Encapsulation of β-Galactosidase into Polyallylamine/Polystyrene Sulphonate Polyelectrolyte Microcapsules
Abstract
1. Introduction
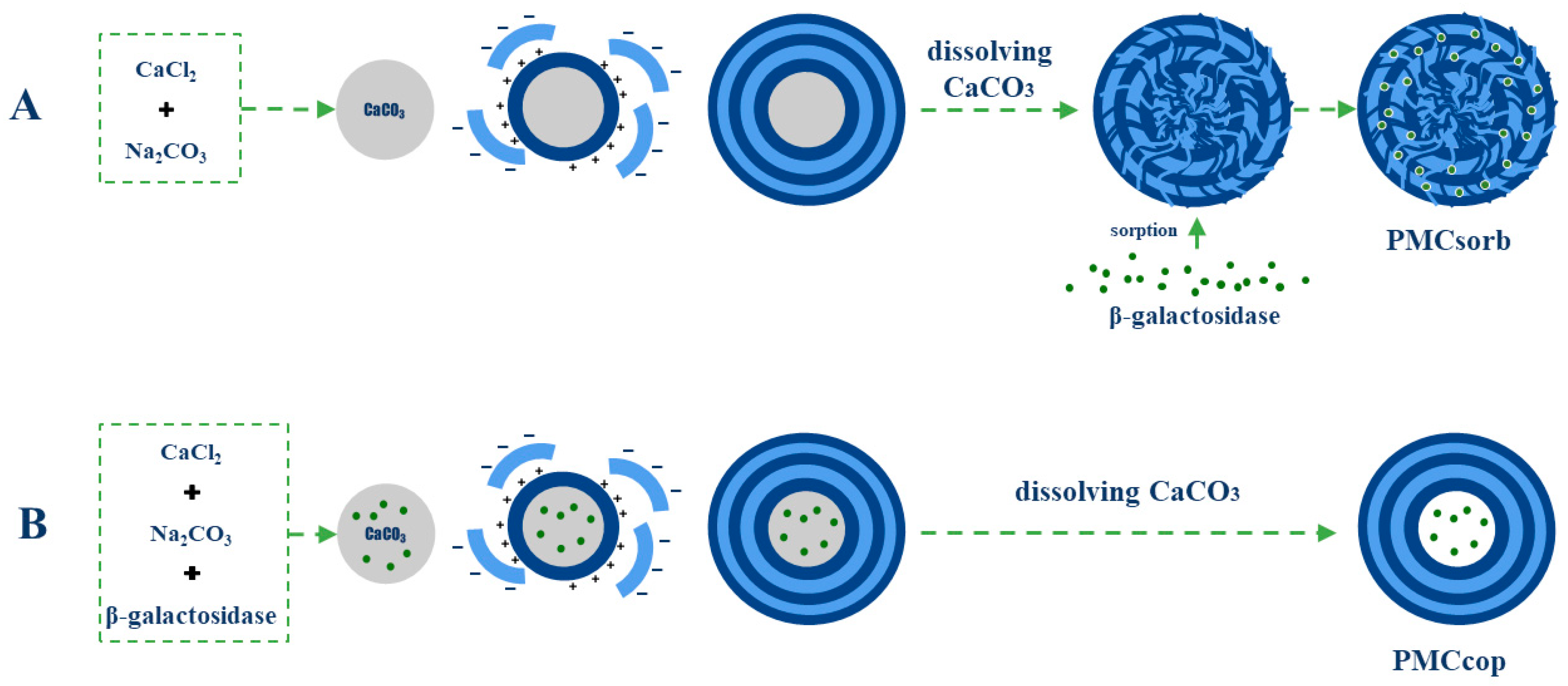
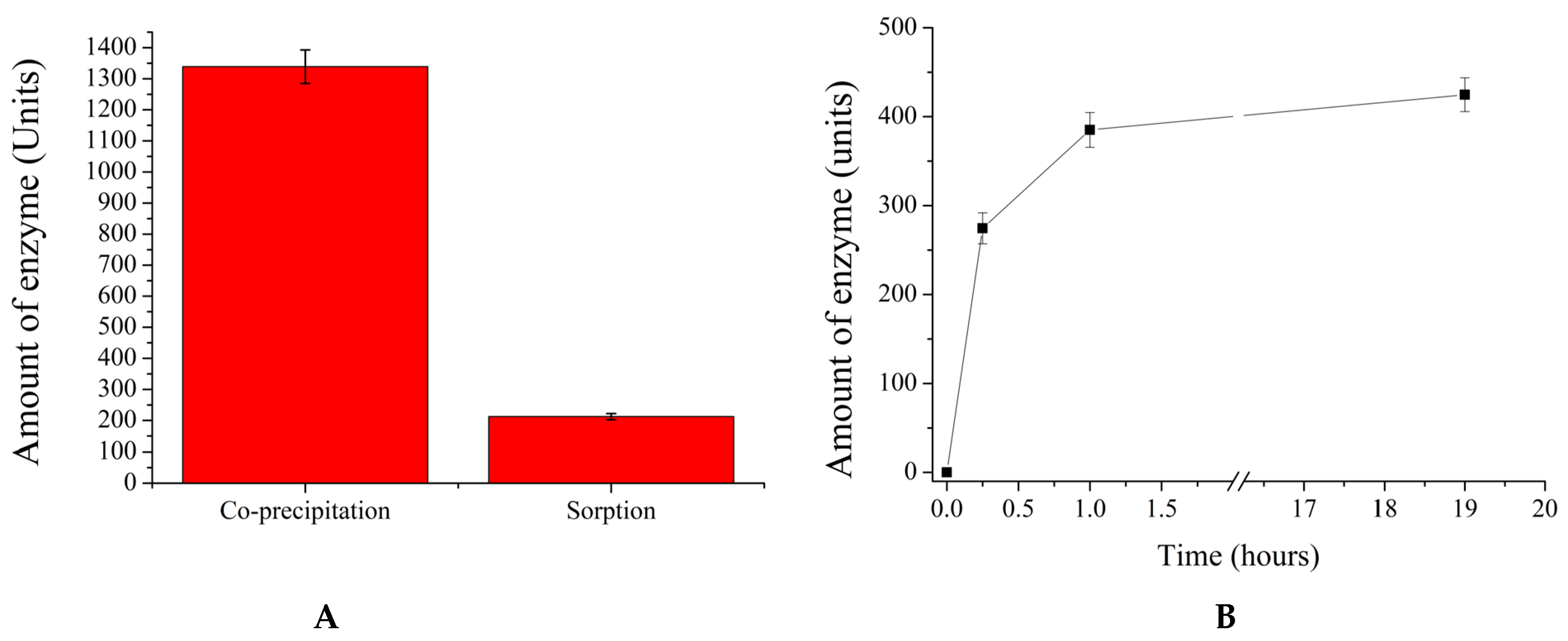
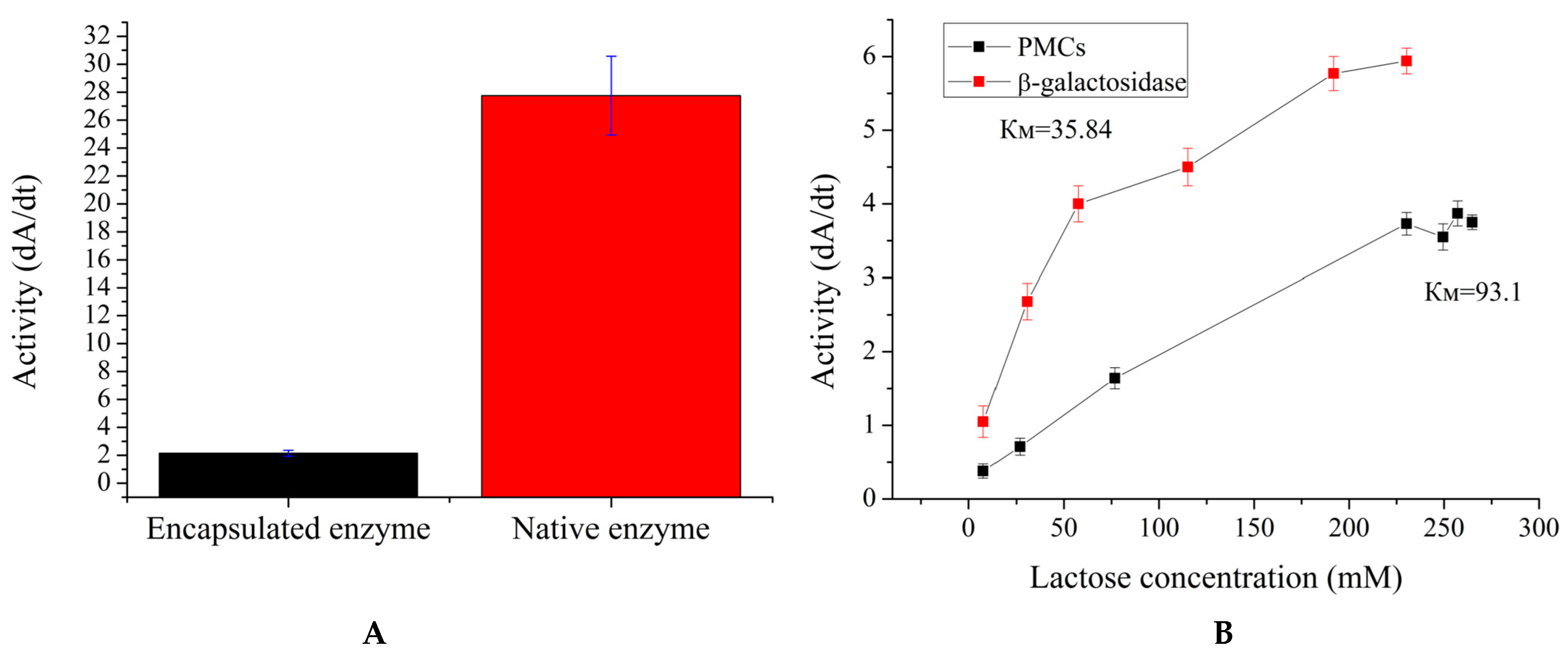
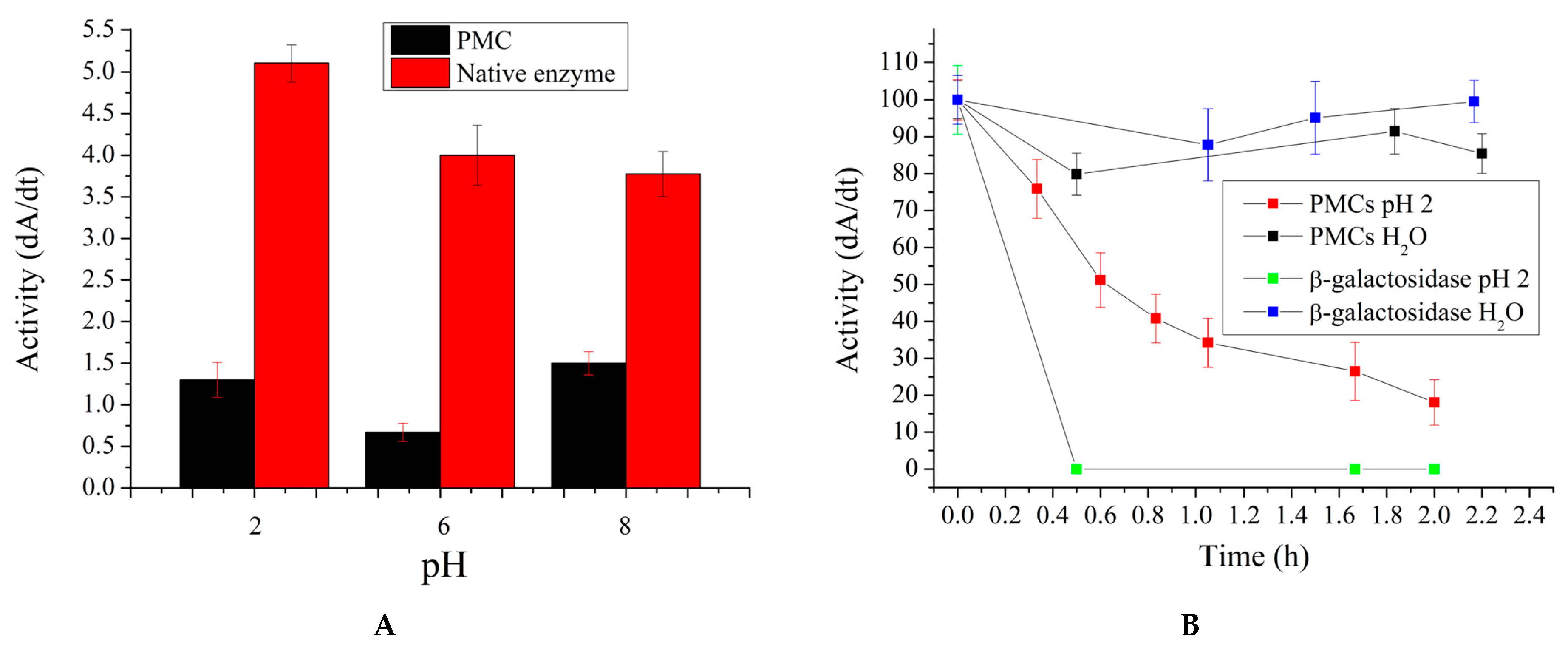
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Encapsulation of Enzymes in Polyelectrolyte Microcapsules (Co-Precipitation Method)
3.3. Encapsulation of Enzymes in Polyelectrolyte Microcapsules (Adsorption Method)
3.4. Measurement of β-Galactosidase Activity
3.5. Determination of Km of β-Galactosidase
3.6. Determination of β-Galactosidase Concentration
3.7. Statistical Data Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Haug, A.; Høstmark, A.T.; Harstad, O.M. Bovine milk in human nutrition—A review. Lipids Health Dis. 2007, 6–25. [Google Scholar] [CrossRef] [PubMed]
- Visioli, F.; Strata, A. Milk, Dairy Products, and Their Functional Effects in Humans: A Narrative Review of Recent Evidence. Adv. Nutr. Int. Rev. J. 2014, 5, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Rizzoli, R. Dairy products, yogurts, and bone health. Am. J. Clin. Nutr. 2014, 99, 1256S–1262S. [Google Scholar] [CrossRef] [PubMed]
- E Black, R.; Williams, S.M.; E Jones, I.; Goulding, A. Children who avoid drinking cow milk have low dietary calcium intakes and poor bone health. Am. J. Clin. Nutr. 2002, 76, 675–680. [Google Scholar] [CrossRef]
- Bailey, R.L.; Dodd, K.W.; Goldman, J.A.; Gahche, J.J.; Dwyer, J.T.; Moshfegh, A.J.; Sempos, C.T.; Picciano, M.F. Estimation of Total Usual Calcium and Vitamin D Intakes in the United States. J. Nutr. 2010, 140, 817–822. [Google Scholar] [CrossRef]
- Bechthold, A.; Boeing, H.; Schwedhelm, C.; Hoffmann, G.; Knüppel, S.; Iqbal, K.; De Henauw, S.; Michels, N.; Devleesschauwer, B.; Schlesinger, S.; et al. Food groups and risk of coronary heart disease, stroke and heart failure: A systematic review and dose-response meta-analysis of prospective studies. Crit. Rev. Food Sci. Nutr. 2019, 59, 1071–1090. [Google Scholar] [CrossRef]
- Franzoi, M.; Niero, G.; Penasa, M.; Cassandro, M.; De Marchi, M. Technical note: Development and validation of a new method for the quantification of soluble and micellar calcium, magnesium, and potassium in milk. J. Dairy Sci. 2018, 101, 1883–1888. [Google Scholar] [CrossRef]
- Storhaug, C.L.; Fosse, S.K.; Fadnes, L.T. Country, regional, and global estimates for lactose malabsorption in adults: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2017, 2, 738–746. [Google Scholar] [CrossRef]
- Szilagyi, A.; Ishayek, N. Lactose Intolerance, Dairy Avoidance, and Treatment Options. Nutrients 2018, 10, 1994. [Google Scholar] [CrossRef]
- De Gregorio, P.R.; Gennari, A.; Nied, C.V.; Volpato, G.; Volken de Souza, C.F. Low-lactose milk production using β-galactosidases. In Enzymes beyond Traditional Applications in Dairy Science and Technology; Elsevier: Amsterdam, The Netherlands, 2023; pp. 361–381. [Google Scholar] [CrossRef]
- Dominici, S.; Marescotti, F.; Sanmartin, C.; Macaluso, M.; Taglieri, I.; Venturi, F.; Zinnai, A.; Facioni, M.S. Lactose: Characteristics, Food and Drug-Related Applications, and Its Possible Substitutions in Meeting the Needs of People with Lactose Intolerance. Foods 2022, 11, 1486. [Google Scholar] [CrossRef]
- Hebbink, G.A.; Dickhoff, B.H.J. Application of lactose in the pharmaceutical industry. In Lactose; Elsevier: Amsterdam, The Netherlands, 2019; pp. 175–229. [Google Scholar] [CrossRef]
- Li, A.; Zheng, J.; Han, X.; Jiang, Z.; Yang, B.; Yang, S.; Zhou, W.; Li, C.; Sun, M. Health implication of lactose intolerance and updates on its dietary management. Int. Dairy J. 2023, 140. [Google Scholar] [CrossRef]
- Szilagyi, A. Adult Lactose Digestion Status and Effects on Disease. Can. J. Gastroenterol. Hepatol. 2015, 29, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Jackson, K.A.; Savaiano, D.A. Lactose Maldigestion, Calcium Intake and Osteoporosis in African-, Asian-, and Hispanic-Americans. J. Am. Coll. Nutr. 2001, 20, 198S–207S. [Google Scholar] [CrossRef] [PubMed]
- La, J.-H.; Feng, B.; Schwartz, E.S.; Brumovsky, P.R.; Gebhart, G.F.; Siri, S.; Maier, F.; Chen, L.; Santos, S.; Pierce, D.M.; et al. Luminal hypertonicity and acidity modulate colorectal afferents and induce persistent visceral hypersensitivity. Am. J. Physiol. Liver Physiol. 2012, 303, G802–G809. [Google Scholar] [CrossRef][Green Version]
- Almeida, C.C.; Lorena, S.L.S.; Pavan, C.R.; Akasaka, H.M.I.; Mesquita, M.A. Beneficial Effects of Long-Term Consumption of a Probiotic Combination of Lactobacillus casei Shirota and Bifidobacterium breve Yakult May Persist After Suspension of Therapy in Lactose-Intolerant Patients. Nutr. Clin. Pract. 2012, 27, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Pakdaman, M.N.; Udani, J.K.; Molina, J.P.; Shahani, M. The effects of the DDS-1 strain of lactobacillus on symptomatic relief for lactose intolerance—A randomized, double-blind, placebo-controlled, crossover clinical trial. Nutr. J. 2015, 15, 56. [Google Scholar] [CrossRef]
- Arnold, J.W.; Simpson, J.B.; Roach, J.; Bruno-Barcena, J.M.; Azcarate-Peril, M.A. Prebiotics for Lactose Intolerance: Variability in Galacto-Oligosaccharide Utilization by Intestinal Lactobacillus rhamnosus. Nutrients 2018, 10, 1517. [Google Scholar] [CrossRef]
- Maksimainen, M.M.; Lampio, A.; Mertanen, M.; Turunen, O.; Rouvinen, J. The crystal structure of acidic β-galactosidase from Aspergillus oryzae. Int. J. Biol. Macromol. 2013, 60, 109–115. [Google Scholar] [CrossRef]
- Lima, P.C.; Gazoni, I.; de Carvalho, A.M.G.; Bresolin, D.; Cavalheiro, D.; de Oliveira, D.; Rigo, E. β-galactosidase from Kluyveromyces lactis in genipin-activated chitosan: An investigation on immobilization, stability, and application in diluted UHT milk. Food Chem. 2021, 349, 129050. [Google Scholar] [CrossRef]
- Fuhrmann, G.; Leroux, J.-C. Improving the Stability and Activity of Oral Therapeutic Enzymes—Recent Advances and Perspectives. Pharm. Res. 2013, 31, 1099–1105. [Google Scholar] [CrossRef]
- Fraile-Gutiérrez, I.; Iglesias, S.; Acosta, N.; Revuelta, J. Chitosan-based oral hydrogel formulations of β-galactosidase to improve enzyme supplementation therapy for lactose intolerance. Int. J. Biol. Macromol. 2024, 255, 127755. [Google Scholar] [CrossRef] [PubMed]
- Facin, B.R.; Moret, B.; Baretta, D.; Belfiore, L.A.; Paulino, A.T. Immobilization and controlled release of β-galactosidase from chitosan-grafted hydrogels. Food Chem. 2015, 179, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Hanauer, D.C.; Paulino, A.T. Anchoring lactase in pectin-based hydrogels for lactose hydrolysis reactions. Process. Biochem. 2022, 122, 50–59. [Google Scholar] [CrossRef]
- Cargnin, M.A.; Gasparin, B.C.; Rosa, D.d.S.; Paulino, A.T. Performance of lactase encapsulated in pectin-based hydrogels during lactose hydrolysis reactions. LWT 2021, 150. [Google Scholar] [CrossRef]
- Bai, Y.; Gilbert, R.G. Mechanistic Understanding of the Effects of Pectin on In Vivo Starch Digestion: A Review. Nutrients 2022, 14, 5107. [Google Scholar] [CrossRef]
- Cao, W.; Guan, S.; Yuan, Y.; Wang, Y.; Nushrat, Y.M.; Liu, Y.; Tong, Y.; Yu, S.; Hua, X. The digestive behavior of pectin in human gastrointestinal tract: a review on fermentation characteristics and degradation mechanism. Crit. Rev. Food Sci. Nutr. 2023, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Zhou, J.; Xuan, R.; Chen, J.; Han, H.; Liu, J.; Niu, T.; Chen, H.; Wang, F. Dietary κ-carrageenan facilitates gut microbiota-mediated intestinal inflammation. Carbohydr. Polym. 2022, 277, 118830. [Google Scholar] [CrossRef]
- Petrov, A.I.; Volodkin, D.V.; Sukhorukov, G.B. Protein-Calcium Carbonate Coprecipitation: A Tool for Protein Encapsulation. Biotechnol. Prog. 2005, 21, 918–925. [Google Scholar] [CrossRef]
- Balabushevitch, N.G.; Sukhorukov, G.B.; Moroz, N.A.; Volodkin, D.V.; Larionova, N.I.; Donath, E.; Mohwald, H. Encapsulation of proteins by layer-by-layer adsorption of polyelectrolytes onto protein aggregates: Factors regulating the protein release. Biotechnol. Bioeng. 2001, 76, 207–213. [Google Scholar] [CrossRef]
- Tong, W.; Dong, W.; Gao, C.; Möhwald, H. Charge-Controlled Permeability of Polyelectrolyte Microcapsules. J. Phys. Chem. B 2005, 109, 13159–13165. [Google Scholar] [CrossRef]
- Plekhanova, Y.V.; Tikhonenko, S.A.; Dubrovsky, A.V.; Kim, A.L.; Musin, E.V.; Wang, G.-J.; Kuznetsova, I.E.; Kolesov, V.V.; Reshetilov, A.N. Comparative Study of Electrochemical Sensors Based on Enzyme Immobilized into Polyelectrolyte Microcapsules and into Chitosan Gel. Anal. Sci. 2019, 35, 1037–1043. [Google Scholar] [CrossRef] [PubMed]
- Reshetilov, A.; Plekhanova, Y.; Tarasov, S.; Tikhonenko, S.; Dubrovsky, A.; Kim, A.; Kashin, V.; Machulin, A.; Wang, G.-J.; Kolesov, V.; et al. Bioelectrochemical Properties of Enzyme-Containing Multilayer Polyelectrolyte Microcapsules Modified with Multiwalled Carbon Nanotubes. Membranes 2019, 9, 53. [Google Scholar] [CrossRef] [PubMed]
- Kazakova, L.I.; Shabarchina, L.I.; Anastasova, S.; Pavlov, A.M.; Vadgama, P.; Skirtach, A.G.; Sukhorukov, G.B. Chemosensors and biosensors based on polyelectrolyte microcapsules containing fluorescent dyes and enzymes. Anal. Bioanal. Chem. 2012, 405, 1559–1568. [Google Scholar] [CrossRef]
- Sukhorukov, B.I.; Tikhonenko, S.A.; Saburova, E.A.; Dubrovskii, A.V.; Dybovskaya, Y.N.; Shabarchina, L.I. Protein-filled polyelectrolyte microcapsules in the design of enzymic microdiagnostics. Biophysics 2007, 52, 575–581. [Google Scholar] [CrossRef]
- Lvov, Y.; Antipov, A.A.; Mamedov, A.; Möhwald, H.; Sukhorukov, G.B. Urease Encapsulation in Nanoorganized Microshells. Nano Lett. 2001, 1, 125–128. [Google Scholar] [CrossRef]
- I Sukhorukov, B.; A Tikhonenko, S.; A Saburova, E.; Dubrovskiĭ, A.V.; Dybovskaia, I.N.; I Shabarchina, L. Incapsulation of enzymes into polyelectrolyte nano- and microcapsules and the problem of the development of enzymatic microdiagnostics. Biofizika 2008, 52, 1041–1048. [Google Scholar]
- Kim, A.L.; Musin, E.V.; Dubrovskii, A.V.; Tikhonenko, S.A. Effect of Pollyallylamine on Alcoholdehydrogenase Structure and Activity. Polymers 2020, 12, 832. [Google Scholar] [CrossRef]
- Gupta, M.; Pandey, H.; Sivakumar, S. Intracellular Delivery of β-Galactosidase Enzyme Using Arginase-Responsive Dextran Sulfate/Polyarginine Capsule for Lysosomal Storage Disorder. ACS Omega 2017, 2, 9002–9012. [Google Scholar] [CrossRef]
- Kim, A.L.; Musin, E.V.; Chebykin, Y.S.; Tikhonenko, S.A. Characterization of Polyallylamine/Polystyrene Sulfonate Polyelectrolyte Microcapsules Formed on Solid Cores: Morphology. Polymers 2024, 16, 1521. [Google Scholar] [CrossRef]
- Musin, E.V.; Kim, A.L.; Dubrovskii, A.V.; Tikhonenko, S.A. New sight at the organization of layers of multilayer polyelectrolyte microcapsules. Sci. Rep. 2021, 11, 14040. [Google Scholar] [CrossRef]
- Volodkin, D.V.; Petrov, A.I.; Prevot, M.; Sukhorukov, G.B. Matrix Polyelectrolyte Microcapsules: New System for Macromolecule Encapsulation. Langmuir 2004, 20, 3398–3406. [Google Scholar] [CrossRef] [PubMed]
- Stamatopoulos, K.; O’Farrell, C.; Simmons, M.; Batchelor, H. In vivo models to evaluate ingestible devices: Present status and current trends. Adv. Drug Deliv. Rev. 2021, 177, 113915. [Google Scholar] [CrossRef] [PubMed]
- Fujimori, S. Gastric acid level of humans must decrease in the future. World J. Gastroenterol. 2020, 26, 6706–6709. [Google Scholar] [CrossRef]
- Lu, P.-J.; Hsu, P.I.; Chen, C.H.; Hsiao, M.; Chang, W.C.; Tseng, H.H.; Lin, K.H.; Chuah, S.K.; Chen, H.C. Gastric juice acidity in upper gastrointestinal diseases. World J. Gastroenterol. 2010, 16, 5496–5501. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.; Zhang, M.; Liu, K. Colon-targeted drug delivery of polysaccharide-based nanocarriers for synergistic treatment of inflammatory bowel disease: A review. Carbohydr. Polym. 2021, 272, 118530. [Google Scholar] [CrossRef]
- Konturek, S.J.; Konturek, P.C.; Pawlik, T.; Sliwowski, Z.; Ochmański, W.; Hahn, E.G. Duodenal mucosal protection by bicarbonate secretion and its mechanisms. J. Physiol. Pharmacol. 2004, 2, 5–17. [Google Scholar]
- Yuan, Q.; Pan, A.; Fu, Y.; Dai, Y. Anatomy and physiology of the pancreas. In Integrative Pancreatic Intervention Therapy; Elsevier: Amsterdam, The Netherlands, 2021; pp. 3–21. [Google Scholar] [CrossRef]
- Antipov, A.A.; Sukhorukov, G.B. Polyelectrolyte multilayer capsules as vehicles with tunable permeability. Adv. Colloid Interface Sci. 2004, 111, 49–61. [Google Scholar] [CrossRef]
- Pechenkin, M.A.; Möhwald, H.; Volodkin, D.V. pH- and salt-mediated response of layer-by-layer assembled PSS/PAH microcapsules: Fusion and polymer exchange. Soft Matter 2012, 8, 8659–8665. [Google Scholar] [CrossRef]
- Dubrovskii, A.V.; Kim, A.L.; Musin, E.V.; Ramazanov, B.R.; Tikhonenko, S.A. The Discovery of the Buffer Capacity of Various Types of Polyelectrolyte Microcapsules. Polymers 2021, 13, 4026. [Google Scholar] [CrossRef]
- The Composition of Intestinal Secretions. Available online: https://www.sciencedirect.com/science/article/pii/S0021925818753143?ref=pdf_download&fr=RR-2&rr=8d136494bf5ece44 (accessed on 31 August 2024).
- Powell, J.J.; Greenfield, S.M.; Thompson, R.P. Concentrations of metals in gastric juice in health and peptic ulcer disease. Gut 1992, 33, 1617–1620. [Google Scholar] [CrossRef]
- Song, C.; Chai, Z.; Chen, S.; Zhang, H.; Zhang, X.; Zhou, Y. Intestinal mucus components and secretion mechanisms: What we do and do not know. Exp. Mol. Med. 2023, 55, 681–691. [Google Scholar] [CrossRef] [PubMed]
- A Antipov, A.; Shchukin, D.; Fedutik, Y.; I Petrov, A.; Sukhorukov, G.B.; Möhwald, H. Carbonate microparticles for hollow polyelectrolyte capsules fabrication. Colloids Surf. A Physicochem. Eng. Asp. 2003, 224, 175–183. [Google Scholar] [CrossRef]
- Ibrahim, N.A.; Eid, B.M.; Amin, H.A. Sustainable textile finishing processes and pollution control based on enzyme technology. In Green Chemistry for Sustainable Textiles; Elsevier: Amsterdam, The Netherlands, 2021; pp. 385–415. [Google Scholar] [CrossRef]
- Cornish-Bowden, A. Current IUBMB recommendations on enzyme nomenclature and kinetics. Perspect. Sci. 2014, 1, 74–87. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chebykin, Y.S.; Musin, E.V.; Kim, A.L.; Tikhonenko, S.A. Encapsulation of β-Galactosidase into Polyallylamine/Polystyrene Sulphonate Polyelectrolyte Microcapsules. Int. J. Mol. Sci. 2024, 25, 10978. https://doi.org/10.3390/ijms252010978
Chebykin YS, Musin EV, Kim AL, Tikhonenko SA. Encapsulation of β-Galactosidase into Polyallylamine/Polystyrene Sulphonate Polyelectrolyte Microcapsules. International Journal of Molecular Sciences. 2024; 25(20):10978. https://doi.org/10.3390/ijms252010978
Chicago/Turabian StyleChebykin, Yuri S., Egor V. Musin, Aleksandr L. Kim, and Sergey A. Tikhonenko. 2024. "Encapsulation of β-Galactosidase into Polyallylamine/Polystyrene Sulphonate Polyelectrolyte Microcapsules" International Journal of Molecular Sciences 25, no. 20: 10978. https://doi.org/10.3390/ijms252010978
APA StyleChebykin, Y. S., Musin, E. V., Kim, A. L., & Tikhonenko, S. A. (2024). Encapsulation of β-Galactosidase into Polyallylamine/Polystyrene Sulphonate Polyelectrolyte Microcapsules. International Journal of Molecular Sciences, 25(20), 10978. https://doi.org/10.3390/ijms252010978





