Genome-Wide Identification and Expression Analysis of WRKY Gene Family in Neolamarckia cadamba
Abstract
1. Introduction
2. Results
2.1. Identification of WRKY Genes in Neolamarckia Cadamba
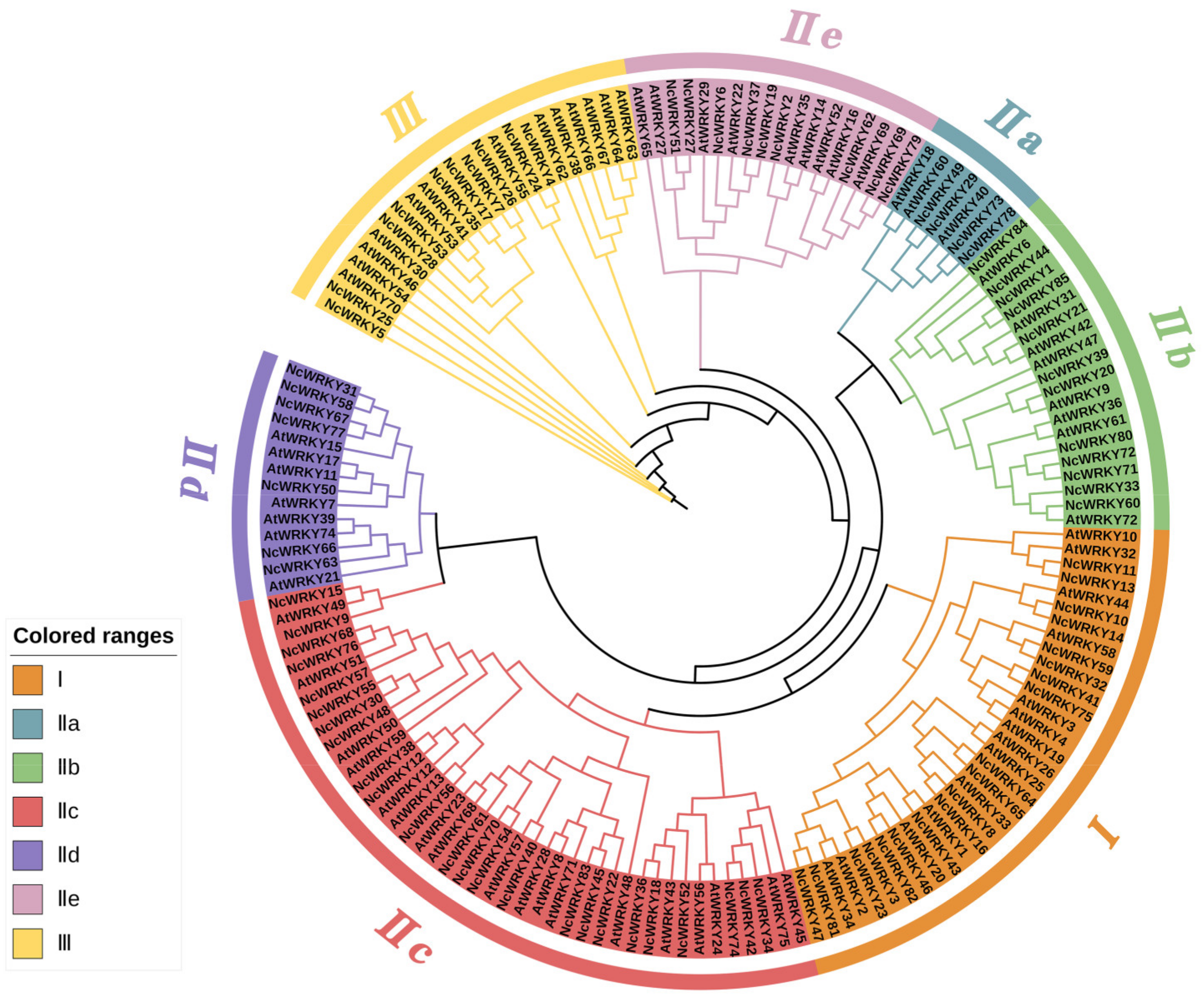
2.2. Phylogenetic Analysis and Multiple Sequence Alignment of NcWRKYs
2.3. Gene Structure and Conserved Motif Analysis
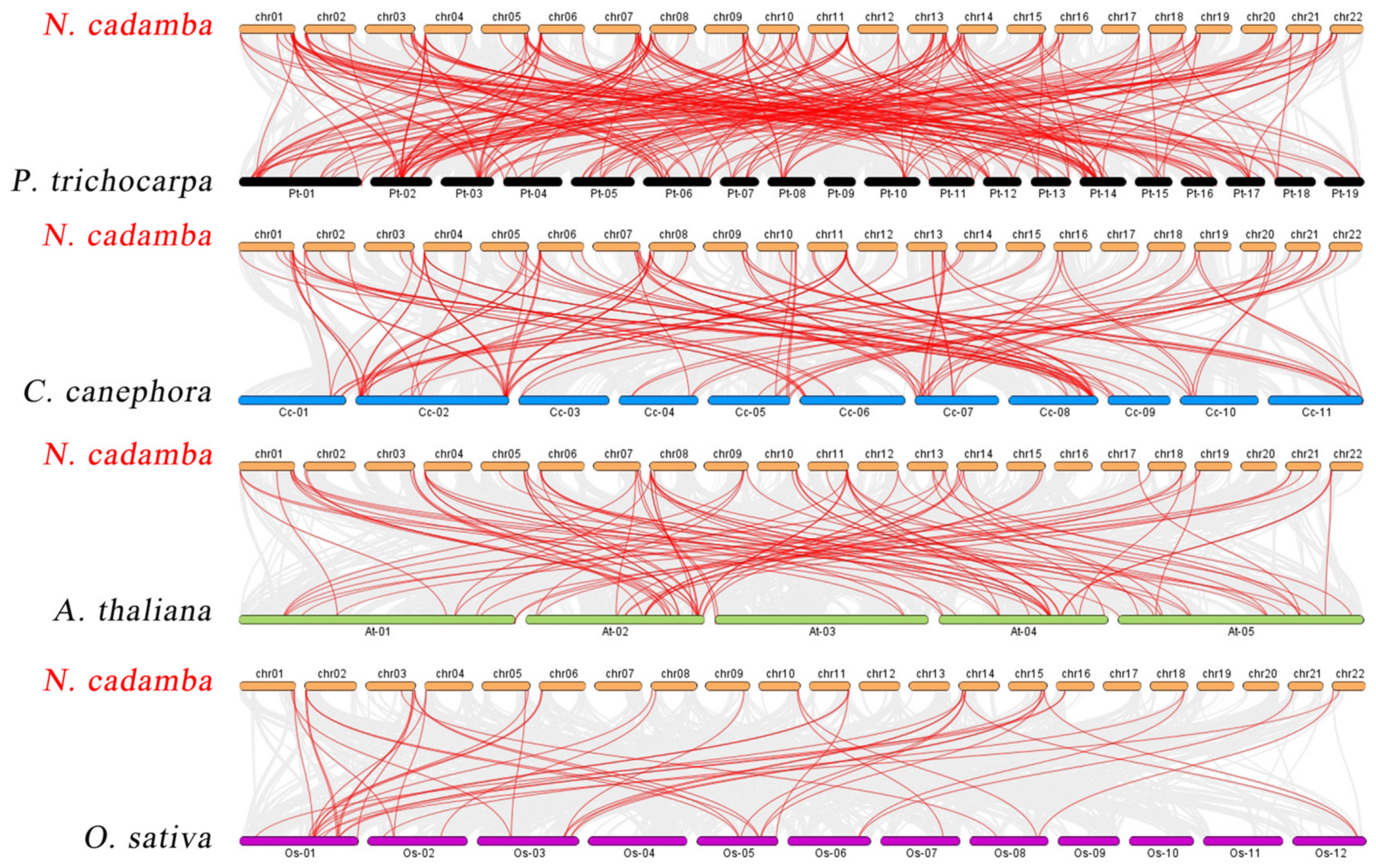
2.4. Chromosomal Distribution and Synteny Analysis of NcWRKYs
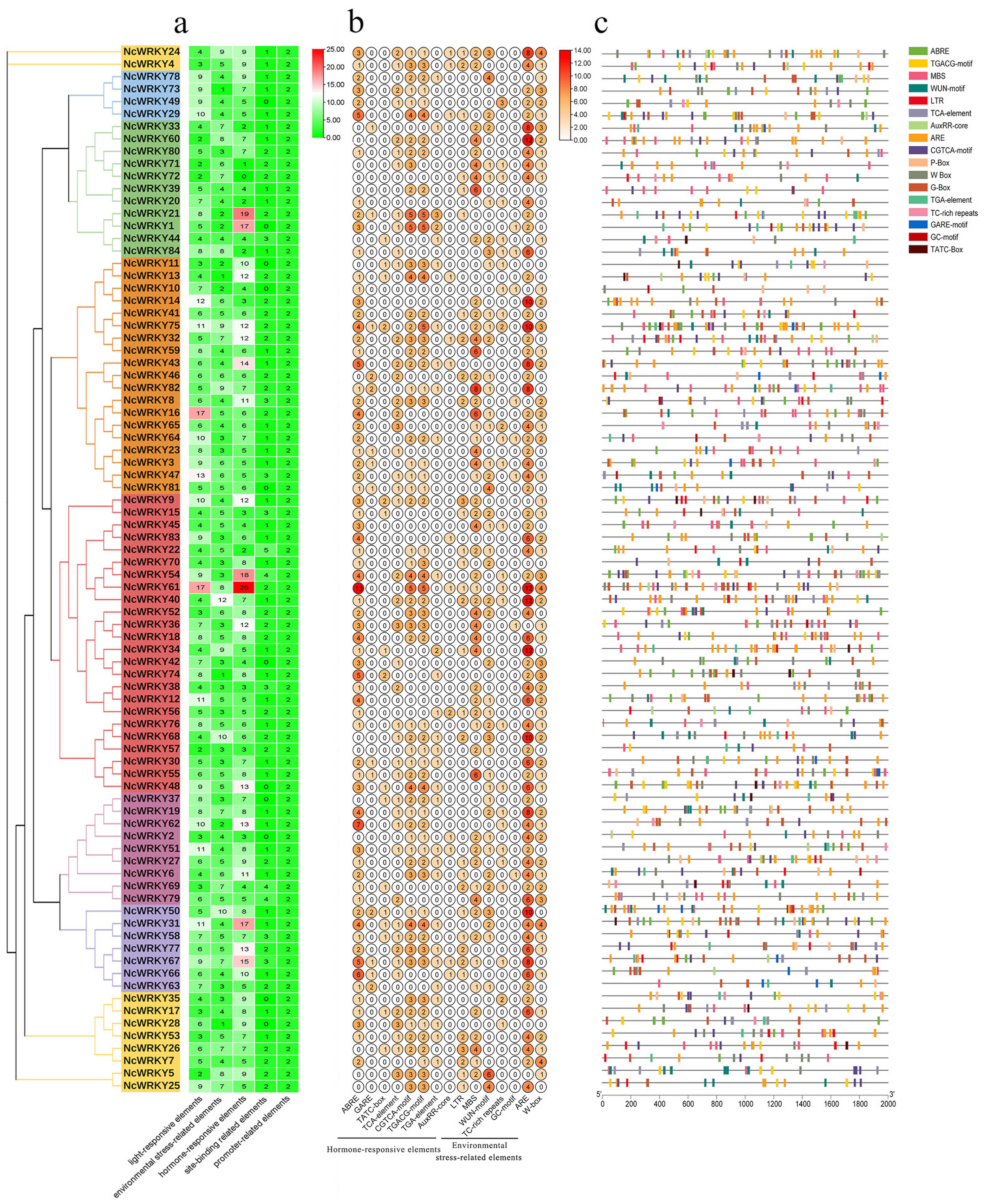
2.5. Analysis of Promoter Cis-Acting Elements
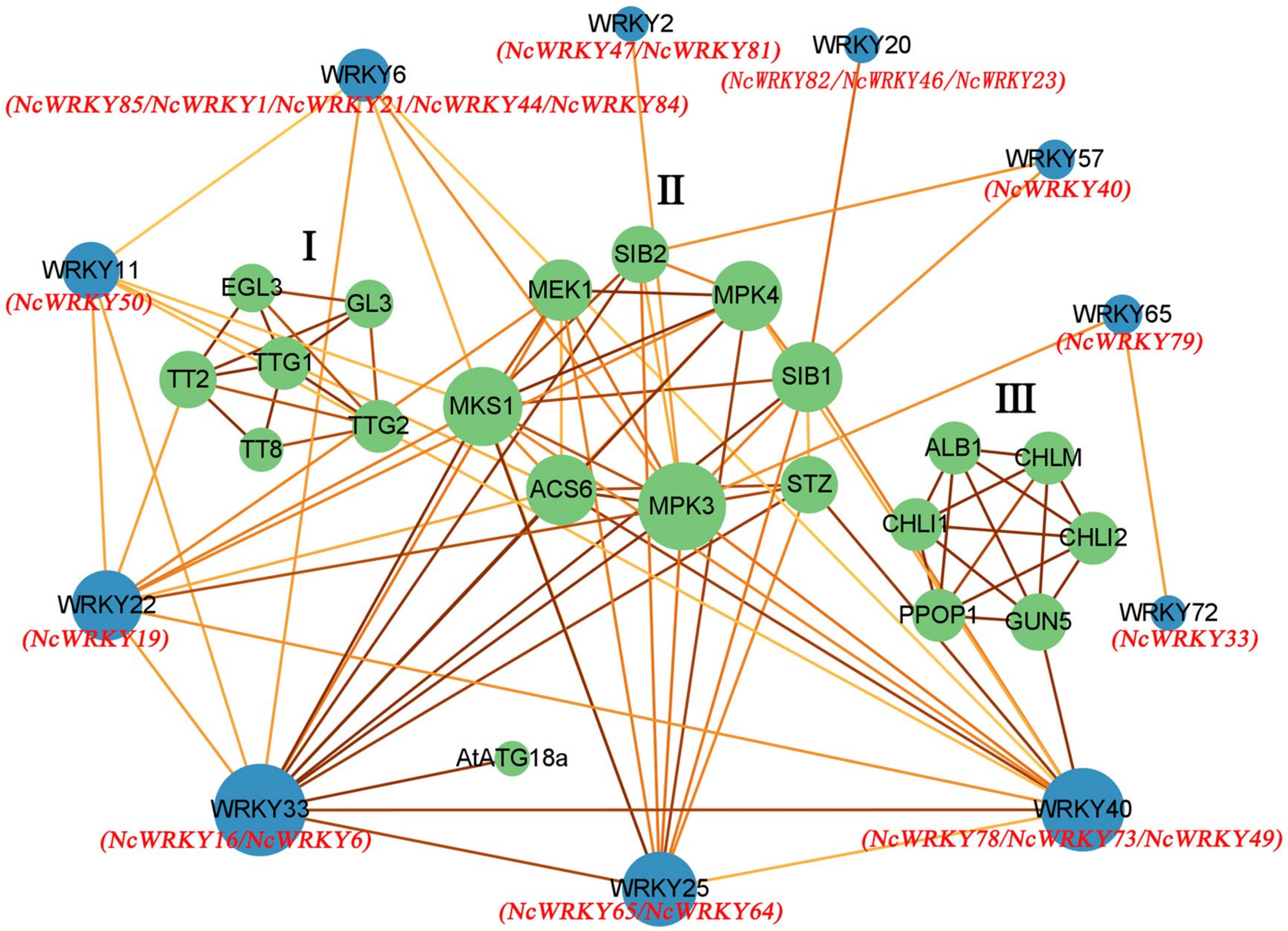
2.6. Protein Interaction Network of NcWRKY
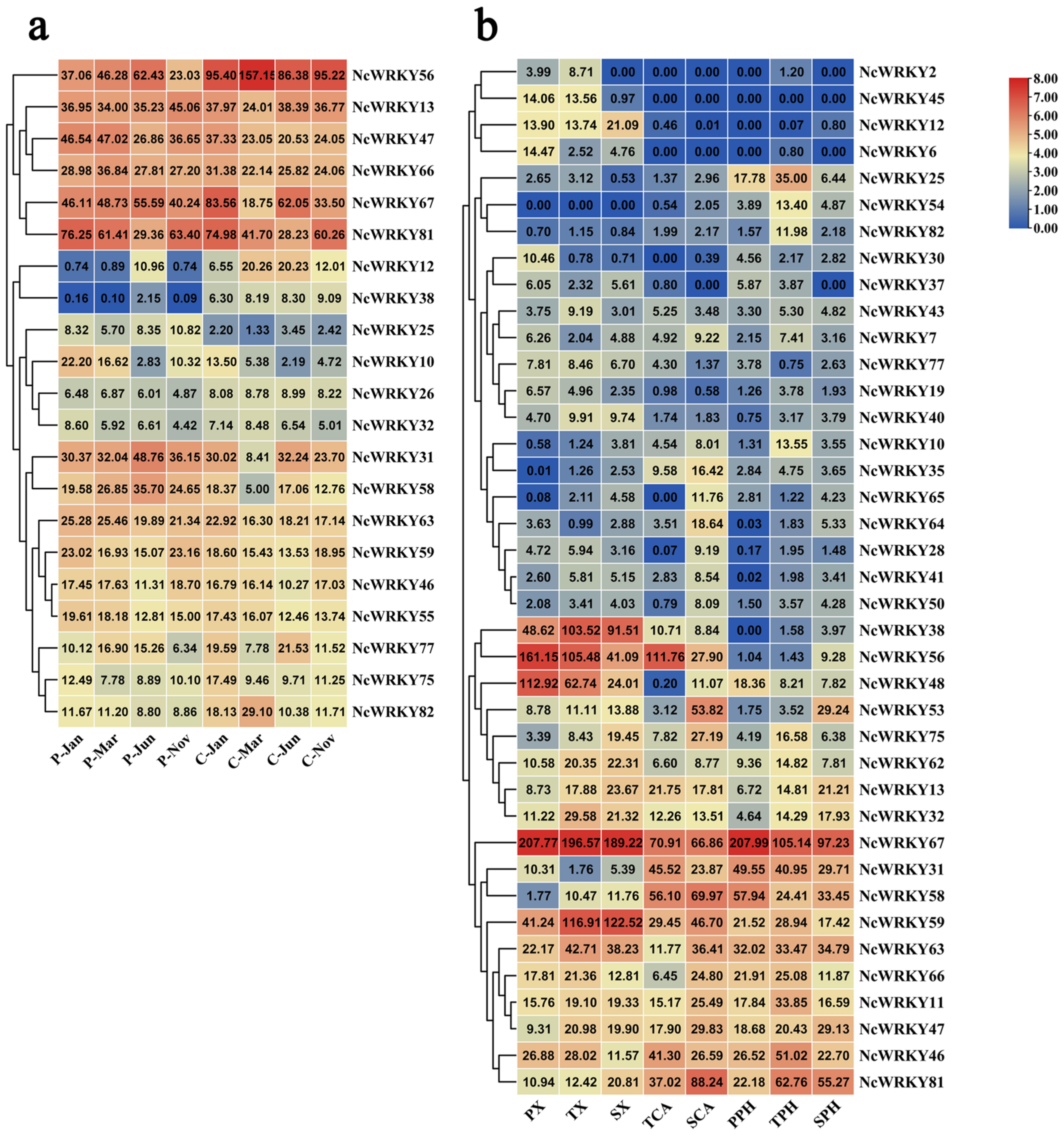
2.7. Expression Patterns of NcWRKYs Gene in N. cadamba Tissues by RNA-Seq
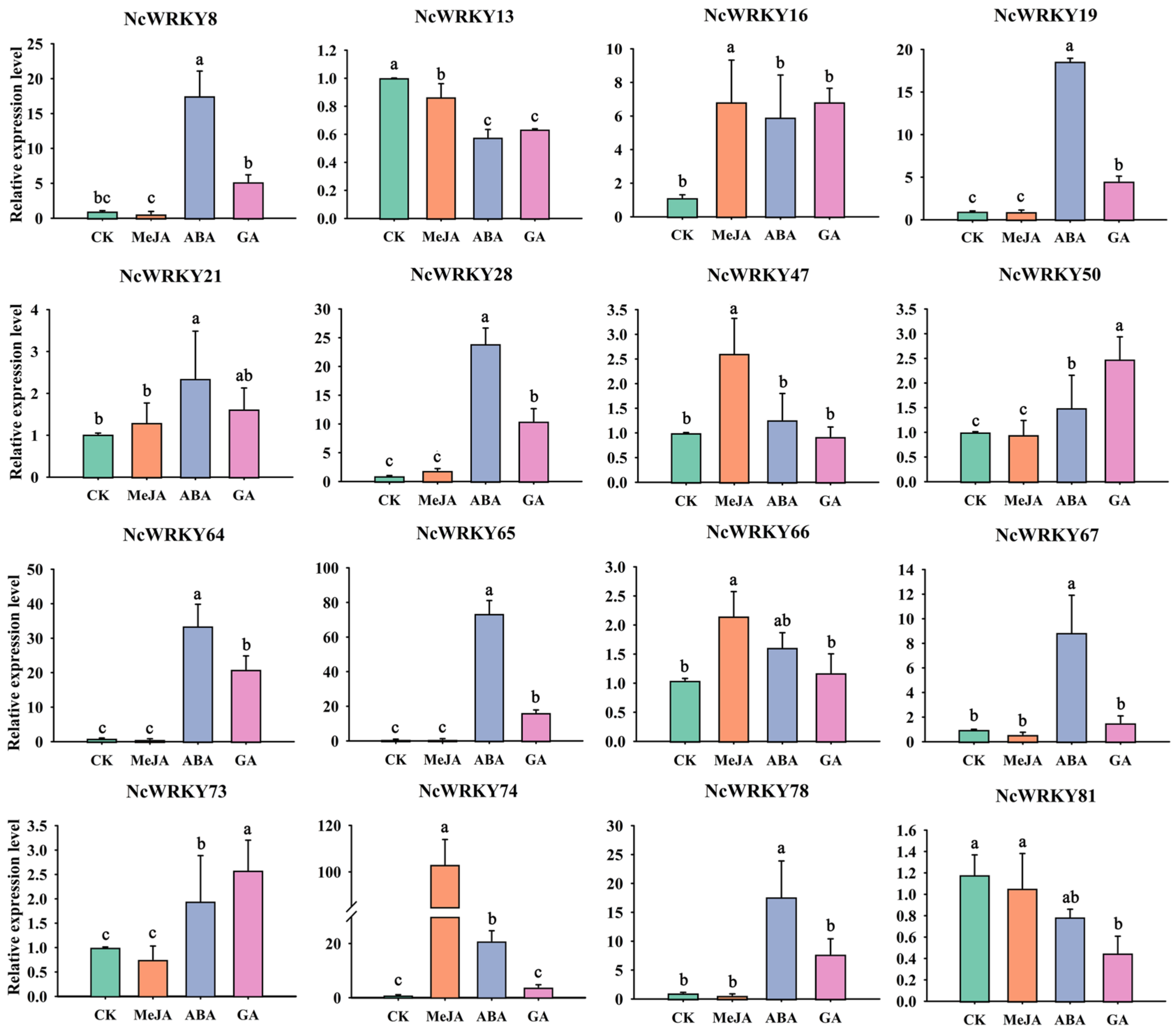
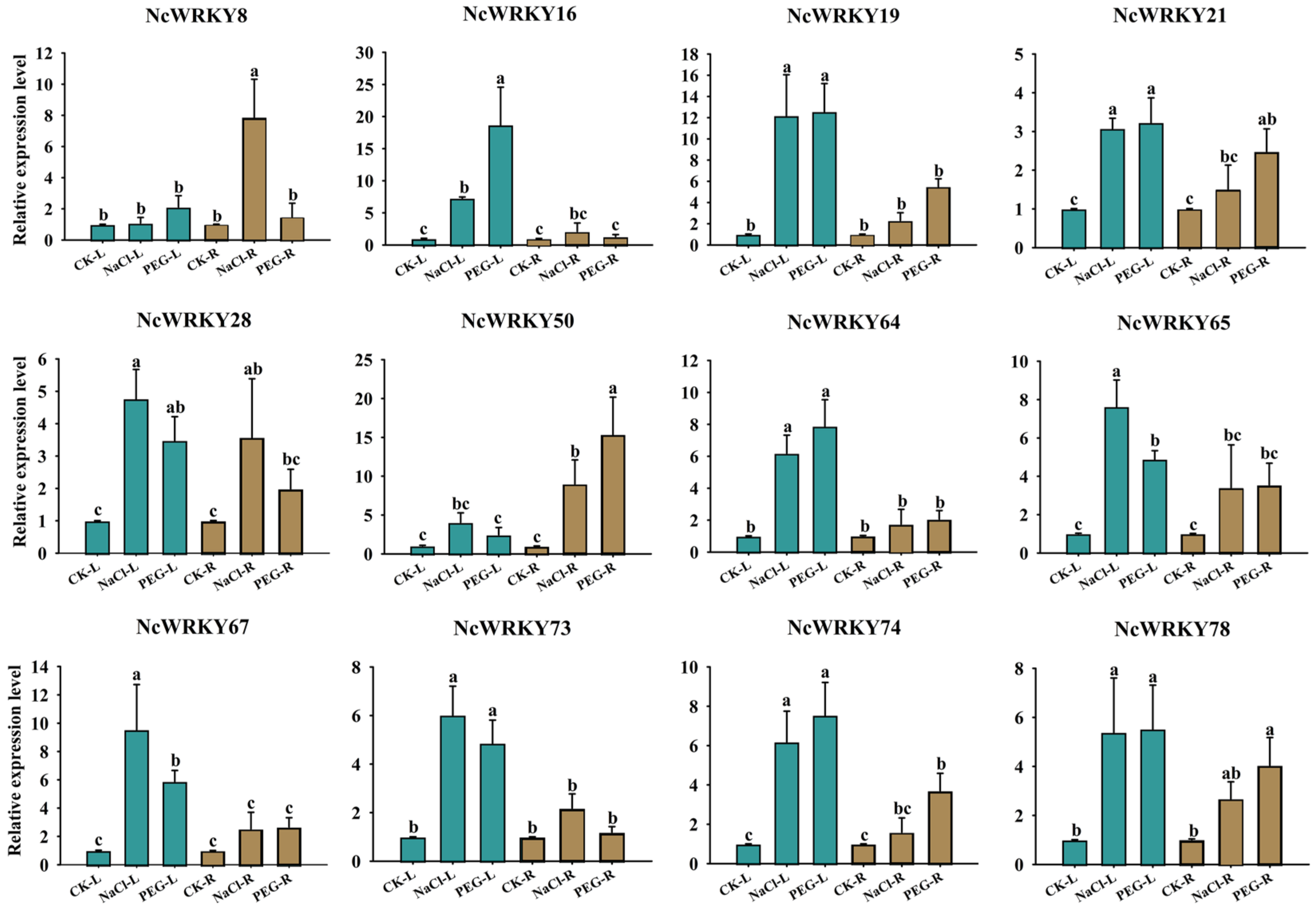
2.8. Expression Patterns of NcWRKYs in Response to Different Treatments
2.9. MeJA Promotes Cadambine Biosynthesis and NcWRKYs Expression
3. Discussion
3.1. Diverse Characterization of WRKY in N. cadamba
3.2. The Potential Function of NcWRKYs in Vascular Development and Response to Hormone and Abiotic Stress
3.3. The Involvement of NcWRKYs in Cadambine Biosynthesis
4. Materials and Methods
4.1. Data Sources
4.2. Identification of WRKY Genes in N. cadamba
4.3. Phylogenetic Analysis of WRKY Family Members and Sequence Alignment
4.4. Analysis of Conserved Motifs, Conserved Domains, and Gene Structures
4.5. Promoter Cis-Regulatory Element Analysis and Chromosomal Localization
4.6. Colinear Analysis and Selective Pressure
4.7. Homology Analyses and Protein Interaction Network Analysis
4.8. Plant Material and Stress Treatments
4.9. Expression Patterns of NcWRKYs
4.10. Extraction and Quantitative Determination of Tryptamine and Cadambine
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ulker, B.; Somssich, I.E. WRKY transcription factors: From DNA binding towards biological function. Curr. Opin. Plant Biol. 2004, 7, 491–498. [Google Scholar] [CrossRef]
- Brand, L.H.; Fischer, N.M.; Harter, K.; Kohlbacher, O.; Wanke, D. Elucidating the evolutionary conserved DNA-binding specificities of WRKY transcription factors by molecular dynamics and in vitro binding assays. Nucleic Acids Res. 2013, 41, 9764–9778. [Google Scholar] [CrossRef] [PubMed]
- Eulgem, T.; Rushton, P.J.; Robatzek, S.; Somssich, I.E. The WRKY superfamily of plant transcription factors. Trends Plant Sci. 2000, 5, 199–206. [Google Scholar] [CrossRef]
- Jiang, J.J.; Ma, S.H.; Ye, N.H.; Jinag, M.; Cao, J.S.; Zhang, J.H. WRKY transcription factors in plant responses to stresses. J. Integr. Plant Biol. 2017, 59, 86–101. [Google Scholar] [CrossRef]
- Rushton, P.J.; Somssich, I.E.; Ringler, P.; Shen, Q.J. WRKY transcription factors. Trends Plant Sci. 2010, 15, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Zhang, Z.L.; Zou, X.; Yang, G.; Komatsu, S.; Shen, Q.J. Interactions of two abscisic-acid induced WRKY genes in repressing gibberellin signaling in aleurone cells. Plant J. 2006, 46, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.H.; Kwon, S.I.; Jang, J.Y.; Fang, I.L.; Lee, H.; Choi, C.; Park, S.; Ahn, I.; Bae, S.C.; Hwang, D.J. OsWRKY51, a rice transcription factor, functions as a positive regulator in defense response against Xanthomonas oryzae pv. oryzae. Plant Cell Rep. 2016, 35, 1975–1985. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Lai, Z.; Shi, J.; Xiao, Y.; Chen, Z.; Xu, X. Roles of Arabidopsis WRKY18, WRKY40 and WRKY60 transcription factors in plant responses to abscisic acid and abiotic stress. BMC Plant Biol. 2010, 10, 281. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.X.; Ma, K.B.; Lu, Z.G.; Chen, G.; Cui, J.W.; Tong, X.P.; Wang, L.; Teng, N.J.; Jin, B. Transcriptomic and metabolomic analysis of the heat-stress response of Populus tomentosa Carr. Forests 2019, 10, 383. [Google Scholar] [CrossRef]
- Wu, M.; Zhang, K.; Xu, Y.; Wang, L.; Liu, H.; Qin, Z.; Xiang, Y. The moso bamboo WRKY transcription factor, PheWRKY86, regulates drought tolerance in transgenic plants. Plant Physiol. Biochem. 2022, 170, 180–191. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.P.; Ju, H.L.; Jae, H.Y.; Byeong, C.M.; Man, S.C.; Yun, H.K.; Sang, M.L.; Ho, S.K.; Kyu, Y.K.; Woo, S.C.; et al. WRKY group IId transcription factors interact with calmodulin. FEBS Lett. 2005, 579, 1545–1550. [Google Scholar] [CrossRef]
- Li, S.; Fu, Q.; Chen, L.; Huang, W.; Yu, D. Arabidopsis thaliana WRKY25, WRKY26, and WRKY33 coordinate induction of plant thermotolerance. Planta 2011, 233, 1237–1252. [Google Scholar] [CrossRef] [PubMed]
- Liang, Q.; Wu, Y.; Wang, K.; Bai, Z.; Liu, Q.; Pan, Y.; Zhang, L.; Jiang, B. Chrysanthemum WRKY gene DgWRKY5 enhances tolerance to salt stress in transgenic chrysanthemum. Sci. Rep. 2017, 7, 4799. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Li, C.; He, X.; Zhang, X.; Zhu, L. ABA signaling is negatively regulated by GbWRKY1 through JAZ1 and ABI1 to affect salt and drought tolerance. Plant Cell Rep. 2020, 39, 181–194. [Google Scholar] [CrossRef]
- Yong, F.G.; Ji, K.L.; Feng, M.Y.; Guo, Y.Z.; Dan, W.; Lin, Z.; Yong, B.O.; Yin, A.Y. The WRKY transcription factor WRKY8 promotes resistance to pathogen infection and mediates drought and salt stress tolerance in Solanum lycopersicum. Physiol. Plant. 2020, 168, 98–117. [Google Scholar] [CrossRef]
- Hao, X.; Xie, C.; Ruan, Q.; Zhang, X.; Wu, C.; Han, B.; Qian, J.; Zhou, W.; Nutzmann, H.W.; Kai, G. The transcription factor OpWRKY2 positively regulates the biosynthesis of the anticancer drug camptothecin in Ophiorrhiza pumila. Hortic. Res. 2021, 8, 7. [Google Scholar] [CrossRef] [PubMed]
- Can, W.; Chao, W.; Yao, W.; Chenhong, X.; Min, S.; Shivraj, N.; Zhigang, Z.; Guoyin, K. Transcription factor OpWRKY3 is involved in the development and biosynthesis of camptothecin and its precursors in Ophiorrhiza pumila hairy roots. Int. J. Mol. Sci. 2019, 20, 3996. [Google Scholar] [CrossRef]
- Wang, C.; Hao, X.; Wang, Y.; Maoz, I.; Zhou, W.; Zhou, Z.; Kai, G. Identification of WRKY transcription factors involved in regulating the biosynthesis of the anti-cancer drug camptothecin in Ophiorrhiza pumila. Hortic. Res. 2022, 9, uhac099. [Google Scholar] [CrossRef]
- Sun, P.W.; Xu, Y.H.; Yu, C.C.; Lv, F.F.; Tang, X.L.; Gao, Z.H.; Zhang, Z.; Wang, H.; Liu, Y.; Wei, J.H. WRKY44 represses expression of the wound-induced sesquiterpene biosynthetic gene ASS1 in Aquilaria sinensis. J. Exp. Bot. 2020, 71, 1128–1138. [Google Scholar] [CrossRef]
- Xu, Y.H.; Wang, J.W.; Wang, S.; Wang, J.Y.; Chen, X.Y. Characterization of GaWRKY1, a cotton transcription factor that regulates the sesquiterpene synthase gene (+)-delta-cadinene synthase-A. Plant Physiol. 2004, 135, 507–515. [Google Scholar] [CrossRef]
- Ma, D.; Pu, G.; Lei, C.; Ma, L.; Wang, H.; Guo, Y.; Chen, J.; Du, Z.; Wang, H.; Li, G.; et al. Isolation and characterization of AaWRKY1, an Artemisia annua transcription factor that regulates the amorpha-4,11-diene synthase gene, a key gene of artemisinin biosynthesis. Plant Cell Physiol. 2009, 50, 2146–2161. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Juan, W.; Jiachen, S.; Junping, H.; Kee-Yoeup, P.; So-Young, P.; Luqi, H.; Wenyuan, G. A WRKY transcription factor, PgWRKY4X, positively regulates ginsenoside biosynthesis by activating squalene epoxidase transcription in Panax ginseng. Ind. Crops Prod. 2020, 154, 112671. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, Y.; Nie, L.; Jin, X.; Liao, W.; Zhao, S.; Fu, C.; Yu, L. Transcriptome-wide identification and screening of WRKY factors involved in the regulation of taxol biosynthesis in Taxus chinensis. Sci. Rep. 2018, 8, 5197. [Google Scholar] [CrossRef] [PubMed]
- Suttipanta, N.; Pattanaik, S.; Kulshrestha, M.; Patra, B.; Singh, S.K.; Yuan, L. The transcription factor CrWRKY1 positively regulates the terpenoid indole alkaloid biosynthesis in Catharanthus roseus. Plant Physiol. 2011, 157, 2081–2093. [Google Scholar] [CrossRef]
- Pandey, A.; Negi, P.S. Traditional uses, phytochemistry and pharmacological properties of Neolamarckia cadamba: A review. J. Ethnopharmacol. 2016, 181, 118–135. [Google Scholar] [CrossRef] [PubMed]
- Isao, K.; Hong, W.; Sanae, N.; Taifo, M.; Kazuyuki, H.; Motomasa, K.; Tahan, U.; Hirotaka, S. Indonesian Medicinal Plants. XIV. Characterization of 3′-0-Caffeoylswerosicle, a new secoiridoid glucoside, and kelampayosides A and B, two new phenolic apioglucosides, from the bark of Anthocephalus chinensis (Rubiaceae). Chem. Pharm. Bull. 1996, 44, 1162–1167. [Google Scholar] [CrossRef]
- Ashish, K.; Somenath, R.C.; Kumar, K.J.; Tulika, C.; Hemanta, K.M.; Tarun, J.; Sibabrata, M. Anthocephaline, a new indole alkaloid and cadambine, a potent inhibitor of DNA topoisomerase IB of leishmania donovani (LdTOP1LS), isolated from Anthocephalus cadamba. Nat. Prod. Commun. 2015, 10, 297–299. [Google Scholar] [CrossRef]
- Deepak, K.; Chilukuri, T.; Saiprasanna, R.; Sumana, M.; Bikas, C.P. Bio-assay guided isolation of anti-cancer compounds from Anthocephalus cadamba bark. Nat. Prod. Commun. 2015, 10, 1349–1350. [Google Scholar] [CrossRef]
- Zhao, X.; Hu, X.; OuYang, K.; Yang, J.; Que, Q.; Long, J.; Zhang, J.; Zhang, T.; Wang, X.; Gao, J.; et al. Chromosome-level assembly of the Neolamarckia cadamba genome provides insights into the evolution of cadambine biosynthesis. Plant J. 2022, 109, 891–908. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Wang, C.; Wang, H.; Li, L.; Wang, C. The function of MAPK cascades in response to various stresses in horticultural Plants. Front. Plant Sci. 2020, 11, 952. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Zhang, S. MAPK cascades in plant disease resistance signaling. Annu. Rev. Phytopathol. 2013, 51, 245–266. [Google Scholar] [CrossRef] [PubMed]
- Pitzschke, A.; Schikora, A.; Hirt, H. MAPK cascade signalling networks in plant defence. Curr. Opin. Plant Biol. 2009, 12, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Krishnamurthy, P.; Vishal, B.; Ho, W.J.; Lok, F.; Lee, F.; Kumar, P.P. Regulation of a cytochrome P450 gene CYP94B1 by WRKY33 transcription factor controls apoplastic barrier formation in roots to confer salt tolerance. Plant Physiol. 2020, 184, 2199–2215. [Google Scholar] [CrossRef] [PubMed]
- Mao, G.; Meng, X.; Liu, Y.; Zheng, Z.; Chen, Z.; Zhang, S. Phosphorylation of a WRKY transcription factor by two pathogen-responsive MAPKs drives phytoalexin biosynthesis in Arabidopsis. Plant Cell 2011, 23, 1639–1653. [Google Scholar] [CrossRef]
- Hu, P.; Zhou, W.; Cheng, Z.; Fan, M.; Wang, L.; Xie, D. JAV1 controls jasmonate-regulated plant defense. Mol. Cell 2013, 50, 504–515. [Google Scholar] [CrossRef]
- Wasternack, C. Action of jasmonates in plant stress responses and development—Applied aspects. Biotechnol. Adv. 2014, 32, 31–39. [Google Scholar] [CrossRef]
- Tang, Y.; Guo, J.; Zhang, T.; Bai, S.; He, K.; Wang, Z. Genome-wide analysis of WRKY gene family and the dynamic responses of key WRKY genes involved in Ostrinia furnacalis attack in Zea mays. Int. J. Mol. Sci. 2021, 22, 13045. [Google Scholar] [CrossRef]
- Huang, S.; Gao, Y.; Liu, J.; Peng, X.; Niu, X.; Fei, Z.; Cao, S.; Liu, Y. Genome-wide analysis of WRKY transcription factors in Solanum lycopersicum. Mol. Genet. Genom. 2012, 287, 495–513. [Google Scholar] [CrossRef]
- Di, P.; Wang, P.; Yan, M.; Han, P.; Huang, X.; Yin, L.; Yan, Y.; Xu, Y.; Wang, Y. Genome-wide characterization and analysis of WRKY transcription factors in Panax ginseng. BMC Genom. 2021, 22, 834. [Google Scholar] [CrossRef]
- Jiang, Y.; Duan, Y.; Yin, J.; Ye, S.; Zhu, J.; Zhang, F.; Lu, W.; Fan, D.; Luo, K. Genome-wide identification and characterization of the Populus WRKY transcription factor family and analysis of their expression in response to biotic and abiotic stresses. J. Exp. Bot. 2014, 65, 6629–6644. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, X.; Xue, J.; Meng, L.; Li, R. Identification and expression analysis of WRKY transcription factors in medicinal plant Catharanthus roseus. Sheng Wu Gong Cheng Xue Bao Chin. J. Biotechnol. 2013, 29, 785–802. [Google Scholar]
- Baillo, E.H.; Kimotho, R.N.; Zhang, Z.; Xu, P. Transcription factors associated with abiotic and biotic stress tolerance and their potential for crops improvement. Genes 2019, 10, 771. [Google Scholar] [CrossRef] [PubMed]
- Van Verk, M.C.; Pappaioannou, D.; Neeleman, L.; Bol, J.F.; Linthorst, H.J. A novel WRKY transcription factor is required for induction of PR-1a gene expression by salicylic acid and bacterial elicitors. Plant Physiol. 2008, 146, 1983–1995. [Google Scholar] [CrossRef] [PubMed]
- Xie, T.; Chen, C.; Li, C.; Liu, J.; Liu, C.; He, Y. Genome-wide investigation of WRKY gene family in pineapple: Evolution and expression profiles during development and stress. BMC Genom. 2018, 19, 490. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, X.; Han, J.; Lu, W.; Ren, Z. Genome-wide analysis of the WRKY gene family in the cucumber genome and transcriptome-wide identification of WRKY transcription factors that respond to biotic and abiotic stresses. BMC Plant Biol. 2020, 20, 443. [Google Scholar] [CrossRef]
- He, H.; Dong, Q.; Shao, Y.; Jiang, H.; Zhu, S.; Cheng, B.; Xiang, Y. Genome-wide survey and characterization of the WRKY gene family in Populus trichocarpa. Plant Cell Rep. 2012, 31, 1199–1217. [Google Scholar] [CrossRef]
- Yu, Y.; Hu, R.; Wang, H.; Cao, Y.; He, G.; Fu, C.; Zhou, G. MlWRKY12, a novel Miscanthus transcription factor, participates in pith secondary cell wall formation and promotes flowering. Plant Sci. 2013, 212, 1–9. [Google Scholar] [CrossRef]
- Yang, L.; Zhao, X.; Yang, F.; Fan, D.; Jiang, Y.; Luo, K. PtrWRKY19, a novel WRKY transcription factor, contributes to the regulation of pith secondary wall formation in Populus trichocarpa. Sci. Rep. 2016, 6, 18643. [Google Scholar] [CrossRef]
- Li, W.; Tian, Z.; Yu, D. WRKY13 acts in stem development in Arabidopsis thaliana. Plant Sci. 2015, 236, 205–213. [Google Scholar] [CrossRef]
- Wei, Y.; Jin, J.; Liang, D.; Gao, J.; Li, J.; Xie, Q.; Lu, C.; Yang, F.; Zhu, G. Genome-wide identification of Cymbidium sinense WRKY gene family and the importance of its Group III members in response to abiotic stress. Front. Plant Sci. 2022, 13, 969010. [Google Scholar] [CrossRef]
- Li, S.; Zhou, X.; Chen, L.; Huang, W.; Yu, D. Functional characterization of Arabidopsis thaliana WRKY39 in heat stress. Mol. Cells 2010, 29, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Rushton, D.L.; Tripathi, P.; Rabara, R.C.; Lin, J.; Ringler, P.; Boken, A.K.; Langum, T.J.; Smidt, L.; Boomsma, D.D.; Emme, N.J.; et al. WRKY transcription factors: Key components in abscisic acid signalling. Plant Biotechnol. J. 2012, 10, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Deyholos, M.K. Functional characterization of Arabidopsis NaCl-inducible WRKY25 and WRKY33 transcription factors in abiotic stresses. Plant Mol. Biol. 2009, 69, 91–105. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Yu, D. Expression profiles of AtWRKY25, AtWRKY26 and AtWRKY33 under abiotic stresses. Heredity 2010, 32, 848–856. [Google Scholar] [CrossRef]
- Van der Fits, L.; Memelink, J. The jasmonate-inducible AP2/ERF-domain transcription factor ORCA3 activates gene expression via interaction with a jasmonate-responsive promoter element. Plant J. 2001, 25, 43–53. [Google Scholar] [CrossRef]
- De Geyter, N.; Gholami, A.; Goormachtig, S.; Goossens, A. Transcriptional machineries in jasmonate-elicited plant secondary metabolism. Trends Plant Sci. 2012, 17, 349–359. [Google Scholar] [CrossRef]
- Sibéril, Y.; Benhamron, S.; Memelink, J.; Giglioli-Guivarc’H, N.; Thiersault, M.; Boisson, B.; Doireau, P.; Gantet, P. Catharanthus roseus G-box binding factors 1 and 2 act as repressors of strictosidine synthase gene expression in cell cultures. Plant Mol. Biol. 2001, 45, 477–488. [Google Scholar] [CrossRef]
- Chini, A.; Boter, M.; Solano, R. Plant oxylipins: COI1/JAZs/MYC2 as the core jasmonic acid-signalling module. FEBS J. 2009, 276, 4682–4692. [Google Scholar] [CrossRef]
- Skibbe, M.; Qu, N.; Galis, I.; Baldwin, I.T. Induced plant defenses in the natural environment: Nicotiana attenuata WRKY3 and WRKY6 coordinate responses to herbivory. Plant Cell 2008, 20, 1984–2000. [Google Scholar] [CrossRef]
- Duan, S.; Wang, J.; Gao, C.; Jin, C.; Li, D.; Peng, D.; Du, G.; Li, Y.; Chen, M. Functional characterization of a heterologously expressed Brassica napus WRKY41-1 transcription factor in regulating anthocyanin biosynthesis in Arabidopsis thaliana. Plant Sci. 2018, 268, 47–53. [Google Scholar] [CrossRef]
- Kishi-Kaboshi, M.; Takahashi, A.; Hirochika, H. MAMP-responsive MAPK cascades regulate phytoalexin biosynthesis. Plant Signal. Behav. 2010, 5, 1653–1656. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.L.; Fiil, B.K.; Petersen, K.; Nielsen, H.B.; Botanga, C.J.; Thorgrimsen, S.; Palma, K.; Suarez-Rodriguez, M.C.; Sandbech-Clausen, S.; Lichota, J.; et al. Arabidopsis MAP kinase 4 regulates gene expression through transcription factor release in the nucleus. EMBO J. 2008, 27, 2214–2221. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Rui, X. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant. 2020, 13, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Letunic, I.; Bork, P. Interactive Tree of Life (iTOL) v4: Recent updates and new developments. Nucleic Acids Res. 2019, 47, W256–W259. [Google Scholar] [CrossRef]
- Higo, K.; Ugawa, Y.; Iwamoto, M.; Korenaga, T. Plant cis-acting regulatory DNA elements (PLACE) database: 1999. Nucleic Acids Res. 1999, 27, 297–300. [Google Scholar] [CrossRef]
- Wang, X.; Long, J.; Dong, T.; Zheng, D.; Zhang, L.; Peng, C. Establishment of vascular tissue cells capture system by laser microdissection in Neolamarckia cadamba. Guihaia 2021, 41, 1226–1236, (In Chinese with English abstract). [Google Scholar]
- Huang, T.; Long, J.; Liu, S.W.; Yang, Z.; Zhu, Q.; Zhao, X.; Peng, C. Selection and validation of reference genes for mRNA expression by quantitative Real-Time PCR analysis in Neolamarckia cadamba. Sci. Rep. 2018, 8, 9931. [Google Scholar] [CrossRef]
- Zhang, D.; Li, J.J.; Zhang, M.J.; Bao, Y.T.; Yang, X.; Xu, W.Y.; Quyang, K.X.; Chen, X.Y. Selection and validation of reference genes for quantitative RT-PCR analysis in Neolamarckia cadamba. Chin. Bull. Bot. 2018, 53, 829–839. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Z.; Liu, Y.; Fang, H.; Wen, Y.; Wang, Y.; Zhang, J.; Peng, C.; Long, J. Genome-Wide Identification and Expression Analysis of WRKY Gene Family in Neolamarckia cadamba. Int. J. Mol. Sci. 2023, 24, 7537. https://doi.org/10.3390/ijms24087537
Xu Z, Liu Y, Fang H, Wen Y, Wang Y, Zhang J, Peng C, Long J. Genome-Wide Identification and Expression Analysis of WRKY Gene Family in Neolamarckia cadamba. International Journal of Molecular Sciences. 2023; 24(8):7537. https://doi.org/10.3390/ijms24087537
Chicago/Turabian StyleXu, Zuowei, Yutong Liu, Huiting Fang, Yanqiong Wen, Ying Wang, Jianxia Zhang, Changcao Peng, and Jianmei Long. 2023. "Genome-Wide Identification and Expression Analysis of WRKY Gene Family in Neolamarckia cadamba" International Journal of Molecular Sciences 24, no. 8: 7537. https://doi.org/10.3390/ijms24087537
APA StyleXu, Z., Liu, Y., Fang, H., Wen, Y., Wang, Y., Zhang, J., Peng, C., & Long, J. (2023). Genome-Wide Identification and Expression Analysis of WRKY Gene Family in Neolamarckia cadamba. International Journal of Molecular Sciences, 24(8), 7537. https://doi.org/10.3390/ijms24087537



