Therapeutic Effectiveness of Interferon-α2b against COVID-19 with Community-Acquired Pneumonia: The Ukrainian Experience
Abstract
1. Introduction
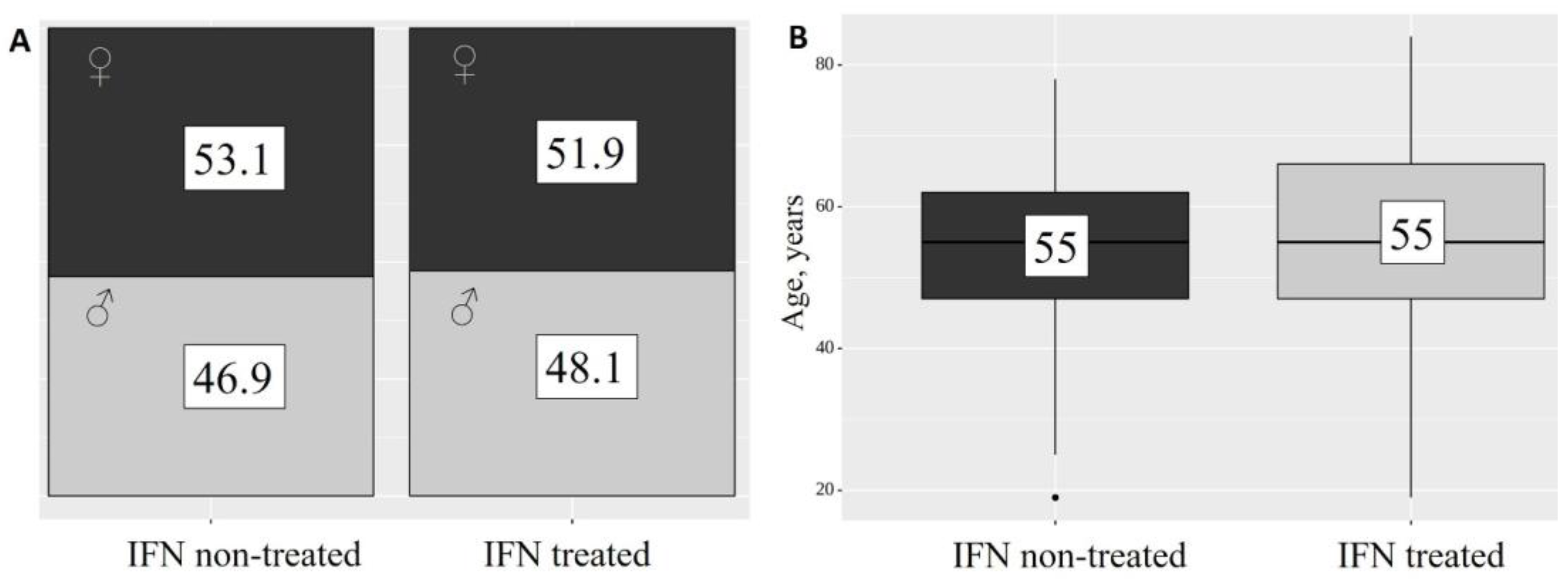
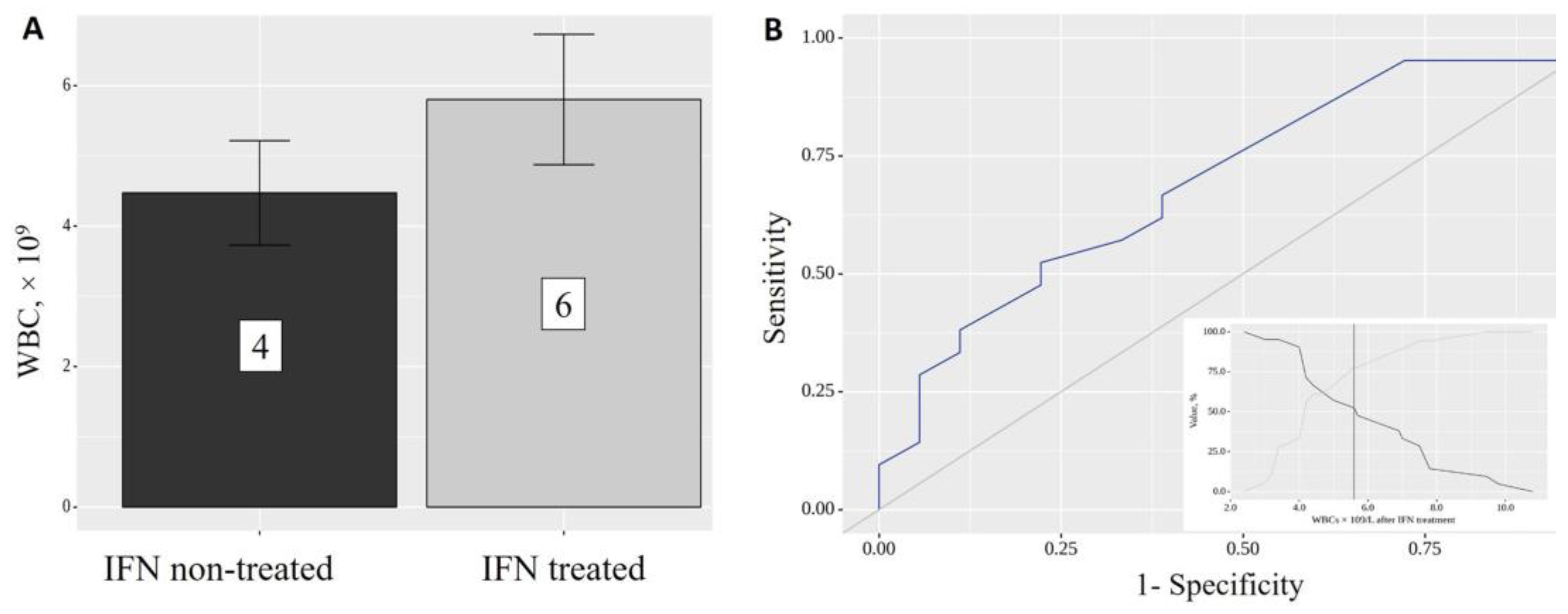
2. Results
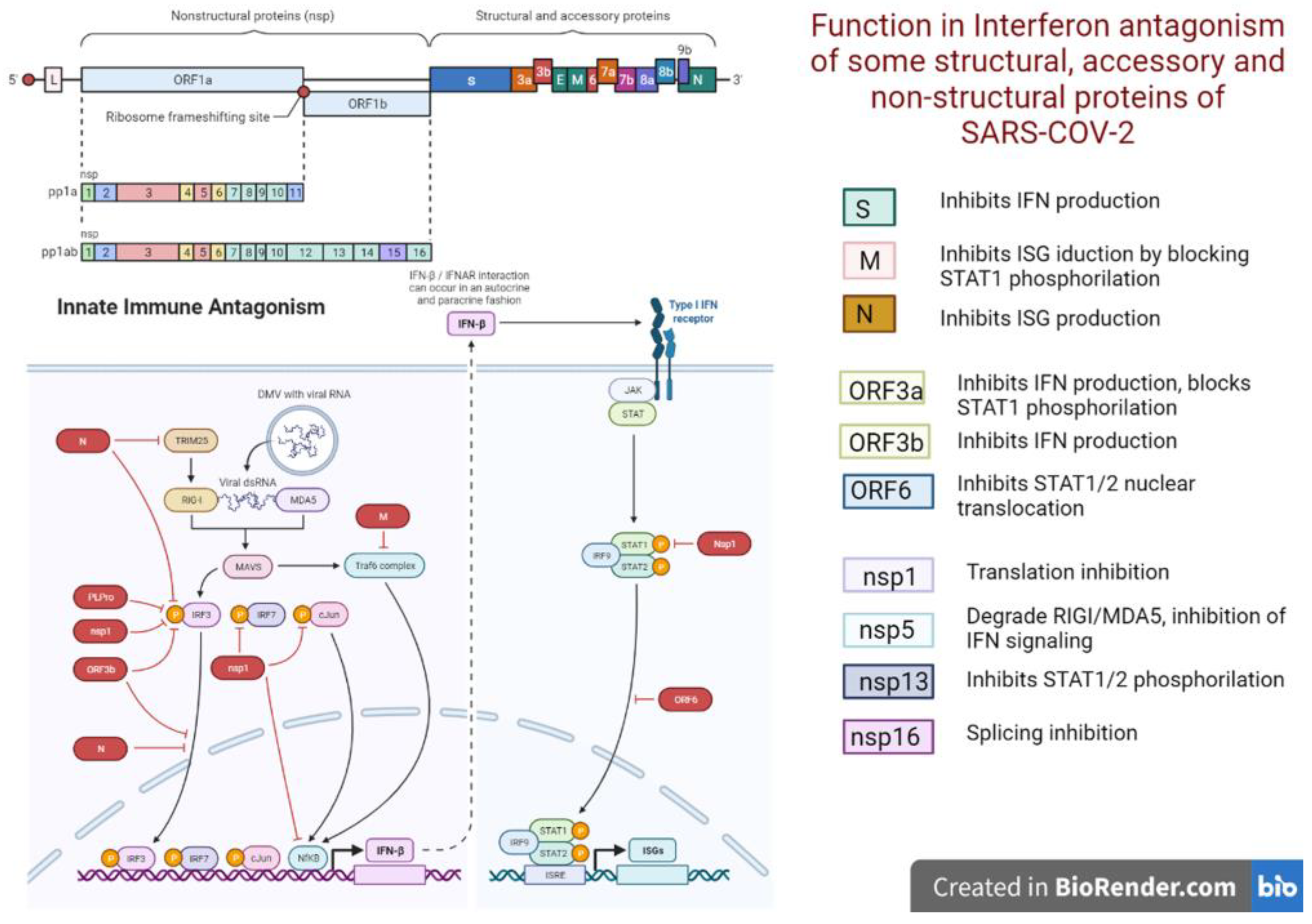
3. Discussion
4. Materials and Methods
4.1. Study Design and Data Collection
4.2. Clinical Characteristics of the Patients
4.3. Randomization, IFN-α Treatment and Outcomes
4.4. Statistical Analysis
5. Conclusions
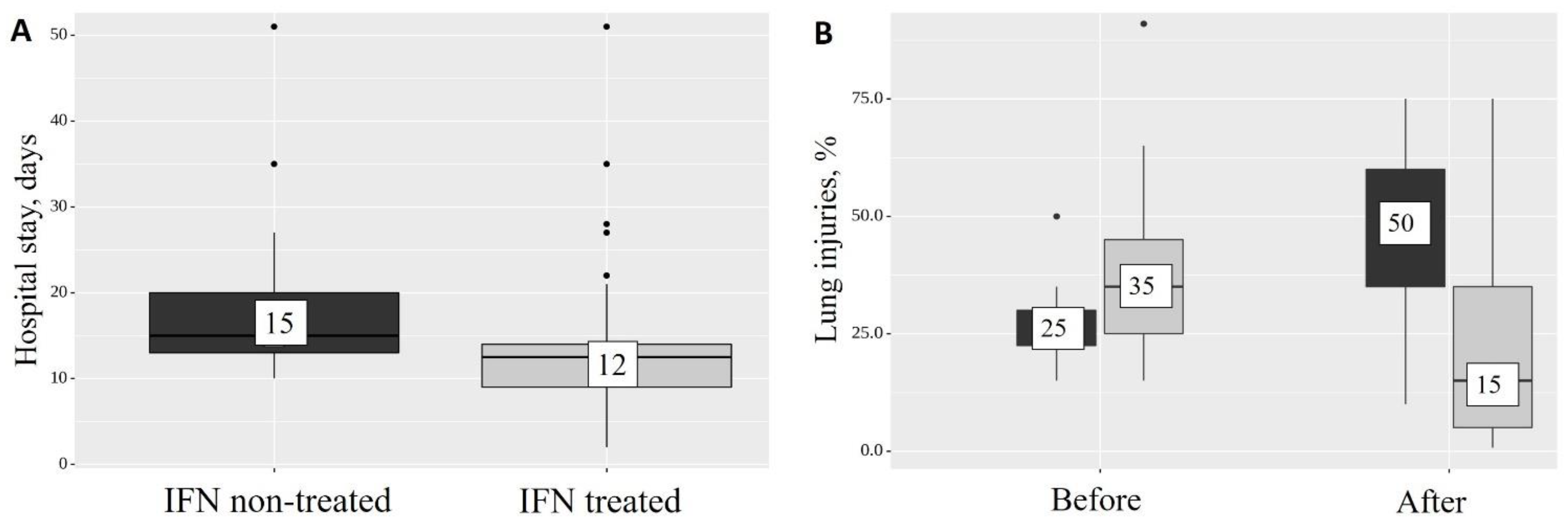
- Adding IFN-α2b to the standard therapy for patients with severe COVID-19 reduces the length of the hospital stay by 3 days (p < 0.001).
- Treatment with IFN-α2b reduces the level of CT-diagnosed lung injuries from 35% to 15% (p = 0.011) at the time of discharge and, compared with the group of patients who did not receive IFN-α2b, the percentage of CT injuries decreases at the end of the hospital stay from 50 to 15% (p = 0.017).
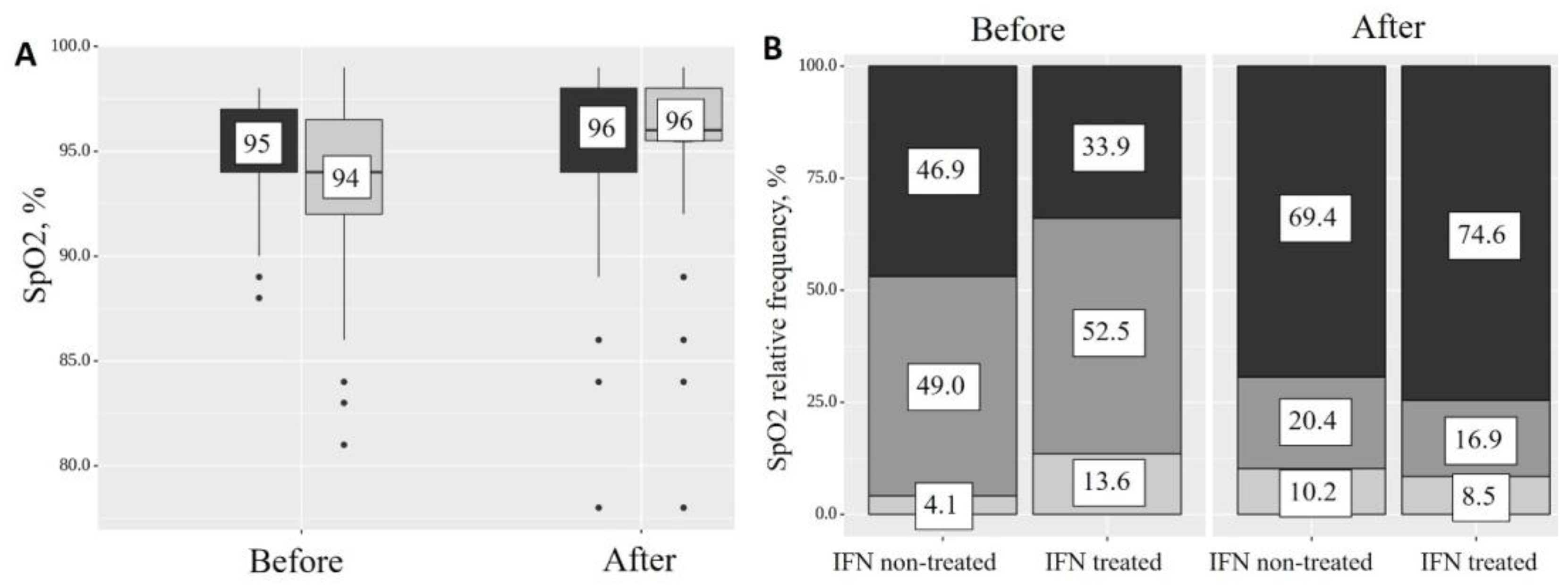
- In the group of patients receiving IFN-α2b, the SpO2 index before and after treatment increased from 94 (92–96, Q1–Q3) to 96 (96–98, Q1–Q3) (p < 0.001), while the percentage of patients with normal saturation increased (from 33.9% to 74.6, p < 0.05), but the level of SpO2 decreased in the low (from 52.5% to 16.9%) and very low (from 13.6% to 8.5%) categories.
- Considering the contradictory results obtained regarding the strength of the response to type I IFNs in patients with severe COVID-19, more accurate information is required for the appropriate therapeutic use of type I IFNs.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO Director-General’s Opening Remarks at the Media Briefing on COVID-19—11 March 2020. Available online: https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 (accessed on 15 February 2023).
- Aktar, M.A.; Alam, M.M.; Al-Amin, A.Q. Global Economic Crisis, energy use, CO(2) emissions, and policy roadmap amid COVID-19. Sustain. Prod. Consum. 2021, 26, 770–781. [Google Scholar] [CrossRef]
- Jaroń, A.; Borucka, A.; Parczewski, R. Analysis of the impact of the COVID-19 pandemic on the value of CO2 emissions from electricity generation. Energies 2022, 15, 4514. [Google Scholar] [CrossRef]
- Morgenstern, B.; Michaelis, M.; Baer, P.C.; Doerr, H.W.; Cinatl, J.J. Ribavirin and interferon-beta synergistically inhibit SARS-associated coronavirus replication in animal and human cell lines. Biochem. Biophys. Res. Commun. 2005, 326, 905–908. [Google Scholar] [CrossRef]
- Chu, C.M.; Cheng, V.C.C.; Hung, I.F.N.; Wong, M.M.L.; Chan, K.H.; Chan, K.S.; Kao, R.Y.T.; Poon, L.L.M.; Wong, C.L.P.; Guan, Y.; et al. Role of Lopinavir/Ritonavir in the treatment of SARS: Initial virological and clinical findings. Thorax 2004, 59, 252–256. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Cao, R.; Zhang, L.; Yang, X.; Liu, J.; Xu, M.; Shi, Z.; Hu, Z.; Zhong, W.; Xiao, G. Remdesivir and Chloroquine effectively inhibit the recently emerged novel coronavirus (2019-NCoV) in Vitro. Cell Res. 2020, 30, 269–271. [Google Scholar] [CrossRef]
- Yamamoto, N.; Yang, R.; Yoshinaka, Y.; Amari, S.; Nakano, T.; Cinatl, J.; Rabenau, H.; Doerr, H.W.; Hunsmann, G.; Otaka, A.; et al. HIV protease inhibitor nelfinavir inhibits replication of SARS-associated coronavirus. Biochem. Biophys. Res. Commun. 2004, 318, 719–725. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Lu, Z.; Xu, T.; Chen, C.; Yang, G.; Zha, T.; Lu, J.; Xue, Y. Arbidol monotherapy is superior to lopinavir/ritonavir in treating COVID-19. J. Infect. 2020, 81, e21–e23. [Google Scholar] [CrossRef]
- Li, H.; Wang, Y.M.; Xu, J.Y.; Cao, B. Potential antiviral therapeutics for 2019 Novel Coronavirus. Zhonghua Jie He He Hu Xi Za Zhi Zhonghua Jiehe He Huxi Zazhi Chin. J. Tuberc. Respir. Dis. 2020, 43, E002. [Google Scholar] [CrossRef]
- Li, D.; Sempowski, G.D.; Saunders, K.O.; Acharya, P.; Haynes, B.F. SARS-CoV-2 neutralizing antibodies for COVID-19 prevention and treatment. Annu. Rev. Med. 2022, 73, 1–16. [Google Scholar] [CrossRef]
- Thümmler, L.; Lindemann, M.; Horn, P.A.; Lenz, V.; Konik, M.; Gäckler, A.; Boss, K.; Theodoropoulos, F.; Besa, V.; Taube, C.; et al. Early treatment with monoclonal antibodies or convalescent plasma reduces mortality in non-vaccinated COVID-19 high-risk patients. Viruses 2023, 15, 119. [Google Scholar] [CrossRef]
- O’Horo, J.C.; Challener, D.W.; Speicher, L.; Bosch, W.; Seville, M.T.; Bierle, D.M.; Ganesh, R.; Wilker, C.G.; Arndt, R.F.; Arndt, L.L.; et al. Effectiveness of monoclonal antibodies in preventing severe COVID-19 with emergence of the delta variant. Mayo Clin. Proc. 2022, 97, 327–332. [Google Scholar] [CrossRef]
- Wen, W.; Chen, C.; Tang, J.; Wang, C.; Zhou, M.; Cheng, Y.; Zhou, X.; Wu, Q.; Zhang, X.; Feng, Z.; et al. Efficacy and safety of three new oral antiviral treatment (Molnupiravir, Fluvoxamine and Paxlovid) for COVID-19: A Meta-Analysis. Ann. Med. 2022, 54, 516–523. [Google Scholar] [CrossRef]
- Jaeckel, E.; Cornberg, M.; Wedemeyer, H.; Santantonio, T.; Mayer, J.; Zankel, M.; Pastore, G.; Dietrich, M.; Trautwein, C.; Manns, M.P. Treatment of acute hepatitis C with interferon Alfa-2b. N. Engl. J. Med. 2001, 345, 1452–1457. [Google Scholar] [CrossRef]
- Feld, J.J.; Hoofnagle, J.H. mechanism of action of interferon and Ribavirin in treatment of hepatitis C. Nature 2005, 436, 967–972. [Google Scholar] [CrossRef]
- Falzarano, D.; de Wit, E.; Rasmussen, A.L.; Feldmann, F.; Okumura, A.; Scott, D.P.; Brining, D.; Bushmaker, T.; Martellaro, C.; Baseler, L.; et al. Treatment with interferon-α2b and ribavirin improves outcome in MERS-CoV-infected rhesus macaques. Nat. Med. 2013, 19, 1313–1317. [Google Scholar] [CrossRef]
- Hijano, D.R.; Vu, L.D.; Kauvar, L.M.; Tripp, R.A.; Polack, F.P.; Cormier, S.A. Role of type I interferon (IFN) in the respiratory syncytial virus (RSV) immune response and disease severity. Front. Immunol. 2019, 10, 566. [Google Scholar] [CrossRef]
- Guo, K.; Barrett, B.S.; Morrison, J.H.; Mickens, K.L.; Vladar, E.K.; Hasenkrug, K.J.; Poeschla, E.M.; Santiago, M.L. Interferon resistance of emerging SARS-CoV-2 variants. Proc. Natl. Acad. Sci. USA 2022, 119, e2203760119. [Google Scholar] [CrossRef]
- Ianevski, A.; Yao, R.; Zusinaite, E.; Lello, L.S.; Wang, S.; Jo, E.; Yang, J.; Ravlo, E.; Wang, W.; Lysvand, H.; et al. Synergistic interferon-Alpha-Based combinations for treatment of SARS-CoV-2 and other viral infections. Viruses 2021, 13, 2489. [Google Scholar] [CrossRef]
- Ianevski, A.; Yao, R.; Lysvand, H.; Grødeland, G.; Legrand, N.; Oksenych, V.; Zusinaite, E.; Tenson, T.; Bjørås, M.; Kainov, D.E. Nafamostat-interferon-α combination suppresses SARS-CoV-2 infection in vitro and in vivo by cooperatively targeting host TMPRSS2. Viruses 2021, 13, 1768. [Google Scholar] [CrossRef]
- Gong, W.J.; Zhou, T.; Wu, S.-L.; Ye, J.-L.; Xu, J.-Q.; Zeng, F.; Su, Y.-Y.; Han, Y.; Lv, Y.-N.; Zhang, Y.; et al. A retrospective analysis of clinical efficacy of Ribavirin in adults hospitalized with severe COVID-19. J. Infect. Chemother. Off. J. Japan Soc. Chemother. 2021, 27, 876–881. [Google Scholar] [CrossRef]
- Li, X.; Zhou, L.; Zhang, L.; Zheng, S.; Huang, F.; Pan, X.; Wang, F. The combined regimens of antiviral therapy might not be useful for the viral clearance of severe COVID-19 cases. J. Infect. Public Health 2021, 14, 1693–1700. [Google Scholar] [CrossRef]
- Zhou, Q.; MacArthur, M.R.; He, X.; Wei, X.; Zarin, P.; Hanna, B.S.; Wang, Z.-H.; Xiang, X.; Fish, E.N. interferon-α2b treatment for COVID-19 is associated with improvements in lung abnormalities. Viruses 2020, 13, 44. [Google Scholar] [CrossRef] [PubMed]
- Yin, P.; Meng, J.; Chen, J.; Gao, J.; Wang, D.; Liu, S.; Guo, Q.; Zhu, M.; Zhang, G.; Liu, Y.; et al. Antiviral drugs Arbidol and interferon alpha-1b contribute to reducing the severity of COVID-19 patients: A retrospective cohort study. Virol. J. 2021, 18, 142. [Google Scholar] [CrossRef] [PubMed]
- Pandit, A.; Bhalani, N.; Bhushan, B.L.S.; Koradia, P.; Gargiya, S.; Bhomia, V.; Kansagra, K. Efficacy and safety of pegylated interferon alfa-2b in moderate COVID-19: A phase II, randomized, controlled, open-label study. Int. J. Infect. Dis. 2021, 105, 516–521. [Google Scholar] [CrossRef]
- Bhushan, B.L.; Wanve, S.; Koradia, P.; Bhomia, V.; Soni, P.; Chakraborty, S.; Khobragade, A.; Joshi, S.; Mendiratta, S.K.; Kansagra, K.K.; et al. Efficacy and safety of pegylated interferon-α2b in moderate COVID-19: A phase 3, randomized, comparator-controlled, open-label study. Int. J. Infect. Dis. 2021, 111, 281–287. [Google Scholar] [CrossRef]
- Buchynskyi, M.; Kamyshna, I.; Lyubomirskaya, K.; Moshynets, O.; Kobyliak, N.; Oksenych, V.; Kamyshnyi, A. efficacy of interferon alpha for the treatment of hospitalized patients with COVID-19: A meta-analysis. Front. Immunol. 2023, 14, 250. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Zhong, M.; Liu, Y.; Zhang, Y.; Zhang, K.; Su, D.; Meng, X.; Zhang, Y. The characteristics and outcomes of 681 severe cases with COVID-19 in China. J. Crit. Care 2020, 60, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Li, Z.; Yang, B.; Wang, P.; Zhou, Q.; Zhang, Z.; Zhu, J.; Chen, X.; Yang, P.; Zhou, H. Delayed-phase thrombocytopenia in patients with coronavirus disease 2019 (COVID-19). Br. J. Haematol. 2020, 190, 179–184. [Google Scholar] [CrossRef]
- Huang, R.; Zhu, L.; Wang, J.; Xue, L.; Liu, L.; Yan, X.; Huang, S.; Li, Y.; Yan, X.; Zhang, B.; et al. Clinical features of patients with COVID-19 with nonalcoholic fatty liver disease. Hepatol. Commun. 2020, 4, 1758–1768. [Google Scholar] [CrossRef]
- Lombardi, A.; Tsomos, E.; Hammerstad, S.S.; Tomer, Y. Interferon alpha: The key trigger of type 1 diabetes. J. Autoimmun. 2018, 94, 7–15. [Google Scholar] [CrossRef]
- Park, A.; Iwasaki, A. Type I and type III interferons-induction, signaling, evasion, and application to combat COVID-19. Cell Host Microbe 2020, 27, 870–878. [Google Scholar] [CrossRef] [PubMed]
- Schoggins, J.W. Interferon-stimulated genes: What Do They All Do? Annu. Rev. Virol. 2019, 6, 567–584. [Google Scholar] [CrossRef] [PubMed]
- Mantlo, E.; Bukreyeva, N.; Maruyama, J.; Paessler, S.; Huang, C. Antiviral activities of type I interferons to SARS-CoV-2 infection. Antivir. Res. 2020, 179, 104811. [Google Scholar] [CrossRef] [PubMed]
- Fisher, T.; Gluck, A.; Narayanan, K.; Kuroda, M.; Nachshon, A.; Hsu, J.C.; Halfmann, P.J.; Yahalom-Ronen, Y.; Tamir, H.; Finkel, Y.; et al. Parsing the role of NSP1 in SARS-CoV-2 infection. Cell Rep. 2022, 39, 110954. [Google Scholar] [CrossRef]
- Glanz, A.; Chakravarty, S.; Varghese, M.; Kottapalli, A.; Fan, S.; Chakravarti, R.; Chattopadhyay, S. Transcriptional and non-transcriptional activation, posttranslational modifications, and antiviral functions of interferon regulatory factor 3 and viral antagonism by the SARS-coronavirus. Viruses 2021, 29, 575. [Google Scholar] [CrossRef]
- Zhou, Q.; Chen, V.; Shannon, C.P.; Wei, X.-S.; Xiang, X.; Wang, X.; Wang, Z.-H.; Tebbutt, S.J.; Kollmann, T.R.; Fish, E.N. Interferon-α2b treatment for COVID-19. Front. Immunol. 2020, 11, 1061. [Google Scholar] [CrossRef]
- Meng, Z.; Wang, T.; Chen, L.; Chen, X.; Li, L.; Qin, X.; Li, H.; Luo, J. The effect of recombinant human interferon alpha nasal drops to prevent COVID-19 pneumonia for medical staff in an epidemic area. Curr. Top. Med. Chem. 2021, 21, 920–927. [Google Scholar] [CrossRef]
- Pereda, R.; González, D.; Rivero, H.B.; Rivero, J.C.; Pérez, A.; Lopez, L.D.R.; Mezquia, N.; Venegas, R.; Betancourt, J.R.; Domínguez, R.E.; et al. Therapeutic effectiveness of interferon alpha 2b treatment for COVID-19 patient recovery. J. Interf. Cytokine Res. 2020, 40, 578–588. [Google Scholar] [CrossRef]
- Wang, N.; Zhan, Y.; Zhu, L.; Hou, Z.; Liu, F.; Song, P.; Qiu, F.; Wang, X.; Zou, X.; Wan, D.; et al. retrospective multicenter cohort study shows early interferon therapy is associated with favorable clinical responses in COVID-19 patients. Cell Host Microbe 2020, 28, 455–464.e2. [Google Scholar] [CrossRef]
- Zhou, Z.; Ren, L.; Zhang, L.; Zhong, J.; Xiao, Y.; Jia, Z.; Guo, L.; Yang, J.; Wang, C.; Jiang, S.; et al. Heightened innate immune responses in the respiratory tract of COVID-19 patients. Cell Host Microbe 2020, 27, 883–890.e2. [Google Scholar] [CrossRef]
- Lopez, L.; Sang, P.C.; Tian, Y.; Sang, Y. dysregulated interferon response underlying severe COVID-19. Viruses 2020, 12, 1433. [Google Scholar] [CrossRef]
- Shaabani, N.; Duhan, V.; Khairnar, V.; Gassa, A.; Ferrer-Tur, R.; Häussinger, D.; Recher, M.; Zelinskyy, G.; Liu, J.; Dittmer, U.; et al. CD169(+) macrophages regulate PD-L1 expression via type I interferon and thereby prevent severe immunopathology after LCMV infection. Cell Death Dis. 2016, 7, e2446. [Google Scholar] [CrossRef]
- Piconese, S.; Pacella, I.; Timperi, E.; Barnaba, V. Divergent Effects of Type-I Interferons on Regulatory T Cells. Cytokine Growth Factor Rev. 2015, 26, 133–141. [Google Scholar] [CrossRef]
- Kuka, M.; De Giovanni, M.; Iannacone, M. The role of type I interferons in CD4+ T cell differentiation. Immunol. Lett. 2019, 215, 19–23. [Google Scholar] [CrossRef]
- Wilson, E.B.; Yamada, D.H.; Elsaesser, H.; Herskovitz, J.; Deng, J.; Cheng, G.; Aronow, B.J.; Karp, C.L.; Brooks, D.G. Blockade of chronic type I interferon signaling to control persistent LCMV infection. Science 2013, 340, 202–207. [Google Scholar] [CrossRef]
- Zhang, L.; Yuan, S.; Cheng, G.; Guo, B. Type I IFN promotes IL-10 production from T cells to suppress Th17 cells and Th17-associated autoimmune inflammation. PLoS ONE 2011, 6, e28432. [Google Scholar] [CrossRef]
- Pan, H.; Peto, R.; Henao-Restrepo, A.-M.; Preziosi, M.-P.; Sathiyamoorthy, V.; Abdool Karim, Q.; Alejandria, M.M.; Hernández García, C.; Kieny, M.-P.; Malekzadeh, R.; et al. Repurposed antiviral drugs for COVID-19—Interim WHO Solidarity Trial Results. N. Engl. J. Med. 2021, 384, 497–511. [Google Scholar] [CrossRef]
- Davoudi-Monfared, E.; Rahmani, H.; Khalili, H.; Hajiabdolbaghi, M.; Salehi, M.; Abbasian, L.; Kazemzadeh, H.; Yekaninejad, M.S. A randomized clinical trial of the efficacy and safety of interferon β-1a in treatment of severe COVID-19. Antimicrob. Agents Chemother. 2020, 64, e01061-20. [Google Scholar] [CrossRef]
- Walz, L.; Cohen, A.J.; Rebaza, A.P.; Vanchieri, J.; Slade, M.D.; Dela Cruz, C.S.; Sharma, L. JAK-inhibitor and type i interferon ability to produce favorable clinical outcomes in COVID-19 patients: A systematic review and meta-analysis. Res. Sq. 2020, 21, 47. [Google Scholar] [CrossRef]
- Nakhlband, A.; Fakhari, A.; Azizi, H. interferon-alpha position in combating with COVID-19: A systematic review. J. Med. Virol. 2021, 93, 5277–5284. [Google Scholar] [CrossRef]
- Lu, L.-Y.; Feng, P.-H.; Yu, M.-S.; Chen, M.-C.; Lin, A.J.-H.; Chen, J.L.; Yu, L.H.-L. current utilization of interferon alpha for the treatment of coronavirus disease 2019: A comprehensive review. Cytokine Growth Factor Rev. 2022, 63, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Melo, D.; Nilsson-Payant, B.E.; Liu, W.-C.; Uhl, S.; Hoagland, D.; Møller, R.; Jordan, T.X.; Oishi, K.; Panis, M.; Sachs, D.; et al. imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell 2020, 181, 1036–1045.e9. [Google Scholar] [CrossRef] [PubMed]
- Hadjadj, J.; Yatim, N.; Barnabei, L.; Corneau, A.; Boussier, J.; Smith, N.; Péré, H.; Charbit, B.; Bondet, V.; Chenevier-Gobeaux, C.; et al. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science 2020, 369, 718–724. [Google Scholar] [CrossRef] [PubMed]
- Wilk, A.J.; Rustagi, A.; Zhao, N.Q.; Roque, J.; Martinez-Colon, G.J.; McKechnie, J.L.; Ivison, G.T.; Ranganath, T.; Vergara, R.; Hollis, T.; et al. A single-cell atlas of the peripheral immune response to severe COVID-19. Nat. Med. 2020, 26, 1070–1076. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Park, S.; Jeong, H.W.; Ahn, J.Y.; Choi, S.J.; Lee, H.; Choi, B.; Nam, S.K.; Sa, M.; Kwon, J.S.; et al. Immunophenotyping of COVID-19 and influenza highlights the role of type I interferons in development of severe COVID-19. Sci. Immunol. 2020, 5, 1554. [Google Scholar] [CrossRef]
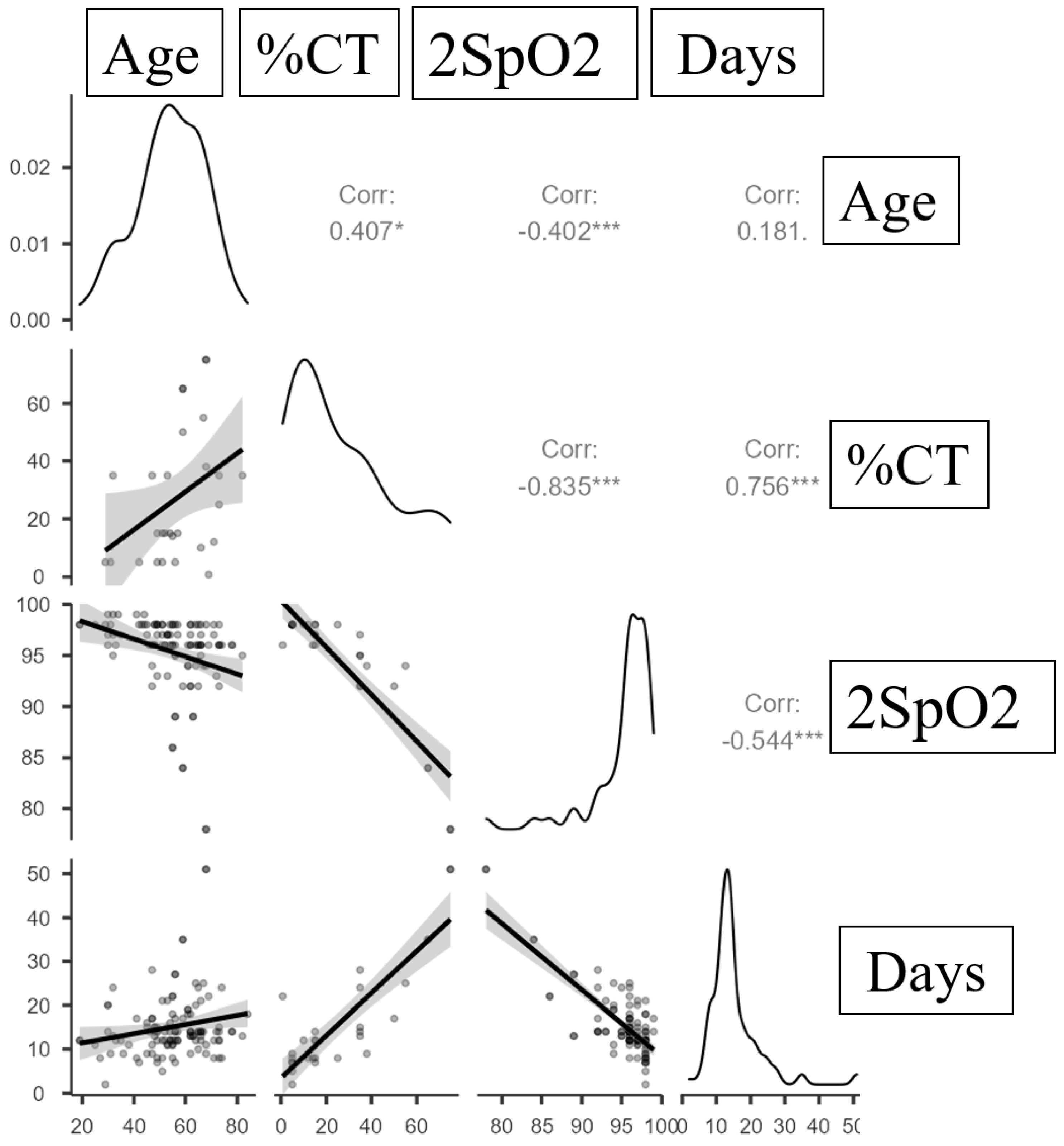
| Quantitative Variables | M ± SD/Me | 95% CI/Q1–Q3 | n | min | max |
| Age, M ± SD | 54 ± 14 | 52–56 | 130 | 19 | 84 |
| Days in hospital, Me | 14 | 12–17 | 117 | 7 | 51 |
| Initial CT% injuries, Me | 35 | 25–45 | 83 | 5 | 91 |
| Final CT% injuries, Me | 15 | 9–36 | 28 | 1 | 75 |
| Initial SpO2, Me | 95 | 93–97 | 116 | 81 | 99 |
| Final SpO2, Me | 96 | 95–98 | 108 | 78 | 99 |
| Categorical Variables | Categories | Abs. | % | ||
| Sex | women | 68 | 52.3 | ||
| men | 62 | 47.7 | |||
| IFN-α2b treatment | IFN non-treated | 49 | 37.7 | ||
| IFN treated | 81 | 62.3 | |||
| Initial SpO2 | normal | 48 | 41.4 | ||
| low | 56 | 48.3 | |||
| very low | 12 | 10.3 | |||
| Final SpO2 | normal | 78 | 72.2 | ||
| low | 20 | 18.5 | |||
| very low | 10 | 9.3 | |||
| IFN Treatment | Follow-Up Periods | p Initial vs. Final | |||
|---|---|---|---|---|---|
| %CT Injuries 1 (Initial) | %CT Injuries 2 (Final) | ||||
| Me | Q1–Q3 | Me | Q1–Q3 | ||
| IFN-α2b non-treated | 25 (n = 7) | 22–30 | 50 (n = 7) | 35–60 | 0.078 |
| IFN-α2b treated | 35 (n = 21) | 25–45 | 15 (n = 21) | 5–35 | 0.011 * |
| p non-treated vs. treated | 0.197 | 0.017 * | – | ||
| IFN-α2b Treatment | Variables | Follow-Up Periods | p Initial vs. Final | |||
|---|---|---|---|---|---|---|
| Initial SpO2 | Final SpO2 | |||||
| Abs. | % | Abs. | % | |||
| IFN-α2b non-treated | normal | 23 | 46.9 | 34 | 69.4 | 0.193 |
| low | 24 | 49.0 | 10 | 20.4 | ||
| very low | 2 | 4.1 | 5 | 10.2 | ||
| IFN-α2b treated | normal | 20 | 33.9 | 44 | 74.6 | <0.001 * |
| low | 31 | 52.5 | 10 | 16.9 | ||
| very low | 8 | 13.6 | 5 | 8.5 | ||
| p non-treated vs. treated | 0.149 | 0.836 | – | |||
| Variables | Correlation Characteristics | ||
|---|---|---|---|
| ρ | Strength of the Association Assessed Using the Chaddock Scale | p | |
| %CT injuries—Age | 0.407 | Moderate | 0.032 * |
| %CT injuries—Days in hospital | 0.756 | Strong | <0.001 * |
| 2SpO2—Days in hospital | −0.544 | Close | <0.001 * |
| %CT injuries—2SpO2 | −0.835 | Strong | <0.001 * |
| 2SpO2—Age | −0.402 | Moderate | <0.001 * |
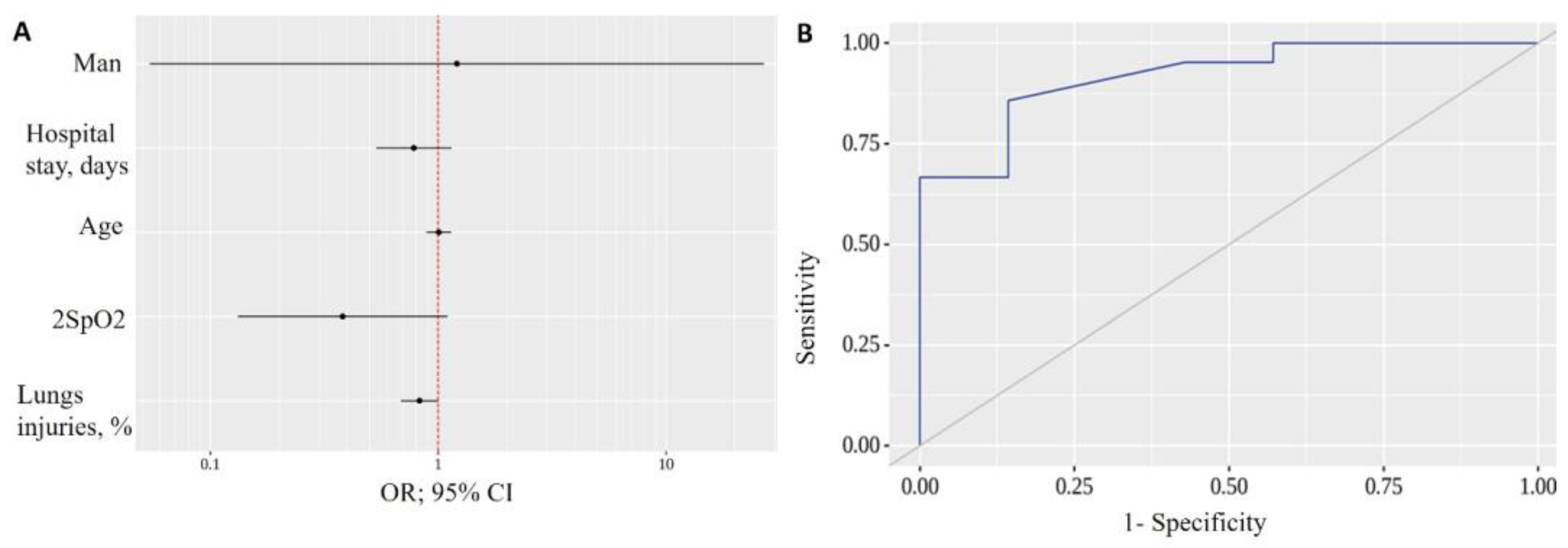
| Predictors | Unadjusted | Adjusted | ||
|---|---|---|---|---|
| COR; 95% CI | p | AOR; 95% CI | p | |
| Sex: men | 1.000; 0.177–5.635 | 1.000 | 1.208; 0.054–26.924 | 0.905 |
| Age | 0.993; 0.929–1.061 | 0.827 | 1.006; 0.889–1.139 | 0.923 |
| Days in hospital | 0.934; 0.870–1.002 | 0.057 | 0.782; 0.534–1.145 | 0.205 |
| % CT injuries | 0.950; 0.908–0.992 | 0.022 * | 0.828; 0.687–0.998 | 0.047 * |
| 2SpO2 | 1.083; 0.946–1.241 | 0.251 | 0.381; 0.132–1.100 | 0.074 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamyshnyi, A.; Koval, H.; Kobevko, O.; Buchynskyi, M.; Oksenych, V.; Kainov, D.; Lyubomirskaya, K.; Kamyshna, I.; Potters, G.; Moshynets, O. Therapeutic Effectiveness of Interferon-α2b against COVID-19 with Community-Acquired Pneumonia: The Ukrainian Experience. Int. J. Mol. Sci. 2023, 24, 6887. https://doi.org/10.3390/ijms24086887
Kamyshnyi A, Koval H, Kobevko O, Buchynskyi M, Oksenych V, Kainov D, Lyubomirskaya K, Kamyshna I, Potters G, Moshynets O. Therapeutic Effectiveness of Interferon-α2b against COVID-19 with Community-Acquired Pneumonia: The Ukrainian Experience. International Journal of Molecular Sciences. 2023; 24(8):6887. https://doi.org/10.3390/ijms24086887
Chicago/Turabian StyleKamyshnyi, Aleksandr, Halyna Koval, Olha Kobevko, Mykhailo Buchynskyi, Valentyn Oksenych, Denis Kainov, Katerina Lyubomirskaya, Iryna Kamyshna, Geert Potters, and Olena Moshynets. 2023. "Therapeutic Effectiveness of Interferon-α2b against COVID-19 with Community-Acquired Pneumonia: The Ukrainian Experience" International Journal of Molecular Sciences 24, no. 8: 6887. https://doi.org/10.3390/ijms24086887
APA StyleKamyshnyi, A., Koval, H., Kobevko, O., Buchynskyi, M., Oksenych, V., Kainov, D., Lyubomirskaya, K., Kamyshna, I., Potters, G., & Moshynets, O. (2023). Therapeutic Effectiveness of Interferon-α2b against COVID-19 with Community-Acquired Pneumonia: The Ukrainian Experience. International Journal of Molecular Sciences, 24(8), 6887. https://doi.org/10.3390/ijms24086887











