Differential Effects of Hypoxia versus Hyperoxia or Physoxia on Phenotype and Energy Metabolism in Human Chondrocytes from Osteoarthritic Compared to Macroscopically Normal Cartilage
Abstract
1. Introduction
2. Results
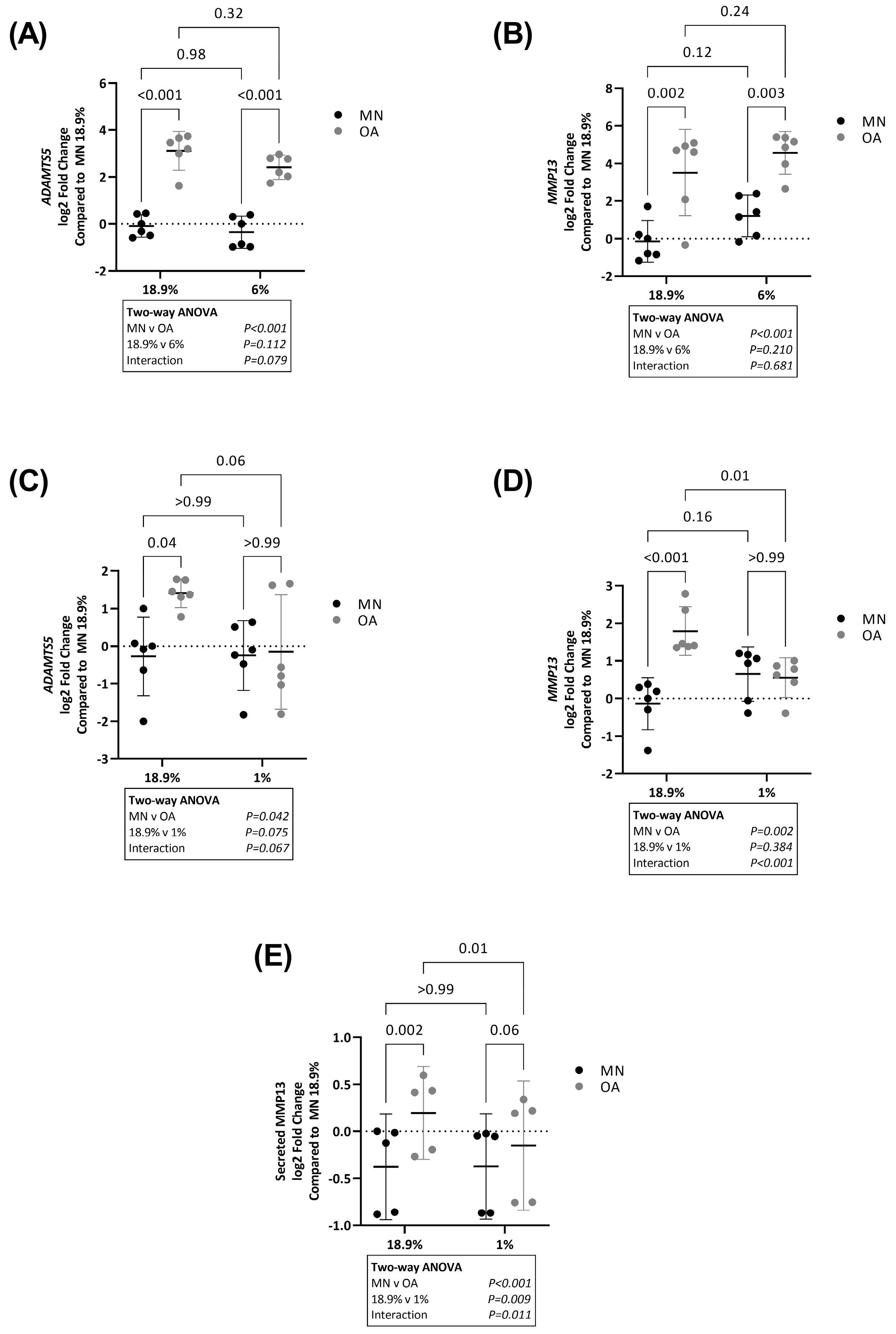
2.1. ADAMTS5 and MMP13 Expression Is Higher in Chondrocytes from Osteoarthritic Cartilage in Hyperoxia and Physoxia but Not Hypoxia
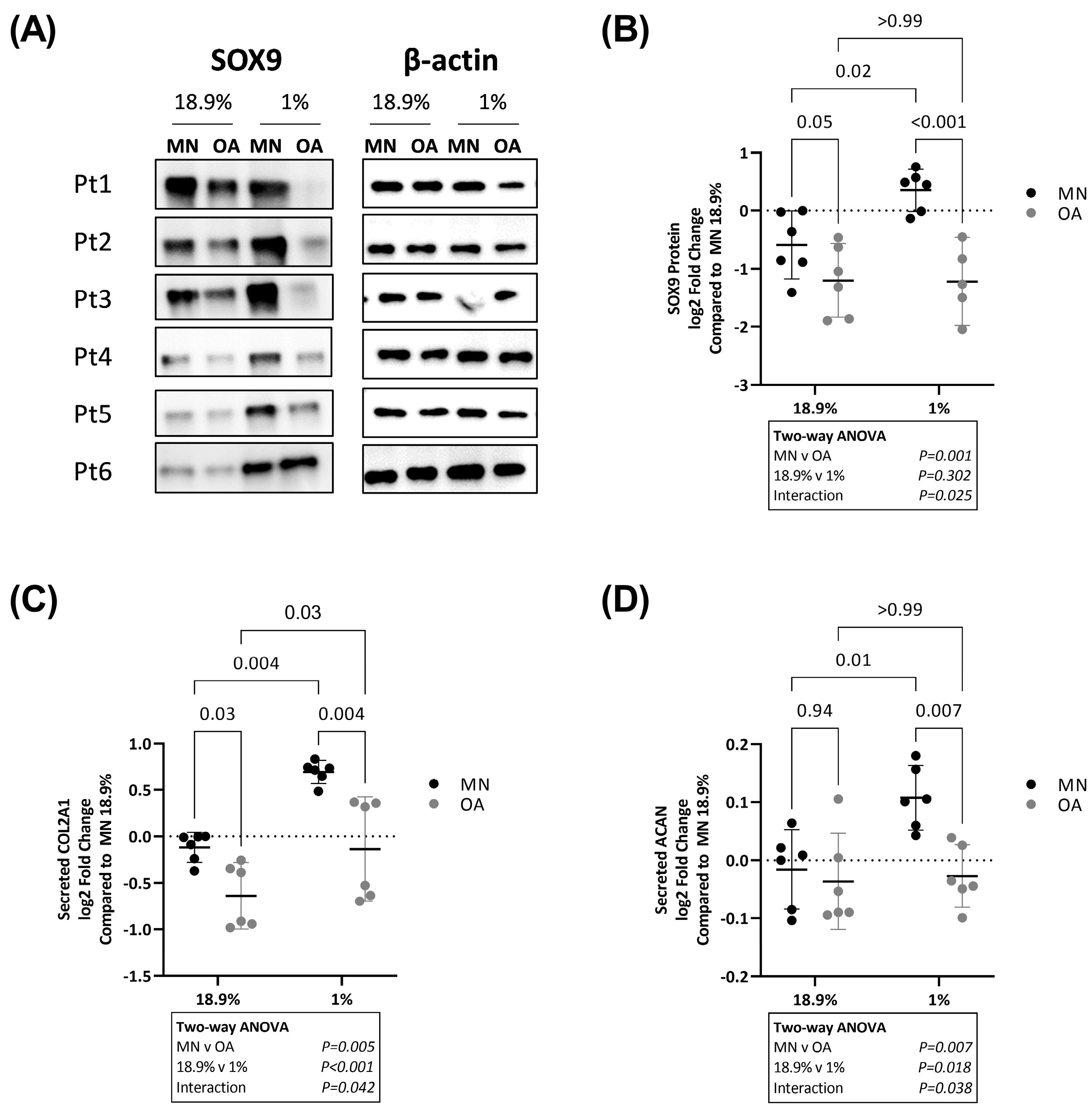
2.2. SOX9, COL2A1 and ACAN Are Upregulated in Chondrocytes from Macroscopically Normal but Not Osteoarthritic Cartilage in Hypoxia
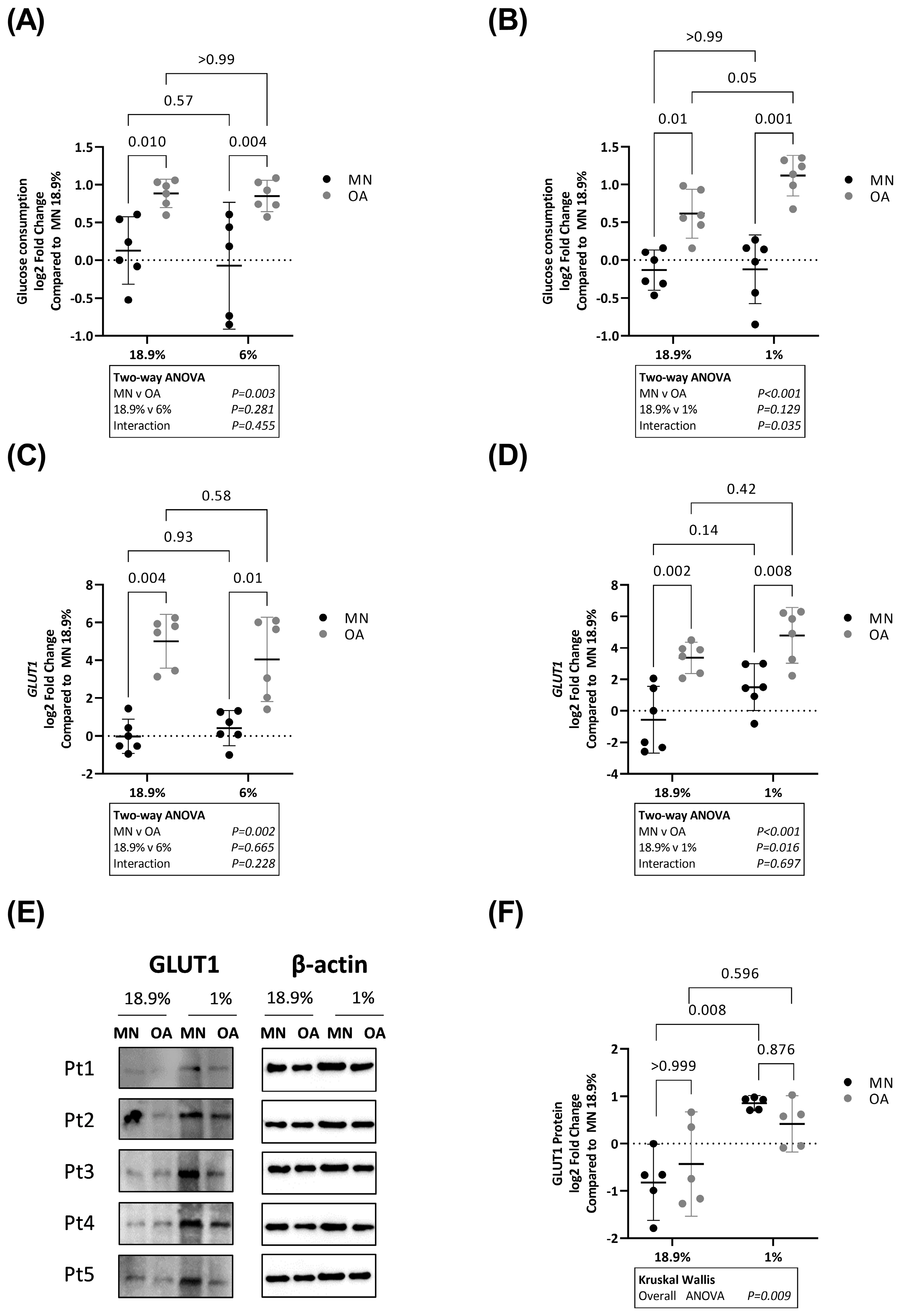
2.3. Glucose Consumption Is Higher in Chondrocytes from Osteoarthritic Cartilage in Hyperoxia, Physoxia and Hypoxia
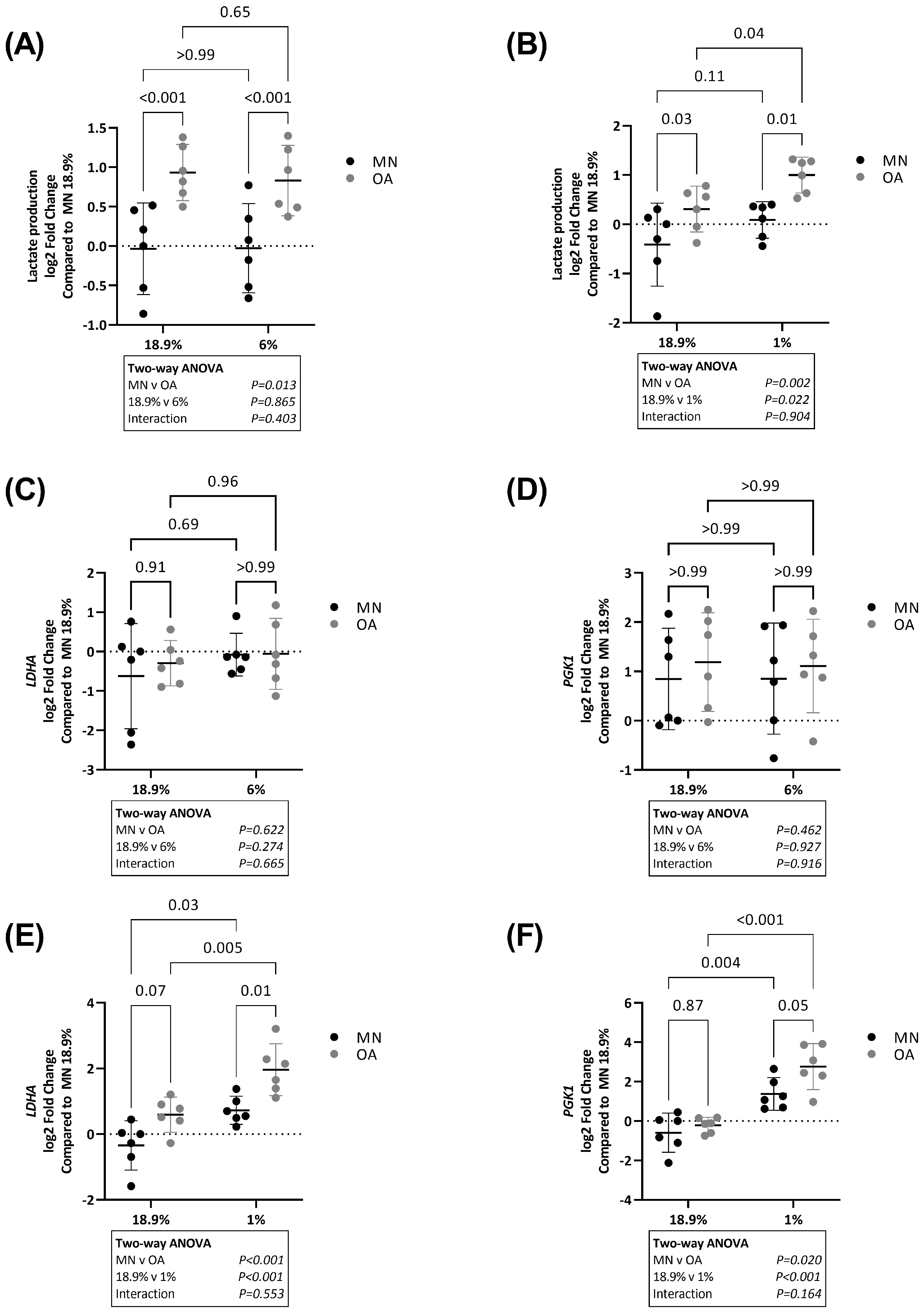
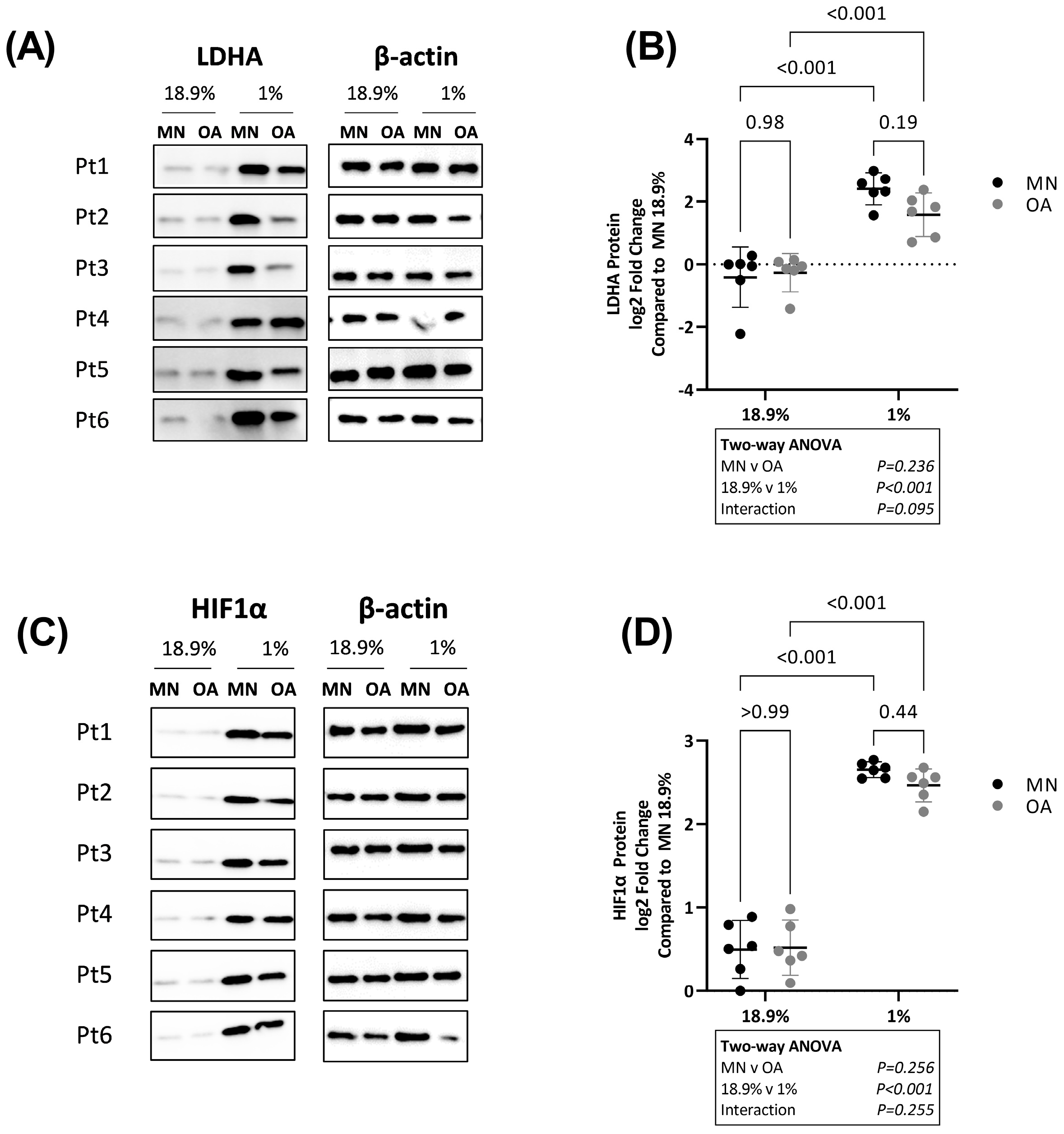
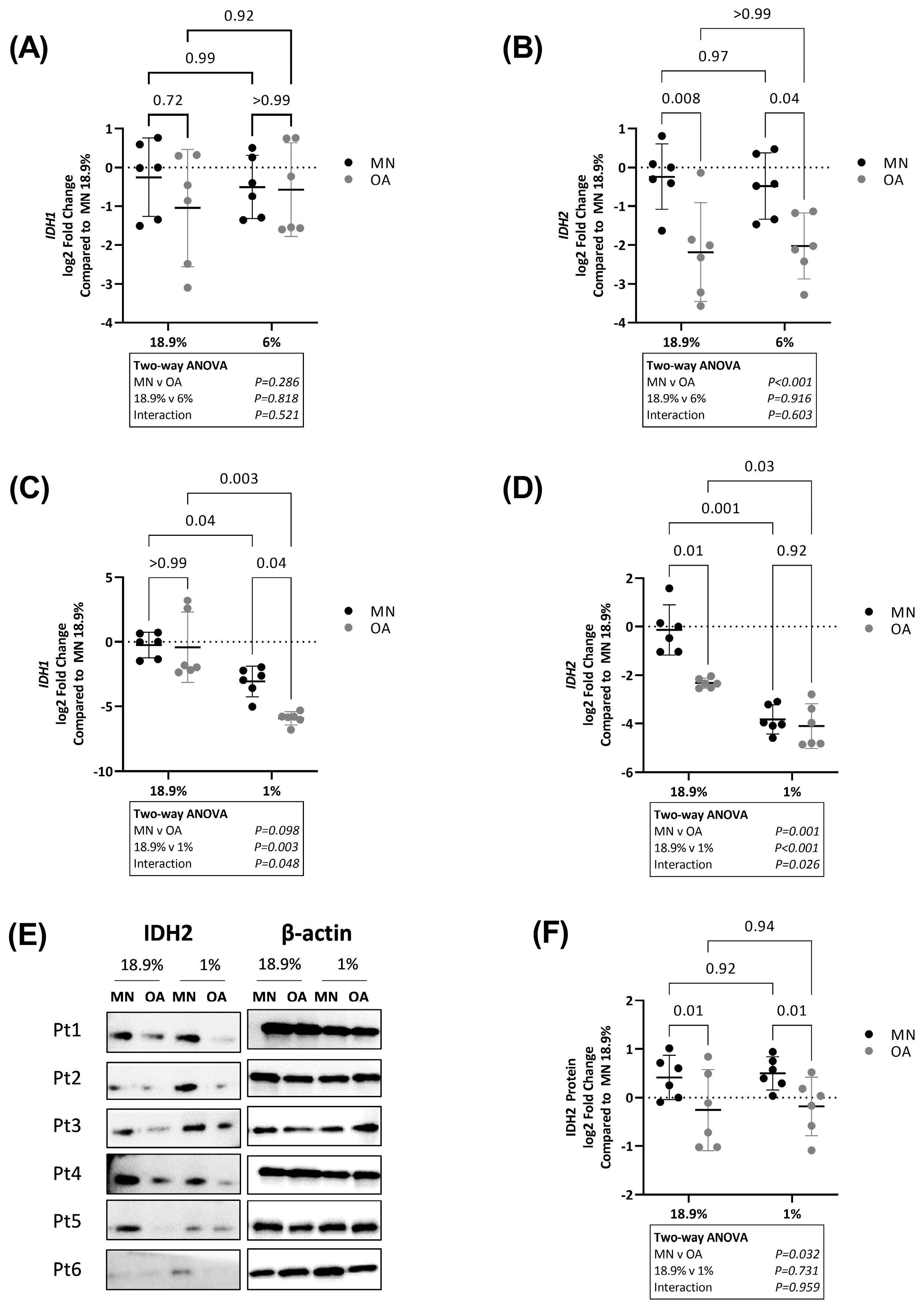
2.4. Chondrocytes from Osteoarthritic Cartilage Utilise More Glycolysis Than Chondrocytes from Macroscopically Normal Cartilage in Hyperoxia, Physoxia and Hypoxia
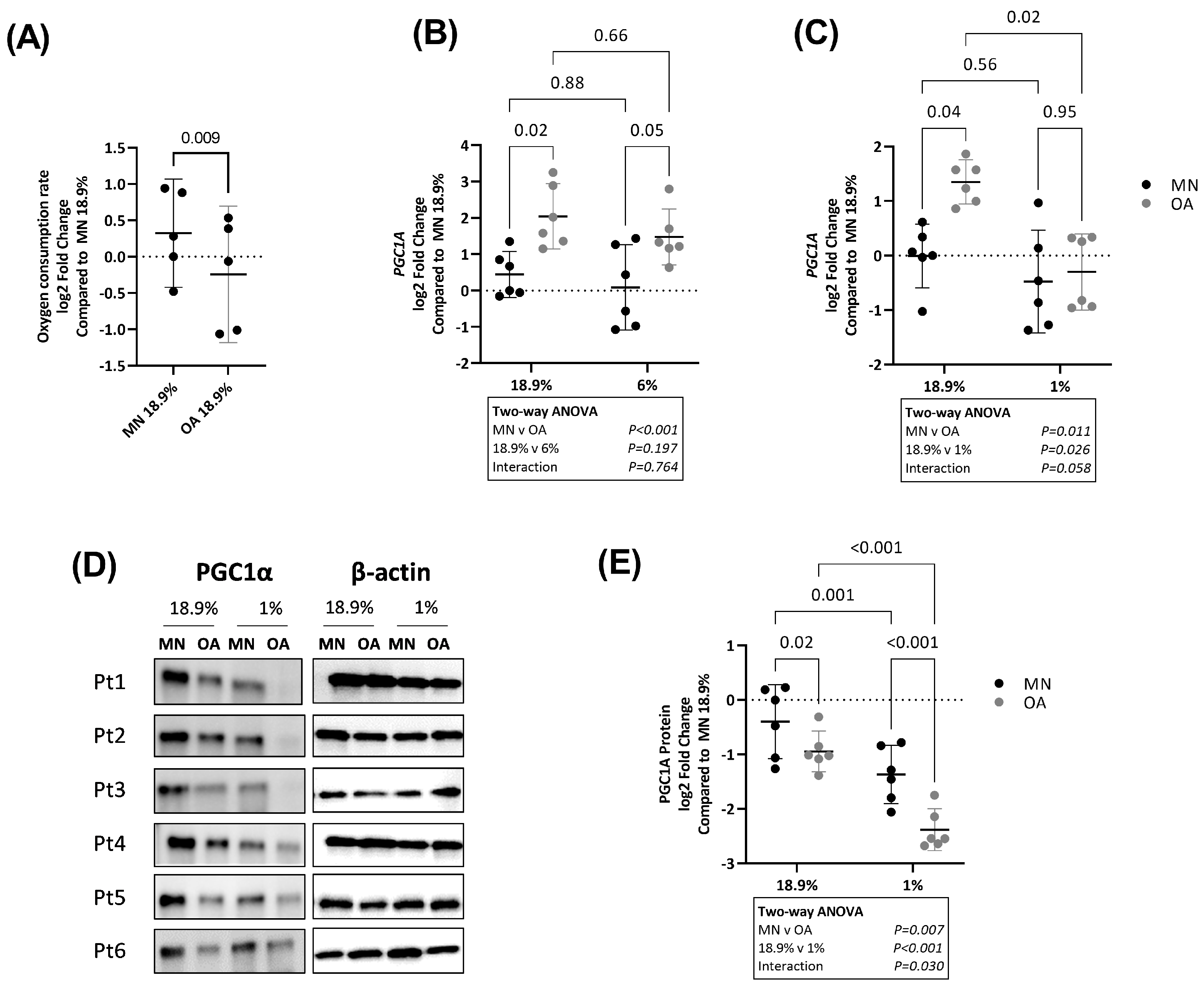
2.5. Chondrocytes from Macroscopically Normal Cartilage Utilise More Oxidative Phosphorylation
3. Discussion
4. Materials and Methods
4.1. Primary Human Chondrocyte Isolation
4.2. Cell Culture
4.3. Real Time PCR
4.4. Western Blotting
4.5. ELISA
4.6. Glucose Consumption and Lactate Production Assays
4.7. Oxygen Consumption Rate (OCR)
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hunter, D.J.; Felson, D.T. Osteoarthritis. BMJ 2006, 332, 639. [Google Scholar] [CrossRef] [PubMed]
- Goldring, M.B. The Role of the Chondrocyte in Osteoarthritis. Arthritis Rheum. 2000, 43, 1916–1926. [Google Scholar] [CrossRef] [PubMed]
- Goldring, M.B. Chondrogenesis, Chondrocyte Differentiation, and Articular Cartilage Metabolism in Health and Osteoarthritis. Ther. Adv. Musculoskelet. Dis. 2012, 4, 269–285. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Marcu, K.B.; Goldring, M.B.; Otero, M. Phenotypic Instability of Chondrocytes in Osteoarthritis: On a Path to Hypertrophy. Ann. N. Y. Acad. Sci. 2019, 1442, 17–34. [Google Scholar] [CrossRef]
- Snelling, S.; Rout, R.; Davidson, R.; Clark, I.; Carr, A.; Hulley, P.A.; Price, A.J. A Gene Expression Study of Normal and Damaged Cartilage in Anteromedial Gonarthrosis, a Phenotype of Osteoarthritis. Osteoarthr. Cartil. 2014, 22, 334–343. [Google Scholar] [CrossRef]
- Rong, J.; Pool, B.; Zhu, M.; Munro, J.; Cornish, J.; McCarthy, G.M.; Dalbeth, N.; Poulsen, R. Basic Calcium Phosphate Crystals Induce Osteoarthritis-Associated Changes in Phenotype Markers in Primary Human Chondrocytes by a Calcium/Calmodulin Kinase 2-Dependent Mechanism. Calcif. Tissue Int. 2019, 104, 331–343. [Google Scholar] [CrossRef]
- Lefebvre, V.; Huang, W.; Harley, V.R.; Goodfellow, P.N.; de Crombrugghe, B. SOX9 Is a Potent Activator of the Chondrocyte-Specific Enhancer of the pro Alpha1(II) Collagen Gene. Mol. Cell. Biol. 1997, 17, 2336–2346. [Google Scholar] [CrossRef]
- Lian, C.; Wang, X.; Qiu, X.; Wu, Z.; Gao, B.; Liu, L.; Liang, G.; Zhou, H.; Yang, X.; Peng, Y.; et al. Collagen Type II Suppresses Articular Chondrocyte Hypertrophy and Osteoarthritis Progression by Promoting Integrin Β1−SMAD1 Interaction. Bone Res. 2019, 7, 8. [Google Scholar] [CrossRef]
- Haq, I.; Murphy, E.; Dacre, J. Osteoarthritis. Postgrad. Med. J. 2003, 79, 377–383. [Google Scholar] [CrossRef]
- Deveza, L.A.; Loeser, R.F. Is Osteoarthritis One Disease or a Collection of Many? Rheumatology 2018, 57, iv34–iv42. [Google Scholar] [CrossRef]
- Mora, J.C.; Przkora, R.; Cruz-Almeida, Y. Knee Osteoarthritis: Pathophysiology and Current Treatment Modalities. J. Pain Res. 2018, 11, 2189. [Google Scholar] [CrossRef]
- Burrage, P.S.; Mix, K.S.; Brinckerhoff, C.E. Matrix Metalloproteinases: Role in Arthritis. Front. Biosci. 2006, 11, 529–543. [Google Scholar] [CrossRef]
- Blanco, F.J.; June, R.K. Cartilage Metabolism, Mitochondria, and Osteoarthritis. J. Am. Acad. Orthop. Surg. 2020, 28, e242–e244. [Google Scholar] [CrossRef]
- Zhai, G. Alteration of Metabolic Pathways in Osteoarthritis. Metabolites 2019, 9, 11. [Google Scholar] [CrossRef]
- Courties, A.; Sellam, J.; Berenbaum, F. Metabolic Syndrome-Associated Osteoarthritis. Curr. Opin. Rheumatol. 2017, 29, 214–222. [Google Scholar] [CrossRef]
- Da Poian, A.T.; El-Bacha, T.; Luz, M.R.M.P. Nutrient Utilization in Humans: Metabolism Pathways. Available online: https://www.nature.com/scitable/topicpage/nutrient-utilization-in-humans-metabolism-pathways-14234029/ (accessed on 1 March 2023).
- Zheng, L.; Zhang, Z.; Sheng, P.; Mobasheri, A. The Role of Metabolism in Chondrocyte Dysfunction and the Progression of Osteoarthritis. Ageing Res. Rev. 2021, 66, 101249. [Google Scholar] [CrossRef]
- Wu, X.; Fan, X.; Crawford, R.; Xiao, Y.; Prasadam, I. The Metabolic Landscape in Osteoarthritis. Aging Dis. 2022, 13, 1166–1182. [Google Scholar] [CrossRef]
- Yang, X.; Chen, W.; Zhao, X.; Chen, L.; Li, W.; Ran, J.; Wu, L. Pyruvate Kinase M2 Modulates the Glycolysis of Chondrocyte and Extracellular Matrix in Osteoarthritis. DNA Cell Biol. 2018, 37, 271–277. [Google Scholar] [CrossRef]
- Mennan, C.; Garcia, J.; McCarthy, H.; Owen, S.; Perry, J.; Wright, K.; Banerjee, R.; Richardson, J.B.; Roberts, S. Human Articular Chondrocytes Retain Their Phenotype in Sustained Hypoxia While Normoxia Promotes Their Immunomodulatory Potential. Cartilage 2019, 10, 467–479. [Google Scholar] [CrossRef]
- Li, H.; Li, X.; Jing, X.; Li, M.; Ren, Y.; Chen, J.; Yang, C.; Wu, H.; Guo, F. Hypoxia Promotes Maintenance of the Chondrogenic Phenotype in Rat Growth Plate Chondrocytes through the HIF-1α/YAP Signaling Pathway. Int. J. Mol. Med. 2018, 42, 3181–3192. [Google Scholar] [CrossRef]
- Li, M.; Ning, J.; Wang, J.; Yan, Q.; Zhao, K.; Jia, X. SETD7 Regulates Chondrocyte Differentiation and Glycolysis via the Hippo Signaling Pathway and HIF-1α. Int. J. Mol. Med. 2021, 48, 210. [Google Scholar] [CrossRef] [PubMed]
- Fermor, B.; Christensen, S.E.; Youn, I.; Cernanec, J.M.; Davies, C.M.; Weinberg, J.B. Oxygen, Nitric Oxide and Articular Cartilage. Eur. Cell. Mater. 2007, 13, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Liyanage, C.; Plan, M.; Stark, T.; McCubbin, T.; Barrero, R.A.; Batra, J.; Crawford, R.; Xiao, Y.; Prasadam, I. Dysregulated Energy Metabolism Impairs Chondrocyte Function in Osteoarthritis. Osteoarthr. Cartil. 2022. [Google Scholar] [CrossRef] [PubMed]
- Salinas, D.; Minor, C.A.; Carlson, R.P.; McCutchen, C.N.; Mumey, B.M.; June, R.K. Combining Targeted Metabolomic Data with a Model of Glucose Metabolism: Toward Progress in Chondrocyte Mechanotransduction. PLoS ONE 2017, 12, e0168326. [Google Scholar] [CrossRef]
- Thoms, B.L.; Dudek, K.A.; Lafont, J.E.; Murphy, C.L. Hypoxia Promotes the Production and Inhibits the Destruction of Human Articular Cartilage. Arthritis Rheum. 2013, 65, 1302–1312. [Google Scholar] [CrossRef]
- Markway, B.D.; Cho, H.; Johnstone, B. Hypoxia Promotes Redifferentiation and Suppresses Markers of Hypertrophy and Degeneration in Both Healthy and Osteoarthritic Chondrocytes. Arthritis Res. Ther. 2013, 15, R92. [Google Scholar] [CrossRef]
- Robins, J.C.; Akeno, N.; Mukherjee, A.; Dalal, R.R.; Aronow, B.J.; Koopman, P.; Clemens, T.L. Hypoxia Induces Chondrocyte-Specific Gene Expression in Mesenchymal Cells in Association with Transcriptional Activation of Sox9. Bone 2005, 37, 313–322. [Google Scholar] [CrossRef]
- Parker, S.J.; Metallo, C.M. Metabolic Consequences of Oncogenic IDH Mutations. Pharmacol. Ther. 2015, 152, 54–62. [Google Scholar] [CrossRef]
- Kierans, S.J.; Taylor, C.T. Regulation of Glycolysis by the Hypoxia-Inducible Factor (HIF): Implications for Cellular Physiology. J. Physiol. 2021, 599, 23–37. [Google Scholar] [CrossRef]
- Liang, H.; Ward, W.F. PGC-1alpha: A Key Regulator of Energy Metabolism. Adv. Physiol. Educ. 2006, 30, 145–151. [Google Scholar] [CrossRef]
- MB Goldring, S.G. Osteoarthritis. J. Cell Physiol. 2007, 213, 626–634. [Google Scholar] [CrossRef]
- Duval, E.; Leclercq, S.; Elissalde, J.M.; Demoor, M.; Galéra, P.; Boumédiene, K. Hypoxia-Inducible Factor 1α Inhibits the Fibroblast-like Markers Type I and Type III Collagen during Hypoxia-Induced Chondrocyte Redifferentiation: Hypoxia Not Only Induces Type II Collagen and Aggrecan, but It Also Inhibits Type I and Type III Collagen in the Hypoxia-Inducible Factor 1α-Dependent Redifferentiation of Chondrocytes. Arthritis Rheum. 2009, 60, 3038–3048. [Google Scholar] [CrossRef]
- Rankin, K.S.; Lakey, R.L.; Gerrand, C.H.; Sprowson, A.P.; McCaskie, A.W.; Birch, M.A. A Novel in Vitro Model to Investigate Behavior of Articular Chondrocytes in Osteoarthritis. J. Rheumatol. 2010, 37, 426–431. [Google Scholar] [CrossRef]
- Grimmer, C.; Balbus, N.; Lang, U.; Aigner, T.; Cramer, T.; Müller, L.; Swoboda, B.; Pfander, D. Regulation of Type II Collagen Synthesis during Osteoarthritis by Prolyl-4-Hydroxylases: Possible Influence of Low Oxygen Levels. Am. J. Pathol. 2006, 169, 491–502. [Google Scholar] [CrossRef]
- Tew, S.R.; Clegg, P.D. Analysis of Post Transcriptional Regulation of SOX9 MRNA during in Vitro Chondrogenesis. Tissue Eng. Part A 2011, 17, 1801–1807. [Google Scholar] [CrossRef]
- Asopa, V.; Vincent, T.; Saklatvala, J. The Effects of Age and Cell Isolation on Collagen II Synthesis by Articular Chondrocytes: Evidence for Transcriptional and Posttranscriptional Regulation. Biomed Res. Int. 2020, 2020, 4060135. [Google Scholar] [CrossRef]
- Nahir, A.M. Aerobic Glycolysis: A Study of Human Articular Cartilage. Cell Biochem. Funct. 1987, 5, 109–112. [Google Scholar] [CrossRef]
- DeBerardinis, R.J.; Lum, J.J.; Hatzivassiliou, G.; Thompson, C.B. The Biology of Cancer: Metabolic Reprogramming Fuels Cell Growth and Proliferation. Cell Metab. 2008, 7, 11–20. [Google Scholar] [CrossRef]
- Heywood, H.K.; Knight, M.M.; Lee, D.A. Both Superficial and Deep Zone Articular Chondrocyte Subpopulations Exhibit the Crabtree Effect but Have Different Basal Oxygen Consumption Rates. J. Cell. Physiol. 2010, 223, 630–639. [Google Scholar] [CrossRef]
- Okada, K.; Mori, D.; Makii, Y.; Nakamoto, H.; Murahashi, Y.; Yano, F.; Chang, S.H.; Taniguchi, Y.; Kobayashi, H.; Semba, H.; et al. Hypoxia-Inducible Factor-1 Alpha Maintains Mouse Articular Cartilage through Suppression of NF-ΚB Signaling. Sci. Rep. 2020, 10, 5425. [Google Scholar] [CrossRef]
- Pfander, D.; Cramer, T.; Swoboda, B. Hypoxia and HIF-1alpha in Osteoarthritis. Int. Orthop. 2005, 29, 6–9. [Google Scholar] [CrossRef] [PubMed]
- Clérigues, V.; Murphy, C.L.; Guillén, M.I.; Alcaraz, M.J. Haem Oxygenase-1 Induction Reverses the Actions of Interleukin-1β on Hypoxia-Inducible Transcription Factors and Human Chondrocyte Metabolism in Hypoxia. Clin. Sci. 2013, 125, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Garnero, P.; Ayral, X.; Rousseau, J.-C.; Christgau, S.; Sandell, L.J.; Dougados, M.; Delmas, P.D. Uncoupling of Type II Collagen Synthesis and Degradation Predicts Progression of Joint Damage in Patients with Knee Osteoarthritis. Arthritis Rheum. 2002, 46, 2613–2624. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Zhang, C.; Ni, L.; Huang, C.; Chen, D.; Shi, K.; Jin, H.; Zhang, K.; Li, Y.; Xie, L.; et al. Stabilization of HIF-1α Alleviates Osteoarthritis via Enhancing Mitophagy. Cell Death Dis. 2020, 11, 481. [Google Scholar] [CrossRef]
- Zhuo, Q.; Yang, W.; Chen, J.; Wang, Y. Metabolic Syndrome Meets Osteoarthritis. Nat. Rev. Rheumatol. 2012, 8, 729–737. [Google Scholar] [CrossRef]
- Pauli, C.; Whiteside, R.; Heras, F.L.; Nesic, D.; Koziol, J.; Grogan, S.P.; Matyas, J.; Pritzker, K.P.H.; D’Lima, D.D.; Lotz, M.K. Comparison of Cartilage Histopathology Assessment Systems on Human Knee Joints at All Stages of Osteoarthritis Development. Osteoarthr. Cartil. 2012, 20, 476–485. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jain, L.; Bolam, S.M.; Monk, A.P.; Munro, J.T.; Chen, E.; Tamatea, J.; Dalbeth, N.; Poulsen, R.C. Differential Effects of Hypoxia versus Hyperoxia or Physoxia on Phenotype and Energy Metabolism in Human Chondrocytes from Osteoarthritic Compared to Macroscopically Normal Cartilage. Int. J. Mol. Sci. 2023, 24, 7532. https://doi.org/10.3390/ijms24087532
Jain L, Bolam SM, Monk AP, Munro JT, Chen E, Tamatea J, Dalbeth N, Poulsen RC. Differential Effects of Hypoxia versus Hyperoxia or Physoxia on Phenotype and Energy Metabolism in Human Chondrocytes from Osteoarthritic Compared to Macroscopically Normal Cartilage. International Journal of Molecular Sciences. 2023; 24(8):7532. https://doi.org/10.3390/ijms24087532
Chicago/Turabian StyleJain, Lekha, Scott M. Bolam, A. Paul Monk, Jacob T. Munro, Even Chen, Jade Tamatea, Nicola Dalbeth, and Raewyn C. Poulsen. 2023. "Differential Effects of Hypoxia versus Hyperoxia or Physoxia on Phenotype and Energy Metabolism in Human Chondrocytes from Osteoarthritic Compared to Macroscopically Normal Cartilage" International Journal of Molecular Sciences 24, no. 8: 7532. https://doi.org/10.3390/ijms24087532
APA StyleJain, L., Bolam, S. M., Monk, A. P., Munro, J. T., Chen, E., Tamatea, J., Dalbeth, N., & Poulsen, R. C. (2023). Differential Effects of Hypoxia versus Hyperoxia or Physoxia on Phenotype and Energy Metabolism in Human Chondrocytes from Osteoarthritic Compared to Macroscopically Normal Cartilage. International Journal of Molecular Sciences, 24(8), 7532. https://doi.org/10.3390/ijms24087532





