The Apelinergic System in Pregnancy
Abstract
1. Overview of the Apelinergic System
2. The Apelinergic System in the Reproductive System—Pregnancy and Postpartum
2.1. Reproductive System
2.2. Development of the Embryo
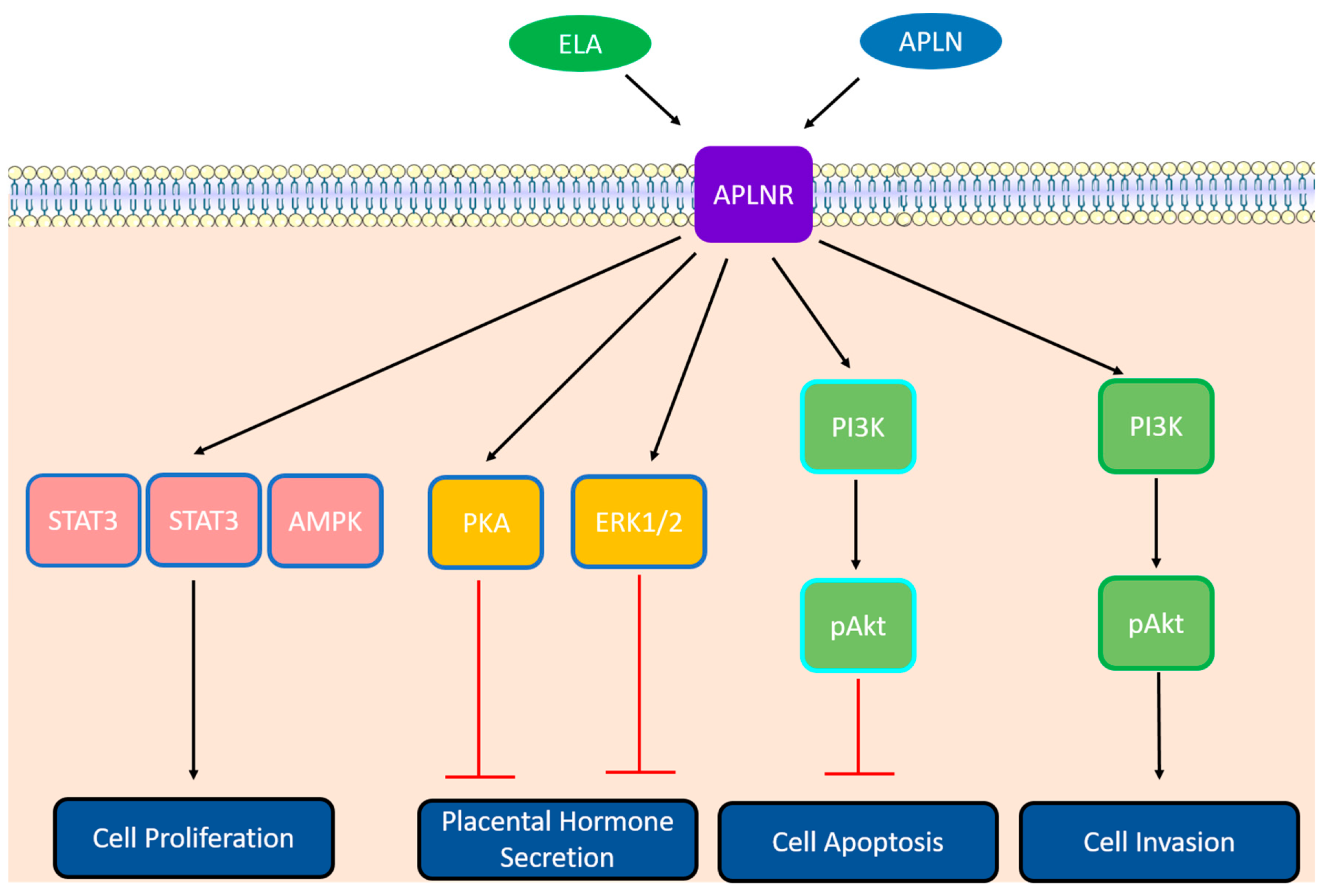
2.3. The Apelinergic System in Placenta
2.3.1. Differentiation
2.3.2. Cell Cycle and Proliferation
2.3.3. Cell Survival
2.3.4. Trophoblastic Invasion
2.3.5. Placental Hormone Secretion
2.4. Labor
2.5. The Apelinergic System and Postpartum/Breastfeeding
3. Placenta-Related Complications
3.1. Preeclampsia (PE)
3.2. Intrauterine Growth Restriction (IUGR)
3.3. Gestational Diabetes Mellitus (GDM)
3.4. Miscarriage
4. From Research to the Clinical Setting: Challenges and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- O’Carroll, A.-M.; Lolait, S.J.; Harris, L.E.; Pope, G.R. The apelin receptor APJ: Journey from an orphan to a multifaceted regulator of homeostasis. J. Endocrinol. 2013, 219, R13–R35. [Google Scholar] [CrossRef] [PubMed]
- O’Dowd, B.F.; Heiber, M.; Chan, A.; Heng, H.H.Q.; Tsui, L.-C.; Kennedy, J.L.; Shi, X.; Petronis, A.; George, S.R.; Nguyen, T. A human gene that shows identity with the gene encoding the angiotensin receptor is located on chromosome 11. Gene 1993, 136, 355–360. [Google Scholar] [CrossRef]
- Tatemoto, K.; Hosoya, M.; Habata, Y.; Fujii, R.; Kakegawa, T.; Zou, M.-X.; Kawamata, Y.; Fukusumi, S.; Hinuma, S.; Kitada, C.; et al. Isolation and Characterization of a Novel Endogenous Peptide Ligand for the Human APJ Receptor. Biochem. Biophys. Res. Commun. 1998, 251, 471–476. [Google Scholar] [CrossRef]
- Charo, D.N.; Ho, M.; Fajardo, G.; Kawana, M.; Kundu, R.K.; Sheikh, A.Y.; Finsterbach, T.P.; Leeper, N.J.; Ernst, K.V.; Chen, M.M.; et al. Endogenous regulation of cardiovascular function by apelin-APJ. Am. J. Physiol.-Heart Circ. Physiol. 2009, 297, H1904–H1913. [Google Scholar] [CrossRef]
- Kang, Y.; Kim, J.; Anderson, J.P.; Wu, J.; Gleim, S.R.; Kundu, R.K.; McLean, D.L.; Kim, J.; Park, H.; Jin, S.; et al. Apelin-APJ Signaling Is a Critical Regulator of Endothelial MEF2 Activation in Cardiovascular Development. Circ. Res. 2013, 113, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Kasai, A.; Shintani, N.; Kato, H.; Matsuda, S.; Gomi, F.; Haba, R.; Hashimoto, H.; Kakuda, M.; Tano, Y.; Baba, A. Retardation of retinal vascular development in apelin-deficient mice. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 1717–1722. [Google Scholar] [CrossRef]
- Mayeur, S.; Wattez, J.-S.; Lukaszewski, M.-A.; Lecoutre, S.; Butruille, L.; Drougard, A.; Eberlé, D.; Bastide, B.; Laborie, C.; Storme, L.; et al. Apelin Controls Fetal and Neonatal Glucose Homeostasis and Is Altered by Maternal Undernutrition. Diabetes 2015, 65, 554–560. [Google Scholar] [CrossRef] [PubMed]
- Hosoya, M.; Kawamata, Y.; Fukusumi, S.; Fujii, R.; Habata, Y.; Hinuma, S.; Kitada, C.; Honda, S.; Kurokawa, T.; Onda, H.; et al. Molecular and functional characteristics of APJ. Tissue distribution of mRNA and interaction with the endogenous ligand apelin. J. Biol. Chem. 2000, 275, 21061–21067. [Google Scholar] [CrossRef] [PubMed]
- Kleinz, M.J.; Davenport, A.P. Immunocytochemical localization of the endogenous vasoactive peptide apelin to human vascular and endocardial endothelial cells. Regul. Pept. 2004, 118, 119–125. [Google Scholar] [CrossRef]
- Kleinz, M.J.; Skepper, J.N.; Davenport, A.P. Immunocytochemical localisation of the apelin receptor, APJ, to human cardiomyocytes, vascular smooth muscle and endothelial cells. Regul. Pept. 2005, 126, 233–240. [Google Scholar] [CrossRef]
- O’Carroll, A.M.; Selby, T.L.; Palkovits, M.; Lolait, S.J. Distribution of mRNA encoding B78/apj, the rat homologue of the human APJ receptor, and its endogenous ligand apelin in brain and peripheral tissues. Biochim. Et Biophys. Acta (BBA)-Gene Struct. Expr. 2000, 1492, 72–80. [Google Scholar] [CrossRef]
- Medhurst, A.D.; Jennings, C.A.; Robbins, M.J.; Davis, R.P.; Ellis, C.; Winborn, K.Y.; Lawrie, K.W.M.; Hervieu, G.; Riley, G.; Bolaky, J.E.; et al. Pharmacological and immunohistochemical characterization of the APJ receptor and its endogenous ligand apelin. J. Neurochem. 2003, 84, 1162–1172. [Google Scholar] [CrossRef] [PubMed]
- Dray, C.; Debard, C.; Jager, J.; Disse, E.; Daviaud, D.; Martin, P.; Attané, C.; Wanecq, E.; Guigné, C.; Bost, F.; et al. Apelin and APJ regulation in adipose tissue and skeletal muscle of type 2 diabetic mice and humans. Am. J. Physiol. Endocrinol. Metab. 2010, 298, E1161–E1169. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Xu, J.; Chen, L.; Li, L. Apelin/APJ signaling in hypoxia-related diseases. Clin. Chim. Acta 2015, 451 Pt B, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.M.; Ashley, E.A.; Deng, D.X.F.; Tsalenko, A.; Deng, A.; Tabibiazar, R.; Ben-Dor, A.; Fenster, B.; Yang, E.; King, J.Y.; et al. Novel role for the potent endogenous inotrope apelin in human cardiac dysfunction. Circulation 2003, 108, 1432–1439. [Google Scholar] [CrossRef] [PubMed]
- Kawamata, Y.; Habata, Y.; Fukusumi, S.; Hosoya, M.; Fujii, R.; Hinuma, S.; Nishizawa, N.; Kitada, C.; Onda, H.; Nishimura, O.; et al. Molecular properties of apelin: Tissue distribution and receptor binding. Biochim. Et Biophys. Acta (BBA)-Mol. Cell Res. 2001, 1538, 162–171. [Google Scholar] [CrossRef]
- Nyimanu, D.; Kay, R.G.; Sulentic, P.; Kuc, R.E.; Ambery, P.; Jermutus, L.; Reimann, F.; Gribble, F.M.; Cheriyan, J.; Maguire, J.J.; et al. Development and validation of an LC-MS/MS method for detection and quantification of in vivo derived metabolites of [Pyr1]apelin-13 in humans. Sci. Rep. 2019, 9, 19934. [Google Scholar] [CrossRef]
- Shin, K.; Landsman, M.; Pelletier, S.; Alamri, B.N.; Anini, Y.; Rainey, J.K. Proapelin is processed extracellularly in a cell line-dependent manner with clear modulation by proprotein convertases. Amino Acids 2019, 51, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Maguire, J.J.; Kleinz, M.J.; Pitkin, S.L.; Davenport, A.P. [Pyr1]apelin-13 identified as the predominant apelin isoform in the human heart: Vasoactive mechanisms and inotropic action in disease. Hypertens. Dallas Tex 1979 2009, 54, 598–604. [Google Scholar] [CrossRef]
- Chng, S.C.; Ho, L.; Tian, J.; Reversade, B. ELABELA: A hormone essential for heart development signals via the apelin receptor. Dev. Cell 2013, 27, 672–680. [Google Scholar] [CrossRef]
- Pauli, A.; Norris, M.L.; Valen, E.; Chew, G.-L.; Gagnon, J.A.; Zimmerman, S.; Mitchell, A.; Ma, J.; Dubrulle, J.; Reyon, D.; et al. Toddler: An embryonic signal that promotes cell movement via Apelin receptors. Science 2014, 343, 1248636. [Google Scholar] [CrossRef]
- Couvineau, P.; Llorens-Cortes, C.; Iturrioz, X. Elabela/Toddler and apelin bind differently to the apelin receptor. FASEB J. 2020, 34, 7989–8000. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yu, D.; Wang, M.; Wang, Q.; Kouznetsova, J.; Yang, R.; Qian, K.; Wu, W.; Shuldiner, A.; Sztalryd, C.; et al. Elabela-apelin receptor signaling pathway is functional in mammalian systems. Sci. Rep. 2015, 5, 8170. [Google Scholar] [CrossRef] [PubMed]
- Ho, L.; van Dijk, M.; Chye, S.T.J.; Messerschmidt, D.M.; Chng, S.C.; Ong, S.; Yi, L.K.; Boussata, S.; Goh, G.H.-Y.; Afink, G.B.; et al. ELABELA deficiency promotes preeclampsia and cardiovascular malformations in mice. Science 2017, 357, 707–713. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Read, C.; Kuc, R.E.; Buonincontri, G.; Southwood, M.; Torella, R.; Upton, P.D.; Crosby, A.; Sawiak, S.J.; Carpenter, T.A.; et al. Elabela/Toddler Is an Endogenous Agonist of the Apelin APJ Receptor in the Adult Cardiovascular System, and Exogenous Administration of the Peptide Compensates for the Downregulation of Its Expression in Pulmonary Arterial Hypertension. Circulation 2017, 135, 1160–1173. [Google Scholar] [CrossRef]
- Flahault, A.; Couvineau, P.; Alvear-Perez, R.; Iturrioz, X.; Llorens-Cortes, C. Role of the Vasopressin/Apelin Balance and Potential Use of Metabolically Stable Apelin Analogs in Water Metabolism Disorders. Front. Endocrinol. 2017, 8, 120. [Google Scholar] [CrossRef]
- Ma, Y.; Yue, Y.; Ma, Y.; Zhang, Q.; Zhou, Q.; Song, Y.; Shen, Y.; Li, X.; Ma, X.; Li, C.; et al. Structural Basis for Apelin Control of the Human Apelin Receptor. Structure 1993 2017, 25, 858–866.e4. [Google Scholar] [CrossRef]
- Murza, A.; Sainsily, X.; Coquerel, D.; Côté, J.; Marx, P.; Besserer-Offroy, É.; Longpré, J.-M.; Lainé, J.; Reversade, B.; Salvail, D.; et al. Discovery and Structure-Activity Relationship of a Bioactive Fragment of ELABELA that Modulates Vascular and Cardiac Functions. J. Med. Chem. 2016, 59, 2962–2972. [Google Scholar] [CrossRef]
- Pitkin, S.L.; Maguire, J.J.; Bonner, T.I.; Davenport, A.P. International Union of Basic and Clinical Pharmacology. LXXIV. Apelin receptor nomenclature, distribution, pharmacology, and function. Pharmacol. Rev. 2010, 62, 331–342. [Google Scholar] [CrossRef]
- Reaux, A.; Gallatz, K.; Palkovits, M.; Llorens-Cortes, C. Distribution of apelin-synthesizing neurons in the adult rat brain. Neuroscience 2002, 113, 653–662. [Google Scholar] [CrossRef]
- Newson, M.J.F.; Roberts, E.M.; Pope, G.R.; Lolait, S.J.; O’Carroll, A.-M. The effects of apelin on hypothalamic-pituitary-adrenal axis neuroendocrine function are mediated through corticotrophin-releasing factor- and vasopressin-dependent mechanisms. J. Endocrinol. 2009, 202, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Kurowska, P.; Barbe, A.; Różycka, M.; Chmielińska, J.; Dupont, J.; Rak, A. Apelin in Reproductive Physiology and Pathology of Different Species: A Critical Review. Int. J. Endocrinol. 2018, 2018, 9170480. [Google Scholar] [CrossRef] [PubMed]
- Pope, G.R.; Roberts, E.M.; Lolait, S.J.; O’Carroll, A.-M. Central and peripheral apelin receptor distribution in the mouse: Species differences with rat. Peptides 2012, 33, 139–148. [Google Scholar] [CrossRef]
- Roche, J.; Ramé, C.; Reverchon, M.; Mellouk, N.; Rak, A.; Froment, P.; Dupont, J. Apelin (APLN) regulates progesterone secretion and oocyte maturation in bovine ovarian cells. Reprod. Camb. Engl. 2017, 153, 589–603. [Google Scholar] [CrossRef] [PubMed]
- Rak, A.; Drwal, E.; Rame, C.; Knapczyk-Stwora, K.; Słomczyńska, M.; Dupont, J.; Gregoraszczuk, E.L. Expression of apelin and apelin receptor (APJ) in porcine ovarian follicles and in vitro effect of apelin on steroidogenesis and proliferation through APJ activation and different signaling pathways. Theriogenology 2017, 96, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Roche, J.; Ramé, C.; Reverchon, M.; Mellouk, N.; Cornuau, M.; Guerif, F.; Froment, P.; Dupont, J. Apelin (APLN) and Apelin Receptor (APLNR) in Human Ovary: Expression, Signaling, and Regulation of Steroidogenesis in Primary Human Luteinized Granulosa Cells. Biol. Reprod. 2016, 95, 104. [Google Scholar] [CrossRef]
- Wang, X.; Liu, X.; Song, Z.; Shen, X.; Lu, S.; Ling, Y.; Kuang, H. Emerging roles of APLN and APELA in the physiology and pathology of the female reproductive system. PeerJ 2020, 8, e10245. [Google Scholar] [CrossRef] [PubMed]
- Ho, L.; Tan, S.Y.X.; Wee, S.; Wu, Y.; Tan, S.J.C.; Ramakrishna, N.B.; Chng, S.C.; Nama, S.; Szczerbinska, I.; Sczerbinska, I.; et al. ELABELA Is an Endogenous Growth Factor that Sustains hESC Self-Renewal via the PI3K/AKT Pathway. Cell Stem Cell 2015, 17, 435–447. [Google Scholar] [CrossRef]
- Wang, C.; Liu, X.; Kong, D.; Qin, X.; Li, Y.; Teng, X.; Huang, X. Apelin as a novel drug for treating preeclampsia. Exp. Ther. Med. 2017, 14, 5917–5923. [Google Scholar] [CrossRef]
- Freyer, L.; Hsu, C.-W.; Nowotschin, S.; Pauli, A.; Ishida, J.; Kuba, K.; Fukamizu, A.; Schier, A.F.; Hoodless, P.A.; Dickinson, M.E.; et al. Loss of Apela Peptide in Mice Causes Low Penetrance Embryonic Lethality and Defects in Early Mesodermal Derivatives. Cell Rep. 2017, 20, 2116–2130. [Google Scholar] [CrossRef]
- Hassan, A.S.; Hou, J.; Wei, W.; Hoodless, P.A. Expression of two novel transcripts in the mouse definitive endoderm. Gene Expr. Patterns GEP 2010, 10, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Devic, E.; Paquereau, L.; Vernier, P.; Knibiehler, B.; Audigier, Y. Expression of a new G protein-coupled receptor X-msr is associated with an endothelial lineage in Xenopus laevis. Mech. Dev. 1996, 59, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.-X.I.; Wilm, T.P.; Sepich, D.S.; Solnica-Krezel, L. Apelin and Its Receptor Control Heart Field Formation during Zebrafish Gastrulation. Dev. Cell 2007, 12, 391–402. [Google Scholar] [CrossRef]
- Helker, C.S.M.; Schuermann, A.; Pollmann, C.; Chng, S.C.; Kiefer, F.; Reversade, B.; Herzog, W. The hormonal peptide Elabela guides angioblasts to the midline during vasculogenesis. Elife 2015, 4, e06726. [Google Scholar] [CrossRef]
- Lu, L.; Cao, J.; Li, L.; Chen, L. Elabela, a new endogenous ligand of APJ, functions in embryos and adults organisms. Acta Biochim. Biophys. Sin. 2017, 49, 378–381. [Google Scholar] [CrossRef] [PubMed]
- Scott, I.C.; Masri, B.; D’Amico, L.A.; Jin, S.-W.; Jungblut, B.; Wehman, A.M.; Baier, H.; Audigier, Y.; Stainier, D.Y.R. The G Protein-Coupled Receptor Agtrl1b Regulates Early Development of Myocardial Progenitors. Dev. Cell 2007, 12, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Paskaradevan, S.; Scott, I.C. The Aplnr GPCR regulates myocardial progenitor development via a novel cell-non-autonomous, Gαi/o protein-independent pathway. Biol. Open 2012, 1, 275–285. [Google Scholar] [CrossRef]
- Kuba, K.; Zhang, L.; Imai, Y.; Arab, S.; Chen, M.; Maekawa, Y.; Leschnik, M.; Leibbrandt, A.; Markovic, M.; Schwaighofer, J.; et al. Impaired heart contractility in Apelin gene-deficient mice associated with aging and pressure overload. Circ. Res. 2007, 101, e32–e42. [Google Scholar] [CrossRef]
- Deshwar, A.R.; Chng, S.C.; Ho, L.; Reversade, B.; Scott, I.C. The Apelin receptor enhances Nodal/TGFβ signaling to ensure proper cardiac development. Elife 2016, 5, e13758. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhang, K.; Guo, Y.; Chen, L.; Li, L. Elabela-APJ axis contributes to embryonic development and prevents pre-eclampsia in pregnancy. Acta Biochim. Biophys. Sin. 2018, 50, 319–321. [Google Scholar] [CrossRef]
- Liu, W.; Yan, J.; Pan, W.; Tang, M. Apelin/Elabela-APJ: A novel therapeutic target in the cardiovascular system. Ann. Transl. Med. 2020, 8, 243. [Google Scholar] [CrossRef] [PubMed]
- Piairo, P.; Moura, R.S.; Nogueira-Silva, C.; Correia-Pinto, J. The apelinergic system in the developing lung: Expression and signaling. Peptides 2011, 32, 2474–2483. [Google Scholar] [CrossRef]
- Tatemoto, K.; Takayama, K.; Zou, M.X.; Kumaki, I.; Zhang, W.; Kumano, K.; Fujimiya, M. The novel peptide apelin lowers blood pressure via a nitric oxide-dependent mechanism. Regul. Pept. 2001, 99, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Yao, F.; Raizada, M.K.; O’Rourke, S.T.; Sun, C. Apelin gene transfer into the rostral ventrolateral medulla induces chronic blood pressure elevation in normotensive rats. Circ. Res. 2009, 104, 1421–1428. [Google Scholar] [CrossRef]
- Kidoya, H.; Ueno, M.; Yamada, Y.; Mochizuki, N.; Nakata, M.; Yano, T.; Fujii, R.; Takakura, N. Spatial and temporal role of the apelin/APJ system in the caliber size regulation of blood vessels during angiogenesis. EMBO J. 2008, 27, 522–534. [Google Scholar] [CrossRef] [PubMed]
- Antushevich, H.; Wójcik, M. Review: Apelin in disease. Clin. Chim. Acta Int. J. Clin. Chem. 2018, 483, 241–248. [Google Scholar] [CrossRef]
- Georgiadou, D.; Boussata, S.; Ranzijn, W.H.M.; Root, L.E.A.; Hillenius, S.; de Weg, J.M.b.; Abheiden, C.N.H.; de Boer, M.A.; de Vries, J.I.P.; Vrijkotte, T.G.M.; et al. Peptide hormone ELABELA enhances extravillous trophoblast differentiation, but placenta is not the major source of circulating ELABELA in pregnancy. Sci. Rep. 2019, 9, 19077. [Google Scholar] [CrossRef]
- Inuzuka, H.; Nishizawa, H.; Inagaki, A.; Suzuki, M.; Ota, S.; Miyamura, H.; Miyazaki, J.; Sekiya, T.; Kurahashi, H.; Udagawa, Y. Decreased expression of apelin in placentas from severe pre-eclampsia patients. Hypertens. Pregnancy 2013, 32, 410–421. [Google Scholar] [CrossRef]
- Mlyczyńska, E.; Kurowska, P.; Drwal, E.; Opydo-Chanek, M.; Tworzydło, W.; Kotula-Balak, M.; Rak, A. Apelin and apelin receptor in human placenta: Expression, signalling pathway and regulation of trophoblast JEG-3 and BeWo cells proliferation and cell cycle. Int. J. Mol. Med. 2020, 45, 691–702. [Google Scholar] [CrossRef]
- Baczyk, D.; Drewlo, S.; Proctor, L.; Dunk, C.; Lye, S.; Kingdom, J. Glial cell missing-1 transcription factor is required for the differentiation of the human trophoblast. Cell Death Differ. 2009, 16, 719–727. [Google Scholar] [CrossRef]
- Renaud, S.J.; Kubota, K.; Rumi, M.A.K.; Soares, M.J. The FOS transcription factor family differentially controls trophoblast migration and invasion. J. Biol. Chem. 2014, 289, 5025–5039. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Damsky, C.H.; Chiu, K.; Roberts, J.M.; Fisher, S.J. Preeclampsia is associated with abnormal expression of adhesion molecules by invasive cytotrophoblasts. J. Clin. Investig. 1993, 91, 950–960. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Wang, R.; Zhu, C.; Wang, H.; Lin, H.-Y.; Gu, Y.; Cross, J.C.; Wang, H. Fine-Tuned and Cell-Cycle-Restricted Expression of Fusogenic Protein Syncytin-2 Maintains Functional Placental Syncytia. Cell Rep. 2018, 23, 3979. [Google Scholar] [CrossRef] [PubMed]
- Kreis, N.-N.; Friemel, A.; Jennewein, L.; Hoock, S.C.; Hentrich, A.E.; Nowak, T.; Louwen, F.; Yuan, J. Functional Analysis of p21Cip1/CDKN1A and Its Family Members in Trophoblastic Cells of the Placenta and Its Roles in Preeclampsia. Cells 2021, 10, 2214. [Google Scholar] [CrossRef]
- Xiao, Z.; Yan, L.; Liang, X.; Wang, H. Progress in deciphering trophoblast cell differentiation during human placentation. Curr. Opin. Cell Biol. 2020, 67, 86–91. [Google Scholar] [CrossRef]
- DeLoia, J.A.; Burlingame, J.M.; Krasnow, J.S. Differential expression of g1, cyclins during human placentogenesis. Placenta 1997, 18, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Hu, H.; Lin, M.; Chen, L.; Liu, M.; Li, H.; Quan, S. ELABELA alleviates syncytiotrophoblast hypoxia/reoxygenation injury and preeclampsia-like symptoms in mice by reducing apoptosis. Placenta 2021, 106, 30–39. [Google Scholar] [CrossRef]
- Dawid, M.; Mlyczynska, E.; Kurowska, P.; Sierpowski, M.; Rak, A. Apelin decreased placental hormone secretion by human trophoblast BeWo cells via apelin receptor, protein kinase A and extracellular signal-regulated kinases 1/2 activation. J. Physiol. Pharmacol. 2019, 70, 895–907. [Google Scholar] [CrossRef]
- Mlyczyńska, E.; Myszka, M.; Kurowska, P.; Dawid, M.; Milewicz, T.; Bałajewicz-Nowak, M.; Kowalczyk, P.; Rak, A. Anti-Apoptotic Effect of Apelin in Human Placenta: Studies on BeWo Cells and Villous Explants from Third-Trimester Human Pregnancy. Int. J. Mol. Sci. 2021, 22, 2760. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, X.; Ding, Y.; Xiong, H.; Xiang, S.; Wang, Y.; Li, H.; Liu, Z.; He, J.; Tao, Y.; et al. Elabela: Negative Regulation of Ferroptosis in Trophoblasts via the Ferritinophagy Pathway Implicated in the Pathogenesis of Preeclampsia. Cells 2023, 12, 99. [Google Scholar] [CrossRef]
- Zhou, L.; Sun, H.; Cheng, R.; Fan, X.; Lai, S.; Deng, C. ELABELA, as a potential diagnostic biomarker of preeclampsia, regulates abnormally shallow placentation via APJ. Am. J. Physiol. Endocrinol. Metab. 2019, 316, E773–E781. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, Y.; Qu, H.; Xu, F.; Hu, H.; Zhang, Q.; Ye, Y. Reduced ELABELA expression attenuates trophoblast invasion through the PI3K/AKT/mTOR pathway in early onset preeclampsia. Placenta 2019, 87, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Dawid, M.; Mlyczyńska, E.; Jurek, M.; Respekta, N.; Pich, K.; Kurowska, P.; Gieras, W.; Milewicz, T.; Kotula-Balak, M.; Rak, A. Apelin, APJ, and ELABELA: Role in Placental Function, Pregnancy, and Foetal Development—An Overview. Cells 2021, 11, 99. [Google Scholar] [CrossRef]
- Guo, Y.-Y.; Li, T.; Liu, H.; Tang, L.; Li, Y.-C.; Hu, H.-T.; Su, Y.-F.; Lin, Y.; Wang, Y.-Y.; Li, C.; et al. Circulating levels of Elabela and Apelin in the second and third trimesters of pregnancies with gestational diabetes mellitus. Gynecol. Endocrinol. 2020, 36, 890–894. [Google Scholar] [CrossRef]
- Hehir, M.P.; Morrison, J.J. The adipokine apelin and human uterine contractility. Am. J. Obstet. Gynecol. 2012, 206, 359.e1–359.e5. [Google Scholar] [CrossRef]
- Kacar, E.; Ercan, Z.; Serhatlioglu, I.; Sumer, A.; Kelestimur, H.; Kutlu, S. The effects of apelin on myometrium contractions in pregnant rats. Cell. Mol. Biol. Noisy-Gd. Fr. 2018, 64, 74–79. [Google Scholar] [CrossRef]
- Carvajal, J.A.; Oporto, J.I. The Myometrium in Pregnant Women with Obesity. Curr. Vasc. Pharmacol. 2021, 19, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Habata, Y.; Fujii, R.; Hosoya, M.; Fukusumi, S.; Kawamata, Y.; Hinuma, S.; Kitada, C.; Nishizawa, N.; Murosaki, S.; Kurokawa, T.; et al. Apelin, the natural ligand of the orphan receptor APJ, is abundantly secreted in the colostrum. Biochim. Et Biophys. Acta (BBA)-Mol. Cell Res. 1999, 1452, 25–35. [Google Scholar] [CrossRef]
- Aydin, S. The presence of the peptides apelin, ghrelin and nesfatin-1 in the human breast milk, and the lowering of their levels in patients with gestational diabetes mellitus. Peptides 2010, 31, 2236–2240. [Google Scholar] [CrossRef]
- Marousez, L.; Hanssens, S.; Butruille, L.; Petit, C.; Pourpe, C.; Besengez, C.; Rakza, T.; Storme, L.; Deruelle, P.; Lesage, J.; et al. Breast milk apelin level increases with maternal obesity and high-fat feeding during lactation. Int. J. Obes. 2005 2021, 45, 1052–1060. [Google Scholar] [CrossRef]
- Khan, K.S.; Wojdyla, D.; Say, L.; Gülmezoglu, A.M.; Van Look, P.F. WHO analysis of causes of maternal death: A systematic review. Lancet 2006, 367, 1066–1074. [Google Scholar] [CrossRef] [PubMed]
- Say, L.; Chou, D.; Gemmill, A.; Tunçalp, Ö.; Moller, A.-B.; Daniels, J.; Gülmezoglu, A.M.; Temmerman, M.; Alkema, L. Global causes of maternal death: A WHO systematic analysis. Lancet Glob. Health 2014, 2, e323–e333. [Google Scholar] [CrossRef] [PubMed]
- Binder, J.; Kalafat, E.; Palmrich, P.; Pateisky, P.; Khalil, A. Should angiogenic markers be included in diagnostic criteria of superimposed pre-eclampsia in women with chronic hypertension? Ultrasound Obstet. Gynecol. 2022, 59, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, L.; Shi, H. The biological function of ELABELA and APJ signaling in the cardiovascular system and pre-eclampsia. Hypertens. Res. 2019, 42, 928–934. [Google Scholar] [CrossRef] [PubMed]
- Yallampalli, C.; Garfield, R.E. Inhibition of nitric oxide synthesis in rats during pregnancy produces signs similar to those of preeclampsia. Am. J. Obstet. Gynecol. 1993, 169, 1316–1320. [Google Scholar] [CrossRef]
- Khalil, R.A.; Crews, J.K.; Novak, J.; Kassab, S.; Granger, J.P. Enhanced vascular reactivity during inhibition of nitric oxide synthesis in pregnant rats. Hypertension 1979 1998, 31, 1065–1069. [Google Scholar] [CrossRef]
- Osol, G.; Barron, C.; Gokina, N.; Mandala, M. Inhibition of nitric oxide synthases abrogates pregnancy-induced uterine vascular expansive remodeling. J. Vasc. Res. 2009, 46, 478–486. [Google Scholar] [CrossRef]
- Zuo, J.; Jiang, Z. Melatonin attenuates hypertension and oxidative stress in a rat model of L-NAME-induced gestational hypertension. Vasc. Med. 2020, 25, 295–301. [Google Scholar] [CrossRef]
- Motta, C.; Grosso, C.; Zanuzzi, C.; Molinero, D.; Picco, N.; Bellingeri, R.; Alustiza, F.; Barbeito, C.; Vivas, A.; Romanini, M. Effect of Sildenafil on Pre-Eclampsia-Like Mouse Model Induced By L-Name. Reprod. Domest. Anim. 2015, 50, 611–616. [Google Scholar] [CrossRef]
- de Souza, C.O.; Peraçoli, M.T.S.; Weel, I.C.; Bannwart, C.F.; Romão, M.; Nakaira-Takahagi, E.; de Medeiros, L.T.L.; Silva, M.G.d.; Peraçoli, J.C. Hepatoprotective and anti-inflammatory effects of silibinin on experimental preeclampsia induced by L-NAME in rats. Life Sci. 2012, 91, 159–165. [Google Scholar] [CrossRef]
- Pritchard, N.; Kaitu’u-Lino, T.J.; Gong, S.; Dopierala, J.; Smith, G.C.S.; Charnock-Jones, D.S.; Tong, S. ELABELA/APELA levels are not decreased in the maternal circulation or placenta among women with preeclampsia. Am. J. Pathol. 2018, 188, 1749–1753. [Google Scholar] [CrossRef] [PubMed]
- Panaitescu, B.; Romero, R.; Gomez-Lopez, N.; Pacora, P.; Erez, O.; Vadillo-Ortega, F.; Yeo, L.; Hassan, S.S.; Hsu, C.-D. ELABELA Plasma Concentrations are Increased in Women with Late-Onset Preeclampsia. J. Matern.-Fetal Neonatal Med. 2020, 33, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Para, R.; Romero, R.; Gomez-Lopez, N.; Tarca, A.L.; Panaitescu, B.; Done, B.; Hsu, R.; Pacora, P.; Hsu, C.-D. Maternal circulating concentrations of soluble Fas and Elabela in early- and late-onset preeclampsia. J. Matern.-Fetal Neonatal Med. 2022, 35, 316–329. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Bian, Y.; Wan, J.; Li, L.; Yang, P.; Zhao, S.; Zhao, H. Variants in the 5’-UTR of APELA gene in women with preeclampsia. Prenat. Diagn. 2019, 39, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Villie, P.; Lefevre, G.; Arrestier, R.; Rousseau, A.; Berkane, N.; Hertig, A. ELABELA concentration is not decreased in maternal plasma before the onset of preeclampsia. Am. J. Obstet. Gynecol. 2019, 220, 284–285. [Google Scholar] [CrossRef]
- Eberlé, D.; Marousez, L.; Hanssens, S.; Knauf, C.; Breton, C.; Deruelle, P.; Lesage, J. Elabela and Apelin actions in healthy and pathological pregnancies. Cytokine Growth Factor Rev. 2019, 46, 45–53. [Google Scholar] [CrossRef]
- Hamza, R.Z.; Diab, A.A.A.; Zahra, M.H.; Asalah, A.K.; Moursi, S.M.M.; Al-Baqami, N.M.; Al-Salmi, F.A.; Attia, M.S. Correlation between Apelin and Some Angiogenic Factors in the Pathogenesis of Preeclampsia: Apelin-13 as Novel Drug for Treating Preeclampsia and Its Physiological Effects on Placenta. Int. J. Endocrinol. 2021, 2021, 5017362. [Google Scholar] [CrossRef]
- Simsek, Y.; Celik, O.; Yilmaz, E.; Karaer, A.; Dogan, C.; Aydin, S.; Ozer, A. Serum levels of apelin, salusin-alpha and salusin-beta in normal pregnancy and preeclampsia. J. Matern.-Fetal Neonatal Med. 2012, 25, 1705–1708. [Google Scholar] [CrossRef]
- Kucur, M.; Tuten, A.; Oncul, M.; Acikgoz, A.S.; Yuksel, M.A.; Imamoglu, M.; Balci Ekmekci, O.; Yilmaz, N.; Madazli, R. Maternal serum apelin and YKL-40 levels in early and late-onset pre-eclampsia. Hypertens. Pregnancy 2014, 33, 467–475. [Google Scholar] [CrossRef]
- Deniz, R.; Baykus, Y.; Ustebay, S.; Ugur, K.; Yavuzkir, Ş.; Aydin, S. Evaluation of elabela, apelin and nitric oxide findings in maternal blood of normal pregnant women, pregnant women with pre-eclampsia, severe pre-eclampsia and umbilical arteries and venules of newborns. J. Obstet. Gynaecol. 2019, 39, 907–912. [Google Scholar] [CrossRef]
- Temur, M.; Yilmaz, Ö.; Taşgöz, F.N.; Kume, T. The evaluation of serum apelin levels in patients complicated with preeclampsia. J. Matern.-Fetal Neonatal Med. 2022, 35, 1848–1852. [Google Scholar] [CrossRef]
- Sattar Taha, A.; Zahraei, Z.; Al-Hakeim, H.K. Serum apelin and galectin-3 in preeclampsia in Iraq. Hypertens. Pregnancy 2020, 39, 379–386. [Google Scholar] [CrossRef]
- Bortoff, K.D.; Qiu, C.; Runyon, S.; Williams, M.A.; Maitra, R. Decreased Maternal Plasma Apelin Concentrations in Preeclampsia. Hypertens. Pregnancy 2012, 31, 398–404. [Google Scholar] [CrossRef]
- Gandham, R.; Dayanand, C.D.; Sheela, S.R.; Kiranmayee, P. Maternal serum Apelin 13 and APLN gene promoter variant -1860T > C in preeclampsia. J. Matern.-Fetal Neonatal Med. 2022, 35, 5008–5016. [Google Scholar] [CrossRef] [PubMed]
- Gürlek, B.; Yılmaz, A.; Durakoğlugil, M.E.; Karakaş, S.; Kazaz, İ.M.; Önal, Ö.; Şatıroğlu, Ö. Evaluation of serum apelin-13 and apelin-36 concentrations in preeclamptic pregnancies. J. Obstet. Gynaecol. Res. 2020, 46, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Fuentes-Carrasco, M.; Ruíz-Román, R.; Savirón-Cornudella, R.; Pérez-Roncero, G.; López-Baena, M.T.; Pérez-López, F.R. Systematic review and meta-analysis regarding maternal apelin in pregnant women with and without preeclampsia. Gynecol. Endocrinol. 2022, 38, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Burton, G.J.; Jauniaux, E. Pathophysiology of placental-derived fetal growth restriction. Am. J. Obstet. Gynecol. 2018, 218, S745–S761. [Google Scholar] [CrossRef] [PubMed]
- Resnik, R. Intrauterine growth restriction. Obstet. Gynecol. 2002, 99, 490–496. [Google Scholar] [CrossRef] [PubMed]
- Gordijn, S.J.; Beune, I.M.; Ganzevoort, W. Building consensus and standards in fetal growth restriction studies. Best Pract. Res. Clin. Obs. Gynaecol. 2018, 49, 117–126. [Google Scholar] [CrossRef]
- Malamitsi-Puchner, A.; Gourgiotis, D.; Boutsikou, M.; Baka, S.; Hassiakos, D.; Briana, D.D. Circulating apelin concentrations in mother/infant pairs at term. Acta Paediatr. 2007, 96, 1751–1754. [Google Scholar] [CrossRef] [PubMed]
- Van Mieghem, T.; Doherty, A.; Baczyk, D.; Drewlo, S.; Baud, D.; Carvalho, J.; Kingdom, J. Apelin in Normal Pregnancy and Pregnancies Complicated by Placental Insufficiency. Reprod. Sci. 2016, 23, 1037–1043. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.-Y.; Xie, H.; Yuan, L.-Q.; Luo, X.-H.; Huang, J.; Cui, R.-R.; Zhou, H.-D.; Wu, X.-P.; Liao, E.-Y. Apelin stimulates proliferation and suppresses apoptosis of mouse osteoblastic cell line MC3T3-E1 via JNK and PI3-K/Akt signaling pathways. Peptides 2007, 28, 708–718. [Google Scholar] [CrossRef] [PubMed]
- Behram, M.; Oğlak, S.C.; Dağ, İ. Circulating levels of Elabela in pregnant women complicated with intrauterine growth restriction. J. Gynecol. Obstet. Hum. Reprod. 2021, 50, 102127. [Google Scholar] [CrossRef] [PubMed]
- Yener, G.; Kavak, S.B.; Gul, Y.; Celik Kavak, E.; Gulcu Bulmus, F.; Sanli, C.; Batmaz, I.; Bulu, G. Elabela levels in pregnancies with intrauterine growth retardation. Ginekol. Pol. 2022, 94, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Aslan, M.; Celik, O.; Celik, N.; Turkcuoglu, I.; Yilmaz, E.; Karaer, A.; Simsek, Y.; Celik, E.; Aydin, S. Cord blood nesfatin-1 and apelin-36 levels in gestational diabetes mellitus. Endocrine 2012, 41, 424–429. [Google Scholar] [CrossRef]
- Akinci, B.; Celtik, A.; Tunali, S.; Genc, S.; Yuksel, F.; Secil, M.; Ozcan, M.A.; Bayraktar, F. Circulating apelin levels are associated with cardiometabolic risk factors in women with previous gestational diabetes. Arch. Gynecol. Obstet. 2014, 289, 787–793. [Google Scholar] [CrossRef] [PubMed]
- Boyadzhieva, M.; Atanasova, I.; Zacharieva, S.; Kedikova, S. Adipocytokines during pregnancy and postpartum in women with gestational diabetes and healthy controls. J. Endocrinol. Invest. 2013, 36, 944–949. [Google Scholar] [CrossRef]
- Mierzyński, R.; Poniedziałek-Czajkowska, E.; Dłuski, D.; Kamiński, M.; Mierzyńska, A.; Leszczyńska-Gorzelak, B. The Potential Role of Chemerin, Lipocalin 2, and Apelin in the Diagnosis and Pathophysiology of Gestational Diabetes Mellitus. J. Diabetes Res. 2021, 2021, 5547228. [Google Scholar] [CrossRef] [PubMed]
- Oncul, M.; Tuten, A.; Erman, H.; Gelisgen, R.; Benian, A.; Uzun, H. Maternal and cord blood apelin, resistin and visfatin levels in gestational diabetes mellitus. Minerva Med. 2013, 104, 527–535. [Google Scholar]
- Telejko, B.; Kuzmicki, M.; Zonenberg, A.; Modzelewska, A.; Niedziolko-Bagniuk, K.; Ponurkiewicz, A.; Wawrusiewicz-Kurylonek, N.; Nikolajuk, A.; Szamatowicz, J.; Laudanski, P.; et al. Ghrelin in gestational diabetes: Serum level and mRNA expression in fat and placental tissue. Exp. Clin. Endocrinol. Diabetes 2010, 118, 87–92. [Google Scholar] [CrossRef]
- Sun, J.; Ren, J.; Zuo, C.; Deng, D.; Pan, F.; Chen, R.; Zhu, J.; Chen, C.; Ye, S. Circulating apelin, chemerin and omentin levels in patients with gestational diabetes mellitus: A systematic review and meta-analysis. Lipids Health Dis. 2020, 19, 26. [Google Scholar] [CrossRef]
- Dasgupta, S.; Banerjee, U.; Mukhopadhyay, P.; Maity, P.; Saha, S.; Das, B. Clinicopathological study and immunohistochemical analysis of expression of annexin A5 and apelin in human placentae of gestational diabetes mellitus. Diabetes Metab. Syndr. 2022, 16, 102435. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.-D.; Huang, Y.; Li, H. Regulatory role of Apelin-13-mediated PI3K/AKT signaling pathway in the glucose and lipid metabolism of mouse with gestational diabetes mellitus. Immunobiology 2021, 226, 152135. [Google Scholar] [CrossRef] [PubMed]
- Romero, R.; Kusanovic, J.P.; Chaiworapongsa, T.; Hassan, S.S. Placental bed disorders in preterm labor, preterm PROM, spontaneous abortion and abruptio placentae. Best Pract. Res. Clin. Obstet. Gynaecol. 2011, 25, 313–327. [Google Scholar] [CrossRef] [PubMed]
- Manolea, M.M.; Dijmărescu, A.L.; Popescu, F.C.; Novac, M.B.; DiŢescu, D. Evaluation of the implantation site morphology in spontaneous abortion. Romanian J. Morphol. Embryol. Rev. Roum. Morphol. Embryol. 2015, 56, 125–131. [Google Scholar]
- Tug, E.; Yirmibes Karaoguz, M.; Nas, T. Expression of the syncytin-1 and syncytin-2 genes in the trophoblastic tissue of the early pregnancy losses with normal and abnormal karyotypes. Gene 2020, 741, 144533. [Google Scholar] [CrossRef]
- Qi, Y.; Hou, Y.; Ma, M.; Li, X.; Wu, J. Circulating levels of Elabela in pregnant women with missed abortion. Gynecol. Endocrinol. Off. J. Int. Soc. Gynecol. Endocrinol. 2022, 38, 693–696. [Google Scholar] [CrossRef]
- Georgiadou, D.; Boussata, S.; van Dijk, M. ELABELA measurements by commercial ELISA kits require sample extraction. Am. J. Physiol.-Endocrinol. Metab. 2019, 317, E1218–E1219. [Google Scholar] [CrossRef]
- Marsault, E.; Llorens-Cortes, C.; Iturrioz, X.; Chun, H.J.; Lesur, O.; Oudit, G.Y.; Auger-Messier, M. The apelinergic system: A perspective on challenges and opportunities in cardiovascular and metabolic disorders. Ann. N. Y. Acad. Sci. 2019, 1455, 12–33. [Google Scholar] [CrossRef]
- Zhen, E.Y.; Higgs, R.E.; Gutierrez, J.A. Pyroglutamyl apelin-13 identified as the major apelin isoform in human plasma. Anal. Biochem. 2013, 442, 1–9. [Google Scholar] [CrossRef]
- De Mota, N.; Reaux-Le Goazigo, A.; El Messari, S.; Chartrel, N.; Roesch, D.; Dujardin, C.; Kordon, C.; Vaudry, H.; Moos, F.; Llorens-Cortes, C. Apelin, a potent diuretic neuropeptide counteracting vasopressin actions through inhibition of vasopressin neuron activity and vasopressin release. Proc. Natl. Acad. Sci. USA 2004, 101, 10464–10469. [Google Scholar] [CrossRef] [PubMed]
- Reaux, A.; De Mota, N.; Skultetyova, I.; Lenkei, Z.; El Messari, S.; Gallatz, K.; Corvol, P.; Palkovits, M.; Llorens-Cortès, C. Physiological role of a novel neuropeptide, apelin, and its receptor in the rat brain. J. Neurochem. 2001, 77, 1085–1096. [Google Scholar] [CrossRef] [PubMed]
- Azizi, M.; Iturrioz, X.; Blanchard, A.; Peyrard, S.; De Mota, N.; Chartrel, N.; Vaudry, H.; Corvol, P.; Llorens-Cortes, C. Reciprocal Regulation of Plasma Apelin and Vasopressin by Osmotic Stimuli. J. Am. Soc. Nephrol. 2008, 19, 1015–1024. [Google Scholar] [CrossRef]
- Brash, L.; Barnes, G.D.; Brewis, M.J.; Church, A.C.; Gibbs, S.J.; Howard, L.S.G.E.; Jayasekera, G.; Johnson, M.K.; McGlinchey, N.; Onorato, J.; et al. Short-Term Hemodynamic Effects of Apelin in Patients With Pulmonary Arterial Hypertension. JACC Basic Transl. Sci. 2018, 3, 176–186. [Google Scholar] [CrossRef] [PubMed]
- Mesmin, C.; Dubois, M.; Becher, F.; Fenaille, F.; Ezan, E. Liquid chromatography/tandem mass spectrometry assay for the absolute quantification of the expected circulating apelin peptides in human plasma. Rapid Commun. Mass Spectrom. 2010, 24, 2875–2884. [Google Scholar] [CrossRef]
- Cobellis, L.; De Falco, M.; Mastrogiacomo, A.; Giraldi, D.; Dattilo, D.; Scaffa, C.; Colacurci, N.; De Luca, A. Modulation of apelin and APJ receptor in normal and preeclampsia-complicated placentas. Histol. Histopathol. 2007, 22, 1–8. [Google Scholar]
- Hanssens, S.; Marousez, L.; Pécheux, O.; Besengez, C.; Storme, L.; Deruelle, P.; Eberlé, D.; Lesage, J. Maternal obesity reduces apelin level in cord blood without altering the placental apelin/elabela-APJ system. Placenta 2022, 128, 112–115. [Google Scholar] [CrossRef]
- Telejko, B.; Kuzmicki, M.; Wawrusiewicz-Kurylonek, N.; Szamatowicz, J.; Nikolajuk, A.; Zonenberg, A.; Zwierz-Gugala, D.; Jelski, W.; Laudański, P.; Wilczynski, J.; et al. Plasma apelin levels and apelin/APJ mRNA expression in patients with gestational diabetes mellitus. Diabetes Res. Clin. Pract. 2010, 87, 176–183. [Google Scholar] [CrossRef]
- Karagoz, Z.K.; Aydin, S.; Ugur, K.; Tigli, A.; Deniz, R.; Baykus, Y.; Sahin, I.; Yalcin, M.H.; Yavuz, A.; Aksoy, A.; et al. Molecular communication between Apelin-13, Apelin-36, Elabela, and nitric oxide in gestational diabetes mellitus. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 3289–3300. [Google Scholar]
- Yamaleyeva, L.M.; Chappell, M.C.; Brosnihan, K.B.; Anton, L.; Caudell, D.L.; Shi, S.; McGee, C.; Pirro, N.; Gallagher, P.E.; Taylor, R.N.; et al. Downregulation of apelin in the human placental chorionic villi from preeclamptic pregnancies. Am. J. Physiol. Endocrinol. Metab. 2015, 309, E852–E860. [Google Scholar] [CrossRef]
- Li, Y.; Yang, X.; Ouyang, S.; He, J.; Yu, B.; Lin, X.; Zhang, Q.; Tao, J. Declined circulating Elabela levels in patients with essential hypertension and its association with impaired vascular function: A preliminary study. Clin. Exp. Hypertens. 2020, 42, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Chapman, F.A.; Nyimanu, D.; Maguire, J.J.; Davenport, A.P.; Newby, D.E.; Dhaun, N. The therapeutic potential of apelin in kidney disease. Nat. Rev. Nephrol. 2021, 17, 840–853. [Google Scholar] [CrossRef] [PubMed]
- Ba, H.-J.; Chen, H.-S.; Su, Z.; Du, M.-L.; Chen, Q.-L.; Li, Y.-H.; Ma, H.-M. Associations between serum apelin-12 levels and obesity-related markers in Chinese children. PLoS ONE 2014, 9, e86577. [Google Scholar] [CrossRef] [PubMed]
- El Wakeel, M.A.; El-Kassas, G.M.; Kamhawy, A.H.; Galal, E.M.; Nassar, M.S.; Hammad, E.M.; El-Zayat, S.R. Serum Apelin and Obesity-Related Complications in Egyptian Children. Open Access Maced. J. Med. Sci. 2018, 6, 1354–1358. [Google Scholar] [CrossRef]
- Vinel, C.; Lukjanenko, L.; Batut, A.; Deleruyelle, S.; Pradère, J.-P.; Le Gonidec, S.; Dortignac, A.; Geoffre, N.; Pereira, O.; Karaz, S.; et al. The exerkine apelin reverses age-associated sarcopenia. Nat. Med. 2018, 24, 1360–1371. [Google Scholar] [CrossRef]
- Meral, C.; Tascilar, E.; Karademir, F.; Tanju, I.A.; Cekmez, F.; Ipcioglu, O.M.; Ercin, C.N.; Gocmen, I.; Dogru, T. Elevated plasma levels of apelin in children with type 1 diabetes mellitus. J. Pediatr. Endocrinol. Metab. 2010, 23, 497–502. [Google Scholar] [CrossRef]
- Castan-Laurell, I.; El Boustany, R.; Pereira, O.; Potier, L.; Marre, M.; Fumeron, F.; Valet, P.; Gourdy, P.; Velho, G.; Roussel, R. Plasma Apelin and Risk of Type 2 Diabetes in a Cohort From the Community. Diabetes Care 2019, 43, e15–e16. [Google Scholar] [CrossRef]
- Capo, A.; Di Nicola, M.; Costantini, E.; Reale, M.; Amerio, P. Circulating levels of Apelin-36 in patients with mild to moderate psoriasis. G. Ital. Di Dermatol. E Venereol. Organo Uff. Soc. Ital. Di Dermatol. E Sifilogr. 2020, 155, 646–651. [Google Scholar] [CrossRef]
- Zorlu, M.; Kiskac, M.; Karatoprak, C.; Kesgin, S.; Cakirca, M.; Yildiz, K.; Ardic, C.; Cikrikcioglu, M.A.; Erkoc, R. Assessment of serum apelin and lipocalin-2 levels in patients with subclinical hypothyroidism. Ann. Endocrinol. 2014, 75, 10–14. [Google Scholar] [CrossRef]
- Gürel, A.; Doğantekin, A.; Özkan, Y.; Aydın, S. Serum apelin levels in patients with thyroid dysfunction. Int. J. Clin. Exp. Med. 2015, 8, 16394–16398. [Google Scholar]
- Kourtis, A.; Gkiomisi, A.; Mouzaki, M.; Makedou, K.; Anastasilakis, A.D.; Toulis, K.A.; Gerou, S.; Gavana, E.; Agorastos, T. Apelin levels in normal pregnancy. Clin. Endocrinol. 2011, 75, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Van Mieghem, T.; van Bree, R.; Van Herck, E.; Pijnenborg, R.; Deprest, J.; Verhaeghe, J. Maternal apelin physiology during rat pregnancy: The role of the placenta. Placenta 2010, 31, 725–730. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, M.; Rezaei, H.; Eskandari-Nasab, E.; Kaykhaei, M.A.; Taheri, M. Association between the apelin rs2235306 gene polymorphism and metabolic syndrome. Turk. J. Med. Sci. 2014, 44, 775–780. [Google Scholar] [CrossRef]
- Wu, X.-D.; Zhang, N.; Liang, M.; Liu, W.-L.; Lin, B.-B.; Xiao, Y.-R.; Li, Y.-Z.; Zeng, K.; Lin, C.-Z. Gender-specific association between Apelin/APJ gene polymorphisms and hypertension risk in Southeast China. Gene 2018, 669, 63–68. [Google Scholar] [CrossRef]
- Laudermilk, L.T.; Harper, K.M.; Moy, S.S.; Runyon, S.; Zhou, B.; Koller, B.; Maitra, R. Aplnr knockout mice display sex-specific changes in conditioned fear. Behav. Brain Res. 2021, 400, 113059. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Zhao, L.; Zhang, Y.-P.; Zhong, J.-C.; Yang, X.-C. Declined ELABELA plasma levels in hypertension patients with atrial fibrillation: A case control study. BMC Cardiovasc. Disord. 2021, 21, 390. [Google Scholar] [CrossRef] [PubMed]
- Chae, S.A.; Son, J.S.; Du, M. Prenatal exercise in fetal development: A placental perspective. FEBS J. 2022, 289, 3058–3071. [Google Scholar] [CrossRef]
- Ma, Z.; Zhao, L.; Martin, S.; Zhang, Y.; Dong, Y.; Zhong, J.-C.; Yang, X.-C. Lower Plasma Elabela Levels in Hypertensive Patients With Heart Failure Predict the Occurrence of Major Adverse Cardiac Events: A Preliminary Study. Front. Cardiovasc. Med. 2021, 8, 638468. [Google Scholar] [CrossRef]

| Variable | Tissue/Fluid | Expression | Methods | Pathologies | Reference |
|---|---|---|---|---|---|
| Pregnancy | |||||
| Molecule: APLNR | |||||
| delivery | placenta | mRNA, protein | Real-time PCR, IHC | Late onset PE (↘) | [71] |
| delivery | placenta | protein | IHC | PE (≈) | [58] |
| delivery | placenta | protein | IHC | PE (↗) | [136] |
| delivery | placenta | mRNA | RT-qPCR | Maternal obesity (≈) | [137] |
| delivery | placenta, adipose tissue | mRNA | Real-time PCR | GDM (≈) | [138] |
| Molecule: APLN | |||||
| delivery | serum, cord blood | protein | ELISA | GDM (≈in cord blood, ↗ in serum) | [115] |
| 24–28 WG | serum | protein | ELISA, EIA | GDM (↘) | [117] |
| 2nd and 3rd trimester | serum | protein | ELISA | GDM (↗only in the 2nd trimester ) | [74] |
| delivery | serum | protein | ELISA | GDM (≈) | [118] |
| delivery | maternal and cord blood | protein | ELISA | GDM (↘ in cord blood, ≈ in maternal blood ) | [119] |
| 24–32 WG and delivery | plasma, adipose tissue, placenta | protein | ELISA, Real-time PCR | GDM (≈mRNA and circulating level) | [138] |
| delivery | placenta | protein | IHC | GDM (↘) | [122] |
| 2nd trimester | serum | protein | ELISA | GDM (↗) | [139] |
| 20–34 WG and delivery | serum, placenta | protein, mRNA | ELISA, IHC, RT-PCR | Preterm ≈, IUGR and PE (↘ prot, ≈mRNA) | [111] |
| delivery | placenta | protein | RIA | PE (↘) | [140] |
| delivery | plasma | protein | ELISA | PE (↘) | [103] |
| time of diagnosis | serum | protein | EIA | PE (↗) | [98] |
| time of diagnosis | serum | protein | EIA | PE (↘) | [101] |
| delivery | maternal and cord blood | protein | ELISA | PE (↘ in both maternal and cord blood) | [100] |
| delivery | placenta | protein | IHC | PE (↘) | [122] |
| delivery | serum | protein | ELISA | PE (↘) | [104] |
| delivery | placenta, serum | protein, mRNA | ELISA, IHC, WB, RT-qPCR | PE (↘ in placenta, ↗ in maternal circulation) | [58] |
| delivery | serum | protein | ELISA | PE (↘) | [105] |
| delivery | placenta | protein | IHC | PE (↗) | [136] |
| time of diagnosis | serum | protein | ELISA | PE (↗) | [99] |
| delivery | plasma, cord blood, placenta | protein, mRNA | ELISA, RT-qPCR | Maternal obesity (≈mRNA and plasma, ↘ in cord blood) | [137] |
| Molecule: ELA | |||||
| time of diagnosis | serum | protein | ELISA | IUGR (↘) | [113] |
| delivery | serum | protein | ELISA | IUGR (↗) | [114] |
| delivery | maternal and cord blood | protein | ELISA | PE (↘ in both maternal and cord blood) | [100] |
| delivery | plasma | protein | ELISA | Early-onset PE (≈), late-onset PE (↗) | [92] |
| delivery | serum, urine, placenta | protein, mRNA | ELISA, IHC, Real-time PCR | Late-onset PE (circulating and placental level ↘) | [71] |
| delivery | plasma, placenta | protein, mRNA | ELISA, RNA sequencing | PE (≈ placental mRNA and circulating protein) | [91] |
| 1st trimester | serum | protein | ELISA | GH/PE (≈) | [67] |
| 2nd trimester | serum | protein | ELISA | GDM (↗) | [139] |
| 2nd and 3rd trimester | serum | protein | ELISA | GDM (↘ during second trimester) | [74] |
| delivery | plasma, cord blood, placenta | protein, mRNA | ELISA, RT-qPCR | Maternal obesity (≈mRNA and protein) | [137] |
| time of diagnosis | serum | protein | ELISA | MA (↘) | [127] |
| Post-partum | |||||
| Molecule: APLN | |||||
| serum, colostrum and mature milk | protein | ELISA | GDM (↘ in colostrum and milk) | [79] | |
| plasma | protein | ELISA | GDM (↘) | [116] | |
| plasma, breast milk | protein | ELISA | Obesity (↗with BMI) | [80] | |
| Model | Peptide | Output | Reference |
|---|---|---|---|
| Obesogenic diet mice | Apelin | Improvement in placental function of obese dams | [157] |
| L-NAME-induced PE in rats | Apelin-13 | Reno-protective effects | [97] |
| PE rat model (TGA-PE) | Pyr- Apelin-13 | Improvement in hemodynamic response and renal injury without fetal toxicity | [140] |
| PE rat model by reduced uterine perfusion pressure | Apelin | Amelioration of PE symptoms | [39] |
| L-NAME-induced PE in rats | ELA | Reversion of the phenotypes of L-NAME-induced PE | [158] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pécheux, O.; Correia-Branco, A.; Cohen, M.; Martinez de Tejada, B. The Apelinergic System in Pregnancy. Int. J. Mol. Sci. 2023, 24, 8014. https://doi.org/10.3390/ijms24098014
Pécheux O, Correia-Branco A, Cohen M, Martinez de Tejada B. The Apelinergic System in Pregnancy. International Journal of Molecular Sciences. 2023; 24(9):8014. https://doi.org/10.3390/ijms24098014
Chicago/Turabian StylePécheux, Océane, Ana Correia-Branco, Marie Cohen, and Begoῆa Martinez de Tejada. 2023. "The Apelinergic System in Pregnancy" International Journal of Molecular Sciences 24, no. 9: 8014. https://doi.org/10.3390/ijms24098014
APA StylePécheux, O., Correia-Branco, A., Cohen, M., & Martinez de Tejada, B. (2023). The Apelinergic System in Pregnancy. International Journal of Molecular Sciences, 24(9), 8014. https://doi.org/10.3390/ijms24098014







