Towards Protection of Nucleic Acids from Herbicide Attack: Self-Assembly of Betaines Based on Pillar[5]arene with Glyphosate and DNA
Abstract
1. Introduction
2. Results and Discussion
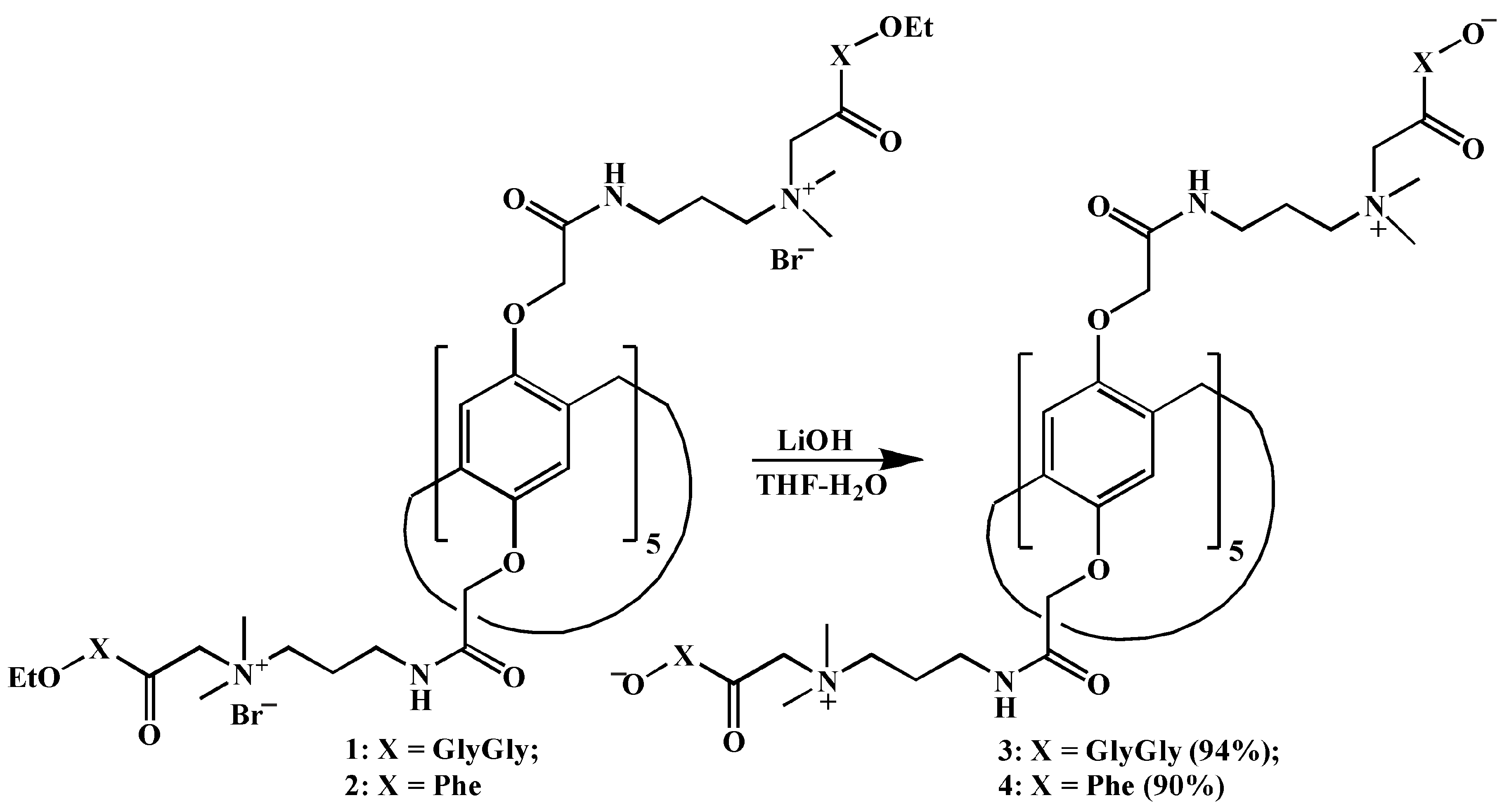
2.1. Synthesis of Decasubstituted Pillar[5]arenes Containing Betaine Fragments
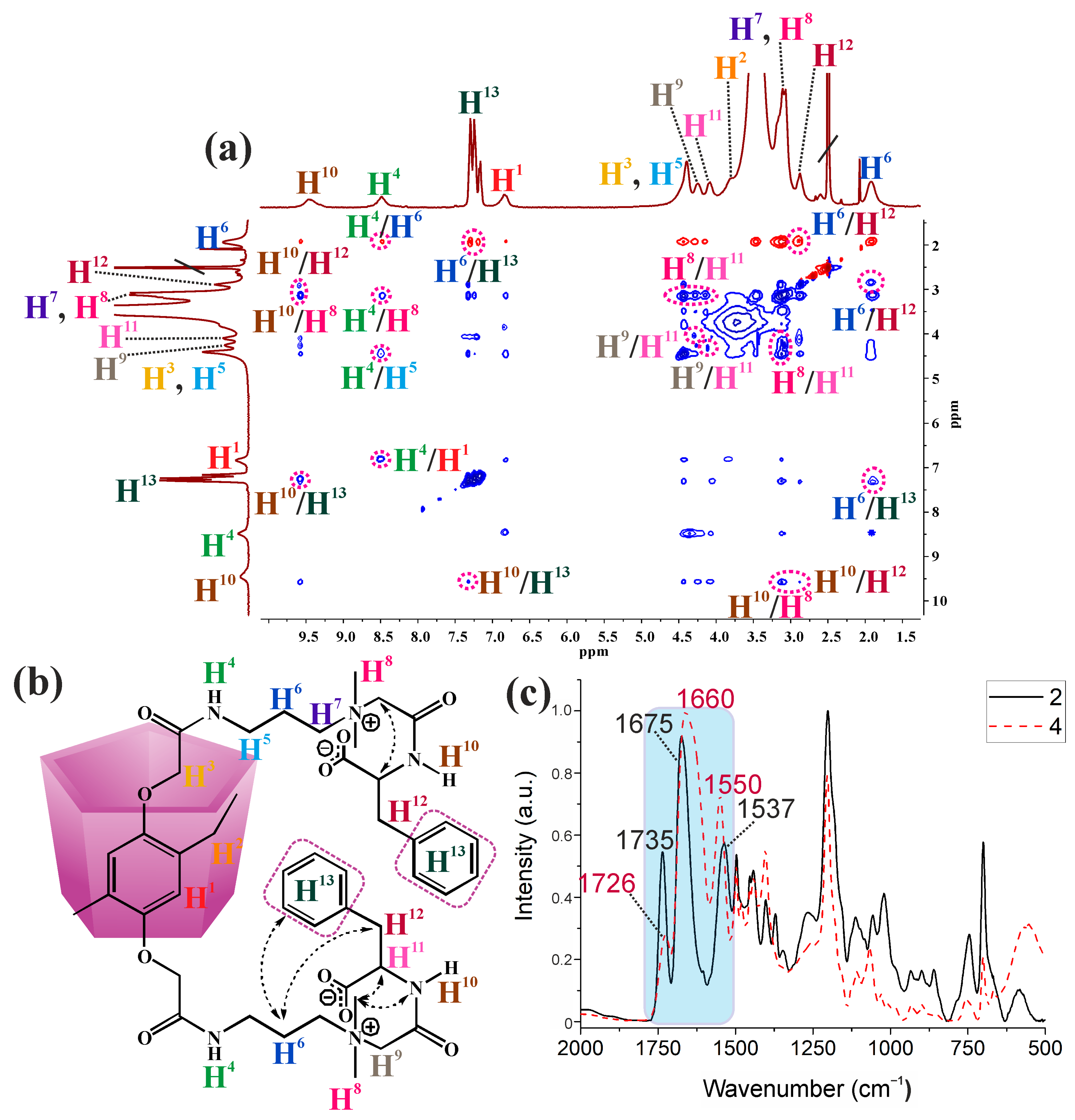
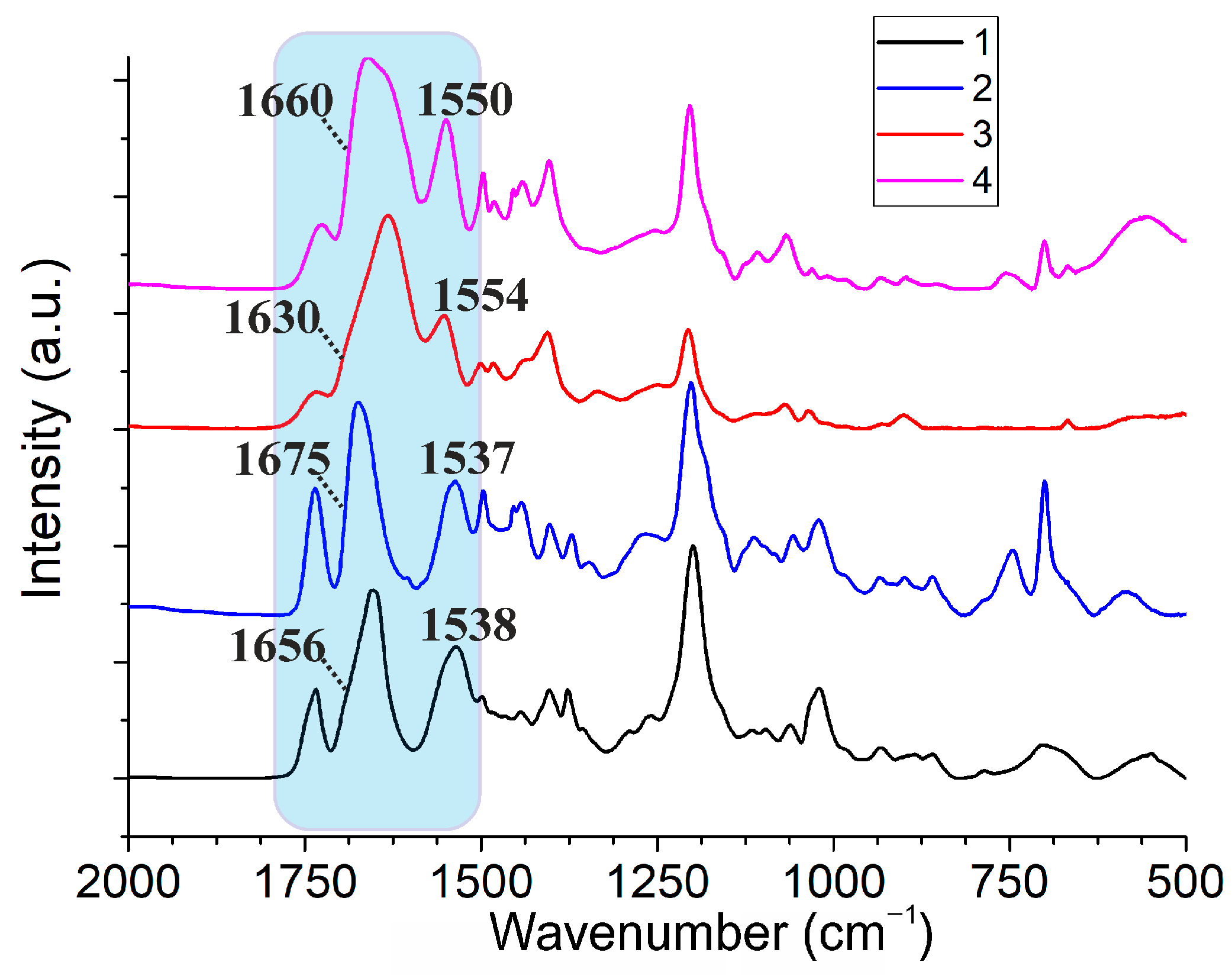
2.2. Aggregation Properties of the Synthesized Macrocycles
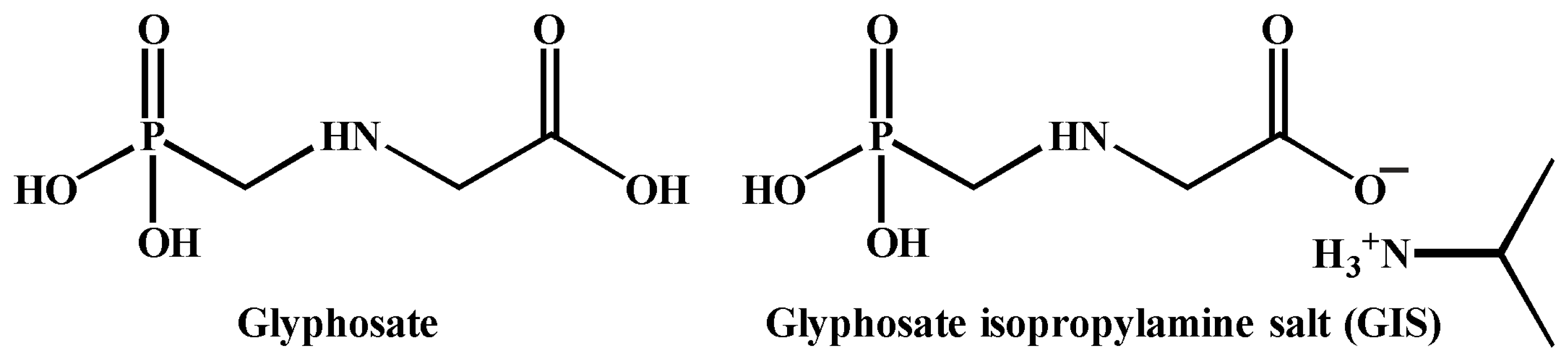
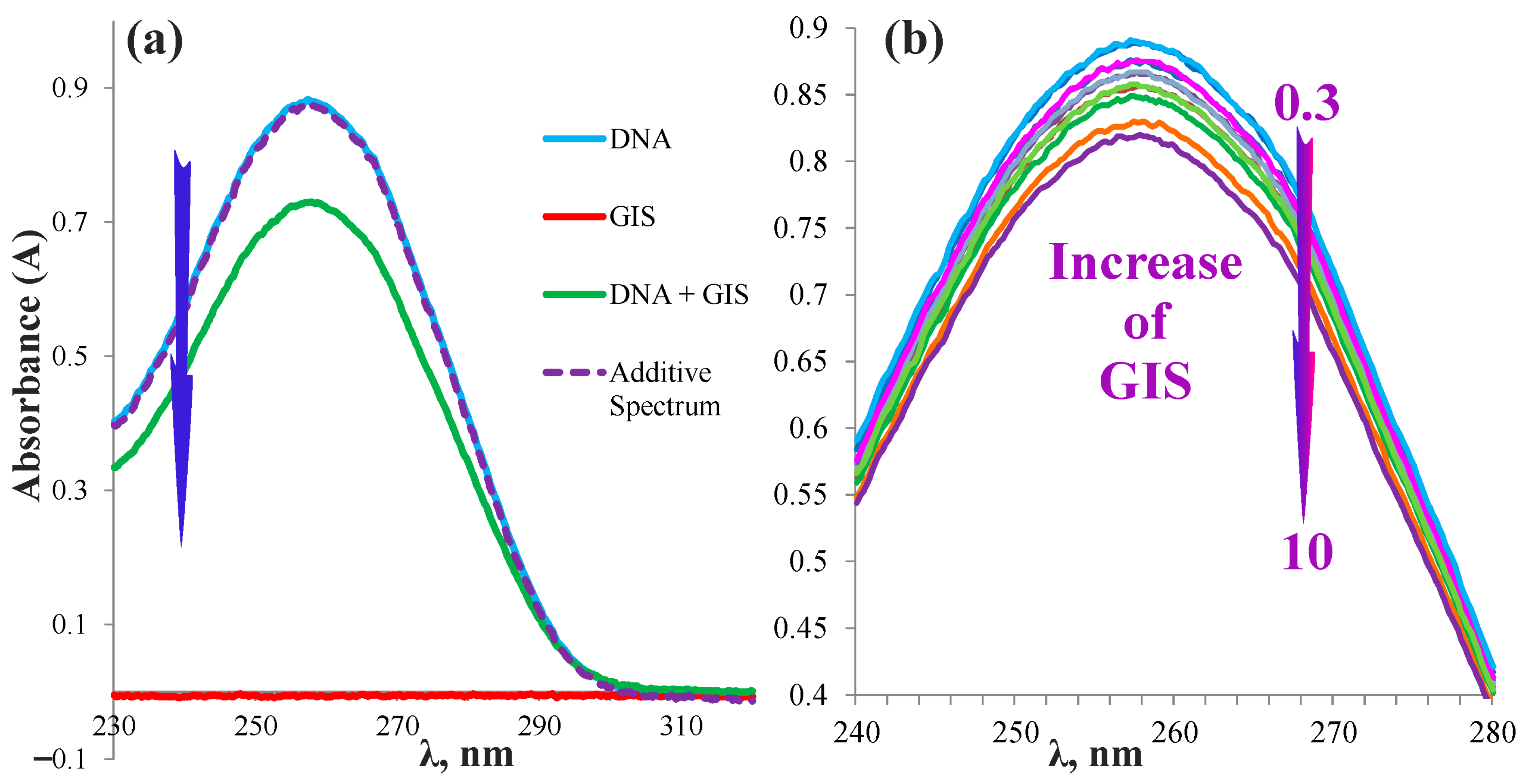
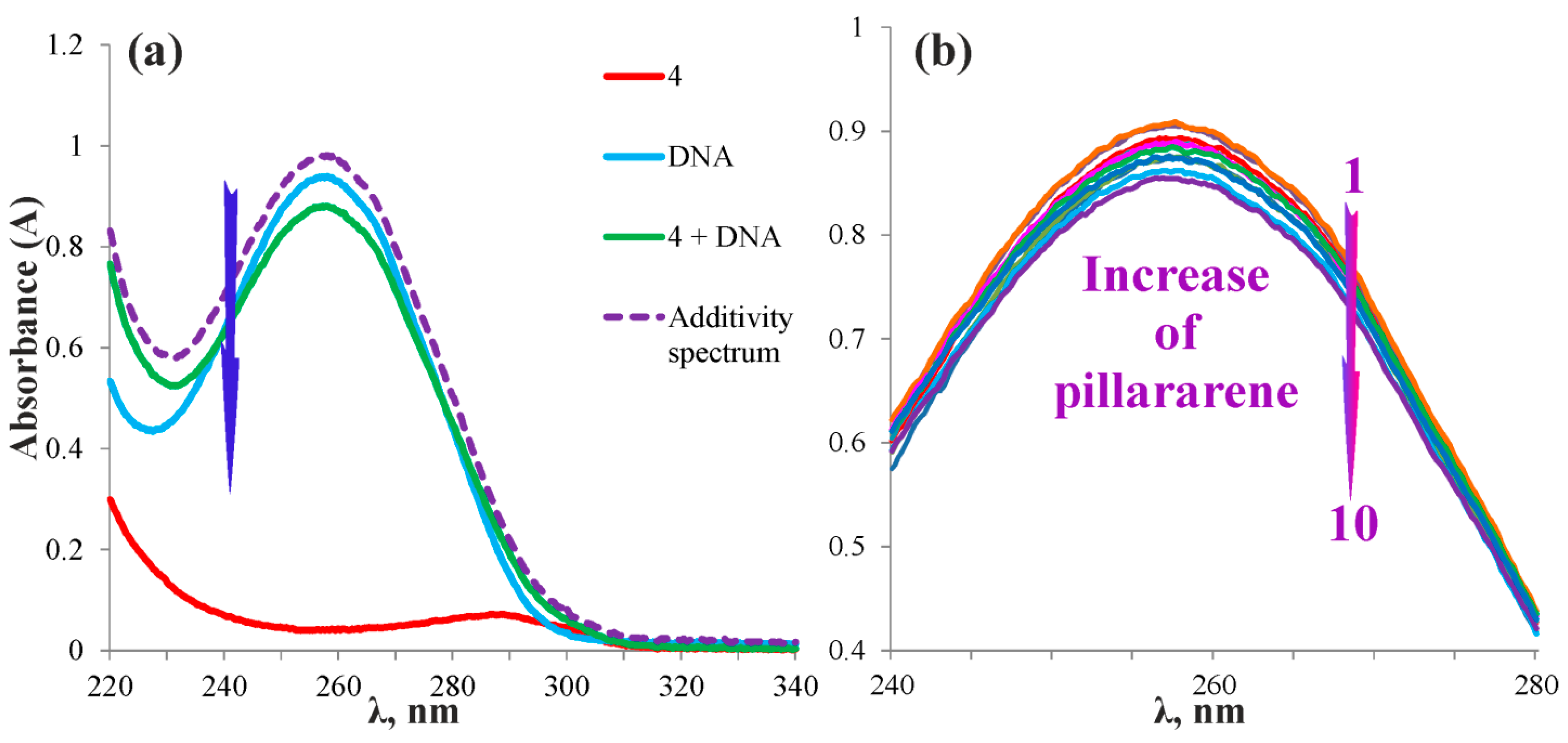
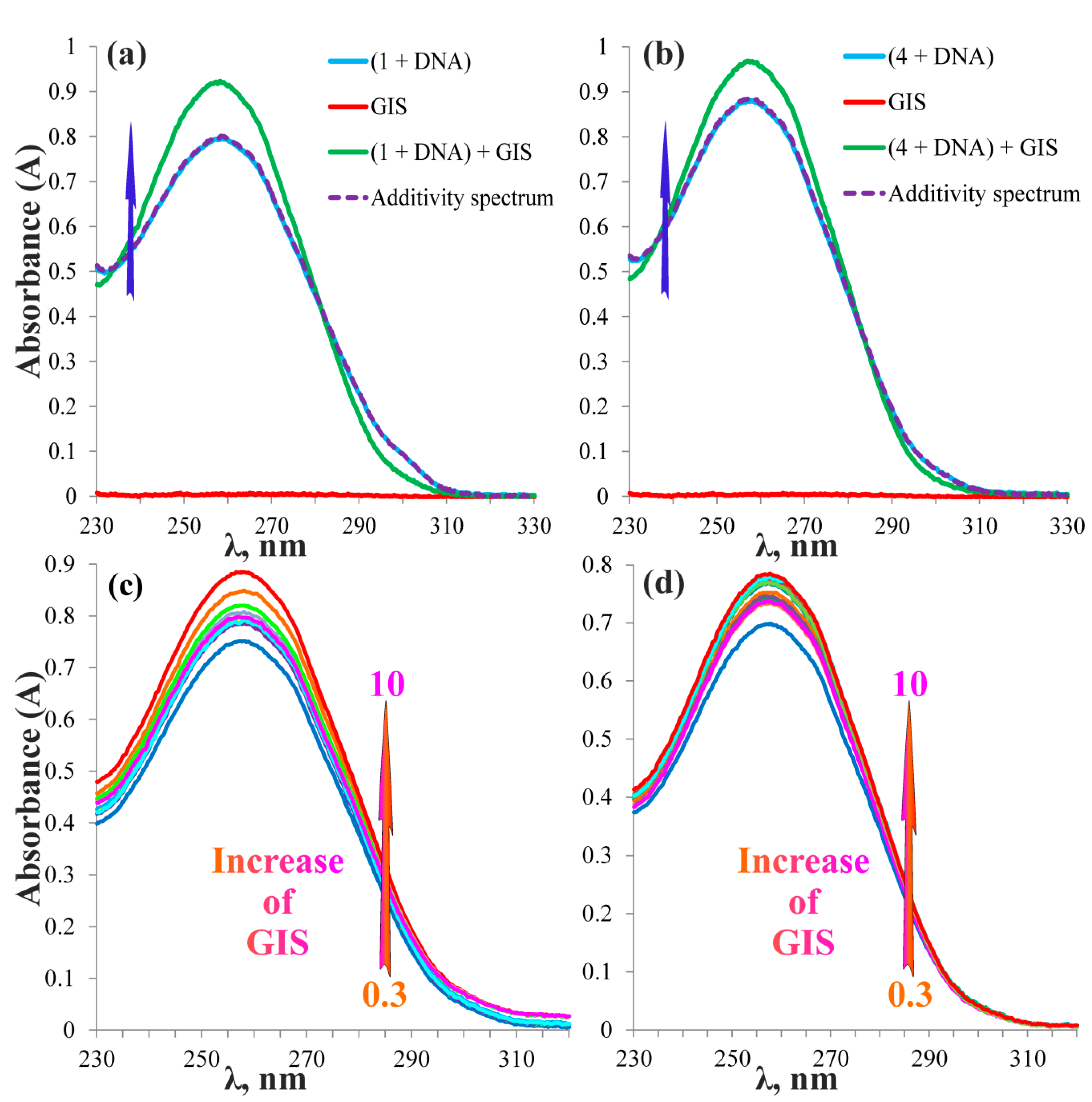
2.3. Complexation of the Pillar[5]arenes Containing Peptide and Betaine Fragments with DNA and Glyphosate Salt
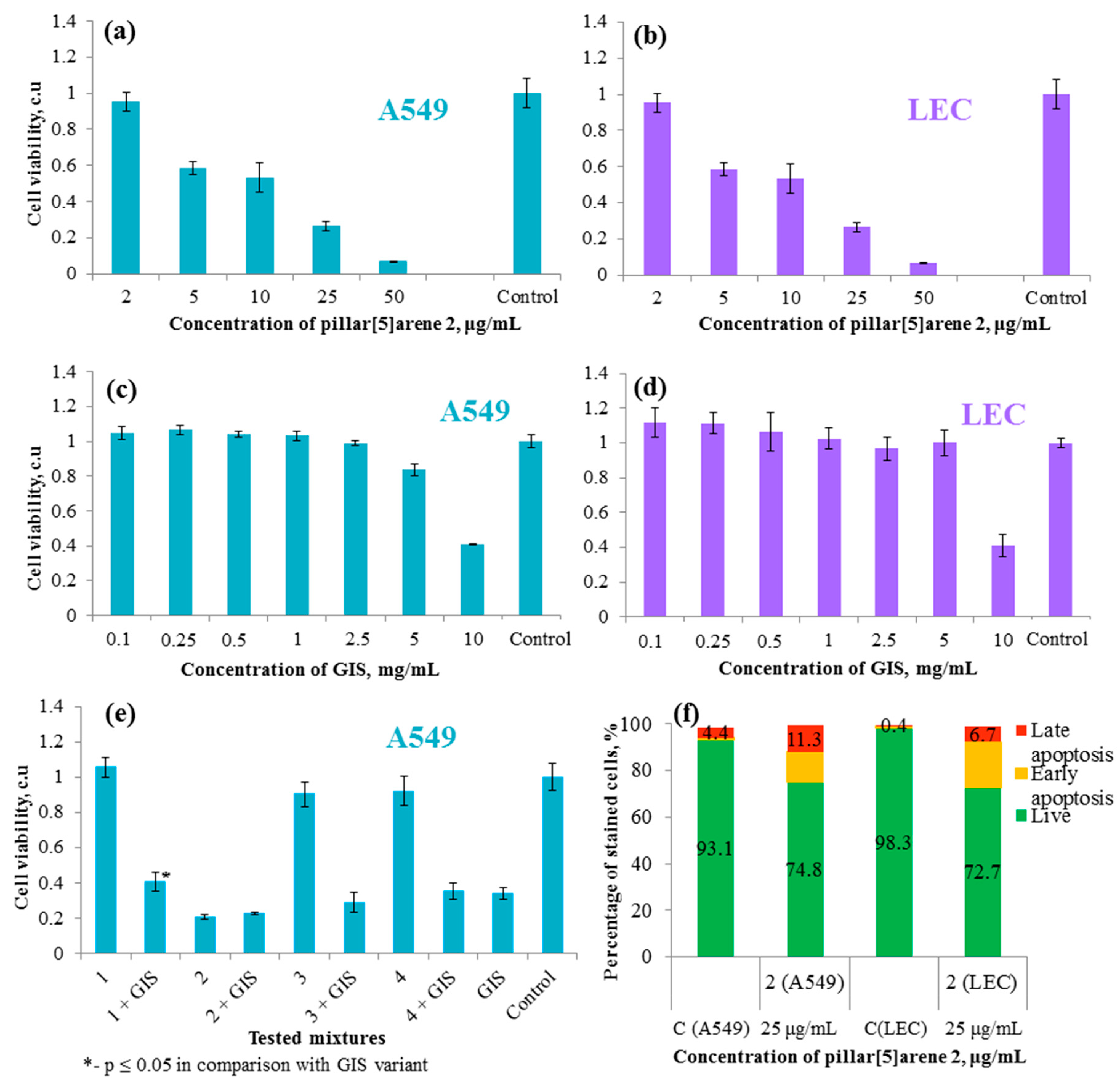
2.4. Cytotoxicity and Apoptosis-Inducing Ability of the Synthesized Macrocycles in the Absence and Presence of GIS
3. Materials and Methods
3.1. General Experimental Information
3.2. General Procedure for the Synthesis of Compounds 3 and 4
3.2.1. 4,8,14,18,23,26,28,31,32,35-Decakis-[(N-[3’-(dimethyl{[(oxidocarbonylmethyl)aminocarbonylmethyl]aminocarbonylmethyl}ammonio)propyl]aminocarbonylmethoxy]-pillar[5]arene (3)
3.2.2. 4,8,14,18,23,26,28,31,32,35-Decakis-[(N-[3’-dimethyl({oxidocarbonyl[S-benzyl]methyl}aminocarbonylmethyl)ammonio]propyl)aminocarbonylmethoxy]-pillar[5]arene (4)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Available online: https://www.un.org/en/global-issues/population (accessed on 29 March 2023).
- Hossain, A.; Krupnik, T.J.; Timsina, J.; Mahboob, M.G.; Chaki, A.K.; Farooq, M.; Bhatt, R.; Fahad, S.; Hasanuzzaman, M. Agricultural land degradation: Processes and problems undermining future food security. In Environment, Climate, Plant and Vegetation Growth; Springer International Publishing: Cham, Switzerland, 2020; pp. 17–61. [Google Scholar] [CrossRef]
- Kopittke, P.M.; Menzies, N.W.; Wang, P.; McKenna, B.A.; Lombi, E. Soil and the intensification of agriculture for global food security. Environ. Int. 2019, 132, 105078. [Google Scholar] [CrossRef] [PubMed]
- Gupta, G.S. Land degradation and challenges of food security. Rev. Eur. Stud. 2019, 11, 63. [Google Scholar] [CrossRef]
- Tudi, M.; Daniel Ruan, H.; Wang, L.; Lyu, J.; Sadler, R.; Connell, D.; Chu, C.; Phung, D.T. Agriculture development, pesticide application and its impact on the environment. Int. J. Environ. Res. Public Health. 2021, 18, 1112. [Google Scholar] [CrossRef] [PubMed]
- Galon, L.; Bragagnolo, L.; Korf, E.P.; dos Santos, J.B.; Barroso, G.M.; Ribeiro, V.H.V. Mobility and environmental monitoring of pesticides in the atmosphere—A review. Environ. Sci. Pollut. Res. 2021, 28, 32236–32255. [Google Scholar] [CrossRef] [PubMed]
- Duke, S.O.; Dayan, F.E. The search for new herbicide mechanisms of action: Is there a ‘holy grail’? Pest Manag. Sci. 2021, 78, 1303–1313. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Kumar, V.; Shahzad, B.; Tanveer, M.; Sidhu, G.P.S.; Handa, N.; Kohli, S.K.; Yadav, P.; Bali, A.S.; Parihar, R.D.; et al. Worldwide pesticide usage and its impacts on ecosystem. SN Appl. Sci. 2019, 1, 1446. [Google Scholar] [CrossRef]
- Mehdizadeh, M.; Mushtaq, W.; Anusha Siddiqui, S.; Ayadi, S.; Kaur, P.; Yeboah, S.; Mazraedoost, S.; K.A.AL-Taey, D.; Tampubolon, K. Herbicide residues in agroecosystems: Fate, detection, and effect on non-target plants. RAS 2021, 9, 157–167. [Google Scholar] [CrossRef]
- Krimsky, S. Can glyphosate-based herbicides contribute to sustainable agriculture? Sustainability 2021, 13, 2337. [Google Scholar] [CrossRef]
- Kanissery, R.; Gairhe, B.; Kadyampakeni, D.; Batuman, O.; Alferez, F. Glyphosate: Its environmental persistence and impact on crop health and nutrition. Plants 2019, 8, 499. [Google Scholar] [CrossRef]
- Annett, R.; Habibi, H.R.; Hontela, A. Impact of glyphosate and glyphosate-based herbicides on the freshwater environment. J. Appl. Toxicol. 2014, 34, 458–479. [Google Scholar] [CrossRef]
- Falfushynska, H.; Khatib, I.; Kasianchuk, N.; Lushchak, O.; Horyn, O.; Sokolova, I.M. Toxic effects and mechanisms of common pesticides (Roundup and chlorpyrifos) and their mixtures in a zebrafish model (Danio rerio). Sci. Total Environ. 2022, 833, 155236. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Zhang, Y.; Cheng, J.; Xu, W.; Xu, Z.; Gao, J.; Tao, L. Adjuvant contributes Roundup’s unexpected effects on A549 cells. Environ. Res. 2020, 184, 109306. [Google Scholar] [CrossRef] [PubMed]
- Congur, G. Monitoring of glyphosate-DNA interaction and synergistic genotoxic effect of glyphosate and 2,4-dichlorophenoxyacetic acid using an electrochemical biosensor. Environ. Pollut. 2021, 271, 116360. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, W.F.; Ruiz de Arcaute, C.; Pérez-Iglesias, J.M.; Laborde, M.R.R.; Soloneski, S.; Larramendy, M.L. DNA Damage exerted by mixtures of commercial formulations of glyphosate and imazethapyr herbicides in Rhinella arenarum (Anura, Bufonidae) tadpoles. Ecotoxicology 2019, 28, 367–377. [Google Scholar] [CrossRef]
- Hong, Y.; Yang, X.; Yan, G.; Huang, Y.; Zuo, F.; Shen, Y.; Ding, Y.; Cheng, Y. Effects of glyphosate on immune responses and haemocyte DNA damage of Chinese mitten Crab, Eriocheir sinensis. Fish Shellfish Immun. 2017, 71, 19–27. [Google Scholar] [CrossRef]
- Woźniak, E.; Sicińska, P.; Michałowicz, J.; Woźniak, K.; Reszka, E.; Huras, B.; Zakrzewski, J.; Bukowska, B. The mechanism of DNA damage induced by Roundup 360 PLUS, glyphosate and AMPA in human peripheral blood mononuclear cells-genotoxic risk assessement. Food Chem. Toxicol. 2018, 120, 510–522. [Google Scholar] [CrossRef]
- Hao, Y.; Chen, H.; Xu, W.; Gao, J.; Yang, Y.; Zhang, Y.; Tao, L. Roundup® confers cytotoxicity through DNA damage and mitochondria-associated apoptosis induction. Environ. Pollut. 2019, 252, 917–923. [Google Scholar] [CrossRef]
- Alvarez-Moya, C.; Sámano-León, A.G.; Reynoso-Silva, M.; Ramírez-Velasco, R.; Ruiz-López, M.A.; Villalobos-Arámbula, A.R. Antigenotoxic effect of ascorbic acid and resveratrol in erythrocytes of Ambystoma mexicanum, Oreochromis niloticus and human lymphocytes exposed to glyphosate. Curr. Issues Mol. Biol. 2022, 44, 2230–2242. [Google Scholar] [CrossRef]
- Yang, X.; Li, S.; Wang, Z.; Lee, S.M.Y.; Wang, L.-H.; Wang, R. Constraining the teratogenicity of pesticide pollution by a synthetic nanoreceptor. Chem. Asian J. 2017, 13, 41–45. [Google Scholar] [CrossRef]
- Kanth, P.C.; Trivedi, M.U.; Patel, K.; Misra, N.M.; Pandey, M.K. Cucurbituril-functionalized nanocomposite as a promising industrial adsorbent for rapid cationic dye removal. ACS Omega 2021, 6, 3024–3036. [Google Scholar] [CrossRef]
- Guo, D.-S.; Wang, L.-H.; Liu, Y. Highly effective binding of methyl viologen dication and its radical cation by p-sulfonatocalix[4,5]arenes. J. Org. Chem. 2007, 72, 7775–7778. [Google Scholar] [CrossRef] [PubMed]
- Hassanzadeh-Afruzi, F.; Ranjbar, G.; Salehi, M.M.; Esmailzadeh, F.; Maleki, A. Thiacalix[4]arene-functionalized magnetic xanthan gum (TC4As-XG@Fe3O4) as a hydrogel adsorbent for removal of dye and pesticide from water medium. Sep. Purif. Technol. 2023, 306, 122700. [Google Scholar] [CrossRef]
- Ogoshi, T.; Kanai, S.; Fujinami, S.; Yamagishi, T.; Nakamoto, Y. Para-Bridged Symmetrical pillar[5]arenes: Their Lewis acid catalyzed synthesis and host–guest property. J. Am. Chem. Soc. 2008, 130, 5022–5023. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-F.; Ding, J.-D.; Wei, T.-B. Pillararenes: Fascinating planar chiral macrocyclic arenes. Chem. Commun. 2021, 57, 9029–9039. [Google Scholar] [CrossRef]
- Zhu, H.; Li, Q.; Khalil-Cruz, L.E.; Khashab, N.M.; Yu, G.; Huang, F. Pillararene-based supramolecular systems for theranostics and bioapplications. Sci. China Chem. 2021, 64, 688–700. [Google Scholar] [CrossRef]
- Yan, M.; Zhou, J. Pillararene-based supramolecular polymers for cancer therapy. Molecules 2023, 28, 1470. [Google Scholar] [CrossRef]
- Chi, X.; Yu, G.; Ji, X.; Li, Y.; Tang, G.; Huang, F. Redox-responsive amphiphilic macromolecular [2]pseudorotaxane constructed from a water-soluble pillar[5]arene and a paraquat-containing homopolymer. ACS Macro Lett. 2015, 4, 996–999. [Google Scholar] [CrossRef]
- Sun, J.; Guo, F.; Shi, Q.; Wu, H.; Sun, Y.; Chen, M.; Diao, G. Electrochemical detection of paraquat based on silver nanoparticles/water-soluble pillar[5]arene functionalized graphene oxide modified glassy carbon electrode. J. Electroanal. Chem. 2019, 847, 113221. [Google Scholar] [CrossRef]
- Wang, P.; Yao, Y.; Xue, M. A novel fluorescent probe for detecting paraquat and cyanide in water based on pillar[5]arene/10-methylacridinium iodide molecular recognition. Chem. Commun. 2014, 50, 5064–5067. [Google Scholar] [CrossRef]
- Sun, Y.; Fu, W.; Chen, C.; Wang, J.; Yao, Y. Water-soluble pillar[5]arene induced the morphology transformation of self-assembled nanostructures and had further application in paraquat detection. Chem. Commun. 2017, 53, 3725–3728. [Google Scholar] [CrossRef]
- Luo, L.; Nie, G.; Tian, D.; Deng, H.; Jiang, L.; Li, H. Dynamic self-assembly adhesion of a paraquat droplet on a pillar[5]arene surface. Angew. Chem. Int. Ed. 2016, 55, 12713–12716. [Google Scholar] [CrossRef] [PubMed]
- Shamagsumova, R.V.; Shurpik, D.N.; Kuzin, Y.I.; Stoikov, I.I.; Rogov, A.M.; Evtugyn, G.A. Pillar[6]arene: Electrochemistry and application in electrochemical (bio)sensors. J. Electroanal. Chem. 2022, 913, 116281. [Google Scholar] [CrossRef]
- Tan, X.; Zhang, Z.; Cao, T.; Zeng, W.; Huang, T.; Zhao, G. Control assembly of pillar[6]arene-modified Ag nanoparticles on covalent organic framework surface for enhanced sensing performance toward paraquat. ACS Sustain. Chem. Eng. 2019, 7, 20051–20059. [Google Scholar] [CrossRef]
- Zhang, H.; Huang, K.-T.; Ding, L.; Yang, J.; Yang, Y.-W.; Liang, F. Electrochemical determination of paraquat using a glassy carbon electrode decorated with pillararene-coated nitrogen-doped carbon dots. Chinese Chem. Lett. 2022, 33, 1537–1540. [Google Scholar] [CrossRef]
- Shangguan, L.; Shi, B.; Chen, Q.; Li, Y.; Zhu, H.; Liu, Y.; Yao, H.; Huang, F. Water-soluble pillar[5]arenes: A new class of plant growth regulators. Tetrahedron Lett. 2019, 60, 150949. [Google Scholar] [CrossRef]
- Tang, Z.-D.; Sun, X.-M.; Huang, T.-T.; Liu, J.; Shi, B.; Yao, H.; Zhang, Y.-M.; Wei, T.-B.; Lin, Q. Pillar[n]arenes-based materials for detection and separation of pesticides. Chinese Chem. Lett. 2023, 34, 107698. [Google Scholar] [CrossRef]
- Tang, M.; Bian, Q.; Zhang, Y.-M.; Arif, M.; Luo, Q.; Men, S.; Liu, Y. Sequestration of pyridinium herbicides in plants by carboxylated pillararenes possessing different alkyl chains. RSC Adv. 2020, 10, 35136–35140. [Google Scholar] [CrossRef]
- Li, T.; Lu, X.-M.; Zhang, M.-R.; Hu, K.; Li, Z. Peptide-based nanomaterials: Self-assembly, properties and applications. Bioact. Mater. 2022, 11, 268–282. [Google Scholar] [CrossRef]
- Hamley, I.W. The amyloid beta peptide: A chemist’s perspective. Role in Alzheimer’s and fibrillization. Chem. Rev. 2012, 112, 5147–5192. [Google Scholar] [CrossRef]
- Shibaeva, K.S.; Nazarova, A.A.; Kuznetsova, D.I.; Stoikov, I.I. Synthesis of aminobismethylenephosphonic acids on a platform of p-tert-butylthiacalix[4]arene in 1,3-alternate configuration. Russ. J. Gen. Chem. 2016, 86, 579–583. [Google Scholar] [CrossRef]
- Nazarova, A.; Padnya, P.; Cragg, P.J.; Stoikov, I. [1]Rotaxanes based on phosphorylated pillar[5]arenes. New J. Chem. 2022, 46, 2033–2037. [Google Scholar] [CrossRef]
- Wang, R.; Tian, Y.; Wang, J.; Song, W.; Cong, Y.; Wei, X.; Mei, Y.; Miyatake, H.; Ito, Y.; Chen, Y.M. Biomimetic glucose trigger-insulin release system based on hydrogel loading bidentate β-cyclodextrin. Adv. Funct. Mater. 2021, 31, 2104488. [Google Scholar] [CrossRef]
- Nazarova, A.; Yakimova, L.; Mostovaya, O.; Kulikova, T.; Mikhailova, O.; Evtugyn, G.; Ganeeva, I.; Bulatov, E.; Stoikov, I. Encapsulation of the quercetin with interpolyelectrolyte complex based on pillar[5]arenes. J. Mol. Liq. 2022, 368, 120807. [Google Scholar] [CrossRef]
- Shurpik, D.N.; Nazarova, A.A.; Makhmutova, L.I.; Kizhnyaev, V.N.; Stoikov, I.I. Uncharged water-soluble amide derivatives of pillar[5]arene: Synthesis and supramolecular self-assembly with tetrazole-containing polymers. Russ. Chem. Bull. 2020, 69, 97–104. [Google Scholar] [CrossRef]
- Guo, J.; Lin, L.; Wang, Y.; Zhang, W.; Diao, G.; Piao, Y. Supramolecular design strategy of a water-soluble diphenylguanidine-cyclodextrin polymer inclusion complex. Molecules 2022, 27, 6919. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Dai, X.; Sun, Y.; Liu, Y. Organic supramolecular aggregates based on water-soluble cyclodextrins and calixarenes. Aggregate 2020, 1, 31–44. [Google Scholar] [CrossRef]
- Yakimova, L.S.; Padnya, P.L.; Kunafina, A.F.; Nugmanova, A.R.; Stoikov, I.I. Sulfobetaine derivatives of thiacalix[4]arene: Synthesis and supramolecular self-Assembly of submicron aggregates with AgI cations. Mendeleev Commun. 2019, 29, 86–88. [Google Scholar] [CrossRef]
- Huang, Y.; Yang, Y.; Liang, B.; Lu, S.; Yuan, X.; Jia, Z.; Liu, J.; Liu, Y. Green nanopesticide: pH-responsive eco-friendly pillar[5]arene-modified selenium nanoparticles for smart delivery of carbendazim to suppress sclerotinia diseases. ACS Appl. Mater. Inter. 2023, 15, 16448–16459. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Li, X.; Ning, L.; Tan, C.-H.; Mu, Y.; Wang, R. Hyperfast water transport through biomimetic nanochannels from peptide-attached (pR)-pillar[5]arene. Small 2019, 15, 1804678. [Google Scholar] [CrossRef]
- Terenteva, O.; Bikmukhametov, A.; Gerasimov, A.; Padnya, P.; Stoikov, I. Macrocyclic ionic liquids with amino acid residues: Synthesis and influence of thiacalix[4]arene conformation on thermal stability. Molecules 2022, 27, 8006. [Google Scholar] [CrossRef]
- Nazarova, A.; Shurpik, D.; Padnya, P.; Mukhametzyanov, T.; Cragg, P.; Stoikov, I. Self-assembly of supramolecular architectures by the effect of amino acid residues of quaternary ammonium pillar[5]arenes. Int. J. Mol. Sci. 2020, 21, 7206. [Google Scholar] [CrossRef] [PubMed]
- Butterfield, S.M.; Patel, P.R.; Waters, M.L. Contribution of aromatic interactions to α-helix stability. J. Am. Chem. Soc. 2002, 124, 9751–9755. [Google Scholar] [CrossRef] [PubMed]
- Solemanifar, A.; Nguyen, T.A.H.; Laycock, B.; Shewan, H.M.; Donose, B.C.; Creasey, R.C.G. Assessing the effect of aromatic residue placement on the α-helical peptide structure and nanofibril formation of 21-mer Peptides. Mol. Syst. Des. Eng. 2020, 5, 521–531. [Google Scholar] [CrossRef]
- Dudarev, A.N.; Gorodetskay, A.Y.; Tkachenko, T.A.; Usynin, I.F. Effects of cortisol and tetrahydrocortisol on the secondary structure of apolipoprotein A-I as measured by fourier transform infrared spectroscopy. Russ. J. Bioorg. Chem. 2022, 48, 96–104. [Google Scholar] [CrossRef]
- Duke, S.O. The history and current status of glyphosate. Pest. Manag. Sci. 2017, 74, 1027–1034. [Google Scholar] [CrossRef]
- Freifelder, D. Physical Blochemistry, Applications to Biochemistry and Molecular Biology, 2nd ed.; W. H. Freeman and Company: San Francisco, CA, USA, 1982. [Google Scholar]
- Yin, H.; Zhang, X.; Wei, J.; Lu, S.; Bardelang, D.; Wang, R. Recent advances in supramolecular antidotes. Theranostics 2021, 11, 1513–1526. [Google Scholar] [CrossRef]
- Zhou, S.; Chen, Y.; Xu, J.; Yin, Y.; Yu, J.; Liu, W.; Chen, S.; Wang, L. Supramolecular detoxification of nitrogen mustard via host–guest encapsulation by carboxylatopillar[5]arene. J. Mater. Chem. B 2023, 11, 2706–2713. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Pozarowski, P.; Grabarek, J.; Darzynkiewicz, Z. Flow cytometry of apoptosis. Curr. Protoc. Cytom. 2003, 21, 18–28. [Google Scholar] [CrossRef]
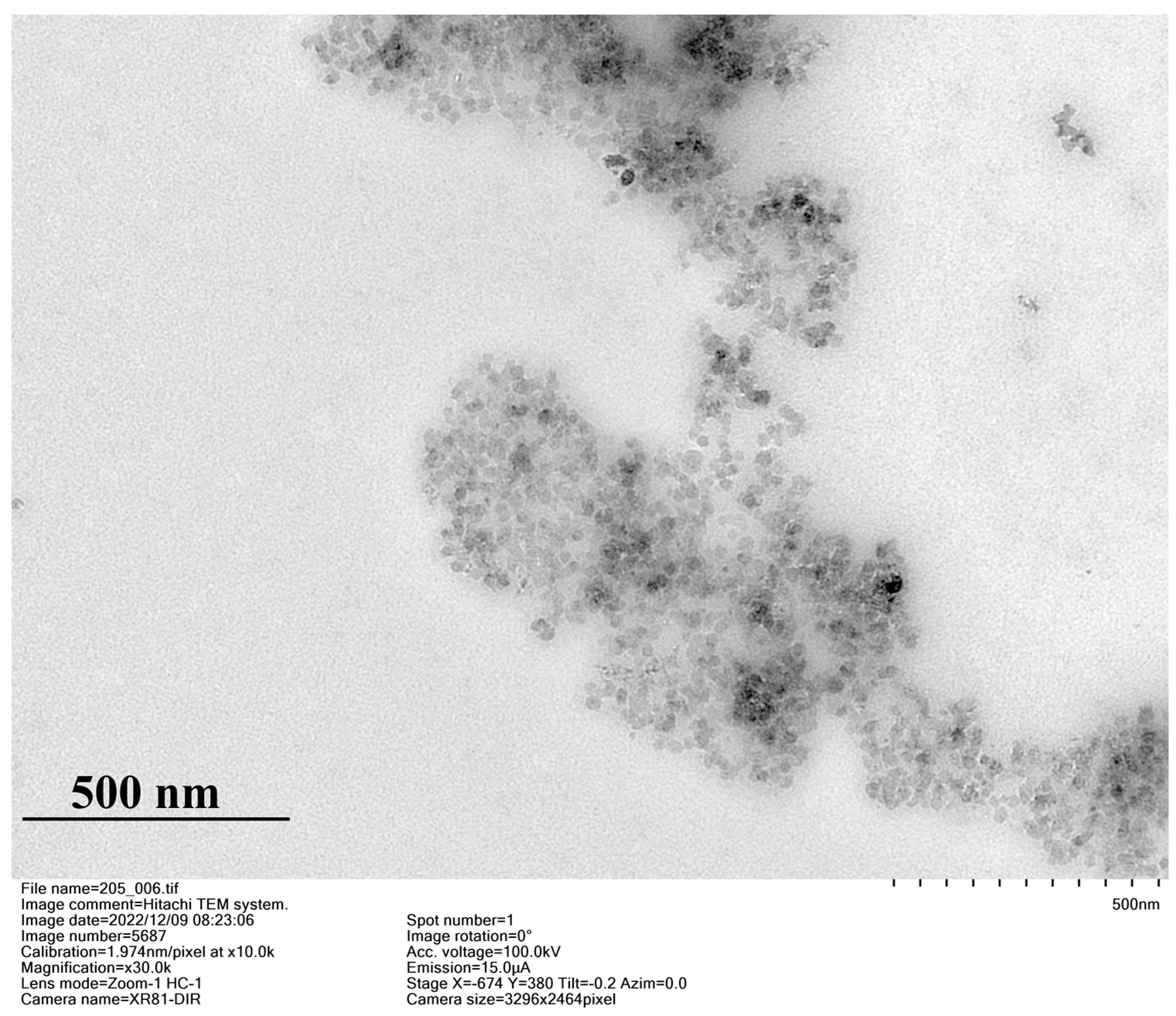

| C, M | d, nm (PDI); ζ, mV | |||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| 1 × 10–6 | 441 ± 81 (0.39) | 144 ± 7 (0.28); ζ = +12.5 | 276 ± 63 (0.39) | 457 ± 65 (0.42) |
| 1 × 10–5 | 286 ± 57 (0.35) | 157 ± 2 (0.21); ζ = +31.6 | 290 ± 38 (0.43) | 218 ± 10 (0.41) |
| 1 × 10–4 | 249 ± 17 (0.37) | 179 ± 8 (0.24); ζ = +15.9 | 565 ± 12 (0.55) | 127 ± 16 (0.36) |
| Associate | (1-DNA)-GIS | (2-DNA)-GIS | (3-DNA)-GIS | (4-DNA)-GIS |
| lgKa | 5.31 | 5.43 | 5.42 | 3.55 |
| Macrocycle | A549 | LEC |
|---|---|---|
| 1 | >100 * | >100 * |
| 2 | 29.67 | 28.16 |
| 3 | >100 * | >100 * |
| 4 | >100 * | >100 * |
| GIS | 10,180 | 11,430 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nazarova, A.; Padnya, P.; Khannanov, A.; Khabibrakhmanova, A.; Zelenikhin, P.; Stoikov, I. Towards Protection of Nucleic Acids from Herbicide Attack: Self-Assembly of Betaines Based on Pillar[5]arene with Glyphosate and DNA. Int. J. Mol. Sci. 2023, 24, 8357. https://doi.org/10.3390/ijms24098357
Nazarova A, Padnya P, Khannanov A, Khabibrakhmanova A, Zelenikhin P, Stoikov I. Towards Protection of Nucleic Acids from Herbicide Attack: Self-Assembly of Betaines Based on Pillar[5]arene with Glyphosate and DNA. International Journal of Molecular Sciences. 2023; 24(9):8357. https://doi.org/10.3390/ijms24098357
Chicago/Turabian StyleNazarova, Anastasia, Pavel Padnya, Arthur Khannanov, Aleksandra Khabibrakhmanova, Pavel Zelenikhin, and Ivan Stoikov. 2023. "Towards Protection of Nucleic Acids from Herbicide Attack: Self-Assembly of Betaines Based on Pillar[5]arene with Glyphosate and DNA" International Journal of Molecular Sciences 24, no. 9: 8357. https://doi.org/10.3390/ijms24098357
APA StyleNazarova, A., Padnya, P., Khannanov, A., Khabibrakhmanova, A., Zelenikhin, P., & Stoikov, I. (2023). Towards Protection of Nucleic Acids from Herbicide Attack: Self-Assembly of Betaines Based on Pillar[5]arene with Glyphosate and DNA. International Journal of Molecular Sciences, 24(9), 8357. https://doi.org/10.3390/ijms24098357











