1,25(OH)2D3 Differently Modulates the Secretory Activity of IFN-DC and IL4-DC: A Study in Cells from Healthy Donors and MS Patients
Abstract
1. Introduction
2. Results
2.1. IFN-DC Express Higher Levels of VDR mRNA Than IL4-DC
2.2. 1,25(OH)2D3 Differently Modulates the Secretion of Pro- and Anti-Inflammatory Mediators in IFN-DC and IL4-DC
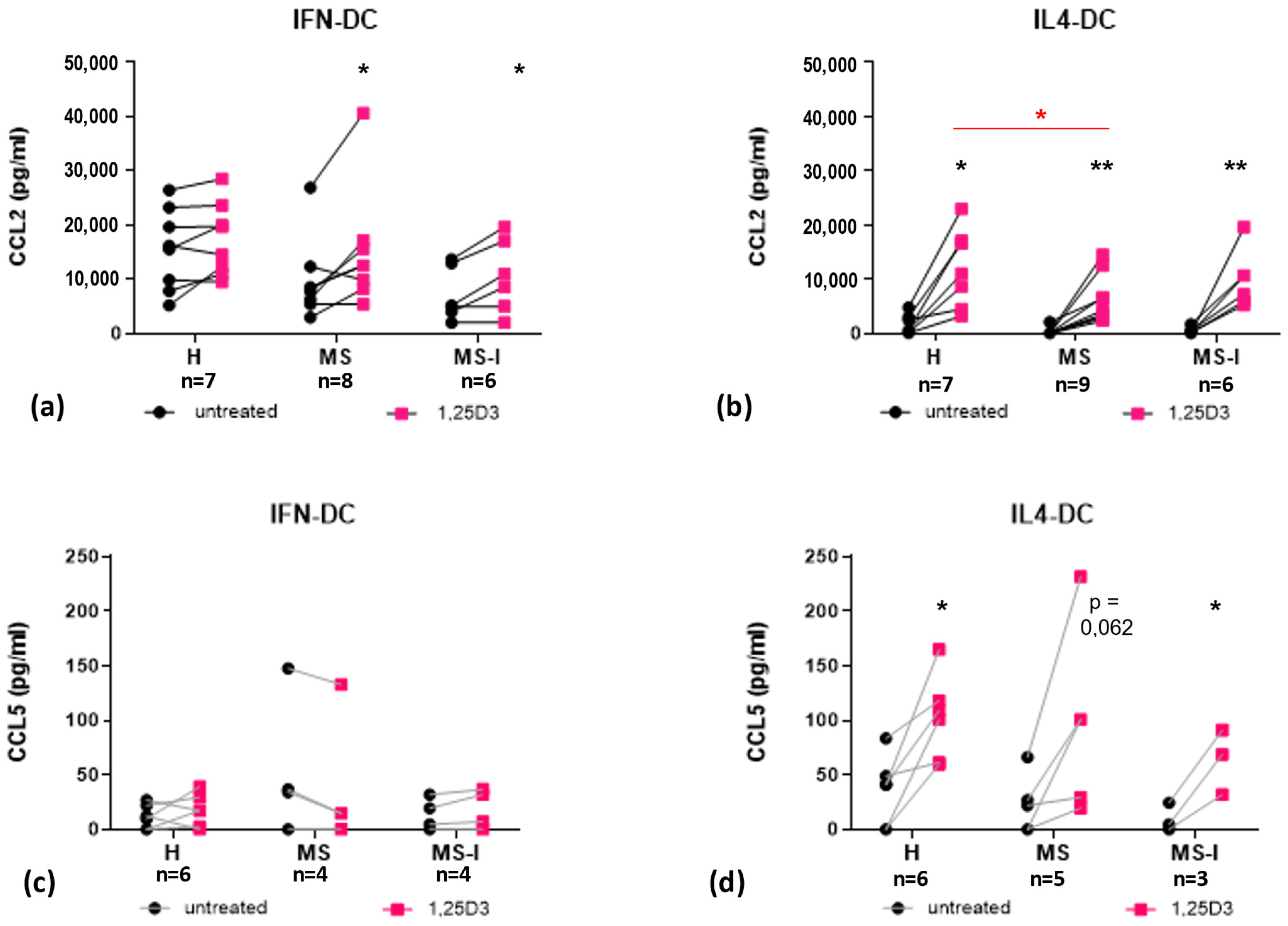
2.3. 1,25(OH)2D3 Differently Modulates Secretion of CCR2 and CCR5 Ligands in IFN-DC and IL4-DC
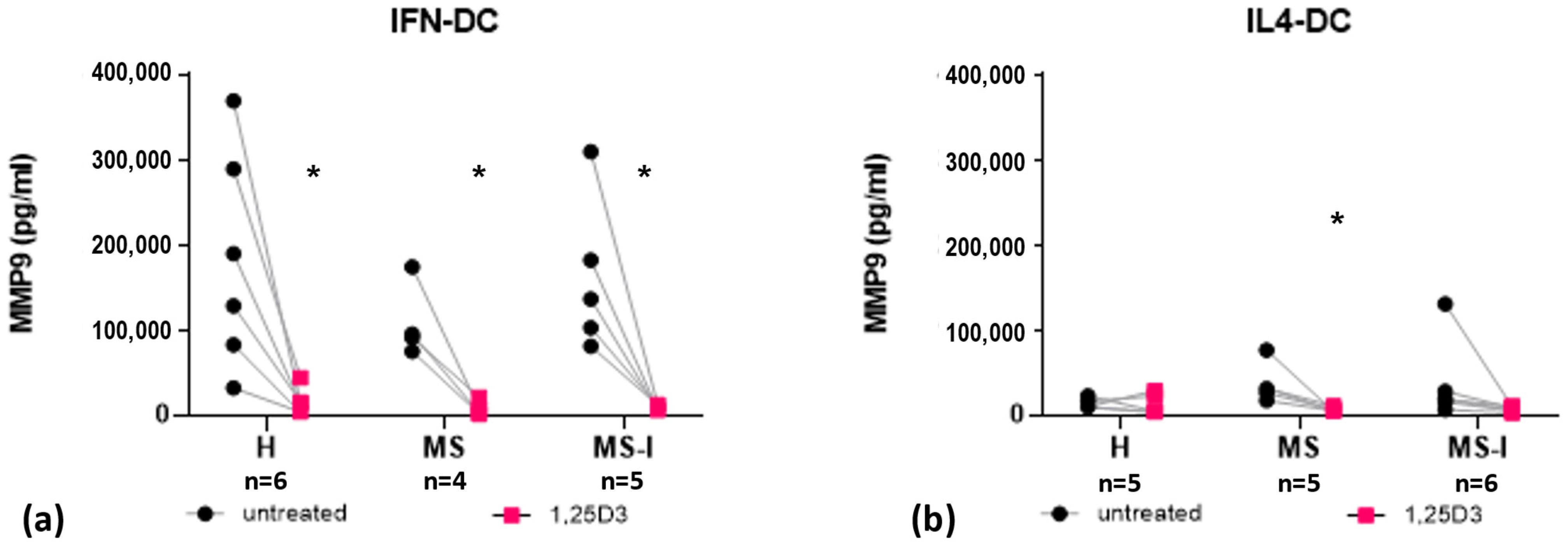
2.4. IFN-DC Secrete High Levels of MMP9, Which Are Strongly Reduced by 1,25(OH)2D3
3. Discussion
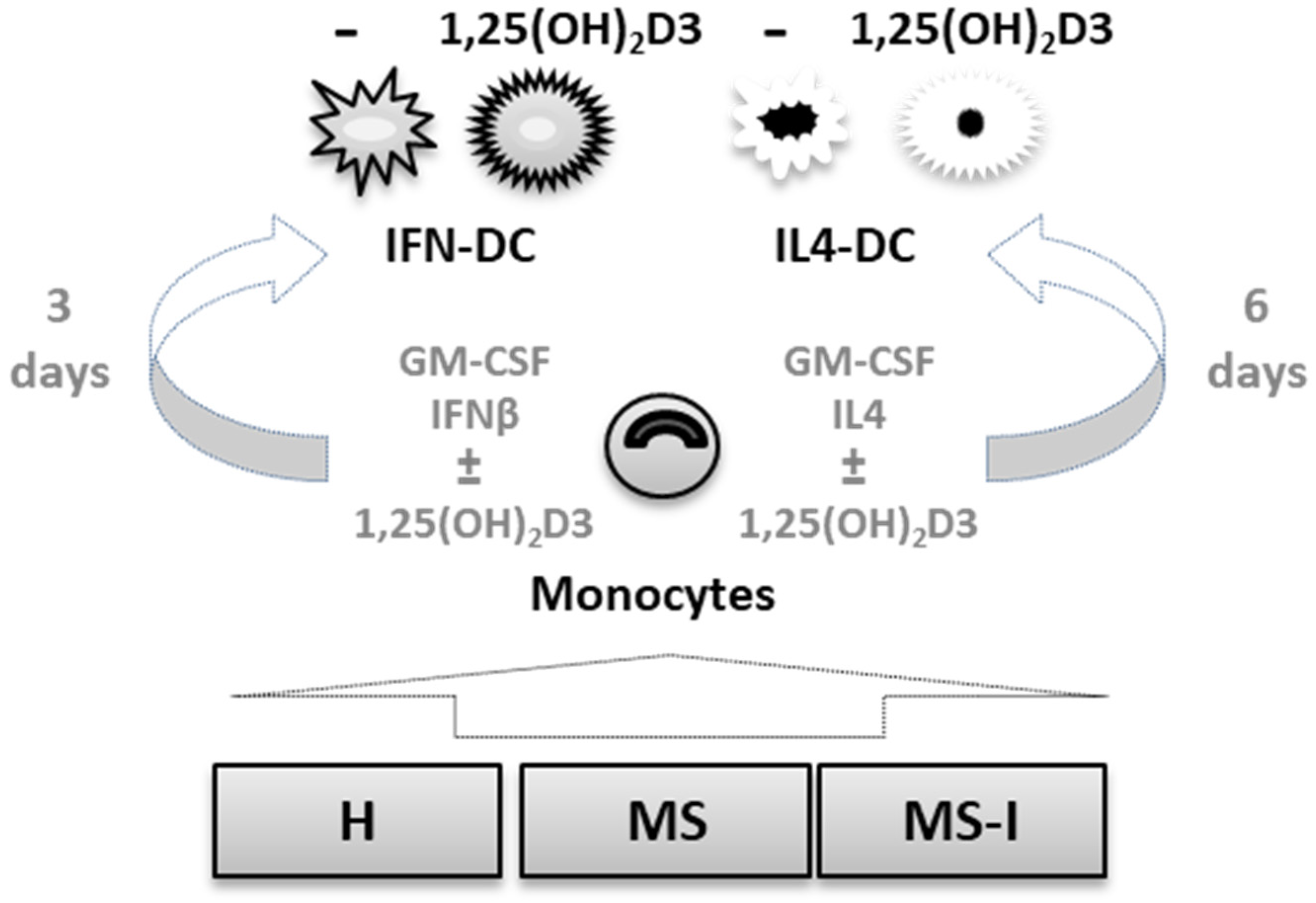
4. Materials and Methods
4.1. Blood Samples
- MS: RRMS patients free of disease modifying therapies;
- MS-I: RRMS patients undergoing high dose treatment with IFNβ-1a and/or 1b;
- H: healthy volunteers with a comparable gender and age distribution.
4.2. Monocyte Separation and DC Culture
4.3. RNA Isolation and Polymerase Chain-Reaction
4.4. ELISA
4.5. Statistical Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thompson, A.J.; Baranzini, S.E.; Geurts, J.; Hemmer, B.; Ciccarelli, O. Multiple Sclerosis. Lancet 2018, 391, 1622–1636. [Google Scholar] [CrossRef] [PubMed]
- Simon, K.C.; Munger, K.L.; Ascherio, A. Vitamin D and Multiple Sclerosis: Epidemiology, Immunology, and Genetics. Curr. Opin. Neurol. 2012, 25, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Sintzel, M.B.; Rametta, M.; Reder, A.T. Vitamin D and Multiple Sclerosis: A Comprehensive Review. Neurol. Ther. 2018, 7, 59–85. [Google Scholar] [CrossRef] [PubMed]
- Vandebergh, M.; Degryse, N.; Dubois, B.; Goris, A. Environmental Risk Factors in Multiple Sclerosis: Bridging Mendelian Randomization and Observational Studies. J. Neurol. 2022, 269, 4565–4574. [Google Scholar] [CrossRef]
- Jagannath, V.A.; Filippini, G.; Di Pietrantonj, C.; Asokan, G.V.; Robak, E.W.; Whamond, L.; Robinson, S.A. Vitamin D for the Management of Multiple Sclerosis. Cochrane Database Syst. Rev. 2018, 9, CD008422. [Google Scholar] [CrossRef]
- Camu, W.; Lehert, P.; Pierrot-Deseilligny, C.; Hautecoeur, P.; Besserve, A.; Jean Deleglise, A.S.; Payet, M.; Thouvenot, E.; Souberbielle, J.C. Cholecalciferol in Relapsing-Remitting MS: A Randomized Clinical Trial (CHOLINE). Neurol. Neuroimmunol. Neuroinflamm. 2019, 6, e597. [Google Scholar] [CrossRef]
- Hupperts, R.; Smolders, J.; Vieth, R.; Holmoy, T.; Marhardt, K.; Schluep, M.; Killestein, J.; Barkhof, F.; Beelke, M.; Grimaldi, L.M.E.; et al. Randomized Trial of Daily High-Dose Vitamin D3 in Patients with RRMS Receiving Subcutaneous Interferon Beta-1a. Neurology 2019, 93, e1906–e1916. [Google Scholar]
- Fatima, M.; Lamis, A.; Siddiqui, S.W.; Ashok, T.; Patni, N.; Fadiora, O.E. Therapeutic Role of Vitamin D in Multiple Sclerosis: An Essentially Contested Concept. Cureus 2022, 14, e26186. [Google Scholar] [CrossRef]
- Pashenkov, M.; Huang, Y.M.; Kostulas, V.; Haglund, M.; Soderstrom, M.; Link, H. Two Subsets of Dendritic Cells are Present in Human Cerebrospinal Fluid. Brain 2001, 124, 480–492. [Google Scholar] [CrossRef]
- Plumb, J.; Armstrong, M.A.; Duddy, M.; Mirakhur, M.; McQuaid, S. CD83-Positive Dendritic Cells are Present in Occasional Perivascular Cuffs in Multiple Sclerosis Lesions. Mult. Scler. 2003, 9, 142–147. [Google Scholar] [CrossRef]
- Serafini, B.; Rosicarelli, B.; Magliozzi, R.; Stigliano, E.; Capello, E.; Mancardi, G.L.; Aloisi, F. Dendritic Cells in Multiple Sclerosis Lesions: Maturation Stage, Myelin Uptake, and Interaction with Proliferating T Cells. J. Neuropathol. Exp. Neurol. 2006, 65, 124–141. [Google Scholar] [CrossRef]
- Ifergan, I.; Kebir, H.; Bernard, M.; Wosik, K.; Dodelet-Devillers, A.; Cayrol, R.; Arbour, N.; Prat, A. The Blood-Brain Barrier Induces Differentiation of Migrating Monocytes into Th17-Polarizing Dendritic Cells. Brain 2008, 131, 785–799. [Google Scholar] [CrossRef]
- Cheng, Y.; Sun, L.; Xie, Z.; Fan, X.; Cao, Q.; Han, J.; Zhu, J.; Jin, T. Diversity of Immune Cell Types in Multiple Sclerosis and its Animal Model: Pathological and Therapeutic Implications. J. Neurosci. Res. 2017, 95, 1973–1983. [Google Scholar] [CrossRef]
- Kim, S.H.; Jung, H.H.; Lee, C.K. Generation, Characteristics and Clinical Trials of Ex Vivo Generated Tolerogenic Dendritic Cells. Yonsei Med. J. 2018, 59, 807–815. [Google Scholar] [CrossRef]
- Phillips, B.E.; Garciafigueroa, Y.; Trucco, M.; Giannoukakis, N. Clinical Tolerogenic Dendritic Cells: Exploring Therapeutic Impact on Human Autoimmune Disease. Front. Immunol. 2017, 8, 1279. [Google Scholar] [CrossRef]
- Willekens, B.; Presas-Rodriguez, S.; Mansilla, M.J.; Derdelinckx, J.; Lee, W.P.; Nijs, G.; De Laere, M.; Wens, I.; Cras, P.; Parizel, P.; et al. Tolerogenic Dendritic Cell-Based Treatment for Multiple Sclerosis (MS): A Harmonised Study Protocol for Two Phase I Clinical Trials Comparing Intradermal and Intranodal Cell Administration. BMJ Open 2019, 9, e030309. [Google Scholar] [CrossRef] [PubMed]
- Conti, L.; Gessani, S. GM-CSF in the Generation of Dendritic Cells from Human Blood Monocyte Precursors: Recent Advances. Immunobiology 2008, 213, 859–870. [Google Scholar] [CrossRef]
- Santini, S.M.; Lapenta, C.; Belardelli, F. Type I Interferons as Regulators of the Differentiation/Activation of Human Dendritic Cells: Methods for the Evaluation of IFN-Induced Effects. Methods Mol. Med. 2005, 116, 167–181. [Google Scholar] [PubMed]
- Santini, S.M.; Lapenta, C.; Logozzi, M.; Parlato, S.; Spada, M.; Di Pucchio, T.; Belardelli, F. Type I Interferon as a Powerful Adjuvant for Monocyte-Derived Dendritic Cell Development and Activity in Vitro and in Hu-PBL-SCID Mice. J. Exp. Med. 2000, 191, 1777–1788. [Google Scholar] [CrossRef] [PubMed]
- Santini, S.M.; Lapenta, C.; Santodonato, L.; D’Agostino, G.; Belardelli, F.; Ferrantini, M. IFN-Alpha in the Generation of Dendritic Cells for Cancer Immunotherapy. Handb. Exp. Pharmacol. 2009, 188, 295–317. [Google Scholar] [CrossRef]
- Parlato, S.; Santini, S.M.; Lapenta, C.; Di Pucchio, T.; Logozzi, M.; Spada, M.; Giammarioli, A.M.; Malorni, W.; Fais, S.; Belardelli, F. Expression of CCR-7, MIP-3beta, and Th-1 Chemokines in Type I IFN-Induced Monocyte-Derived Dendritic Cells: Importance for the Rapid Acquisition of Potent Migratory and Functional Activities. Blood 2001, 98, 3022–3029. [Google Scholar] [CrossRef] [PubMed]
- Lapenta, C.; Gabriele, L.; Santini, S.M. IFN-Alpha-Mediated Differentiation of Dendritic Cells for Cancer Immunotherapy: Advances and Perspectives. Vaccines 2020, 8, 617. [Google Scholar] [CrossRef] [PubMed]
- Cohan, S.L.; Hendin, B.A.; Reder, A.T.; Smoot, K.; Avila, R.; Mendoza, J.P.; Weinstock-Guttman, B. Interferons and Multiple Sclerosis: Lessons from 25 Years of Clinical and Real-World Experience with Intramuscular Interferon Beta-1a (Avonex). CNS Drugs 2021, 35, 743–767. [Google Scholar] [CrossRef]
- Gauzzi, M.C.; Purificato, C.; Donato, K.; Jin, Y.; Wang, L.; Daniel, K.C.; Maghazachi, A.A.; Belardelli, F.; Adorini, L.; Gessani, S. Suppressive Effect of 1alpha,25-Dihydroxyvitamin D3 on Type I IFN-Mediated Monocyte Differentiation into Dendritic Cells: Impairment of Functional Activities and Chemotaxis. J. Immunol. 2005, 174, 270–276. [Google Scholar] [CrossRef]
- Nurminen, V.; Seuter, S.; Carlberg, C. Primary Vitamin D Target Genes of Human Monocytes. Front. Physiol. 2019, 10, 194. [Google Scholar] [CrossRef] [PubMed]
- Gobel, K.; Ruck, T.; Meuth, S.G. Cytokine Signaling in Multiple Sclerosis: Lost in Translation. Mult. Scler. 2018, 24, 432–439. [Google Scholar] [CrossRef]
- Lobo-Silva, D.; Carriche, G.M.; Castro, A.G.; Roque, S.; Saraiva, M. Balancing the Immune Response in the Brain: IL-10 and its Regulation. J. Neuroinflamm. 2016, 13, 297. [Google Scholar] [CrossRef]
- Fantuzzi, L.; Tagliamonte, M.; Gauzzi, M.C.; Lopalco, L. Dual CCR5/CCR2 Targeting: Opportunities for the Cure of Complex Disorders. Cell Mol. Life Sci. 2019, 76, 4869–4886. [Google Scholar] [CrossRef] [PubMed]
- Sanseverino, I.; Rinaldi, A.O.; Purificato, C.; Cortese, A.; Millefiorini, E.; Gessani, S.; Gauzzi, M.C. CCL2 Induction by 1,25(OH)2D3 in Dendritic Cells from Healthy Donors and Multiple Sclerosis Patients. J. Steroid Biochem. Mol. Biol. 2014, 144, 102–105. [Google Scholar] [CrossRef] [PubMed]
- Benesova, Y.; Vasku, A.; Novotna, H.; Litzman, J.; Stourac, P.; Beranek, M.; Kadanka, Z.; Bednarik, J. Matrix Metalloproteinase-9 and Matrix Metalloproteinase-2 as Biomarkers of various Courses in Multiple Sclerosis. Mult. Scler. 2009, 15, 316–322. [Google Scholar] [CrossRef]
- Hannocks, M.; Zhang, X.; Gerwien, H.; Chashchina, A.; Burmeister, M.; Korpos, E.; Song, J.; Sorokin, L. The Gelatinases, MMP-2 and MMP-9, as Fine Tuners of Neuroinflammatory Processes. Matrix Biol. 2019, 75–76, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Zettl, U.K.; Hecker, M.; Aktas, O.; Wagner, T.; Rommer, P.S. Interferon Beta-1a and Beta-1b for Patients with Multiple Sclerosis: Updates to Current Knowledge. Expert Rev. Clin. Immunol. 2018, 14, 137–153. [Google Scholar] [CrossRef]
- Adorini, L.; Penna, G. Dendritic Cell Tolerogenicity: A Key Mechanism in Immunomodulation by Vitamin D Receptor Agonists. Hum. Immunol. 2009, 70, 345–352. [Google Scholar] [CrossRef]
- Bscheider, M.; Butcher, E.C. Vitamin D Immunoregulation through Dendritic Cells. Immunology 2016, 148, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Tough, D.F. Type I Interferon as a Link between Innate and Adaptive Immunity through Dendritic Cell Stimulation. Leuk. Lymphoma 2004, 45, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Soilu-Hanninen, M.; Aivo, J.; Lindstrom, B.; Elovaara, I.; Sumelahti, M.; Farkkila, M.; Tienari, P.; Atula, S.; Sarasoja, T.; Herrala, L.; et al. A Randomised, Double Blind, Placebo Controlled Trial with Vitamin D3 as an Add on Treatment to Interferon Beta-1b in Patients with Multiple Sclerosis. J. Neurol. Neurosurg. Psychiatry 2012, 83, 565–571. [Google Scholar] [CrossRef]
- Sommer, A.; Fabri, M. Vitamin D Regulates Cytokine Patterns Secreted by Dendritic Cells to Promote Differentiation of IL-22-Producing T Cells. PLoS ONE 2015, 10, e0130395. [Google Scholar] [CrossRef]
- Chamorro, S.; Garcia-Vallejo, J.J.; Unger, W.W.J.; Fernandes, R.J.; Bruijns, S.C.M.; Laban, S.; Roep, B.O.; ‘t Hart, B.A.; van Kooyk, Y. TLR Triggering on Tolerogenic Dendritic Cells Results in TLR2 Up-Regulation and a Reduced Proinflammatory Immune Program. J. Immunol. 2009, 183, 2984–2994. [Google Scholar] [CrossRef]
- Galoppin, M.; Kari, S.; Soldati, S.; Pal, A.; Rival, M.; Engelhardt, B.; Astier, A.; Thouvenot, E. Full Spectrum of Vitamin D Immunomodulation in Multiple Sclerosis: Mechanisms and Therapeutic Implications. Brain Commun. 2022, 4, fcac171. [Google Scholar] [CrossRef]
- Wobke, T.K.; Sorg, B.L.; Steinhilber, D. Vitamin D in Inflammatory Diseases. Front. Physiol. 2014, 5, 244. [Google Scholar]
- Ismailova, A.; White, J.H. Vitamin D, Infections and Immunity. Rev. Endocr. Metab. Disord. 2022, 23, 265–277. [Google Scholar] [CrossRef]
- Rose-John, S.; Heinrich, P.C. Soluble Receptors for Cytokines and Growth Factors: Generation and Biological Function. Biochem. J. 1994, 300, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Stampanoni Bassi, M.; Iezzi, E.; Drulovic, J.; Pekmezovic, T.; Gilio, L.; Furlan, R.; Finardi, A.; Marfia, G.A.; Sica, F.; Centonze, D.; et al. IL-6 in the Cerebrospinal Fluid Signals Disease Activity in Multiple Sclerosis. Front. Cell. Neurosci. 2020, 14, 120. [Google Scholar] [CrossRef] [PubMed]
- Stampanoni Bassi, M.; Iezzi, E.; Mori, F.; Simonelli, I.; Gilio, L.; Buttari, F.; Sica, F.; De Paolis, N.; Mandolesi, G.; Musella, A.; et al. Interleukin-6 Disrupts Synaptic Plasticity and Impairs Tissue Damage Compensation in Multiple Sclerosis. Neurorehabil. Neural Repair 2019, 33, 825–835. [Google Scholar] [CrossRef] [PubMed]
- Kleiter, I.; Ayzenberg, I.; Araki, M.; Yamamura, T.; Gold, R. Tocilizumab, MS, and NMOSD. Mult. Scler. 2016, 22, 1891–1892. [Google Scholar] [CrossRef] [PubMed]
- Rothaug, M.; Becker-Pauly, C.; Rose-John, S. The Role of Interleukin-6 Signaling in Nervous Tissue. Biochim. Biophys. Acta 2016, 1863, 1218–1227. [Google Scholar] [CrossRef]
- Garbers, C.; Heink, S.; Korn, T.; Rose-John, S. Interleukin-6: Designing Specific Therapeutics for a Complex Cytokine. Nat. Rev. Drug Discov. 2018, 17, 395–412. [Google Scholar] [CrossRef]
- Catala-Moll, F.; Ferrete-Bonastre, A.G.; Godoy-Tena, G.; Morante-Palacios, O.; Ciudad, L.; Barbera, L.; Fondelli, F.; Martinez-Caceres, E.M.; Rodriguez-Ubreva, J.; Li, T.; et al. Vitamin D Receptor, STAT3, and TET2 Cooperate to Establish Tolerogenesis. Cell Rep. 2022, 38, 110244. [Google Scholar] [CrossRef]
- Chauss, D.; Freiwald, T.; McGregor, R.; Yan, B.; Wang, L.; Nova-Lamperti, E.; Kumar, D.; Zhang, Z.; Teague, H.; West, E.E.; et al. Autocrine Vitamin D Signaling Switches Off Pro-Inflammatory Programs of TH1 Cells. Nat. Immunol. 2022, 23, 62–74. [Google Scholar] [CrossRef]
- Fresegna, D.; Bullitta, S.; Musella, A.; Rizzo, F.R.; De Vito, F.; Guadalupi, L.; Caioli, S.; Balletta, S.; Sanna, K.; Dolcetti, E.; et al. Re-Examining the Role of TNF in MS Pathogenesis and Therapy. Cells 2020, 9, 2290. [Google Scholar] [CrossRef]
- Lund, B.T.; Ashikian, N.; Ta, H.Q.; Chakryan, Y.; Manoukian, K.; Groshen, S.; Gilmore, W.; Cheema, G.S.; Stohl, W.; Burnett, M.E.; et al. Increased CXCL8 (IL-8) Expression in Multiple Sclerosis. J. Neuroimmunol. 2004, 155, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Kwilasz, A.J.; Grace, P.M.; Serbedzija, P.; Maier, S.F.; Watkins, L.R. The Therapeutic Potential of Interleukin-10 in Neuroimmune Diseases. Neuropharmacology 2015, 96, 55–69. [Google Scholar] [CrossRef] [PubMed]
- Penna, G.; Adorini, L. 1 Alpha,25-Dihydroxyvitamin D3 Inhibits Differentiation, Maturation, Activation, and Survival of Dendritic Cells Leading to Impaired Alloreactive T Cell Activation. J. Immunol. 2000, 164, 2405–2411. [Google Scholar] [CrossRef] [PubMed]
- Mahad, D.J.; Ransohoff, R.M. The Role of MCP-1 (CCL2) and CCR2 in Multiple Sclerosis and Experimental Autoimmune Encephalomyelitis (EAE). Semin. Immunol. 2003, 15, 23–32. [Google Scholar] [CrossRef]
- Szczucinski, A.; Losy, J. Chemokines and Chemokine Receptors in Multiple Sclerosis. Potential Targets for New Therapies. Acta Neurol. Scand. 2007, 115, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Yong, V.W.; Power, C.; Forsyth, P.; Edwards, D.R. Metalloproteinases in Biology and Pathology of the Nervous System. Nat. Rev. Neurosci. 2001, 2, 502–511. [Google Scholar] [CrossRef]
- Mohammadhosayni, M.; Khosrojerdi, A.; Lorian, K.; Aslani, S.; Imani, D.; Razi, B.; Babaie, F.; Torkamandi, S. Matrix Metalloproteinases (MMPs) Family Gene Polymorphisms and the Risk of Multiple Sclerosis: Systematic Review and Meta-Analysis. BMC Neurol. 2020, 20, 218. [Google Scholar] [CrossRef] [PubMed]
- Gearing, A.J.; Beckett, P.; Christodoulou, M.; Churchill, M.; Clements, J.M.; Crimmin, M.; Davidson, A.H.; Drummond, A.H.; Galloway, W.A.; Gilbert, R. Matrix Metalloproteinases and Processing of Pro-TNF-Alpha. J. Leukoc. Biol. 1995, 57, 774–777. [Google Scholar] [CrossRef]
- Denney, H.; Clench, M.R.; Woodroofe, M.N. Cleavage of Chemokines CCL2 and CXCL10 by Matrix Metalloproteinases-2 and -9: Implications for Chemotaxis. Biochem. Biophys. Res. Commun. 2009, 382, 341–347. [Google Scholar] [CrossRef]
- Coussens, A.; Timms, P.M.; Boucher, B.J.; Venton, T.R.; Ashcroft, A.T.; Skolimowska, K.H.; Newton, S.M.; Wilkinson, K.A.; Davidson, R.N.; Griffiths, C.J.; et al. 1alpha,25-Dihydroxyvitamin D3 Inhibits Matrix Metalloproteinases Induced by Mycobacterium Tuberculosis Infection. Immunology 2009, 127, 539–548. [Google Scholar] [CrossRef]
- Das, L.M.; Binko, A.M.; Traylor, Z.P.; Peng, H.; Lu, K.Q. Vitamin D Improves Sunburns by Increasing Autophagy in M2 Macrophages. Autophagy 2019, 15, 813–826. [Google Scholar] [CrossRef] [PubMed]
- Suuring, M.; Moreau, A. Regulatory Macrophages and Tolerogenic Dendritic Cells in Myeloid Regulatory Cell-Based Therapies. Int. J. Mol. Sci. 2021, 22, 7970. [Google Scholar] [CrossRef] [PubMed]
- Mansilla, M.J.; Presas-Rodriguez, S.; Teniente-Serra, A.; Gonzalez-Larreategui, I.; Quirant-Sanchez, B.; Fondelli, F.; Djedovic, N.; Iwaszkiewicz-Grzes, D.; Chwojnicki, K.; Miljkovic, D.; et al. Paving the Way towards an Effective Treatment for Multiple Sclerosis: Advances in Cell Therapy. Cell. Mol. Immunol. 2021, 18, 1353–1374. [Google Scholar] [CrossRef] [PubMed]
- Nuyts, A.H.; Ponsaerts, P.; Van Tendeloo, V.F.I.; Lee, W.; Stein, B.; Nagels, G.; D’hooghe, M.B.; Willekens, B.; Cras, P.; Wouters, K.; et al. Except for C-C Chemokine Receptor 7 Expression, Monocyte-Derived Dendritic Cells from Patients with Multiple Sclerosis are Functionally Comparable to those of Healthy Controls. Cytotherapy 2014, 16, 1024–1030. [Google Scholar] [CrossRef]
- Raiotach-Regue, D.; Grau-Lopez, L.; Naranjo-Gomez, M.; Ramo-Tello, C.; Pujol-Borrell, R.; Martinez-Caceres, E.; Borras, F.E. Stable Antigen-Specific T-Cell Hyporesponsiveness Induced by Tolerogenic Dendritic Cells from Multiple Sclerosis Patients. Eur. J. Immunol. 2012, 42, 771–782. [Google Scholar] [CrossRef]
- Lee, W.; Willekens, B.; Cras, P.; Goossens, H.; Martinez-Caceres, E.; Berneman, Z.N.; Cools, N. Immunomodulatory Effects of 1,25-Dihydroxyvitamin D(3) on Dendritic Cells Promote Induction of T Cell Hyporesponsiveness to Myelin-Derived Antigens. J. Immunol. Res. 2016, 2016, 5392623. [Google Scholar] [CrossRef]
- Florez-Grau, G.; Zubizarreta, I.; Cabezon, R.; Villoslada, P.; Benitez-Ribas, D. Tolerogenic Dendritic Cells as a Promising Antigen-Specific Therapy in the Treatment of Multiple Sclerosis and Neuromyelitis Optica from Preclinical to Clinical Trials. Front. Immunol. 2018, 9, 1169. [Google Scholar] [CrossRef]
- Soldan, S.S.; Lieberman, P.M. Epstein-Barr Virus and Multiple Sclerosis. Nat. Rev. Microbiol. 2023, 21, 51–64. [Google Scholar] [CrossRef]
- Aloisi, F.; Cross, A.H. MINI-Review of Epstein-Barr Virus Involvement in Multiple Sclerosis Etiology and Pathogenesis. J. Neuroimmunol. 2022, 371, 577935. [Google Scholar] [CrossRef]
- Milo, R.; Korczyn, A.D.; Manouchehri, N.; Stuve, O. The Temporal and Causal Relationship between Inflammation and Neurodegeneration in Multiple Sclerosis. Mult. Scler. 2020, 26, 876–886. [Google Scholar] [CrossRef]
- Prinz, M.; Schmidt, H.; Mildner, A.; Knobeloch, K.; Hanisch, U.; Raasch, J.; Merkler, D.; Detje, C.; Gutcher, I.; Mages, J.; et al. Distinct and Nonredundant in Vivo Functions of IFNAR on Myeloid Cells Limit Autoimmunity in the Central Nervous System. Immunity 2008, 28, 675–686. [Google Scholar] [CrossRef] [PubMed]
- Cauwels, A.; Van Lint, S.; Catteeuw, D.; Pang, S.; Paul, F.; Rogge, E.; Verhee, A.; Prinz, M.; Kley, N.; Uze, G.; et al. Targeting Interferon Activity to Dendritic Cells Enables in Vivo Tolerization and Protection Against EAE in Mice. J. Autoimmun. 2019, 97, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Kundu, R.; Theodoraki, A.; Haas, C.T.; Zhang, Y.; Chain, B.; Kriston-Vizi, J.; Noursadeghi, M.; Khoo, B. Cell-Type-Specific Modulation of Innate Immune Signalling by Vitamin D in Human Mononuclear Phagocytes. Immunology 2017, 150, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Polman, C.H.; Reingold, S.C.; Banwell, B.; Clanet, M.; Cohen, J.A.; Filippi, M.; Fujihara, K.; Havrdova, E.; Hutchinson, M.; Kappos, L.; et al. Diagnostic Criteria for Multiple Sclerosis: 2010 Revisions to the McDonald Criteria. Ann. Neurol. 2011, 69, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Law, S.P.L.; Gatt, P.N.; Schibeci, S.D.; McKay, F.C.; Vucic, S.; Hart, P.; Byrne, S.N.; Brown, D.; Stewart, G.J.; Liddle, C.; et al. Expression of CYP24A1 and Other Multiple Sclerosis Risk Genes in Peripheral Blood Indicates Response to Vitamin D in Homeostatic and Inflammatory Conditions. Genes Immun. 2021, 22, 227–233. [Google Scholar] [CrossRef]
- Perriard, G.; Mathias, A.; Enz, L.; Canales, M.; Schluep, M.; Gentner, M.; Schaeren-Wiemers, N.; Du Pasquier, R.A. Interleukin-22 is Increased in Multiple Sclerosis Patients and Targets Astrocytes. J. Neuroinflamm. 2015, 12, 119. [Google Scholar] [CrossRef] [PubMed]



| IL6 | sIL6R | TNFα | IL8 | IL10 | CCL2 | CCL5 | MMP9 | ||
|---|---|---|---|---|---|---|---|---|---|
| untreated | MS vs. H | 0.4065 | 0.6535 | 0.9357 | 0.3676 | 0.0387 * | 0.3192 | 0.1659 | 0.2711 |
| MS-I vs. H | 0.1846 | 0.9118 | 0.8376 | 0.9122 | 0.9967 | 0.1255 | 0.9947 | 0.8946 | |
| MS-I vs. MS | 0.8473 | 0.4632 | 0.9793 | 0.219 | 0.0614 | 0.7955 | 0.2532 | 0.5121 | |
| 1,25(OH)2D3 | MS vs. H | 0.3271 | 0.3788 | 0.8572 | 0.318 | 0.8991 | 0.8471 | 0.5774 | 0.9854 |
| MS-I vs. H | 0.193 | 0.922 | 0.1451 | 0.3011 | 0.9938 | 0.2422 | 0.9978 | 0.9884 | |
| MS-I vs. MS | 0.9326 | 0.6469 | 0.091 | 0.9808 | 0.8655 | 0.5023 | 0.6669 | 0.9996 |
| IL6 | sIL6R | TNFα | IL8 | IL10 | CCL2 | CCL5 | MMP9 | ||
|---|---|---|---|---|---|---|---|---|---|
| untreated | MS vs. H | >0.9999 | 0.5604 | 0.4878 | 0.883 | 0.9501 | 0.783 | 0.8905 | 0.273 |
| MS-I vs. H | >0.9999 | 0.2107 | 0.2376 | 0.9975 | 0.9996 | 0.8536 | 0.7051 | 0.2509 | |
| MS-I vs. MS | >0.9999 | 0.7309 | 0.9049 | 0.9244 | 0.9556 | 0.9971 | 0.9174 | 0.9998 | |
| 1,25(OH)2D3 | MS vs. H | 0.5091 | 0.1504 | 0.9871 | 0.6982 | 0.115 | 0.0219 * | 0.9753 | 0.906 |
| MS-I vs. H | 0.9369 | 0.0607 | 0.1278 | 0.8339 | 0.7146 | 0.6219 | 0.4758 | 0.9174 | |
| MS-I vs. MS | 0.2502 | 0.8743 | 0.1996 | 0.3968 | 0.3817 | 0.2261 | 0.6042 | 0.9988 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanseverino, I.; Rinaldi, A.O.; Purificato, C.; Cortese, A.; Millefiorini, E.; Gauzzi, M.C. 1,25(OH)2D3 Differently Modulates the Secretory Activity of IFN-DC and IL4-DC: A Study in Cells from Healthy Donors and MS Patients. Int. J. Mol. Sci. 2023, 24, 6717. https://doi.org/10.3390/ijms24076717
Sanseverino I, Rinaldi AO, Purificato C, Cortese A, Millefiorini E, Gauzzi MC. 1,25(OH)2D3 Differently Modulates the Secretory Activity of IFN-DC and IL4-DC: A Study in Cells from Healthy Donors and MS Patients. International Journal of Molecular Sciences. 2023; 24(7):6717. https://doi.org/10.3390/ijms24076717
Chicago/Turabian StyleSanseverino, Isabella, Arturo Ottavio Rinaldi, Cristina Purificato, Antonio Cortese, Enrico Millefiorini, and Maria Cristina Gauzzi. 2023. "1,25(OH)2D3 Differently Modulates the Secretory Activity of IFN-DC and IL4-DC: A Study in Cells from Healthy Donors and MS Patients" International Journal of Molecular Sciences 24, no. 7: 6717. https://doi.org/10.3390/ijms24076717
APA StyleSanseverino, I., Rinaldi, A. O., Purificato, C., Cortese, A., Millefiorini, E., & Gauzzi, M. C. (2023). 1,25(OH)2D3 Differently Modulates the Secretory Activity of IFN-DC and IL4-DC: A Study in Cells from Healthy Donors and MS Patients. International Journal of Molecular Sciences, 24(7), 6717. https://doi.org/10.3390/ijms24076717





