Are Voltage Sensors Really Embedded in Muscarinic Receptors?
Abstract
1. Introduction
2. Discussion
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sheoy, S.K. Seven-transmembrane receptors and ubiquitination. Circ. Res. 2007, 100, 1142–1154. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.A. Regulation of ion channels by muscarinic receptors. Neuropharmacology 2018, 136, 383–400. [Google Scholar] [CrossRef] [PubMed]
- Kruse, A.C.; Kobilka, B.K.; Gautam, D.; Sexton, P.M.; Christopoulos, A.; Wess, J. Muscarinic acetylcholine receptors: Novel opportunities for drug development. Nat. Rev. Drug Discov. 2014, 13, 549–560. [Google Scholar] [CrossRef]
- Birdsall, N.J.M.; Hulme, E.C. Muscarinic receptor subclasses. Trends Pharmacol. Sci. 1983, 4, 459–463. [Google Scholar] [CrossRef]
- Cohen-Armon, M.; Sokolovsky, M. Depolarization-induced changes in the muscarinic receptor in rat brain and heart are mediated by pertussis-toxin- sensitive G-proteins. J. Biol. Chem. 1991, 266, 2595–2605. [Google Scholar] [CrossRef] [PubMed]
- Ben-Chaim, Y.; Chanda, B.; Dascal, N.; Bezanilla, F.; Parnas, I.; Parnas, H. Movement of ‘gating charge’ is coupled to ligand binding in a G-protein-coupled receptor. Nature 2006, 444, 106–109. [Google Scholar] [CrossRef]
- Rinne, A.; Mobarec, J.C.; Mahaut-Smith, M.; Kolb, P.; Bünemann, M. The mode of agonist binding to a G-protein-coupled receptor switches the effect that voltage changes have on signaling. Sci. Signal. 2015, 8, 110–1118. [Google Scholar] [CrossRef]
- Barchad-Avitzur, O.; Priest, M.F.; Dekel, N.; Bezanilla, F.; Parnas, H.; Ben-Chaim, Y. A novel voltage sensor in the orthosteric binding site of the M2 muscarinic receptor. Biophys. J. 2016, 111, 1396–1408. [Google Scholar] [CrossRef]
- Stanfield, P. Voltage sparks a GPCR. Nat. Cell Biol. 2006, 8, 1323–1325. [Google Scholar] [CrossRef]
- Dekel, N.; Priest, M.F.; Parnas, H.; Parnas, I.; Bezanilla, F. Depolarization induces a conformational change in the binding site region of the M2 muscarinic receptor. Proc. Natl. Acad. Sci. USA 2012, 1091, 285–290. [Google Scholar] [CrossRef]
- Mahaut-Smith, M.P.; Martinez-Pinna, J.; Gurung, I.S. A role of membrane potential in regulating GPCR? Cell 2008, 29, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Cohen-Armon, M.; Sokolovsky, M. Evidence for involvement of the voltage-dependent Na+ channel gating in depolarization-induced activation of G-proteins. J. Biol. Chem. 1993, 268, 9824–9838. [Google Scholar] [CrossRef] [PubMed]
- Cohen-Armon, M.; Kloog, Y.; Henis, Y.I.; Sokolovsky, M. Batrachotoxin changes the properties of the muscarinic receptor in rat brain and heart: Possible interaction(s) between muscarinic receptors and sodium channels. Proc. Natl. Acad. Sci. USA 1985, 82, 3524–3527. [Google Scholar] [CrossRef] [PubMed]
- Anis, Y.; Nürnberg, B.; Visochek, L.; Reiss, N.; Naor, Z.; Cohen-Armon, M. Activation of Go-proteins by membrane depolarization traced by in-situ photoaffinity labeling of Gao-proteins with [a32P]GTP-azidoanilide. J. Biol. Chem. 1999, 274, 7431–7440. [Google Scholar] [CrossRef] [PubMed]
- Cohen-Armon, M.; Garty, H.; Sokolovsky, M. G-protein mediates voltage regulation of agonist binding to muscarinic receptors: Effects on receptor-Na+ channel interaction. Biochemistry 1988, 27, 368–374. [Google Scholar] [CrossRef]
- Hollingaworth, E.B.; McNeal, E.T.; Burton, J.L.; Williams, R.J.; Daly, J.W.; Creveling, C.R. Biochemical characterization of a filtered synaptoneurosome preparation from guinea pig cerebral cortex: Cyclic adenosine 3’:5’-monophosphate- generating systems, receptors, and enzymes. J. Neurosci. 1985, 5, 2240–2253. [Google Scholar] [CrossRef]
- Catterall, W.A.; Cestele, S.; Yarov-Yarovoy, V.; Yu, F.H.; Konoki, K.; Scheuer, T. Voltage-gated ion-channels and gating modifier toxins. Toxicon 2007, 49, 124–141. [Google Scholar] [CrossRef]
- Khodorov, B.I. Batrachotoxin as a tool to study voltage-sensitive sodium channels of excitable membranes. Prog. Biophys. Mol. Biol. 1985, 45, 57–148. [Google Scholar] [CrossRef]
- De Lera Ruiz, M.; Kraus, R.L. Voltage-gated Sodium Channels: Structure, function, pharmacology and clinical indications. J. Med. Chem. 2015, 58, 7093–7118. [Google Scholar] [CrossRef]
- Romey, G.; Quast, U.; Pauron, D.; Frelin, C.; Renaud, J.F.; Lazdunski, M. Na+ channels as sites of action of the cardioactive agent DPI 201-106 with agonist and antagonist enantiomer. Proc. Natl. Acad. Sci. USA 1987, 84, 896–900. [Google Scholar] [CrossRef]
- Yarov-Yarovoya, V.; Decaena, P.G.; Westenbroeka, R.E.; Chien-Yuan, P.; Scheuera, T.S.; Bakerb, D.; Catterall, W.A. Structural basis for gating charge movement in the voltage sensor of a sodium channel. Proc. Natl. Acad. Sci. USA 2012, 109, E93–E102. [Google Scholar] [CrossRef] [PubMed]
- Dekel, D.; Bentulila, Z.; Tauber, M.; Ben-Chaim, Y. G-protein coupled receptors regulated by membrane potential. Int. J. Mol. Sci. 2022, 23, 13988. [Google Scholar]
- Jiang, M.; Bajpayee, N.S. Molecular mechanisms of Go signaling. Neurosignals 2009, 17, 23–41. [Google Scholar] [CrossRef] [PubMed]
- Navaroli, V.L.; Zao, Y.; Boguszewski, P.; Brown, T.H. Muscarinic receptor activation enables persistent firing in Pyramidal neurons from superficial layers of dorsal peririhinal cortex. Hippocampus 2012, 22, 1392–1404. [Google Scholar] [CrossRef]
- Tikhonova, T.B.; Miyamae, T.; Gulchina, Y.; Lewis, D.A.; Gonzalez-Burgos, G. Cell type and layer-specific muscarinic potentiation of excitatory synaptic drive onto parvalbumin neurons in mouse prefrontal cortex. Eneuro 2018, 5, eneuro.0208-0218. [Google Scholar] [CrossRef]
 ,
,  ) and absence (
) and absence ( ,
,  ) of atropine (0.1 µM). The specific carbamylcholine-induced binding of [3H]BTX was measured in the presence of TTX (1 µM). The non-specific carbamylcholine-induced 22Na+ uptake, both in the presence and absence of atropine, was measured in the presence of TTX (1 µM) [15].
) of atropine (0.1 µM). The specific carbamylcholine-induced binding of [3H]BTX was measured in the presence of TTX (1 µM). The non-specific carbamylcholine-induced 22Na+ uptake, both in the presence and absence of atropine, was measured in the presence of TTX (1 µM) [15].
 ,
,  ) and absence (
) and absence ( ,
,  ) of atropine (0.1 µM). The specific carbamylcholine-induced binding of [3H]BTX was measured in the presence of TTX (1 µM). The non-specific carbamylcholine-induced 22Na+ uptake, both in the presence and absence of atropine, was measured in the presence of TTX (1 µM) [15].
) of atropine (0.1 µM). The specific carbamylcholine-induced binding of [3H]BTX was measured in the presence of TTX (1 µM). The non-specific carbamylcholine-induced 22Na+ uptake, both in the presence and absence of atropine, was measured in the presence of TTX (1 µM) [15].
 ,
,  ) and upon depolarization (
) and upon depolarization ( ,
,  ). [3H]Ach binding measured in untreated synaptoneurosomes (black lines, circles) and in synaptoneurosomes treated (grey lines, triangles) with the S-enantiomer of DPI (5 µM) in the presence of 1 µM TTX (A) or with the R-enantiomer of DPI (5 µM) (B), are presented. The non-specific binding of [3H]ACh was measured in the presence of 1 µM atropine. (Ref. [12]).
). [3H]Ach binding measured in untreated synaptoneurosomes (black lines, circles) and in synaptoneurosomes treated (grey lines, triangles) with the S-enantiomer of DPI (5 µM) in the presence of 1 µM TTX (A) or with the R-enantiomer of DPI (5 µM) (B), are presented. The non-specific binding of [3H]ACh was measured in the presence of 1 µM atropine. (Ref. [12]).
 ,
,  ) and upon depolarization (
) and upon depolarization ( ,
, 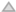 ). [3H]Ach binding measured in untreated synaptoneurosomes (black lines, circles) and in synaptoneurosomes treated (grey lines, triangles) with the S-enantiomer of DPI (5 µM) in the presence of 1 µM TTX (A) or with the R-enantiomer of DPI (5 µM) (B), are presented. The non-specific binding of [3H]ACh was measured in the presence of 1 µM atropine. (Ref. [12]).
). [3H]Ach binding measured in untreated synaptoneurosomes (black lines, circles) and in synaptoneurosomes treated (grey lines, triangles) with the S-enantiomer of DPI (5 µM) in the presence of 1 µM TTX (A) or with the R-enantiomer of DPI (5 µM) (B), are presented. The non-specific binding of [3H]ACh was measured in the presence of 1 µM atropine. (Ref. [12]).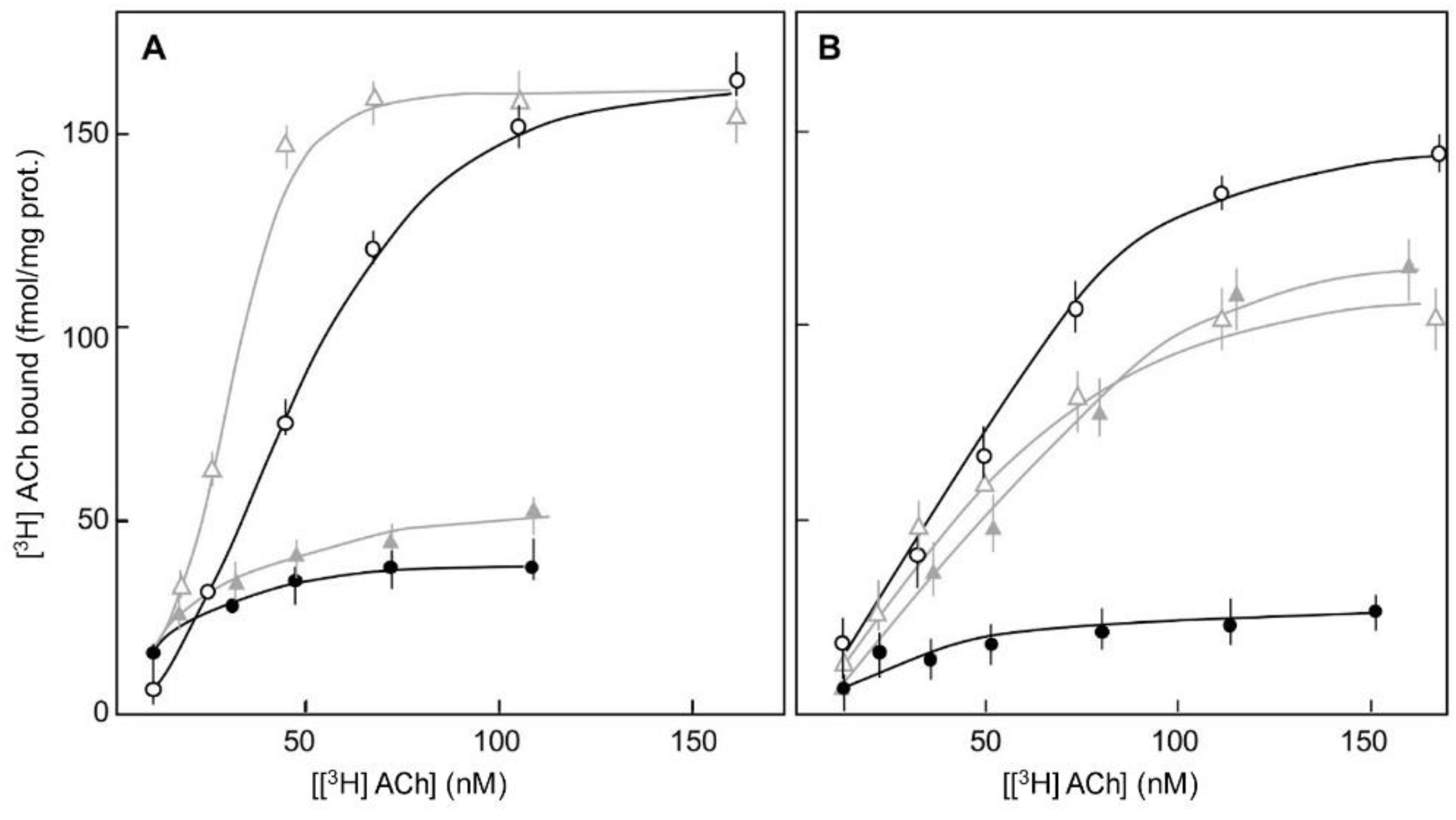
 ) and in the absence of Gpp(NH)p (
) and in the absence of Gpp(NH)p ( ). (B). [3H] ACh binding was measured after treatment with the DPI R-enantiomer (5 μM) (grey curves, triangles) or with the racemic mixture of the cardio-tonic drug DPI (5 μM) (black curves, circles) in the presence (
). (B). [3H] ACh binding was measured after treatment with the DPI R-enantiomer (5 μM) (grey curves, triangles) or with the racemic mixture of the cardio-tonic drug DPI (5 μM) (black curves, circles) in the presence ( ,
,  ) and in the absence of Gpp(NH)p (
) and in the absence of Gpp(NH)p ( ,
,  ). The non-specific binding of [3H]ACh was measured in the presence of 1 μM atropine. (Ref. [12]).
). The non-specific binding of [3H]ACh was measured in the presence of 1 μM atropine. (Ref. [12]).
 ) and in the absence of Gpp(NH)p (
) and in the absence of Gpp(NH)p ( ). (B). [3H] ACh binding was measured after treatment with the DPI R-enantiomer (5 μM) (grey curves, triangles) or with the racemic mixture of the cardio-tonic drug DPI (5 μM) (black curves, circles) in the presence (
). (B). [3H] ACh binding was measured after treatment with the DPI R-enantiomer (5 μM) (grey curves, triangles) or with the racemic mixture of the cardio-tonic drug DPI (5 μM) (black curves, circles) in the presence ( ,
,  ) and in the absence of Gpp(NH)p (
) and in the absence of Gpp(NH)p ( ,
,  ). The non-specific binding of [3H]ACh was measured in the presence of 1 μM atropine. (Ref. [12]).
). The non-specific binding of [3H]ACh was measured in the presence of 1 μM atropine. (Ref. [12]).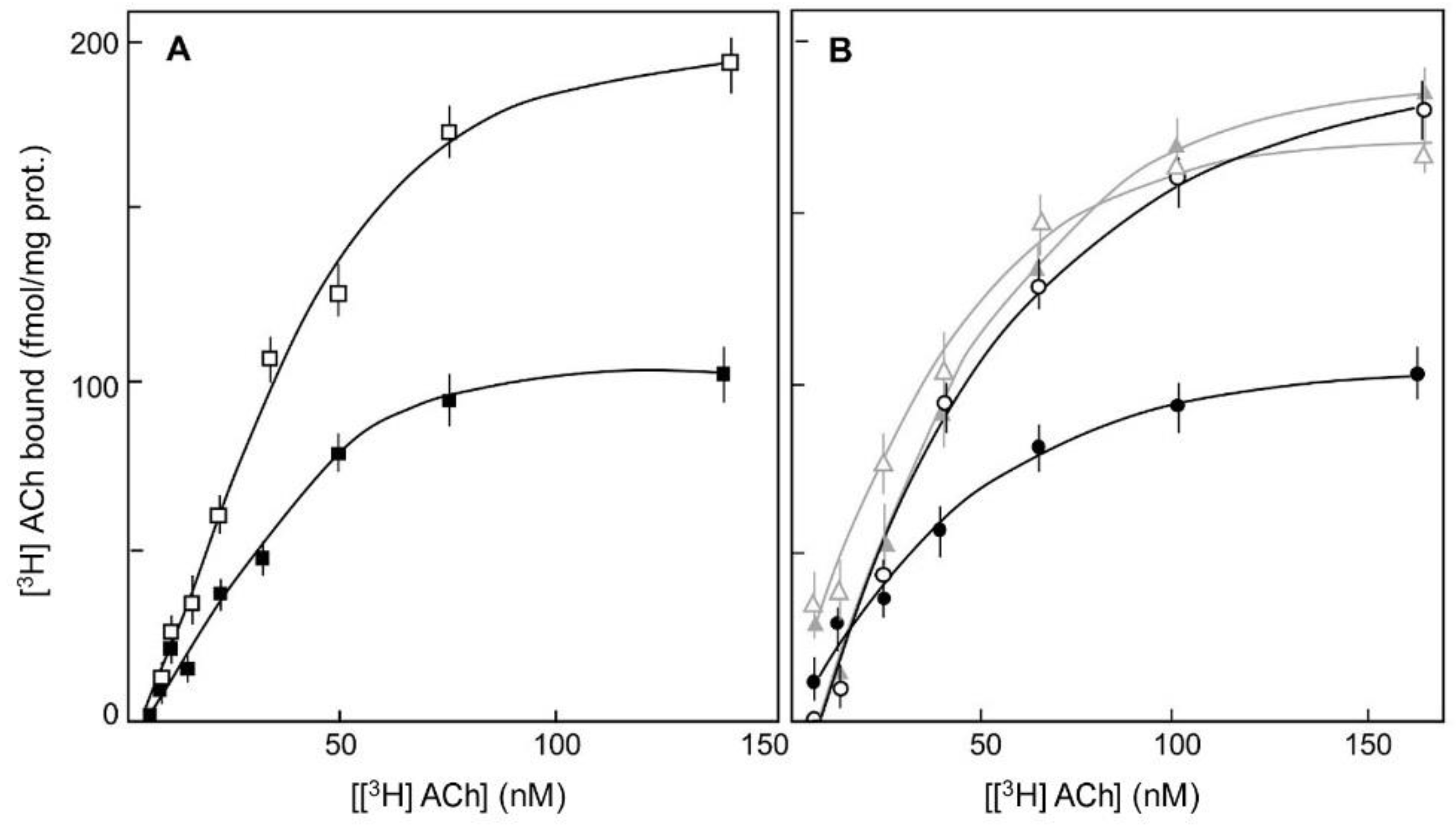
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cohen-Armon, M. Are Voltage Sensors Really Embedded in Muscarinic Receptors? Int. J. Mol. Sci. 2023, 24, 7538. https://doi.org/10.3390/ijms24087538
Cohen-Armon M. Are Voltage Sensors Really Embedded in Muscarinic Receptors? International Journal of Molecular Sciences. 2023; 24(8):7538. https://doi.org/10.3390/ijms24087538
Chicago/Turabian StyleCohen-Armon, Malka. 2023. "Are Voltage Sensors Really Embedded in Muscarinic Receptors?" International Journal of Molecular Sciences 24, no. 8: 7538. https://doi.org/10.3390/ijms24087538
APA StyleCohen-Armon, M. (2023). Are Voltage Sensors Really Embedded in Muscarinic Receptors? International Journal of Molecular Sciences, 24(8), 7538. https://doi.org/10.3390/ijms24087538





