Abstract
Breast cancer is a growing disease, with a high worldwide incidence and mortality rate among women. Among the various types, the treatment of triple-negative breast cancer (TNBC) remains a challenge. Considering the recent advances in cold atmospheric plasma (CAP) cancer research, our goal was to evaluate efficacy data from studies based on chemotherapy and CAP in TNBC cell lines and animal models. A search of the literature was carried out in the PubMed, Web of Science, Cochrane Library, and Embase databases. Of the 10,999 studies, there were fifty-four in vitro studies, three in vivo studies, and two in vitro and in vivo studies included. MDA-MB-231 cells were the most used. MTT, MTS, SRB, annexin-V/propidium iodide, trypan blue, and clonogenic assay were performed to assess efficacy in vitro, increasing the reliability and comprehensiveness of the data. There was found to be a decrease in cell proliferation after both chemotherapy and CAP; however, different protocol settings, including an extensive range of drug doses and CAP exposure times, were reported. For both therapies, a considerable reduction in tumor volume was observed in vivo compared with that of the untreated group. The treatment of TNBC cell lines with CAP proved successful, with apoptosis emerging as the predominant type of cellular death. This systematic review presents a comprehensive overview of the treatment landscape in chemotherapy and CAP regarding their efficacy in TNBC cell lines.
1. Introduction
Cancer is a growing disease worldwide. In 2020, breast cancer (BC) had highest incidence among cancers in women and was responsible for the largest number of deaths across all age groups [1]. The expressions of estrogen receptor-, progesterone receptor-, and human epidermal growth factor receptor-related protein are primary determinants of BC biology. This profile, which is associated with various high-throughput techniques, is used for BC stratification, prognosis, and treatment [2]. Triple-negative breast cancer (TNBC) represents approximately 15–20% of all breast cancer molecular subtypes [3]. It is characterized by the absence of these three types of receptors [4] and tends to have a worse prognosis [5]. Accurate molecular classification of TNBC is crucial for risk stratification [6,7]. Six TNBC molecular subtypes have been proposed, each one with its own features and responses to standard treatment: two different basal-like types (basal-like 1 and basal-like 2), immunomodulatory, mesenchymal, mesenchymal stem-like, and luminal androgen receptors [8,9]. These updates have allowed for personalized treatment with enhanced specificity. Currently, TNBC therapy responds to conventional chemotherapy and monoclonal antibodies, for example, pembrolizumab and avelumab, in the presence of specific biological markers such as programmed death-ligand 1 [10,11]. According to the European Society for Medical Oncology (ESMO) and American Society for Clinical Oncology (ASCO) guidelines for the treatment of TNBC, (neo)adjuvant chemotherapy drugs are used in almost all cases [11,12]. There are different regimens in use, including doxorubicin or epirubicin in combination with cyclophosphamide and paclitaxel or docetaxel in combination with carboplatin. If residual disease or the presence of the BRCA gene mutations is positive, capecitabine or olaparib is also included as an option [11,12]. Unfortunately, TNBC treatment continues to be a challenge, and new approaches are still needed. Some patients present insufficient response, others develop resistance, and treatments are frequently associated with adverse effects [13].
The plasma state, also known as the fourth state of matter, has enough energy to ionize a significant amount of positive and negative particles [14]. There are two types of plasma, namely thermal and non-thermal; the latter is frequently referred to as “cold atmospheric plasma” (CAP). CAP is characterized as non-thermal because the heavy particles are at room temperature. Several methods for producing it have been described [15]. CAP has recently been studied as a potential cancer treatment, with evidence obtained in several malign neoplasms both in vitro and in vivo [16,17,18,19,20]. Some authors have demonstrated CAP selectivity to tumoral cells compared to non-malignant counterparts, highlighting its potential in cancer treatment [21,22,23]. Some mechanisms have been proposed to explain the effects of CAP, namely properties of ultraviolet radiation [24], electric fields that may affect cellular permeabilization by increasing calcium permeability [25], and reactive species that may alter the intracellular redox state, triggering critical cellular responses [20]. Thus, it is crucial to understand the potential of CAP when compared to chemotherapy drugs. Based on the population, intervention, comparison, outcome, and study design (PICOS) criteria, this study aimed to systematically review the literature to determine whether CAP can be as effective as chemotherapy in the treatment of TNBC. Since CAP therapy is not yet a clinically approved treatment, TNBC cell lines and animal models were chosen to compare the cytotoxic effects of CAP with chemotherapeutic agents selected according to ESMO and ASCO guidelines [11,12]. Specifically, doxorubicin, epirubicin, cyclophosphamide, paclitaxel, docetaxel, carboplatin, capecitabine, and olaparib were considered for this study. These chemotherapeutic drugs are the most commonly used in clinical practice. Therefore, the main aim of this systematic review was to answer the PICO question: Is cold atmospheric plasma as effective as chemotherapy in the treatment of triple-negative breast cancer?
2. Methods
This systematic review was developed and reported following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [26], and its protocol was registered in the International Prospective Register of Systematic Reviews—PROSPERO—with the number CRD42023414394. The research question was built according to the PICO methodology, as described in Table 1.

Table 1.
Population, intervention, comparison, and outcome (PICO) research strategy used in this systematic review.
2.1. Search Strategy
The literature search was performed in four databases, namely Medline (through PubMed), Web of Science (all databases), Embase, and Cochrane Library. The search formulas used for each database are presented in the Supplemental Materials. No restriction on publication date was applied, and the English, Portuguese, Spanish, and French language filters were used. The search was completed on 13 February 2023. A manual search of the reference lists of relevant studies was performed to find additional potentially relevant studies. The search results were imported to the reference management program Mendeley Reference Manager© v2.80.1 (Mendeley Ltd., London, UK), and duplicate results were removed.
2.2. Inclusion and Exclusion Criteria
Two independent reviewers critically assessed the eligibility of studies for inclusion, first by title and abstract and later by evaluating the full text. In case of uncertainty or discrepancies regarding eligibility, a third reviewer was consulted, and a decision was made by consensus. In the eligibility phase, only in vitro and in vivo studies were considered, according to the following inclusion criteria: (1) cell lines and animal models of TNBC; (2) CAP treatment; (3) treatment with chemotherapy drugs selected according to the most recent European and American Society for Medical Oncology Clinical Practice Guidelines (ESMO and ASCO 2021), specifically doxorubicin or epirubicin or cyclophosphamide or paclitaxel or docetaxel or carboplatin or capecitabine or olaparib, and (4) papers whose main goal was to study the cell viability/proliferation of both treatments alone or in combination or in vivo tumor regression, measured as volume or histopathological changes. The exclusion criteria were as follows: (1) other study types, (2) new drugs, (3) resistant cell lines, (4) delivery methods or other formulations, (5) patient samples, (6) non-approved pharmacological combinations, (7) no report of cell viability/proliferation or tumor regression, (8) other experimental models, and (9) other types of studies whose main aim was not to evaluate the efficacy of treatments.
2.3. Data Extraction
For studies that met the inclusion criteria, the following information was collected: (1) authors and year of publication; (2) study model (i.e., triple-negative cell line(s) or animal model(s)), (3) chemotherapy treatment (i.e., chemotherapy agent, concentration drug, periodicity of drug administration and/or how it was carried out), (4) CAP treatment (i.e., plasma source, features of machine as voltage, and exposure times), (5) combination treatments with chemotherapy and CAP, (6) methods for cytotoxicity assay and/or histopathological assessment, and (7) cell viability/proliferation and in vivo tumor regression measurements.
2.4. Quality Assessment
The risk of bias of the in vitro studies was evaluated with Toxicological Data Reliability Assessment Tool (ToxRTool), which provides guidance on assessing the consistency and quality of toxicologic data [27]. The methodological quality of in vivo studies was checked by assessing the risk of bias with the Systematic Review Centre for Laboratory Animal Experimentation (SYRCLE) risk-of-bias tool [28]. Two independent authors evaluated the quality assessment methodology of eligible studies included in this systematic review.
3. Results
3.1. Study Selection
A total of 19,364 studies were obtained for analysis, with 4614 articles from PubMed (4507 regarding chemotherapy drugs plus 107 on CAP treatment), 6308 articles from Web of Science (6016 regarding chemotherapy drugs plus 292 on CAP treatment), 7308 articles from Embase (7219 regarding chemotherapy drugs plus 89 on CAP treatment), and 1134 from Cochrane Library (1107 regarding chemotherapy drugs plus 27 on CAP treatment). Before the screening, duplicate articles (8365) were removed using Web Manager (Clarivate™), leading to 10,999 records. Of these, 10,927 were excluded based on title and abstract analysis, resulting in 72 studies for full-text reading. Despite attempts to obtain all full-text records, only 66 were available to assess eligibility. Of these, seven articles were excluded for not meeting the inclusion criteria (two did not examine any drugs included in our review, one performed a mathematical analysis not encompassed in the inclusion criteria, and four did not evaluate cell viability/proliferation as defined in the PICO strategy). Thus, 59 articles were included in this systematic review for full-text reading and analysis, with publication dates from 1986 to 2023.
The PRISMA flow diagram, which summarizes the study selection performed in this systematic review, is shown in Figure 1.
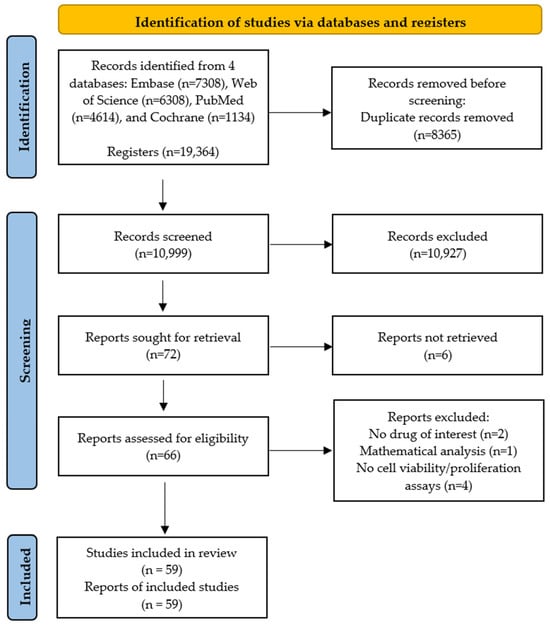
Figure 1.
PRISMA flow diagram summarizing study selection in this systematic review.
3.2. Studies’ Characteristics
Table 2 summarizes the main results observed in the studies included. In this systematic review, 59 studies were considered. Of these, 54 were in vitro studies, 3 were in vivo, and 2 articles included in vitro and in vivo experiments. The outcomes regarding the different types of studies are reported separately. We found articles on all chemotherapy drugs considered, as well as CAP treatment. Concerning CAP therapy, we identified two distinct methods: directly administered CAP and plasma-activated media (PAM), an indirect approach consisting of previously exposed solutions. Moreover, a table quantifying the percentage of inhibition induced by the therapies administered was compiled and included in the Supplemental Materials (Table S1).

Table 2.
Studies’ details and outcomes.
3.2.1. In Vitro Studies
The TNBC cell line MDA-MB-231 was employed in most of the research. The BT-20, BT-549, CAL51, CAL148, DU4475, HCC1143, HCC1395, HCC1806, HCC1937, HCC28, HCC38, HCC70, Hs578T, MDA-MB-157, MDA-MB-436, MDA-MB-453, MDA-MB-468, MFM223, MUM51, SUM52, SUM102, SUM149, SUM159, and SUM185 cell lines were also used. MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide], MTS [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium], sulforhodamine B (SRB) assay, annexin-V/propidium iodide (PI) for flow cytometry (FC), trypan blue, and clonogenic assay were performed as described in Table 2. Detailed values can be consulted in Table S1.
Chemotherapy
Paclitaxel, docetaxel, doxorubicin, olaparib, cyclophosphamide, and carboplatin concentrations ranged from 0.1 nM to 10,000 nM, from 0.1 nM to 500 nM, from 0.1 µM to 100 µM, from 0.001 µM to 250 µM, from 0.01 µM to 1 µM, and from 1 µM to 20 µM, respectively. The concentrations of capecitabine and epirubicin were not reported for in vitro studies [40,66]. Survival, assessed via the formation of colonies after 14 days, was inhibited by paclitaxel concentrations higher than 5 nM [83]. Cell viability was reduced to 80% with the exposure to 6 µM of doxorubicin for 24 hours [30]. The half-maximal inhibitory concentration (IC50) of doxorubicin was 0.3 µM in MDA-MB-231 and BT-20 cells [47,49]; however, other studies reported different values regarding MDA-MB-231 cells (1 µM [67], 6602 nM [64], 888.75 ± 65.26 nM [73], 45–50 µM and 5–10 µM [74]). The concentration of docetaxel that reduced cell proliferation by 75% in MDA-MB-231 cells was 2 nM when a 24-hour incubation was performed [42]. Cyclophosphamide induced a decrease in cell proliferation at 1 μM. Olaparib demonstrated an IC50 > 100 µM and an IC50 = 18 µM in MDA-MB-231 and MDA-MB-468 cells, respectively [37]. However, a different study in MDA-MB-231 and MDA-MB-468 cells reported values of IC50 = 13.5 µM and IC50 = 5.2 µM, respectively [45]. Concentrations from 1 µM to 10 µM (olaparib) were insufficient to induce significant alterations in the cell viability after 72 hours [78]. Carboplatin showed an IC50 = 10 µM after 24 h, and 1, 4, 8, 10, and 20 µM significantly reduced cell proliferation (MTT assay) [60]. The IC50 of capecitabine in MDA-MB-231 cells was 5150 µM and 2790 µM after incubations of 24 and 72 hours [66]. Epirubicin, in combination with other drugs [40,50], namely paclitaxel, demonstrated an antagonistic effect [50]. Doses of carboplatin lower than 10 µM, and paclitaxel showed additive interactions in MDA-MB-231 cells [50]. Despite the vast number of strategies used for chemotherapy studies, doxorubicin, paclitaxel, and docetaxel seemed to be the most promising drugs, exhibiting greater cell viability reduction at lower concentrations. Paclitaxel was capable of inhibiting 50% of cell viability at lower concentrations (0.07 nM–SRB assay) in contrast to olaparib (13.5 µM–MTT assay), with all IC50 values for the MDA-MB-231 cell line being compared.
CAP Treatment
This section describes the analysis of 19 studies. Regarding PAM treatment, the solutions used were millipore water, cell culture medium, and Ringer’s solution. A decrease of cell proliferation to 20% (p = 0.001) was observed in a volume of 150 µL and 200 µL of PAM (millipore water-based) [72]. The cell viability was reduced to 0.41 and 0.46 in MDA-MB-231 and MDA-MB-468 cells, respectively, compared to control cells after 5 minutes of PAM treatment (medium-based) [79]. The viable cells significantly decreased from 80.50 ± 1.59% to 65.00 ± 3.39% after 120 seconds of CAP treatment in HCC1806 cells [20], while another study demonstrated a reduction of more than 50% in the MDA-MB-231 cell line [52]. Chen et al. reported a reduction of cell viability of approximately 27.4% and 14.7%, respectively, with argon or helium gas flow. CAP decreased cell viability in a dose-dependent manner with statistical significance, as described in Ma et al. [55]. The viability of the MDA-MB-231 cell line decreased to under 40% or 50% after 5 minutes of CAP or PAM exposure, respectively [44]. Furthermore, apoptosis seemed to be the most prevalent type of cell death [20,55,65]. In general, CAP and PAM demonstrated an effective anti-tumoral effect in short exposure times, such as 60 and 120 seconds; however, some authors tested longer exposure times, mainly in PAM treatment.
CAP Treatment and Chemotherapy
Interestingly, CAP treatment and chemotherapy drugs, specifically paclitaxel and olaparib, were combined in two studies [19,51]. Olaparib showed a tendency to improve the efficacy of CAP in all cell lines [51]. Moreover, the chemosensitivity to paclitaxel (0.01 µM) was improved after 15 seconds of CAP exposure. According to clonogenic assay, the combined treatment decreased the number of colonies to a number similar to or even smaller than that specific to PAM or paclitaxel [19].
3.2.2. In Vivo Studies
To obtain animal models, the strains CB-17 of severe combined immunodeficiency mice [56,61,70] and Balb/c were used [79,84] and were inoculated with the TNBC cell line MDA-MB-231.
Chemotherapy
As described by Man et al., cyclophosphamide was administered following two regimens. The group continuously administered 25 mg/kg of cyclophosphamide (low concentration) via drinking water showed an initial reduction of tumor size with no weight loss for 50 days. However, the group of mice treated in cycles of 6 days with 450 mg/kg/cycle (150 mg/kg/injection every other day) demonstrated severe weight loss and death one week after starting the therapy [56]. Munõz et al., demonstrated that 20 mg/kg/day via drinking water starting on the 14th day reduced the volume of tumor compared to the control group. The percentage of necrosis increased from the control group (78%) to the treated group (85%), and no weight loss or other signs of toxicity were observed [61]. Another study demonstrated that cyclophosphamide (20 mg/kg/day) added to capecitabine (100 mg/kg) significantly increased survival compared to the control [70].
CAP Treatment
CAP treatment was administered directly into animal tumors, and PAM injection of PBS showed significant inhibition of tumor growth (p = 0.044 and p = 0.017, respectively). In the comparison of both approaches, the survival of mice in the CAP treatment group was significantly higher than that in the PAM group (p = 4.9 × 10−4). In addition, all control mice died within 27 days, while all mice from the CAP group survived until the end of the experiment (30 days) [84]. Another study showed that the tumor volume was inhibited by PAM injection, and tumor weight dropped considerably after treatment (from 4.053 g to 0.787 g, p = 4.69 × 10−4). No visible adverse effects were observed [79].
3.3. Quality Assessment
In terms of quality assessment, most studies presented an unclear bias, determined by the SYRCLE tool regarding in vivo studies, as described in Figure 2 and detailed in Supplemental Table S2. According to these bias tool guidelines, only three parameters were correctly reported for all studies, specifically, incomplete outcome data, selective outcome reporting, and other sources of bias. Moreover, 20% of articles presented no data on random outcome assessment, as shown in Figure 2. For other studies, the ToxRTool protocol was used, and articles were categorized based on this score. We observed scores between 11 and 14 as the most prevalent, where the studies were considered reliable with restrictions. Although five articles were classified as not reliable, eleven studies were classified under the criterion of reliability without restrictions. The risk assessment for individual study bias is represented in Figure 3 and detailed in Supplemental Table S3.
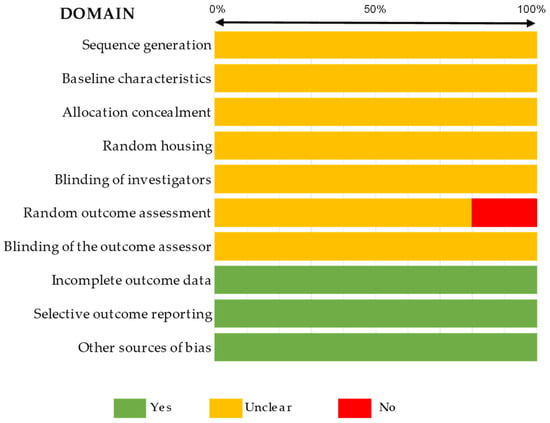
Figure 2.
Summary of the quality assessment of the in vivo studies included in the systematic review completed with the SYRCLE tool.
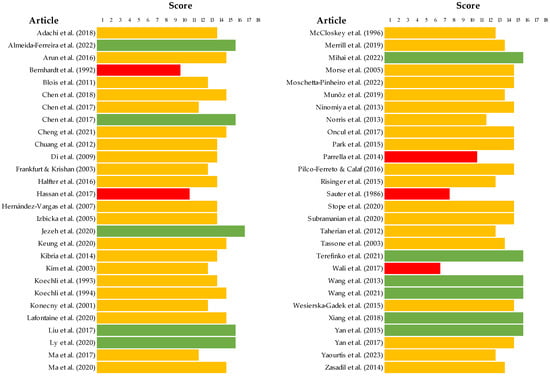
Figure 3.
Score of the quality assessment of the in vitro studies included in the systematic review completed with the ToxRTool [19,20,21,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,57,58,59,60,61,62,63,64,65,66,67,68,69,71,72,73,74,75,76,77,78,79,80,81,82,83]. Articles with scores <11 were represented in red, scores between 11–14 were represented in yellow, and scores between 15–18 were represented in green.
4. Discussion
The clinical complexity of treating TNBC often requires a tailored approach due to its aggressive nature and poor prognosis compared to other molecular subtypes [85,86,87]. Chemotherapy is a well-established treatment indicated in clinical practice regarding TNBC [10,11]; however, it has associated adverse effects. CAP has been investigated across a vast array of medical fields, specifically in dental medicine, regeneration of tissues, and tumor therapy, without causing significant harm to healthy cells, as well as having antimicrobial effects and an impact on stem cells and nitric oxide levels [88]. It has been explored as a new emerging medical approach for several types of cancer, including TNBC, with promising results in terms of cell death [20,44,75,77]. As CAP is not a clinically approved treatment, being under preclinical research, a comparison was conducted to determine the efficacy of both treatments without associated interventions.
The chemotherapeutic drugs explored—paclitaxel, docetaxel, cyclophosphamide, doxorubicin, olaparib, carboplatin, and capecitabine—were selected based on ESMO and ASCO guidelines [11,12], ensuring a broad and complete assessment of cellular effects [52,79]. Currently, chemotherapy drugs are used in clinical practice and are heavily used as a positive control in most experiments [89,90]. For all treatments, the outcomes depended on the protocol definitions, including the dose of chemotherapy drugs administered and the time of exposure to CAP. Several assays, such as MTT, MTS, SRB, annexin-V/propidium iodide, trypan blue, and clonogenic assay, were performed to prove the in vitro efficacy of CAP and chemotherapy drugs.
We found a wide range of chemotherapy concentrations tested in in vitro assays. The papers showed a reduction in cell proliferation, which is supported by the mechanisms of action of the drugs on tumor cells [91,92,93,94,95]. There were additive interactions between paclitaxel and carboplatin in vitro [50]. In fact, in patients whose TNBC disease progressed after taxane administration, carboplatin was one of the recommended chemotherapy agents [11,12]. According to the guidelines, taxane-anthracycline-based combinations are options for treatment [11,12]. The results obtained corroborated our expectations about the efficacy of chemotherapy in TNBC cell lines. However, the evidence available was often insufficient to statistically compare several studies since the data were not described quantitatively, and the various strategies do not allow for a comparative evaluation.
CAP therapy is an emerging therapeutic approach in cancer although the mechanism of action remains unclear. Recent studies have shown its effects on cell proliferation in several types of cancer, leading to cell death [35,44,96,97,98]. Here, we examine two different strategies concerning the application of CAP in TNBC cell lines, namely CAP and PAM, which used different exposure times and solutions. Both strategies are also reported in preclinical studies regarding other types of cancer [17,18,19,44,99,100,101]. Different types of equipment that are able to generate CAP are described. Some authors used a flow of gases such as argon or helium [34,44,53,62,77], and the frequency and high voltage were also dependent on the equipment of each research group. Generally, the exposure times were 60 seconds or 120 seconds [19,20,21,33,35,44,51,52,53,55,71,80,81]. The reduction of cell viability in TNBC cells was time dependent in all studies. Apoptosis seemed to be the predominant type of cell death [19,20]. Studies on different types of cancer demonstrated a close correlation with the abovementioned, supporting the anti-tumoral potential of CAP as a promising strategy. A vast number of cell lines representing melanoma, brain tumor and leukemia, cervical, breast, colorectal, gastric, lung, ovarian, head and neck, and pancreatic cancers have already been used for CAP studies, demonstrating its anti-proliferative effect [88]. Moreover, some authors reported the selectivity of CAP to tumor cell lines [22,102,103]. The efficacy of olaparib or paclitaxel combined with CAP tended to improve the cytotoxicity of treatment in five distinct TNBC cell lines [51]. PAM improved the chemosensitivity to the lowest concentration of paclitaxel and reduced the metabolic activity compared to isolated therapies [19].
Translating cell line models into animal models is a crucial stage in assessing the efficacy of therapy in a biologically complex organism. In the studies, the methods used were tumor growth or volume, animal weight, or survival monitoring. Cyclophosphamide was able to reduce the volume of tumor with no weight loss and no signs of toxicity [61]. The combination of cyclophosphamide and capecitabine increased survival [70]. Similarly, CAP and PAM inhibited tumor growth significantly, and no noticeable adverse effects were observed [52,84]. CAP was applied locally to the tumor in both plasma approaches. Although we only analyzed two papers with CAP treatment in vivo, the results proved the efficacy in TNBC. In vivo studies with different types of cancer have been performed in recent years [104,105]. Nevertheless, further studies should include cell lines representative of other TNBC molecular subtypes and consider other animal models to encompass as many of the characteristics observed in clinical practice as possible. Unfortunately, in vivo studies regarding CAP and chemotherapy combination are unavailable. In the future, to maximize the anti-tumor potential of CAP, combining it with chemotherapy drugs should also be studied in vivo, promoting a potential coordinated translation to the clinical setting. Furthermore, patient-derived xenograft models could be an enriched research option for evaluating CAP efficacy in human BC tissue samples.
As regards CAP, it could be a promising option for clinical practice. From the results obtained, we hypothesize a reduction of the side effects associated with chemotherapy with a lowering of the concentrations of the drugs administered.
One of the limitations observed was the heterogeneity between the studies due to methodological approaches, namely exposure, intervals and doses of drugs, voltages of equipment, times of exposure, and evaluation times. Moreover, the lack of important information, such as concentrations and detailed and quantitative results, impeded meta-analysis studies. In addition, we observed differences between the number of studies for each drug, which means that the sample included in some groups was reduced, limiting the conclusions. The bias assessment, based on a set of well-defined guidelines, proved the difficulty in obtaining all the information necessary to proceed to the planned statistical study, as illustrated in Figure 2 and Figure 3. CAP studies provided a more detailed description of the conditions of treatment used. Table 3 summarizes key aspects of this systematic review, including the main considerations of the study and the next steps. In the future, based on our results, we suggest creating a list of standard methodologies to address the limitations found. Moreover, correlating studies with human tissue samples of TNBC after CAP treatment with clinical reports of patients will enhance the potential translational value of therapy. Nevertheless, combining chemotherapy and CAP or PAM in vivo appears to be a strategic option.

Table 3.
Key aspects of this systematic review.
5. Conclusions
Chemotherapy agents effectively reduced cell proliferation in most TNBC cell lines, depending on a wide range of concentrations and experimenting conditions. CAP treatment successfully treated TNBC cell lines, with apoptosis being the most prevalent type of cell death. Paclitaxel or olaparib combined with CAP in vitro should be further investigated. Our results suggest that other combinations should be considered and evaluated. In the in vivo studies, the selection of different chemotherapeutic regimens influenced the obtained results, with both CAP and PAM effectively reducing tumor volume without visible side effects. However, the lack of information on both treatments and the diversity of experimental conditions underscore the need for more research. Despite the weaknesses observed, our results indicate that CAP is as effective as chemotherapy in TNBC. Innovative studies should be performed to increase the knowledge of CAP treatment and enhance future medical options on TNBC.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/ijms25063254/s1.
Author Contributions
Conceptualization, C.A.-F., C.M.M., C.C. and J.A.-F.; methodology, C.A.-F., C.M.M., J.A.-F. and C.C.; software, C.A.-F. and C.M.M.; validation, C.M.M. and M.L.; formal analysis, C.M.M., M.L., C.F. and M.J.C.; investigation, C.A.-F., C.M.M., C.C. and J.A.-F.; resources, C.M.M.; data curation, C.A.-F., C.C. and J.A.-F.; writing—original draft preparation, C.A.-F.; writing—review and editing, C.M.M., M.L., C.F., M.J.C. and M.F.B.; visualization, C.M.M., M.J.C., C.F., M.L. and M.F.B.; supervision, M.L. and M.F.B.; project administration, M.L. and M.F.B.; funding acquisition, C.A.-F. All authors have read and agreed to the published version of the manuscript.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and publication of this work. FCT (Foundation for Science and Technology) supports the Center for Innovative Biomedicine and Biotechnology (CIBB) through the Strategic Projects UIDB/04539/2020 (https://doi.org/10.54499/UIDB/04539/2020) and UIDP/04539/2020 (https://doi.org/10.54499/UIDP/04539/2020) and the Associated Laboratory funding LA/P/0058/2020 (https://doi.org/10.54499/LA/P/0058/2020). This work was supported by the Project CARBONCT, 2022.03596.PTDC (https://doi.org/10.54499/2022.03596.PTDC). A PhD fellowship granted by FCT supports Catarina Almeida-Ferreira (2022.12228.BD).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Rakha, E.A.; Green, A.R. Molecular classification of breast cancer: What the pathologist needs to know. Pathology 2017, 49, 111–119. [Google Scholar] [CrossRef]
- Palomba, G.; Budroni, M.; Olmeo, N.; Atzori, F.; Ionta, M.T.; Pisano, M.; Tanda, F.; Cossu, A.; Palmieri, G. Triple-negative breast cancer frequency and type of BRCA mutation: Clues from Sardinia. Oncol. Lett. 2014, 7, 948–952. [Google Scholar] [CrossRef][Green Version]
- Provenzano, E.; Ulaner, G.A.; Chin, S.-F. Molecular Classification of Breast Cancer. PET Clin. 2018, 13, 325–338. [Google Scholar] [CrossRef]
- Cosar, R.; Sut, N.; Ozen, A.; Tastekin, E.; Topaloglu, S.; Cicin, I.; Nurlu, D.; Ozler, T.; Demir, S.; Yıldız, G.; et al. Breast Cancer Subtypes and Prognosis: Answers to Subgroup Classification Questions, Identifying the Worst Subgroup in Our Single-Center Series. Breast Cancer Targets Ther. 2022, 14, 259–280. [Google Scholar] [CrossRef]
- Lu, B.; Natarajan, E.; Balaji Raghavendran, H.R.; Markandan, U.D. Molecular Classification, Treatment, and Genetic Biomarkers in Triple-Negative Breast Cancer: A Review. Technol. Cancer Res. Treat. 2023, 22, 15330338221145246. [Google Scholar] [CrossRef]
- Sakach, E.; O’Regan, R.; Meisel, J.; Li, X. Molecular Classification of Triple Negative Breast Cancer and the Emergence of Targeted Therapies. Clin. Breast Cancer 2021, 21, 509–520. [Google Scholar] [CrossRef]
- Lehmann, B.D.; Jovanović, B.; Chen, X.; Estrada, M.V.; Johnson, K.N.; Shyr, Y.; Moses, H.L.; Sanders, M.E.; Pietenpol, J.A. Refinement of Triple-Negative Breast Cancer Molecular Subtypes: Implications for Neoadjuvant Chemotherapy Selection. PLoS ONE 2016, 11, e0157368. [Google Scholar] [CrossRef]
- Lehmann, B.D.; Bauer, J.A.; Chen, X.; Sanders, M.E.; Chakravarthy, A.B.; Shyr, Y.; Pietenpol, J.A. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J. Clin. Investig. 2011, 121, 2750. [Google Scholar] [CrossRef]
- Harbeck, N.; Penault-Llorca, F.; Cortes, J.; Gnant, M.; Houssami, N.; Poortmans, P.; Ruddy, K.; Tsang, J.; Cardoso, F. Breast cancer. Nat. Rev. Dis. Primers 2019, 5, 66. [Google Scholar] [CrossRef]
- Gennari, A.; André, F.; Barrios, C.H.; Cortés, J.; de Azambuja, E.; DeMichele, A.; Dent, R.; Fenlon, D.; Gligorov, J.; Hurvitz, S.A.; et al. ESMO Clinical Practice Guideline for the diagnosis, staging and treatment of patients with metastatic breast cancer. Ann. Oncol. 2021, 32, 1475–1495. [Google Scholar] [CrossRef]
- Korde, L.A.; Somerfield, M.R.; Carey, L.A.; Crews, J.R.; Denduluri, N.; Hwang, E.S.; Khan, S.A.; Loibl, S.; Morris, E.A.; Perez, A.; et al. Neoadjuvant Chemotherapy, Endocrine Therapy, and Targeted Therapy for Breast Cancer: ASCO Guideline. J. Clin. Oncol. 2020, 39, 1485–1505. [Google Scholar] [CrossRef]
- Lainetti, P.d.F.; Leis-Filho, A.F.; Laufer-Amorim, R.; Battazza, A.; Fonseca-Alves, C.E. Mechanisms of Resistance to Chemotherapy in Breast Cancer and Possible Targets in Drug Delivery Systems. Pharmaceutics 2020, 12, 1193. [Google Scholar] [CrossRef]
- Izadjoo, M.; Zack, S.; Kim, H.; Skiba, J. Medical applications of cold atmospheric plasma: State of the science. J. Wound. Care 2018, 27 (Suppl. S9), S4–S10. [Google Scholar] [CrossRef]
- Hoffmann, C.; Berganza, C.; Zhang, J. Cold Atmospheric Plasma: Methods of production and application in dentistry and oncology. Med. Gas Res. 2013, 3, 21. [Google Scholar] [CrossRef]
- Ikeda, J.; Tanaka, H.; Ishikawa, K.; Sakakita, H.; Ikehara, Y.; Hori, M. Plasma-activated medium (PAM) kills human cancer-initiating cells. Pathol. Int. 2018, 68, 23–30. [Google Scholar] [CrossRef]
- Yan, D.; Sherman, J.H.; Keidar, M. The Application of the Cold Atmospheric Plasma-Activated Solutions in Cancer Treatment. Anticancer Agents Med. Chem. 2018, 18, 769–775. [Google Scholar] [CrossRef]
- Ishaq, M.; Kumar, S.; Varinli, H.; Han, Z.J.; Rider, A.E.; Evans, M.D.M.; Murphy, A.B.; Ostrikov, K. Atmospheric gas plasma-induced ROS production activates TNF-ASK1 pathway for the induction of melanoma cancer cell apoptosis. Mol. Biol. Cell 2014, 25, 1523–1531. [Google Scholar] [CrossRef]
- Mihai, C.-T.; Mihaila, I.; Pasare, M.A.; Pintilie, R.M.; Ciorpac, M.; Topala, I. Cold Atmospheric Plasma-Activated Media Improve Paclitaxel Efficacy on Breast Cancer Cells in a Combined Treatment Model. Curr. Issues Mol. Biol. 2022, 44, 1995–2014. [Google Scholar] [CrossRef]
- Almeida-Ferreira, C.; Silva-Teixeira, R.; Gonçalves, A.C.; Marto, C.M.; Sarmento-Ribeiro, A.B.; Caramelo, F.; Botelho, M.F.; Laranjo, M. Cold Atmospheric Plasma Apoptotic and Oxidative Effects on MCF7 and HCC1806 Human Breast Cancer Cells. Int. J. Mol. Sci. 2022, 23, 1698. [Google Scholar] [CrossRef]
- Wang, M.; Holmes, B.; Cheng, X.; Zhu, W.; Keidar, M.; Zhang, L.G. Cold Atmospheric Plasma for Selectively Ablating Metastatic Breast Cancer Cells. PLoS ONE 2013, 8, e73741. [Google Scholar] [CrossRef]
- Silva-Teixeira, R.; Laranjo, M.; Lopes, B.; Almeida-Ferreira, C.; Gonçalves, A.C.; Rodrigues, T.; Matafome, P.; Sarmento-Ribeiro, A.B.; Caramelo, F.; Botelho, M.F. Plasma activated media and direct exposition can selectively ablate retinoblastoma cells. Free Radic. Biol. Med. 2021, 171, 302–313. [Google Scholar] [CrossRef]
- Guerrero-Preston, R.; Ogawa, T.; Uemura, M.; Shumulinsky, G.; Valle, B.L.; Pirini, F.; Ravi, R.; Sidransky, D.; Keidar, M.; Trink, B. Cold atmospheric plasma treatment selectively targets head and neck squamous cell carcinoma cells. Int. J. Mol. Med. 2014, 34, 941–946. [Google Scholar] [CrossRef]
- Keidar, M.; Shashurin, A.; Volotskova, O.; Ann Stepp, M.; Srinivasan, P.; Sandler, A.; Trink, B. Cold atmospheric plasma in cancer therapy. Phys. Plasmas 2013, 20, 057101. [Google Scholar] [CrossRef]
- Schneider, C.; Gebhardt, L.; Arndt, S.; Karrer, S.; Zimmermann, J.L.; Fischer, M.J.M.; Bosserhoff, A.-K. Cold atmospheric plasma causes a calcium influx in melanoma cells triggering CAP-induced senescence. Sci. Rep. 2018, 8, 10048. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gotzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ 2009, 339, b2700. [Google Scholar] [CrossRef]
- Schneider, K.; Schwarz, M.; Burkholder, I.; Kopp-Schneider, A.; Edler, L.; Kinsner-Ovaskainen, A.; Hartung, T.; Hoffmann, S. “ToxRTool”, a new tool to assess the reliability of toxicological data. Toxicol. Lett. 2009, 189, 138–144. [Google Scholar] [CrossRef]
- Hooijmans, C.R.; Rovers, M.M.; de Vries, R.B.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s risk of bias tool for animal studies. BMC Med. Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef] [PubMed]
- Adachi, Y.; Yoshimura, M.; Nishida, K.; Usuki, H.; Shibata, K.; Hattori, M.; Kondo, N.; Yatabe, Y.; Iwata, H.; Kikumori, T.; et al. Acute phase dynamics of circulating tumor cells after paclitaxel and doxorubicin chemotherapy in breast cancer mouse models. Breast Cancer Res. Treat. 2018, 167, 439–450. [Google Scholar] [CrossRef]
- Arun, R.; Dhivya, S.; Abraham, S.K.; Premkumar, K. Low-dose chemotherapeutic drugs induce reactive oxygen species and initiate apoptosis-mediated genomic instability. Toxicol. Res. 2016, 5, 547–556. [Google Scholar] [CrossRef]
- Bernhardt, G.; Reile, H.; Birnbiick, H.; Sprufl, T.; Schiinenberger, H. Standardized kinetic microassay to quantify differential chemosensitivity on the basis of proliferative activity. J. Cancer Res. Clin. Oncol. 1992, 118, 35–43. [Google Scholar] [CrossRef]
- Blois, J.; Smith, A.; Josephson, L. The slow cell death response when screening chemotherapeutic agents. Cancer Chemother. Pharmacol. 2011, 68, 795–803. [Google Scholar] [CrossRef]
- Chen, Z.; Lin, L.; Zheng, Q.; Sherman, J.H.; Canady, J.; Trink, B.; Keidar, M. Micro-Sized Cold Atmospheric Plasma Source for Brain and Breast Cancer Treatment. Plasma Med. 2018, 8, 203–215. [Google Scholar] [CrossRef]
- Chen, Z.; Cheng, X.; Lin, L.; Keidar, M. Cold atmospheric plasma discharged in water and its potential use in cancer therapy. J. Phys. D Appl. Phys. 2017, 50, 015208. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, S.; Levchenko, I.; Beilis, I.I.; Keidar, M. In vitro Demonstration of Cancer Inhibiting Properties from Stratified Self-Organized Plasma-Liquid Interface. Sci. Rep. 2017, 7, 12163. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Murthy, S.R.K.; Zhuang, T.; Ly, L.; Jones, O.; Basadonna, G.; Keidar, M.; Kanaan, Y.; Canady, J. Canady helios cold plasma induces breast cancer cell death by oxidation of histone mRNA. Int. J. Mol. Sci. 2021, 22, 9578. [Google Scholar] [CrossRef]
- Chuang, H.C.; Kapuriya, N.; Kulp, S.K.; Chen, C.S.; Shapiro, C.L. Differential anti-proliferative activities of poly(ADP-ribose) polymerase (PARP) inhibitors in triple-negative breast cancer cells. Breast Cancer Res. Treat. 2012, 134, 649–659. [Google Scholar] [CrossRef]
- Di, X.; Shiu, R.P.; Newsham, I.F.; Gewirtz, D.A. Apoptosis, autophagy, accelerated senescence and reactive oxygen in the response of human breast tumor cells to Adriamycin. Biochem. Pharmacol. 2009, 77, 1139–1150. [Google Scholar] [CrossRef]
- Frankfurt, O.S.; Krishan, A. Apoptosis-based drug screening and detection of selective toxicity to cancer cells. Anti-Cancer Drugs 2003, 14, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Halfter, K.; Hoffmann, O.; Ditsch, N.; Ahne, M.; Arnold, F.; Paepke, S.; Grab, D.; Bauerfeind, I.; Mayer, B. Testing chemotherapy efficacy in HER2 negative breast cancer using patient-derived spheroids. J. Transl. Med. 2016, 14, 112. [Google Scholar] [CrossRef]
- Hassan, S.; Esch, A.; Liby, T.; Gray, J.W.; Heiser, L.M. Pathway-enriched gene signature associated with 53BP1 response to PARP inhibition in triple-negative breast cancer. Mol. Cancer Ther. 2017, 16, 2892–2901. [Google Scholar] [CrossRef]
- Hernández-Vargas, H.; Palacios, J.; Moreno-Bueno, G. Molecular profiling of docetaxel cytotoxicity in breast cancer cells: Uncoupling of aberrant mitosis and apoptosis. Oncogene 2007, 26, 2902–2913. [Google Scholar] [CrossRef]
- Izbicka, E.; Campos, D.; Carrizales, G.; Tolcher, A. Biomarkers for sensitivity to docetaxel and paclitaxel in human tumor cell lines in vitro. Cancer Genom. Proteom. 2005, 2, 219–226. [Google Scholar]
- Jezeh, M.A.; Tayebi, T.; Khani, M.R.; Niknejad, H.; Shokri, B. Direct cold atmospheric plasma and plasma-activated medium effects on breast and cervix cancer cells. Plasma Process. Polym. 2020, 17, 1900241. [Google Scholar] [CrossRef]
- Keung, M.Y.; Wu, Y.; Badar, F.; Vadgama, J.V. Response of breast cancer cells to PARP inhibitors is independent of BRCA status. J. Clin. Med. 2020, 9, 940. [Google Scholar] [CrossRef] [PubMed]
- Kibria, G.; Hatakeyama, H.; Akiyama, K.; Hida, K.; Harashima, H. Comparative Study of the Sensitivities of Cancer Cells to Doxorubicin, and Relationships between the Effect of the Drug-Efflux Pump P-gp. Biol. Pharm. Bull. 2014, 37, 1926–1935. [Google Scholar] [CrossRef]
- Kim, R.; Tanabe, K.; Emi, M.; Uchida, Y.; Toge, T. Death receptor-dependent and -independent pathways in anticancer drug-induced apoptosis of breast cancer cells. Oncol. Rep. 2003, 10, 1925–1930. [Google Scholar] [CrossRef]
- Koechli, O.R.; Sevin, B.-U.; Perras, J.R.; Chao Chou, T.; Angioli, R.; Steren, A.; Untch, M.; Averette, H.E. Characteristics of the combination paclitaxel plus doxorubicin in breast cancer cell lines analyzed with the ATP-Cell Viability Assay. Breast Cancer Res. Treat. 1993, 28, 21–27. [Google Scholar] [CrossRef]
- Koechli, O.R.; Sevin, B.-U.; Perras, J.P.; Angioli, R.; Untch, M.; Steren, A.; Ramachandran, C.; Averette, H.E. Comparative chemosensitivity profiles in three human breast cancer cell lines with the ATP-cell viability assay. Oncology 1994, 51, 552–558. [Google Scholar] [CrossRef]
- Konecny, G.; Untch, M.; Slamon, D.; Beryt, M.; Kahlert, S.; Felber, M.; Langer, E.; Lude, S.; Hepp, H.; Pegram, M. Drug interactions and cytotoxic effects of paclitaxel in combination with carboplatin, epirubicin, gemcitabine or vinorelbine in breast cancer cell lines and tumor samples. Breast Cancer Res. Treat. 2001, 67, 223–233. [Google Scholar] [CrossRef]
- Lafontaine, J.; Boisvert, J.-S.; Glory, A.; Coulombe, S.; Wong, P. Synergy between non-thermal plasma with radiation therapy and olaparib in a panel of breast cancer cell lines. Cancers 2020, 12, 348. [Google Scholar] [CrossRef]
- Liu, Y.; Tan, S.; Zhang, H.; Kong, X.; Ding, L.; Shen, J.; Lan, Y.; Cheng, C.; Zhu, T.; Xia, W. Selective effects of non-thermal atmospheric plasma on triple-negative breast normal and carcinoma cells through different cell signaling pathways. Sci. Rep. 2017, 7, 7980. [Google Scholar] [CrossRef]
- Ly, L.; Cheng, X.; Murthy, S.R.K.; Zhuang, T.; Jones, O.Z.; Basadonna, G.; Keidar, M.; Canady, J. Canady cold plasma conversion system treatment: An effective inhibitor of cell viability in breast cancer molecular subtypes. Clin. Plasma Med. 2020, 19–20, 100109. [Google Scholar] [CrossRef]
- Ma, J.; Zhang, Q.; Huang, Y.; Xu, Z. Study on inhibitory effect of paclitaxel on MEK and ERK protein overexpression and activation in different breast cancer cell lines. Int. J. Clin. Exp. Med. 2017, 10, 2986–2991. [Google Scholar]
- Ma, J.; Yu, K.N.; Zhang, H.; Nie, L.; Cheng, C.; Cui, S.; Chen, G.; Han, W. Non-Thermal Plasma Induces Apoptosis Accompanied by Protective Autophagy via Activating JNK/Sestrin2 Pathway. J. Phys. D Appl. Phys. 2020, 53, 465201. [Google Scholar] [CrossRef]
- Man, S.; Bocci, G.; Francia, G.; Green, S.; Jothy, S.; Hanahan, D.; Bohlen, P.; Hicklin, D.J.; Bergers, G.; Kerbel, R.S. Antitumor effects in mice of low-dose (metronomic) cyclophosphamide administered continuously through the drinking water. Cancer Res. 2002, 62, 2731–2735. [Google Scholar] [PubMed]
- Mccloskey, D.E.; Kaufmann, S.H.; Prestigiacomo, L.J.; Davidson, N.E. Paclitaxel Induces Programmed Cell Death in MDAMB-468 Human Breast Cancer Cells. Clin. Cancer Res. 1996, 2, 847–854. [Google Scholar] [PubMed]
- Merrill, N.M.; Lachacz, E.J.; Vandecan, N.M.; Ulintz, P.J.; Bao, L.; Lloyd, J.P.; Yates, J.A.; Morikawa, A.; Merajver, S.D.; Soellner, M.B. Molecular determinants of drug response in TNBC cell lines. Breast Cancer Res. Treat. 2020, 179, 337–347. [Google Scholar] [CrossRef]
- Morse, D.L.; Gray, H.; Payne, C.M.; Gillies, R.J. Docetaxel induces cell death through mitotic catastrophe in human breast cancer cells. Mol. Cancer Ther. 2005, 4, 1495–1504. [Google Scholar] [CrossRef]
- Moschetta-Pinheiro, M.G.; Colombo, J.; Tuckumantel, M.S.; Rebolho, G.K.; Zuccari, D.A.P.C. Treatment of Triple Negative Cell Lines with Olaparib to Block DNA Repair. Anticancer Agents Med. Chem. 2022, 22, 2036–2045. [Google Scholar] [CrossRef]
- Muñoz, R.; Hileeto, D.; Cruz-Muñoz, W.; Wood, G.A.; Xu, P.; Man, S.; Viloria-Petit, A.; Kerbel, R.S. Suppressive impact of metronomic chemotherapy using UFT and/or cyclophosphamide on mediators of breast cancer dissemination and invasion. PLoS ONE 2019, 14, e0222580. [Google Scholar] [CrossRef]
- Ninomiya, K.; Ishijima, T.; Imamura, M.; Yamahara, T.; Enomoto, H.; Takahashi, K.; Tanaka, Y.; Uesugi, Y.; Shimizu, N. Evaluation of extra- and intracellular OH radical generation, cancer cell injury, and apoptosis induced by a non-thermal atmospheric-pressure plasma jet. J. Phys. D Appl. Phys. 2013, 46, 425401. [Google Scholar] [CrossRef]
- Norris, R.E.; Adamson, P.C.; Nguyen, V.T.; Fox, E. Preclinical evaluation of the PARP inhibitor, olaparib, in combination with cytotoxic chemotherapy in pediatric solid tumors. Pediatr. Blood Cancer 2014, 61, 145–150. [Google Scholar] [CrossRef]
- Oncul, S.; Ercan, A. Discrimination of the Effects of Doxorubicin on Two Different Breast Cancer Cell Lines on Account of Multidrug Resistance and Apoptosis. Indian J. Pharm. Sci. 2017, 79, 599–607. [Google Scholar] [CrossRef]
- Park, S.B.; Kim, B.; Bae, H.; Lee, H.; Lee, S.; Choi, E.H.; Kim, S.J. Differential epigenetic effects of atmospheric cold plasma on MCF-7 and MDA-MB-231 breast cancer cells. PLoS ONE 2015, 10, e0129931. [Google Scholar] [CrossRef] [PubMed]
- Parrella, A.; Lavorgna, M.; Criscuolo, E.; Russo, C.; Isidori, M. Estrogenic activity and cytotoxicity of six anticancer drugs detected in water systems. Sci. Total Environ. 2014, 485–486, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Pilco-Ferreto, N.; Calaf, G.M. Influence of doxorubicin on apoptosis and oxidative stress in breast cancer cell lines. Int. J. Oncol. 2016, 49, 753–762. [Google Scholar] [CrossRef]
- Risinger, A.L.; Dybdal-Hargreaves, N.F.; Mooberry, S.L. Breast cancer cell lines exhibit differential sensitivities to microtubule-targeting drugs independent of doubling time. Anticancer Res. 2015, 35, 5845–5850. [Google Scholar] [PubMed]
- Sauter, C.; Cogoli, M.; Arrenbrecht, S. Interactions of Cytotoxic and Other Drugs: Rapid Cell Culture Assay. Oncology 1986, 43, 46–49. [Google Scholar] [CrossRef]
- Shaked, Y.; Pham, E.; Hariharan, S.; Magidey, K.; Beyar-Katz, O.; Xu, P.; Man, S.; Wu, F.T.H.; Miller, V.; Andrews, D.; et al. Evidence implicating immunological host effects in the efficacy of metronomic low-dose chemotherapy. Cancer Res. 2016, 76, 5983–5993. [Google Scholar] [CrossRef]
- Stope, M.B.; Benouahi, R.; Sander, C.; Haralambiev, L.; Nitsch, A.; Egger, E.; Mustea, A. Protherapeutic effects and inactivation of mammary carcinoma cells by a medical argon plasma device. Anticancer Res. 2020, 40, 6205–6212. [Google Scholar] [CrossRef]
- Subramanian, P.S.G.; Jain, A.; Shivapuji, A.M.; Sundaresan, N.R.; Dasappa, S.; Rao, L. Plasma-activated water from a dielectric barrier discharge plasma source for the selective treatment of cancer cells. Plasma Process. Polym. 2020, 17, 1900260. [Google Scholar] [CrossRef]
- Taherian, A.; Mazoochi, T. Different expression of extracellular signal-regulated kinases (ERK) 1/2 and phospho-Erk proteins in MBA-MB-231 and MCF-7 cells after chemotherapy with doxorubicin or docetaxel. Iran. J. Basic Med. Sci. 2012, 15, 669–677. [Google Scholar] [PubMed]
- Tassone, P.; Tagliaferri, P.; Perricelli, A.; Blotta, S.; Quaresima, B.; Martelli, M.L.; Goel, A.; Barbieri, V.; Costanzo, F.; Boland, C.R.; et al. BRCA1 expression modulates chemosensitivity of BRCA1-defective HCC1937 human breast cancer cells. Br. J. Cancer 2003, 88, 1285–1291. [Google Scholar] [CrossRef] [PubMed]
- Terefinko, D.; Dzimitrowicz, A.; Bielawska-Pohl, A.; Klimczak, A.; Pohl, P.; Jamroz, P. The influence of cold atmospheric pressure plasma-treated media on the cell viability, motility, and induction of apoptosis in human non-metastatic (Mcf7) and metastatic (mda-mb-231) breast cancer cell lines. Int. J. Mol. Sci. 2021, 22, 3855. [Google Scholar] [CrossRef]
- Wali, V.B.; Langdon, C.G.; Held, M.A.; Platt, J.T.; Patwardhan, G.A.; Safonov, A.; Aktas, B.; Pusztai, L.; Stern, D.F.; Hatzis, C. Systematic drug screening identifies tractable targeted combination therapies in triple-negative breast cancer. Cancer Res. 2017, 77, 566–578. [Google Scholar] [CrossRef]
- Wang, P.; Zhou, R.; Thomas, P.; Zhao, L.; Zhou, R.; Mandal, S.; Jolly, M.K.; Richard, D.J.; Rehm, B.H.A.; Ostrikov, K.; et al. Epithelial-to-mesenchymal transition enhances cancer cell sensitivity to cytotoxic effects of zcold atmospheric plasmas in breast and bladder cancer systems. Cancers 2021, 13, 2889. [Google Scholar] [CrossRef]
- Węsierska-Gądek, J.; Mauritz, M.; Mitulovic, G.; Cupo, M. Differential Potential of Pharmacological PARP Inhibitors for Inhibiting Cell Proliferation and Inducing Apoptosis in Human Breast Cancer Cells. J. Cell Biochem. 2015, 116, 2824–2839. [Google Scholar] [CrossRef]
- Xiang, L.; Xu, X.; Zhang, S.; Cai, D.; Dai, X. Cold atmospheric plasma conveys selectivity on triple negative breast cancer cells both in vitro and in vivo. Free Radic. Biol. Med. 2018, 124, 205–213. [Google Scholar] [CrossRef]
- Yan, D.; Talbot, A.; Nourmohammadi, N.; Cheng, X.; Canady, J.; Sherman, J.; Keidar, M. Principles of using Cold Atmospheric Plasma Stimulated Media for Cancer Treatment. Sci. Rep. 2015, 5, 18339. [Google Scholar] [CrossRef]
- Yan, D.; Cui, H.; Zhu, W.; Nourmohammadi, N.; Milberg, J.; Zhang, L.G.; Sherman, J.H.; Keidar, M. The Specific Vulnerabilities of Cancer Cells to the Cold Atmospheric Plasma-Stimulated Solutions. Sci. Rep. 2017, 7, 4479. [Google Scholar] [CrossRef] [PubMed]
- Yaourtis, A.M.; Levina, A.; Lay, P.A. Tumour cell heterogeneity in triple-negative breast cancer cells affects response to cisplatin, but not doxorubicin. J. Inorg. Biochem. 2023, 239, 112082. [Google Scholar] [CrossRef] [PubMed]
- Zasadil, L.M.; Andersen, K.A.; Yeum, D.; Rocque, G.B.; Wilke, L.G.; Tevaarwerk, A.J.; Raines, R.T.; Burkard, M.E.; Weaver, B.A. Cytotoxicity of Paclitaxel in Breast Cancer Is due to Chromosome Missegregation on Multipolar Spindles. Sci. Transl. Med. 2014, 6, 229ra43. [Google Scholar] [CrossRef]
- Zhou, X.; Cai, D.; Xiao, S.; Ning, M.; Zhou, R.; Zhang, S.; Chen, X.; Ostrikov, K.; Dai, X. Invivopen: A novel plasma source for in vivo cancer treatment. J. Cancer 2020, 11, 2273–2282. [Google Scholar] [CrossRef] [PubMed]
- Bosch, A.; Eroles, P.; Zaragoza, R.; Viña, J.R.; Lluch, A. Triple-negative breast cancer: Molecular features, pathogenesis, treatment and current lines of research. Cancer Treat. Rev. 2010, 36, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Gregório, A.C.; Lacerda, M.; Figueiredo, P.; Simões, S.; Dias, S.; Moreira, J.N. Therapeutic Implications of the Molecular and Immune Landscape of Triple-Negative Breast Cancer. Pathol. Oncol. Res. 2017, 24, 701–716. [Google Scholar] [CrossRef]
- Parsons, H.A.; Beaver, J.A.; Cimino-Mathews, A.; Ali, S.M.; Axilbund, J.; Chu, D.; Connolly, R.M.; Cochran, R.L.; Croessmann, S.; Clark, T.A.; et al. Individualized Molecular Analyses Guide Efforts (IMAGE): A prospective study of molecular profiling of tissue and blood in metastatic triple-negative breast cancer. Clin. Cancer Res. 2017, 23, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Braný, D.; Dvorská, D.; Halašová, E.; Škovierová, H. Molecular Sciences Cold Atmospheric Plasma: A Powerful Tool for Modern Medicine. Int. J. Mol. Sci. 2020, 21, 2932. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hou, H.; Zhang, P.; Zhang, Z. Co-delivery of doxorubicin and paclitaxel by reduction/pH dual responsive nanocarriers for osteosarcoma therapy. Drug Deliv. 2020, 27, 1044–1053. [Google Scholar] [CrossRef]
- Li, H.; Xu, W.; Li, F.; Zeng, R.; Zhang, X.; Wang, X.; Zhao, S.; Weng, J.; Li, Z.; Sun, L. Amplification of anticancer efficacy by co-delivery of doxorubicin and lonidamine with extracellular vesicles. Drug Deliv. 2022, 29, 192–202. [Google Scholar] [CrossRef]
- Pazdur, R.; Kudelka, A.P.; Kavanagh, J.J.; Cohen, P.R.; Raber, M.N. The taxoids: Paclitaxel (Taxol®) and docetaxel (Taxotere®). Cancer Treat. Rev. 1993, 19, 351–386. [Google Scholar] [CrossRef]
- Goulooze, S.C.; Cohen, A.F.; Rissmann, R. Olaparib. Br. J. Clin. Pharmacol. 2016, 81, 171–173. [Google Scholar] [CrossRef]
- de Sousa, G.F.; Wlodarczyk, S.R.; Monteiro, G. Carboplatin: Molecular mechanisms of action associated with chemoresistance. Braz. J. Pharm. Sci. 2014, 50, 693–701. [Google Scholar] [CrossRef]
- Mills, K.A.; Chess-Williams, R.; McDermott, C. Novel insights into the mechanism of cyclophosphamide-induced bladder toxicity: Chloroacetaldehyde’s contribution to urothelial dysfunction in vitro. Arch. Toxicol. 2019, 93, 3291–3303. [Google Scholar] [CrossRef]
- Walko, C.M.; Lindley, C. Capecitabine: A review. Clin. Ther. 2005, 27, 23–44. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Xiong, Z.; Zou, F.; Zhao, S.; Lu, X.; Yang, G.; He, G.; Ostrikov, K.K. Plasma-Induced Death of HepG2 Cancer Cells: Intracellular Effects of Reactive Species. In Plasma Processes and Polymers; Wiley: New York, NY, USA, 2012; Volume 9, pp. 59–66. [Google Scholar]
- Kumara, M.H.S.R.; Piao, M.J.; Kang, K.A.; Ryu, Y.S.; Park, J.E.; Shilnikova, K.; Jo, J.O.; Mok, Y.S.; Shin, J.H.; Park, Y.; et al. Non-thermal gas plasma-induced endoplasmic reticulum stress mediates apoptosis in human colon cancer cells. Oncol. Rep. 2016, 36, 2268–2274. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, N.; Nakamura, K.; Kajiyama, H. Current understanding of plasma-activated solutions for potential cancer therapy. Free Radic. Res. 2023, 57, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Ahn, H.J.; Kim, K.I.; Hoan, N.N.; Kim, C.H.; Moon, E.; Choi, K.S.; Yang, S.S.; Lee, J.S. Targeting cancer cells with reactive oxygen and nitrogen species generated by atmospheric-pressure air plasma. PLoS ONE 2014, 9, e86173. [Google Scholar] [CrossRef]
- Almeida-Ferreira, C.; Silva-Teixeira, R.; Laranjo, M.; Almeida, N.; Brites, G.; Dias-Ferreira, J.; Marques, I.; Neves, R.; Serambeque, B.; Teixo, R.; et al. Open-Air Cold Plasma Device Leads to Selective Tumor Cell Cytotoxicity. Appl. Sci. 2021, 11, 4171. [Google Scholar] [CrossRef]
- Tavares-da-Silva, E.; Pereira, E.; Pires, A.S.; Neves, A.R.; Braz-Guilherme, C.; Marques, I.A.; Abrantes, A.M.; Gonçalves, A.C.; Caramelo, F.; Silva-Teixeira, R.; et al. Cold Atmospheric Plasma, a Novel Approach against Bladder Cancer, with Higher Sensitivity for the High-Grade Cell Line. Biology 2021, 10, 41. [Google Scholar] [CrossRef]
- Hamouda, I.; Labay, C.; Cvelbar, U.; Ginebra, M.-P.; Canal, C. Selectivity of direct plasma treatment and plasma-conditioned media in bone cancer cell lines. Sci. Rep. 2021, 11, 17521. [Google Scholar] [CrossRef] [PubMed]
- Canal, C.; Fontelo, R.; Hamouda, I.; Guillem-Marti, J.; Cvelbar, U.; Ginebra, M.-P. Plasma-induced selectivity in bone cancer cells death. Free Radic. Biol. Med. 2017, 110, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Vaquero, J.; Judée, F.; Vallette, M.; Decauchy, H.; Arbelaiz, A.; Aoudjehane, L.; Scatton, O.; Gonzalez-Sanchez, E.; Merabtene, F.; Augustin, J.; et al. Cold-atmospheric plasma induces tumor cell death in preclinical in vivo and in vitro models of human cholangiocarcinoma. Cancers 2020, 12, 1280. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.M.; Yoon, H.K.; Kim, S.Y.; Yun, M.R.; Kim, G.H.; Lee, W.J.; Lee, M.W.; Chang, S.E.; Won, C.H. Anticancer Effect of Cold Atmospheric Plasma in Syngeneic Mouse Models of Melanoma and Colon Cancer. Molecules 2023, 28, 4171. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).