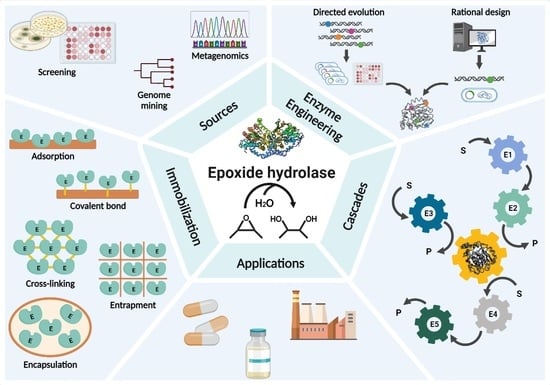
Epoxide Hydrolases: Multipotential Biocatalysts
Abstract
1. Introduction
2. Epoxides and Diols as Chiral Precursors and Their Applications
3. Natural and Recombinant EHs
4. Improvement of EHs by Enzyme Engineering
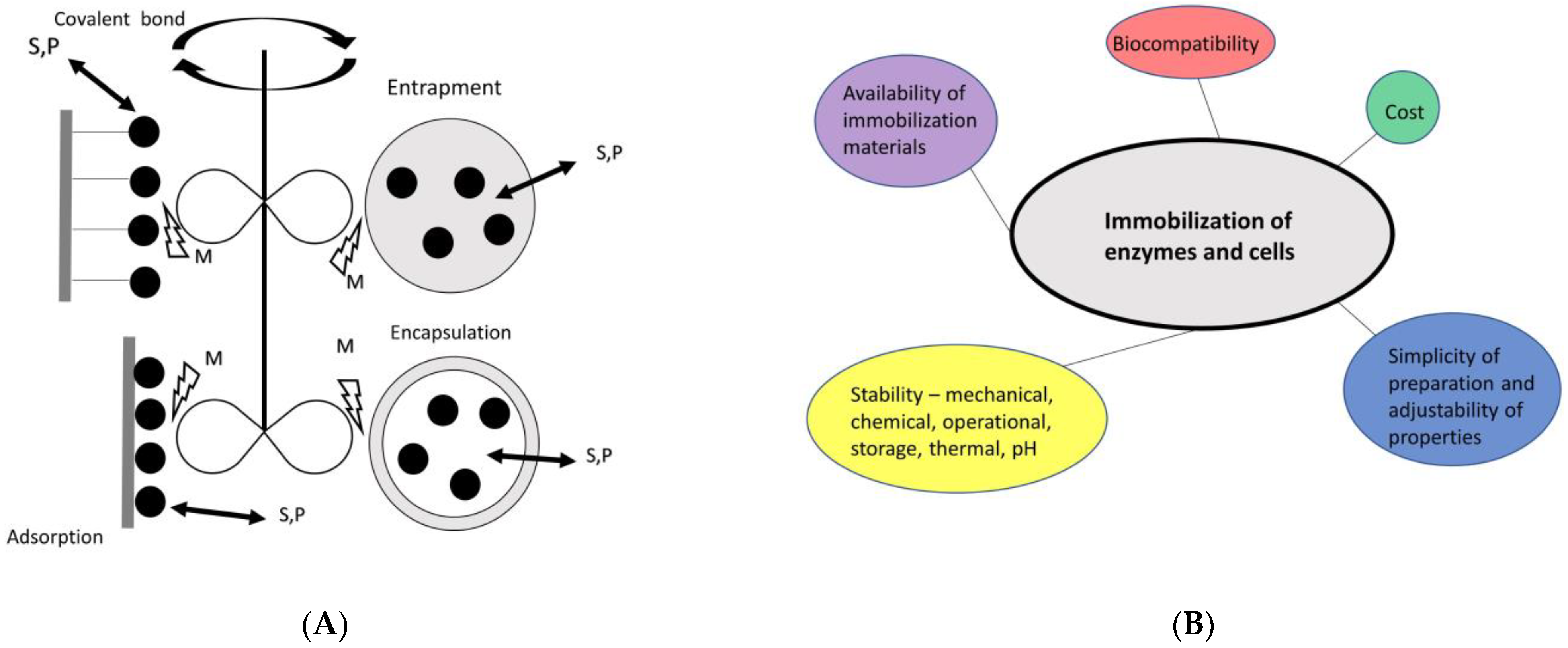
5. Immobilization of EHs
6. Whole-Cell Cascade Biotransformations Using Microbial Epoxide Hydrolases
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Choi, W.J.; Choi, C.Y. Production of chiral epoxides: Epoxide hydrolase-catalyzed enantioselective hydrolysis. Biotechnol. Bioprocess. Eng. 2005, 10, 167. [Google Scholar] [CrossRef]
- BRENDA. Available online: www.brenda-enzymes.org (accessed on 20 March 2023).
- Morisseau, C.; Hammock, B.D. Gerry Brooks and epoxide hydrolases: Four decades to a pharmaceutical. Pest Manag. Sci. 2008, 64, 594–609. [Google Scholar] [CrossRef]
- Decker, M.; Arand, M.; Cronin, A. Mammalian epoxide hydrolases in xenobiotic metabolism and signalling. Arch. Toxicol. 2009, 83, 297–318. [Google Scholar] [CrossRef]
- Orru, R.V.A.; Archelas, A.; Furstoss, R.; Faber, K. Epoxide Hydrolases and Their Synthetic Applications. In Biotransformations; Faber, K., Ed.; Springer: Berlin/Heidelberg, Germany, 1999; pp. 145–167. [Google Scholar]
- Archelas, A.; Furstoss, R. Epoxide hydrolases: New tools for the synthesis of fine organic chemicals. Trends Biotechnol. 1998, 16, 108–116. [Google Scholar] [CrossRef]
- Saini, P.; Sareen, D. An Overview on the Enhancement of Enantioselectivity and Stability of Microbial Epoxide Hydrolases. Mol. Biotechnol. 2017, 59, 98–116. [Google Scholar] [CrossRef]
- Choi, W.J. Biotechnological production of enantiopure epoxides by enzymatic kinetic resolution. Appl. Microbiol. Biotechnol. 2009, 84, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Bala, N.; Chimni, S.S. Recent developments in the asymmetric hydrolytic ring opening of epoxides catalysed by microbial epoxide hydrolase. Tetrahedron Asymmetry 2010, 21, 2879–2898. [Google Scholar] [CrossRef]
- Archelas, A.; Iacazio, G.; Kotik, M. Epoxide Hydrolases and their Application in Organic Synthesis. In Green Biocatalysis; Patel, R.N., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; pp. 179–229. [Google Scholar]
- Jiang, W.; Fang, B. Synthesizing Chiral Drug Intermediates by Biocatalysis. Appl. Biochem. Biotechnol. 2020, 192, 146–179. [Google Scholar] [CrossRef] [PubMed]
- Research, V.M. Global Chiral Chemicals Market Size by Technology, by Application, by Geographic Scope and Forecast. Available online: https://www.verifiedmarketresearch.com/product/chiral-chemicals-market/ (accessed on 11 April 2023).
- Saini, P.; Sareen, D. Epoxide Hydrolase for the Synthesis of Chiral Drugs. In Nanoscience and Biotechnology for Environmental Applications, 1st ed.; Gothandam, K.M., Ranjan, S., Dasgupta, N., Lichtfouse, E., Eds.; Environmental Chemistry for a Sustainable World; Springer: Cham, Switzerland, 2019; Volume 22, pp. 141–198. [Google Scholar]
- Xuan, J.; Feng, Y. Enantiomeric Tartaric Acid Production Using cis-Epoxysuccinate Hydrolase: History and Perspectives. Molecules 2019, 24, 903. [Google Scholar] [CrossRef]
- Chen, X.J.; Archelas, A.; Furstoss, R. Microbiological transformations. 27. The first examples for preparative-scale enantioselective or diastereoselective epoxide hydrolyses using microorganisms. An unequivocal access to all four bisabolol stereoisomers. J. Org. Chem. 1993, 58, 5528–5532. [Google Scholar] [CrossRef]
- Jacobs, M.H.J.; van den Wijngaard, A.J.; Pentenga, M.; Janssen, D.B. Characterization of the epoxide hydrolase from an epichlorohydrin-degrading Pseudomonas sp. Eur. J. Biochem. 1991, 202, 1217–1222. [Google Scholar] [CrossRef] [PubMed]
- Weijers, C.A.G.M. Enantioselective hydrolysis of aryl, alicyclic and aliphatic epoxides by Rhodotorula glutinis. Tetrahedron Asymmetry 1997, 8, 639–647. [Google Scholar] [CrossRef]
- Chen, L.; Shen, H.; Wei, C.; Zhu, Q. Bioresolution of (R)-glycidyl azide by Aspergillus niger ZJUTZQ208: A new and concise synthon for chiral vicinal amino alcohols. Appl. Microbiol. Biotechnol. 2013, 97, 2609–2616. [Google Scholar] [CrossRef] [PubMed]
- Li, F.-L.; Zheng, Y.-C.; Li, H.; Chen, F.-F.; Yu, H.-L.; Xu, J.-H. Preparing β-blocker (R)-Nifenalol based on enantioconvergent synthesis of (R)-p-nitrophenylglycols in continuous packed bed reactor with epoxide hydrolase. Tetrahedron 2019, 75, 1706–1710. [Google Scholar] [CrossRef]
- Pedragosa-Moreau, S.; Morisseau, C.; Baratti, J.; Zylber, J.; Archelas, A.; Furstoss, R. Microbiological transformations 37. An enantioconvergent synthesis of the β-blocker (R)-Nifénalol® using a combined chemoenzymatic approach. Tetrahedron 1997, 53, 9707–9714. [Google Scholar] [CrossRef]
- Manoj, K.M.; Archelas, A.; Baratti, J.; Furstoss, R. Microbiological transformations. Part 45: A green chemistry preparative scale synthesis of enantiopure building blocks of Eliprodil: Elaboration of a high substrate concentration epoxide hydrolase-catalyzed hydrolytic kinetic resolution process. Tetrahedron 2001, 57, 695–701. [Google Scholar] [CrossRef]
- Zhang, J.; Reddy, J.; Roberge, C.; Senanayake, C.; Greasham, R.; Chartrain, M. Chiral bio-resolution of racemic indene oxide by fungal epoxide hydrolases. J. Ferment. Bioeng. 1995, 80, 244–246. [Google Scholar] [CrossRef]
- Cleij, M.; Archelas, A.; Furstoss, R. Microbiological Transformations 43. Epoxide Hydrolases as Tools for the Synthesis of Enantiopure α-Methylstyrene Oxides: A New and Efficient Synthesis of (S)-Ibuprofen. J. Org. Chem. 1999, 64, 5029–5035. [Google Scholar] [CrossRef]
- Li, C.; Liu, Q.; Song, X.; Ding, D.; Ji, A.; Qu, Y. Epoxide hydrolase-catalyzed resolution of ethyl 3-phenylglycidate using whole cells of Pseudomonas sp. Biotechnol. Lett. 2003, 25, 2113–2116. [Google Scholar] [CrossRef] [PubMed]
- Ueberbacher, B.J.; Osprian, I.; Mayer, S.F.; Faber, K. A Chemoenzymatic, Enantioconvergent, Asymmetric Total Synthesis of(R)-Fridamycin E. Eur. J. Org. Chem. 2005, 2005, 1266–1270. [Google Scholar] [CrossRef]
- Kong, X.-D.; Yuan, S.; Li, L.; Chen, S.; Xu, J.-H.; Zhou, J. Engineering of an epoxide hydrolase for efficient bioresolution of bulky pharmaco substrates. Proc. Natl. Acad. Sci. USA 2014, 111, 15717–15722. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.J.; Puah, S.M.; Tan, L.L.; Ng, S.S. Production of (R)-ethyl-3,4-epoxybutyrate by newly isolated Acinetobacter baumannii containing epoxide hydrolase. Appl. Microbiol. Biotechnol. 2008, 79, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Wang, Z.; Li, Z. Preparation of (S)-2-, 3-, and 4-chlorostyrene oxides with the epoxide hydrolase from Sphingomonas sp. HXN-200. Tetrahedron Asymmetry 2008, 19, 407–415. [Google Scholar] [CrossRef]
- Monterde, M.I.; Lombard, M.; Archelas, A.; Cronin, A.; Arand, M.; Furstoss, R. Enzymatic transformations. Part 58: Enantioconvergent biohydrolysis of styrene oxide derivatives catalysed by the Solanum tuberosum epoxide hydrolase. Tetrahedron Asymmetry 2004, 15, 2801–2805. [Google Scholar] [CrossRef]
- Monfort, N.; Archelas, A.; Furstoss, R. Enzymatic transformations. Part 53: Epoxide hydrolase-catalysed resolution of key synthons for azole antifungal agents. Tetrahedron Asymmetry 2002, 13, 2399–2401. [Google Scholar] [CrossRef]
- Monfort, N.; Archelas, A.; Furstoss, R. Enzymatic transformations. Part 55: Highly productive epoxide hydrolase catalysed resolution of an azole antifungal key synthon. Tetrahedron 2004, 60, 601–605. [Google Scholar] [CrossRef]
- Fujino, A.; Asano, M.; Yamaguchi, H.; Shirasaka, N.; Sakoda, A.; Ikunaka, M.; Obata, R.; Nishiyama, S.; Sugai, T. Bacillus subtilis epoxide hydrolase-catalyzed preparation of enantiopure 2-methylpropane-1,2,3-triol monobenzyl ether and its application to expeditious synthesis of (R)-bicalutamide. Tetrahedron Lett. 2007, 48, 979–983. [Google Scholar] [CrossRef]
- Roiban, G.-D.; Sutton, P.W.; Splain, R.; Morgan, C.; Fosberry, A.; Honicker, K.; Homes, P.; Boudet, C.; Dann, A.; Guo, J.; et al. Development of an Enzymatic Process for the Production of (R)-2-Butyl-2-ethyloxirane. Org. Process Res. Dev. 2017, 21, 1302–1310. [Google Scholar] [CrossRef]
- Bottalla, A.-L.; Ibrahim-Ouali, M.; Santelli, M.; Furstoss, R.; Archelas, A. Epoxide Hydrolase-Catalysed Kinetic Resolution of a Spiroepoxide, a Key Building Block of Various 11-Heterosteroids. Adv. Synth. Catal. 2007, 349, 1102–1110. [Google Scholar] [CrossRef]
- Goswami, A.; Totleben, M.J.; Singh, A.K.; Patel, R.N. Stereospecific enzymatic hydrolysis of racemic epoxide: A process for making chiral epoxide. Tetrahedron Asymmetry 1999, 10, 3167–3175. [Google Scholar] [CrossRef]
- Patel, R.N. Chapter 11—Applications of Biocatalysis for Pharmaceuticals and Chemicals. In Organic Synthesis Using Biocatalysis; Goswami, A., Stewart, J.D., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 339–411. [Google Scholar]
- Pienaar, D.P.; Mitra, R.K.; Deventer, T.I.v.; Botes, A.L. Synthesis of a variety of optically active hydroxylated heterocyclic compounds using epoxide hydrolase technology. Tetrahedron Lett. 2008, 49, 6752–6755. [Google Scholar] [CrossRef]
- Halama, A.; Kruliš, R.; Rymeš, J. A Convenient Synthesis of Rivaroxaban from (S)-Epichlorohydrin. Org. Prep. Proced. Int. 2020, 52, 201–211. [Google Scholar] [CrossRef]
- Halama, A.; Krulis, R.; Dammer, O.; Kalasek, S. A Process for the Preparation of Rivaroxaban Based on the Use of (S)-Epichlorohydrin. W.O. Patent No. 2013/120465, 22 August 2013. [Google Scholar]
- Kasai, N.; Suzuki, T.; Furukawa, Y. Chiral C3 epoxides and halohydrins: Their preparation and synthetic application. J. Mol. Catal. B Enzym. 1998, 4, 237–252. [Google Scholar] [CrossRef]
- Gu, S.; Wang, X.; Li, Q. A Preparation Method of high-Purity L-Carnitine. W.O. Patent No. 2010/043110, 22 April 2010. [Google Scholar]
- Chang, J.-Y.; Lee, H.S.; Kim, D.J.; Ko, M.-S.; Kim, N.D.; Chang, Y.K.; Kim, M.S. Method for Preparing Sitagliptin and Amine Salt Intermediates Used Therein. W.O. Patent No. 2011/102640, 25 August 2011. [Google Scholar]
- Kitaori, K.; Takehira, Y.; Furukawa, Y.; Yoshimoto, H.; Otera, J. A Pratical Synthesis of Optically Active Atenolol from Chiral Epichlorohydrin. Chem. Pharm. Bull. 1997, 45, 412–414. [Google Scholar] [CrossRef]
- Narina, S.V.; Sudalai, A. Enantioselective synthesis of (S)-timolol via kinetic resolution of terminal epoxides and dihydroxylation of allylamines. Tetrahedron 2007, 63, 3026–3030. [Google Scholar] [CrossRef]
- Ling, X.; Lu, D.; Wang, J.; Chen, J.; Ding, L.; Chen, J.; Chai, H.; Ouyang, P. Kinetic investigation on enantioselective hydrolytic resolution of epichlorohydrin by crude epoxide hydrolase from domestic duck liver. Afr. J. Biotechnol. 2011, 10, 3436–3443. [Google Scholar] [CrossRef]
- Zhang, Z.; Sheng, Y.; Jiang, K.; Wang, Z.; Zheng, Y.; Zhu, Q. Bio-resolution of glycidyl (o, m, p)-methylphenyl ethers by Bacillus megaterium. Biotechnol. Lett. 2010, 32, 513–516. [Google Scholar] [CrossRef]
- Kotik, M.; Brichac, J.; Kyslík, P. Novel microbial epoxide hydrolases for biohydrolysis of glycidyl derivatives. J. Biotechnol. 2005, 120, 364–375. [Google Scholar] [CrossRef]
- Zocher, F.; Enzelberger, M.M.; Bornscheuer, U.T.; Hauer, B.; Wohlleben, W.; Schmid, R.D. Epoxide hydrolase activity of Streptomyces strains. J. Biotechnol. 2000, 77, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Moussou, P.; Archelas, A.; Furstoss, R. Microbiological transformations 41.: Screening for novel fungal epoxide hydrolases. J. Mol. Catal. B Enzym. 1998, 5, 447–458. [Google Scholar] [CrossRef]
- Yeates, C.A.; van Dyk, M.S.; Botes, A.L.; Breytenbach, J.C.; Krieg, H.M. Biocatalysis of nitro substituted styrene oxides by non-conventional yeasts. Biotechnol. Lett. 2003, 25, 675–680. [Google Scholar] [CrossRef] [PubMed]
- Osprian, I.; Kroutil, W.; Mischitz, M.; Faber, K. Biocatalytic resolution of 2-methyl-2-(aryl)alkyloxiranes using novel bacterial epoxide hydrolases. Tetrahedron Asymmetry 1997, 8, 65–71. [Google Scholar] [CrossRef]
- Woo, J.-H.; Kang, K.-M.; Kwon, T.-H.; Park, N.-H.; Lee, E.Y. Isolation, identification and characterization of marine bacteria exhibiting complementary enantioselective epoxide hydrolase activity for preparing chiral chlorinated styrene oxide derivatives. J. Ind. Eng. Chem. 2015, 28, 225–228. [Google Scholar] [CrossRef]
- Woo, J.-H.; Kwon, T.-H.; Kim, J.-T.; Kim, C.-G.; Lee, E.Y. Identification and characterization of epoxide hydrolase activity of polycyclic aromatic hydrocarbon-degrading bacteria for biocatalytic resolution of racemic styrene oxide and styrene oxide derivatives. Biotechnol. Lett. 2013, 35, 599–606. [Google Scholar] [CrossRef]
- Woo, J.-H.; Kim, H.-S.; Park, N.-H.; Suk, H.Y. Isolation of a novel strain, Sphingorhabdus sp. YGSMI21 and characterization of its enantioselective epoxide hydrolase activity. J. Microbiol. 2021, 59, 675–680. [Google Scholar] [CrossRef] [PubMed]
- Zocher, F.; Enzelberger, M.M.; Bornscheuer, U.T.; Hauer, B.; Schmid, R.D. A colorimetric assay suitable for screening epoxide hydrolase activity. Anal. Chim. Acta 1999, 391, 345–351. [Google Scholar] [CrossRef]
- Wahler, D.; Reymond, J.-L. The Adrenaline Test for Enzymes. Angew. Chem. Int. Ed. 2002, 41, 1229–1232. [Google Scholar] [CrossRef]
- Mateo, C.; Archelas, A.; Furstoss, R. A spectrophotometric assay for measuring and detecting an epoxide hydrolase activity. Anal. Biochem. 2003, 314, 135–141. [Google Scholar] [CrossRef]
- Saini, P.; Wani, S.I.; Kumar, R.; Chhabra, R.; Chimni, S.S.; Sareen, D. Trigger factor assisted folding of the recombinant epoxide hydrolases identified from C. pelagibacter and S. nassauensis. Protein Expr. Purif. 2014, 104, 71–84. [Google Scholar] [CrossRef]
- van Loo, B.; Kingma, J.; Arand, M.; Wubbolts, M.G.; Janssen, D.B. Diversity and Biocatalytic Potential of Epoxide Hydrolases Identified by Genome Analysis. Appl. Environ. Microbiol. 2006, 72, 2905–2917. [Google Scholar] [CrossRef]
- Stojanovski, G.; Dobrijevic, D.; Hailes, H.C.; Ward, J.M. Identification and catalytic properties of new epoxide hydrolases from the genomic data of soil bacteria. Enzym. Microb. Technol. 2020, 139, 109592. [Google Scholar] [CrossRef] [PubMed]
- Robinson, S.L.; Piel, J.; Sunagawa, S. A roadmap for metagenomic enzyme discovery. Nat. Prod. Rep. 2021, 38, 1994–2023. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, D.J.; Dini-Andreote, F.; Ottoni, J.R.; de Oliveira, V.M.; van Elsas, J.D.; Andreote, F.D. Compositional profile of α/β-hydrolase fold proteins in mangrove soil metagenomes: Prevalence of epoxide hydrolases and haloalkane dehalogenases in oil-contaminated sites. Microb. Biotechnol. 2015, 8, 604–613. [Google Scholar] [CrossRef] [PubMed]
- Kotik, M.; Štěpánek, V.; Marešová, H.; Kyslík, P.; Archelas, A. Environmental DNA as a source of a novel epoxide hydrolase reacting with aliphatic terminal epoxides. J. Mol. Catal. B Enzym. 2009, 56, 288–293. [Google Scholar] [CrossRef]
- Kotik, M.; Štěpánek, V.; Grulich, M.; Kyslík, P.; Archelas, A. Access to enantiopure aromatic epoxides and diols using epoxide hydrolases derived from total biofilter DNA. J. Mol. Catal. B Enzym. 2010, 65, 41–48. [Google Scholar] [CrossRef]
- Ferrandi, E.E.; Sayer, C.; Isupov, M.N.; Annovazzi, C.; Marchesi, C.; Iacobone, G.; Peng, X.; Bonch-Osmolovskaya, E.; Wohlgemuth, R.; Littlechild, J.A.; et al. Discovery and characterization of thermophilic limonene-1,2-epoxide hydrolases from hot spring metagenomic libraries. FEBS J. 2015, 282, 2879–2894. [Google Scholar] [CrossRef]
- Ferrandi, E.E.; Sayer, C.; De Rose, S.A.; Guazzelli, E.; Marchesi, C.; Saneei, V.; Isupov, M.N.; Littlechild, J.A.; Monti, D. New Thermophilic α/β Class Epoxide Hydrolases Found in Metagenomes from Hot Environments. Front. Bioeng. Biotechnol. 2018, 6, 144. [Google Scholar] [CrossRef]
- Reetz, M.T. Laboratory Evolution of Stereoselective Enzymes: A Prolific Source of Catalysts for Asymmetric Reactions. Angew. Chem. Int. Ed. 2011, 50, 138–174. [Google Scholar] [CrossRef]
- Basheer, S.M.; Chellappan, S. Enzyme Engineering. In Bioresources and Bioprocess in Biotechnology: Volume 2: Exploring Potential Biomolecules; Sugathan, S., Pradeep, N.S., Abdulhameed, S., Eds.; Springer: Singapore, 2017; pp. 151–168. [Google Scholar]
- Nardini, M.; Rink, R.; Janssen, D.B.; Dijkstra, B.W. Structure and mechanism of the epoxide hydrolase from Agrobacterium radiobacter AD1. J. Mol. Catal. B Enzym. 2001, 11, 1035–1042. [Google Scholar] [CrossRef]
- Zou, J.; Hallberg, B.M.; Bergfors, T.; Oesch, F.; Arand, M.; Mowbray, S.L.; Jones, T.A. Structure of Aspergillus niger epoxide hydrolase at 1.8 Å resolution: Implications for the structure and function of the mammalian microsomal class of epoxide hydrolases. Structure 2000, 8, 111–122. [Google Scholar] [CrossRef]
- Arand, M.; Hallberg, B.M.; Zou, J.; Bergfors, T.; Oesch, F.; van der Werf, M.J.; de Bont, J.A.; Jones, T.A.; Mowbray, S.L. Structure of Rhodococcus erythropolis limonene-1,2-epoxide hydrolase reveals a novel active site. EMBO J. 2003, 22, 2583–2592. [Google Scholar] [CrossRef]
- Johansson, P.; Unge, T.; Cronin, A.; Arand, M.; Bergfors, T.; Jones, T.A.; Mowbray, S.L. Structure of an atypical epoxide hydrolase from Mycobacterium tuberculosis gives insights into its function. J. Mol. Biol. 2005, 351, 1048–1056. [Google Scholar] [CrossRef]
- Reetz, M.T.; Zonta, A.; Schimossek, K.; Jaeger, K.-E.; Liebeton, K. Creation of Enantioselective Biocatalysts for Organic Chemistry by in vitro Evolution. Angew. Chem. Int. Ed. Engl. 1997, 36, 2830–2832. [Google Scholar] [CrossRef]
- Cedrone, F.; Niel, S.; Roca, S.; Bhatnagar, T.; Ait-abdelkader, N.; Torre, C.; Krumm, H.; Maichele, A.; Reetz, M.T.; Baratti, J.C. Directed Evolution of the Epoxide Hydrolase from Aspergillus niger. Biocatal. Biotransf. 2003, 21, 357–364. [Google Scholar] [CrossRef]
- Reetz, M.T.; Torre, C.; Eipper, A.; Lohmer, R.; Hermes, M.; Brunner, B.; Maichele, A.; Bocola, M.; Arand, M.; Cronin, A.; et al. Enhancing the Enantioselectivity of an Epoxide Hydrolase by Directed Evolution. Org. Lett. 2004, 6, 177–180. [Google Scholar] [CrossRef]
- Rui, L.; Cao, L.; Chen, W.; Reardon, K.F.; Wood, T.K. Active Site Engineering of the Epoxide Hydrolase from Agrobacterium radiobacter AD1 to Enhance Aerobic Mineralization of cis-1,2-Dichloroethylene in Cells Expressing an Evolved Toluene ortho-Monooxygenase. J. Biol. Chem. 2004, 279, 46810–46817. [Google Scholar] [CrossRef]
- Rui, L.; Cao, L.; Chen, W.; Reardon, K.F.; Wood, T.K. Protein Engineering of Epoxide Hydrolase from Agrobacterium radiobacter AD1 for Enhanced Activity and Enantioselective Production of (R)-1-Phenylethane-1,2-Diol. Appl. Environ. Microbiol. 2005, 71, 3995–4003. [Google Scholar] [CrossRef]
- Sun, Z.; Lonsdale, R.; Kong, X.-D.; Xu, J.-H.; Zhou, J.; Reetz, M.T. Reshaping an Enzyme Binding Pocket for Enhanced and Inverted Stereoselectivity: Use of Smallest Amino Acid Alphabets in Directed Evolution. Angew. Chem. Int. Ed. 2015, 54, 12410–12415. [Google Scholar] [CrossRef]
- Sun, Z.; Lonsdale, R.; Li, G.; Reetz, M.T. Comparing Different Strategies in Directed Evolution of Enzyme Stereoselectivity: Single- versus Double-Code Saturation Mutagenesis. ChemBioChem 2016, 17, 1865–1872. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Lonsdale, R.; Wu, L.; Li, G.; Li, A.; Wang, J.; Zhou, J.; Reetz, M.T. Structure-Guided Triple-Code Saturation Mutagenesis: Efficient Tuning of the Stereoselectivity of an Epoxide Hydrolase. ACS Catal. 2016, 6, 1590–1597. [Google Scholar] [CrossRef]
- Arabnejad, H.; Bombino, E.; Colpa, D.I.; Jekel, P.A.; Trajkovic, M.; Wijma, H.J.; Janssen, D.B. Computational Design of Enantiocomplementary Epoxide Hydrolases for Asymmetric Synthesis of Aliphatic and Aromatic Diols. ChemBioChem 2020, 21, 1893–1904. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Zong, X.-C.; Xue, F.; Li, C.; Hu, B.-C.; Wu, M.-C. Manipulating regioselectivity of an epoxide hydrolase for single enzymatic synthesis of (R)-1,2-diols from racemic epoxides. Chem. Commun. 2020, 56, 2799–2802. [Google Scholar] [CrossRef]
- Li, G.; Qin, Y.; Fontaine, N.T.; Ng Fuk Chong, M.; Maria-Solano, M.A.; Feixas, F.; Cadet, X.F.; Pandjaitan, R.; Garcia-Borràs, M.; Cadet, F.; et al. Machine Learning Enables Selection of Epistatic Enzyme Mutants for Stability against Unfolding and Detrimental Aggregation. ChemBioChem 2021, 22, 904–914. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.-D.; Ma, Q.; Zhou, J.; Zeng, B.-B.; Xu, J.-H. A Smart Library of Epoxide Hydrolase Variants and the Top Hits for Synthesis of (S)-β-Blocker Precursors. Angew. Chem. Int. Ed. 2014, 53, 6641–6644. [Google Scholar] [CrossRef] [PubMed]
- van Loo, B.; Spelberg, J.H.L.; Kingma, J.; Sonke, T.; Wubbolts, M.G.; Janssen, D.B. Directed Evolution of Epoxide Hydrolase from A. radiobacter toward Higher Enantioselectivity by Error-Prone PCR and DNA Shuffling. Chem. Biol. 2004, 11, 981–990. [Google Scholar] [CrossRef]
- van Loo, B.; Kingma, J.; Heyman, G.; Wittenaar, A.; Lutje Spelberg, J.H.; Sonke, T.; Janssen, D.B. Improved enantioselective conversion of styrene epoxides and meso-epoxides through epoxide hydrolases with a mutated nucleophile-flanking residue. Enzym. Microb. Technol. 2009, 44, 145–153. [Google Scholar] [CrossRef]
- Xue, F.; Liu, Z.-Q.; Wan, N.-W.; Zhu, H.-Q.; Zheng, Y.-G. Engineering the epoxide hydrolase from Agromyces mediolanus for enhanced enantioselectivity and activity in the kinetic resolution of racemic epichlorohydrin. RSC Adv. 2015, 5, 31525–31532. [Google Scholar] [CrossRef]
- Reetz, M.T.; Wang, L.-W.; Bocola, M. Directed Evolution of Enantioselective Enzymes: Iterative Cycles of CASTing for Probing Protein-Sequence Space. Angew. Chem. Int. Ed. 2006, 45, 1236–1241. [Google Scholar] [CrossRef]
- Reetz, M.T.; Zheng, H. Manipulating the Expression Rate and Enantioselectivity of an Epoxide Hydrolase by Using Directed Evolution. ChemBioChem 2011, 12, 1529–1535. [Google Scholar] [CrossRef]
- Gumulya, Y.; Sanchis, J.; Reetz, M.T. Many Pathways in Laboratory Evolution Can Lead to Improved Enzymes: How to Escape from Local Minima. ChemBioChem 2012, 13, 1060–1066. [Google Scholar] [CrossRef]
- Hu, D.; Hu, B.-C.; Hou, X.-D.; Zhang, D.; Lei, Y.-Q.; Rao, Y.-J.; Wu, M.-C. Structure-Guided Regulation in the Enantioselectivity of an Epoxide Hydrolase to Produce Enantiomeric Monosubstituted Epoxides and Vicinal Diols via Kinetic Resolution. Org. Lett. 2022, 24, 1757–1761. [Google Scholar] [CrossRef]
- Zheng, H.; Reetz, M.T. Manipulating the Stereoselectivity of Limonene Epoxide Hydrolase by Directed Evolution Based on Iterative Saturation Mutagenesis. J. Am. Chem. Soc. 2010, 132, 15744–15751. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Salas, P.T.; Siirola, E.; Lonsdale, R.; Reetz, M.T. Exploring productive sequence space in directed evolution using binary patterning versus conventional mutagenesis strategies. Bioresour. Bioprocess 2016, 3, 44. [Google Scholar] [CrossRef]
- Li, G.; Zhang, H.; Sun, Z.; Liu, X.; Reetz, M.T. Multiparameter Optimization in Directed Evolution: Engineering Thermostability, Enantioselectivity, and Activity of an Epoxide Hydrolase. ACS Catal. 2016, 6, 3679–3687. [Google Scholar] [CrossRef]
- Li, A.; Acevedo-Rocha, C.G.; Sun, Z.; Cox, T.; Xu, J.L.; Reetz, M.T. Beating Bias in the Directed Evolution of Proteins: Combining High-Fidelity on-Chip Solid-Phase Gene Synthesis with Efficient Gene Assembly for Combinatorial Library Construction. ChemBioChem 2018, 19, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Wijma, H.J.; Floor, R.J.; Bjelic, S.; Marrink, S.J.; Baker, D.; Janssen, D.B. Enantioselective Enzymes by Computational Design and in silico Screening. Angew. Chem. Int. Ed. 2015, 54, 3726–3730. [Google Scholar] [CrossRef] [PubMed]
- Gurell, A.; Widersten, M. Modification of Substrate Specificity Resulting in an Epoxide Hydrolase with Shifted Enantiopreference for (2,3-Epoxypropyl)benzene. ChemBioChem 2010, 11, 1422–1429. [Google Scholar] [CrossRef]
- Carlsson, Å.J.; Bauer, P.; Ma, H.; Widersten, M. Obtaining Optical Purity for Product Diols in Enzyme-Catalyzed Epoxide Hydrolysis: Contributions from Changes in both Enantio- and Regioselectivity. Biochemistry 2012, 51, 7627–7637. [Google Scholar] [CrossRef]
- Li, Y.; Ou, X.; Guo, Z.; Zong, M.; Lou, W. Using multiple site-directed modification of epoxide hydrolase to significantly improve its enantioselectivity in hydrolysis of rac-glycidyl phenyl ether. Chin. J. Chem. Eng. 2020, 28, 2181–2189. [Google Scholar] [CrossRef]
- Kotik, M.; Zhao, W.; Iacazio, G.; Archelas, A. Directed evolution of metagenome-derived epoxide hydrolase for improved enantioselectivity and enantioconvergence. J. Mol. Catal. B Enzym. 2013, 91, 44–51. [Google Scholar] [CrossRef]
- Kotik, M.; Archelas, A.; Faměrová, V.; Oubrechtová, P.; Křen, V. Laboratory evolution of an epoxide hydrolase—Towards an enantioconvergent biocatalyst. J. Biotechnol. 2011, 156, 1–10. [Google Scholar] [CrossRef]
- Liu, Y.-Y.; Wu, M.-D.; Zhu, X.-X.; Zhang, X.-D.; Zhang, C.; Xu, Y.-H.; Wu, M.-C. Remarkable improvement in the regiocomplementarity of a Glycine max epoxide hydrolase by reshaping its substrate-binding pocket for the enantioconvergent preparation of (R)-hexane-1,2-diol. Mol. Catal. 2021, 514, 111851. [Google Scholar] [CrossRef]
- Ye, H.-H.; Hu, D.; Shi, X.-L.; Wu, M.-C.; Deng, C.; Li, J.-F. Directed modification of a novel epoxide hydrolase from Phaseolus vulgaris to improve its enantioconvergence towards styrene epoxides. Catal. Commun. 2016, 87, 32–35. [Google Scholar] [CrossRef]
- Zong, X.-C.; Li, C.; Xu, Y.-H.; Hu, D.; Hu, B.-C.; Zang, J.; Wu, M.-C. Substantially improving the enantioconvergence of PvEH1, a Phaseolus vulgaris epoxide hydrolase, towards m-chlorostyrene oxide by laboratory evolution. Microb. Cell Fact. 2019, 18, 202. [Google Scholar] [CrossRef]
- Zhu, X.-X.; Hu, B.-C.; Lin, W.-Q.; Zhang, D.; Zhao, J.; Wu, M.-C. Engineering the regiocomplementarity of an epoxide hydrolase from Rhodotorula paludigena by means of computer-aided design for the scale-up enantioconvergent hydrolysis of racemic m-nitrostyrene oxide. Biochem. Eng. J. 2022, 180, 108359. [Google Scholar] [CrossRef]
- Li, F.-L.; Kong, X.-D.; Chen, Q.; Zheng, Y.-C.; Xu, Q.; Chen, F.-F.; Fan, L.-Q.; Lin, G.-Q.; Zhou, J.; Yu, H.-L.; et al. Regioselectivity Engineering of Epoxide Hydrolase: Near-Perfect Enantioconvergence through a Single Site Mutation. ACS Catal. 2018, 8, 8314–8317. [Google Scholar] [CrossRef]
- Li, F.-L.; Qiu, Y.-Y.; Zheng, Y.-C.; Chen, F.-F.; Kong, X.D.; Xu, J.-H.; Yu, H.-L. Reprogramming Epoxide Hydrolase to Improve Enantioconvergence in Hydrolysis of Styrene Oxide Scaffolds. Adv. Synth. Catal. 2020, 362, 4699–4706. [Google Scholar] [CrossRef]
- Wijma, H.J.; Floor, R.J.; Jekel, P.A.; Baker, D.; Marrink, S.J.; Janssen, D.B. Computationally designed libraries for rapid enzyme stabilization. Protein Eng. Des. Sel. 2014, 27, 49–58. [Google Scholar] [CrossRef]
- Gumulya, Y.; Reetz, M.T. Enhancing the Thermal Robustness of an Enzyme by Directed Evolution: Least Favorable Starting Points and Inferior Mutants Can Map Superior Evolutionary Pathways. ChemBioChem 2011, 12, 2502–2510. [Google Scholar] [CrossRef]
- Qiao, P.; Wu, M.; Zhu, L.; Zhang, Y.; Yang, L.; Fei, H.; Lin, J. Enhancing the thermal tolerance of a cis-epoxysuccinate hydrolase via combining directed evolution with various semi-rational redesign methods. J. Mol. Catal. B Enzym. 2015, 121, 96–103. [Google Scholar] [CrossRef]
- Jochens, H.; Stiba, K.; Savile, C.; Fujii, R.; Yu, J.-G.; Gerassenkov, T.; Kazlauskas, R.J.; Bornscheuer, U.T. Converting an Esterase into an Epoxide Hydrolase. Angew. Chem. Int. Ed. 2009, 48, 3532–3535. [Google Scholar] [CrossRef] [PubMed]
- Nagel, B.; Dellweg, H.; Gierasch, L.M. Glossary for chemists of terms used in biotechnology (IUPAC Recommendations 1992). Pure Appl. Chem. 1992, 64, 143–168. [Google Scholar] [CrossRef]
- Hechtberger, P.; Wirnsberger, G.; Mischitz, M.; Klempier, N.; Faber, K. Asymmetric hydrolysis of epoxides using an immobilized enzyme preparation from Rhodococcus sp. Tetrahedron Asymmetry 1993, 4, 1161–1164. [Google Scholar] [CrossRef]
- Lin, H.; Liu, J.-Y.; Wang, H.-B.; Ahmed, A.A.Q.; Wu, Z.-L. Biocatalysis as an alternative for the production of chiral epoxides: A comparative review. J. Mol. Catal. B Enzym. 2011, 72, 77–89. [Google Scholar] [CrossRef]
- Vikartovská, A.; Bučko, M.; Gemeiner, P.; Nahálka, J.; Pätoprstý, V.; Hrabárová, E. Flow Calorimetry—A Useful Tool for Determination of Immobilized cis-Epoxysuccinate Hydrolase Activity from Nocardia tartaricans. Artif. Cells Blood Substit. Biotechnol. 2004, 32, 77–89. [Google Scholar] [CrossRef]
- Bučko, M.; Vikartovská, A.; Lacík, I.; Kolláriková, G.; Gemeiner, P.; Pätoprstý, V.; Brygin, M. Immobilization of a whole-cell epoxide-hydrolyzing biocatalyst in sodium alginate-cellulose sulfate-poly(methylene-co-guanidine) capsules using a controlled encapsulation process. Enzym. Microb. Technol. 2005, 36, 118–126. [Google Scholar] [CrossRef]
- Rosenberg, M.; Miková, H.; Krištofíková, L. Production of L-tartaric acid by immobilized bacterial cells Nocardia tartaricans. Biotechnol. Lett. 1999, 21, 491–495. [Google Scholar] [CrossRef]
- Bučko, M.; Vikartovská, A.; Gemeiner, P.; Lacík, I.; Kolláriková, G.; Marison, I.W. Nocardia tartaricans cells immobilized in sodium alginate–cellulose sulfate–poly (methylene-co-guanidine)capsules: Mechanical resistance and operational stability. J. Chem. Technol. Biotechnol. 2006, 81, 500–504. [Google Scholar] [CrossRef]
- Huang, R.; Wu, M.; Goldman, M.J.; Li, Z. Encapsulation of enzyme via one-step template-free formation of stable organic–inorganic capsules: A simple and efficient method for immobilizing enzyme with high activity and recyclability. Biotechnol. Bioeng. 2015, 112, 1092–1101. [Google Scholar] [CrossRef]
- Zhou, R.; Dong, S.; Feng, Y.; Cui, Q.; Xuan, J. Development of highly efficient whole-cell catalysts of cis-epoxysuccinic acid hydrolase by surface display. Bioresour. Bioprocess 2022, 9, 92. [Google Scholar] [CrossRef]
- Zou, S.-P.; Wang, Z.-C.; Qin, C.; Zheng, Y.-G. Covalent immobilization of Agrobacterium radiobacter epoxide hydrolase on ethylenediamine functionalised epoxy supports for biocatalytical synthesis of (R)-epichlorohydrin. Biotechnol. Lett. 2016, 38, 1579–1585. [Google Scholar] [CrossRef] [PubMed]
- Zou, S.-P.; Xuan, X.-L.; Wang, Z.-J.; Zheng, Y.-G. Conjugation of Agrobacterium radiobacter epoxide hydrolase with ficoll: Catalytic, kinetic and thermodynamic analysis. Int. J. Biol. Macromol. 2018, 119, 1098–1105. [Google Scholar] [CrossRef] [PubMed]
- Grulich, M.; Maršálek, J.; Kyslík, P.; Štěpánek, V.; Kotik, M. Production, enrichment and immobilization of a metagenome-derived epoxide hydrolase. Process Biochem. 2011, 46, 526–532. [Google Scholar] [CrossRef]
- Mateo, C.; Archelas, A.; Fernandez-Lafuente, R.; Manuel Guisan, J.; Furstoss, R. Enzymatic transformations. Immobilized A. niger epoxide hydrolase as a novel biocatalytic tool for repeated-batch hydrolytic kinetic resolution of epoxides. Org. Biomol. Chem. 2003, 1, 2739–2743. [Google Scholar] [CrossRef]
- Yildirim, D.; Tükel, S.S.; Alagöz, D.; Alptekin, Ö. Preparative-scale kinetic resolution of racemic styrene oxide by immobilized epoxide hydrolase. Enzym. Microb. Technol. 2011, 49, 555–559. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, D.; Tükel, S.S.; Alptekin, Ö.; Alagöz, D. Immobilized Aspergillus niger epoxide hydrolases: Cost-effective biocatalysts for the prepation of enantiopure styrene oxide, propylene oxide and epichlorohydrin. J. Mol. Catal. B Enzym. 2013, 88, 84–90. [Google Scholar] [CrossRef]
- Petri, A.; Marconcini, P.; Salvadori, P. Efficient immobilization of epoxide hydrolase onto silica gel and use in the enantioselective hydrolysis of racemic para-nitrostyrene oxide. J. Mol. Catal. B Enzym. 2005, 32, 219–224. [Google Scholar] [CrossRef]
- Mateo, C.; Fernandez-Lafuente, R.; Archelas, A.; Guisan, J.M.; Furstoss, R. Preparation of a very stable immobilized Solanum tuberosum epoxide hydrolase. Tetrahedron Asymmetry 2007, 18, 1233–1238. [Google Scholar] [CrossRef]
- Ibrahim, M.; Hubert, P.; Dellacherie, E.; Magdalou, J.; Siest, G. Immobilization of epoxide hydrolase purified from rat liver microsomes. Biotechnol. Lett. 1984, 6, 771–776. [Google Scholar] [CrossRef]
- Ibrahim, M.; Hubert, P.; Dellacherie, E.; Magdalou, J.; Muller, J.; Siest, G. Covalent attachment of epoxide hydrolase to dextran. Enzym. Microb. Technol. 1985, 7, 66–72. [Google Scholar] [CrossRef]
- Kamble, M.; Salvi, H.; Yadav, G.D. Preparation of amino-functionalized silica supports for immobilization of epoxide hydrolase and cutinase: Characterization and applications. J. Porous Mater. 2020, 27, 1559–1567. [Google Scholar] [CrossRef]
- Cassimjee, K.E.; Kourist, R.; Lindberg, D.; Wittrup Larsen, M.; Thanh, N.H.; Widersten, M.; Bornscheuer, U.T.; Berglund, P. One-step enzyme extraction and immobilization for biocatalysis applications. Biotechnol. J. 2011, 6, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.H.; Kim, H.S.; Lee, I.S.; Lee, E.Y. Functional expression and magnetic nanoparticle-based Immobilization of a protein-engineered marine fish epoxide hydrolase of Mugil cephalus for enantioselective hydrolysis of racemic styrene oxide. Biotechnol. Lett. 2010, 32, 1685–1691. [Google Scholar] [CrossRef]
- Wang, Z.; Su, M.; Li, Y.; Wang, Y.; Su, Z. Production of tartaric acid using immobilized recominant cis-epoxysuccinate hydrolase. Biotechnol. Lett. 2017, 39, 1859–1863. [Google Scholar] [CrossRef]
- Karboune, S.; Archelas, A.; Baratti, J.C. Free and immobilized Aspergillus niger epoxide hydrolase-catalyzed hydrolytic kinetic resolution of racemic p-chlorostyrene oxide in a neat organic solvent medium. Process Biochem. 2010, 45, 210–216. [Google Scholar] [CrossRef]
- Karboune, S.; Archelas, A.; Furstoss, R.; Baratti, J. Immobilization of epoxide hydrolase from Aspergillus niger onto DEAE-cellulose: Enzymatic properties and application for the enantioselective resolution of a racemic epoxide. J. Mol. Catal. B Enzym. 2005, 32, 175–183. [Google Scholar] [CrossRef]
- Karboune, S.; Amourache, L.; Nellaiah, H.; Morisseau, C.; Baratti, J. Immobilization of the epoxide hydrolase from Aspergillus niger. Biotechnol. Lett. 2001, 23, 1633–1639. [Google Scholar] [CrossRef]
- Kroutil, W.; Genzel, Y.; Pietzsch, M.; Syldatk, C.; Faber, K. Purification and characterization of a highly selective epoxide hydrolase from Nocardia sp. EH1. J. Biotechnol. 1998, 61, 143–150. [Google Scholar] [CrossRef]
- Jin, H.-X.; Liu, Z.-Q.; Hu, Z.-C.; Zheng, Y.-G. Production of (R)-epichlorohydrin from 1,3-dichloro-2-propanol by two-step biocatalysis using haloalcohol dehalogenase and epoxide hydrolase in two-phase system. Biochem. Eng. J. 2013, 74, 1–7. [Google Scholar] [CrossRef]
- Kim, Y.H.; Lee, I.; Choi, S.H.; Lee, O.K.; Shim, J.; Lee, J.; Kim, J.; Lee, E.Y. Enhanced stability and reusability of marine epoxide hydrolase using ship-in-a-bottle approach with magnetically-separable mesoporous silica. J. Mol. Catal. B Enzym. 2013, 89, 48–51. [Google Scholar] [CrossRef]
- Yu, C.-Y.; Li, X.-F.; Lou, W.-Y.; Zong, M.-H. Cross-linked enzyme aggregates of Mung bean epoxide hydrolases: A highly active, stable and recyclable biocatalyst for asymmetric hydrolysis of epoxides. J. Biotechnol. 2013, 166, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.-Y.; Wei, P.; Li, X.-F.; Zong, M.-H.; Lou, W.-Y. Using Ionic Liquid in a Biphasic System to Improve Asymmetric Hydrolysis of Styrene Oxide Catalyzed by cross-Linked Enzyme Aggregates (CLEAs) of Mung Bean Epoxide Hydrolases. Ind. Eng. Chem. Res. 2014, 53, 7923–7930. [Google Scholar] [CrossRef]
- Kronenburg, N.A.E.; de Bont, J.A.M.; Fischer, L. Improvement of enantioselectivity by immobilized imprinting of epoxide hydrolase from Rhodotorula glutinis. J. Mol. Catal. B Enzym. 2001, 16, 121–129. [Google Scholar] [CrossRef]
- Salvi, H.M.; Yadav, G.D. Organic-inorganic epoxide hydrolase hybrid nanoflowers with enhanced catalytic activity: Hydrolysis of styrene oxide to 1-phenyl-1,2-ethanediol. J. Biotechnol. 2021, 341, 113–120. [Google Scholar] [CrossRef]
- Cao, S.-L.; Yue, D.-M.; Li, X.-H.; Smith, T.J.; Li, N.; Zong, M.-H.; Wu, H.; Ma, Y.-Z.; Lou, W.-Y. Novel Nano-/Micro-Biocatalyst: Soybean Epoxide Hydrolase Immobilized on UiO-66-NH2 MOF for Efficient Biosynthesis of Enantiopure (R)-1, 2-Octanediol in Deep Eutectic Solvents. ACS Sustain. Chem. Eng. 2016, 4, 3586–3595. [Google Scholar] [CrossRef]
- Onur, H.; Tülek, A.; Aslan, E.S.; Binay, B.; Yildirim, D. A new highly enantioselective stable epoxide hydrolase from Hypsibius dujardini: Expression in Pichia pastoris and immobilization in ZIF-8 for asymmetric hydrolysis of racemic styrene oxide. Biochem. Eng. J. 2022, 189, 108726. [Google Scholar] [CrossRef]
- Maritz, J.; Krieg, H.M.; Yeates, C.A.; Botes, A.L.; Breytenbach, J.C. Calcium alginate entrapment of the yeast Rhodosporidium toruloides for the kinetic resolution of 1,2-epoxyoctane. Biotechnol. Lett. 2003, 25, 1775–1781. [Google Scholar] [CrossRef]
- Bao, W.; Pan, H.; Zhang, Z.; Cheng, Y.; Xie, Z.; Zhang, J. Isolation of the stable strain Labrys sp. BK-8 for l(+)-tartaric acid production. J. Biosci. Bioeng. 2015, 119, 538–542. [Google Scholar] [CrossRef] [PubMed]
- Buchholz, K.; Kasche, V.; Bornscheuer, U.T. Enzymes in Organic Chemistry. In Biocatalysts and Enzyme Technology, 1st ed.; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2005; pp. 141–145. [Google Scholar]
- Faber, K.; Mischitz, M.; Kroutil, W. Microbial Epoxide Hydrolases. Acta Chem. Scand. 1996, 50, 249–258. [Google Scholar] [CrossRef]
- Mayer, S.F.; Steinreiber, A.; Orru, R.V.A.; Faber, K. Enzyme-Triggered Enantioconvergent Transformation of Haloalkyl Epoxides. Eur. J. Org. Chem. 2001, 2001, 4537–4542. [Google Scholar] [CrossRef]
- Xu, Y.; Jia, X.; Panke, S.; Li, Z. Asymmetric dihydroxylation of arylolefins by sequential enantioselective epoxidation and regioselective hydrolysis with tandem biocatalysts. Chem. Commun. 2009, 12, 1481–1483. [Google Scholar] [CrossRef]
- Pandey, R.K.; Fernandes, R.A.; Kumar, P. An asymmetric dihydroxylation route to enantiomerically pure norfluoxetine and fluoxetine. Tetrahedron Lett. 2002, 43, 4425–4426. [Google Scholar] [CrossRef]
- Schrittwieser, J.H.; Velikogne, S.; Hall, M.; Kroutil, W. Artificial Biocatalytic Linear Cascades for Preparation of Organic Molecules. Chem. Rev. 2018, 118, 270–348. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, S.; Wu, J.; Li, Z. Enantioselective Cascade Biocatalysis via Epoxide Hydrolysis and Alcohol Oxidation: One-Pot Synthesis of (R)-α-Hydroxy Ketones from Meso- or Racemic Epoxides. ACS Catal. 2015, 5, 51–58. [Google Scholar] [CrossRef]
- Wu, S.; Chen, Y.; Xu, Y.; Li, A.; Xu, Q.; Glieder, A.; Li, Z. Enantioselective trans-Dihydroxylation of Aryl Olefins by Cascade Biocatalysis with Recombinant Escherichia coli Coexpressing Monooxygenase and Epoxide Hydrolase. ACS Catal. 2014, 4, 409–420. [Google Scholar] [CrossRef]
- Xue, F.; Liu, Z.-Q.; Wang, Y.-J.; Zhu, H.-Q.; Wan, N.-W.; Zheng, Y.-G. Efficient synthesis of (S)-epichlorohydrin in high yield by cascade biocatalysis with halohydrin dehalogenase and epoxide hydrolase mutants. Catal. Commun. 2015, 72, 147–149. [Google Scholar] [CrossRef]
- Gao, P.; Wu, S.; Praveen, P.; Loh, K.-C.; Li, Z. Enhancing productivity for cascade biotransformation of styrene to (S)-vicinal diol with biphasic system in hollow fiber membrane bioreactor. Appl. Microbiol. Biotechnol. 2017, 101, 1857–1868. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Parnell, A.; Williams, C.; Bakar, N.A.; Challand, M.R.; van der Kamp, M.W.; Simpson, T.J.; Race, P.R.; Crump, M.P.; Willis, C.L. A Rieske oxygenase/epoxide hydrolase-catalysed reaction cascade creates oxygen heterocycles in mupirocin biosynthesis. Nat. Catal. 2018, 1, 968–976. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, X.; Liu, F.; Xu, M.; Yang, T.; Long, M.; Zhou, J.; Osire, T.; Yang, S.; Rao, Z. Designing of a Cofactor Self-Sufficient Whole-Cell Biocatalyst System for Production of 1,2-Amino Alcohols from Epoxides. ACS Synth. Biol. 2019, 8, 734–743. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-D.; Yang, X.-X.; Jia, Q.; Zhao, J.-W.; Gao, L.-L.; Gao, W.-C.; Chang, H.-H.; Wei, W.-L.; Xu, J.-H. Asymmetric ring opening of racemic epoxides for enantioselective synthesis of (S)-β-amino alcohols by a cofactor self-sufficient cascade biocatalysis system. Catal. Sci. Technol. 2019, 9, 70–74. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, X.; Dong, R.; Gao, L.; Li, J.; Li, X.; Huang, S.; Zhang, C.; Chang, H. Cascade Biocatalysis for Regio- and Stereoselective Aminohydroxylation of Styrenyl Olefins to Enantiopure Arylglycinols. ACS Sustain. Chem. Eng. 2020, 8, 18277–18285. [Google Scholar] [CrossRef]
- See, W.W.L.; Choo, J.P.S.; Jung, D.-Y.; Zhi, L. Recent developments in oxidative biocatalytic cascades. Curr. Opin. Green Sustain. Chem. 2022, 38, 100700. [Google Scholar] [CrossRef]

| Chiral Precursor | Final Product | Application of Final Product | Reaction Comment | Ref. |
|---|---|---|---|---|
 | 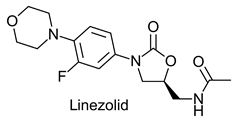 | Synthetic oxazolidinone antibiotic effective against Gram positive bacteria | Selective hydrolysis of (S)-enantiomer | [18] |
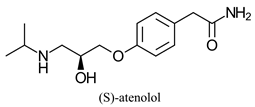 | Cardio selective β-blocker for treatment of high blood pressure and heart associated chest pain | |||
 | Dietary supplement, involved in long-chain fatty acid transport in cells | |||
 | 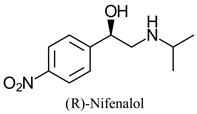 | β-adrenergic blocker with antianginal and antiarrhythmic properties. | Chemo-enzymatic enantioconvergent synthesis | [19,20] |
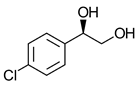 | 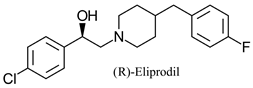 | Neuroprotective agent (aspartate receptor antagonist) | Sequential bi-enzymatic hydrolysis using 2 enantiocomplementary EHs | [21] |
 | 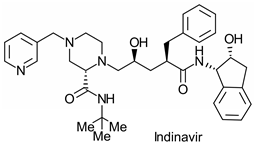 | HIV protease inhibitor MK 639 | Selective hydrolysis of 1(R),2(S)-enantiomer | [22] |
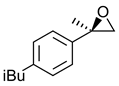 | 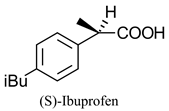 | Non-steroidal anti-inflammatory drug | Selective hydrolysis of (R)-enantiomer | [23] |
 | 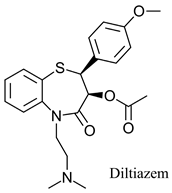 | Calcium channel blocker | Kinetic resolution | [24] |
 | 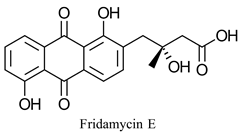 | Anthracycline antibiotic with chemotherapeutic properties | Enzymatic deracemization for production of (S)-diol used for chemical synthesis | [25] |
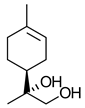 | 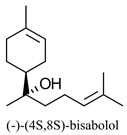 | Major constituent of Matricaria chamomilla essential oil; ingredient for skin creams, lotions, ointments with anti-inflammatory, bactericidal and antimycotic properties | Chemo-enzymatic process for production of all 4 stereoisomers of bisabolol | [15] |
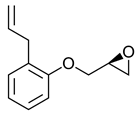 | 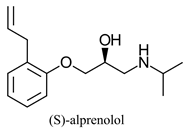 | β-adrenergic receptor blocking drugs | Selective hydrolysis of (R)-enantiomer | [26] |
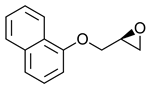 | 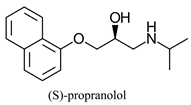 | |||
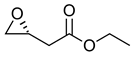 | 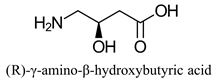 | Neuromediator with antiepileptic and antihypertensive activities | Selective hydrolysis of (S)-enantiomer | [27] |
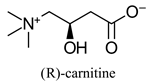 | Dietary supplement, involved in long-chain fatty acid transport in cells | |||
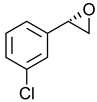 | 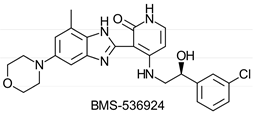 | IGF-1R kinase inhibitor | Selective hydrolysis of (R)-enantiomer | [28] |
 | β3-adrenergic receptor agonists | Enantioconvergent hydrolysis of racemic epoxide | [29] | |
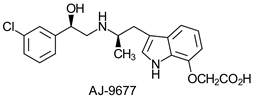 | ||||
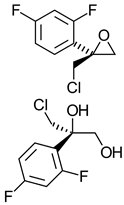 | 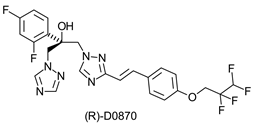 | Antifungal triazole drug | Production of optically pure epoxide and diol that can be used for chemical synthesis of optically pure triazole derivatives | [30,31] |
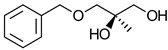 |  | Non-steroidal antiandrogen drug used for treatment of prostate cancer | Chemo-enzymatic synthesis of optically pure diol | [32] |
 |  | Ileal bile acid transport (iBAT) inhibitor indicated for diabetes type II | Kinetic resolution | [33] |
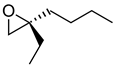
 | 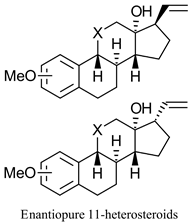 | Chiral precursors for synthesis of various steroidal compounds | Kinetic resolution to produce both enantiomers of spiroepoxide, using 2 different EHs | [34] |
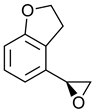 | 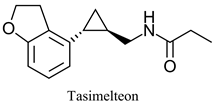 | Melatonin receptor agonist used for treatment of sleep disorders | Selective hydrolysis of (R)-enantiomer | [35,36] |
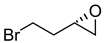 | 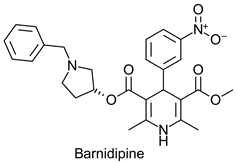 | Calcium channel blocker used for treatment of hypertension | Selective hydrolysis of (R)-enantiomer | [37] |
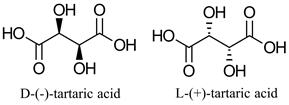 | Chiral chemical building block with broad applications in chemical, pharmaceutical, food industries | Asymmetric hydrolysis to produce optically pure diol | [14] | |
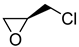 | 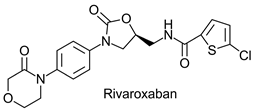 | Anticoagulant; direct factor Xa inhibitor developed by Bayer and marketed as Xarelto | - 1 | [38,39] |
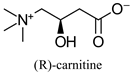 | Dietary supplement, involved in long-chain fatty acid transport in cells | - | [40,41] | |
 | Antidiabetic drug | - | [42] | |
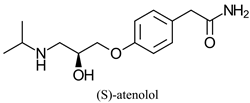 | β-adrenergic receptor blocking drug | - | [43] | |
 | β-adrenergic antagonist drug | - | [44,45] | |
| Modified Property | Source of EH Used as Template for Mutagenesis | Enzyme Engineering Method | Mutant | Substrate | E (-) | eeP (%) | C (%) | Ref. | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mutant | WT | Mutant | WT | Mutant | WT | ||||||
| Enantioselectivity | Agrobacterium radiobacter AD1 | Directed evolution—Error-prone PCR and DNA shuffling | F108I/P205H/Y215H/E271V | styrene oxide | >50 | 16 | NI 1 | NI | [85] | ||
| p-nitrostyrene oxide | 81 | 56 | NI | NI | |||||||
| p-nitrophenyl glycidyl ether | 32 | 3.4 | NI | NI | |||||||
| epichlorohydrin | 40 | <2 | NI | NI | |||||||
| 1,2-epoxyhexane | 27 | 3.6 | NI | NI | |||||||
| Directed evolution—DNA shuffling and site-saturation mutagenesis | I219F | styrene oxide | 91 (R) | 17 (R) | NI | NI | [77] | ||||
| Rational design—Site-saturation mutagenesis | F108I | p-nitrophenyl glycidyl ether | 20 (S) | 3.4 (S) | NI | NI | [86] | ||||
| F108T | 22 (S) | 3.4 (S) | NI | NI | |||||||
| Agromyces mediolanus ZJB120203 | Rational design—Structure-based site saturation and site-directed mutagenesis | W182F/S207V/N240D | epichlorohydrin | 90.0 (R) | 12.9 (R) | NI | NI | [87] | |||
| Aspergillus niger LCP 521 | Directed evolution—one round of error-prone PCR | A217V/K332E/A390E | phenyl glycidyl ether | 10.8 (S) | 4.6 (S) | 74 (S) | 56 (S) | 39 | 33 | [75] | |
| Semi-rational design —Directed evolution using ISM with combinatorial active site saturation (CASTing) | L215F/A217N/R219S/L249Y/T317W/T318V/M329P/L330Y/C350V | phenyl glycidyl ether | 115 (S) | 4.6 (S) | 95 (S) | 56 (S) | 48 | 33 | [88] | ||
| Semi-rational design—Directed evolution using ISM and optimalization of expression in recombinant cells | P221S/F244C/L249F/L215F/T317F/T318V(ProThrAlaSerAlaProHisThrTyrArgGluPheIle)-L349V 2 | phenyl glycidyl ether | 160 (S) | 4.6 (S) | 97 (S) | 56 (S) | 45 | 33 | [89] | ||
| Semi-rational design—Directed evolution using all 24 possible pathways using 4 randomization sites for ISM | L215F/R219V/L249F/T317F/T318C/L349D/C350Y | phenyl glycidyl ether | 158 (S) | 4 (S) | 98 (S) | 61 (S) | 30 | 28 | [90] | ||
| Aspergillus usamii (AuEH2) | Semi-rational design—Microtuning of the substrate-binding pocket | A214C/A250I | styrene oxide | 202 | 16 | >99 (R) | NI | 50.2 | 40 | [91] | |
| A250I | o-nitrostyrene oxide | 341 | 96 | 98.0 (R) | NI | 50.5 | NI | ||||
| A250W | isopropyl glycidyl ether | 204 | 6.8 | 80.2 (S) | NI | 55.2 | NI | ||||
| Rhodococcus erythropolis DCL14 | Directed evolution—ISM using NDT codon degeneracy | M32C/I80F/L114C/I116V | cyclopentene oxide | NI | 93 (S,S) | 13 (R,R) | 65 | 72 | [92] | ||
| cyclohexene oxide | NI | 97 (S,S) | 4 (S,S) | 94 | 84 | ||||||
| cycloheptene oxide | NI | 98 (S,S) | 17 (S,S) | 75 | 74 | ||||||
| cis-2,3-butene oxide | NI | 93 (R,R) | 5 (S,S) | NI | |||||||
| phenyl glycidyl ether | 32 (R) | 2.6 (R) | 92 (R) | 37 (R) | 31 | 33 | |||||
| styrene oxide | 44 (S) | 2.8 (R) | 91 (S) | 40 (R) | 43 | 36 | |||||
| M32C/L74I/M78F/I80C/V83I | cyclopentene oxide | NI | 80 (R,R) | 13 (R,R) | 81 | 72 | |||||
| cyclohexene oxide | NI | 90 (R,R) | 4 (S,S) | 74 | 84 | ||||||
| cycloheptene oxide | NI | 77 (R,R) | 17 (S,S) | 84 | 74 | ||||||
| cis-2,3-butene oxide | NI | 83 (R,R) | 5 (S,S) | NI | |||||||
| styrene oxide | 36 (R) | 2.8 (R) | 91 (R) | 40 (R) | 30 | 36 | |||||
| Directed evolution—ISM using a single-code saturation mutagenesis (SCSM) | L74F/M78F/L103V/L114V/I116V/F139V/L147V | cyclohexene oxide | NI | 92 (S,S) | 4(S,S) | >99 | 84 | [78] | |||
| cycloheptene oxide | NI | 94 (S,S) | 17 (S,S) | 52 | 97 | ||||||
| L74F/M78F/I80V/L114F | cyclohexene oxide | NI | 96 (R,R) | 4 (S,S) | 83 | 84 | |||||
| cycloheptene oxide | NI | 94 (R,R) | 17 (S,S) | 66 | 97 | ||||||
| Directed evolution—ISM using double-code saturation mutagenesis (DCSM) | L74F/M78F/I80F/L114V/I116V/F138V | cyclopentene oxide | NI | 85 (S,S) | 13 (R,R) | 13 | 84 | [79] | |||
| cyclohexene oxide | NI | 97 (S,S) | 4 (S,S) | 98 | >99 | ||||||
| cycloheptene oxide | NI | 97 (S,S) | 17 (S,S) | 73 | 97 | ||||||
| M78V/I80V/L114F | cyclohexene oxide | NI | 92 (R,R) | 13 (R,R) | 99 | >99 | |||||
| cycloheptene oxide | NI | 85 (R,R) | 4 (S,S) | 40 | 97 | ||||||
| styrene oxide | NI | 57 (S) | 21 (R) | 7 | 46 | ||||||
| Directed evolution—ISM using triple-code saturation mutagenesis (TCSM) | I80Y/L114V/I116V | cyclohexene oxide | NI | 99 (S,S) | 4 (S,S) | 97 | >99 | [80] | |||
| cycloheptene oxide | NI | 98 (S,S) | 17 (S,S) | 81 | 97 | ||||||
| styrene oxide | 28.0 | 1.8 | 92 (S) | 26 (R) | 15 | 17 | |||||
| M32V/M78V/I80V/L114F | cyclohexene oxide | NI | 97 (R,R) | 4 (S,S) | >99 | >99 | |||||
| cycloheptene oxide | NI | 94 (R,R) | 17 (S,S) | 83 | 97 | ||||||
| Semi-rational design—Directed evolution using ISM with reduced AA alphabets using binary pattern based on choosing hydrophobic and hydrophilic amino acids | I80F/V83I/L114 V/I116V | cyclopentene oxide | NI | 94 (S,S) | 7 (R,R) | 34 | 69 | [93] | |||
| cyclohexene oxide | NI | 97 (S,S) | 3 (S,S) | 93 | 87 | ||||||
| cycloheptene oxide | NI | 97 (S,S) | 22 (S,S) | 96 | 99 | ||||||
| I80V/V83I/L114 V | cyclopentene oxide | NI | 51 (R,R) | 7 (R,R) | 48 | 69 | |||||
| cyclohexene oxide | NI | 79 (R,R) | 3 (S,S) | 97 | 87 | ||||||
| cycloheptene oxide | NI | 53 (R,R) | 22 (S,S) | 99 | 99 | ||||||
| Semi-rational design—Directed evolution using ISM with the aim to improve thermostability, enantioselectivity and activity | T76K/L114V/I116V/N92K/F139V/L147F/S15D/A19K/L74F/M78F/E45D | cyclohexene oxide | NI | 94 (S,S) | 2 (S,S) | 100 | 100 | [94] | |||
| S15P/M78F/N92K/F139V/T76K/T85K/E45D/I80V/E124D | cyclohexene oxide | NI | 80 (R,R) | 2 (S,S) | 100 | 100 | |||||
| Semi-rational design—Directed evolution using saturation mutagenesis, mutants were prepared by high-fidelity solid-phase chemical gene synthesis on silicon chips followed by efficient gene assembly instead of PCR to overcome AA bias | M78F/I80Y/L114V/I116V | cyclohexene oxide | NI | >98 (S,S) | NI | >98 | NI | [95] | |||
| R. erythropolis DCL14 (mutant LEH-P) 3 | Rational design—Computational design of mutant library using CASCO strategy | M32L/L74I/I80V/L103F/F139L | cyclopentene oxide | NI | 85.5 (R,R) | 23.9 (R,R) | NI | [96] | |||
| M32L/L35W/L74F/M78F/I80A/I116V/F139L | NI | 90.2 (S,S) | 23.9 (R,R) | NI | |||||||
| Rational design—Use of Rosetta enzyme design to computationally predict enantioselective mutants and high-throughput-multiple independent molecular docking simulations for in silico screening of the generated mutant libraries | M32A/M78I/I80F/L103I/I116V/F139L | cyclopentene oxide | NI | 85 (S,S) | 14 (R,R) | NI | [81] | ||||
| L35W/L74F/I80G/I116V/F139L | cis-2,3-butane oxide | NI | 82 (S,S) | 2 (S,S) | NI | ||||||
| M32L/L35G/I80W/L103V/F139L | cis-stilbene oxide | NI | >99 (R,R) | 92 (R,R) | 98 | NI | |||||
| M32L/L35M/L103I/L114M/I116F/F139L | NI | 88 (S,S) | 92 (R,R) | 63 | NI | ||||||
| Solanum tuberosum (StEH1) | Semi-rational design—Directed evolution—ISM targeting AA residues around active site of enzyme | W106L/L109Y/V141K/I155V | (2,3-epoxypropyl)benzene | 15 (R) | 0.4 (R) | 60 (R) | 32 (S) | NI | [97] | ||
| Semi-rational design—Directed evolution with 2 rounds of iterative saturation mutagenesis | W106L/L109Y/V141K/I155W/F189C | styrene oxide | 5800 (S) | 69 (S) | NI | NI | [98] | ||||
| trans-2-methylstyrene oxide | 770 (S) | 84 (S) | NI | NI | |||||||
| Sphingomonas sp. HXN-200 | Semi-rational design—Site-directed mutagenesis of selected AA residues in active site based on homology modelling | V196A/N226A/M332A | phenyl glycidyl ether | 21.2 (R) | 2.2 (R) | 79.2 (S) | 61.9 (S) | 50 | 50 | [99] | |
| metagenomic DNA (Kau2EH) | Semi-rational design—Directed evolution by randomizing selected sites within substrate binding pocket | V290Y | p-chlorostyrene oxide | 130 | NI | 97 (R) | NI | 50 | NI | [100] | |
| Enantioconvergence | A. niger M200 | Semi-rational design—ISM, mutated sites were chosen on structural similarity with EH from A. niger LCP 521 | L349V/C350W/T317W/T318V/M218W/R219E/L215M/A217G/M245A | styrene oxide | 22 | 10 | 70.1 (R) | 3.0 (R) | 100 | 100 | [101] |
| p-chlorostyrene oxide | 20 | 40 | 70.5 (R) | 4.4 (R) | 100 | 100 | |||||
| Glycine max (GmEH3) | Semi-rational design —Site-saturation and combinatorial mutagenesis used for reshaping substrate-binding pocket | W102V/P187F | 1,2-epoxyhexane | NI | 83.8 (R) | 47.2 (R) | >99 | >99 | [102] | ||
| Phaseolus vulgaris (PvEH1) | Rational design—Site-directed mutagenesis based on molecular docking simulations and multiple alignment | L105I/M160A/M175I | styrene oxide | 3.6 | 1.5 | 87.8 (R) | 33.6 (R) | NI | [103] | ||
| m-chlorostyrene oxide | NI | 69.7 (R) | 1.0 (R) | NI | |||||||
| p-nitrostyrene oxide | NI | 64.7 (R) | 50.3 (R) | NI | |||||||
| m-nitrostyrene oxide | NI | 52.3 (R) | 14.7 (R) | NI | |||||||
| p-chlorostyrene oxide | NI | 70.9 (R) | 51.4 (R) | NI | |||||||
| Rational design—Leucine scanning used for identification of AA residues at sites lining the enzyme’s binding pocket responsible for enantioconvergence and subsequent saturation mutagenesis | L105I/M160A/M175I/Y149L/P184W | m-chlorostyrene oxide | NI | 96.1 (R) | 1.0 (R) | >99 | >99 | [104] | |||
| Rational design—Reshaping of substrate binding pocket | L105I/V106I/M160A/M175I/S178T/P184W | styrene oxide | NI | 90.3 (R,R) | 33.6 (R,R) | >99.9 | 99.1 | [82] | |||
| p-nitrostyrene oxide | NI | 86.7 (R,R) | 50.3 (R,R) | 84.2 | 99.3 | ||||||
| m-nitrostyrene oxide | NI | 85.1 (R,R) | 14.7 (R,R) | >99.9 | 99.7 | ||||||
| p-fluorostyrene oxide | NI | 90.6 (R,R) | 13.6 (R,R) | >99.9 | 98.7 | ||||||
| m-chlorostyrene oxide | 6 | 2 | 96.2 (R,R) | 1.0 (R,R) | 99.2 | 99.9 | |||||
| Rhodotorula paludigena JNU001 | Rational design—Microtuning substrate-binding pocket of EH by computer-aided design using valine scanning mutagenesis | L360C | m-nitrostyrene oxide | NI | 93.4 (R) | 85.7 (R) | 99 | >99 | [105] | ||
| Vigna radiata (VrEH2) | Rational design—Creation of smart library by site-directed mutagenesis using reduced AA alphabet to prepare enantioconvergent EH | M263N | p-nitrostyrene oxide | NI | 98 (R) | 84 (R) | 99.5 | NI | [106] | ||
| m-nitrostyrene oxide | NI | 90 (R) | 20 (R) | >99 | >99 | ||||||
| Rational design—Creation of smart library by site-directed mutagenesis using reduced AA alphabet to prepare enantioconvergent EH | M263Q | m-chlorostyrene oxide | NI | 90 (R) | 20 (R) | NI | [107] | ||||
| M263V | 2-naphthyloxirane | NI | 90 | 60 | NI | ||||||
| metagenomic DNA (Kau2EH) | Semi-rational design —Directed evolution by randomizing selected sites within substrate binding pocket | W110L/F113L/F161Y/P193G/V290W | p-chlorostyrene oxide | 17 | 23 | 93 (R) | 84 (R) | 100 | 100 | [100] | |
| Immobilization Technique | EH (Source) | Immobilized Biocatalyst | Support | Benefit of Immobilization | Ref. |
|---|---|---|---|---|---|
| Covalent bond | ArEH (Agrobacterium radiobacter AD1) | Crude enzyme extract | LX-1000EP modified by EDA LX-1000EP | Operational stability, reusability, increased thermal stability as compared to free enzyme | [121] |
| Purified enzyme | Dextran activated with NaIO4 and ethylene glycol Ficoll activated with NaIO4 and ethylene glycol Amylopectin activated with NaIO4 and ethylene glycol Carboxymethyl cellulose activated with NaIO4 and ethylene glycol | Improved tolerance to the inhibitory effects of Co2+, Fe3+ and EDTA | [122] | ||
| Kau2EH (metagenomic DNA) | Purified enzyme | Eupergit C 250L Eupergit C Eupergit C modified by IDA and CuSO4 Sepabeads EC-EP Sepabeads EC-EP modified by IDA and CuSO4 | Significantly higher thermal stability as compared to free enzyme | [123] | |
| VrEH2M263N (Vigna radiata) | Purified enzyme | ECR8205F (Epoxy) ECR4204F (Epoxy) ECR8215F (Epoxy) ES-103 (Epoxy) ESR-1 (Amino) ESQ-1 (Amino) ECR8405F (Amino) | Improvement of thermal and operational stability as compared to free enzyme | [19] | |
| AnEH (Aspergillus niger LCP 521) | Purified enzyme (lyophilized powder) | Eupergit C Eupergit C 250L Eupergit C 250L modified by EDA Eupergit C modified by IDA and CuSO4 | Improvement of enzyme stability and enantioselectivity | [124] | |
| Eupergit C 250L modified by EDA and glutaraldehyde | Improvement of enzyme storage and thermal stability and enantioselectivity | [125] | |||
| Eupergit C modified by EDA and glutaraldehyde; Florisil® silanized with 3-APTES and activated with glutaraldehyde | Improvement of enzyme reusability and enantioselectivity | [126] | |||
| Epoxide-derived silica gel | Enhancement of enzyme stability in the presence of DMSO | [127] | |||
| StEH (Solanum tuberosum) | Crude enzyme extract (lyophilized powder) | Sepabeads EP—Epoxy modified by IDA and CuSO4 Glyoxyl–agarose (agarose modified by glycidol and oxidized by NaIO4) | Stabilization of enzyme | [128] | |
| mEH (rat liver) | Purified enzyme | Sephadex G-150 activated by 1,1′-carbonyldiimidazole | Enhancement of stability and repeated use of the enzyme | [129] | |
| Dextran activated by 1,1′-carbonyldiimidazole | Increasement of enzyme stability | [130] | |||
| VaEH (Vigna angularis) | Partially purified enzyme | Mesocellular foam silica (MCF) amino modified and activated by glutaraldehyde; Santa Barbara Amorphous (SBA-15) amino modified and activated by glutaraldehyde | Enhancement of enzyme operational stability and thermal stability | [131] | |
| Ionic bond/Affinity bond (His-tag) | StEH (Solanum tuberosum) | Crude enzyme extract | Silica oxide powder modified by resacetophenone and Co2+ | Observation of enzyme activity in organic solvents | [132] |
| mMcEH (triple mutant) (Mugil cephalus) | Purified enzyme | NiO presenting magnetic nanoparticles | Reusability of enzyme | [133] | |
| CESH (Nocardia tartaricans CAS-52) | Purified enzyme | Metal ion affinity chromatography media Ni-IDA QZT 6FF | Enhancement of enzyme activity | [134] | |
| Adsorption | AnEH (Aspergillus niger LCP 521) | Purified enzyme | Accurel EP 100 (polypropylene resin) | Enhancement of enzyme operational stability using nonporous DEAE-cellulose | [135] |
| DEAE cellulose (ionic bond) | Reusability of enzyme | [135,136] | |||
| Porous polypropylene | Immobilized for preparative purposes (reuse, continuous reactor) | [137] | |||
| Lewatit® VP OC 1600 | Enzyme reusability, enhancement of enantioselectivity | [126] | |||
| Nsp.EH (Nocardia sp. EH1) | Partially purified enzyme | DEAE cellulose (ionic bond) | Enzyme stabilization | [138] | |
| ArEH (Agrobacterium radiobacter AD1 expressed in E. coli) | Whole cells | Perlite | Immobilized for preparative purposes | [139] | |
| McEH (Mugil cephalus) | Purified enzyme | Magnetically separable silica Mag-MSU-F (adsorption) + cross-linking with glutaraldehyde | Enhancement of enzyme stability and reusability | [140] | |
| CLEA | VrEH (Vigna radiata) | Partially purified enzyme extract | Cross-linker: glutaraldehyde | Enhancement of catalytic efficiency, enantioselectivity and product yield | [141] |
| Enhancement of initial reaction rate, product yield, enantioselectivity, operational stability | [142] | ||||
| Co-polymerization | RgEH (Rhodotorula glutinis CIMW 147 (ATCC 201718)) | Partially purified enzyme | Acylation of enzyme by itaconic acid, bio-imprinted with substrate and copolymerized with ethylene glycol dimethacrylate | Enzyme stabilization, reusability and product separation, improvement of enantioselectivity | [143] |
| Nanoflowers | GmEH (Glycine max) | Purified enzyme | Organic–inorganic nanoflowers formed with Ca2+ ions | High catalytic activity and stability | [144] |
| Metal–organic framework (MOFs) | GmEH (Glycine max) | Crude enzyme preparation (extract) | UiO-66-NH2 metal−organic framework (MOF) cross-linked with glutaraldehyde | Higher enzyme pH stability, thermostability and tolerance to organic solvents as compared to free enzyme | [145] |
| HdEH (Hypsibius dujardini) | Purified enzyme | Zeolitic imidazole frameworks (ZIF-8) Zeolitic imidazole frameworks treated with glutaraldehyde (Glu/ZIF-8) | Enhancement of stability, enantioselectivity, reusability of enzyme | [146] | |
| Encapsulation | CESH (Nocardia tartaricans ATCC 31191) | Whole cells | Polyelectrolyte complex microcapsules from sodium alginate−cellulose sulfate−poly(methylene-co-guanidine) | Enhanced enzyme activity, storage stability and decreased reaction time using immobilized whole cells as compared to free cells | [116] |
| Enhancement of operational stability | [118] | ||||
| Entrapment | RtEH (Rhodosporidium toruloides UOFS Y-0471) | Whole cells | Calcium alginate | Stabilization of cells | [147] |
| CESH (Labrys sp. BK-8) | Whole cells | κ-carrageenan | Stabilization of cells | [148] | |
| not mentioned | NOVO SP409 (Rhodococcus sp. commercial preparation) | Crude enzyme | Not mentioned | Preparative purposes | [113] |
| Enzymes in the Cascade including EH and Enzyme Source/GMO Cells | Substrate(s) | Product(s) | Note to the Role of EH in the Cascade | Ref. |
|---|---|---|---|---|
Epoxide hydrolase SpEH from Sphingomonas sp. HXN-200 and butanediol dehydrogenase BDHA from Bacillus subtilis BGSC1A1 and NADH oxidase NOX from Lactobacillus brevis DSM 20054/
| Meso- or racemic epoxides | R-(α)-hydroxyketones | No significant influence of using separately expressed vs. co-expressed enzymes of the cascade on ee and conversion | [155] |
Epoxide hydrolase SpEH from Sphingomonas sp. HXN-200 or Epoxide hydrolase StEH from Solanum tuberosum and styrene monooxygenase SMO/
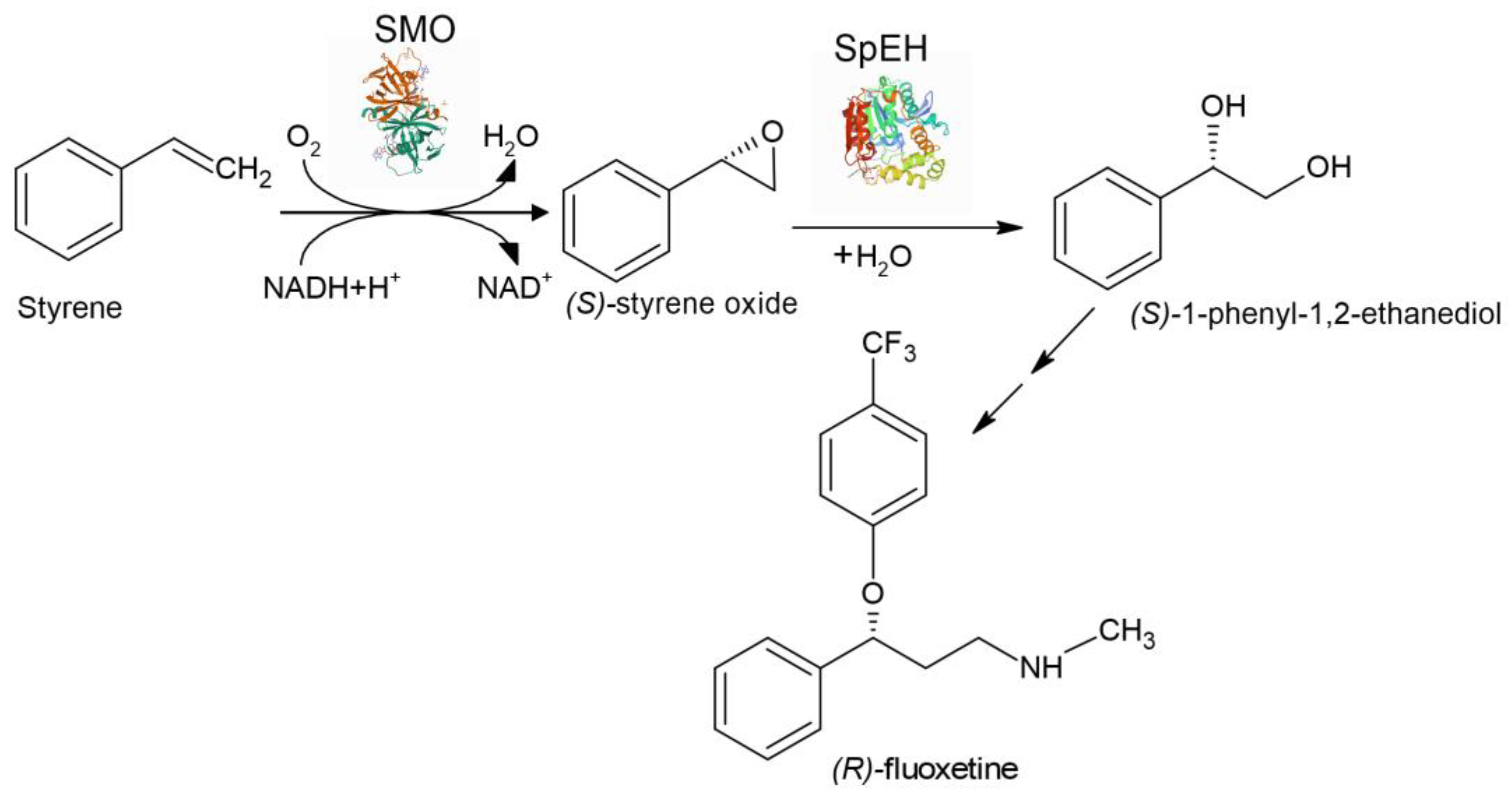
| Aryl olefins | Chiral vicinal diols | The first enzyme cascade which enabled reversing enantioselectivity of dihydroxylation using StEH instead of SpEH | [156] |
Epoxide hydrolase AmEH from Agromyces mediolanus and halohydrin dehalogenase HheC from Agrobacterium radiobacter AD1/
| 1,3-dichloro-2-propanol | Chiral epichlorohydrin | Effect of co-expressed vs. separately expressed enzymes on the enantioselectivity of the cascade | [157] |
Epoxide hydrolase SpEH from Sphingomonas sp. and styrene monooxygenase SMO from Pseudomonas sp./
| Styrene | (S)-1-phenyl-1,2-ethanediol | Aqueous/organic biphasic reaction system was used for the first time for cascade biotransformation to enhance productivity | [158] |
Epoxide hydrolase MupZ from Pseudomonas fluorescens NCIMB 10586 and Rieske non-heme oxygenase MupW Pseudomonas fluorescens NCIMB 10586/
| Mupirocins | Hydroxylated tetrahydropyrans and tetrahydrofurans | Cascade containing epoxide hydrolase and Rieske non-heme oxygenase enabled formation of heterocyclic THP ring, which is difficult to achieve biosynthetically | [159] |
| Epoxide hydrolase SpEH from Sphingomonas sp. HXN-200, alcohol dehydrogenase MnADH from Mycobacterium neoaurum VKM AC-1815D, ω-transaminase PAKω-TA from Pseudomonas aeruginosa and glutamate dehydrogenase GluDH from E. coli BL21/ E. coli BL21 (SGMP) co-expressing 4-enzyme self-sufficient cascade system SpEH-MnADH-PAKω-TA-GluDH | (S)-epoxides | Chiral 1,2-aminoalcohols | The first one-step synthesis of optically pure 1,2-amino alcohols from (S)-epoxides employing a synthetic redox-self-sufficient enzyme cascade in recombinant cells | [160] |
Epoxide hydrolase SpEH from Sphingomonas sp. HXN-200, 2,3-butanediol dehydrogenase BDHA from Bacillus subtilis, polyol dehydrogenase GoSCR from Gluconobacter oxydans, (R)-ω-transaminase MVTA from Mycobacterium vanbaalenii/
| Racemic epoxides | Enantiopure β-amino alcohols | General access to variety of chiral β-amino alcohols starting from inexpensive racemic epoxides using designed enzyme cascade process in recombinant cells | [161] |
Styrene monooxygenase SMO from Pseudomonas sp., epoxide hydrolase SpEH from Sphingomonas sp. HXN-200, polyol dehydrogenase GoSCR from Gluconobacter oxydans, (R)-ω-transaminase MVTA from Mycobacterium vanbaalenii or transaminase BMTA from Bacillus megaterium SC6394/
| Styrenyl olefins | 2-amino-2-phenyl ethanols | Challenging direct regio- and stereoselective aminohydroxylation of olefins to unprotected enantioenriched β-amino alcohols was enabled by novel one-pot four-enzyme biocatalytic cascade in good yields and excellent enantioselectivity | [162] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bučko, M.; Kaniaková, K.; Hronská, H.; Gemeiner, P.; Rosenberg, M. Epoxide Hydrolases: Multipotential Biocatalysts. Int. J. Mol. Sci. 2023, 24, 7334. https://doi.org/10.3390/ijms24087334
Bučko M, Kaniaková K, Hronská H, Gemeiner P, Rosenberg M. Epoxide Hydrolases: Multipotential Biocatalysts. International Journal of Molecular Sciences. 2023; 24(8):7334. https://doi.org/10.3390/ijms24087334
Chicago/Turabian StyleBučko, Marek, Katarína Kaniaková, Helena Hronská, Peter Gemeiner, and Michal Rosenberg. 2023. "Epoxide Hydrolases: Multipotential Biocatalysts" International Journal of Molecular Sciences 24, no. 8: 7334. https://doi.org/10.3390/ijms24087334
APA StyleBučko, M., Kaniaková, K., Hronská, H., Gemeiner, P., & Rosenberg, M. (2023). Epoxide Hydrolases: Multipotential Biocatalysts. International Journal of Molecular Sciences, 24(8), 7334. https://doi.org/10.3390/ijms24087334