Enhanced Antioxidant and Neuroprotective Properties of Pterostilbene (Resveratrol Derivative) in Amorphous Solid Dispersions
Abstract
1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Preparation of Physical Mixtures and Amorphous Solid Dispersion (ASD)
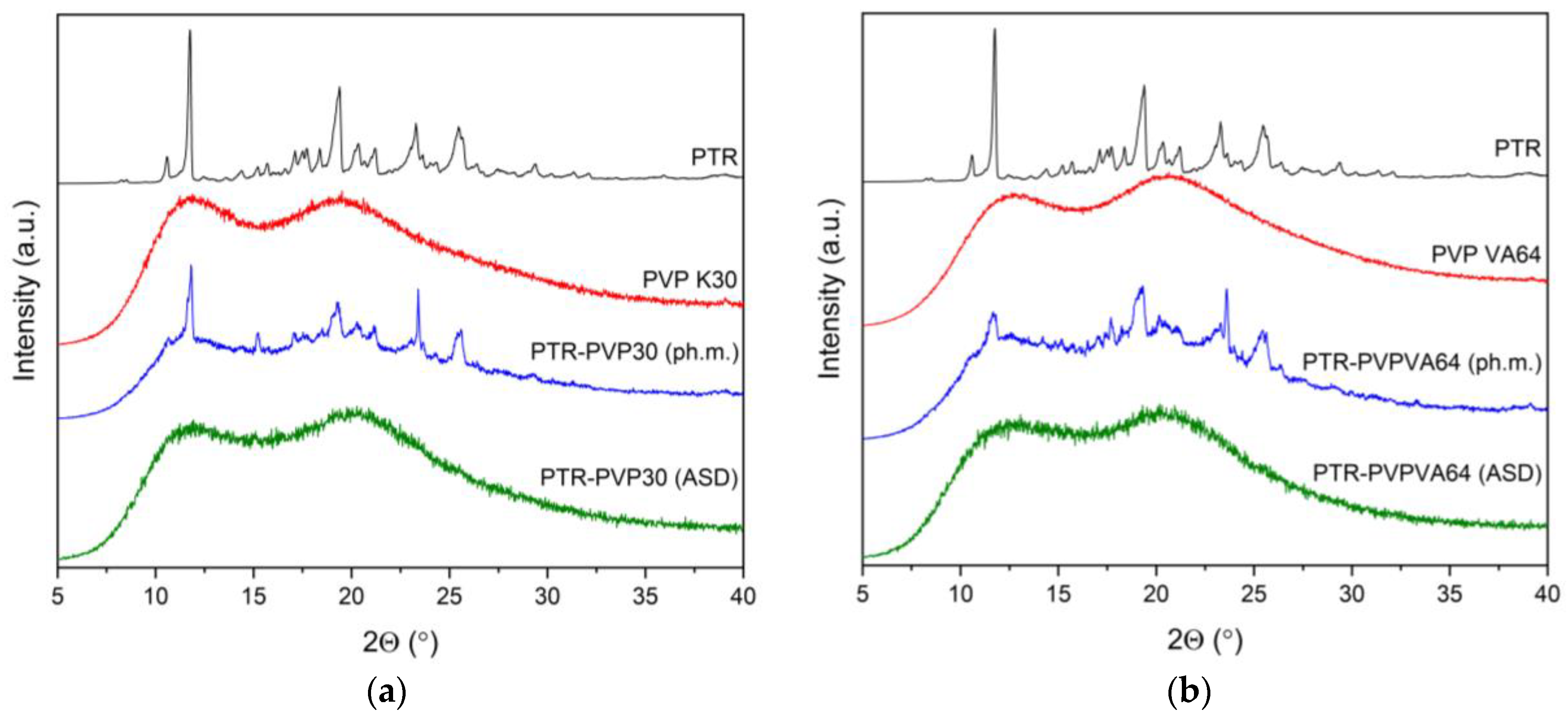
3.3. X-ray Powder Diffraction (XRPD)
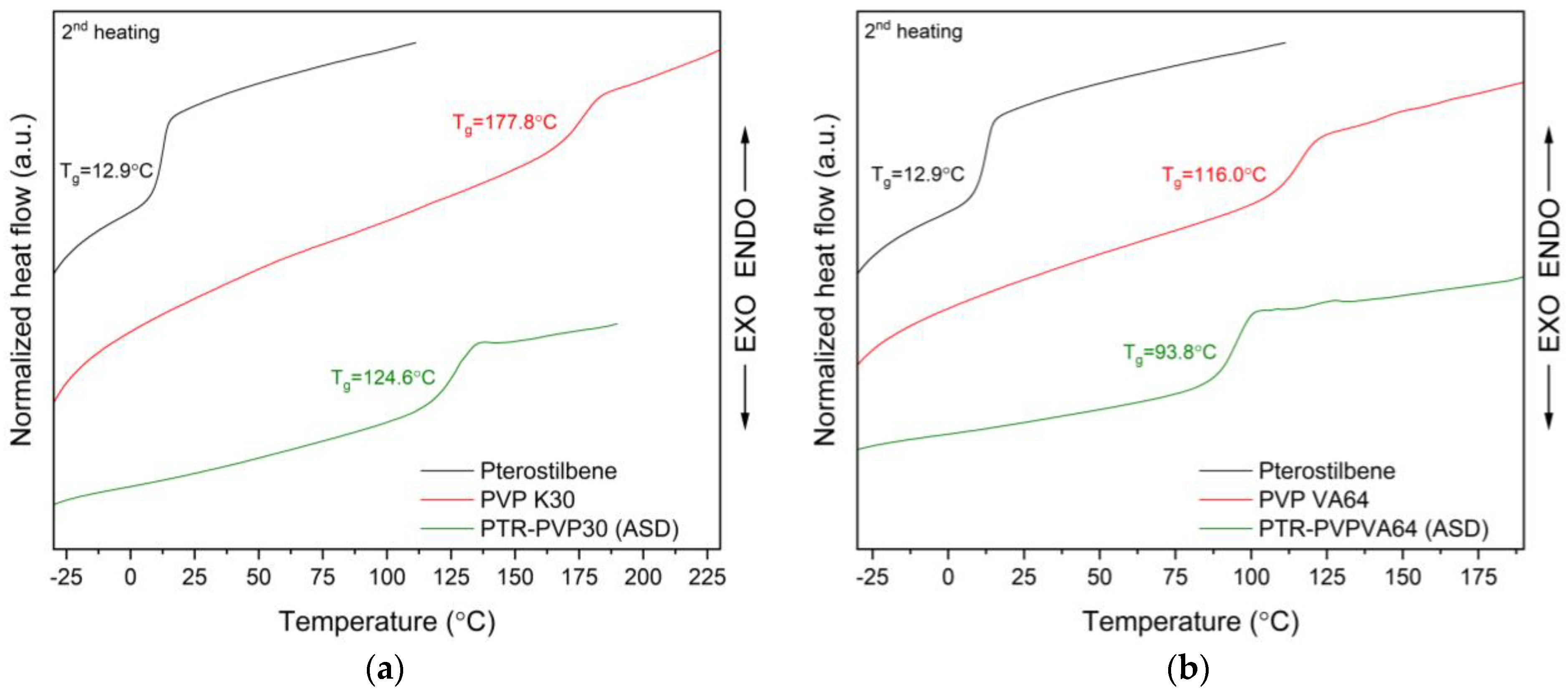
3.4. Thermal Analysis of Amorphous Solid Dispersions
3.4.1. Thermogravimetric Analysis (TG)
3.4.2. Differential Scanning Calorimetry (DSC)
3.5. ATR-FTIR Spectroscopy
3.6. Studies of PTR Properties after Introduction to ASD
3.6.1. Physical Stability
3.6.2. HPLC Analysis
3.6.3. Apparent Solubility
3.6.4. Dissolution Rate Studies
3.6.5. Permeability Studies
3.6.6. Antioxidant Activity
3.6.7. Anticholinesterase Activity
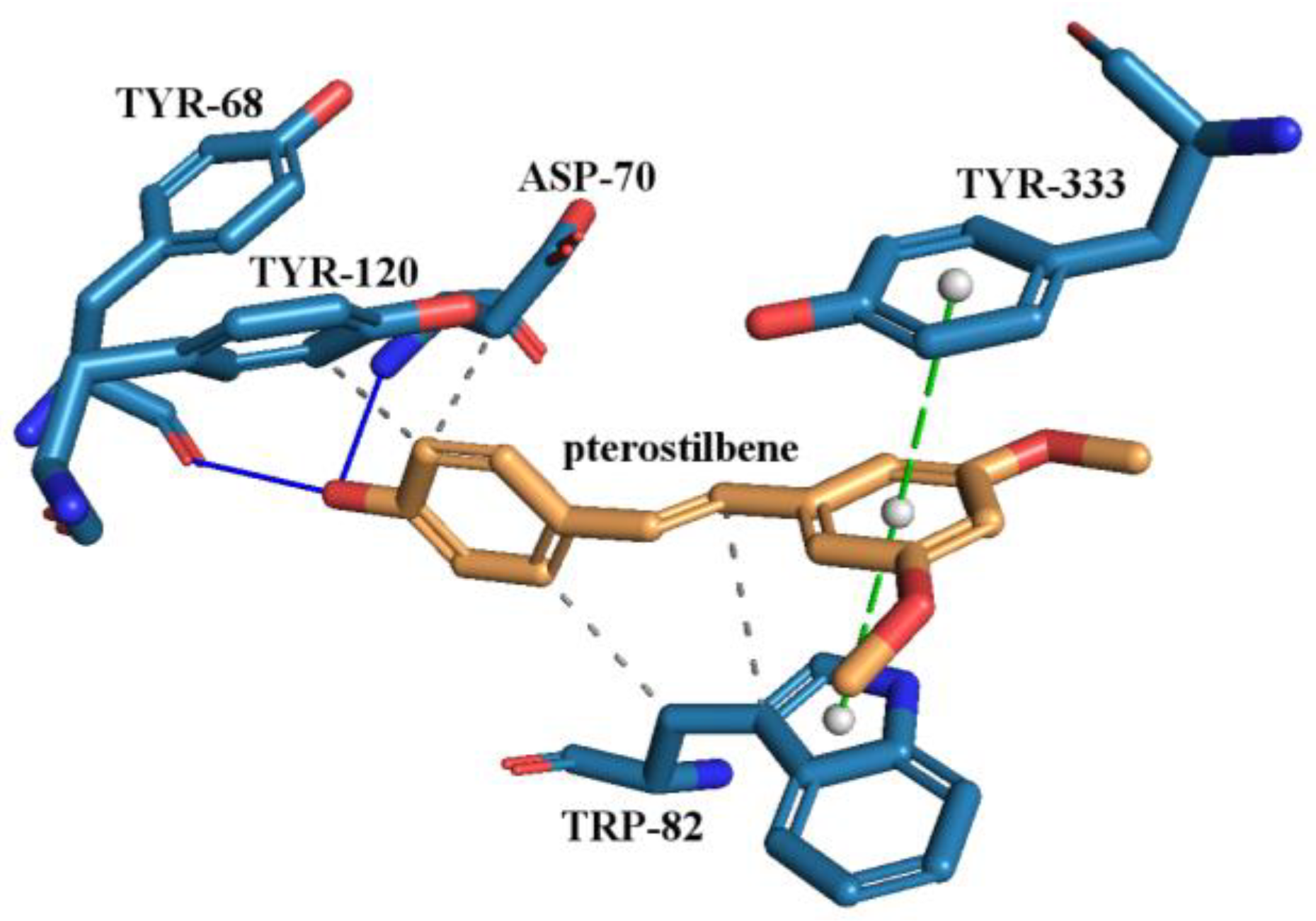
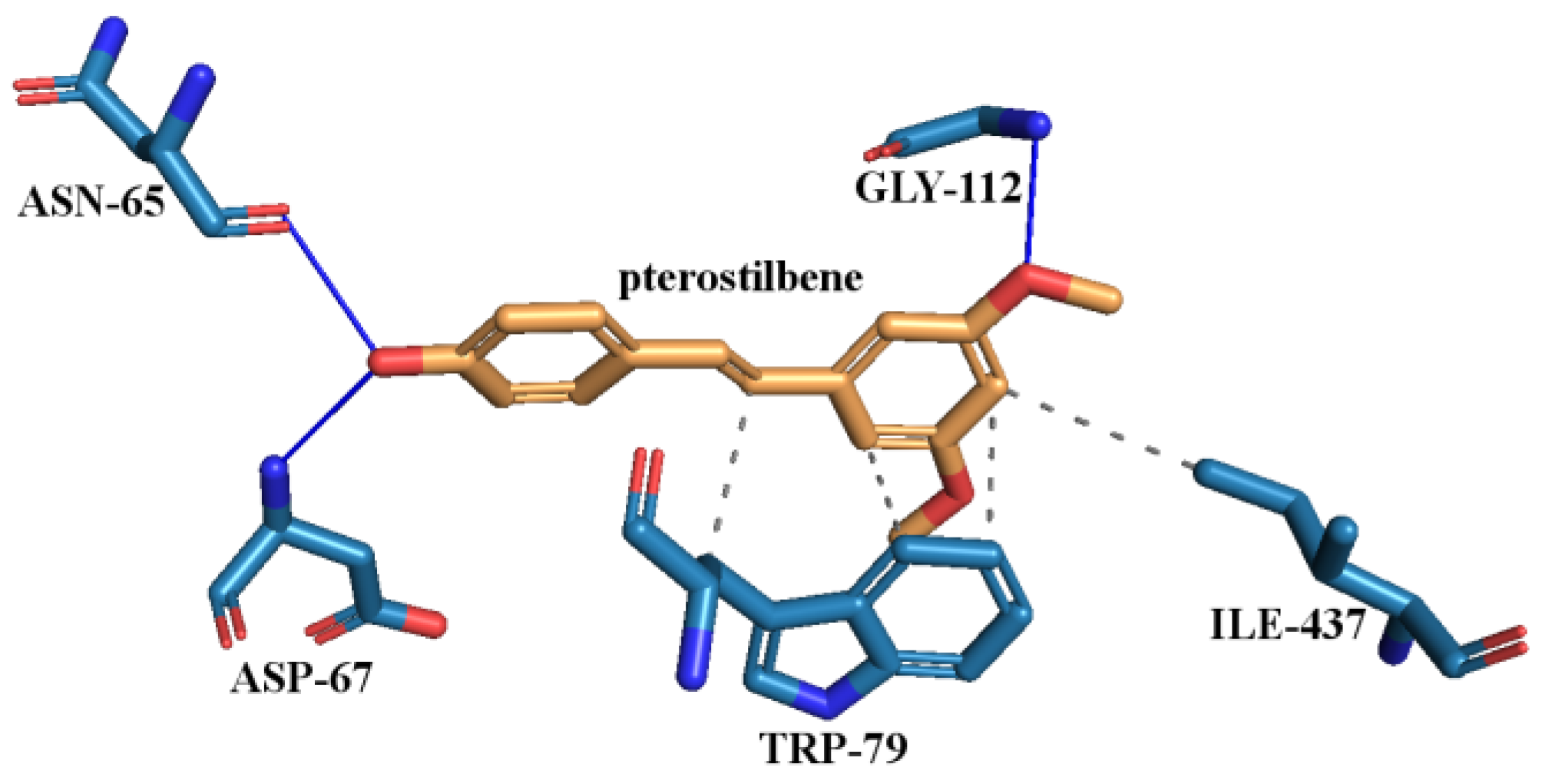
3.6.8. Molecular Docking Study
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, W.-C.; Liu, J.-C.; Hsia, C.-W.; Fong, T.-H.; Hsia, C.-H.; Tran, O.-T.; Velusamy, M.; Yang, C.-H.; Sheu, J.-R. Pterostilbene, a Dimethylether Analogue of Resveratrol, Possesses High Potency in the Prevention of Platelet Activation in Humans and the Reduction of Vascular Thrombosis in Mice. J. Agric. Food Chem. 2021, 69, 4697–4707. [Google Scholar] [CrossRef]
- Liu, Y.; You, Y.; Lu, J.; Chen, X.; Yang, Z. Recent advances in synthesis, bioactivity, and pharmacokinetics of pterostilbene, an important analog of resveratrol. Molecules 2020, 25, 5166. [Google Scholar] [CrossRef]
- Gómez-Zorita, S.; González-Arceo, M.; Trepiana, J.; Aguirre, L.; Crujeiras, A.B.; Irles, E.; Segues, N.; Bujanda, L.; Portillo, M.P. Comparative Effects of Pterostilbene and Its Parent Compound Resveratrol on Oxidative Stress and Inflammation in Steatohepatitis Induced by High-Fat High-Fructose Feeding. Antioxidants 2020, 9, 1042. [Google Scholar] [CrossRef]
- Chiou, Y.-S.; Tsai, M.-L.; Nagabhushanam, K.; Wang, Y.-J.; Wu, C.-H.; Ho, C.-T.; Pan, M.-H. Pterostilbene is more potent than resveratrol in preventing azoxymethane (AOM)-induced colon tumorigenesis via activation of the NF-E2-related factor 2 (Nrf2)-mediated antioxidant signaling pathway. J. Agric. Food Chem. 2011, 59, 2725–2733. [Google Scholar] [CrossRef]
- Wang, P.; Sang, S. Metabolism and pharmacokinetics of resveratrol and pterostilbene. Biofactors 2018, 44, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Pecyna, P.; Wargula, J.; Murias, M.; Kucinska, M. More than resveratrol: New insights into stilbene-based compounds. Biomolecules 2020, 10, 1111. [Google Scholar] [CrossRef] [PubMed]
- Dvorakova, M.; Landa, P. Anti-inflammatory activity of natural stilbenoids: A review. Pharmacol. Res. 2017, 124, 126–145. [Google Scholar] [CrossRef] [PubMed]
- Perecko, T.; Drabikova, K.; Lojek, A.; Ciz, M.; Ponist, S.; Bauerova, K.; Nosal, R.; Harmatha, J.; Jancinova, V. The Effects of Pterostilbene on Neutrophil Activity in Experimental Model of Arthritis. BioMed Res. Int. 2013, 2013, 106041. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, Y. Pterostilbene, a novel natural plant conduct, inhibits high fat-induced atherosclerosis inflammation via NF-κB signaling pathway in Toll-like receptor 5 (TLR5) deficient mice. BioMed Pharmacother. 2016, 81, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Abd-Elmawla, M.A.; Abdelalim, E.; Ahmed, K.A.; Rizk, S.M. The neuroprotective effect of pterostilbene on oxaliplatin-induced peripheral neuropathy via its anti-inflammatory, anti-oxidative and anti-apoptotic effects: Comparative study with celecoxib. Life Sci. 2023, 315, 121364. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Rimando, A.; Pallas, M.; Camins, A.; Porquet, D.; Reeves, J.; Shukitt-Hale, B.; Smith, M.A.; Joseph, J.A.; Casadesus, G. Low-dose pterostilbene, but not resveratrol, is a potent neuromodulator in aging and Alzheimer’s disease. Neurobiol. Aging 2012, 33, 2062–2071. [Google Scholar] [CrossRef]
- Hou, Y.; Xie, G.; Miao, F.; Ding, L.; Mou, Y.; Wang, L.; Su, G.; Chen, G.; Yang, J.; Wu, C. Pterostilbene attenuates lipopolysaccharide-induced learning and memory impairment possibly via inhibiting microglia activation and protecting neuronal injury in mice. Prog. Neuro Psychopharmacol. Biol. Psychiatry 2014, 54, 92–102. [Google Scholar] [CrossRef]
- Sun, H.; Liu, X.; Long, S.R.; Wang, T.; Ge, H.; Wang, Y.; Yu, S.; Xue, Y.; Zhang, Y.; Li, X.; et al. Antidiabetic effects of pterostilbene through PI3K/Akt signal pathway in high fat diet and STZ-induced diabetic rats. Eur. J. Pharmacol. 2019, 859, 172526. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, S.; Lin, C. Effect of resveratrol and pterostilbene on aging and longevity. BioFactors 2018, 44, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Lange, K.W.; Li, S. Resveratrol, pterostilbene, and dementia. Biofactors 2018, 44, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Majeed, M.; Majeed, S.; Jain, R.; Mundkur, L.; Rajalakshmi, H.R.; Lad, P.; Neupane, P. A Randomized Study to Determine the Sun Protection Factor of Natural Pterostilbene from Pterocarpus Marsupium. Cosmetics 2020, 7, 16. [Google Scholar] [CrossRef]
- Chakraborty, A.; Gupta, N.; Ghosh, K.; Roy, P. In vitro evaluation of the cytotoxic, anti-proliferative and anti-oxidant properties of pterostilbene isolated from Pterocarpus marsupium. Toxicol. Vitr. 2010, 24, 1215–1228. [Google Scholar] [CrossRef] [PubMed]
- Suh, N.; Paul, S.; Hao, X.; Simi, B.; Xiao, H.; Rimando, A.M.; Reddy, B.S. Pterostilbene, an Active Constituent of Blueberries, Suppresses Aberrant Crypt Foci Formation in the Azoxymethane-Induced Colon Carcinogenesis Model in Rats. Clin. Cancer Res. 2007, 13, 350–355. [Google Scholar] [CrossRef]
- Rimando, A.M.; Kalt, W.; Magee, J.B.; Dewey, J.; Ballington, J.R. Resveratrol, Pterostilbene, and Piceatannol in Vaccinium Berries. J. Agric. Food Chem. 2004, 52, 4713–4719. [Google Scholar] [CrossRef]
- Obrador, E.; Salvador-Palmer, R.; Jihad-Jebbar, A.; López-Blanch, R.; Dellinger, T.H.; Dellinger, R.W.; Estrela, J.M. Pterostilbene in Cancer Therapy. Antioxidants 2021, 10, 492. [Google Scholar] [CrossRef]
- Lin, W.-S.; Leland, J.V.; Ho, C.-T.; Pan, M.-H. Occurrence, bioavailability, anti-inflammatory, and anticancer effects of pterostilbene. J. Agric. Food Chem. 2020, 68, 12788–12799. [Google Scholar] [CrossRef]
- Riche, D.M.; McEwen, C.L.; Riche, K.D.; Sherman, J.J.; Wofford, M.R.; Deschamp, D.; Griswold, M. Analysis of safety from a human clinical trial with pterostilbene. J. Toxicol. 2013, 2013, 463595. [Google Scholar] [CrossRef]
- Rosiak, N.; Tykarska, E.; Cielecka-Piontek, J. Amorphous Pterostilbene Delivery Systems Preparation—Innovative Approach to Preparation Optimization. Pharmaceutics 2023, 15, 1231. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.R.I.; Preshaw, P.M.; Lim, L.P.; Ong, M.M.A.; Lin, H.-S.; Tan, K.S. Pterostilbene complexed with cyclodextrin exerts antimicrobial and anti-inflammatory effects. Sci. Rep. 2020, 10, 9072. [Google Scholar] [CrossRef] [PubMed]
- Bin Jardan, Y.A.; Ahad, A.; Raish, M.; Al-Mohizea, A.M.; Al-Jenoobi, F.I. Microwave-Assisted Formation of Ternary Inclusion Complex of Pterostilbene. Pharmaceuticals 2023, 16, 1641. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Shi, A.; Ma, Q.; Yan, X.; Bian, L.; Zhang, P.; Wu, J. Nanoparticles prepared from pterostilbene reduce blood glucose and improve diabetes complications. J. Nanobiotechnol. 2021, 19, 191. [Google Scholar] [CrossRef]
- Bethune, S.J.; Schultheiss, N.; Henck, J.-O. Improving the Poor Aqueous Solubility of Nutraceutical Compound Pterostilbene through Cocrystal Formation. Cryst. Growth Des. 2011, 11, 2817–2823. [Google Scholar] [CrossRef]
- Bofill, L.; Prohens, R.; Barbas, R.; Frontera, A. DFT Analysis of Uncommon π···H-Bond Array Interaction in a New Pterostilbene/Theophylline Cocrystal. Cryst. Growth Des. 2020, 20, 6691–6698. [Google Scholar] [CrossRef]
- Bofill, L.; Barbas, R.; de Sande, D.; Font-Bardia, M.; Ràfols, C.; Albertí, J.; Prohens, R. A Novel, Extremely Bioavailable Cocrystal of Pterostilbene. Cryst. Growth Des. 2021, 21, 2315–2323. [Google Scholar] [CrossRef]
- Zhang, Y.; Shang, Z.; Gao, C.; Du, M.; Xu, S.; Song, H.; Liu, T. Nanoemulsion for Solubilization, Stabilization, and In Vitro Release of Pterostilbene for Oral Delivery. AAPS PharmSciTech 2014, 15, 1000–1008. [Google Scholar] [CrossRef]
- Shi, Q.; Zhao, G.; Wei, S.; Guo, C.; Wu, X.; Zhao, R.C.; Di, G. Pterostilbene alleviates liver ischemia/reperfusion injury via PINK1-mediated mitophagy. J. Pharmacol. Sci. 2022, 148, 19–30. [Google Scholar] [CrossRef]
- Rimando, A.M.; Nagmani, R.; Feller, D.R.; Yokoyama, W. Pterostilbene, a New Agonist for the Peroxisome Proliferator-Activated Receptor α-Isoform, Lowers Plasma Lipoproteins and Cholesterol in Hypercholesterolemic Hamsters. J. Agric. Food Chem. 2005, 53, 3403–3407. [Google Scholar] [CrossRef]
- El-Sayed, E.M.; Mansour, A.M.; Nady, M.E. Protective Effects of Pterostilbene against Acetaminophen-Induced Hepatotoxicity in Rats. J. Biochem. Mol. Toxicol. 2015, 29, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Riche, D.M.; Riche, K.D.; Blackshear, C.T.; McEwen, C.L.; Sherman, J.J.; Wofford, M.R.; Griswold, M.E. Pterostilbene on metabolic parameters: A randomized, double-blind, and placebo-controlled trial. Evidence-Based Complement. Altern. Med. 2014, 2014, 459165. [Google Scholar] [CrossRef]
- Wu, J.; Li, M.; He, J.; Lv, K.; Wang, M.; Guan, W.; Liu, J.; Tao, Y.; Li, S.; Ho, C.-T.; et al. Protective effect of pterostilbene on concanavalin A-induced acute liver injury. Food Funct. 2019, 10, 7308–7314. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, J.; Zhang, Y.; Wu, D.; Li, S.; Jiang, A.; Du, C.; Xie, G. Pterostilbene Exerts Hepatoprotective Effects through Ameliorating LPS/D-Gal-Induced Acute Liver Injury in Mice. Inflammation 2021, 44, 526–535. [Google Scholar] [CrossRef] [PubMed]
- Joseph, J.A.; Fisher, D.R.; Cheng, V.; Rimando, A.M.; Shukitt-Hale, B. Cellular and Behavioral Effects of Stilbene Resveratrol Analogues: Implications for Reducing the Deleterious Effects of Aging. J. Agric. Food Chem. 2008, 56, 10544–10551. [Google Scholar] [CrossRef] [PubMed]
- Naik, B.; Nirwane, A.; Majumdar, A. Pterostilbene ameliorates intracerebroventricular streptozotocin induced memory decline in rats. Cogn. Neurodyn. 2017, 11, 35–49. [Google Scholar] [CrossRef] [PubMed]
- Surien, O.; Masre, S.F.; Basri, D.F.; Ghazali, A.R. Chemopreventive Effects of Oral Pterostilbene in Multistage Carcinogenesis of Skin Squamous Cell Carcinoma Mouse Model Induced by DMBA/TPA. Biomedicines 2022, 10, 2743. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Haldar, S.; Ghosh, S.; Chauhan, S.; Sharma, A.; Dhankhar, P.; Kumar, A.; Jaiswal, S.; Saini, S.; Gupta, S.; et al. Pterostilbene-isothiocyanate impedes RANK/TRAF6 interaction to inhibit osteoclastogenesis, promoting osteogenesis in vitro and alleviating glucocorticoid induced osteoporosis in rats. Biochem. Pharmacol. 2022, 206, 115284. [Google Scholar] [CrossRef]
- Lv, C.; Ma, X.; Liang, C.; Chen, Y.; Qin, F.; Zhou, C.; Huang, W.; Liu, Q.; Wang, Y.; Liu, Z.; et al. The interaction of pterostilbene with Kelch-like ECH-associated protein 1 and its regulation on the nuclear factor erythroid 2-related factor 2/antioxidant response element pathway. Process Biochem. 2023, 132, 228–235. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, Y.; Ban, W.; Liu, H.; Lv, L.; Zhang, B.; Liu, A.; Hou, Z.; Lu, J.; Chen, X.; et al. Pterostilbene alleviated cerebral ischemia/reperfusion-induced blood–brain barrier dysfunction via inhibiting early endothelial cytoskeleton reorganization and late basement membrane degradation. Food Funct. 2023, 14, 8291–8308. [Google Scholar] [CrossRef]
- Tippani, R.; Prakhya, L.J.S.; Porika, M.; Sirisha, K.; Abbagani, S.; Thammidala, C. Pterostilbene as a potential novel telomerase inhibitor: Molecular docking studies and its in vitro evaluation. Curr. Pharm. Biotechnol. 2014, 14, 1027–1035. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, J.; You, S.; Zhang, J.; Zhang, Y.; Akram, Z.; Sun, S. Pterostilbene upregulates MICA/B via the PI3K/AKT signaling pathway to enhance the capability of natural killer cells to kill cervical cancer cells. Exp. Cell Res. 2024, 435, 113933. [Google Scholar] [CrossRef]
- Yang, S.-C.; Tseng, C.-H.; Wang, P.-W.; Lu, P.-L.; Weng, Y.-H.; Yen, F.-L.; Fang, J.-Y. Pterostilbene, a Methoxylated Resveratrol Derivative, Efficiently Eradicates Planktonic, Biofilm, and Intracellular MRSA by Topical Application. Front. Microbiol. 2017, 8, 1103. [Google Scholar] [CrossRef]
- Rahmani, A.H.; Anwar, S.; Raut, R.; Almatroudi, A.; Babiker, A.Y.; Khan, A.A.; Alsahli, M.A.; Almatroodi, S.A. Therapeutic Potential of Myrrh, a Natural Resin, in Health Management through Modulation of Oxidative Stress, Inflammation, and Advanced Glycation End Products Formation Using In Vitro and In Silico Analysis. Appl. Sci. 2022, 12, 9175. [Google Scholar] [CrossRef]
- Studzińska-Sroka, E.; Majchrzak-Celińska, A.; Bańdurska, M.; Rosiak, N.; Szwajgier, D.; Baranowska-Wójcik, E.; Szymański, M.; Gruszka, W.; Cielecka-Piontek, J. Is Caperatic Acid the Only Compound Responsible for Activity of Lichen Platismatia glauca within the Nervous System? Antioxidants 2022, 11, 2069. [Google Scholar] [CrossRef]
- Studzińska-Sroka, E.; Bulicz, M.; Henkel, M.; Rosiak, N.; Paczkowska-Walendowska, M.; Szwajgier, D.; Baranowska-Wójcik, E.; Korybalska, K.; Cielecka-Piontek, J. Pleiotropic Potential of Evernia prunastri Extracts and Their Main Compounds Evernic Acid and Atranorin: In Vitro and In Silico Studies. Molecules 2024, 29, 233. [Google Scholar] [CrossRef] [PubMed]
- Alhaithloul, H.A.S.; Alqahtani, M.M.; Abdein, M.A.; Ahmed, M.A.I.; Hesham, A.E.-L.; Aljameeli, M.M.E.; Al Mozini, R.N.; Gharsan, F.N.; Hussien, S.M.; El-Amier, Y.A. Rosemary and neem methanolic extract: Antioxidant, cytotoxic, and larvicidal activities supported by chemical composition and molecular docking simulations. Front. Plant Sci. 2023, 14, 1155698. [Google Scholar] [CrossRef] [PubMed]
- Wdowiak, K.; Pietrzak, R.; Tykarska, E.; Cielecka-Piontek, J. Hot-Melt Extrusion as an Effective Technique for Obtaining an Amorphous System of Curcumin and Piperine with Improved Properties Essential for Their Better Biological Activities. Molecules 2023, 28, 3848. [Google Scholar] [CrossRef]
- Kim, N.A.; Oh, H.K.; Lee, J.C.; Choi, Y.H.; Jeong, S.H. Comparison of solubility enhancement by solid dispersion and micronized butein and its correlation with in vivo study. J. Pharm. Investig. 2021, 51, 53–60. [Google Scholar] [CrossRef]
- Rosiak, N.; Tykarska, E.; Cielecka-Piontek, J. The Study of Amorphous Kaempferol Dispersions Involving FT-IR Spectroscopy. Int. J. Mol. Sci. 2023, 24, 17155. [Google Scholar] [CrossRef] [PubMed]
- Altamimi, M.A.; Elzayat, E.M.; Alshehri, S.M.; Mohsin, K.; Ibrahim, M.A.; Al Meanazel, O.T.; Shakeel, F.; Alanazi, F.K.; Alsarra, I.A. Utilizing spray drying technique to improve oral bioavailability of apigenin. Adv. Powder Technol. 2018, 29, 1676–1684. [Google Scholar] [CrossRef]
- Sip, S.; Rosiak, N.; Sip, A.; Żarowski, M.; Hojan, K.; Cielecka-Piontek, J. A Fisetin Delivery System for Neuroprotection: A Co-Amorphous Dispersion Prepared in Supercritical Carbon Dioxide. Antioxidants 2023, 13, 24. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Teng, J.; Selbo, J. Amorphous solid dispersion of epigallocatechin gallate for enhanced physical stability and controlled release. Pharmaceuticals 2017, 10, 88. [Google Scholar] [CrossRef]
- Kanaze, F.I.; Kokkalou, E.; Niopas, I.; Georgarakis, M.; Stergiou, A.; Bikiaris, D. Dissolution enhancement of flavonoids by solid dispersion in PVP and PEG matrixes: A comparative study. J. Appl. Polym. Sci. 2006, 102, 460–471. [Google Scholar] [CrossRef]
- Li, B.; Harich, K.; Wegiel, L.; Taylor, L.S.; Edgar, K.J. Stability and solubility enhancement of ellagic acid in cellulose ester solid dispersions. Carbohydr. Polym. 2013, 92, 1443–1450. [Google Scholar] [CrossRef]
- Li, J.; Wang, X.; Li, C.; Fan, N.; Wang, J.; He, Z.; Sun, J. Viewing Molecular and Interface Interactions of Curcumin Amorphous Solid Dispersions for Comprehending Dissolution Mechanisms. Mol. Pharm. 2017, 14, 2781–2792. [Google Scholar] [CrossRef]
- Lee, S.H.; Lee, Y.; Song, J.G.; Han, H.-K. Improved In vivo Effect of Chrysin as an Absorption Enhancer Via the Preparation of Ternary Solid Dispersion with Brij®L4 and Aminoclay. Curr. Drug Deliv. 2018, 16, 86–92. [Google Scholar] [CrossRef]
- Rosiak, N.; Wdowiak, K.; Tykarska, E.; Cielecka-Piontek, J. Amorphous Solid Dispersion of Hesperidin with Polymer Excipients for Enhanced Apparent Solubility as a More Effective Approach to the Treatment of Civilization Diseases. Int. J. Mol. Sci. 2022, 23, 15198. [Google Scholar] [CrossRef]
- Garbiec, E.; Rosiak, N.; Tykarska, E.; Zalewski, P.; Cielecka-Piontek, J. Sinapic Acid Co-Amorphous Systems with Amino Acids for Improved Solubility and Antioxidant Activity. Int. J. Mol. Sci. 2023, 24, 5533. [Google Scholar] [CrossRef]
- Hatwar, P.; Pathan, I.B.; Chishti, N.A.H.; Ambekar, W. Pellets containing quercetin amino acid co-amorphous mixture for the treatment of pain: Formulation, optimization, in-vitro and in-vivo study. J. Drug Deliv. Sci. Technol. 2021, 62, 102350. [Google Scholar] [CrossRef]
- Han, J.; Tong, M.; Li, S.; Yu, X.; Hu, Z.; Zhang, Q.; Xu, R.; Wang, J. Surfactant-free amorphous solid dispersion with high dissolution for bioavailability enhancement of hydrophobic drugs: A case of quercetin. Drug Dev. Ind. Pharm. 2021, 47, 153–162. [Google Scholar] [CrossRef]
- Nowak, P.; Krupa, A.; Kubat, K.; Węgrzyn, A.; Harańczyk, H.; Ciułkowska, A.; Jachowicz, R. Water vapour sorption in tadalafil-Soluplus co-milled amorphous solid dispersions. Powder Technol. 2019, 346, 373–384. [Google Scholar] [CrossRef]
- Bakhtiari, S.E.; Zhu, Z.; Magdysyuk, O.V.; Brocchini, S.; Williams, G.R. Amorphous solid dispersions of lidocaine and lidocaine HCl produced by ball milling with well-defined RAFT-synthesised methacrylic acid polymers. Int. J. Pharm. 2023, 644, 123291. [Google Scholar] [CrossRef] [PubMed]
- Bezzon, V.D.N.; Pinto, R.d.S.; de Araújo, G.L.B.; de Lima, J.C.; Ferreira, F.F. D escribing the Influence of Ball-milling on the Amorphization of Flubendazole Using the PDF and RMC Methods with X-ray Powder Diffraction Data. J. Pharm. Sci. 2022, 111, 3054–3063. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, Q.; Wang, J.-R.; Lin, K.-L.; Mei, X. Amino acids as co-amorphous excipients for tackling the poor aqueous solubility of valsartan. Pharm. Dev. Technol. 2017, 22, 69–76. [Google Scholar] [CrossRef]
- Tzeng, W.-S.; Teng, W.-L.; Huang, P.-H.; Lin, T.-C.; Yen, F.-L.; Shiue, Y.-L. Pterostilbene Nanoparticles Downregulate Hypoxia-Inducible Factors in Hepatoma Cells Under Hypoxic Conditions. Int. J. Nanomed. 2021, 16, 867–879. [Google Scholar] [CrossRef] [PubMed]
- Molaei, M.-A.; Osouli-Bostanabad, K.; Adibkia, K.; Shokri, J.; Asnaashari, S.; Javadzadeh, Y. Enhancement of ketoconazole dissolution rate by the liquisolid technique. Acta Pharm. 2018, 68, 325–336. [Google Scholar] [CrossRef]
- Wdowiak, K.; Miklaszewski, A.; Pietrzak, R.; Cielecka-Piontek, J. Amorphous System of Hesperetin and Piperine—Improvement of Apparent Solubility, Permeability, and Biological Activities. Int. J. Mol. Sci. 2023, 24, 4859. [Google Scholar] [CrossRef]
- Nadal, J.M.; Gomes, M.L.S.; Borsato, D.M.; Almeida, M.A.; Barboza, F.M.; Zawadzki, S.F.; Farago, P.V.; Zanin, S.M.W. Spray-dried solid dispersions containing ferulic acid: Comparative analysis of three carriers, in vitro dissolution, antioxidant potential and in vivo anti-platelet effect. Drug Dev. Ind. Pharm. 2016, 42, 1813–1824. [Google Scholar] [CrossRef]
- He, Y.; Liu, H.; Bian, W.; Liu, Y.; Liu, X.; Ma, S.; Zheng, X.; Du, Z.; Zhang, K.; Ouyang, D. Molecular Interactions for the Curcumin-Polymer Complex with Enhanced Anti-Inflammatory Effects. Pharmaceutics 2019, 11, 442. [Google Scholar] [CrossRef]
- Panizzon, G.P.; Giacomini Bueno, F.; Ueda-Nakamura, T.; Nakamura, C.V.; Dias Filho, B.P. Manufacturing Different Types of Solid Dispersions of BCS Class IV Polyphenol (Daidzein) by Spray Drying: Formulation and Bioavailability. Pharmaceutics 2019, 11, 492. [Google Scholar] [CrossRef]
- Zaini, E.; Putri, V.Z.; Octavia, M.D.; Ismed, F. Peningkatan Laju Disolusi Dispersi Padat Amorf Genistein dengan PVP K-30. J. Sains Farm. Klin. 2017, 4, 67. [Google Scholar] [CrossRef]
- Lacerda, D.S.; Bianchi, S.E.; Pinós, W.L.; Campos-Carraro, C.; Türck, P.; Hickmann, A.R.; Pittol, V.; Teixeira, R.B.; Belló-Klein, A.; Bassani, V.L.; et al. Effect of pterostilbene complexed with cyclodextrin on rat liver: Potential reduction of oxidative damage and modulation redox-sensitive proteins. Med. Chem. Res. 2018, 27, 2265–2278. [Google Scholar] [CrossRef]
- Catenacci, L.; Vicatos, A.I.; Sorrenti, M.; Bonferoni, M.C.; Caira, M.R. Native Cyclodextrins as Complexation Agents for Pterostilbene: Complex Preparation and Characterization in Solution and in the Solid State. Pharmaceutics 2021, 14, 8. [Google Scholar] [CrossRef] [PubMed]
- Löbmann, K.; Grohganz, H.; Laitinen, R.; Strachan, C.; Rades, T. Amino acids as co-amorphous stabilizers for poorly water soluble drugs—Part 1: Preparation, stability and dissolution enhancement. Eur. J. Pharm. Biopharm. 2013, 85, 873–881. [Google Scholar] [CrossRef] [PubMed]
- Garbiec, E.; Rosiak, N.; Zalewski, P.; Tajber, L.; Cielecka-Piontek, J. Genistein Co-Amorphous Systems with Amino Acids: An Investigation into Enhanced Solubility and Biological Activity. Pharmaceutics 2023, 15, 2653. [Google Scholar] [CrossRef] [PubMed]
- Teng, J.; Bates, S.; Engers, D.A.; Leach, K.; Schields, P.; Yang, Y. Effect of Water Vapor Sorption on Local Structure of Poly(vinylpyrrolidone). J. Pharm. Sci. 2010, 99, 3815–3825. [Google Scholar] [CrossRef]
- van Drooge, D.J.; Hinrichs, W.L.J.; Visser, M.R.; Frijlink, H.W. Characterization of the molecular distribution of drugs in glassy solid dispersions at the nano-meter scale, using differential scanning calorimetry and gravimetric water vapour sorption techniques. Int. J. Pharm. 2006, 310, 220–229. [Google Scholar] [CrossRef]
- Shamblin, S.L.; Taylor, L.S.; Zografi, G. Mixing Behavior of Colyophilized Binary Systems. J. Pharm. Sci. 1998, 87, 694–701. [Google Scholar] [CrossRef]
- Barmpalexis, P.; Karagianni, A.; Kachrimanis, K. Molecular simulations for amorphous drug formulation: Polymeric matrix properties relevant to hot-melt extrusion. Eur. J. Pharm. Sci. 2018, 119, 259–267. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, S.; Wang, S.; Liu, C.; Su, C.; Hageman, M.; Hussain, M.; Haskell, R.; Stefanski, K.; Qian, F. Initial Drug Dissolution from Amorphous Solid Dispersions Controlled by Polymer Dissolution and Drug-Polymer Interaction. Pharm. Res. 2016, 33, 2445–2458. [Google Scholar] [CrossRef]
- Monschke, M.; Wagner, K.G. Impact of HPMCAS on the Dissolution Performance of Polyvinyl Alcohol Celecoxib Amorphous Solid Dispersions. Pharmaceutics 2020, 12, 541. [Google Scholar] [CrossRef]
- Budiman, A.; Nurani, N.V.; Laelasari, E.; Muchtaridi, M.; Sriwidodo, S.; Aulifa, D.L. Effect of Drug–Polymer Interaction in Amorphous Solid Dispersion on the Physical Stability and Dissolution of Drugs: The Case of Alpha-Mangostin. Polymers 2023, 15, 3034. [Google Scholar] [CrossRef]
- de Mello Costa, A.R.; Marquiafável, F.S.; de Oliveira Lima Leite Vaz, M.M.; Rocha, B.A.; Pires Bueno, P.C.; Amaral, P.L.M.; da Silva Barud, H.; Berreta-Silva, A.A. Quercetin-PVP K25 solid dispersions. J. Therm. Anal. Calorim. 2011, 104, 273–278. [Google Scholar] [CrossRef]
- Gayo, Z.; Lucida, H.; Zaini, E. Solid dispersion of quercetin-PVP K-30 and its effects on the antioxidant activity. J. Ilm. Farm. 2020, 16, 144–154. [Google Scholar] [CrossRef]
- Wang, Y.; Fang, Y.; Zhou, F.; Liang, Q.; Deng, Y. The amorphous quercetin/hydroxypropylmethylcellulose acetate succinate solid dispersions prepared by co-precipitation method to enhance quercetin dissolution. J. Pharm. Sci. 2021, 110, 3230–3237. [Google Scholar] [CrossRef] [PubMed]
- Dudhedia, M.S.; Agrawal, A.M. Rheological study of copovidone and solid dispersion blend used for hot melt extrusion. J. Appl. Polym. Sci. 2016, 133, 43278. [Google Scholar] [CrossRef]
- Van den Mooter, G. The use of amorphous solid dispersions: A formulation strategy to overcome poor solubility and dissolution rate. Drug Discov. Today Technol. 2012, 9, e79–e85. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Ocando, J.E.; Trombetta, L.; Chatterjee, P. Molecular Interaction Studies of Amorphous Solid Dispersions of the Antimelanoma Agent Betulinic Acid. AAPS PharmSciTech 2015, 16, 384–397. [Google Scholar] [CrossRef] [PubMed]
- Freitas, D.F.S.; Mattos, G.C.; Mendes, L.C. The role of octadecylamine as zirconium phosphate intercalating agent on poly(vinyl alcohol)/poly(N-vinyl-2-pyrrolidone) biodegradable systems. J. Therm. Anal. Calorim. 2022, 147, 315–325. [Google Scholar] [CrossRef]
- Teoh, X.-Y.; Yeoh, Y.; Yoong, L.-K.; Chan, S.-Y. Sustainable Dissolution Performance of a Carrier Tailored Electrospun. Pharm. Res. 2020, 37, 28. [Google Scholar] [CrossRef] [PubMed]
- Dumitraşcu, M.; Meltzer, V.; Sima, E.; Virgolici, M.; Albu, M.G.; Ficai, A.; Moise, V.; Minea, R.; Vancea, C.; Scărişoreanu, A. Characterization of electron beam irradiated collagen-polyvinylpyrrolidone (PVP) and collagen-dextran (DEX) blends. Dig. J. Nanomater. Biostruct 2011, 6, 1793–1803. [Google Scholar]
- Erizal, E.; Tjahyono, T.; Dian, P.P.; Darmawan, D. Synthesis of Polyvinyl Pirrolidone (PVC)/Κ-Carrageenan Hydrogel Prepared by Gamma Radiation Processing As a Function of Dose and PVP Concentration. Indones. J. Chem. 2013, 13, 41–46. [Google Scholar] [CrossRef]
- Dong, L.; Mai, Y.; Liu, Q.; Zhang, W.; Yang, J. Mechanism and Improved Dissolution of Glycyrrhetinic Acid Solid Dispersion by Alkalizers. Pharmaceutics 2020, 12, 82. [Google Scholar] [CrossRef]
- Jia, S.; Ning, S.; Leng, Y.; Jing, Q.; Xu, Z.; Ren, F. Stabilizing Effect of Soluplus on Erlotinib Metastable Crystal Form in Microparticles and Amorphous Solid Dispersions. Polymers 2022, 14, 1241. [Google Scholar] [CrossRef]
- Karavas, E.; Koutris, E.; Papadopoulos, A.G.; Sigalas, M.P.; Nanaki, S.; Papageorgiou, G.Z.; Achilias, D.Z.; Bikiaris, D.N. Application of density functional theory in combination with FTIR and DSC to characterise polymer drug interactions for the preparation of sustained release formulations between fluvastatin and carrageenans. Int. J. Pharm. 2014, 466, 211–222. [Google Scholar] [CrossRef]
- Yang, J.; Grey, K.; Doney, J. An improved kinetics approach to describe the physical stability of amorphous solid dispersions. Int. J. Pharm. 2010, 384, 24–31. [Google Scholar] [CrossRef]
- Rumondor, A.C.F.; Wikström, H.; Van Eerdenbrugh, B.; Taylor, L.S. Understanding the Tendency of Amorphous Solid Dispersions to Undergo Amorphous–Amorphous Phase Separation in the Presence of Absorbed Moisture. AAPS PharmSciTech 2011, 12, 1209–1219. [Google Scholar] [CrossRef]
- Rumondor, A.C.F.; Marsac, P.J.; Stanford, L.A.; Taylor, L.S. Phase Behavior of Poly(vinylpyrrolidone) Containing Amorphous Solid Dispersions in the Presence of Moisture. Mol. Pharm. 2009, 6, 1492–1505. [Google Scholar] [CrossRef]
- Fitzpatrick, S.; McCabe, J.F.; Petts, C.R.; Booth, S.W. Effect of moisture on polyvinylpyrrolidone in accelerated stability testing. Int. J. Pharm. 2002, 246, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Liang, Q.; Wang, Y.; Zhang, X.; Yan, C.; He, Y. The inhibiting role of hydroxypropylmethylcellulose acetate succinate on piperine crystallization to enhance its dissolution from its amorphous solid dispersion and permeability. RSC Adv. 2019, 9, 39523–39531. [Google Scholar] [CrossRef] [PubMed]
- Stasiłowicz-Krzemień, A.; Gołębiewski, M.; Płazińska, A.; Płaziński, W.; Miklaszewski, A.; Żarowski, M.; Adamska-Jernaś, Z.; Cielecka-Piontek, J. The Systems of Naringenin with Solubilizers Expand Its Capability to Prevent Neurodegenerative Diseases. Int. J. Mol. Sci. 2022, 23, 755. [Google Scholar] [CrossRef] [PubMed]
- Stasiłowicz-Krzemień, A.; Rosiak, N.; Miklaszewski, A.; Cielecka-Piontek, J. Screening of the Anti-Neurodegenerative Activity of Caffeic Acid after Introduction into Inorganic Metal Delivery Systems to Increase Its Solubility as the Result of a Mechanosynthetic Approach. Int. J. Mol. Sci. 2023, 24, 9218. [Google Scholar] [CrossRef]
- Stasiłowicz-Krzemień, A.; Rosiak, N.; Płazińska, A.; Płaziński, W.; Miklaszewski, A.; Tykarska, E.; Cielecka-Piontek, J. Cyclodextrin Derivatives as Promising Solubilizers to Enhance the Biological Activity of Rosmarinic Acid. Pharmaceutics 2022, 14, 2098. [Google Scholar] [CrossRef]
- Acharya, J.D.; Ghaskadbi, S.S. Protective effect of Pterostilbene against free radical mediated oxidative damage. BMC Complement. Altern. Med. 2013, 13, 238. [Google Scholar] [CrossRef]
- Ververis, A.; Savvidou, G.; Ioannou, K.; Nicolaou, P.; Christodoulou, K.; Plioukas, M. Greek Sage Exhibits Neuroprotective Activity against Amyloid Beta-Induced Toxicity. Evid. Based Complement. Altern. Med. 2020, 2020, 2975284. [Google Scholar] [CrossRef]
- Cásedas, G.; Moliner, C.; Maggi, F.; Mazzara, E.; López, V. Evaluation of two different Cannabis sativa L. extracts as antioxidant and neuroprotective agents. Front. Pharmacol. 2022, 13, 1009868. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.; Ahmed, S.; Elasbali, A.M.; Adnan, M.; Alam, S.; Hassan, M.I.; Pasupuleti, V.R. Therapeutic Implications of Caffeic Acid in Cancer and Neurological Diseases. Front. Oncol. 2022, 12, 860508. [Google Scholar] [CrossRef]
- Colonnello, A.; Aguilera-Portillo, G.; Rubio-López, L.C.; Robles-Bañuelos, B.; Rangel-López, E.; Cortez-Núñez, S.; Evaristo-Priego, Y.; Silva-Palacios, A.; Galván-Arzate, S.; García-Contreras, R.; et al. Comparing the Neuroprotective Effects of Caffeic Acid in Rat Cortical Slices and Caenorhabditis elegans: Involvement of Nrf2 and SKN-1 Signaling Pathways. Neurotox. Res. 2020, 37, 326–337. [Google Scholar] [CrossRef] [PubMed]
- Metz, C.N.; Pavlov, V.A. Treating disorders across the lifespan by modulating cholinergic signaling with galantamine. J. Neurochem. 2021, 158, 1359–1380. [Google Scholar] [CrossRef] [PubMed]
- Sie, Y.-Y.; Chen, L.-C.; Li, C.-J.; Yuan, Y.-H.; Hsiao, S.-H.; Lee, M.-H.; Wang, C.-C.; Hou, W.-C. Inhibition of Acetylcholinesterase and Amyloid-β Aggregation by Piceatannol and Analogs: Assessing In Vitro and In Vivo Impact on a Murine Model of Scopolamine-Induced Memory Impairment. Antioxidants 2023, 12, 1362. [Google Scholar] [CrossRef] [PubMed]
- Makarian, M.; Gonzalez, M.; Salvador, S.M.; Lorzadeh, S.; Hudson, P.K.; Pecic, S. Synthesis, kinetic evaluation and molecular docking studies of donepezil-based acetylcholinesterase inhibitors. J. Mol. Struct. 2022, 1247, 131425. [Google Scholar] [CrossRef] [PubMed]
- Jang, C.; Yadav, D.K.; Subedi, L.; Venkatesan, R.; Venkanna, A.; Afzal, S.; Lee, E.; Yoo, J.; Ji, E.; Kim, S.Y.; et al. Identification of novel acetylcholinesterase inhibitors designed by pharmacophore-based virtual screening, molecular docking and bioassay. Sci. Rep. 2018, 8, 14921. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.-Q.; Peng, S.; He, W.-J.; Liu, Y.-H.; Wang, J.-F.; Zhou, X.-J. Antitubercular and cytotoxic tigliane-type diterpenoids from Croton tiglium. Bioorg. Med. Chem. Lett. 2016, 26, 4996–4999. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Polli, J. Identification of inhibitor concentrations to efficiently screen and measure inhibition Ki values against solute carrier transporters. Eur. J. Pharm. Sci. 2010, 41, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Terefe, E.M.; Ghosh, A. Molecular Docking, Validation, Dynamics Simulations, and Pharmacokinetic Prediction of Phytochemicals Isolated From Croton dichogamus Against the HIV-1 Reverse Transcriptase. Bioinform. Biol. Insights 2022, 16, 117793222211256. [Google Scholar] [CrossRef]
- Mesallati, H.; Tajber, L. Polymer/Amorphous Salt Solid Dispersions of Ciprofloxacin. Pharm. Res. 2017, 34, 2425–2439. [Google Scholar] [CrossRef]
- Fischer, H.; Kansy, M.; Avdeef, A.; Senner, F. Permeation of permanently positive charged molecules through artificial membranes—Influence of physico-chemical properties. Eur. J. Pharm. Sci. 2007, 31, 32–42. [Google Scholar] [CrossRef]
- Sip, S.; Sip, A.; Miklaszewski, A.; Żarowski, M.; Cielecka-Piontek, J. Zein as an Effective Carrier for Hesperidin Delivery Systems with Improved Prebiotic Potential. Molecules 2023, 28, 5209. [Google Scholar] [CrossRef] [PubMed]
- Stasiłowicz-Krzemień, A.; Cielecka-Piontek, J. Hop Flower Supercritical Carbon Dioxide Extracts Coupled with Carriers with Solubilizing Properties—Antioxidant Activity and Neuroprotective Potential. Antioxidants 2023, 12, 1722. [Google Scholar] [CrossRef]
- Gościniak, A.; Szulc, P.; Zielewicz, W.; Walkowiak, J.; Cielecka-Piontek, J. Multidirectional Effects of Red Clover (Trifolium pratense L.) in Support of Menopause Therapy. Molecules 2023, 28, 5178. [Google Scholar] [CrossRef]
- Sip, S.; Gościniak, A.; Szulc, P.; Walkowiak, J.; Cielecka-Piontek, J. Assisted Extraction with Cyclodextrins as a Way of Improving the Antidiabetic Activity of Actinidia Leaves. Pharmaceutics 2022, 14, 2473. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef]
- Jakubec, D.; Skoda, P.; Krivak, R.; Novotny, M.; Hoksza, D. PrankWeb 3: Accelerated ligand-binding site predictions for experimental and modelled protein structures. Nucleic Acids Res. 2022, 50, W593–W597. [Google Scholar] [CrossRef] [PubMed]
- Krivák, R.; Hoksza, D. P2Rank: Machine learning based tool for rapid and accurate prediction of ligand binding sites from protein structure. J. Cheminform. 2018, 10, 39. [Google Scholar] [CrossRef]
- Jendele, L.; Krivak, R.; Skoda, P.; Novotny, M.; Hoksza, D. PrankWeb: A web server for ligand binding site prediction and visualization. Nucleic Acids Res. 2019, 47, W345–W349. [Google Scholar] [CrossRef]
- O’Boyle, N.M.; Banck, M.; James, C.A.; Morley, C.; Vandermeersch, T.; Hutchison, G.R. Open Babel: An open chemical toolbox. J. Cheminform. 2011, 3, 33. [Google Scholar] [CrossRef]
- Salentin, S.; Schreiber, S.; Haupt, V.J.; Adasme, M.F.; Schroeder, M. PLIP: Fully automated protein–ligand interaction profiler. Nucleic Acids Res. 2015, 43, W443–W447. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Adasme, M.F.; Linnemann, K.L.; Bolz, S.N.; Kaiser, F.; Salentin, S.; Haupt, V.J.; Schroeder, M. PLIP 2021: Expanding the scope of the protein–ligand interaction profiler to DNA and RNA. Nucleic Acids Res. 2021, 49, W530–W534. [Google Scholar] [CrossRef] [PubMed]
- DeLano, W.L.; Bolea, I. The PyMOL Molecular Graphics System, Version 1.3; Schrçdinger: New York, NY, USA, 2002. [Google Scholar]
- Sugimoto, H.; Ogura, H.; Arai, Y.; Iimura, Y.; Yamanishi, Y. Research and development of donepezil hydrochloride, a new type of acetylcholinesterase inhibitor. Jpn. J. Pharmacol. 2002, 89, 7–20. [Google Scholar] [CrossRef] [PubMed]




| Mass (mg) | ΔCp °C)−1) | Tg,exp (°C) | Tg,G-T (°C) | Tg,C-K (°C) | Deviation | |
|---|---|---|---|---|---|---|
| PTR | 7.20 | 0.461 | 12.9 | - | - | |
| PVP K30 | 7.58 | 0.154 | 177.8 | - | - | |
| PVP VA64 | 8.40 | 0.311 | 115.3 | - | - | |
| PTR-PVP30 ASD | 7.50 | 0.329 | 124.6 | 51.1 | 107.2 | + |
| PTR-PVPVA64 ASD | 7.08 | 0.404 | 93.8 | 44.4 | 87.6 | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosiak, N.; Tykarska, E.; Cielecka-Piontek, J. Enhanced Antioxidant and Neuroprotective Properties of Pterostilbene (Resveratrol Derivative) in Amorphous Solid Dispersions. Int. J. Mol. Sci. 2024, 25, 2774. https://doi.org/10.3390/ijms25052774
Rosiak N, Tykarska E, Cielecka-Piontek J. Enhanced Antioxidant and Neuroprotective Properties of Pterostilbene (Resveratrol Derivative) in Amorphous Solid Dispersions. International Journal of Molecular Sciences. 2024; 25(5):2774. https://doi.org/10.3390/ijms25052774
Chicago/Turabian StyleRosiak, Natalia, Ewa Tykarska, and Judyta Cielecka-Piontek. 2024. "Enhanced Antioxidant and Neuroprotective Properties of Pterostilbene (Resveratrol Derivative) in Amorphous Solid Dispersions" International Journal of Molecular Sciences 25, no. 5: 2774. https://doi.org/10.3390/ijms25052774
APA StyleRosiak, N., Tykarska, E., & Cielecka-Piontek, J. (2024). Enhanced Antioxidant and Neuroprotective Properties of Pterostilbene (Resveratrol Derivative) in Amorphous Solid Dispersions. International Journal of Molecular Sciences, 25(5), 2774. https://doi.org/10.3390/ijms25052774







