Flavonols in Action: Targeting Oxidative Stress and Neuroinflammation in Major Depressive Disorder
Abstract
1. Introduction
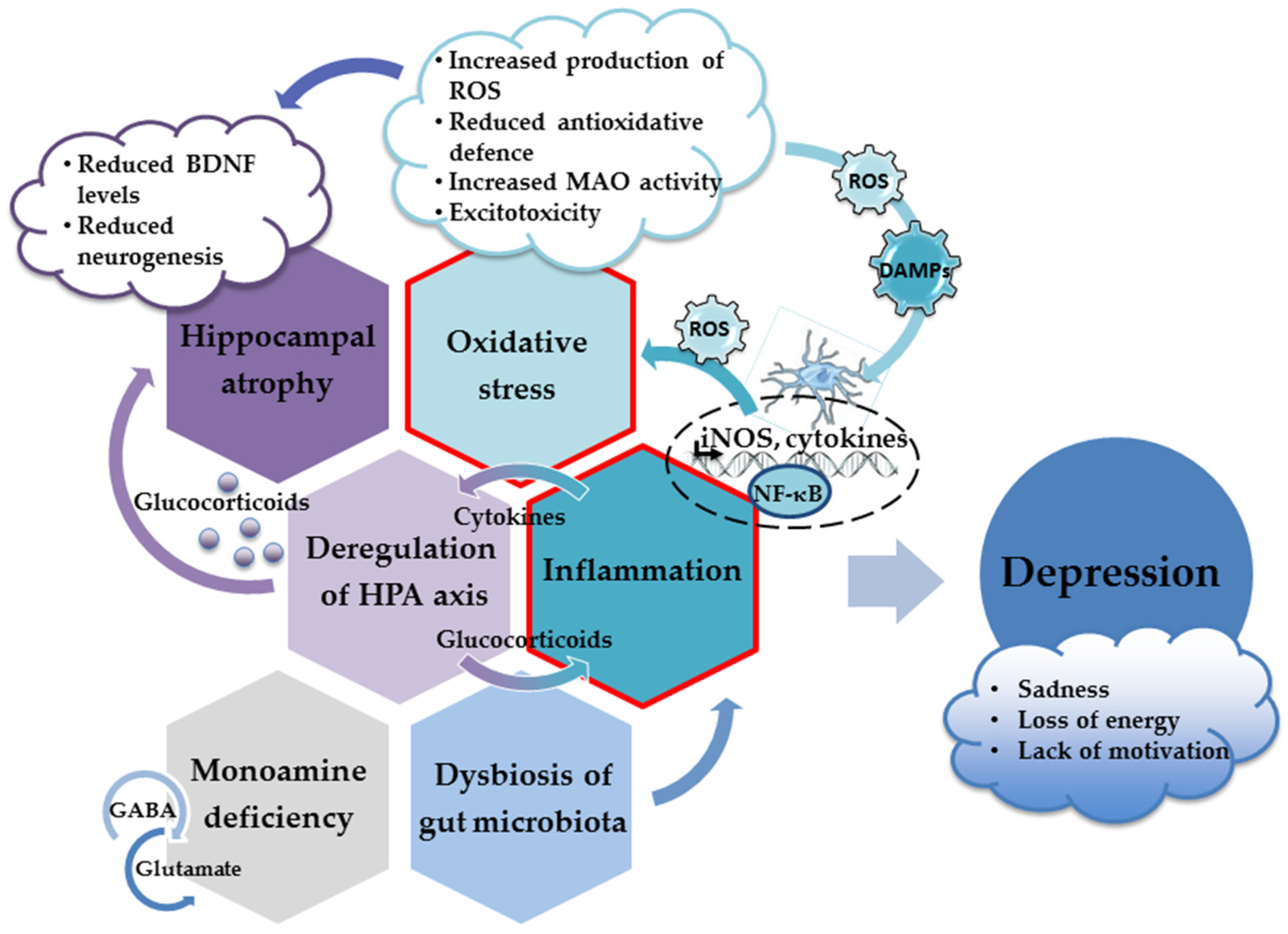
2. Neuropathological Mechanisms Underlying the Development of Depression
2.1. Monoamine Hypothesis of Depression
2.2. Hippocampus and Depression
Adult Neurogenesis and Depression
2.3. HPA Axis and Depression
2.4. Oxidative Stress and Depression
2.5. Neuroinflammation, Cytokines, and Depression
3. Experimental Approaches and Behavioral Tests for Studying Depressive-like Behavior in Animals
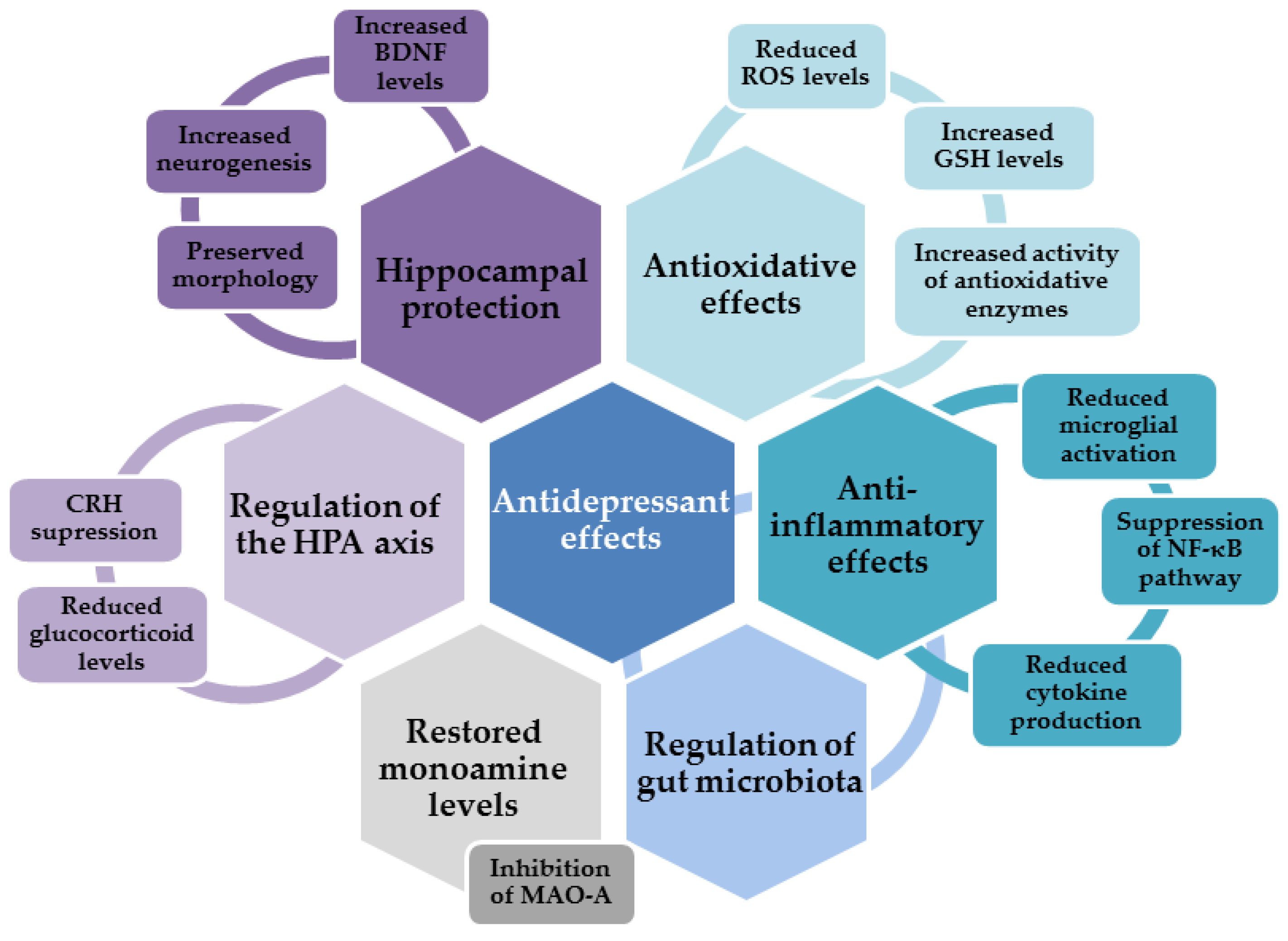
4. Antidepressant Effects of Flavonols
4.1. Antidepressant Effects of Quercetin
4.2. Antidepressant Effects of Myricetin and Myricitrin
4.3. Antidepressant Effects of Rutin
4.4. Antidepressant Effects of Avicularin
4.5. Antidepressant Effects of Fisetin
4.6. Antidepressant Effects of Kaempferol
4.7. Antidepressant Effects of Morin
4.8. Antidepressant Effects of Other Flavonols
5. Flavonols and Gut–Brain Axis
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Otte, C.; Gold, S.M.; Penninx, B.W.; Pariante, C.M.; Etkin, A.; Fava, M.; Mohr, D.C.; Schatzberg, A.F. Major depressive disorder. Nat. Rev. Dis. Prim. 2016, 2, 16065. [Google Scholar] [CrossRef]
- Christensen, M.C.; Wong, C.M.J.; Baune, B.T. Symptoms of Major Depressive Disorder and Their Impact on Psychosocial Functioning in the Different Phases of the Disease: Do the Perspectives of Patients and Healthcare Providers Differ? Front. Psychiatry 2020, 11, 280. [Google Scholar] [CrossRef]
- Lim, G.Y.; Tam, W.W.; Lu, Y.; Ho, C.S.; Zhang, M.W.; Ho, R.C. Prevalence of Depression in the Community from 30 Countries between 1994 and 2014. Sci. Rep. 2018, 8, 2861. [Google Scholar] [CrossRef]
- Campbell, S.; Macqueen, G. The role of the hippocampus in the pathophysiology of major depression. J. Psychiatry Neurosci. 2004, 29, 417–426. [Google Scholar] [PubMed]
- Palmer, S.M.; Crewther, S.G.; Carey, L.M. START Project Team A meta-analysis of changes in brain activity in clinical depression. Front. Hum. Neurosci. 2015, 8, 1045. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.F.; Peng, W.; Sweeney, J.A.; Jia, Z.Y.; Gong, Q.Y. Brain structure alterations in depression: Psychoradiological evidence. CNS Neurosci. Ther. 2018, 24, 994–1003. [Google Scholar] [CrossRef] [PubMed]
- Helm, K.; Viol, K.; Weiger, T.M.; Tass, P.A.; Grefkes, C.; Del Monte, D.; Schiepek, G. Neuronal connectivity in major depressive disorder: A systematic review. Neuropsychiatr. Dis. Treat. 2018, 14, 2715–2737. [Google Scholar] [CrossRef]
- Sindermann, L.; Redlich, R.; Opel, N.; Böhnlein, J.; Dannlowski, U.; Leehr, E.J. Systematic transdiagnostic review of magnetic-resonance imaging results: Depression, anxiety disorders and their co-occurrence. J. Psychiatr. Res. 2021, 142, 226–239. [Google Scholar] [CrossRef]
- Baek, S.J.; Park, J.S.; Kim, J.; Yamamoto, Y.; Tanaka-Yamamoto, K. VTA-projecting cerebellar neurons mediate stress-dependent depression-like behaviors. eLife 2022, 11, e72981. [Google Scholar] [CrossRef]
- Vermeiren, Y.; Van Dam, D.; Aerts, T.; Engelborghs, S.; De Deyn, P.P. Monoaminergic neurotransmitter alterations in postmortem brain regions of depressed and aggressive patients with Alzheimer’s disease. Neurobiol. Aging 2014, 35, 2691–2700. [Google Scholar] [CrossRef]
- Kunugi, H.; Hori, H.; Ogawa, S. Biochemical markers subtyping major depressive disorder. Psychiatry Clin. Neurosci. 2015, 69, 597–608. [Google Scholar] [CrossRef]
- Ogawa, S.; Tsuchimine, S.; Kunugi, H. Cerebrospinal fluid monoamine metabolite concentrations in depressive disorder: A meta-analysis of historic evidence. J. Psychiatr. Res. 2018, 105, 137–146. [Google Scholar] [CrossRef]
- Silić, A.; Vukojević, J.; Peitl, V.; De Hert, M.; Karlović, D. Major depressive disorder: A possible typisation according to serotonin, inflammation, and metabolic syndrome. Acta Neuropsychiaty 2022, 34, 15–23. [Google Scholar] [CrossRef]
- Shao, X.; Zhu, G. Associations Among Monoamine Neurotransmitter Pathways, Personality Traits, and Major Depressive Disorder. Front. Psychiatry 2020, 11, 381. [Google Scholar] [CrossRef]
- Jacobsen, J.P.; Medvedev, I.O.; Caron, M.G. The 5-HT deficiency theory of depression: Perspectives from a naturalistic 5-HT deficiency model, the tryptophan hydroxylase 2Arg439His knockin mouse. Philos. Trans R. Soc. Lond. B Biol. Sci. 2012, 367, 2444–2459. [Google Scholar] [CrossRef]
- Correia, A.S.; Vale, N. Tryptophan Metabolism in Depression: A Narrative Review with a Focus on Serotonin and Kynurenine Pathways. Int. J. Mol. Sci. 2022, 23, 8493. [Google Scholar] [CrossRef]
- Jiang, Y.; Zou, D.; Li, Y.; Gu, S.; Dong, J.; Ma, X.; Xu, S.; Wang, F.; Huang, J.H. Monoamine Neurotransmitters Control Basic Emotions and Affect Major Depressive Disorders. Pharmaceuticals 2022, 15, 1203. [Google Scholar] [CrossRef]
- Delgado, P.L. Depression: The case for a monoamine deficiency. J. Clin. Psychiatry 2000, 61 (Suppl. S6), 7–11. [Google Scholar]
- Gabriel, F.C.; de Melo, D.O.; Fráguas, R.; Leite-Santos, N.C.; Mantovani da Silva, R.A.; Ribeiro, E. Pharmacological treatment of depression: A systematic review comparing clinical practice guideline recommendations. PLoS ONE 2020, 15, e0231700. [Google Scholar] [CrossRef]
- Elias, E.; Zhang, A.Y.; Manners, M.T. Novel Pharmacological Approaches to the Treatment of Depression. Life 2022, 12, 196. [Google Scholar] [CrossRef]
- Kessler, R.C.; Berglund, P.; Demler, O.; Jin, R.; Koretz, D.; Merikangas, K.R.; Rush, A.J.; Walters, E.E.; Wang, P.S. National Comorbidity Survey Replication The epidemiology of major depressive disorder: Results from the National Comorbidity Survey Replication (NCS-R). JAMA 2003, 289, 3095–3105. [Google Scholar] [CrossRef] [PubMed]
- Braund, T.A.; Tillman, G.; Palmer, D.M.; Gordon, E.; Rush, A.J.; Harris, A.W.F. Antidepressant side effects and their impact on treatment outcome in people with major depressive disorder: An iSPOT-D report. Transl. Psychiatry 2021, 11, 417. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.F. A hypothesis of monoamine (5-HT)—Glutamate/GABA long neural circuit: Aiming for fast-onset antidepressant discovery. Pharmacol. Ther. 2020, 208, 107494. [Google Scholar] [CrossRef] [PubMed]
- Duman, R.S.; Sanacora, G.; Krystal, J.H. Altered Connectivity in Depression: GABA and Glutamate Neurotransmitter Deficits and Reversal by Novel Treatments. Neuron 2019, 102, 75–90. [Google Scholar] [CrossRef] [PubMed]
- Taillefer de Laportalière, T.; Yrondi, A.; Jullien, A.; Cestac, P.; Montastruc, F. How to deprescribe esketamine in resistant depression? A point of view after first clinical uses. Epidemiol. Psychiatr. Sci. 2022, 31, e4. [Google Scholar] [CrossRef] [PubMed]
- Kantrowitz, J.T.; Dong, Z.; Milak, M.S.; Rashid, R.; Kegeles, L.S.; Javitt, D.C.; Lieberman, J.A.; John Mann, J. Ventromedial prefrontal cortex/anterior cingulate cortex Glx, glutamate, and GABA levels in medication-free major depressive disorder. Transl. Psychiatry 2021, 11, 419. [Google Scholar] [CrossRef]
- Pandya, M.; Altinay, M.; Malone, D.A., Jr.; Anand, A. Where in the brain is depression? Curr. Psychiatry Rep. 2012, 14, 634–642. [Google Scholar] [CrossRef]
- Malberg, J.E.; Schechter, L.E. Increasing hippocampal neurogenesis: A novel mechanism for antidepressant drugs. Curr. Pharm. Des. 2005, 11, 145–155. [Google Scholar] [CrossRef]
- Malykhin, N.V.; Carter, R.; Seres, P.; Coupland, N.J. Structural changes in the hippocampus in major depressive disorder: Contributions of disease and treatment. J. Psychiatry Neurosci. 2010, 35, 337–343. [Google Scholar] [CrossRef]
- MacQueen, G.; Frodl, T. The hippocampus in major depression: Evidence for the convergence of the bench and bedside in psychiatric research? Mol. Psychiatry 2011, 16, 252–264. [Google Scholar] [CrossRef]
- Roddy, D.W.; Farrell, C.; Doolin, K.; Roman, E.; Tozzi, L.; Frodl, T.; O’Keane, V.; O’Hanlon, E. The Hippocampus in Depression: More Than the Sum of Its Parts? Advanced Hippocampal Substructure Segmentation in Depression. Biol. Psychiatry 2019, 85, 487–497. [Google Scholar] [CrossRef]
- Hsieh, M.H.; McQuoid, D.R.; Levy, R.M.; Payne, M.E.; MacFall, J.R.; Steffens, D.C. Hippocampal volume and antidepressant response in geriatric depression. Int. J. Geriatr. Psychiatry 2002, 17, 519–525. [Google Scholar] [CrossRef]
- Boku, S.; Nakagawa, S.; Toda, H.; Hishimoto, A. Neural basis of major depressive disorder: Beyond monoamine hypothesis. Psychiatry Clin. Neurosci. 2018, 72, 3–12. [Google Scholar] [CrossRef]
- Kobayashi, K.; Ikeda, Y.; Sakai, A.; Yamasaki, N.; Haneda, E.; Miyakawa, T.; Suzuki, H. Reversal of hippocampal neuronal maturation by serotonergic antidepressants. Proc. Natl. Acad. Sci. USA 2010, 107, 8434–8439. [Google Scholar] [CrossRef]
- Anacker, C.; Zunszain, P.A.; Cattaneo, A.; Carvalho, L.A.; Garabedian, M.J.; Thuret, S.; Price, J.; Pariante, C.M. Antidepressants increase human hippocampal neurogenesis by activating the glucocorticoid receptor. Mol. Psychiatry 2011, 16, 738–750. [Google Scholar] [CrossRef]
- Foltran, R.B.; Diaz, S.L. BDNF isoforms: A round trip ticket between neurogenesis and serotonin? J. Neurochem. 2016, 138, 204–221. [Google Scholar] [CrossRef]
- Colucci-D’Amato, L.; Speranza, L.; Volpicelli, F. Neurotrophic Factor BDNF, Physiological Functions and Therapeutic Potential in Depression, Neurodegeneration and Brain Cancer. Int. J. Mol. Sci. 2020, 21, 7777. [Google Scholar] [CrossRef]
- Baydyuk, M.; Xu, B. BDNF signaling and survival of striatal neurons. Front. Cell. Neurosci. 2014, 8, 254. [Google Scholar] [CrossRef]
- Wang, C.S.; Kavalali, E.T.; Monteggia, L.M. BDNF signaling in context: From synaptic regulation to psychiatric disorders. Cell 2022, 185, 62–76. [Google Scholar] [CrossRef]
- Ying, S.W.; Futter, M.; Rosenblum, K.; Webber, M.J.; Hunt, S.P.; Bliss, T.V.; Bramham, C.R. Brain-derived neurotrophic factor induces long-term potentiation in intact adult hippocampus: Requirement for ERK activation coupled to CREB and upregulation of Arc synthesis. J. Neurosci. 2002, 22, 1532–1540. [Google Scholar] [CrossRef]
- Rex, C.S.; Lin, C.Y.; Kramár, E.A.; Chen, L.Y.; Gall, C.M.; Lynch, G. Brain-derived neurotrophic factor promotes long-term potentiation-related cytoskeletal changes in adult hippocampus. J. Neurosci. 2007, 27, 3017–3029. [Google Scholar] [CrossRef] [PubMed]
- Lau, A.G.; Irier, H.A.; Gu, J.; Tian, D.; Ku, L.; Liu, G.; Xia, M.; Fritsch, B.; Zheng, J.Q.; Dingledine, R.; et al. Distinct 3′UTRs differentially regulate activity-dependent translation of brain-derived neurotrophic factor (BDNF). Proc. Natl. Acad. Sci. USA 2010, 107, 15945–15950. [Google Scholar] [CrossRef] [PubMed]
- Pruunsild, P.; Kazantseva, A.; Aid, T.; Palm, K.; Timmusk, T. Dissecting the human BDNF locus: Bidirectional transcription, complex splicing, and multiple promoters. Genomics 2007, 90, 397–406. [Google Scholar] [CrossRef]
- Kowiański, P.; Lietzau, G.; Czuba, E.; Waśkow, M.; Steliga, A.; Moryś, J. BDNF: A Key Factor with Multipotent Impact on Brain Signaling and Synaptic Plasticity. Cell. Mol. Neurobiol. 2018, 38, 579–593. [Google Scholar] [CrossRef] [PubMed]
- Karlović, D.; Serretti, A.; Jevtović, S.; Vrkić, N.; Serić, V.; Peleš, A.M. Diagnostic accuracy of serum brain derived neurotrophic factor concentration in antidepressant naïve patients with first major depression episode. J. Psychiatr. Res. 2013, 47, 162–167. [Google Scholar] [CrossRef]
- Molendijk, M.L.; Bus, B.A.; Spinhoven, P.; Penninx, B.W.; Kenis, G.; Prickaerts, J.; Voshaar, R.C.; Elzinga, B.M. Serum levels of brain-derived neurotrophic factor in major depressive disorder: State-trait issues, clinical features and pharmacological treatment. Mol. Psychiatry 2011, 16, 1088–1095. [Google Scholar] [CrossRef]
- Miyanishi, H.; Nitta, A. A Role of BDNF in the Depression Pathogenesis and a Potential Target as Antidepressant: The Modulator of Stress Sensitivity “Shati/Nat8l-BDNF System” in the Dorsal Striatum. Pharmaceuticals 2021, 14, 889. [Google Scholar] [CrossRef]
- Sakata, K.; Woo, N.H.; Martinowich, K.; Greene, J.S.; Schloesser, R.J.; Shen, L.; Lu, B. Critical role of promoter IV-driven BDNF transcription in GABAergic transmission and synaptic plasticity in the prefrontal cortex. Proc. Natl. Acad. Sci. USA 2009, 106, 5942–5947. [Google Scholar] [CrossRef]
- Sakata, K.; Duke, S.M. Lack of BDNF expression through promoter IV disturbs expression of monoamine genes in the frontal cortex and hippocampus. Neuroscience 2014, 260, 265–275. [Google Scholar] [CrossRef]
- Sakata, K.; Jin, L.; Jha, S. Lack of promoter IV-driven BDNF transcription results in depression-like behavior. Genes Brain Behav. 2010, 9, 712–721. [Google Scholar] [CrossRef]
- Lieb, K.; Dreimüller, N.; Wagner, S.; Schlicht, K.; Falter, T.; Neyazi, A.; Müller-Engling, L.; Bleich, S.; Tadić, A.; Frieling, H. BDNF Plasma Levels and BDNF Exon IV Promoter Methylation as Predictors for Antidepressant Treatment Response. Front. Psychiatry 2018, 9, 511. [Google Scholar] [CrossRef]
- Jevtović, S.; Karlović, D.; Mihaljević-Peleš, A.; Šerić, V.; Vrkić, N.; Jakšić, N. Serum Brain-derived neurotrophic factor (BDNF): The severity and symptomatic dimensions of depression. Psychiatr. Danub. 2011, 23, 363–369. [Google Scholar]
- Kreinin, A.; Lisson, S.; Nesher, E.; Schneider, J.; Bergman, J.; Farhat, K.; Farah, J.; Lejbkowicz, F.; Yadid, G.; Raskin, L.; et al. Blood BDNF level is gender specific in severe depression. PLoS ONE 2015, 10, e0127643. [Google Scholar] [CrossRef]
- Caldieraro, M.A.; Vares, E.A.; Souza, L.H.; Spanemberg, L.; Guerra, T.A.; Wollenhaupt-Aguiar, B.; Ferrari, P.; Nierenberg, A.A.; Fleck, M.P. Illness severity and biomarkers in depression: Using a unidimensional rating scale to examine BDNF. Compr. Psychiatry 2017, 75, 46–52. [Google Scholar] [CrossRef]
- Katsuki, A.; Yoshimura, R.; Kishi, T.; Hori, H.; Umene-Nakano, W.; Ikenouchi-Sugita, A.; Hayashi, K.; Atake, K.; Iwata, N.; Nakamura, J. Serum levels of brain-derived neurotrophic factor (BDNF), BDNF gene Val66Met polymorphism, or plasma catecholamine metabolites, and response to mirtazapine in Japanese patients with major depressive disorder (MDD). CNS Spectr. 2012, 17, 155–163. [Google Scholar] [CrossRef]
- Brunoni, A.R.; Lopes, M.; Fregni, F. A systematic review and meta-analysis of clinical studies on major depression and BDNF levels: Implications for the role of neuroplasticity in depression. Int. J. Neuropsychopharmacol. 2008, 11, 1169–1180. [Google Scholar] [CrossRef]
- Martinac, M.; Pehar, D.; Karlović, D.; Babić, D.; Marcinko, D.; Jakovljević, M. Metabolic syndrome, activity of the hypothalamic-pituitary-adrenal axis and inflammatory mediators in depressive disorder. Acta Clin. Croat. 2014, 53, 55–71. [Google Scholar] [CrossRef]
- Varghese, F.P.; Brown, E.S. The Hypothalamic-Pituitary-Adrenal Axis in Major Depressive Disorder: A Brief Primer for Primary Care Physicians. Prim. Care Companion J. Clin. Psychiatry 2001, 3, 151–155. [Google Scholar] [CrossRef]
- Iob, E.; Kirschbaum, C.; Steptoe, A. Persistent depressive symptoms, HPA-axis hyperactivity, and inflammation: The role of cognitive-affective and somatic symptoms. Mol. Psychiatry 2020, 25, 1130–1140. [Google Scholar] [CrossRef]
- Zhu, L.J.; Liu, M.Y.; Li, H.; Liu, X.; Chen, C.; Han, Z.; Wu, H.Y.; Jing, X.; Zhou, H.H.; Suh, H.; et al. The different roles of glucocorticoids in the hippocampus and hypothalamus in chronic stress-induced HPA axis hyperactivity. PLoS ONE 2014, 9, e97689. [Google Scholar] [CrossRef]
- Kino, T. Stress, glucocorticoid hormones, and hippocampal neural progenitor cells: Implications to mood disorders. Front. Physiol. 2015, 6, 230. [Google Scholar] [CrossRef] [PubMed]
- Cole, A.B.; Montgomery, K.; Bale, T.L.; Thompson, S.M. What the hippocampus tells the HPA axis: Hippocampal output attenuates acute stress responses via disynaptic inhibition of CRF+ PVN neurons. Neurobiol. Stress 2022, 20, 100473. [Google Scholar] [CrossRef] [PubMed]
- Keller, J.; Gomez, R.; Williams, G.; Lembke, A.; Lazzeroni, L.; Murphy, G.M., Jr.; Schatzberg, A.F. HPA axis in major depression: Cortisol, clinical symptomatology and genetic variation predict cognition. Mol. Psychiatry 2017, 22, 527–536. [Google Scholar] [CrossRef] [PubMed]
- Gjerstad, J.K.; Lightman, S.L.; Spiga, F. Role of glucocorticoid negative feedback in the regulation of HPA axis pulsatility. Stress 2018, 21, 403–416. [Google Scholar] [CrossRef]
- Menke, A. Is the HPA Axis as Target for Depression Outdated, or Is There a New Hope? Front. Psychiatry 2019, 10, 101. [Google Scholar] [CrossRef]
- McEwen, B.S. Glucocorticoids, depression, and mood disorders: Structural remodeling in the brain. Metabolism 2005, 54 (Suppl. S1), 20–23. [Google Scholar] [CrossRef]
- Krugers, H.J.; Lucassen, P.J.; Karst, H.; Joëls, M. Chronic stress effects on hippocampal structure and synaptic function: Relevance for depression and normalization by anti-glucocorticoid treatment. Front. Synaptic Neurosci. 2010, 2, 24. [Google Scholar] [CrossRef]
- Liston, C.; Gan, W.B. Glucocorticoids are critical regulators of dendritic spine development and plasticity in vivo. Proc. Natl. Acad. Sci. USA 2011, 108, 16074–16079. [Google Scholar] [CrossRef]
- Tata, D.A.; Anderson, B.J. The effects of chronic glucocorticoid exposure on dendritic length, synapse numbers and glial volume in animal models: Implications for hippocampal volume reductions in depression. Physiol. Behav. 2010, 99, 186–193. [Google Scholar] [CrossRef]
- Bhatt, S.; Nagappa, A.N.; Patil, C.R. Role of oxidative stress in depression. Drug Discover. Today 2020, 25, 1270–1276. [Google Scholar] [CrossRef]
- Juszczyk, G.; Mikulska, J.; Kasperek, K.; Pietrzak, D.; Mrozek, W.; Herbet, M. Chronic Stress and Oxidative Stress as Common Factors of the Pathogenesis of Depression and Alzheimer’s Disease: The Role of Antioxidants in Prevention and Treatment. Antioxidants 2021, 10, 1439. [Google Scholar] [CrossRef]
- Salim, S. Oxidative Stress and the Central Nervous System. J. Pharmacol. Exp. Ther. 2017, 360, 201–205. [Google Scholar] [CrossRef]
- Wang, X.; Michaelis, E.K. Selective neuronal vulnerability to oxidative stress in the brain. Front. Aging Neurosci. 2010, 2, 12. [Google Scholar] [CrossRef]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative Stress: A Key Modulator in Neurodegenerative Diseases. Molecules 2019, 24, 1583. [Google Scholar] [CrossRef]
- Lee, K.H.; Cha, M.; Lee, B.H. Neuroprotective Effect of Antioxidants in the Brain. Int. J. Mol. Sci. 2020, 21, 7152. [Google Scholar] [CrossRef]
- Cobley, J.N.; Fiorello, M.L.; Bailey, D.M. 13 reasons why the brain is susceptible to oxidative stress. Redox Biol. 2018, 15, 490–503. [Google Scholar] [CrossRef]
- Bakunina, N.; Pariante, C.M.; Zunszain, P.A. Immune mechanisms linked to depression via oxidative stress and neuroprogression. Immunology 2015, 144, 365–373. [Google Scholar] [CrossRef]
- Morris, G.; Berk, M.; Klein, H.; Walder, K.; Galecki, P.; Maes, M. Nitrosative Stress, Hypernitrosylation, and Autoimmune Responses to Nitrosylated Proteins: New Pathways in Neuroprogressive Disorders Including Depression and Chronic Fatigue Syndrome. Mol. Neurobiol. 2017, 54, 4271–4291. [Google Scholar] [CrossRef]
- Lopresti, A.L.; Maker, G.L.; Hood, S.D.; Drummond, P.D. A review of peripheral biomarkers in major depression: The potential of inflammatory and oxidative stress biomarkers. Prog. Neuropsychopharmacol. Biol. Psychiatry 2014, 48, 102–111. [Google Scholar] [CrossRef]
- Correia, A.S.; Cardoso, A.; Vale, N. Oxidative Stress in Depression: The Link with the Stress Response, Neuroinflammation, Serotonin, Neurogenesis and Synaptic Plasticity. Antioxidants 2023, 12, 470. [Google Scholar] [CrossRef]
- Zhong, J.; Li, G.; Xu, H.; Wang, Y.; Shi, M. Baicalin ameliorates chronic mild stress-induced depression-like behaviors in mice and attenuates inflammatory cytokines and oxidative stress. Braz. J. Med. Biol. Res. 2019, 52, e8434. [Google Scholar] [CrossRef] [PubMed]
- Vaváková, M.; Ďuračková, Z.; Trebatická, J. Markers of Oxidative Stress and Neuroprogression in Depression Disorder. Oxid. Med. Cell. Longev. 2015, 2015, 898393. [Google Scholar] [CrossRef] [PubMed]
- Forlenza, M.J.; Miller, G.E. Increased serum levels of 8-hydroxy-2′-deoxyguanosine in clinical depression. Psychosom. Med. 2006, 68, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Lindqvist, D.; Dhabhar, F.S.; James, S.J.; Hough, C.M.; Jain, F.A.; Bersani, F.S.; Reus, V.I.; Verhoeven, J.E.; Epel, E.S.; Mahan, L.; et al. Oxidative stress, inflammation and treatment response in major depression. Psychoneuroendocrinology 2017, 76, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Mazereeuw, G.; Herrmann, N.; Andreazza, A.C.; Khan, M.M.; Lanctôt, K.L. A meta-analysis of lipid peroxidation markers in major depression. Neuropsychiatr. Dis. Treat. 2015, 11, 2479–2491. [Google Scholar] [CrossRef]
- Liu, T.; Zhong, S.; Liao, X.; Chen, J.; He, T.; Lai, S. A Meta-Analysis of Oxidative Stress Markers in Depression. PLoS ONE 2015, 10, e0138904. [Google Scholar] [CrossRef]
- Islam, M.R.; Islam, M.R.; Ahmed, I.; Moktadir, A.A.; Nahar, Z.; Islam, M.S.; Shahid, S.F.B.; Islam, S.N.; Islam, M.S.; Hasnat, A. Elevated serum levels of malondialdehyde and cortisol are associated with major depressive disorder: A case-control study. SAGE Open Med. 2018, 6, 2050312118773953. [Google Scholar] [CrossRef]
- Nobis, A.; Zalewski, D.; Waszkiewicz, N. Peripheral Markers of Depression. J. Clin. Med. 2020, 9, 3793. [Google Scholar] [CrossRef]
- Camkurt, M.A.; Fındıklı, E.; İzci, F.; Kurutaş, E.B.; Tuman, T.C. Evaluation of malondialdehyde, superoxide dismutase and catalase activity and their diagnostic value in drug naïve, first episode, non-smoker major depression patients and healthy controls. Psychiatry Res. 2016, 238, 81–85. [Google Scholar] [CrossRef]
- Michel, T.M.; Camara, S.; Tatschner, T.; Frangou, S.; Sheldrick, A.J.; Riederer, P.; Grünblatt, E. Increased xanthine oxidase in the thalamus and putamen in depression. World J. Biol. Psychiatry 2010, 11 Pt 2, 314–320. [Google Scholar] [CrossRef]
- Xu, M.; Tian, P.; Zhu, H.; Zou, R.; Zhao, J.; Zhang, H.; Wang, G.; Chen, W. Lactobacillus paracasei CCFM1229 and Lactobacillus rhamnosus CCFM1228 Alleviated Depression- and Anxiety-Related Symptoms of Chronic Stress-Induced Depression in Mice by Regulating Xanthine Oxidase Activity in the Brain. Nutrients 2022, 14, 1294. [Google Scholar] [CrossRef]
- Sarandol, A.; Sarandol, E.; Eker, S.S.; Erdinc, S.; Vatansever, E.; Kirli, S. Major depressive disorder is accompanied with oxidative stress: Short-term antidepressant treatment does not alter oxidative-antioxidative systems. Hum. Psychopharmacol. 2007, 22, 67–73. [Google Scholar] [CrossRef]
- Katrenčíková, B.; Vaváková, M.; Paduchová, Z.; Nagyová, Z.; Garaiova, I.; Muchová, J.; Ďuračková, Z.; Trebatická, J. Oxidative Stress Markers and Antioxidant Enzymes in Children and Adolescents with Depressive Disorder and Impact of Omega-3 Fatty Acids in Randomised Clinical Trial. Antioxidants 2021, 10, 1256. [Google Scholar] [CrossRef]
- Tsai, M.C.; Huang, T.L. Increased activities of both superoxide dismutase and catalase were indicators of acute depressive episodes in patients with major depressive disorder. Psychiatry Res. 2016, 235, 38–42. [Google Scholar] [CrossRef]
- Sabade, S.B.; Gagare, D.B.; Vikhe, B.B.; Bhalerao, M.M. Study of serum catalase in depression at Pravara institute of medical sciences. Indian J. Clin. Anat. Physiol. 2022, 9, 279–282. [Google Scholar] [CrossRef]
- Maes, M.; Mihaylova, I.; Kubera, M.; Uytterhoeven, M.; Vrydags, N.; Bosmans, E. Lower whole blood glutathione peroxidase (GPX) activity in depression, but not in myalgic encephalomyelitis/chronic fatigue syndrome: Another pathway that may be associated with coronary artery disease and neuroprogression in depression. Neuro. Endocrinol. Lett. 2011, 32, 133–140. [Google Scholar]
- Bilici, M.; Efe, H.; Köroğlu, M.A.; Uydu, H.A.; Bekaroğlu, M.; Değer, O. Antioxidative enzyme activities and lipid peroxidation in major depression: Alterations by antidepressant treatments. J. Affect. Disord. 2001, 64, 43–51. [Google Scholar] [CrossRef]
- da Cruz Jung, I.E.; da Cruz, I.B.M.; Barbisan, F.; Trott, A.; Houenou, L.J.; Osmarin Turra, B.; Duarte, T.; de Souza Praia, R.; Maia-Ribeiro, E.A.; da Costa Escobar Piccoli, J.; et al. Superoxide imbalance triggered by Val16Ala-SOD2 polymorphism increases the risk of depression and self-reported psychological stress in free-living elderly people. Mol. Gen. Genom. Med. 2019, 8, e1080. [Google Scholar] [CrossRef]
- Wigner, P.; Czarny, P.; Synowiec, E.; Bijak, M.; Białek, K.; Talarowska, M.; Galecki, P.; Szemraj, J.; Sliwinski, T. Variation of genes involved in oxidative and nitrosative stresses in depression. Eur. Psychiatry 2018, 48, 38–48. [Google Scholar] [CrossRef]
- Huang, Q.; Liu, H.; Suzuki, K.; Ma, S.; Liu, C. Linking What We Eat to Our Mood: A Review of Diet, Dietary Antioxidants, and Depression. Antioxidants 2019, 8, 376. [Google Scholar] [CrossRef]
- Maes, M.; De Vos, N.; Pioli, R.; Demedts, P.; Wauters, A.; Neels, H.; Christophe, A. Lower serum vitamin E concentrations in major depression. Another marker of lowered antioxidant defenses in that illness. J. Affect. Disord. 2000, 58, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Gautam, M.; Agrawal, M.; Gautam, M.; Sharma, P.; Gautam, A.S.; Gautam, S. Role of antioxidants in generalised anxiety disorder and depression. Indian J. Psychiatry 2012, 54, 244–247. [Google Scholar] [CrossRef] [PubMed]
- Moylan, S.; Berk, M.; Dean, O.M.; Samuni, Y.; Williams, L.J.; O’Neil, A.; Hayley, A.C.; Pasco, J.A.; Anderson, G.; Jacka, F.N.; et al. Oxidative & nitrosative stress in depression: Why so much stress? Neurosci. Biobehav. Rev. 2014, 45, 46–62. [Google Scholar] [CrossRef] [PubMed]
- Abshirini, M.; Siassi, F.; Koohdani, F.; Qorbani, M.; Mozaffari, H.; Aslani, Z.; Soleymani, M.; Entezarian, M.; Sotoudeh, G. Dietary total antioxidant capacity is inversely associated with depression, anxiety and some oxidative stress biomarkers in postmenopausal women: A cross-sectional study. Ann. Gen. Psychiatry 2019, 18, 3. [Google Scholar] [CrossRef]
- Wu, S.-X.; Li, J.; Zhou, D.-D.; Xiong, R.-G.; Huang, S.-Y.; Saimaiti, A.; Shang, A.; Li, H.-B. Possible Effects and Mechanisms of Dietary Natural Products and Nutrients on Depression and Anxiety: A Narrative Review. Antioxidants 2022, 11, 2132. [Google Scholar] [CrossRef]
- Winiarska-Mieczan, A.; Kwiecień, M.; Jachimowicz-Rogowska, K.; Donaldson, J.; Tomaszewska, E.; Baranowska-Wójcik, E. Anti-Inflammatory, Antioxidant, and Neuroprotective Effects of Polyphenols—Polyphenols as an Element of Diet Therapy in Depressive Disorders. Int. J. Mol. Sci. 2023, 24, 2258. [Google Scholar] [CrossRef]
- Moritz, B.; Schmitz, A.E.; Rodrigues, A.L.S.; Dafre, A.L.; Cunha, M.P. The role of vitamin C in stress-related disorders. J. Nutr. Biochem. 2020, 85, 108459. [Google Scholar] [CrossRef]
- Yosaee, S.; Keshtkaran, Z.; Abdollahi, S.; Shidfar, F.; Sarris, J.; Soltani, S. The effect of vitamin C supplementation on mood status in adults: A systematic review and meta-analysis of randomized controlled clinical trials. Gen. Hosp. Psychiatry 2021, 71, 36–42. [Google Scholar] [CrossRef]
- Sahraian, A.; Ghanizadeh, A.; Kazemeini, F. Vitamin C as an adjuvant for treating major depressive disorder and suicidal behavior, a randomized placebo-controlled clinical trial. Trials 2015, 16, 94. [Google Scholar] [CrossRef]
- Amr, M.; El-Mogy, A.; Shams, T.; Vieira, K.; Lakhan, S.E. Efficacy of vitamin C as an adjunct to fluoxetine therapy in pediatric major depressive disorder: A randomized, double-blind, placebo-controlled pilot study. Nutr. J. 2013, 12, 31. [Google Scholar] [CrossRef]
- Manosso, L.M.; Camargo, A.; Dafre, A.L.; Rodrigues, A.L.S. Vitamin E for the management of major depressive disorder: Possible role of the anti-inflammatory and antioxidant systems. Nutr. Neurosci. 2020, 25, 1310–1324. [Google Scholar] [CrossRef]
- Lee, A.R.Y.B.; Tariq, A.; Lau, G.; Tok, N.W.K.; Tam, W.W.S.; Ho, C.S.H. Vitamin E, Alpha-Tocopherol, and Its Effects on Depression and Anxiety: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 656. [Google Scholar] [CrossRef]
- Berk, M.; Dean, O.M.; Cotton, S.M.; Jeavons, S.; Tanious, M.; Kohlmann, K.; Hewitt, K.; Moss, K.; Allwang, C.; Schapkaitz, I.; et al. The efficacy of adjunctive N-acetylcysteine in major depressive disorder: A double-blind, randomized, placebo-controlled trial. J. Clin. Psychiatry 2014, 75, 628–636. [Google Scholar] [CrossRef]
- Fernandes, B.S.; Dean, O.M.; Dodd, S.; Malhi, G.S.; Berk, M. N-Acetylcysteine in depressive symptoms and functionality: A systematic review and meta-analysis. J. Clin. Psychiatry 2016, 77, e457–e466. [Google Scholar] [CrossRef]
- Ooi, S.L.; Green, R.; Pak, S.C. N-Acetylcysteine for the Treatment of Psychiatric Disorders: A Review of Current Evidence. Biomed. Res. Int. 2018, 2469486. [Google Scholar] [CrossRef]
- Woo, J.; Cho, H.; Seol, Y.; Kim, S.H.; Park, C.; Yousefian-Jazi, A.; Hyeon, S.J.; Lee, J.; Ryu, H. Power Failure of Mitochondria and Oxidative Stress in Neurodegeneration and Its Computational Models. Antioxidants 2021, 10, 229. [Google Scholar] [CrossRef]
- Tsang, Y.-L.; Kao, C.-L.; Lin, S.-C.A.; Li, C.-J. Mitochondrial Dysfunction and Oxidative Stress in Aging and Disease. Biomedicines 2022, 10, 2872. [Google Scholar] [CrossRef]
- Wang, Q.; Dwivedi, Y. Transcriptional profiling of mitochondria associated genes in prefrontal cortex of subjects with major depressive disorder. World J. Biol. Psychiatry 2017, 18, 592–603. [Google Scholar] [CrossRef]
- Allen, J.; Romay-Tallon, R.; Brymer, K.J.; Caruncho, H.J.; Kalynchuk, L.E. Mitochondria and Mood: Mitochondrial Dysfunction as a Key Player in the Manifestation of Depression. Front. Neurosci. 2018, 12, 386. [Google Scholar] [CrossRef]
- Tobe, E.H. Mitochondrial dysfunction, oxidative stress, and major depressive disorder. Neuropsychiatr. Dis. Treat. 2013, 9, 567–573. [Google Scholar] [CrossRef]
- Bansal, Y.; Kuhad, A. Mitochondrial Dysfunction in Depression. Curr. Neuropharmacol. 2016, 14, 610–618. [Google Scholar] [CrossRef]
- Lindqvist, D.; Wolkowitz, O.M.; Picard, M.; Ohlsson, L.; Bersani, F.S.; Fernström, J.; Westrin, Å.; Hough, C.M.; Lin, J.; Reus, V.I.; et al. Circulating cell-free mitochondrial DNA, but not leukocyte mitochondrial DNA copy number, is elevated in major depressive disorder. Neuropsychopharmacology 2018, 43, 1557–1564. [Google Scholar] [CrossRef] [PubMed]
- Meyer, J.H.; Ginovart, N.; Boovariwala, A.; Sagrati, S.; Hussey, D.; Garcia, A.; Young, T.; Praschak-Rieder, N.; Wilson, A.A.; Houle, S. Elevated monoamine oxidase a levels in the brain: An explanation for the monoamine imbalance of major depression. Arch. Gen. Psychiatry 2006, 63, 1209–1216. [Google Scholar] [CrossRef] [PubMed]
- Chiuccariello, L.; Houle, S.; Miler, L.; Cooke, R.G.; Rusjan, P.M.; Rajkowska, G.; Levitan, R.D.; Kish, S.J.; Kolla, N.J.; Ou, X.; et al. Elevated monoamine oxidase a binding during major depressive episodes is associated with greater severity and reversed neurovegetative symptoms. Neuropsychopharmacology 2014, 39, 973–980. [Google Scholar] [CrossRef] [PubMed]
- Naoi, M.; Maruyama, W.; Shamoto-Nagai, M. Type A monoamine oxidase and serotonin are coordinately involved in depressive disorders: From neurotransmitter imbalance to impaired neurogenesis. J. Neural. Transm. 2018, 125, 53–66. [Google Scholar] [CrossRef] [PubMed]
- Stahl, S.M.; Felker, A. Monoamine oxidase inhibitors: A modern guide to an unrequited class of antidepressants. CNS Spectr. 2008, 13, 855–870. [Google Scholar] [CrossRef]
- Flockhart, D.A. Dietary restrictions and drug interactions with monoamine oxidase inhibitors: An update. J. Clin. Psychiatry 2012, 73 (Suppl. S1), 17–24. [Google Scholar] [CrossRef]
- Shulman, K.I.; Herrmann, N.; Walker, S.E. Current place of monoamine oxidase inhibitors in the treatment of depression. CNS Drugs 2013, 27, 789–797. [Google Scholar] [CrossRef]
- Haroon, E.; Miller, A.H.; Sanacora, G. Inflammation, Glutamate, and Glia: A Trio of Trouble in Mood Disorders. Neuropsychopharmacology 2017, 42, 193–215. [Google Scholar] [CrossRef]
- Jazvinšćak Jembrek, M.; Radovanović, V.; Vlainić, J.; Vuković, L.; Hanžić, N. Neuroprotective effect of zolpidem against glutamate-induced toxicity is mediated via the PI3K/Akt pathway and inhibited by PK11195. Toxicology 2018, 406–407, 58–69. [Google Scholar] [CrossRef]
- Olloquequi, J.; Cornejo-Córdova, E.; Verdaguer, E.; Soriano, F.X.; Binvignat, O.; Auladell, C.; Camins, A. Excitotoxicity in the pathogenesis of neurological and psychiatric disorders: Therapeutic implications. J. Psychopharmacol. 2018, 32, 265–275. [Google Scholar] [CrossRef]
- Kuhn, D.M.; Geddes, T.J. Peroxynitrite inactivates tryptophan hydroxylase via sulfhydryl oxidation. Coincident nitration of enzyme tyrosyl residues has minimal impact on catalytic activity. J. Biol. Chem. 1999, 274, 29726–29732. [Google Scholar] [CrossRef]
- Wang, H.; He, Y.; Sun, Z.; Ren, S.; Liu, M.; Wang, G.; Yang, J. Microglia in depression: An overview of microglia in the pathogenesis and treatment of depression. J. Neuroinflamm. 2022, 19, 132. [Google Scholar] [CrossRef]
- Atagun, M.İ.; Atay, O.C.; Balaban, O.D.; Ipekcioglu, D.; Alpugan, B.; Yalcin, S.; Senat, A.; Karamustafalioglu, N.; Ilnem, M.C.; Erel, O. Serum nitric oxide levels are depleted in depressed patients treated with electroconvulsive therapy. Indian J. Psychiatry 2021, 63, 456–461. [Google Scholar] [CrossRef]
- Savitz, J. Role of Kynurenine Metabolism Pathway Activation in Major Depressive Disorders. Curr. Top. Behav. Neurrosci. 2017, 31, 249–267. [Google Scholar] [CrossRef]
- Marx, W.; McGuinness, A.J.; Rocks, T.; Ruusunen, A.; Cleminson, J.; Walker, A.J.; Gomes-da-Costa, S.; Lane, M.; Sanches, M.; Diaz, A.P.; et al. The kynurenine pathway in major depressive disorder, bipolar disorder, and schizophrenia: A meta-analysis of 101 studies. Mol. Psychiatry 2021, 26, 4158–4178. [Google Scholar] [CrossRef]
- Hacioglu, G.; Senturk, A.; Ince, I.; Alver, A. Assessment of oxidative stress parameters of brain-derived neurotrophic factor heterozygous mice in acute stress model. Iran. J. Basic Med. Sci. 2016, 19, 388–393. [Google Scholar]
- Afridi, R.; Suk, K. Neuroinflammatory Basis of Depression: Learning from Experimental Models. Front. Cell. Neurosci. 2021, 15, 691067. [Google Scholar] [CrossRef]
- Crnković, D.; Buljan, D.; Karlović, D.; Krmek, M. Connection between inflammatory markers, antidepressants and depression. Acta Clin. Croat. 2012, 51, 25–33. [Google Scholar]
- Berk, M.; Williams, L.J.; Jacka, F.N.; O’Neil, A.; Pasco, J.A.; Moylan, S.; Allen, N.B.; Stuart, A.L.; Hayley, A.C.; Byrne, M.L.; et al. So depression is an inflammatory disease, but where does the inflammation come from? BMC Med. 2013, 11, 200. [Google Scholar] [CrossRef]
- Himmerich, H.; Patsalos, O.; Lichtblau, N.; Ibrahim, M.A.A.; Dalton, B. Cytokine Research in Depression: Principles, Challenges, and Open Questions. Front. Psychiatry 2019, 10, 30. [Google Scholar] [CrossRef] [PubMed]
- Beurel, E.; Toups, M.; Nemeroff, C.B. The Bidirectional Relationship of Depression and Inflammation: Double Trouble. Neuron 2020, 107, 234–256. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.-B.; Lee, J.-H.; Park, S.-C. The Relationship between Stress, Inflammation, and Depression. Biomedicines 2022, 10, 1929. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Giuliani, F. The Role of Inflammation in Depression and Fatigue. Front. Immunol. 2019, 10, 1696. [Google Scholar] [CrossRef]
- Hannestad, J.; DellaGioia, N.; Bloch, M. The effect of antidepressant medication treatment on serum levels of inflammatory cytokines: A meta-analysis. Neuropsychopharmacology 2011, 36, 2452–2459. [Google Scholar] [CrossRef]
- Felger, J.C.; Lotrich, F.E. Inflammatory cytokines in depression: Neurobiological mechanisms and therapeutic implications. Neuroscience 2013, 246, 199–229. [Google Scholar] [CrossRef]
- Capuron, L.; Miller, A.H. Immune system to brain signaling: Neuropsychopharmacological implications. Pharmacol. Ther. 2011, 130, 226–238. [Google Scholar] [CrossRef]
- Jeenger, J.; Singroha, V.; Sharma, M.; Mathur, D.; Mathur, D.M. C-reactive protein, brain-derived neurotrophic factor, interleukin-2, and stressful life events in drug-naive first-episode and recurrent depression: A cross-sectional study. Indian J. Psychiatry 2018, 60, 334–339. [Google Scholar] [CrossRef]
- Rengasamy, M.; Marsland, A.; Spada, M.; Hsiung, K.; Kovats, T.; Price, R.B. A chicken and egg scenario in psychoneuroimmunology: Bidirectional linking cytokines and depression. J. Affect. Disord. Rep. 2021, 6, 100177. [Google Scholar] [CrossRef]
- Zunszain, P.A.; Anacker, C.; Cattaneo, A.; Carvalho, L.A.; Pariante, C.M. Glucocorticoids, cytokines and brain abnormalities in depression. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2011, 35, 722–729. [Google Scholar] [CrossRef]
- Karlović, D.; Serretti, A.; Vrkić, N.; Martinac, M.; Marčinko, D. Serum concentrations of CRP, IL-6, TNF-α and cortisol in major depressive disorder with melancholic or atypical features. Psychiatry Res. 2012, 198, 74–80. [Google Scholar] [CrossRef]
- Leonard, B.E. The immune system, depression and the action of antidepressants. Prog. Neuropsychopharmacol. Biol. Psychiatry 2001, 25, 767–780. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Vikash, V.; Ye, Q.; Wu, D.; Liu, Y.; Dong, W. ROS and ROS-Mediated Cellular Signaling. Oxid. Med. Cell. Longev. 2016, 2016, 4350965. [Google Scholar] [CrossRef]
- Simpson, D.S.A.; Oliver, P.L. ROS Generation in Microglia: Understanding Oxidative Stress and Inflammation in Neurodegenerative Disease. Antioxidants 2020, 9, 743. [Google Scholar] [CrossRef]
- Lee, K.H.; Cha, M.; Lee, B.H. Crosstalk between Neuron and Glial Cells in Oxidative Injury and Neuroprotection. Int. J. Mol. Sci. 2021, 22, 13315. [Google Scholar] [CrossRef]
- Koo, J.W.; Russo, S.J.; Ferguson, D.; Nestler, E.J.; Duman, R.S. Nuclear factor-kappaB is a critical mediator of stress-impaired neurogenesis and depressive behavior. Proc. Natl. Acad. Sci. USA 2010, 107, 2669–2674. [Google Scholar] [CrossRef]
- Li, Y.; Song, W.; Tong, Y.; Zhang, X.; Zhao, J.; Gao, X.; Yong, J.; Wang, H. Isoliquiritin ameliorates depression by suppressing NLRP3-mediated pyroptosis via miRNA-27a/SYK/NF-κB axis. J. Neuroinflamm. 2021, 18, 1. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, J.; Liu, Y.; Li, Z.; Li, X. TLR4-NF-κB Signal Involved in Depressive-like Behaviors and Cytokine Expression of Frontal Cortex and Hippocampus in Stressed C57BL/6 and ob/ob Mice. Neural Plast. 2018, 2018, 7254016. [Google Scholar] [CrossRef]
- Chen, H.; Ma, Y.; Chen, M.; Chen, J.; Chen, J. Safflower extract improves depression in mice by inhibiting the TLR4-NLRP3 inflammation signaling pathway. Ann. Palliat. Med. 2021, 10, 8015–8023. [Google Scholar] [CrossRef]
- Nazari, M.; Khodadadi, H.; Fathalizadeh, J.; Hassanshahi, G.; Bidaki, R.; Ayoobi, F.; Hajebrahimi, B.; Bagheri, F.; Arababadi, M.K. Defective NF-kB transcription factor as the mediator of inflammatory responses: A study on depressed Iranian medical students. Clin. Lab. 2013, 59, 827–830. [Google Scholar] [CrossRef]
- Miklowitz, D.J.; Portnoff, L.C.; Armstrong, C.C.; Keenan-Miller, D.; Breen, E.C.; Muscatell, K.A.; Eisenberger, N.I.; Irwin, M.R. Inflammatory cytokines and nuclear factor-kappa B activation in adolescents with bipolar and major depressive disorders. Psychiatry Res. 2016, 241, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Müller, N. COX-2 Inhibitors, Aspirin, and Other Potential Anti-Inflammatory Treatments for Psychiatric Disorders. Front. Psychiatry 2019, 10, 375. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; Feng, Y.B.; Wang, L.; Shen, J.; Li, Y.; Fan, C.; Wang, P.; Yu, S.Y. COX-2 inhibition rescues depression-like behaviors via suppressing glial activation, oxidative stress and neuronal apoptosis in rats. Neuropharmacology 2019, 160, 107779. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Han, Y.; Liao, X.; Zou, M.; Wang, Y. Biology of cyclooxygenase-2: An application in depression therapeutics. Front. Psychiatry 2022, 13, 1037588. [Google Scholar] [CrossRef]
- Gałecki, P.; Florkowski, A.; Bieńkiewicz, M.; Szemraj, J. Functional polymorphism of cyclooxygenase-2 gene (G-765C) in depressive patients. Neuropsychobiology 2010, 62, 116–120. [Google Scholar] [CrossRef]
- Seo, J.S.; Park, J.Y.; Choi, J.; Kim, T.K.; Shin, J.H.; Lee, J.K.; Han, P.L. NADPH oxidase mediates depressive behavior induced by chronic stress in mice. J. Neurosci. 2012, 32, 9690–9699. [Google Scholar] [CrossRef]
- Mehta, V.; Parashar, A.; Udayabanu, M. Quercetin prevents chronic unpredictable stress induced behavioral dysfunction in mice by alleviating hippocampal oxidative and inflammatory stress. Physiol. Behav. 2017, 171, 69–78. [Google Scholar] [CrossRef]
- Quraishi, M.; Mokale, S.N.; Sakle, N.S. Ameliorative effect of quercetin and rutin via modulation of hypothalamic–Pituitary–Adrenal axis and regulation of fasting glucose in chronic stress-induced prediabetes. Phcog. Mag. 2018, 14 (Suppl. S1), 65–71. Available online: http://www.phcog.com/text.asp?2018/14/55/65/235260 (accessed on 28 June 2018).
- Khan, K.; Najmi, A.; Akhtar, M. A Natural Phenolic Compound Quercetin Showed the Usefulness by Targeting Inflammatory, Oxidative Stress Markers and Augment 5-HT Levels in One of the Animal Models of Depression in Mice. Drug Res. 2019, 69, 392–400. [Google Scholar] [CrossRef]
- Wang, Q.; Timberlake, M.A., 2nd; Prall, K.; Dwivedi, Y. The recent progress in animal models of depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 2017, 77, 99–109. [Google Scholar] [CrossRef]
- D’Aquila, P.S.; Brain, P.; Willner, P. Effects of chronic mild stress on performance in behavioural tests relevant to anxiety and depression. Physiol. Behav. 1994, 56, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Belovicova, K.; Bogi, E.; Csatlosova, K.; Dubovicky, M. Animal tests for anxiety-like and depression-like behavior in rats. Interdiscip. Toxicol. 2017, 10, 40–43. [Google Scholar] [CrossRef] [PubMed]
- Powell, T.R.; Fernandes, C.; Schalkwyk, L.C. Depression-Related Behavioral Tests. Curr. Protoc. Mouse Biol. 2012, 2, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Machado, D.G.; Bettio, L.E.; Cunha, M.P.; Santos, A.R.; Pizzolatti, M.G.; Brighente, I.M.; Rodrigues, A.L. Antidepressant-like effect of rutin isolated from the ethanolic extract from Schinus molle L. in mice: Evidence for the involvement of the serotonergic and noradrenergic systems. Eur. J. Pharmacol. 2008, 587, 163–168. [Google Scholar] [CrossRef]
- Merzoug, S.; Toumi, M.L.; Tahraoui, A. Quercetin mitigates Adriamycin-induced anxiety- and depression-like behaviors, immune dysfunction, and brain oxidative stress in rats. Naunyn Schmiedebergs Arch. Pharmacol. 2014, 387, 921–933. [Google Scholar] [CrossRef]
- Parashar, A.; Mehta, V.; Udayabanu, M. Rutin alleviates chronic unpredictable stress-induced behavioral alterations and hippocampal damage in mice. Neurosci. Lett. 2017, 656, 65–71. [Google Scholar] [CrossRef]
- Antunes, M.; Biala, G. The novel object recognition memory: Neurobiology, test procedure, and its modifications. Cogn. Process. 2012, 13, 93–110. [Google Scholar] [CrossRef]
- Pellow, S.; Chopin, P.; File, S.E.; Briley, M. Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J. Neurosci. Methods 1985, 14, 149–167. [Google Scholar] [CrossRef]
- Moore, A.; Beidler, J.; Hong, M.Y. Resveratrol and Depression in Animal Models: A Systematic Review of the Biological Mechanisms. Molecules 2018, 23, 2197. [Google Scholar] [CrossRef]
- Singh, A.; Yau, Y.F.; Leung, K.S.; El-Nezami, H.; Lee, J.C.-Y. Interaction of Polyphenols as Antioxidant and Anti-Inflammatory Compounds in Brain–Liver–Gut Axis. Antioxidants 2020, 9, 669. [Google Scholar] [CrossRef]
- Mucha, P.; Skoczyńska, A.; Małecka, M.; Hikisz, P.; Budzisz, E. Overview of the Antioxidant and Anti-Inflammatory Activities of Selected Plant Compounds and Their Metal Ions Complexes. Molecules 2021, 26, 4886. [Google Scholar] [CrossRef]
- Jazvinšćak Jembrek, M.; Oršolić, N.; Mandić, L.; Sadžak, A.; Šegota, S. Anti-Oxidative, Anti-Inflammatory and Anti-Apoptotic Effects of Flavonols: Targeting Nrf2, NF-κB and p53 Pathways in Neurodegeneration. Antioxidants 2021, 10, 1628. [Google Scholar] [CrossRef]
- Herraiz, T.; Guillén, H. Monoamine Oxidase-A Inhibition and Associated Antioxidant Activity in Plant Extracts with Potential Antidepressant Actions. Biomed. Res. Int. 2018, 2018, 4810394. [Google Scholar] [CrossRef]
- Dhiman, P.; Malik, N.; Sobarzo-Sánchez, E.; Uriarte, E.; Khatkar, A. Quercetin and Related Chromenone Derivatives as Monoamine Oxidase Inhibitors: Targeting Neurological and Mental Disorders. Molecules 2019, 24, 418. [Google Scholar] [CrossRef]
- Kim, K.; Vance, T.M.; Chun, O.K. Estimated intake and major food sources of flavonoids among US adults: Changes between 1999–2002 and 2007–2010 in NHANES. Eur. J. Nutr. 2016, 55, 833–843. [Google Scholar] [CrossRef]
- Boots, A.W.; Haenen, G.R.; Bast, A. Health effects of quercetin: From antioxidant to nutraceutical. Eur. J. Pharmacol. 2008, 585, 325–337. [Google Scholar] [CrossRef]
- Dabeek, W.M.; Marra, M.V. Dietary Quercetin and Kaempferol: Bioavailability and Potential Cardiovascular-Related Bioactivity in Humans. Nutrients 2019, 11, 2288. [Google Scholar] [CrossRef]
- Zubčić, K.; Radovanović, V.; Vlainić, J.; Hof, P.R.; Oršolić, N.; Šimić, G.; Jazvinšćak Jembrek, M. PI3K/Akt and ERK1/2 Signalling Are Involved in Quercetin-Mediated Neuroprotection against Copper-Induced Injury. Oxid. Med. Cell. Longev. 2020, 2020, 9834742. [Google Scholar] [CrossRef]
- Islam, M.S.; Quispe, C.; Hossain, R.; Islam, M.T.; Al-Harrasi, A.; Al-Rawahi, A.; Martorell, M.; Mamurova, A.; Seilkhan, A.; Altybaeva, N.; et al. Neuropharmacological Effects of Quercetin: A Literature-Based Review. Front. Pharmacol. 2021, 12, 665031. [Google Scholar] [CrossRef]
- Xu, D.; Hu, M.-J.; Wang, Y.-Q.; Cui, Y.-L. Antioxidant Activities of Quercetin and Its Complexes for Medicinal Application. Molecules 2019, 24, 1123. [Google Scholar] [CrossRef]
- Yang, D.; Wang, T.; Long, M.; Li, P. Quercetin: Its Main Pharmacological Activity and Potential Application in Clinical Medicine. Oxid. Med. Cell. Longev. 2020, 2020, 8825387. [Google Scholar] [CrossRef] [PubMed]
- Jazvinšćak Jembrek, M.; Vuković, L.; Puhović, J.; Erhardt, J.; Oršolić, N. Neuroprotective effect of quercetin against hydrogen peroxide-induced oxidative injury in P19 neurons. J. Mol. Neurosci. 2012, 47, 286–299. [Google Scholar] [CrossRef] [PubMed]
- Suematsu, N.; Hosoda, M.; Fujimori, K. Protective effects of quercetin against hydrogen peroxide-induced apoptosis in human neuronal SH-SY5Y cells. Neurosci. Lett. 2011, 504, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Godoy, J.A.; Lindsay, C.B.; Quintanilla, R.A.; Carvajal, F.J.; Cerpa, W.; Inestrosa, N.C. Quercetin Exerts Differential Neuroprotective Effects Against H2O2 and Aβ Aggregates in Hippocampal Neurons: The Role of Mitochondria. Mol. Neurobiol. 2017, 54, 7116–7128. [Google Scholar] [CrossRef]
- Bandaruk, Y.; Mukai, R.; Terao, J. Cellular uptake of quercetin and luteolin and their effects on monoamine oxidase-A in human neuroblastoma SH-SY5Y cells. Toxicol. Rep. 2014, 1, 639–649. [Google Scholar] [CrossRef]
- Lee, M.H.; Lin, R.D.; Shen, L.Y.; Yang, L.L.; Yen, K.Y.; Hou, W.C. Monoamine oxidase B and free radical scavenging activities of natural flavonoids in Melastoma candidum D. Don. J. Agric. Food Chem. 2001, 49, 5551–5555. [Google Scholar] [CrossRef]
- Han, X.; Xu, T.; Fang, Q.; Zhang, H.; Yue, L.; Hu, G.; Sun, L. Quercetin hinders microglial activation to alleviate neurotoxicity via the interplay between NLRP3 inflammasome and mitophagy. Redox Biol. 2021, 44, 102010. [Google Scholar] [CrossRef]
- Silvestro, S.; Bramanti, P.; Mazzon, E. Role of Quercetin in Depressive-Like Behaviors: Findings from Animal Models. Appl. Sci. 2021, 11, 7116. [Google Scholar] [CrossRef]
- Chen, S.; Tang, Y.; Gao, Y.; Nie, K.; Wang, H.; Su, H.; Wang, Z.; Lu, F.; Huang, W.; Dong, H. Antidepressant Potential of Quercetin and its Glycoside Derivatives: A Comprehensive Review and Update. Front. Pharmacol. 2022, 13, 865376. [Google Scholar] [CrossRef]
- Guan, Y.; Wang, J.; Wu, X.; Song, L.; Wang, Y.; Gong, M.; Li, B. Quercetin reverses chronic unpredictable mild stress-induced depression-like behavior in vivo by involving nuclear factor-E2-related factor 2. Brain Res. 2021, 1772, 147661. [Google Scholar] [CrossRef]
- Kawabata, K.; Kawai, Y.; Terao, J. Suppressive effect of quercetin on acute stress-induced hypothalamic-pituitary-adrenal axis response in Wistar rats. J. Nutr. Biochem. 2010, 21, 374–380. [Google Scholar] [CrossRef]
- Bhutada, P.; Mundhada, Y.; Bansod, K.; Ubgade, A.; Quazi, M.; Umathe, S.; Mundhada, D. Reversal by quercetin of corticotrophin releasing factor induced anxiety- and depression-like effect in mice. Prog. Neuropsychopharmacol. Biol. Psychiatry 2010, 34, 955–960. [Google Scholar] [CrossRef]
- Kosari-Nasab, M.; Shokouhi, G.; Ghorbanihaghjo, A.; Mesgari-Abbasi, M.; Salari, A.A. Quercetin mitigates anxiety-like behavior and normalizes hypothalamus-pituitary-adrenal axis function in a mouse model of mild traumatic brain injury. Behav. Pharmacology 2019, 30, 282–289. [Google Scholar] [CrossRef]
- Demir, E.A.; Gergerlioglu, H.S.; Oz, M. Antidepressant-like effects of quercetin in diabetic rats are independent of hypothalamic-pituitary-adrenal axis. Acta Neuropsychiatry 2016, 28, 23–30. [Google Scholar] [CrossRef]
- Fang, K.; Li, H.R.; Chen, X.X.; Gao, X.R.; Huang, L.L.; Du, A.Q.; Jiang, C.; Li, H.; Ge, J.F. Quercetin Alleviates LPS-Induced Depression-like Behavior in Rats via Regulating BDNF-Related Imbalance of Copine 6 and TREM1/2 in the Hippocampus and PFC. Front. Pharmacol. 2019, 10, 1544. [Google Scholar] [CrossRef]
- Rinwa, P.; Kumar, A. Quercetin suppress microglial neuroinflammatory response and induce antidepressent-like effect in olfactory bulbectomized rats. Neuroscience 2013, 255, 86–98. [Google Scholar] [CrossRef]
- Singh, V.; Chauhan, G.; Shri, R. Anti-depressant like effects of quercetin 4′-O-glucoside from Allium cepa via regulation of brain oxidative stress and monoamine levels in mice subjected to unpredictable chronic mild stress. Nutr. Neurosci. 2021, 24, 35–44. [Google Scholar] [CrossRef]
- Sakakibara, H.; Yoshino, S.; Kawai, Y.; Terao, J. Antidepressant-like effect of onion (Allium cepa L.) powder in a rat behavioral model of depression. Biosci. Biotechnol. Biochem. 2008, 72, 94–100. [Google Scholar] [CrossRef]
- Sakakibara, H.; Ishida, K.; Grundmann, O.; Nakajima, J.; Seo, S.; Butterweck, V.; Minami, Y.; Saito, S.; Kawai, Y.; Nakaya, Y.; et al. Antidepressant effect of extracts from Ginkgo biloba leaves in behavioral models. Biol. Pharm. Bull. 2006, 29, 1767–1770. [Google Scholar] [CrossRef]
- Zhang, J.; Ning, L.; Wang, J. Dietary quercetin attenuates depressive-like behaviors by inhibiting astrocyte reactivation in response to stress. Biochem. Biophys. Res. Commun. 2020, 533, 1338–1346. [Google Scholar] [CrossRef]
- Taheri, Y.; Suleria, H.; Martins, N.; Sytar, O.; Beyatli, A.; Yeskaliyeva, B.; Seitimova, G.; Salehi, B.; Semwal, P.; Painuli, S.; et al. Myricetin bioactive effects: Moving from preclinical evidence to potential clinical applications. BMC Complement. Med. Ther. 2020, 20, 241. [Google Scholar] [CrossRef] [PubMed]
- Semwal, D.K.; Semwal, R.B.; Combrinck, S.; Viljoen, A. Myricetin: A Dietary Molecule with Diverse Biological Activities. Nutrients 2016, 8, 90. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Yue, Y.; Peng, A.; Zhang, L.; Xiang, J.; Cao, X.; Ding, H.; Yin, S. Myricetin ameliorates brain injury and neurological deficits via Nrf2 activation after experimental stroke in middle-aged rats. Food Func. 2016, 7, 2624–2634. [Google Scholar] [CrossRef] [PubMed]
- Ramezani, M.; Darbandi, N.; Khodagholi, F.; Hashemi, A. Myricetin protects hippocampal CA3 pyramidal neurons and improves learning and memory impairments in rats with Alzheimer’s disease. Neural Regen Res. 2016, 11, 1976–1980. [Google Scholar] [CrossRef] [PubMed]
- Sadžak, A.; Vlašić, I.; Kiralj, Z.; Batarelo, M.; Oršolić, N.; Jazvinšćak Jembrek, M.; Kušen, I.; Šegota, S. Neurotoxic effect of flavonol myricetin in the presence of excess copper. Molecules 2021, 26, 845. [Google Scholar] [CrossRef]
- Ni, M.; You, Y.; Chen, J.; Zhang, L. Copper in depressive disorder: A systematic review and meta-analysis of observational studies. Psychiatry Res. 2018, 267, 506–515. [Google Scholar] [CrossRef]
- Jazvinšćak Jembrek, M.; Vlainić, J.; Radovanović, V.; Erhardt, J.; Oršolić, N. Effects of copper overload in P19 neurons: Impairment of glutathione redox homeostasis and crosstalk between caspase and calpain protease systems in ROS-induced apoptosis. Biometals 2014, 27, 1303–1322. [Google Scholar] [CrossRef]
- Ma, Z.; Wang, G.; Cui, L.; Wang, Q. Myricetin Attenuates Depressant-like Behavior in Mice Subjected to Repeated Restraint Stress. Int. J. Mol. Sci. 2015, 16, 28377–28385. [Google Scholar] [CrossRef]
- Wang, Q.M.; Wang, G.L.; Ma, Z.G. Protective effects of myricetin on chronic stress-induced cognitive deficits. Neuroreport 2016, 27, 652–658. [Google Scholar] [CrossRef]
- Sur, B.; Lee, B. Myricetin Inhibited Fear and Anxiety-Like Behaviors by HPA Axis Regulation and Activation of the BDNF-ERK Signaling Pathway in Posttraumatic Stress Disorder Rats. Evid. Based Complement. Alternat. Med. 2022, 2022, 8320256. [Google Scholar] [CrossRef]
- Shimada, Y.; Sato, Y.; Kumazoe, M.; Kitamura, R.; Fujimura, Y.; Tachibana, H. Myricetin improves cognitive function in SAMP8 mice and upregulates brain-derived neurotrophic factor and nerve growth factor. Biochem. Biophys. Res. Commun. 2022, 616, 33–40. [Google Scholar] [CrossRef]
- Shashikumara, S.; Purushotham, K.; Darshan, C.L.; Kalal, B.S. Characterization of antidepressant activity of Saraca asoca flower (Roxb.) Wilde in mice subjected to acute restraint stress. Am. J. Transl. Res. 2022, 14, 5014–5023. [Google Scholar]
- Kim, H.D.; Jeong, K.H.; Jung, U.J.; Kim, S.R. Myricitrin ameliorates 6-hydroxydopamine-induced dopaminergic neuronal loss in the substantia nigra of mouse brain. J. Med. Food 2016, 19, 374–382. [Google Scholar] [CrossRef]
- Meyer, E.; Mori, M.A.; Campos, A.C.; Andreatini, R.; Guimarães, F.S.; Milani, H.; de Oliveira, R.M. Myricitrin induces antidepressant-like effects and facilitates adult neurogenesis in mice. Behav. Brain Res. 2017, 316, 59–65. [Google Scholar] [CrossRef]
- Pereira, M.; Siba, I.P.; Acco, A.; Correia, D.; Lapa, F.R.; Santos, A.R.S.; Ruani, A.P.; Pizzolatti, M.G.; Andreatini, R. Myricitrin exhibits antidepressant-like effects and reduces IL-6 hippocampal levels in the chronic mild stress model. Behav. Brain Res. 2022, 429, 113905. [Google Scholar] [CrossRef]
- Dimpfel, W. Rat electropharmacograms of the flavonoids rutin and quercetin in comparison to those of moclobemide and clinically used reference drugs suggest antidepressive and/or neuroprotective action. Phytomedicine 2009, 16, 287–294. [Google Scholar] [CrossRef]
- Khan, M.M.; Ahmad, A.; Ishrat, T.; Khuwaja, G.; Srivastawa, P.; Khan, M.B.; Raza, S.S.; Javed, H.; Vaibhav, K.; Khan, A.; et al. Rutin protects the neural damage induced by transient focal ischemia in rats. Brain Res. 2009, 1292, 123–135. [Google Scholar] [CrossRef]
- Koda, T.; Kuroda, Y.; Imai, H. Rutin supplementation in the diet has protective effects against toxicant-induced hippocampal injury by suppression of microglial activation and pro-inflammatory cytokines: Protective effect of rutin against toxicant-induced hippocampal injury. Cell. Mol. Neurobiol. 2009, 29, 523–531. [Google Scholar] [CrossRef]
- Çelik, H.; Kandemir, F.M.; Caglayan, C.; Özdemir, S.; Çomaklı, S.; Kucukler, S.; Yardım, A. Neuroprotective effect of rutin against colistin-induced oxidative stress, inflammation and apoptosis in rat brain associated with the CREB/BDNF expressions. Mol. Biol. Rep. 2020, 47, 2023–2034. [Google Scholar] [CrossRef]
- Javed, H.; Khan, M.M.; Ahmad, A.; Vaibhav, K.; Ahmad, M.E.; Khan, A.; Ashafaq, M.; Islam, F.; Siddiqui, M.S.; Safhi, M.M.; et al. Rutin prevents cognitive impairments by ameliorating oxidative stress and neuroinflammation in rat model of sporadic dementia of Alzheimer type. Neuroscience 2012, 210, 340–352. [Google Scholar] [CrossRef] [PubMed]
- Yusha’u, Y.; Muhammad, U.A.; Nze, M.; Egwuma, J.M.; Igomu, O.J.; Abdulkadir, M. Modulatory Role of Rutin Supplement on Open Space Forced Swim Test Murine Model of Depression. Niger. J. Physiol. Sci. 2017, 32, 201–205. [Google Scholar] [PubMed]
- Oboh, G.; Adebayo, A.A.; Ademosun, A.O.; Olowokere, O.G. Rutin alleviates cadmium-induced neurotoxicity in Wistar rats: Involvement of modulation of nucleotide-degrading enzymes and monoamine oxidase. Metab. Brain Dis. 2019, 34, 1181–1190. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.M.; Hussein, M.M.A. Neurotoxic effects of silver nanoparticles and the protective role of rutin. Biomed. Pharmacother. 2017, 90, 731–739. [Google Scholar] [CrossRef] [PubMed]
- Azam, F.; Abodabos, H.S.; Taban, I.M.; Rfieda, A.R.; Mahmood, D.; Anwar, M.J.; Khan, S.; Sizochenko, N.; Poli, G.; Tuccinardi, T. Rutin as promising drug for the treatment of Parkinson’s disease: An assessment of MAO-B inhibitory potential by docking, molecular dynamics and DFT studies. Mol. Simul. 2019, 45, 1563–1571. [Google Scholar] [CrossRef]
- Foudah, A.I.; Alqarni, M.H.; Alam, A.; Devi, S.; Salkini, M.A.; Alam, P. Rutin Improves Anxiety and Reserpine-Induced Depression in Rats. Molecules 2022, 27, 7313. [Google Scholar] [CrossRef]
- Barauna, S.C.; Delwing-Dal Magro, D.; Brueckheimer, M.B.; Maia, T.P.; Sala, G.A.B.N.; Döhler, A.W.; Harger, M.C.; de Melo, D.F.M.; de Gasper, A.L.; Alberton, M.D.; et al. Antioxidant and antidepressant-like effects of Eugenia catharinensis D. Legrand in an animal model of depression induced by corticosterone. Metab. Brain Dis. 2018, 33, 1985–1994. [Google Scholar] [CrossRef]
- Daodee, S.; Monthakantirat, O.; Ruengwinitwong, K.; Gatenakorn, K.; Maneenet, J.; Khamphukdee, C.; Sekeroglu, N.; Chulikhit, Y.; Kijjoa, A. Effects of the Ethanol Extract of Dipterocarpus alatus Leaf on the Unpredictable Chronic Mild Stress-Induced Depression in ICR Mice and Its Possible Mechanism of Action. Molecules 2019, 24, 3396. [Google Scholar] [CrossRef]
- Zhang, X.Q.; Xu, L.S. Determination of avicularin in Polygonum aviculare L. by square wave polarography. Proc. Chin. Acad. Med. Sci. Peking Union Med. Coll. 1998, 4, 193–195. [Google Scholar]
- Shen, Z.; Xu, Y.; Jiang, X.; Wang, Z.; Guo, Y.; Pan, W.; Hou, J. Avicularin Relieves Depressive-like Behaviors Induced by Chronic Unpredictable Mild Stress in Mice. Med. Sci. Monit. 2019, 25, 2777–2784. [Google Scholar] [CrossRef]
- Vo, V.A.; Lee, J.W.; Chang, J.E.; Kim, J.Y.; Kim, N.H.; Lee, H.J.; Kim, S.S.; Chun, W.; Kwon, Y.S. Avicularin Inhibits Lipopolysaccharide-Induced Inflammatory Response by Suppressing ERK Phosphorylation in RAW 264.7 Macrophages. Biomol. Ther. 2012, 20, 532–537. [Google Scholar] [CrossRef]
- Maher, P.; Dargusch, R.; Bodai, L.; Gerard, P.E.; Purcell, J.M.; Marsh, J.L. ERK activation by the polyphenols fisetin and resveratrol provides neuroprotection in multiple models of Huntington’s disease. Human Mol. Gen. 2011, 20, 261–270. [Google Scholar] [CrossRef]
- Chiruta, C.; Schubert, D.; Dargusch, R.; Maher, P. Chemical modification of the multitarget neuroprotective compound fisetin. J. Med. Chem. 2012, 55, 378–389. [Google Scholar] [CrossRef]
- Nabavi, S.F.; Braidy, N.; Habtemariam, S.; Sureda, A.; Manayi, A.; Nabavi, S.M. Neuroprotective Effects of Fisetin in Alzheimer’s and Parkinson’s Diseases: From Chemistry to Medicine. Curr. Top. Med. Chem. 2016, 16, 1910–1915. [Google Scholar] [CrossRef]
- Wang, T.H.; Wang, S.Y.; Wang, X.D.; Jiang, H.Q.; Yang, Y.Q.; Wang, Y.; Cheng, J.L.; Zhang, C.T.; Liang, W.W.; Feng, H.L. Fisetin Exerts Antioxidant and Neuroprotective Effects in Multiple Mutant hSOD1 Models of Amyotrophic Lateral Sclerosis by Activating ERK. Neuroscience 2018, 379, 152–166. [Google Scholar] [CrossRef]
- Hassan, S.S.U.; Samanta, S.; Dash, R.; Karpiński, T.M.; Habibi, E.; Sadiq, A.; Ahmadi, A.; Bunagu, S. The neuroprotective effects of fisetin, a natural flavonoid in neurodegenerative diseases: Focus on the role of oxidative stress. Front. Pharmacol. 2022, 13, 1015835. [Google Scholar] [CrossRef]
- Cordaro, M.; D’Amico, R.; Fusco, R.; Peritore, A.F.; Genovese, T.; Interdonato, L.; Franco, G.; Arangia, A.; Gugliandolo, E.; Crupi, R.; et al. Discovering the Effects of Fisetin on NF-κB/NLRP-3/NRF-2 Molecular Pathways in a Mouse Model of Vascular Dementia Induced by Repeated Bilateral Carotid Occlusion. Biomedicines 2022, 10, 1448. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Cui, J. Neuroprotective Effect of Fisetin against the Cerebral Ischemia-Reperfusion Damage via Suppression of Oxidative Stress and Inflammatory Parameters. Inflammation 2021, 44, 1490–1506. [Google Scholar] [CrossRef]
- Cui, J.; Fan, J.; Li, H.; Zhang, J.; Tong, J. Neuroprotective potential of fisetin in an experimental model of spinal cord injury: Via modulation of NF-κB/IκBα pathway. Neuroreport 2021, 32, 296–305. [Google Scholar] [CrossRef]
- Zhen, L.; Zhu, J.; Zhao, X.; Huang, W.; An, Y.; Li, S.; Du, X.; Lin, M.; Wang, Q.; Xu, Y.; et al. The antidepressant-like effect of fisetin involves the serotonergic and noradrenergic system. Behav. Brain Res. 2012, 228, 359–366. [Google Scholar] [CrossRef]
- Mazzio, E.A.; Harris, N.; Soliman, K.F. Food constituents attenuate monoamine oxidase activity and peroxide levels in C6 astrocyte cells. Planta Med. 1998, 64, 603–606. [Google Scholar] [CrossRef]
- Yu, X.; Jiang, X.; Zhang, X.; Chen, Z.; Xu, L.; Chen, L.; Wang, G.; Pan, J. The effects of fisetin on lipopolysaccharide-induced depressive-like behavior in mice. Metab. Brain Dis. 2016, 31, 1011–1021. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, B.; Lu, J.; Shi, H.; Gong, S.; Wang, Y.; Hamdy, R.C.; Chua, B.H.L.; Yang, L.; Xu, X. Fisetin provides antidepressant effects by activating the tropomyosin receptor kinase B signal pathway in mice. J. Neurochem. 2018, 143, 561–568. [Google Scholar] [CrossRef]
- Choubey, P.; Kwatra, M.; Pandey, S.N.; Kumar, D.; Dwivedi, D.K.; Rajput, P.; Mishra, A.; Lahkar, M.; Jangra, A. Ameliorative effect of fisetin against lipopolysaccharide and restraint stress-induced behavioral deficits via modulation of NF-κB and IDO-1. Psychopharmacology 2018, 236, 741–752. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Li, L.; Kandhare, A.D.; Mukherjee-Kandhare, A.A.; Bodhankar, S.L. Attenuation of reserpine-induced fibromyalgia via ROS and serotonergic pathway modulation by fisetin, a plant flavonoid polyphenol. Exp. Ther. Med. 2020, 19, 1343–1355. [Google Scholar] [CrossRef]
- Silva Dos Santos, J.; Gonçalves Cirino, J.P.; de Oliveira Carvalho, P.; Ortega, M.M. The Pharmacological Action of Kaempferol in Central Nervous System Diseases: A Review. Front. Pharmacol. 2021, 11, 565700. [Google Scholar] [CrossRef]
- Gao, W.; Wang, W.; Peng, Y.; Deng, Z. Antidepressive effects of kaempferol mediated by reduction of oxidative stress, proinflammatory cytokines and up-regulation of AKT/β-catenin cascade. Metab. Brain Dis. 2019, 34, 485–494. [Google Scholar] [CrossRef]
- Park, S.H.; Sim, Y.B.; Han, P.L.; Lee, J.K.; Suh, H.W. Antidepressant-like Effect of Kaempferol and Quercitirin, Isolated from Opuntia ficus-indica var. saboten. Exp. Neurobiol. 2010, 19, 30–38. [Google Scholar] [CrossRef]
- de la Garza, A.L.; Garza-Cuellar, M.A.; Silva-Hernandez, I.A.; Cardenas-Perez, R.E.; Reyes-Castro, L.A.; Zambrano, E.; Gonzalez-Hernandez, B.; Garza-Ocañas, L.; Fuentes-Mera, L.; Camacho, A. Maternal Flavonoids Intake Reverts Depression-like Behaviour in Rat Female Offspring. Nutrients 2019, 11, 572. [Google Scholar] [CrossRef]
- Li, H.-Y.; Wang, J.; Liang, L.-F.; Shen, S.-Y.; Li, W.; Chen, X.-R.; Li, B.; Zhang, Y.-Q.; Yu, J. Sirtuin 3 Plays a Critical Role in the Antidepressant- and Anxiolytic-like Effects of Kaempferol. Antioxidants 2022, 11, 1886. [Google Scholar] [CrossRef]
- Zarei, M.; Mohammadi, S.; Komaki, A.; Golipour Choshali, Z. Antidepressant-like Effects of Intra-cerebroventricular Microinjection of Kaempferol in Male Rats: Involvement of 5-HT2 Receptors. Avicenna J. Neuro. Psycho. Physiol. 2022, 9, 23–30. Available online: http://ajnpp.umsha.ac.ir/article-1-379-en.html (accessed on 1 February 2022).
- Zhang, Z.T.; Cao, X.B.; Xiong, N.; Wang, H.C.; Huang, J.S.; Sun, S.G.; Wang, T. Morin exerts neuroprotective actions in Parkinson disease models in vitro and in vivo. Acta Pharmacol. Sin. 2010, 31, 900–906. [Google Scholar] [CrossRef] [PubMed]
- Alberdi, E.; Sánchez-Gómez, M.V.; Ruiz, A.; Cavaliere, F.; Ortiz-Sanz, C.; Quintela-López, T.; Capetillo-Zarate, E.; Solé-Domènech, S.; Matute, C. Mangiferin and morin attenuate oxidative stress, mitochondrial dysfunction, and neurocytotoxicity, induced by amyloid beta oligomers. Oxid. Med. Cell. Longev. 2018, 2018, 2856063. [Google Scholar] [CrossRef]
- Olonode, E.T.; Aderibigbe, A.O.; Adeoluwa, O.A.; Ajayi, A.M. Protective Effects of Morin Hydrate on Acute Stress-Induced Behavioral and Biochemical Alterations in Mice. Basic Clin. Neurosci. 2018, 9, 195–208. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.M.; Gad, A.M.; Menze, E.T.; Badary, O.A.; El-Naga, R.N. Protective effects of morin against depressive-like behavior prompted by chronic unpredictable mild stress in rats: Possible role of inflammasome-related pathways. Biochem. Pharmacol. 2020, 180, 114140. [Google Scholar] [CrossRef]
- Ben-Azu, B.; Aderibigbe, A.O.; Ajayi, A.M.; Umukoro, S.; Iwalewa, E.O. Involvement of l-arginine-nitric oxide pathway in the antidepressant and memory promoting effects of morin in mice. Drug Dev. Res. 2019, 80, 1071–1079. [Google Scholar] [CrossRef]
- Salem, H.A.; Elsherbiny, N.; Alzahrani, S.; Alshareef, H.M.; Abd Elmageed, Z.Y.; Ajwah, S.M.; Hamdan, A.M.E.; Abdou, Y.S.; Galal, O.O.; El Azazy, M.K.A.; et al. Neuroprotective Effect of Morin Hydrate against Attention-Deficit/Hyperactivity Disorder (ADHD) Induced by MSG and/or Protein Malnutrition in Rat Pups: Effect on Oxidative/Monoamines/Inflammatory Balance and Apoptosis. Pharmaceuticals 2022, 15, 1012. [Google Scholar] [CrossRef]
- Kalkunte, S.S.; Singh, A.P.; Chaves, F.C.; Gianfagna, T.J.; Pundir, V.S.; Jaiswal, A.K.; Vorsa, N.; Sharma, S. Antidepressant and antistress activity of GC-MS characterized lipophilic extracts of Ginkgo biloba leaves. Phytother. Res. 2007, 21, 1061–1065. [Google Scholar] [CrossRef]
- Yang, J.H.; Shin, B.Y.; Han, J.Y.; Kim, M.G.; Wi, J.E.; Kim, Y.W.; Cho, I.J.; Kim, S.C.; Shin, S.M.; Ki, S.H. Isorhamnetin protects against oxidative stress by activating Nrf2 and inducing the expression of its target genes. Toxicol. Appl. Pharmacol. 2014, 274, 293–301. [Google Scholar] [CrossRef]
- Gong, G.; Guan, Y.Y.; Zhang, Z.L.; Rahman, K.; Wang, S.J.; Zhou, S.; Luan, X.; Zhang, H. Isorhamnetin: A review of pharmacological effects. Biomed. Pharmacother. 2020, 128, 110301. [Google Scholar] [CrossRef]
- Pérez-González, A.; Rebollar-Zepeda, A.M.; León-Carmona, J.R.; Galano, A. Reactivity indexes and O-H bond dissociation energies of a large series of polyphenols: Implications for their free radical scavenging activity. J. Mex. Chem. Soc. 2012, 56, 241–249. [Google Scholar] [CrossRef]
- Mohammadi, S.; Golshani, Y. Neuroprotective effects of rhamnazin as a flavonoid on chronic stress-induced cognitive impairment. J. Adv. Neurosci. Res. 2017, 4, 30–37. [Google Scholar] [CrossRef]
- Gulsheen Kumar, A.; Sharma, A. Antianxiety and antidepressant activity guided isolation and characterization of gossypetin from Hibiscus sabdariffa Linn. Calyces. J. Biol. Act. Prod. Nat. 2019, 9, 205–214. [Google Scholar] [CrossRef]
- García-Durán, L.; Flores-Burgess, A.; Cantero-García, N.; Puigcerver, A.; Narváez, J.Á.; Fuxe, K.; Santín, L.; Millón, C.; Díaz-Cabiale, Z. Galanin(1–15) Potentiates the Antidepressant-like Effects Induced by Escitalopram in a Rat Model of Depression. Int. J. Mol. Sci. 2021, 22, 10848. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Zhang, H.; Zhang, J.; Yan, M. Isoquercetin attenuates oxidative stress and neuronal apoptosis after ischemia/reperfusion injury via Nrf2-mediated inhibition of the NOX4/ROS/NF-κB pathway. Chem. Biol. Interact. 2018, 284, 32–40. [Google Scholar] [CrossRef]
- Butterweck, V.; Hegger, M.; Winterhoff, H. Flavonoids of St. John’s Wort reduce HPA axis function in the rat. Planta Med. 2004, 70, 1008–1011. [Google Scholar] [CrossRef]
- Song, A.; Wu, Z.; Zhao, W.; Shi, W.; Cheng, R.; Jiang, J.; Ni, Z.; Qu, H.; Qiaolongbatu, X.; Fan, G.; et al. The Role and Mechanism of Hyperoside against Depression-like Behavior in Mice via the NLRP1 Inflammasome. Medicina 2022, 58, 1749. [Google Scholar] [CrossRef]
- Haas, J.S.; Stolz, E.D.; Betti, A.H.; Stein, A.C.; Schripsema, J.; Poser, G.L.; Rates, S.M. The anti-immobility effect of hyperoside on the forced swimming test in rats is mediated by the D2-like receptors activation. Planta Med. 2011, 77, 334–339. [Google Scholar] [CrossRef]
- Portincasa, P.; Bonfrate, L.; Vacca, M.; De Angelis, M.; Farella, I.; Lanza, E.; Khalil, M.; Wang, D.Q.-H.; Sperandio, M.; Di Ciaula, A. Gut Microbiota and Short Chain Fatty Acids: Implications in Glucose Homeostasis. Int. J. Mol. Sci. 2022, 23, 1105. [Google Scholar] [CrossRef]
- Jiang, H.; Ling, Z.; Zhang, Y.; Mao, H.; Ma, Z.; Yin, Y.; Wang, W.; Tang, W.; Tan, Z.; Shi, J.; et al. Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav. Immun. 2015, 48, 186–194. [Google Scholar] [CrossRef]
- Song, J.; Ma, W.; Gu, X.; Zhao, L.; Jiang, J.; Xu, Y.; Zhang, L.; Zhou, M.; Yang, L. Metabolomic signatures and microbial community profiling of depressive rat model induced by adrenocorticotrophic hormone. J. Transl. Med. 2019, 17, 224. [Google Scholar] [CrossRef]
- Ait Chait, Y.; Mottawea, W.; Tompkins, T.A.; Hammami, R. Unravelling the antimicrobial action of antidepressants on gut commensal microbes. Sci. Rep. 2020, 10, 17878. [Google Scholar] [CrossRef]
- Caldara, M.; Marmiroli, N. Antimicrobial Properties of Antidepressants and Antipsychotics—Possibilities and Implications. Pharmaceuticals 2021, 14, 915. [Google Scholar] [CrossRef]
- Liu, S.; Guo, R.; Liu, F.; Yuan, Q.; Yu, Y.; Ren, F. Gut Microbiota Regulates Depression-like Behavior in Rats through the Neuroendocrine-Immune-Mitochondrial Pathway. Neuropsychiatr. Dis. Treat. 2020, 16, 859–869. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, J.; Chen, Y. Regulation of Neurotransmitters by the Gut Microbiota and Effects on Cognition in Neurological Disorders. Nutrients 2021, 13, 2099. [Google Scholar] [CrossRef]
- Yano, J.M.; Yu, K.; Donaldson, G.P.; Shastri, G.G.; Ann, P.; Ma, L.; Nagler, C.R.; Ismagilov, R.F.; Mazmanian, S.K.; Hsiao, E.Y. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 2015, 161, 264–276. [Google Scholar] [CrossRef]
- Donati Zeppa, S.; Ferrini, F.; Agostini, D.; Amatori, S.; Barbieri, E.; Piccoli, G.; Sestili, P.; Stocchi, V. Nutraceuticals and Physical Activity as Antidepressants: The Central Role of the Gut Microbiota. Antioxidants 2022, 11, 236. [Google Scholar] [CrossRef]
- Wang, X.; Yu, J.; Zhang, X. Dietary Polyphenols as Prospective Natural-Compound Depression Treatment from the Perspective of Intestinal Microbiota Regulation. Molecules 2022, 27, 7637. [Google Scholar] [CrossRef]
- Inserra, A.; Rogers, G.B.; Licinio, J.; Wong, M. The microbiota-inflammasome hypothesis of major depression. Bioessays 2018, 40, 1800027. [Google Scholar] [CrossRef]
- Carlessi, A.S.; Borba, L.A.; Zugno, A.I.; Quevedo, J.; Réus, G.Z. Gut microbiota-brain axis in depression: The role of neuroinflammation. Eur. J. Neurosci. 2021, 53, 222–235. [Google Scholar] [CrossRef]
- Yang, J.; Zheng, P.; Li, Y.; Wu, J.; Tan, X.; Zhou, J.; Sun, Z.; Chen, X.; Zhang, G.; Zhang, H.; et al. Landscapes of bacterial and metabolic signatures and their interaction in major depressive disorders. Sci. Adv. 2020, 6, eaba8555. [Google Scholar] [CrossRef]
- Strandwitz, P.; Kim, K.H.; Terekhova, D.; Liu, J.K.; Sharma, A.; Levering, J.; McDonald, D.; Dietrich, D.; Ramadhar, T.R.; Lekbua, A.; et al. GABA-modulating bacteria of the human gut microbiota. Nat. Microbiol. 2019, 4, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Aizawa, E.; Tsuji, H.; Asahara, T.; Takahashi, T.; Teraishi, T.; Yoshida, S.; Ota, M.; Koga, N.; Hattori, K.; Kunugi, H. Possible association of Bifidobacterium and Lactobacillus in the gut microbiota of patients with major depressive disorder. J. Affect. Disord. 2016, 202, 254–257. [Google Scholar] [CrossRef] [PubMed]
- Filosa, S.; Di Meo, F.; Crispi, S. Polyphenols-gut microbiota interplay and brain neuromodulation. Neural Regen. Res. 2018, 13, 2055–2059. [Google Scholar] [CrossRef] [PubMed]
- Kumar Singh, A.; Cabral, C.; Kumar, R.; Ganguly, R.; Kumar Rana, H.; Gupta, A.; Rosaria Lauro, M.; Carbone, C.; Reis, F.; Pandey, A.K. Beneficial Effects of Dietary Polyphenols on Gut Microbiota and Strategies to Improve Delivery Efficiency. Nutrients 2019, 11, 2216. [Google Scholar] [CrossRef]
- Espín, J.C.; González-Sarrías, A.; Tomás-Barberán, F.A. The gut microbiota: A key factor in the therapeutic effects of (poly)phenols. Biochem. Pharmacol. 2017, 139, 82–93. [Google Scholar] [CrossRef]
- Westfall, S.; Pasinetti, G.M. The Gut Microbiota Links Dietary Polyphenols with Management of Psychiatric Mood Disorders. Front. Neurosci. 2019, 13, 1196. [Google Scholar] [CrossRef]
- Zhou, N.; Gu, X.; Zhuang, T.; Xu, Y.; Yang, L.; Zhou, M. Gut Microbiota: A Pivotal Hub for Polyphenols as Antidepressants. J. Agric. Food Chem. 2020, 68, 6007–6020. [Google Scholar] [CrossRef]
- Feng, J.; Li, Z.; Ma, H.; Yue, Y.; Hao, K.; Li, J.; Xiang, Y.; Min, Y. Quercetin alleviates intestinal inflammation and improves intestinal functions via modulating gut microbiota composition in LPS-challenged laying hens. Poult. Sci. 2023, 102, 102433. [Google Scholar] [CrossRef]
- Sun, L.; Guo, L.; Xu, G.; Li, Z.; Appiah, M.O.; Yang, L.; Lu, W. Quercetin Reduces Inflammation and Protects Gut Microbiota in Broilers. Molecules 2022, 27, 3269. [Google Scholar] [CrossRef]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The Role of Short-Chain Fatty Acids from Gut Microbiota in Gut-Brain Communication. Front. Endocrinol. 2020, 11, 25. [Google Scholar] [CrossRef]
- Lu, X.Y.; Kim, C.S.; Frazer, A.; Zhang, W. Leptin: A potential novel antidepressant. Proc. Natl. Acad. Sci. USA 2006, 103, 1593–1598. [Google Scholar] [CrossRef]
- Abdel-Latif, M.A.; Elbestawy, A.R.; El-Far, A.H.; Noreldin, A.E.; Emam, M.; Baty, R.S.; Albadrani, G.M.; Abdel-Daim, M.M.; Abd El-Hamid, H.S. Quercetin Dietary Supplementation Advances Growth Performance, Gut Microbiota, and Intestinal mRNA Expression Genes in Broiler Chickens. Animals 2021, 11, 2302. [Google Scholar] [CrossRef]
- Gu, F.; Wu, Y.; Liu, Y.; Dou, M.; Jiang, Y.; Liang, H. Lactobacillus casei improves depression-like behavior in chronic unpredictable mild stress-induced rats by the BDNF-TrkB signal pathway and the intestinal microbiota. Food Funct. 2020, 11, 6148–6157. [Google Scholar] [CrossRef]
- Tamura, M.; Hoshi, C.; Kobori, M.; Takahashi, S.; Tomita, J.; Nishimura, M.; Nishihira, J. Quercetin metabolism by fecal microbiota from healthy elderly human subjects. PLoS ONE 2017, 12, e0188271. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jazvinšćak Jembrek, M.; Oršolić, N.; Karlović, D.; Peitl, V. Flavonols in Action: Targeting Oxidative Stress and Neuroinflammation in Major Depressive Disorder. Int. J. Mol. Sci. 2023, 24, 6888. https://doi.org/10.3390/ijms24086888
Jazvinšćak Jembrek M, Oršolić N, Karlović D, Peitl V. Flavonols in Action: Targeting Oxidative Stress and Neuroinflammation in Major Depressive Disorder. International Journal of Molecular Sciences. 2023; 24(8):6888. https://doi.org/10.3390/ijms24086888
Chicago/Turabian StyleJazvinšćak Jembrek, Maja, Nada Oršolić, Dalibor Karlović, and Vjekoslav Peitl. 2023. "Flavonols in Action: Targeting Oxidative Stress and Neuroinflammation in Major Depressive Disorder" International Journal of Molecular Sciences 24, no. 8: 6888. https://doi.org/10.3390/ijms24086888
APA StyleJazvinšćak Jembrek, M., Oršolić, N., Karlović, D., & Peitl, V. (2023). Flavonols in Action: Targeting Oxidative Stress and Neuroinflammation in Major Depressive Disorder. International Journal of Molecular Sciences, 24(8), 6888. https://doi.org/10.3390/ijms24086888






