Triterpenoid Saponins and Flavonoid Glycosides from the Flower of Camellia flavida and Their Cytotoxic and α-Glycosidase Inhibitory Activities
Abstract
1. Introduction
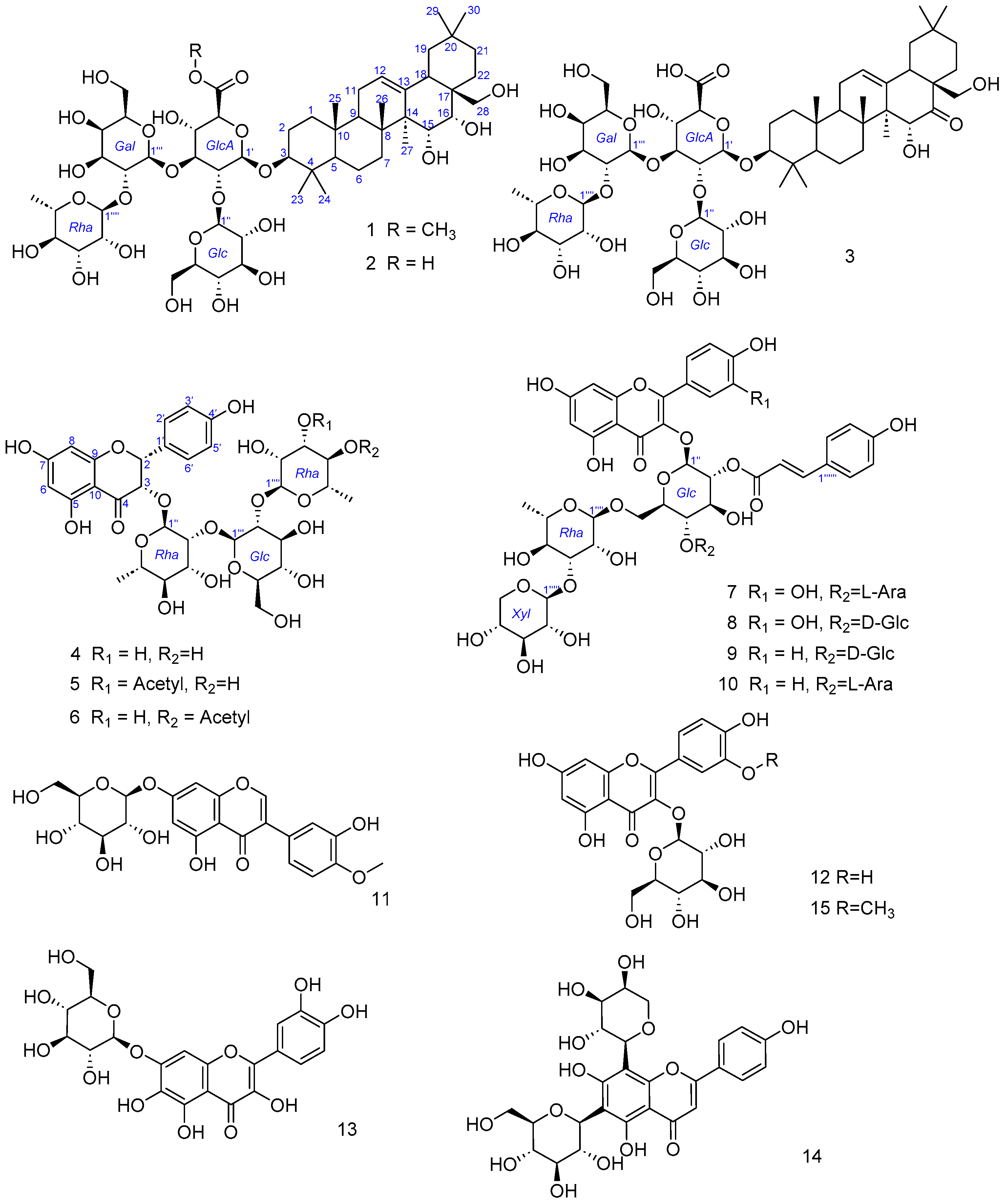
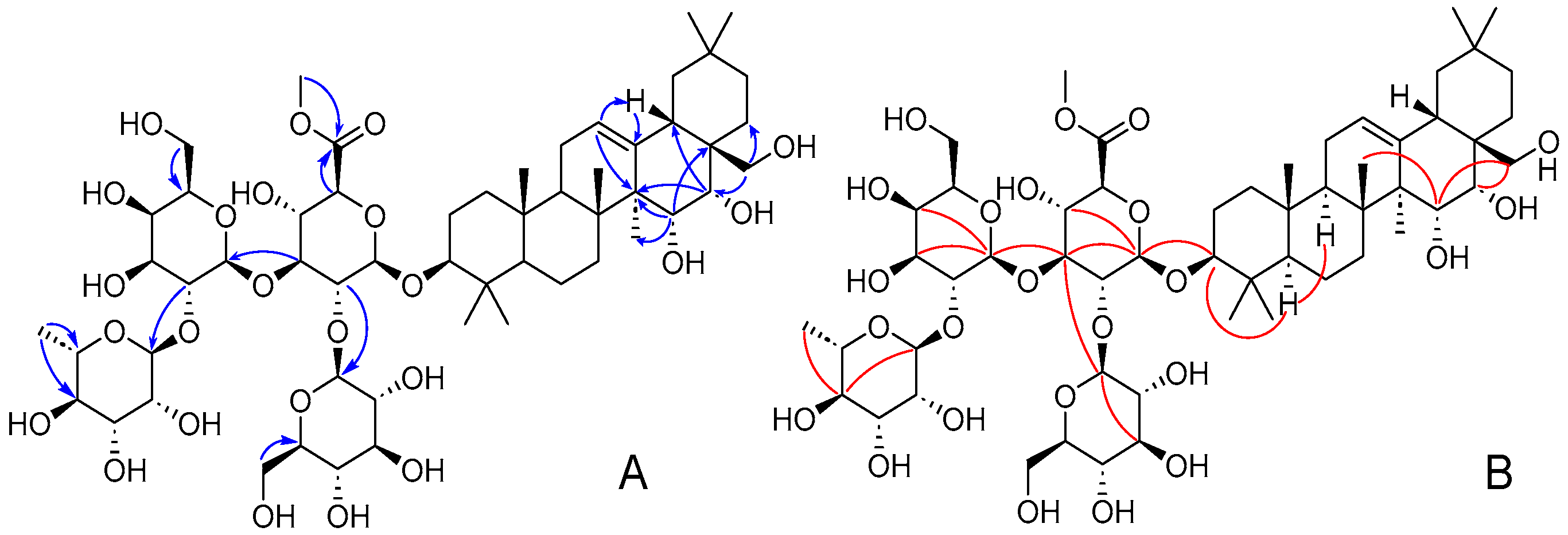
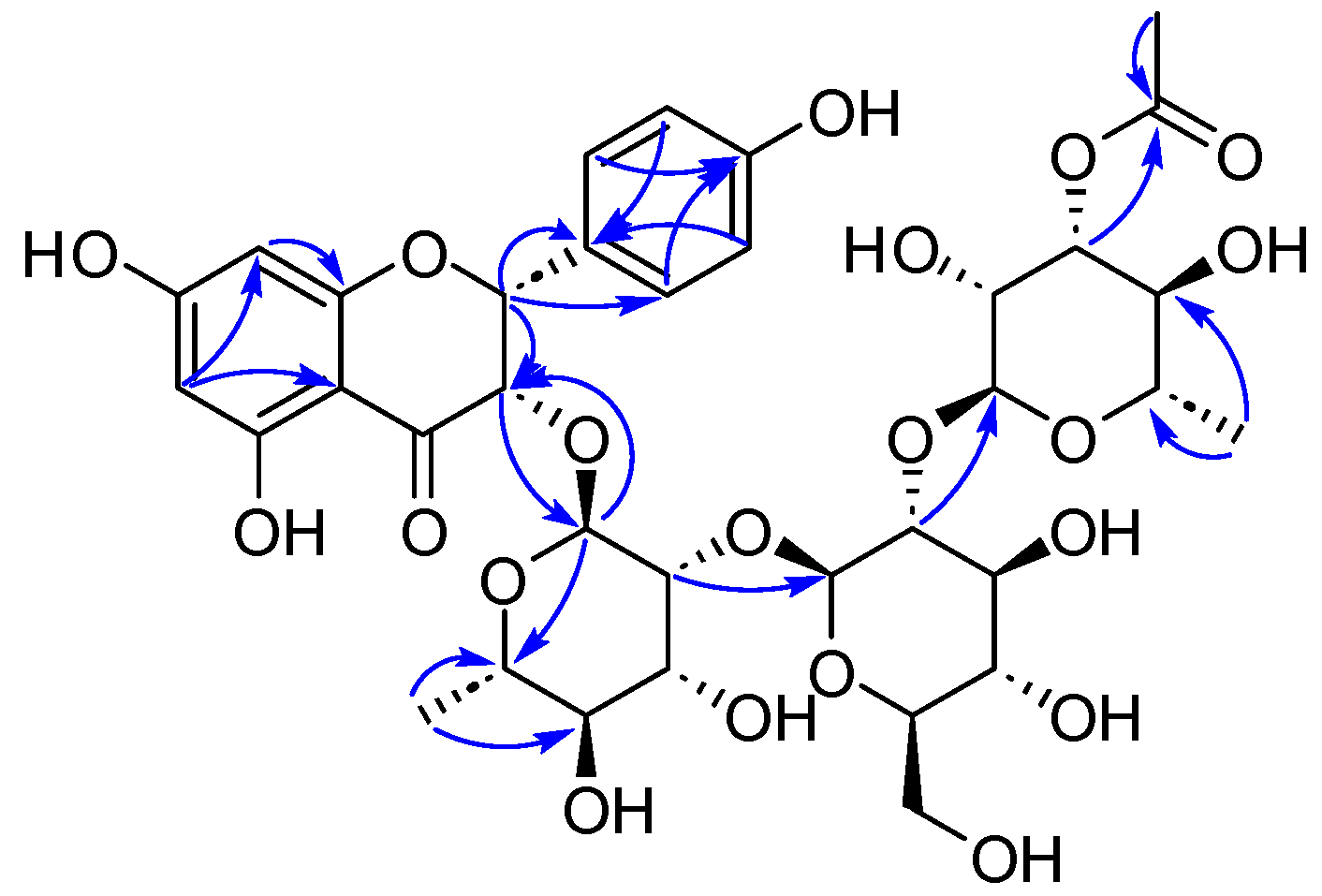
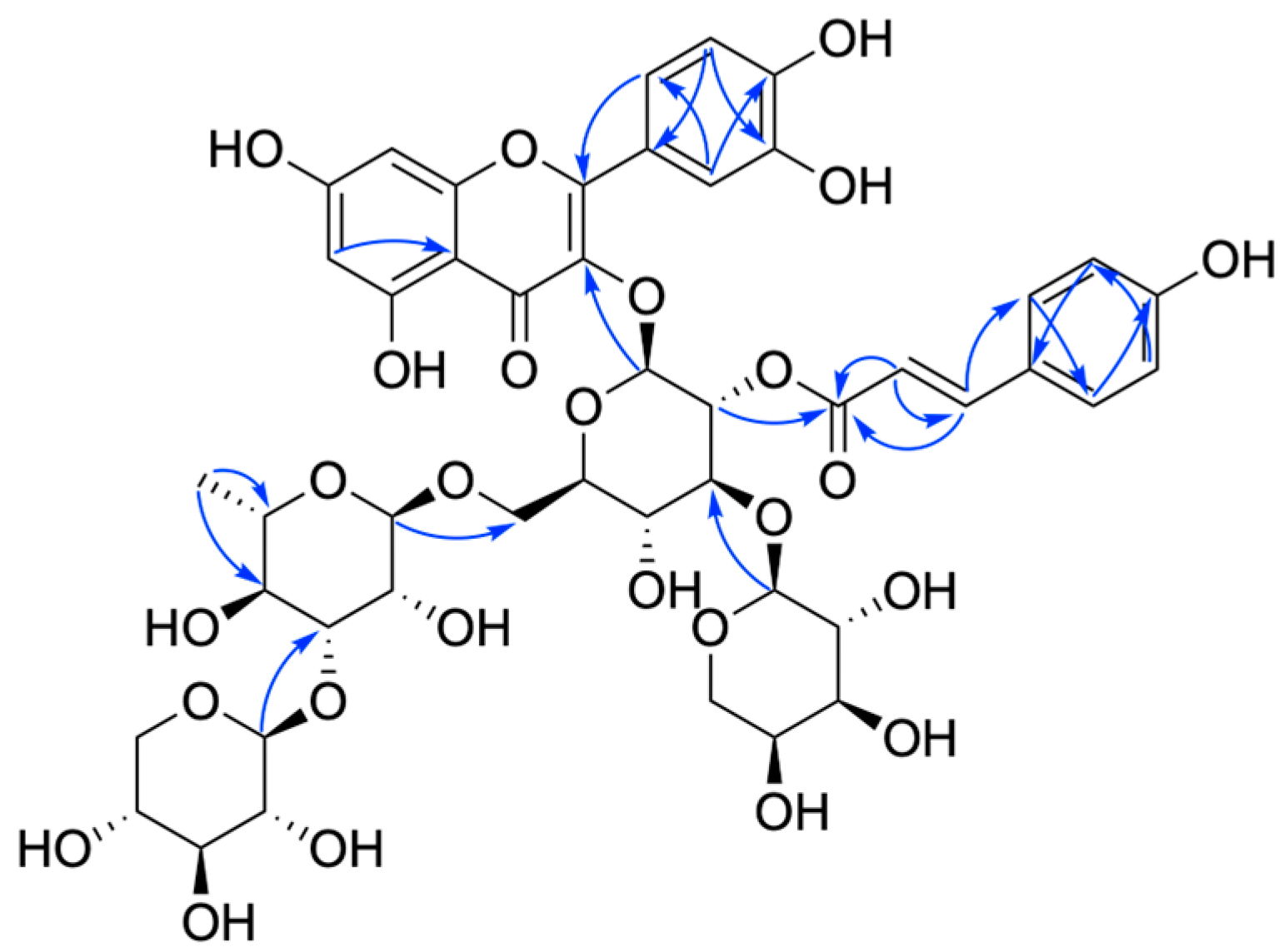
2. Results and Discussion
3. Experimental Procedures
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Extraction and Isolation
3.3.1. Compound 1
3.3.2. Compound 2
3.3.3. Compound 3
3.3.4. Compound 4
3.3.5. Compound 5
3.3.6. Compound 6
3.3.7. Compound 7
3.3.8. Compound 8
3.3.9. Compound 9
3.3.10. Compound 10
3.3.11. Purity Statement
3.4. Acid Hydrolysis
3.5. ECD Calculation
3.6. Cytotoxicity Assays
3.6.1. Cell Culture and Treatment
3.6.2. Cell Viability Assay
3.7. α-Glycosidase Inhibitory Assays
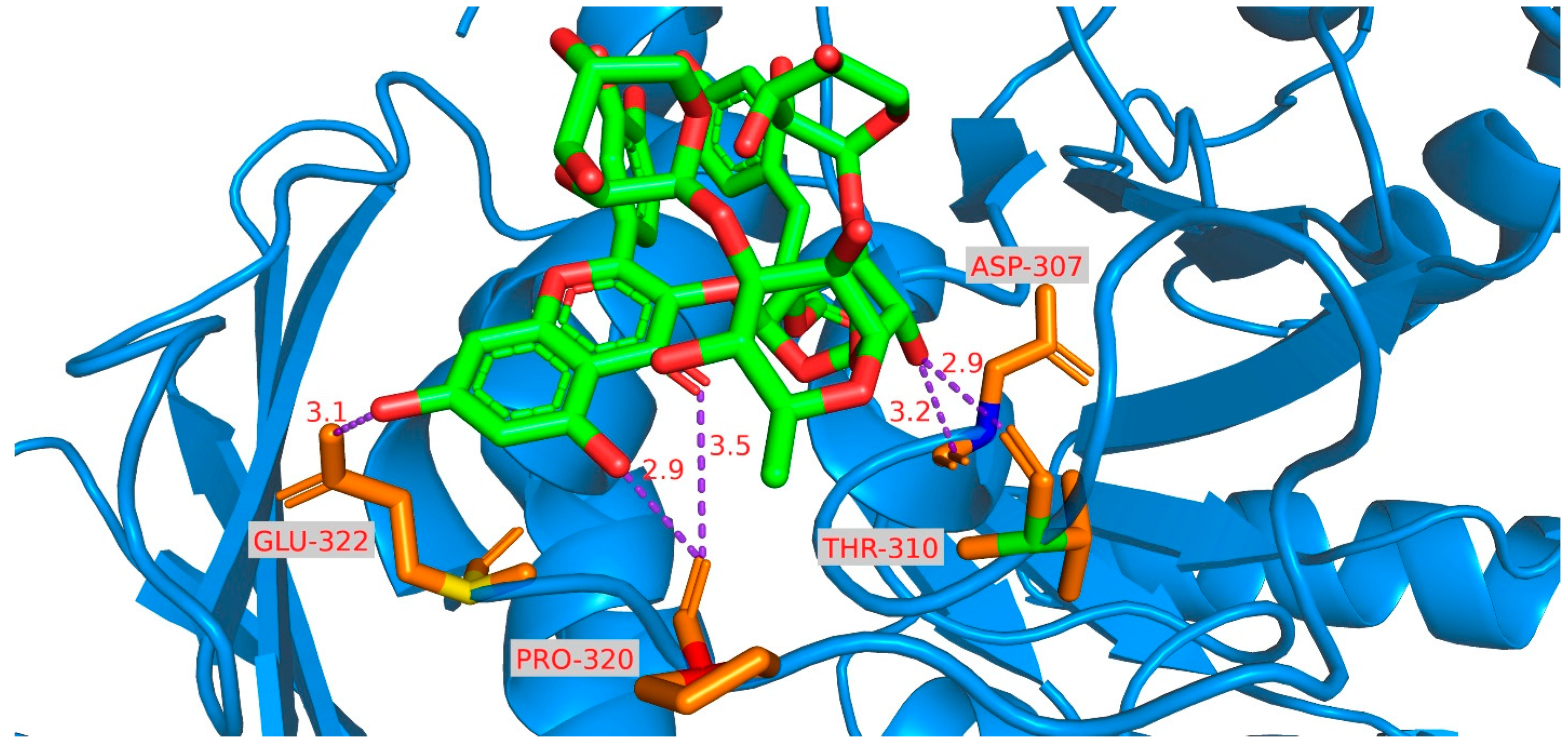
3.8. Molecular Docking Investigation
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, R.; Guan, Y.; Zhou, J.; Sun, B.; Wang, Z.; Chen, H.; He, Z.; Jia, A. Phytochemicals from Camellia nitidissima Chi flowers reduce the pyocyanin production and motility of Pseudomonas aeruginosa PAO1. Front. Microbiol. 2018, 8, 2640. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Feng, J.; Su, S.; Huang, L. Hepatoprotective effects of Camellia nitidissima aqueous ethanol extract against CCl4-induced acute liver injury in SD rats related to Nrf2 and NF-κB signalling. Pharm. Biol. 2020, 58, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Liu, H.; Wang, Z.; Qi, J.; Yuan, S.; Zhang, W.; Chen, H.; Finley, J.W.; Gu, L.; Jia, A. Phytochemicals from Camellia nitidissima Chi inhibited the formation of advanced glycation end-products by scavenging methylglyoxal. Food Chem. 2016, 205, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Guan, Y.; Wang, W.; Chen, H.; He, Z.; Jia, A. Antioxidant capacity of phenolics in Camellia nitidissima Chi flowers and their identification by HPLC Triple TOF MS/MS. PLoS ONE 2018, 13, e0195508. [Google Scholar] [CrossRef]
- Hou, X.; Du, H.; Yang, R.; Qi, J.; Huang, Y.; Feng, S.; Wu, Y.; Lin, S.; Liu, Z.; Jia, A.; et al. The antitumor activity screening of chemical constituents from Camellia nitidissima Chi. Int. J. Mol. Med. 2018, 41, 2793–2801. [Google Scholar] [CrossRef]
- Ma, S.; Weng, M.; Yang, T.; Ge, L.; Yang, K. Triterpenes and pheophorbides from Camellia ptilosperma and their cytotoxicity, photocytotoxicity, and photodynamic antibacterial activity. Molecules 2023, 28, 7058. [Google Scholar] [CrossRef]
- Ma, S.; Ge, L.; Li, Y.; Liao, N.; Xie, J.; Yang, K. Ursane-type triterpenes with a phenylpropanoid unit from Camellia ptilosperma and evaluation of their cytotoxic activities. J. Nat. Prod. 2023, 86, 1793–1800. [Google Scholar] [CrossRef]
- Wang, Z.; Hou, X.; Li, M.; Ji, R.; Li, Z.; Wang, Y.; Guo, Y.; Liu, D.; Huang, B.; Du, H. Active fractions of golden-flowered tea (Camellia nitidissima Chi) inhibit epidermal growth factor receptor mutated non-small cell lung cancer via multiple pathways and targets in vitro and in vivo. Front. Nutr. 2022, 9, 1014414. [Google Scholar] [CrossRef]
- Zhou, A.; Zhou, C.; Liu, M.; He, J.; Huang, L. Anticancer effects of Camellia nitidissima Chi polysaccharide on hepa-tocellular carcinoma cells via apoptosis. J. Herb. Med. 2023, 42, 100800. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, F.; Du, Z.; Xie, J.; Xia, L.; Hou, X.; Hao, E.; Deng, J. Proteome analysis of Camellia nitidissima Chi revealed its role in colon cancer through the apoptosis and ferroptosis pathway. Front. Oncol. 2021, 11, 727130. [Google Scholar] [CrossRef]
- Naeem, A.; Hu, P.; Yang, M.; Zhang, J.; Liu, Y.; Zhu, W.; Zheng, Q. Natural products as anticancer agents: Current status and future perspectives. Molecules 2022, 27, 8367. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wu, Q.; Qin, X. Camellia nitidissima Chi flower extracts inhibit α-amylase and α-glucosidase: In vitro by analysis of optimization of addition methods, inhibitory kinetics and mechanisms. Process Biochem. 2019, 86, 177–185. [Google Scholar] [CrossRef]
- Song, X.; Cao, P.; Bai, X.; Zhao, Y.; Zhang, Y.; Kong, H.; Zhao, Y.; Qu, H. The effects of carbon dots from hordei fructus Germinatus Carbonisatus on Glycometabolism and α-glycosidase activity. J. Biomed. Nanotechnol. 2022, 18, 2750–2758. [Google Scholar] [CrossRef]
- Xiong, J.; Wan, J.; Ding, J.; Wang, P.; Ma, G.; Li, J.; Hu, J. Camellianols A–G, barrigenol-like triterpenoids with PTP1B inhibitory effects from the endangered ornamental plant Camellia crapnelliana. J. Nat. Prod. 2017, 80, 2874–2882. [Google Scholar] [CrossRef]
- Zhang, H.; Li, X.; Wu, K.; Wang, M.; Liu, P.; Wang, X.; Deng, R. Antioxidant activities and chemical constituents of flavonoids from the flower of Paeonia ostii. Molecules 2016, 22, 5. [Google Scholar] [CrossRef]
- William, G.; Anthony, C.; Waiss, J. Structural relationships and interconversions of isomeric astilbins. J. Org. Chem. 1975, 40, 1057–1061. [Google Scholar] [CrossRef]
- Linh, N.T.T.; Thuy, T.T.; Tam, N.T.; Cham, B.T.; Tam, K.T.; Sa, N.H.; Thao, D.T.; Chinh, V.T.; Anh, N.T.H. Chemical constituents of Impatiens chapaensis Tard. and their α-glucosidase inhibition activities. Nat. Prod. Res. 2021, 36, 3229–3233. [Google Scholar] [CrossRef]
- Kim, S.; Li, Y.; Lin, L.; Sayasith, P.R.; Tarr, A.T.; Wright, E.B.; Yasmin, S.; Lannigan, D.A.; O’Doherty, G.A. Synthesis and biological evaluation of 4′-substituted kaempfer-3-ols. J. Org. Chem. 2020, 85, 4279–4288. [Google Scholar] [CrossRef]
- Noccioli, C.; Luciardi, L.; Barsellini, S. Flavonoids from two Italian Genista species: Genista cilentina and Genista sulcitana. Chem. Nat. Compd. 2012, 48, 672–673. [Google Scholar] [CrossRef]
- de Beer, D.; Joubert, E.; Malherbe, C.J.; Jacobus Brand, D. Use of countercurrent chromatography during isolation of 6-hydroxyluteolin-7-O-β-glucoside, a major antioxidant of Athrixia phylicoides. J. Chromatogr. A 2011, 1218, 6179–6186. [Google Scholar] [CrossRef]
- Jin, H.; Chen, G.; Li, X.; Shen, Y.; Yan, S.; Zhang, L.; Yang, M.; Zhang, W. Flavonoids from Rhododendron decorum. Chem. Nat. Compd. 2009, 45, 85–86. [Google Scholar] [CrossRef]
- de Oliveira, A.P.; Coppede, J.S.; Bertoni, B.W.; Crotti, A.E.M.; França, S.C.; Pereira, A.M.S.; Taleb-Contini, S.H. Costus spiralis (Jacq.) roscoe: A novel source of flavones with α-glycosidase inhibitory activity. Chem. Biodivers. 2017, 15, el700421. [Google Scholar] [CrossRef] [PubMed]
- Benahmed, M.; Akkal, S.; Elomri, A.; Laouer, H.; Vérité, P.; Seguin, E. Constituents from Bupleurum montanum (Coss. & Dur.) (Apiaceae). Arab. J. Chem. 2014, 7, 1065–1069. [Google Scholar] [CrossRef]
- Sporn, M.B.; Liby, K.T.; Yore, M.M.; Fu, L.; Lopchuk, J.M.; Gribble, G.W. New Synthetic Triterpenoids: Potent Agents for Prevention and Treatment of Tissue Injury Caused by Inflammatory and Oxidative Stress. J. Nat. Prod. 2011, 74, 537–545. [Google Scholar] [CrossRef]
- Huyen, L.T.; Bao, D.G.; Son, N.T.; Anh, N.T.V.; Huu Tai, B.H.; Huong, P.T.T.; Kiem, P.V.; Thuy, N.T.K.; Hanh, N.T.; Park, S.; et al. Cytotoxic Effects of Oleanane-type Saponins from Lysimachia laxa. Chem. Biodivers. 2024, e202401024. [Google Scholar] [CrossRef]
- Han, L.; Shi, L.; Yu, D.; Tang, Q.; Tang, L.; Feng, B.; Wang, Y. Inhibitive Effect of Seeds of Camellia chrysantha (Hu) Tuyama on Gonadal Hormones Dependent Tumour in vitro. Lishizhen Med. Mater. Medica Res. 2009, 20, 3146–3148. [Google Scholar]
- Luo, Z.; Zeng, J.; Yu, H.; Huang, H.; Bao, X.; Qin, S.; Chen, G.; Zhou, Z.; Zhi, H.; Yao, X. Astramalabaricosides A–T, highly oxygenated malabaricane triterpenoids with migratory inhibitory activity from Astragalus membranaceus var. mongholicus. J. Nat. Prod. 2022, 85, 2312–2331. [Google Scholar] [CrossRef]
- Zhang, Y.; Lv, P.; Ma, J.; Chen, N.; Guo, H.; Chen, Y.; Gan, X.; Wang, R.; Liu, X.; Fan, S.; et al. Antrodia cinnamomea exerts an anti-hepatoma effect by targeting PI3K/AKT-mediated cell cycle progression in vitro and in vivo. Acta Pharm. Sin. B 2022, 12, 890–906. [Google Scholar] [CrossRef]



| No. | 1 | 2 | 3 |
|---|---|---|---|
| 1 | 0.83 m 1.42 m | 0.81 m 1.37 m | 0.76 m 1.35 m |
| 2 | 1.68 m 1.97 m | 1.71 m 2.06 m | 1.72 m 2.05 m |
| 3 | 3.17 dd (11.7, 4.1) | 3.20 dd (11.3, 3.9) | 3.17 dd (11.5, 3.8) |
| 5 | 0.77 br d (12.3) | 0.77 br d (11.9) | 0.72 br d (11.6) |
| 6 | 1.33 m 1.53 m | 1.34 m 1.54 m | 1.31 m 1.50 m |
| 7 | 2.03 m 2.15 m | 2.03 m 2.15 m | 1.85–1.97 m |
| 9 | 1.68 m | 1.68 m | 1.49, m |
| 11 | 1.82, m | 1.84, m | 1.85, m |
| 12 | 5.45 t (3.4) | 5.44 t (3.4) | 5.46 t (3.4) |
| 15 | 4.48 overlapped | 4.48 overlapped | 5.09 s |
| 16 | 4.55 d (3.8) | 4.54 d (3.7) | |
| 18 | 2.45 dd (13.5, 3.8) | 2.44 dd (13.5, 3.8) | 2.71 dd, 13.9, 3.4) |
| 19 | 1.30 overlapped 2.70 dd (13.5, 12.9) | 1.29 overlapped 2.70 dd (13.5, 12.9) | 1.21 overlapped 1.56 t-like (13.9) |
| 21 | 1.40 m 2.39 m | 1.39 m 2.39 m | 1.29 m 1.76 m |
| 22 | 2.17 m 2.27 m | 2.16 m 2.27 m | 1.53 overlapped 2.88 br d (13.7) |
| 23 | 1.12 s | 1.13, s | 1.10, s |
| 24 | 1.02 s | 1.04, s | 1.04, s |
| 25 | 0.82 s | 0.81, s | 0.79, s |
| 26 | 1.05 s | 1.04, s | 1.13, s |
| 27 | 1.81 s | 1.82, s | 1.34, s |
| 28 | 3.60 d (10.5) 3.79 d (10.5) | 3.60, d (10.5) 3.79, d (10.5) | 3.81, d (10.8) 4.42, overlapped |
| 29 | 1.05 s | 1.04, s | 0.85, s |
| 30 | 1.10 s | 1.10, s | 0.89, s |
| GlcA-1 | 4.86 overlapped | 4.86, overlapped | 4.82, d (7.1) |
| 2 | 4.68 m | 4.73, m | 4.71, m |
| 3 | 4.67 m | 4.74, m | 4.74, m |
| 4 | 4.52 m | 4.64, m | 4.63, dd (9.5, 9.0) |
| 5 | 4.45 overlapped | 4.57, m | 4.57, d (9.5) |
| 6-OCH3 | 3.69 s | ||
| Glc-1 | 5.87 d (7.4) | 5.90 d (7.4) | 5.90 d (7.4) |
| 2 | 4.10 dd (9.0, 7.4) | 4.12 dd (9.3, 7.4) | 4.10 dd (9.0, 7.4) |
| 3 | 4.40 m | 4.41 m | 4.39 m |
| 4 | 4.07 t-like (9.1) | 4.07 t-like (9.0) | 4.04 t-like (9.1) |
| 5 | 4.40 m | 4.41 m | 4.39 m |
| 6 | 4.31 m 4.66 m | 4.31 m 4.66 m | 4.28 m 4.66 m |
| Gal-1 | 6.10 d (7.7) | 6.18 d (7.6) | 6.21 overlapped |
| 2 | 4.69 m | 4.71 m | 4.71 m |
| 3 | 4.45 m | 4.48 m | 4.49 br d (9.2) |
| 4 | 4.45 m | 4.46 m | 4.44 br s |
| 5 | 4.23 m | 4.26 m | 4.27 m |
| 6 | 4.31 m | 4.32 m | 4.33 m |
| Rha-1 | 6.23 br s | 6.23 br s | 6.21 br s |
| 2 | 4.77 br s | 4.78 br s | 4.76 br s |
| 3 | 4.71 m | 4.72 m | 4.69 m |
| 4 | 4.20 t-like (9.2) | 4.21 t-like (9.1) | 4.20 t-like (9.1) |
| 5 | 4.84 m | 4.88 m | 4.89 m |
| 6 | 1.41 d (6.1) | 1.42 d (5.8) | 1.40 d (5.8) |
| No. | 1 | 2 | 3 |
|---|---|---|---|
| 1 | 39.0 | 39.0 | 39.0 |
| 2 | 26.4 | 26.5 | 26.6 |
| 3 | 89.7 | 89.8 | 89.8 |
| 4 | 39.6 | 39.7 | 39.7 |
| 5 | 55.6 | 55.6 | 55.6 |
| 6 | 18.8 | 18.9 | 18.7 |
| 7 | 36.8 | 36.8 | 36.1 |
| 8 | 41.4 | 41.5 | 41.8 |
| 9 | 47.4 | 47.3 | 47.1 |
| 10 | 37.0 | 37.1 | 37.0 |
| 11 | 24.0 | 24.0 | 24.1 |
| 12 | 123.9 | 123.9 | 126.0 |
| 13 | 146.0 | 146.0 | 142.0 |
| 14 | 48.0 | 48.0 | 54.3 |
| 15 | 67.4 | 67.4 | 73.8 |
| 16 | 78.7 | 78.7 | 216.1 |
| 17 | 41.1 | 41.1 | 53.2 |
| 18 | 43.3 | 43.3 | 48.1 |
| 19 | 48.0 | 48.0 | 48.0 |
| 20 | 31.3 | 31.3 | 31.0 |
| 21 | 37.1 | 37.1 | 35.8 |
| 22 | 31.0 | 31.0 | 27.1 |
| 23 | 28.1 | 28.0 | 28.0 |
| 24 | 16.8 | 16.8 | 16.9 |
| 25 | 15.9 | 15.9 | 15.8 |
| 26 | 17.6 | 17.6 | 17.7 |
| 27 | 21.0 | 21.0 | 21.7 |
| 28 | 69.6 | 69.6 | 70.9 |
| 29 | 33.5 | 33.5 | 33.1 |
| 30 | 24.6 | 24.6 | 23.6 |
| GlcA-1 | 105.2 | 105.4 | 105.4 |
| 2 | 79.5 | 79.5 | 79.6 |
| 3 | 82.8 | 82.8 | 82.7 |
| 4 | 70.9 | 71.3 | 71.3 |
| 5 | 76.6 | 77.4 | 77.4 |
| 6 | 170.3 | 172.9 | 172.2 |
| 6-OCH3 | 52.1 | ||
| Glc-1 | 102.9 | 102.7 | 102.8 |
| 2 | 76.4 | 76.4 | 76.5 |
| 3 | 78.5 | 78.5 | 78.5 |
| 4 | 72.6 | 72.6 | 72.7 |
| 5 | 78.2 | 78.1 | 78.2 |
| 6 | 63.5 | 63.6 | 63.7 |
| Gal-1 | 101.6 | 101.4 | 101.4 |
| 2 | 76.2 | 76.4 | 76.5 |
| 3 | 76.1 | 76.0 | 76.1 |
| 4 | 71.1 | 71.2 | 71.3 |
| 5 | 77.0 | 77.0 | 77.1 |
| 6 | 62.0 | 62.0 | 62.1 |
| Rha-1 | 102.3 | 102.4 | 102.4 |
| 2 | 72.6 | 72.6 | 72.7 |
| 3 | 72.6 | 72.6 | 72.7 |
| 4 | 73.9 | 74.0 | 74.0 |
| 5 | 69.8 | 69.8 | 69.9 |
| 6 | 18.3 | 18.3 | 18.3 |
| No. | δH (J in Hz) | δC | No. | δH (J in Hz) | δC |
|---|---|---|---|---|---|
| 2 | 5.19 d (10.4) | 83.5 | 4 | 3.29 overlapped | 73.9 |
| 3 | 4.55 d (10.4) | 81.2 | 5 | 4.18 m | 70.9 |
| 4 | 195.7 | 6 | 1.20 d (6.2) | 17.9 | |
| 5 | 165.5 | Glc-1 | 4.02 d (7.6) | 105.3 | |
| 6 | 5.92 d (1.9) | 97.4 | 2 | 3.30 overlapped | 78.9 |
| 7 | 168.6 | 3 | 3.41 t-like (9.1) | 78.3 | |
| 8 | 5.90 d (1.9) | 96.3 | 4 | 3.33 overlapped | 70.9 |
| 9 | 164.0 | 5 | 3.10 dt (9.5, 3.1) | 77.2 | |
| 10 | 102.5 | 6 | 3.68 d (3.1) | 62.1 | |
| 1′ | 128.7 | Rha2-1 | 5.21 d (1.5) | 101.8 | |
| 2′, 6′ | 7.36 d (8.4) | 130.1 | 2 | 3.88 dd (3.3, 1.5) | 71.9 |
| 3′, 5′ | 6.85 d (8.4) | 116.5 | 3 | 3.79 dd (9.5, 3.3) | 71.8 |
| 4′ | 159.2 | 4 | 3.33 overlapped | 74.2 | |
| Rha1-1 | 4.71 d (1.4) | 102.1 | 5 | 4.02 m | 70.3 |
| 2 | 3.46 dd (3.2, 1.4) | 82.4 | 6 | 1.26 d (6.2) | 17.9 |
| 3 | 3.72 dd (9.8, 3.2) | 71.6 |
| No. | 5 1 | 6 2 | ||
|---|---|---|---|---|
| δH (J in Hz) | δC | δH (J in Hz) | δC | |
| 2 | 5.48 d (2.3) | 82.1 | 5.42 d (8.4) | 80.9 |
| 3 | 4.17 d (2.3) | 75.8 | 4.71 d (8.4) | 76.9 |
| 4 | 194.4 | 193.8 | ||
| 5 | 166.1 | 163.4 | ||
| 6 | 5.93 d (1.7) | 97.5 | 5.90 s | 96.0 |
| 7 | 168.9 | 167.1 | ||
| 8 | 5.97 d (1.7) | 96.3 | 5.90 s | 95.1 |
| 9 | 164.6 | 161.9 | ||
| 10 | 101.9 | 101.0 | ||
| 1′ | 128.0 | 126.2 | ||
| 2′, 6′ | 7.31 d (8.5) | 129.0 | 7.25 d (8.4) | 128.8 |
| 3′, 5′ | 6.82 d (8.5) | 116.3 | 6.78 d (8.4) | 115.3 |
| 4′ | 158.7 | 157.7 | ||
| 5-OH | 11.71 br s | |||
| 7-OH | 10.96 br s | |||
| 4′-OH | 9.62 br s | |||
| Rha1-1 | 5.36 d (1.2) | 99.6 | 4.77 br s | 99.7 |
| 2 | 3.73 br s | 81.8 | 3.49 br s | 77.9 |
| 3 | 3.53 dd (9.7 3.4) | 71.6 | 3.09 m | 70.1 |
| 4 | 3.18 t-like (9.7) | 73.4 | 3.15 m | 72.1 |
| 5 | 2.38 dq (9.7 6.1) | 70.3 | 3.60 m | 69.0 |
| 6 | 0.88 d (6.1) | 17.9 | 1.07 d (6.0) | 17.6 |
| Glc-1 | 4.47 d (7.7) | 105.5 | 4.31 d (7.8) | 102.5 |
| 2 | 3.41 overlapped | 78.4 | 3.17 m | 75.6 |
| 3 | 3.46 t-like (9.0) | 78.7 | 3.28 m | 77.5 |
| 4 | 3.39 overlapped | 71.1 | 3.46 m | 70.0 |
| 5 | 3.20 m | 77.4 | 3.07 m | 76.6 |
| 6 | 3.74 m 3.85 m | 62.4 | 3.48 m 3.64 m | 60.8 |
| Rha2-1 | 5.27 d (1.8) | 101.3 | 5.18 br s | 99.5 |
| 2 | 4.00 dd (3.2 1.8) | 69.8 | 3.70 br s | 70.3 |
| 3 | 5.03 dd (9.8 3.2) | 75.5 | 3.75 m | 67.7 |
| 4 | 3.50 t-like (9.8) | 71.6 | 4.72 t-like (9.8) | 74.1 |
| 5 | 4.19 m | 70.1 | 4.07 m | 65.7 |
| 6 | 1.24 d (6.2) | 17.9 | 0.94 d (6.1) | 17.4 |
| Acetyl-1 | 172.8 | 170.2 | ||
| 2 | 2.08 s | 21.1 | 2.00 s | 21.0 |
| No. | 7 1 | 8 2 | 9 1 | 10 1 |
|---|---|---|---|---|
| 6 | 6.13 br s | 6.15 br s | 6.17 br s | 6.15 br s |
| 8 | 6.32 br s | 6.33 br s | 6.35 br s | 6.35 br s |
| 2′ | 7.60 br s | 7.51 overlapped | 7.98 d (8.3) | 7.98 d (8.6) |
| 3′ | 6.89 d (8.3) | 6.90 d (8.6) | ||
| 5′ | 6.88 d (7.8) | 6.84 d (8.8) | 6.89 d (8.3) | 6.90 d (8.6) |
| 6′ | 7.55 br d (7.8) | 7.51 overlapped | 7.98 d (8.3) | 7.98 d (8.6) |
| 5-OH | 12.58 br s | |||
| Glc1-1 | 5.58 d (7.8) | 5.57 d (7.9) | 5.53 d (8.0) | 5.57 d (8.0) |
| 2 | 5.24 dd (9.5, 7.8) | 5.09 dd (9.5, 7.9) | 5.20 dd (9.5, 8.0) | 5.19 dd (9.5, 8.0) |
| 3 | 3.88 m | 3.82 t-like (9.0) | 3.90 t-like (9.2) | 3.87 t-like (9.0) |
| 4 | 3.48 m | 3.29 m | 3.43 m | 3.42 t-like (9.2) |
| 5 | 3.58 m | 3.52 m | 3.52 m | 3.54 m |
| 6 | 3.51 m 3.88 m | 3.41 overlapped 3.73 br d (11.3) | 3.47 m 3.86 m | 3.47 m 3.87 m |
| Ara-1 | 4.34 d (6.8) | 4.34 d (7.0) | ||
| 2 | 3.54 m | 3.53 m | ||
| 3 | 3.49 m | 3.47 m | ||
| 4 | 3.77 br s | 3.77 br s | ||
| 5 | 3.56 m 3.85 m | 3.56 m 3.87 m | ||
| Glc2-1 | 4.31 d (7.9) | 4.42 d (7.7) | ||
| 2 | 2.95 m | 3.18 dd (9.0, 7.7) | ||
| 3 | 3.20 m | 3.28 m | ||
| 4 | 3.26 m | 3.27 m | ||
| 5 | 3.10 m | 3.32 m | ||
| 6 | 3.41 overlapped 3.68 m | 3.62 dd (11.6, 6.3) 3.86 m | ||
| Rha-1 | 4.58 br s | 4.38 br s | 4.54 br s | 4.54 d (1.5) |
| 2 | 3.85 br s | 3.57 br s | 3.80 br s | 3.81 dd (3.1, 1.5) |
| 3 | 3.60 dd (9.2, 3.0) | 3.32 m | 3.55 dd (9.3, 3.1) | 3.56 m |
| 4 | 3.44 t-like (9.4) | 3.26 m | 3.42 t-like (9.4) | 3.43 t-like (9.4) |
| 5 | 3.53 m | 3.32 m | 3.49 m | 3.51 m |
| 6 | 1.13 d (6.0) | 0.96 d (6.0) | 1.11 d (6.1) | 1.12 d (6.1) |
| Xyl-1 | 4.39 d (7.4) | 4.24 d (7.5) | 4.35 d (7.5) | 4.36 d (7.5) |
| 2 | 3.26 dd (9.1, 7.4) | 3.02 m | 3.26 dd (9.0, 7.5) | 3.25 dd (9.1, 7.5) |
| 3 | 3.37 t-like, (9.0) | 3.12 m | 3.35 t-like (9.0) | 3.35 t-like (9.0) |
| 4 | 3.49 m | 3.03 m | 3.49 m | 3.49 m |
| 5 | 3.17 t-like (10.5) 3.80 m | 3.00 m 3.60 dd (11.1, 5.0) | 3.15 t-like (10.7) 3.79 m | 3.15 t-like (10.8) 3.79 m |
| Coumaroyl-2, 6 | 7.45 d (8.0) | 7.51 d (8.4) | 7.47 d (8.2) | 7.46 d (8.2) |
| 3, 5 | 6.80 d (8.0) | 6.79 d (8.4) | 6.81 d (8.2) | 6.80 d (8.2) |
| 7 | 7.68 d (15.9) | 7.54 d (15.9) | 7.70 d (15.9) | 7.69 d (15.9) |
| 8 | 6.42 d (15.9) | 6.36 d (15.9) | 6.39 d (15.9) | 6.39 d (15.9) |
| No. | 7 1 | 8 2 | 9 1 | 10 1 |
|---|---|---|---|---|
| 2 | 158.9 | 156.8 | 159.1 | 159.1 |
| 3 | 134.8 | 132.8 | 134.6 | 134.7 |
| 4 | 178.9 | 177.0 | 179.0 | 179.0 |
| 5 | 162.9 | 161.2 | 163.0 | 163.0 |
| 6 | 99.9 | 98.6 | 99.9 | 99.9 |
| 7 | 165.5 | 164.1 | 165.6 | 165.6 |
| 8 | 94.8 | 93.6 | 94.9 | 94.9 |
| 9 | 158.3 | 156.4 | 158.4 | 158.5 |
| 10 | 105.9 | 104.0 | 105.8 | 105.9 |
| 1′ | 123.3 | 120.9 | 122.8 | 122.9 |
| 2′ | 117.5 | 116.3 | 132.3 | 132.3 |
| 3′ | 145.8 | 144.8 | 116.2 | 116.2 |
| 4′ | 149.6 | 148.6 | 161.3 | 161.3 |
| 5′ | 116.2 | 115.2 | 116.2 | 116.2 |
| 6′ | 123.5 | 121.7 | 132.3 | 132.3 |
| Glc1-1 | 100.7 | 98.8 | 100.7 | 100.7 |
| 2 | 74.5 | 72.2 | 74.4 | 74.5 |
| 3 | 84.5 | 83.1 | 84.6 | 84.3 |
| 4 | 70.3 | 69.2 | 70.4 | 70.4 |
| 5 | 76.8 | 75.3 | 76.9 | 76.9 |
| 6 | 68.4 | 67.9 | 68.6 | 68.5 |
| Ara-1 | 105.2 | 105.3 | ||
| 2 | 72.2 | 72.2 | ||
| 3 | 73.9 | 73.9 | ||
| 4 | 69.5 | 69.5 | ||
| 5 | 67.2 | 67.2 | ||
| Glc2-1 | 103.3 | 104.8 | ||
| 2 | 73.2 | 74.7 | ||
| 3 | 76.8 | 77.6 | ||
| 4 | 69.5 | 71.3 | ||
| 5 | 76.4 | 78.0 | ||
| 6 | 61.0 | 62.5 | ||
| Rha-1 | 102.2 | 101.3 | 102.2 | 102.2 |
| 2 | 71.6 | 69.8 | 71.7 | 71.7 |
| 3 | 82.4 | 81.1 | 82.4 | 82.4 |
| 4 | 72.7 | 70.7 | 72.6 | 72.7 |
| 5 | 69.5 | 68.0 | 69.5 | 69.5 |
| 6 | 17.9 | 17.6 | 17.9 | 17.9 |
| Xyl-1 | 106.2 | 105.4 | 106.3 | 106.3 |
| 2 | 75.3 | 73.9 | 75.2 | 75.3 |
| 3 | 77.5 | 76.0 | 77.5 | 77.5 |
| 4 | 71.1 | 69.9 | 71.0 | 71.1 |
| 5 | 66.8 | 65.5 | 66.8 | 66.8 |
| Coumaroyl-1 | 127.3 | 125.3 | 127.3 | 127.4 |
| 2, 6 | 131.3 | 130.2 | 131.3 | 131.3 |
| 3, 5 | 116.8 | 115.8 | 116.8 | 116.8 |
| 4 | 161.1 | 159.7 | 161.2 | 161.2 |
| 7 | 147.3 | 144.6 | 147.2 | 147.3 |
| 8 | 115.1 | 114.4 | 115.2 | 115.2 |
| 9 | 168.8 | 165.7 | 168.6 | 168.6 |
| Hela | MCF-7 | BEL-7402 | A549 | Hep G2 | MB-231 | MRC-5 | HNEpC | |
|---|---|---|---|---|---|---|---|---|
| 1 | 15.07 ± 1.06 | 9.06 ± 0.45 | 7.62 ± 1.06 | >30 | 25.60 ± 3.46 | >30 | >30 | >30 |
| 2 | 4.17 ± 0.85 | 19.24 ± 3.71 | >30 | >30 | 14.04 ± 3.29 | 26.53 ± 3.07 | >30 | >30 |
| 3 | >30 | 1.65 ± 0.39 | 4.94 ± 0.41 | 23.42 ± 7.26 | 8.77 ± 1.56 | 10.08 ± 2.28 | >30 | >30 |
| 4 | >30 | >30 | >30 | >30 | >30 | >30 | >30 | >30 |
| 5 | >30 | >30 | >30 | >30 | 20.76 ± 3.79 | >30 | >30 | >30 |
| 6 | >30 | >30 | 11.40 ± 3.11 | >30 | >30 | >30 | >30 | >30 |
| 7 | >30 | >30 | >30 | >30 | >30 | >30 | >30 | >30 |
| 8 | >30 | >30 | >30 | >30 | >30 | >30 | >30 | >30 |
| 9 | 24.72 ± 4.39 | >30 | >30 | >30 | 28.80 ± 5.55 | >30 | >30 | >30 |
| 10 | >30 | >30 | >30 | 17.38 ± 2.81 | >30 | >30 | >30 | >30 |
| PC | 11.06 ± 0.72 1 | 3.79 ± 0.26 2 | 9.94 ± 0.80 1 | 4.20 ± 0.37 1 | 6.31 ± 0.18 1 | 9.06 ± 1.01 1 | ND | ND |
| IC50 | IC50 | ||
|---|---|---|---|
| 1 | >20 | 7 | 2.38 ± 0.22 |
| 2 | >20 | 8 | 1.17 ± 0.30 |
| 3 | >20 | 9 | 3.33 ± 0.48 |
| 4 | 6.15 ± 0.43 | 10 | 2.26 ± 0.48 |
| 5 | 12.73 ± 1.08 | acarbose | 2.04 ± 0.27 |
| 6 | 8.09 ± 2.61 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, S.; Wu, Y.; Min, H.; Ge, L.; Yang, K. Triterpenoid Saponins and Flavonoid Glycosides from the Flower of Camellia flavida and Their Cytotoxic and α-Glycosidase Inhibitory Activities. Int. J. Mol. Sci. 2024, 25, 10977. https://doi.org/10.3390/ijms252010977
Ma S, Wu Y, Min H, Ge L, Yang K. Triterpenoid Saponins and Flavonoid Glycosides from the Flower of Camellia flavida and Their Cytotoxic and α-Glycosidase Inhibitory Activities. International Journal of Molecular Sciences. 2024; 25(20):10977. https://doi.org/10.3390/ijms252010977
Chicago/Turabian StyleMa, Siyuan, Yuxin Wu, Hanfeng Min, Li Ge, and Kedi Yang. 2024. "Triterpenoid Saponins and Flavonoid Glycosides from the Flower of Camellia flavida and Their Cytotoxic and α-Glycosidase Inhibitory Activities" International Journal of Molecular Sciences 25, no. 20: 10977. https://doi.org/10.3390/ijms252010977
APA StyleMa, S., Wu, Y., Min, H., Ge, L., & Yang, K. (2024). Triterpenoid Saponins and Flavonoid Glycosides from the Flower of Camellia flavida and Their Cytotoxic and α-Glycosidase Inhibitory Activities. International Journal of Molecular Sciences, 25(20), 10977. https://doi.org/10.3390/ijms252010977






