Biologic Mechanisms of Macrophage Phenotypes Responding to Infection and the Novel Therapies to Moderate Inflammation
Abstract
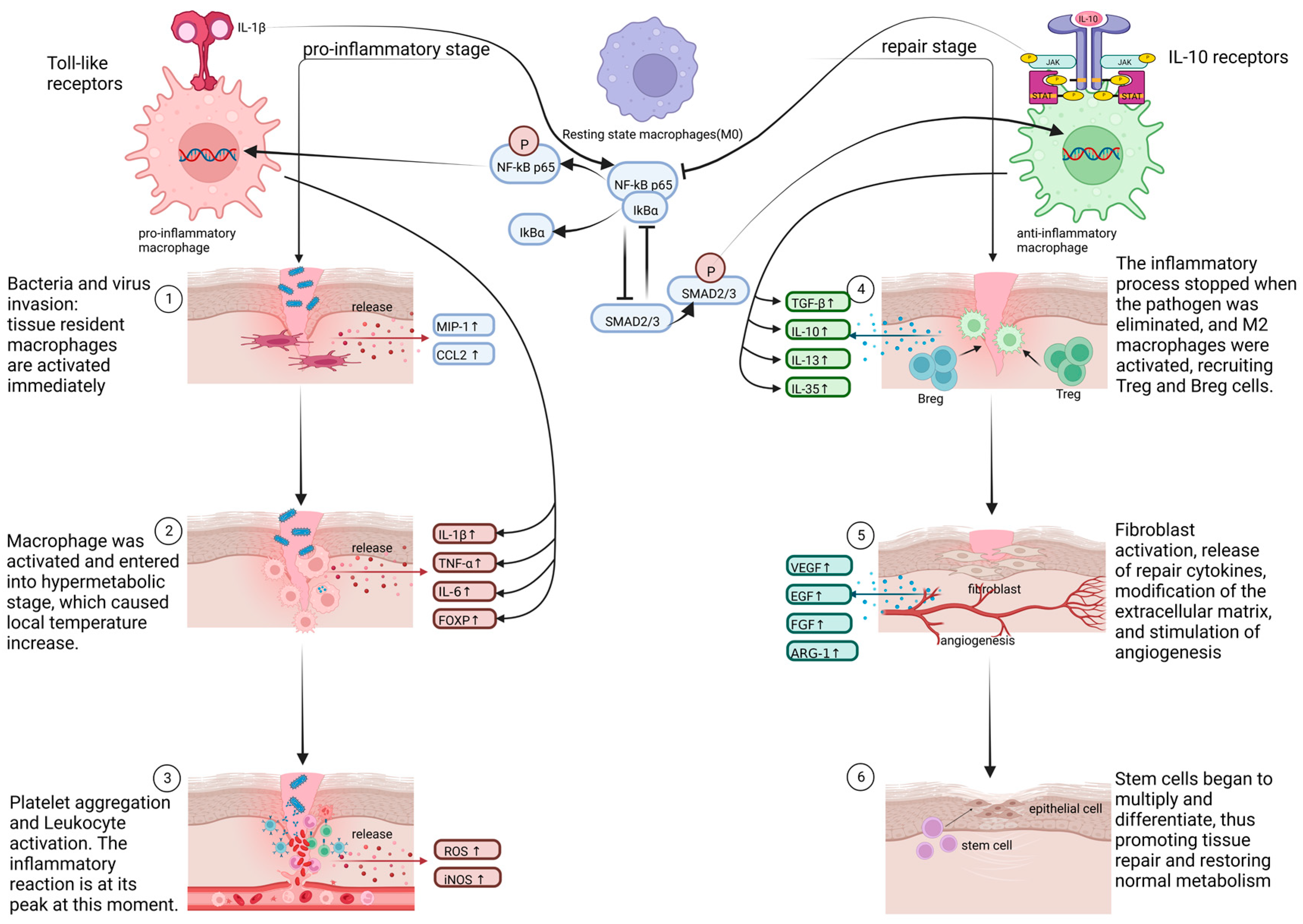
1. Introduction
2. Significance of Anti-Inflammation in Disease Treatments
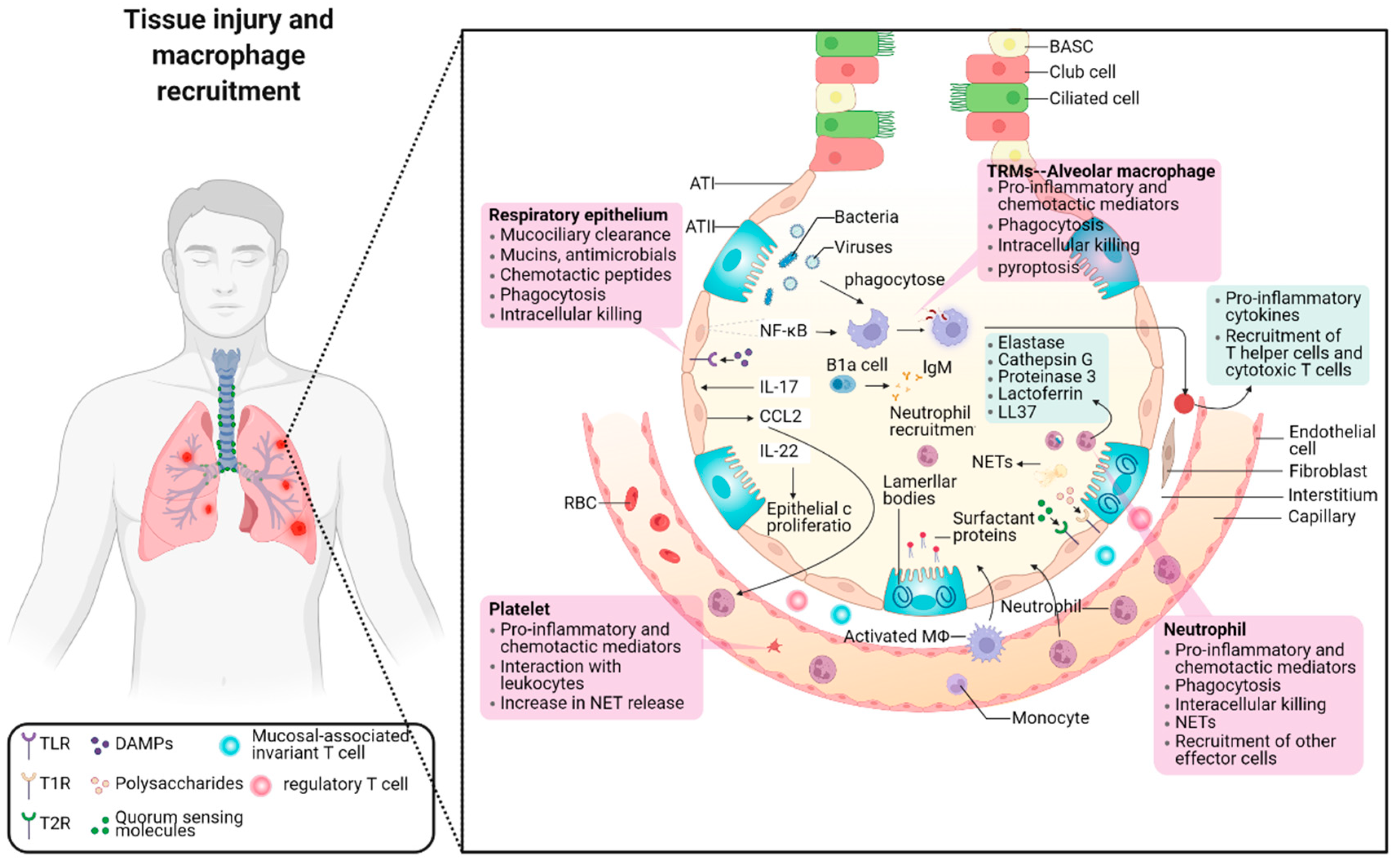
2.1. The Balance between Pro-Inflammation and Anti-Inflammation in Pneumonia
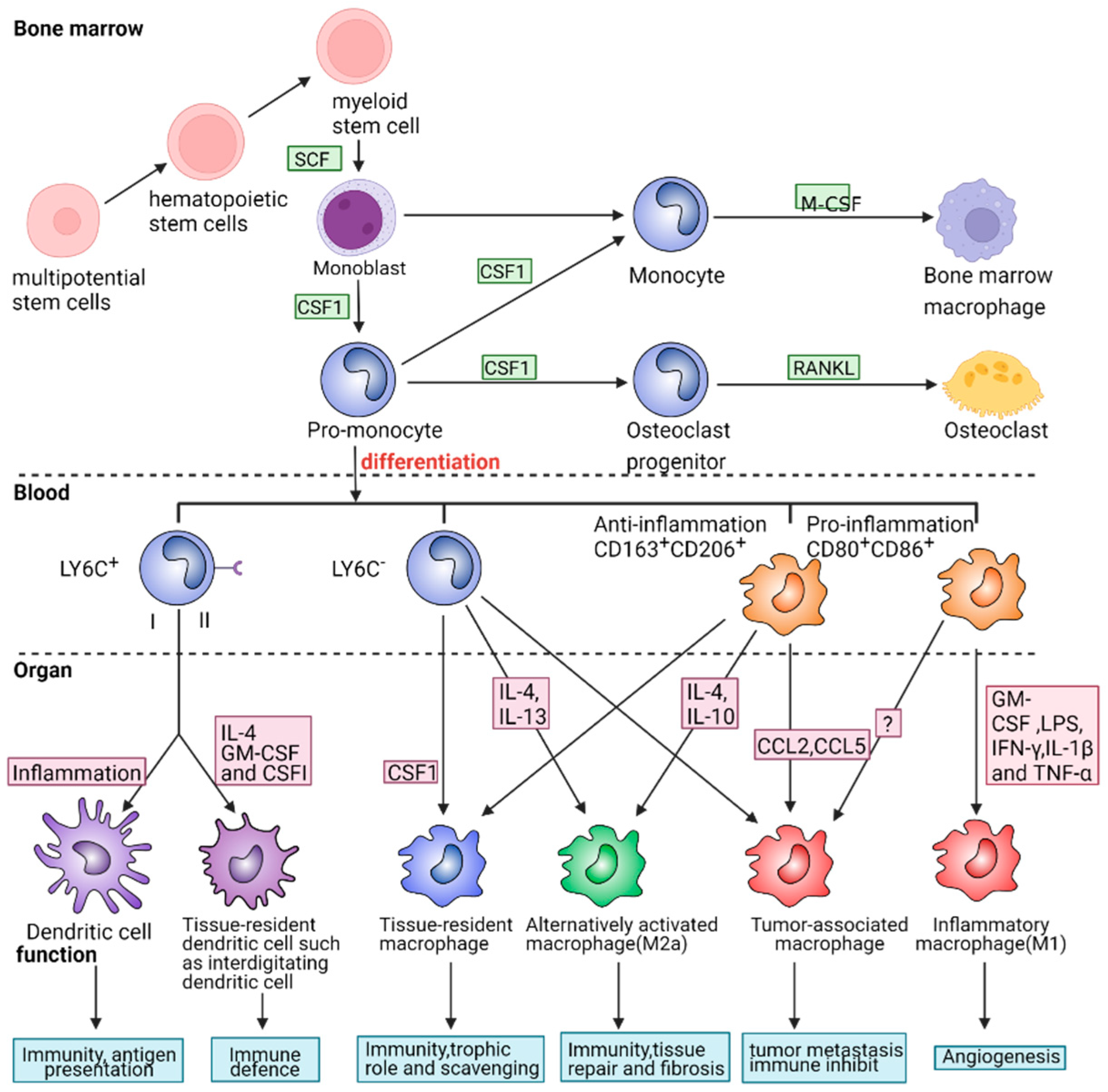
2.2. The Role of Macrophage Phenotypes in Response to Inflammation
2.2.1. Pro-Inflammatory and Cytotoxic Macrophages
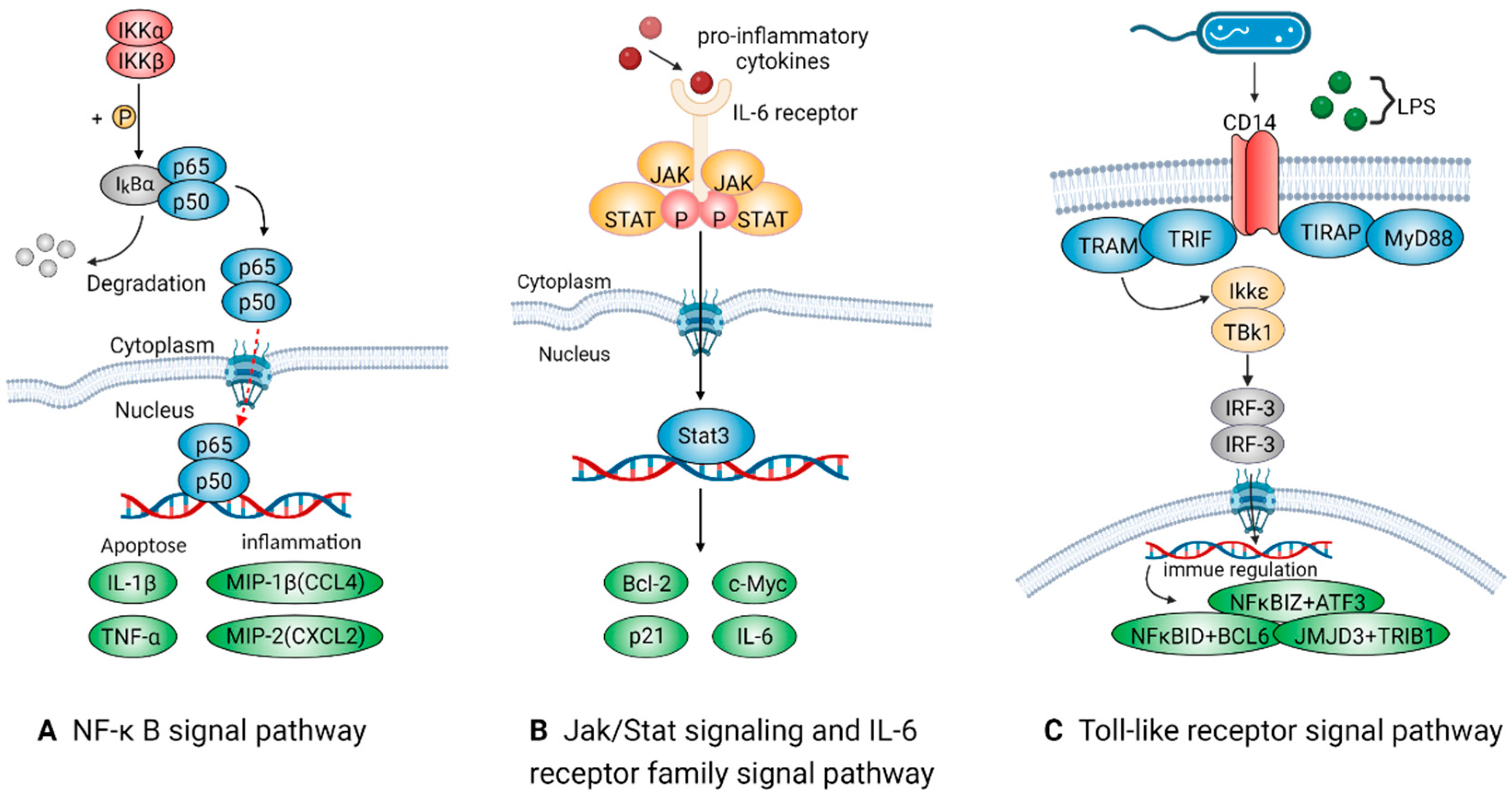
2.2.2. Signal Pathways Closely Associated with Pro-Inflammatory Macrophages
NF-Kappa B Signal Pathway
Jak/Stat and IL-6 Receptor Family Signal Pathways
Toll-Like Receptor Signal Pathway
2.2.3. Anti-Inflammatory and Restorative Macrophage
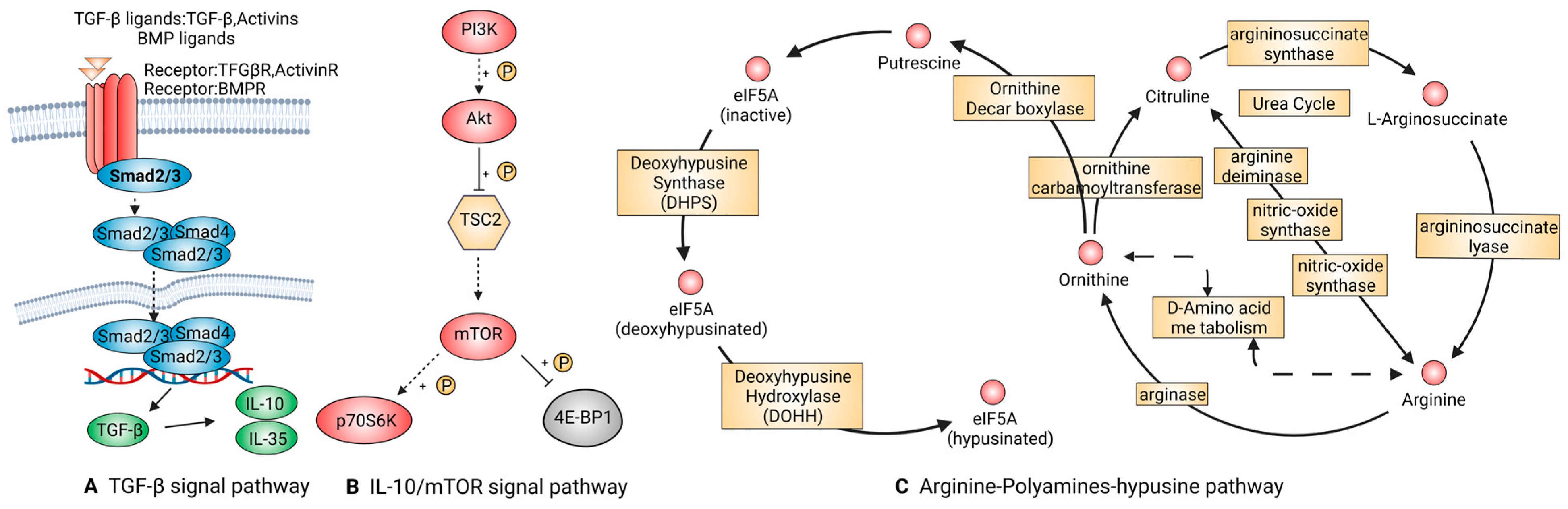
2.2.4. Signal Pathways That Are Tightly Linked to Anti-Inflammatory Macrophages
TGF-β Signal Pathway
IL-10/mTOR Signal Pathway
Arginine-Polyamines-Hypusine Pathway
2.2.5. Crosstalk between Various Signal Pathways
3. Recent Research on Inflammation Control
3.1. Why Do We Interfere with Inflammation Response
3.2. Adverse Effects of Long-Term Application of Anti-Inflammatory Drugs
3.3. dECM-Related Material Is a Novel Therapy to Moderate the Inflammation
| Materials | Seeding Cells | Diseases | Function | References |
|---|---|---|---|---|
| Decellularized lung organ | Lung epithelial and endothelial cells | Chronic obstructive pulmonary | Establishing a model of the lung’s physiological microenvironment to carry out gas exchange. | [97] |
| Decellularized liver matrix | Hepatocytes | Acute and chronic liver damage | Supporting hepatocyte survival and function like albumin secretion, urea production, and cytochrome P450 expression. | [98] |
| Decellularized kidney | Renal endothelial cells | Chronic kidney disease, end-stage renal disease | Clearing metabolites, reabsorbing electrolytes, and generating concentrated urine. | [99,100] |
| Perfusion-decellularized whole-heart scaffolds | Myocardial cells induced by iPSC | Heart failure, myocardial infarction | Regulating angiogenic growth factors and guiding anisotropic microvascular growth and development towards maintaining heart homeostasis and remodeling. | [101,102] |
| Decellularized pig skin combined with gelatin/hyaluronic acid | Fibroblasts | Diabetic foot ulcers, large area skin trauma | Promoting granulation tissue formation, epithelial regeneration and pro-angiogenesis activity. Reducing scar formation by shortening the inflammatory stage. | [95,103] |
| Decellularized cow and human cadaveric bone | Bone marrow stromal cells | Bone fracture, osteoarthritis | Promoting chondrocytes differentiation, maturation and osteogenics to improve repairing long bone defects. | [104,105] |
4. Conclusions and Prospective
Funding
Acknowledgments
Conflicts of Interest
References
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Grant, R.A.; Morales-Nebreda, L.; Markov, N.S.; Swaminathan, S.; Querrey, M.; Guzman, E.R.; Abbott, D.A.; Donnelly, H.K.; Donayre, A.; Goldberg, I.A.; et al. Circuits between infected macrophages and T cells in SARS-CoV-2 pneumonia. Nature 2021, 590, 635–641. [Google Scholar] [CrossRef]
- Sefik, E.; Qu, R.; Junqueira, C.; Kaffe, E.; Mirza, H.; Zhao, J.; Brewer, J.R.; Han, A.; Steach, H.R.; Israelow, B.; et al. Inflammasome activation in infected macrophages drives COVID-19 pathology. Nature 2022, 606, 585–593. [Google Scholar] [CrossRef]
- Saghazadeh, A.; Rezaei, N. Insights on Mpox virus infection immunopathogenesis. Rev. Med. Virol. 2023, 33, e2426. [Google Scholar] [CrossRef]
- Mitjà, O.; Alemany, A.; Marks, M.; Lezama Mora, J.I.; Rodríguez-Aldama, J.C.; Torres Silva, M.S.; Corral Herrera, E.A.; Crabtree-Ramirez, B.; Blanco, J.L.; Girometti, N.; et al. Mpox in people with advanced HIV infection: A global case series. Lancet 2023, 401, 939–949. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Chan, C.N.; Rovira-Clave, X.; Chen, H.; Bai, Y.; Zhu, B.; McCaffrey, E.; Greenwald, N.F.; Liu, C.; Barlow, G.L.; et al. Combined protein and nucleic acid imaging reveals virus-dependent B cell and macrophage immunosuppression of tissue microenvironments. Immunity 2022, 55, 1118–1134.e8. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, O.; Akira, S. Pattern recognition receptors and inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef]
- Mantovani, A.; Sica, A.; Sozzani, S.; Allavena, P.; Vecchi, A.; Locati, M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004, 25, 677–686. [Google Scholar] [CrossRef]
- Locati, M.; Curtale, G.; Mantovani, A. Diversity, Mechanisms, and Significance of Macrophage Plasticity. Annu. Rev. Pathol. 2020, 15, 123–147. [Google Scholar] [CrossRef]
- Funes, S.C.; Rios, M.; Escobar-Vera, J.; Kalergis, A.M. Implications of macrophage polarization in autoimmunity. Immunology 2018, 154, 186–195. [Google Scholar] [CrossRef]
- Li, L.; Cao, J.; Li, S.; Cui, T.; Ni, J.; Zhang, H.; Zhu, Y.; Mao, J.; Gao, X.; Midgley, A.C.; et al. M2 Macrophage-Derived sEV Regulate Pro-Inflammatory CCR2(+) Macrophage Subpopulations to Favor Post-AMI Cardiac Repair. Adv. Sci. 2023, 22, e2202964. [Google Scholar] [CrossRef]
- Cron, R.Q.; Goyal, G.; Chatham, W.W. Cytokine Storm Syndrome. Annu. Rev. Med. 2023, 74, 321–337. [Google Scholar] [CrossRef]
- Fadok, V.A.; Bratton, D.L.; Konowal, A.; Freed, P.W.; Westcott, J.Y.; Henson, P.M. Macrophages That Have Ingested Apoptotic Cells In Vitro Inhibit Proinflammatory Cytokine Production Through Autocrine/Paracrine Mechanisms Involving TGF-β, PGE2, and PAF. J. Clin. Investig. 1998, 101, 890–898. [Google Scholar] [CrossRef] [PubMed]
- Chazaud, B. Inflammation and Skeletal Muscle Regeneration: Leave It to the Macrophages! Trends Immunol. 2020, 41, 481–492. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Guo, J.; Wang, C.; Luo, F.; Yu, X.; Zhang, W.; Li, J.; Zhao, D.; Xu, D.; Gong, Q.; et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: A retrospective review of medical records. Lancet 2020, 395, 809–815. [Google Scholar] [CrossRef] [PubMed]
- Denorme, F.; Portier, I.; Rustad, J.L.; Cody, M.J.; de Araujo, C.V.; Hoki, C.; Alexander, M.D.; Grandhi, R.; Dyer, M.R.; Neal, M.D.; et al. Neutrophil extracellular traps regulate ischemic stroke brain injury. J. Clin. Investig. 2022, 132. [Google Scholar] [CrossRef]
- Lafuse, W.P.; Wozniak, D.J.; Rajaram, M.V.S. Role of Cardiac Macrophages on Cardiac Inflammation, Fibrosis and Tissue Repair. Cells 2020, 10, 51. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Savill, J. Resolution of inflammation: The beginning programs the end. Nat. Immunol. 2005, 6, 1191–1197. [Google Scholar] [CrossRef]
- Serhan, C.N.; Chiang, N.; Van Dyke, T.E. Resolving inflammation: Dual anti-inflammatory and pro-resolution lipid mediators. Nat. Rev. Immunol. 2008, 8, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.S.; Han, W.; Chen, S.M.; Sherrill, T.P.; Chont, M.; Park, G.Y.; Sheller, J.R.; Polosukhin, V.V.; Christman, J.W.; Yull, F.E.; et al. Airway epithelium controls lung inflammation and injury through the NF-kappa B pathway. J. Immunol. 2007, 178, 6504–6513. [Google Scholar] [CrossRef] [PubMed]
- Quinton, L.J.; Jones, M.R.; Simms, B.T.; Kogan, M.S.; Robson, B.E.; Skerrett, S.J.; Mizgerd, J.P. Functions and regulation of NF-kappaB RelA during pneumococcal pneumonia. J. Immunol. 2007, 178, 1896–1903. [Google Scholar] [CrossRef]
- Nayak, A.; Dodagatta-Marri, E.; Tsolaki, A.G.; Kishore, U. An Insight into the Diverse Roles of Surfactant Proteins, SP-A and SP-D in Innate and Adaptive Immunity. Front. Immunol. 2012, 3, 131. [Google Scholar] [CrossRef]
- Han, S.; Mallampalli, R.K. The Role of Surfactant in Lung Disease and Host Defense against Pulmonary Infections. Ann. Am. Thorac Soc. 2015, 12, 765–774. [Google Scholar] [CrossRef]
- Torres, A.; Cilloniz, C.; Niederman, M.S.; Menendez, R.; Chalmers, J.D.; Wunderink, R.G.; van der Poll, T. Pneumonia. Nat. Rev. Dis. Prim. 2021, 7, 25. [Google Scholar] [CrossRef]
- Zhang, F.; Mears, J.R.; Shakib, L.; Beynor, J.I.; Shanaj, S.; Korsunsky, I.; Nathan, A.; Accelerating Medicines Partnership Rheumatoid Arthritis and Systemic Lupus Erythematosus (AMP RA/SLE) Consortium; Donlin, L.T.; Raychaudhuri, S. IFN-gamma and TNF-alpha drive a CXCL10+ CCL2+ macrophage phenotype expanded in severe COVID-19 lungs and inflammatory diseases with tissue inflammation. Genome Med. 2021, 13, 64. [Google Scholar] [CrossRef]
- Wu, D.D.; Pan, P.H.; Liu, B.; Su, X.L.; Zhang, L.M.; Tan, H.Y.; Cao, Z.; Zhou, Z.R.; Li, H.T.; Li, H.S.; et al. Inhibition of Alveolar Macrophage Pyroptosis Reduces Lipopolysaccharide-induced Acute Lung Injury in Mice. Chin. Med. J. 2015, 128, 2638–2645. [Google Scholar] [CrossRef]
- Tao, Q.; Du, J.; Li, X.; Zeng, J.; Tan, B.; Xu, J.; Lin, W.; Chen, X.L. Network pharmacology and molecular docking analysis on molecular targets and mechanisms of Huashi Baidu formula in the treatment of COVID-19. Drug Dev. Ind. Pharm. 2020, 46, 1345–1353. [Google Scholar] [CrossRef]
- Li, D.; Ren, W.; Jiang, Z.; Zhu, L. Regulation of the NLRP3 inflammasome and macrophage pyroptosis by the p38 MAPK signaling pathway in a mouse model of acute lung injury. Mol. Med. Rep. 2018, 18, 4399–4409. [Google Scholar] [CrossRef] [PubMed]
- Fan, E.K.Y.; Fan, J. Regulation of alveolar macrophage death in acute lung inflammation. Respir. Res. 2018, 19, 50. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Sun, T. Glycyrrhizin administration ameliorates Streptococcus aureus-induced acute lung injury. Int. Immunopharmacol. 2019, 70, 504–511. [Google Scholar] [CrossRef] [PubMed]
- Xiao, K.; He, W.; Guan, W.; Hou, F.; Yan, P.; Xu, J.; Zhou, T.; Liu, Y.; Xie, L. Mesenchymal stem cells reverse EMT process through blocking the activation of NF-kappaB and Hedgehog pathways in LPS-induced acute lung injury. Cell Death Dis. 2020, 11, 863. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, D.; Wang, L.; Wang, S.; Roden, A.C.; Zhao, H.; Li, X.; Prakash, Y.S.; Matteson, E.L.; Tschumperlin, D.J.; et al. Profibrotic effect of IL-17A and elevated IL-17RA in idiopathic pulmonary fibrosis and rheumatoid arthritis-associated lung disease support a direct role for IL-17A/IL-17RA in human fibrotic interstitial lung disease. Am. J. Physiol. Lung Cell. Mol. Physiol. 2019, 316, L487–L497. [Google Scholar] [CrossRef] [PubMed]
- Andrews, K.; Abdelsamed, H.; Yi, A.K.; Miller, M.A.; Fitzpatrick, E.A. TLR2 regulates neutrophil recruitment and cytokine production with minor contributions from TLR9 during hypersensitivity pneumonitis. PLoS ONE 2013, 8, e73143. [Google Scholar] [CrossRef]
- Hasan, S.A.; Eksteen, B.; Reid, D.; Paine, H.V.; Alansary, A.; Johannson, K.; Gwozd, C.; Goring, K.A.; Vo, T.; Proud, D.; et al. Role of IL-17A and neutrophils in fibrosis in experimental hypersensitivity pneumonitis. J. Allergy Clin. Immunol. 2013, 131, 1663–1673. [Google Scholar] [CrossRef]
- Kawamura, S.; Onai, N.; Miya, F.; Sato, T.; Tsunoda, T.; Kurabayashi, K.; Yotsumoto, S.; Kuroda, S.; Takenaka, K.; Akashi, K.; et al. Identification of a Human Clonogenic Progenitor with Strict Monocyte Differentiation Potential: A Counterpart of Mouse cMoPs. Immunity 2017, 46, 835–848.e4. [Google Scholar] [CrossRef] [PubMed]
- Swirski, F.K.; Hilgendorf, I.; Robbins, C.S. From proliferation to proliferation: Monocyte lineage comes full circle. Semin. Immunopathol. 2014, 36, 137–148. [Google Scholar] [CrossRef]
- Kuroda, Y.; Hisatsune, C.; Nakamura, T.; Matsuo, K.; Mikoshiba, K. Osteoblasts induce Ca2+ oscillation-independent NFATc1 activation during osteoclastogenesis. Proc. Natl. Acad. Sci. USA 2008, 105, 8643–8648. [Google Scholar] [CrossRef] [PubMed]
- Menezes, S.; Melandri, D.; Anselmi, G.; Perchet, T.; Loschko, J.; Dubrot, J.; Patel, R.; Gautier, E.L.; Hugues, S.; Longhi, M.P.; et al. The Heterogeneity of Ly6C(hi) Monocytes Controls Their Differentiation into iNOS(+) Macrophages or Monocyte-Derived Dendritic Cells. Immunity 2016, 45, 1205–1218. [Google Scholar] [CrossRef]
- Gao, Y.; Zhao, H.; Wang, P.; Wang, J.; Zou, L. The roles of SOCS3 and STAT3 in bacterial infection and inflammatory diseases. Scand. J. Immunol. 2018, 88, e12727. [Google Scholar] [CrossRef]
- Qin, H.; Holdbrooks, A.T.; Liu, Y.; Reynolds, S.L.; Yanagisawa, L.L.; Benveniste, E.N. SOCS3 deficiency promotes M1 macrophage polarization and inflammation. J. Immunol. 2012, 189, 3439–3448. [Google Scholar] [CrossRef]
- Marie de Lumley, D.J.H.; Matthew, A. Cooper, Stefan Symeonides, Jonathan M. Blackburn. Genetic analysis of NF-kB and Rel transcription factors. J. Mol. Biol. 2004, 339, 1059–1075. [Google Scholar]
- Mitchell, S.; Vargas, J.; Hoffmann, A. Signaling via the NFkappaB system. Wiley Interdiscip. Rev. Syst. Biol. Med. 2016, 8, 227–241. [Google Scholar] [CrossRef] [PubMed]
- Remels, A.H.; Gosker, H.R.; Langen, R.C.; Polkey, M.; Sliwinski, P.; Galdiz, J.; van den Borst, B.; Pansters, N.A.; Schols, A.M. Classical NF-kappaB activation impairs skeletal muscle oxidative phenotype by reducing IKK-alpha expression. Biochim. Biophys. Acta 2014, 1842, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Beinke, S.; Robinson, M.J.; Hugunin, M.; Ley, S.C. Lipopolysaccharide activation of the TPL-2/MEK/extracellular signal-regulated kinase mitogen-activated protein kinase cascade is regulated by IkappaB kinase-induced proteolysis of NF-kappaB1 p105. Mol. Cell. Biol. 2004, 24, 9658–9667. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.T.; Papoutsopoulou, S.; Belich, M.; Brender, C.; Janzen, J.; Gantke, T.; Handley, M.; Ley, S.C. Coordinate regulation of TPL-2 and NF-kappaB signaling in macrophages by NF-kappaB1 p105. Mol. Cell. Biol. 2012, 32, 3438–3451. [Google Scholar] [CrossRef]
- O’Neill, L.A.; Bowie, A.G. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat. Rev. Immunol. 2007, 7, 353–364. [Google Scholar] [CrossRef]
- Speciale, A.; Muscara, C.; Molonia, M.S.; Toscano, G.; Cimino, F.; Saija, A. In Vitro Protective Effects of a Standardized Extract from Cynara Cardunculus L. Leaves Against TNF-alpha-Induced Intestinal Inflammation. Front. Pharmacol. 2022, 13, 809938. [Google Scholar] [CrossRef]
- Hunter, C.A.; Jones, S.A. IL-6 as a keystone cytokine in health and disease. Nat. Immunol. 2015, 16, 448–457. [Google Scholar] [CrossRef]
- McConnell, M.J.; Kawaguchi, N.; Kondo, R.; Sonzogni, A.; Licini, L.; Valle, C.; Bonaffini, P.A.; Sironi, S.; Alessio, M.G.; Previtali, G.; et al. Liver injury in COVID-19 and IL-6 trans-signaling-induced endotheliopathy. J. Hepatol. 2021, 75, 647–658. [Google Scholar] [CrossRef]
- Rose-John, S. IL-6 trans-signaling via the soluble IL-6 receptor: Importance for the pro-inflammatory activities of IL-6. Int. J. Biol. Sci. 2012, 8, 1237–1247. [Google Scholar] [CrossRef]
- Smith, M.A.; Choudhary, G.S.; Pellagatti, A.; Choi, K.; Bolanos, L.C.; Bhagat, T.D.; Gordon-Mitchell, S.; Von Ahrens, D.; Pradhan, K.; Steeples, V.; et al. U2AF1 mutations induce oncogenic IRAK4 isoforms and activate innate immune pathways in myeloid malignancies. Nat. Cell Biol. 2019, 21, 640–650. [Google Scholar] [CrossRef]
- Gantke, T.; Sriskantharajah, S.; Ley, S.C. Regulation and function of TPL-2, an IkappaB kinase-regulated MAP kinase kinase kinase. Cell Res. 2011, 21, 131–145. [Google Scholar] [CrossRef]
- Kayama, H.; Ramirez-Carrozzi, V.R.; Yamamoto, M.; Mizutani, T.; Kuwata, H.; Iba, H.; Matsumoto, M.; Honda, K.; Smale, S.T.; Takeda, K. Class-specific regulation of pro-inflammatory genes by MyD88 pathways and IkappaBzeta. J. Biol. Chem. 2008, 283, 12468–12477. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Zhang, R.; Li, C.; Jiang, L.; Xiang, M.; Ye, Z.; Kita, H.; Melnick, A.M.; Dent, A.L.; Sun, J. BCL6 modulates tissue neutrophil survival and exacerbates pulmonary inflammation following influenza virus infection. Proc. Natl. Acad. Sci. USA 2019, 116, 11888–11893. [Google Scholar] [CrossRef] [PubMed]
- Raines, L.N.; Zhao, H.; Wang, Y.; Chen, H.Y.; Gallart-Ayala, H.; Hsueh, P.C.; Cao, W.; Koh, Y.; Alamonte-Loya, A.; Liu, P.S.; et al. PERK is a critical metabolic hub for immunosuppressive function in macrophages. Nat. Immunol. 2022, 23, 431–445. [Google Scholar] [CrossRef]
- Satoh, T.; Takeuchi, O.; Vandenbon, A.; Yasuda, K.; Tanaka, Y.; Kumagai, Y.; Miyake, T.; Matsushita, K.; Okazaki, T.; Saitoh, T.; et al. The Jmjd3-Irf4 axis regulates M2 macrophage polarization and host responses against helminth infection. Nat. Immunol. 2010, 11, 936–944. [Google Scholar] [CrossRef] [PubMed]
- Jaguin, M.; Houlbert, N.; Fardel, O.; Lecureur, V. Polarization profiles of human M-CSF-generated macrophages and comparison of M1-markers in classically activated macrophages from GM-CSF and M-CSF origin. Cell Immunol. 2013, 281, 51–61. [Google Scholar] [CrossRef]
- Barros, M.H.; Hauck, F.; Dreyer, J.H.; Kempkes, B.; Niedobitek, G. Macrophage polarisation: An immunohistochemical approach for identifying M1 and M2 macrophages. PLoS ONE 2013, 8, e80908. [Google Scholar] [CrossRef] [PubMed]
- Daassi, D.; Hamada, M.; Jeon, H.; Imamura, Y.; Nhu Tran, M.T.; Takahashi, S. Differential expression patterns of MafB and c-Maf in macrophages in vivo and in vitro. Biochem. Biophys. Res. Commun. 2016, 473, 118–124. [Google Scholar] [CrossRef]
- Conejo-Garcia, J.R.; Rodriguez, P.C. c-Maf: A bad influence in the education of macrophages. J. Clin. Investig. 2020, 130, 1629–1631. [Google Scholar] [CrossRef]
- Tzavlaki, K.; Moustakas, A. TGF-beta Signaling. Biomolecules 2020, 10, 487. [Google Scholar] [CrossRef]
- Xu, H.; Wu, L.; Nguyen, H.H.; Mesa, K.R.; Raghavan, V.; Episkopou, V.; Littman, D.R. Arkadia-SKI/SnoN signaling differentially regulates TGF-beta-induced iTreg and Th17 cell differentiation. J. Exp. Med. 2021, 218, e20210777. [Google Scholar] [CrossRef] [PubMed]
- Carvajal Alegria, G.; Cornec, D.; Saraux, A.; Devauchelle-Pensec, V.; Jamin, C.; Hillion, S.; Pers, J.O.; Pochard, P. Abatacept Promotes Regulatory B Cell Functions, Enhancing Their Ability to Reduce the Th1 Response in Rheumatoid Arthritis Patients through the Production of IL-10 and TGF-beta. J. Immunol. 2021, 207, 470–482. [Google Scholar] [CrossRef]
- Tanaka, Y. A review of Janus kinase inhibitors for the treatment of COVID-19 pneumonia. Inflamm. Regen. 2023, 43, 3. [Google Scholar] [CrossRef]
- Liu, Z.Z.; Xu, N.Y.; Wang, M.L.; Tang, R.Z.; Liu, X.Q. Physical confinement in alginate cryogels determines macrophage polarization to a M2 phenotype by regulating a STAT-related mRNA transcription pathway. Biomater. Sci. 2022, 10, 2315–2327. [Google Scholar] [CrossRef] [PubMed]
- Mia, S.; Warnecke, A.; Zhang, X.M.; Malmstrom, V.; Harris, R.A. An optimized protocol for human M2 macrophages using M-CSF and IL-4/IL-10/TGF-beta yields a dominant immunosuppressive phenotype. Scand. J. Immunol. 2014, 79, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Ma, Q.; Li, C.; Liu, R.; Zhao, L.; Wang, W.; Zhang, P.; Liu, X.; Gao, G.; Liu, F.; et al. Profiling serum cytokines in COVID-19 patients reveals IL-6 and IL-10 are disease severity predictors. Emerg. Microbes. Infect. 2020, 9, 1123–1130. [Google Scholar] [CrossRef]
- Van den Bossche, J.; Baardman, J.; Otto, N.A.; van der Velden, S.; Neele, A.E.; van den Berg, S.M.; Luque-Martin, R.; Chen, H.J.; Boshuizen, M.C.; Ahmed, M.; et al. Mitochondrial Dysfunction Prevents Repolarization of Inflammatory Macrophages. Cell Rep. 2016, 17, 684–696. [Google Scholar] [CrossRef]
- Ji, L.; Zhao, X.; Zhang, B.; Kang, L.; Song, W.; Zhao, B.; Xie, W.; Chen, L.; Hu, X. Slc6a8-Mediated Creatine Uptake and Accumulation Reprogram Macrophage Polarization via Regulating Cytokine Responses. Immunity 2019, 51, 272–284.e7. [Google Scholar] [CrossRef]
- Anderson-Baucum, E.; Pineros, A.R.; Kulkarni, A.; Webb-Robertson, B.J.; Maier, B.; Anderson, R.M.; Wu, W.; Tersey, S.A.; Mastracci, T.L.; Casimiro, I.; et al. Deoxyhypusine synthase promotes a pro-inflammatory macrophage phenotype. Cell Metab. 2021, 33, 1883–1893 e1887. [Google Scholar] [CrossRef]
- Puleston, D.J.; Buck, M.D.; Klein Geltink, R.I.; Kyle, R.L.; Caputa, G.; O’Sullivan, D.; Cameron, A.M.; Castoldi, A.; Musa, Y.; Kabat, A.M.; et al. Polyamines and eIF5A Hypusination Modulate Mitochondrial Respiration and Macrophage Activation. Cell Metab. 2019, 30, 352–363 e358. [Google Scholar] [CrossRef] [PubMed]
- Simon, B.; Brown, J.S. Fas Ligand and Induces Apoptosis of Phagocytosis Triggers Macrophage Release of Bystander Leukocytes. J. Immunol. 1998, 162, 480–485. [Google Scholar]
- Luo, K. Signaling Cross Talk between TGF-beta/Smad and Other Signaling Pathways. Cold Spring Harb. Perspect. Biol. 2017, 9, a022137. [Google Scholar] [CrossRef] [PubMed]
- Vannella, K.M.; Wynn, T.A. Mechanisms of Organ Injury and Repair by Macrophages. Annu. Rev. Physiol. 2017, 79, 593–617. [Google Scholar] [CrossRef]
- Klompas, M. Overuse of Broad-Spectrum Antibiotics for Pneumonia. JAMA Intern. Med. 2020, 180, 485–486. [Google Scholar] [CrossRef]
- Blaser, M.J.; Melby, M.K.; Lock, M.; Nichter, M. Accounting for variation in and overuse of antibiotics among humans. Bioessays 2021, 43, e2000163. [Google Scholar] [CrossRef]
- Wang, J.; Wang, P.; Wang, X.; Zheng, Y.; Xiao, Y. Use and prescription of antibiotics in primary health care settings in China. JAMA Intern. Med. 2014, 174, 1914–1920. [Google Scholar] [CrossRef]
- Almeida, M.Q.; Mendonca, B.B. Adrenal Insufficiency and Glucocorticoid Use During the COVID-19 Pandemic. Clinics 2020, 75, e2022. [Google Scholar] [CrossRef]
- Oray, M.; Abu Samra, K.; Ebrahimiadib, N.; Meese, H.; Foster, C.S. Long-term side effects of glucocorticoids. Expert Opin. Drug Saf. 2016, 15, 457–465. [Google Scholar] [CrossRef]
- Gurnell, M.; Heaney, L.G.; Price, D.; Menzies-Gow, A. Long-term corticosteroid use, adrenal insufficiency and the need for steroid-sparing treatment in adult severe asthma. J. Intern. Med. 2021, 290, 240–256. [Google Scholar] [CrossRef]
- Van Raalte, D.H.; Ouwens, D.M.; Diamant, M. Novel insights into glucocorticoid-mediated diabetogenic effects: Towards expansion of therapeutic options? Eur. J. Clin. Investig. 2009, 39, 81–93. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.M.; Carvalho, J.F.; Canalis, E. Glucocorticoid-induced osteoporosis in rheumatic diseases. Clinics 2010, 65, 1197–1205. [Google Scholar] [CrossRef]
- Patel, V.; Fisher, M.; Voelker, M.; Gessner, U. Gastrointestinal effects of the addition of ascorbic acid to aspirin. Pain Pract. 2012, 12, 476–484. [Google Scholar] [CrossRef] [PubMed]
- Potaczek, D.P.; Trad, G.; Sanak, M.; Garn, H.; Mastalerz, L. Local and Systemic Production of Pro-Inflammatory Eicosanoids Is Inversely Related to Sensitization to Aeroallergens in Patients with Aspirin-Exacerbated Respiratory Disease. J. Pers. Med. 2022, 12, 447. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Nowicki, M.; Sun, H.; Hann, S.Y.; Cui, H.; Esworthy, T.; Lee, J.D.; Plesniak, M.; Zhang, L.G. 3D Bioprinting-Tunable Small-Diameter Blood Vessels with Biomimetic Biphasic Cell Layers. ACS Appl. Mater. Interfaces 2020, 12, 45904–45915. [Google Scholar] [CrossRef]
- Aachoui, Y.; Ghosh, S.K. Extracellular matrix from porcine small intestinal submucosa (SIS) as immune adjuvants. PLoS ONE 2011, 6, e27083. [Google Scholar] [CrossRef] [PubMed]
- Paramasivam, T.; Maiti, S.K.; Palakkara, S.; Rashmi; Mohan, D.; Manjunthaachar, H.V.; Karthik, K.; Kumar, N. Effect of PDGF-B Gene-Activated Acellular Matrix and Mesenchymal Stem Cell Transplantation on Full Thickness Skin Burn Wound in Rat Model. Tissue Eng. Regen Med. 2021, 18, 235–251. [Google Scholar] [CrossRef]
- Keane, T.J.; Londono, R.; Turner, N.J.; Badylak, S.F. Consequences of ineffective decellularization of biologic scaffolds on the host response. Biomaterials 2012, 33, 1771–1781. [Google Scholar] [CrossRef]
- Brown, B.N.; Valentin, J.E.; Stewart-Akers, A.M.; McCabe, G.P.; Badylak, S.F. Macrophage phenotype and remodeling outcomes in response to biologic scaffolds with and without a cellular component. Biomaterials 2009, 30, 1482–1491. [Google Scholar] [CrossRef]
- Dziki, J.L.; Wang, D.S.; Pineda, C.; Sicari, B.M.; Rausch, T.; Badylak, S.F. Solubilized extracellular matrix bioscaffolds derived from diverse source tissues differentially influence macrophage phenotype. J. Biomed. Mater. Res. A 2017, 105, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Sadtler, K.; Estrellas, K.; Allen, B.W.; Wolf, M.T.; Fan, H.; Tam, A.J.; Patel, C.H.; Luber, B.S.; Wang, H.; Wagner, K.R.; et al. Developing a pro-regenerative biomaterial scaffold microenvironment requires T helper 2 cells. Science 2016, 352, 366–370. [Google Scholar] [CrossRef] [PubMed]
- Huleihel, L.H.G.; Naranjo, J.D.; Zhang, L.; Dziki, J.L.; Turner, N.J.; Stolz, D.B.; Badylak, S.F. Matrix-bound nanovesicles within ECM bioscaffolds. Sci. Adv. 2016, 2, e1600502. [Google Scholar] [CrossRef]
- Shen, Q.; Shi, P.; Gao, M.; Yu, X.; Liu, Y.; Luo, L.; Zhu, Y. Progress on materials and scaffold fabrications applied to esophageal tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 1860–1866. [Google Scholar] [CrossRef]
- Shi, P.; Gao, M.; Shen, Q.; Hou, L.; Zhu, Y.; Wang, J. Biocompatible surgical meshes based on decellularized human amniotic membrane. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 54, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Wang, W.; Ye, Q.; Bu, S.; Shen, Z.; Zhu, Y. The repairing of full-thickness skin deficiency and its biological mechanism using decellularized human amniotic membrane as the wound dressing. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 77, 739–747. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Shen, M.; Feng, P.; Qiu, H.; Wu, X.; Yang, L.; Zhu, Y. Various administration forms of decellularized amniotic membrane extract towards improving corneal repair. J. Mater. Chem. B 2021, 9, 9347–9357. [Google Scholar] [CrossRef] [PubMed]
- Uhl, F.E.; Zhang, F.; Pouliot, R.A.; Uriarte, J.J.; Rolandsson Enes, S.; Han, X.; Ouyang, Y.; Xia, K.; Westergren-Thorsson, G.; Malmström, A.; et al. Functional role of glycosaminoglycans in decellularized lung extracellular matrix. Acta Biomater. 2020, 102, 231–246. [Google Scholar] [CrossRef]
- Willemse, J.; van Tienderen, G.; van Hengel, E.; Schurink, I.; van der Ven, D.; Kan, Y.; de Ruiter, P.; Rosmark, O.; Westergren-Thorsson, G.G.; Schneeberger, K.; et al. Hydrogels derived from decellularized liver tissue support the growth and differentiation of cholangiocyte organoids. Biomaterials 2022, 284, 121473. [Google Scholar] [CrossRef]
- Song, J.J.; Guyette, J.P.; Gilpin, S.E.; Gonzalez, G.; Vacanti, J.P.; Ott, H.C. Regeneration and experimental orthotopic transplantation of a bioengineered kidney. Nat. Med. 2013, 19, 646–651. [Google Scholar] [CrossRef]
- Kim, J.W.; Nam, S.A.; Yi, J.; Kim, J.Y.; Lee, J.Y.; Park, S.Y.; Sen, T.; Choi, Y.M.; Lee, J.Y.; Kim, H.L.; et al. Kidney Decellularized Extracellular Matrix Enhanced the Vascularization and Maturation of Human Kidney Organoids. Adv. Sci. 2022, 9, e2103526. [Google Scholar] [CrossRef]
- Li, C.; Zhou, Y.; Liu, S.; Guo, R.; Lu, C.; Yin, D.; Zhang, Y.; Xu, X.; Dong, N.; Shi, J. Surface Modification of Decellularized Heart Valve by the POSS-PEG Hybrid Hydrogel to Prepare a Composite Scaffold Material with Anticalcification Potential. ACS Appl. Bio Mater. 2022, 5, 3923–3935. [Google Scholar] [CrossRef] [PubMed]
- Snyder, Y.; Jana, S. Strategies for development of decellularized heart valve scaffolds for tissue engineering. Biomaterials 2022, 288, 121675. [Google Scholar] [CrossRef]
- Holl, J.; Pawlukianiec, C.; Corton Ruiz, J.; Groth, D.; Grubczak, K.; Hady, H.R.; Dadan, J.; Reszec, J.; Czaban, S.; Kowalewski, C.; et al. Skin Substitute Preparation Method Induces Immunomodulatory Changes in Co-Incubated Cells through Collagen Modification. Pharmaceutics 2021, 13, 2164. [Google Scholar] [CrossRef]
- Wang, X.; Ma, Y.; Chen, J.; Liu, Y.; Liu, G.; Wang, P.; Wang, B.; Taketo, M.M.; Bellido, T.; Tu, X. A novel decellularized matrix of Wnt signaling-activated osteocytes accelerates the repair of critical-sized parietal bone defects with osteoclastogenesis, angiogenesis, and neurogenesis. Bioact. Mater. 2023, 21, 110–128. [Google Scholar] [CrossRef] [PubMed]
- Liang, K.; Zhao, C.; Song, C.; Zhao, L.; Qiu, P.; Wang, S.; Zhu, J.; Gong, Z.; Liu, Z.; Tang, R.; et al. In Situ Biomimetic Mineralization of Bone-Like Hydroxyapatite in Hydrogel for the Acceleration of Bone Regeneration. ACS Appl. Mater. Interfaces 2023, 15, 292–308. [Google Scholar] [CrossRef]
| Phenotype of Macrophage | Signal Pathway | Factors | Functions | References |
|---|---|---|---|---|
| M1(pro-inflammatory) | NF-κB | NF-κB, TNF-α, IL-1β | Relating to inflammation, apoptosis and tumorigenesis. Activated by TNF receptor and IL receptor (IL-1R). | [41,42] |
| Jak/Stat and IL-6 receptor family | STAT3, IL-6 | Inducing fever in autoimmune diseases, acute inflammatory response and infections. IL-6ST/GP130 could trigger the intracellular STAT3 signal pathway, exacerbating inflammation. | [48,50] | |
| Toll-like receptor | TLR4, CCL2 | LPS in Gram-negative bacterial walls could trigger this signaling system and take part in the tardive immune response or recruit pro-inflammatory macrophages. | [52,53] | |
| M2(anti-inflammatory) | TGF-β | TGF-β1, BMP-2, Smad2/3 | Regulating cell growth and differentiation and promoting transformation from pro-inflammatory into anti-inflammatory macrophages. | [55,56,57] |
| IL-10/mTOR | IL-10 | Taking actions on T, B, and dendritic cells in the immune system and exerting a powerful anti-inflammatory effect, limiting tissue destruction caused by inflammatory responses. | [59,60] | |
| Arginine-polyamines-hypusine | ARG, eIF5A | ARG is related to the transformation of macrophages from M1 to M2 polarization. eIF5A suppresses oxidative phosphorylation-dependent macrophage activation. | [66,67,68] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ni, R.; Jiang, L.; Zhang, C.; Liu, M.; Luo, Y.; Hu, Z.; Mou, X.; Zhu, Y. Biologic Mechanisms of Macrophage Phenotypes Responding to Infection and the Novel Therapies to Moderate Inflammation. Int. J. Mol. Sci. 2023, 24, 8358. https://doi.org/10.3390/ijms24098358
Ni R, Jiang L, Zhang C, Liu M, Luo Y, Hu Z, Mou X, Zhu Y. Biologic Mechanisms of Macrophage Phenotypes Responding to Infection and the Novel Therapies to Moderate Inflammation. International Journal of Molecular Sciences. 2023; 24(9):8358. https://doi.org/10.3390/ijms24098358
Chicago/Turabian StyleNi, Renhao, Lingjing Jiang, Chaohai Zhang, Mujie Liu, Yang Luo, Zeming Hu, Xianbo Mou, and Yabin Zhu. 2023. "Biologic Mechanisms of Macrophage Phenotypes Responding to Infection and the Novel Therapies to Moderate Inflammation" International Journal of Molecular Sciences 24, no. 9: 8358. https://doi.org/10.3390/ijms24098358
APA StyleNi, R., Jiang, L., Zhang, C., Liu, M., Luo, Y., Hu, Z., Mou, X., & Zhu, Y. (2023). Biologic Mechanisms of Macrophage Phenotypes Responding to Infection and the Novel Therapies to Moderate Inflammation. International Journal of Molecular Sciences, 24(9), 8358. https://doi.org/10.3390/ijms24098358







