Effectively Enhancing the Physiological Activity and Sensory Quality of Whole Calamondin Puree via Yeast Fermentation
Abstract
1. Introduction
2. Results and Discussion
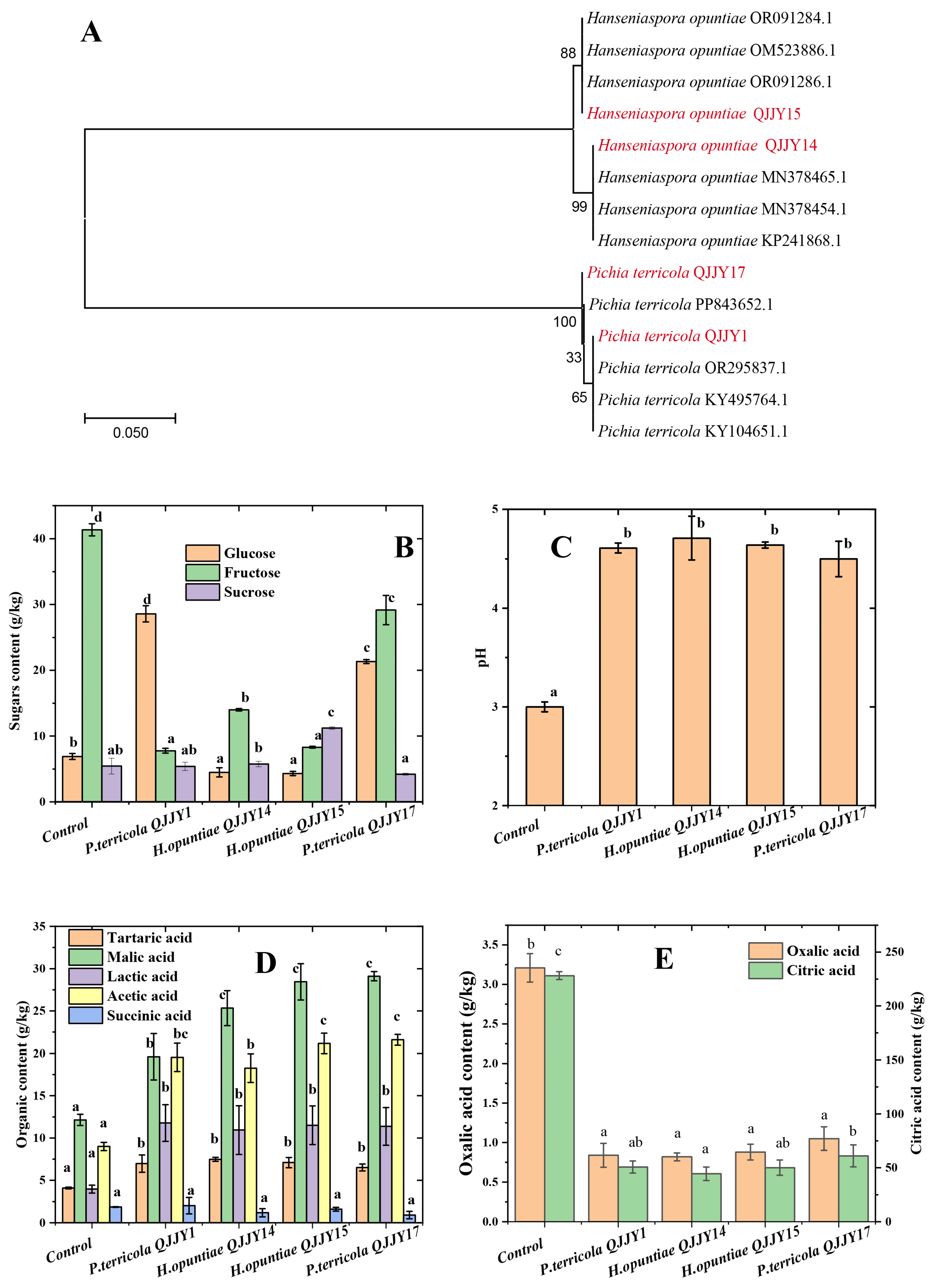
2.1. Yeast Isolation and Identification
2.2. pH, Sugar, and Organic Acid Profile of the Fermented Fruit Puree
2.3. Free Amino Acid Profile of the Fermented Fruit Puree
2.4. Phenolic Compound and Limonoid Profile of Fermented Fruit Puree
2.5. Volatile Compound Profile of the Fermented Fruit Puree
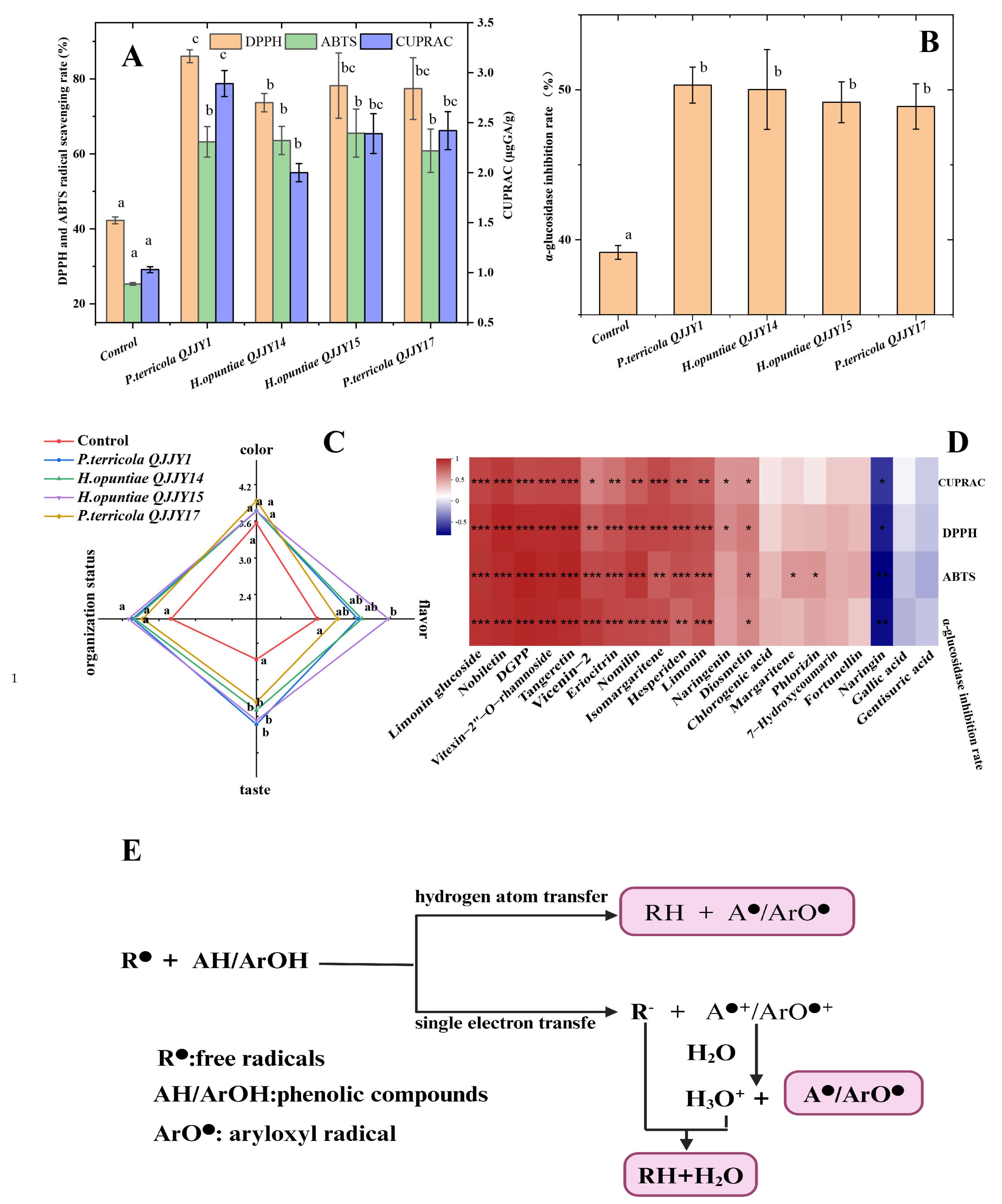
2.6. Sensory Analysis
2.7. Evaluation of Antioxidant Activity and α-Glucosidase Inhibition Rate
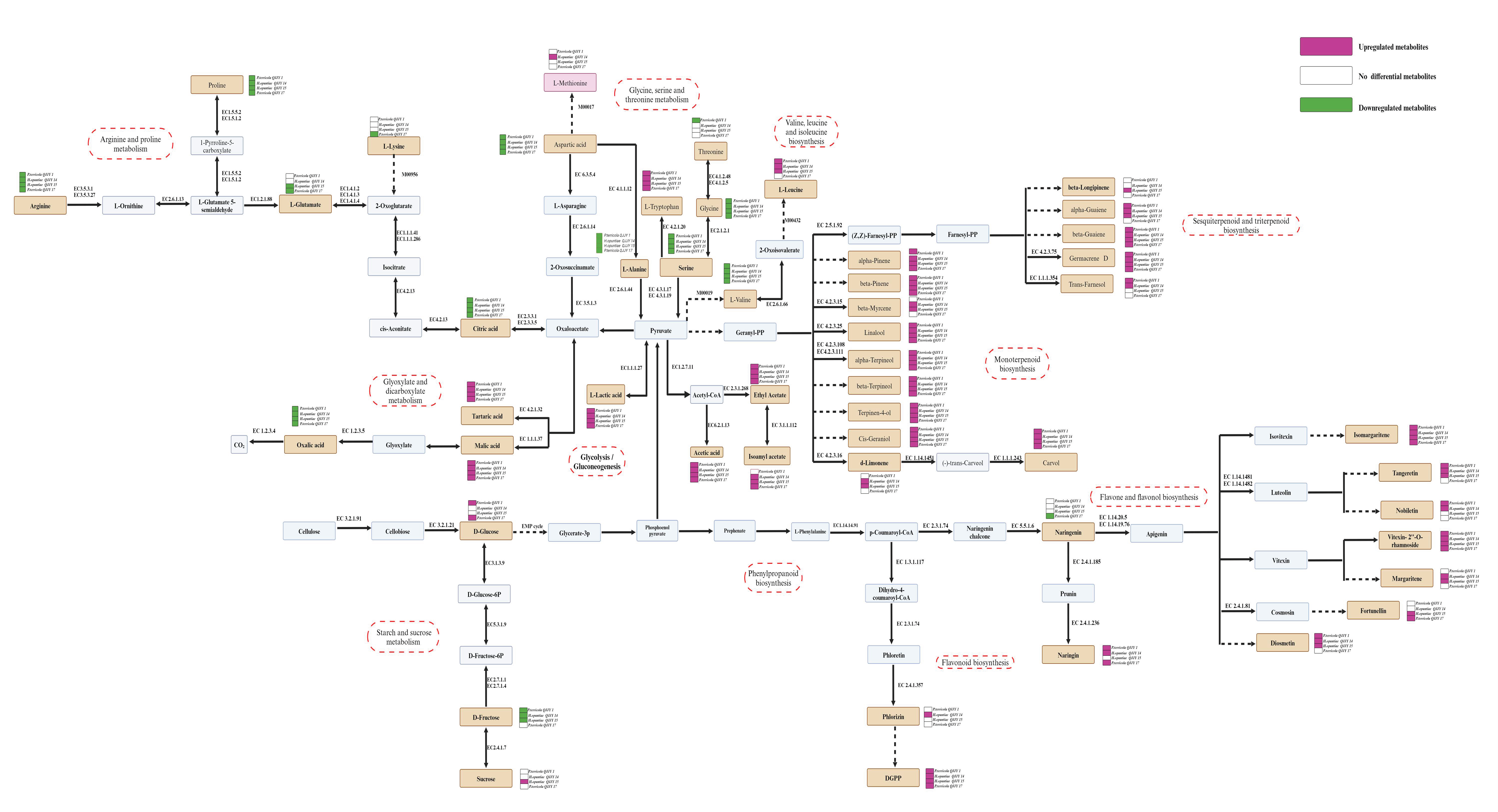
2.8. Characteristic Metabolites and Metabolic Pathway Prediction
3. Material and Methods
3.1. Samples and Chemical Reagents
3.2. Yeast Isolation and Identification
3.3. Use of Isolated Yeast Stains in Whole Fruit Puree Fermentation
3.4. Determination of Sugars, Organic Acids, and Free Amino Acids
3.4.1. Preparation of the Sugar, Organic Acid, and Amino Acid Extracts
3.4.2. Determination of Sugars
3.4.3. Determination of Organic Acids
3.4.4. Determination of Free Amino Acids
3.5. Determination of Phenolic Compounds and Limonoids
3.5.1. Preparation of Phenolic Compound and Limonoid Extracts
3.5.2. Determination of Phenolic Compounds and Limonins
3.6. Determination of Volatile Compounds
3.7. Evaluation of the Antioxidant Activity and A-Glucosidase Inhibition Rate
3.7.1. DPPH (2,2-Diphenyl-1-picrylhydrazyl) Radical Scavenging Activity
3.7.2. ABTS Radical Cation Scavenging Activity
3.7.3. Cupric Reducing Antioxidant Capacity
3.7.4. Determination of the α-Glucosidase Inhibition Rate
3.8. Sensory Assessment
3.9. Data Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lou, S.N.; Ho, C.T. Phenolic compounds and biological activities of small-size citrus: Kumquat and calamondin. J. Food Drug Anal. 2017, 25, 162–175. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Zhang, H.; Zheng, L. Physicochemical Characteristics of Calamondin (Citrus microcarpa) from Hainan. Asian Agric. Res. 2020, 12, 58–62. [Google Scholar]
- Lou, S.N.; Lin, Y.S.; Hsu, Y.S.; Chiu, E.M.; Ho, C.T. Soluble and insoluble phenolic compounds and antioxidant activity of immature calamondin affected by solvents and heat treatment. Food Chem. 2014, 161, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Zeng, S.; Li, S.; Xiao, P.; Cai, Y.; Chu, C.; Chen, B.; Li, P.; Li, J.; Liu, E. Citrus polymethoxyflavones attenuate metabolic syndrome by regulating gut microbiome and amino acid metabolism. Sci. Adv. 2020, 6, eaax6208. [Google Scholar] [CrossRef]
- Singh, A.; Sharma, V.; Banerjee, R.; Sharma, S.; Kuila, A. Perspectives of cell-wall degrading enzymes in cereal polishing. Food Biosci. 2016, 15, 81–86. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, Y.; Bai, Y.; Fu, C.; Zhou, M.; Gao, B.; Wang, C.; Li, D.; Hu, Y.; Xu, N. Effects of mixed cultures of Saccharomyces cerevisiae and Lactobacillus plantarum in alcoholic fermentation on the physicochemical and sensory properties of citrus vinegar. LWT-Food Sci. Technol. 2017, 84, 753–763. [Google Scholar] [CrossRef]
- Hu, X.; Zeng, J.; Shen, F.; Xia, X.; Tian, X.; Wu, Z. Citrus pomace fermentation with autochthonous probiotics improves its nutrient composition and antioxidant activities. LWT-Food Sci. Technol. 2022, 157, 113076. [Google Scholar] [CrossRef]
- Tao, R.; Chen, Q.; Li, Y.; Guo, L.; Zhou, Z. Physicochemical, nutritional, and phytochemical profile changes of fermented citrus puree from enzymatically hydrolyzed whole fruit under cold storage. LWT-Food Sci. Technol. 2022, 169, 114009. [Google Scholar] [CrossRef]
- Macêdo, E.d.L.C.; Pimentel, T.C.; de Sousa Melo, D.; de Souza, A.C.; de Morais, J.S.; dos Santos Lima, M.; Dias, D.R.; Schwan, R.F.; Magnani, M. Yeasts from fermented Brazilian fruits as biotechnological tools for increasing phenolics bioaccessibility and improving the volatile profile in derived pulps. Food Chem. 2023, 401, 134200. [Google Scholar] [CrossRef]
- Pimentel, T.C.; de Oliveira, L.I.G.; Macedo, E.d.L.C.; Costa, G.N.; Dias, D.R.; Schwan, R.F.; Magnani, M. Understanding the potential of fruits, flowers, and ethnic beverages as valuable sources of techno-functional and probiotics strains: Current scenario and main challenges. Trends Food Sci. Technol. 2021, 114, 25–59. [Google Scholar] [CrossRef]
- Karim, A.; Gerliani, N.; Aïder, M. Kluyveromyces marxianus: An emerging yeast cell factory for applications in food and biotechnology. Int. J. Food Microbiol. 2020, 333, 108818. [Google Scholar] [CrossRef]
- Giuffrè, D.; Giuffrè, A.M. Fermentation technology and functional foods. Front. Biosci. -Elite 2024, 16, 8. [Google Scholar] [CrossRef]
- Jiang, Y.; Luo, T.; Tang, Y.; Chen, S.; Ni, H.; Chen, Q.; Song, X.; Bao, Y.; Deng, Z.; Wang, J. Isolation of a novel characterized Issatchenkia terricola from red raspberry fruits on the degradation of citric acid and enrichment of flavonoid and volatile profiles in fermented red raspberry juice. Food Sci. Hum. Wellness 2022, 11, 1018–1027. [Google Scholar] [CrossRef]
- Arroyo-López, F.; Romero-Gil, V.; Bautista-Gallego, J.; Rodríguez-Gómez, F.; Jiménez-Díaz, R.; García-García, P.; Querol, A.; Garrido-Fernández, A. Yeasts in table olive processing: Desirable or spoilage microorganisms? Int. J. Food Microbiol. 2012, 160, 42–49. [Google Scholar] [CrossRef]
- Multari, S.; Carafa, I.; Barp, L.; Caruso, M.; Licciardello, C.; Larcherb, R.; Tuohy, K.; Martens, S. Effects of Lactobacillus spp. on the phytochemical composition of juices from two varieties of Citrus sinensis L. Osbeck: ‘Tarocco’ and ‘Washington navel’. LWT-Food Sci. Technol. 2020, 125, 109205. [Google Scholar] [CrossRef]
- He, Y.; Zhu, Y.; Lv, J.; Gu, Y.; Wang, T.; Chen, J. Effects of lactic acid bacteria fermentation on the bioactive composition, volatile compounds and antioxidant activity of Huyou (Citrus aurantium ‘Changshan-huyou’) peel and pomace. Food Qual. Saf. 2023, 7, fyad003. [Google Scholar] [CrossRef]
- Fang, S.; Chen, J.; Zhou, J.; Sha, R.; Mao, J. Analysis of organic acids and antioxidant activity in vitro of citrus medica Jiaosu during fermentation process. Sci. Technol. Food Ind. 2020, 41, 68–74. [Google Scholar]
- Rêgo, E.S.B.; Rosa, C.A.; Freire, A.L.; Machado, A.M.d.R.; Gomes, F.d.C.O.; Costa, A.S.P.d.; Mendonça, M.d.C.; Hernández-Macedo, M.L.; Padilha, F.F. Cashew wine and volatile compounds produced during fermentation by non-Saccharomyces and Saccharomyces yeast. LWT-Food Sci. Technol. 2020, 126, 109291. [Google Scholar] [CrossRef]
- Ferreira, I.M.; Guido, L.F. Impact of wort amino acids on beer flavour: A review. Fermentation 2018, 4, 23. [Google Scholar] [CrossRef]
- Shen, F.; Wang, T.; Zhang, R.; Zhong, B.; Wu, Z. Metabolism and release of characteristic components and their enzymatic mechanisms in Pericarpium Citri Reticulatae co-fermentation. Food Chem. 2024, 432, 137227. [Google Scholar] [CrossRef]
- Waskiw-Ford, M.; Hannaian, S.; Duncan, J.; Kato, H.; Sawan, S.A.; Locke, M.; Kumbhare, D.; Moore, D. Leucine-Enriched Essential Amino Acids Improve Recovery from Post-Exercise Muscle Damage Independent of Increases in Integrated Myofibrillar Protein Synthesis in Young Men. Nutrients 2020, 12, 1061. [Google Scholar] [CrossRef]
- Luo, Z.; Liu, Y.; Wang, X.; Fan, F.; Yang, Z.; Luo, D. Exploring tryptophan metabolism: The transition from disturbed balance to diagnostic and therapeutic potential in metabolic diseases. Biochem. Pharmacol. 2024, 230, 116554. [Google Scholar] [CrossRef]
- Lou, S.N.; Yu, M.W.; Ho, C.T. Tyrosinase inhibitory components of immature calamondin peel. Food Chem. 2012, 135, 1091–1096. [Google Scholar] [CrossRef]
- Kurowska, E.; Hasgawa, S.; Manners, G. Regulation of apo B production in HepG2 cells by citrus limonoids. In Citrus Limonoids: Functional Chemicals in Agriculture and Food; American Chemical Society: Washington, DC, USA, 2000. [Google Scholar]
- Cheong, M.W.; Chong, Z.S.; Liu, S.Q.; Zhou, W.; Curran, P.; Yu, B. Characterisation of calamansi (Citrus microcarpa). Part I: Volatiles, aromatic profiles and phenolic acids in the peel. Food Chem. 2012, 134, 686–695. [Google Scholar] [CrossRef]
- Withers, S.T.; Keasling, J.D. Biosynthesis and engineering of isoprenoid small molecules. Appl. Microbiol. Biotechnol. 2007, 73, 980–990. [Google Scholar] [CrossRef]
- Daenen, L.; Sterckx, F.; Delvaux, F.R.; Verachtert, H.; Derdelinckx, G. Evaluation of the glycoside hydrolase activity of a Brettanomyces strain on glycosides from sour cherry (Prunus cerasus L.) used in the production of special fruit beers. FEMS Yeast Res. 2008, 8, 1103–1114. [Google Scholar] [CrossRef]
- Yan, S.; Chen, X.; Xiang, X. Improvement of the aroma of lily rice wine by using aroma-producing yeast strain Wickerhamomyces anomalus HN006. AMB Express 2019, 9, 89. [Google Scholar] [CrossRef]
- Hočevar, B.; Grilc, M.; Huš, M.; Likozar, B. Mechanism, ab initio calculations and microkinetics of straight-chain alcohol, ether, ester, aldehyde and carboxylic acid hydrodeoxygenation over Ni-Mo catalyst. Chem. Eng. J. 2019, 359, 1339–1351. [Google Scholar] [CrossRef]
- Liu, G.; Huang, L.; Lian, J. Alcohol acyltransferases for the biosynthesis of esters. Biotechnol. Biofuels 2023, 16, 93. [Google Scholar] [CrossRef]
- Ponnampalam, E.N.; Kiani, A.; Santhiravel, S.; Holman, B.W.; Lauridsen, C.; Dunshea, F.R. The importance of dietary antioxidants on oxidative stress, meat and milk production, and their preservative aspects in farm animals: Antioxidant action, animal health, and product quality—Invited review. Animals 2022, 12, 3279. [Google Scholar] [CrossRef]
- Yan, T.; Yue, Z.; Gu, Y.; Zheng, H.; Cao, J. Screening of lipase inhibitors in citrus fruits by electrophoretically-mediated microanalysis combined with molecular docking. J. Food Compos. Anal. 2022, 105, 104185. [Google Scholar] [CrossRef]
- Zheng, Z.; Xu, Y.; Qu, H.; Zhou, H.; Yang, H. Enhancement of anti-diabetic activity of pomelo peel by the fermentation of Aspergillus oryzae CGMCC23295: In vitro and in silico docking studies. Food Chem. 2024, 432, 137195. [Google Scholar] [CrossRef]
- Chen, J.; Wu, X.; Zhou, Y.; He, J. Camellia nitidissima Chi leaf as pancreatic lipase inhibitors: Inhibition potentials and mechanism. J. Food Biochem. 2021, 45, e13837. [Google Scholar] [CrossRef]
- Huang, R.; Zhang, Y.; Shen, S.; Zhi, Z.; Cheng, H.; Chen, S.; Ye, X. Antioxidant and pancreatic lipase inhibitory effects of flavonoids from different citrus peel extracts: An in vitro study. Food Chem. 2020, 326, 126785. [Google Scholar] [CrossRef]
- Munteanu, I.G.; Apetrei, C. Analytical methods used in determining antioxidant activity: A review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef]
- Panda, P.K.; Yang, J.M.; Chang, Y.H.; Su, W.W. Modification of different molecular weights of chitosan by p-Coumaric acid: Preparation, characterization and effect of molecular weight on its water solubility and antioxidant property. Int. J. Biol. Macromol. 2019, 136, 661–667. [Google Scholar] [CrossRef]
- Schoch, C.L.; Seifert, K.A.; Huhndorf, S.; Robert, V.; Spouge, J.L.; Levesque, C.A.; Chen, W.; Consortium, F.B.; List, F.B.C.A.; Bolchacova, E. Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi. Proc. Natl. Acad. Sci. USA 2012, 109, 6241–6246. [Google Scholar] [CrossRef]
- Georgelis, N.; Fencil, K.; Richael, C.M. Validation of a rapid and sensitive HPLC/MS method for measuring sucrose, fructose and glucose in plant tissues. Food Chem. 2018, 262, 191–198. [Google Scholar] [CrossRef]
- Zeppa, G.; Conterno, L.; Gerbi, V. Determination of organic acids, sugars, diacetyl, and acetoin in cheese by high-performance liquid chromatography. J. Agric. Food Chem. 2001, 49, 2722–2726. [Google Scholar] [CrossRef]
- Li, P.; Liu, M.; Hu, J.; Su, W. Systematic chemical profiling of Citrus grandis ‘Tomentosa’ by ultra-fast liquid chromatography/diode-array detector/quadrupole time-of-flight tandem mass spectrometry. J. Pharm. Biomed. Anal. 2014, 90, 167–179. [Google Scholar] [CrossRef]
- Hwang, Y.S.; Yang, J.S.; Lee, H.W.; Ha, J.H. Optimization and comparison of headspace hot injection and trapping, headspace solid-phase microextraction, and static headspace sampling techniques with gas chromatography–mass spectrometry for the analysis of volatile compounds in kimchi. LWT-Food Sci. Technol. 2020, 134, 110155. [Google Scholar] [CrossRef]
- Xia, A.; Liu, L.; Tang, X.; Lei, S.; Meng, X.; Liu, Y. Dynamics of microbial communities, physicochemical factors and flavor in rose jam during fermentation. LWT-Food Sci. Technol. 2022, 155, 112920. [Google Scholar] [CrossRef]
- Fan, H.; Sun, L.; Yang, L.; Zhou, J.; Yin, P.; Li, K.; Xue, Q.; Li, X.; Liu, Y. Assessment of the bioactive phenolic composition of Acer truncatum seed coat as a byproduct of seed oil. Ind. Crops Prod. 2018, 118, 11–19. [Google Scholar] [CrossRef]
- Esin Çelik, S.; Özyürek, M.; Güçlü, K.; Çapanoğlu, E.; Apak, R. Identification and Anti-oxidant Capacity Determination of Phenolics and their Glycosides in Elderflower by On-line HPLC–CUPRAC Method. Phytochem. Anal. 2014, 25, 147–154. [Google Scholar] [CrossRef]
- Fu, W.; Zhao, G.; Liu, J. Effect of preparation methods on physiochemical and functional properties of yeast β-glucan. LWT-Food Sci. Technol. 2022, 160, 113284. [Google Scholar] [CrossRef]
| Free Amino Acid (mg/g) | Control | P. terricola QJJY1 | H. opuntiae QJJY14 | H. opuntiae QJJY15 | P. terricola QJJY17 |
|---|---|---|---|---|---|
| Asp | 6.41 ± 0.20 c | 2.30 ± 0.07 b | 1.43 ± 0.09 a | 1.17 ± 0.19 a | 1.36 ± 0.30 a |
| Glu | 1.74 ± 0.13 d | 1.27 ± 0.03 c | 0.96 ± 0.03 b | 0.59 ± 0.04 a | 0.63 ± 0.12 a |
| Ser | 1.80 ± 0.04 d | 0.26 ± 0.02 c | 0.15 ± 0.07 b | 0.06 ± 0.00 a | 0.10 ± 0.04 ab |
| Gly | 0.22 ± 0.02 b | 0.09 ± 0.01 a | 0.09 ± 0.03 a | 0.07 ± 0.01 a | 0.08 ± 0.01 a |
| His | 0.33 ± 0.05 ab | 0.40 ± 0.02 bc | 0.31 ± 0.01 a | 0.25 ± 0.08 a | 0.42 ± 0.02 c |
| Arg | 0.33 ± 0.02 b | 0.06 ± 0.01 a | 0.08 ± 0.02 a | 0.08 ± 0.02 a | 0.06 ± 0.02 a |
| Thr | 0.32 ± 0.02 bc | 0.06 ± 0.01 a | 0.33 ± 0.01 c | 0.29 ± 0.03 b | 0.32 ± 0.03 bc |
| Ala | 0.87 ± 0.02 c | 0.43 ± 0.04 b | 0.21 ± 0.01 a | 0.23 ± 0.05 a | 0.27 ± 0.02 a |
| Pro | 1.96 ± 0.05 c | 0.32 ± 0.06 b | 0.19 ± 0.01 a | 0.20 ± 0.02 a | 0.34 ± 0.09 b |
| Tyr | 0.18 ± 0.00 b | 0.07 ± 0.01 a | 0.09 ± 0.04 a | 0.05 ± 0.03 a | 0.08 ± 0.01 a |
| Val | 0.28 ± 0.01 b | 0.07 ± 0.01 a | 0.11 ± 0.06 a | 0.06 ± 0.01 a | 0.06 ± 0.02 a |
| Met | 0.03 ± 0.00 a | 0.04 ± 0.01 a | 0.06 ± 0.02 a | 0.04 ± 0.01 a | 0.04 ± 0.01 a |
| Cys | 0.36 ± 0.00 a | 0.38 ± 0.01 ab | 0.38 ± 0.01 ab | 0.38 ± 0.01 ab | 0.39 ± 0.01 b |
| Ile | 0.10 ± 0.00 a | 0.08 ± 0.02 a | 0.11 ± 0.03 a | 0.09 ± 0.02 a | 0.09 ± 0.02 a |
| Leu | 0.09 ± 0.00 a | 0.13 ± 0.01 abc | 0.19 ± 0.06 c | 0.15 ± 0.03 bc | 0.12 ± 0.03 ab |
| Phe | 0.59 ± 0.01 b | 0.19 ± 0.00 a | 0.20 ± 0.03 a | 0.17 ± 0.03 a | 0.18 ± 0.00 a |
| Trp | 0.05 ± 0.00 a | 0.15 ± 0.01 b | 0.18 ± 0.00 b | 0.16 ± 0.03 b | 0.17 ± 0.02 b |
| Lys | 0.29 ± 0.00 d | 0.13 ± 0.01 a | 0.25 ± 0.05 cd | 0.19 ± 0.03 bc | 0.14 ± 0.02 ab |
| Total amino acids | 15.96 ± 0.30 d | 6.44 ± 0.17 c | 5.31 ± 0.39 b | 4.24 ± 0.36 a | 4.84 ± 0.52 ab |
| No. | Volatile Compounds | LRI | CAS | μg/kg | Odor Description | ||||
|---|---|---|---|---|---|---|---|---|---|
| Control | P. terricola QJJY1 | H. opuntiae QJJY14 | H. opuntiae QJJY15 | P. terricola QJJY17 | |||||
| Higher alcohols | 3668.57 ± 296.87 a | 7164.43 ± 893.14 b | 7423.03 ± 754.98 b | 7699.94 ± 1629.31 b | 6762.57 ± 230.07 b | ||||
| 1 | 2-methyl-3-Buten-2-ol A | 659 | 115-18-4 | 12.93 ± 1.23 a | 149.22 ± 29.07 b | 181.11 ± 12.3 bc | 224.21 ± 52.41 c | 193.93 ± 7.28 bc | Herb |
| 2 | Linalool | 1098 | 78-70-6 | 132.14 ± 21.57 a | 985.6 ± 204.04 b | 963.46 ± 34.27 b | 1068.83 ± 262.64 b | 865.23 ± 38.23 b | Coriander, floral, lemon, rose |
| 3 | β-Terpineol A | 1146 | 138-87-4 | 237.2 ± 19.61 a | 577.04 ± 63.63 b | 581.2 ± 90.47 b | 597.44 ± 127.67 b | 528.45 ± 15.64 b | Coriander |
| 4 | 1-Nonanol | 1171 | 143-08-8 | 0.08 ± 0.14 a | 5.58 ± 5.67 a | n.d. | n.d. | n.d. | Fat, floral, green, oil |
| 5 | Terpinen-4-ol | 1176 | 562-74-3 | 188.48 ± 21.91 a | 667.28 ± 104.09 b | 686.58 ± 50.95 b | 729.83 ± 162.67 b | 629.52 ± 26.04 b | Earth, must, nutmeg, wood |
| 6 | α-Terpineol | 1190 | 98-55-5 | 2982.44 ± 220.97 a | 4447.84 ± 434.86 b | 4694.05 ± 537.97 b | 4784.78 ± 979.69 b | 4304.11 ± 122.17 b | Anise, fresh, mint, oil |
| 7 | Ageratriol | 1215 | 38022-97-8 | n.d. | 2.81 ± 4.86 ab | 8.42 ± 0.55 b | 6.78 ± 2.49 ab | 5.93 ± 6.36 ab | n.a. |
| 8 | cis-Geraniol A | 1250 | 106-25-2 | n.d. | 24.36 ± 7.4 b | 16.87 ± 13.94 ab | 2.49 ± 4.32 a | 9.76 ± 16.91 ab | Sweet |
| 9 | trans-Farnesol A | 1347 | 106-28-5 | 8.22 ± 0.72 a | 26.8 ± 5.83 b | 13.98 ± 5.24 a | 7.8 ± 1.29 a | 8.98 ± 2.28 a | Muguet |
| 10 | 2-Naphthalenemethanol | 1646 | 1592-38-7 | 107.07 ± 10.99 a | 277.9 ± 47.29 b | 277.37 ± 38.97 b | 277.77 ± 44.66 b | 216.67 ± 14.31 b | n.a. |
| Aldehydes | 207.97 ± 38.51 b | 21.55 ± 14.35 a | 0 ± 0 a | 0 ± 0 a | 0 ± 0 a | ||||
| 11 | Nonanal | 1102 | 124-19-6 | 100.01 ± 21.96 b | 6.49 ± 1.83 a | n.d. | n.d. | n.d. | Fat, floral, green, lemon |
| 12 | Decanal | 1203 | 112-31-2 | 105.26 ± 16.52 b | 15.07 ± 13.05 a | n.d. | n.d. | n.d. | Floral, fried, orange peel, penetrating, tallow |
| 13 | (E)-2-Decenal A | 1259 | 3913-81-3 | 2.7 ± 0.02 b | n.d. | n.d. | n.d. | n.d. | Tallow |
| Esters | 395.91 ± 33.4 a | 649.12 ± 109.28 b | 357.93 ± 63.73 a | 392.78 ± 117.57 a | 239.12 ± 107.8 a | ||||
| 14 | Ethyl acetate | 668 | 141-78-6 | 0.12 ± 0.05 a | 1.11 ± 0.53 b | 0.71 ± 0.45 ab | 0.51 ± 0.13 ab | 0.33 ± 0.1 a | Aromatic, brandy, grape |
| 15 | Isoamyl acetateA | 726 | 123-92-2 | n.d. | n.d. | 213.84 ± 37.86 ab | 280.86 ± 67.85 c | 130.53 ± 100.94 a | Banana |
| 16 | 1-Octanol acetate B | 1209 | 112-14-1 | 38.25 ± 5.34 a | 34.23 ± 9.7 a | n.d. | n.d. | n.d. | Green, earthy, mushroom |
| 17 | Geranyl propionate | 1356 | 105-90-8 | 44.1 ± 2.81 b | 92.75 ± 13.91 c | 35.96 ± 13.9 ab | 19.38 ± 8.78 a | 19.53 ± 4.7 a | Floral |
| 18 | Geranyl isovalerate | 1375 | 109-20-6 | 313.43 ± 25.2 b | 521.04 ± 86.34 c | 107.41 ± 36.62 a | 92.04 ± 43.79 a | 88.73 ± 36.7 a | Apple, fruit, rose |
| Terpene hydrocarbons | 339,719.56 ± 79,433.24 a | 455,810.93 ± 72,571.69 ab | 512,905.53 ± 48,108.58 ab | 560,466.59 ± 13,6886.41 b | 465,057.92 ± 50,295.93 ab | ||||
| 19 | α-Pinene | 929 | 80-56-8 | 2138.49 ± 599.44 a | 3508.87 ± 590.55 b | 4207.59 ± 341.72 b | 4716.36 ± 1188.87 b | 3856.71 ± 450.9 b | Cedarwood, pine, sharp |
| 20 | Camphene | 944 | 79-92-5 | 48.13 ± 12.64 a | 32.18 ± 7.87 a | 39.18 ± 4.25 a | 44.52 ± 9.67 a | 37.19 ± 5.21 a | Camphor, mothball, oil, warm |
| 21 | β-Phellandrene A | 969 | 555-10-2 | 71.35 ± 21.65 a | 66.34 ± 11.75 a | 75.82 ± 16.92 a | 79.31 ± 16.6 a | 72.81 ± 6.11 a | Mint, turpentine |
| 22 | β-Pinene | 972 | 127-91-3 | 168.06 ± 51.24 a | 341.76 ± 66.02 b | 410.66 ± 19.49 b | 440.61 ± 124.2 b | 356.7 ± 37.99 b | Pine, polish, wood |
| 23 | β-Myrcene | 989 | 123-35-3 | 6148.43 ± 1616.86 a | 8312.27 ± 1374.4 ab | 9640.49 ± 986.98 b | 10650.3 ± 2643.51 b | 8680.54 ± 1032.66 ab | Balsamic, fruit, geranium, herb, must |
| 24 | α-Terpinen | 1014 | 99-86-5 | 423.28 ± 104.83 a | 435.78 ± 67.46 a | 387.54 ± 66.98 a | 408.52 ± 84.93 a | 341.62 ± 45.4 a | Lemon |
| 25 | 1,3,8-p-Menthatriene A | 1021 | 18368-95-1 | 68.46 ± 17.58 a | 40.47 ± 39.58 a | 64.13 ± 12.75 a | 69.81 ± 22.56 a | 52.59 ± 2.96 a | Turpentine |
| 26 | D-Limonene | 1030 | 5989-27-5 | 329,499.45 ± 76,769.22 a | 441,446.1 ± 70181.22 ab | 496,630.38 ± 46,441.31 b | 542,599.63 ± 132,418.24 b | 450,571.98 ± 48,612.59 ab | Citrus, mint |
| 27 | 3-Carene | 1046 | 13466-78-9 | 70.99 ± 19.34 a | 99.55 ± 21.21 a | 94.91 ± 13.89 a | 102.69 ± 26.78 a | 79.88 ± 10.91 a | Lemon |
| 28 | γ-Terpinene | 1056 | 99-85-4 | 221.98 ± 52.59 a | 283.46 ± 44.08 a | 290.35 ± 36.6 a | 301.85 ± 72.18 a | 245.66 ± 24.92 a | Bitter, citrus |
| 29 | 3,7-dimethyl-1-Octene B | 1072 | 4984-01-4 | 13.91 ± 1.89 a | 39.23 ± 11.48 b | 50.94 ± 9.27 b | 55.14 ± 15.47 b | 49.61 ± 2.67 b | Woody, piney, herbaceous |
| 30 | Terpinolene | 1082 | 586-62-9 | 615.94 ± 133.24 b | 534.6 ± 73.55 ab | 490.57 ± 93.1 ab | 488.73 ± 93.78 ab | 402.34 ± 36.95 a | Pine |
| 31 | 2-ethenyl-1,1-dimethyl-3-methylene- Cyclohexane | 1112 | 95452-08-7 | 7.77 ± 1.13 ab | 4.97 ± 5.82 a | 12.7 ± 2.33 b | 9.17 ± 3.52 ab | 6.42 ± 2.4 ab | n.a. |
| 32 | 1,1-bis(dodecyloxy-Hexadecane | 1407 | 56554-64-4 | 14.84 ± 1.29 b | 29.9 ± 6.18 c | 2.94 ± 5.09 a | n.d. | n.d. | n.a. |
| 33 | β-Longipinene | 1412 | 39703-25-8 | 4.08 ± 0.49 a | 2.88 ± 3.53 a | 5.77 ± 5 a | 8.52 ± 7.8 a | n.d. | n.a. |
| 34 | Germacrene D | 1474 | 24150-39-8 | 125.84 ± 20.49 a | 468.72 ± 87.5 c | 339.87 ± 49.21 b | 331.87 ± 100.4 b | 195.26 ± 60.46 a | Floral, soapy, green |
| 35 | α-Guaiene | 1482 | 53863-54-0 | 47.37 ± 6.33 a | 94.27 ± 19.39 b | 79.19 ± 13.41 b | 80.67 ± 24.17 b | 47.74 ± 13.76 a | n.a. |
| 36 | Alloaromadendrene A | 1489 | 25246-27-9 | 9.57 ± 0.57 a | 31.49 ± 16.16 b | 26.38 ± 11.19 ab | 29.73 ± 8.88 b | 20.62 ± 5.89 ab | Woody |
| 37 | β-Acorenol | 1542 | 28400-11-5 | 6.45 ± 0.07 ab | n.d. | 17.74 ± 4.75 c | 17.08 ± 6.63 c | 12.71 ± 1.07 bc | n.a. |
| 38 | β-Guaiene A | 1614 | 88-84-6 | 15.18 ± 0.12 a | 41.07 ± 10.02 b | 38.38 ± 10.38 b | 32.07 ± 11.48 b | 27.55 ± 3.17 ab | Woody, spice |
| Ketones | 10.79 ± 1.12 a | 44.99 ± 8.25 b | 56.86 ± 4.99 b | 60.28 ± 18.22 b | 43.82 ± 2.54 b | ||||
| 39 | Carvol | 1239 | 2244-16-8 | 10.79 ± 1.12 a | 44.99 ± 8.25 b | 56.86 ± 4.99 b | 60.28 ± 18.22 b | 43.82 ± 2.54 b | Basil, bitter, caraway, fennel, mint |
| No. | Physicochemical Parameter (mg/g) | Control | P. terricola QJJY1 | H. opuntiae QJJY14 | H. opuntiae QJJY15 | P. terricola QJJY17 |
|---|---|---|---|---|---|---|
| Total polyphenols | 15.03 ± 0.26 a | 22.97 ± 0.94 b | 22.75 ± 0.75 b | 22.73 ± 0.67 b | 22.15 ± 0.26 b | |
| Phenolic acids | ||||||
| 1 | Gallic acid | 0.09 ± 0.02 a | 0.09 ± 0.01 a | 0.07 ± 0.02 a | 0.07 ± 0.01 a | 0.14 ± 0.02 b |
| 2 | Chlorogenic acid | 0.28 ± 0.04 a | 0.29 ± 0.04 a | 0.32 ± 0.05 a | 0.32 ± 0.03 a | 0.30 ± 0.05 a |
| 3 | Gentisuric acid | 0.09 ± 0.05 a | 0.12 ± 0.07 a | 0.07 ± 0.02 a | 0.07 ± 0.02 a | 0.07 ± 0.01 a |
| Flavone glycosides | ||||||
| 4 | Vicenin-2 | 1.13 ± 0.17 a | 1.47 ± 0.04 b | 1.53 ± 0.08 b | 1.44 ± 0.05 b | 1.41 ± 0.02 b |
| 5 | Vitexin-2″-O-rhamnoside | 0.68 ± 0.06 a | 1.42 ± 0.04 c | 1.32 ± 0.06 b | 1.29 ± 0.05 b | 1.35 ± 0.02 bc |
| 6 | Eriocitrin | 1.42 ± 0.02 a | 1.94 ± 0.19 b | 2.00 ± 0.1 b | 1.85 ± 0.12 b | 1.82 ± 0.05 b |
| 7 | DGPP | 8.02 ± 0.113 a | 12.58 ± 0.34 c | 12.43 ± 0.20 bc | 12.44 ± 0.19 bc | 12.15 ± 0.12 b |
| 8 | Naringin | 0.13 ± 0.03 a | 0.22 ± 0.04 c | 0.20 ± 0.04 bc | 0.15 ± 0.03 ab | 0.22 ± 0.02 c |
| 9 | Diosmetin | 0.14 ± 0.02 a | 0.32 ± 0.07 c | 0.29 ± 0.03 c | 0.25 ± 0.04 bc | 0.19 ± 0.06 ab |
| 10 | Margaritene | 0.17 ± 0.05 a | 0.22 ± 0.05 a | 0.28 ± 0.13 ab | 0.39 ± 0.08 b | 0.23 ± 0.03 a |
| 11 | Hesperiden | 0.82 ± 0.03 a | 1.17 ± 0.19 b | 1.09 ± 0.13 b | 1.19 ± 0.06 b | 1.19 ± 0.05 b |
| 12 | Phlorizin | 0.33 ± 0.05 a | 0.44 ± 0.11 a | 0.62 ± 0.07 b | 0.48 ± 0.08 ab | 0.47 ± 0.09 ab |
| 13 | Isomargaritene | 0.17 ± 0.03 a | 0.39 ± 0.04 c | 0.31 ± 0.01 b | 0.29 ± 0.06 b | 0.26 ± 0.03 b |
| 14 | Fortunellin | 0.27 ± 0.03 a | 0.34 ± 0.15 a | 0.35 ± 0.05 a | 0.69 ± 0.09 b | 0.61 ± 0.02 b |
| Aglycons | ||||||
| 15 | Naringenin | 0.09 ± 0.01 b | 0.07 ± 0.04 ab | 0.06 ± 0.00 ab | 0.05 ± 0.01 b | 0.04 ± 0.00 b |
| 16 | Nobiletin | 0.56 ± 0.01 a | 0.91 ± 0.04 d | 0.84 ± 0.03 c | 0.82 ± 0.02 bc | 0.78 ± 0.01 b |
| 17 | Tangeretin | 0.56 ± 0.00 a | 0.86 ± 0.06 b | 0.85 ± 0.02 b | 0.84 ± 0.02 b | 0.81 ± 0.02 b |
| 18 | 7-Hydroxycoumarin | 0.07 ± 0.01 a | 0.1 ± 0.02 a | 0.09 ± 0.01 a | 0.11 ± 0.03 a | 0.08 ± 0.00 a |
| Total limonoids | 2.89 ± 0.07 a | 3.61 ± 0.08 bc | 3.61 ± 0.04 bc | 3.50 ± 0.24 b | 3.76 ± 0.10 c | |
| 19 | Limonin glucoside | 1.88 ± 0.04 a | 2.18 ± 0.05 b | 2.19 ± 0.05 b | 2.08 ± 0.10 b | 2.16 ± 0.08 b |
| 20 | Limonin | 0.97 ± 0.02 a | 1.35 ± 0.10 b | 1.34 ± 0.01 b | 1.33 ± 0.20 b | 1.52 ± 0.04 b |
| 21 | Nomilin | 0.04 ± 0.00 a | 0.08 ± 0.01 b | 0.08 ± 0.00 b | 0.09 ± 0.02 b | 0.07 ± 0.00 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Liu, S.; Ma, Z.; Huang, H.; Zheng, L.; Tian, Y.; Zhong, Q. Effectively Enhancing the Physiological Activity and Sensory Quality of Whole Calamondin Puree via Yeast Fermentation. Int. J. Mol. Sci. 2024, 25, 11984. https://doi.org/10.3390/ijms252211984
Zhang H, Liu S, Ma Z, Huang H, Zheng L, Tian Y, Zhong Q. Effectively Enhancing the Physiological Activity and Sensory Quality of Whole Calamondin Puree via Yeast Fermentation. International Journal of Molecular Sciences. 2024; 25(22):11984. https://doi.org/10.3390/ijms252211984
Chicago/Turabian StyleZhang, Hongjian, Shuaiguang Liu, Zewei Ma, Huan Huang, Lianhe Zheng, Yan Tian, and Qiuping Zhong. 2024. "Effectively Enhancing the Physiological Activity and Sensory Quality of Whole Calamondin Puree via Yeast Fermentation" International Journal of Molecular Sciences 25, no. 22: 11984. https://doi.org/10.3390/ijms252211984
APA StyleZhang, H., Liu, S., Ma, Z., Huang, H., Zheng, L., Tian, Y., & Zhong, Q. (2024). Effectively Enhancing the Physiological Activity and Sensory Quality of Whole Calamondin Puree via Yeast Fermentation. International Journal of Molecular Sciences, 25(22), 11984. https://doi.org/10.3390/ijms252211984





