Characterization of the Difference between Day and Night Temperatures on the Growth, Photosynthesis, and Metabolite Accumulation of Tea Seedlings
Abstract
1. Introduction
2. Results
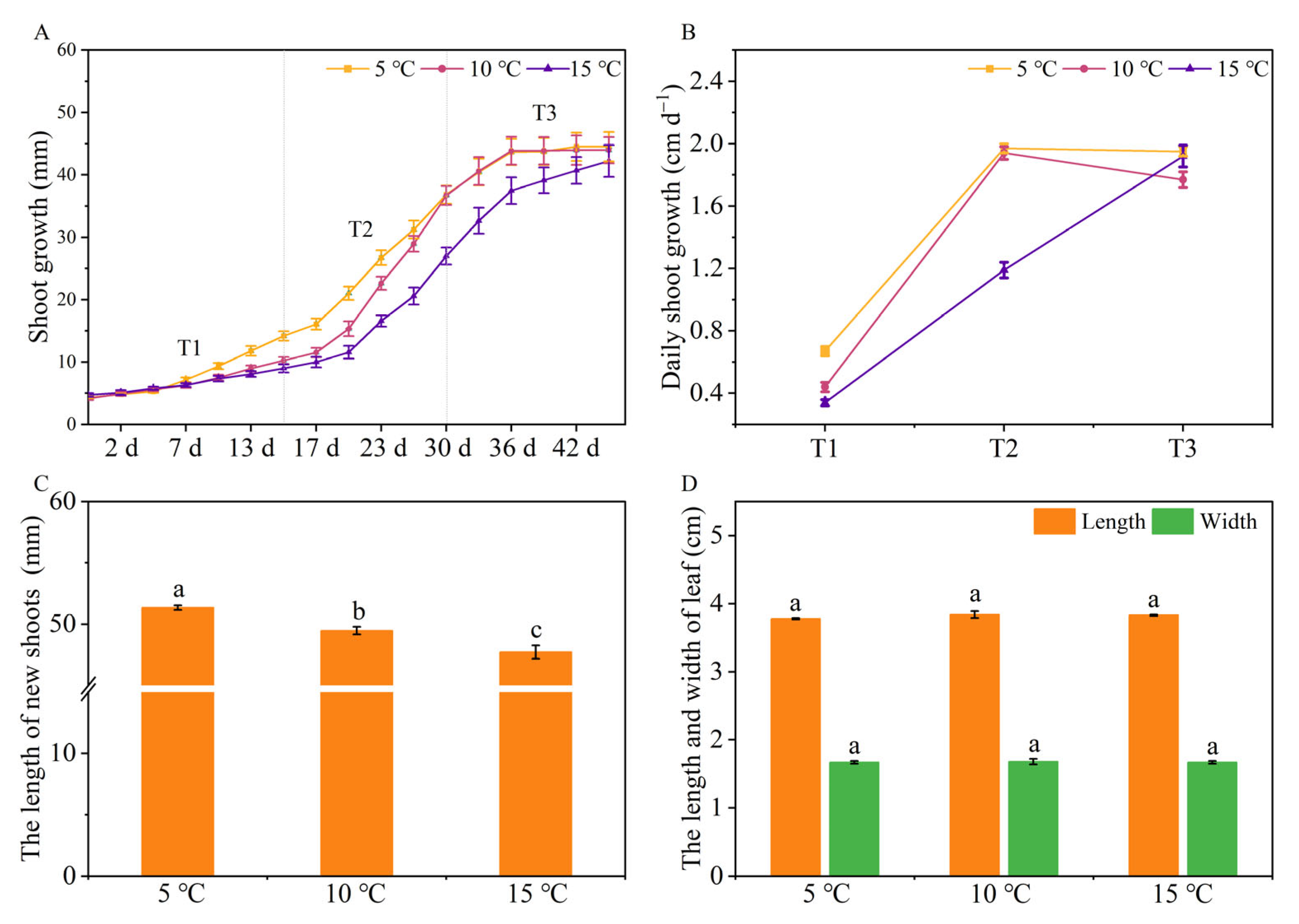
2.1. The Effects of Different DIFs on Tea Plant Growth
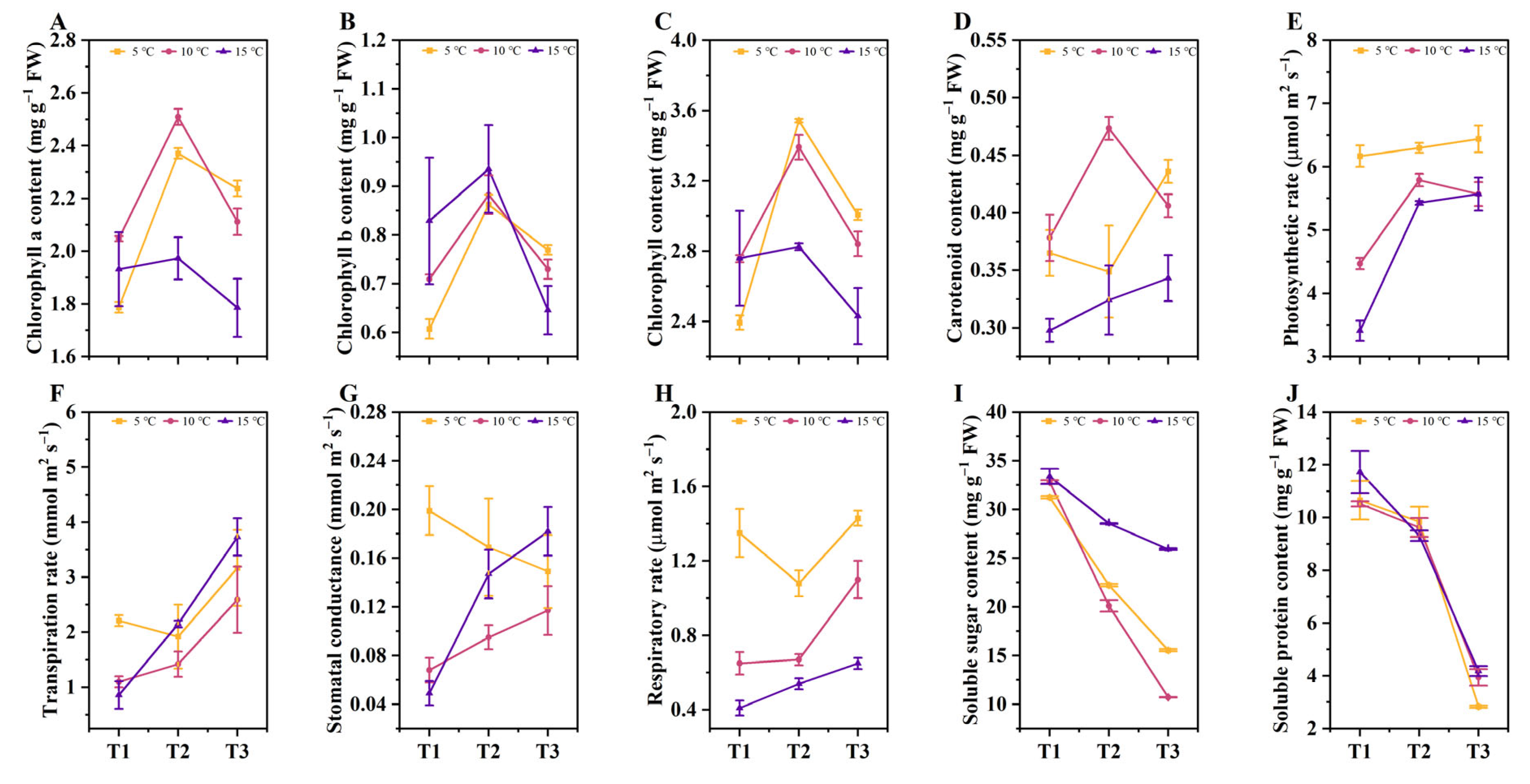
2.2. The Effects of Different DIFs on the Photosynthetic Indicators, Soluble Sugars, and Protein Contents
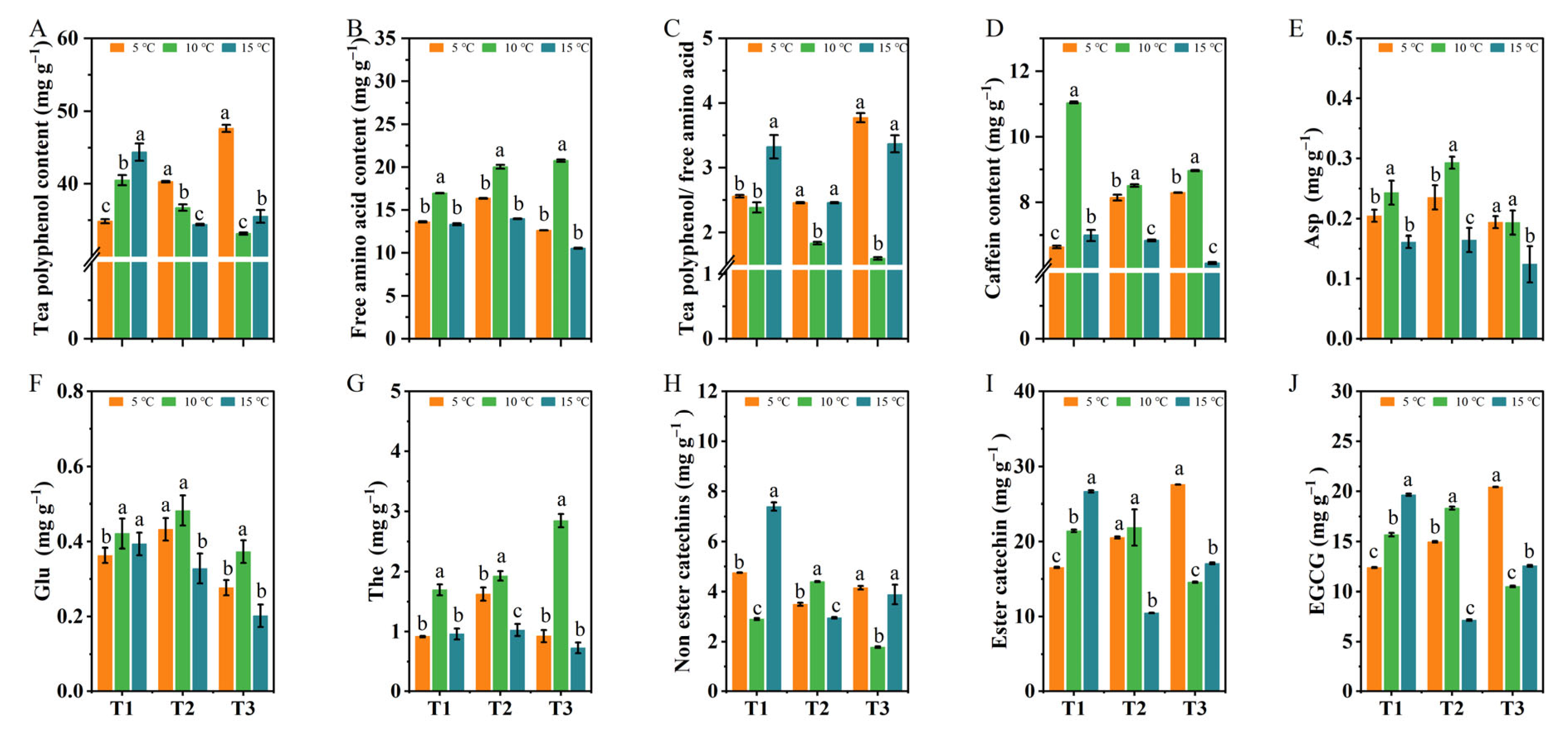
2.3. The Effects of Different DIFs on the Contents of Tea Polyphenols, Caffeine, Free Amino Acids, and Catechin Components
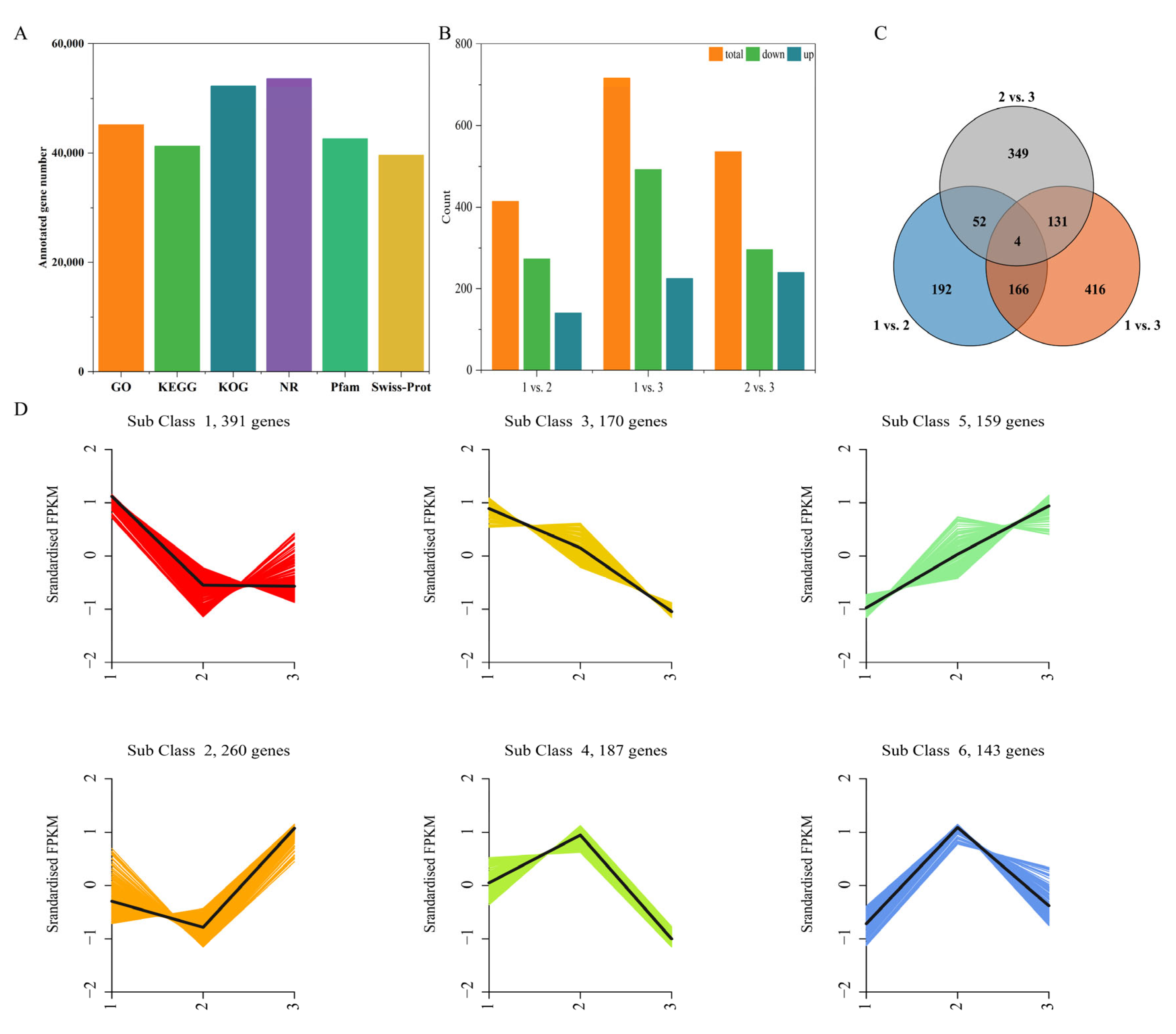
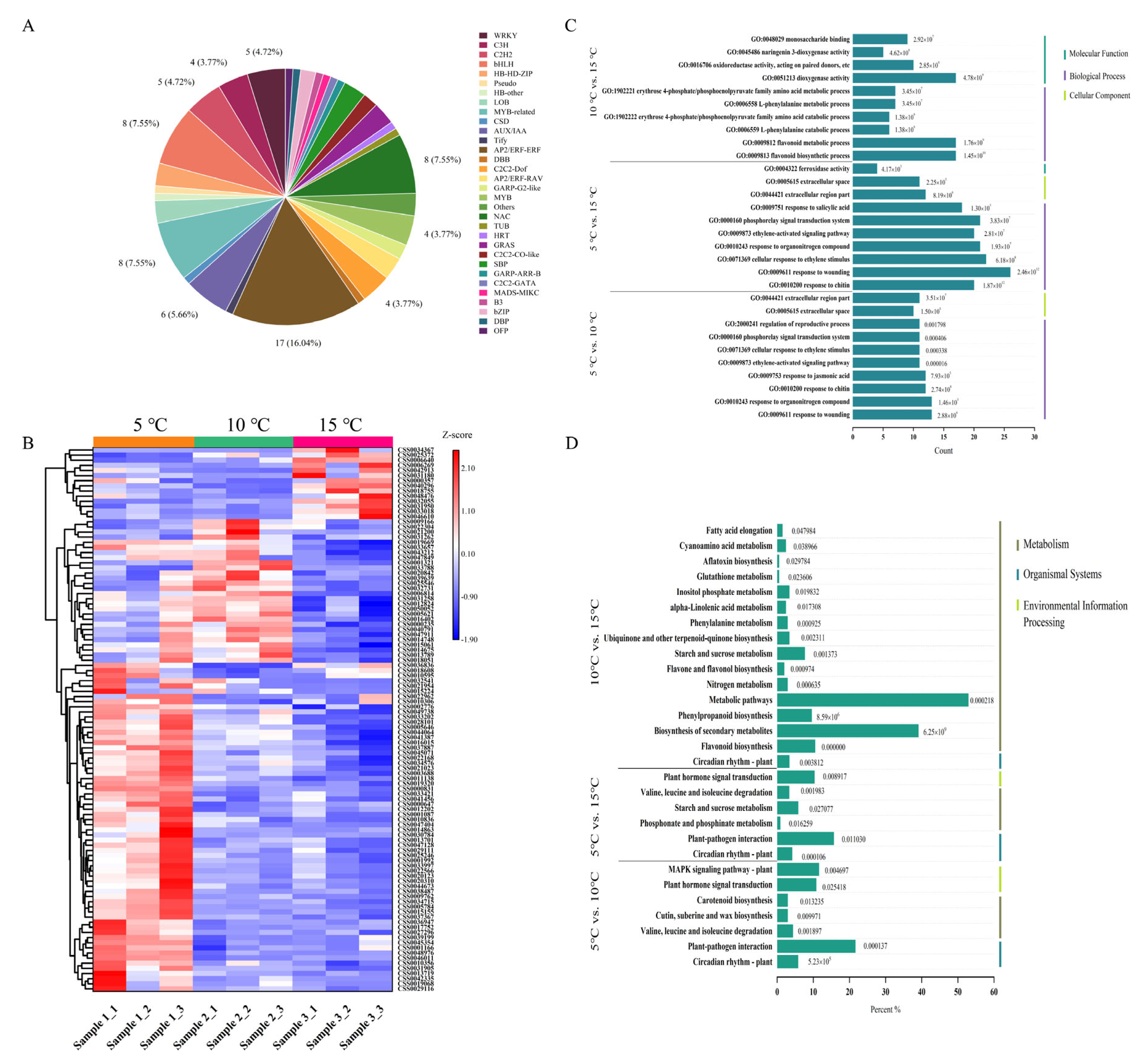
2.4. Quality Control Analysis of the Transcriptome Data and the Identification of DEGs
2.5. Analysis of the Differentially Expressed Transcription Factors (TFs) and the Function of the DEGs
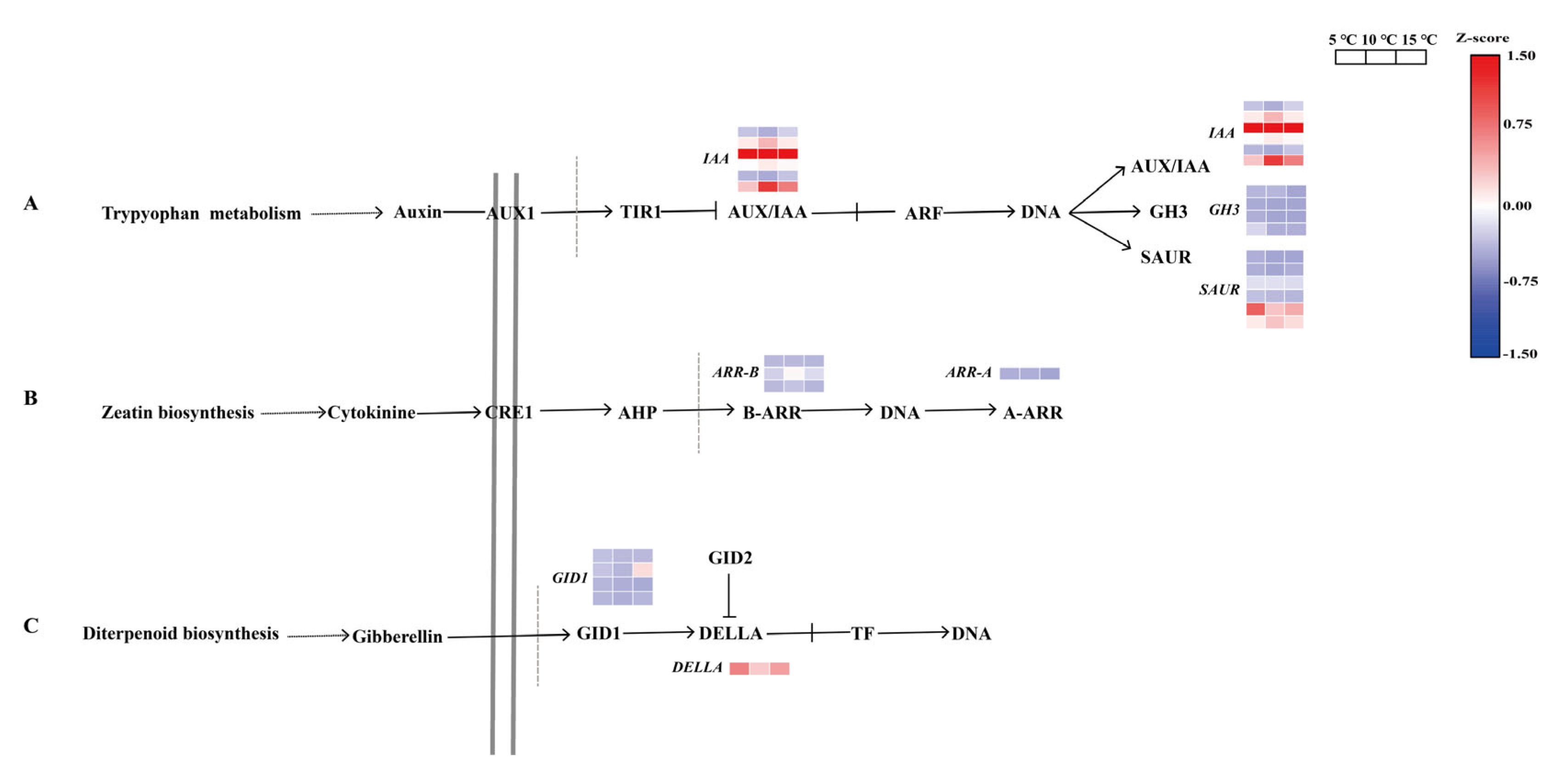
2.6. Regulation of the DEGs in the Plant Hormone Signal Transduction
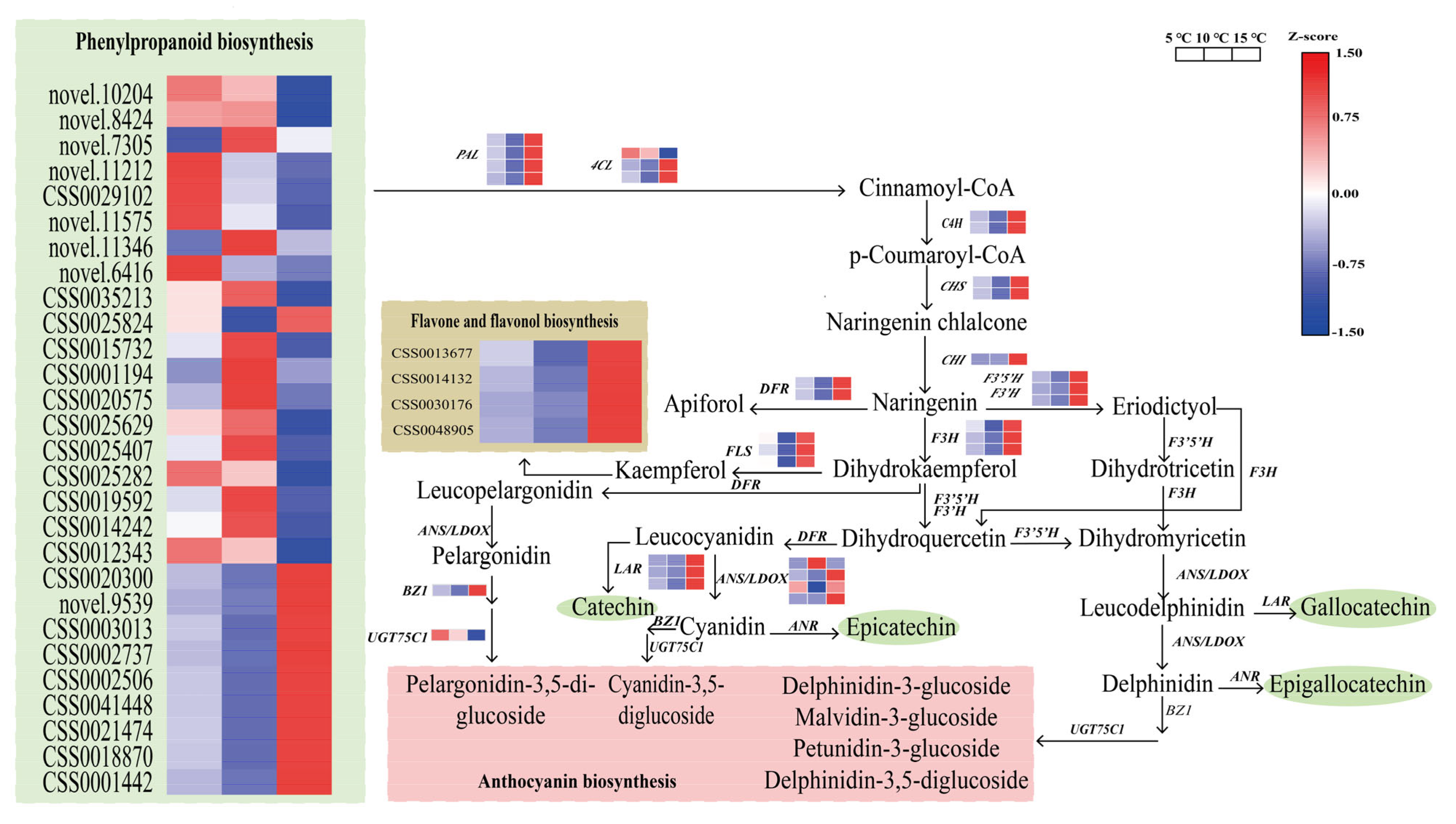
2.7. Regulation of the DEGs in the Catechin Biosynthesis Pathway
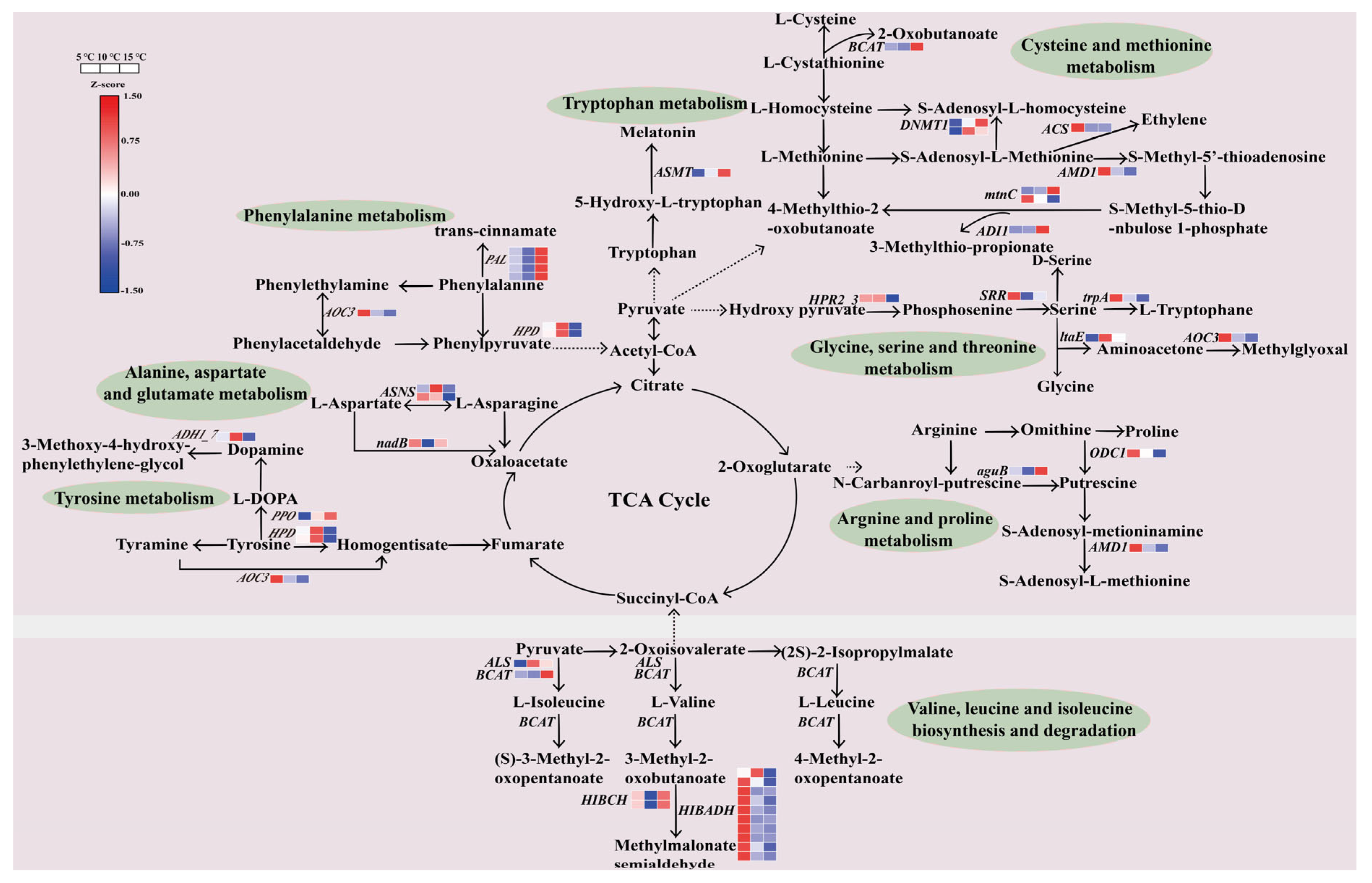
2.8. Regulation of the DEGs in the Amino Acid Metabolic Pathway
2.9. Regulation of the DEGs in the Caffeine Metabolic Pathway
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Treatments
4.2. Measurement of Phenotypic Characteristic of Tea Seedlings
4.3. Analysis of Physiological Indicators
4.3.1. Determination of Photosynthetic Parameters and Dark Respiration Rate of Mature Leaves
4.3.2. Determination of Photosynthetic Pigment, Soluble Sugar, and Soluble Protein Contents
4.4. Analysis of Biochemical Indicators
4.4.1. Determination of Free Amino Acid and Component Contents
4.4.2. Determination of Tea Polyphenol, Catechin, and Caffeine Contents
4.5. Analysis of Gene Expressions Related to Theanine Metabolism
4.6. RNA-Sequencing
4.7. Genome Alignment
4.8. Differential Expression Analysis and Functional Enrichment Analysis
4.9. The qRT-PCR Validation
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, X.C.; Feng, H.; Chang, Y.X.; Ma, C.L.; Wang, L.Y.; Hao, X.Y.; Li, A.L.; Cheng, H.; Wang, L.; Cui, P.; et al. Population sequencing enhances understanding of tea plant evolution. Nat. Commun. 2020, 11, 4447. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.Y.; Nie, Q.; Tai, H.C.; Song, X.L.; Tong, Y.F.; Zhuang, L.J.F.; Wu, X.W.; Lin, Z.H.; Zhang, Y.Y.; Ye, D.Y.; et al. Tea and tea drinking: China’s outstanding contributions to the mankind. Chin. Med. 2022, 17, 27. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Cao, Q.Q.; Granato, D.; Xu, Y.Q.; Ho, C.T. Association between chemistry and taste of tea: A review. Trends Food Sci. Technol. 2020, 101, 139–149. [Google Scholar] [CrossRef]
- Zhu, M.Z.; Li, N.; Zhou, F.; Ouyang, J.; Lu, D.M.; Xu, W.; Li, J.; Lin, H.Y.; Zhang, Z.; Xiao, J.; et al. Microbial bioconversion of the chemical components in dark tea. Food Chem. 2020, 312, 126043. [Google Scholar] [CrossRef]
- Zhu, M.Z.; Zhou, F.; Ouyang, J.; Wang, Q.Y.; Li, Y.L.; Wu, J.L.; Huang, J.A.; Liu, Z.H. Combined use of epigallocatechin-3-gallate (EGCG) and caffeine in low doses exhibits marked anti-obesity synergy through regulation of gut microbiota and bile acid metabolism. Food Funct. 2021, 12, 4105–4116. [Google Scholar] [CrossRef]
- Xiang, P.; Zhu, Q.F.; Tukhvatshin, M.; Cheng, B.S.; Tan, M.; Liu, J.H.; Wang, X.J.; Huang, J.X.; Gao, S.L.; Lin, D.Y.; et al. Light control of catechin accumulation is mediated by photosynthetic capacity in tea plant (Camellia sinensis). BMC Plant Biol. 2021, 21, 478. [Google Scholar] [CrossRef]
- Wang, M.; Yang, J.; Li, J.L.; Zhou, X.C.; Xiao, Y.Y.; Liao, Y.Y.; Tang, J.C.; Dong, F.; Zeng, L.T. Effects of temperature and light on quality-related metabolites in tea (Camellia sinensis (L.) Kuntze) leaves. Food Res. Int. 2022, 161, 111882. [Google Scholar] [CrossRef]
- Rigden, A.J.; Ongoma, V.; Huybers, P. Kenyan tea is made with heat and water: How will climate change influence its yield? Environ. Res. Lett. 2020, 15, 4. [Google Scholar] [CrossRef]
- Yan, P.; Wu, L.Q.; Wang, D.H.; Fu, J.Y.; Shen, C.; Li, X.; Zhang, L.P.; Zhang, L.; Fan, L.C.; Han, W.Y. Soil acidification in Chinese tea plantations. Sci. Total Environ. 2020, 715, 136963. [Google Scholar] [CrossRef]
- Jiang, C.J.; Li, Y.Y.; Ding, Z.T.; Fang, W.P.; Guo, Y.Q.; Huang, Y.H.; Jiang, J.Y.; Li, D.H.; Liu, S.Q.; Lu, J.L.; et al. Tea Breeding. Tea Breeding, 3rd ed.; Chinese Agricultural Press: Beijing, China, 2021; pp. 89–90. [Google Scholar]
- Sánchez, M.; Velásquez, Y.; González, M.; Cuevás, J. Activity and foraging behaviour of the hoverfly Eristalinus aeneus (Scopoli, 1763) in protected cultivation of mango (Mangifera indica L.). Bull. Entomol. Res. 2022, 112, 101–109. [Google Scholar] [CrossRef]
- Pachiyappan, P.; Kumar, P.; Reddy, K.V.; Kumar, K.N.R.; Konduru, S.; Paramesh, V.; Rajanna, G.A.; Shankarappa, S.K.; Jaganathan, D.; Immanuel, S.; et al. Protected cultivation of horticultural crops as a livelihood opportunity in western India: An economic assessment. Sustainability 2022, 14, 7430. [Google Scholar] [CrossRef]
- Sánchez, M.; Velásquez, Y.; González, M.; Cuevas, J. Hoverfly pollination enhances yield and fruit quality in mango under protected cultivation. Sci. Hortic. 2022, 304, 111320. [Google Scholar] [CrossRef]
- Jayasinghe, S.L.; Kumar, L. Climate change may imperil tea production in the four major tea producers according to climate prediction models. Agronomy 2020, 10, 1536. [Google Scholar] [CrossRef]
- Yan, Y.L.; Jeong, S.; Park, C.E.; Mueller, N.D.; Piao, S.L.; Park, H.; Joo, J.; Chen, X.; Wang, X.H.; Liu, J.G.; et al. Effects of extreme temperature on China’s tea production. Environ. Res. Lett. 2021, 16, 044040. [Google Scholar] [CrossRef]
- Duncan, J.M.A.; Saikia, S.D.; Gupta, N.; Biggs, E.M. Observing climate impacts on tea yield in Assam, India. Appl. Geogr. 2016, 77, 64–71. [Google Scholar] [CrossRef]
- Wijeratne, M.A.; Anandacoomaraswamy, A.; Amarathunga, S.; Ratnasiri, J.; Basnayake, B.R.S.; Kalra, N. Assessment of impact of climate change on productivity of tea (Camellia sinensis L.) plantations in Sri Lanka. J. Natl. Sci. Found. Sri Lanka 2011, 35, 119–126. [Google Scholar] [CrossRef]
- Huang, J.; Wang, Y.; Yu, J.; Li, F.; Yi, L.; Li, Y.; Xie, N.; Wu, Q.; Samarina, L.; Tong, W.; et al. Evolutionary landscape of tea circular RNAs and its contribution to chilling tolerance of tea plant. Int. J. Mol. Sci. 2023, 24, 1478. [Google Scholar] [CrossRef]
- Yang, Y.Z.; Li, T.; Teng, R.M.; Han, M.H.; Zhuang, J. Low temperature effects on carotenoids biosynthesis in the leaves of green and albino tea plant (Camellia sinensis (L.) O. Kuntze). Sci. Hortic. 2021, 285, 110164. [Google Scholar] [CrossRef]
- Liu, K.H.; Li, S.Y.; Han, J.D.; Zeng, X.Y.; Ling, M.L.; Mao, J.L.; Li, Y.Y.; Jiang, J.Y. Effect of selenium on tea (Camellia sinensis) under low temperature: Changes in physiological and biochemical responses and quality. Environ. Exp. Bot. 2021, 188, 104475. [Google Scholar] [CrossRef]
- Lu, J.Z.; Guan, P.X.; Gu, J.M.; Yang, X.L.; Wang, F.; Qi, M.F.; Li, T.L.; Liu, Y.F. Exogenous DA-6 improves the low night temperature tolerance of tomato through regulating cytokinin. Front. Plant Sci. 2021, 11, 599111. [Google Scholar] [CrossRef]
- Hao, X.Y.; Wang, B.; Wang, L.; Zeng, J.M.; Yang, Y.J.; Wang, X.C. Comprehensive transcriptome analysis reveals common and specific genes and pathways involved in cold acclimation and cold stress in tea plant leaves. Sci. Hortic. 2018, 240, 354–368. [Google Scholar] [CrossRef]
- Jing, P.A.; Li, N.N.; Di, T.M.; Ding, C.Q.; Li, X.M.; Wu, Y.D.; Hao, X.Y.; Wang, Y.C.; Yang, Y.J.; Wang, X.C. The interaction of CsWRKY4 and CsOCP3 with CsICE1 regulates CsCBF1/3 and mediates stress response in tea plant (Camellia sinensis). Environ. Exp. Bot. 2022, 199, 104892. [Google Scholar] [CrossRef]
- Opole, R.A.; Prasad, P.V.V.; Djanaguiraman, M.; Vimala, K.; Kirkham, M.B.; Upadhyaya, H.D. Thresholds, sensitive stages and genetic variability of finger millet to high temperature stress. J. Agron. Crop Sci. 2018, 204, 477–492. [Google Scholar] [CrossRef]
- Lizaso, J.I.; Ruiz-Ramos, M.L.; Rodríguez, L.; Gabaldon-Leal, C.; Oliveira, J.A.; Lorite, I.J.; Sanchez, D.; Garcia, E.; Rodriguez, A. Impact of high temperatures in maize: Phenology and yield components. Field Crop Res. 2018, 216, 129–140. [Google Scholar] [CrossRef]
- Lin, G.Q.; Yang, Y.; Chen, X.Y.; Yu, X.R.; Wu, Y.F.; Xiong, F. Effects of high temperature during two growth stages on caryopsis development and physicochemical properties of starch in rice. Int. J. Biol. Macromol. 2020, 14, 301–310. [Google Scholar] [CrossRef]
- Impa, M.S.; Sunoj, V.S.J.; Krassovskaya, I.; Raju, B.R.; Obata, T.; Jagadish, S.V.K. Carbon balance and source-sink metabolic changes in winter wheat exposed to high night-time temperature. Plant Cell Environ. 2018, 42, 1233–1246. [Google Scholar] [CrossRef]
- Snider, J.L.; Thangthong, N.; Rossi, C.; Pilon, C. Root system growth and anatomy of cotton seedlings under suboptimal temperature. J. Agron. Crop Sci. 2022, 208, 372–383. [Google Scholar] [CrossRef]
- Singh, B.; Norvell, E.; Wijewardana, C.; Wallace, T.; Chastain, D.; Reddy, K.R. Assessing morphological characteristics of elite cotton lines from different breeding programmes for low temperature and drought tolerance. J. Agron. Crop Sci. 2018, 204, 467–476. [Google Scholar] [CrossRef]
- Reddy, K.R.; Brand, D.; Wijewardana, C.; Gao, W. Temperature effects on cotton seedling emergence, growth, and development. Agron. J. 2017, 109, 1379–1387. [Google Scholar] [CrossRef]
- Herritt, M.T.; Fritschi, F.B. Characterization of photosynthetic phenotypes and chloroplast ultrastructural changes of Soybean (Glycine max) in response to elevated air temperatures. Front. Plant Sci. 2020, 11, 153. [Google Scholar] [CrossRef]
- Kim, Y.U.; Lee, B.W. Differential mechanisms of potato yield loss induced by high day and night temperatures during tuber initiation and bulking: Photosynthesis and tuber growth. Front. Plant Sci. 2019, 10, 300. [Google Scholar] [CrossRef]
- Tombesi, S.; Cincera, I.; Frioni, T.; Ughini, V.; Gatti, M.; Palliotti, A.; Poni, S. Relationship among night temperature, carbohydrate translocation and inhibition of grapevine leaf photosynthesis. Environ. Exp. Bot. 2018, 157, 293–298. [Google Scholar] [CrossRef]
- Sunoj, V.S.J.; Shroyer, K.J.; Jagadish, S.V.K.; Prasad, P.V.V. Diurnal temperature amplitude alters physiological and growth response of maize (Zea mays L.) during the vegetative stage. Environ. Exp. Bot. 2016, 130, 113–121. [Google Scholar] [CrossRef]
- Liu, Y.; Ren, X.X.; Jeong, B.R. Night temperature affects the growth, metabolism, and photosynthetic gene expression in Astragalus Membranaceus and Codonopsis lanceolata plug seedlings. Plants 2019, 8, 10. [Google Scholar] [CrossRef]
- Binenbaum, J.; Weinstain, R.; Shani, E. Gibberellin localization and transport in plants. Trends Plant Sci. 2018, 23, 410–421. [Google Scholar] [CrossRef] [PubMed]
- Ferrero, L.V.; Viola, I.L.; Ariel, F.D.; Gonzalez, D.H. Class I TCP transcription factors target the gibberellin biosynthesis gene GA20ox1 and the growth-promoting genes HBI1 and PRE6 during thermomorphogenic growth in Arabidopsis. Plant Cell Physiol. 2019, 60, 1633–1645. [Google Scholar] [CrossRef] [PubMed]
- Ohtaka, K.; Yoshida, A.; Kakei, Y.; Fukui, K.; Kojima, M.; Takebayashi, Y.; Yano, K.; Imanishi, S.; Sakakibara, H. Difference between day and night temperatures affects stem elongation in tomato (Solanum lycopersicum) seedlings via regulation of gibberellin and auxin synthesis. Front. Plant Sci. 2020, 11, 577235. [Google Scholar] [CrossRef]
- Wu, X.Y.; Han, W.; Yang, Z.Q.; Zhang, Y.X.; Zheng, Y.J. The difference in temperature between day and night affects the strawberry soluble sugar content by influencing the photosynthesis, respiration and sucrose phosphatase synthase. Hort. Sci. 2021, 48, 174–182. [Google Scholar] [CrossRef]
- Ihsan, M.Z.; Khaliq, A.; Siddiqui, M.H.; Ali, L.; Kumar, R.; Ali, H.M.; Matloob, A.; Fahad, S. The response of Triticum aestivum treated with plant growth regulators to acute day/night temperature rise. J. Plant Growth Regul. 2022, 41, 5. [Google Scholar] [CrossRef]
- Kettler, B.A.; Carrera, C.S.; Songzogni, F.D.N.; Trachsel, S.; Andrade, F.H.; Neiff, N. High night temperature during maize post-flowering increases night respiration and reduces photosynthesis, growth and kernel number. J. Agron. Crop Sci. 2022, 208, 335–347. [Google Scholar] [CrossRef]
- Wang, L.H.; Zhai, Y.N.; Wu, J.X.; Stevanato, P.; Sha, S.S.; Geng, G.; Xu, Y.; Yu, L.H.; Wang, Y.G. Low night temperature-induced feedback inhibition of photosynthesis through sucrose accumulation in sugar beet (Beta vulgaris L.) leaves. Environ. Exp. Bot. 2022, 204, 105083. [Google Scholar] [CrossRef]
- Wu, D.; Liu, Y.; Pang, J.; Yong, J.W.H.; Chen, Y.L.; Bai, C.; Han, X.R.; Liu, X.Y.; Sun, Z.Y.; Zhang, S.W.; et al. Exogenous calcium alleviates night chilling-induced feedback inhibition of photosynthesis by improving sink demand in peanut (Arachis hypogaea). Front. Plant Sci. 2020, 11, 607029. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.W.; Kim, G.S.; Lee, W.S.; Kim, Y.H.; Kang, N.J.; Jin, J.S.; Lee, G.M.; Kim, S.T.; Abd El-Aty, A.M.; Shim, J.H.; et al. The effects of different night-time temperatures and cultivation durations on the polyphenolic contents of lettuce: Application of principal component analysis. J. Adv. Res. 2015, 6, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Li, P.H.; Xia, T.; Wan, X.C. Exploring plant metabolic genomics: Chemical diversity, metabolic complexity in the biosynthesis and transport of specialized metabolites with the tea plant as a model. Crit. Rev. Biotechnol. 2020, 40, 667–688. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.L.; Yang, H.; Wang, S.B.; Zhao, J.; Liu, C.; Gao, L.P.; Xia, E.H.; Lu, Y.; Tai, Y.L.; She, G.B.; et al. Draft genome sequence of Camellia sinensis var sinensis provides insights into the evolution of the tea genome and tea quality. Proc. Natl. Acad. Sci. USA 2018, 115, E4151–E4158. [Google Scholar] [CrossRef]
- Wang, W.L.; Wang, Y.X.; Li, H.; Liu, Z.W.; Cui, X.; Zhuang, J. Two MYB transcription factors (CsMYB2 and CsMYB26) are involved in flavonoid biosynthesis in tea plant (Camellia sinensis (L.) O. Kuntze). BMC Plant Biol. 2018, 18, 288. [Google Scholar] [CrossRef]
- Xu, W.L.; Li, J.; Zhang, L.Y.; Zhang, X.Y.; Zhao, H.; Guo, F.; Wang, Y.; Wang, P.; Chen, Y.Q.; Ni, D.J.; et al. Metabolome and RNA-seq analysis of responses to nitrogen deprivation and resupply in tea plant (Camellia sinensis) roots. Front. Plant Sci. 2022, 13, 932720. [Google Scholar] [CrossRef]
- Yue, C.N.; Peng, H.; Li, W.J.; Tong, Z.F.; Wang, Z.H.; Yang, P.X. Untargeted metabolomics and transcriptomics reveal the mechanism of metabolite differences in spring tender Shoots of tea plants of different ages. Foods 2022, 11, 2303. [Google Scholar] [CrossRef]
- Zheng, C.; Ma, J.Q.; Chen, J.D.; Ma, C.L.; Chen, W.; Yao, M.Z.; Chen, L. Gene coexpression networks reveal key drivers of flavonoid variation in eleven tea cultivars (Camellia sinensis). J. Agric. Food Chem. 2019, 67, 9967–9978. [Google Scholar] [CrossRef]
- Liu, J.W.; Liu, M.Y.; Fang, H.H.; Zhang, Q.F.; Ruan, J.Y. Accumulation of amino acids and flavonoids in young tea shoots is highly correlated with carbon and nitrogen metabolism in roots and mature leaves. Front. Plant Sci. 2021, 12, 756433. [Google Scholar] [CrossRef]
- Li, W.; Xiang, F.; Zhong, M.C.; Zhou, L.Y.; Liu, H.Y.; Li, S.J.; Wang, X.W. Transcriptome and metabolite analysis identifies nitrogen utilization genes in tea plant (Camellia sinensis). Sci. Rep. 2017, 7, 1. [Google Scholar] [CrossRef] [PubMed]
- Momayyezi, M.; McKown, A.D.; Bell, S.C.S.; Guy, R.D. Emerging roles for carbonic anhydrase in mesophyll conductance and photosynthesis. Plant J. 2020, 101, 831–844. [Google Scholar] [CrossRef] [PubMed]
- Li, H.F.; Li, L.; Yu, L.Y.; Yang, X.H.; Shi, X.G.; Wang, J.R.; Li, J.S.; Lin, S.J. Transcriptome profiling reveals versatile dissolved organic nitrogen utilization, mixotrophy, and N conservation in the dinoflagellate Prorocentrum shikokuense under N deficiency. Sci. Total Environ. 2020, 763, 143013. [Google Scholar] [CrossRef]
- Sun, Z.; Chen, D.; Zhu, L.Y.; Zhao, Y.N.; Lin, Z.; Li, X.Z.; Dai, W.D. A comprehensive study of the differences in protein expression and chemical constituents in tea leaves (Camellia sinensis var sinensis) with different maturity using a combined proteomics and metabolomics method. Food Res. Int. 2022, 157, 111397. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.K.; Lin, W.D.; Zhu, J.X.; Li, J.M. Amino acids profiling and transcriptomic data integration demonstrates the dynamic regulation of amino acids synthesis in the leaves of Cyclocarya paliurus. PeerJ 2022, 10, e13689. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.H.; Fu, X.M.; Wang, X.Q.; Liao, Y.Y.; Zeng, L.T.; Dong, F.; Yang, Z.Y. Studies on the biochemical formation pathway of the amino acid L-theanine in tea (Camellia sinensis) and other plants. J. Agric. Food Chem. 2017, 65, 7210–7216. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.M.; Yang, Z.Y. Understanding different regulatory mechanisms of proteinaceous and non-proteinaceous amino acid formation in tea (Camellia sinensis) provides new insights into the safe and effective alteration of tea flavor and function. Crit. Rev. Food Sci. Nutr. 2020, 60, 844–858. [Google Scholar] [CrossRef]
- Dong, C.X.; Li, F.; Yang, T.Y.; Feng, L.; Zhang, S.P.; Li, F.D.; Li, W.H.; Xu, G.H.; Bao, S.L.; Wan, S.C.; et al. Theanine transporters identified in tea plants (Camellia sinensis L.). Plant J. 2020, 101, 57–70. [Google Scholar] [CrossRef]
- She, G.B.; Yu, S.W.; Li, Z.G.; Peng, A.Q.; Li, P.H.; Li, Y.Y.; Chang, M.M.; Liu, L.L.; Chen, Q.; Shi, C.Y.; et al. Characterization of CsTSI in the biosynthesis of theanine in tea plants (Camellia sinensis). J. Agric. Food Chem. 2022, 70, 826–836. [Google Scholar] [CrossRef]
- Jin, J.Q.; Yao, M.Z.; Ma, C.L.; Ma, J.Q.; Chen, L. Natural allelic variations of TCS1 play a crucial role in caffeine biosynthesis of tea plant and its related species. Plant Physiol. Biochem. 2016, 100, 18–26. [Google Scholar] [CrossRef]
- Zhang, Y.R.; Li, P.H.; She, G.B.; Xu, Y.J.; Peng, A.Q.; Wan, X.C.; Zhao, J. Molecular basis of the distinct metabolic features in shoot tips and roots of tea plants (Camellia sinensis): Characterization of MYB regulator for root theanine synthesis. J. Agric. Food Chem. 2021, 69, 3415–3429. [Google Scholar] [CrossRef] [PubMed]
- Li, P.H.; Ye, Z.L.; Fu, J.M.; Xu, Y.J.; Shen, Y.H.; Zhang, Y.R.; Tang, D.K.; Li, P.; Zuo, H.; Tong, W.; et al. CsMYB184 regulates caffeine biosynthesis in tea plants. Plant Biotechnol. J. 2022, 20, 1012–1014. [Google Scholar] [CrossRef] [PubMed]
- Li, P.H.; Xia, E.H.; Fu, J.M.; Xu, Y.J.; Zhao, X.C.; Tong, W.; Tang, Q.; Tadege, M.; Fernie, A.R.; Zhao, J. Diverse roles of MYB transcription factors in regulating secondary metabolite biosynthesis, shoot development, and stress responses in tea plants (Camellia sinensis). Plant J. 2022, 110, 1144–1165. [Google Scholar] [CrossRef]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, X.; Li, H.; Zhang, Z.; Yang, Y.; Jin, Z.; Chen, W.; Tang, D.; Wei, C.; Tang, Q. Characterization of the Difference between Day and Night Temperatures on the Growth, Photosynthesis, and Metabolite Accumulation of Tea Seedlings. Int. J. Mol. Sci. 2023, 24, 6718. https://doi.org/10.3390/ijms24076718
Tan X, Li H, Zhang Z, Yang Y, Jin Z, Chen W, Tang D, Wei C, Tang Q. Characterization of the Difference between Day and Night Temperatures on the Growth, Photosynthesis, and Metabolite Accumulation of Tea Seedlings. International Journal of Molecular Sciences. 2023; 24(7):6718. https://doi.org/10.3390/ijms24076718
Chicago/Turabian StyleTan, Xiaoqin, Huili Li, Zhongyue Zhang, Yanjuan Yang, Zhen Jin, Wei Chen, Dandan Tang, Chaoling Wei, and Qian Tang. 2023. "Characterization of the Difference between Day and Night Temperatures on the Growth, Photosynthesis, and Metabolite Accumulation of Tea Seedlings" International Journal of Molecular Sciences 24, no. 7: 6718. https://doi.org/10.3390/ijms24076718
APA StyleTan, X., Li, H., Zhang, Z., Yang, Y., Jin, Z., Chen, W., Tang, D., Wei, C., & Tang, Q. (2023). Characterization of the Difference between Day and Night Temperatures on the Growth, Photosynthesis, and Metabolite Accumulation of Tea Seedlings. International Journal of Molecular Sciences, 24(7), 6718. https://doi.org/10.3390/ijms24076718






