Study on Physicochemical Properties of Biocomposite Films with Spent Coffee Grounds as a Filler and Their Influence on Physiological State of Growing Plants
Abstract
1. Introduction
2. Results and Discussion
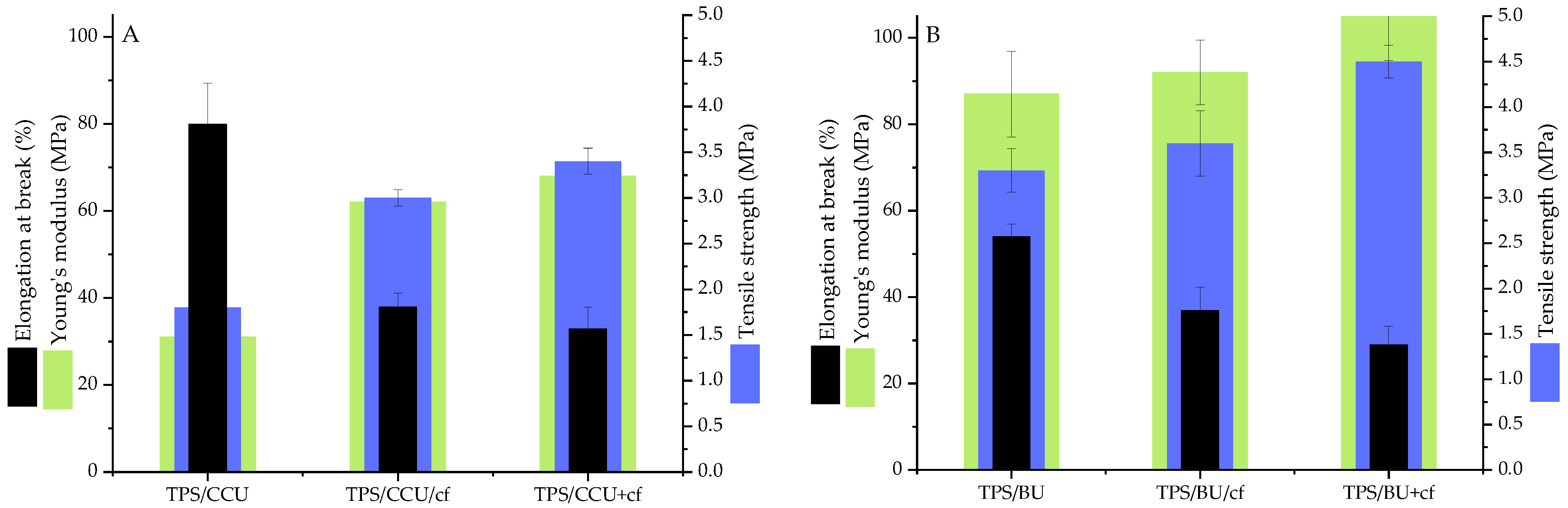
2.1. Mechanical Tests Results
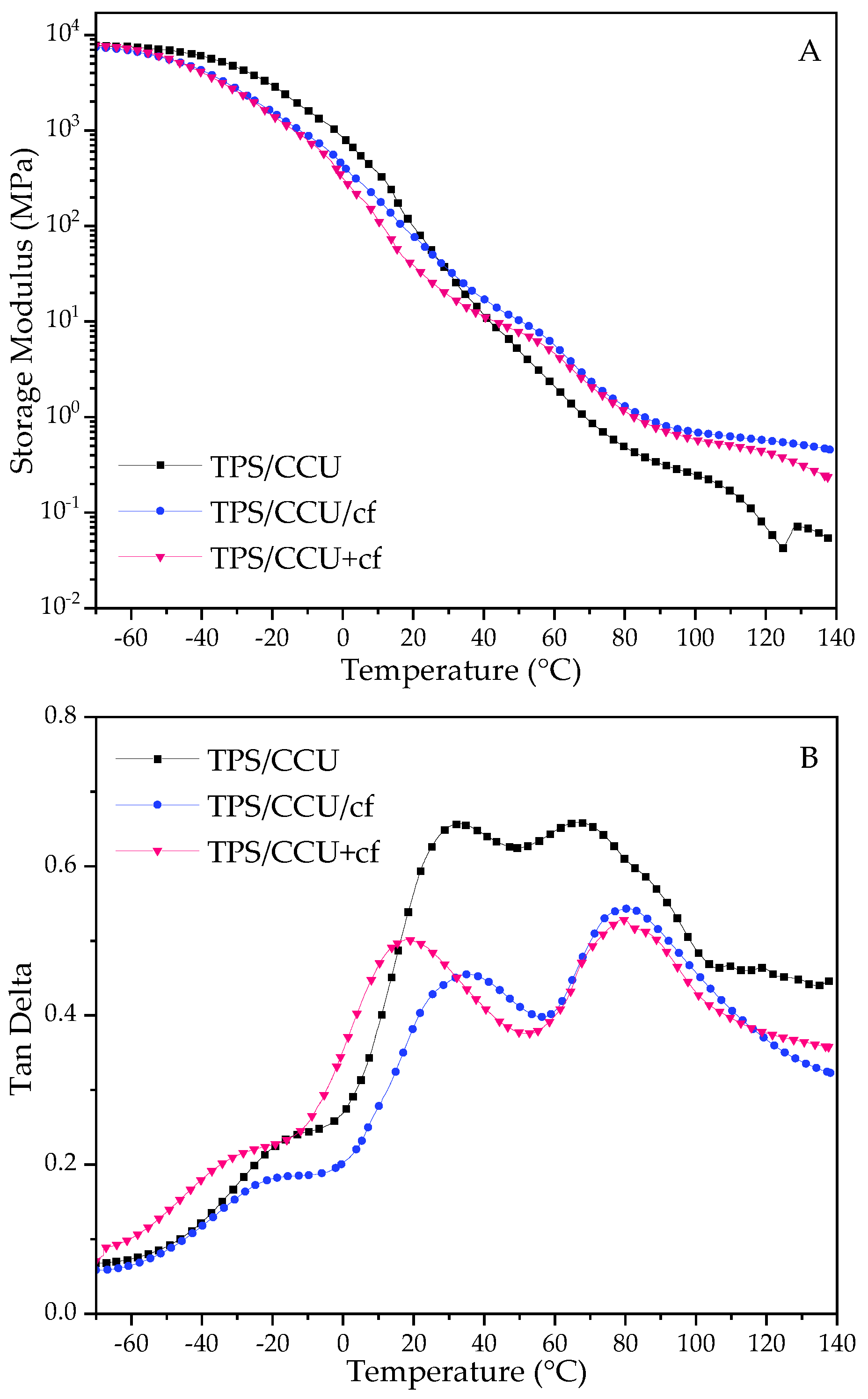
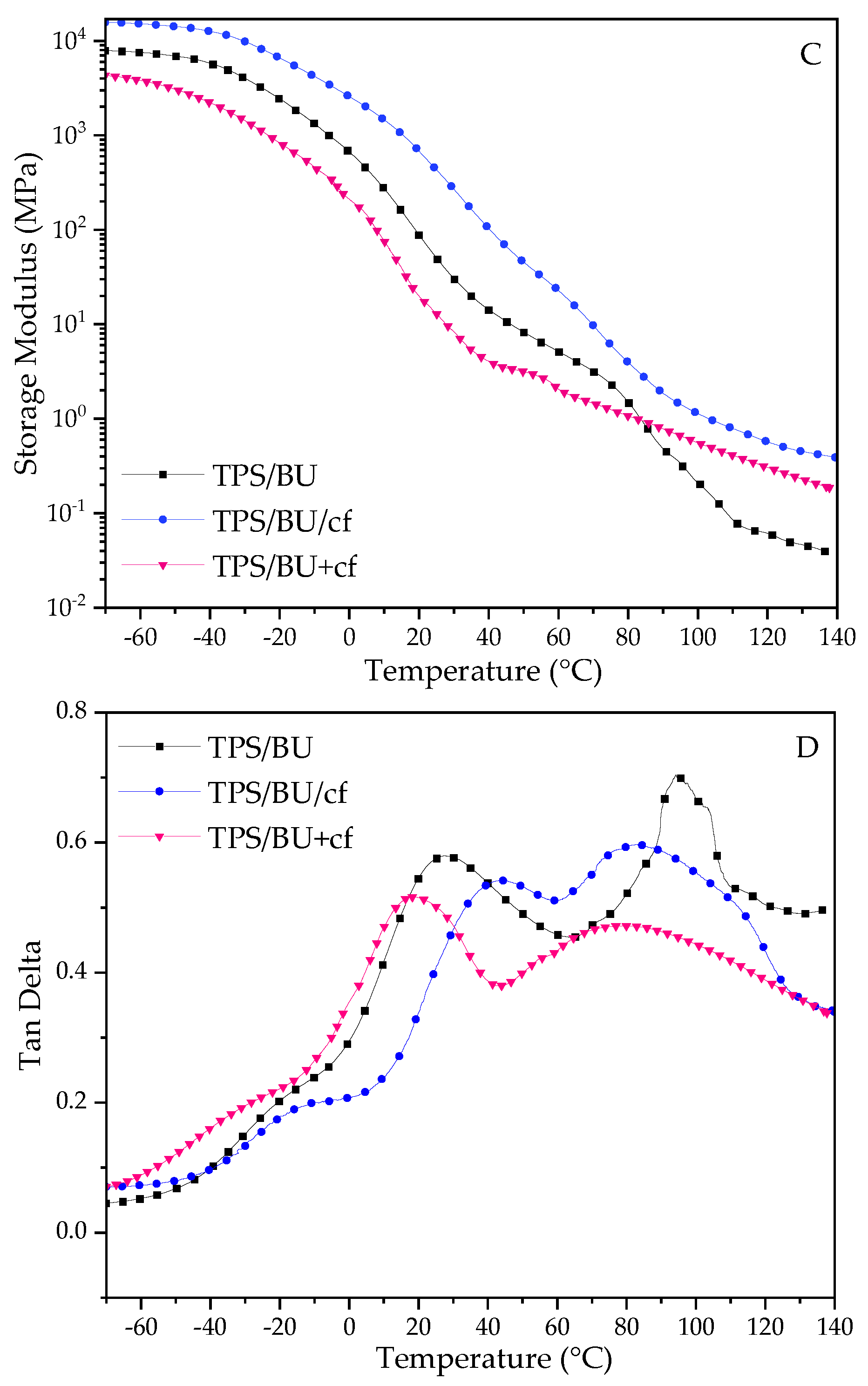
2.2. Dynamical Mechanical Thermal Analysis (DMTA)
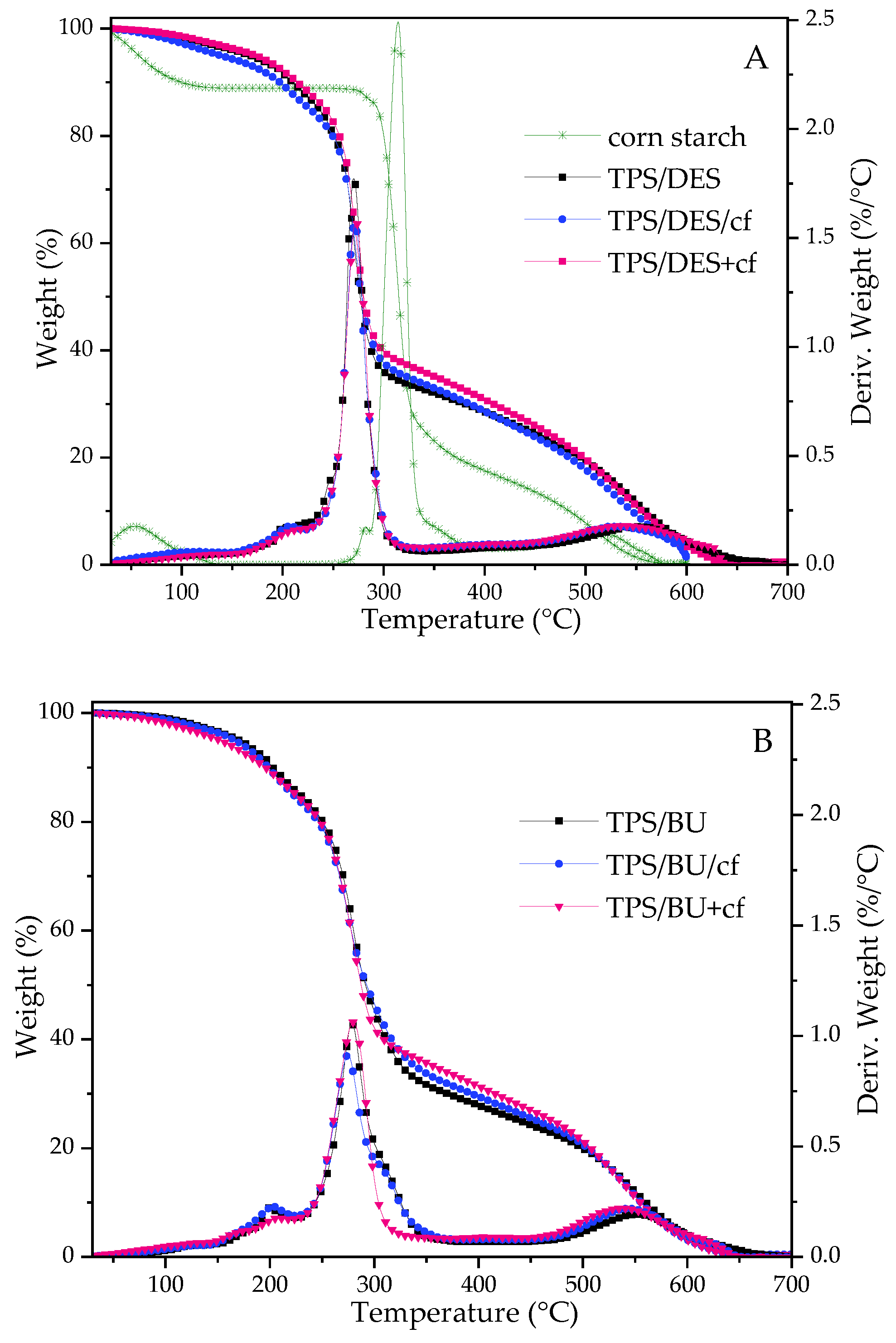
2.3. Thermal Stability of TPS Films (TGA)
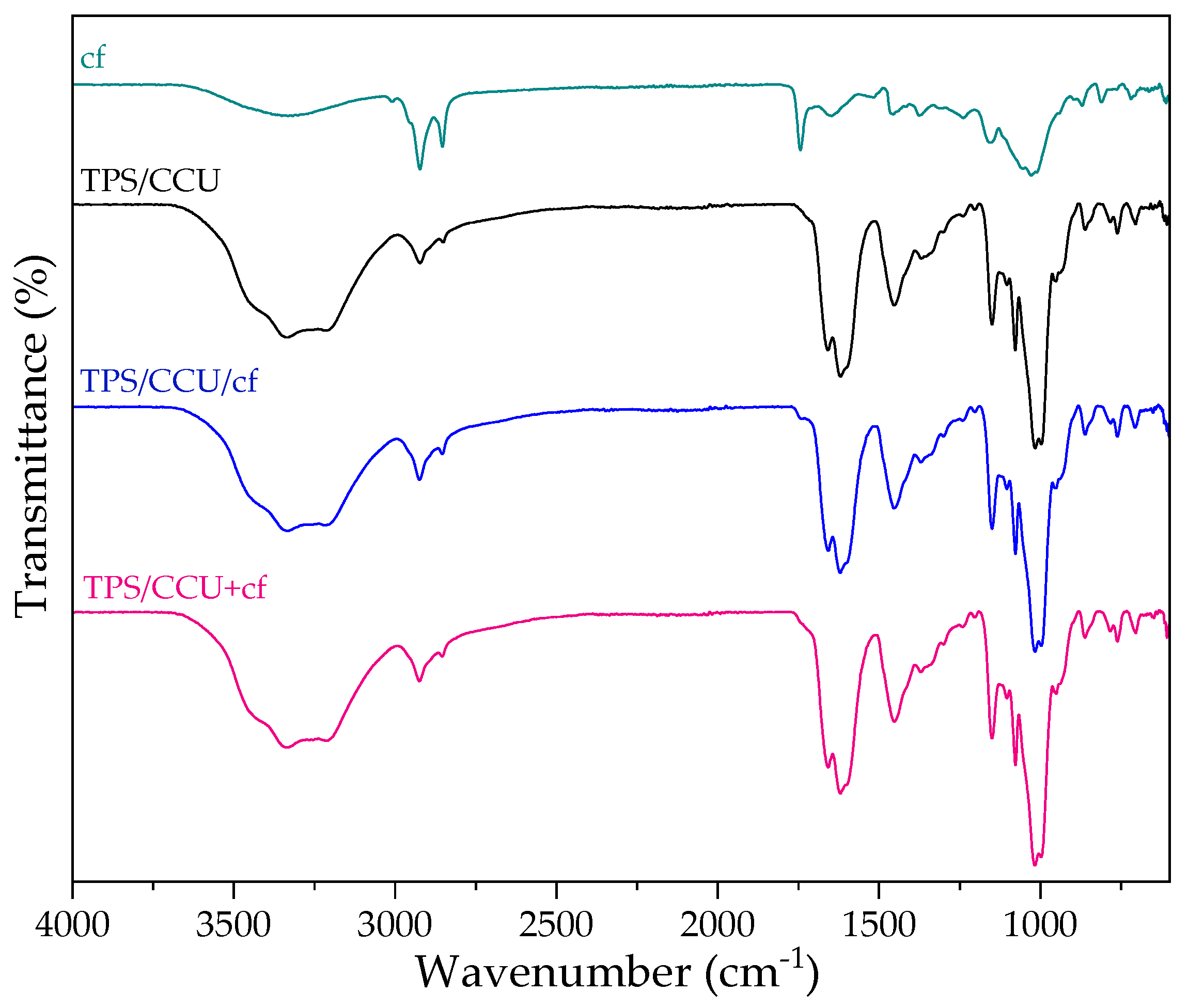
2.4. FTIR Characterization
2.5. Moisture Sorption, Swelling and Dissolution Degrees
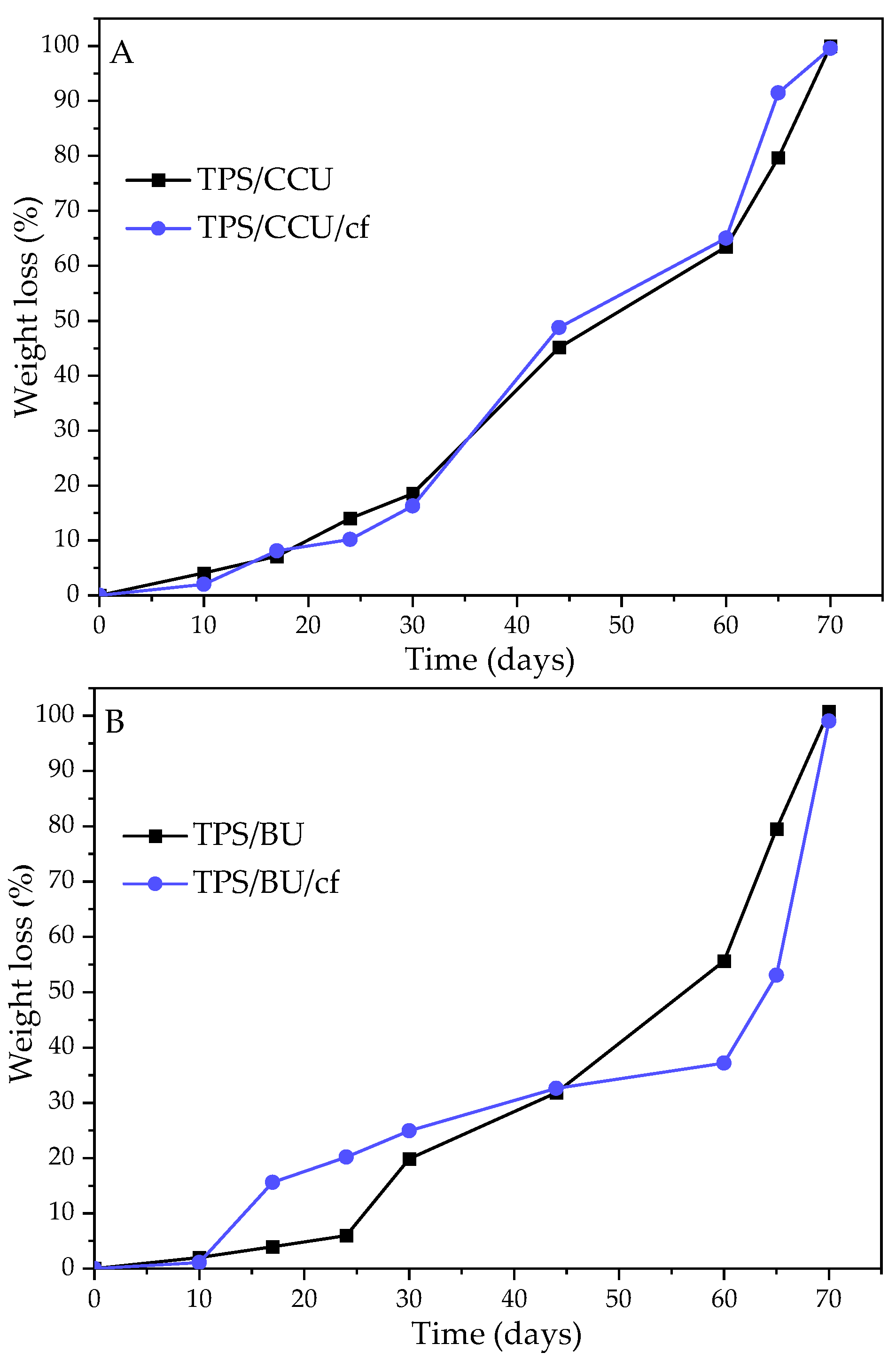
2.6. Biodegradation in Soil
2.7. Influence of TPS Materials Presence on Physicochemical State of Model Plants
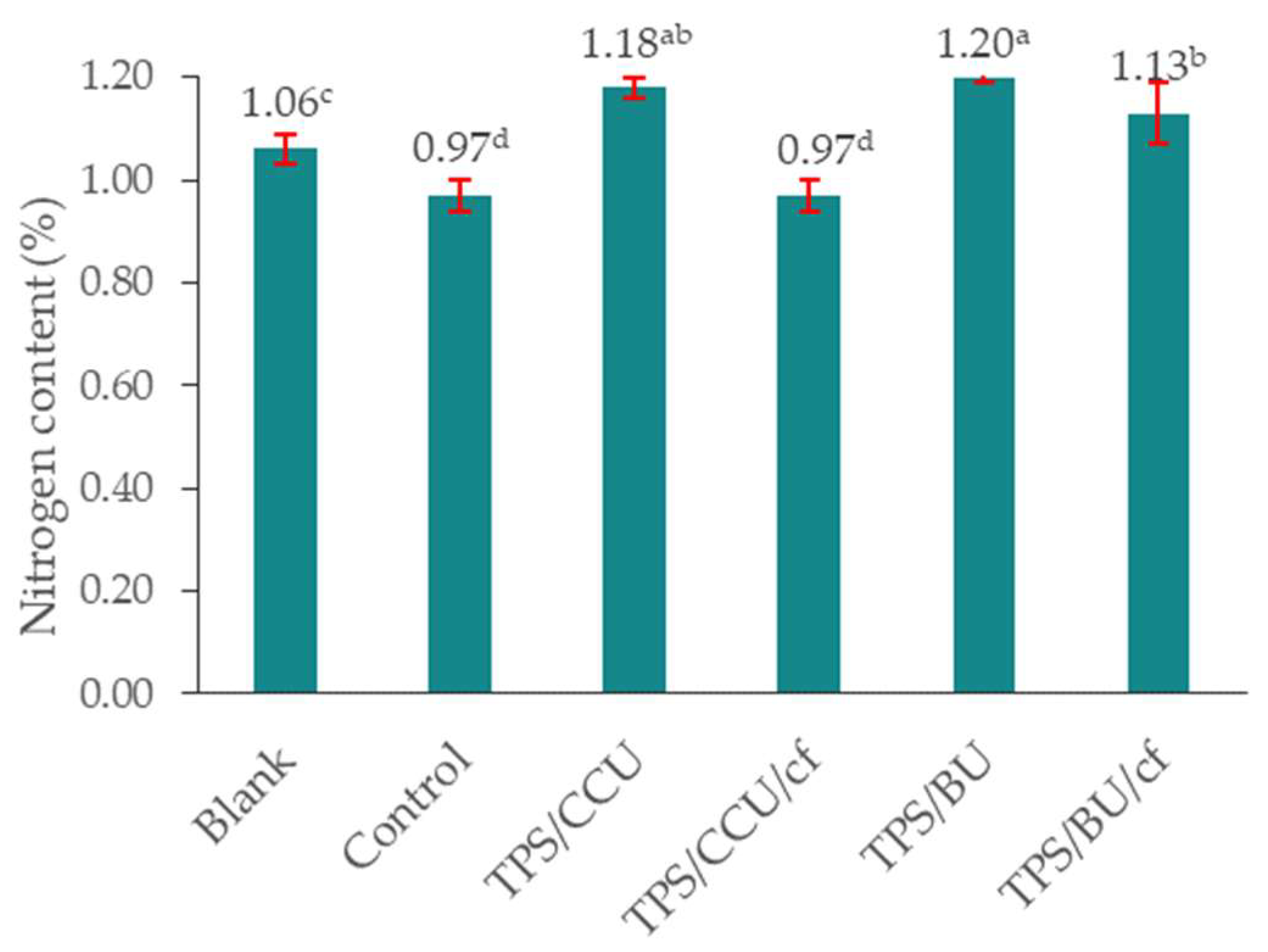
2.8. Remaining of Nitrogen in the Soil Substrate from TPS Materials
3. Materials and Methods
3.1. Materials
3.2. TPS Films Preparation
3.3. Mechanical Tests
3.4. Dynamical Mechanical Thermal Analysis—DMTA
3.5. Thermal Gravimetry Analysis—TGA
3.6. FTIR-ATR Spectroscopy of the Films
3.7. Evaluation of the Samples’ Behavior in Moisture and Water
3.8. Determination of Biodegradability in Soil
3.9. Investigation of the Toxicity and Influence of the TPS-Based Films on Growing Plants’ Physiological State
3.9.1. Gas Exchange Parameters
3.9.2. Chlorophyll “a” Fluorescence Parameters
3.9.3. Content of Photosynthetic Pigments
3.9.4. Determination of Proline Content
3.10. Nitrogen Content in Soil Substrate
3.11. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ellis, R.P.; Cochrane, M.P.; Dale, M.F.B.; Duffus, C.M.; Lynn, A.; Morrison, I.M.; Prentice, R.D.M.; Swanston, J.S.; Tiller, S.A. Starch Production and Industrial Use. J. Sci. Food Agric. 1998, 77, 289–311. [Google Scholar] [CrossRef]
- Montilla-Buitrago, C.E.; Gómez-López, R.A.; Solanilla-Duque, J.F.; Serna-Cock, L.; Villada-Castillo, H.S. Effect of Plasticizers on Properties, Retrogradation, and Processing of Extrusion-Obtained Thermoplastic Starch: A Review. Starch-Stärke 2021, 73, 2100060. [Google Scholar] [CrossRef]
- Nafchi, A.M.; Moradpour, M.; Saeidi, M.; Alias, A.K. Thermoplastic starches: Properties, challenges and prospects. Starch 2013, 65, 61–72. [Google Scholar] [CrossRef]
- Yu, J.; Liu, X.; Xu, S.; Shao, P.; Li, J.; Chen, Z.; Wang, X.; Lin, Y.; Renard, C.M.G.C. Advances in Green Solvents for Production of Polysaccharide-based Packaging Films: Insights of Ionic Liquids and Deep Eutectic Solvents. Comp. Rev. Food Sci. Food Safe 2023, 22, 1030–1057. [Google Scholar] [CrossRef] [PubMed]
- Ptak, S.; Zarski, A.; Kapuśniak, J. The Importance of Ionic Liquids in the Modification of Starch and Processing of Starch-Based Materials. Materials 2020, 13, 4479. [Google Scholar] [CrossRef]
- Lončarić, M.; Jakobek, L.; Molnar, M. Deep Eutectic Solvents in the Production of Biopolymer-Based Materials. Croat. Chem. Acta 2021, 94, P1–P8. [Google Scholar] [CrossRef]
- Zdanowicz, M.; Wilpiszewska, K.; Spychaj, T. Deep eutectic solvents for polysaccharides processing: A review. Carbohydr. Polym. 2018, 200, 361–380. [Google Scholar] [CrossRef] [PubMed]
- Zdanowicz, M. Starch Treatment with Deep Eutectic Solvents, Ionic Liquids and Glycerol. A Comparative Study. Carbohydr. Polym. 2020, 229, 115574. [Google Scholar] [CrossRef] [PubMed]
- Zdanowicz, M. Deep Eutectic Solvents Based on Urea, Polyols and Sugars for Starch Treatment. Int. J. Biol. Macromol. 2021, 176, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Zdanowicz, M. Influence of Urea Content in Deep Eutectic Solvents on Thermoplastic Starch Films’ Properties. Appl. Sci. 2023, 13, 1383. [Google Scholar] [CrossRef]
- Chen, Y.; Shull, K.R. Controlling the Properties of Thermoplastic Starch Films with Hydrogen Bonding Plasticizers. Carbohydr. Polym. Technol. App. 2023, 5, 100291. [Google Scholar] [CrossRef]
- Xiaofei, M.; Jiugao, Y.; Jin, F. Urea and Formamide as a Mixed Plasticizer for Thermoplastic Starch. Polym. Int. 2004, 53, 1780–1785. [Google Scholar] [CrossRef]
- Ma, X.F.; Yu, J.G.; Wan, J.J. Urea and Ethanolamine as a Mixed Plasticizer for Thermoplastic Starch. Carbohydr. Polym. 2006, 64, 267–273. [Google Scholar] [CrossRef]
- De Souza Gamarano, D.; Pereira, I.M.; Mottin, A.C.; Ayres, E. Thermoplastic Starch—Urea, a Feasible Alternative to Release Nitrogen as Fertilizer. Macromol. Sym. 2022, 406, 2200043. [Google Scholar] [CrossRef]
- Bijla, L.; Aissa, R.; Laknifli, A.; Bouyahya, A.; Harhar, H.; Gharby, S. Spent Coffee Grounds: A Sustainable Approach toward Novel Perspectives of Valorization. J. Food Biochem. 2022, 46, e14190. [Google Scholar] [CrossRef]
- McNutt, J.; He, Q. (Sophia) Spent Coffee Grounds: A Review on Current Utilization. J. Ind. Eng. Chem. 2019, 71, 78–88. [Google Scholar] [CrossRef]
- Kovalcik, A.; Obruca, S.; Marova, I. Valorization of Spent Coffee Grounds: A Review. Food Bioprod. Process. 2018, 110, 104–119. [Google Scholar] [CrossRef]
- Morikawa, C.K.; Saigusa, M. Recycling Coffee Grounds and Tea Leaf Wastes to Improve the Yield and Mineral Content of Grains of Paddy Rice. J. Sci. Food Agric. 2011, 91, 2108–2111. [Google Scholar] [CrossRef]
- de Bomfim, A.S.C.; de Oliveira, D.M.; Walling, E.; Babin, A.; Hersant, G.; Vaneeckhaute, C.; Dumont, M.-J.; Rodrigue, D. Spent Coffee Grounds Characterization and Reuse in Composting and Soil Amendment. Waste 2022, 1, 2–20. [Google Scholar] [CrossRef]
- Ciesielczuk, T.; Rosik-Dulewska, C.; Poluszyńska, J.; Miłek, D.; Szewczyk, A.; Sławińska, I. Acute Toxicity of Experimental Fertilizers Made of Spent Coffee Grounds. Waste Biomass Valor. 2018, 9, 2157–2164. [Google Scholar] [CrossRef]
- Bomfim, A.; Oliveira, D.; Voorwald, H.; Benini, K.; Dumont, M.-J.; Rodrigue, D. Valorization of Spent Coffee Grounds as Precursors for Biopolymers and Composite Production. Polymers 2022, 14, 437. [Google Scholar] [CrossRef] [PubMed]
- Hejna, A. Potential Applications of By-Products from the Coffee Industry in Polymer Technology—Current State and Perspectives. Waste Manag. 2021, 121, 296–330. [Google Scholar] [CrossRef]
- Chen, Y.M.; Phang, S.W.; Tee, T.T.; Lee, T.S.; Soo, B.S. Preliminary Study of Mechanical Properties in Thermoplastic Starch (TPS)/Coffee-Waste-Derived Fillers Composites. In Proceedings of the Engineering Undergraduate Research Catalyst Conference (EURECA), Sunway, Malaysia, 1–2 July 2015; Available online: https://expert.taylors.edu.my/file/rems/publication/104770_1817_1.pdf (accessed on 1 January 2020).
- Nguyen, V.H.T.; Prabhakar, M.N.; Lee, D.; Song, J. Spent Coffee Grounds: An Intriguing Biowaste Reinforcement of Thermoplastic Starch with Potential Application in Green Packaging. Polym. Comp. 2022, 43, 5488–5499. [Google Scholar] [CrossRef]
- Adamus, J.; Spychaj, T.; Zdanowicz, M.; Jędrzejewski, R. Thermoplastic starch with deep eutectic solvents and montmorillonite as a base for composite materials. Ind. Crops Prod. 2018, 123, 278–284. [Google Scholar] [CrossRef]
- Zdanowicz, M.; Sałasińska, K.; Lewandowski, K.; Skórczewska, K. Thermoplastic Starch/Ternary Deep Eutectic Solvent/Lignin Materials: Study of Physicochemical Properties and Fire Behavior. ACS Sustain. Chem. Eng. 2022, 10, 4579–4587. [Google Scholar] [CrossRef]
- Zdanowicz, M.; Sałasińska, K. Characterization of Thermoplastic Starch Plasticized with Ternary Urea-Polyols Deep Eutectic Solvent with Two Selected Fillers: Microcrystalline Cellulose and Montmorillonite. Polymers 2023, 15, 972. [Google Scholar] [CrossRef]
- Wang, J.; Cheng, F.; Zhu, P. Structure and Properties of Urea-Plasticized Starch Films with Different Urea Contents. Carbohydr. Polym. 2014, 101, 1109–1115. [Google Scholar] [CrossRef]
- Zdanowicz, M.; Johansson, C. Mechanical and Barrier Properties of Starch-Based Films Plasticized with Two- or Three Component Deep Eutectic Solvents. Carbohydr. Polym. 2016, 151, 103–112. [Google Scholar] [CrossRef]
- Schutz, G.F.; Alves, R.M.V.; Vieira, R.P. Development of Starch-Based Films Reinforced with Coffee Husks for Packaging Applications. J. Polym. Environ. 2023, 31, 1955–1966. [Google Scholar] [CrossRef]
- Grylewicz, A.; Spychaj, T.; Zdanowicz, M. Thermoplastic starch/wood biocomposites processed with deep eutectic solvents. Compos. Part A 2019, 121, 517–524. [Google Scholar] [CrossRef]
- Collazo-Bigliardi, S.; Ortega-Toro, R.; Chiralt, A. Improving Properties of Thermoplastic Starch Films by Incorporating Active Extracts and Cellulose Fibres Isolated from Rice or Coffee Husk. Food Pack. Shelf Life 2019, 22, 100383. [Google Scholar] [CrossRef]
- Zdanowicz, M.; Staciwa, P.; Spychaj, T. Low Transition Temperature Mixtures (LTTM) Containing Sugars as Potato Starch Plasticizers. Starch-Stärke 2019, 71, 1900004. [Google Scholar] [CrossRef]
- Rosales, Z.G.; Solano, J.K.; Orjuela, D.; Ilarri, J.R.; Clavero, M.E.R. Rodrigo Clavero Maria Elena Synthesis and Characterization of a Cassava Starch (Manihot Esculenta) and Dried Coffee Pulp Mixture to Produce Biofilms. Chem. Eng. Trans. 2022, 92, 439–444. [Google Scholar] [CrossRef]
- Chou, W.-L.; Wang, C.-T.; Huang, K.-Y.; Chang, Y.-C.; Shu, C.-M. Investigation of Indium Ions Removal from Aqueous Solutions Using Spent Coffee Grounds. Int. J. Phys. Sci. 2012, 7, 2445–2454. [Google Scholar] [CrossRef]
- Ballesteros, L.F.; Cerqueira, M.A.; Teixeira, J.A.; Mussatto, S.I. Characterization of Polysaccharides Extracted from Spent Coffee Grounds by Alkali Pretreatment. Carbohydr. Polym. 2015, 127, 347–354. [Google Scholar] [CrossRef]
- Zdanowicz, M.; Staciwa, P.; Jędrzejewski, R.; Spychaj, T. Sugar Alcohol-Based Deep Eutectic Solvents as Potato Starch Plasticizers. Polymers 2019, 11, 1385. [Google Scholar] [CrossRef]
- Cetner, M.D.; Dąbrowski, P.; Samborska, I.A.; Łukasik, I.; Swoczyna, T.; Pietkiewicz, S.; Bąba, W.; Kalaji, H.M. Zastosowanie pomiarów fluorescencji chlorofilu w badaniach środowiskowych. Kosmos 2016, 65, 197–205. [Google Scholar]
- Angelini, G.; Ragni, P.; Esposito, D.; Giardi, P.; Pompili, M.L.; Moscardelli, R.; Giardi, M.T. A device to study the effect of space radiation on photosynthetic organisms. Phys. Med. 2001, 17, 267–268. [Google Scholar]
- Kalaji, M.H.; Łoboda, T. Fluorescencja Chlorofilu w Badaniach Stanu Fizjologicznego Roślin; Wyd. SGGW: Warsaw, Poland, 2010. [Google Scholar]
- Ozturk, M.; Turkyilmaz Unal, B.; García-Caparrós, P.; Khursheed, A.; Gul, A.; Hasanuzzaman, M. Osmoregulation and its actions during the drought stress in plants. Physiol. Plant. 2021, 172, 1321–1335. [Google Scholar] [CrossRef]
- Almasi, H.; Ghanbarzadeh, B.; Entezami, A.A. Physicochemical Properties of Starch–CMC–Nanoclay Biodegradable Films. Int. J. Biol. Macromol. 2010, 46, 1–5. [Google Scholar] [CrossRef]
- Avella, M.; De Vlieger, J.J.; Errico, M.E.; Fischer, S.; Vacca, P.; Volpe, M.G. Biodegradable Starch/Clay Nanocomposite Films for Food Packaging Applications. Food Chem. 2005, 93, 467–474. [Google Scholar] [CrossRef]
- Arnon, D.I.; Hoagland, D.R. Crop production in artificial culture solutions and in soils with special reference to factors influencing yields and absorption of inorganic nutrients. Soil Sci. 1940, 50, 463–485. [Google Scholar]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophyll a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Hager, A.; Mayer-Berthenrath, T. Die Isolierung und quantative Bestimung der Carotenoide und Chlorophyll von Blatern, Algen und isolierten Chloroplasten mit Hilfe Dunnschichtchromatographischer Methoden. Planta 1966, 69, 198–217. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]


| Sample | At RH50% (%) | Sw (%) | Sol (%) |
|---|---|---|---|
| TPS/CCU | 13.2 (±0.001) | 375 (±0.06) | 46 (±0.01) |
| TPS/CCU/cf | 14.4 (±0.000) | 275 (±0.22) | 48 (±0.01) |
| TPS/CCU+cf | 14.7 (±0.000) | 240 (±0.21) | 45 (±0.05) |
| TPS/BU | 14.2 (±0.004) | 349 (±0.53) | 48 (±0.01) |
| TPS/BU/cf | 15.8 (±0.000) | 283 (±0.07) | 46 (±0.02) |
| TPS/BU+cf | 15.5 (±0.004) | 277 (±0.02) | 43 (±0.02) |
| Sample | Transpiration Intensity (mmol H2O∙m−2∙s−1) | Stomal Conductivity H2O (mol H2O∙m−2∙s−1) | Assimilation Intensity CO2 Net (mmol H2O∙m−2∙s−1) | Substomatal CO2 Concentration (μmol CO2∙mol−1) |
|---|---|---|---|---|
| Control | 0.393 (±0.093) b | 0.036 (±0.015) ab | 2.508 (±0.775) bc | 414.67 (±37.31) a |
| TPS/CCU | 0.489 (±0.121) ab | 0.042 (±0.013) a | 2.633 (±0.735) bc | 312.83 (±53.90) b |
| TPS/CCU/cf | 0.348 (±0.092) b | 0.025 (±0.006) b | 2.217 (±0.904) c | 372.58 (±77.34) a |
| TPS/BU | 0.405 (±0.087) a | 0.032 (±0.010) ab | 2.792 (±1.133) a | 284.17 (±72.82) c |
| TPS/BU/cf | 0.372 (±0.092) a | 0.031 (±0.009) a | 3.000 (±0.283) bc | 269.09 (±41.98) b |
| Sample | Initial (zero) Fluorescence F0 | Maximum Fluorescence FM | Variable Fluorescence FV | FV/FM | Chlorophyll Fluorescence Growth Time (ms) | Area above the Fluorescence Induction Curve (kbms) |
|---|---|---|---|---|---|---|
| Control | 239.50 (±18.75) b | 1119.08 (±120.12) a | 895.25 (±99.22) a | 0.782 (±0.046) a | 866.67 (±49.24) a | 52.89 (±73.81) ab |
| TPS/CCU | 224.46 (±9.90) b | 1190.54 (±53.16) ab | 966.08 (±53.33) a | 0.811(±0.011) a | 900.00 (±0.00) a | 64.20 (±55.18) a |
| TPS/CCU/cf | 226.09 (±11.00) b | 1119.00 (±103.09) a | 917.27 (±124.69) a | 0.800 (±0.022) a | 872.73 (±64.67) a | 51.97 (±77.45) a |
| TPS/BU | 268.88 (±53.46) a | 1084.38 (±242.47) a | 815.50 (±278.63) a | 0.733 (±0.109) a | 862.50 (±51.75) a | 40.51 (±18.51) b |
| TPS/BU/cf | 232.17 (±19.06) b | 1179.08 (±102.33) a | 946.92 (±110.28) a | 0.801 (±0.027) a | 883.33 (±38.92) a | 59.83 (±10.73) a |
| Sample | Chlorophyll “a” (mg g−1 FM) | Chlorophyll “b” (mg g−1 FM) | Total Chlorophyll (mg g−1 FM) | Carotenoids (mg g−1 FM) | Proline (mg g−1 FM) |
|---|---|---|---|---|---|
| Control | 2.88 (±0.19) abc | 1.37 (±0.20) ab | 4.25 (±0.22) abc | 2.20 (±0.02) a | 0.58 (±0.05) a |
| TPS/CCU | 2.60 (±0.73) ac | 1.09 (±0.30) c | 3.69 (±1.01) c | 1.76 (±0.72) a | 0.64 (±0.04) a |
| TPS/CCU/cf | 3.45 (±0.16) ab | 1.41 (±0.05) b | 4.86 (±0.18) ab | 2.56 (±0.29) a | 0.62 (±0.03) a |
| TPS/BU | 2.63 (±0.40) bc | 1.12 (±0.23) bc | 3.75 (±0.62) bc | 1.62(±0.39) a | 0.63 (±0.04) a |
| TPS/BU/cf | 3.50 (±0.17) a | 1.51 (±0.12) a | 5.01 (±0.19) a | 2.76 (±0.74) a | 0.65 (±0.07) a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zdanowicz, M.; Rokosa, M.; Pieczykolan, M.; Antosik, A.K.; Chudecka, J.; Mikiciuk, M. Study on Physicochemical Properties of Biocomposite Films with Spent Coffee Grounds as a Filler and Their Influence on Physiological State of Growing Plants. Int. J. Mol. Sci. 2023, 24, 7864. https://doi.org/10.3390/ijms24097864
Zdanowicz M, Rokosa M, Pieczykolan M, Antosik AK, Chudecka J, Mikiciuk M. Study on Physicochemical Properties of Biocomposite Films with Spent Coffee Grounds as a Filler and Their Influence on Physiological State of Growing Plants. International Journal of Molecular Sciences. 2023; 24(9):7864. https://doi.org/10.3390/ijms24097864
Chicago/Turabian StyleZdanowicz, Magdalena, Marta Rokosa, Magdalena Pieczykolan, Adrian Krzysztof Antosik, Justyna Chudecka, and Małgorzata Mikiciuk. 2023. "Study on Physicochemical Properties of Biocomposite Films with Spent Coffee Grounds as a Filler and Their Influence on Physiological State of Growing Plants" International Journal of Molecular Sciences 24, no. 9: 7864. https://doi.org/10.3390/ijms24097864
APA StyleZdanowicz, M., Rokosa, M., Pieczykolan, M., Antosik, A. K., Chudecka, J., & Mikiciuk, M. (2023). Study on Physicochemical Properties of Biocomposite Films with Spent Coffee Grounds as a Filler and Their Influence on Physiological State of Growing Plants. International Journal of Molecular Sciences, 24(9), 7864. https://doi.org/10.3390/ijms24097864









