Abstract
Although diagnosis and treatment of vestibular schwannomas (VSs) improved in recent years, no factors have yet been identified as being capable of predicting tumor growth. Molecular rearrangements occur in neoplasms before any macroscopic morphological changes become visible, and the former are the underlying cause of disease behavior. Tumor microenvironment (TME) encompasses cellular and non-cellular elements interacting together, resulting in a complex and dynamic key of tumorigenesis, drug response, and treatment outcome. The aim of this systematic, narrative review was to assess the level of knowledge on TME implicated in the biology, behavior, and prognosis of sporadic VSs. A search (updated to November 2022) was run in Scopus, PubMed, and Web of Science electronic databases according to the PRISMA guidelines, retrieving 624 titles. After full-text evaluation and application of inclusion/exclusion criteria, 37 articles were included. VS microenvironment is determined by the interplay of a dynamic ecosystem of stromal and immune cells which produce and remodel extracellular matrix, vascular networks, and promote tumor growth. However, evidence is still conflicting. Further studies will enhance our understanding of VS biology by investigating TME-related biomarkers able to predict tumor growth and recognize immunological and molecular factors that could be potential therapeutic targets for medical treatment.
1. Introduction
Vestibular schwannomas (VSs) are uncommon, slow-growing benign tumors that originate from the Schwann cells lining of the axons of the eighth cranial nerve (vestibulo-cochlear nerve). Most of them arise from the vestibular nerve itself or one of its branches [1]. VSs represent about 8% of all intracranial tumors, being the most common neoplasm of the cerebellopontine angle (CPA) in adults [2]. Over the past years, VS incidence rate has been steadily increasing to about 34 VS/million/year, most probably due to easier access to improved diagnostics (e.g., Magnetic Resonance Imaging) [3]. In about 95% of cases, the VS is sporadic and unilateral, while bilateral lesions are encountered in the framework of genetic disorders such as neurofibromatosis type 2 (NF-2) or schwannomatosis [2].
The optimal management of sporadic VSs is to offer long-term cure of the disease with preservation of function, which is a realistic goal in small tumors. The choice of treatment depends on the balance between tumor and patient factors at diagnosis, such as size, growth, hearing, age, comorbidities, feasibility of sparing-function surgery and rehabilitation, and patients’ expectations. A multi-option strategy is shared with the patient, and involves an observation policy, or active treatments such as microsurgery or stereotactic radiosurgery [4]. The goal of observation is to preserve hearing and facial nerve function as long as possible in non-growing small tumors. Radiation therapy aims to stop the growth of VSs that are getting bigger. Surgery focuses on the functional outcomes (facial nerve function and hearing) and definitive cure [5,6,7]. Observational policy might expose patients to tumor growth and consequently, if surgery is then indicated, to the risks of a microsurgical resection in a bigger tumor, with potentially worse postoperative outcomes [5,6,7]. SRS aims to offer tumor stability in growing tumors, but this approach is not free of complications nor long-term functional impairments [8,9,10].
To date, the only alternative or complementary medical treatment approved for VSs is Bevacizumab, an anti-vascular endothelial growth factor (VEGF), available since 2009 [11]. It is indicated to treat growing or hearing-compromised VS in NF-2 patients; its aim is to control tumor growth over the longest period of treatment, allowing surgery to be avoided or postponed. However, Bevacizumab demonstrated less efficacy in children and its efficacy on residual VS after partial resection is low [12,13]. Therefore, there is an urgent need for novel medical treatment modalities in VSs, which preliminarily requires a better knowledge of the tumor environment.
Nowadays, there is increasing evidence that the role of the tumor microenvironment (TME) dictates tumor characteristics and evolutive features, which may play a role in the selection of the most appropriate treatment. Specifically, TME consists of cellular and non-cellular elements (e.g., the extracellular matrix components, metabolites, and cytokines) interacting together, and influencing tumor behavior [14]. The cellular compartment of TME is composed of both cells that are present in normal tissue before tumor development, and cells that are recruited from distal sites, including fibroblasts, adipocytes, immune cells, and endothelial cells [15]. Awareness of VS molecular biology and microenvironment may be of the utmost importance in investigating alternative treatments. Several attempts have been made, identifying—among others—acetylsalicylic acid and COX-2 inhibitors as potential drugs against VS growth, with debatable results [16,17,18]. However, tumor biology in sporadic VSs is still poorly understood.
The purpose of this systematic, narrative review was to assess the level of current knowledge on the role of TME in VS. A high awareness of the TME in VS would be useful to: (i) investigate biomarkers predicting tumor evolution, and (ii) recognize immunological and molecular factors that could be potential targets for medical treatment in the near future.
2. Materials and Methods
2.1. Protocol Registration
The protocol of this systematic review and meta-analysis was registered on PROSPERO, an international database of prospectively registered systematic reviews in health and social care (Center for Reviews and Dissemination, University of York, York, UK), in November 2022 (registry number CRD42022366609).
2.2. Search Strategy
A systematic literature review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) recommendations [19]. The electronic databases Scopus, Pubmed, and Web of Science were searched from database inception to 15 November 2022. A combination of MeSH terms and free-text words were utilized to search for: “vestibular schwannoma”; “acoustic neuroma”; “microenvironment”; “immunohistochemistry”; “growth factor” (Supplementary Materials). The reference lists of all the included articles were thoroughly screened to find other relevant articles. References were exported to Zotero bibliography manager (v6.0.10, Center for History and New Media, George Mason University, Fairfax, VA, USA). After duplicates removal, two reviewers (A.D. and D.C.) independently screened all titles and abstracts and then evaluated the full texts of the eligible articles based on the inclusion criteria. Any disagreement between the reviewers involved in the literature search was resolved through discussion with all authors to reach a consensus.
2.3. Selection Criteria
Studies were deemed eligible when the following inclusion criteria were met: (i) confirmed pathological diagnosis of sporadic VS; (ii) tissue specimen analysis performed through immunohistochemistry (IHC) or molecular methods; (iii) tumors primarily treated with surgery. Exclusion criteria were as follows: (i) retrospective series with less than 10 cases; (ii) NF-2 patients; (iii) previously irradiated tumors; (iv) lack of relevant data; (v) non-original studies (i.e., reviews, recommendations, letters, editorials, or book chapters); (vi) animal model studies, (vii) non-English studies. The papers were thoroughly screened for duplicates.
2.4. Data Extraction and Quality Assessment
Extracted data were collected in an electronic database including first author, year of publication, country of origin, study design, sample size, number of patients included, mean age of the patients, sex ratio, mean tumor size, investigated biomarkers, methods applied for biomarkers detection, study aim, key findings. The quality of the studies eligible for inclusion was categorized as Poor, Fair, and Good, in agreement with the National Institutes of Health quality assessment tool for Observational Cohorts and Cross-Sectional Studies (https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools, accessed on 15 November 2022) [20]. Two reviewers (A.D. and D.C.) independently evaluated the papers, and any disagreement was resolved by consensus.
3. Results and Discussion
3.1. Search Results and Quality Assessment
A total of 624 titles were collected from our literature search. After duplicates removal and exclusion of 336 records due to coherence with the inclusion/exclusion criteria, 103 articles relevant to the topic were examined. Five records were unavailable for retrieving. Ninety-four articles were assessed for eligibility, and, in the end, 37 were included in the review [21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57]. A detailed flowchart of the search process is shown in Figure 1. Available data in the included manuscripts were inadequate to perform a quantitative analysis.
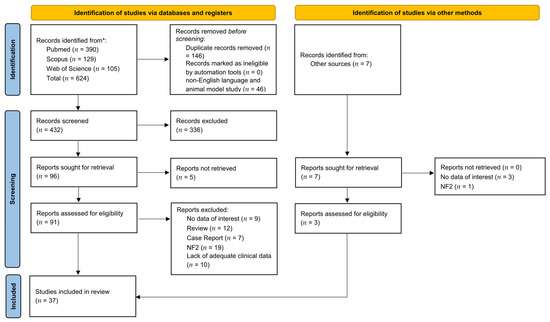
Figure 1.
PRISMA diagram summarizing Electronic Database Search and Inclusion/Exclusion process of the review. * date of last search 15 November 2022.
In accordance with the National Institutes of Health quality assessment tool for Observational Cohorts and Cross-Sectional Studies [20], 12 studies were deemed of Good quality (32.5%), 16 Fair (43.2%), and only 9 (24.3%) were classified as Poor, due to the lack in reporting information on the series’ features (Table S1).
3.2. Included Studies’ Characteristics
All 37 studies included in the qualitative analysis had an observational retrospective design, and were ex vivo tissue investigations based on histopathological analysis of surgical specimens [21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57]. Studies were published from 1990 to 2022. The median number of patients per study was 32 (range 10–923).
Major findings of the retrieved articles are discussed in dedicated paragraphs, and data on patients’ demographics, study design, tumor characteristics, and relevant conclusions of each article included, are reported in Table 1, Table 2, Table 3, Table 4, Table 5 and Table 6.
The current knowledge and debate on TME in VS presented in the eligible articles focused on: (i) angiogenesis; (ii) immune cells infiltration; (iii) molecular regulators; (iv) growth factors; (v) matrix metalloproteinases; and (vi) hormone receptors.
3.3. Angiogenesis
Tumor cells secrete several factors leading to the transformation of a previously anti-tumorigenic milieu into a pro-tumorigenic one [58]. Tumors interact with numerous stromal cells, including local and infiltrating fibroblasts, macrophages, mesenchymal stem cells, endothelial cells, pericytes, secreted factors, and the extracellular matrix within the TME [59]. Angiogenesis is a major prerequisite for the proliferation and progression of several neoplasms [60]. Judah Folkman, considered the “father of angiogenesis”, proposed the first model of tumor angiogenesis according to which tumor cells are able to sense their increasing distance from existing vasculature and, in response, release angiogenic signals [61]. Currently, tumor angiogenesis is considered the result of an imbalance between pro- and anti-angiogenic factors produced by tumor and physiological cells [62].
Among the various pro-angiogenic growth factors, the vascular endothelial growth factor (VEGF) is a major regulator of angiogenesis [63]. Previous studies analyzed different types of brain tumors for VEGF expression [64,65]. Furthermore, VEGF has been reported to be a survival factor for Schwann cells [66]. This result suggests a crosstalk between tumor cells and vessels in a paracrine-stimulating manner.
Even though VS are generally slow-growing tumors, a functional vascular system still remains important for tumor growth [39]. In 1996, Matsugana et al. [48] hypothesized that VSs might shrink spontaneously or remain silent for a long time if there was poor angiogenesis. Whereas they might grow or regrow rapidly in the presence of significant angiogenesis [48].
Investigating the angiogenic effect of VS on the adjacent vestibulocochlear nerve, an increased vascularization was found showing delicate blood vessels, susceptible to mechanical stress [48]. These data suggested that one of the most plausible explanations for hearing loss in VS was edema of the cochlear nerve due to circulatory disturbance and that postoperative hearing loss was due to surgical injury of the vessels running between tumor and nerve [48].
In VS, a relationship was found between micro-vessel density (MVD), tumor size, and growth rate with an ultrastructural approach [41,67], while other studies demonstrated tumor expression of VEGF with immunohistochemical methods [68,69]. Brieger et al. [26] analyzed angiogenesis in 34 VS specimens, concluding that VEGF, VEGF-R1, VEGF-R2, and TGF-β1 were unexpressed in tumor tissue, with the exception of one case. MVD investigated through CD31 staining revealed low expression in the specimens [26]. On the other hand, other studies on MVD in VS showed divergent results, with MVD being positively correlated with tumor size and growth [29,44]. Furthermore, these studies evidenced an association between angiogenic factors and MVD with CD68 positive cells and macrophage density in tumor tissue [29,44].
Cayè-Thomasen et al. [28] found that the high-affinity receptor VEGFR-1 was expressed in VS, and also that the tumor homogenate concentration of both VEGF and VEGFR-1 correlated positively with tumor growth rate but not with tumor size. These findings suggested that VEGF might also play a role in benign neoplasms’ growth. Accordingly, VS growth could be associated with high levels of VEGF in both blood and cerebrospinal fluid, therefore providing a growth indicator. Consequently, VEGF measurement may become a valuable tool for therapeutic approach choice [28].
Koutsimpelas et al. [38] tried to clarify the role of VEGF and basic fibroblast growth factor (bFGF) in VS vascularization and growth. They found a significant association between VEGF, bFGF, MVD, and tumor growth index. Furthermore, bFGF was associated with tumor volume in VS. They hypothesized that accelerated growth could be related to higher bFGF levels, resulting in larger tumors.
It was found that recurrent VS and tumors treated with radiosurgery prior to operating had a considerably elevated VEGF expression in comparison to the primary tumors, probably because of surgical trauma or an intrinsic propensity [39]. It was suggested that these tumors express high levels of VEGF per se, and the increased VEGF levels might have a role in protecting the endothelial and/or tumor cells during the radiation. Consequently, the failure of radiosurgery to control VS tumors might be due to high VEGF levels [39]. It was hypothesized that recurrent VS might be targeted using antiangiogenic active compounds. Furthermore, if it was proven that VEGF mediated VS protection through reduction of radiation sensitivity, then a combination of radiotherapy and anti-VEGF treatment might improve therapeutic outcome [39].

Table 1.
Studies examining the role of angiogenetic factors in sporadic vestibular schwannomas.
Table 1.
Studies examining the role of angiogenetic factors in sporadic vestibular schwannomas.
| Author | Year | Country | Study Design | Cases (n) | Mean Age ± SD (Range) | Sex (M/F) | Tumor Size, Mean ± SD (Range) | Method of Marker Detection | Marker Studied | Key Findings |
|---|---|---|---|---|---|---|---|---|---|---|
| Brieger et al. [26] | 2003 | Germany | Ex vivo tissue study | 34 | 49 (19–72) | 20/14 | 16.5 mm (5–36) | IHC | VEGF VEGF-R1 VEGF-R2 TGF-β1 CD31 CD68 |
|
| Cayè-Thomasen et al. [28] | 2005 | Denmark | Ex vivo tissue study | 27 | 53 (35–61) | 11/16 | 1.431 cm2 (0.198–4.589) | ELISA | VEGF VEGF-R1 |
|
| Koutsimpelas et al. [38] | 2007 | Germany | Ex vivo tissue study | 17 | 51.5 ± 12.2 (28–71) | 11/6 | 424 ± 658 mm3 (36–2556) | IHC qPCR | VEGF bFGF |
|
| Koutsimpelas et al. [39] | 2012 | Germany | Ex vivo tissue study | 182 | 52 ± 10.6 (18–78) | 79/ 103 | 2.404 ± 2.329 mm3 (24–37.679) | Tissue microarray IHC | VEGF VEGFR-1 VEGFR-2 NP1 |
|
| Marioni et al. [46] | 2019 | Italy | Ex vivo tissue study | 71 | 52.8 ± 13.0 | 37/34 | 10 intra-meatal; 21 small (<1 cm); 27 medium-sized (1–2.5 cm); 13 large (>2.5 cm) | IHC | Endoglin (CD105) |
|
| Matsunaga et al. [48] | 1996 | Japan | Ex vivo tissue study | 29 | 51.38 ± 12.18 (8–66) | 13/16 | 19.03 ± 10.32 mm (5–40) | IHC | Blood vessels |
|
| Xia et al. [57] | 2020 | China | Ex vivo tissue study | 38 | NR | NR | 31.95 ± 1.74 (NR) | IHC | MMP-14 VEGF |
|
F: female; M: male; MVD: microvessel density; n: number of cases; SD: standard deviation; NR: not reported; VS: vestibular schwannoma.
Marioni et al. [46] first investigated neoangiogenesis in sporadic VS using CD105 staining. CD105 resulted in a useful marker to identify proliferating endothelium involved in tumor angiogenesis [70]. They found a significant positive correlation between tumor size at the time of surgery and (i) vessel cross-sectional area as well as (ii) CD105-assessed vessel density.
Quite recently, the different expression of matrix metalloproteinases (MMPs) and VEGF in the Antoni A and B areas was investigated [57]. The authors evidenced a significantly higher MMP-14 and VEGF expression in the Antoni B than in the Antoni A areas, and concluded that the upregulation of MMP-14 induced the degradation of loose collagen in the Antoni B area, thus contributing to cystic formation. Moreover, the overexpression of VEGF (which is also enhanced by MMP-14) increased the permeability of tumor vessels and caused cyst fluid leakage from the fragile neo-vasculature in the Antoni B area, which determined cystic expansion and intra-tumoral hemorrhage.
Based on these results, angiogenesis seems to have an impact on TME, for instance by influencing tumor growth index and prognosis [29,39,48]. Overall, VS progression may be decreased by modulating the characteristics of TME through the inhibition of cytokines and growth factors (e.g., VEGF, bFGF, M-CSF, IL-34, etc.) [28,29,38]. In vivo VS models are necessary to clarify the biological mechanisms that are involved in the associations of TME mutations and VS behavior.
3.4. Immune Cells Infiltrates
Immune system functions are involved in several processes in tumor tissues, from immunosurveillance to immune escape, when the tumor overcomes immunological control, leading to tumor growth [71]. Immune cell infiltration in the VS tissue microenvironment has long been observed, in particular for T and B lymphocytes and macrophages; however, their definitive role in tumorigenesis, growth, or patients’ symptoms such as hearing deterioration remains to be established.
One of the first studies examining the inflammatory phenotype in VSs revealed a positive correlation between tumor tissue inflammation (investigated through CD45 staining) and the duration of clinical symptoms [41]. Having observed that the degree of tumor tissue inflammation increased with time, it was postulated that inflammation could be a degenerative process in TME [41].
The relationship between angiogenesis and immune cells infiltration in VS received increased attention when the role of tumor-associated macrophages (TAMs) was investigated. TAMs came into play in TME of solid tumors with a prominent role in the regulation of tumor growth, invasion, and angiogenesis [14,72]. Specifically, among the heterogeneous population of TAMs, M2-type macrophages—identified through the cell surface marker CD68—seem to have tumor-promoting features and seem to be associated with poor outcomes in different gastro-intestinal malignancies [73]. de Vries et al. [29] found a significant MVD increase in VSs with a high number of CD68 positive cells (macrophage markers). More recently, the same research group investigated the role in VS of the macrophage colony stimulating factor M-CSF, a cytokine that regulates macrophage recruitment, proliferation, and differentiation, polarizing macrophages towards a pro-tumoral M2-like phenotype in TME. de Vries et al. [30] reported a significantly higher expression of M-CSF in fast-growing VSs, as well as in cystic tumors. Analogously, CD163 expression was significantly elevated in tumors with strong M-CSF staining [30]. This evidence shed light on the potential role of M-CSF as a target for specific inhibitors.
The existing association between sporadic VS vascularity and TAM infiltration was further corroborated by Lewis et al. [44]. Higher MVD and fibrinogen density (marker of vessel permeability) were found in high-TAM-density tumor regions. Furthermore, a spatial correlation of areas of high TAM density and (i) VEGF and (ii) VEGFR-1 expression was observed. Thus, it is likely that VEGF/VEGFR-1 signaling promotes chemo-attraction of VEGFR-1-expressing TAMs in the TME. In this context, treatment directed against the VEGF pathway might reduce both TAM effect and angiogenesis.
Many attempts have been made to identify potential immune cell factors influencing the growth of sporadic VS. A series of 67 VSs was evaluated for the leukocyte antigen CD45 and for CD68 positive cells, revealing a positive correlation among their expression, tumor size, and tumor growth index [29]. M2 macrophages involvement was analyzed by the same researchers [30] in two groups of 10 slow- and 10 fast-growing VSs. The expression of CD163 was found to be significantly higher in fast-growing tumors, highlighting the potential involvement of M2 TAMs in growth progression. In this sense, Leisz et al. [43] investigated the role of TME in association with VS volume and growth rate. They detected increased macrophage markers expression (CD163 and CD68) in volumetrically larger tumors, an observation that was confirmed by Gonçalves et al. [34] considering 923 VSs. In the Leisz series, growth rate was determined in a subset of 74 patients, revealing significantly higher expression of CD68 in fast-growing tumors (> 1 cm3/year) [43]. These findings were confirmed on a subset of randomly selected growing tumors, where fast tumor growth was associated with higher levels of CD163 and CD68 positive cells. Similar observations were reported by Lewis et al. [44] demonstrating higher TAM density in rapidly growing VSs. Contrarily to the previous results, Gonçalves et al. [34] proved an inverse relationship between tumor growth rate and tissue inflammation. The authors elaborated an inflammatory score encompassing CD3, CD8, CD68, and CD163 expressions. Surprisingly, higher inflammatory score levels were independently associated with slower volumetric tumor growth. Thus, for the first time, growth rate in VS and its inflammatory microenvironment were found to be inversely correlated, demonstrating the need for further detailed studies on the role of immune response in VS.
Two investigations [23,53] tried to define the immune signature of VSs that underwent sub-total microsurgical resection and observed the evolution of tumor residuals. Amit et al. [23] found a different tumor-immune environment between the collected specimens of rapidly progressing and slowly or not-progressing residuals, in both the innate and adaptive immune compartments. Particularly, rapidly progressing tumors presented a significant enrichment of CD68+ macrophages, CD4+ and CD8+ T lymphocytes, and CD20+ B cells, whereas a lower density of dendritic cells positive for CD1a. To further explore these findings, a transcriptomic analysis was conducted revealing differences in gene expression and pathways dysregulation. Early progressing residuals revealed immune signaling downregulation and enriched cellular senescence pathways linked to viral infection (e.g., NF-kB pathway); thus, leading the authors to hypothesize a possible viral etiology in fast progressing VSs.
On a cohort of operated VSs, a significantly increased TAM density in tumors that progressed after sub-total resection was confirmed [53]. Remarkably, this research group reported contrasting results to what had previously been observed by other teams. Significantly higher CD163 positive cell density and increased M2 index were encountered in VSs that remained stable after sub-total resection, than in progressive tumors. Given these results, the authors concluded that TAM phenotype in VS might be far more elaborate than ordinary M1/M2 polarization. High density of M2-like macrophages in stable tumors could reasonably be considered as the result of the anti-tumor host response.

Table 2.
Studies examining the role of immune cell populations in sporadic vestibular schwannomas.
Table 2.
Studies examining the role of immune cell populations in sporadic vestibular schwannomas.
| Author | Year | Country | Study Design | Cases (n) | Mean Age ± SD (Range) | Sex (M/F) | Tumor Size, Mean ± SD (Range) | Method of Marker Detection | Marker Studied | Key Findings |
|---|---|---|---|---|---|---|---|---|---|---|
| Amit et al. [23] | 2022 | USA | Ex vivo tissue study | 17 | 51.3 ± 11.22 | 6/11 | NR | CD4/CD8 CD20 CD68 CD1A | IF |
|
| De Vries et al. [29] | 2012 | Netherlands | Ex vivo tissue study | 67 | 49.04 ± 14.06 (15–72) | 26/41 | 24.03 ± 11.52 mm (5–50) | Histone H3 Ki-67 CD31 CD45 CD68 Hemosiderin | IHC |
|
| De Vries et al. [30] | 2019 | Netherlands | Ex vivo tissue study | 20 | 56.75 (39–81) | 4/16 | 13 ± 7.44 mL | M-CSF IL-34 | IHC |
|
| Gonçalves et al. [34] | 2021 | Germany | Ex vivo tissue study | 923 | NR | NR | 4.73 cm3 (0.04–52.14) | CD3 CD8 CD68 CD163 | IHC |
|
| Labit-Bouvier et al. [41] | 2000 | France | Ex vivo tissue study | 69 | 53 (median) (20–77) | 37/32 | 18 mm (median) (6–50) | CD34 CD45 ER, PR | IHC |
|
| Leisz et al. [43] | 2022 | Germany | Ex vivo tissue study | 74 | 53 (28–77) | 32/42 | 2.44 cm3 (0.1–18.8) | Ki-67 COX2 VEGF M-CSF GM-CSF CD163 CD68 | RT-PCR |
|
| Lewis et al. [44] | 2021 | UK | Ex vivo tissue study | 17 | 49.4 (median) (41.3–55-8) | 7/10 | 2.51 cm3 (median) (1.56–5-91) | Iba+ CD31 Fibrinogen TAM VEGF VEGFR-1 Ki-67 | IHC IF DCE-MRI |
|
| Perry et al. [53] | 2020 | USA | Ex vivo tissue study | 46 | 57 (median) (25–83) | 21/25 | Stable disease 3.3 cm (1.8–5.2) Tumor progression 2.9 cm (1.8–5.0) (median) | CD68 CD163 PD-L1 | IHC |
|
ceMRI: contrast-enhanced magnetic resonance imaging; F: female; M: male; MVD: microvessel density; n: number of cases; NR: not reported; SD: standard deviation; TAM: tumor-associated macrophage; VS: vestibular schwannoma.
3.5. Molecular Regulators
The VS microenvironment is finely modulated by the interplay of several cellular pathways leading to dysregulation of the natural physiological balance between programmed cell death and cell proliferation. The primary molecular events inducing VS, in both sporadic and NF-2 related VS, are the occurrence of Nf2 gene mutations on chromosome 22 and subsequent loss of the tumor suppressor protein Merlin. Merlin is a protein of FERM (4.1 protein/Ezrin/radixin/moesin) that plays a role in cell attachment, cell motility, membrane receptor availability, signal transduction, and then in cell proliferation. Merlin’s antitumor activity is anti-proliferative. Its overexpression induces cell cycle arrest in G1 phase, decreasing P21 expression and inhibiting the cell cycle regulators as cyclins [35]. The inactivation of Merlin detected in VS results from the phosphorylation of serine 518 that is exerted by the activity of P21 activated kinase (PAK), which in turn is activated by ras-related C3 botulinum toxin substrate 1 (Rac1) [74,75]. Rac1 drives the progression of the cell cycle, from G1 to the synthesis phase, promoting the cyclin D1 expression. Among the cyclins in VS patients, it has been demonstrated that both cyclin D1 and cyclin D3 play a role in cell proliferation [35,42,52]. There is no consistency in the evidence reported about a direct correlation between cyclin D1 and cyclin D3 expression and the proliferation index Ki-67 [35,42,52]. Ki-67 is a nuclear protein expressed during cell replication. This evidence suggests that VS event undergoes different mechanisms that regulate cell proliferation [35,52]. Cyclin D1 activity is expressed early in the G1 phase; it binds to cyclin-dependent kinases CDK4 or CDK6, that once activated phosphorylate the target protein retinoblastoma Rb. The latter induce E2F transcription factors to activate the expression of S-phase genes and thus the progression of the cell cycle [42]. Based on the foregoing, a possible predictor of VS aggression may be the deregulation of a factor involved in cyclin D-CDK binding during the transition from G1 to S as P27 protein. The P27 together with P21e P57 are included in the Cip/Kip family; they influence the cyclin-CDK complexes formation during the progression from G1- to S-phase. In fact, the p27 and p21 are cyclin-dependent kinase inhibitors and the p27 expression was observed inversely related to the Ki-67 proliferation index [55].
Another gene involved in cell cycle progression is the p53 transcription factor. This tumor suppressor acts at the control point of the cell cycle, where, in response to specific cellular stress stimuli, p53 binds to a specific tract of DNA to induce the arrest in G1 or apoptosis [55]. Its involvement in VS is still unclear. Several authors have reported that its dysfunction is given by the loss of heterozygosity in the region of the first gene intron, others attribute it to a phosphorylation (resulting age-related), others have not found a correlation with tumor aggressiveness in terms of p53 expression [21,55]. A homologous gene of p53 is P73. It is very similar (60% homology) in terms of domain structure, conformation and functions. The P73 can be translated into two different isoforms, one full length and active and one truncated and inactive. The former acts as an onco-suppressor like P53, whereas the latter isoform has an oncogenic action. The latter, by blocking the transactivation of p53 and the active form of P73, hinders its apoptotic activity. On the other hand, both p53 and the active form of P73 induce expression of the truncated form of P73, producing a complex feedback loop [21]. In VS, the expression of P73 was demonstrated in 40% of the samples analyzed, but which isoform corresponded was not defined. In other epigenetic studies in schwannomas, an aberrant DNA methylation of various genes including P73 was reported, explaining the deregulation of tumor suppressors [21].

Table 3.
Studies examining the role of molecular regulators in sporadic vestibular schwannomas.
Table 3.
Studies examining the role of molecular regulators in sporadic vestibular schwannomas.
| Author | Year | Country | Study Design | Cases (n) | Mean Age ± SD (Range) | Sex (M/F) | Tumor Size, Mean ± SD (Range) | Method of Marker Detection | Marker Studied | Key Findings |
|---|---|---|---|---|---|---|---|---|---|---|
| Ahmad et al. [21] | 2009 | Spain | Ex vivo tissue study | 34 | 49.5 (25–72) | 19/15 | 17 mm (3–40) | IHC, cell culture WB, IF, Flow citometry | p73 |
|
| Breun et al. [24] | 2018 | Germany | Ex vivo tissue study | 30 | 51 (NR) | 12/18 | 10 small tumors (T3A or smaller) 20 large tumors (T3B of larger) | IHC WB RT-qPCR | CXCR4 CXCL12 |
|
| Jabbour et al. [35] | 2016 | Australia | Ex vivo tissue study | 180 | 54 ± 13.9 (14–81) | 96/84 | NR | IHC | cyclin D1 cyclin D3 Ki-67 |
|
| Lassaletta et al. [42] | 2011 | Spain | Ex vivo tissue study | 64 | 49 (16–78) | 27/37 | 23 mm (5–55) | IHC | Cyclin D1 |
|
| Martini et al. [47] | 2017 | Italy | Ex vivo tissue study | 36 | 51.8 ± 12.4 (NR) | 17/19 | 4 intra-meatal; 8 small-sized (<1 cm); 19 medium-sized (1–2.5 cm); 5 large-sized (>2.5 cm) | IHC ceMRI | YAP TAZ AREG |
|
| Mawrin et al. [49] | 2002 | Germany | Ex vivo tissue study | 14 | 58.36 ± 5.35 (52–73) | 7/7 | NR | IHC | Fas-Fas-L Bcl-2 Bax MIB-1 |
|
| Neff et al. [52] | 2006 | USA | Ex vivo tissue study | 15 | NR | NR | NR | IHC | Cyclin D1 Cyclin D3 |
|
| Seol et al. [55] | 2005 | Korea | Ex vivo tissue study | 12 | NR | NR | NR | IHC | p53 Bax Bcl-2 Fas Fas-L Caspase-3 p27 p21 |
|
ceMRI: contrast-enhanced magnetic resonance imaging; F: female; M: male; n: number of cases; NR: not reported; SD: standard deviation; VS: vestibular schwannoma.
The loss of Merlin function in the VS occurrence could activate other two main signaling pathways, Ras/Raf/MEK pathway and PI3K/Akt/mTor pathway, which, by inducing the inhibition of apoptosis, lead to increased cell survival and proliferation [24]. The tumorigenesis is linked to the activation of IP3/Akt and MAP signaling, downstream of the activation of Chemokine-4 receptor (CXCR4) [24]. In a recent study, it was reported that CXCR4 was similarly overexpressed in both sporadic and NF2-associated VS, with no significant association with the size or extent of the tumor. Interestingly, a positive correlation has been seen between its expression and the level of hearing impairment, from which the authors suggested an association with the increasing invasiveness of the tumor [24]. The CXCR4 is a G protein coupled receptor known for its activity during embryogenesis and used in various therapies, including cancer. Its only ligand is CXCL12; their binding leads to the activation of IP3/Akt and MAP signaling, phosphorylation ERK1/2, and calcium release, resulting in increased tumor invasiveness and proliferation [24].
Based on the above considerations, it appears clear that TME promotes its development as a consequence of the deregulation of cell proliferation and/or apoptosis. Intrinsic apoptosis is activated by two main pathways: the first involves activation of cell surface receptors (such as Fas/Fas-L), while the second involves release of mitochondrial cytochrome C into the cytosol. Available evidence has made it possible to exclude the first route. As for the second, a balance between the expression of the apoptosis inhibitor Bcl-2 and the pro-apoptotic factor Bax was detected [55]. It should be noted that Bcl-2 can exert its anti-apoptotic action in two ways, on the one hand interacting with Fas induces the lack of Fas expression, on the other forming heterodimers with Bax inhibits its pro-apoptotic effect [49,55]. It has been suggested that in VS a kind of balance exists between proliferation and cell death maintained by the pro- and anti-apoptotic factor expression and the related increase of the Ki-67 proliferation index [49,55].
The Merlin’s loss of function also influences other pathways, like the Hippo pathway and the VEGF-mediated signaling pathway. The first downstream effectors of the Hippo signaling pathway are Yes-associated protein (YAP) and its paralog transcriptional co-activator with PDZ-binding motif (TAZ) [47,76]. Translocation of TAZ and YAP from the cytoplasm to the nucleus leads to activation of various transcription factors including p73, T-box transcription 5, SMAD family proteins and several members of the TEA domain (TEAD) family. The TEAD family is known to be the most common in the regulation of gene expression related to cell proliferation and apoptosis [47,76,77]. Another target gene of YAP and TAZ is Amphiregulin (AREG), a member of the epidermal growth factor family that has been identified as a schwannoma-derived growth factor. In a recent study, a significant positive correlation between VS volume and the expression of TAZ, not of YAP or AREG, was found. For this reason, the authors assumed that TAZ likely promoted the transcription of other target genes [47]. By an analysis of gene expression profiling, it was demonstrated that the increased nuclear expression of YAP was positively correlated with the Ki-67 proliferation index in VS [77].
3.6. Growth Factors
In VS, the TME that then initiates progression of the tumor is given by the cascade of molecular death or cell proliferation pathways, which implement downstream of Merlin dysregulation. Regarding the growth and aggressiveness of cancer, deregulation of the expression of growth factors and their receptors plays a fundamental role. Intriguingly, a positive correlation between the proliferation index and the gene expression of different neurotrophic factor was detected in VS microenvironment [40,56]. The brain-derived neurotrophic factor (BDNF), co-receptor Ret and the transforming growth factor (TGF)-β1 were found overexpressed in VS, but only BDNF was positively correlated to the Ki-67 proliferation index [40]. The BDNF and NT-3 are involved in the myelination of axons and their correspondent receptors are p75NTR, tyrosine kinase (Trk)-B and Trk-C. It is not yet clear how BDNF and p75NTR and Trk-B pathways are involved in VS, but it was hypothesized that the binding of BDNF-p75NTR leads to myelination, which is reflected in the loss of axonal contact in benign schwannomas. In the absence of p75NTR, myelination is inhibited due to Trk-B expression, which blocks Trk-C signaling leading to suppression of NT-3 mediated myelination [40]. The TGF-β1 is activated by two receptors, the threonine/kinase transmembrane receptor 1(TGF-βR1) and TGF-βR2 [31,45,56]. The binding to the first receptor has a proliferative activity, on the contrary, the binding to the type 2 receptor leads to an anti-proliferative action [45,56]. It has been suggested that TGF-β1 may also be indirectly involved in tumor progression by promoting angiogenesis mediated by VEGF overexpression [56]. VEGF induces angiogenesis through the proliferation and migration of endothelial cells. This is activated by VEGFR-1 and VEGFR-2 receptors. Their binding leads to the extravasation of plasma proteins from the tumor vessels, which leads to the formation of a temporary extra-vascular matrix that results in the formation of new blood vessels due to migration and proliferation of endothelial cells [56]. Note that angiogenic activity of the VEGF pathway has also been shown to be related to loss of Merlin function [47,56,76]. Expression of TGF-β1 was also analyzed in synergy with the growth factor glial-cell-derived growth factor (GDNF) [31]. The two factors belong to the same superfamily of growth factors and are involved in the neurotrophic action. In particular, their co-expression has been reported in most of the VS samples analyzed, reinforcing the theory that they act in concert to stimulate neurotropicity in sympathetic neurons [31].
Among other growth factors involved in VS together with the loss of Merlin function, neuroregulin 1 and Platelet Derived Growth Factor (PDGF) were studied [22,78,79]. The binding of neuregulin 1 to its receptor ERBB2 induces the mitogenic action of Schwann cells followed by a cascade of phosphorylation involving the Pl3k and MAPK system [18,19,20], leading to the mitogenic effect. Regarding hearing loss following tumor development in patients with VS, it has been shown that while PDGF expression is positively related to age and hearing loss; it is not related to tumor size [22,33]. At the same time, the Fibroblast Growth Factor 2 (FGF2) appears to have a protective effect against hearing loss despite its expression being directly related to the size of the tumor [33]. Finally, the expression of ERBB2 having proliferative effects is not related to hearing loss [33,78,79]. In conclusion, all this information gathered about the VS microenvironment could help to obtain customized therapeutic protocols dosing inhibitory molecules with stimulants, allowing the tumor to be reduced and hearing preserved [33].

Table 4.
Studies examining the role of growth factors in sporadic vestibular schwannomas.
Table 4.
Studies examining the role of growth factors in sporadic vestibular schwannomas.
| Author | Year | Country | Study Design | Cases (n) | Mean Age ± SD (Range) | Sex (M/F) | Tumor Size, Mean ± SD (Range) | Method of Marker Detection | Marker Studied | Key Findings |
|---|---|---|---|---|---|---|---|---|---|---|
| Altuna et al. [22] | 2011 | Spain | Ex vivo tissue study | 34 | 49.5 (25–72) | 19/15 | 17 mm (3–40) | IHC, cell culture and WB, IF, Colony Formation Assay | PDGF-R c-Kit |
|
| Diensthuber et al. [31] | 2004 | Germany | Ex vivo tissue study | 22 | 55.3 ± 11.8 (27–77) | 13/9 | 17 ± 6.8 mm (7–30) | IHC | TGF-β1 Glial Cell Line-Derived Neurotrophic Factor Ki-67 TβR II, GFRα-1 and Ret |
|
| Dilwali et al. [33] | 2013 | USA | Ex vivo tissue study | 16 gh 19 ph | NR | NR | NR | Cytokine Array ELISA | FGF2 |
|
| Kramer et al. [40] | 2010 | Germany | Ex vivo tissue study | 18 | 54 (20–77) | NR | NR | RT-qPCR IHC | BDNF, GDNF TGF-β1/β2 Ki-67 |
|
| Löttrich et al. [45] | 2007 | Germany | Ex vivo tissue study | 40 | NR | NR | NR | IHC qRT-PCR | TGF-β R1 TGF-β R2 |
|
| Taurone et al. [56] | 2015 | Italy | Ex vivo tissue study | 10 | NR (45–69) | 6/4 | NR | IHC | TGF-β1 IL-1β IL-6 TNF-α ICAM-1 VEGF |
|
F: female; M: male; n: number of cases; NR: not reported; SD: standard deviation; VS: vestibular schwannoma.
3.7. Matrix Metalloproteinases
Matrix metalloproteinases (MMPs) are a family of more than 20 zinc endopeptidases that degrade various components of the basal membrane and extracellular matrix [80]. Metalloproteinases and Tissue Inhibitors MetalloProteinases (TIMPs) are secreted by normal cells in various tissues and have a crucial role in physiological activities [81]. An abnormal regulation and secretion of MMPs and TIMPs can lead to pathological conditions (including diabetes mellitus, altered wound healing, renal disorders, neurodegenerative diseases, carcinogenesis) by promoting cell adhesion, proliferation, migration, and apoptosis [82]. In the tumor microenvironment, MMPs have important functions in cell migration and invasion, by degrading basal membrane and extracellular matrix protein components. Moreover, MMPs play roles in other steps of tumor progression, including neo-angiogenesis, modification of signaling pathways, and regulation of the immune response.
Moller et al. [50] investigated MMP-2, MMP-9, and TIMP-1 expressions in non-cystic VSs and their possible correlations with clinic-pathological parameters. They found that all tumors expressed MMP-2, MMP-9, and TIMP-1. A significant correlation was described between the tumor concentration of MMP-9 and the absolute growth rate but not with preoperative tumor size.
Cystic VSs are tumors with a different behavior compared to the solid variant, regarding the growth rate and adhesion to surrounding structures and the facial nerve [51]. It has been hypothesized that MMPs 2 and 14 might play a relevant role in the pathogenesis of cyst formation and peritumoral adhesion. In fact, MMP2 can be found ubiquitously in cyst fluid, and it is localized in the inner luminal surface cells adjacent to the cyst cavity [51]. This suggested that cyst expansion and enlargement could be related to proteolytic activity of MMP-2. Similarly, a higher expression of MMP-14 has been found in the Antoni B compared to the Antoni A area [57].
ADAMs (A disintegrin and metalloproteinase) are a family of trans-membrane and secreted metallo-endopeptidases [83]. One member of this family is ADAM9, which comes into play in cell adhesion and cell signaling and is overexpressed in several tumors [84,85,86]. Breun et al. [25] found that ADAM9 was overexpressed by Schwann cells of VS and its expression was higher in sporadic VSs compared to NF2-associated ones. Moreover, ADAM9 levels significantly correlated with hearing loss.
The cause of sensorineural hearing loss in patients with VS is mechanical compression of the cochlear nerve, as well as the presence of tumor-secreted factors, which contain pro-inflammatory cytokines that can directly cause cochlear damage [87]. Sagers et al. [54] demonstrated this latter mechanism by studying NLRP3 inflammasome expression in a group of VSs. NLRP3 inflammasome is a multi-protein complex made up of apoptosis-associated Speck-like protein with a caspase-recruitment domain, pro-caspase-1, and cryopyrin [88]. In the Sagers et al. [54] investigation, overexpression of multiple key genes associated with the NLRP3 inflammasome was observed in VS compared with control nerves; in addition, two associated proteins (NLRP3, IL-1β) were present in patients with worse hearing loss. These observations confirmed the hypothesis that an important inflammatory response in VS tissue might be associated with cochlear damage and poor hearing levels.

Table 5.
Studies examining the role of local inflammation proteins in sporadic vestibular schwannomas.
Table 5.
Studies examining the role of local inflammation proteins in sporadic vestibular schwannomas.
| Author | Year | Country | Study Design | Cases (n) | Mean Age ± SD (Range) | Sex (M/F) | Tumor Size, Mean ± SD (Range) | Method of Marker Detection | Marker Studied | Key Findings |
|---|---|---|---|---|---|---|---|---|---|---|
| Breun et al. [25] | 2020 | Germany | Ex vivo tissue study | 30 | 51 (NR) | 12/18 | 10 small tumors (T3A or smaller) 20 large tumors (T3B of larger) | IHC WB RT-qPCR | ADAM9 |
|
| Møller et al. [50] | 2010 | Denmark | Ex vivo tissue study | 34 | 53.61 ± 12.06 (25–75) | 14/20 | 1.686 ± 1.247 cm3 (0.11–4.716) | IHC ELISA | MMP-2 MMP-9 TIMP-1 |
|
| Moon et al. [51] | 2007 | South Korea | Ex vivo tissue study | 24 | 40.5 (16–71) | 12/12 | 43.8 mm (22–60) | Gelatin zymography IHC | MMP-2 |
|
| Sagers et al. [54] | 2019 | USA | Ex vivo tissue study | 80 | NR | NR | NR | IHC qRT-PCR | NLRP3 (CASP1, PYCARD, IL-18, NLRP3, NAIP, NLRC4, AIM2, IL-1β) |
|
F: female; M: male; n: number of cases; NR: not reported; SD: standard deviation; VS: vestibular schwannoma.
3.8. Hormone Receptors
TME is a dynamic network of stromal and immune cells that, under the effect of tumor cells, control biochemical and mechanical signaling via extracellular matrix production and remodeling, formation of vascular networks, and promotion of tumor growth. In other well-known tumors (e.g., breast cancer), hormone receptor-mediated signaling is a key controller of cancer cell proliferation and invasiveness, which occurs both through cell-autonomous means and via cancer cell–stroma crosstalk. In the absence of hormone receptors, a different microenvironment landscape emerges with its own challenges for therapy [89].
Estrogen receptors (ER) may be detected in intracranial tumors as well as VSs [90,91]. VSs are more frequent in females, and an increased growth during pregnancy has been described [92]. In 1981, Kasantikul and Brown [90] first demonstrated estradiol receptors in 8 VSs. Many diverging studies have been published on the contents of sex hormone receptors in VSs. They assumed estrogen and progesterone association with proliferation of VSs [37,90,93,94,95,96]. These studies tried to ascertain a role of sex hormones in considering endocrinological therapy, especially in recurrent and residual cases where complete excision was not feasible. However, the clinical significance of estrogen and progesterone receptors (PR) in VS is still controversial. As hypothesized by Jailswal et al. [36], these discrepancies were probably due to the divergent methodologies that have been used by various research groups, ranging from immunohistochemical methods to molecular techniques. Kasantikul and Brown [90] stated that estrogens might promote tumor growth by inducing vascular endothelium proliferation with a resultant increase in tumor vascularization. Monsell and Wiet [93] investigated 37 cases of VS for estrogen and PR by radioimmunoassay. They found no correlation between ER positivity and the sex of the patient. In a consecutive series of 18 VSs, Klinken et al. [37] found neither estrogen nor PR in clinically relevant quantities.
In 59 cases of VS, Cafer et al. [27] investigated the presence of Ki-67, estrogen and progesterone hormone receptors as well as their clinical correlates. All samples were positive for PR and negative for ER staining. The authors concluded that estrogen is not relevant in VS due to its absence in the tissue samples. Regarding progesterone, since its receptor was expressed in all samples, further studies are needed to evaluate the potential inhibitory effect of anti-progesterone treatment on growth. Labit-Bouvier et al. [41] analyzed 69 cases of VSs and found that 5 were focally positive for PR and none for ER. Curley et al. [96] found no evidence to support the clinical hypothesis that VS might be a hormone-dependent tumor. Dalgorf et al. [97] studied 9 female patients with VS for expression of estrogen, progesterone and VEGF. Their result for estrogen and PR was negative in all cases, while VEGF was positive in 8 out of 9 cases. Jailswal et al. [36] did not find estrogen and PR positivity in any of their 100 VS cases, showing no evidence to support the clinical hypothesis that VS might be a hormone-dependent tumor.
Regarding other hormones, there is a dearth of knowledge about the roles of erythropoietin (EPO) or erythropoietin-receptor (EPO-R) in VS. EPO and EPO-R seem to play important roles in brain’s development and homeostasis [98]. Dillard et al. [32] found that 13 of 14 VS cases showed positive staining for EPO, 9 positive staining for EPO-R. Tumors with high EPO-R levels tended to be larger than those with low or no staining.

Table 6.
Studies examining the role of hormone receptors in sporadic vestibular schwannomas.
Table 6.
Studies examining the role of hormone receptors in sporadic vestibular schwannomas.
| Author | Year | Country | Study Design | Cases (n) | Mean Age ± SD (Range) | Sex (M/F) | Tumor Size, Mean ± SD (Range) | Method of Marker Detection | Marker Studied | Key Findings |
|---|---|---|---|---|---|---|---|---|---|---|
| Cafer et al. [27] | 2008 | Turkey | Ex vivo tissue study | 59 | 46.8 (14–75) | 27/32 | 21 small size tumors (<19 mm) 35 medium size tumors (20–39 mm) 3 large size tumors (>40 mm) | IHC | Ki-67 ER PR |
|
| Dillard et al. [32] | 2001 | USA | Ex vivo tissue study | 14 | NR | NR | 2.41 ± 1.53 cm (1–6) | IHC | EPO EPO-R |
|
| Jaiswal et al. [36] | 2016 | India | Ex vivo tissue study | 100 | 37.5 (12–77) | 63/37 | NR | IHC | ER PR |
|
| Klinken et al. [37] | 1990 | Denmark | Ex vivo tissue study | 18 | 52 (26–73) | 7/11 | NR | IHC | ER PR |
|
ER: estrogen receptor; F: female; M: male; n: number of cases; NR: not reported; PR: progesterone receptor; SD: standard deviation; VS: vestibular schwannoma. They concluded that EPO and EPO-R might play a growth-promoting role in the pathogenesis of VSs.
The possibility that VS could be hormone dependent is worthy of investigation because of the success of hormonal manipulation treatments in other areas (e.g., breast and prostatic malignancies). Although some studies identified progesterone and EPO levels in VS, the presence of these hormones in the TME of VS is still controversial.
4. Conclusions
The sporadic VS microenvironment is determined by the interplay of several cellular and molecular factors at different levels. A dynamic network of stromal and immune cells interplays under the influence of tumor cells, producing and remodeling extracellular matrix and vascular networks and promoting tumor growth. However, results are sometimes conflicting, which sheds light on how far we are from understanding the complex interactions in the VS microenvironment. Mostly due to the rarity of VS but also to the high costs of molecular-based research, the literature on this topic is based on retrospective ex vivo studies with limited sample size.
Future investigations into the role of TME in VS will further enhance our understanding of the tumor biology, investigating biomarkers predicting tumor growth and recognizing immunological and molecular factors that could be potential therapeutic targets for medical treatment.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms24076522/s1.
Author Contributions
Conceptualization, D.C., G.M., E.Z. and A.D.; methodology, D.C. and A.D.; data curation, D.C. and A.D.; writing—original draft preparation, D.C., L.A., A.D., G.T., E.S., E.Z. and G.M.; writing—review and editing, D.C., L.A., A.D., G.T., E.S., A.M., E.Z. and G.M.; supervision, G.M., E.Z. and A.M. All authors have read and agreed to the published version of the manuscript.
Funding
This study was partly supported by grant No. DOR2215418/22 (G. Marioni) from the University of Padova, Italy.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets generated and analyzed during the current study are available on reasonable request.
Acknowledgments
The authors thank Alison Garside for correcting the English version of this paper and Rosalinda Russo for the literature search.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Roosli, C.; Linthicum, F.H.; Cureoglu, S.; Merchant, S.N. What is the site of origin of cochleovestibular schwannomas? Audiol. Neurootol. 2012, 17, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Carlson, M.L.; Link, M.J. Vestibular schwannomas. N. Engl. J. Med. 2021, 384, 1335–1348. [Google Scholar] [CrossRef] [PubMed]
- Reznitsky, M.; Petersen, M.M.B.S.; West, N.; Stangerup, S.E.; Cayé-Thomasen, P. Epidemiology of vestibular schwannomas—Prospective 40-year data from an unselected national cohort. Clin. Epidemiol. 2019, 11, 981–986. [Google Scholar] [CrossRef]
- Zanoletti, E.; Cazzador, D.; Faccioli, C.; Gallo, S.; Denaro, L.; D’Avella, D.; Martini, A.; Mazzoni, A. Multi-option therapy vs observation for small acoustic neuroma: Hearing-focused management. Acta Otorhinol. Ital. 2018, 38, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Zanoletti, E.; Concheri, S.; Tealdo, G.; Cazzador, D.; Denaro, L.; d’Avella, D.; Mazzoni, A. Early surgery and definitive cure in small sporadic vestibular schwannoma. Acta Otorhinol. Ital. 2022, 42, 481–486. [Google Scholar] [CrossRef]
- Zanoletti, E.; Mazzoni, A.; Chiumenti, F.A.; d’Avella, D.; Cazzador, D. Early Translabyrinthine surgery for small- and medium-sized vestibular schwannomas: Consecutive cohort analysis of outcomes. Otol. Neurotol. 2022, 43, 962–967. [Google Scholar] [CrossRef]
- Schwartz, M.S.; Lekovic, G.P.; Miller, M.E.; Slattery, W.H.; Wilkinson, E.P. Translabyrinthine microsurgical resection of small vestibular schwannomas. J. Neurosurg. 2018, 129, 128–136. [Google Scholar] [CrossRef]
- Carlson, M.L.; Jacob, J.T.; Pollock, B.E.; Neff, B.A.; Tombers, N.M.; Driscoll, C.L.; Link, M.J. Long-term hearing outcomes following stereotactic radiosurgery for vestibular schwannoma: Patterns of hearing loss and variables influencing audiometric decline. J. Neurosurg. 2013, 118, 579–587. [Google Scholar] [CrossRef]
- Lee, D.Y.; Lerner, D.K.; Naples, J.G.; Brant, J.A.; Bigelow, D.C.; Lee, J.Y.K.; Alonso-Basanta, M.; Ruckenstein, M.J. Vestibular schwannoma tumor size is associated with acute vestibular symptoms after gamma knife therapy. Otol. Neurotol. 2019, 40, 1088–1093. [Google Scholar] [CrossRef]
- Lerner, D.K.; Lee, D.; Naples, J.G.; Brant, J.A.; Bigelow, D.; Alonso-Basanta, M.; Ruckenstein, M.J. Factors associated with facial nerve paresis following gamma knife for vestibular schwannoma. Otol. Neurotol. 2020, 41, e83–e88. [Google Scholar] [CrossRef]
- Lu, V.M.; Ravindran, K.; Graffeo, C.S.; Perry, A.; Van Gompel, J.J.; Daniels, D.J.; Link, M.J. Efficacy and safety of bevacizumab for vestibular schwannoma in neurofibromatosis type 2: A systematic review and meta-analysis of treatment outcomes. J. Neurooncol. 2019, 144, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Goutagny, S.; Kalamarides, M. Medical treatment in neurofibromatosis type 2. Review of the literature and presentation of clinical reports. Neurochirurgie 2018, 64, 370–374. [Google Scholar] [CrossRef] [PubMed]
- Gugel, I.; Kluwe, L.; Zipfel, J.; Teuber, C.; Tatagiba, M.; Mautner, V.F.; Schuhmann, M.U.; Grimm, F. Minimal effect of bevacizumab treatment on residual vestibular schwannomas after partial resection in young neurofibromatosis type 2 patients. Cancers 2019, 11, 1862. [Google Scholar] [CrossRef]
- Fridman, W.H.; Dieu-Nosjean, M.C.; Pagès, F.; Cremer, I.; Damotte, D.; Sautès-Fridman, C.; Galon, J. The immune microenvironment of human tumors: General significance and clinical impact. Cancer Microenviron. 2013, 6, 117–122. [Google Scholar] [CrossRef]
- Hanahan, D.; Coussens, L.M. Accessories to the crime: Functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012, 21, 309–322. [Google Scholar] [CrossRef]
- Kandathil, C.K.; Dilwali, S.; Wu, C.C.; Ibrahimov, M.; McKenna, M.J.; Lee, H.; Stankovic, K.M. Aspirin intake correlates with halted growth of sporadic vestibular schwannoma in vivo. Otol. Neurotol. 2014, 35, 353–357. [Google Scholar] [CrossRef] [PubMed]
- MacKeith, S.; Wasson, J.; Baker, C.; Guilfoyle, M.; John, D.; Donnelly, N.; Mannion, R.; Jefferies, S.; Axon, P.; Tysome, J.R. Aspirin does not prevent growth of vestibular schwannomas: A case-control study. Laryngoscope 2018, 128, 2139–2144. [Google Scholar] [CrossRef] [PubMed]
- Dilwali, S.; Kao, S.Y.; Fujita, T.; Landegger, L.D.; Stankovic, K.M. Nonsteroidal anti-inflammatory medications are cytostatic against human vestibular schwannomas. Transl. Res. 2015, 166, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. PLoS Med. 2021, 18, e1003583. [Google Scholar] [CrossRef]
- NHLBI NIH. Study Quality Assessment Tools. Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed on 15 November 2022).
- Ahmad, Z.K.; Altuna, X.; Lopez, J.P.; An, Y.; Wang-Rodriguez, J.; Juneja, V.R.; Chen, J.S.; Arandazi, M.J.; Aguilera, J.; Harris, J.P.; et al. p73 expression and function in vestibular schwannoma. Arch. Otolaryngol. Head Neck Surg. 2009, 135, 662–669. [Google Scholar] [CrossRef]
- Altuna, X.; Lopez, J.P.; Yu, M.A.; Arandazi, M.J.; Harris, J.P.; Wang-Rodriguez, J.; An, Y.; Dobrow, R.; Doherty, J.K.; Ongkeko, W.M. Potential role of imatinib mesylate (Gleevec, STI-571) in the treatment of vestibular schwannoma. Otol. Neurotol. 2011, 32, 163–170. [Google Scholar] [CrossRef]
- Amit, M.; Xie, T.; Gleber-Netto, F.O.; Hunt, P.J.; Mehta, G.U.; Bell, D.; Silverman, D.A.; Yaman, I.; Ye, Y.; Burks, J.K.; et al. Distinct immune signature predicts progression of vestibular schwannoma and unveils a possible viral etiology. J. Exp. Clin. Cancer Res. 2022, 41, 292. [Google Scholar] [CrossRef] [PubMed]
- Breun, M.; Schwerdtfeger, A.; Martellotta, D.D.; Kessler, A.F.; Perez, J.M.; Monoranu, C.M.; Ernestus, R.I.; Matthies, C.; Löhr, M.; Hagemann, C. CXCR4: A new player in vestibular schwannoma pathogenesis. Oncotarget 2018, 9, 9940–9950. [Google Scholar] [CrossRef] [PubMed]
- Breun, M.; Schwerdtfeger, A.; Martellotta, D.D.; Kessler, A.F.; Monoranu, C.M.; Matthies, C.; Löhr, M.; Hagemann, C. ADAM9: A novel player in vestibular schwannoma pathogenesis. Oncol. Lett. 2020, 19, 1856–1864. [Google Scholar] [CrossRef] [PubMed]
- Brieger, J.; Bedavanija, A.; Lehr, H.; Maurer, J.; Mann, W. Expression of angiogenic growth factors in acoustic neurinoma. Acta Oto-Laryngol. 2003, 123, 1040–1045. [Google Scholar] [CrossRef]
- Cafer, S.; Bayramoglu, I.; Uzum, N.; Memis, L.; Uygur, K. Expression and clinical significance of Ki-67, oestrogen and progesterone receptors in acoustic neuroma. J. Laryngol. Otol. 2008, 122, 125–127. [Google Scholar] [CrossRef]
- Cayé-Thomasen, P.; Werther, K.; Nalla, A.; Bøg-Hansen, T.C.; Nielsen, H.J.; Stangerup, S.E.; Thomsen, J. VEGF and VEGF receptor-1 concentration in vestibular schwannoma homogenates correlates to tumor growth rate. Otol. Neurotol. 2005, 26, 98–101. [Google Scholar] [CrossRef]
- De Vries, W.M.; Hogendoorn, P.C.W.; Briaire-de Bruyn, I.H.; Malessy, M.J.A.; van der Mey, A.G.L. Intratumoral hemorrhage, vessel density, and the inflammatory reaction contribute to volume increase of sporadic vestibular schwannomas. Virchows Arch. 2012, 460, 629–636. [Google Scholar] [CrossRef]
- De Vries, W.M.; Briaire-de Bruijn, I.H.; van Benthem, P.P.G.; van der Mey, A.G.L.; Hogendoorn, P.C.W. M-CSF and IL-34 expression as indicators for growth in sporadic vestibular schwannoma. Virchows Arch. 2019, 474, 375–381. [Google Scholar] [CrossRef]
- Diensthuber, M.; Brandis, A.; Lenarz, T.; Stöver, T. Co-expression of transforming growth factor-beta1 and glial cell line-derived neurotrophic factor in vestibular schwannoma. Otol. Neurotol. 2004, 25, 359–365. [Google Scholar] [CrossRef]
- Dillard, D.G.; Venkatraman, G.; Cohen, C.; Delgaudio, J.; Gal, A.A.; Mattox, D.E. Immunolocalization of erythropoietin and erythropoietin receptor in vestibular schwannoma. Acta Otolaryngol. 2001, 121, 149–152. [Google Scholar] [CrossRef]
- Dilwali, S.; Lysaght, A.; Roberts, D.; Barker, F.G.; McKenna, M.J.; Stankovic, K.M. Sporadic vestibular schwannomas associated with good hearing secrete higher levels of fibroblast growth factor 2 than those associated with poor hearing irrespective of tumor size. Otol. Neurotol. 2013, 34, 748–754. [Google Scholar] [CrossRef]
- Gonçalves, V.M.; Suhm, E.M.; Ries, V.; Skardelly, M.; Tabatabai, G.; Tatagiba, M.; Schittenhelm, J.; Behling, F. Macrophage and lymphocyte infiltration is associated with volumetric tumor size but not with volumetric growth in the Tübingen schwannoma cohort. Cancers 2021, 13, 466. [Google Scholar] [CrossRef] [PubMed]
- Jabbour, J.; Earls, P.; Biggs, N.; Gracie, G.; Fagan, P.; Bova, R. Role of cyclins D1 and D3 in vestibular schwannoma. J. Laryngol. Otol. 2016, 130, S2–S10. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, S.; Agrawal, V.; Jaiswal, A.K.; Pandey, R.; Mahapatra, A.K. Expression of estrogen and progesterone receptors in vestibular schwannomas and their clinical significance. J. Negat. Res. Biomed. 2009, 8, 9. [Google Scholar] [CrossRef]
- Klinken, L.; Thomsen, J.; Rasmussen, B.B.; Wiet, R.J.; Tos, M. Estrogen and progesterone receptors in acoustic neuromas. Arch. Otolaryngol. Head Neck Surg. 1990, 116, 202–204. [Google Scholar] [CrossRef]
- Koutsimpelas, D.; Stripf, T.; Heinrich, U.R.; Mann, W.J.; Brieger, J. Expression of vascular endothelial growth factor and basic fibroblast growth factor in sporadic vestibular schwannomas correlates to growth characteristics. Otol. Neurotol. 2007, 28, 1094–1099. [Google Scholar] [CrossRef] [PubMed]
- Koutsimpelas, D.; Bjelopavlovic, M.; Yetis, R.; Frauenknecht, K.; Adryan, B.; Schmidtmann, I.; Gouveris, H.; Fruth, K.; Heinrich, U.R.; Stauber, R.H.; et al. The VEGF/VEGF-R axis in sporadic vestibular schwannomas correlates with irradiation and disease recurrence. ORL J. Otorhinolaryngol. Relat. Spec. 2012, 74, 330–338. [Google Scholar] [CrossRef]
- Kramer, F.; Stöver, T.; Warnecke, A.; Diensthuber, M.; Lenarz, T.; Wissel, K. BDNF mRNA expression is significantly upregulated in vestibular schwannomas and correlates with proliferative activity. J. Neurooncol. 2010, 98, 31–39. [Google Scholar] [CrossRef]
- Labit-Bouvier, C.; Crebassa, B.; Bouvier, C.; Andrac-Meyer, L.; Magnan, J.; Charpin, C. Clinicopathologic growth factors in vestibular schwannomas: A morphologic and immunohistochemical study of 69 tumors. Acta Otolaryngol. 2000, 120, 950–954. [Google Scholar] [CrossRef]
- Lassaletta, L.; Del Rio, L.; Torres-Martin, M.; Rey, J.A.; Patrón, M.; Madero, R.; Roda, J.M.; Gavilan, J. Cyclin D1 expression and facial function outcome after vestibular schwannoma surgery. Otol Neurotol. 2011, 32, 136–140. [Google Scholar] [CrossRef]
- Leisz, S.; Klause, C.H.; Vital Dos Santos, T.; Haenel, P.; Scheer, M.; Simmermacher, S.; Mawrin, C.; Strauss, C.; Scheller, C.; Rampp, S. Vestibular schwannoma volume and tumor growth correlates with macrophage marker expression. Cancers 2022, 14, 4429. [Google Scholar] [CrossRef] [PubMed]
- Lewis, D.; Donofrio, C.A.; O’Leary, C.; Li, K.L.; Zhu, X.; Williams, R.; Djoukhadar, I.; Agushi, E.; Hannan, C.J.; Stapleton, E.; et al. The microenvironment in sporadic and neurofibromatosis type II-related vestibular schwannoma: The same tumor or different? A comparative imaging and neuropathology study. J. Neurosurg. 2020, 134, 1419–1429. [Google Scholar] [CrossRef] [PubMed]
- Löttrich, M.; Mawrin, C.; Chamaon, K.; Kirches, E.; Dietzmann, K.; Freigang, B. Expression of transforming growth factor-beta receptor type 1 and type 2 in human sporadic vestibular Schwannoma. Pathol. Res. Pract. 2007, 203, 245–249. [Google Scholar] [CrossRef] [PubMed]
- Marioni, G.; Blandamura, S.; Nicolè, L.; Denaro, L.; Cazzador, D.; Pavone, C.; Giacomelli, L.; Guzzardo, V.; Fassina, A.; Mazzoni, A.; et al. Endoglin-based assessment of neoangiogenesis in sporadic VIII cranial nerve schwannoma. Pathol. Res. Pract. 2019, 215, 152648. [Google Scholar] [CrossRef]
- Martini, A.; Marioni, G.; Zanoletti, E.; Cappellesso, R.; Stramare, R.; Fasanaro, E.; Faccioli, C.; Giacomelli, L.; Denaro, L.; D’Avella, D.; et al. YAP, TAZ and AREG expression in eighth cranial nerve schwannoma. Int. J. Biol. Markers 2017, 32, e319–e324. [Google Scholar] [CrossRef] [PubMed]
- Matsunaga, T.; Kanzaki, J.; Hosoda, Y. Angiogenesis from the eighth cranial nerve to vestibular schwannomas. Acta Otolaryngol. 1996, 116, 52–58. [Google Scholar] [CrossRef]
- Mawrin, C.; Kirches, E.; Dietzmann, K.; Roessner, A.; Boltze, C. Expression pattern of apoptotic markers in vestibular schwannomas. Pathol. Res. Pract. 2002, 198, 813–819. [Google Scholar] [CrossRef]
- Møller, M.N.; Werther, K.; Nalla, A.; Stangerup, S.E.; Thomsen, J.; Bøg-Hansen, T.C.; Nielsen, H.J.; Cayé-Thomasen, P. Angiogenesis in vestibular schwannomas: Expression of extracellular matrix factors MMP-2, MMP-9, and TIMP-1. Laryngoscope 2010, 120, 657–662. [Google Scholar] [CrossRef] [PubMed]
- Moon, K.S.; Jung, S.; Seo, S.K.; Jung, T.Y.; Kim, I.Y.; Ryu, H.H.; Jin, Y.H.; Jin, S.G.; Jeong, Y.I.; Kim, K.K.; et al. Cystic vestibular schwannomas: A possible role of matrix metalloproteinase-2 in cyst development and unfavorable surgical outcome. J. Neurosurg. 2007, 106, 866–871. [Google Scholar] [CrossRef]
- Neff, B.A.; Oberstien, E.; Lorenz, M.; Chaudhury, A.R.; Welling, D.B.; Chang, L.S. Cyclin D(1) and D(3) expression in vestibular schwannomas. Laryngoscope 2006, 116, 423–426. [Google Scholar] [CrossRef] [PubMed]
- Perry, A.; Graffeo, C.S.; Carlstrom, L.P.; Raghunathan, A.; Driscoll, C.L.W.; Neff, B.A.; Carlson, M.L.; Parney, I.F.; Link, M.J.; Van Gompel, J.J. Predominance of M1 subtype among tumor-associated macrophages in phenotypically aggressive sporadic vestibular schwannoma. J. Neurosurg. 2019, 133, 1637–1645. [Google Scholar] [CrossRef] [PubMed]
- Sagers, J.E.; Sahin, M.I.; Moon, I.; Ahmed, S.G.; Stemmer-Rachamimov, A.; Brenner, G.J.; Stankovic, K.M. NLRP3 inflammasome activation in human vestibular schwannoma: Implications for tumor-induced hearing loss. Hear. Res. 2019, 381, 107770. [Google Scholar] [CrossRef] [PubMed]
- Seol, H.J.; Jung, H.W.; Park, S.H.; Hwang, S.K.; Kim, D.G.; Paek, S.H.; Chung, Y.S.; Sub Lee, C. Aggressive vestibular schwannomas showing postoperative rapid growth—Their association with decreased p27 expression. J. Neurooncol. 2005, 75, 203–207. [Google Scholar] [CrossRef]
- Taurone, S.; Bianchi, E.; Attanasio, G.; di Gioia, C.; Ierinó, R.; Carubbi, C.; Galli, D.; Pastore, F.S.; Giangaspero, F.; Filipo, R.; et al. Immunohistochemical profile of cytokines and growth factors expressed in vestibular schwannoma and in normal vestibular nerve tissue. Mol. Med. Rep. 2015, 12, 737–745. [Google Scholar] [CrossRef]
- Xia, L.; Yang, S.; Wang, C.; Yu, E.; Zhang, H.; Zhang, Y.; Ruan, L.; Shi, L.; Ni, J.; Luo, J.; et al. Immunohistochemical profiles of matrix metalloproteinases and vascular endothelial growth factor overexpression in the Antoni B area of vestibular schwannomas. World Neurosurg. 2020, 144, e72–e79. [Google Scholar] [CrossRef]
- Dzobo, K.; Senthebane, D.A.; Dandara, C. The tumor microenvironment in tumorigenesis and therapy resistance revisited. Cancers 2023, 15, 376. [Google Scholar] [CrossRef]
- Alessandrini, L.; Ferrari, M.; Taboni, S.; Sbaraglia, M.; Franz, L.; Saccardo, T.; del Forno, B.M.; Agugiaro, F.; Frigo, A.C.; Dei Tos, A.P.; et al. Tumor-stroma ratio, neoangiogenesis and prognosis in laryngeal carcinoma. A pilot study on preoperative biopsies and matched surgical specimens. Oral Oncol. 2022, 132, 105982. [Google Scholar] [CrossRef]
- Folkman, J. Tumor angiogenesis: Therapeutic implications. N. Engl. J. Med. 1971, 285, 1182–1186. [Google Scholar] [CrossRef]
- Folkman, J.; Merler, E.; Abernathy, C.; Williams, G. Isolation of a tumor factor responsible for angiogenesis. J. Exp. Med. 1971, 133, 275–288. [Google Scholar] [CrossRef]
- Marioni, G.; Marino, F.; Blandamura, S.; D’Alessandro, E.; Giacomelli, L.; Guzzardo, V.; Lionello, M.; De Filippis, C.; Staffieri, A. Neoangiogenesis in laryngeal carcinoma: Angiogenin and CD105 expression is related to carcinoma recurrence rate and disease-free survival. Histopathology 2010, 57, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Folkman, J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell 1996, 9, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Pietsch, T.; Valter, M.M.; Wolf, H.K.; von Deimling, A.; Huang, H.J.; Cavenee, W.K.; Wiestler, O.D. Expression and distribution of vascular endothelial growth factor protein in human brain tumors. Acta Neuropathol. 1997, 93, 109–117. [Google Scholar] [CrossRef]
- Nishikawa, R.; Cheng, S.Y.; Nagashima, R.; Huang, H.J.; Cavenee, W.K.; Matsutani, M. Expression of vascular endothelial growth factor in human brain tumors. Acta Neuropathol. 1998, 96, 453–462. [Google Scholar] [CrossRef]
- Schratzberger, P.; Schratzberger, G.; Silver, M.; Curry, C.; Kearney, M.; Magner, M.; Alroy, J.; Adelman, L.S.; Weinberg, D.H.; Ropper, A.H.; et al. Favorable effect of VEGF gene transfer on ischemic peripheral neuropathy. Nat. Med. 2000, 6, 405–413. [Google Scholar] [CrossRef]
- Charabi, S. Acoustic neuroma/vestibular schwannoma in vivo and in vitro growth models. A clinical and experimental study. Acta Otolaryngol. 1997, 530, 1–27. [Google Scholar]
- Saito, K.; Kato, M.; Susaki, N.; Nagatani, T.; Nagasaka, T.; Yoshida, J. Expression of Ki-67 antigen and vascular endothelial growth factor in sporadic and neurofibromatosis type 2-associated schwannomas. Clin. Neuropathol. 2003, 22, 30–34. [Google Scholar] [PubMed]
- Caye-Thomasen, P.; Baandrup, L.; Jacobsen, G.K.; Thomsen, J.; Stangerup, S.E. Immunohistochemical demonstration of VEGF in vestibular schwannomas correlates to tumor growth rate. Laryngoscope 2003, 113, 2129–2134. [Google Scholar] [CrossRef] [PubMed]
- Marioni, G.; Gaio, E.; Giacomelli, L.; Marchese-Ragona, R.; Staffieri, C.; Staffieri, A.; Marino, F. Endoglin (CD105) expression in head and neck basaloid squamous cell carcinoma. Acta Otolaryngol. 2005, 125, 307–311. [Google Scholar] [CrossRef] [PubMed]
- Archibald, D.J.; Neff, B.A.; Voss, S.G.; Splinter, P.L.; Driscoll, C.L.; Link, M.J.; Dong, H.; Kwon, E.D. B7-H1 expression in vestibular schwannomas. Otol. Neurotol. 2010, 31, 991–997. [Google Scholar] [CrossRef]
- Solinas, G.; Germano, G.; Mantovani, A.; Allavena, P. Tumor-associated macrophages (TAM) as major players of the cancer-related inflammation. J. Leuk. Biol. 2009, 86, 1065–1073. [Google Scholar] [CrossRef] [PubMed]
- Lahmar, Q.; Keirsse, J.; Laoui, D.; Movahedi, K.; van Overmeire, E.; van Ginderachter, J.A. Tissue-resident versus monocyte-derived macrophages in the tumor microenvironment. Biochim. Biophys. Acta. 2016, 1865, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Kissil, J.L.; Johnson, K.C.; Eckman, M.S.; Jacks, T. Merlin phosphorylation by p21-activated kinase 2 and effects of phosphorylation on merlin localization. J. Biol. Chem. 2002, 277, 10394–10399. [Google Scholar] [CrossRef] [PubMed]
- Okada, T.; Lopez-Lago, M.; Giancotti, F.G. Merlin/NF-2 mediates contact inhibition of growth by suppressing recruitment of Rac to the plasma membrane. J. Cell Biol. 2005, 171, 361–371. [Google Scholar] [CrossRef]
- Meng, Z.; Moroishi, T.; Guan, K.L. Mechanisms of Hippo pathway regulation. Genes Dev. 2016, 30, 1–17. [Google Scholar] [CrossRef]
- Zhao, F.; Yang, Z.; Chen, Y.; Zhou, Q.; Zhang, J.; Liu, J.; Wang, B.; He, Q.; Zhang, L.; Yu, Y.; et al. Deregulation of the Hippo pathway promotes tumor cell proliferation through YAP activity in human sporadic vestibular schwannoma. World Neurosurg. 2018, 117, e269–e279. [Google Scholar] [CrossRef]
- Hilton, D.A.; Hanemann, C.O. Schwannomas and their pathogenesis. Brain Pathol. 2014, 24, 205–220. [Google Scholar] [CrossRef]
- Boerboom, A.; Dion, V.; Chariot, A.; Franzen, R. Molecular mechanisms involved in schwann cell plasticity. Front. Mol. Neurosci. 2017, 10, 38. [Google Scholar] [CrossRef]
- Rundhaug, J.E. Matrix metalloproteinases and angiogenesis. J. Cell Mol. Med. 2005, 9, 267–285. [Google Scholar] [CrossRef]
- Cabral-Pacheco, G.A.; Garza-Veloz, I.; La Rosa, C.C.-D.; Ramirez-Acuña, J.M.; Perez-Romero, B.A.; Guerrero-Rodriguez, J.F.; Martinez-Avila, N.; Martinez-Fierro, M.L. The roles of matrix metalloproteinases and their inhibitors in human diseases. Int. J. Mol. Sci. 2020, 21, 9739. [Google Scholar] [CrossRef]
- Nagase, H.; Visse, R.; Murphy, G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc. Res. 2006, 69, 562–573. [Google Scholar] [CrossRef]
- Weskamp, G.; Krätzschmar, J.; Reid, M.S.; Blobel, C.P. MDC9, a widely expressed cellular disintegrin containing cytoplasmic SH3 ligand domains. J. Cell Biol. 1996, 132, 717–726. [Google Scholar] [CrossRef]
- Mazzocca, A.; Coppari, R.; de Franco, R.; Cho, J.Y.; Libermann, T.A.; Pinzani, M.; Toker, A. A secreted form of ADAM9 promotes carcinoma invasion through tumor-stromal interactions. Cancer Res. 2005, 65, 4728–4738. [Google Scholar] [CrossRef]
- Fan, X.; Wang, Y.; Zhang, C.; Liu, L.; Yang, S.; Wang, Y.; Lu, X.; Qian, Z.; Fang, S.; Qiao, H.; et al. ADAM9 expression is associated with glioma tumor grade and histological type, and acts as a prognostic factor in lower-grade gliomas. Int. J. Mol. Sci. 2016, 17, 1276. [Google Scholar] [CrossRef]
- Oria, V.O.; Lopatta, P.; Schmitz, T.; Preca, B.T.; Nyström, A.; Conrad, C.; Bartsch, J.W.; Kulemann, B.; Hoeppner, J.; Maurer, J.; et al. ADAM9 contributes to vascular invasion in pancreatic ductal adenocarcinoma. Mol. Oncol. 2019, 13, 456–479. [Google Scholar] [CrossRef] [PubMed]
- Dilwali, S.; Landegger, L.D.; Soares, V.Y.; Deschler, D.G.; Stankovic, K.M. Secreted factors from human vestibular schwannomas can cause cochlear damage. Sci. Rep. 2015, 5, 18599. [Google Scholar] [CrossRef] [PubMed]
- Baroja-Mazo, A.; Martín-Sánchez, F.; Gomez, A.I.; Martínez, C.M.; Amores-Iniesta, J.; Compan, V.; Barberà-Cremades, M.; Yagüe, J.; Ruiz-Ortiz, E.; Antón, J.; et al. The NLRP3 inflammasome is released as a particulate danger signal that amplifies the inflammatory response. Nat. Immunol. 2014, 15, 738–748. [Google Scholar] [CrossRef] [PubMed]
- Boyle, S.T. RISING STARS: Hormonal regulation of the breast cancer microenvironment. J. Mol. Endocrinol. 2023, 70, e220174. [Google Scholar] [CrossRef]
- Kasantikul, V.; Brown, W.J. Estrogen receptors in acoustic neurilemmomas. Surg. Neurol. 1981, 15, 105–109. [Google Scholar] [CrossRef]
- Whittle, I.; Hawkins, R.A.; Miller, J.D. Sex hormone receptors in intracranial tumors and normal brain. Eur. J. Surg. Oncol. 1987, 13, 303–307. [Google Scholar]
- Allen, J.; Eldridge, R.; Koerber, T. Acoustic neuroma in the last months of pregnancy. Am. J. Obstet. Gynecol. 1974, 119, 516–520. [Google Scholar] [CrossRef]
- Monsell, E.W.; Wiet, R.J. Estrogen and progesterone binding in acoustic neuroma tissue. Otolaryngol. Head Neck Surg. 1990, 103, 377–379. [Google Scholar] [CrossRef]
- Beatty, C.W.; Scheithauer, B.W.; Katzmann, J.A.; Roche, P.C.; Kjeldahl, K.S.; Ebsersold, M.J. Acoustic schwannomas in pregnancy: A DNA flow cytometric, steroid hormone receptor and proliferation marker study. Laryngoscope 1995, 105, 693–700. [Google Scholar] [CrossRef]
- Carroll, R.S.; Zhang, J.P.; Black, P.M.L. Hormone receptors in vestibular schwannomas. Acta Neurochir. 1997, 139, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Curley, J.W.; Ramsden, R.T.; Howell, A.; Healy, K.; Lye, R.H. Oestrogen and progesterone receptors in acoustic neuroma. J. Laryngol. Otol. 1990, 104, 865–867. [Google Scholar] [CrossRef] [PubMed]
- Dalgorf, D.M.; Rowsell, C.; Bilbao, J.M.; Chen, J.M. Immunohistochemical investigation of hormonal receptors and vascular endothelial growth factor concentration in vestibular schwannoma. Skull Base 2008, 18, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Juul, S.E.; Yachnis, A.T.; Rojiani, A.M.; Christensen, R.D. Immunohistochemical localization of erythropoietin and its receptor in the developing human brain. Ped. Dev. Path. 1999, 2, 148–158. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).