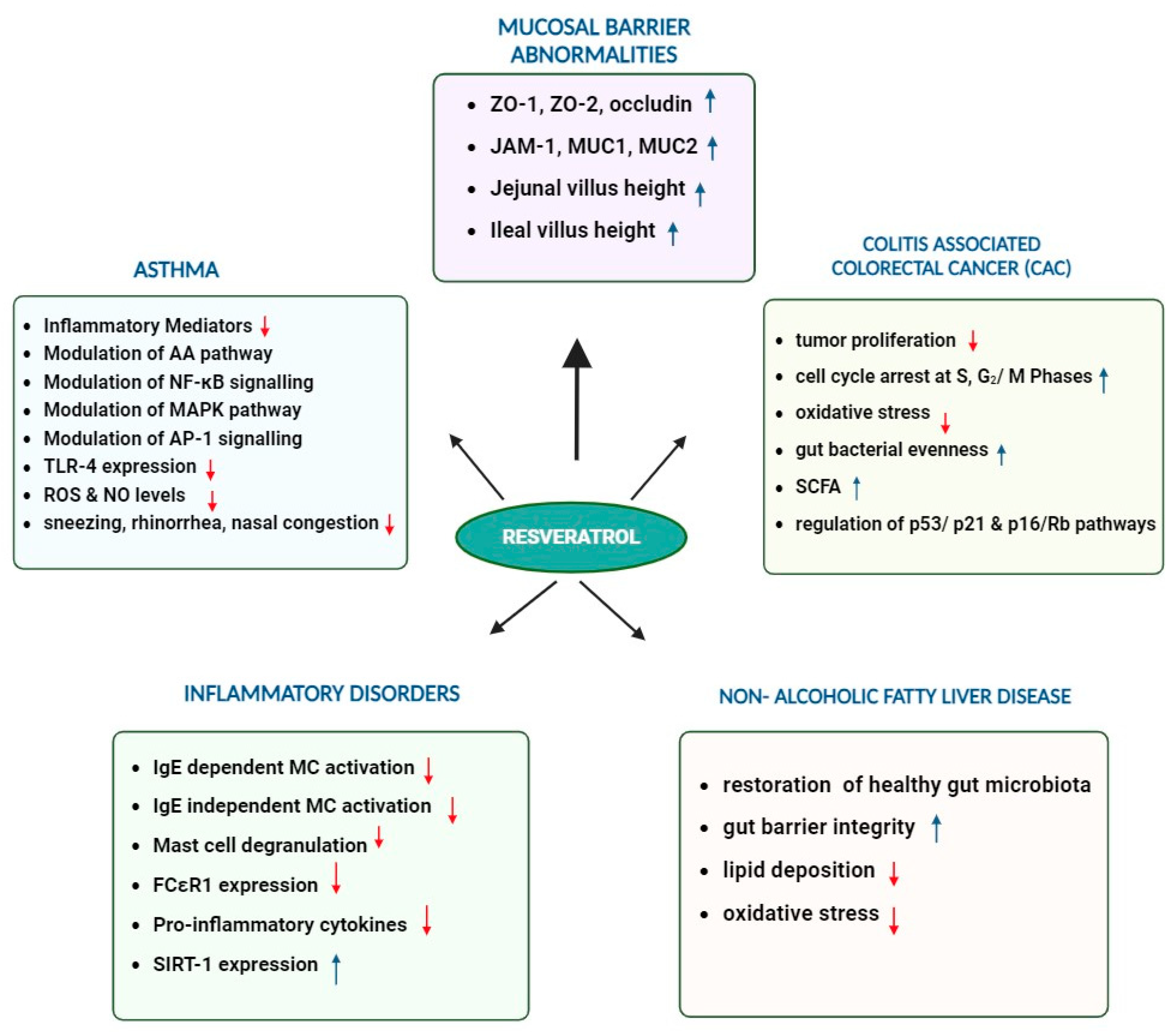
3. Multifaceted Role of Resveratrol in Enhancing Gut-Barrier Integrity, Modulating Microbiota, and Combating Metabolic Disorders
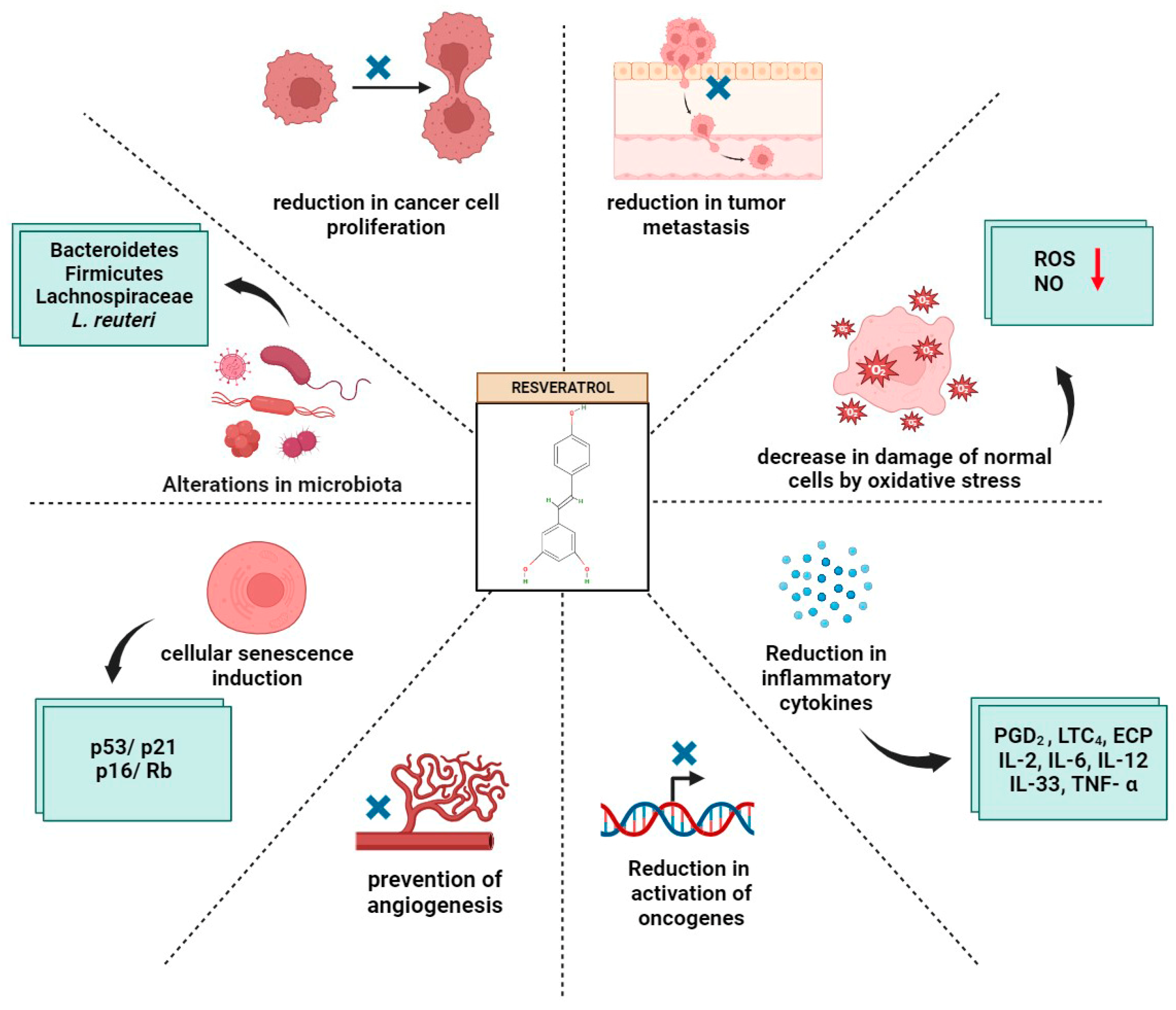
4. Potential of Resveratrol and the Gut Microbiome in Cancer Prevention
5. Anti-Inflammatory and Immunomodulatory Effects of Resveratrol in Asthma and other Inflammatory Diseases
6. Synergistic Effects of Resveratrol with Other Compounds
7. Formulations to Improve the Bioavailability of Resveratrol
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tian, B.; Liu, J. Resveratrol: A Review of Plant Sources, Synthesis, Stability, Modification and Food Application. J. Sci. Food Agric. 2020, 100, 1392–1404. [Google Scholar] [CrossRef] [PubMed]
- Jang, M.; Cai, L.; Udeani, G.O.; Slowing, K.V.; Thomas, C.F.; Beecher, C.W.W.; Fong, H.H.S.; Farnsworth, N.R.; Kinghorn, A.D.; Mehta, R.G.; et al. Cancer Chemopreventive Activity of Resveratrol, a Natural Product Derived from Grapes. Science 1997, 275, 218–220. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Zhou, J.; Zhao, C.N.; Gan, R.Y.; Li, H. Bin Health Benefits and Molecular Mechanisms of Resveratrol: A Narrative Review. Foods 2020, 9, 340. [Google Scholar] [CrossRef] [PubMed]
- Etxeberria, U.; Arias, N.; Boqué, N.; Macarulla, M.T.; Portillo, M.P.; Martínez, J.A.; Milagro, F.I. Reshaping Faecal Gut Microbiota Composition by the Intake of Trans-Resveratrol and Quercetin in High-Fat Sucrose Diet-Fed Rats. J. Nutr. Biochem. 2015, 26, 651–660. [Google Scholar] [CrossRef]
- Bonnefont-Rousselot, D. Resveratrol and Cardiovascular Diseases. Nutrients 2016, 8, 250. [Google Scholar] [CrossRef] [PubMed]
- Talebi, M.; Talebi, M.; Farkhondeh, T.; Samarghandian, S. Therapeutic Effects of Resveratrol in Inflammatory Bowel Diseases: Shedding Light on the Role of Cellular and Molecular Pathways. Rev. Bras. Farmacogn. 2022, 32, 160–173. [Google Scholar] [CrossRef]
- Ko, J.-H.; Sethi, G.; Um, J.-Y.; Shanmugam, M.K.; Arfuso, F.; Kumar, A.P.; Bishayee, A.; Ahn, K.S. The Role of Resveratrol in Cancer Therapy. Int. J. Mol. Sci. 2017, 18, 2589. [Google Scholar] [CrossRef]
- Izzo, C.; Annunziata, M.; Melara, G.; Sciorio, R.; Dallio, M.; Masarone, M.; Federico, A.; Persico, M. The Role of Resveratrol in Liver Disease: A Comprehensive Review from In Vitro to Clinical Trials. Nutrients 2021, 13, 933. [Google Scholar] [CrossRef]
- Mahjabeen, W.; Khan, D.A.; Mirza, S.A. Role of Resveratrol Supplementation in Regulation of Glucose Hemostasis, Inflammation and Oxidative Stress in Patients with Diabetes Mellitus Type 2: A Randomized, Placebo-Controlled Trial. Complement. Ther. Med. 2022, 66, 102819. [Google Scholar] [CrossRef]
- Barber, T.M.; Kabisch, S.; Randeva, H.S.; Pfeiffer, A.F.H.; Weickert, M.O. Implications of Resveratrol in Obesity and Insulin Resistance: A State-of-the-Art Review. Nutrients 2022, 14, 2870. [Google Scholar] [CrossRef]
- Singh, N.; Nagar, E.; Gautam, A.; Kapoor, H.; Arora, N. Resveratrol Mitigates MiR-212-3p Mediated Progression of Diesel Exhaust-Induced Pulmonary Fibrosis by Regulating SIRT1/FoxO3. Sci. Total Environ. 2023, 902, 166063. [Google Scholar] [CrossRef]
- Wenzel, E.; Somoza, V. Metabolism and Bioavailability of Trans-Resveratrol. Mol. Nutr. Food Res. 2005, 49, 472–481. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, Y.; Liu, X.; Yin, J.; Li, X.; Zhang, X.; Xing, X.; Wang, J.; Wang, S. Differential Protective Effect of Resveratrol and Its Microbial Metabolites on Intestinal Barrier Dysfunction Is Mediated by the AMPK Pathway. J. Agric. Food Chem. 2022, 70, 11301–11313. [Google Scholar] [CrossRef]
- Ren, Z.; Yang, J.; Zhang, M.; Huang, Y.W.; Song, D.; Sun, X.; Pan, L.; Sun, J. A Novel Resveratrol Analog Upregulates Sirtuin 1 and Inhibits Inflammatory Cell Infiltration in Acute Pancreatitis. Acta Pharmacol. Sin. 2022, 43, 1264–1273. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Xu, Y.; Lv, H.; Pang, W.; Wang, J.; Ma, H.; Wang, S. Intestinal Pharmacokinetics of Resveratrol and Regulatory Effects of Resveratrol Metabolites on Gut Barrier and Gut Microbiota. Food Chem. 2021, 357, 129532. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.M.; Bae, H. An Overview of Stress-Induced Resveratrol Synthesis in Grapes: Perspectives for Resveratrol-Enriched Grape Products. Molecules 2017, 22, 294. [Google Scholar] [CrossRef] [PubMed]
- Cosme, P.; Rodríguez, A.B.; Espino, J.; Garrido, M. Plant Phenolics: Bioavailability as a Key Determinant of Their Potential Health-Promoting Applications. Antioxidants 2020, 9, 1263. [Google Scholar] [CrossRef] [PubMed]
- Meresman, G.F.; Götte, M.; Laschke, M.W. Plants as Source of New Therapies for Endometriosis: A Review of Preclinical and Clinical Studies. Hum. Reprod. Update 2021, 27, 367–392. [Google Scholar] [CrossRef] [PubMed]
- Dull, A.M.; Moga, M.A.; Dimienescu, O.G.; Sechel, G.; Burtea, V.; Anastasiu, C.V. Therapeutic Approaches of Resveratrol on Endometriosis via Anti-Inflammatory and Anti-Angiogenic Pathways. Molecules 2019, 24, 667. [Google Scholar] [CrossRef] [PubMed]
- Cione, E.; La Torre, C.; Cannataro, R.; Caroleo, M.C.; Plastina, P.; Gallelli, L. Quercetin, Epigallocatechin Gallate, Curcumin, and Resveratrol: From Dietary Sources to Human MicroRNA Modulation. Molecules 2019, 25, 63. [Google Scholar] [CrossRef]
- Gu, C.; Howell, K.; Dunshea, F.R.; Suleria, H.A.R. LC-ESI-QTOF/MS Characterisation of Phenolic Acids and Flavonoids in Polyphenol-Rich Fruits and Vegetables and Their Potential Antioxidant Activities. Antioxidants 2019, 8, 405. [Google Scholar] [CrossRef] [PubMed]
- Fraga, C.G.; Croft, K.D.; Kennedy, D.O.; Tomás-Barberán, F.A. The Effects of Polyphenols and Other Bioactives on Human Health. Food Funct. 2019, 10, 514–528. [Google Scholar] [CrossRef] [PubMed]
- Abo-Kadoum, M.A.; Abouelela, M.E.; Al Mousa, A.A.; Abo-Dahab, N.F.; Mosa, M.A.; Helmy, Y.A.; Hassane, A.M.A. Resveratrol Biosynthesis, Optimization, Induction, Bio-Transformation and Bio-Degradation in Mycoendophytes. Front. Microbiol. 2022, 13, 1010332. [Google Scholar] [CrossRef] [PubMed]
- Thapa, S.B.; Pandey, R.P.; Il Park, Y.; Sohng, J.K. Biotechnological Advances in Resveratrol Production and Its Chemical Diversity. Molecules 2019, 24, 2571. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, L.; Zheng, Y.; Zhou, C.; Huang, P.; Xiao, X.; Zhao, Y.; Hao, X.; Hu, Z.; Chen, Q.; et al. Assembly and Annotation of a Draft Genome of the Medicinal Plant Polygonum Cuspidatum. Front. Plant Sci. 2019, 10, 1274. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Inokuma, K.; Matsuda, M.; Kondo, A.; Hasunuma, T. Resveratrol Production from Several Types of Saccharide Sources by a Recombinant Scheffersomyces Stipitis Strain. Metab. Eng. Commun. 2021, 13, e00188. [Google Scholar] [CrossRef]
- Sáez-Sáez, J.; Wang, G.; Marella, E.R.; Sudarsan, S.; Cernuda Pastor, M.; Borodina, I. Engineering the Oleaginous Yeast Yarrowia Lipolytica for High-Level Resveratrol Production. Metab. Eng. 2020, 62, 51–61. [Google Scholar] [CrossRef]
- Quideau, S.; Deffieux, D.; Douat-Casassus, C.; Pouységu, L. Plant Polyphenols: Chemical Properties, Biological Activities, and Synthesis. Angew. Chem. Int. Ed. 2011, 50, 586–621. [Google Scholar] [CrossRef] [PubMed]
- Braga, A.; Oliveira, J.; Silva, R.; Ferreira, P.; Rocha, I.; Kallscheuer, N.; Marienhagen, J.; Faria, N. Impact of the Cultivation Strategy on Resveratrol Production from Glucose in Engineered Corynebacterium glutamicum. J. Biotechnol. 2018, 265, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Mei, Y.-Z.; Liu, R.-X.; Wang, D.-P.; Wang, X.; Dai, C.-C. Biocatalysis and Biotransformation of Resveratrol in Microorganisms. Biotechnol. Lett. 2015, 37, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Halls, C.; Yu, O. Potential for Metabolic Engineering of Resveratrol Biosynthesis. Trends Biotechnol. 2008, 26, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wu, B.-H.; Liu, Z.-N.; Qiao, J.; Zhao, G.-R. Combinatorial Optimization of Resveratrol Production in Engineered E. coli. J. Agric. Food Chem. 2018, 66, 13444–13453. [Google Scholar] [CrossRef] [PubMed]
- Irnidayanti, Y.; Sutiono, D.R. Tempeh & Soybean Seed Coat: The Alternative Sources of Trans-Resveratrol as Neuroprotective Agents. Int. J. Morphol. 2019, 37, 1164–1171. [Google Scholar] [CrossRef]
- Crăciun, A.L.; Gutt, G. Study on Kinetics of Trans-Resveratrol, Total Phenolic Content, and Antioxidant Activity Increase in Vine Waste during Post-Pruning Storage. Appl. Sci. 2022, 12, 1450. [Google Scholar] [CrossRef]
- Abdelhafiz, Y.; Hussain Gora, A.; Rehman, S.; Chowdhury, S.; Park, Y.; Bisa, S.; Verlhac Trichet, V.; Fernandes, J.M.O.; Sørensen, M.; Kiron, V. Fish as the Lesser-Known Counterpart to Mammalian Models to Explore the Biofunctionality of Polyphenols. J. Funct. Foods 2023, 107, 105654. [Google Scholar] [CrossRef]
- Vesely, O.; Baldovska, S.; Kolesarova, A. Enhancing Bioavailability of Nutraceutically Used Resveratrol and Other Stilbenoids. Nutrients 2021, 13, 95. [Google Scholar] [CrossRef]
- Goldberg, D.M.; Yan, J.; Soleas, G.J. Absorption of Three Wine-Related Polyphenols in Three Different Matrices by Healthy Subjects. Clin. Biochem. 2003, 36, 79–87. [Google Scholar] [CrossRef]
- Walle, T.; Hsieh, F.; DeLegge, M.H.; Oatis, J.E.; Walle, U.K. High Absorption but Very Low Bioavailability of Oral Resveratrol in Humans. Drug Metab. Dispos. 2004, 32, 1377–1382. [Google Scholar] [CrossRef]
- La Porte, C.; Voduc, N.; Zhang, G.; Seguin, I.; Tardiff, D.; Singhal, N.; Cameron, D.W. Steady-State Pharmacokinetics and Tolerability of Trans-Resveratrol 2000 Mg Twice Daily with Food, Quercetin and Alcohol (Ethanol) in Healthy Human Subjects. Clin. Pharmacokinet. 2010, 49, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Ortuño, J.; Covas, M.I.; Farre, M.; Pujadas, M.; Fito, M.; Khymenets, O.; Andres-Lacueva, C.; Roset, P.; Joglar, J.; Lamuela-Raventós, R.M.; et al. Matrix Effects on the Bioavailability of Resveratrol in Humans. Food Chem. 2010, 120, 1123–1130. [Google Scholar] [CrossRef]
- Rotches-Ribalta, M.; Andres-Lacueva, C.; Estruch, R.; Escribano, E.; Urpi-Sarda, M. Pharmacokinetics of Resveratrol Metabolic Profile in Healthy Humans after Moderate Consumption of Red Wine and Grape Extract Tablets. Pharmacol. Res. 2012, 66, 375–382. [Google Scholar] [CrossRef]
- Lam, C.-S.; Xia, Y.-X.; Chen, B.-S.; Du, Y.-X.; Liu, K.-L.; Zhang, H.-J. Dihydro-Resveratrol Attenuates Oxidative Stress, Adipogenesis and Insulin Resistance in In Vitro Models and High-Fat Diet-Induced Mouse Model via AMPK Activation. Nutrients 2023, 15, 3006. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.; Fei, Y.; Zhang, S.; Qiu, B.; Zhu, L.; Li, F.; Berglund, B.; Xiao, H.; Li, L. Gut Microbiota Composition in Relation to the Metabolism of Oral Administrated Resveratrol. Nutrients 2022, 14, 1013. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, J.; Yin, N.; Wele, P.; Li, F.; Dave, S.; Lin, J.; Xiao, H.; Wu, X. Resveratrol in Disease Prevention and Health Promotion: A Role of the Gut Microbiome. Crit. Rev. Food Sci. Nutr. 2023, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Wang, J.; Li, D.; Ke, W.; Chen, F.; Hu, X. Targeting the Gut Microbiota with Resveratrol: A Demonstration of Novel Evidence for the Management of Hepatic Steatosis. J. Nutr. Biochem. 2020, 81, 108363. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Yang, J.; Wang, L.; Yang, X.; Gao, K.; Zhu, C.; Jiang, Z. Dietary Resveratrol Attenuation of Intestinal Inflammation and Oxidative Damage Is Linked to the Alteration of Gut Microbiota and Butyrate in Piglets Challenged with Deoxynivalenol. J. Anim. Sci. Biotechnol. 2021, 12, 71. [Google Scholar] [CrossRef] [PubMed]
- Kai, Y. Intestinal Villus Structure Contributes to Even Shedding of Epithelial Cells. Biophys. J. 2021, 120, 699–710. [Google Scholar] [CrossRef]
- Cai, T.T.; Ye, X.L.; Li, R.R.; Chen, H.; Wang, Y.Y.; Yong, H.J.; Pan, M.L.; Lu, W.; Tang, Y.; Miao, H.; et al. Resveratrol Modulates the Gut Microbiota and Inflammation to Protect Against Diabetic Nephropathy in Mice. Front. Pharmacol. 2020, 11, 1249. [Google Scholar] [CrossRef]
- Wang, P.; Li, D.; Ke, W.; Liang, D.; Hu, X.; Chen, F. Resveratrol-Induced Gut Microbiota Reduces Obesity in High-Fat Diet-Fed Mice. Int. J. Obes. 2020, 44, 213–225. [Google Scholar] [CrossRef]
- Song, Q.; Wang, Y.; Huang, L.; Shen, M.; Yu, Y.; Yu, Q.; Chen, Y.; Xie, J. Review of the Relationships among Polysaccharides, Gut Microbiota, and Human Health. Food Res. Int. 2021, 140, 109858. [Google Scholar] [CrossRef]
- Ding, R.; Goh, W.-R.; Wu, R.; Yue, X.; Luo, X.; Khine, W.W.T.; Wu, J.; Lee, Y.-K. Revisit Gut Microbiota and Its Impact on Human Health and Disease. J. Food Drug Anal. 2019, 27, 623–631. [Google Scholar] [CrossRef]
- Hsu, M.-H.; Huang, Y.-C.; Chen, Y.-C.; Sheen, J.-M.; Huang, L.-T. Increased Circulating ADMA in Young Male Rats Caused Cognitive Deficits and Increased Intestinal and Hippocampal NLRP3 Inflammasome Expression and Microbiota Composition Alterations: Effects of Resveratrol. Pharmaceuticals 2023, 16, 825. [Google Scholar] [CrossRef]
- Alghetaa, H.; Mohammed, A.; Zhou, J.; Singh, N.; Nagarkatti, M.; Nagarkatti, P. Resveratrol-Mediated Attenuation of Superantigen-Driven Acute Respiratory Distress Syndrome Is Mediated by Microbiota in the Lungs and Gut. Pharmacol. Res. 2021, 167, 105548. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Dong, J.; Wang, M.; Yan, J.; Hu, Y.; Liu, Y.; Pan, Y.; Li, H. Resveratrol Ameliorates Liver Fibrosis Induced by Nonpathogenic Staphylococcus in BALB/c Mice through Inhibiting Its Growth. Mol. Med. 2022, 28, 52. [Google Scholar] [CrossRef] [PubMed]
- Alharris, E.; Mohammed, A.; Alghetaa, H.; Zhou, J.; Nagarkatti, M.; Nagarkatti, P. The Ability of Resveratrol to Attenuate Ovalbumin-Mediated Allergic Asthma Is Associated with Changes in Microbiota Involving the Gut-Lung Axis, Enhanced Barrier Function and Decreased Inflammation in the Lungs. Front. Immunol. 2022, 13, 805770. [Google Scholar] [CrossRef] [PubMed]
- Skroza, D.; Šimat, V.; Smole Možina, S.; Katalinić, V.; Boban, N.; Generalić Mekinić, I. Interactions of Resveratrol with Other Phenolics and Activity against Food-Borne Pathogens. Food Sci. Nutr. 2019, 7, 2312–2318. [Google Scholar] [CrossRef]
- Berretta, M.; Bignucolo, A.; Di Francia, R.; Comello, F.; Facchini, G.; Ceccarelli, M.; Iaffaioli, R.V.; Quagliariello, V.; Maurea, N. Resveratrol in Cancer Patients: From Bench to Bedside. Int. J. Mol. Sci. 2020, 21, 2945. [Google Scholar] [CrossRef]
- El-Readi, M.Z.; Eid, S.; Abdelghany, A.A.; Al-Amoudi, H.S.; Efferth, T.; Wink, M. Resveratrol Mediated Cancer Cell Apoptosis, and Modulation of Multidrug Resistance Proteins and Metabolic Enzymes. Phytomedicine 2019, 55, 269–281. [Google Scholar] [CrossRef]
- Xiao, Q.; Zhu, W.; Feng, W.; Lee, S.S.; Leung, A.W.; Shen, J.; Gao, L.; Xu, C. A Review of Resveratrol as a Potent Chemoprotective and Synergistic Agent in Cancer Chemotherapy. Front. Pharmacol. 2019, 9, 1534. [Google Scholar] [CrossRef]
- Cao, Y.; Fu, Z.-D.; Wang, F.; Liu, H.-Y.; Han, R. Anti-Angiogenic Activity of Resveratrol, a Natural Compound from Medicinal Plants. J. Asian Nat. Prod. Res. 2005, 7, 205–213. [Google Scholar] [CrossRef]
- Farooqi, A.A.; Khalid, S.; Ahmad, A. Regulation of Cell Signaling Pathways and MiRNAs by Resveratrol in Different Cancers. Int. J. Mol. Sci. 2018, 19, 652. [Google Scholar] [CrossRef]
- Liang, Z.J.; Wan, Y.; Zhu, D.D.; Wang, M.X.; Jiang, H.M.; Huang, D.L.; Luo, L.F.; Chen, M.J.; Yang, W.P.; Li, H.M.; et al. Resveratrol Mediates the Apoptosis of Triple Negative Breast Cancer Cells by Reducing POLD1 Expression. Front. Oncol. 2021, 11, 569295. [Google Scholar] [CrossRef] [PubMed]
- Al Bander, Z.; Nitert, M.D.; Mousa, A.; Naderpoor, N. The Gut Microbiota and Inflammation: An Overview. Int. J. Env. Res. Public Health 2020, 17, 7618. [Google Scholar] [CrossRef] [PubMed]
- Klimesova, K.; Kverka, M.; Zakostelska, Z.; Hudcovic, T.; Hrncir, T.; Stepankova, R.; Rossmann, P.; Ridl, J.; Kostovcik, M.; Mrazek, J.; et al. Altered Gut Microbiota Promotes Colitis-Associated Cancer in IL-1 Receptor-Associated Kinase M-Deficient Mice. Inflamm. Bowel Dis. 2013, 19, 1266–1277. [Google Scholar] [CrossRef]
- Zhao, Y.; Nakatsu, C.; Jones-Hall, Y.; Jiang, Q. Supplementation of Polyphenol-Rich Grapes Attenuates Colitis, Colitis-Associated Colon Cancer, and Disease-Associated Dysbiosis in Mice, but Fails to Mitigate Colitis in Antibiotic-Treated Mice. J. Nutr. Biochem. 2022, 109, 109124. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Jiang, Q. Roles of the Polyphenol-Gut Microbiota Interaction in Alleviating Colitis and Preventing Colitis-Associated Colorectal Cancer. Adv. Nutr. 2021, 12, 546–565. [Google Scholar] [CrossRef] [PubMed]
- Alrafas, H.R.; Busbee, P.B.; Chitrala, K.N.; Nagarkatti, M.; Nagarkatti, P. Alterations in the Gut Microbiome and Suppression of Histone Deacetylases by Resveratrol Are Associated with Attenuation of Colonic Inflammation and Protection against Colorectal Cancer. J. Clin. Med. 2020, 9, 1796. [Google Scholar] [CrossRef] [PubMed]
- Long, A.G.; Lundsmith, E.T.; Hamilton, K.E. Inflammation and Colorectal Cancer. Curr. Color. Cancer Rep. 2017, 13, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Sahu, R.K.; Verma, V.V.; Kumar, A.; Tandon, S.; Chandra Das, B.; Hedau, S.T. In Silico Prediction and Interaction of Resveratrol on Methyl-CpG Binding Proteins by Molecular Docking and MD Simulations Study. RSC Adv. 2022, 12, 11493–11504. [Google Scholar] [CrossRef]
- Zhong, Z.; Guo, X.; Zheng, Y. Network Pharmacology-Based and Molecular Docking Analysis of Resveratrol’s Pharmacological Effects on Type I Endometrial Cancer. Anti-Cancer Agents Med. Chem. 2021, 22, 1933–1944. [Google Scholar] [CrossRef]
- Kumar Bukhya, C.; Manchineela, S.; L, C.K.; Nallari, P. An Insilico Approach of Resveratrol and Its Derivative Analog 3E Binding Patterns with SIRT1, COX, ER, and NF-ΚB Proteins. Int. J. Creat. Res. Thoughts 2022, 10, d921–d930. [Google Scholar]
- Furlan, V.; Bren, U. Insight into Inhibitory Mechanism of Pde4d by Dietary Polyphenols Using Molecular Dynamics Simulations and Free Energy Calculations. Biomolecules 2021, 11, 479. [Google Scholar] [CrossRef]
- González-Sarrías, A.; Espín-Aguilar, J.C.; Romero-Reyes, S.; Puigcerver, J.; Alajarín, M.; Berná, J.; Selma, M.V.; Espín, J.C. Main Determinants Affecting the Antiproliferative Activity of Stilbenes and Their Gut Microbiota Metabolites in Colon Cancer Cells: A Structure–Activity Relationship Study. Int. J. Mol. Sci. 2022, 23, 5102. [Google Scholar] [CrossRef]
- Li, F.; Han, Y.; Wu, X.; Cao, X.; Gao, Z.; Sun, Y.; Wang, M.; Xiao, H. Gut Microbiota-Derived Resveratrol Metabolites, Dihydroresveratrol and Lunularin, Significantly Contribute to the Biological Activities of Resveratrol. Front. Nutr. 2022, 9, 912591. [Google Scholar] [CrossRef]
- Trayes, K.P.; Cokenakes, S.E.H. Breast Cancer Treatment. Am. Fam. Physician 2021, 104, 171–178. [Google Scholar] [CrossRef]
- Giménez-Bastida, J.A.; Ávila-Gálvez, M.Á.; Espín, J.C.; González-Sarrías, A. Conjugated Physiological Resveratrol Metabolites Induce Senescence in Breast Cancer Cells: Role of P53/P21 and P16/Rb Pathways, and ABC Transporters. Mol. Nutr. Food Res. 2019, 63, 1900629. [Google Scholar] [CrossRef]
- Sunilkumar, D.; Drishya, G.; Chandrasekharan, A.; Shaji, S.K.; Bose, C.; Jossart, J.; Perry, J.J.P.; Mishra, N.; Kumar, G.B.; Nair, B.G. Oxyresveratrol Drives Caspase-Independent Apoptosis-like Cell Death in MDA-MB-231 Breast Cancer Cells through the Induction of ROS. Biochem. Pharmacol. 2020, 173, 113724. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Yang, T.; Xu, J.; Yang, L.; Zhao, J.; Zhang, X.; Bai, C.; Kang, J.; Ran, P.; Shen, H.; et al. Prevalence, Risk Factors, and Management of Asthma in China: A National Cross-Sectional Study. Lancet 2019, 394, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Barcik, W.; Boutin, R.C.T.; Sokolowska, M.; Finlay, B.B. The Role of Lung and Gut Microbiota in the Pathology of Asthma. Immunity 2020, 52, 241–255. [Google Scholar] [CrossRef]
- Kuo, C.-H.S.; Pavlidis, S.; Loza, M.; Baribaud, F.; Rowe, A.; Pandis, I.; Sousa, A.; Corfield, J.; Djukanovic, R.; Lutter, R.; et al. T-Helper Cell Type 2 (Th2) and Non-Th2 Molecular Phenotypes of Asthma Using Sputum Transcriptomics in U-BIOPRED. Eur. Respir. J. 2017, 49, 1602135. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Yao, Q.; Teng, F.; Cui, J.; Dong, J.; Wei, Y. Active Ingredients from Chinese Medicine Plants as Therapeutic Strategies for Asthma: Overview and Challenges. Biomed. Pharmacother. 2021, 137, 111383. [Google Scholar] [CrossRef]
- Gillissen, A.; Paparoupa, M. Inflammation and Infections in Asthma. Clin. Respir. J. 2015, 9, 257–269. [Google Scholar] [CrossRef]
- Meng, T.; Xiao, D.; Muhammed, A.; Deng, J.; Chen, L.; He, J. Anti-Inflammatory Action and Mechanisms of Resveratrol. Molecules 2021, 26, 229. [Google Scholar] [CrossRef]
- Li, J.; Zhang, F.; Li, J. The Immunoregulatory Effects of Traditional Chinese Medicine on Treatment of Asthma or Asthmatic Inflammation. Am. J. Chin. Med. 2015, 43, 1059–1081. [Google Scholar] [CrossRef]
- Gao, X.; Xu, Y.X.; Janakiraman, N.; Chapman, R.A.; Gautam, S.C. Immunomodulatory Activity of Resveratrol: Suppression of Lymphocyte Proliferation, Development of Cell-Mediated Cytotoxicity, and Cytokine Production11Abbreviations: CTLs, Cytotoxic T Lymphocytes; LAK Cells, Lymphokine Activated Killer Cells; IL-2, Interl. Biochem. Pharmacol. 2001, 62, 1299–1308. [Google Scholar] [CrossRef]
- Fuggetta, M.P.; Bordignon, V.; Cottarelli, A.; Macchi, B.; Frezza, C.; Cordiali-Fei, P.; Ensoli, F.; Ciafrè, S.; Marino-Merlo, F.; Mastino, A.; et al. Downregulation of Proinflammatory Cytokines in HTLV-1-Infected T Cells by Resveratrol. J. Exp. Clin. Cancer Res. 2016, 35, 118. [Google Scholar] [CrossRef] [PubMed]
- Corren, J.; Castro, M.; O’Riordan, T.; Hanania, N.A.; Pavord, I.D.; Quirce, S.; Chipps, B.E.; Wenzel, S.E.; Thangavelu, K.; Rice, M.S.; et al. Dupilumab Efficacy in Patients with Uncontrolled, Moderate-to-Severe Allergic Asthma. J. Allergy Clin. Immunol. Pract. 2020, 8, 516–526. [Google Scholar] [CrossRef]
- Ramratnam, S.K.; Bacharier, L.B.; Guilbert, T.W. Severe Asthma in Children. J. Allergy Clin. Immunol. Pract. 2017, 5, 889–898. [Google Scholar] [CrossRef]
- Rietschel, E.T.; Kirikae, T.; Schade, F.U.; Mamat, U.; Schmidt, G.; Loppnow, H.; Ulmer, A.J.; Zähringer, U.; Seydel, U.; Di Padova, F.; et al. Bacterial Endotoxin: Molecular Relationships of Structure to Activity and Function. FASEB J. 1994, 8, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.-M.; Zong, Y.; Sun, L.; Guo, J.-Z.; Zhang, W.; He, Y.; Song, R.; Wang, W.-M.; Xiao, C.-J.; Lu, D. Resveratrol Inhibits Inflammatory Responses via the Mammalian Target of Rapamycin Signaling Pathway in Cultured LPS-Stimulated Microglial Cells. PLoS ONE 2012, 7, e32195. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, D.M.L.; de Oliveira, A.H.S.; Coutinho, L.G.; Fontes, F.L.; de Medeiros Oliveira, R.K.; Oliveira, T.T.; Faustino, A.L.F.; Lira da Silva, V.; de Melo Campos, J.T.A.; Lajus, T.B.P.; et al. Resveratrol Decreases the Expression of Genes Involved in Inflammation through Transcriptional Regulation. Free Radic. Biol. Med. 2019, 130, 8–22. [Google Scholar] [CrossRef]
- Panaro, M.A.; Carofiglio, V.; Acquafredda, A.; Cavallo, P.; Cianciulli, A. Anti-Inflammatory Effects of Resveratrol Occur via Inhibition of Lipopolysaccharide-Induced NF-ΚB Activation in Caco-2 and SW480 Human Colon Cancer Cells. Br. J. Nutr. 2012, 108, 1623–1632. [Google Scholar] [CrossRef] [PubMed]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative Stress, Inflammation, and Cancer: How Are They Linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef] [PubMed]
- Adhami, V.M.; Afaq, F.; Ahmad, N. Suppression of Ultraviolet B Exposure-Mediated Activation of NF-ΚB in Normal Human Keratinocytes by Resveratrol. Neoplasia 2003, 5, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Pirola, L.; Fröjdö, S. Resveratrol: One Molecule, Many Targets. IUBMB Life 2008, 60, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Manna, S.K.; Mukhopadhyay, A.; Aggarwal, B.B. Resveratrol Suppresses TNF-Induced Activation of Nuclear Transcription Factors NF-ΚB, Activator Protein-1, and Apoptosis: Potential Role of Reactive Oxygen Intermediates and Lipid Peroxidation1. J. Immunol. 2000, 164, 6509–6519. [Google Scholar] [CrossRef] [PubMed]
- Yi, C.-O.; Jeon, B.T.; Shin, H.J.; Jeong, E.A.; Chang, K.C.; Lee, J.E.; Lee, D.H.; Kim, H.J.; Kang, S.S.; Cho, G.J.; et al. Resveratrol Activates AMPK and Suppresses LPS-Induced NF-ΚB-Dependent COX-2 Activation in RAW 264.7 Macrophage Cells. ACB 2011, 44, 194–203. [Google Scholar] [CrossRef]
- Jiang, H.; Duan, J.; Xu, K.; Zhang, W. Resveratrol Protects against Asthma-Induced Airway Inflammation and Remodeling by Inhibiting the HMGB1/TLR4/NF-ΚB Pathway. Exp. Ther. Med. 2019, 18, 459–466. [Google Scholar] [CrossRef]
- Saiko, P.; Szakmary, A.; Jaeger, W.; Szekeres, T. Resveratrol and Its Analogs: Defense against Cancer, Coronary Disease and Neurodegenerative Maladies or Just a Fad? Mutat. Res. /Rev. Mutat. Res. 2008, 658, 68–94. [Google Scholar] [CrossRef]
- Nakajima, S.; Ishimaru, K.; Kobayashi, A.; Yu, G.; Nakamura, Y.; Oh-oka, K.; Suzuki-Inoue, K.; Kono, K.; Nakao, A. Resveratrol Inhibits IL-33–Mediated Mast Cell Activation by Targeting the MK2/3–PI3K/Akt Axis. Sci. Rep. 2019, 9, 18423. [Google Scholar] [CrossRef] [PubMed]
- Alam, S.B.; Wagner, A.; Willows, S.; Kulka, M. Quercetin and Resveratrol Differentially Decrease Expression of the High-Affinity IgE Receptor (FcεRI) by Human and Mouse Mast Cells. Molecules 2022, 27, 6704. [Google Scholar] [CrossRef] [PubMed]
- Civelek, M.; Bilotta, S.; Lorentz, A. Resveratrol Attenuates Mast Cell Mediated Allergic Reactions: Potential for Use as a Nutraceutical in Allergic Diseases? Mol. Nutr. Food Res. 2022, 66, 2200170. [Google Scholar] [CrossRef]
- Bilotta, S.; Arbogast, J.; Schart, N.; Frei, M.; Lorentz, A. Resveratrol Treatment Prevents Increase of Mast Cells in Both Murine OVA Enteritis and IL-10−/− Colitis. Int. J. Mol. Sci. 2022, 23, 1213. [Google Scholar] [CrossRef]
- Bilotta, S.; Paruchuru, L.B.; Feilhauer, K.; Köninger, J.; Lorentz, A. Resveratrol Is a Natural Inhibitor of Human Intestinal Mast Cell Activation and Phosphorylation of Mitochondrial Erk1/2 and Stat3. Int. J. Mol. Sci. 2021, 22, 7640. [Google Scholar] [CrossRef]
- Liu, H.; Hou, C.; Wang, G.; Jia, H.; Yu, H.; Zeng, X.; Thacker, P.A.; Zhang, G.; Qiao, S. Lactobacillus reuteri I5007 Modulates Intestinal Host Defense Peptide Expression in the Model of IPEC-J2 Cells and Neonatal Piglets. Nutrients 2017, 9, 559. [Google Scholar] [CrossRef]
- Chimento, A.; De Amicis, F.; Sirianni, R.; Sinicropi, M.S.; Puoci, F.; Casaburi, I.; Saturnino, C.; Pezzi, V. Progress to Improve Oral Bioavailability and Beneficial Effects of Resveratrol. Int. J. Mol. Sci. 2019, 20, 1381. [Google Scholar] [CrossRef] [PubMed]
- Anusmitha, K.M.; Aruna, M.; Job, J.T.; Narayanankutty, A.; Benil, P.B.; Rajagopal, R.; Alfarhan, A.; Barcelo, D. Phytochemical Analysis, Antioxidant, Anti-Inflammatory, Anti-Genotoxic, and Anticancer Activities of Different Ocimum Plant Extracts Prepared by Ultrasound-Assisted Method. Physiol. Mol. Plant Pathol. 2022, 117, 101746. [Google Scholar] [CrossRef]
- del Valle, P.; García-Armesto, M.R.; de Arriaga, D.; González-Donquiles, C.; Rodríguez-Fernández, P.; Rúa, J. Antimicrobial Activity of Kaempferol and Resveratrol in Binary Combinations with Parabens or Propyl Gallate against Enterococcus faecalis. Food Control 2016, 61, 213–220. [Google Scholar] [CrossRef]
- Berman, A.Y.; Motechin, R.A.; Wiesenfeld, M.Y.; Holz, M.K. The Therapeutic Potential of Resveratrol: A Review of Clinical Trials. NPJ Precis. Oncol. 2017, 1, 35. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Xu, C.; Li, N.; Wang, F.; Wang, F.; Li, Z.; Yu, Q.; Zhang, G. Synergistic Anti-Inflammatory Effects of Resveratrol and Vitamin E in Lipopolysaccharide-Induced RAW264.7 Cells. Food Sci. Technol. 2022, 42, e24122. [Google Scholar] [CrossRef]
- Prakash, V.; Krishnan, A.S.; Ramesh, R.; Bose, C.; Pillai, G.G.; Nair, B.G.; Pal, S. Synergistic Effects of Limosilactobacillus fermentum ASBT-2 with Oxyresveratrol Isolated from Coconut Shell Waste. Foods 2021, 10, 2548. [Google Scholar] [CrossRef] [PubMed]
- Najafi, M.; Cheki, M.; Amini, P.; Javadi, A.; Shabeeb, D.; Eleojo Musa, A. Evaluating the Protective Effect of Resveratrol, Q10, and Alpha-Lipoic Acid on Radiation-Induced Mice Spermatogenesis Injury: A Histopathological Study. Int. J. Reprod. Biomed. 2019, 17, 907–914. [Google Scholar] [CrossRef]
- Sarkar, F.H.; Li, Y.; Wang, Z.; Kong, D. Cellular Signaling Perturbation by Natural Products. Cell Signal 2009, 21, 1541–1547. [Google Scholar] [CrossRef]
- Zahid, M.; Saeed, M.; Beseler, C.; Rogan, E.G.; Cavalieri, E.L. Resveratrol and N-Acetylcysteine Block the Cancer-Initiating Step in MCF-10F Cells. Free Radic. Biol. Med. 2011, 50, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Komina, O.; Węsierska-Gądek, J. Action of Resveratrol Alone or in Combination with Roscovitine, a CDK Inhibitor, on Cell Cycle Progression in Human HL-60 Leukemia Cells. Biochem. Pharmacol. 2008, 76, 1554–1562. [Google Scholar] [CrossRef]
- Zheng, Y.; Jia, R.; Li, J.; Tian, X.; Qian, Y. Curcumin- and Resveratrol-Co-Loaded Nanoparticles in Synergistic Treatment of Hepatocellular Carcinoma. J. Nanobiotechnol. 2022, 20, 339. [Google Scholar] [CrossRef]
- Qian, W.; Xiao, Q.; Wang, L.; Qin, T.; Xiao, Y.; Li, J.; Yue, Y.; Zhou, C.; Duan, W.; Ma, Q.; et al. Resveratrol Slows the Tumourigenesis of Pancreatic Cancer by Inhibiting NFκB Activation. Biomed. Pharmacother. 2020, 127, 110116. [Google Scholar] [CrossRef] [PubMed]
- Bostan, M.; Mihaila, M.; Petrica-Matei, G.G.; Radu, N.; Hainarosie, R.; Stefanescu, C.D.; Roman, V.; Diaconu, C.C. Resveratrol Modulation of Apoptosis and Cell Cycle Response to Cisplatin in Head and Neck Cancer Cell Lines. Int. J. Mol. Sci. 2021, 22, 6322. [Google Scholar] [CrossRef]
- Muhanmode, Y.; Wen, M.K.; Maitinuri, A.; Shen, G. Curcumin and resveratrol inhibit chemoresistance in cisplatin-resistant epithelial ovarian cancer cells via targeting P13K pathway. Hum. Exp. Toxicol. 2022, 41, 09603271221095929. [Google Scholar] [CrossRef]
- Liu, Y.; Song, X.; Wu, M.; Wu, J.; Liu, J. Synergistic Effects of Resveratrol and Temozolomide Against Glioblastoma Cells: Underlying Mechanism and Therapeutic Implications. Cancer Manag. Res. 2020, 12, 8341–8354. [Google Scholar] [CrossRef]
- Bahadur, S.; Sahu, A.K.; Baghel, P.; Saha, S. Current Promising Treatment Strategy for Glioblastoma Multiform: A Review. Oncol. Rev. 2019, 13, 417. [Google Scholar] [CrossRef]
- Hou, C.-Y.; Tain, Y.-L.; Yu, H.-R.; Huang, L.-T. The Effects of Resveratrol in the Treatment of Metabolic Syndrome. Int. J. Mol. Sci. 2019, 20, 535. [Google Scholar] [CrossRef]
- Cortés-Rojo, C.; Vargas-Vargas, M.A.; Olmos-Orizaba, B.E.; Rodríguez-Orozco, A.R.; Calderón-Cortés, E. Interplay between NADH Oxidation by Complex I, Glutathione Redox State and Sirtuin-3, and Its Role in the Development of Insulin Resistance. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2020, 1866, 165801. [Google Scholar] [CrossRef]
- Li, H.; Zheng, F.; Zhang, Y.; Sun, J.; Gao, F.; Shi, G. Resveratrol, Novel Application by Preconditioning to Attenuate Myocardial Ischemia/Reperfusion Injury in Mice through Regulate AMPK Pathway and Autophagy Level. J. Cell Mol. Med. 2022, 26, 4216–4229. [Google Scholar] [CrossRef]
- Abo Alrob, O.; Al-Horani, R.A.; Altaany, Z.; Nusair, M.B. Synergistic Beneficial Effects of Resveratrol and Diet on High-Fat Diet-Induced Obesity. Medicina 2022, 58, 1301. [Google Scholar] [CrossRef]
- Gray, B.P.; Brown, K.C. Combinatorial Peptide Libraries: Mining for Cell-Binding Peptides. Chem. Rev. 2014, 114, 1020–1081. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Lee, S.S.; Chen, J.; Sun, H.; Zhao, Y.; Chai, Z.; Hu, Y. High-Throughput Screening of Substrate Specificity for Protein Tyrosine Phosphatases (PTPs) on Phosphopeptide Microarrays. Methods Mol. Biol. 2016, 1368, 181–196. [Google Scholar] [CrossRef] [PubMed]
- Amri, A.; Chaumeil, J.C.; Sfar, S.; Charrueau, C. Administration of Resveratrol: What Formulation Solutions to Bioavailability Limitations? J. Control. Release 2012, 158, 182–193. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, N.K.; Nargi, D.; Randolph, C.; Narayanan, B.A. Liposome encapsulation of curcumin and resveratrol in combination reduces prostate cancer incidence in PTEN knockout mice. Int. J. Cancer. 2009, 125, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Teskac, K.; Kristl, J. The evidence for solid lipid nanoparticles mediated cell uptake of resveratrol. Int. J. Pharm. 2010, 390, 61–69. [Google Scholar] [CrossRef] [PubMed]
- López-Nicolás, J.M.; Núñez-Delicado, E.; Pérez-López, A.J.; Barrachina, A.C.; Cuadra-Crespo, P. Determination of stoichiometric coefficients and apparent formation constants for beta-cyclodextrin complexes of trans-resveratrol using reversed-phase liquid chromatography. J. Chromatogr. A. 2006, 1135, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Li, X.; Lu, X.; Jiang, C.; Hu, Y.; Li, Q.; You, Y.; Fu, Z. Enhanced growth inhibition effect of resveratrol incorporated into biodegradable nanoparticles against glioma cells is mediated by the induction of intracellular reactive oxygen species levels. Colloids Surf. B Biointerfaces 2009, 72, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.F.; Chen, J.K.; Liao, M.H.; Lo, H.M.; Fang, J.Y. Development and evaluation of emulsion-liposome blends for resveratrol delivery. J. Nanosci. Nanotechnol. 2006, 6, 2950–2958. [Google Scholar] [CrossRef] [PubMed]
| Microorganism | Associated Molecule | Observations/Inferences | Ref. |
|---|---|---|---|
| Bacteroidetes, Actinobacteria, Cyanobacteria | Lunularin | Metabolizes resveratrol to lunularin. | [35] |
| Slackia equolifaciens, Adlercreutzia equolifaciens | Dihydroresveratrol, lunularin | Chemopreventive effects in renal and colon cancer cell lines in association with the production of dihydroresveratrol and lunularin. | [35] |
| Desulfovibrio | Resveratrol | Resveratrol supplementation reduced bacterial counts, and downregulated inflammation. | [45] |
| Alistipes | Resveratrol | Decreased abundance of this specific bacterium has been linked to obesity and metabolic disorders. | [45] |
| Allobaculum, Blautia | Resveratrol | Elevation of these bacterial levels resulted in enhanced synthesis of SCFAs in the gut. | [45] |
| Roseburia | Resveratrol | Significant increase in levels of this bacteria, which was established to alleviate intestinal inflammation. | [46] |
| Anaerotruncus | Resveratrol | Resveratrol supplementation effectively rescued the bacterial levels to the homeostatic state. | [52] |
| Lactobacillus reuteri | Resveratrol | Significant augmentation of the levels of this well-known probiotic. | [53] |
| Staphylococcus xylosus, Staphylococcus lentus | Resveratrol | Reduction in levels resulted in the alleviation of CCl4-induced liver fibrosis. | [54] |
| Verrucomicrobia | Lunularin | Metabolic conversion of resveratrol to lunularin. | [55] |
| Bacteroides acidifaciens | Resveratrol | An increase in these bacteria resulted in enhanced production of SCFAs in gut. | [55] |
| Staphylococcus aureus, Bacillus cereus, Escherichia coli | Resveratrol + kaempferol | The combinatorial supplementation of these compounds demonstrated a synergistic antimicrobial impact. | [56] |
| Cells Involved | Downregulated Molecules/Signaling Pathways | Ref. |
|---|---|---|
| Monocytes | PGE2, COX-2, IL-1, IL-8, TNF α, monocyte chemoattractant protein-1 ROS and NO species | [90,91] |
| Macrophages | IL-2, TNF α | [85] |
| Lymphocytes | IL-2, IFN γ | [85] |
| Spleen cells | IL-2, Con A, and allo-antigen-induced proliferation, IL-17 mRNA expression and protein release | [85] |
| Mast cells | MK2/3-PI3K/AKT signaling, FCεR1 expression | [101,103] |
| Compounds | Observations | Inferences | Ref. |
|---|---|---|---|
| Resveratrol + Kaempferol Resveratrol + Rutin | Inhibited S. aureus, B. cereus, and E. coli | Inhibitory potential against Gram-positive bacteria. | [56] |
| Resveratrol + Vitamin E | Downregulated TLR4, pN-Bp65, and pIB signaling | Demonstration of anti-inflammatory synergistic effect in murine macrophage cell lines. | [110] |
| Oxyresveratrol + L. fermentum ASBT-2 | Inhibited Salmonella enterica in the gut | Enhancement of probiotic activity targeting pathogens in the gut. | [111] |
| Resveratrol + N-acetyl cysteine | Decrease in estrogen–adduct formation | Downregulates adduct formation, which is a crucial factor in breast cancer initiation. | [114] |
| Resveratrol + ROSC | Increase in cell-cycle arrest of leukemia cells at the G1 phase | Prominent synergistic impact against the cancer of blood-forming tissues. | [115] |
| Resveratrol + Cisplatin | Effective treatment in many malignancies. Reduction in cisplatin chemoresistance. | A suitable adjunct therapy, which can be considered along with the current standard of care to reduce side effects. | [116,118,119] |
| Resveratrol + Temozolamide | Lowered MGMT expression. Induction of apoptosis and cell-cycle arrest in glioma cells | An exemplary synergistic combination against glioblastoma. | [120] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prakash, V.; Bose, C.; Sunilkumar, D.; Cherian, R.M.; Thomas, S.S.; Nair, B.G. Resveratrol as a Promising Nutraceutical: Implications in Gut Microbiota Modulation, Inflammatory Disorders, and Colorectal Cancer. Int. J. Mol. Sci. 2024, 25, 3370. https://doi.org/10.3390/ijms25063370
Prakash V, Bose C, Sunilkumar D, Cherian RM, Thomas SS, Nair BG. Resveratrol as a Promising Nutraceutical: Implications in Gut Microbiota Modulation, Inflammatory Disorders, and Colorectal Cancer. International Journal of Molecular Sciences. 2024; 25(6):3370. https://doi.org/10.3390/ijms25063370
Chicago/Turabian StylePrakash, Vidhya, Chinchu Bose, Damu Sunilkumar, Robin Mathew Cherian, Shwetha Susan Thomas, and Bipin G. Nair. 2024. "Resveratrol as a Promising Nutraceutical: Implications in Gut Microbiota Modulation, Inflammatory Disorders, and Colorectal Cancer" International Journal of Molecular Sciences 25, no. 6: 3370. https://doi.org/10.3390/ijms25063370
APA StylePrakash, V., Bose, C., Sunilkumar, D., Cherian, R. M., Thomas, S. S., & Nair, B. G. (2024). Resveratrol as a Promising Nutraceutical: Implications in Gut Microbiota Modulation, Inflammatory Disorders, and Colorectal Cancer. International Journal of Molecular Sciences, 25(6), 3370. https://doi.org/10.3390/ijms25063370






