Abstract
Basic helix–loop–helix (bHLH)/HLH transcription factors are involved in various aspects of the growth and development of plants. Here, we identified four HLH genes, PePRE1-4, in moso bamboo plants that are homologous to Arabidopsis PRE genes. In bamboo seedlings, PePRE1/3 were found to be highly expressed in the internode and lamina joint by using quantitative RT-PCR analysis. In the elongating internode of bamboo shoots, PePRE genes are expressed at higher levels in the basal segment than in the mature top segment. Overexpression of PePREs (PePREs-OX) in Arabidopsis showed longer petioles and hypocotyls, as well as earlier flowering. PePRE1 overexpression restored the phenotype due to the deficiency of AtPRE genes caused by artificial micro-RNA. PePRE1-OX plants showed hypersensitivity to propiconazole treatment compared with the wild type. In addition, PePRE1/3 but not PePRE2/4 proteins accumulated as punctate structures in the cytosol, which was disrupted by the vesicle recycling inhibitor brefeldin A (BFA). PePRE genes have a positive function in the internode elongation of moso bamboo shoots, and overexpression of PePREs genes promotes flowering and growth in Arabidopsis. Our findings provided new insights about the fast-growing mechanism of bamboo shoots and the application of PRE genes from bamboo.
1. Introduction
Moso bamboo (Phyllostachys edulis) is one of the fastest growing non-timber tree plants all over the world, with considerable ecological, economic, and cultural value [1,2]. In spring, it can grow up to 1 m in less than 24 h and reach a final height of 20 m in 45–60 days [3]. The rapid expansion of bamboo stems is driven by the cell division and elongation of internodes, which are regulated by a combination of endogenous phytohormones and environmental factors such as auxin, gibberellin acid (GA), brassinosteroids (BRs), and light [4]. Recent studies demonstrate that the BR, auxin, GA, and phytochrome pathways converge through direct interactions among their transcription factors/regulators, then pass to a tripartite module of helix-loop-helix (HLH) and basic helix–loop–helix (bHLH) factors, which is named the HHbH module [5]. The HLH/bHLH cascade regulates cell elongation downstream of various hormonal and environmental signaling pathways [6,7].
Paclobutrazol-resistant (PRE) proteins are a typical bHLH transcription factors, homologs to the human Id-1 (inhibitor of DNA binding 1) protein which lacks the basic domain required for DNA binding. PRE proteins dimerize with other bHLH factors to inhibit their DNA-binding activity [8,9]. IBH1 (ILI1 binding bHLH protein) interacts with HBI1 (homolog of BEE2 interacting with IBH1), a positive regulator of cell elongation, and inhibits its transcriptional activity, thereby promoting the hypocotyl elongation by inhibiting the DNA-binding activity of HBI1 [10,11]. PRE1 and ILI1 promote cell elongation both in Arabidopsis and rice by interacting with IBH1 and forming a pair of antagonistic HLH/bHLH transcriptional factors that function downstream of BZR1 (brassinazole resistant 1) to mediate BRs regulation of cell elongation [12]. PIF4 (phytochrome-interacting factor 4) had a major role in the multiple signal integration for plant growth regulation [13]. PIF4 and BZR1 are direct targets of PRE1, PRE5, and PRE6/KIDARI [14]. PRE3/TOM7 is involved in regulating a plant’s growth response to light signals [9]. PAR1 (phytochrome rapidly regulated 1)–PRE1 and PAR1–PIF4 heterodimers form a complex HLH/bHLH network that controls cell elongation and plant development in response to light [15]. PRE6 regulates photomorphogenesis by inhibiting the activity of HFR1 (the long hypocotyl in far-red 1) [16,17]. Another study showed the CIB1 (cryptochrome-interacting bHLH 1)–PAR1 and PIF4–PAR1/HFR1 systems antagonistically regulate cell elongation in response to light and high temperature [18]. Auxin works independently of and in conjunction with the PIF and GA pathways to regulate expression of growth-associated genes in cell elongation [19,20].
On the other hand, GA, BR, and auxin enhance cell elongation by inhibiting many inhibitory bHLH factors by inducing the expression of PRE family genes [6,16]. Overexpression of PRE1 suppressed GA-deficient phenotypes of the ga2 mutant and impacted several aspects of GA-dependent response, indicating that PRE1 plays a regulatory function in GA-dependent development in Arabidopsis [21]. Overexpression of PRE3 suppresses the dwarf phenotype of the bri1-301 mutant, indicating PREs are involved and functional redundancy in BR signaling and response [9]. PRE1 acts downstream of ARF10 (auxin response factor 10) in regulating hypocotyl elongation, whereas PRE6 is a transcriptional repressor that is directly regulated by ARF5 and ARF8 in Arabidopsis [22].
PRE homologs are also involved in the regulation of plant development in other species. In rice, the PRE homologous gene OsILI1 (increased leaf inclination 1) stimulates cell elongation in the lamina joint and OsILI1 overexpression results in a large leaf angle phenotype [10]. FaPRE (Fragaria × ananassa paclobutrazol resistant) promotes the expression of genes involved in the ripening process while suppressing the expression of growth-promoting genes in the receptacle of octoploid strawberries [23]. GhPRE1 (Gossypium hirsutum paclobutrazol resistant 1) overexpression results in longer fibers with higher quality characteristics in cotton [24]. In a recent study, moso bamboo PRE homolog genes were downregulated by a BR biosynthesis inhibitor (propiconazole (PPZ)) treatment, according to the transcriptome profile [25].
Recently, transcriptome sequencing and epigenetic-modification profiling in fast-growing moso bamboo shoots identified a large number of putative fast-growing genes [3]. Among these genes, the transcription factors act as regulatory switches for gene expression, which will deepen and expand understanding the fast-growth mechanism. However, the role of moso bamboo PRE genes, putative growth-promoting transcription factors, remains unclear. In this study, we identified four PRE genes from the moso bamboo genome. Specific expression patterns of PePRE genes were observed in the seedling and elongating bamboo shoots, as well as in the different parts of the internodes. Overexpressing PePRE1 in Arabidopsis resulted in phenotypes including a long petiole, slightly pale green leaves, and early flowering. PRE1 and PRE3 proteins accumulated as punctate structures in the cytosol. Our study suggests that PePREs may play a positive role in the fast-growth processes of moso bamboo. Our results will benefit the future identification of more growth-promoting genes from moso bamboo genome and will provide basis for further functional characterizations of PRE family genes.
2. Results
2.1. Identification of PePREs in Moso Bamboo
Four homologous PRE genes were identified from the moso bamboo genome by BLAST-P searching in the moso bamboo protein database (http://www.bamboogdb.org/, accessed on 8 May 2017) [26] with AtPREs amino acids sequences. These were named as PePRE1 (PH01000065G2010), PePRE2 (PH01000068G1560), PePRE3 (PH01000519G0840), and PePRE4 (PH01000960G0260). When compared with rice and Arabidopsis PREs, all bamboo PRE proteins are clustered with rice PREs but not AtPREs (Figure 1b). Protein sequence alignment of the PREs showed that all bamboo PREs proteins have highly conserved helix–loop–helix (HLH) domains but not basic domains that are critical for DNA binding (Figure 1a). The expression patterns of PePRE genes were investigated in different tissues of moso bamboo seedlings by quantitative reverse transcription PCR (qRT-PCR) (Figure 1c,d). PePRE2 and PePRE3 are found highly expressed in roots, whereas PePRE1 and PePRE4 were hardly detected (Figure 1d). Interestingly, only the PePRE1 gene was detected in leaves, suggesting its unique function in leaf development (Figure 1d). Consistent with a previous report about the critical function of PRE genes on the lamina joint bending [12], four bamboo PRE genes were found to be expressed in the lamina joint (Figure 1d). As all PePREs were expressed in the internode and sheath, PePRE1 and PePRE3 showed predominant expressions in the sheath and internode, respectively (Figure 1d). Together, these results suggested that PePRE genes showed tissue-specific patterns in moso bamboo seedlings.
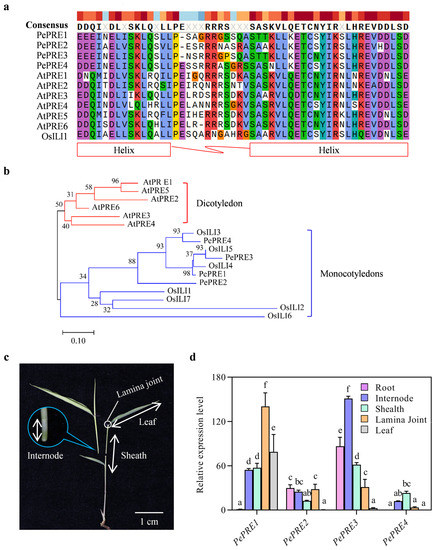
Figure 1.
PePREs in moso bamboo. (a) Sequences alignment of PRE proteins. Identical or similar amino acid residues are indicated by colorful shading and the helix and loop domains are highlighted. (b) Phylogenetic analysis of PRE proteins from rice and Arabidopsis. Full amino acid sequences were used. Bootstrap values of 1000 replications were shown. Pe, Phyllostachys edulis; Os, Oryza sativa; At, Arabidopsis thaliana. (c) The tissue of the aerial part of moso bamboo seedling. Scale bar represents 1 cm. (d) Expression level of PePREs in different tissues of 3-week-old seedlings by qRT-PCR analysis. Different letters above the data columns indicate significant differences (p < 0.05; Duncan’s test).
2.2. Expression of PePRE Genes in Elongating Bamboo Shoots
Bamboo shoot growth is attributed to cell proliferation in the intercalary meristem and subsequent cell elongation in the elongation zone of the internodes. The expression patterns of bamboo PRE genes were investigated in the elongating bamboo shoot (Figure 2a). PePRE1 and PePRE3 transcript levels were highest in the elongating internode (EIN) and scale leaves, whereas PePRE2 and PePRE4 transcript levels were highest in the scale leaves (Figure 2b). All PePREs showed higher expression levels in the internodes than in the nodes (Figure 2b). To further explore the role of PePRE genes in internode growth, the elongating internode of bamboo shoots was divided into top, middle, and basal regions (Figure 2a bottom right). Generally, expression of PePRE genes accumulated in the middle and basal regions, whereas PePREs were hardly detected in the upper parts. PePRE1 and PePRE3 were predominantly expressed in the middle and basal regions (Figure 2c). There was high PePRE2 expression in scale leaves, followed by the unelongated internodes. Interestingly, PePRE4 showed the lowest expression levels in the internodes and had relatively higher expression in the upper parts. Collectively, these data suggest that PePREs have a prevalent accumulation in the elongating tissue of bamboo shoots.
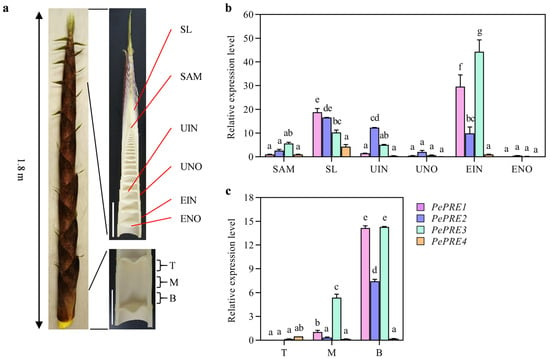
Figure 2.
Expression pattern of PePREs in the elongating bamboo shoot. (a) Structure of an elongating moso bamboo shoot. Samples are collected from a 1.8 m bamboo shoot. SAM, shoot apical meristem; SL, scale leaf; UIN, unelongated internode; UNO, unelongated node; EIN, elongating internode; ENO, elongating node. Scale bar represents 10 cm. (b) Expression levels of PePRE genes in the elongating bamboo shoot. (c) Expression levels of PePRE genes in different parts of an elongating internode. T, top part; M, middle part; B, bottom part. Different letters above the data columns indicate significant differences (p < 0.05; Duncan’s test).
2.3. Overexpressing PePREs in Arabidopsis
To further understand the biological function of PePREs, we generated overexpression transgenic lines with the 35S promoter driving the PePRE cDNAs fused with green fluorescent protein (GFP) in a Col-0 background. A total of 59, 42, 64, and 14 transgenic lines for PePRE1, 2, 3, and 4 were generated, respectively, with 31 (52.5%), 34 (81%), 22 (34.4%), and 3 (21.4%) lines for each gene, respectively, showing an early flowering phenotype. Days to bolting for PePRE1-OX #19 and PePRE2-OX #21 were found to be reduced by 13.3% and 14.0%, respectively, whereas the total rosette leaf numbers of PePRE-OX plants were also reduced with exception of PePRE4-OX. Under long-day conditions, PePRE1-OX transgenic plants exhibited longer petioles and had paler green leaves than control plants (Figure 3a), which were similar to the AtPRE1 overexpression lines reported in a previous study [27]. This suggested that PePREs have a conserved function similar to AtPRE1 on flowering control. A total of 45% and 82.5% of PePRE1 and PePRE3 overexpression transgenic lines, respectively, showed a stem-bending phenotype, which was mainly due to a broken stem with a longitudinal crack (Figure A2). We further crossed PePRE1-OX transgenic plants with pre-amiR transgenic lines, in which four AtPREs (AtPRE1/2/5/6) were knocked-down using artificial microRNA [14]. Due to the lack of AtPREs, pre-amiR exhibited extreme dwarfism, delayed flowering, and had a reduced fertility phenotype [21]. All F1 plants showed a normal phenotype similar to the wild type (Figure 3d). Overexpression of PePRE1 rescued the AtPRE (1/2/5/6)-deficient phenotypes of the pre-amiR mutant, including late flowering and curled leaves. To exclude AtPRE1 expression interference, the expression level of AtPRE1 was detected in all plants. Only in the AtPRE1-OX line did the AtPRE1 expression level increase; there was no difference in the Col-0 and PePRE1-OX lines (Figure 3e). These studies suggest that PePRE genes play a conserved role similar to AtPRE genes on flowering promotion and cell elongation.
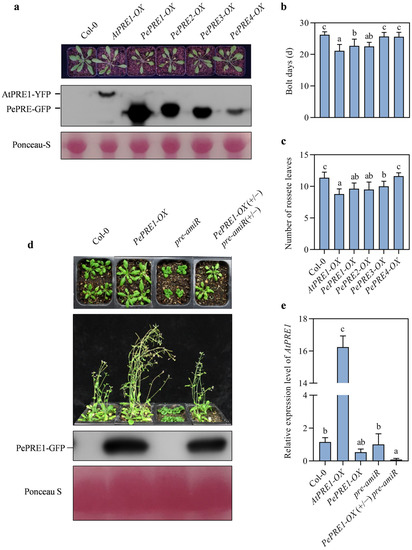
Figure 3.
Overexpression of PePREs promoted plant flowering and growth in Arabidopsis. (a) Overexpression of PePREs in Arabidopsis increased petiole elongation. Panels from top to bottom are pictures of plants grown in soil for 4 weeks (left to right: Col-0, AtPRE1-OX (35S::AtPRE1-YFP), and PePREs-OX (35S::PePREs-GFP)), Western bolt of PRE proteins using GFP antibody, and protein loading control by Ponceau S. Days of bolting (b) and rosette leaf numbers (c) when plants started bolting. A total of 10 plants were used for calculations. (d) Overexpression of PePRE1 suppressed the dwarf phenotype of pre-amiR. Panels from top to bottom are morphology of 4-week-old plants of Col-0, PePRE1-OX, pre-amiR, and PePRE1-OX (+/−) pre-amiR (+/−), 5-weeks-old plants of Col-0, PePRE1-OX, pre-amiR, and PePRE1-OX (+/−) pre-amiR (+/−), expression level of PRE1 using GFP antibody, and the protein loading control by Ponceau S. (e) Quantitative analysis of AtPRE1 in Col-0, PePRE1-OX, pre-amiR, and PePRE1-OX (+/−) pre-amiR (+/−). Different letters above the data columns indicate significant differences compared between Col-0 and transgenic lines (p < 0.05; Duncan’s test).
2.4. Subcellular Localization of PePREs in Arabidopsis
To determine the intracellular localization of PePRE proteins, the fluorescence signals of 35S::PePRE-GFP were examined in transgenic Arabidopsis plants. PePRE-GFP signals were mainly located in the nucleus, cytosol, and plasma membranes, whereas 4′, 6-diamidino-2-phenylindole dihydrochloride (DAPI) staining was used as the indicator of nuclear area. Interestingly, PePRE1-GFP and PePRE3-GFP proteins specifically displayed small punctate structures in the cytosol (Figure 4a), which are different from the AtPRE1 protein. When treated with BFA, a vesicle trafficking inhibitor [28], aggregation of PePRE1 fusion proteins in the punctate structures was reduced. Moreover, these signals were recovered by removing BFA (Figure 4b). Taken together, PePREs are located in the nucleus, cytosol, and membrane, whereas PePRE1 and PePRE3 are distributed in the cytosol in punctate structures.
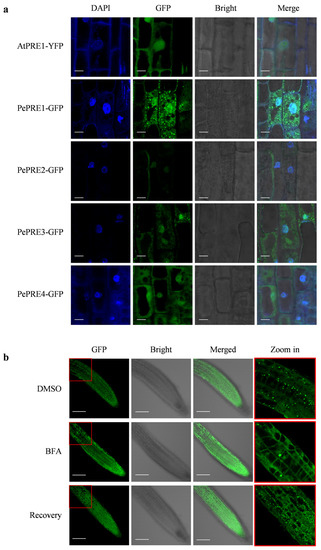
Figure 4.
Subcellular localization of PRE proteins in Arabidopsis. (a) The root tip of the PePREs’ transgenic lines was observed by confocal microscope. Seedlings were grown on half MS medium with constant light for 7 days. Cells were stained with DAPI. Scale bar indicates 10 μm. (b) Subcellular localization of PePRE1-GFP by treating with or without BFA. The root tips of PePRE1-OX #19 were observed by confocal microscope. Seedlings were grown on half MS medium with constant light for 4 days vertically. Scale bar indicates 100 μm.
2.5. BR Regulates the Expression Levels of PePREs
The expression levels of PePRE1 and PePRE2 dramatically decreased in the aerial part, whereas BR increased the expression of PePRE1 and PePRE2 in bamboo shoot [25]. We further examined the expression profile of PRE homologous genes in various tissues of bamboo seedlings with PPZ and eBL (Figure 5). Expression of PRE1, 3, and 4 was decreased by PPZ but recovered by subsequent BR treatment in most tissues. However, mRNA levels of PePRE2 increased in the internode and sheath and decreased in the lamina joint and root. In addition, PePRE2 did not further respond to eBL treatment. Overexpression of the PePRE1 gene resulted in longer hypocotyl in comparison with the wild type (Figure 6a,b; Figure A1). Compared with the wild type, PePRE1 overexpressing plants have longer hypocotyls and roots (Figure 6a,b), similar to AtPRE1 overexpressing plants. Additionally, compared with AtPRE1-OX, the shoot and root parts of PePRE1-OX plants showed more sensitivity and less sensitivity to PPZ, respectively (Figure 6c,d; Figure A3).
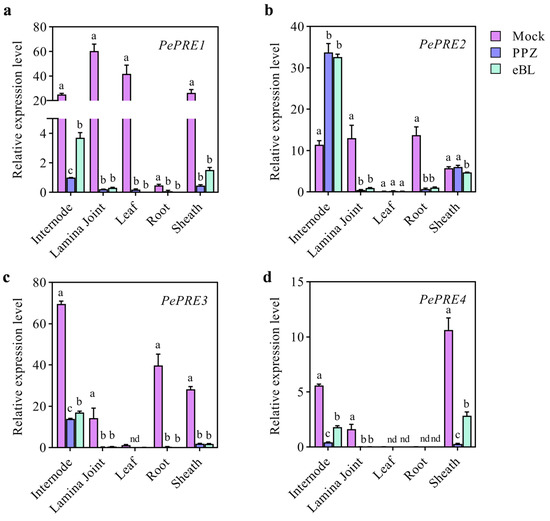
Figure 5.
The expression levels of PePREs after eBL or PPZ treatment in bamboo seedlings. Quantitative RT-PCR analysis of PePREs (a–d) in different bamboo seedling organs after PPZ or eBL treatment. The bamboo seedlings were grown on half MS medium for 18 days. For PPZ treatment, plants were transferred to half MS medium containing 50 μM PPZ for another 4 days. For eBL treatment, the seedlings were transferred to half MS medium containing 10 μM PPZ and 1 μM eBL solution for 4 h. Different lowercase letters above the data columns indicate significant differences (p < 0.05; Duncan’s test); nd, no data available.
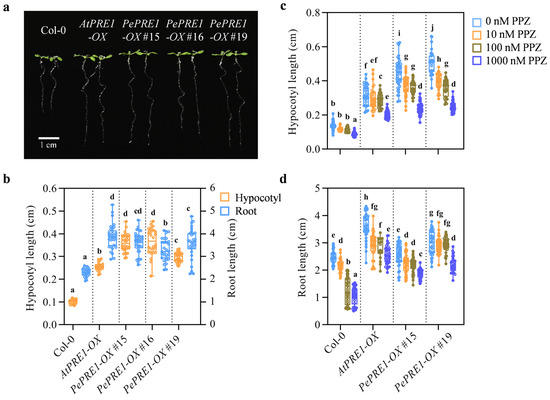
Figure 6.
PePRE1-OX transgenic Arabidopsis exhibited longer hypocotyls and roots that were hypersensitive to PPZ treatment. (a) Representative seedlings of Col-0, AtPRE1-OX, and PePRE1-OX. The seeds were sown on half MS medium and grown for 7 days vertically. Scale bar presents 1 cm. (b) The hypocotyl and root lengths of the control and PePRE1-OX plants. Hypocotyl length (c) and root length (d) of different lines of PePRE1-OX compared with Col-0 with a gradient concentration (0 nM, 10 nM, 100 nM, and 1000 nM) of PPZ treatment. Values are the means calculated from at least 20 seedlings. Error bars represent mix/max values. Different letters above the data column indicate significant differences (p < 0.05; Duncan’s test).
3. Discussion
Although the rapid growth of woody bamboo plants has been widely studied, little is known about the molecular mechanism underlying the elongation of moso bamboo. In this study, we investigated the unique patterns of PePRE genes, the conserved growth-promoting function in Arabidopsis, and the potential important role for PePREs in the elongation of moso bamboo.
3.1. PePREs Have Tissue-Specific Expression Patterns in Moso Bamboo
PePRE1 and PePRE4 are highly expressed in the shoot, whereas PePRE2 and PePRE4 are specifically expressed in the roots of seedlings. Consistent with these tissue-specific expressions, a rice homologous gene of PePRE, PGL1 (positive regulator of grain length 1), was expressed in the floral organs, young panicle, and predominantly in the root but not the leaf [29]. The expression level of BU1 (brassinosteroid upregulated 1) is high in the lamina joint in vegetative organs and the panicle at the heading stage [30]. OsILI1 is ubiquitously expressed in rice, whereas the highest expression of OsILI1 was observed in the lamina joint [12]. OsBUL1 (O. sativa brassinosteroid upregulated 1-like1), an AtPRE homolog in rice, is preferentially expressed in the lamina joint where it controls cell elongation and positively affects leaf angles [31,32]. FaPRE1 was proposed as a ripening-associated gene and showed a rapid increase in expression in the receptacle during fruit enlargement [33]. In our study, all four PRE genes were highly expressed in the elongating tissues but not in the mature tissues such as the nodes of the bamboo shoot (Figure 1 and Figure 2), which is consistent with their function in promoting cell elongation. During the rapid growth of monocots, elongation generally occurs from top to bottom in each individual internode [34]. From our results, PePRE1 is highly expressed in the basal part of the elongating internodes, with a gradient distribution from the basal to the top parts (Figure 7), which may contribute to the fast growth of bamboo shoot. A previous report showed that GRFs (growth-regulating factors) and ARF genes were highly expressed in the basal region of the elongating internode, in which ARF6 and two ARF8s targeted by miR167 and 11 GRFs targeted by miR396 were in the top region [35]. The DsEXLA2 (Dendrocalamus sinicus expansin-like A2) gene is highly expressed in the elongating internode and accelerates the plant growth rate of Arabidopsis [36]. PeGT43s (P. edulis Glycosyltransferase 43) and lignin biosynthesis are significantly upregulated within the shoot [3]. Considering the roles for PRE in Arabidopsis, PePRE genes may work downstream of ARF or GRF genes to regulate target genes in the elongating bamboo.
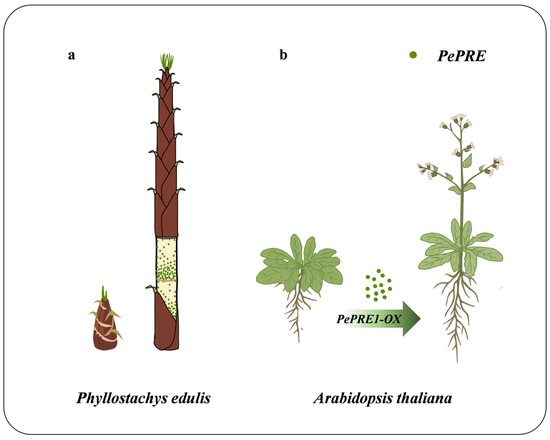
Figure 7.
Diagram of PePRE1 gene expression and function. (a) Gradient expression levels of PePRE1 in the elongating internodes of moso bamboo shoots. (b) Overexpression of PePRE1 genes leads to early flowering, longer hypocotyl, and growth promotion in Arabidopsis. Green dots indicate PePRE genes.
3.2. Phytohormones Regulates PRE Function in Bamboo Elongation
The stem elongation was contributed to by the division and expansion of individual internodes [37]. Transcriptome analysis revealed that multiple signaling pathways, including GA, auxin, and ABA, may play a role in regulating internode elongation [38]. GA plays an antagonistic regulatory role in regulating internode stem elongation in rice [39]. OsbHLH073 encodes an atypical bHLH protein and regulates plant height, internode elongation, and panicle extension by regulating GA biosynthesis genes [40]. The content of GAs in dwarf bamboo varieties is also lower than that in normal bamboos, implying that GA plays a major role in the height of Shidu bamboo [41]. Exogenous application of GA resulted in a significant increase in internode length in bamboo seedlings [42]. Those results hint that GA may play a dual role in internode elongation between shoots and seedlings of bamboo. PREs were reported to act as hostile antagonists of the bHLH family of transcription factors, which positively regulate cell elongation via multiple signaling pathways [6,7].
Bamboo PRE proteins showed different subcellular localizations, and only PRE1 and PRE3 showed punctate structures that were disturbed by BFA treatment. BFA treatment may lead to rapid protein aggregation within the endoplasmic reticulum and collapse of the Golgi apparatus [28]. The specific localizations of PePRE1 and PePRE3 may be related to the tuning of protein stability or function. PePREs are localized in both the nucleus and cytoplasm, whereas the punctate structures in cytoplasm were only observed in PePRE1 and PePRE3 in transgenic lines (Figure 4). Considering synchrony in phenotype, expression pattern, and subcellular localization, we proposed that the difference in subcellular localization may contribute to its functional variation. Certain PePRE overexpressing plants were easily dislodged caused by a broken stem (Figure A2). PRE1 was reported to promote cell elongation by preventing IBH1 from inhibiting HBI1, which directly activates genes encoding cell-wall-loosening enzymes (e.g., EXP, etc.) [10,43]. The accumulation of PePRE may change the stem segment structure, especially the composition of the cell wall, leading to the rupture and hollowness of the stem (Figure A2).
3.3. Various Functions of PRE in Regulating Plant Growth through Multiple Signaling Pathways
PRE proteins are important parts of the signaling pathways involved in physiological development and reproduction [21,27,44]. Due to the limitation of the transformation of moso bamboo, the function of PePREs was checked in Arabidopsis in this work. The overexpression of PePREs was associated with early flowering and promoted growth, such as longer petioles, hypocotyls, and roots (Figure 7). PREs are involved in regulating the growth of floral organs in Arabidopsis [27,45]. FaPRE1 antagonistically modulates the transcription of genes related to both receptacle growth and ripening [23,33]. GhPRE1 has contributed to spinnable fiber formation in cotton; overexpressing GhPRE1 leads to longer fibers with improved quality parameters, indicating that this bHLH gene is useful for improving cotton fiber quality [24]. SlPRE2 was reported to regulate fruit development via the gibberellin pathway and tomato fruit pigment accumulation in tomatoes [46]. Those results suggest that PRE affects multiple aspects of the development of plants. PRE6 is a positive regulator of shade avoidance and interacts with a number of negative growth regulators (PAR1, etc.) [17]. Transcriptional regulators (ARFs and BZR1) and post-transcriptional regulators (HFR1, etc.) were key modules of the signaling network controlling shade avoidance [47,48]. Interestingly, PePRE genes are regulated by brassinosteroid levels (Figure 5) in the elongating bamboo, and the transgenic lines also shown an altered response to PPZ treatment (Figure 6). Shade avoidance syndrome (SAS) allows plants that are grown in densely populated environments to maximize their sunlight access [48]. As mentioned above in the relationship between those TFs and PRE, PePREs may play a positive role in rhizome elongation underground and shoot elongation aboveground. Functional analyses of PePREs will help to elucidate the mechanism of fast growth in plants as well. Light affects the dynamic growth and development of the P. pygmaeus rhizome–root system [49]. Taken together, PePRE genes show a conserved function in controlling flowering and promoting growth by responding to multiple signaling pathways. This is similar to the PRE genes from other species.
Our analysis identified PePRE genes with specific expression patterns in the seedling and shooting stage and demonstrated that PePREs are brassinosteroid-regulated genes. Overexpression of the PePRE1 gene will promote flowering, hypocotyl elongation, and root growth. The current results indicate a key role for PePRE genes in bamboo growth and development. Our findings will provide the basis for further functional characterizations of PRE family genes and the molecular mechanism of bamboo fast growth and will benefit the application of growth-promoting gene resources from bamboo.
4. Materials and Methods
4.1. Plant Material and Growth Conditions
The moso bamboo shoots (approximately 1.8 m above ground height) were obtained from the Bamboo Garden of Fujian Agriculture and Forestry University, Fuzhou (coordinate 119°14′ E, 26°50′ N). Moso bamboo seeds were collected from Gong city, Guanxi province, China (118°48′ E, 24°51′ N). The seeds of moso bamboo were soaked in tap water for 24 h to induce seed germination and then sown on the soil. Seedlings were cultivated for 3 weeks in a greenhouse (long-day conditions, 22 °C).
Columbia (Col-0) wild-type seeds of Arabidopsis were germinated on half MS medium (pH = 5.8) with 1% sucrose, then transferred to a greenhouse (long-day conditions, 22 °C). PePRE-OX was crossed with pre-amiR to generate PePRE-OX pre-amiR plants.
4.2. Protein Sequence Alignment and Phylogenic Tree Construction
Alignment of the protein sequences was performed using Clustal Omega and analyzed in the GENEDOC program with the default settings. A phylogenic tree based on the sequence alignment was generated using MEGA-X by the neighbor-joining method [50]. Accession number: The protein sequences reported in this article can be found in the database (http://www.bamboogdb.org/, accessed on 8 May 2017) [26], the rice genome database (http://rice.plantbiology.msu.edu/, accessed on 8 May 2017), and TAIR (http://www.arabidopsis.org/, accessed on 8 May 2017) under the following accession numbers: OsILI1 (Os04g54900.1), OsILI2 (Os11g39000.1), OsILI3 (Os03g07540.1), OsILI4 (Os06g12210.1), OsILI5 (Os02g51320.1), OsILI6 (Os03g07510.1), OsILI7 (Os10g26460.1), PePRE1 (PH01000065G2010), PePRE2 PH01000068G1560), PePRE3 (PH01000519G0840), PePRE4 (PH01000960G0260), AtPRE1 (AT5G39860.1), AtPRE2 (AT5G15160.1), AtPRE3 (AT1G74500.1), AtPRE4 (AT3G47710.1), AtPRE5 (AT3G28857.1), and AtPRE6 (AT1G26945.1).
4.3. Gene Expression Analysis
Total RNA was isolated from various tissues with the Plant Total RNA Kit (Sigma, ATRN50) and extensively treated with RNase-free DNase I (Sigma, St. Louis, MO, USA, DNASE70-1SET). cDNA was generated by RT-PCR using the PrimeScriptTM RT reagent Kit (Takara, Kusatsu, Japan, RR047A). qRT-PCR analysis was performed with SYBR Premix Ex Taq II (Takara, RR820) on a QuanStudio 6 Flex instrument (Applied Biosystems, Waltham, MA, USA). For each sample, qPCR was performed with three technical replicates on three biological replicates. The bamboo PeTIP41 gene [51] was used as an internal control for the qRT-PCR. The relative expression levels were calculated as E−ΔCq and normalized to PeTIP41. The primer sequences used in this study are listed in Table A1.
4.4. Vector Construction and Transformation
The full-length PePRE1 sequence was amplified and ligated into pAGM1311. A 35S-promotor-driven PePRE C-terminal fused with a GFP tag was constructed into a pAGM4673 backbone. Through Agrobacterium tumefaciens strain GV3101, these constructions were transformed into Arabidopsis (Col-0). T1 seeds were screened by an RFP selection marker and then further confirmed by qRT-PCR and Western blot.
4.5. Hypocotyl Measurements and Statistical Analysis
For hypocotyl and root length measurements, seedlings were grown for 7 days on vertically oriented plates. Seedlings were flattened and photographed before taking quantitative measurements using ImageJ software (http://rsb.info.nih.gov/, accessed on 8 April 2018) to analyze the scanned images of the seedlings. The differences among groups were assessed by one-way ANOVA and Duncan’s multiple comparisons test using SPSS 23.0. GraphPad Prism 8.0 (http://www.graphpad.com/, accessed on 26 April 2018) was used to plot figures. At least 20 seedlings were measured, and experiments were repeated more than two times.
4.6. BFA Treatment
For BFA treatment, 4-day-old seedlings of PePRE1-OX were incubated in 50 μM BFA for 90 min before viewing the seedlings; control seedlings were incubated in a solution without BFA but with DMSO at the same concentration as the BFA-treated seedlings. After BFA treatment, the seedlings were washed with half MS liquid media several times and the root tips were observed for the formation of BFA compartments using a confocal laser scanning microscope (Leica Microsystems, Wetzlar, Germany). For recovery, BFA was removed from seedlings and supplied with half MS media for another 40 min followed by immediate observation with the confocal microscope.
5. Conclusions
Our study identified an atypical bHLH transcription factor (PRE homologs) in moso bamboo via gene expression analysis, heterologous overexpression, etc. We verified that PePREs function as a positive regulator in the promotion of internode elongation during the fast-growth process. Overexpressing PePRE promoted Arabidopsis growth and petiole/hypocotyl elongation. Our findings shed light on bHLH-mediated fast growth to provide preliminary knowledge for fast-growing plants.
Author Contributions
Conceptualization, S.Z., K.S., W.W. and X.Y.; methodology, data curation, and validation, S.Z., K.S., W.W. and X.Y.; writing-original draft preparation, S.Z., W.W. and X.Y.; writing-review and editing, K.S., W.L. and X.Y.; funding acquisition, W.W.; All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Science and Technology Innovation Program of FAFU (CXZX2020046A) and the program of First-class Ecology Disciplines Construction of FAFU to W.W.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We thank Mingyi Bai (Shandong University) for providing the Arabidopsis seeds of the AtPRE1-OX and pre-amiR plants.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Table A1.
The primers used for quantitative PCR (RT-qPCR) and amplification of full-length coding sequences of PePREs in Phyllostachys edulis.
Table A1.
The primers used for quantitative PCR (RT-qPCR) and amplification of full-length coding sequences of PePREs in Phyllostachys edulis.
| Primers | Sequences (5′-3′) | Note |
|---|---|---|
| PePRE1 qPCR F1 | ATTATGTCCATGGATCTCTG | Gene expression level detection of PePRE1 |
| PePRE1 qPCR R1 | ACTTAGTCCTTAGGTATCGA | Gene expression level detection of PePRE1 |
| PePRE2 qPCR F1 | CCCGCAGGCAGACATCAT | Gene expression level detection of PePRE2 |
| PePRE2 qPCR R1 | GTGCTTCGAACTGAGTAAAT | Gene expression level detection of PePRE2 |
| PePRE3 qPCR F1 | CAAGACCTCACCCAGCTTAG | Gene expression level detection of PePRE3 |
| PePRE3 qPCR R1 | TGCCGCGCGACGACCTTC | Gene expression level detection of PePRE3 |
| PePRE4 qPCR F1 | TGAGTTTAAGCTCACACTACTC | Gene expression level detection of PePRE4 |
| PePRE4 qPCR R1 | AGAGGTCGCGATCTCCTAGC | Gene expression level detection of PePRE4 |
| qPeTIP41-F1 | AAAATCATTGTAGGCCATTGTCG | Internal control gene in Ph. edulis |
| qPeTIP41-R1 | ACTAAATTAAGCCAGCGGGAGTG | Internal control gene in Ph. edulis |
| AtPRE1 qPCR F | GTTCTGATAAGGCATCAGCCTCG | Gene expression level detection of AtPRE1 |
| AtPRE1 qPCR R | CATGAGTAGGCTTCTAATAACGG | Gene expression level detection of AtPRE1 |
| UBQ10-F | ATCACCCTTGAAGTGGA | Internal control gene in Arabidopsis |
| UBQ10-F | ATCACCCTTGAAGTGGA | Internal control gene in Arabidopsis |
| PePRE1 MoClo F | tttgaagacaaAATGTCGAGCCGGAGGTCGCG | For amplifying CDS of PePRE1 |
| PePRE1 MoClo R | tttgaagacaacgaaccGCGGAGGAGGCTGCGGATGA | For amplifying CDS of PePRE1 |
| PePRE2 MoClo F1 | tttgaagacaaAATGTCGAGGCGGCGGGGG | For amplifying CDS of PePRE2 |
| PePRE2 MoClo R | tttgaagacaacgaaccGCTAGGAGGACCGGAGCTGA | For amplifying CDS of PePRE2 |
| PePRE3 MoClo F | tttgaagacaaAATGTCGGGCCGAAGGTCGTC | For amplifying CDS of PePRE3 |
| PePRE3 MoClo R | tttgaagacaacgaaccGGAGCGGAGGATGTTGCGGA | For amplifying CDS of PePRE3 |
| PePRE4 MoClo F | tttgaagacaaAATGTCGGGGCGCAGAGCCGG | For amplifying CDS of PePRE4 |
| PePRE4 MoClo R | tttgaagacaacgaaccGGATGAGCGGAGGAGACTGC | For amplifying CDS of PePRE4 |
| PePRE4 MoClo F(-R)1 | tttgaagacaaGTCaTCGGCGTCGAACCTGCTG | For amplifying CDS of PePRE4 |
| PePRE4 MoClo (F-)R | tttgaagacaatGACTGCAACAAAAACATTA | For amplifying CDS of PePRE4 |
| level 0 F | CGTTATCCCCTGATTCTGTGGATAAC | MoClo cloning general primer |
| level 0 R | GTCTCATGAGCGGATACATATTTGAATG | MoClo cloning general primer |
| level 1 F | GAACCCTGTGGTTGGCATGCACATAC | MoClo cloning general primer |
| level 1 R | CTGGTGGCAGGATATATTGTGGTG | MoClo cloning general primer |
| level 2 F | GTGGTGTAAACAAATTGACGC | MoClo cloning general primer |
| level 2 R | GGATAAACCTTTTCACGCCC | MoClo cloning general primer |
Appendix B
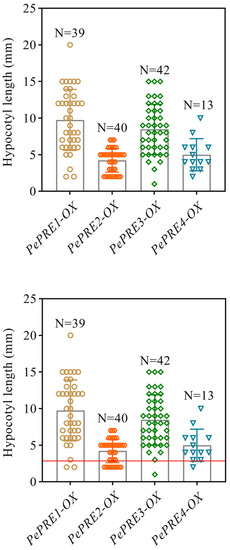
Figure A1.
The phenotypes of T1 transgenic plants overexpressing PePREs in Col-0 background. Overexpression PePRE-GFP caused longer hypocotyls in Col-0. Hypocotyl measurement of light-grown PePRE-GFP transgenic plants compared with Col-0 (the red line indicates the average hypocotyl length of Col-0).
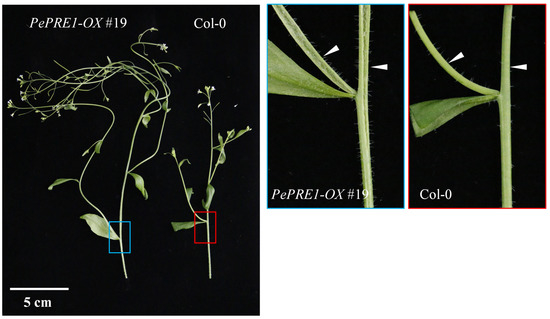
Figure A2.
The morphology of T1 transgenic plants overexpressing PePREs (35S::PePRE1-GFP #19 phenotype) in Arabidopsis. The plants were grown in a growth room for 4 weeks.
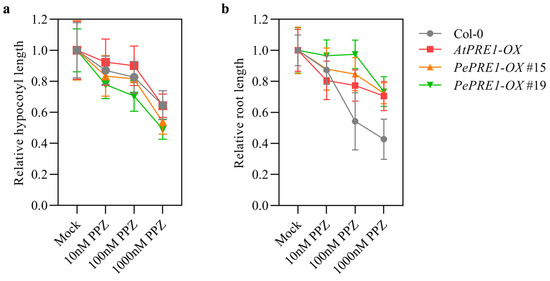
Figure A3.
The PePRE1-OX transgenic seedlings were treated with PPZ. The relative hypocotyl length (a) and relative root length (b) of PePRE1-OX and control plants were treated with mock solution and a gradient concentration of PPZ. Seedlings were grown on medium containing different concentrations of PPZ for 7 days under constant light. The relative hypocotyl/root length was the average of at least 20 seedlings and normalized to the untreated seedlings. Error bars represent standard deviations.
References
- Kelchner, S.A.; Bamboo Phylogeny, G. Higher level phylogenetic relationships within the bamboos (Poaceae: Bambusoideae) based on five plastid markers. Mol. Phylogenet. Evol. 2013, 67, 404–413. [Google Scholar] [CrossRef] [PubMed]
- Wysocki, W.P.; Clark, L.G.; Attigala, L.; Ruiz-Sanchez, E.; Duvall, M.R. Evolution of the bamboos (Bambusoideae; Poaceae): A full plastome phylogenomic analysis. BMC Evol. Biol. 2015, 15, 50. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Wang, H.; Zhang, Y.; Wang, H.; Zhang, Z.; Liu, X.; Zhang, Z.; Liu, K.; Yang, D.; Zhang, H.; et al. Comprehensive profiling of epigenetic modifications in fast-growing Moso bamboo shoots. Plant Physiol. 2023, 191, 1017–1035. [Google Scholar] [CrossRef] [PubMed]
- Gamuyao, R.; Nagai, K.; Ayano, M.; Mori, Y.; Minami, A.; Kojima, M.; Suzuki, T.; Sakakibara, H.; Higashiyama, T.; Ashikari, M.; et al. Hormone distribution and transcriptome profiles in bamboo shoots provide insights on bamboo stem emergence and growth. Plant Cell Physiol. 2017, 58, 702–716. [Google Scholar] [CrossRef] [PubMed]
- Chaiwanon, J.; Wang, W.; Zhu, J.Y.; Oh, E.; Wang, Z.Y. Information integration and communication in plant growth regulation. Cell 2016, 164, 1257–1268. [Google Scholar] [CrossRef]
- Oh, E.; Zhu, J.Y.; Bai, M.Y.; Arenhart, R.A.; Sun, Y.; Wang, Z.Y. Cell elongation is regulated through a central circuit of interacting transcription factors in the Arabidopsis hypocotyl. Elife 2014, 3, e03031. [Google Scholar] [CrossRef]
- Zhiponova, M.K.; Morohashi, K.; Vanhoutte, I.; Machemer-Noonan, K.; Revalska, M.; Van Montagu, M.; Grotewold, E.; Russinova, E. Helix-loop-helix/basic helix-loop-helix transcription factor network represses cell elongation in Arabidopsis through an apparent incoherent feed-forward loop. Proc. Natl. Acad. Sci. USA 2014, 111, 2824–2829. [Google Scholar] [CrossRef]
- Ruzinova, M.B.; Benezra, R. Id proteins in development, cell cycle and cancer. Trends Cell Biol. 2003, 13, 410–418. [Google Scholar] [CrossRef]
- Castelain, M.; Le Hir, R.; Bellini, C. The non-DNA-binding bHLH transcription factor PRE3/bHLH135/ATBS1/TMO7 is involved in the regulation of light signaling pathway in Arabidopsis. Physiol. Plant 2012, 145, 450–460. [Google Scholar] [CrossRef]
- Bai, M.Y.; Fan, M.; Oh, E.; Wang, Z.Y. A triple Helix-Loop-Helix/basic Helix-Loop-Helix cascade controls cell elongation downstream of multiple hormonal and environmental signaling pathways in Arabidopsis. Plant Cell 2012, 24, 4917–4929. [Google Scholar] [CrossRef]
- Malinovsky, F.G.; Batoux, M.; Schwessinger, B.; Youn, J.H.; Stransfeld, L.; Win, J.; Kim, S.K.; Zipfel, C. Antagonistic regulation of growth and immunity by the Arabidopsis basic helix-loop-helix transcription factor homolog of brassinosteroid enhanced expression2 interacting with increased leaf inclination1 binding bHLH1. Plant Physiol. 2014, 164, 1443–1455. [Google Scholar] [CrossRef]
- Zhang, L.Y.; Bai, M.Y.; Wu, J.; Zhu, J.Y.; Wang, H.; Zhang, Z.; Wang, W.; Sun, Y.; Zhao, J.; Sun, X.; et al. Antagonistic HLH/bHLH transcription factors mediate brassinosteroid regulation of cell elongation and plant development in rice and Arabidopsis. Plant Cell 2009, 21, 3767–3780. [Google Scholar] [CrossRef]
- Choi, H.; Oh, E. PIF4 integrates multiple environmental and hormonal signals for plant growth regulation in Arabidopsis. Mol. Cells 2016, 39, 587–593. [Google Scholar] [CrossRef]
- Oh, E.; Zhu, J.Y.; Wang, Z.Y. Interaction between BZR1 and PIF4 integrates brassinosteroid and environmental responses. Nat. Cell Biol. 2012, 14, 802–809. [Google Scholar] [CrossRef]
- Hao, Y.; Oh, E.; Choi, G.; Liang, Z.; Wang, Z.Y. Interactions between HLH and bHLH factors modulate light-regulated plant development. Mol. Plant 2012, 5, 688–697. [Google Scholar] [CrossRef]
- Hyun, Y.; Lee, I. KIDARI, encoding a non-DNA Binding bHLH protein, represses light signal transduction in Arabidopsis thaliana. Plant Mo.l Biol. 2006, 61, 283–296. [Google Scholar] [CrossRef]
- Buti, S.; Pantazopoulou, C.K.; van Gelderen, K.; Hoogers, V.; Reinen, E.; Pierik, R. A Gas-and-Brake mechanism of bHLH proteins modulates shade avoidance. Plant Physiol. 2020, 184, 2137–2153. [Google Scholar] [CrossRef]
- Ikeda, M.; Mitsuda, N.; Ishizuka, T.; Satoh, M.; Ohme-Takagi, M. The CIB1 transcription factor regulates light- and heat-inducible cell elongation via a two-step HLH/bHLH system. J. Exp. Bot. 2021, 72, 1795–1808. [Google Scholar] [CrossRef]
- Chapman, E.J.; Greenham, K.; Castillejo, C.; Sartor, R.; Bialy, A.; Sun, T.P.; Estelle, M. Hypocotyl transcriptome reveals auxin regulation of growth-promoting genes through GA-dependent and -independent pathways. PLoS ONE 2012, 7, e36210. [Google Scholar] [CrossRef]
- Reed, J.W.; Wu, M.F.; Reeves, P.H.; Hodgens, C.; Yadav, V.; Hayes, S.; Pierik, R. Three auxin response factors promote hypocotyl elongation. Plant Physiol. 2018, 178, 864–875. [Google Scholar] [CrossRef]
- Lee, S.; Lee, S.; Yang, K.Y.; Kim, Y.M.; Park, S.Y.; Kim, S.Y.; Soh, M.S. Overexpression of PRE1 and its homologous genes activates Gibberellin-dependent responses in Arabidopsis thaliana. Plant Cell Physiol. 2006, 47, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Lu, Q.; Wang, J.; Wang, L.; Xiang, F.; Liu, Z. MiR160 and its target genes ARF10, ARF16 and ARF17 modulate hypocotyl elongation in a light, BRZ, or PAC-dependent manner in Arabidopsis: miR160 promotes hypocotyl elongation. Plant Sci. 2021, 303, 110686. [Google Scholar] [CrossRef] [PubMed]
- Medina-Puche, L.; Martinez-Rivas, F.J.; Molina-Hidalgo, F.J.; Garcia-Gago, J.A.; Mercado, J.A.; Caballero, J.L.; Munoz-Blanco, J.; Blanco-Portales, R. Ectopic expression of the atypical HLH FaPRE1 gene determines changes in cell size and morphology. Plant Sci. 2021, 305, 110830. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Cao, J.F.; Hu, G.J.; Chen, Z.W.; Wang, L.Y.; Shangguan, X.X.; Wang, L.J.; Mao, Y.B.; Zhang, T.Z.; Wendel, J.F.; et al. Core cis-element variation confers subgenome-biased expression of a transcription factor that functions in cotton fiber elongation. New Phytol. 2018, 218, 1061–1075. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, X.; Cheng, L.; Guo, Z.; Wang, H.; Wu, W.; Shin, K.; Zhu, J.; Zheng, X.; Bian, J.; et al. Physiological and transcriptomic analyses of brassinosteroid function in moso bamboo (Phyllostachys edulis) seedlings. Planta 2020, 252, 27. [Google Scholar] [CrossRef]
- Zhao, H.; Peng, Z.; Fei, B.; Li, L.; Hu, T.; Gao, Z.; Jiang, Z. BambooGDB: A bamboo genome database with functional annotation and an analysis platform. Database 2014, 2014, bau006. [Google Scholar] [CrossRef]
- Mara, C.D.; Huang, T.; Irish, V.F. The Arabidopsis floral homeotic proteins APETALA3 and PISTILLATA negatively regulate the BANQUO genes implicated in light signaling. Plant Cell 2010, 22, 690–702. [Google Scholar] [CrossRef]
- Klausner, R.D.; Donaldson, J.G.; Lippincott-Schwartz, J. Brefeldin A: Insights into the control of membrane traffic and organelle structure. J. Cell Biol. 1992, 116, 1071–1080. [Google Scholar] [CrossRef]
- Heang, D.; Sassa, H. Antagonistic actions of HLH/bHLH proteins are involved in grain length and weight in rice. PLoS ONE 2012, 7, e31325. [Google Scholar] [CrossRef]
- Tanaka, A.; Nakagawa, H.; Tomita, C.; Shimatani, Z.; Ohtake, M.; Nomura, T.; Jiang, C.J.; Dubouzet, J.G.; Kikuchi, S.; Sekimoto, H.; et al. BRASSINOSTEROID UPREGULATED1, encoding a Helix-Loop-Helix protein, is a novel gene involved in brassinosteroid signaling and controls bending of the lamina joint in rice. Plant Physiol. 2009, 151, 669–680. [Google Scholar] [CrossRef]
- Jang, S.; Cho, J.Y.; Do, G.R.; Kang, Y.; Li, H.Y.; Song, J.; Kim, H.Y.; Kim, B.G.; Hsing, Y.I. Modulation of rice leaf angle and grain size by expressing OsBCL1 and OsBCL2 under the control of OsBUL1 promoter. Int. J. Mol. Sci. 2021, 22, 7792. [Google Scholar] [CrossRef]
- Jang, S.; An, G.; Li, H.Y. Rice leaf angle and grain size are affected by the OsBUL1 transcriptional activator complex. Plant Physiol. 2017, 173, 688–702. [Google Scholar] [CrossRef]
- Medina-Puche, L.; Martinez-Rivas, F.J.; Molina-Hidalgo, F.J.; Mercado, J.A.; Moyano, E.; Rodriguez-Franco, A.; Caballero, J.L.; Munoz-Blanco, J.; Blanco-Portales, R. An atypical HLH transcriptional regulator plays a novel and important role in strawberry ripened receptacle. BMC Plant Biol. 2019, 19, 586. [Google Scholar] [CrossRef]
- McKim, S.M. Moving on up—Controlling internode growth. New Phytol. 2020, 226, 672–678. [Google Scholar] [CrossRef]
- Wang, K.L.; Zhang, Y.; Zhang, H.M.; Lin, X.C.; Xia, R.; Song, L.; Wu, A.M. MicroRNAs play important roles in regulating the rapid growth of the Phyllostachys edulis culm internode. New Phytol. 2021, 231, 2215–2230. [Google Scholar] [CrossRef]
- Li, J.; Liu, Z.; Gao, C.; Miao, Y.; Cui, K. Overexpression of DsEXLA2 gene from Dendrocalamus sinicus accelerates the plant growth rate of Arabidopsis. Phytochemistry 2022, 199, 113178. [Google Scholar] [CrossRef]
- McKim, S.M. How plants grow up. J. Integr. Plant Biol. 2019, 61, 257–277. [Google Scholar] [CrossRef]
- Yeh, S.H.; Lee, B.H.; Liao, S.C.; Tsai, M.H.; Tseng, Y.H.; Chang, H.C.; Yang, C.C.; Jan, H.C.; Chiu, Y.C.; Wang, A.Y. Identification of genes differentially expressed during the growth of Bambusa oldhamii. Plant Physiol. Biochem. 2013, 63, 217–226. [Google Scholar] [CrossRef]
- Nagai, K.; Mori, Y.; Ishikawa, S.; Furuta, T.; Gamuyao, R.; Niimi, Y.; Hobo, T.; Fukuda, M.; Kojima, M.; Takebayashi, Y.; et al. Antagonistic regulation of the gibberellic acid response during stem growth in rice. Nature 2020, 584, 109–114. [Google Scholar] [CrossRef]
- Lee, J.; Moon, S.; Jang, S.; Lee, S.; An, G.; Jung, K.-H.; Park, S.K. OsbHLH073 negatively regulates internode elongation and plant height by modulating ga homeostasis in rice. Plants 2020, 9, 547. [Google Scholar] [CrossRef]
- Wang, Y.; Qiao, G.; Xu, J.; Jin, K.; Fan, M.; Ding, Y.; Wei, Q.; Zhuo, R. Anatomical characteristics and variation mechanisms on the thick-walled and dwarfed culm of shidu bamboo (Phyllostachys nidularia f. farcta). Front. Plant Sci. 2022, 13, 876658. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Guo, L.; Ramakrishnan, M.; Fei, Z.; Vinod, K.K.; Ding, Y.; Jiao, C.; Gao, Z.; Zha, R.; Wang, C.; et al. Rapid growth of Moso bamboo (Phyllostachys edulis): Cellular roadmaps, transcriptome dynamics, and environmental factors. Plant Cell 2022, 34, 3577–3610. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, N.R. A tripartite growth regulatory cascade of basic Helix-Loop-Helix transcription factors. Plant Cell 2012, 24, 4774. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, M.; Fujiwara, S.; Mitsuda, N.; Ohme-Takagi, M. A triantagonistic basic helix-loop-helix system regulates cell elongation in Arabidopsis. Plant Cell 2012, 24, 4483–4497. [Google Scholar] [CrossRef] [PubMed]
- Shin, K.; Lee, I.; Kim, E.; Park, S.K.; Soh, M.S.; Lee, S. PACLOBUTRAZOL-RESISTANCE gene family regulates floral organ growth with unequal genetic redundancy in Arabidopsis thaliana. Int. J. Mol. Sci. 2019, 20, 869. [Google Scholar] [CrossRef]
- Zhu, Z.; Liang, H.; Chen, G.; Li, F.; Wang, Y.; Liao, C.; Hu, Z. The bHLH transcription factor SlPRE2 regulates tomato fruit development and modulates plant response to gibberellin. Plant Cell Rep. 2019, 38, 1053–1064. [Google Scholar] [CrossRef]
- Casal, J.J.; Fankhauser, C. Shade avoidance in the context of climate change. Plant Physiol. 2023, 191, 1475–1491. [Google Scholar] [CrossRef]
- Galstyan, A.; Cifuentes-Esquivel, N.; Bou-Torrent, J.; Martinez-Garcia, J.F. The shade avoidance syndrome in Arabidopsis: A fundamental role for atypical basic helix-loop-helix proteins as transcriptional cofactors. Plant J. 2011, 66, 258–267. [Google Scholar] [CrossRef]
- Huang, W.; Ding, Y.; Wang, S.; Song, C.; Wang, F. Growth and development responses of the Rhizome-Root system in Pleioblastus pygmaeus to light intensity. Plants 2022, 11, 2204. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Fan, C.; Ma, J.; Guo, Q.; Li, X.; Wang, H.; Lu, M. Selection of reference genes for quantitative real-time PCR in bamboo (Phyllostachys edulis). PLoS ONE 2013, 8, e56573. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).