Structural and Interactional Analysis of the Flavonoid Pathway Proteins: Chalcone Synthase, Chalcone Isomerase and Chalcone Isomerase-like Protein
Abstract
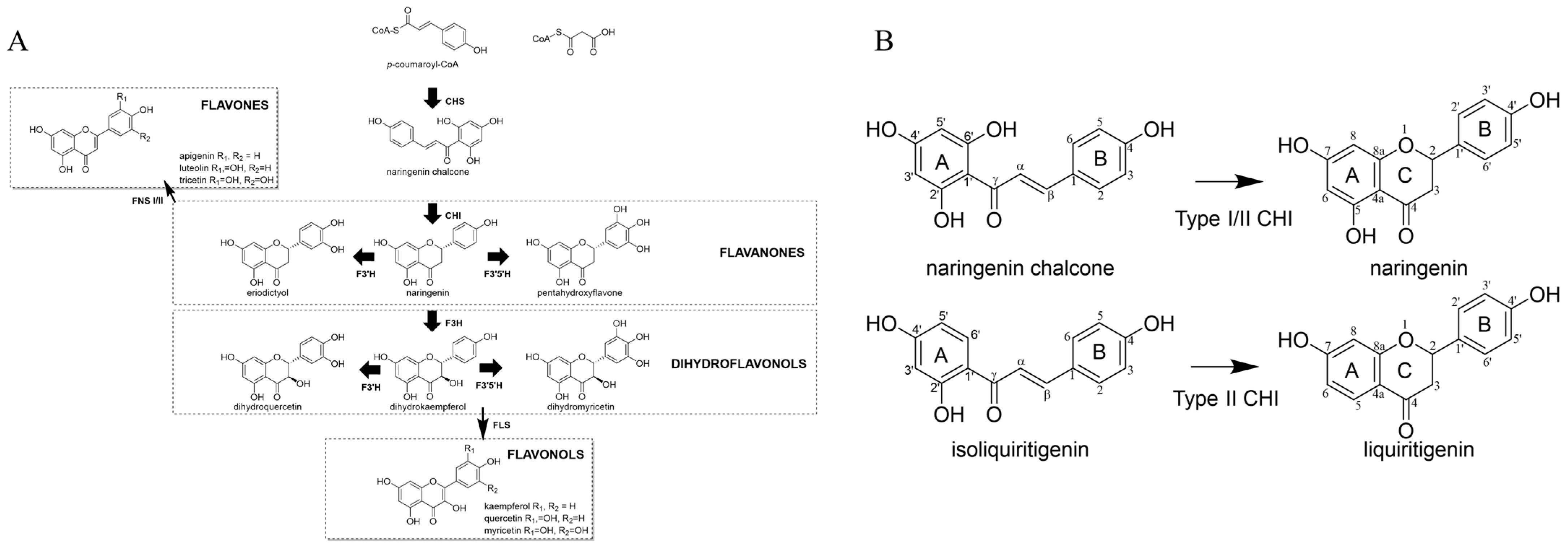
1. Introduction
2. Results
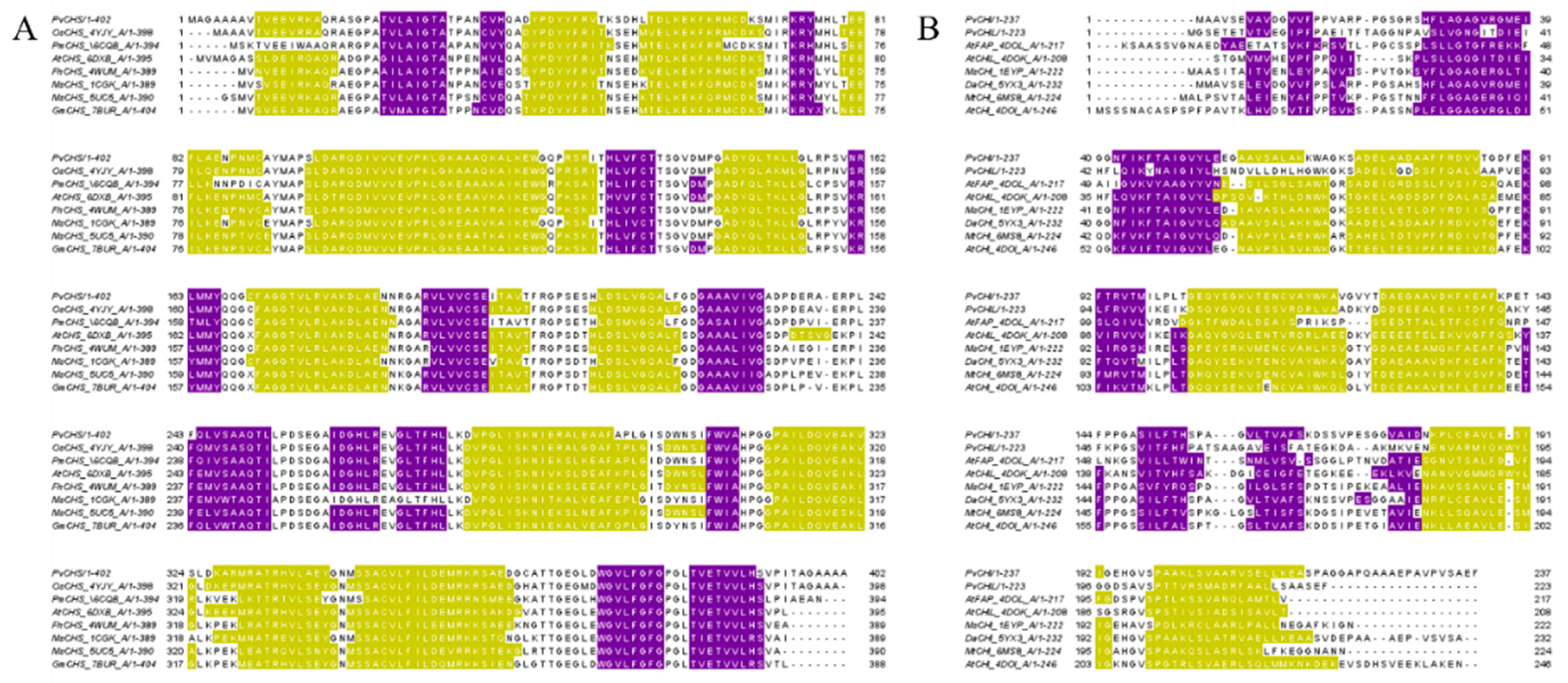
2.1. Overall Structure of PvCHS
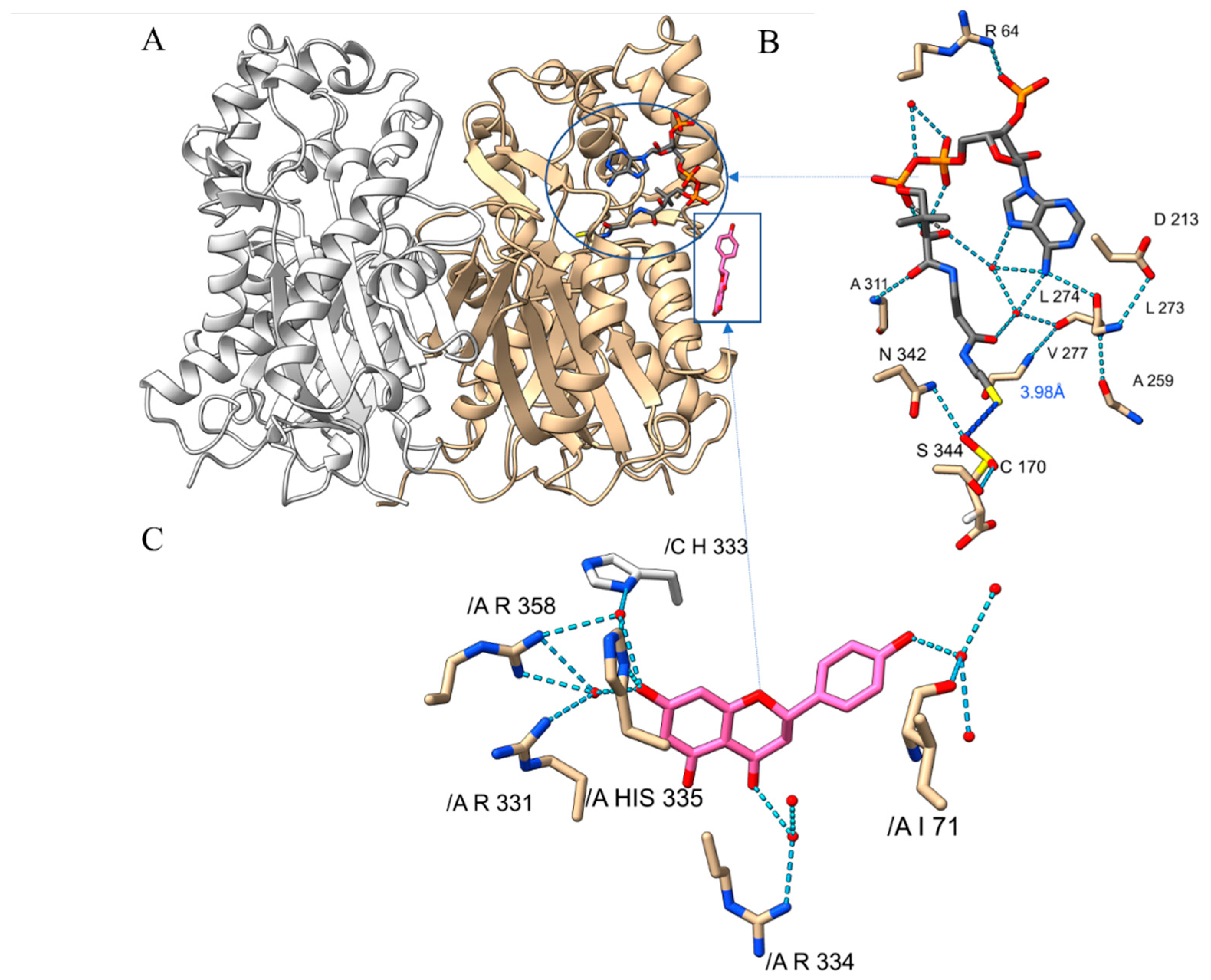
2.2. Substrate-Binding Pocket of PvCHS
2.3. Naringenin-Binding of PvCHS
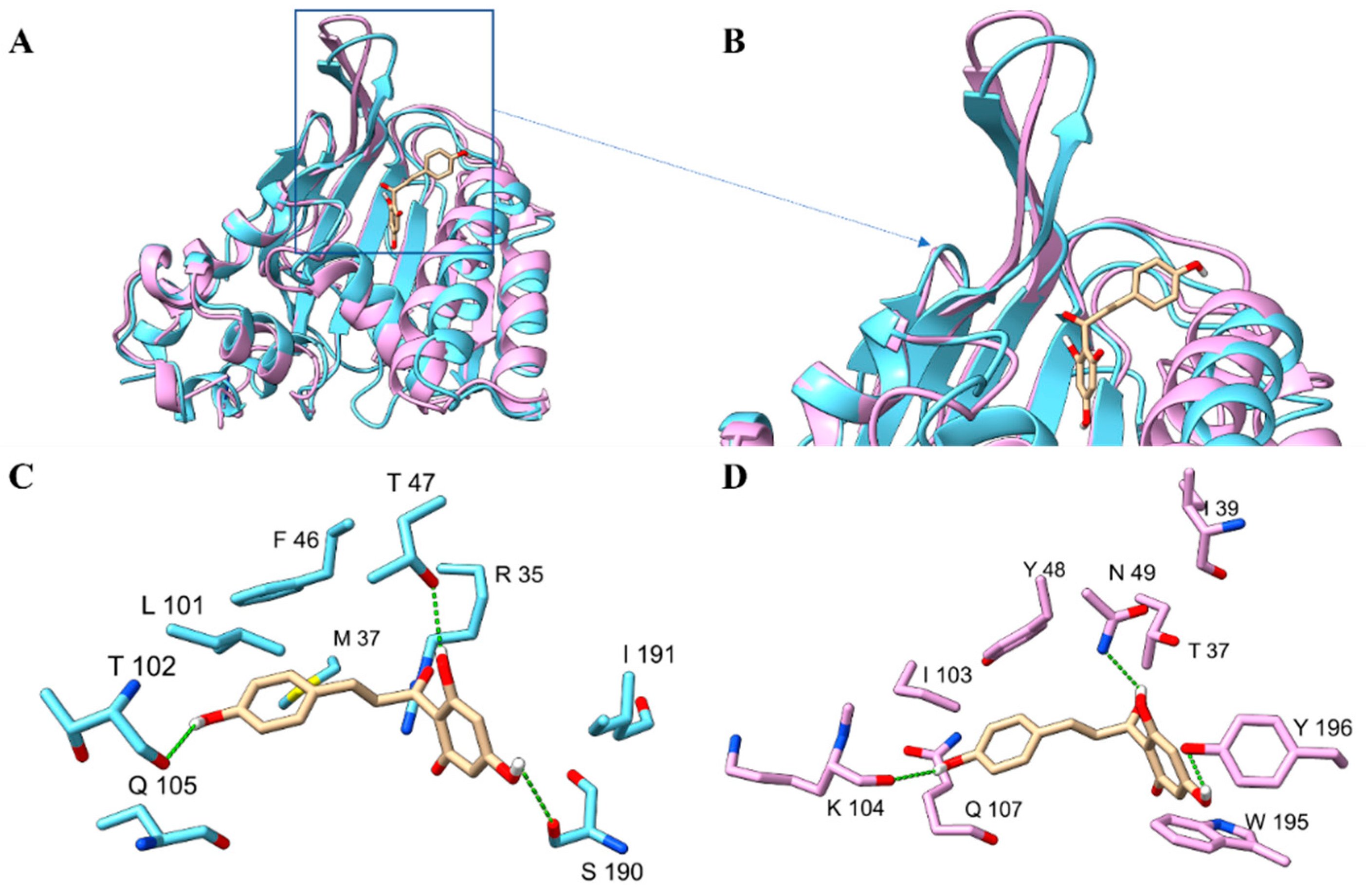
2.4. Overall Structure of PvCHI and SbCHI
2.5. The Substrate-Binding Pocket of SbCHI
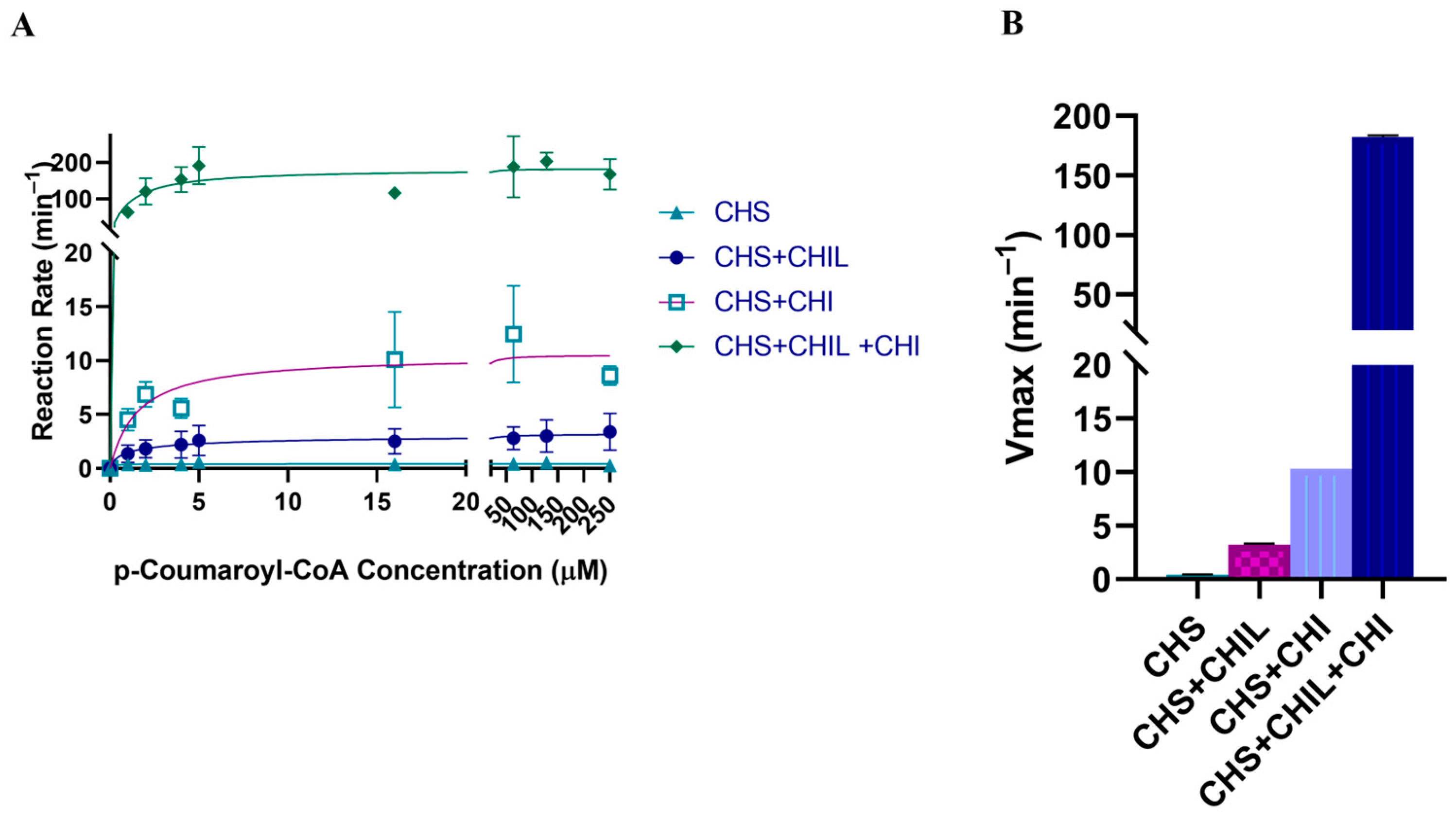
2.6. Activity of CHS with and without CHIL
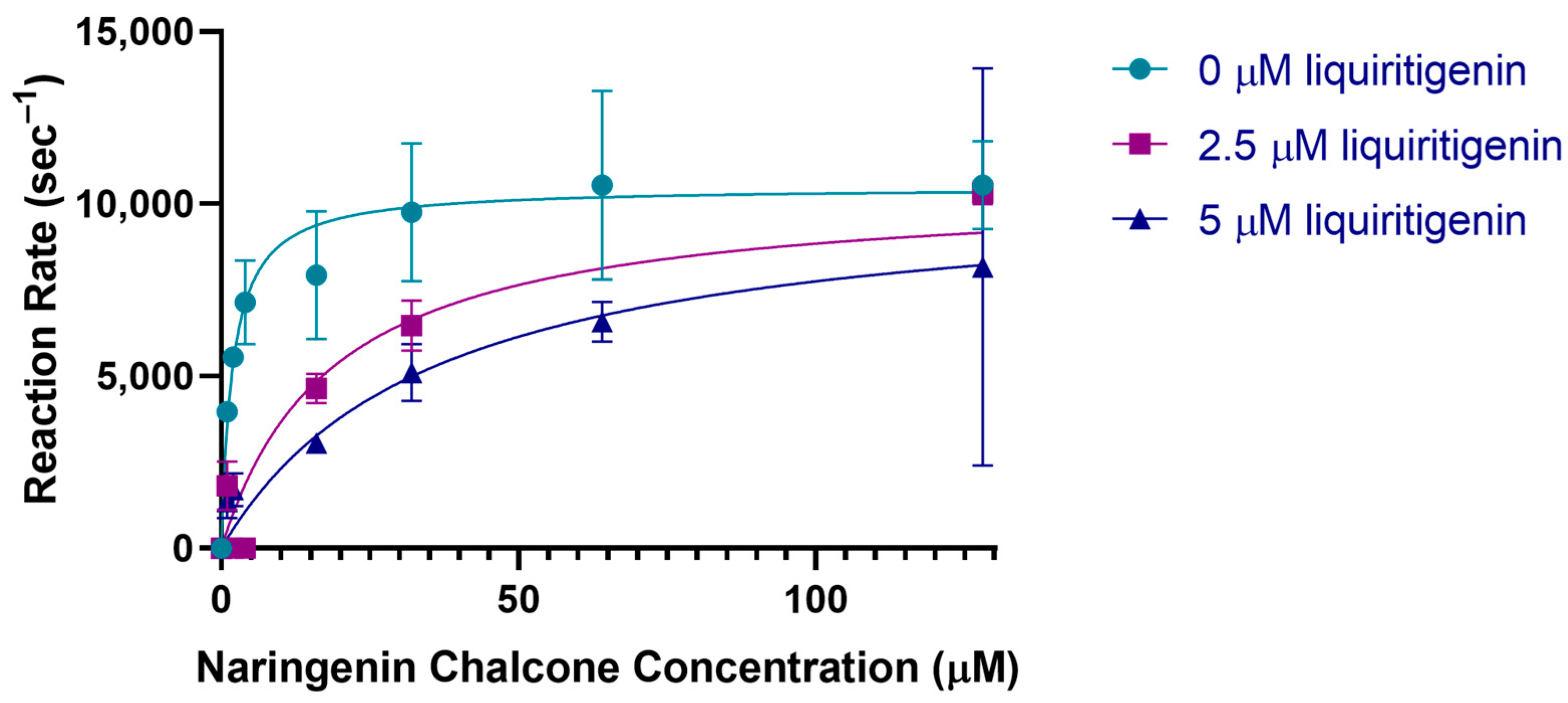
2.7. Activity of CHI
2.8. Activity of CHS with CHI and CHIL
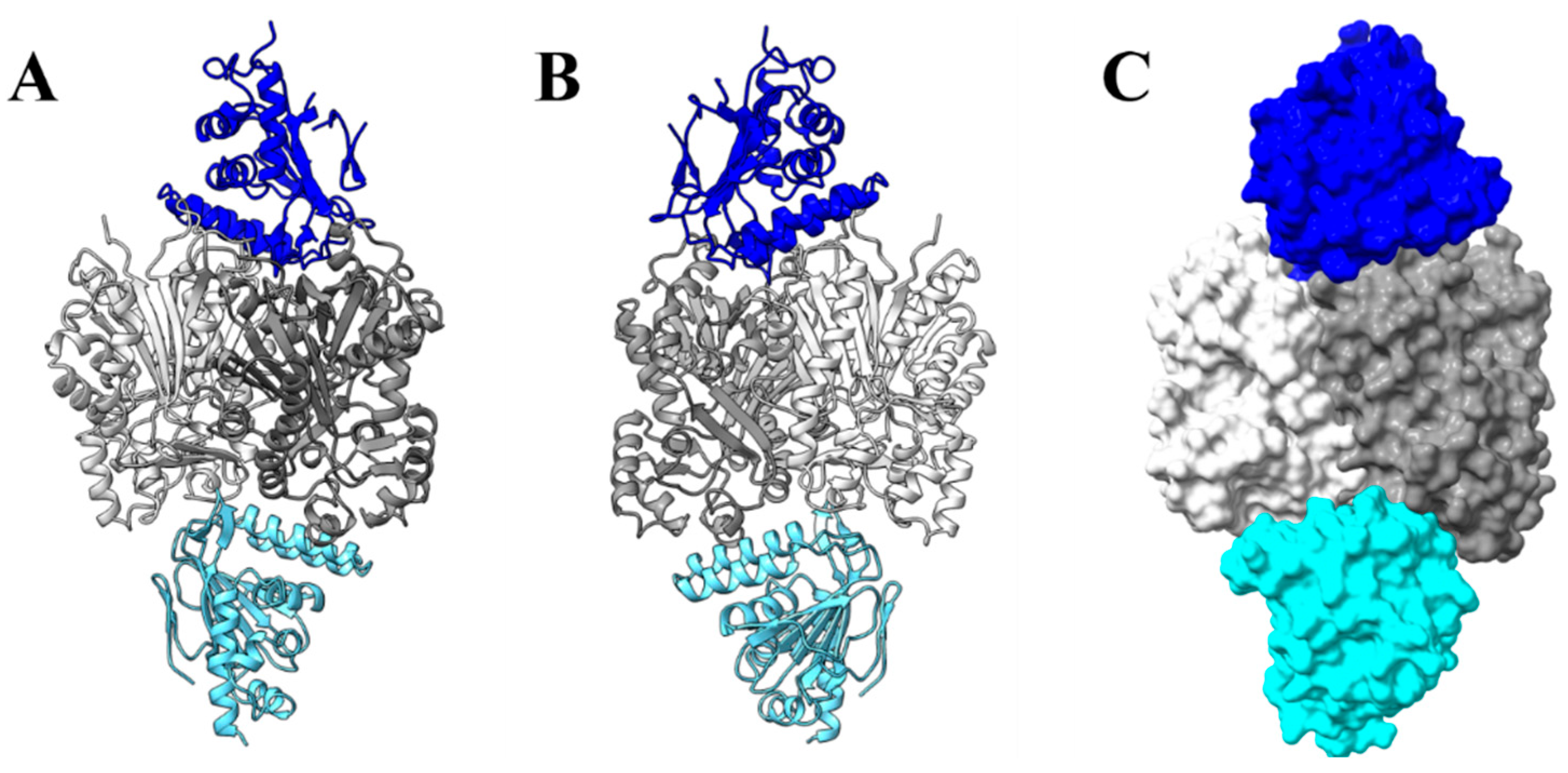
2.9. Analysis of Interactions among CHS, CHI, and CHIL
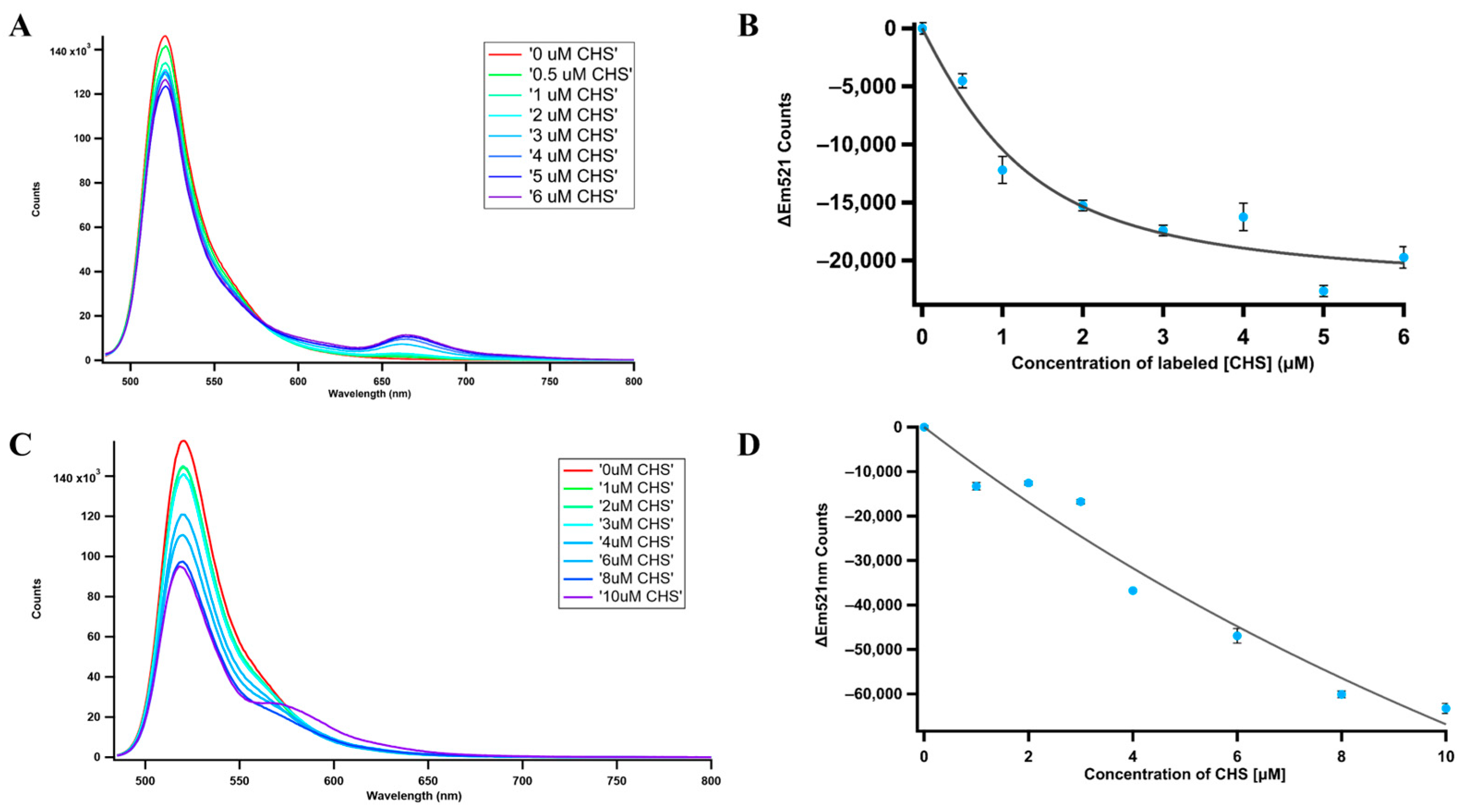
2.10. Isothermal Titration Calorimetry
2.11. Fluorescence Resonance Energy Transfer (FRET)
2.12. Substrate Molecular Docking
2.13. Molecular Docking between PvCHIL and CHS, PvCHI, and PvCHS
3. Discussion
3.1. Catalytic and Inhibition Mechanisms of PvCHS
3.2. Binding and Catalytic Mechanism of PvCHI
3.3. Type II Activity of PvCHI
3.4. Affinity and Effect of CHIL/CHI on the CHS
4. Materials and Methods
4.1. Chemicals and Software
4.2. Expression and Purification of Recombinant PvCHS and Recombinant CHIs
4.3. Crystallization and Structure Determination
4.4. Kinetic Assay of PvCHS
4.5. Kinetic Assay of PvCHI
4.6. Isothermal Titration Calorimetry
4.7. Molecular Docking
4.8. FRET
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Christensen, A.B.; Gregersen, P.L.; Schröder, J.; Collinge, D.B. A chalcone synthase with an unusual substrate preference is expressed in barley leaves in response to UV light and pathogen attack. Plant Mol. Biol. 1998, 37, 849–857. [Google Scholar] [CrossRef] [PubMed]
- Guetsky, R.; Kobiler, I.; Wang, X.; Perlman, N.; Gollop, N.; Avila-Quezada, G.; Hadar, I.; Prusky, D. Metabolism of the Flavonoid Epicatechin by Laccase of Colletotrichum gloeosporioides and Its Effect on Pathogenicity on Avocado Fruits. Phytopathology 2005, 95, 1341–1348. [Google Scholar] [CrossRef] [PubMed]
- Francis, G.J.L. Jack Natural Food Colorants: Science and Technology; CRC Press: Boca Raton, FL, USA, 2014; ISBN 978-0-429-07911-5. [Google Scholar]
- Sun, W.; Meng, X.; Liang, L.; Jiang, W.; Huang, Y.; He, J.; Hu, H.; Almqvist, J.; Gao, X.; Wang, L. Molecular and Biochemical Analysis of Chalcone Synthase from Freesia hybrid in Flavonoid Biosynthetic Pathway. PLoS ONE 2015, 10, e0119054. [Google Scholar] [CrossRef] [PubMed]
- Weng, J.; Chapple, C. The origin and evolution of lignin biosynthesis. New Phytol. 2010, 187, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Liou, G.; Chiang, Y.-C.; Wang, Y.; Weng, J.-K. Mechanistic basis for the evolution of chalcone synthase catalytic cysteine reactivity in land plants. J. Biol. Chem. 2018, 293, 18601–18612. [Google Scholar] [CrossRef] [PubMed]
- Koga, T.; Meydani, M. Effect of plasma metabolites of (+)-catechin and quercetin on monocyte adhesion to human aortic endothelial cells. Am. J. Clin. Nutr. 2001, 73, 941–948. [Google Scholar] [CrossRef] [PubMed]
- Overman, A.; Chuang, C.-C.; McIntosh, M. Quercetin attenuates inflammation in human macrophages and adipocytes exposed to macrophage-conditioned media. Int. J. Obes. 2011, 35, 1165–1172. [Google Scholar] [CrossRef]
- Scarano, A.; Chieppa, M.; Santino, A. Looking at Flavonoid Biodiversity in Horticultural Crops: A Colored Mine with Nutritional Benefits. Plants 2018, 7, 98. [Google Scholar] [CrossRef]
- Arroyo, B.J.; Santos, A.P.; de Almeida de Melo, E.; Campos, A.; Lins, L.; Boyano-Orozco, L.C. Chapter 8—Bioactive Compounds and Their Potential Use as Ingredients for Food and Its Application in Food Packaging. In Bioactive Compounds; Campos, M.R.S., Ed.; Woodhead Publishing: Sawston, UK, 2019; pp. 143–156. ISBN 978-0-12-814774-0. [Google Scholar]
- Septembre-Malaterre, A.; Boumendjel, A.; Seteyen, A.-L.S.; Boina, C.; Gasque, P.; Guiraud, P.; Sélambarom, J. Focus on the high therapeutic potentials of quercetin and its derivatives. Phytomed. Plus 2022, 2, 100220. [Google Scholar] [CrossRef]
- Weng, J.-K.; Noel, J.P. Structure–Function Analyses of Plant Type III Polyketide Synthases. In Methods in Enzymology; Hopwood, D.A., Ed.; Natural Product Biosynthesis by Microorganisms and Plants, Part A; Academic Press: Cambridge, MA, USA, 2012; Volume 515, pp. 317–335. [Google Scholar]
- Austin, M.B.; Noel, J.P. The chalcone synthase superfamily of type III polyketide synthases. Nat. Prod. Rep. 2002, 20, 79–110. [Google Scholar] [CrossRef]
- Abe, I.; Morita, H. Structure and function of the chalcone synthase superfamily of plant type III polyketide synthases. Nat. Prod. Rep. 2010, 27, 809–838. [Google Scholar] [CrossRef] [PubMed]
- de Souza, L.P.; Garbowicz, K.; Brotman, Y.; Tohge, T.; Fernie, A.R. The Acetate Pathway Supports Flavonoid and Lipid Biosynthesis in Arabidopsis. Plant Physiol. 2019, 182, 857–869. [Google Scholar] [CrossRef]
- Jez, J.M.; Ferrer, J.-L.; Bowman, M.E.; Austin, M.B.; A Dixon, R.; Noel, J.P.; Schröder, J. Structure and mechanism of chalcone synthase-like polyketide synthases. J. Ind. Microbiol. Biotechnol. 2001, 27, 393–398. [Google Scholar] [CrossRef]
- Harborne, J.B. The Flavonoids Advances in Research Since 1986; Chapman & Hall: New York, NY, USA, 1994. [Google Scholar]
- Hinderer, W.; Seitz, H.U. Chalcone synthase from cell suspension cultures of Daucus carota L. Arch. Biochem. Biophys. 1985, 240, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, I.M.; Dixon, R.A. Chalcone synthase from cell suspension cultures of Phaseolus vulgaris L. Biochim. Biophys. Acta (BBA)—Protein Struct. Mol. Enzym. 1983, 747, 298–303. [Google Scholar] [CrossRef]
- Weng, J.K.; Noel, J.P. The Remarkable Pliability and Promiscuity of Specialized Metabolism. Cold Spring Harb. Symp. Quant. Biol. 2012, 77, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.A.; Zhang, B.; Harza, R.; Palmer, N.; Sarath, G.; Sattler, S.E.; Twigg, P.; Vermerris, W.; Kang, C. Structural Similarities and Overlapping Activities among Dihydroflavonol 4-Reductase, Flavanone 4-Reductase, and Anthocyanidin Reductase Offer Metabolic Flexibility in the Flavonoid Pathway. Int. J. Mol. Sci. 2023, 24, 13901. [Google Scholar] [CrossRef] [PubMed]
- Waki, T.; Mameda, R.; Nakano, T.; Yamada, S.; Terashita, M.; Ito, K.; Tenma, N.; Li, Y.; Fujino, N.; Uno, K.; et al. A conserved strategy of chalcone isomerase-like protein to rectify promiscuous chalcone synthase specificity. Nat. Commun. 2020, 11, 870. [Google Scholar] [CrossRef]
- Wolf-Saxon, E.R.; Moorman, C.C.; Castro, A.; Ruiz-Rivera, A.; Mallari, J.P.; Burke, J.R. Regulatory ligand binding in plant chalcone isomerase–like (CHIL) proteins. J. Biol. Chem. 2023, 299, 104804. [Google Scholar] [CrossRef]
- A Bednar, R.; Hadcock, J.R. Purification and characterization of chalcone isomerase from soybeans. J. Biol. Chem. 1988, 263, 9582–9588. [Google Scholar] [CrossRef]
- Yin, Y.-C.; Zhang, X.-D.; Gao, Z.-Q.; Hu, T.; Liu, Y. The Research Progress of Chalcone Isomerase (CHI) in Plants. Mol. Biotechnol. 2018, 61, 32–52. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Li, C.; Li, H.; Lu, S. Identification and Characterization of Flavonoid Biosynthetic Enzyme Genes in Salvia miltiorrhiza (Lamiaceae). Molecules 2018, 23, 1467. [Google Scholar] [CrossRef] [PubMed]
- Ralston, L.; Subramanian, S.; Matsuno, M.; Yu, O. Partial Reconstruction of Flavonoid and Isoflavonoid Biosynthesis in Yeast Using Soybean Type I and Type II Chalcone Isomerases. Plant Physiol. 2005, 137, 1375–1388. [Google Scholar] [CrossRef] [PubMed]
- Ngaki, M.N.; Louie, G.V.; Philippe, R.N.; Manning, G.; Pojer, F.; Bowman, M.E.; Li, L.; Larsen, E.; Wurtele, E.S.; Noel, J.P. Evolution of the chalcone-isomerase fold from fatty-acid binding to stereospecific catalysis. Nature 2012, 485, 530–533. [Google Scholar] [CrossRef] [PubMed]
- Burbulis, I.E.; Winkel-Shirley, B. Interactions among enzymes of the Arabidopsis flavonoid biosynthetic pathway. Proc. Natl. Acad. Sci. USA 1999, 96, 12929–12934. [Google Scholar] [CrossRef]
- Jiang, W.; Yin, Q.; Wu, R.; Zheng, G.; Liu, J.; Dixon, R.A.; Pang, Y. Role of a chalcone isomerase-like protein in flavonoid biosynthesis in Arabidopsis thaliana. J. Exp. Bot. 2015, 66, 7165–7179. [Google Scholar] [CrossRef]
- Ban, Z.; Qin, H.; Mitchell, A.J.; Liu, B.; Zhang, F.; Weng, J.-K.; Dixon, R.A.; Wang, G. Noncatalytic chalcone isomerase-fold proteins in Humulus lupulus are auxiliary components in prenylated flavonoid biosynthesis. Proc. Natl. Acad. Sci. USA 2018, 115, E5223–E5232. [Google Scholar] [CrossRef] [PubMed]
- Shih, C.H.; Chu, H.; Tang, L.K.; Sakamoto, W.; Maekawa, M.; Chu, I.K.; Wang, M.; Lo, C. Functional characterization of key structural genes in rice flavonoid biosynthesis. Planta 2008, 228, 1043–1054. [Google Scholar] [CrossRef] [PubMed]
- Crosby, K.C.; Pietraszewska-Bogiel, A.; Gadella, T.W.; Winkel, B.S. Förster resonance energy transfer demonstrates a flavonoid metabolon in living plant cells that displays competitive interactions between enzymes. FEBS Lett. 2011, 585, 2193–2198. [Google Scholar] [CrossRef]
- Diharce, J.; Golebiowski, J.; Fiorucci, S.; Antonczak, S. Fine-tuning of microsolvation and hydrogen bond interaction regulates substrate channeling in the course of flavonoid biosynthesis. Phys. Chem. Chem. Phys. 2016, 18, 10337–10345. [Google Scholar] [CrossRef]
- Fujino, N.; Tenma, N.; Waki, T.; Ito, K.; Komatsuzaki, Y.; Sugiyama, K.; Yamazaki, T.; Yoshida, S.; Hatayama, M.; Yamashita, S.; et al. Physical interactions among flavonoid enzymes in snapdragon and torenia reveal the diversity in the flavonoid metabolon organization of different plant species. Plant J. 2018, 94, 372–392. [Google Scholar] [CrossRef]
- D’Odorico, P.; Carr, J.A.; Laio, F.; Ridolfi, L.; Vandoni, S. Feeding humanity through global food trade. Earth’s Future 2014, 2, 458–469. [Google Scholar] [CrossRef]
- Lam, L.P.Y.; Wang, L.; Lui, A.C.W.; Liu, H.; Umezawa, T.; Tobimatsu, Y.; Lo, C. Flavonoids in major cereal grasses: Distribution, functions, biosynthesis, and applications. Phytochem. Rev. 2023, 22, 1399–1438. [Google Scholar] [CrossRef]
- Cloutier, M.; Chatterjee, D.; Elango, D.; Cui, J.; Bruns, M.A.; Chopra, S. Sorghum Root Flavonoid Chemistry, Cultivar, and Frost Stress Effects on Rhizosphere Bacteria and Fungi. Phytobiomes J. 2021, 5, 39–50. [Google Scholar] [CrossRef]
- Nakabayashi, R.; Mori, T.; Saito, K. Alternation of flavonoid accumulation under drought stress in Arabidopsis thaliana. Plant Signal. Behav. 2014, 9, e29518. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-C.; Liu, J.-M.; Lu, H.-Y.; Gao, S.-L. Enhanced flavonoid production in hairy root cultures of Glycyrrhiza uralensis Fisch by combining the over-expression of chalcone isomerase gene with the elicitation treatment. Plant Cell Rep. 2009, 28, 1205–1213. [Google Scholar] [CrossRef] [PubMed]
- Sheng, H.; Sun, X.; Yan, Y.; Yuan, Q.; Wang, J.; Shen, X. Metabolic Engineering of Microorganisms for the Production of Flavonoids. Front. Bioeng. Biotechnol. 2020, 8, 589069. [Google Scholar] [CrossRef] [PubMed]
- Peng, B.; Zhang, L.; He, S.; Oerlemans, R.; Quax, W.J.; Groves, M.R.; Haslinger, K. Engineering a Plant Polyketide Synthase for the Biosynthesis of Methylated Flavonoids. J. Agric. Food Chem. 2023, 72, 529–539. [Google Scholar] [CrossRef] [PubMed]
- IJMS|Free Full-Text|Dynamic Reconfiguration of Switchgrass Proteomes in Response to Rust (Puccinia Novopanici) Infection. Available online: https://www.mdpi.com/1422-0067/24/19/14630 (accessed on 14 May 2024).
- IJMS|Free Full-Text|Differential Defense Responses of Upland and Lowland Switchgrass Cultivars to a Cereal Aphid Pest. Available online: https://www.mdpi.com/1422-0067/21/21/7966 (accessed on 14 May 2024).
- Koch, K.G.; Palmer, N.A.; Donze-Reiner, T.; Scully, E.D.; Seravalli, J.; Amundsen, K.; Twigg, P.; Louis, J.; Bradshaw, J.D.; Heng-Moss, T.M.; et al. Aphid-Responsive Defense Networks in Hybrid Switchgrass. Front. Plant Sci. 2020, 11, 1145. [Google Scholar] [CrossRef]
- Tetreault, H.M.; Gries, T.; Liu, S.; Toy, J.; Xin, Z.; Vermerris, W.; Ralph, J.; Funnell-Harris, D.L.; Sattler, S.E. The Sorghum (Sorghum bicolor) Brown Midrib 30 Gene Encodes a Chalcone Isomerase Required for Cell Wall Lignification. Front. Plant Sci. 2021, 12, 732307. [Google Scholar] [CrossRef]
- Gaut, B.S. Evolutionary dynamics of grass genomes. New Phytol. 2002, 154, 15–28. [Google Scholar] [CrossRef]
- Krissinel, E.; Henrick, K. Inference of Macromolecular Assemblies from Crystalline State. J. Mol. Biol. 2007, 372, 774–797. [Google Scholar] [CrossRef] [PubMed]
- Holm, L. Using Dali for Protein Structure Comparison. In Structural Bioinformatics: Methods and Protocols; Gáspári, Z., Ed.; Methods in Molecular Biology; Springer US: New York, NY, USA, 2020; pp. 29–42. ISBN 978-1-07-160270-6. [Google Scholar]
- Go, M.K.; Wongsantichon, J.; Cheung, V.W.N.; Chow, J.Y.; Robinson, R.C.; Yew, W.S. Synthetic Polyketide Enzymology: Platform for Biosynthesis of Antimicrobial Polyketides. ACS Catal. 2015, 5, 4033–4042. [Google Scholar] [CrossRef]
- Ferrer, J.L.; Jez, J.M.; Bowman, M.E.; Dixon, R.A.; Noel, J.P. Structure of chalcone synthase and the molecular basis of plant polyketide biosynthesis. Nat. Struct. Biol. 1999, 6, 775–784. [Google Scholar] [CrossRef] [PubMed]
- Stewart, C.; Woods, K.; Macias, G.; Allan, A.C.; Hellens, R.P.; Noel, J.P. Molecular architectures of benzoic acid-specific type III polyketide synthases. Acta Crystallogr. Sect. D Struct. Biol. 2017, 73, 1007–1019. [Google Scholar] [CrossRef] [PubMed]
- Imaizumi, R.; Mameda, R.; Takeshita, K.; Kubo, H.; Sakai, N.; Nakata, S.; Takahashi, S.; Kataoka, K.; Yamamoto, M.; Nakayama, T.; et al. Crystal structure of chalcone synthase, a key enzyme for isoflavonoid biosynthesis in soybean. Proteins Struct. Funct. Bioinform. 2021, 89, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Madden, T.L.; Schäffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef]
- Tian, W.; Chen, C.; Lei, X.; Zhao, J.; Liang, J. CASTp 3.0: Computed atlas of surface topography of proteins. Nucleic Acids Res. 2018, 46, W363–W367. [Google Scholar] [CrossRef]
- Jez, J.M.; Noel, J.P. Mechanism of Chalcone Synthase. J. Biol. Chem. 2000, 275, 39640–39646. [Google Scholar] [CrossRef]
- Peters, A.; Schneider-Poetsch, H.A.; Schwarz, H.; Weissenböck, G. Biochemical and Immunological Characterization of Chalcone Synthase from Rye Leaves. J. Plant Physiol. 1988, 133, 178–182. [Google Scholar] [CrossRef]
- Noel, J.P.; Jez, J.M.; Bowman, M.E.; Dixon, R.A. Structure and mechanism of the evolutionarily unique plant enzyme chalcone isomerase. Nat. Struct. Biol. 2000, 7, 786–791. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-H.; Lee, C.W.; Cho, S.M.; Lee, H.; Park, H.; Lee, J.; Lee, J.H. Crystal structure and enzymatic properties of chalcone isomerase from the Antarctic vascular plant Deschampsia antarctica Desv. PLoS ONE 2018, 13, e0192415. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, T.; Takahashi, S.; Waki, T. Formation of Flavonoid Metabolons: Functional Significance of Protein-Protein Interactions and Impact on Flavonoid Chemodiversity. Front. Plant Sci. 2019, 10, 821. [Google Scholar] [CrossRef]
- Waki, T.; Yoo, D.; Fujino, N.; Mameda, R.; Denessiouk, K.; Yamashita, S.; Motohashi, R.; Akashi, T.; Aoki, T.; Ayabe, S.-I.; et al. Identification of protein–protein interactions of isoflavonoid biosynthetic enzymes with 2-hydroxyisoflavanone synthase in soybean (Glycine max (L.) Merr.). Biochem. Biophys. Res. Commun. 2016, 469, 546–551. [Google Scholar] [CrossRef]
- Mameda, R.; Waki, T.; Kawai, Y.; Takahashi, S.; Nakayama, T. Involvement of chalcone reductase in the soybean isoflavone metabolon: Identification of GmCHR5, which interacts with 2-hydroxyisoflavanone synthase. Plant J. 2018, 96, 56–74. [Google Scholar] [CrossRef]
- Neese, F. The ORCA program system. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2012, 2, 73–78. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef]
- Yan, Y.; Tao, H.; He, J.; Huang, S.-Y. The HDOCK server for integrated protein–protein docking. Nat. Protoc. 2020, 15, 1829–1852. [Google Scholar] [CrossRef]
- Zhang, Y.; Fernie, A.R. Metabolons, enzyme-enzyme assemblies that mediate substrate channeling, and their roles in plant metabolism. Plant Commun. 2021, 2, 100081. [Google Scholar] [CrossRef]
- Jez, J.M.; Ferrer, J.-L.; Bowman, M.E.; Dixon, R.A.; Noel, J.P. Dissection of Malonyl-Coenzyme A Decarboxylation from Polyketide Formation in the Reaction Mechanism of a Plant Polyketide Synthase. Biochemistry 2000, 39, 890–902. [Google Scholar] [CrossRef]
- Pan, X.; Wang, H.; Li, C.; Zhang, J.Z.H.; Ji, C. MolGpka: A Web Server for Small Molecule pKa Prediction Using a Graph-Convolutional Neural Network. J. Chem. Inf. Model. 2021, 61, 3159–3165. [Google Scholar] [CrossRef]
- Cheng, A.; Zhang, X.; Han, X.; Zhang, Y.; Gao, S.; Liu, C.; Lou, H. Identification of chalcone isomerase in the basal land plants reveals an ancient evolution of enzymatic cyclization activity for synthesis of flavonoids. New Phytol. 2017, 217, 909–924. [Google Scholar] [CrossRef] [PubMed]
- Otwinowski, Z.; Minor, W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 1997, 276, 307–326. [Google Scholar] [PubMed]
- Liebschner, D.; Afonine, P.V.; Baker, M.L.; Bunkóczi, G.; Chen, V.B.; Croll, T.I.; Hintze, B.; Hung, L.-W.; Jain, S.; McCoy, A.J.; et al. Macromolecular structure determination using X-rays, neutrons and electrons: Recent developments in Phenix. Acta Crystallogr. Sect. D Struct. Biol. 2019, 75, 861–877. [Google Scholar] [CrossRef]
- Emsley, P.; Lohkamp, B.; Scott, W.G.; Cowtan, K. Features and development of Coot. Acta Crystallogr. Sect. D Biol. Crystallogr. 2010, 66, 486–501. [Google Scholar] [CrossRef] [PubMed]
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Hutchison, G.R. Avogadro: An advanced semantic chemical editor, visualization, and analysis platform. J. Cheminform. 2012, 4, 17. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef]
- Yan, Y.; Zhang, D.; Zhou, P.; Li, B.; Huang, S.-Y. HDOCK: A web server for protein–protein and protein–DNA/RNA docking based on a hybrid strategy. Nucleic Acids Res. 2017, 45, W365–W373. [Google Scholar] [CrossRef]
- Liao, J.; Madahar, V.; Dang, R.; Jiang, L. Quantitative FRET (qFRET) Technology for the Determination of Protein–Protein Interaction Affinity in Solution. Molecules 2021, 26, 6339. [Google Scholar] [CrossRef]
- Dykes, L.; Rooney, W.L.; Rooney, L.W. Evaluation of phenolics and antioxidant activity of black sorghum hybrids. J. Cereal Sci. 2013, 58, 278–283. [Google Scholar] [CrossRef]



| PvCHI | PvCHS (CoA and Naringenin Complex) | PvCHS C170S | PvCHIL | SbCHI (Liquiritigenin Complex) | |
|---|---|---|---|---|---|
| PDBID | 8V8L | 8V8M | 8V8N | 8V8O | 8V8P |
| Wavelength | 1Å | 1Å | 1Å | 1Å | 1Å |
| Resolution range | 36.39–1.74 (1.802–1.74) | 46.59–2.29 (2.372–2.29) | 39.33–2.124 (2.2–2.124) | 41.92–3.21 (3.325–3.21) | 46.2–3.18 (3.294–3.18) |
| Space group | C 1 2 1 | P 1 | P 21 21 21 | P 43 21 2 | P 1 21 1 |
| Unit cell a, b, c (Å) α, β, γ (°) | 139.09 46.521 59.054 90 106.372 90 | 51.05 83.085 106.45 79.431 76.253 77.211 | 55.757 61.722 204.087 90 90 90 | 100.171 100.171 118.93 90 90 90 | 92.453 127.825 95.311 90 116.271 90 |
| Total reflections | 120,786 (11,568) | 121,592 (11,993) | 148,323 (12,456) | 130,626 (11,969) | 112,695 (10,017) |
| Unique reflections | 36,857 (3640) | 71,129 (6990) | 39,995 (3675) | 10,355 (998) | 33,435 (2812) |
| Multiplicity | 3.3 (3.2) | 1.7 (1.7) | 3.7 (3.4) | 12.6 (11.8) | 3.4 (3.0) |
| Completeness (%) | 98.27 (98.27) | 95.94 (94.15) | 97.62 (91.99) | 98.65 (98.52) | 92.07 (84.88) |
| Mean I/sigma (I) | 11.90 (1.23) | 6.64 (3.23) | 6.06 (1.51) | 7.19 (1.88) | 5.48 (1.61) |
| Wilson B-factor | 27.14 | 9.93 | 25.96 | 54.38 | 39.17 |
| R-merge | 0.05583 (0.7246) | 0.07506 (0.1528) | 0.1643 (0.6189) | 0.3151 (1.119) | 0.1969 (0.5945) |
| R-meas | 0.06661 (0.8691) | 0.1062 (0.2161) | 0.1955 (0.7378) | 0.3284 (1.171) | 0.2344 (0.7241) |
| R-pim | 0.03587 (0.4737) | 0.07506 (0.1528) | 0.1034 (0.3917) | 0.09113 (0.3398) | 0.126 (0.4087) |
| Wavelength (Å) | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 |
| CC1/2 | 0.998 (0.615) | 0.959 (0.954) | 0.968 (0.619) | 0.984 (0.68) | 0.958 (0.613) |
| CC* | 0.999 (0.873) | 0.989 (0.988) | 0.992 (0.874) | 0.996 (0.9) | 0.989 (0.872) |
| Reflections used in refinement | 36,812 (3640) | 70,877 (6981) | 39,715 (3650) | 10,263 (998) | 30,826 (2812) |
| Reflections used for R-free | 1991 (197) | 1017 (100) | 1999 (184) | 1027 (100) | 1880 (175) |
| R-work | 0.1959 (0.2770) | 0.2014 (0.2400) | 0.2095 (0.2973) | 0.2407 (0.3088) | 0.2165 (0.3072) |
| R-free | 0.2396 (0.3288) | 0.1954 (0.2434) | 0.2112 (0.2666) | 0.2539 (0.3232) | 0.2735 (0.3836) |
| CC (work) | 0.955 (0.758) | 0.932 (0.903) | 0.954 (0.804) | 0.921 (0.684) | 0.913 (0.780) |
| CC (free) | 0.930 (0.756) | 0.936 (0.872) | 0.962 (0.831) | 0.944 (0.765) | 0.872 (0.621) |
| Number of non-hydrogen atoms | 3400 | 12,930 | 6320 | 3234 | 12,441 |
| Macromolecules | 3127 | 11,866 | 5932 | 3234 | 12,346 |
| Ligands | 28 | 136 | 14 | 0 | 155 |
| Solvent | 261 | 928 | 382 | 0 | 0 |
| Protein residues | 426 | 1554 | 778 | 418 | 1679 |
| 1RMS (bonds) | 0.006 | 0.008 | 0.003 | 0.003 | 0.005 |
| RMS (angles) | 0.75 | 0.99 | 0.52 | 0.59 | 0.55 |
| Ramachandran favored (%) | 99.05 | 96.35 | 96.64 | 92.2 | 90.2 |
| Ramachandran allowed (%) | 0.95 | 3.32 | 2.71 | 7.07 | 8.36 |
| Ramachandran outliers (%) | 0 | 0.33 | 0.65 | 0.73 | 1.44 |
| Rotamer outliers (%) | 0.31 | 2.55 | 1.91 | 1.46 | 0.16 |
| Clashscore | 7.18 | 12.25 | 11 | 7.31 | 26.06 |
| Average B-factor | 34.94 | 15.41 | 29.02 | 53.66 | 44.46 |
| Macromolecules | 34.5 | 15.06 | 28.99 | 53.66 | 44.54 |
| Ligands | 50.72 | 28.16 | 42.95 | 34.12 | |
| Solvent | 39.54 | 17.99 | 29.4 |
| Enzyme | Substrate | Km (µM) | kcat (s−1) | kcat Km−1 (s−1 µM−1) |
|---|---|---|---|---|
| PvCHI | Naringenin chalcone | 16.04 ± 6.28 | 8012 ± 1185 | 499.5 |
| Isoliquiritigenin | 17.60 ± 5.09 | 0.6344 ± 0.07195 | 0.03579 | |
| SbCHI | Naringenin chalcone | 16.55 ± 2.08 | 2889 ± 143.9 | 174.5 |
| Isoliquiritigenin | 1.585 ± 0.929 | 0.2387 ± 0.0274 | 0.1506 | |
| PvCHS | p-coumaroyl-CoA | 0.1303 ± 0.0319 | 0.00695 ± 0.00077 | 0.0533 |
| PvCHS + CHI | p-coumaroyl-CoA | 1.545 ± 1.061 | 0.175 ± 0.017 | 0.1133 |
| PvCHS + CHIL | p-coumaroyl-CoA | 1.321 ± 0.263 | 0.0505 ± 0.0018 | 0.0382 |
| PvCHS + CHI + CHIL | p-coumaroyl-CoA | 1.121 ± 0.561 | 3.040 ± 0.261 | 2.711 |
| Enzyme | Substrate | Kd (µM) | ∆H (kcal mol−1) | ∆S (cal mol−1 K−1) |
|---|---|---|---|---|
| PvCHS | PvCHIL | 76.3 | 31.84 ± 7.44 | 127 |
| PvCHIL with Naringenin | 7.46 | 5.34 ± 0.51 | 41.4 | |
| PvCHIL with naringenin chalcone | 170.35 | 36.38 ± 3.68 | 140 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lewis, J.A.; Jacobo, E.P.; Palmer, N.; Vermerris, W.; Sattler, S.E.; Brozik, J.A.; Sarath, G.; Kang, C. Structural and Interactional Analysis of the Flavonoid Pathway Proteins: Chalcone Synthase, Chalcone Isomerase and Chalcone Isomerase-like Protein. Int. J. Mol. Sci. 2024, 25, 5651. https://doi.org/10.3390/ijms25115651
Lewis JA, Jacobo EP, Palmer N, Vermerris W, Sattler SE, Brozik JA, Sarath G, Kang C. Structural and Interactional Analysis of the Flavonoid Pathway Proteins: Chalcone Synthase, Chalcone Isomerase and Chalcone Isomerase-like Protein. International Journal of Molecular Sciences. 2024; 25(11):5651. https://doi.org/10.3390/ijms25115651
Chicago/Turabian StyleLewis, Jacob A., Eric P. Jacobo, Nathan Palmer, Wilfred Vermerris, Scott E. Sattler, James A Brozik, Gautam Sarath, and ChulHee Kang. 2024. "Structural and Interactional Analysis of the Flavonoid Pathway Proteins: Chalcone Synthase, Chalcone Isomerase and Chalcone Isomerase-like Protein" International Journal of Molecular Sciences 25, no. 11: 5651. https://doi.org/10.3390/ijms25115651
APA StyleLewis, J. A., Jacobo, E. P., Palmer, N., Vermerris, W., Sattler, S. E., Brozik, J. A., Sarath, G., & Kang, C. (2024). Structural and Interactional Analysis of the Flavonoid Pathway Proteins: Chalcone Synthase, Chalcone Isomerase and Chalcone Isomerase-like Protein. International Journal of Molecular Sciences, 25(11), 5651. https://doi.org/10.3390/ijms25115651






