In Vitro Screening of Ecotoxic and Cytotoxic Activities of Ailanthus altissima Leaf Extract against Target and Non-Target Plant and Animal Cells
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of Phenolic Compounds of A. altissima Leaf Extract by HPLC-DAD
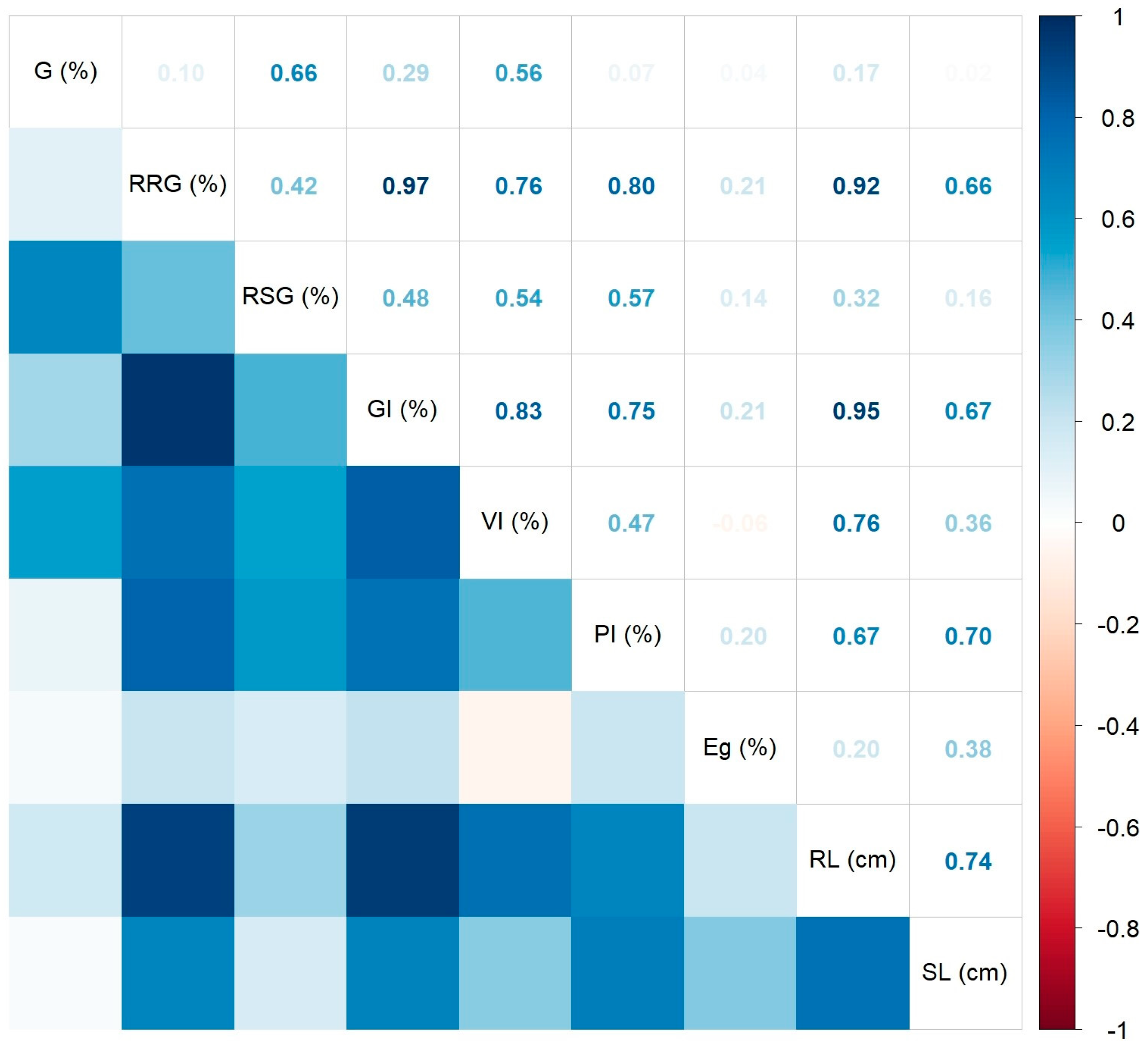
2.2. Ecotoxicity of A. altissima Leaf Extract
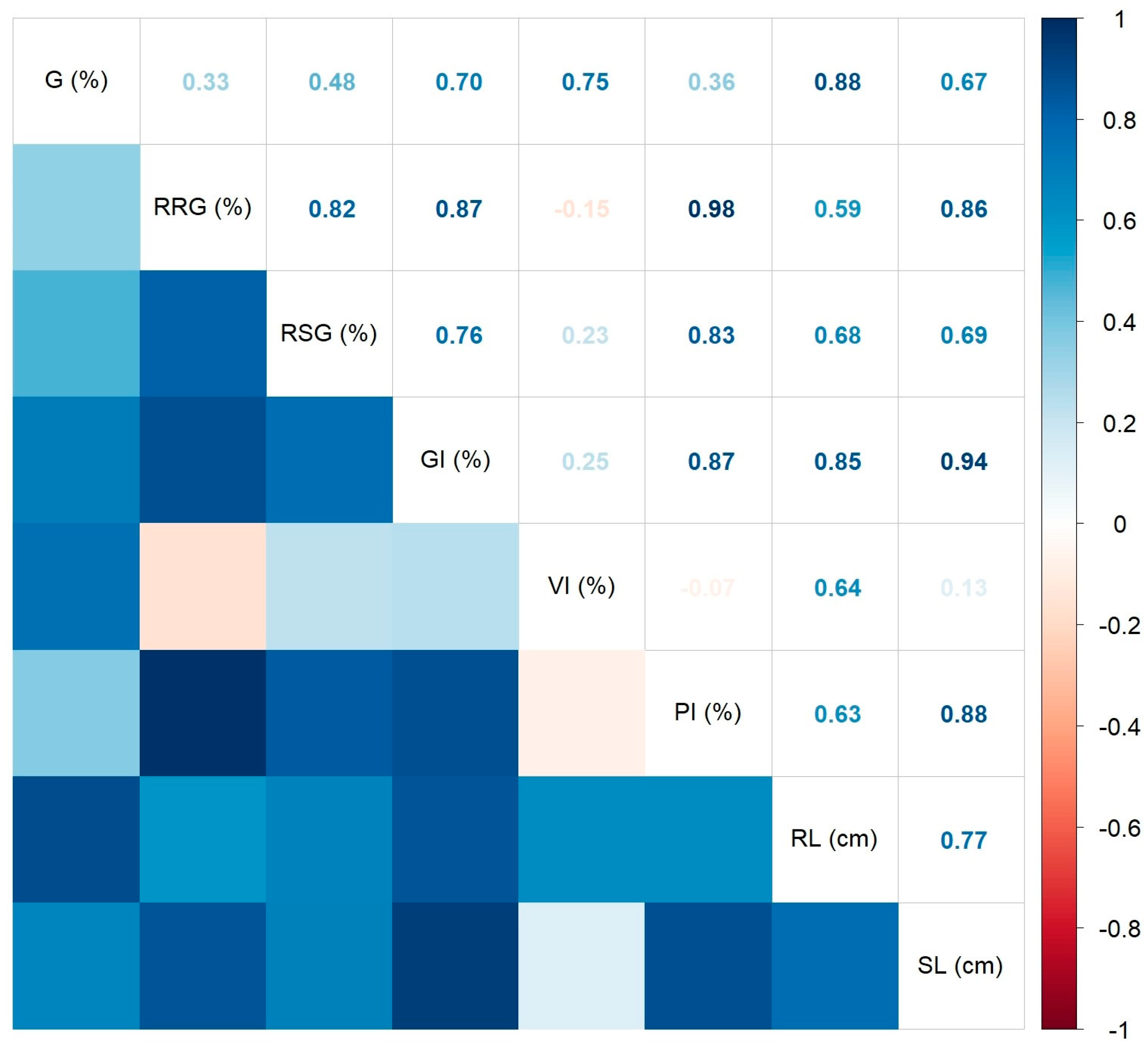
2.2.1. Inhibitory Effects of A. altissima Leaf Extract on Germination of Seeds Used in Agricultural Crops
Wheat Caryopsis Germination Test
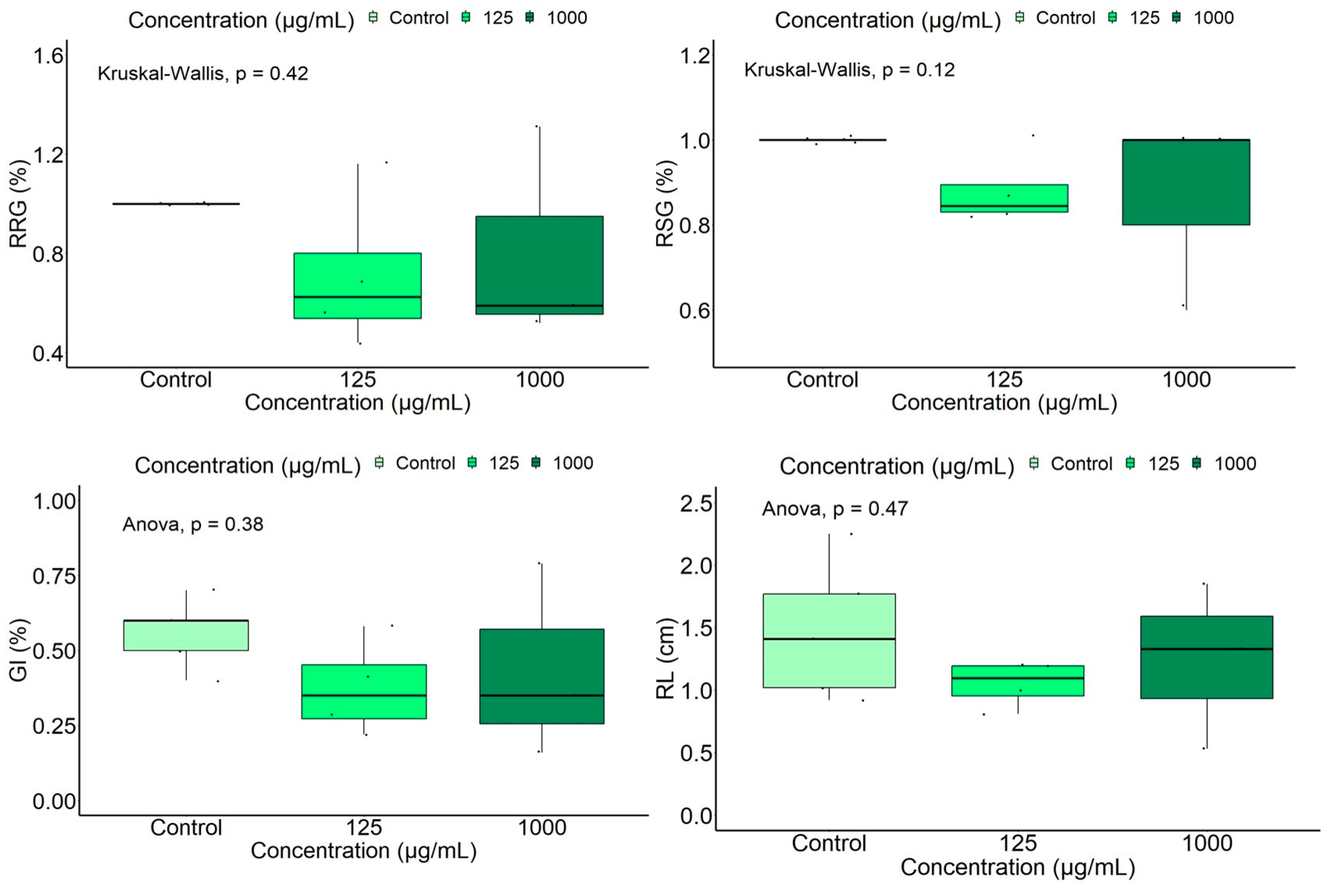
Tomato Seed Germination Test
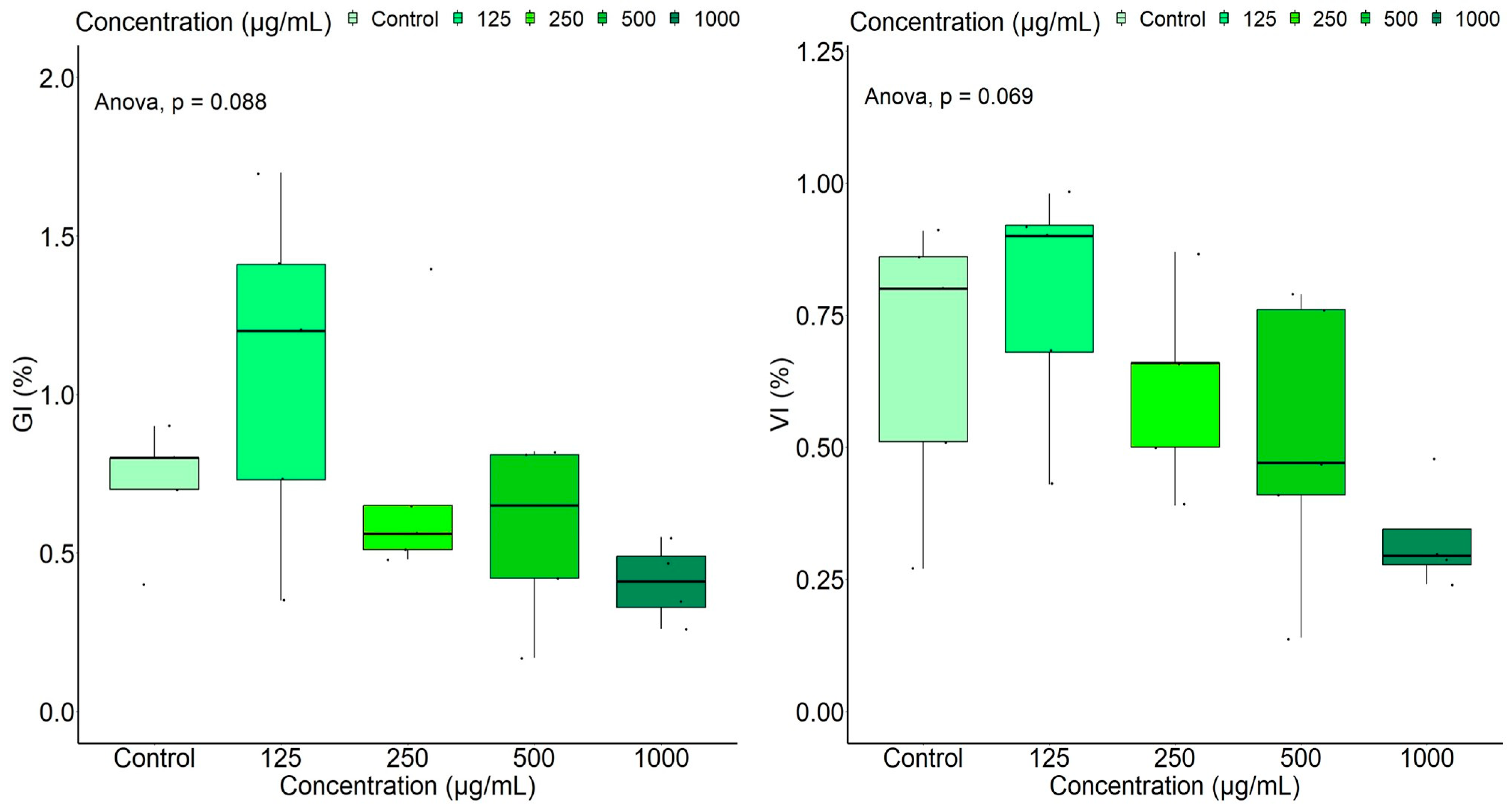
Parsley Seed Germination Test
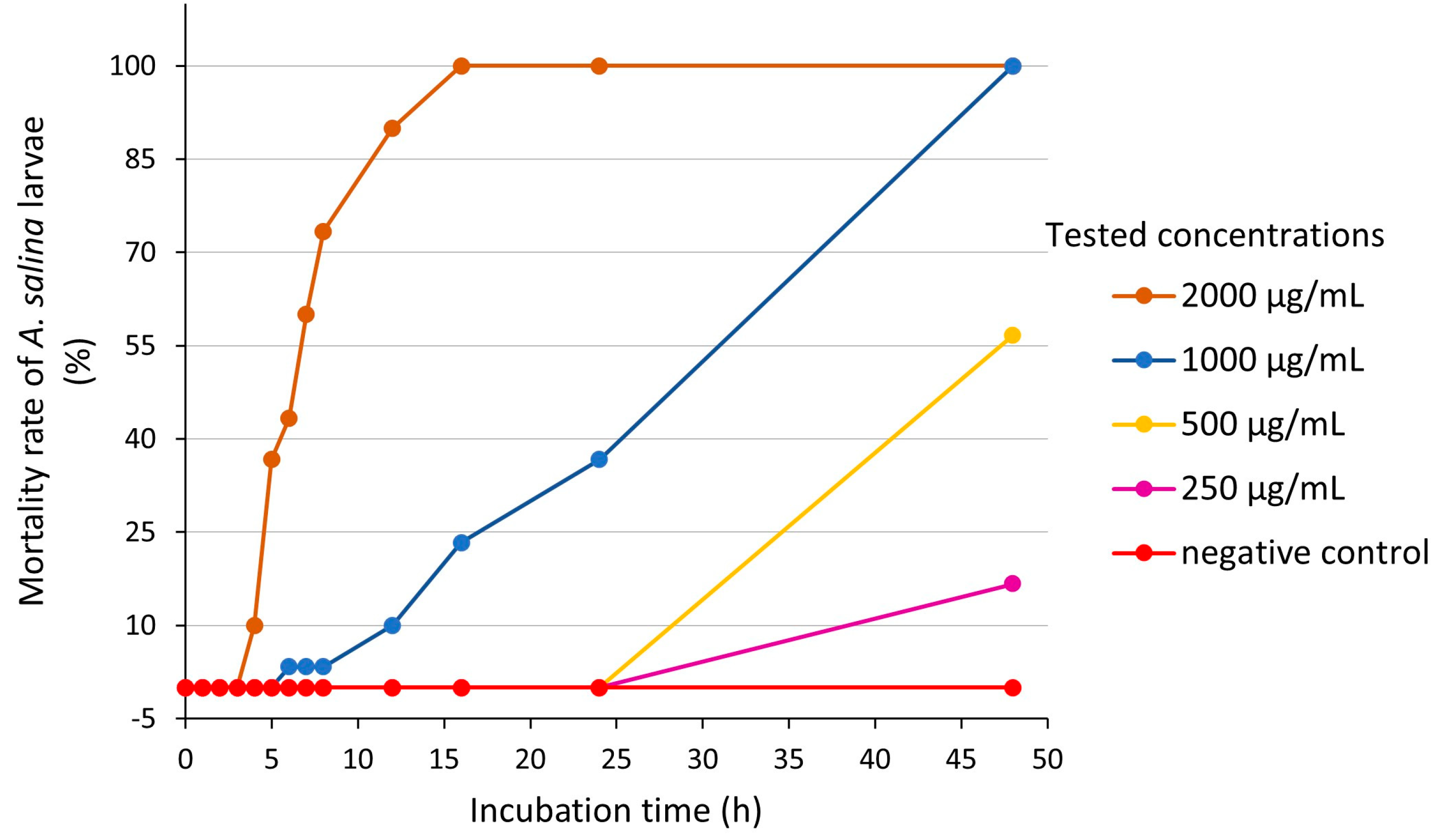
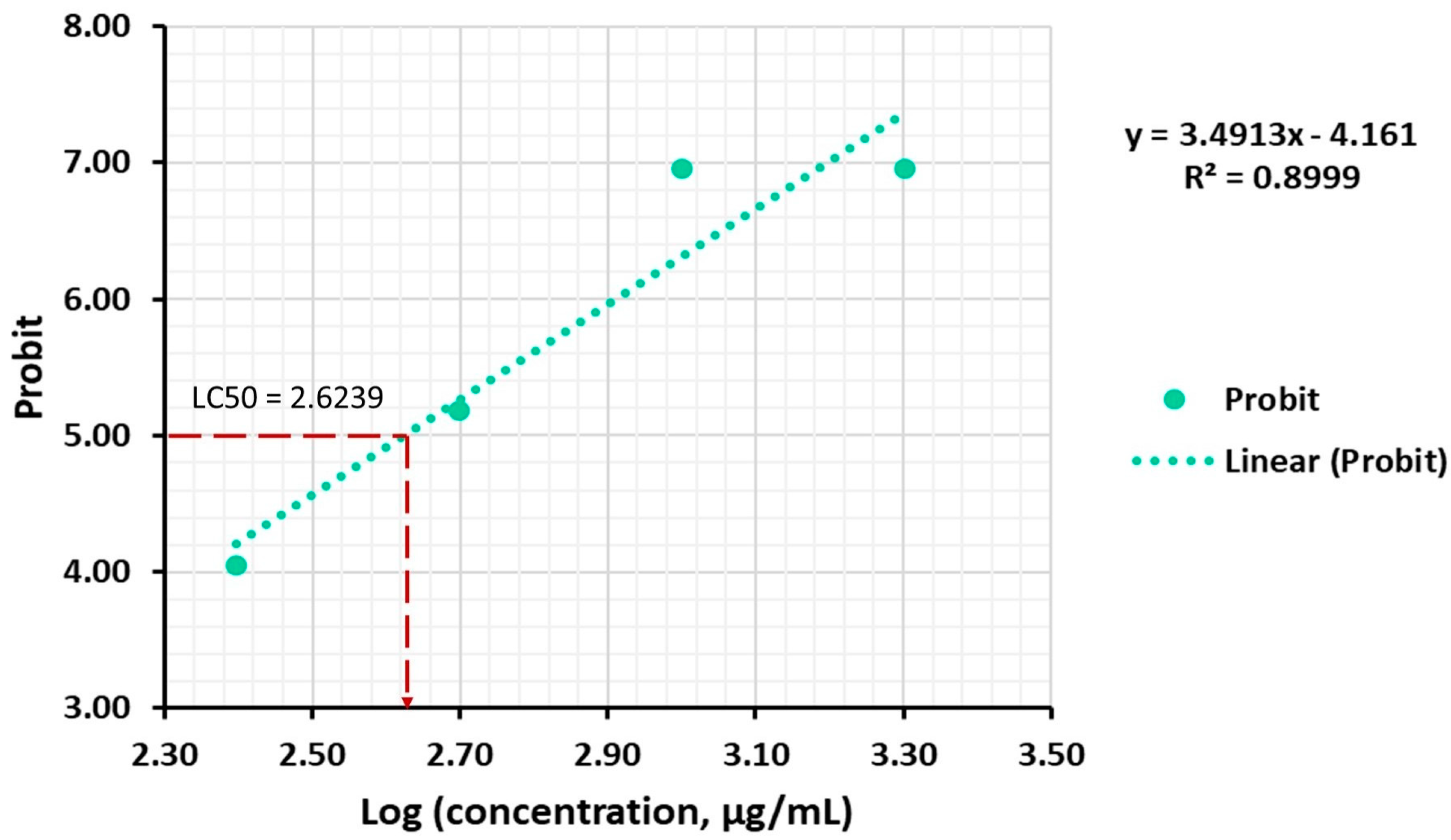
2.2.2. Inhibitory Effects of A. altissima Leaf Extract on A. salina Hatching
2.3. Cytotoxicity of A. altissima Leaf Extract
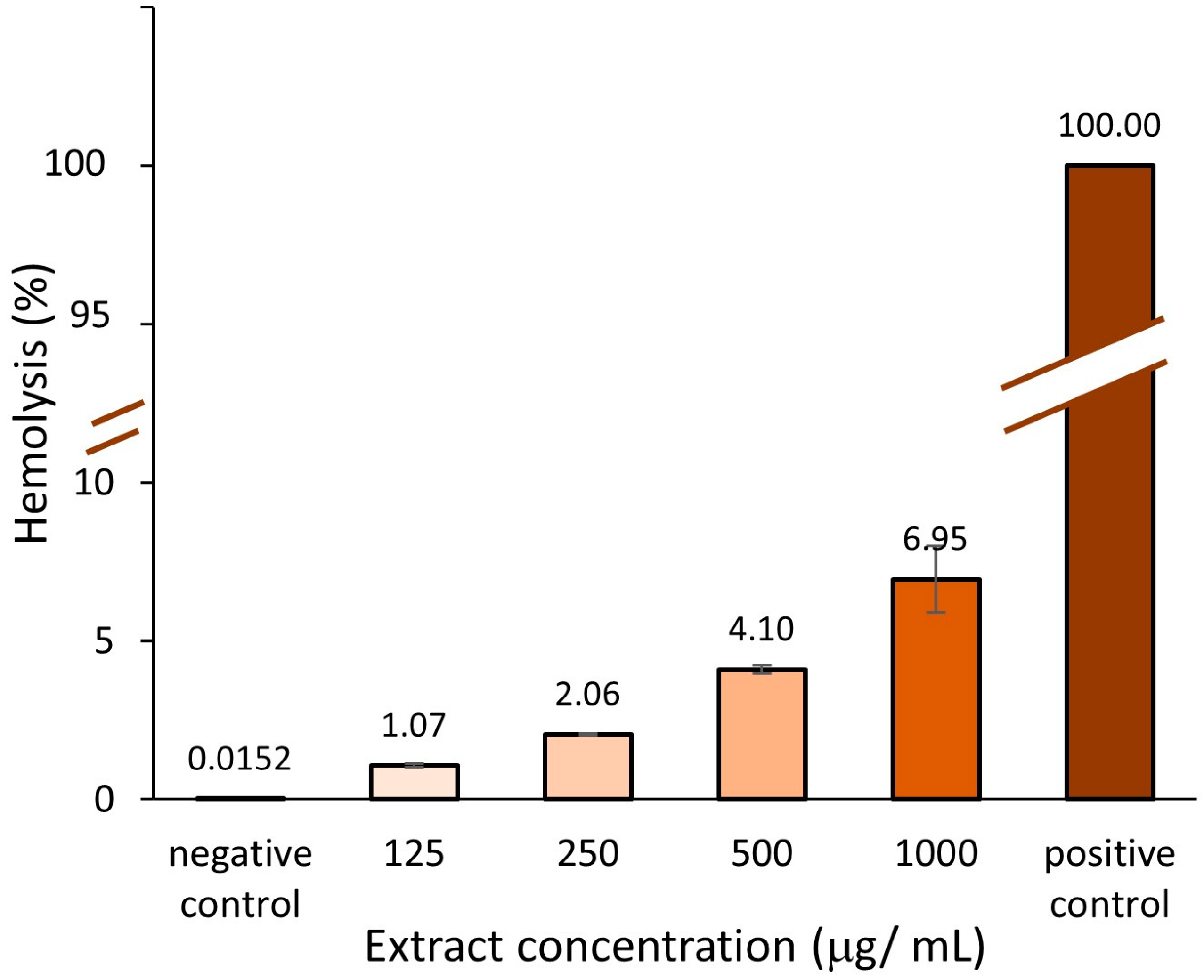
2.3.1. Hemolytic Activity
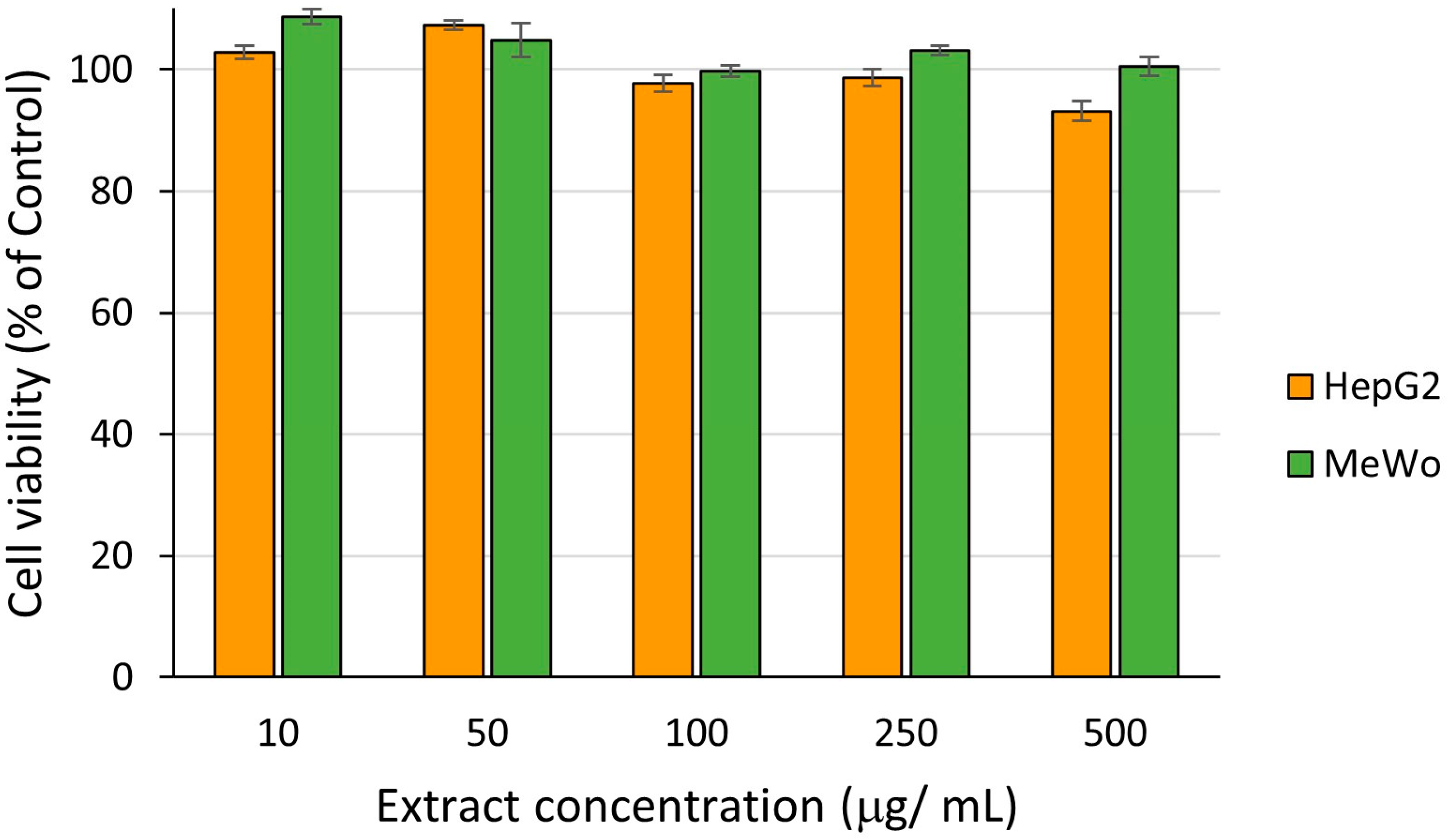
2.3.2. In Vitro Biocompatibility and Cytotoxicity of Leaf Extract at Different Concentrations
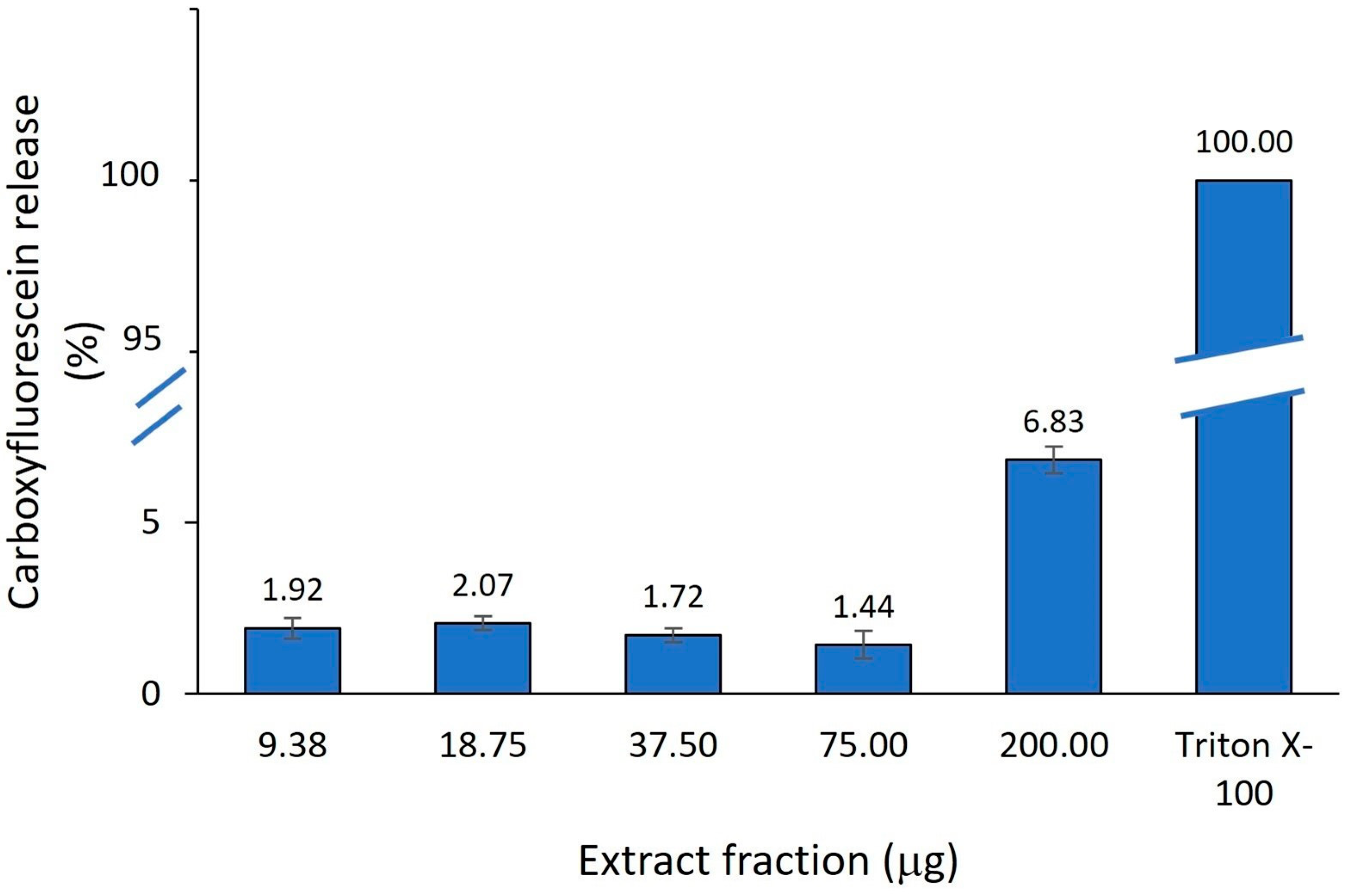
2.3.3. Model Membrane-Modifying Properties of A. altissima Leaf Extract
3. Materials and Methods
3.1. Plant Material and Extract Preparation
3.2. Characterization of A. altissima Leaf Extract
3.3. Determination of Ecotoxicity of A. altissima Leaf Extract
3.3.1. Germination Bioassay of Wheat (Triticum aestivum L.) Caryopsis
3.3.2. Germination Bioassay of Tomato (Lycopersicum esculentum Mill.) Caryopsis
3.3.3. Germination Bioassay of Parsley (Petroselinum crispum (Mill.) var. Crispum) Seed
3.3.4. Brine Shrimp (Artemia salina) Assay
3.4. Determination of Cytotoxicity of A. altissima Leaf Extract
3.4.1. Hemolytic Activity Assay (Erythrocyte Viability)
- Asample = absorbance of the test sample (RBC in the presence of extract)
- Acontrol= absorbance of the control (RBC and PBS)
- A100 = absorbance in case of total lysis (RBC and distilled water)
3.4.2. In Vitro Biocompatibility and Cytotoxicity Assessment (MTS Assay)
3.4.3. Membrane Leakage Assay from Lipid Vesicle
SUV Preparation
Leakage from SUV
- F0 = fluorescence intensity of vesicles in the absence of extract,
- Ft = fluorescence intensity of vesicles at time t in the presence of extract,
- FT = total fluorescence intensity determined by disrupting the vesicles by addition of 50 µL of a Triton X-100 solution.
3.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Montecchiari, S.; Tesei, G.; Allegrezza, M. Ailanthus altissima forests determine a shift in herbaceous layer richness: A paired comparison with hardwood native forests in sub-Mediterranean Europe. Plants 2020, 9, 1404. [Google Scholar] [CrossRef]
- EPPO. EPPO Global Database. 2024. Available online: https://gd.eppo.int (accessed on 21 March 2024).
- Brundu, G. Information on Measures and Related Costs in Relation to Species Considered for Inclusion on the Union List: Ailanthus altissima. Technical Note Prepared by IUCN for the European Commission. 2017. Available online: https://circabc.europa.eu (accessed on 21 March 2024).
- Huo, Q.; Shao, J.; Lin, Q. Study on the antibacterial and bactericidal effects of Ailanthus altissima leaves extract. Asian J. Chem. 2012, 24, 3545. [Google Scholar]
- Poljuha, D.; Sladonja, B.; Šola, I.; Dudaš, S.; Bilić, J.; Rusak, G.; Motlhatlego, K.E.; Eloff, J.N. Phenolic composition of leaf extracts of Ailanthus altissima (Simaroubaceae) with antibacterial and antifungal activity equivalent to standard antibiotics. Nat. Prod. Commun. 2017, 12, 1609–1612. [Google Scholar] [CrossRef]
- Rahman, H.M.A.; Javaid, S.; Ashraf, W.; Rasool, M.F.; Saleem, H.; Khan, S.A.; Ul-Haq, Z.; Anjum, S.M.M.; Ahmad, T.; Alqahtani, F.; et al. Effects of long-term Ailanthus altissima extract supplementation on fear, cognition and brain an-tioxidant levels. Saudi. Pharm. J. 2023, 31, 191–206. [Google Scholar] [CrossRef] [PubMed]
- Caramelo, D.; Pedro, S.I.; Marques, H.; Simão, A.Y.; Rosado, T.; Barroca, C.; Gominho, J.; Anjos, O.; Gallardo, E. Insights into the bioactivities and chemical analysis of Ailanthus altissima (Mill.) Swingle. Appl. Sci. 2021, 11, 11331. [Google Scholar] [CrossRef]
- Andonova, T.; Muhovski, Y.; Slavov, I.; Vrancheva, R.; Georgiev, V.; Apostolova, E.; Naimov, S.; Mladenov, R.; Pavlov, A.; Dimitrova-Dyulgerova, I. Phenolic profile, antioxidant and DNA-protective capacity, and microscopic characters of Ailanthus altissima aerial substances. Plants 2023, 12, 920. [Google Scholar] [CrossRef]
- He, Q.; Xiao, H.; Li, J.; Liu, Y.; Jia, M.; Wang, F.; Zhang, Y.; Wang, W.; Wang, S. Fingerprint analysis and pharmacological evaluation of Ailanthus altissima. Int. J. Mol. Med. 2018, 41, 3024–3032. [Google Scholar] [CrossRef] [PubMed]
- Kundu, P.; Laskar, S. A brief resume on the genus Ailanthus: Chemical and pharmacological aspects. Phytochem. Rev. 2010, 9, 379–412. [Google Scholar] [CrossRef]
- Zhang, D.D.; Bai, M.; Yan, Z.Y.; Huang, X.X.; Song, S.J. Chemical constituents from Ailanthus altissima (Mill.) Swingle and chemotaxonomic significance. Biochem. Syst. Ecol. 2020, 93, 104174. [Google Scholar] [CrossRef]
- Kožuharova, E.; Lebanova, H.; Getov, I.; Benbassat, N.; Kochmarov, V. Ailanthus altissima (Mill.) Swingle-a terrible invasive pest in Bulgaria or potential useful medicinal plant. Bothalia J. 2014, 44, 213–230. [Google Scholar]
- Shin, Y.; Masami, I.; Takakiko, T.; Takahashi, T.; Matsushita, K. Constituents of Ailanthus altissima Swingle. Isolation and structures of shinjuglycosides A, B, C, and D. Bull. Chem. Soc. Jpn. 1984, 57, 2496–2501. [Google Scholar]
- Zhao, C.C.; Shao, J.H.; Li, X.; Xu, J.; Zhang, P. Antimicrobial constituents from fruits of Ailanthus altissima Swingle. Arch. Pharm. Res. 2005, 28, 1147–1151. [Google Scholar] [CrossRef]
- Ansari, S.H.; Ali, M. Two new phytosterols from Ailanthus altissima (Mill) Swingle. Acta Hort. 2003, 597, 91–94. [Google Scholar] [CrossRef]
- Ni, J.C.; Shi, J.T.; Tan, Q.W.; Chen, Q.J. Phenylpropionamides, piperidine, and phenolic derivatives from the fruit of Ailanthus altissima. Molecules 2017, 22, 2107. [Google Scholar] [CrossRef]
- Anfossi, L.; Giovannoli, C.; Di Nardo, F.; Cavalera, S.; Chiarello, M.; Trotta, F.; Baggiani, C. Selective enrichment of ailanthone from leaves of Ailanthus altissima by tandem reverse phase/molecularly imprinted solid phase extraction. Microchem. J. 2020, 158, 105198. [Google Scholar] [CrossRef]
- Lee, Y.S. Analysis of components in the different parts of Ailanthus altissima. Korean J. Food Preserv. 2008, 15, 261–268. [Google Scholar]
- Cocîrlea, M.D.; Oancea, S. Impact of invasive plant species on ecosystems, biodiversity loss and their potential industrial applications. Curr. Trends Nat. Sci. 2022, 11, 88–103. [Google Scholar] [CrossRef]
- Ferraz, C.A.; Pastorinho, M.R.; Palmeira-de-Oliveira, A.; Sousa, A.C. Ecotoxicity of plant extracts and essential oils: A review. Environ. Pollut. 2022, 292, 118319. [Google Scholar] [CrossRef]
- Ahmad, Z.; Khan, K.R.; Farooq, M.; Shah, A.H.; Mehmood, A.; Jabeen, T.; Zohra, L. Evaluation of allelopathic potential of agricultural land associated trees on germination attributes of wheat (Triticum aestivum L.). Proc. Int. Acad. Ecol. Environ. Sci. 2020, 10, 38–44. [Google Scholar]
- Albouchi, F.; Hassen, I.; Casabianca, H.; Hosni, K. Phytochemicals, antioxidant, antimicrobial and phytotoxic activities of Ailanthus altissima (Mill.) Swingle leaves. S. Afr. J. Bot. 2013, 87, 164–174. [Google Scholar] [CrossRef]
- Bostan, C.; Borlea, F.; Mihoc, C.; Selesan, M. Ailanthus altissima species invasion on biodiversity caused by potential allelopathy. Res. J. Agric. Sci. 2014, 46, 95–103. [Google Scholar]
- Sladonja, B.; Poljuha, D.; Sušek, M.; Dudaš, S. Herbicidal effect of Ailanthus altissima leaves water extracts on Medicago sativa seeds germination. In Proceedings of the Conference VIVUS: Transmission of Innovations, Knowledge and Practical Experience into Everyday Practice, Naklo, Slovenia, 14–15 November 2014. [Google Scholar]
- Tsao, R.; Romanchuk, F.E.; Peterson, C.J.; Coats, J.R. Plant growth regulatory effect and insecticidal activity of the extracts of the tree of heaven (Ailanthus altissima L.). BMC Ecol. 2002, 2, 1. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, J.G.; Colwell, A.; Sexton, O.J. The ecological impact of allelopathy in Ailanthus altissima (Simaroubaceae). Am. J. Bot. 1991, 78, 948–958. [Google Scholar] [CrossRef]
- Alonso, A.; Vázquez de Aldana, B.R.; Castro-Díez, P.; Medina-Villar, S.; Pérez-Corona, M.E. Effects of leaf litter extracts from four tree species on aquatic invertebrates: An ecotoxicological risk assessment approach. Aquat. Ecol. 2020, 54, 1155–1168. [Google Scholar] [CrossRef]
- Sæbø, I.P.; Bjørås, M.; Franzyk, H.; Helgesen, E.; Booth, J.A. Optimization of the hemolysis assay for the assessment of cytotoxicity. Int. J. Mol. Sci. 2023, 24, 2914. [Google Scholar] [CrossRef] [PubMed]
- Peetla, C.; Stine, A.; Labhasetwar, V. Biophysical interactions with model lipid membranes: Applications in drug discovery and drug delivery. Mol. Pharm. 2009, 6, 1264–1276. [Google Scholar] [CrossRef]
- Ding, H.; Yu, X.; Hang, C.; Gao, K.; Lao, X.; Jia, Y.; Yan, Z. Ailanthone: A novel potential drug for treating human cancer. Oncol. Lett. 2020, 20, 1489–1503. [Google Scholar] [CrossRef] [PubMed]
- Bai, W.; Yang, H.Y.; Jiao, X.Z.; Feng, K.N.; Chen, J.J.; Gao, K. Structurally diverse highly oxygenated triterpenoids from the roots of Ailanthus altissima and their cytotoxicity. J. Nat. Prod. 2018, 81, 1777–1785. [Google Scholar] [CrossRef]
- Mohamed, H.R.; El-Wakil, E.A.; Abdel-Hameed, E.S.S.; El-Hashash, M.M.; Shemis, M. Evaluation of total phenolics, flavonoids, and antioxidant and cytotoxic potential of Ailanthus altissima (Mill.) swingle leaves. J. Rep. Pharm. Sci. 2021, 10, 130–136. [Google Scholar] [CrossRef]
- De Feo, V.; Martino, L.D.; Santoro, A.; Leone, A.; Pizza, C.; Franceschelli, S.; Pascale, M. Antiproliferative effects of tree-of-heaven (Ailanthus altissima Swingle). Phytother. Res. 2005, 19, 226–230. [Google Scholar] [CrossRef]
- Yang, X.L.; Yuan, Y.L.; Zhang, D.M.; Li, F.; Ye, W.C. Shinjulactone O, a new quassinoid from the root bark of Ailanthus altissima. Nat. Prod. Res. 2014, 28, 1432–1437. [Google Scholar] [CrossRef]
- Hwang, Y.J.; Woo, H.I.; Kim, I.H.; Park, D.S.; Kim, J.H.; Om, A.S.; Hwang, K.A. Effect of apoptosis induction of Ailanthus altissima on human lung carcinoma cells. JALS 2011, 45, 91–96. [Google Scholar]
- Rosati, A.; Quaranta, E.; Ammirante, M.; Turco, M.C.; Leone, A.; De Feo, V. Quassinoids can induce mitochondrial membrane depolarisation and caspase 3 activation in human cells. Cell Death Differ. 2004, 11, S216–S218. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, W.J.; Su, C.; Zhang, D.M.; Xu, L.P.; He, R.R.; Wang, L.; Zhang, J.; Zhang, X.Q.; Ye, W.C. Cytotoxic quassinoids from Ailanthus altissima. BMCL 2013, 23, 654–657. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Lu, Y.; Li, H.; Sun, L.; Yang, N.; Zhao, M.; Zhang, M.; Shi, Q. Antitumor activity of the Ailanthus altissima bark phytochemical ailanthone against breast cancer MCF-7 cells. Oncol. Lett. 2018, 15, 6022–6028. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhong, Z.; Ma, M.; Zhao, Y.; Zhang, C.; Qian, Z.; Wang, B. The role played by ailanthone in inhibiting bone metastasis of breast cancer by regulating tumor-bone microenvironment through the RANKL-dependent pathway. Front. Pharmacol. 2023, 13, 1081978. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, Y.; Ma, S.; Zhao, Q.; Wu, J.; Duan, L.; Xie, Y.; Wang, S. Traditional uses, phytochemistry, and pharmacology of Ailanthus altissima (Mill.) Swingle bark: A comprehensive review. J. Ethnopharmacol. 2021, 275, 114121. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhu, L.; Yang, X.; Wei, C.; Chen, C.; He, Y.; Ji, Z. Ailanthone induces G2/M cell cycle arrest and apoptosis of SGC-7901 human gastric cancer cells. Mol. Med. Rep. 2017, 16, 6821–6827. [Google Scholar] [CrossRef] [PubMed]
- Tanasković, S.; Gvozdenac, S.; Kolarov, R.; Bursić, V.; Konstantinović, B.; Prvulović, D. Antifeeding and insecticidal activity of Ailanthus altissima and Morus alba extracts against gipsy moth (Lymantria dispar (L.), Lepidoptera, Lymantridae) larvae under laboratory conditions. J. Entomol. Res. Soc. 2021, 23, 197–212. [Google Scholar] [CrossRef]
- Marinaș, I.C.; Dinu, M.; Ancuceanu, R.; Hovaneț, M.V.; Oprea, E.; Geană, E.I.; Lazăr, V. The phenols content and phytotoxic capacity of various invasive plants. Rom. Biotechnol. Lett. 2018, 23, 13887. [Google Scholar]
- Jităreanu, A.; Ioana-Cezara, C.A.B.A.; Trifan, A.; Pădureanu, S.; Agoroaei, L. Triticum aestivum assay-a useful tool for environmental monitoring and toxicity assessment. Not. Bot. Horti. Agrobo. 2019, 47, 1005–1018. [Google Scholar] [CrossRef]
- Ostroumov, S.A. Toxicity testing of chemicals without use of animals. Russ. J. Gen. Chem. 2016, 86, 2933–2941. [Google Scholar] [CrossRef]
- Wang, W.; Keturi, P.H. Comparative seed germination tests using ten plant species for toxicity assessment of a metal engraving effluent sample. Water Air Soil Pollut. 1990, 52, 369–376. [Google Scholar] [CrossRef]
- Hou, J.; Liu, G.N.; Xue, W.; Fu, W.J.; Liang, B.C.; Liu, X.H. Seed germination, root elongation, root-tip mitosis, and micronucleus induction of five crop plants exposed to chromium in fluvo-aquic soil. Environ. Toxicol. Chem. 2014, 33, 671–676. [Google Scholar] [CrossRef] [PubMed]
- Igrejas, G.; Branlard, G. The importance of wheat. In Wheat Quality for Improving Processing and Human Health; Igrejas, G., Ikeda, T.M., Guzmán, C., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 1–7. ISBN 978-3-030-34162-6. [Google Scholar]
- An, J.; Zhou, Q.; Sun, Y.; Xu, Z. Ecotoxicological effects of typical personal care products on seed germination and seedling development of wheat (Triticum aestivum L.). Chemosphere 2009, 76, 1428–1434. [Google Scholar] [CrossRef] [PubMed]
- Behboudi, F.; Sarvestani, Z.T.; Kassaee, M.Z.; Sanavi, S.A.M.M.; Sorooshzadeh, A. Phytotoxicity of chitosan and SiO2 nanoparticles to seed germination of wheat (Triticum aestivum L.) and barley (Hordeum vulgare L.) plants. Not. Sci. Biol. 2017, 9, 242–249. [Google Scholar] [CrossRef]
- Novak, M.; Novak, N.; Milinović, B. Differences in allelopathic effect of tree of heaven root extracts and isolated ailanthone on test-species. JCEA 2021, 22, 611–622. [Google Scholar] [CrossRef]
- Caser, M.; Demasi, S.; Caldera, F.; Dhakar, N.K.; Trotta, F.; Scariot, V. Activity of Ailanthus altissima (Mill.) swingle extract as a potential bioherbicide for sustainable weed management in horticulture. Agronomy 2020, 10, 965. [Google Scholar] [CrossRef]
- Mahdavikia, F.; Saharkhiz, M.J. Phytotoxic activity of essential oil and water extract of peppermint (Mentha × piperita L. CV. Mitcham). J. Appl. Res. Med. Aromat. Plants 2015, 2, 146–153. [Google Scholar] [CrossRef]
- Lara-Núñez, A.; Sánchez-Nieto, S.; Luisa Anaya, A.; Cruz-Ortega, R. Phytotoxic effects of Sicyos deppei (Cucurbitaceae) in germinating tomato seeds. Physiol. Plant. 2009, 136, 180–192. [Google Scholar] [CrossRef]
- Miranda-Arámbula, M.; Reyes-Chilpa, R.; Anaya L, A.L. Phytotoxic activity of aqueous extracts of ruderal plants and its potential application to tomato crop. Bot. Sci. 2021, 99, 487–498. [Google Scholar] [CrossRef]
- Bellino, A.; Lofrano, G.; Carotenuto, M.; Libralato, G.; Baldantoni, D. Antibiotic effects on seed germination and root development of tomato (Solanum lycopersicum L.). Ecotoxicol. Environ. Saf. 2018, 148, 135–141. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, G.; Li, G.; Ma, R.; Kong, Y.; Yuan, J. Selection of sensitive seeds for evaluation of compost maturity with the seed germination index. Waste Manag. 2021, 136, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Heisey, R.M.; Kish Heisey, T. Herbicidal effects under field conditions of Ailanthus altissima bark extract, which contains ailanthone. Plant Soil. 2023, 256, 85–99. [Google Scholar] [CrossRef]
- Ganea, M.; Vicaș, L.G.; Gligor, O.; Sarac, I.; Onisan, E.; Nagy, C.; Moisa, C.; Ghitea, T.C. Exploring the Therapeutic Efficacy of Parsley (Petroselinum crispum Mill.) as a Functional Food: Implications in Immunological Tolerability, Reduction of Muscle Cramps, and Treatment of Dermatitis. Molecules 2024, 29, 608. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, S.; Hussain, S.; Malik, F. Critique of medicinal conspicuousness of Parsley (Petroselinum crispum): A culinary herb of Mediterranean region. Pak. J. Pharm. Sci. 2014, 27, 193–202. [Google Scholar] [PubMed]
- van Dijk, N.; Konings, J.; van der Spek, R. Effects of Micosat F Granulate in Ocimum basilicum (Basil) and Petroselinum crispum (Parsley). 21 January 2015, pp. 1–17. Available online: https://micosat.nl/wp-content/uploads/2021/07/Effects-of-Micosat-F-granulate-in-Ocimum-basilicum-and-Petroselinum-crispum-3.pdf (accessed on 21 March 2024).
- Dehkourdi, E.H.; Mosavi, M. Effect of anatase nanoparticles (TiO2) on parsley seed germination (Petroselinum crispum) in vitro. Biol. Trace Elem. Res. 2013, 155, 283–286. [Google Scholar] [CrossRef] [PubMed]
- Momeni, K.; Moradi, A.; Mahmoudi, S.; Latif Manesh, H. The effect of biopriming and gibberellin on the quality and germination properties of parsley seed (Petroselinum crispum). Iran. J. Seed Res. 2023, 10, 1–17. [Google Scholar] [CrossRef]
- Rabin, J.; Berkowitz, G.A.; Akers, S.W. Field performance of osmotically primed parsley seed. Hortic. Sci. 1988, 23, 554–555. [Google Scholar] [CrossRef]
- Vidotto, F.; Tesio, F.; Ferrero, A. Allelopathic effects of Ambrosia artemisiifolia L. in the invasive process. Crop Prot. 2013, 54, 161–167. [Google Scholar] [CrossRef]
- Asgharipour, M.R.; Armin, M. Inhibitory effects of Sorghum halepens root and leaf extracts on germination and early seedling growth of widely used medicinal plants. Adv. Environ. Biol. 2010, 4, 316–325. [Google Scholar]
- ASTM E1193–97; Standard Guide for Daphnia Magna Life-Cycle Toxicity Tests. Annual Book of ASTM Standards. American Society for Testing and Materials: Philadelphia, PA, USA, 1997.
- OECD, 2004. Test No. 202: Daphnia sp. Acute Immobilisation Test. Available online: https://doi.org/10.1787/9789264069947-en (accessed on 21 March 2024).
- Regulation (EC) No 1907/2006 of the European Parliament and of the Council of 18 December 2006 Concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH), Establishing a European Chemicals Agency, Amending Directive 1999/45/EC and Repealing Council Regulation (EEC) No 793/93 and Commission Regulation (EC) No 1488/94 as Well as Council Directive 76/769/EEC and Commission Directives 91/155/EEC, 93/67/EEC, 93/105/EC and 2000/21/EC. Available online: https://eur-lex.europa.eu/legal-content/RO/TXT/HTML/?uri=CELEX:32006R1907 (accessed on 21 March 2024).
- Weber, C.I. Methods for Measuring the Acute Toxicity of Effluents and Receiving Waters to Freshwater and Marine Organisms, 4th ed.; US-Environmental Protection Agency: Cincinnati, OH, USA, 1993; pp. 117–254.
- Meyer, B.; Ferrigni, N.R.; Putnam, J.E.; Jacobsen, L.B.; Nichols, D.E.; McLaughlin, J.L. Brine Shrimp: A Convinient General Bioassay for Active Plant Constituents. J. Med. Plant Res. 1982, 45, 31–34. [Google Scholar] [CrossRef] [PubMed]
- Persoone, G.; Wells, P.G. Artemia in aquatic toxicology: A review. In Artemia Research and Its Applications. Morphology, Genetics, Strain Characterization Toxicology; Sorgeloos, P., Ed.; Universita Press: Wetteren, Belgium, 1987; pp. 259–275. [Google Scholar]
- Salawu, K.M.; Ajaiyeoba, E.O.; Ogbole, O.O.; Adeniji, J.A.; Faleye, T.C.; Agunu, A. Antioxidant, brine shrimp lethality, and antiproliferative properties of gel and leaf extracts of Aloe schweinfurthii and Aloe vera. J. Herbs Spices Med. Plants 2017, 23, 263–271. [Google Scholar] [CrossRef]
- McLaughlin, J.L.; Rogers, L.L.; Anderson, J.E. The Use of Biological Assays to Evaluate Botanicals. Drug Inf. J. 1998, 32, 513–524. [Google Scholar] [CrossRef]
- Manure, J.Y. Evaluation of anticancer activity of leaves of Rumex vesicarius Linn and Symplocos racemosa Roxb. by brine shrimp lethality and (3-(4,5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide) methods. IJGP 2017, 11. [Google Scholar] [CrossRef]
- Ferraz Filha, Z.S.; Lombardi, J.A.; Guzzo, L.S. Brine shrimp (Artemia salina Leach) bioassay of extracts from Lychnophoriopsis candelabrum and different Lychnophora species. RBPM 2012, 14, 358–361. [Google Scholar] [CrossRef]
- Jeyaratnam, N.; Nour, A.H.; Kanthasamy, R.; Nour, A.H.; Yuvaraj, A.R.; Akindoyo, J.O. Essential oil from Cinnamomum cassia bark through hydrodistillation and advanced microwave assisted hydrodistillation. Ind. Crops Prod. 2016, 92, 57–66. [Google Scholar] [CrossRef]
- Miller, L.C.; Tainter, M. Estimation of the ED50 and its error by means of logarithmic-probit graph paper. Proc. Soc. Exp. Biol. Med. 1944, 57, 261–264. [Google Scholar] [CrossRef]
- Randhawa, M.A. Calculation of LD50 values from the method of Miller and Tainter, 1944. J. Ayub. Med. Coll. Abbottabad 2009, 21, 184–185. [Google Scholar] [PubMed]
- Clarkson, C.; Maharaj, V.J.; Crouch, N.R.; Grace, O.M.; Pillay, P.; Matsabisa, M.G.; Bhagwandin, N.; Smith, P.J.; Folb, P.I. In vitro antiplasmodial activity of medicinal plants native to or naturalised in South Africa. J. Ethnopharmacol. 2004, 92, 177–191. [Google Scholar] [CrossRef]
- Carballo, J.L.; Hernández-Inda, Z.L.; Pérez, P.; García-Grávalos, M.D. A comparison between two brine shrimp assays to detect in vitrocytotoxicity in marine natural products. BMC Biotechnol. 2002, 2, 17. [Google Scholar] [CrossRef]
- Krishnaraju, A.V.; Rao, T.V.; Sundararaju, D.; Vanisree, M.; Tsay, H.S.; Subbaraju, G.V. Biological screening of medicinal plants collected from Eastern Ghats of India using Artemia salina (brine shrimp test). IJASE 2006, 4, 115–125. [Google Scholar] [CrossRef]
- Šturm, L.; Poklar Ulrih, N. Basic methods for preparation of liposomes and studying their interactions with different compounds, with the emphasis on polyphenols. Int. J. Mol. Sci. 2021, 22, 6547. [Google Scholar] [CrossRef] [PubMed]
- Loganayaki, N.; Siddhuraju, P.; Manian, S. Antioxidant activity and free radical scavenging capacity of phenolic extracts from Helicteres isora L. and Ceiba pentandra L. J. Food Sci. Technol. 2013, 50, 687–695. [Google Scholar] [CrossRef]
- Ran, Q.; Xiang, Y.; Liu, Y.; Xiang, L.; Li, F.; Deng, X.; Xiao, Y.; Chen, L.; Chen, L.; Li, Z. Eryptosis indices as a novel predictive parameter for biocompatibility of Fe3O4 magnetic nanoparticles on erythrocytes. Sci. Rep. 2015, 5, 16209. [Google Scholar] [CrossRef]
- Greco, I.; Molchanova, N.; Holmedal, E.; Jenssen, H.; Hummel, B.D.; Watts, J.L.; Håkansson, J.; Hansen, P.R.; Svenson, J. Correlation between hemolytic activity, cytotoxicity and systemic in vivo toxicity of synthetic antimicrobial peptides. Sci. Rep. 2020, 10, 13206. [Google Scholar] [CrossRef]
- Cuervo-Rodríguez, R.; Muñoz-Bonilla, A.; López-Fabal, F.; Fernández-García, M. Hemolytic and antimicrobial activities of a series of cationic amphiphilic copolymers comprised of same centered comonomers with thiazole moieties and polyethylene glycol derivatives. Polymers 2020, 12, 972. [Google Scholar] [CrossRef] [PubMed]
- ASTM. Standard Test Method for Analysis of Hemolytic Properties of Nanoparticles; ASTM: West Conshohocken, PA, USA, 2013. [Google Scholar]
- Silva, A.C.; de Moraes, D.C.; do Carmo, D.C.; Gomes, G.C.C.; Ganesan, A.; Lopes, R.S.C.; Ferreira-Pereira, A.; Lopes, C.C. Synthesis of altissimacoumarin D and other prenylated coumarins and their ability to reverse the multidrug resistance phenotype in Candida albicans. J. Fungi. 2023, 9, 758. [Google Scholar] [CrossRef] [PubMed]
- Paiva-Martins, F.; Fernandes, J.; Santos, V.; Silva, L.; Borges, F.; Rocha, S.; Belo, L.; Santos-Silva, A. Powerful protective role of 3, 4-dihydroxyphenylethanol− elenolic acid dialdehyde against erythrocyte oxidative-induced hemolysis. J. Agric. Food Chem. 2010, 58, 135–140. [Google Scholar] [CrossRef]
- Bashir, A.; Farooq, S.; Bhat, Z.A. Pharmacognostic standardization and phytochemical evaluation of Ailanthus altissima (Mill.) Swingle leaves. JDDT 2019, 9, 179–183. [Google Scholar]
- Djeridane, A.; Yousfi, M.; Nadjemi, B.; Vidal, N.; Lesgards, J.F.; Stocker, P. Screening of some Algerian medicinal plants for the phenolic compounds and their antioxidant activity. Eur. Food Res. Technol. 2007, 224, 801–809. [Google Scholar] [CrossRef]
- Olchowik-Grabarek, E.; Swiecicka, I.; Andreeva-Kovaleskaya, Z.; Solonin, A.; Bonarska-Kujawa, D.; Kleszczyńska, H.; Mavlyanov, S.; Zamaraeva, M. Role of structural changes induced in biological membranes by hydrolysable tannins from sumac leaves (Rhus typhina L.) in their antihemolytic and antibacterial effects. J. Membr. Biol. 2014, 247, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.H.; Duan, Z.K.; Ma, Z.T.; Ye, L.; Yao, G.D.; Huang, X.X.; Song, S.J. Chouchunsteride A–D, four new steroids from the leaves of Ailanthus altissima (Mill.) Swingle. Steroids 2022, 188, 109117. [Google Scholar] [CrossRef]
- Salgado, M.T.S.F.; Fernandes e Silva, E.; Nascimento, M.A.D.; Lopes, A.C.; Paiva, L.S.D.; Votto, A.P.D.S. Potential Therapeutic Targets of Quercetin in the Cutaneous Melanoma Model and Its Cellular Regulation Pathways: A Systematic Review. Nutr. Cancer 2023, 75, 1687–1709. [Google Scholar] [CrossRef]
- Morita, S.; Sakai, C.; Sakamoto, M.; Nishimoto, M. Evaluating pesticide ecotoxicity using a stimuli-response model in liposomes. JCIS Open 2023, 10, 100082. [Google Scholar] [CrossRef]
- Silva, D.R.; Cabello, M.C.; Severino, D.; Baptista, M.D.S. Performance of cosmetic ingredients evaluated by their membrane protection efficiency. JCDSA 2021, 11, 169–185. [Google Scholar] [CrossRef]
- Rodrigues, D.; Viotto, A.C.; Checchia, R.; Gomide, A.; Severino, D.; Itri, R.; Baptista, M.S.; Martins, W.K. Mechanism of Aloe Vera extract protection against UVA: Shelter of lysosomal membrane avoids photodamage. PPS 2016, 15, 334–350. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.R.; Park, Y.; Li, M.; Kim, Y.K.; Lee, S.; Son, S.Y.; Lee, J.S.; Lee, C.H.; Park, H.H.; Lee, J.Y.; et al. Anti-inflammatory effect of Ailanthus altissima (Mill.) Swingle leaves in lipopolysaccharide-stimulated astrocytes. J. Ethnopharmacol. 2022, 286, 114258. [Google Scholar] [CrossRef] [PubMed]
- Luís, Â.; Gil, N.; Amaral, M.E.; Domingues, F.; Duartea, A.P. Ailanthus altissima (Miller) Swingle: A source of bioactive compounds with antioxidant activity. BioResources 2012, 7, 2105–2120. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolyb-dic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Price, M.L.; Van Scoyoc, S.; Butler, L.G. A critical evaluation of the vanillin reaction as an assay for tannin in sorghum grain. Agric. Food Chem. 1978, 26, 1214–1218. [Google Scholar] [CrossRef]
- Scott, K.J. Detection and measurement of carotenoids by UV/VIS spectrophotometry. Curr. Protoc. Food Anal. Chem. 2001, 1, F2.2.1–F2.2.10. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Lindsey, B.E., III; Rivero, L.; Calhoun, C.S.; Grotewold, E.; Brkljacic, J. Standardized method for high-throughput sterilization of Arabidopsis seeds. JoVE 2017, 128, e56587. [Google Scholar] [CrossRef] [PubMed]
- Dal Cortivo, C.; Barion, G.; Visioli, G.; Mattarozzi, M.; Mosca, G.; Vamerali, T. Increased root growth and nitrogen accumulation in common wheat following PGPR inoculation: Assessment of plant-microbe interactions by ESEM. Agric. Ecosyst. Environ. 2017, 247, 396–408. [Google Scholar] [CrossRef]
- Veisz, O.; Galiba, G.; Sutka, J. Effect of abscisic acid on the cold hardiness of wheat seedlings. J. Plant Physiol. 1996, 149, 439–443. [Google Scholar] [CrossRef]
- Amooaghaie, R.; Tabatabaei, F.; Ahadi, A.M. Role of hematin and sodium nitroprusside in regulating Brassica nigra seed germination under nanosilver and silver nitrate stresses. Ecotoxicol. Environ. Saf. 2015, 113, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Cudalbeanu, M.; Furdui, B.; Cârâc, G.; Barbu, V.; Iancu, A.V.; Marques, F.; Leitão, J.H.; Sousa, S.A.; Dinica, R.M. Antifungal, antitumoral and antioxidant potential of the Danube Delta Nymphaea alba extracts. Antibiotics 2019, 9, 7. [Google Scholar] [CrossRef] [PubMed]
- González-Feijoo, R.; Rodríguez-Seijo, A.; Fernández-Calviño, D.; Arias-Estévez, M.; Arenas-Lago, D. Use of three different nanoparticles to reduce Cd availability in soils: Effects on germination and early growth of Sinapis alba L. Plants 2023, 12, 801. [Google Scholar] [CrossRef] [PubMed]
- Gopinathan, M.; Thirumurthy, M. Evaluation of phytotoxicity for compost from organic fraction of municipal solid waste and paper & pulp mill sludge. EREM 2012, 59, 47–51. [Google Scholar]
- Luo, Y.; Liang, J.; Zeng, G.; Chen, M.; Mo, D.; Li, G.; Zhang, D. Seed germination test for toxicity evaluation of compost: Its roles, problems and prospects. Waste Manag. 2018, 71, 109–114. [Google Scholar] [CrossRef]
- Ugolini, F.; Crisci, A.; Albanese, L.; Cencetti, G.; Maienza, A.; Michelozzi, M.; Zabini, F.; Meneguzzo, F. Effects of Silver Fir (Abies alba Mill.) needle extract produced via hydrodynamic cavitation on seed germination. Plants 2021, 10, 1399. [Google Scholar] [CrossRef] [PubMed]
- Apetroaei, M.R.; Pădureţu, C.; Rău, I.; Schroder, V. New-chitosan characterization and its bioassay in different salinity solutions using Artemia salina as bio tester. Chem. Pap. 2018, 72, 1853–1860. [Google Scholar] [CrossRef]
- Nerdy, N.; Lestari, P.; Sinaga, J.P.; Ginting, S.; Zebua, N.F.; Mierza, V.; Bakri, T.K. Brine shrimp (Artemia salina Leach.) lethality test of ethanolic extract from green betel (Piper betle Linn.) and red betel (Piper crocatum Ruiz and Pav.) through the soxhletation method for cytotoxicity test. Open Access Maced. J. Med. Sci. 2021, 9, 407–412. [Google Scholar] [CrossRef]
- Manfra, L.; Savorelli, F.; Pisapia, M.; Magaletti, E.; Cicero, A.M. Long-term lethal toxicity test with the crustacean Artemia franciscana. JoVE 2012, 62, e3790. [Google Scholar] [CrossRef] [PubMed]
- Lam, P.H.; Le, M.T.; Dang, D.M.T.; Doan, T.C.D.; Tu, N.P.C.; Dang, C.M. Safe concentration of silver nanoparticles in solution for white leg shrimp (Litopeaneus vannamei) farming. Biol. Chem. Res. 2020, 7, 35–45. [Google Scholar]
- Pohan, D.J.; Marantuan, R.S.; Djojosaputro, M. Toxicity test of strong drug using the BSLT (brine shrimp lethality test) method. IJHSR 2023, 13, 203–209. [Google Scholar] [CrossRef]
- Nigar, S.; Gupta, N.; Trivedi, A.; Gupta, V. Lead nitrate induced acute toxicity in the freshwater fishes Channa punctatus and Heteropneustes fossilis. Appl. Ecol. Environ. Sci. 2021, 9, 735–743. [Google Scholar]
- Yamada, K.; Natori, S. Characterization of the antimicrobial peptide derived from sapecin B, an antibacterial protein of Sarcophaga peregrina (flesh fly). Biochem. J. 1994, 298, 623–628. [Google Scholar] [CrossRef]
- El Hajji, M.; Rebuffat, S.; Le Doan, T.; Klein, G.; Satre, M.; Bodo, B. Interaction of trichorzianines A and B with model membranes and with the amoeba Dictyostelium. BBA-Biomembranes 1989, 978, 97–104. [Google Scholar] [CrossRef]
- R Core Development Team. R: A Language and Environment for Statistical Computing, Version 4.3.1; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: http://www.r-project.org (accessed on 30 September 2023).
- Dinno, A. _dunn.test: Dunn’s Test of Multiple Comparisons Using Rank Sums_. R Package Version 1.3.5. 2017. Available online: https://CRAN.R-project.org/package=dunn.test (accessed on 27 January 2024).
- Harrell, F., Jr. _Hmisc: Harrell Miscellaneous_. R Package Version 5.1-0. 2023. Available online: https://CRAN.R-project.org/package=Hmisc (accessed on 27 January 2024).
- Kassambara, A. _ggpubr: ‘ggplot2’ Based Publication Ready Plots_. R Package Version 0.6.0. 2023. Available online: https://CRAN.R-project.org/package=ggpubr (accessed on 27 January 2024).
- Wei, T.; Simko, V.R. R Package ‘Corrplot’: Visualization of a Correlation Matrix (Version 0.92). 2021. Available online: https://github.com/taiyun/corrplot (accessed on 27 January 2024).
- Finney, D.J. Probit Analysis; Cambridge University Press: Cambridge, UK, 1971. [Google Scholar]
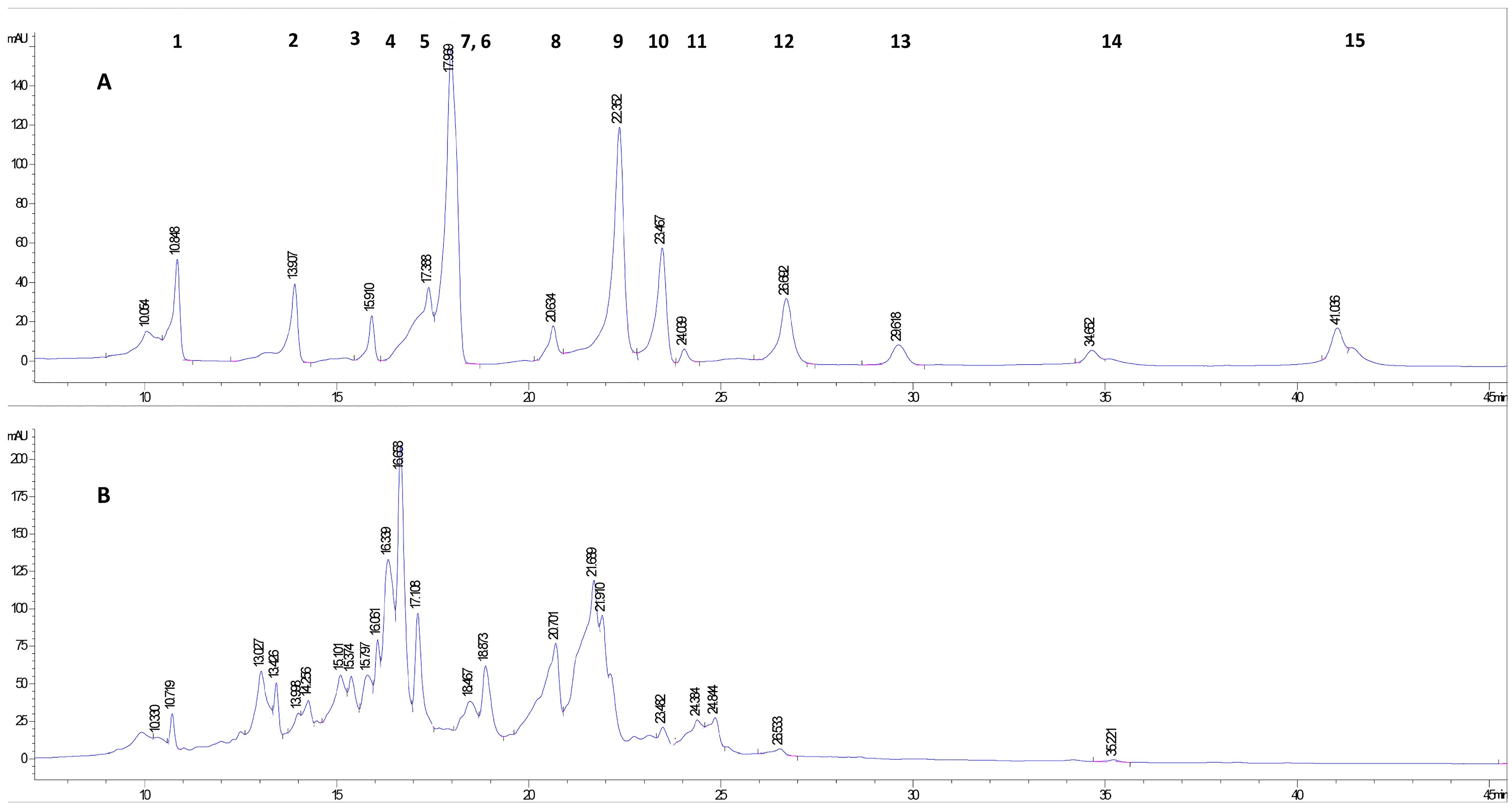




| Polyphenols | RT (min) | ||
|---|---|---|---|
| Polyphenols as Individual Run | Polyphenols in Mixture Run | A. altissima Leaf Extract | |
| Gallic acid | 10.97 | 10.84 | 10.71 |
| Protocatechuic acid | 13.98 | 13.9 | 13.99 |
| Catechin | 15.63 | 15.91 | 15.79 |
| Vanillic acid | 16.74 | overlapped with epicatechin | 16.65 |
| Epicatechin | 17.28 | 17.38 | - |
| Caffeic acid | 17.49 | 17.96 | - |
| Syringic acid | 18.09 | overlapped with caffeic acid | - |
| Rutin | 20.42 | 20.63 | 20.7 |
| Ferulic acid | 22.38 | 22.35 | - |
| p-Coumaric acid | 23.1 | 23.46 | 23.48 |
| Hesperidin | 24.01 | 24.03 | 24.38 |
| Rosmarinic acid | 26.33 | 26.69 | 26.53 |
| Salicylic acid | 29.31 | 29.61 | - |
| Quercetin | 34.72 | 34.65 | 35.22 |
| Kaempferol | 39.71 | 41.0 | - |
| Physiological Parameters * | Leaf Extract Concentration (μg/mL) | Control | |||
|---|---|---|---|---|---|
| 125 | 250 | 500 | 1000 | ||
| Eg (%) | 62.00 ± 0.27 | 42.00 ± 0.19 | 40.00 ± 0.16 | 60.00 ± 0.18 | 48.00 ± 0.18 |
| G (%) | 86.00 ± 0.15 | 76.00 ± 0.13 | 68.00 ± 0.24 | 58.00 ± 0.17 | 72.00 ± 0.19 |
| RL (cm) | 7.89 ± 2.53 | 6.17 ±1.66 | 5.57 ± 1.65 | 5.10 ± 0.44 | 6.94 ± 1.46 |
| SL (cm) | 8.68 ± 1.76 | 7.68 ± 1.25 | 7.68 ± 2.39 | 8.91 ± 1.27 | 7.89 ± 1.65 |
| RRG (%) | 121.00 ± 0.54 | 91.00 ± 0.29 | 82.00 ± 0.25 | 71.00 ± 0.15 | 100.00 ± 0.00 |
| RSG (%) | 131.00 ± 0.57 | 121.00 ± 0.73 | 107.00 ± 0.62 | 72.00 ± 0.21 | 100.00 ± 0.00 |
| GI (%) | 108.03 ± 0.54 | 72.25 ± 0.39 | 57.48 ± 0.28 | 40.77 ± 0.13 | 72.00 ± 0.19 |
| VI (%) | 78.21 ± 0.23 | 61.59 ± 0.18 | 51.46 ± 0.27 | 33.14 ± 0.10 | 67.02 ± 0.27 |
| PI (%) | 152.00 ± 0.82 | 125.00 ± 0.87 | 113.00 ± 0.78 | 89.00 ± 0.45 | 100.00 ± 0.00 |
| Physiological Parameters * | Leaf Extract Concentration (μg/mL) | Control | |
|---|---|---|---|
| 125 | 1000 | ||
| Eg (%) | 66.00 ± 0.05 | 60.00 ± 0.10 | 56.00 ± 0.05 |
| G (%) | 88.00 ± 0.13 | 84.00 ± 0.15 | 92.00 ± 0.08 |
| RL (cm) | 3.77 ± 1.05 | 1.12 ± 0.45 | 3.72 ± 0.69 |
| SL (cm) | 2.46 ± 0.47 | 2.14 ± 0.48 | 2.29 ± 0.43 |
| RRG (%) | 107.87 ± 0.45 | 32.12 ± 0.19 | 100.00 ± 0.00 |
| RSG (%) | 96.28 ± 0.17 | 91.56 ± 0.16 | 100.00 ± 0.00 |
| GI (%) | 96.25 ± 0.45 | 26.14 ± 0.14 | 92.00 ± 0.08 |
| VI (%) | 133.64 ± 0.27 | 44.01 ± 0.14 | 149.96 ± 0.15 |
| PI (%) | 111.64 ± 0.51 | 87.96 ± 0.34 | 100.00 ± 0.00 |
| Physiological Parameters * | Leaf Extract Concentration (μg/mL) | Control | |
|---|---|---|---|
| 125 | 1000 | ||
| G (%) | 52.50 ± 0.05 | 50.00 ± 0.17 | 56.00 ± 0.11 |
| RL (cm) | 1.05 ± 0.18 | 1.24 ± 0.66 | 1.47 ± 0.55 |
| SL (cm) | 0.77 ± 0.25 | 0.85 ± 0.65 | 0.90 ± 0.22 |
| RRG (%) | 71.52 ± 0.31 | 80.65 ± 0.44 | 100.00 ± 0.00 |
| RSG (%) | 88.01 ± 0.08 | 86.67 ± 0.23 | 100.00 ± 0.00 |
| GI (%) | 37.46 ± 0.16 | 43.19 ± 0.32 | 56.00 ± 0.11 |
| VI (%) | 73.94 ± 0.12 | 81.54 ± 0.44 | 92.64 ± 0.36 |
| PI (%) | 78.90 ± 0.43 | 84.36 ± 0.66 | 100.00 ± 0.00 |
| Concentration (μg/mL) | Concentration Logs (X) | Number of Organisms | Mortality Rate (%) | Probit (Y) | Probit (Y) Corrected * | LC50 (μg/mL) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| 24 h | 48 h | 24 h | 48 h | 24 h | 48 h | 24 h | 48 h | |||
| 250 | 2.40 | 10 | 0 | 17.00 ± 0.05 | 2.60 | 4.05 | 3.04 * | 4.05 | 951.04 ± 28.26 | 420.65 ± 8.56 |
| 500 | 2.70 | 10 | 0 | 57.00 ± 0.05 | 2.60 | 5.18 | 3.04 * | 5.18 | ||
| 1000 | 3.00 | 10 | 37.00 ± 0.06 | 100.00 ± 0.00 | 4.67 | 7.40 | 4.67 | 6.96 * | ||
| 2000 | 3.30 | 10 | 100.00 ± 0.00 | 100.00 ± 0.00 | 7.40 | 7.40 | 6.96 * | 6.96 * | ||
| % B | 1 | 5 | 20 | 45 | 70 | 100 | 1 | 1 |
| Time | 0 | 2 | 10 | 40 | 55 | 75 | 80 | 90 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cocîrlea, M.D.; Simionescu, N.; Petrovici, A.R.; Silion, M.; Biondi, B.; Lastella, L.; Oancea, S. In Vitro Screening of Ecotoxic and Cytotoxic Activities of Ailanthus altissima Leaf Extract against Target and Non-Target Plant and Animal Cells. Int. J. Mol. Sci. 2024, 25, 5653. https://doi.org/10.3390/ijms25115653
Cocîrlea MD, Simionescu N, Petrovici AR, Silion M, Biondi B, Lastella L, Oancea S. In Vitro Screening of Ecotoxic and Cytotoxic Activities of Ailanthus altissima Leaf Extract against Target and Non-Target Plant and Animal Cells. International Journal of Molecular Sciences. 2024; 25(11):5653. https://doi.org/10.3390/ijms25115653
Chicago/Turabian StyleCocîrlea, Maria Denisa, Natalia Simionescu, Anca Roxana Petrovici, Mihaela Silion, Barbara Biondi, Luana Lastella, and Simona Oancea. 2024. "In Vitro Screening of Ecotoxic and Cytotoxic Activities of Ailanthus altissima Leaf Extract against Target and Non-Target Plant and Animal Cells" International Journal of Molecular Sciences 25, no. 11: 5653. https://doi.org/10.3390/ijms25115653
APA StyleCocîrlea, M. D., Simionescu, N., Petrovici, A. R., Silion, M., Biondi, B., Lastella, L., & Oancea, S. (2024). In Vitro Screening of Ecotoxic and Cytotoxic Activities of Ailanthus altissima Leaf Extract against Target and Non-Target Plant and Animal Cells. International Journal of Molecular Sciences, 25(11), 5653. https://doi.org/10.3390/ijms25115653








