Therapeutic Lymphangiogenesis Is a Promising Strategy for Secondary Lymphedema
Abstract
1. Introduction
2. Lymphatic Vessel
3. Lymphedema
3.1. Causes
3.2. Epidemiology
3.3. Symptoms and Complications
3.4. Conventional Treatment
3.4.1. Skin Care
3.4.2. Compression Garments
3.4.3. Lymphatic Massage
3.4.4. Multi-Layer Inelastic Lymphedema Bandaging
3.4.5. Intermittent Pneumatic Compression
3.4.6. Exercise
3.4.7. Psychosocial Support
3.4.8. Palliative Care
3.4.9. Surgery
3.4.10. Other Treatments
4. Animal Lymphedema Model
4.1. Primary Lymphedema Animal Model
4.2. Secondary Lymphedema Animal Model
4.2.1. Mouse Model
4.2.2. Rat Model
4.2.3. Rabbit Model
4.2.4. Large Animal Models
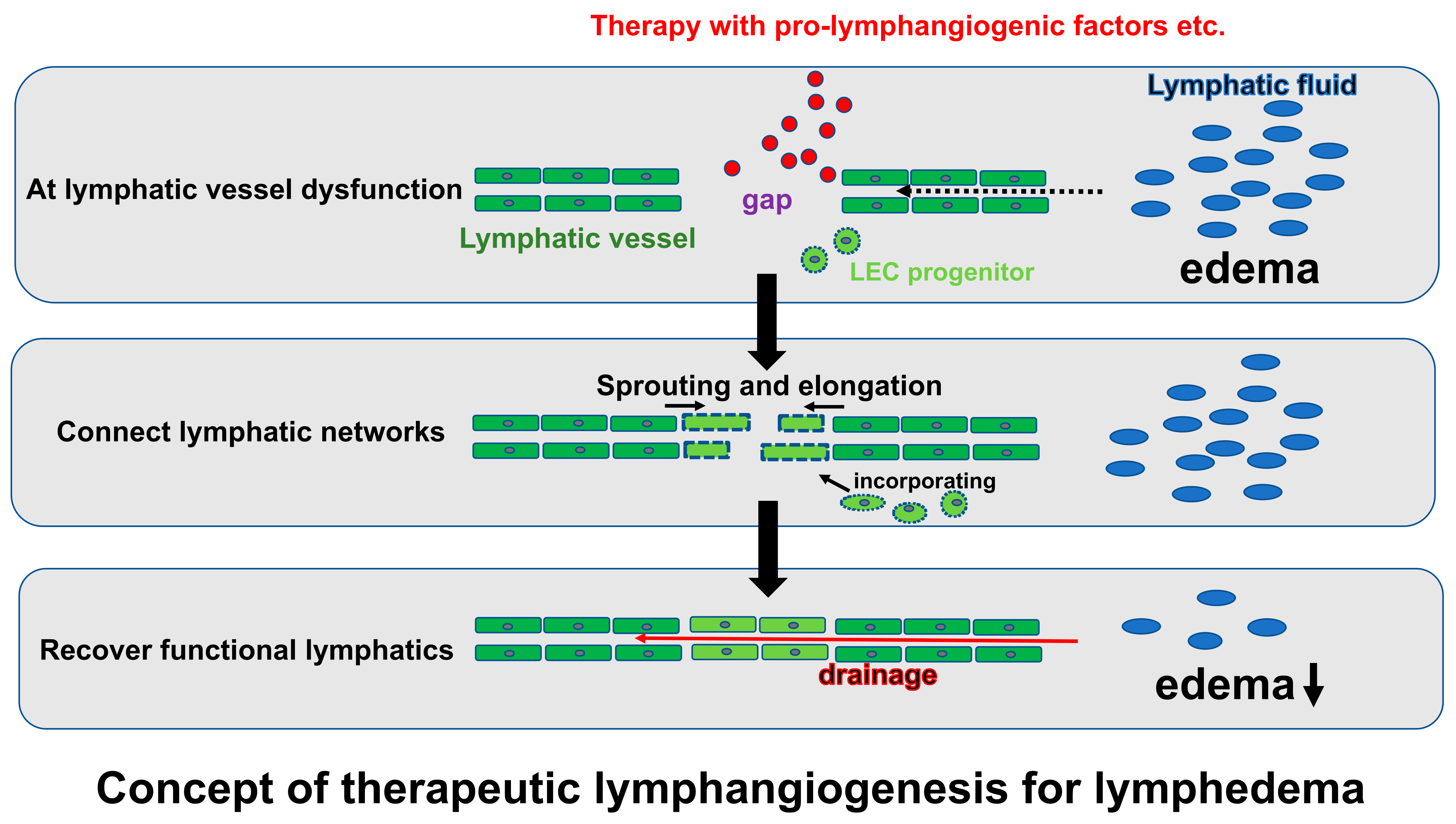
5. The Mechanism of Lymphangiogenesis
6. Therapeutic Lymphangiogenesis
6.1. Cell Therapy
6.2. Gene Therapy (Growth Factors)
6.3. Proteins, Peptides, miRNA, Drugs
6.4. Others
7. Clinical Study
8. Limitation and Future Perspective
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Petrova, T.V.; Koh, G.Y. Biological functions of lymphatic vessels. Science 2020, 369, eaax4063. [Google Scholar] [CrossRef] [PubMed]
- Dayan, J.H.; Ly, C.L.; Kataru, R.P.; Mehrara, B.J. Lymphedema: Pathogenesis and Novel Therapies. Annu. Rev. Med. 2018, 69, 263–276. [Google Scholar] [CrossRef]
- Oliver, G.; Alitalo, K. The Lymphatic Vasculature: Recent Progress and Paradigms. Annu. Rev. Cell Dev. Biol. 2005, 21, 457–483. [Google Scholar] [CrossRef] [PubMed]
- Landau, S.; Newman, A.; Edri, S.; Michael, I.; Ben-Shaul, S.; Shandalov, Y.; Ben-Arye, T.; Kaur, P.; Zheng, M.H.; Levenberg, S. Investigating lymphangiogenesis in vitro and in vivo using engineered human lymphatic vessel networks. Proc. Natl. Acad. Sci. USA 2021, 118, e2101931118. [Google Scholar] [CrossRef]
- Karkkainen, M.J.; Haiko, P.; Sainio, K.; Partanen, J.; Taipale, J.; Petrova, T.V.; Jeltsch, M.; Jackson, D.G.; Talikka, M.; Rauvala, H.; et al. Vascular endothelial growth factor C is required for sprouting of the first lymphatic vessels from embryonic veins. Nat. Immunol. 2004, 5, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Wigle, J.T.; Oliver, G. Prox1 Function Is Required for the Development of the Murine Lymphatic System. Cell 1999, 98, 769–778. [Google Scholar] [CrossRef]
- Albrecht, I.; Christofori, G. Molecular mechanisms of lymphangiogenesis in development and cancer. Int. J. Dev. Biol. 2011, 55, 483–494. [Google Scholar] [CrossRef]
- Pfeiffer, F.; Kumar, V.; Butz, S.; Vestweber, D.; Imhof, B.A.; Stein, J.V.; Engelhardt, B. Distinct molecular composition of blood and lymphatic vascular endothelial cell junctions establishes specific functional barriers within the peripheral lymph node. Eur. J. Immunol. 2008, 38, 2142–2155. [Google Scholar] [CrossRef]
- Norrmén, C.; Tammela, T.; Petrova, T.V.; Alitalo, K. Biological Basis of Therapeutic Lymphangiogenesis. Circulation 2011, 123, 1335–1351. [Google Scholar] [CrossRef]
- Stacker, S.A.; Williams, S.P.; Karnezis, T.; Shayan, R.; Fox, S.B.; Achen, M.G. Lymphangiogenesis and lymphatic vessel remodelling in cancer. Nat. Rev. Cancer 2014, 14, 159–172. [Google Scholar] [CrossRef]
- Wechman, S.L.; Emdad, L.; Sarkar, D.; Das, S.K.; Fisher, P.B. Vascular mimicry: Triggers, molecular interactions and in vivo models. Adv. Cancer Res. 2020, 148, 27–67. [Google Scholar] [CrossRef] [PubMed]
- Tammela, T.; Alitalo, K. Lymphangiogenesis: Molecular Mechanisms and Future Promise. Cell 2010, 140, 460–476. [Google Scholar] [CrossRef] [PubMed]
- Warren, A.G.; Brorson, H.; Borud, L.J.; Slavin, S.A. Lymphedema: A comprehensive review. Ann. Plast. Surg. 2007, 59, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Connell, F.; Brice, G.; Mortimer, P. Phenotypic Characterization of Primary Lymphedema. Ann. N. Y. Acad. Sci. 2008, 1131, 140–146. [Google Scholar] [CrossRef]
- Szuba, A.; Rockson, S.G. Lymphedema: Classification, diagnosis and therapy. Vasc. Med. 1998, 3, 145–156. [Google Scholar] [CrossRef]
- Levinson, K.L.; Feingold, E.; Ferrell, R.E.; Glover, T.W.; Traboulsi, E.I.; Finegold, D.N. Age of onset in hereditary lymphedema. J. Pediatr. 2003, 142, 704–708. [Google Scholar] [CrossRef]
- Ho, B.; Gordon, K.; Mortimer, P.S. A Genetic Approach to the Classification of Primary Lymphoedema and Lymphatic Malformations. Eur. J. Vasc. Endovasc. Surg. 2018, 56, 465–466. [Google Scholar] [CrossRef]
- Grada, A.A.; Phillips, T.J. Lymphedema: Pathophysiology and clinical manifestations. J. Am. Acad. Dermatol. 2017, 77, 1009–1020. [Google Scholar] [CrossRef]
- Maguire, A.; Brogi, E. Sentinel lymph nodes for breast carcinoma: An update on current practice. Histopathology 2016, 68, 152–167. [Google Scholar] [CrossRef]
- Smeltzer, D.M.; Stickler, G.B.; Schirger, A. Primary lymphedema in children and adolescents: A follow-up study and review. Pediatrics 1985, 76, 206–218. [Google Scholar]
- Rockson, S.G. Lymphedema. Curr. Treat. Options Cardiovasc. Med. 2000, 2, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Schook, C.C.; Mulliken, J.B.; Fishman, S.J.; Grant, F.D.; Zurakowski, D.; Greene, A.K. Primary Lymphedema: Clinical Features and Management in 138 Pediatric Patients. Plast. Reconstr. Surg. 2011, 127, 2419–2431. [Google Scholar] [CrossRef] [PubMed]
- Burgos, J.A.M.; Luginbuhl, A. Lymphedema Tarda. N. Engl. J. Med. 2009, 360, 1015. [Google Scholar] [CrossRef] [PubMed]
- Rockson, S.G.; Rivera, K.K. Estimating the Population Burden of Lymphedema. Ann. N. Y. Acad. Sci. 2008, 1131, 147–154. [Google Scholar] [CrossRef]
- DiSipio, T.; Rye, S.; Newman, B.; Hayes, S. Incidence of unilateral arm lymphoedema after breast cancer: A systematic review and meta-analysis. Lancet Oncol. 2013, 14, 500–515. [Google Scholar] [CrossRef] [PubMed]
- Cooper, G.; Bagnall, A. Prevalence of lymphoedema in the UK: Focus on the southwest and West Midlands. Br. J. Community Nurs. 2016, 21, S6–S14. [Google Scholar] [CrossRef]
- Kitamura, K.; Iwase, S.; Komoike, Y.; Ogawa, Y.; Utsugi, K.; Yamamoto, D.; Odagiri, H. Evidence-Based Practice Guideline for the Management of Lymphedema Proposed by the Japanese Lymphedema Society. Lymphat. Res. Biol. 2022, 20, 539–547. [Google Scholar] [CrossRef]
- Arié, A.; Yamamoto, T. Lymphedema secondary to melanoma treatments: Diagnosis, evaluation, and treatments. Glob. Health Med. 2020, 2, 227–234. [Google Scholar] [CrossRef]
- Kim, J.H.; Choi, J.H.; Ki, E.Y.; Lee, S.J.; Yoon, J.H.; Lee, K.H.; Park, T.C.; Park, J.S.; Bae, S.N.; Hur, S.Y. Incidence and Risk Factors of Lower-Extremity Lymphedema after Radical Surgery with or without Adjuvant Radiotherapy in Patients with FIGO Stage I to Stage IIA Cervical Cancer. Int. J. Gynecol. Cancer 2012, 22, 686–691. [Google Scholar] [CrossRef]
- Salani, R.; Preston, M.M.; Hade, E.; Johns, J.; Fowler, J.M.; Paskett, E.P.; Katz, M.L. Swelling among Women Who Need Education about Leg Lymphedema: A Descriptive Study of Lymphedema in Women Undergoing Surgery for Endometrial Cancer. Int. J. Gynecol. Cancer 2014, 24, 1507–1512. [Google Scholar] [CrossRef]
- Deng, J.; Ridner, S.H.; Dietrich, M.S.; Wells, N.; Wallston, K.A.; Sinard, R.J.; Cmelak, A.J.; Murphy, B.A. Prevalence of Secondary Lymphedema in Patients with Head and Neck Cancer. J. Pain Symptom Manag. 2012, 43, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Murphy, B.A.; Dietrich, M.S.; Wells, N.; Wallston, K.A.; Sinard, R.J.; Cmelak, A.J.; Gilbert, J.; Ridner, S.H. Impact of secondary lymphedema after head and neck cancer treatment on symptoms, functional status, and quality of life. Head Neck 2013, 35, 1026–1035. [Google Scholar] [CrossRef]
- Smith, B.G.; Hutcheson, K.A.; Little, L.G.; Skoracki, R.J.; Rosenthal, D.I.; Lai, S.Y.; Lewin, J.S. Lymphedema Outcomes in Patients with Head and Neck Cancer. Otolaryngol. Neck Surg. 2015, 152, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Mitra, D.; Catalano, P.J.; Cimbak, N.; Damato, A.L.; Muto, M.G.; Viswanathan, A.N. The risk of lymphedema after postoperative radiation therapy in endometrial cancer. J. Gynecol. Oncol. 2016, 27, e4. [Google Scholar] [CrossRef] [PubMed]
- Shaitelman, S.F.; Cromwell, K.D.; Rasmussen, J.C.; Stout, N.L.; Armer, J.M.; Lasinski, B.B.; Cormier, J.N. Recent progress in the treatment and prevention of cancer-related lymphedema. CA Cancer J. Clin. 2015, 65, 55–81. [Google Scholar] [CrossRef]
- Stolldorf, D.P.; Dietrich, M.S.; Ridner, S.H. A Comparison of the Quality of Life in Patients with Primary and Secondary Lower Limb Lymphedema: A Mixed-Methods Study. West. J. Nurs. Res. 2016, 38, 1313–1334. [Google Scholar] [CrossRef]
- Kitsiou-Tzeli, S.; Vrettou, C.; Leze, E.; Makrythanasis, P.; Kanavakis, E.; Willems, P. Milroy’s primary congenital lymphedema in a male infant and review of the literature. In Vivo 2010, 24, 309–314. [Google Scholar]
- Rockson, S.G. Lymphedema. Am. J. Med. 2001, 110, 288–295. [Google Scholar] [CrossRef]
- Beer, D.J.; Pereira, W.; Snider, G.L. Pleural effusion associated with primary lymphedema: A perspective on the yellow nail syndrome. Am. Rev. Respir. Dis. 1978, 117, 595–599. [Google Scholar] [CrossRef]
- Williams, W.H.; Witte, C.L.; Witte, M.H.; McNEILL, G.C. Radionuclide Lymphangioscintigraphy in the Evaluation of Peripheral Lymphedema. Clin. Nucl. Med. 2000, 25, 451–464. [Google Scholar] [CrossRef]
- Arrault, M.; Vignes, S. Risk factors for developing upper limb lymphedema after breast cancer treatment. Bull. Cancer 2006, 93, 1001–1006. [Google Scholar] [PubMed]
- Teerachaisakul, M.; Ekataksin, W.; Durongwatana, S.; Taneepanichskul, S. Risk factors for cellulitis in patients with lymphedema: A case-controlled study. Lymphology 2013, 46, 150–156. [Google Scholar]
- Cohen, S.R.; Payne, D.K.; Tunkel, R.S. Lymphedema: Strategies for management. Cancer 2001, 92, 980–987. [Google Scholar] [CrossRef] [PubMed]
- Kerchner, K.; Fleischer, A.; Yosipovitch, G. Lower extremity lymphedema: Update: Pathophysiology, diagnosis, and treatment guidelines. J. Am. Acad. Dermatol. 2008, 59, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Chopra, K.; Tadisina, K.K.; Brewer, M.; Holton, L.H.; Banda, A.K.; Singh, D.P. Massive Localized Lymphedema Revisited: A quickly rising complication of the obesity epidemic. Ann. Plast. Surg. 2015, 74, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Fife, C.E.; Farrow, W.; Hebert, A.A.; Armer, N.C.; Stewart, B.R.; Cormier, J.N.; Armer, J.M. Skin and Wound Care in Lymphedema Patients: A Taxonomy, Primer, and Literature Review. Adv. Ski. Wound Care 2017, 30, 305–318. [Google Scholar] [CrossRef]
- King, M.; Deveaux, A.; White, H.; Rayson, D. Compression garments versus compression bandaging in decongestive lymphatic therapy for breast cancer-related lymphedema: A randomized controlled trial. Support. Care Cancer 2012, 20, 1031–1036. [Google Scholar] [CrossRef]
- Thompson, B.; Gaitatzis, K.; De Jonge, X.J.; Blackwell, R.; Koelmeyer, L.A. Manual lymphatic drainage treatment for lymphedema: A systematic review of the literature. J. Cancer Surviv. 2021, 15, 244–258. [Google Scholar] [CrossRef]
- de Godoy, J.M.; Batigalia, F.; Godoy Mde, F. Preliminary evaluation of a new, more simplified physiotherapy technique for lymphatic drainage. Lymphology 2002, 35, 91–93. [Google Scholar]
- Moffatt, C.J.; Burian, E.; Karlsmark, T.; Keeley, V.; Vignes, S.; Doiron, S.; Tilley, A.; Liebl, M.; Reißhauer, A.; Murray, S.; et al. Factors Predicting Limb Volume Reduction Using Compression Bandaging within Decongestive Lymphatic Therapy in Lymphedema: A Multicountry Prospective Study. Lymphat. Res. Biol. 2021, 19, 412–422. [Google Scholar] [CrossRef]
- Karafa, M.; Karafova, A.; Szuba, A. The effect of different compression pressure in therapy of secondary upper extremity lymphedema in women after breast cancer surgery. Lymphology 2018, 51, 28–37. [Google Scholar]
- Dunn, N.; Williams, E.M.; Dolan, G.; Davies, J.H. Intermittent Pneumatic Compression for the Treatment of Lower Limb Lymphedema: A Pilot Trial of Sequencing to Mimic Manual Lymphatic Drainage Versus Traditional Graduated Sequential Compression. Lymphat. Res. Biol. 2022, 20, 514–521. [Google Scholar] [CrossRef]
- Phillips, J.J.; Gordon, S.J. Intermittent Pneumatic Compression Dosage for Adults and Children with Lymphedema: A Systematic Review. Lymphat. Res. Biol. 2019, 17, 2–18. [Google Scholar] [CrossRef]
- Zaleska, M.; Olszewski, W.L.; Durlik, M. The Effectiveness of Intermittent Pneumatic Compression in Long-Term Therapy of Lymphedema of Lower Limbs. Lymphat. Res. Biol. 2014, 12, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Morris, C.; Wonders, K.Y. Concise review on the safety of exercise on symptoms of lymphedema. World J. Clin. Oncol. 2015, 6, 43–44. [Google Scholar] [CrossRef] [PubMed]
- Moseley, A.L.; Piller, N.B.; Carati, C.J. The effect of gentle arm exercise and deep breathing on secondary arm lymphedema. Lymphology 2005, 38, 136–145. [Google Scholar]
- Fu, M.R.; Ridner, S.H.; Hu, S.H.; Stewart, B.R.; Cormier, J.N.; Armer, J.M. Psychosocial impact of lymphedema: A systematic review of literature from 2004 to 2011. Psycho-Oncol. 2012, 22, 1466–1484. [Google Scholar] [CrossRef] [PubMed]
- Eaton, L.H.; Narkthong, N.; Hulett, J.M. Psychosocial Issues Associated with Breast Cancer-Related Lymphedema: A Literature Review. Curr. Breast Cancer Rep. 2020, 12, 216–224. [Google Scholar] [CrossRef]
- Beck, M.; Wanchai, A.; Stewart, B.R.; Cormier, J.N.; Armer, J.M. Palliative Care for Cancer-Related Lymphedema: A Systematic Review. J. Palliat. Med. 2012, 15, 821–827. [Google Scholar] [CrossRef]
- Granzow, J.W.; Soderberg, J.M.; Kaji, A.H.; Dauphine, C. Review of Current Surgical Treatments for Lymphedema. Ann. Surg. Oncol. 2014, 21, 1195–1201. [Google Scholar] [CrossRef]
- Schaverien, M.V.; Coroneos, C.J. Surgical Treatment of Lymphedema. Plast. Reconstr. Surg. 2019, 144, 738–758. [Google Scholar] [CrossRef]
- Forte, A.J.; Khan, N.; Huayllani, M.T.; Boczar, D.; Saleem, H.Y.; Lu, X.; Manrique, O.J.; Ciudad, P.; McLaughlin, S.A. Lymphaticovenous Anastomosis for Lower Extremity Lymphedema: A Systematic Review. Indian J. Plast. Surg. 2020, 53, 17–24. [Google Scholar] [CrossRef]
- Granzow, J.W.; Soderberg, J.M.; Dauphine, C. A Novel Two-Stage Surgical Approach to Treat Chronic Lymphedema. Breast J. 2014, 20, 420–422. [Google Scholar] [CrossRef]
- Forte, A.J.; Boczar, D.; Huayllani, M.T.; Lu, X.; McLaughlin, S.A. Pharmacotherapy Agents in Lymphedema Treatment: A Systematic Review. Cureus 2019, 11, e6300. [Google Scholar] [CrossRef] [PubMed]
- Koo, J.H.; Song, S.H.; Oh, H.S.; Oh, S.H. Comparison of the short-term effects of hyperbaric oxygen therapy and complex decongestive therapy on breast cancer-related lymphedema: A pilot study. Medicine 2020, 99, e19564. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, D.; Ahmad, A.; Sharif, F.; Arslan, S.A. Clinical application of low-level laser therapy (Photo-biomodulation therapy) in the management of breast cancer-related lymphedema: A systematic review. BMC Cancer 2022, 22, 937. [Google Scholar] [CrossRef] [PubMed]
- Geng, X.; Cha, B.; Mahamud, R.; Lim, K.-C.; Silasi-Mansat, R.; Uddin, M.K.; Miura, N.; Xia, L.; Simon, A.M.; Engel, J.D.; et al. Multiple mouse models of primary lymphedema exhibit distinct defects in lymphovenous valve development. Dev. Biol. 2016, 409, 218–233. [Google Scholar] [CrossRef] [PubMed]
- Karkkainen, M.J.; Saaristo, A.; Jussila, L.; Karila, K.A.; Lawrence, E.C.; Pajusola, K.; Bueler, H.; Eichmann, A.; Kauppinen, R.; Kettunen, M.I.; et al. A model for gene therapy of human hereditary lymphedema. Proc. Natl. Acad. Sci. USA 2001, 98, 12677–12682. [Google Scholar] [CrossRef] [PubMed]
- Hadrian, R.; Palmes, D. Animal Models of Secondary Lymphedema: New Approaches in the Search for Therapeutic Options. Lymphat. Res. Biol. 2017, 15, 2–16. [Google Scholar] [CrossRef]
- Morfoisse, F.; Tatin, F.; Chaput, B.; Therville, N.; Vaysse, C.; Métivier, R.; Malloizel-Delaunay, J.; Pujol, F.; Godet, A.-C.; De Toni, F.; et al. Lymphatic Vasculature Requires Estrogen Receptor-α Signaling to Protect from Lymphedema. Arter. Thromb. Vasc. Biol. 2018, 38, 1346–1357. [Google Scholar] [CrossRef]
- Mendez, U.; Brown, E.M.; Ongstad, E.L.; Slis, J.R.; Goldman, J. Functional recovery of fluid drainage precedes lymphangiogenesis in acute murine foreleg lymphedema. Am. J. Physiol. Circ. Physiol. 2012, 302, H2250–H2256. [Google Scholar] [CrossRef]
- Komatsu, E.; Nakajima, Y.; Mukai, K.; Urai, T.; Asano, K.; Okuwa, M.; Sugama, J.; Nakatani, T. Lymph Drainage during Wound Healing in a Hindlimb Lymphedema Mouse Model. Lymphat. Res. Biol. 2017, 15, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.; Agollah, G.D.; Wu, G.; Sevick-Muraca, E.M. Spatio-Temporal Changes of Lymphatic Contractility and Drainage Patterns following Lymphadenectomy in Mice. PLoS ONE 2014, 9, e106034. [Google Scholar] [CrossRef] [PubMed]
- Bramos, A.; Perrault, D.; Yang, S.; Jung, E.; Hong, Y.K.; Wong, A.K. Prevention of Postsurgical Lymphedema by 9-cis Retinoic Acid. Ann. Surg. 2016, 264, 353–361. [Google Scholar] [CrossRef]
- Jørgensen, M.G.; Toyserkani, N.M.; Hansen, C.R.; Hvidsten, S.; Baun, C.; Hejbøl, E.K.; Schrøder, H.D.; Sørensen, J.A. Quantification of Chronic Lymphedema in a Revised Mouse Model. Ann. Plast. Surg. 2018, 81, 594–603. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, Y.; Shibata, R.; Ishii, M.; Ohashi, K.; Kambara, T.; Uemura, Y.; Yuasa, D.; Kataoka, Y.; Kihara, S.; Murohara, T.; et al. Adiponectin-Mediated Modulation of Lymphatic Vessel Formation and Lymphedema. J. Am. Hear. Assoc. 2013, 2, e000438. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, J.; Shimizu, Y.; Hayashi, T.; Che, Y.; Pu, Z.; Tsuzuki, K.; Narita, S.; Shibata, R.; Ishii, I.; Calvert, J.W.; et al. Hydrogen Sulfide Attenuates Lymphedema Via the Induction of Lymphangiogenesis through a PI3K/Akt-Dependent Mechanism. J. Am. Hear. Assoc. 2022, 11, e026889. [Google Scholar] [CrossRef]
- Ghanta, S.; Cuzzone, D.A.; Torrisi, J.S.; Albano, N.J.; Joseph, W.J.; Savetsky, I.L.; Gardenier, J.C.; Chang, D.; Zampell, J.C.; Mehrara, B.J. Regulation of inflammation and fibrosis by macrophages in lymphedema. Am. J. Physiol. Heart Circ. Physiol. 2015, 308, H1065–H1077. [Google Scholar] [CrossRef]
- Zampell, J.C.; Aschen, S.; Weitman, E.S.; Yan, A.; Elhadad, S.; De Brot, M.; Mehrara, B.J. Regulation of Adipogenesis by Lymphatic Fluid Stasis: Part I. Adipogenesis, fibrosis, and inflammation. Plast. Reconstr. Surg. 2012, 129, 825–834. [Google Scholar] [CrossRef] [PubMed]
- Gardenier, J.C.; Kataru, R.P.; Hespe, G.E.; Savetsky, I.L.; Torrisi, J.S.; Nores, G.D.G.; Jowhar, D.K.; Nitti, M.D.; Schofield, R.C.; Carlow, D.C.; et al. Topical tacrolimus for the treatment of secondary lymphedema. Nat. Commun. 2017, 8, 14345. [Google Scholar] [CrossRef]
- Cheung, L.; Han, J.; Beilhack, A.; Joshi, S.; Wilburn, P.; Dua, A.; An, A.; Rockson, S.G. An experimental model for the study of lymphedema and its response to therapeutic lymphangiogenesis. Biodrugs 2006, 20, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Choi, I.; Lee, Y.S.; Chung, H.K.; Choi, D.; Ecoiffier, T.; Lee, H.N.; Kim, K.E.; Lee, S.; Park, E.K.; Maeng, Y.S.; et al. Interleukin-8 reduces post-surgical lymphedema formation by promoting lymphatic vessel regeneration. Angiogenesis 2013, 16, 29–44. [Google Scholar] [CrossRef] [PubMed]
- Lynch, L.L.; Mendez, U.; Waller, A.B.; Gillette, A.A.; Ii, R.J.G.; Goldman, J. Fibrosis worsens chronic lymphedema in rodent tissues. Am. J. Physiol. Heart Circ. Physiol. 2015, 308, H1229–H1236. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.-Y.; Zhong, S.-Z. A model of experimental lymphedema in rats’ limbs. Microsurgery 1985, 6, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Park, H.S.; Jung, I.M.; Choi, G.H.; Hahn, S.; Yoo, Y.S.; Lee, T. Modification of a Rodent Hindlimb Model of Secondary Lymphedema: Surgical Radicality versus Radiotherapeutic Ablation. BioMed Res. Int. 2013, 2013, 208912. [Google Scholar] [CrossRef]
- Sommer, T.; Buettner, M.; Bruns, F.; Breves, G.; Hadamitzky, C.; Pabst, R. Improved Regeneration of Autologous Transplanted Lymph Node Fragments by VEGF-C Treatment. Anat. Rec. 2012, 295, 786–791. [Google Scholar] [CrossRef]
- Oashi, K.; Furukawa, H.; Oyama, A.; Funayama, E.; Hayashi, T.; Saito, A.; Yamamoto, Y. A New Model of Acquired Lymphedema in the Mouse Hind Limb: A preliminary report. Ann. Plast. Surg. 2012, 69, 565–568. [Google Scholar] [CrossRef]
- Harb, A.A.; Levi, M.A.; Corvi, J.J.; Nicolas, C.F.; Zheng, Y.; Chaudhary, K.R.; Akelina, Y.; Connolly, E.P.; Ascherman, J.A. Creation of a Rat Lower Limb Lymphedema Model. Ann. Plast. Surg. 2020, 85, S129–S134. [Google Scholar] [CrossRef]
- Kawai, Y.; Shiomi, H.; Abe, H.; Naka, S.; Kurumi, Y.; Tani, T. Cell transplantation therapy for a rat model of secondary lymphedema. J. Surg. Res. 2014, 189, 184–191. [Google Scholar] [CrossRef]
- Serizawa, F.; Ito, K.; Matsubara, M.; Sato, A.; Shimokawa, H.; Satomi, S. Extracorporeal Shock Wave Therapy Induces Therapeutic Lymphangiogenesis in a Rat Model of Secondary Lymphoedema. Eur. J. Vasc. Endovasc. Surg. 2011, 42, 254–260. [Google Scholar] [CrossRef]
- Daneshgaran, G.; Lo, A.Y.; Paik, C.B.; Cooper, M.N.; Sung, C.; Jiao, W.; Park, S.Y.; Ni, P.; Yu, R.P.; Vorobyova, I.; et al. A Pre-clinical Animal Model of Secondary Head and Neck Lymphedema. Sci. Rep. 2019, 9, 18264. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Wang, M.; Hou, C.; Jin, X.; Wu, X. Exogenous VEGF-C Augments the Efficacy of Therapeutic Lymphangiogenesis Induced by Allogenic Bone Marrow Stromal Cells in a Rabbit Model of Limb Secondary Lymphedema. Jpn. J. Clin. Oncol. 2011, 41, 841–846. [Google Scholar] [CrossRef]
- Peñuela, R.F.; Arazo, L.C.; Ayala, J.M. Outcomes in Vascularized Lymph Node Transplantation in Rabbits: A Reliable Model for Improving the Surgical Approach to Lymphedema. Lymphat. Res. Biol. 2019, 17, 413–417. [Google Scholar] [CrossRef] [PubMed]
- Kubo, M.; Li, T.-S.; Kamota, T.; Ohshima, M.; Shirasawa, B.; Hamano, K. Extracorporeal shock wave therapy ameliorates secondary lymphedema by promoting lymphangiogenesis. J. Vasc. Surg. 2010, 52, 429–434. [Google Scholar] [CrossRef]
- Peñuela, R.F.; Playa, G.P.; Arazo, L.C.; Ayala, J.M. An Experimental Lymphedema Animal Model for Assessing the Results of Lymphovenous Anastomosis. Lymphat. Res. Biol. 2018, 16, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Suami, H.; Yamashita, S.; Soto-Miranda, M.A.; Chang, D.W. Lymphatic Territories (Lymphosomes) in a Canine: An Animal Model for Investigation of Postoperative Lymphatic Alterations. PLoS ONE 2013, 8, e69222. [Google Scholar] [CrossRef] [PubMed]
- Das, S.K.; Franklin, J.D.; O’Brien, B.M.; Morrison, W.A. A practical model of secondary lymphedema in dogs. Plast. Reconstr. Surg. 1981, 68, 422–428. [Google Scholar] [CrossRef]
- Chen, H.-C.; Pribaz, J.J.; O’Brien, B.M.; Knight, K.R.; Morrison, W.A. Creation of Distal Canine Limb Lymphedema. Plast. Reconstr. Surg. 1989, 83, 1022–1026. [Google Scholar] [CrossRef] [PubMed]
- Tobbia, D.; Semple, J.; Baker, A.; Dumont, D.; Semple, A.; Johnston, M. Lymphedema Development and Lymphatic Function following Lymph Node Excision in Sheep. J. Vasc. Res. 2009, 46, 426–434. [Google Scholar] [CrossRef]
- Baker, A.; Kim, H.; Semple, J.L.; Dumont, D.; Shoichet, M.; Tobbia, D.; Johnston, M. Experimental assessment of pro-lymphangiogenic growth factors in the treatment of post-surgical lymphedema following lymphadenectomy. Breast Cancer Res. 2010, 12, R70. [Google Scholar] [CrossRef]
- Blum, K.S.; Hadamitzky, C.; Gratz, K.F.; Pabst, R. Effects of autotransplanted lymph node fragments on the lymphatic system in the pig model. Breast Cancer Res. Treat. 2010, 120, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Hadamitzky, C.; Zaitseva, T.S.; Bazalova-Carter, M.; Paukshto, M.V.; Hou, L.; Strassberg, Z.; Ferguson, J.; Matsuura, Y.; Dash, R.; Yang, P.C.; et al. Aligned nanofibrillar collagen scaffolds—Guiding lymphangiogenesis for treatment of acquired lymphedema. Biomaterials 2016, 102, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Ito, R.; Suami, H. Lymphatic Territories (Lymphosomes) in Swine: An Animal Model for Future Lymphatic Research. Plast. Reconstr. Surg. 2015, 136, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Xu, H.; Zhou, W.; Yuan, X.; Yang, Z.; Yang, Q.; Ding, F.; Meng, Z.; Liang, W.; Geng, C.; et al. Rhesus monkey is a new model of secondary lymphedema in the upper limb. Int. J. Clin. Exp. Pathol. 2014, 7, 5665–5673. [Google Scholar]
- Isner, J.M.; Asahara, T. Angiogenesis and vasculogenesis as therapeutic strategies for postnatal neovascularization. J. Clin. Investig. 1999, 103, 1231–1236. [Google Scholar] [CrossRef]
- Qi, S.; Pan, J. Cell-Based Therapy for Therapeutic Lymphangiogenesis. Stem Cells Dev. 2015, 24, 271–283. [Google Scholar] [CrossRef]
- Conrad, C.; Niess, H.; Huss, R.; Huber, S.; Von Luettichau, I.; Nelson, P.J.; Ott, H.C.; Jauch, K.-W.; Bruns, C.J. Multipotent Mesenchymal Stem Cells Acquire a Lymphendothelial Phenotype and Enhance Lymphatic Regeneration In Vivo. Circulation 2009, 119, 281–289. [Google Scholar] [CrossRef]
- Kondo, K.; Shintani, S.; Shibata, R.; Murakami, H.; Murakami, R.; Imaizumi, M.; Kitagawa, Y.; Murohara, T. Implantation of Adipose-Derived Regenerative Cells Enhances Ischemia-Induced Angiogenesis. Arter. Thromb. Vasc. Biol. 2009, 29, 61–66. [Google Scholar] [CrossRef]
- Kato, T.; Kato, K.; Shimizu, Y.; Takefuji, M.; Murohara, T. Treatment with adipose-derived regenerative cells enhances ischemia-induced angiogenesis via exosomal microRNA delivery in mice. Nagoya J. Med. Sci. 2021, 83, 465–476. [Google Scholar] [CrossRef]
- Katagiri, T.; Kondo, K.; Shibata, R.; Hayashida, R.; Shintani, S.; Yamaguchi, S.; Shimizu, Y.; Unno, K.; Kikuchi, R.; Kodama, A.; et al. Therapeutic angiogenesis using autologous adipose-derived regenerative cells in patients with critical limb ischaemia in Japan: A clinical pilot study. Sci. Rep. 2020, 10, 16045. [Google Scholar] [CrossRef]
- Pu, Z.; Shimizu, Y.; Tsuzuki, K.; Suzuki, J.; Hayashida, R.; Kondo, K.; Fujikawa, Y.; Unno, K.; Ohashi, K.; Takefuji, M.; et al. Important Role of Concomitant Lymphangiogenesis for Reparative Angiogenesis in Hindlimb Ischemia. Arter. Thromb. Vasc. Biol. 2021, 41, 2006–2018. [Google Scholar] [CrossRef]
- Shimizu, Y.; Shibata, R.; Shintani, S.; Ishii, M.; Murohara, T. Therapeutic Lymphangiogenesis With Implantation of Adipose-Derived Regenerative Cells. J. Am. Hear. Assoc. 2012, 1, e000877. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Cano, R.; Vranckx, J.; Lasso, J.; Calabrese, C.; Merck, B.; Milstein, A.; Sassoon, E.; DeLay, E.; Weiler-Mithoff, E. Prospective trial of Adipose-Derived Regenerative Cell (ADRC)-enriched fat grafting for partial mastectomy defects: The RESTORE-2 trial. Eur. J. Surg. Oncol. (EJSO) 2012, 38, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, Y.; Kondo, K.; Hayashida, R.; Sasaki, K.-I.; Ohtsuka, M.; Fukumoto, Y.; Takashima, S.; Inoue, O.; Usui, S.; Takamura, M.; et al. Therapeutic angiogenesis for patients with no-option critical limb ischemia by adipose-derived regenerative cells: TACT-ADRC multicenter trial. Angiogenesis 2022, 25, 535–546. [Google Scholar] [CrossRef]
- Zhang, L.; Yuan, J.; Ocansey, D.K.W.; Lu, B.; Wan, A.; Chen, X.; Zhang, X.; Qiu, W.; Mao, F. Exosomes derived from human umbilical cord mesenchymal stem cells regulate lymphangiogenesis via the miR-302d-3p/VEGFR3/AKT axis to ameliorate inflammatory bowel disease. Int. Immunopharmacol. 2022, 110, 109066. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.Z.; Wang, H.J.; Zhang, M.H.; Quan, Z.; Li, T.; He, Q.Z. CD34+ VEGFR-3+ progenitor cells have a potential to differentiate towards lymphatic endothelial cells. J. Cell Mol. Med. 2014, 18, 422–433. [Google Scholar] [CrossRef]
- Lee, S.-J.; Park, C.; Lee, J.Y.; Kim, S.; Kwon, P.J.; Kim, W.; Jeon, Y.H.; Lee, E.; Yoon, Y.-S. Generation of pure lymphatic endothelial cells from human pluripotent stem cells and their therapeutic effects on wound repair. Sci. Rep. 2015, 5, 11019. [Google Scholar] [CrossRef]
- Liersch, R.; Nay, F.; Lu, L.; Detmar, M. Induction of lymphatic endothelial cell differentiation in embryoid bodies. Blood 2006, 107, 1214–1216. [Google Scholar] [CrossRef]
- Kono, T.; Kubo, H.; Shimazu, C.; Ueda, Y.; Takahashi, M.; Yanagi, K.; Fujita, N.; Tsuruo, T.; Wada, H.; Yamashita, J.K. Differentiation of Lymphatic Endothelial Cells from Embryonic Stem Cells on OP9 Stromal Cells. Arter. Thromb. Vasc. Biol. 2006, 26, 2070–2076. [Google Scholar] [CrossRef]
- Yoon, Y.-S.; Murayama, T.; Gravereaux, E.; Tkebuchava, T.; Silver, M.; Curry, C.; Wecker, A.; Kirchmair, R.; Hu, C.S.; Kearney, M.; et al. VEGF-C gene therapy augments postnatal lymphangiogenesis and ameliorates secondary lymphedema. J. Clin. Investig. 2003, 111, 717–725. [Google Scholar] [CrossRef]
- Visuri, M.T.; Honkonen, K.M.; Hartiala, P.; Tervala, T.V.; Halonen, P.J.; Junkkari, H.; Knuutinen, N.; Ylä-Herttuala, S.; Alitalo, K.; Saarikko, A.M. VEGF-C and VEGF-C156S in the pro-lymphangiogenic growth factor therapy of lymphedema: A large animal study. Angiogenesis 2015, 18, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Forte, A.J.; Boczar, D.; Huayllani, M.T.; Anastasiadis, P.Z.; McLaughlin, S.A. Use of Vascular Endothelial Growth Factor-D as a Targeted Therapy in Lymphedema Treatment: A Comprehensive Literature Review. Lymphat. Res. Biol. 2022, 20, 3–6. [Google Scholar] [CrossRef]
- Saito, Y.; Nakagami, H.; Morishita, R.; Takami, Y.; Kikuchi, Y.; Hayashi, H.; Nishikawa, T.; Tamai, K.; Azuma, N.; Sasajima, T.; et al. Transfection of Human Hepatocyte Growth Factor Gene Ameliorates Secondary Lymphedema via Promotion of Lymphangiogenesis. Circulation 2006, 114, 1177–1184. [Google Scholar] [CrossRef]
- Jin, D.; Harada, K.; Ohnishi, S.; Yamahara, K.; Kangawa, K.; Nagaya, N. Adrenomedullin induces lymphangiogenesis and ameliorates secondary lymphoedema. Cardiovasc. Res. 2008, 80, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Onishi, T.; Nishizuka, T.; Kurahashi, T.; Arai, T.; Iwatsuki, K.; Yamamoto, M.; Hirata, H. Topical bFGF Improves Secondary Lymphedema through Lymphangiogenesis in a Rat Tail Model. Plast. Reconstr. Surg.—Glob. Open 2014, 2, e196. [Google Scholar] [CrossRef] [PubMed]
- Morisada, T.; Oike, Y.; Yamada, Y.; Urano, T.; Akao, M.; Kubota, Y.; Maekawa, H.; Kimura, Y.; Ohmura, M.; Miyamoto, T.; et al. Angiopoietin-1 promotes LYVE-1-positive lymphatic vessel formation. Blood 2005, 105, 4649–4656. [Google Scholar] [CrossRef]
- Tervala, T.V.; Hartiala, P.; Tammela, T.; Visuri, M.T.; Ylä-Herttuala, S.; Alitalo, K.; Saarikko, A.M. Growth factor therapy and lymph node graft for lymphedema. J. Surg. Res. 2015, 196, 200–207. [Google Scholar] [CrossRef]
- Clavin, N.W.; Avraham, T.; Fernandez, J.; Daluvoy, S.V.; Soares, M.A.; Chaudhry, A.; Mehrara, B.J. TGF-β1 is a negative regulator of lymphatic regeneration during wound repair. Am. J. Physiol. Heart Circ. Physiol. 2008, 295, H2113–H2127. [Google Scholar] [CrossRef]
- Brown, S.; Dayan, J.H.; Coriddi, M.; Campbell, A.; Kuonqui, K.; Shin, J.; Park, H.J.; Mehrara, B.J.; Kataru, R.P. Pharmacological Treatment of Secondary Lymphedema. Front. Pharmacol. 2022, 13, 828513. [Google Scholar] [CrossRef]
- Ting, Z.; Zhi-Xin, Y.; You-Wen, T.; Fu-Ji, Y.; Hui, S.; Fei, M.; Wei, Z.; Wen-Rong, X.; Hui, Q.; Yong-Min, Y. Exosomes derived from human umbilical cord Wharton’s jelly mesenchymal stem cells ameliorate experimental lymphedema. Clin. Transl. Med. 2021, 11, e384. [Google Scholar] [CrossRef]
- Kimura, T.; Hamazaki, T.S.; Sugaya, M.; Fukuda, S.; Chan, T.; Tamura-Nakano, M.; Sato, S.; Okochi, H. Cilostazol improves lymphatic function by inducing proliferation and stabilization of lymphatic endothelial cells. J. Dermatol. Sci. 2014, 74, 150–158. [Google Scholar] [CrossRef]
- Choi, I.; Lee, S.; Chung, H.K.; Lee, Y.S.; Kim, K.E.; Choi, D.; Park, E.K.; Yang, D.; Ecoiffier, T.; Monahan, J.; et al. 9-Cis Retinoic Acid Promotes Lymphangiogenesis and Enhances Lymphatic Vessel Regeneration: Therapeutic implications of 9-cis retinoic acid for secondary lymphedema. Circulation 2012, 125, 872–882. [Google Scholar] [CrossRef]
- Lee, G.K.; Perrault, D.P.; Bouz, A.; Pourmoussa, A.J.; Yu, R.; Kim, S.J.; Gardner, D.; Johnson, M.; Park, S.Y.; Park, E.K.; et al. Prolymphangiogenic Effects of 9-cis Retinoic Acid Are Enhanced at Sites of Lymphatic Injury and Dependent on Treatment Duration in Experimental Postsurgical Lymphedema. Lymphat. Res. Biol. 2022, 20, 640–650. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.J.; Roh, K.H.; Kim, G.C.; Kim, Y.O.; Lee, J.; Lee, M.J.; Sim, Y.J. Hyaluronidase treatment of acute lymphedema in a mouse tail model. Lymphology 2013, 46, 160–172. [Google Scholar] [PubMed]
- Kashiwagi, S.; Hosono, K.; Suzuki, T.; Takeda, A.; Uchinuma, E.; Majima, M. Role of COX-2 in lymphangiogenesis and restoration of lymphatic flow in secondary lymphedema. Lab. Investig. 2011, 91, 1314–1325. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Radhakrishnan, K.; Wong, Y.M.; Rockson, S.G. Anti-Inflammatory Pharmacotherapy with Ketoprofen Ameliorates Experimental Lymphatic Vascular Insufficiency in Mice. PLoS ONE 2009, 4, e8380. [Google Scholar] [CrossRef]
- Tian, W.; Rockson, S.G.; Jiang, X.; Kim, J.; Begaye, A.; Shuffle, E.M.; Tu, A.B.; Cribb, M.; Nepiyushchikh, Z.; Feroze, A.H.; et al. Leukotriene B4 antagonism ameliorates experimental lymphedema. Sci. Transl. Med. 2017, 9, eaal3920. [Google Scholar] [CrossRef]
- García-Caballero, M.; Zecchin, A.; Souffreau, J.; Truong, A.-C.K.; Teuwen, L.-A.; Vermaelen, W.; Martín-Pérez, R.; de Zeeuw, P.; Bouché, A.; Vinckier, S.; et al. Role and therapeutic potential of dietary ketone bodies in lymph vessel growth. Nat. Metab. 2019, 1, 666–675. [Google Scholar] [CrossRef]
- Chen, H.-C.; O’Brien, B.; Rogers, I.; Pribaz, J.; Eaton, C. Lymph node transfer for the treatment of obstructive lymphoedema in the canine model. Br. J. Plast. Surg. 1990, 43, 578–586. [Google Scholar] [CrossRef]
- Becker, C.; Assouad, J.; Riquet, M.; Hidden, G. Postmastectomy Lymphedema: Long-term results following microsurgical lymph node transplantation. Ann. Surg. 2006, 243, 313–315. [Google Scholar] [CrossRef]
- Nguyen, D.H.; Chou, P.-Y.; Hsieh, Y.-H.; Momeni, A.; Fang, Y.-H.D.; Patel, K.M.; Yang, C.-Y.; Cheng, M.-H. Quantity of lymph nodes correlates with improvement in lymphatic drainage in treatment of hind limb lymphedema with lymph node flap transfer in rats. Microsurgery 2016, 36, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Lähteenvuo, M.; Honkonen, K.; Tervala, T.; Tammela, T.; Suominen, E.; Lähteenvuo, J.; Kholová, I.; Alitalo, K.; Ylä-Herttuala, S.; Saaristo, A. Growth Factor Therapy and Autologous Lymph Node Transfer in Lymphedema. Circulation 2011, 123, 613–620. [Google Scholar] [CrossRef]
- Stanek, K.; Jonas, F.; Ticha, P.; Molitor, M.; Mestak, O. Animal Models Used in the Research of Vascularized Lymph Node Transfer: A Systematic Review. J. Surg. Res. 2022, 272, 1–8. [Google Scholar] [CrossRef]
- Eldaly, A.S.; Avila, F.R.; Torres-Guzman, R.A.; Maita, K.C.; Garcia, J.P.; Serrano, L.P.; Saleem, H.Y.; Forte, A.J. Animal models in lymph node transfer surgery: A systematic review. J. Clin. Transl. Res. 2022, 8, 243–255. [Google Scholar]
- Jang, D.-H.; Song, D.-H.; Chang, E.-J.; Jeon, J.Y. Anti-inflammatory and lymphangiogenetic effects of low-level laser therapy on lymphedema in an experimental mouse tail model. Lasers Med. Sci. 2016, 31, 289–296. [Google Scholar] [CrossRef]
- Hartiala, P.; Suominen, S.; Suominen, E.; Kaartinen, I.; Kiiski, J.; Viitanen, T.; Alitalo, K.; Saarikko, A.M. Phase 1 LymfactinⓇ Study: Short-term Safety of Combined Adenoviral VEGF-C and Lymph Node Transfer Treatment for Upper Extremity Lymphedema. J. Plast. Reconstr. Aesthetic Surg. 2020, 73, 1612–1621. [Google Scholar] [CrossRef] [PubMed]
- Hou, C.; Wu, X.; Jin, X. Autologous Bone Marrow Stromal Cells Transplantation for the Treatment of Secondary Arm Lymphedema: A Prospective Controlled Study in Patients with Breast Cancer Related Lymphedema. Jpn. J. Clin. Oncol. 2008, 38, 670–674. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, G.E.M.; Pérez, C.A.A.; Covarrubias, E.E.A.; Cabriales, S.A.M.; Leyva, L.A.; Pérez, J.C.J.; Almaguer, D.G. Autologous stem cells for the treatment of post-mastectomy lymphedema: A pilot study. Cytotherapy 2011, 13, 1249–1255. [Google Scholar] [CrossRef]
- Toyserkani, N.M.; Jensen, C.H.; Sheikh, S.P.; Sørensen, J.A. Cell-Assisted Lipotransfer Using Autologous Adipose-Derived Stromal Cells for Alleviation of Breast Cancer-Related Lymphedema. STEM CELLS Transl. Med. 2016, 5, 857–859. [Google Scholar] [CrossRef]
- Toyserkani, N.M.; Jensen, C.H.; Andersen, D.C.; Sheikh, S.P.; Sørensen, J.A. Treatment of Breast Cancer-Related Lymphedema with Adipose-Derived Regenerative Cells and Fat Grafts: A Feasibility and Safety Study. STEM CELLS Transl. Med. 2017, 6, 1666–1672. [Google Scholar] [CrossRef]
- Toyserkani, N.; Jensen, C.H.; Tabatabaeifar, S.; Jørgensen, M.; Hvidsten, S.; Simonsen, J.A.; Andersen, D.C.; Sheikh, S.; Sørensen, J.A. Adipose-derived regenerative cells and fat grafting for treating breast cancer-related lymphedema: Lymphoscintigraphic evaluation with 1 year of follow-up. J. Plast. Reconstr. Aesthetic Surg. 2019, 72, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.H.; Chen, S.C.; Henry, S.L.; Tan, B.K.; Chia-Yu Lin, M.; Huang, J.J. Vascularized groin lymph node flap transfer for postmastectomy upper limb lymphedema: Flap anatomy, recipient sites, and outcomes. Plast Reconstr. Surg. 2013, 131, 1286–1298. [Google Scholar] [CrossRef] [PubMed]
- Asuncion, M.O.; Chu, S.Y.; Huang, Y.L.; Lin, C.Y.; Cheng, M.H. Accurate Prediction of Submental Lymph Nodes Using Magnetic Resonance Imaging for Lymphedema Surgery. Plast Reconstr. Surg. Glob. Open 2018, 6, e1691. [Google Scholar] [CrossRef] [PubMed]
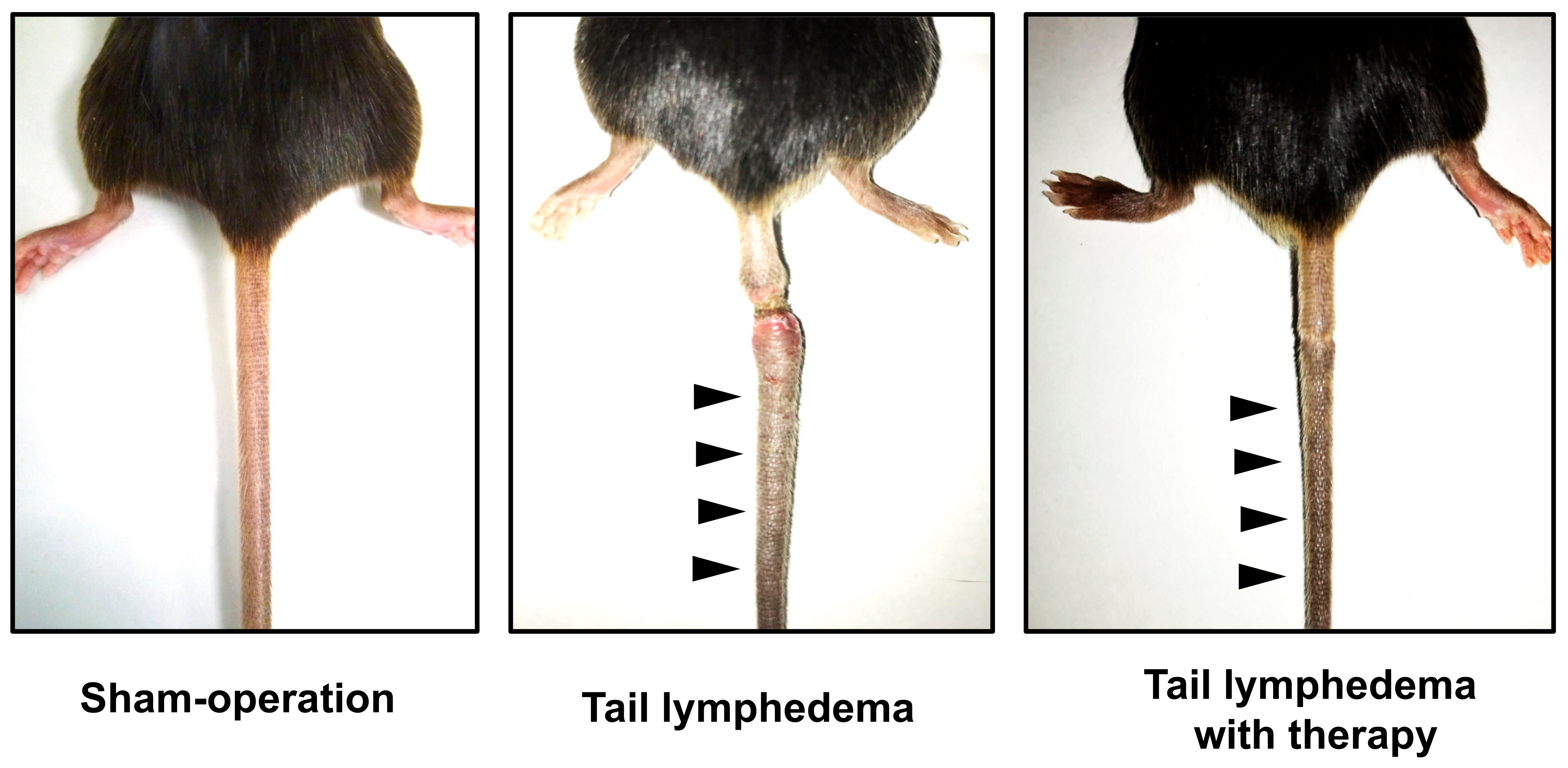
| Target Molecule | Patient Number | Edema Site | Follow-Up | Result | Year | Ref. | |
|---|---|---|---|---|---|---|---|
| Gene therapy | VEGF-C | 15 | Upper limb | 12 months | No dose-limiting toxicities and well tolerated. | 2020 | Hartiala et al. [146] |
| Cell therapy | BM-stromal cell | 15 | Upper limb | 12 months | The BMSC Group had a reduction in lymphedema volume and pain scale and a better long-term cure result. | 2008 | Hou et al. [147] |
| BM-MNC | 10 | Upper limb | 3 months | BM-MNCs reduce lymphedema volume and chronic pain and improve sensitivity. | 2011 | Maldonado et al. [148] | |
| ADSC | 1 | Upper limb | 1 and 4 months | Symptoms in patients were improved over time, and volume of affected arm was reduced. | 2016 | Toyserkani et al. [149] | |
| ADSC | 10 | Upper limb | 1, 3, and 6 months | Non-significant change in volume was observed. Patient outcomes improved significantly over time. Half of the patients reduced their use of conservative management. | 2017 | Toyserkani et al. [150] | |
| ADSC | 10 | Upper limb | 1, 3, 6, and 12 months | No significant change in volume was observed. Patient outcomes improved significantly over time. Half of the patients reduced their use of conservative management. | 2019 | Toyserkani et al. [151] | |
| Others | LN transfer | 20 | Upper limb | 39 months | Patients exhibit decrease in cellulitis, circumferential reduction, and circumferential differentiation. | 2013 | Cheng et al. [152] |
| LN transfer | 15 | Limb | 12 months | Mean episodes of cellulitis and circumferential difference and the overall lymphedema quality of life were improved. | 2018 | Asuncion et al. [153] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shimizu, Y.; Che, Y.; Murohara, T. Therapeutic Lymphangiogenesis Is a Promising Strategy for Secondary Lymphedema. Int. J. Mol. Sci. 2023, 24, 7774. https://doi.org/10.3390/ijms24097774
Shimizu Y, Che Y, Murohara T. Therapeutic Lymphangiogenesis Is a Promising Strategy for Secondary Lymphedema. International Journal of Molecular Sciences. 2023; 24(9):7774. https://doi.org/10.3390/ijms24097774
Chicago/Turabian StyleShimizu, Yuuki, Yiyang Che, and Toyoaki Murohara. 2023. "Therapeutic Lymphangiogenesis Is a Promising Strategy for Secondary Lymphedema" International Journal of Molecular Sciences 24, no. 9: 7774. https://doi.org/10.3390/ijms24097774
APA StyleShimizu, Y., Che, Y., & Murohara, T. (2023). Therapeutic Lymphangiogenesis Is a Promising Strategy for Secondary Lymphedema. International Journal of Molecular Sciences, 24(9), 7774. https://doi.org/10.3390/ijms24097774





