Rosuvastatin Synergistically Enhances the Antinociceptive Efficacy of Duloxetine in Paclitaxel-Induced Neuropathic Pain in Mice
Abstract
1. Introduction
2. Results
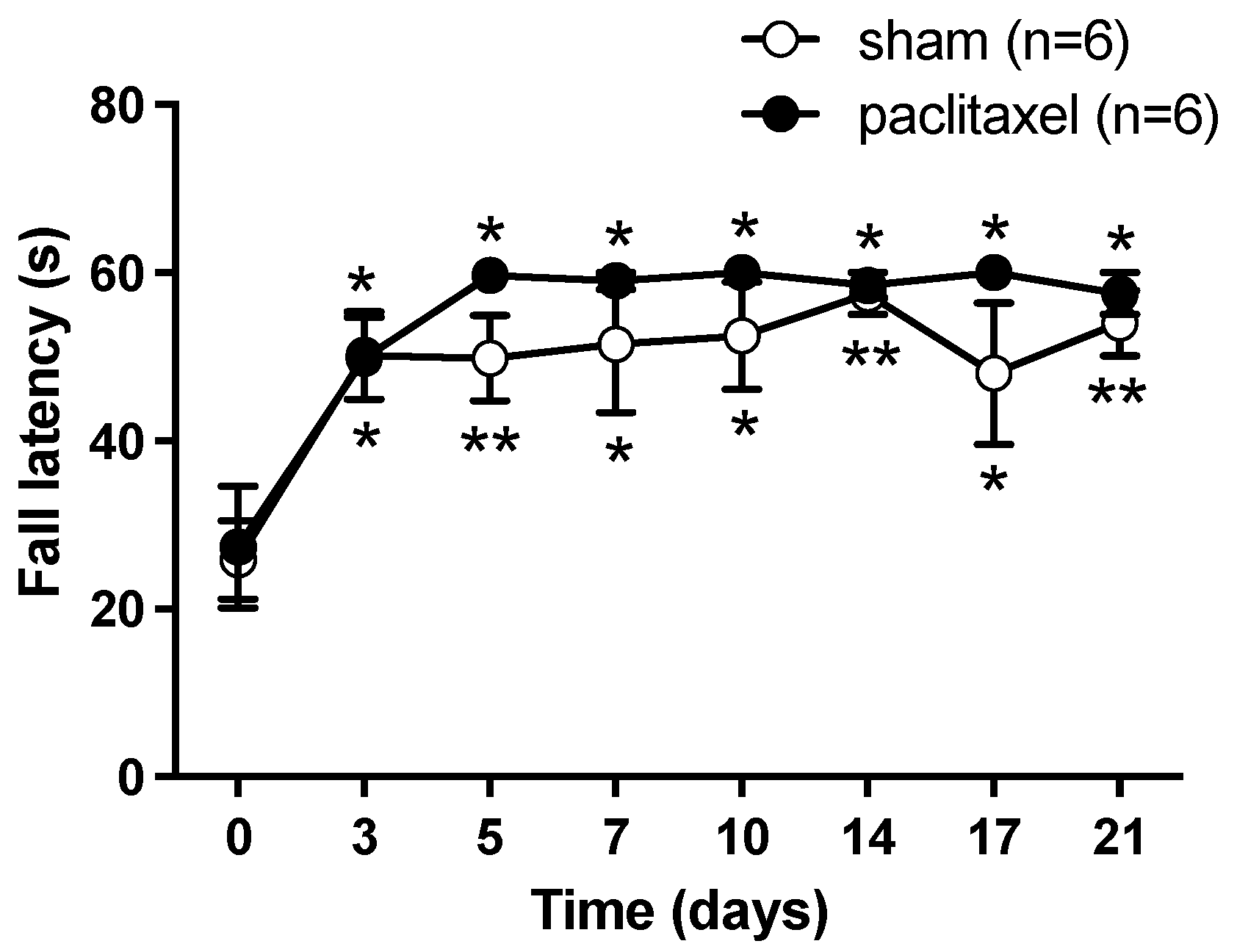
2.1. Algesimetric Evaluation and Motor Performance in Mice with Paclitaxel-Induced Neuropathic Pain
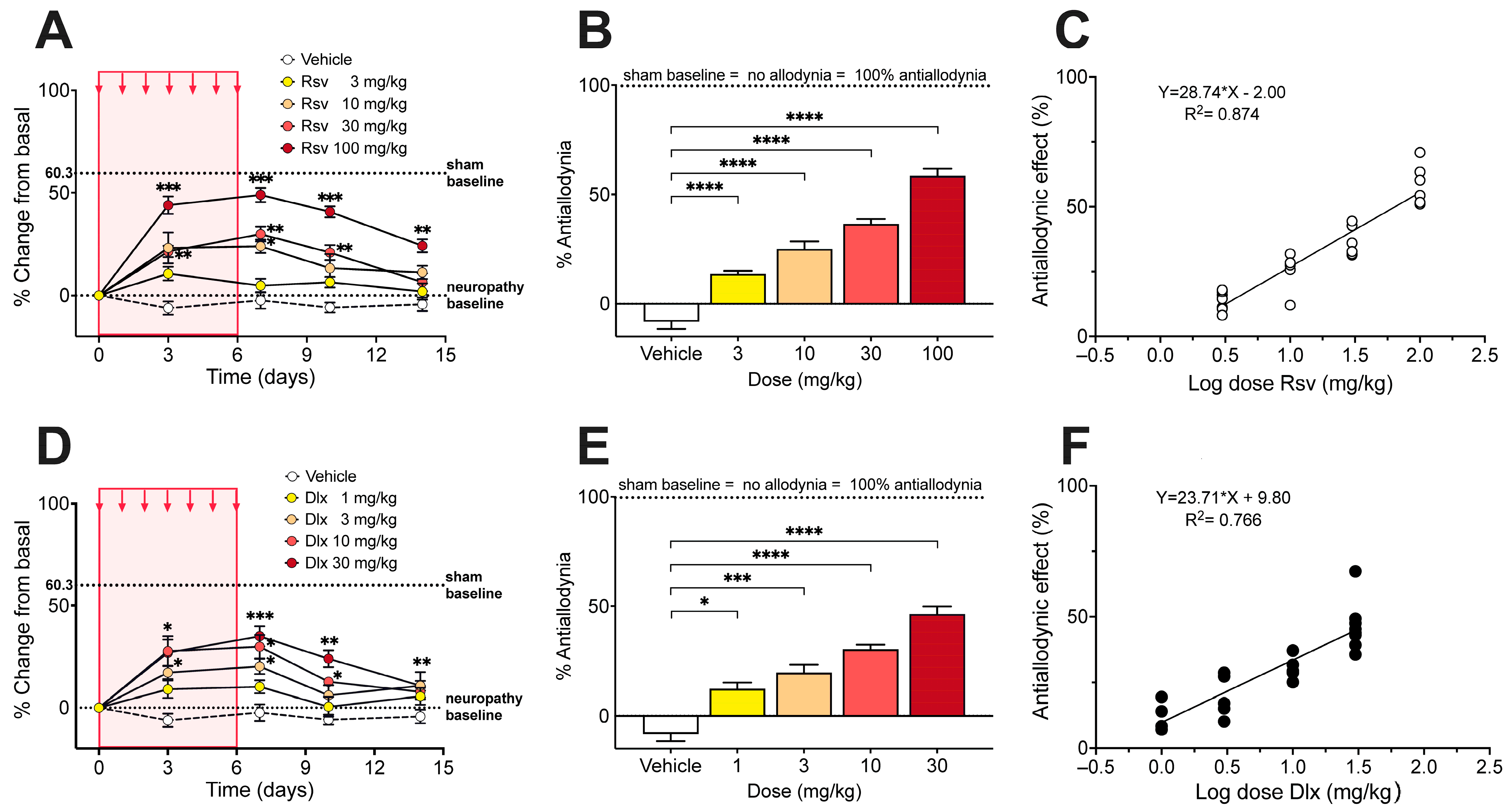
2.2. Antinociceptive Effect of Rosuvastatine, Duloxetine, or Their Combination on Paclitaxel-Induced Neuropathic Pain, as Measured in the Electronic von Frey Test
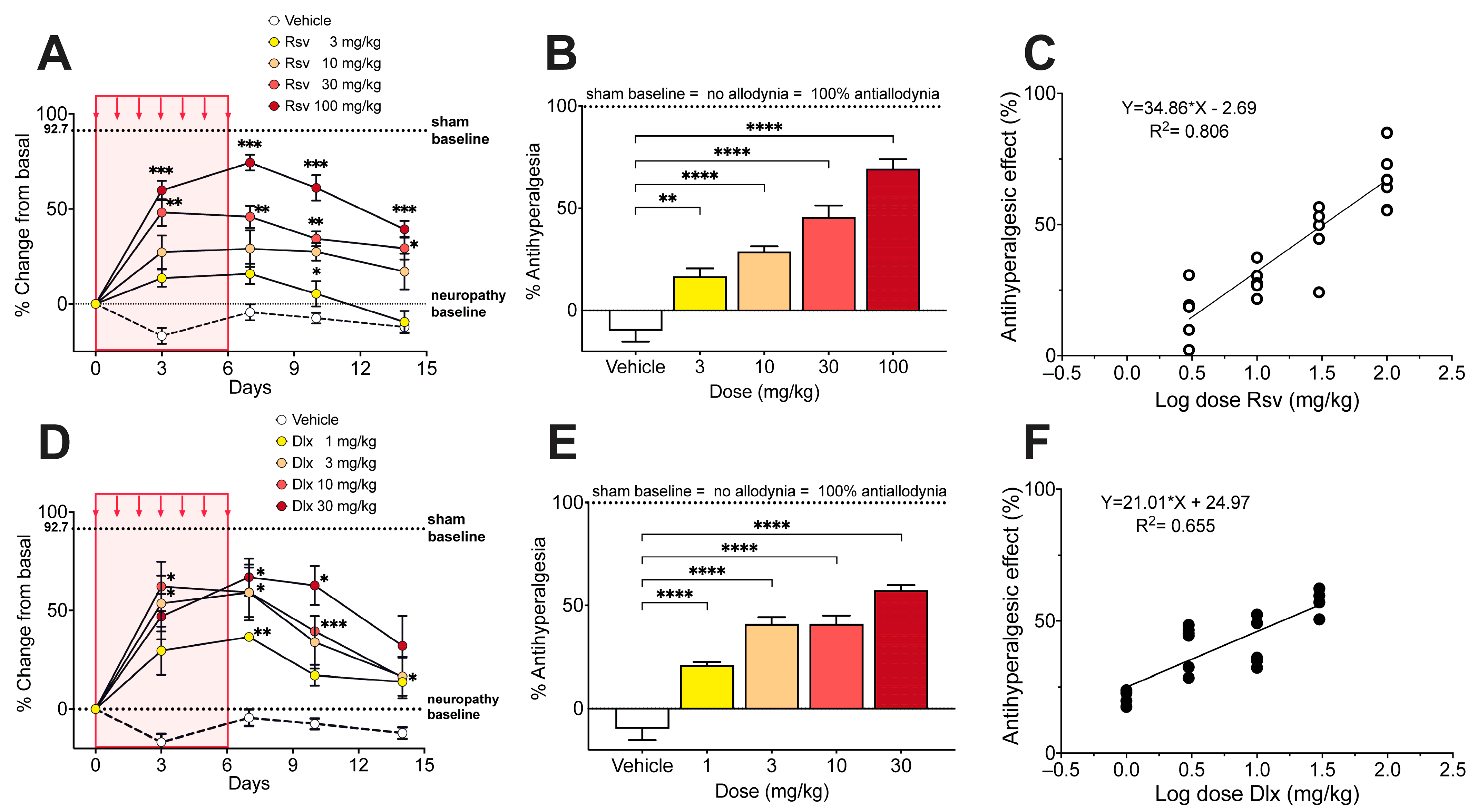
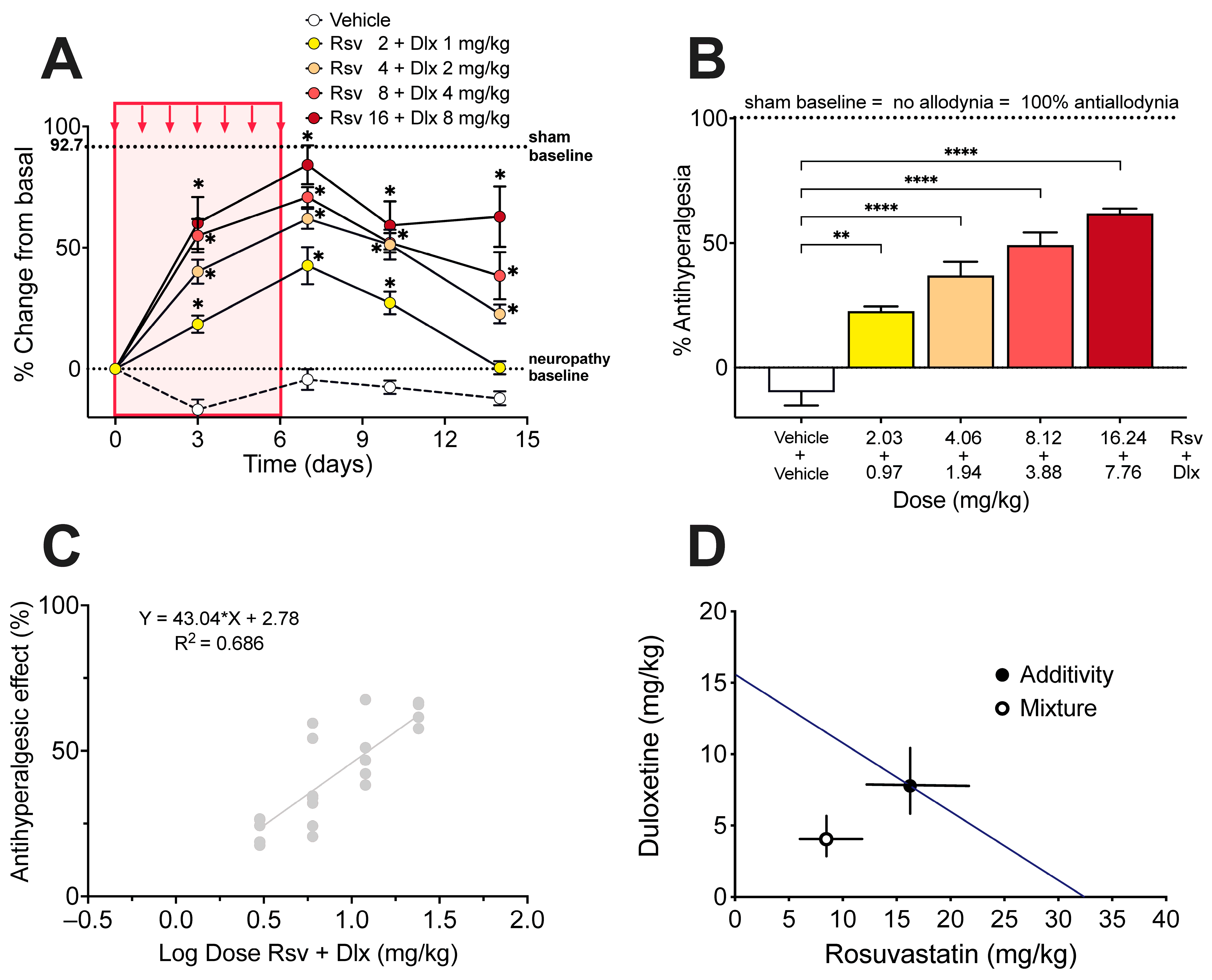
2.3. Antinociceptive Effect of Rosuvastatine, Duloxetine, or Their Combination on Paclitaxel-Induced Neuropathic Pain, as Measured in the Hot Plate Test
2.4. Changes in Expression Levels of GFAP and CD11b Proteins in the Dorsal Horn Tissue of Mice with Paclitaxel-Induced Neuropathy, and the Effect of Rosuvasatatin/Duloxetine Combination Treatment
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Materials and Drugs
4.3. Measurement of Antinociceptive Activity
4.4. Measurement of Motor Coordination and Balance
4.5. Induction of Peripheral Neuropathy by Paclitaxel in Mice
4.6. Dose-Response Curves of Rosuvastatin and Duloxetine on the Paclitaxel-Induced Neuropathy
4.7. Isobolographic Analysis of the Interaction of Rosuvastatin with Duloxetine
4.8. Proteins Determination in Spinal Cord: SDS-PAGE and Western Blott
4.9. Data Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 19 December 2022).
- Cavaletti, G.; Cornblath, D.R.; Merkies, I.S.; Postma, T.J.; Rossi, E.; Frigeni, B.; Alberti, P.; Bruna, J.; Velasco, R.; Argyriou, A.A.; et al. The chemotherapy-induced peripheral neuropathy outcome measures standardization study: From consensus to the first validity and reliability findings. Ann. Oncol. 2013, 24, 454–462. [Google Scholar] [CrossRef]
- Burgess, J.; Ferdousi, M.; Gosal, D.; Boon, C.; Matsumoto, K.; Marshall, A.; Mak, T.; Marshall, A.; Frank, B.; Malik, R.A.; et al. Chemotherapy-induced peripheral neuropathy: Epidemiology, pathomechanisms and treatment. Oncol. Ther. 2021, 9, 385–450. [Google Scholar] [CrossRef]
- Colvin, L.A. Chemotherapy-induced peripheral neuropathy: Where are we now? Pain 2019, 160 (Suppl. S1), S1–S10. [Google Scholar] [CrossRef] [PubMed]
- Zajączkowska, R.; Kocot-Kępska, M.; Leppert, W.; Wrzosek, A.; Mika, J.; Wordliczek, J. Mechanisms of chemotherapy-induced peripheral neuropathy. Int. J. Mol. Sci. 2019, 20, 1451. [Google Scholar] [CrossRef] [PubMed]
- Staff, N.P.; Grisold, A.; Grisold, W.; Windebank, A.J. Chemotherapy-induced peripheral neuropathy: A current review. Ann. Neurol. 2017, 81, 772–781. [Google Scholar] [CrossRef]
- Ibrahim, E.Y.; Ehrlich, B.E. Prevention of chemotherapy-induced peripheral neuropathy: A review of recent findings. Crit. Rev. Oncol. Hematol. 2020, 45, 102831. [Google Scholar] [CrossRef] [PubMed]
- Loprinzi, C.L.; Lacchetti, C.; Bleeker, J.; Cavaletti, G.; Chauhan, C.; Hertz, D.L.; Kelley, M.R.; Lavino, A.; Lustberg, M.B.; Paice, J.A.; et al. Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: ASCO guideline update. J. Clin. Oncol. 2020, 38, 3325–3348. [Google Scholar] [CrossRef]
- Attal, N. Pharmacological treatments of neuropathic pain: The latest recommendations. Rev. Neurol. 2019, 175, 46–50. [Google Scholar] [CrossRef]
- Obata, H. Analgesic Mechanisms of antidepressants for neuropathic pain. Int. J. Mol. Sci. 2017, 18, 2483. [Google Scholar] [CrossRef]
- Majithia, N.; Loprinzi, C.L.; Smith, T.J. New practical approaches to chemotherapy-induced neuropathic pain: Prevention, assessment, and treatment. Oncology 2016, 30, 1020–1029. [Google Scholar]
- Schachter, M. Chemical, pharmacokinetic and pharmacodynamic properties of statins: An update. Fundam. Clin. Pharmacol. 2005, 19, 117–125. [Google Scholar] [CrossRef]
- Miranda, H.F.; Sierralta, F.; Aranda, N.; Poblete, P.; Castillo, R.L.; Noriega, V.; Prieto, J.C. Antinociception induced by rosuvastatin in murine neuropathic pain. Pharmacol. Rep. 2018, 70, 503–508. [Google Scholar] [CrossRef]
- Garcia, G.G.; Miranda, H.F.; Noriega, V.; Sierralta, F.; Olavarría, L.; Zepeda, R.J.; Prieto, J. Antinociception induced by atorvastatin in different pain models. Pharmacol. Biochem. Behav. 2011, 100, 125–129. [Google Scholar] [CrossRef]
- Miranda, H.F.; Noriega, V.; Olavarria, L.; Zepeda, R.J.; Sierralta, F.; Prieto, J.C. Antinociception and anti-inflammation induced by simvastatin in algesiometric assays in mice. Basic Clin. Pharmacol. Toxicol. 2011, 109, 438–442. [Google Scholar] [CrossRef]
- Ohsawa, M.; Aasato, M.; Hayashi, S.S.; Kamei, J. RhoA/Rho kinase pathway contributes to the pathogenesis of thermal hyperalgesia in diabetic mice. Pain 2011, 152, 114–122. [Google Scholar] [CrossRef]
- Shi, X.Q.; Lim, T.K.Y.; Lee, S.; Zhao, Y.Q.; Zhang, J. Statins alleviate experimental nerve injury-induced neuropathic pain. Pain 2011, 152, 1033–1043. [Google Scholar] [CrossRef]
- Pathak, N.N.; Balaganur, V.; Lingaraju, M.C.; More, A.S.; Kant, V.; Kumar, D.; Kumar, D.; Tandan, S.K. Antihyperalgesic and anti-inflammatory effects of atorvastatin in chronic constriction injury-induced neuropathic pain in rats. Inflammation 2013, 36, 1468–1478. [Google Scholar] [CrossRef]
- Hasanvand, A.; Ahmadizar, F.; Abbaszadeh, A.; Amini-Khoei, H.; Goudarzi, M.; Abbasnezhad, A.; Choghakhori, R. The antinociceptive effects of rosuvastatin in chronic constriction injury model of male rats. Basic Clin. Neurosci. 2018, 9, 251–260. [Google Scholar] [CrossRef]
- Peng, Y.; Zhang, X.; Zhang, T.; Grace, P.M.; Li, H.; Wang, Y.; Li, H.; Chen, H.; Watkins, L.R.; Hutchinson, M.R.; et al. Lovastatin inhibits Toll-like receptor 4 signaling in microglia by targeting its co-receptor myeloid differentiation protein 2 and attenuates neuropathic pain. Brain Behav. Immun. 2019, 82, 432–444. [Google Scholar] [CrossRef]
- Gholami, M.R.; Abolhassani, F.; Pasbakhsh, P.; Akbari, M.; Sobhani, A.; Eshraghian, M.R.; Kamalian, N.; Amoli, F.A.; Dehpoor, A.R.; Sohrabi, D. The effects of simvastatin on ischemia-reperfusion injury of sciatic nerve in adult rats. Eur. J. Pharmacol. 2008, 590, 111–114. [Google Scholar] [CrossRef]
- Yavuz, C.; Demirtas, S.; Guclu, O.; Karahan, O.; Caliskan, A.; Yazici, S.; Mavitas, B. Rosuvastatin may have neuroprotective effect on spinal cord ischemia reperfusion injury. CNS Neurol. Disord. Drug Targets 2013, 12, 1011–1016. [Google Scholar] [CrossRef] [PubMed]
- Aghazadeh, J.; Samadi Motlagh, P.; Salehpour, F.; Meshkini, A.; Fatehi, M.; Mirzaei, F.; Alavi, S.A.N. Effects of atorvastatin in patients with acute spinal cord injury. Asian Spine J. 2017, 11, 903–907. [Google Scholar] [CrossRef] [PubMed]
- Tallarida, R.J. Drug Synergism and Dose-Effect Data Analysis, 1st ed.; Chapman & Hall/CRC: Boca Raton, FL, USA, 2000; pp. 1–264. ISBN 978-0-367-398347. [Google Scholar]
- Polomano, R.C.; Mannes, A.J.; Clark, U.S.; Bennett, G.J. A painful peripheral neuropathy in the rat produced by the chemotherapeutic drug, paclitaxel. Pain 2001, 94, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Nieto, F.R.; Entrena, J.M.; Cendán, C.M.; Del Pozo, E.; Vela, J.M.; Baeyens, J.M. Tetrodotoxin inhibits the development and expression of neuropathic pain induced by paclitaxel in mice. Pain 2008, 137, 520–531. [Google Scholar] [CrossRef]
- Ward, S.J.; Ramirez, M.D.; Neelakantan, H.; Walker, E.A. Cannabidiol prevents the development of cold and mechanical allodynia in paclitaxel-treated female C57Bl6 mice. Anesth. Analg. 2011, 113, 947–950. [Google Scholar] [CrossRef]
- Kim, W.; Chung, Y.; Choi, S.; Min, B.I.; Kim, S.K. Duloxetine protects against oxaliplatin-Iinduced neuropathic pain and spinal neuron hyperexcitability in rodents. Int. J. Mol. Sci. 2017, 18, 2626. [Google Scholar] [CrossRef]
- Tawfik, M.K.; Helmy, S.A.; Badran, D.I.; Zaitone, S.A. Neuroprotective effect of duloxetine in a mouse model of diabetic neuropathy: Role of glia suppressing mechanisms. Life Sci. 2018, 205, 113–124. [Google Scholar] [CrossRef]
- Tripathi, C.D.; Mehta, A.K.; Yadav, A.M. Drug combinations in diabetic neuropathic pain: An experimental validation. J. Basic Clin. Physiol. Pharmacol. 2016, 27, 617–624. [Google Scholar] [CrossRef]
- Kearns, B.; Cooper, K.; Orr, M.; Essat, M.; Hamilton, J.; Cantrell, A. The incidence and costs of adverse events associated with antidepressants: Results from a systematic review, network meta-analysis and multi-country economic model. Neuropsychiatr. Dis. Treat. 2022, 18, 1133–1143. [Google Scholar] [CrossRef]
- Sugimoto, M.; Takagi, T.; Suzuki, R.; Konno, N.; Asama, H.; Sato, Y.; Irie, H.; Okubo, Y.; Nakamura, J.; Takasumi, M.; et al. Drug treatment for chemotherapy-induced peripheral neuropathy in patients with pancreatic cancer. Fukushima J. Sci. 2022, 68, 1–10. [Google Scholar] [CrossRef]
- Shukla, R.; Khasbage, S.; Garg, M.K.; Singh, S. Duloxetine-induced hypertensive urgency in type 2 diabetes mellitus with diabetic neuropathy. Indian J. Pharmacol. 2020, 52, 213–215. [Google Scholar] [CrossRef]
- Athanasios, A.; Fitzsimmons, A.; Sheedy, C. Hypertensive emergency and stroke with medication interaction: A case report on metoprolol and duloxetine. J. Clin. Psychopharmacol. 2022, 42, 424–426. [Google Scholar] [CrossRef]
- Park, K.; Kim, S.; Ko, Y.J.; Park, B.J. Duloxetine and cardiovascular adverse events: A systematic review and meta-analysis. J. Psychiatr. Res. 2020, 124, 109–114. [Google Scholar] [CrossRef]
- Ward, N.C.; Watts, G.F.; Eckel, R.H. Statin Toxicity. Circ. Res. 2019, 124, 328–350. [Google Scholar] [CrossRef]
- Tallarida, R.J. An overview of drug combination analysis with isobolograms. J. Pharmacol. Exp. Ther. 2006, 319, 1–7. [Google Scholar] [CrossRef]
- Tallarida, R.J. Drug combinations: Tests and analysis with isoboles. Curr. Protoc. Pharmacol. 2016, 72, 9.19.1–9.19.19. [Google Scholar] [CrossRef]
- Raouf, M.; Glogowski, A.J.; Bettinger, J.J.; Fudin, J. Serotonin-norepinephrine reuptake inhibitors and the influence of binding affinity (Ki) on analgesia. J. Clin. Pharm. Ther. 2017, 42, 513–517. [Google Scholar] [CrossRef]
- Wang, S.Y.; Calderon, J.; Wang, G.K. Block of neuronal Na+ channels by antidepressant duloxetine in a state-dependent manner. Anesthesiology 2010, 113, 655–665. [Google Scholar] [CrossRef]
- Stoetzer, C.; Papenberg, B.; Doll, T.; Völker, M.; Heineke, J.; Stoetzer, M.; Wegner, F.; Leffler, A. Differential inhibition of cardiac and neuronal Na+ channels by the selective serotonin-norepinephrine reuptake inhibitors duloxetine and venlafaxine. Eur. J. Pharmacol. 2016, 783, 1–10. [Google Scholar] [CrossRef]
- Zimova, L.; Ptakova, A.; Mitro, M.; Krusek, J.; Vlachova, V. Activity dependent inhibition of TRPC1/4/5 channels by duloxetine involves voltage sensor-like domain. Biomed. Pharmacother. 2022, 152, 113262. [Google Scholar] [CrossRef]
- Zeiser, R. Immune modulatory effects of statins. Immunology 2018, 154, 69–75. [Google Scholar] [CrossRef]
- Chen, L.W.; Lin, C.-S.; Tsai, M.-C.; Shih, S.-F.; Lim, Z.W.; Chen, S.-J.; Tsui, P.-F.; Ho, L.-J.; Lai, J.-H.; Liou, J.-T. Pitavastatin exerts potent anti-inflammatory and immunomodulatory effects via the suppression of AP-1 signal transduction in human T cells. Int. J. Mol. Sci. 2019, 20, 3534. [Google Scholar] [CrossRef]
- Ling, Q.; Tejada-Simon, M.V. Statins and the brain: New perspective for old drugs. Prog. Neuropsychopharmacol. Biol. Psychiatry 2016, 66, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Sodero, A.O.; Barrantes, F.J. Pleiotropic effects of statins on brain cells. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183340. [Google Scholar] [CrossRef]
- Jo, Y.H.; Choi, G.W.; Kim, M.L.; Sung, K.R. Statins inhibit the gliosis of MIO-M1, a Müller glial cell line Induced by TRPV4 activation. Int. J. Mol. Sci. 2022, 23, 5190. [Google Scholar] [CrossRef]
- Su, K.-H.; Lin, S.-J.; Wei, J.; Lee, K.-I.; Zhao, J.-F.; Shyue, S.-K.; Lee, T.-S. The essential role of transient receptor potential vanilloid 1 in simvastatin-induced activation of endothelial nitric oxide synthase and angiogenesis. Acta Physiol. 2014, 212, 191–204. [Google Scholar] [CrossRef]
- Yam, M.F.; Loh, Y.C.; Oo, C.W.; Basir, R. Overview of neurological mechanism of pain profile used for animal “pain-like” behavioral study with proposed analgesic pathways. Int. J. Mol. Sci. 2020, 21, 4355. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Huang, Z.Z.; Ling, Y.Z.; Wei, J.Y.; Cui, Y.; Zhang, X.Z.; Zhu, H.Q.; Xin, W.J. Up-regulation of CX3CL1 via nuclear factor-κB-dependent histone acetylation is involved in paclitaxel-induced peripheral neuropathy. Anesthesiology 2015, 122, 1142–1151. [Google Scholar] [CrossRef]
- Makker, P.G.; Duffy, S.S.; Lees, J.G.; Perera, C.J.; Tonkin, R.S.; Butovsky, O.; Park, S.B.; Goldstein, D.; Moalem-Taylor, G. Characterisation of immune and neuroinflammatory changes associated with chemotherapy-induced peripheral neuropathy. PLoS ONE 2017, 12, e0170814. [Google Scholar] [CrossRef] [PubMed]
- Huehnchen, P.; Muenzfeld, H.; Boehmerle, W.; Endres, M. Blockade of IL-6 signaling prevents paclitaxel-induced neuropathy in C57Bl/6 mice. Cell Death Dis. 2020, 11, 45. [Google Scholar] [CrossRef]
- Deng, L.; Guindon, J.; Cornett, B.L.; Makriyannis, A.; Mackie, K.; Hohmann, A.G. Chronic cannabinoid receptor 2 activation reverses paclitaxel neuropathy without tolerance or cannabinoid receptor 1-dependent withdrawal. Biol. Psychiatry 2014, 77, 475–487. [Google Scholar] [CrossRef] [PubMed]
- Zheng, F.Y.; Xiao, W.H.; Bennett, G.J. The response of spinal microglia to chemotherapy-evoked painful peripheral neuropathies is distinct from that evoked by traumatic nerve injuries. Neuroscience 2011, 176, 447–454. [Google Scholar] [CrossRef]
- Zhang, H.; Yoon, S.Y.; Zhang, H.; Dougherty, P.M. Evidence that spinal astrocytes but not microglia contribute to the pathogenesis of Paclitaxel-induced painful neuropathy. J. Pain 2012, 13, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Sorci, G.; Agneletti, A.L.; Bianchi, R.; Donato, R. Association of S100B with intermediate filaments and microtubules in glial cells. Biochim. Biophys. Acta 1998, 1448, 277–289. [Google Scholar] [CrossRef] [PubMed]
- Cavaletti, G.; Cavalletti, E.; Oggioni, N.; Sottani, C.; Minoia, C.; D’Incalci, M.; Zucchetti, M.; Marmiroli, P.; Tredici, G. Distribution of paclitaxel within the nervous system of the rat after repeated intravenous administration. Neurotoxicology 2000, 21, 389–393. [Google Scholar] [CrossRef] [PubMed]
- Klein, I.; Lehmann, H.C. Pathomechanisms of paclitaxel-induced peripheral neuropathy. Toxics 2021, 9, 229. [Google Scholar] [CrossRef]
- Quan, J.; Lee, J.Y.; Choi, H.; Kim, Y.C.; Yang, S.; Jeong, J.; Park, H.J. Effect of pregabalin combined with duloxetine and tramadol on allodynia in chronic postischemic pain and spinal nerve ligation mouse models. Pharmaceutics 2022, 14, 670. [Google Scholar] [CrossRef]
- Ohsawa, M.; Ishikura, K.; Mutoh, J.; Hisa, H. Involvement of inhibition of RhoA/Rho kinase signaling in simvastatin-induced amelioration of neuropathic pain. Neuroscience 2016, 333, 204–213. [Google Scholar] [CrossRef]
- Abdelhak, A.; Foschi, M.; Abu-Rumeileh, S.; Yue, J.K.; D’Anna, L.; Huss, A.; Oeckl, P.; Ludolph, A.C.; Kuhle, J.; Petzold, A.; et al. Blood GFAP as an emerging biomarker in brain and spinal cord disorders. Nat. Rev. Neurol. 2022, 18, 158–172. [Google Scholar] [CrossRef]
- De Luca, C.; Virtuoso, A.; Korai, S.A.; Cirillo, R.; Gargano, F.; Papa, M.; Cirillo, G. Altered spinal homeostasis and maladaptive plasticity in GFAP null mice following peripheral nerve injury. Cells 2022, 11, 1224. [Google Scholar] [CrossRef]
- Bi, X.; Baudry, M.; Liu, J.; Yao, Y.; Fu, L.; Brucher, F.; Lynch, G. Inhibition of geranylgeranylation mediates the effects of 3-hydroxy-3-methylglutaryl (HMG)-CoA reductase inhibitors on microglia. J. Biol. Chem. 2004, 279, 48238–48245. [Google Scholar] [CrossRef]
- Van Der Putten, C.; Kuipers, H.F.; Zuiderwijk-Sick, E.A.; Van Straalen, L.; Kondova, I.; Van Den Elsen, P.J.; Bajramovic, J.J. Statins amplify TLR-induced responses in microglia via inhibition of cholesterol biosynthesis. Glia 2012, 60, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Erichsen, H.K.; Hao, J.X.; Xu, X.J.; Blackburn-Munro, G. Comparative actions of the opioid analgesics morphine, methadone and codeine in rat models of peripheral and central neuropathic pain. Pain 2005, 116, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Andoh, T.; Kitamura, R.; Kuraishi, Y. Milnacipran inhibits oxaliplatin-induced mechanical allodynia through spinal action in mice. Biol. Pharm. Bull. 2015, 38, 151–154. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, H.L.; Duggett, N.A.; Flatters, S.J.L. Chemotherapy-induced painful neuropathy: Pain-like behaviours in rodent models and their response to commonly-used analgesics. Curr. Opin. Support. Palliat. Care 2016, 10, 119–128. [Google Scholar] [CrossRef]
- National Research Council. Guide for the Care and Use of Laboratory Animals, 8th ed.; The National Academies Press: Washington, DC, USA, 2011; pp. 1–120. ISBN 978-0-309-38629-6.
- Zimmermann, M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain 1983, 16, 109–110. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Martinov, T.; Mack, M.; Sykes, A.; Chatterjea, D. Measuring changes in tactile sensitivity in the hind paw of mice using an electronic von Frey apparatus. J. Vis. Exp. 2013, 82, e51212. [Google Scholar] [CrossRef]
- Parvathy, S.S.; Masocha, W. Coadministration of indomethacin and minocycline attenuates established paclitaxel-induced neuropathic thermal hyperalgesia: Involvement of cannabinoid CB1 receptors. Sci. Rep. 2015, 5, 10541. [Google Scholar] [CrossRef]
- Dunham, N.W.; Miya, T.S. A note on a simple apparatus for detecting neurological deficit in rats and mice. J. Am. Pharm. Assoc. Am. Pharm. Assoc. 1957, 46, 208–209. [Google Scholar] [CrossRef]
- Shiotsuki, H.; Yoshimi, K.; Shimo, Y.; Funayama, M.; Takamatsu, Y.; Ikeda, K.; Takahashi, R.; Kitazawa, S.; Hattori, N. A rotarod test for evaluation of motor skill learning. J. Neurosci. Methods 2010, 189, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Bomholt, S.F.; Mikkelsen, J.D.; Blackburn-Munro, G. Antinociceptive effects of the antidepressants amitriptyline, duloxetine, mirtazapine and citalopram in animal models of acute, persistent and neuropathic pain. Neuropharmacology 2005, 48, 252–263. [Google Scholar] [CrossRef]
- Pelissier, T.; Infante, C.; Constandil, L.; Espinosa, J.; Lapeyra, C.D.; Hernández, A. Antinociceptive effect and interaction of uncompetitive and competitive NMDA receptor antagonists upon capsaicin and paw pressure testing in normal and monoarthritic rats. Pain 2008, 134, 113–127. [Google Scholar] [CrossRef] [PubMed]
- Cazanga, V.; Hernandez, A.; Morales, B.; Pelissier, T.; Constandil, L. Antinociception induced by copper salt revisited: Interaction with ketamine in formalin-induced intraplantar and orofacial pain in mice. J. Oral Facial Pain Headache 2018, 32, 247–257. [Google Scholar] [CrossRef] [PubMed]
- González, V.; Pelissier, T.; Cazanga, V.; Hernández, A.; Constandil, L. Magnesium salt, a simple strategy to improve methadone analgesia in chronic pain: An isobolographic preclinical study in neuropathic mice. Front. Pharmacol. 2020, 11, 566. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lobos, N.; Lux, S.; Zepeda, R.J.; Pelissier, T.; Marcos, J.L.; Bustos-Quevedo, G.; Hernández, A.; Constandil, L. Rosuvastatin Synergistically Enhances the Antinociceptive Efficacy of Duloxetine in Paclitaxel-Induced Neuropathic Pain in Mice. Int. J. Mol. Sci. 2023, 24, 8359. https://doi.org/10.3390/ijms24098359
Lobos N, Lux S, Zepeda RJ, Pelissier T, Marcos JL, Bustos-Quevedo G, Hernández A, Constandil L. Rosuvastatin Synergistically Enhances the Antinociceptive Efficacy of Duloxetine in Paclitaxel-Induced Neuropathic Pain in Mice. International Journal of Molecular Sciences. 2023; 24(9):8359. https://doi.org/10.3390/ijms24098359
Chicago/Turabian StyleLobos, Nicolás, Sebastián Lux, Ramiro Javier Zepeda, Teresa Pelissier, José Luis Marcos, Gonzalo Bustos-Quevedo, Alejandro Hernández, and Luis Constandil. 2023. "Rosuvastatin Synergistically Enhances the Antinociceptive Efficacy of Duloxetine in Paclitaxel-Induced Neuropathic Pain in Mice" International Journal of Molecular Sciences 24, no. 9: 8359. https://doi.org/10.3390/ijms24098359
APA StyleLobos, N., Lux, S., Zepeda, R. J., Pelissier, T., Marcos, J. L., Bustos-Quevedo, G., Hernández, A., & Constandil, L. (2023). Rosuvastatin Synergistically Enhances the Antinociceptive Efficacy of Duloxetine in Paclitaxel-Induced Neuropathic Pain in Mice. International Journal of Molecular Sciences, 24(9), 8359. https://doi.org/10.3390/ijms24098359






